ABSTRACT
The spikelet is the basic unit of the grass inflorescence. In this study, we show that wheat MADS-box genes VRN1, FUL2 and FUL3 play critical and redundant roles in spikelet and spike development, and also affect flowering time and plant height. In the vrn1ful2ful3-null triple mutant, the inflorescence meristem formed a normal double-ridge structure, but then the lateral meristems generated vegetative tillers subtended by leaves instead of spikelets. These results suggest an essential role of these three genes in the fate of the upper spikelet ridge and the suppression of the lower leaf ridge. Inflorescence meristems of vrn1ful2ful3-null and vrn1ful2-null remained indeterminate and single vrn1-null and ful2-null mutants showed delayed formation of the terminal spikelet and increased number of spikelets per spike. Moreover, the ful2-null mutant showed more florets per spikelet, which together with a higher number of spikelets, resulted in a significant increase in the number of grains per spike in the field. Our results suggest that a better understanding of the mechanisms underlying wheat spikelet and spike development can inform future strategies to improve grain yield in wheat.
KEY WORDS: Wheat, Spike development, Spikelet, Meristem identity, MADS-box
Summary: The wheat MADS-box proteins VRN1, FUL2 and FUL3 are essential for the initial development of the lateral and terminal spikelets, and control the number of spikelets per spike.
INTRODUCTION
The grass family (Poaceae) has approximately 10,000 species, including important food crops such as rice, maize, sorghum, barley and wheat (Kellogg, 2001). The flowers of these species are organized in a unique and diagnostic structure called spikelet (literally ‘little spike’), which is a compact inflorescence developing within the larger inflorescence (Malcomber et al., 2006). A spikelet typically has two sterile bracts (called glumes) enclosing one or more florets. Each floret includes a carpel, three or six stamens and two modified scales (called lodicules), all subtended by two bract-like organs, the palea and the lemma (Preston et al., 2009).
Grass inflorescences have been described as a progressive acquisition of different meristem identities that begins with the transition of the vegetative shoot apical meristem (SAM) to an inflorescence meristem (IM). The IM generates lateral primary branch meristems (PBMs) and secondary branch meristems (SBM) that terminate into spikelet meristems (SMs), which generate glumes and lateral floral meristems (FMs) (McSteen et al., 2000). This model has been a useful phenomenological description but is too rigid to explain some grass branching mutants, so a more flexible model is emerging in which meristem fate is regulated by the genes expressed at discrete signal centers localized adjacent to the meristems (Whipple, 2017).
In wheat, shortening of the inflorescence branches results in spikelets attached directly to the central axis or rachis and the formation of a derived inflorescence, a spike in which spikelets are arranged alternately in opposite vertical rows (a distichous pattern) (Kellogg et al., 2013). In the initial stage, the IM generates a double-ridge structure in which the lower leaf ridges are suppressed and the upper ridges acquire SM identity and form spikelets. The number of spikelets per spike is determined by the number of lateral meristems formed before the transition of the IM into a SM to form the terminal spikelet. In wheat, the growth of the spike is determinate, but the growth of each spikelet is indeterminate, with each SM initiating a variable number of FMs (Ciaffi et al., 2011). The numbers of spikelets per spike and florets per spikelet determine the maximum number of grains per spike and are important components of wheat grain yield potential.
Studies in Arabidopsis, which has a simpler inflorescence than grasses (Malcomber et al., 2006), have shown that MIKC-type MADS-box transcription factors APETALA1 (AP1), CAULIFLOWER (CAL) and FRUITFULL (FUL) are critical in the determination of floral meristem identity. In the triple ap1calful mutant, the IM is not able to produce flowers and reiterates the development of leafy shoots (Ferrándiz et al., 2000). MIKC-type MADS-box proteins have a highly conserved MADS DNA-binding domain, an intervening (I) domain, a keratin-like (K) domain, and a C-terminal domain (C). These proteins bind as dimers to DNA sequences named ‘CArG’ boxes, and organize in tetrameric complexes that can recognize different CArG boxes. The multimeric nature of these complexes generates a large number of combinatorial possibilities with different targets and functions (Honma and Goto, 2001; Theissen et al., 2016).
In rice, combined loss-of-function mutations in MADS14 and MADS15 resulted in inflorescences with leaf-like organs on top of the primary branches (Wu et al., 2017). Simultaneous knockdown of rice MADS14, MADS15 and MADS18 in a pap2 mutant background (PAP2 is also known as MADS34) eliminated the formation of primary branches, and resulted in the formation of lateral vegetative tillers subtended by leaves (Kobayashi et al., 2012). The wheat orthologs of rice MADS14, MADS15 and MAD18 are VERNALIZATION 1 (VRN1), FUL2 and FUL3, respectively. A phylogenetic analysis of the proteins encoded by these genes (Fig. S1) indicates that the Arabidopsis and grass proteins have independent sub-functionalization stories (Preston and Kellogg, 2006). In the grass lineage, the VRN1 and FUL2 clades are closer to each other than to the FUL3 clade (Preston and Kellogg, 2006). Mutations causing large truncations in the proteins encoded by the two VRN1 homeologs in tetraploid wheat delayed heading time, but did not alter spikelet morphology or the ability of flowers to form viable grains (Chen and Dubcovsky, 2012). Since FUL2 and FUL3 are the closest paralogs of VRN1, we hypothesized that they could have redundant spikelet and floral meristem identity functions.
In this study, we combined loss-of-function mutants for the two homeologs of VRN1, FUL2 and FUL3 to generate double- and triple-null mutants in the same tetraploid background. Characterization of these mutants revealed that VRN1, FUL2 and FUL3 have overlapping roles in the regulation of flowering time and stem elongation and, more importantly, that they play critical and redundant roles in spikelet development, suppression of the lower leaf ridge and spike determinacy. Individual vrn1 and ful2 mutants showed significant increases in the number of spikelets and grains per spike, suggesting that manipulations of these genes may contribute to increasing wheat grain yield potential.
RESULTS
Combination of loss-of-function mutations in VRN1, FUL2 and FUL3
We identified point mutations in the A and B genome homeologs of FUL2 and FUL3 in an ethyl methane sulfonate (EMS)-mutagenized population of the tetraploid spring wheat variety Kronos (Krasileva et al., 2017; Uauy et al., 2009). We selected mutations that generated premature stop codons or modified splice sites. The proteins encoded by these mutant alleles are predicted to have large deletions or complete truncations of the K and C domains (Fig. S2; Materials and Methods) and, therefore, are most likely not functional. We backcrossed each individual mutant two to three times into a Kronos vrn-2 null background (Distelfeld et al., 2009b) to reduce background mutations. This genetic background was used to avoid the extremely late flowering of plants carrying the vrn1-null mutation in the presence of the functional VRN2 flowering repressor (Chen and Dubcovsky, 2012). All mutants described in this study are in Kronos vrn2-null background, which is referred to as ‘Control’ in all figures.
We intercrossed the mutants in the A and B homeologs for each gene and selected plants homozygous for both mutations. For simplicity, mutants with loss-of-function mutations in both homeologs will be referred to as null mutants (e.g. vrn1-null). The ful2-null and ful3-null mutants were crossed with vrn1-null (Chen and Dubcovsky, 2012) to generate vrn1ful2-null and vrn1ful3-null mutants, which were intercrossed to generate all eight homozygous VRN1, FUL2 and FUL3 allele combinations including the triple vrn1ful2ful3-null mutant. These eight genotypes were analyzed for stem length (Fig. 1A) and number of leaves (Fig. 1B) using three-way factorial ANOVAs (Fig. 1C).
Fig. 1.
