Abstract
Care for patients transitioning from chronic kidney disease to kidney failure often falls short of meeting patients’ needs. The PREPARE NOW study is a cluster randomized controlled trial studying the effectiveness of a pragmatic health system intervention, ‘Patient Centered Kidney Transition Care,’ a multi-component health system intervention designed to improve patients’ preparation for kidney failure treatment. Patient-Centered Kidney Transition Care provides a suite of new electronic health information tools (including a disease registry and risk prediction tools) to help providers recognize patients in need of Kidney Transitions Care and focus their attention on patients’ values and treatment preferences. Patient-Centered Kidney Transition Care also adds a ‘Kidney Transitions Specialist’ to the nephrology health care team to facilitate patients’ self-management empowerment, shared-decision making, psychosocial support, care navigation, and health care team communication. The PREPARE NOW study is conducted among eight (8) outpatient nephrology clinics at Geisinger, a large integrated health system in rural Pennsylvania. Four randomly selected nephrology clinics employ the Patient Centered Kidney Transitions Care intervention while four clinics employ usual nephrology care. To assess intervention effectiveness, patient reported, biomedical, and health system outcomes are collected annually over a period of 36 months via telephone questionnaires and electronic health records. The PREPARE NOW Study may provide needed evidence on the effectiveness of patient-centered health system interventions to improve nephrology patients’ experiences, capabilities, and clinical outcomes, and it will guide the implementation of similar interventions elsewhere.
Trial registration:
Keywords: Chronic kidney disease, kidney failure, shared decision-making, self-management, care navigation, electronic health tools
Introduction
Over 115,000 patients develop kidney failure each year.1 Patients with kidney failure have high rates of mortality2-4 and must receive treatment to replace their kidney function, such as dialysis or a kidney transplant, to survive. Due to the morbidity of kidney disease and the demands of kidney failure treatments, patients with kidney failure often experience drastic changes in their physical and mental health that are often devastating not only for patients but also for their families.5-12 Ideally, patients should receive substantial advance preparation before initiating kidney failure treatments. Optimal treatment preparation involves educating patients regarding numerous treatment options, assisting patients in treatment decision-making, and ensuring patients receive a number of preparatory clinical evaluations, including evaluations for vascular surgery, kidney transplantation, or home dialysis treatments.
Unfortunately, many patients are unprepared for kidney failure treatments—even when they have been under nephrology specialty care for years.13-15 A number of factors contribute to patients’ poor preparation. For instance, nephrologists are often unable to predict with precision the timing of when patients’ kidney failure will occur, as many patients with advanced kidney disease never progress to kidney failure.16 As a result, nephrologists may feel hesitant to discuss kidney failure with patients too early. However, some patients with advanced kidney disease experience very rapid declines in their kidney function. Most of these patients have no symptoms, and their kidney disease may progress so rapidly that nephrologists have little time to help patients prepare in advance. As a result, many patients experience unplanned, chaotic, and psychologically traumatic treatment initation.17-21 Even when patients are aware of their declining kidney function in advance of treatment, they may fail to obtain recommended consultations to facilitate their advance preparation for dialysis or transplantation.22-25 Hence, interventions are needed to improve the identification of patients in need of advance preparation, educate patients on their treatment options, help patients navigate multiple preparatory clinical evaluations for kidney failure treatment, and help patients obtain adequate psychosocial support for potentially traumatic kidney care transitions.
Efficacious interventions exist to help patients experience better kidney transitions, but their effectiveness in the real world may be limited by their disjointed or piecemeal implementation. For instance, risk prediction tools are now available to help physicians recognize when patients are at risk of kidney failure and could most benefit from kidney transitions care.26-29 Further, randomized trials have shown that patients who receive education and psychosocial support for their kidney disease experience 43% fewer hospitalizations and prolonged time to kidney failure.30-32 Randomized trials in patients at risk of kidney disease have also shown that when patients learn skills to overcome problems they feel empowered and better manage their kidney disease risks.33,34 Studies also suggest that when patients receive assistance to make decisions and navigate complex care plans, they are up to 30% more likely to pursue self-care treatment options such as kidney transplantation or peritoneal dialysis.31,35 To date, these promising interventions have not been implemented in a coordinated fashion to improve patients’ care experiences and outcomes in a comprehensive manner.
Materials and Methods
Overview
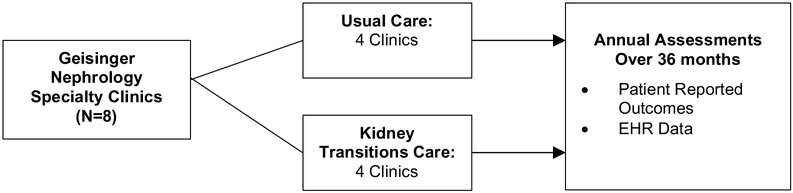
The PREPARE NOW study is a pragmatic cluster randomized controlled trial designed to quantify the effectiveness of integrated health system interventions to improve patients’ preparation for kidney failure treatments. Based in Geisinger health system and working with Geisinger Health Plan, the study takes advantage of existing health system and health plan informatics, clinics, and care-management resources. In PREPARE NOW, a new intervention, called ‘Patient Centered Kidney Transitions Care’ has been deployed among four (4) of eight (8) randomly assigned nephrology clinics at Geisinger. Patient reported, biomedical, and health system outcomes are being measured over 36 months through telephone questionnaires, Geisinger electronic health records (EHR), and administrative data (Figure 1). All study procedures have been approved through single IRB agreement oversight at Duke University.
Figure 1.
Study Design
Study Setting and Patient Population
Health System and Health Plan
Geisinger is a large integrated health system that provides care for over 4 million residents in 50 rural and suburban counties in Pennsylvania and New Jersey. The counties that Geisinger serves are substantially rural (40% population) with moderate to low education (57% with high school or less education), advanced age (20% age >65 years), and low household income (52% with annual household income <$50,000). Outpatient nephrology care is delivered in nine clinical practice sites to approximately 4,000 patients with chronic kidney disease (CKD) not on dialysis. There are one (1) to three (3) nephrology providers per clinic depending on the size of each clinic, and some providers practice in more than one clinic. Patients receiving CKD care in Geisinger have demographic characteristics reflective of the overall health system, but with a higher proportion of persons over the age of 65 years (76% versus 20% in overall health system) since the prevalence of CKD increases with age. Geisinger uses a common electronic health record system (Epic® System) across all nephrology clinical practice sites. About one-third of Geisinger patients are also insured by the health system, through Geisinger Health Plan. Geisinger Health Plan manages care for over 580,000 members, insured through commercial, employer-based, and public payers. It deploys disease and care management programs for over 69,000 members, and it provides wellness programs for over 75,000 members. Geisinger Health Plan features a robust care-management program, providing nurse care management for patients with a number of complex chronic illnesses including congestive heart failure, advanced pulmonary disease, and kidney disease.36
Nephrology Clinics and Patient Population
The PREPARE NOW intervention was piloted at one of nine Geisinger nephrology clinic sites from December 2016 through June 2017. The study is being conducted formally among the remaining other eight (8) nephrology clinic sites, from July 2017 through December 2020. Clinics vary in terms of their size but are largely similar with regard to the distribution of demographic characteristics of patients receiving care in clinics (Table 1). All clinics are located within Central Pennsylvania and draw from a primarily rural population.