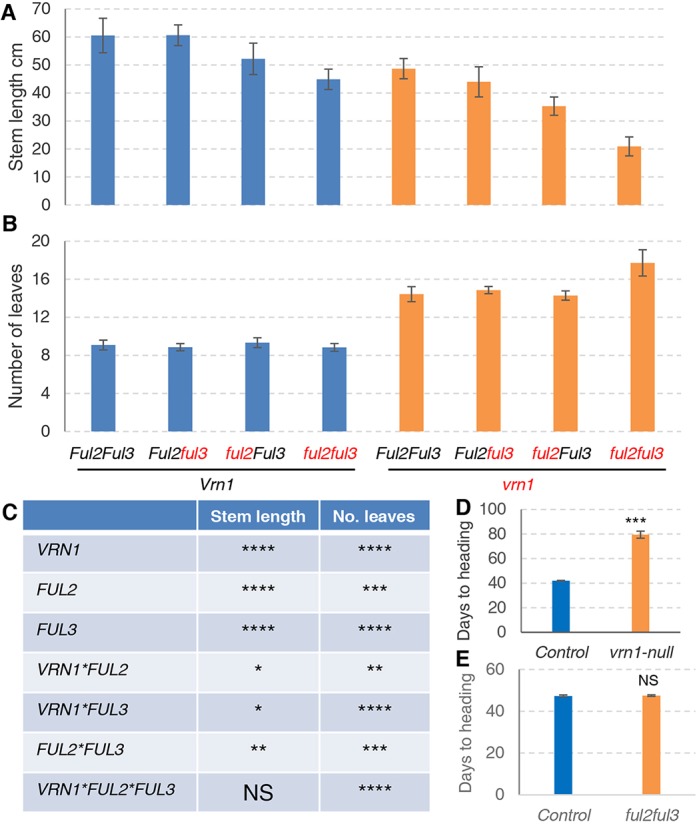
Effect of VRN1, FUL2 and FUL3 on stem length, leaf number and heading time. Kronos plants (vrn2-null background) grown under long-day photoperiod. Stem length was determined from the base of the plant to the base of the spike. (A) Stem length in cm (n=6-12). (B) Number of true leaves (n=6-12). Alleles in red indicate homozygous null mutants and alleles in black homozygous wild-type alleles. (C) P-values from three-way ANOVAs for stem length and leaf number including all eight homozygous VRN1, FUL2 and FUL3 allele combinations (n=59). *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; NS, P>0.05. (D) Heading time of vrn1-null (n=6) versus control (n=6). (E) Heading time of ful2ful3-null (n=15) vs control (n=10) in a Vrn1 background. D and E are separate experiments. Error bars are s.e.m. ***P<0.001; NS, P>0.05, calculated by unpaired, two-tailed t-tests.
VRN1, FUL2 and FUL3 loss-of-function mutations reduce stem elongation
Since some mutant combinations lack real spikes, we determined final stem length from the base of the plant to the base of the spike (or spike-like structure) instead of total plant height. Plants carrying only the ful3-null mutation showed no significant reduction in stem length, but those carrying the vrn1-null or ful2-null mutations were 20% and 14% shorter than the control, respectively (Fig. 1A). A three-way factorial ANOVA for stem length revealed highly significant effects for all three genes (Fig. 1C). All three double-mutant combinations had shorter stems than predicted from combined additive effects of the individual mutations, which was reflected in significant synergistic interactions (Fig. 1C). Taken together, these results indicate that VRN1, FUL2 and FUL3 have redundant roles in the regulation of stem elongation, and that the effect of the individual genes is larger in the absence of the other paralogs.
VRN1, FUL2 and FUL3 mutations delay flowering time
Functional redundancy among VRN1, FUL2 and FUL3 was also observed for heading time. The vrn1-null mutant headed 37.5 days later than the control (Fig. 1D), but differences in heading time for the ful2-null, ful3-null and ful2ful3-null mutants in the presence of the strong Vrn-A1 allele were non-significant (Fig. 1E). For the vrn1ful2-null and vrn1ful2ful3-null mutants, it was not possible to determine heading time accurately because they had short stems and abnormal spikes that interfere with normal ear emergence. Instead, we determined the final number of leaves (Fig. 1B) and the timing of the transition between the vegetative and double-ridge stages (Fig. S3).
A three-way factorial ANOVA for leaf number revealed highly significant effects for the three individual genes, as well as for all the two- and three-way interactions (Fig. 1C). The vrn1-null mutant had on average 14.4 leaves (59%>control; Fig. 1B), which was consistent with its later heading time (Fig. 1D). Similar leaf numbers were detected in vrn1ful2-null (14.3) and vrn1ful3-null (14.9), but the triple vrn1ful2ful3-null mutant had on average 17.7 leaves (Fig. 1B), which was consistent with the 9- to 12-day delay in the transition between the vegetative SAM and the double-ridge stage relative to the vrn1-null control (Fig. S3). These results indicate that FUL3 has a residual ability to accelerate flowering in the absence of VRN1 and FUL2.
Transgenic Kronos plants overexpressing the coding regions of FUL2 fused with a C-terminal 3×HA tag (henceforth Ubi::FUL2; Fig. S4A, events #1 and #6) or FUL3 fused with a C-terminal 4×MYC tag (henceforth Ubi::FUL3; Fig. S4B, events #4 and #5) headed 2-4 days earlier than the non-transgenic sister lines (P<0.0001). The effect of Ubi::FUL2 was further characterized in the F2 progeny from the cross between Ubi::FUL2 (Vrn1Vrn2) and vrn1vrn2-null under greenhouse conditions. A three-way factorial ANOVA for heading time showed significant effects for VRN1, Ubi:FUL2 and VRN2 and for all two- and three-way interactions (P<0.0001, Table S3). In the presence of a functional VRN2 allele, the differences in heading time between FUL2 wild type (FUL2-wt) and Ubi::FUL2 alleles were small in lines homozygous for the functional VRN1 allele (2.6 days; Fig. S4A), intermediate in VRN1 heterozygous lines (11.1 days; Fig. S4C) and large in homozygous vrn1-null mutants (53 days; Fig. S4D). These results indicate that the effect of the Ubi::FUL2 transgene on heading time depends on the particular VRN1 and VRN2 alleles present in the genetic background (Fig. S4C,D).
In summary, the strong effect of VRN1 in the acceleration of wheat flowering time can mask the smaller effects of FUL2 and FUL3, but in the absence of VRN1, both FUL2 and FUL3 have redundant effects on accelerating wheat flowering time.
Flowering delays in VRN1, FUL2 and FUL3 mutants are associated with reduced FT1 transcript levels in leaves
Since there is a known positive feedback regulatory loop between VRN1 and FT1 (Shaw et al., 2019), we compared the FT1 transcript levels in the leaves of the different VRN1, FUL2 and FUL3 mutant combinations. FT1 transcript levels higher than ACTIN were observed in leaves of 4-week-old plants carrying the Vrn1 wild-type allele, but were detected only after 10 weeks in plants carrying the vrn1-null allele (Fig. S5A,B). This result is consistent with the large differences in heading time between these genotypes (Fig. 1D). FT1 transcript levels in the 10-week-old vrn1-null plants was highest in the presence of the FUL2 and FUL3 wild-type alleles and lowest in the triple mutant (Fig. S5C), consistent with the higher number of leaves in this genotype (Fig. 1B). Even in the vrn1ful2ful3-null plants, FT1 transcript levels increased above ACTIN in 14-week-old plants (Fig. S5D). Taken together, these results indicate that FT1 expression levels in the leaves are positively regulated by VRN1, FUL2 and FUL3, but that they can also be upregulated in the absence of all three of these genes.
VRN1, FUL2 and FUL3 play critical and redundant roles in spikelet development
Plants with individual vrn1-null, ful2-null and ful3-null mutations produced normal spikelets and flowers, but vrn1ful2-null or vrn1ful2ful3-null mutants had spike-like structures in which all lateral spikelets were replaced by leafy shoots (Fig. 2A-J), henceforth referred to as ‘inflorescence tillers’. Removal of these inflorescence tillers revealed a thicker and shorter rachis with fewer internodes of variable length, but still retaining the characteristic alternating internode angles typical of a wild-type rachis (Fig. 2B).
Fig. 2.
Phenotypic characterization of the vrn1ful2 and vrn1ful2ful3 mutants. (A) Stems and heads of vrn1-null, vrn1ful2-null and vrn1ful2ful3-null mutants (leaves were removed before photography). (B) Rachises of the different mutants. Arrows indicate the position of the first spikelet before removal. (C-G) vrn1ful2-null mutant. (C) Spike-like structure. Arrow points to the bract subtending a basal inflorescence tiller. (D) Spike-like structure after removal of the inflorescence tillers to show subtending bracts (arrows). (E) Dissection of an inflorescence tiller showing two glumes and one lemma partially transformed into leaves, followed by four leaves. The inset with yellow border shows the meristem transition into an IM with lateral VMs. (F) Detail of white rectangle in E revealing an ovary, two anthers, and leafy-lemma and palea. (G) Leafy palea and lodicules subtending one anther and two ovaries. (H-J) vrn1ful2ful3-null mutant. (H) Normal leaves L11 to L18 with no axillary buds. L19 marks the beginning of the spike-like structure in which spikelets have been replaced by tillers subtended by leaves (L19 and L20) or bracts. (I,J) Detail of the tillers subtended by L19 (I) and L20 (J). Insets in white rectangles are the SAM of these tillers (transitioning into IM with lateral VM) and the yellow rectangle presents the exhausted IM.
In vrn1ful2-null mutants, ∼70% of the central inflorescence tillers had leafy glumes, lemmas and paleas and abnormal floral organs, whereas the rest were fully vegetative. Floral abnormalities included leafy lodicules, reduced number of anthers, anthers fused to ovaries, and multiple ovaries (Fig. 2E-G). After the first modified floret, meristems from these inflorescence tillers developed 2-5 true leaves before transitioning again to an IM generating lateral VMs (Fig. 2E). The combined presence of floral organs and leaves suggests that the originating meristem had an intermediate identity between VM and SM before transitioning to an IM. In the vrn1ful2-null double mutant the inflorescence tillers were subtended by bracts (Fig. 2C,D).
In vrn1ful2ful3-null mutants, the lateral meristems generated inflorescence tillers that had no floral organs, and that were subtended by leaves in the basal positions and bracts in more distal positions (Fig. 2H-J). The presence of well-developed axillary tillers in these basal inflorescence leaves (Fig. 2H, L19 and L20) marked the border of the spike-like structure, because no axillary tillers or developing buds were detected in the true leaves located below this border (Fig. 2H, L11-L18).
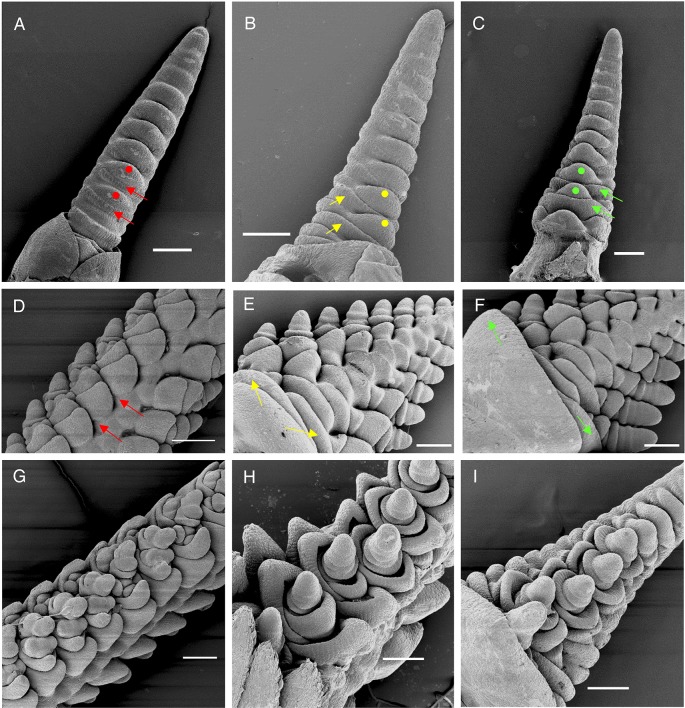
Scanning electron microscopy (SEM) images of the early developing inflorescences in the vrn1ful2-null and vrn1ful2ful3-null mutants revealed elongated double-ridge structures similar to those in Kronos (Fig. 3A) or vrn1-null (Fig. 3C) plants. Suppression of the lower leaf ridge was complete in Kronos (Fig. 3A) and in vrn1-null (Fig. 3D, red arrows), but was incomplete in vrn1ful2-null (Fig. 3B,E, yellow arrows), and even weaker in vrn1ful2ful3-null (Fig. 3C,F, green arrows). As a result of this change, inflorescence tillers were subtended by bracts in vrn1ful2-null (Fig. 2C,D) and by leaves in vrn1ful2ful3-null (Fig. 2H,I). The upper ridges (Fig. 3A-C, dots) transitioned into normal SMs in vrn1-null, with glume and lemma primordia (Fig. 3D,G), but looked like typical vegetative meristems in vrn1ful2-null and vrn1ful2ful3-null plants (Fig. 3E,F,H,I).
Fig. 3.
Scanning electron microscopy images. Early double-ridge stage (A-C) and later stage (D-I) showing the fate of the lateral meristems. (A) Kronos control. (D,G) vrn1-null control. Red arrows indicate the repressed lower leaf ridge, and red dots the upper ridges that develop into normal spikelets (D,G). (B,E,H) vrn1ful2-null mutants. Yellow arrows indicate the partially repressed lower leaf ridges that develop into bracts (see Fig. 2D) and yellow dots indicate the upper ridges that develop into intermediate meristems that generate tiller-like structures with altered floral organs (see Fig. 2E-G). (C,F,I) vrn1ful2ful3-null mutants. Green arrows indicate basal lower leaf ridges that develop into normal leaves (see Fig. 2H) and green dots indicate upper ridges that produce lateral vegetative meristems that generate vegetative tillers with no floral organs (see Fig. 2I-J). Scale bars: 200 µm.
To characterize better the relative effects of VRN1 and FUL2, we examined their individual effects when present as a single functional copy in a heterozygous state (underlined). Both ful2-null/Vrn-A1 vrn-B1-null (functional Vrn-A1 allele for spring growth habit) and ful2-null/vrn-A1-null vrn-B1 (functional vrn-B1 allele for winter growth habit) produced spike-like structures with leafy lateral shoots (Fig. S6A,B) and normal floral organs (Fig. S6C) but no viable seeds. The developing spikes of these plants showed lateral meristems with floral primordia (Fig. S6D), some of which later developed into spikelets with an elongated axis (rachilla) and leafy organs (Fig. S6E-G). By contrast, the presence of a single heterozygous copy of FUL2 (vrn1-null/ful-A2-null Ful-B2) was sufficient to generate more normal-looking spikelets (Fig. S6H-J), some of which were able to set viable seeds. Abnormal spikelets (Fig. S6I) and basal branches with lateral spikelets and fertile florets (Fig. S6J) were also observed in this mutant. Taken together, these results indicate that VRN1, FUL2 and FUL3 have redundant and essential roles in spikelet development, with FUL2 having the strongest effect and FUL3 the weakest.
Transcript levels of SHORT VEGETATIVE PHASE (SVP)-like genes VRT2, BM1 and BM10 are upregulated in the developing spikes of the vrn1ful2-null mutant
A partial reversion of basal spikelets to vegetative tillers, similar to the one described above for the vrn1ful2-null mutant, has been described in barley lines overexpressing SVP-like genes BM1 or BM10 (Trevaskis et al., 2007). To test whether transcript levels of the SVP-like wheat genes were affected in the vrn1ful2-null mutants, we first determined their expression during normal spike development in Kronos. Transcript levels of the three related wheat paralogs BM1, BM10 and VRT2 (RefSeq v1.1 gene designations in Fig. S7) decreased three- to five-fold from the initial stages of spike development (W2, Waddington scale) to the floret primordium stage (W3.5) (Fig. S7A-C).
Next, we compared the transcriptional levels of the SVP-like wheat genes in vrn1ful2-null and vrn1-null mutants. Plants were grown for 53 days in a growth chamber until the developing spikes of vrn1-null reached the terminal spikelet stage and those from vrn1ful2-null had a similar number of lateral meristems. The transcript levels of BM1, BM10 and VRT2 in the developing spikes were roughly ten-fold higher in the vrn1ful2-null mutant than in the vrn1-null and control lines (P<0.0001; Fig. S7D-F). These results suggest that VRN1 and FUL2 are either direct or indirect transcriptional repressors of the three wheat SVP-like genes.
FUL2 and VRN1 have redundant roles in spike determinacy and regulate the number of spikelets per spike
Normal wheat spikes are determinate, with the distal IM transitioning into a terminal spikelet after producing a relatively stable number of lateral meristems (Fig. 4A). In vrn1ful2-null, by contrast, the IM was indeterminate (Fig. 4B) and continued to produce lateral meristems while growing conditions were favorable and eventually died without producing any terminal structure. In the ful2-null background, one functional copy of VRN1 in the heterozygous state was sufficient to generate a determinate spike (Fig. S6D, ful2-null/vrn-A1-null vrn-B1), and the same was true for a single functional copy of FUL2 in a vrn1-null background (Fig. S6K, vrn1-null/full-A2-null Ful-B2).
Fig. 4.
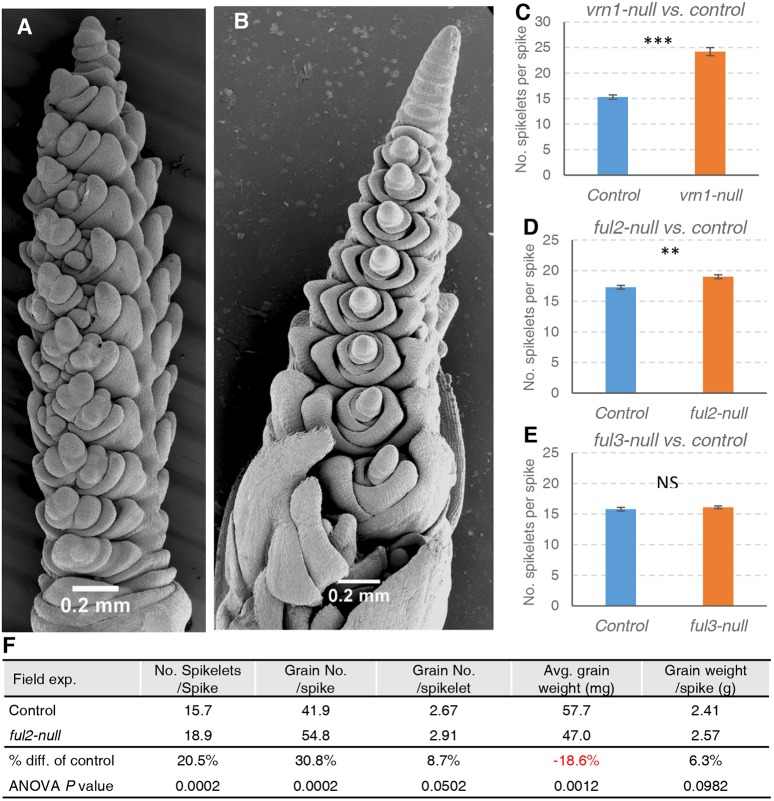
VRN1 and FUL2 play redundant roles in the control of spike determinacy and spikelet number. (A) Scanning electron microscopy of a normal wheat spike with a terminal spikelet in the vrn1-null mutant. (B) vrn1ful2-null mutant spike with indeterminate apical meristem. (C-E) Number of spikelets per spike in a growth chamber experiment (n=6). (C) vrn1-null (58% increase over control), (D) ful2-null (10% increase) and (E) ful3-null (no significant increase). Bars represent mean±s.e.m. and asterisks indicate statistically significant difference to the control line. **P<0.01; ***P<0.001; NS, P>0.05, calculated by unpaired, two-tailed t-test. (F) ANOVA averages and P-values for spike traits in ful2-null and sister control lines in the field (randomized complete block design with 8 blocks).