Table 1.
Demographics of adult patient population in Geisinger nephrology practices
| Practice Name |
Total CKD Patients* |
eGFR < 30 |
White | African American |
Hispanic | Female | Age > 65 years |
|---|---|---|---|---|---|---|---|
| Pilot Site** | 1754 | 593 | 98% | 2% | 0% | 51% | 69% |
| Study Site 1 | 132 | 51 | 99% | 2% | 0% | 58% | 74% |
| Study Site 2 | 126 | 50 | 95% | 4% | 0% | 64% | 71% |
| Study Site 3 | 861 | 248 | 99% | 1% | 0% | 62% | 82% |
| Study Site 4 | 172 | 54 | 98% | 1% | 0% | 53% | 81% |
| Study Site 5 | 221 | 58 | 100% | 0% | 0% | 62% | 82% |
| Study Site 6 | 582 | 112 | 97% | 2% | 0% | 56% | 79% |
| Study Site 7 | 116 | 46 | 99% | 1% | 0% | 51% | 82% |
| Study Site 8 | 76 | 34 | 100% | 0% | 0% | 66% | 82% |
| Grand Total | 4,040 | 1246 | 98% | 2% | 0% | 56% | 76% |
All patients >18 years with 2 eGFR measures <60 ml/min/1.73m2 more than 90 days apart, not on dialysis, with at least 1 visit at each respective study site;
One Geisinger nephrology clinic served only as Pre-Trial Intervention Refinement Site
The PREPARE NOW study targets all adults receiving care in the eight (8) Geisinger nephrology practices who are older than 18 years of age and who have advanced kidney disease (all patients with a “very high risk” prognosis based on Kidney Disease Improving Global Outcomes (KDIGO) classification).37,38 A computer algorithm continuously identifies all eligible patients via a disease registry implemented as part of the study. Patients are not excluded from enrollment in Patient Centered Kidney Transitions Care based on their language preference, however non-English speakers are excluded from telephone questionnaire assessments.
Intervention Overview and Conceptual Framework
Patient-Centered Kidney Transitions Care is designed to help patients overcome obstacles to optimal kidney disease transitions by (1) improving health system infrastructure, (2) employing educational programs and established behavioral approaches (e.g. motivational interviewing and self-management training),33,39 (3) providing patient navigation to help patients make high-quality informed treatment decisions,40 and (4) helping patients achieve their preference-aligned treatment goals in a timely manner.41,42 The Chronic Care Model43,44 provides a framework for the design of Patient-Centered Kidney Transitions Care (Table 2).
Table 2.
Chronic Care Model elements targeted by “Patient Centered Kidney Transitions Care’ intervention
| Chronic Care Model Element |
Patient-centered Kidney Transitions Intervention Component Addressing Model |
|---|---|
| Health System Culture | • Prompt providers to engage in patient-centered transitions care • Broadcast patient preferences for kidney failure treatments as advanced directives • Embed Kidney Transitions Specialists into health care team |
| Clinical Information System | • Kidney Transitions registry • Enable entry and display of patient values and preferences in Electronic Health Record • Enable personalized Kidney Transitions Care planning |
| Health Delivery System Design | • Enable education, psychosocial, and biomedical care coordination support |
| Decision Support | • (For providers): Prompts to engage in shared decision-making and develop plans that are aligned with patients’ preferences • (For patients): Provide resources and support informed shared decision-making |
| Self-management Support | • Provide self-management education • Build self-management skills through Empowerment Training |
| Community Resources | • Facilitate patients’ access to clinic and community resources for professional and peer psychosocial support |
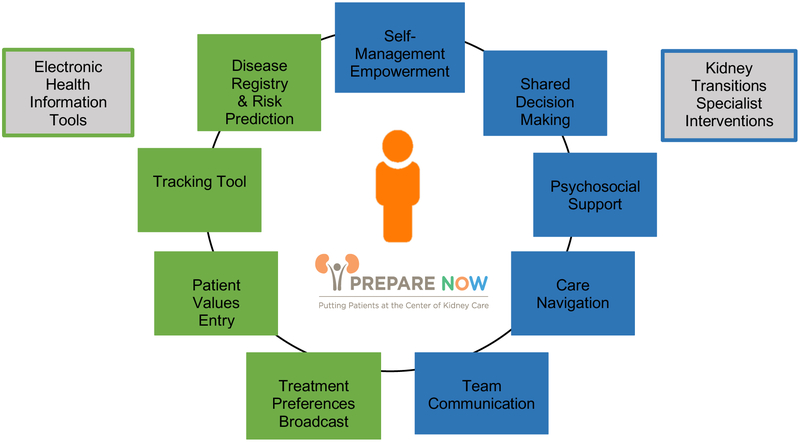
The intervention provides a suite of new electronic health information tools to help health care providers recognize patients in need of Kidney Transitions Care and to help health care providers focus their attention on patients’ values and treatment preferences related to kidney disease. The intervention also adds a ‘Kidney Transitions Specialist’ to the health care team who provides and facilitates integrated delivery of programs that provide patients with knowledge, skills, and assistance to manage their disease, make high-quality treatment decisions, obtain needed psychosocial support, and navigate complex treatment plans (Figure 2). Kidney Transitions Care is driven by the Kidney Transitions Specialist and occurs independent of other providers (e.g., nephrologists and primary care providers) who are not trained to change their usual practice patterns aside from being prompted to utilize the electronic tools available at intervention sites.
Figure 2.
Components of Patient Centered Kidney Transitions Care
Intervention Electronic Health Information Tools
Patient Centered Kidney Transitions Care electronic health information tools include (1) a continually updated disease registry paired with a real-time validated risk prediction tool, (2) a tool to electronically assess patients’ values to facilitate treatment decision-making, (3) a care navigation and tracking tool, and (4) mechanisms for ‘broadcasting’ patients’ kidney failure treatment preferences in advance of kidney failure (Table 3). These tools are only available to be used at intervention sites as part of Kidney Transitions Care.
Table 3.
Features and Goals of New Electronic Health Information Tools
| Feature | Goals |
|---|---|
| Disease Registry and Risk Prediction Tool | • Create a registry that identifies all patients needing Kidney Transitions Care and those at greatest risk of kidney failure • Prompt providers to let them know patients need Kidney Transitions Care |
| Patient Values Tool | • Allow patients to enter their own values and treatment preferences directly into their health records |
| Care Navigation and Tracking Tool | • Create a special place in the Health Information System and Electronic Health Record to plan care for patients’ CKD transitions |
| Treatment Preferences Broadcast | • Make all providers aware of treatments patients want before they develop kidney failure |
Disease Registry and Risk Prediction Electronic Tools
To identify patients at increased risk of progression to kidney failure and therefore at greatest need for intervention, we have implemented a population based kidney disease registry (i.e., continually updated electronic list, called the ‘Kidney Transitions Registry’) which incorporates an automated risk prediction tool alongside the Geisinger electronic health record platform. The Kidney Transitions Registry classifies patients as being on the registry based on staging criteria from Kidney Disease Improving Global Outcomes (KDIGO). The registry is designed to include all patients with a “very high risk” prognosis based on eGFR and albuminuria categories (stages G3aA3, G3bA2-A3, G4A1-A3, and G5A1-A3).37 Outpatient data from the electronic health record are processed nightly to identify qualifying patients. Patients remain in the registry throughout all their care at Geisinger until six (6) months after they transition to care for kidney failure (i.e., for dialysis or kidney transplant).