The individual vrn1-null and ful2-null homozygous mutants showed a larger number of spikelets per spike than the control. This increase was 58% in the vrn1-null mutant (P<0.0001; Fig. 4C) and 10% in the ful2-null mutant (P=0.0014; Fig. 4D). Although no significant increases in the number of spikelets per spike were detected in the ful3-null mutant (P=0.4096; Fig. 4E), two independent transgenic lines overexpressing FUL3 (Ubi::FUL3) showed an average reduction of 1.12 spikelets per spike relative to their non-transgenic sister lines (P=0.0132 and P<0.0001; Fig. S8A), which indicates that FUL3 does play a role in the timing of the transition from IM to terminal spikelet.
A similar reduction in the number of spikelets per spike was observed in two independent Ubi::FUL2 transgenic lines (1.05 spikelets per spike reduction, P<0.03; Fig. S8B). We then investigated the effect of this transgene in the presence of different VRN1 and VRN2 alleles in the Ubi::FUL2×vrn1vrn2-null F2 population. In the vrn2-null F2 plants, the differences in spikelet number between Ubi::FUL2 and wild-type alleles were larger in vrn1-null than in the plants heterozygous for VRN-A1 and VRN-B1 (Vrn1-Het) (interaction P<0.0001; Fig. S8C). In a separate group of F2 plants fixed for Vrn1-Het and segregating for VRN2 and FUL2, we did not detect significant effects for Ubi::FUL2 and the interaction was not significant (Fig. S8D). However, we observed 3.3 more spikelets per spike in plants carrying the Vrn2-wt allele than in those with the vrn2-null allele (P<0.0001; Fig. S8D). These results suggest that the strong Vrn-A1 allele for spring growth habit can mask the effects of the Ubi::FUL2 transgene but not those of VRN2 on the number of spikelets per spike.
Increased transcript levels of CEN2, CEN4 and CEN5 in developing spikes of the vrn1ful2-null mutant
Based on the strong effect observed in the Arabidopsis tfl1 mutant and the Antirrhinum cen mutant on inflorescence determinacy (Bradley et al., 1997; Ratcliffe et al., 1999), we investigated the effect of the vrn1ful2-null mutations on the expression levels of the TFL1/CEN-like wheat homologs in developing spikes. Since no previous nomenclature was available for the wheat CEN paralogs, we assigned them numbers to match their chromosome locations, and designated them as CEN2, CEN4 and CEN5 (RefSeq v1.1 designations can be found in the legend of Fig. S9). The transcript levels of these three genes were downregulated as the developing spike progressed from the double-ridge stage to the floret primordium stage (Waddington scale 2 to 3.5) (Fig. S9A-C).
Comparison of vrn1ful2-null and vrn1-null plants grown for 53 days in the same growth chamber showed that the transcript levels of CEN2, CEN4 and CEN5 were significantly higher (P<0.0001) in the developing spikes of the vrn1ful2-null mutant than in those of the vrn1-null mutant or the Kronos control (all in vrn2-null background). These differences were larger for CEN2 and CEN4 than for CEN5 (Fig. S9D-F). Taken together, these results suggested that VRN1 and FUL2 work as transcriptional repressors of the TFL1/CEN-like wheat homologs.
The ful2-null mutant produces a higher number of florets per spikelet and more grains per spike in the field
In addition to the higher number of spikelets per spike, the ful2-null mutant produced a higher number of florets per spikelet than the Kronos control, an effect that was not observed for vrn1-null (Fig. 2A) or ful3-null (Fig. S10A). The mean increase in floret number was similar in ful2-null (1.3 florets) and ful2ful3-null (0.9 florets), suggesting that FUL3 has a limited effect on this trait. In spite of some heterogeneity in the distribution of spikelets with extra florets among spikes, the differences between the control and the ful2-null mutants were significant at all spike positions (Fig. S10B).
Similar increases in the number of florets per spikelet have previously been reported in Kronos plants overexpressing miRNA172 under the UBIQUITIN promoter (Debernardi et al., 2017). To study the genetic interactions between Ubi::miR172 and ful2-null we crossed the transgenic and mutant lines and studied their effects on floret number in the progeny using a two-way factorial ANOVA. We detected significant differences in mean floret number for both ful2-null and Ubi::miR172 (P<0.01) and a marginally significant interaction (P<0.0435) that can be visualized in the interaction graph in Fig. S10C. The differences in average floret number between ful2-null and the wild-type control were larger (and more variable) in the Ubi::miR172 than in the non-transgenic background (Fig. S10C). This synergistic interaction suggests that miRNA172 and FUL2 may control floret number through a common pathway. Both the mutant and transgenic lines showed heterogeneity among spikes in the location of spikelets with increased numbers of florets (Fig. S10D-F).
Based on its positive effect on the number of florets per spikelet and spikelets per spike (and its small effect on heading time), we selected the ful2-null mutant for evaluation in a replicated field experiment. Relative to the control, the ful2-null mutant produced 20% more spikelets per spike (P=0.0002) and 9% more grains per spikelet (P=0.05), which resulted in a 31% increase in the number of grains per spike (P=0.0002; Fig. 4F). Although part of the positive effect on grain yield was offset by a 19% reduction in average kernel weight (P=0.0012), we observed a slight net increase of 6% in total grain weight per spike (P=0.09; Fig. 4F). This negative correlation between grain number and grain weight suggests that in this particular genotype by environment combination grain yield was more limited by the ‘source’ (produced and transported starch) than by the ‘sink’ (number and size of grains).
DISCUSSION
Results from this study have shown that wheat VRN1, FUL2 and FUL3 genes have overlapping functions in stem elongation, flowering time and spike development, which are discussed separately in the following sections.
Mutations in VRN1, FUL2 and FUL3 reduce stem elongation
We detected highly significant effects of VRN1, FUL2 and FUL3 on plant height and significant synergistic interactions (Fig. 1A,C). These results suggest that VRN1, FUL2 and FUL3 have redundant functions in the regulation of stem elongation, and that their individual effects are magnified in the absence of the other paralogs. Significant reductions in plant height have also been reported for rice mutants mads14 (12.2% reduction) and mads15 (9.0% reduction), and the double mutant (43.8% reduction), suggesting a conserved function in grasses (Wu et al., 2017).
Although the molecular mechanisms by which these genes affect stem elongation are currently unknown, an indirect way by which they may contribute to this trait is through their strong effect on the regulation of FT1 (Fig. S5), which is associated with the upregulation of gibberellic acid (GA) biosynthetic genes in the developing spike (Pearce et al., 2013). A recent study has shown that rice HEADING DATE 3 (Hd3) and RICE FLOWERING LOCUS T 1 (RFT1), the orthologs of wheat FT1, can increase stem responsiveness to GA by reducing PREMATURE INTERNODE ELONGATION 1 (PINE1) expression in the SAM and compressed stem (Gómez-Ariza et al., 2019). In Arabidopsis, a number of genes involved in hormone pathways are direct targets of FUL, which may explain the shorter stem and internodes detected in the ful mutant (Bemer et al., 2017). A characterization of the direct DNA targets and protein interactors of VRN1, FUL2 and FUL3 may shed light on the mechanisms responsible for the conserved role of these genes in plant height in grasses.
Mutations in VRN1, FUL2 and FUL3 delay flowering initiation in wheat
VRN1 is one of the main genes controlling natural variation in wheat flowering time (Fu et al., 2005; Kippes et al., 2016; Yan et al., 2003; Zhang et al., 2008), so it was not surprising that vrn1-null delayed heading time more than ful2-null or ful3-null. Although the strong Vrn-A1 allele for spring growth habit masked the smaller effects of FUL2 and FUL3 (Fig. 1A-C), in the vrn1-null background, the ful2-null and ful3-null mutants showed delayed flowering initiation and increased number of leaves (Fig. 1B,F), indicating that FUL2 and FUL3 have retained some residual functionality in the acceleration of wheat flowering time. This was further confirmed by the accelerated flowering of the Ubi::FUL2 and Ubi::FUL3 transgenic plants (Fig. S4A,B). Similar results have been reported in Brachypodium distachyon and rice. In Brachypodium, overexpression of VRN1 (Ream et al., 2014), FUL2 or FUL3 (Li et al., 2016) accelerates flowering, and downregulation of VRN1 delays flowering relative to non-transgenic controls (Woods et al., 2016). In rice, overexpression of MADS15 also accelerates flowering (Lu et al., 2012). These results suggest a conserved role of these genes in the regulation of flowering time in grasses.
Previous studies have shown a significant genetic interaction between wheat VRN1 and VRN2 in the regulation of heading time (Tranquilli and Dubcovsky, 2000). This study shows that similar interactions exist between FUL2 and VRN2 (Fig. S4C,D). A tetraploid wheat population segregating for VRN1, FUL2 and VRN2 revealed highly significant two-way and three-way interactions among these genes, indicating that the effect of each of these genes on heading time is dependent on the particular combination of alleles present for the other two. Previous studies have shown that part of the ability of VRN1 to accelerate flowering depends on its ability to repress VRN2 (Chen and Dubcovsky, 2012). The larger effect on heading time of the Ubi::FUL2 transgene in the presence of functional Vrn2 allele than in the vrn2-null background (Fig. S4C,D) suggests that FUL2 repression of VRN2 can also contribute to its ability to accelerate heading time.
Interestingly, mutations in VRN1, FUL2 and FUL3 were associated with delayed induction of FT1 even in the absence of functional VRN2 alleles (Fig. S5). Lines carrying the wild-type VRN1 allele showed high levels of FT1 in the leaves six weeks earlier than lines carrying the vrn1-null allele and flowered 37 days earlier. Among the lines carrying the vrn1-null allele, those with mutations in both FUL2 and FUL3 showed the latest induction of FT1 (Fig. S5C,D) and had 3.3 more leaves (excluding leaves in the spike-like structure; Fig. 1B). However, in 14-week-old vrn1ful2ful3-null plants, FT1 transcripts still reached higher levels than ACTIN. These results indicate that VRN1, FUL2 and FUL3 are positive transcriptional regulators of FT1 but they are not essential for its expression in the leaves.
FT1 has also been shown to be a positive regulator of VRN1 expression in both leaves and SAM. Natural variation or transformation experiments that affect FT1 transcript levels in the leaves are always associated with parallel changes in VRN1 expression (Lv et al., 2014; Yan et al., 2006). Taken together, these results suggest the existence of a positive regulatory feedback loop in which each gene acts as a positive regulator of the other. Although this feedback loop can be mediated in some cases by VRN2 (Distelfeld et al., 2009a), results from this study and from Shaw et al. (2019) in a vrn2-null background suggest the existence of a positive feedback loop that operates independently of VRN2. This hypothesis is supported by the ability of the FT1–FDL2–14-3-3C protein complex to bind to the wheat VRN1 promoter in vitro (Li et al., 2015) and of VRN1 to bind to the FT1 promoter in ChIP-seq experiments (Deng et al., 2015). Studies in rice have suggested the possibility of a similar regulatory feedback loop between the orthologous genes (Kobayashi et al., 2012).
VRN1, FUL2 and FUL3 play critical and redundant roles in spikelet development
RNA in situ hybridization studies at the early stages of inflorescence development in Lolium temulentum (Gocal et al., 2001), wheat and oat (Preston and Kellogg, 2008), and early-diverging grasses (Preston et al., 2009) have shown VRN1 and FUL2 expression in the IM, lateral SMs and FMs. Similarly, transgenic barley plants transformed with a VRN-H1 promoter fused with GFP showed fluorescence in the three meristems (Alonso-Peral et al., 2011). VRN1, FUL2 and FUL3 can interact with different MADS-box partners in the IM, SMs and FMs and, therefore, mutations in these genes can alter different functions in different meristems.
The significant effects of VRN1, FUL2 and FUL3 on the timing of the transitions from the vegetative SAM to IM and from IM to terminal spikelet indicate that these genes play important roles in the acquisition and termination of IM identity. During the early stages of spike development both vrn1ful2-null and vrn1ful2ful3-null mutants showed an elongated double-ridge structure with lateral meristems organized in a distichous phyllotaxis that were similar to the Kronos control (Fig. 3A-C), and both mutants had a rachis similar to a normal spike rachis (Fig. 2B). These results suggest that these IM functions were not disrupted by the combined mutations in VRN1, FUL2 and FUL3.
After the double-ridge stage, the development of the lateral meristems diverged drastically in vrn1ful2-null and vrn1ful2ful3-null mutants relative to the vrn1-null control. In vrn1-null, the upper ridges transitioned into SMs that generated normal spikelets, whereas in vrn1ful2ful3-null they transitioned into lateral VMs that generated inflorescence tillers (which we interpret as the default identity of an axillary meristem). The vrn1ful2-null mutant generated an intermediate structure that produced both leafy-floral organs and leaves. Based on these results we concluded that VRN1, FUL2 and FUL3 play essential and redundant roles in spikelet and floral development. However, we currently do not know whether the transition of the upper ridges to SMs requires the expression of functional VRN1, FUL2 and FUL3 proteins in the upper ridge, in the IM, or in both.
Replacement of basal spikelets with inflorescence tillers similar to the ones described for vrn1ful2ful3-null was observed in barley plants overexpressing BM1 and BM10 (Trevaskis et al., 2007). These two genes, together with VRT2, are related to the Arabidopsis MADS-box genes SVP and AGAMOUS-LIKE 24 (AGL24), which play important roles in the formation of floral meristems (Kaufmann et al., 2010; Liu et al., 2007). In Arabidopsis, SVP and AGL24 are directly repressed by AP1 (Kaufmann et al., 2010). In the absence of AP1, ectopic expression of SVP and AGL24 transformed floral meristems into shoot meristems (Liu et al., 2007). The upregulation of VRT2, BM1 and BM10 in the vrn1ful2-null developing wheat spikes (Fig. S7), together with the transgenic barley results, suggest that these genes may have contributed to the observed replacement of spikelets by vegetative tillers in vrn1ful2-null plants.
Both vrn1ful2-null and vrn1ful2ful3-null mutants showed reduced suppression of the lower leaf ridge
An important characteristic of a grass IM is the complete suppression of the lower leaf ridge subtending all branching events, which in the wheat spike is the suppression of the lower leaf ridge subtending the spikelet. This suppression was disrupted in the vrn1ful2-null and vrn1ful2ful3-null mutants, which developed bracts or leaves subtending the inflorescence tillers (Fig. 2C,D,H-J). These results suggest that all three genes contribute to the suppression of the lower leaf ridge, but we do not know whether this suppression requires the expression of VRN1, FUL2 and FUL3 in the lateral meristem, in the IM, or in both. In this case, an indirect IM effect seems more likely because in situ hybridization studies have detected VRN1 and FUL2 (or their grass orthologs) in the upper ridge rather than in the lower leaf ridge (Gocal et al., 2001; Preston and Kellogg, 2008; Preston et al., 2009). However, a more direct effect on the lateral meristem cannot be ruled out because a VRN1–GFP fusion driven by the VRN1 promoter was detected in the lower leaf ridge of the developing spike in barley (Alonso-Peral et al., 2011).
The inflorescence tillers subtended by leaves in vrn1ful2ful3-null were not very different from vegetative tillers, but there was a difference that marked a clear boundary between them. In rice and wheat, true leaves do not show axillary buds or tillers until 4-5 younger leaves are formed (Friend, 1965; Oikawa and Kyozuka, 2009). Then, bud development into tillers proceeds sequentially from older to younger leaves. By contrast, leaves developed from the lower leaf ridge in vrn1ful2ful3-null (Fig. 3C,F) had axillary meristems (the upper ridge) from the beginning, which rapidly developed into axillary tillers (Fig. 2H, L19 and L20). True leaves below the inflorescence (Fig. 2H, L11-L18) showed no visible axillary buds, which is normal for wheat leaves that subtend an elongated internode (Williams and Langer, 1975). In summary, even in the vrn1ful2ful3-null mutant a clear boundary was established between the inflorescence and the vegetative leaves.
Interpretation of observed meristem identity changes
An inflorescence phenotype similar to the one described here for the vrn1ful2ful3-null wheat mutant has been described for the rice mads14mads15mads18pap2 quadruple knockdown, in which the panicle was replaced by tillers subtended by leaves (Kobayashi et al., 2012). The authors of the rice study interpreted this phenotype as the result of an incomplete transition between the vegetative SAM and the IM, and suggested that these genes act redundantly to promote IM identity and, therefore, are IM identity genes.