The automated risk prediction tool is a well-validated computer algorithm designed to help providers identify individuals with a high predicted risk of developing kidney failure within 2 years based on their personal characteristics, including their demographics and their most recent commonly obtained laboratory measures (age, gender, eGFR, urine albumin-to-creatinine ratio, calcium, phosphorus, albumin, and bicarbonate).26,45 The risk prediction algorithm is applied nightly to all patients on the registry to detect any changes in patients’ individual risk profiles based on outpatient laboratory values. When the algorithm identifies a patient at imminent risk of progression within 2 years (i.e., predicted risk >10% to occur within 2 years), the health care team is alerted to the need for interventions to prompt shared decisionmaking about kidney failure treatments and to navigate patients through preparation care.
Patient Values Electronic Tool
The ‘Patient Values Tool’ enables patients to enter their own lifestyle and treatment values directly into their health records. Patients use a secure web-based values clarification tool, adapted from an existing tool developed by Medical Education Institute.46 The tool asks patients a series of questions to help them clarify their lifestyle and treatment values as a starting point for establishing their informed preferences for kidney failure treatment. Patients rate the importance of a set of values previously identified as meaningful to kidney patients, such as fertility, ability to work or travel, quality of life, physical symptoms, and survival.20,42,50 A report is then generated with the patient’s ranked values from most to least important. Patients are able to complete the tool on their own (i.e., at home through the electronic health portal) or with assistance from a Kidney Transitions Specialist (see below). Clinical interpreter services are used to assist non-English speakers in completing the tool. As intervention patients complete the tool, their values are directly transferred into their personal EHRs through a secure electronic interface. Providers are able to view patients’ values during visits, providing a basis for engaging in shared and informed decisions about kidney failure treatments. Patients are invited to complete the tool at any time and to update their treatment preferences if they change.
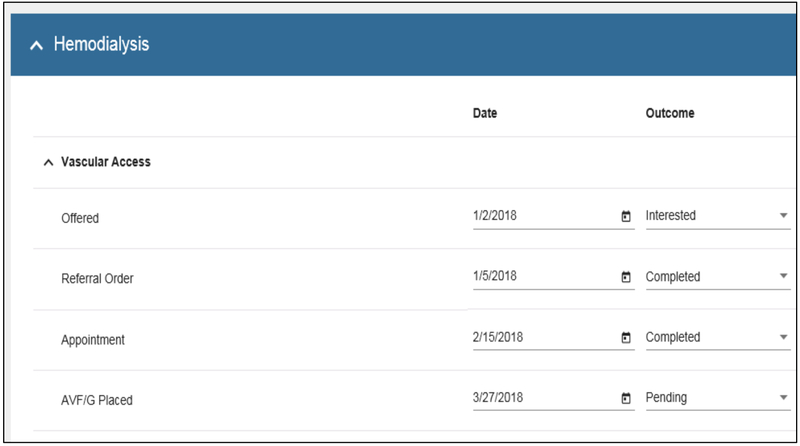
Kidney Transitions Specialist Care Navigation and Tracking Electronic Tool
The ‘Care Navigation and Tracking Tool’ (Figure 3) creates an electronic workspace to facilitate Kidney Transitions Specialists’ comprehensive care planning and tracking for individual patients receiving Patient-Centered Kidney Transitions Care. Kidney Transitions Specialists plan, initiate, and track support they provide to individual patients receiving Patient-Centered Kidney Transitions Care using this tool. The tool enables Kidney Transitions Specialists in the intervention clinics to type free text notes about interactions with patients in reports that are uploaded to patients’ EHRs for other health care providers to view. The tool pairs with the Kidney Transitions Registry which provides Kidney Transitions Specialists with continually updated lists of patients qualifying for Kidney Transitions Care as well as continually updated information on patients’ risk (imminent versus not) of developing kidney failure.
Figure 3.
Sample screenshot from ‘Care Navigation and Tracking Tool”
Treatment Preferences Electronic Broadcast in Patient Health Records
The ‘Treatment Preferences Broadcast’ makes all providers aware of treatments patients want before they develop kidney failure. After patients identify their preferred kidney failure treatment, Kidney Transitions Specialists “broadcast” patients’ preferences widely throughout the EHR by posting preferences on the problem list for all providers to see. Options for treatment preferences include in-center hemodialysis, home hemodialysis, peritoneal dialysis, transplant, conservative care (i.e., no dialysis or transplant therapy), or undecided. The problem list is reviewed during each clinical encounter as part of routine care. In addition, an alert banner displays within the electronic health record for any patient enrolled in Kidney Transitions Care to direct providers to the problem list to view kidney failure treatment preferences. This banner is visible to all providers within the health system, including primary care, subspecialists, and emergency room providers.
Intervention Kidney Transitions Specialist Activities
Kidney Transitions Specialists (N=2) are registered nurses with special behavioral and navigation skills training who are supported jointly through research and Geisinger Health Plan Funding. Their activities are managed completely through Geisinger Health Plan, who partnered with the research team to co-develop the roles, responsibilities, and workflows of the Kidney Transitions Specialists to ensure they would be feasible and sustainable. By leveraging the disease registry to identify the highest risk patients, the target patient census is approximately 100 patients per specialist at any given time, which is consistent with goals for specialty care management services across the health system.
Kidney Transitions Specialists conduct five key activities: (1) empowering patients’ self-management; (2) facilitating patients’ shared and informed decisions about kidney failure treatments; (3) offering patients psychosocial support; (4) providing care navigation; and (5) facilitating team communication. Kidney Transitions Specialists provide and facilitate an individually tailored, multi-component intervention featuring evidence-based47-50 patient support programs to impart patients with knowledge, skills, and assistance throughout kidney transitions care (Table 4). All interactions reinforce principles of self-care to slow kidney disease progression and encourage informed decision-making and planning for the possibility of future kidney failure, when appropriate.
Table 4.