In wheat vrn1ful2ful3-null, the changes observed in the lateral meristems can also be explained by postulating an indirect effect of the IM on the regulation of genes expressed in the signaling centers flanking the lateral meristems. However, the same changes can be explained by a more direct effect of non-functional VRN1, FUL2 and FUL3 proteins in the lateral meristem, where they are normally expressed. If this second interpretation is correct, VRN1, FUL2 and FUL3 should be considered to include SM identity functions, in addition to IM and FM identity functions. This proposed SM identity function is consistent with the role of homologous FM identity genes AP1, CAL and FUL in Arabidopsis (Ferrándiz et al., 2000). Regardless of their direct or indirect effect on SM identity, VRN1, FUL2 and FUL3 are essential for spikelet development in both wheat and rice.
This does not seem to be the case for the rice gene PAP2 or its wheat ortholog AGLG1 (Yan et al., 2003). Kobayashi et al. (2012) suggested that the loss of function of this gene was important for the rice mads14mads15mads18pap2 phenotype. However, the complete suppression of spikelets in the presence of functional PAP2/AGLG1 genes in rice mads14mads15 (Wu et al., 2017) and wheat vrn1ful2ful3-null mutants suggests a less critical role for PAP2 in SM identity.
VRN1 and FUL2 have essential and redundant roles in wheat spike determinacy
The determinate growth of the wheat spike is marked by the transition of the distal IM into a SM and the formation of a terminal spikelet. However, the vrn1ful2-null mutants were unable to form spikelets and the IM remained indeterminate. A single functional copy of VRN1 or FUL2 in a heterozygous state was sufficient to restore spike determinacy (Fig. S5D,K), suggesting that the wheat IM is very sensitive to the activity of these genes.
Loss-of-function mutations in TERMINAL FLOWER 1 (TFL1) in Arabidopsis or in the CENTRORADIALIS (CEN) homolog in Antirrhinum result in the formation of a terminal flower and the transformation of indeterminate into determinate inflorescences (Bradley et al., 1997; Ratcliffe et al., 1999). In rice, knockdowns of the four CEN homologs (RCN1-RCN4) reduced the number of branches, whereas their overexpression increased branch number by competing with rice FT homologs (Kaneko-Suzuki et al., 2018; Nakagawa et al., 2002). In wheat, overexpression of CEN-D2 extended the duration of the double-ridge stage and increased the number of spikelets per spike (Wang et al., 2017), whereas loss-of-function mutations in barley CEN2 reduced the number of spikelets per spike (Bi et al., 2019). Based on these results, we hypothesize that the upregulation of the wheat CEN2, CEN4 and CEN5 homologs in the developing spike of the vrn1ful2-null mutant might have contributed to its indeterminate growth.
The vrn1-null and ful2-null mutants have a higher number of spikelets per spike
We showed in this study that the timing of the transition between the IM and the terminal spikelet is modulated by VRN1 and FUL2 and that this affects the number of spikelets per spike. The stronger effect of vrn1-null (nine additional spikelets; Fig. 4C) relative to ful2-null (two additional spikelets; Fig. 4D) is likely associated with VRN1’s stronger effect on heading time (Fig. 1A-C), which provides more time for the formation of additional spikelets. This seems to be also the case in rice, where overexpression of MADS15 resulted in reduced number of primary branches in the panicle (Lu et al., 2012). Similarly, a premature stop codon mutation in a homolog of AP1 in rapeseed altered plant architecture and increased the number of seeds per plant (Shah et al., 2018). Taken together, these results suggest that mutations in this group of meristem identity genes may be useful to modulate seed number in different plant species.
In addition to its effect on spikelet number, the ful2-null mutation was also associated with an increase in the number of florets per spikelet, which suggests that this gene contributes to maintaining a limited number of florets per spikelet (Fig. S10C-F). This effect was not detected in vrn1-null and ful3-null. Since a higher number of florets per spikelet and increased spikelet number can both contribute to increases in grain yield potential, we explored the effect of the ful2-null mutant on grain yield components. In a field study, the ful2-null plants showed a 30.8% increase in the average number of grains per spike compared with the control sister lines. Although in this experiment the positive increase in grain number was partially offset by a decrease in average grain weight, the total grain weight per spike was still slightly higher (6.3%) in the ful2-null mutant relative to the control. It would be interesting to test whether the introgression of this mutation in genotypes with high biomass (increased ‘source’) grown under optimum agronomic conditions can reduce the negative correlation between grain number and grain weight.
In summary, our results indicate that VRN1, FUL2 and FUL3 play redundant and essential roles in spikelet development, repression of the lower leaf ridge and spike determinacy, and that mutations in VRN1 and FUL2 can be used to increase the number of spikelets per spike, an important component of grain yield. These results suggest that a better understanding of the processes that control the development of grass flowers and inflorescences may contribute to improving the productivity of a group of species that is critical for the global food supply.
MATERIALS AND METHODS
Selected mutations and mutant combinations
An ethyl methane sulphonate (EMS) mutagenized population of the tetraploid wheat variety Kronos was screened for mutations initially using CelI assays (Uauy et al., 2009) and later using BLAST searches in the database of sequenced mutations for the same population (Krasileva et al., 2017). We identified loss-of-function mutations in the A and B genome homologs of FUL2 and FUL3, which were confirmed using genome-specific primers described in Table S1. Single-genome mutants were backcrossed two or three times to Kronos vrn2-null to reduce background mutations. The wild-type Kronos carries a functional VERNALIZATION 2 (VRN2) flowering repressor, which results in extremely late flowering in the presence of the vrn1-null mutation (Chen and Dubcovsky, 2012). To avoid this problem all mutants described in this study were developed in a Kronos vrn2-null background (Distelfeld et al., 2009b), unless indicated otherwise.
For FUL-A2, we selected line T4-837 (henceforth ful-A2), which has a mutation in the splice donor site of the fifth intron. RT-PCR and sequencing analysis of the ful-A2 transcripts revealed two incorrect splicing forms. The most abundant form skipped the fifth exon, which resulted in a deletion of 14 amino acids in the middle of the K-box (Δ144-157). In the other alternative splicing form, the retention and translation of the fifth intron generated a premature stop codon that disrupted the K-box and removed the entire C-terminus (Fig. S2A). For FUL-B2, we selected line T4-2911 that carries a C-to-T change at nucleotide 484 (henceforth ful-B2). The ful-B2 mutation generates a premature stop codon at position 162 (Q162*) that removed the last 13 amino acids of the K-box and the entire C-terminus (Fig. S2A).
For FUL-A3, we selected line T4-2375 that carries a G-to-A mutation in the splice acceptor site of the third intron. Sequencing of ful-A3 transcripts revealed that this mutation generated a new splice acceptor site that shifted the reading frame by one nucleotide. The alternative translation generated a premature stop codon that truncated 72% of the K-box and the entire C-terminus (Fig. S2B). For FUL-B3, we selected line T4-2139 that carries a C-to-T mutation at nucleotide position 394 that generates a premature stop codon at amino acid position 132 (Q132*). This premature stop removed half of the K-box and the complete C-terminus (Fig. S2B). Given the critical roles of the K-domain in protein–protein interactions, and the C-terminal domain in transcriptional activation, these selected mutations are expected to impair the normal function of the FUL2 and FUL3 proteins.
The A and B-genome mutants for each gene were intercrossed to generate double mutants, which for simplicity, are referred to hereafter as null mutants. The ful2-null and ful3-null were intercrossed with a vrn1vrn2-null mutant (vrn-A1-null T4-2268/vrn-B1 T4-2619/vrn2-null) (Chen and Dubcovsky, 2012) to generate vrn1ful2-null and vrn1ful3-null, which were finally intercrossed to generate vrn1ful2ful3-null (all in a vrn2-null background). The vrn1ful2-null and vrn1ful2ful3-null mutants were sterile, so they were maintained and crossed by keeping the ful-B2 mutation in heterozygous state. The single mutants are available as part of the publicly available sequenced TILLING populations (Krasileva et al., 2017) and the mutant combinations are available upon request.
Transgenic Kronos plants overexpressing FUL2 and FUL3 coding regions were generated at the UC Davis Transformation facility using Agrobacterium-mediated transformation. The coding regions of these two genes were cloned from Kronos into the binary vector pLC41 (Japan Tobacco, Tokyo, Japan) downstream of the maize UBIQUITIN promoter. A C-terminal 3×HA tag was added to FUL2 and a C-terminal 4×MYC tag was added to FUL3. Mutant and transgenic wheat plants were grown in PGR15 CONVIRON chambers under LD (16 h light/8 h dark, light intensity ∼330 μM m−2 s−1) at 22°C during the day and 18°C during the night.