Kidney Transition Specialist Activities and Goals
| Activities | Goals |
|---|---|
|
Self-Management Empowerment • Provide and refer for education on CKD self-management • Conduct “Problem Solving Self-Care Empowerment Classes” |
Enable patients’ self-care and activation |
|
Facilitate Shared Decision-Making • Assess patients’ readiness to engage in CKD self-care and CKD decision-making and tailor intervention to patient readiness • Help patients comprehend CKD diagnosis and potential need for long-term planning about kidney failure treatments • Support shared decision-making • Ascertain values and enter in electronic health record • Review educational information on treatment modalities • Refer to kidney failure treatment modality classes • Help patient document their treatment preferences |
Improve patients’ informed decision-making about kidney failure treatments |
|
Offer Psychosocial Support • Connect to behavioral and mental health services • Connect to peer-mentors program (National Kidney Foundation) • Identify caregiver support needs and facilitate support |
Connect patients to mental health and social support |
|
Provide Care Navigation • Promote timely movement through multi-step referrals and tests (education, encouragement, assistance) • Create link between disconnected CKD clinics and dialysis or transplant centers through letters and phone calls |
Navigate patients through multistep medical plans (e.g., referrals and tests) |
|
Facilitate Team Communication • Communicate with care team to encourage alignment of care with patients’ preferences |
Advocate to align patients’ care with their values |
Kidney Transitions Specialists tailor their approach to each individual patient’s readiness for making self-care behavior changes or decisions about kidney failure treatments as well as their risks of imminent kidney failure (i.e., within 2 years based on personalized risk prediction tool). The goal of this tailored approach is to help guide patients toward necessary changes ‘at their own pace’ and without overwhelming them, while also ensuring that patients begin planning for and making decisions about kidney failure treatments when they are most likely to need it. Using motivational interviewing techniques,39 Kidney Transitions Specialists assess patients’ psychological readiness to engage in kidney disease self-care and treatment planning. They also refer to the risk prediction tool (see above) to determine whether discussion of kidney failure treatment modalities should be accelerated (e.g., when patients’ risks of imminent kidney failure are ‘high’).
Self-Management Empowerment
Kidney Transitions Specialists ascertain patients’ knowledge of kidney disease self-care principles or treatment options and provide patient education on kidney disease self-management through a range of avenues. These include: (1) reviewing education materials with patients, emphasizing core aspects of self-management that can slow kidney disease progression and decrease risk (e.g., diabetes and hypertension self-care including monitoring and medication adherence, diet and exercise, and avoidance of medications that are toxic to kidneys); (2) referring patients to dieticians for recommended dietary education,51,52 and (3) conducting nine-week group ‘Living with Kidney Disease’ classes.
‘Living with Kidney Disease’ classes have been rigorously developed, successfully implemented in ambulatory care settings,53 and shown in clinical trials to improve the self-management of patients with risk factors for kidney disease progression, including diabetes and hypertension.33 They have also been shown to improve perceived empowerment and selfefficacy among patients with kidney disease.34 During two-hour sessions held weekly, patients are encouraged to consider a range of self-identified obstacles they face with regard to engaging in or reinforcing personal awareness of kidney disease and kidney disease self-management behaviors, including adherence to lifestyle recommendations, adherence to prescribed medical care, and engagement in treatment decisions. Strategies employed in education and skills training sessions include group instruction, handouts, teaching metaphors, prompting, modeling, behavioral rehearsal, homework assignments (performed in a workbook), reinforcement and feedback. Initial classes review principles of kidney disease self-management behaviors (including monitoring of risk factors for kidney disease progression such as diabetes and hypertension and avoidance of nephrotoxins). Emphasis of the classes is to empower patients to prevent kidney disease progression and to take an active role in treatment decision-making through self-care and by overcoming barriers, including impulsive or careless (e.g., denial) coping behaviors. Class material and content were adapted from prior work in patients with diabetes and hypertension33,53 to focus participants’ attention on managing risk factors to mitigate kidney disease progression. All written materials for patients are developed at a 4th grade reading level to accommodate patients with low health literacy.
Shared and Informed Decision-Making
Support for shared and informed decision-making begins during Living with Kidney Disease classes, when patients receive information about the progressive nature of kidney disease and the often-unpredictable decline of function to kidney failure. Information includes an overview of treatment options for kidney failure, emphasizing the importance that patients begin to consider their lifestyle and treatment values early. When patients are deemed to be at high risk of imminent kidney failure, Kidney Transitions Specialists refer patients to kidney failure treatment modality education classes, where patients learn about differences in kidney failure treatments during a one-time class (two hours) facilitated by the Kidney Transitions Specialist. During this class, patients complete the Values Tool to help clarify their personal lifestyle and treatment values. Groups watch a video and review written materials about treatment options using an evidence-based decision aid (PREPARED Decision Aid).54 This decision aid was rigorously developed following International Patient Decision Aids Standards55 and includes information on all treatment modalities (kidney transplant, in-center hemodialysis, home hemodialysis, peritoneal dialysis, and treatment with no dialysis or transplant). It is available in both English and Spanish. The facilitator provides an overview of the goal of treatments and how they are delivered. They discuss a range of factors patients should consider when selecting a treatment option (including differences in the frequency and intensity of treatments, the amount of self-care they want to perform, concerns about surgery for transplant, quality of life, and financial concerns).
Patients are provided their own copies of decision aids to take home and are encouraged to review materials at home with family members or caregivers. After treatment modality education classes, Kidney Transitions Specialists contact patients to arrange 1-on-1 meetings for individual decision support. In meetings, Kidney Transitions Specialists follow principles of the Ottawa Decision Support Framework40 to (1) help patients clarify the decision to be made regarding kidney failure treatments and their needs for information or support to make a decision; (2) review decision aids with facts and probabilities on risks with different treatments; (3) help patients clarify their values; (4) iteratively guide patients in their deliberation; and (5) monitor and facilitate patients’ progress with decision-making.
Psychosocial Support
Kidney Transitions Specialists refer all patients to an initial mental health evaluation, conducted by a Behavioral Medicine/Adult Psychology group, which provides psychological support for patients transitioning through a number of chronic or terminal disease transitions (e.g., congestive heart failure and cancer). These groups are part of usual care provided by Geisinger. Mental health professionals screen patients for mental health concerns, provide ongoing care for depression or anxiety, and assess social support, coping styles, communication preferences, and barriers to decision-making or adherence. They also provide counseling and grief support as needed. They are available along the continuum of patients’ kidney disease care and maintain ongoing communication with Kidney Transitions Specialists throughout the course of patients’ care.
Kidney Transitions Specialists also connect patients to peer support through a direct partnership with the National Kidney Foundation’s “NKF Peers Program”.56 In this program, patients are linked with trained peer mentors who offer support as patients face challenges with kidney disease self-care or kidney failure treatment decisions. The NKF Peers Program staff conducts a phone evaluation and pair patients with appropriate peer mentors. The NKF currently has approximately 60 trained peer mentors with dialysis and/or transplant experience.
Patient Care Navigation
Kidney Transitions Specialists support patients’ timely accomplishment of complex treatment plans and help them overcome barriers to completing plans. They assist with planning and making appointments for education and to obtain procedures and tests needed to prepare for renal replacement therapy of their choice (e.g., evaluation for transplant, referral for fistula placement, vascular surgery appointments). Kidney Transition Specialists also follow a standard protocol to facilitate goals of care conversations and end-of-life-care. For patients choosing conservative care (i.e., no dialysis or transplant), Kidney Transition Specialists monitor patients’ symptoms, assist with completion of advance directives, and facilitate referrals to palliative medicine and/or hospice as clinically indicated. Kidney Transitions Specialists also assess a number of social and behavioral determinants of health and chronic disease self-management (e.g., transportation needs, environmental risks to health (e.g., lack of heat), food insecurity, and financial needs). Kidney Transitions Specialists are also trained registered nurses (RNs) who play a role in the clinical management of patients’ chronic conditions, as acute situations arise and health status changes. The Kidney Transitions Specialists collaborate with patients’ primary care providers, nephrologists and other healthcare team members to ensure care is coordinated and that non-biomedical quality of life issues are also addressed.
Team Communication
Kidney Transitions Specialists partner with patients and act as ‘champions’ on their behalves to advocate for treatments patients want. With patients’ permission, Kidney Transitions Specialists (1) communicate with inpatient hospital teams if patients are admitted to initiate dialysis and (2) communicate with patients’ non-Geisinger dialysis care teams. If patients are admitted to a Geisinger hospital, the Kidney Transitions Registry list identifies patients, allowing Kidney Transitions Specialists to review inpatient notes to determine if dialysis has been initiated or is being planned during the hospitalization. Kidney Transitions Specialists contact the inpatient hospital care team to let the team know of the patient’s preference and advocate for preference aligned care. If patients initiate dialysis in a non-Geisinger hospital or are discharged to an outpatient dialysis clinic, Kidney Transitions Specialists contact providers (e.g., dialysis facility physician, nurse director, or social worker) via letters and phone calls to alert them about patients’ preferences for care.
Kidney Transitions Specialists also directly communicate with primary care providers to keep them informed about the clinical status of their patients. When a patient is enrolled in Kidney Transitions Care, a letter is sent to the primary care provider notifying them that their patient is now at high-risk for progressing to kidney failure and will begin discussions about renal replacement therapy options. Primary care providers are invited to participate in these conversations and are notified once the patient has made a final decision about their kidney failure treatment choice.
Control Condition: Usual Nephrology Care at Study Practice Sites
Usual Nephrology Care consists of patients’ routine visits with their nephrologists. Patients receive medical care as prescribed by nephrologists with preparation for kidney disease transitions as deemed appropriate by nephrologists. Nephrologists document their care in the EHR. There is currently no Kidney Transitions Registry list, no routine ‘flag’ or prompt for providers to initiate kidney transitions care, no computer application to collect patients’ values, track care, report patients’ preferred care plans or broadcast treatment preferences. As kidney disease progresses, nephrologists refer patients to kidney failure treatment modality group education classes and discuss treatment options on an ad-hoc basis. Classes feature an industry-sponsored educational video and are facilitated by a social worker or nurse. Nephrologists make referrals to prepare patients for kidney transitions (e.g., for fistula placement or transplant evaluation) through routine mechanisms (e.g., referrals through the EHR) without the assistance of dedicated personnel. Peer mentoring and behavioral health services are not routinely offered. Usual nephrology care does not feature any system-supported CKD care coordination.
Clinic Randomization Procedure
In the cluster randomized trial, eight Geisinger nephrology clinics have been randomly assigned to employ Patient Centered Kidney Transitions Care (intervention arm, 4 sites) or Usual Nephrology Care (control arm, 4 sites). Clinics were randomized to the two arms of the study, constrained to be marginally balanced by clinic size (> 500 vs. <200 in 2016) and region of service (Central, Western), using the SURVEYSELECT procedure in the SAS v9. 4 statistical software.
Intervention Implementation and Assessment of Fidelity
Two Kidney Transitions Specialists have been hired and trained to implement the intervention via a standard protocol. Each Kidney Transitions Specialist covers 2 intervention sites, which are assigned based on clinic size and region. Throughout the study, data are collected to measure fidelity to the intervention as an indication of the quality of the implementation of the intervention.57,58 A nurse manager within Geisinger Health Plan reviews documentation of the Kidney Transition Specialists meetings, phone calls, and self-care empowerment classes and assesses the extent to which the Specialists adhere to established protocols. Re-training is provided as needed. Monthly reports are generated from EHR data and the Care Management and Tracking Tool to examine the extent to which Kidney Transitions Specialists coordinate services and the extent to which patients adhere to Kidney Transitions’ Specialists recommendations (i.e., attend scheduled classes or 1-on-1 meetings for decisionmaking).
Outcomes
We hypothesize the integrated components of Patient-Centered Kidney Transitions Care will improve several patient reported, biomedical, and health system outcomes. We will conduct the same outcomes assessments among patients with advanced kidney disease in intervention and control clinics. We will also collect data on potential correlates of intervention effectiveness. All assessments will occur through (a) participating patients' self-reported responses to telephone questionnaires or (b) data extracted from patients’ EHRs (Table 5). In separate analyses, we will investigate the effectiveness of the intervention compared to usual care on each of five primary outcomes. We will seek evidence that the intervention has an effect on any of these outcomes individually.
Table 5.
Measures collected to assess outcomes and correlates in the PREPARE NOW study
| Source | |
|---|---|
| Primary Outcomes | |
| Patient ‘Control’, Decision-Making | |
| • Empowerment Score | Q59 |
| • Confidence (self-efficacy) with self care score | Q60 |
| • % Patients deciding to initiate self-care treatment | Q, EHR |
| Medical | |
| • Hospitalizations | EHR, Q |
| Health System Culture | |
| • % Patients with advance care plans or kidney failure treatment preferences broadcast in EHR | EHR |
| Secondary Outcomes | |
| • % Patients with self-care biomedical care plans (PD, Home Hemodialysis, or Transplant referrals) | EHR |
| • % Patients achieving values aligned care within 6 months of kidney failure treatment initiation | Q |
| • Values and preferences documented in EHR | EHR |
| • Emergency dialysis initiation | EHR, Q |
| • Time to kidney failure | EHR, Q |
| • Vascular access (e.g., fistula) in place at hemodialysis initiation | EHR, Q |
| Demographics | |
| • Age, gender, ethnicity/race, education, health insurance status, employment, income, financial well being | EHR¶, Q61§ |
| Physical and Mental Health Status | |
| • Kidney Function (glomerular filtration rate) | EHR62 |
| • Presence and control of kidney disease progression risk factors [blood pressure, blood glucose, lipids, body mass index] | EHR63-67 |
| • Comorbid Health Conditions | EHR68 |
| • Depression, anxiety, need for mental health support | Q69 |
| • Quality of Life | Q70 |
| Self-Care Behaviors | |
| • Self-management, diet, exercise | Q60 |
| • Medication adherence | Q,71 MPR** |
| Nephrology Care | |
| • Duration and frequency of care | EHR, Q |
| • Patient centeredness of care | Q72 |
| Health Literacy and Numeracy | |
| • Health literacy | Q73-75 |
| Decision-Making | |
| • Decisional conflict | Q76 |
| • Preferred involvement in decisions-making | Q77 |
| • Confidence in decision-making (decision self-efficacy) | Q78 |
| • Control (locus of control) | Q79 |
| Medical Care Plans | |
| • Barriers to complex treatment plans | Q |
| Intervention Fidelity, Feasibility and Sustainability | |
| • Kidney Transitions Specialist adherence to protocol | O¶ |
| • Sustainability (RE-AIM)80** | Q, FG$, DI± |
Annual assessments over 36 months;
EHR=electronic health record;
Q=questionnaire;
O=Observation;
FG=Focus group;
DI-Directed Interview;
MPR=Medication possession ratio from Geisinger Health Plan claims data
Patient Reported Outcomes
Three primary patient reported outcomes will measure patients’ perceptions of their empowerment, capability with self-management, and decisions to initiate self-care kidney failure treatments via telephone questionnaires. Secondarily, we will measure evidence of patients’ enactment of care plans reflecting their preferred kidney treatments that align with their values (Table 5).