To study the effect of Ubi::FUL2 in different genetic backgrounds we crossed the Kronos Ubi::FUL2 with Kronos-vrn1vrn2-null and analyzed the effect of the three genes in the F2 progeny under greenhouse conditions. A field experiment comparing ful2-null and its control line was performed at the University of California, Davis field station during the 2017-2018 growing season (sowed on 12/01/2017 and harvested on 06/25/2018). One-meter rows (30 grains per row) were used as experimental units and the experiment was organized in a randomized complete block design with eight blocks. During the growing season plants received 200 units of N as ammonium sulfate, three irrigations, one application of broad-leaf herbicides (2,4D and Buctril, Bayer Corporation) and alternating applications of fungicides Quadris and Tilt (Syngenta) every 2 weeks.
Effect of the splice site mutations in ful-A2 and ful-B3 mutants
To determine the effect of the splice site mutations in ful-A2 and ful-B3, we extracted total RNA from leaf samples using the Spectrum Plant Total RNA kit. cDNA was synthesized from 2 µg of RNA using the High Capacity Reverse Transcription Kit according to the manufacturer's instructions and used as RT-PCR template. For ful-A2, we used primers FUL2-837-F (5′-CCATACAAAAATGTCACAAGC-3′) and FUL2-837-R (5′-TTCTGC CTCTCCACCAGTT-3′) for RT-PCR. These primers, which are not genome-specific, amplified three fragments of 303 bp, 220 bp and 178 bp. We gel-purified these fragments, cloned them into pGEM-T vector (Promega), and sequenced them. The 220 bp fragment was from the wild-type FUL-B2 allele, whereas the other two fragments corresponded to two alternative splicing forms of ful-A2 that either retained the fifth intron (303 bp) or skipped the fifth exon (178 bp).
For the ful-B3 mutant, we performed RT-PCR using primers FUL3-2375-F (5′-ATGGATGTGATTCTTGAAC-3′) and FUL3-2375-R (5′-TGTCCTGCAGAAGCACCTCGTAGAGA-3′). Sequencing analysis of the PCR products showed that the G-to-A mutation generated a new splice acceptor site with an adjacent G that shifted the reading frame by one nucleotide after 333 bp, and generated a premature stop codon.
Scanning electron microscopy (SEM)
Apices from different developmental stages were dissected and fixed for a minimum of 24 h in FAA [50% ethanol, 5% (v/v) acetic acid, 3.7% (v/v) formaldehyde], and then dehydrated through a graded ethanol series to absolute ethanol. Samples were critical-point-dried in liquid CO2 (tousimis 931 Series critical point drier), mounted on aluminium stubs, sputter-coated with gold (Bio-Rad SEM Coating System Model E5100), and examined with a Philips XL30 scanning electron microscope operating at 5 kV. Images were recorded at slow scan 3 for high definition and saved as TIFF files.
RNA extraction and real-time qPCR analysis
RNAs from apices were extracted using Trizol reagent (Thermo Fisher Scientific, 15596026). A 1 µg aliquot of RNA was used for cDNA synthesis following the manufacturer's instructions for the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, 4368814). The cDNA was then diluted 20 times and 5 µl of the dilution was mixed with 2×VeriQuest Fast SYBR Green qPCR Master Mix (Affymetrix, 75690) and with primers for the real-time qPCR analysis. Primer sequences are listed in Table S2. INITIATION FACTOR 4A (IF4A) and ACTIN were used as endogenous controls. Transcript levels for all genes are expressed as linearized fold-change from IF4A levels calculated by the formula 2(IF4A CT – TARGET CT)±s.e.m. The resulting number indicates the ratio between the initial number of molecules of the target gene and the number of molecules in the endogenous control.
Supplementary Material
Acknowledgements
We thank Dr Alejandra Alvarez and Dr Josh Hegarty for their help with field experiments and Dr Daniel Woods and Dr Juan Debernardi for their valuable comments and suggestions. We thank the anonymous reviewers for their helpful comments and valuable suggestions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.L., J.D.; Methodology: C.L., H.L., A.C., J.J., J.D.; Formal analysis: C.L., H.L., J.D.; Investigation: C.L., H.L., A.C., M.L., J.D.; Resources: C.L., H.L., A.C., M.L., J.D.; Data curation: H.L., J.D.; Writing - original draft: C.L., H.L.; Writing - review & editing: C.L., H.L., A.C., M.L., J.J., J.D.; Visualization: H.L., J.J., J.D.; Supervision: C.L., J.D.; Project administration: J.D.; Funding acquisition: J.D.
Funding
This project was supported by the Howard Hughes Medical Institute, USDA National Institute of Food and Agriculture (NIFA) NRI Competitive Grant 2016-67013-24617 and the International Wheat Yield Partnership Initiative (IWYP). Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.175398.supplemental
References
- Alonso-Peral M. M., Oliver S. N., Casao M. C., Greenup A. A. and Trevaskis B. (2011). The promoter of the cereal VERNALIZATION1 gene is sufficient for transcriptional induction by prolonged cold. PLoS ONE 6, e29456 10.1371/journal.pone.0029456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemer M., van Mourik H., Muiño J. M., Ferrándiz C., Kaufmann K. and Angenent G. C. (2017). FRUITFULL controls SAUR10 expression and regulates Arabidopsis growth and architecture. J. Exp. Bot. 68, 3391-3403. 10.1093/jxb/erx184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X., van Esse W., Mulki M. A., Kirschner G., Zhong J., Simon R. and von Korff M. (2019). CENTRORADIALIS interacts with FLOWERING LOCUS T-like genes to control floret development and grain number. Plant Physiol. 180, 1010-1030. 10.1104/pp.18.01454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D., Ratcliffe O., Vincent C., Carpenter R. and Coen E. (1997). Inflorescence commitment and architecture in Arabidopsis. Science 275, 80-83. 10.1126/science.275.5296.80 [DOI] [PubMed] [Google Scholar]
- Chen A. and Dubcovsky J. (2012). Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet. 8, e1003134 10.1371/journal.pgen.1003134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaffi M., Paolacci A. R., Tanzarella O. A. and Porceddu E. (2011). Molecular aspects of flower development in grasses. Sex. Plant Reprod. 24, 247-282. 10.1007/s00497-011-0175-y [DOI] [PubMed] [Google Scholar]
- Debernardi J. M., Lin H., Chuck G., Faris J. D. and Dubcovsky J. (2017). microRNA172 plays a crucial role in wheat spike morphogenesis and grain threshability. Development 144, 1966-1975. 10.1242/dev.146399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. W., Casao M. C., Wang P. H., Sato K., Hayes P. M., Finnegan E. J. and Trevaskis B. (2015). Direct links between the vernalization response and other key traits of cereal crops. Nat. Commun. 6, 5882 10.1038/ncomms6882 [DOI] [PubMed] [Google Scholar]
- Distelfeld A., Li C. and Dubcovsky J. (2009a). Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 12, 178-184. 10.1016/j.pbi.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Distelfeld A., Tranquilli G., Li C., Yan L. and Dubcovsky J. (2009b). Genetic and molecular characterization of the VRN2 loci in tetraploid wheat. Plant Physiol. 149, 245-257. 10.1104/pp.108.129353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrándiz C., Gu Q., Martienssen R. and Yanofsky M. F. (2000). Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127, 725-734. [DOI] [PubMed] [Google Scholar]
- Friend D. J. C. (1965). Tillering and leaf production in wheat as affected by temperature and light intensity. Can. J. Bot. 43, 1063 10.1139/b65-123 [DOI] [Google Scholar]
- Fu D., Szűcs P., Yan L., Helguera M., Skinner J. S., von Zitzewitz J., Hayes P. M. and Dubcovsky J. (2005). Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genomics 273, 54-65. 10.1007/s00438-004-1095-4 [DOI] [PubMed] [Google Scholar]
- Gocal G. F. W., King R. W., Blundell C. A., Schwartz O. M., Andersen C. H. and Weigel D. (2001). Evolution of floral meristem identity genes. Analysis of Lolium temulentum genes related to APETALA1 and LEAFY of Arabidopsis. Plant Physiol. 125, 1788-1801. 10.1104/pp.125.4.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ariza J., Brambilla V., Vicentini G., Landini M., Cerise M., Carrera E., Shrestha R., Chiozzotto R., Galbiati F., Caporali E. et al. (2019). A transcription factor coordinating internode elongation and photoperiodic signals in rice. Nat. Plants 5, 358-362. 10.1038/s41477-019-0401-4 [DOI] [PubMed] [Google Scholar]
- Honma T. and Goto K. (2001). Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409, 525-529. 10.1038/35054083 [DOI] [PubMed] [Google Scholar]
- Kaneko-Suzuki M., Kurihara-Ishikawa R., Okushita-Terakawa C., Kojima C., Nagano-Fujiwara M., Ohki I., Tsuji H., Shimamoto K. and Taoka K.-I. (2018). TFL1-like proteins in rice antagonize rice FT-like protein in inflorescence development by competition for complex formation with 14-3-3 and FD. Plant Cell Physiol. 59, 458-468. 10.1093/pcp/pcy021 [DOI] [PubMed] [Google Scholar]