Biomedical Outcomes
One primary biomedical outcome will measure patients’ rate of all-cause hospitalizations through EHR data. We will also explore associations between the intervention and cause-specific as well as types of (e.g., planned versus unplanned) hospitalizations. A majority of Geisinger nephrology patients receive hospital care within Geisinger Health System. We will also ask patients to report the presence and number of non-Geisinger health system hospitalizations they have experienced in the 12 months prior via questionnaire. Secondarily, we will measure outcomes reflecting patients’ improved self-management of kidney disease (including time to kidney failure from index registry date) and patients’ less chaotic or risky transitions to kidney failure (e.g., planned versus emergency initiation of dialysis37 or initiation of dialysis with a fistula versus infection-prone catheters81).
Health System Outcomes
One primary health system outcome will measure the proportion of patients in nephrology clinics who have completed plans for possible future kidney failure therapy (preparatory steps for kidney failure treatments such as referrals to vascular surgery or transplant) or have kidney failure treatment preferences broadcast in the EHR. Completion of any of these actions reflects a critical step toward nephrologists’ patient-centered facilitation of patients’ preferred treatments. Currently, physicians in usual care are able to enter kidney failure treatment preferences on the EHR problem list, although this practice is not routinely encouraged in patients’ kidney care plans. The intervention will encourage these behaviors through improved electronic health system tools. We will query the EHR for evidence of kidney failure treatment preferences broadcast across all study patients in intervention and control clinics. Because advance care plans may also be documented in patient progress notes that are not easily queried as discrete data elements using routine computer algorithms, we will also conduct manual reviews of notes in EHRs (performed by a trained study nurse) for all study patients to document evidence of these advance care plans or EHR broadcasts indicating patients’ kidney failure treatment preferences.
Statistical Analysis
Sample size estimates
Among all study clinics, we estimate approximately 1000 patients will qualify for the disease registry and therefore will be eligible for observation and enrollment in PREPARE NOW. Each year, we will attempt to contact the entire sample of patients actively listed on the disease registry within the prior 12 months and invite them to participate in a study questionnaire. Within each year, we expect at least 500 patients will respond to the study telephone questionnaires.82,83 Recruitment of 500 participants for study questionnaires will enable adequate statistical power at the end of the study to detect clinically significant minimum estimated differences between intervention versus control clinics for each of the patient centered primary study outcomes. Because we will have medical records on all disease registry participants, we will also be able to capture and summarize all biomedical and health system outcomes measured through the EHR. Power estimates are based on 0.05 level, two-sided t-test comparisons of study arms, and account for a cluster-randomized design with eight (8) clinics and a 0.05 intra-class correlation coefficient, extrapolating from cluster randomized studies with similar design.84 (Table 6) While this approach is a simplification of the proposed analysis plan for all measurements over time, it should provide a conservative estimate of expected power to detect such overall changes by the end of the study.
Table 6.
Power and sample size estimates and assumptions
| Outcome | Assumed Baseline (SD) |
Previously Observed Change |
Min Diff to Detect |
Sample Size |
Power |
|---|---|---|---|---|---|
| Patient Reported Outcomes | |||||
| Empowerment | |||||
| Intervention | 98.40 (SD 9.19) |
+6.64 points34 | 6.64 | 500 | 91.4% |
| 550 | 91.9% | ||||
| Control | 98.40 (SD 9.19) |
+0 points | 600 | 92.3% | |
| 997 | 93.9% | ||||
| Self-Efficacy with self-care | |||||
| Intervention | 89.56 (SD 14.23) |
+6.96 points34 | 8.8 points | 500 | 81.7% |
| 550 | 82.5% | ||||
| Control | 89.56 (SD 14.23) |
+0 points | 600 | 83.2% | |
| 997 | 86.1% | ||||
| Decision to start self-care treatment | |||||
| Intervention | 17% | +48%31 | +22% | 500 | 82.8% |
| 550 | 83.6% | ||||
| Control | 17% | +5% | +5% | 600 | 84.3% |
| 997 | 87.2% | ||||
| Patient Biomedical Outcomes | |||||
| Hospitalizations (per 1,000 patient months) | |||||
| Intervention | 134 | 767,85(−43%) | 130 (−3%) | 500 | 86.3% |
| 550 | 86.7% | ||||
| Control | 134 | 134(−0%) | 600 | 87.1% | |
| 997 | 88.8% | ||||
| Health System Culture | |||||
| Advance directives or orders documented in EHR | |||||
| Intervention | 12% | +65% 86 | +24% | 500 | 80.5% |
| 550 | 81.3% | ||||
| Control | 12% | +6% | +6% | 600 | 82.1% |
| 997 | 85.2% | ||||
Statistical analysis
The initial exploration of outcome variables (summary measures, graphical displays) will be used to assess the reasonableness of distributional assumptions and observed balance in key predictor variables. All primary analyses comparing the effect of the intervention strategy over time on our primary and secondary outcomes will be by intention-to-treat, including all eligible study participants with relevant and permissible data as appropriate to the outcome. We will also track patient visit locations and address any potential contamination due to patient crossover between clinics. Among the primary outcomes, several are binary (e.g., presence/absence in EHR of advance care plans or EHR broadcasts, and self-care treatment decisions), two are scaled scores (empowerment and self-efficacy with self-care), and time until first hospitalization could be treated as time-to-event, with the first year of eligibility as the start time. If the hospitalization date(s) is not captured in the EHR (e.g., hospitalizations outside the Geisinger system) for a substantial proportion of patients (thus requiring reliance on recall) or if the likelihood of multiple hospitalizations per year in this population is substantial, then we would treat hospitalization as a count variable to obtain rates per year, rather than using a time-to-event analysis over the entire study period.
We will collect all measures at baseline and at nine (9) to twelve (12) month intervals for three years. A questionnaire tracking database will be used to schedule a window in which a participant should receive his/her next call. Thus we will have correlated trajectory data over time at the individual level, and can expect some correlation within clinics as well, due to practice patterns, interactions among health care providers, etc. Generalized linear mixed models will be our preferred methodology, which includes (as special cases) hierarchical models. Participants will be considered nested within clinics, reflective of the intent-to-treat strategy for treatment assignment.