- Kaufmann K., Wellmer F., Muino J. M., Ferrier T., Wuest S. E., Kumar V., Serrano-Mislata A., Madueno F., Krajewski P., Meyerowitz E. M. et al. (2010). Orchestration of floral initiation by APETALA1. Science 328, 85-89. 10.1126/science.1185244 [DOI] [PubMed] [Google Scholar]
- Kellogg E. A. (2001). Evolutionary history of the grasses. Plant Physiol. 125, 1198-1205. 10.1104/pp.125.3.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg E. A., Camara P. E. A. S., Rudall P. J., Ladd P., Malcomber S. T., Whipple C. J. and Doust A. N. (2013). Early inflorescence development in the grasses (Poaceae). Front. Plant Sci. 4, 250 10.3389/fpls.2013.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippes N., Chen A., Zhang X., Lukaszewski A. J. and Dubcovsky J. (2016). Development and characterization of a spring hexaploid wheat line with no functional VRN2 genes. Theor. Appl. Genet. 129, 1417-1428. 10.1007/s00122-016-2713-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Yasuno N., Sato Y., Yoda M., Yamazaki R., Kimizu M., Yoshida H., Nagamura Y. and Kyozuka J. (2012). Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell 24, 1848-1859. 10.1105/tpc.112.097105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasileva K. V., Vasquez-Gross H. A., Howell T., Bailey P., Paraiso F., Clissold L., Simmonds J., Ramirez-Gonzalez R. H., Wang X., Borrill P. et al. (2017). Uncovering hidden variation in polyploid wheat. Proc. Natl. Acad. Sci. USA 114, E913-E921. 10.1073/pnas.1619268114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lin H. and Dubcovsky J. (2015). Factorial combinations of protein interactions generate a multiplicity of florigen activation complexes in wheat and barley. Plant J. 84, 70-82. 10.1111/tpj.12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang Y., Wang F. X., Guo Y. Y., Duan X. Q., Sun J. H. and An H. L. (2016). Functional conservation and diversification of APETALA1/FRUITFULL genes in Brachypodium distachyon. Physiol. Plant 157, 507-518. 10.1111/ppl.12427 [DOI] [PubMed] [Google Scholar]
- Liu C., Zhou J., Bracha-Drori K., Yalovsky S., Ito T. and Yu H. (2007). Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 134, 1901-1910. 10.1242/dev.003103 [DOI] [PubMed] [Google Scholar]
- Lu S.-J., Wei H., Wang Y., Wang H.-M., Yang R.-F., Zhang X.-B. and Tu J.-M. (2012). Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (Oryza sativa L.). Plant Mol. Biol. Reporter 30, 1461-1469. 10.1007/s11105-012-0468-9 [DOI] [Google Scholar]
- Lv B., Nitcher R., Han X., Wang S., Ni F., Li K., Pearce S., Wu J., Dubcovsky J. and Fu D. (2014). Characterization of FLOWERING LOCUS T1 (FT1) gene in Brachypodium and wheat. PLoS ONE 9, e94171 10.1371/journal.pone.0094171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcomber S. T., Preston J. C., Reinheimer R., Kossuth J. and Kellogg E. A. (2006). Developmental gene evolution and the origin of grass inflorescence diversity. Adv. Bot. Res. 44, 425-481. 10.1016/S0065-2296(06)44011-8 [DOI] [Google Scholar]
- McSteen P., Laudencia-Chingcuanco D. and Colasanti J. (2000). A floret by any other name: control of meristem identity in maize. Trends Plant Sci. 5, 61-66. 10.1016/S1360-1385(99)01541-1 [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Shimamoto K. and Kyozuka J. (2002). Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J. 29, 743-750. 10.1046/j.1365-313X.2002.01255.x [DOI] [PubMed] [Google Scholar]
- Oikawa T. and Kyozuka J. (2009). Two-step regulation of LAX PANICLE1 protein accumulation in axillary meristem formation in rice. Plant Cell 21, 1095-1108. 10.1105/tpc.108.065425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S., Vanzetti L. S. and Dubcovsky J. (2013). Exogenous gibberellins induce wheat spike development under short days only in the presence of VERNALIZATION. Plant Physiol. 163, 1433-1445. 10.1104/pp.113.225854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. C. and Kellogg E. A. (2006). Reconstructing the evolutionary history of paralogous APETALA1/FRUITFULL-like genes in grasses (Poaceae). Genetics 174, 421-437. 10.1534/genetics.106.057125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. C. and Kellogg E. A. (2008). Discrete developmental roles for temperate cereal grass VERNALIZATION1/FRUITFULL-like genes in flowering competency and the transition to flowering. Plant Physiol. 146, 265-276. 10.1104/pp.107.109561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. C., Christensen A., Malcomber S. T. and Kellogg E. A. (2009). MADS-box gene expression and implications for developmental origins of the grass spikelet. Am. J. Bot. 96, 1419-1429. 10.3732/ajb.0900062 [DOI] [PubMed] [Google Scholar]
- Ratcliffe O. J., Bradley D. J. and Coen E. S. (1999). Separation of shoot and floral identity in Arabidopsis. Development 126, 1109-1120. [DOI] [PubMed] [Google Scholar]
- Ream T. S., Woods D. P., Schwartz C. J., Sanabria C. P., Mahoy J. A., Walters E. M., Kaeppler H. F. and Amasino R. M. (2014). Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon. Plant Physiol. 164, 694-709. 10.1104/pp.113.232678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Karunarathna N. L., Jung C. and Emrani N. (2018). An APETALA1 ortholog affects plant architecture and seed yield component in oilseed rape (Brassica napus L.). BMC Plant Biol. 18, 380 10.1186/s12870-018-1606-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L. M., Lyu B., Turner R., Li C., Chen F., Han X., Fu D. and Dubcovsky J. (2019). FLOWERING LOCUS T2 (FT2) regulates spike development and fertility in temperate cereals. J. Exp. Bot. 70, 193-204. 10.1093/jxb/ery350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen G., Melzer R. and Rümpler F. (2016). MADS-domain transcription factors and the floral quartet model of flower development: linking plant development and evolution. Development 143, 3259-3271. 10.1242/dev.134080 [DOI] [PubMed] [Google Scholar]
- Tranquilli G. and Dubcovsky J. (2000). Epistatic interaction between vernalization genes Vrn-Am1 and Vrn-Am2 in diploid wheat. J. Hered. 91, 304-306. 10.1093/jhered/91.4.304 [DOI] [PubMed] [Google Scholar]
- Trevaskis B., Tadege M., Hemming M. N., Peacock W. J., Dennis E. S. and Sheldon C. (2007). Short Vegetative Phase-like MADS-box genes inhibit floral meristem identity in barley. Plant Physiol. 143, 225-235. 10.1104/pp.106.090860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy C., Paraiso F., Colasuonno P., Tran R. K., Tsai H., Berardi S., Comai L. and Dubcovsky J. (2009). A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biol. 9, 115-128. 10.1186/1471-2229-9-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yu H., Tian C., Sajjad M., Gao C., Tong Y., Wang X. and Jiao Y. (2017). Transcriptome association identifies regulators of wheat spike architecture. Plant Physiol. 175, 746-757. 10.1104/pp.17.00694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple C. J. (2017). Grass inflorescence architecture and evolution: the origin of novel signaling centers. New Phytol. 216, 367-372. 10.1111/nph.14538 [DOI] [PubMed] [Google Scholar]
- Williams R. F. and Langer R. H. M. (1975). Growth and development of the wheat tiller. II. The dynamics of tiller growth. Aust. J. Bot. 23, 745-759. 10.1071/BT9750745 [DOI] [Google Scholar]
- Woods D. P., McKeown M. A., Dong Y., Preston J. C. and Amasino R. M. (2016). Evolution of VRN2/Ghd7-like genes in vernalization-mediated repression of grass flowering. Plant Physiol. 170, 2124-2135. 10.1104/pp.15.01279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Shi X., Lin X., Liu Y., Chong K., Theissen G. and Meng Z. (2017). The ABCs of flower development: mutational analysis of AP1/FUL-like genes in rice provides evidence for a homeotic (A)-function in grasses. Plant J. 89, 310-324. 10.1111/tpj.13386 [DOI] [PubMed] [Google Scholar]
- Yan L., Loukoianov A., Tranquilli G., Helguera M., Fahima T. and Dubcovsky J. (2003). Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 100, 6263-6268. 10.1073/pnas.0937399100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Fu D., Li C., Blechl A., Tranquilli G., Bonafede M., Sanchez A., Valarik M., Yasuda S. and Dubcovsky J. (2006). The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 103, 19581-19586. 10.1073/pnas.0607142103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. K., Xiao Y. G., Zhang Y., Xia X. C., Dubcovsky J. and He Z. H. (2008). Allelic variation at the vernalization genes Vrn-A1, Vrn-B1, Vrn-D1 and Vrn-B3 in Chinese common wheat cultivars and their association with growth habit. Crop Sci. 48, 458-470. 10.2135/cropsci2007.06.0355 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.