We will assume the following for each outcome: (a) the patient-level trajectory over time will vary among patients in both slope and intercept; (b) the average slope and intercept within each clinic could be different. These two assumptions would lead to including both random slopes and intercepts at the patient and clinic levels in initial analyses. Graphical and statistical assessments will be used to check whether such assumptions are reasonable. Likelihood ratio tests will be used to test the reasonableness of those assumptions by testing whether the variance components of those random effects are significantly larger than 0. We expect that clinic size could be a major cluster-level confounder, and could be included in the model as a fixed effect altering the overall average trajectory as function of size.
Baseline patient demographics and health status are also potential confounders. Time-dependent measures of health could be mediators of the impact of the intervention, in that they may be influenced by the intervention and will also influence the impact of the intervention. Initial analyses will assume that changes in these mediators will be randomly distributed across intervention and control patients, aside from the influence of the intervention on these mediators. In other words, these mediators will not be included in initial models. We will obtain the conditional average treatment effect for the first four outcomes by means of generalized linear models, assuming normality when we can for the continuous outcomes (including scaled measures) and either the binomial or Poisson distribution for count variables, accounting for time-on-study. Canonical links will be used (e.g., logit link for binary data). Hypothesis tests will focus on the difference between study groups on the changes observed over time, rather than repeated testing each year. In other words, we will be looking to find changes in the average or “typical” outcome profile over time that differs from one study arm to the other. Two-sided tests will be used, although we are hypothesizing an improvement in these measures over time in the intervention arm.
The above modeling approach will be considered primary, but there are other questions we would like to address that would require enhanced models. While the same basic framework will be used, we want to assess whether time-dependent measures of health (e.g., risk level for kidney failure, blood pressure or serum glucose control) and/or time-stationary basic demographics (age, gender, education), are mediating the impact of the intervention. The modeling approach described allows us to augment the above models with both time-varying and time-stationary covariates and test for a mediation impact. It is possible that we will then be able to create principal strata among which we can use structural equation models to account for mediation in a proposed causal pathway.
A key assumption of our design is that clinics are similar in patient composition and resources. If some clinics care for more predominately elderly patient panels, the types of support and care plans could differ substantially. For example, elderly populations may need more support or make different decisions on care. To test this assumption, we will characterize clinics by panel size and their composition of elderly patients. We will perform sensitivity analyses by employing models with interaction terms accounting for differences we find. We will also assess heterogeneity of treatment effects across various considerations, including clinics’ panel burden of comorbidity.
Discussion
To our knowledge, the PREPARE NOW Study will be among the first U.S. studies to rigorously quantify the effectiveness of a comprehensive and fully integrated health system intervention to improve kidney transitions care among patients with chronic kidney disease as they transition toward kidney failure. As the number of efficacious approaches to improving kidney patients’ self-management and biomedical outcomes continues to increase, effective strategies to implement these interventions in real world clinical settings are needed. PREPARE NOW will provide important evidence on the effectiveness of implementing these real world interventions to improve the experiences, capabilities, and clinical outcomes of patients with chronic kidney disease.
PREPARE NOW interventions address the full Chronic Care Model,43 through programs employing information technologies (e.g., continually updated disease registry and continuous population risk stratification) and through patient centered services that not only address patients’ care coordination needs, but also address a number of other needs to empower patients with knowledge, resources, and skills to improve their own care and clinical outcomes. PREPARE NOW outcomes assessments, which will capture both the effectiveness of interventions through patients’ reports as well as through their health records, will provide a holistic view of programs’ success and value. In addition, PREPARE NOW is also collecting information on the process of implementing the intervention and barriers and facilitators to achieving intervention fidelity. As a result, findings from PREPARE NOW will answer important questions regarding the feasibility and effectiveness of a number of kidney care strategies that have been studied individually but have not routinely been jointly implemented in a coordinated fashion. Finally, since our outcomes assessments will include details of planning and initiation of renal replacement therapy, we will be able to quantify ‘Optimal End Stage Renal Disease Starts’ (receipt of a preemptive kidney transplant, initiating home dialysis, or initiating outpatient incenter hemodialysis via arteriovenous fistula or arteriovenous graft), a measure endorsed by the National Quality Forum.87
We anticipate findings from PREPARE NOW will help inform the implementation of similar interventions among health systems across the U.S. We also anticipate they will provide numerous stakeholders, including patients, their families, health care providers, and payers with critically needed evidence to support the implementation of these interventions in other settings. Nonetheless, we anticipate some potential limitations. First, since Geisinger is an integrated health system which shares a unified electronic health record and informatics platform, some innovations deployed in PREPARE NOW may not be fully portable to nephrology care in other settings. Similarly, not all nephrology practices will have capabilities enabled through the Geisinger Health Plan, such as care management. In addition, since PREPARE NOW is being conducted within a single health system in rural Pennsylvania, our findings may not be generalizable to all populations of patients. While the Geisinger population is not racially diverse, the low education and low income levels are similar to national rates. In addition, the Geisinger CKD population has a larger proportion of patients age >65 which is consistent with national trends showing that the elderly have the highest incident ESRD growth rate. It is also possible that patients may not progress to kidney failure in the time period that we observe them. However, patients’ experiences and preparation for potential kidney care transitions are important proximal outcomes reflecting their likelihood of achieving optimum transitions when they do occur. For this reason, we have chosen to focus on patient-centered outcomes (e.g., perceived empowerment, confidence with care decisions, or achievement of referrals) that occur proximal to the development of kidney failure. Finally, since our program is being implemented within nephrology specialty care, it is possible that we are missing high-risk patients who have never been referred to nephrology. However, primary care providers are encouraged to refer high-risk patients to nephrology through the use of a best-practice alert. Despite these potential limitations, we believe PREPARE NOW will provide critically needed insight into the effectiveness of patient centered interventions to enhance kidney care and patients’ clinical outcomes.
Conclusion
The PREPARE NOW Study may provide key evidence on the effectiveness of comprehensive patient-centered interventions to improve patients’ care as they transition to kidney failure. If they are effective, these interventions could be broadly disseminated to improve the care and outcomes of patients across the U.S. and elsewhere.
Acknowledgements
We would like to acknowledge Elizabeth R. DeLong, PhD, Gary Green and Holly St. Clair for their early work in assisting the design of the study.
Funding source and role of funding source
This work was supported by a Patient-Centered Outcomes Research Institute (PCORI) Award [IHS-1409-20967]. The views in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. The funding source had no involvement in the study design; the collection, analysis or interpretation of data; the writing of the report or in the decision to submit this article for publication.
Abbreviations
- CKD
Chronic kidney disease
- EHR
Electronic health record
Footnotes
Competing interests
We have no competing interests to report.
References
- 1.United States Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2012. http://www.usrds.org/adr.htm. Accessed 2013.
- 2.Szeto CC, Kwan BC, Chow KM, et al. Life expectancy of Chinese patients with chronic kidney disease without dialysis. Nephrology (Carlton). 2011;16(8):715–719. [DOI] [PubMed] [Google Scholar]
- 3.Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4(10):1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussain JA, Mooney A, Russon L. Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med. 2013;27(9):829–839. [DOI] [PubMed] [Google Scholar]
- 5.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16): 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans RW, Manninen DL, Garrison LP Jr., et al. The quality of life of patients with end-stage renal disease N Engl J Med. 1985;12(9):553–559. [DOI] [PubMed] [Google Scholar]
- 7.Mix TC, St peter WL, Ebben J, et al. Hospitalization during advancing chronic kidney disease. Am J Kidney Dis. 2003;42(5):972–981. [DOI] [PubMed] [Google Scholar]
- 8.Lacson E Jr., Bruce L, Li NC, Mooney A, Maddux FW. Depressive affect and hospitalization risk in incident hemodialysis patients. Clin J Am Soc Nephrol. 2014;9(10): 1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulutas O, Farragher J, Chiu E, Cook WL, Jassal SV. Functional Disability in Older Adults Maintained on Peritoneal Dialysis Therapy. Perit Dial Int. 2016;36(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altintepe L, Levendoglu F, Okudan N, et al. Physical disability, psychological status, and health-related quality of life in older hemodialysis patients and age-matched controls. Hemodialysis international International Symposium on Home Hemodialysis. 2006;10(3):260–266. [DOI] [PubMed] [Google Scholar]
- 11.Belasco A, Barbosa D, Bettencourt AR, Diccini S, Sesso R. Quality of life of family caregivers of elderly patients on hemodialysis and peritoneal dialysis. Am J Kidney Dis. 2006;48(6):955–963. [DOI] [PubMed] [Google Scholar]
- 12.Belasco AG, Sesso R. Burden and quality of life of caregivers for hemodialysis patients. Am J Kidney Dis. 2002;39(4):805–812. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein FO, Story K, Firanek C, et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int. 2008;74(9):1178–1184. [DOI] [PubMed] [Google Scholar]
- 14.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients' preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341(22): 1661–1669. [DOI] [PubMed] [Google Scholar]
- 15.Boulwware LE. Shared and Informed Decision-Making in Nephrology Practices. Unpublished Work in Progress: Duke University; 2014. [Google Scholar]
- 16.Levin A, Djurdjev O, Beaulieu M, Er L. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis. 2008;52(4):661–671. [DOI] [PubMed] [Google Scholar]
- 17.Buck J, Baker R, Cannaby AM, Nicholson S, Peters J, Warwick G. Why do patients known to renal services still undergo urgent dialysis initiation? A cross-sectional survey. Nephrol Dial Transplant. 2007;22(11):3240–3245. [DOI] [PubMed] [Google Scholar]
- 18.Hughes SA, Mendelssohn JG, Tobe SW, McFarlane PA, Mendelssohn DC. Factors associated with suboptimal initiation of dialysis despite early nephrologist referral. Nephrol Dial Transplant. 2013;28(2):392–397. [DOI] [PubMed] [Google Scholar]
- 19.Mendelssohn DC, Curtis B, Yeates K, et al. Suboptimal initiation of dialysis with and without early referral to a nephrologist. Nephrol Dial Transplant. 2011;26(9):2959–2965. [DOI] [PubMed] [Google Scholar]
- 20.Sheu J, Ephraim PL, Powe NR, et al. African American and non-African American patients' and families' decision making about renal replacement therapies. Qual Health Res. 2012;22(7):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong A, Lowe A, Sainsbury P, Craig JC. Experiences of parents who have children with chronic kidney disease: a systematic review of qualitative studies. Pediatrics. 2008;121(2):349–360. [DOI] [PubMed] [Google Scholar]
- 22.O'Hare AM, Allon M, Kaufman JS. Whether and when to refer patients for predialysis AV fistula creation: complex decision making in the face of uncertainty. Seminars in dialysis. 2010;23(5):452–455. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Vargas PA, Craig JC, Gallagher MP, et al. Barriers to timely arteriovenous fistula creation: a study of providers and patients. Am J Kidney Dis. 2011;57(6):873–882. [DOI] [PubMed] [Google Scholar]
- 24.Kazley AS, Simpson KN, Chavin KD, Baliga P. Barriers facing patients referred for kidney transplant cause loss to follow-up. Kidney Int. 2012;82(9):1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dageforde LA, Box A, Feurer ID, Cavanaugh KL. Understanding Patient Barriers to Kidney Transplant Evaluation. Transplantation. 2015;99(7):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–1559. [DOI] [PubMed] [Google Scholar]
- 27.Jolly Se, Navaneethan SD, Schold JD, et al. Chronic kidney disease in an electronic health record problem list: quality of care, ESRD, and mortality. Am J Nephrol. 2014;39(4):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navaneethan SD, Jolly SE, Sharp J, et al. Electronic health records: a new tool to combat chronic kidney disease? Clin Nephrol. 2013;79(3):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride D, Dohan D, Handley MA, Powe NR, Tuot DS. Developing a CKD registry in primary care: provider attitudes and input. Am J Kidney Dis. 2014;63(4):577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devins GM, Mendelssohn DC, Barre Pe, Taub K, Binik YM. Predialysis psychoeducational intervention extends survival in CKD: a 20-year follow-up. Am J Kidney Dis. 2005;46(6): 1088–1098. [DOI] [PubMed] [Google Scholar]
- 31.Manns BJ, Taub K, Vanderstraeten C, et al. The impact of education on chronic kidney disease patients' plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int. 2005;68(4):1777–1783. [DOI] [PubMed] [Google Scholar]
- 32.Mason J, Khunti K, Stone M, Farooqi A, Carr S. Educational interventions in kidney disease care: a systematic review of randomized trials. Am J Kidney Dis. 2008;51(6):933–951. [DOI] [PubMed] [Google Scholar]
- 33.Hill-Briggs F, Lazo M, Peyrot M, et al. Effect of problem-solving-based diabetes self-management training on diabetes control in a low income patient sample. J Gen Intern Med. 2011;26(9):972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsay SL, Hung LO. Empowerment of patients with end-stage renal disease--a randomized controlled trial. Int J Nurs Stud. 2004;41(1):59–65. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan C, Leon JB, Sayre SS, et al. Impact of navigators on completion of steps in the kidney transplant process: a randomized, controlled trial. Clin J Am Soc Nephrol. 2012;7(10): 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geisinger. About Geisinger. 2018; https://www.geisinger.org/about-geisinqer Accessed March 23, 2018.
- 37.Kidney Disease Improving Global Outcomes. Guidelines for CKD Evaluation and Management. (n.d.); http://kdigo.org/home/guidelines/ckd-evaluation-management. Accessed 2014.
- 38.Kidney Disease Improving Global Outcomes. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2013; http://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed March 23, 2018
- 39.Miller WR, Rollnick S. Motivational Interviewing: Helping People Change New York, NY: Guilford Press; 2012. [Google Scholar]
- 40.O'Connor A Ottawa Decision Support Framework to Address Decisional Conflict. (n.d.); http://decisionaid.ohri.ca/docs/develop/ODSF.pdf. Accessed Accessed March 15, 2009.
- 41.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3(1):19–30. [PubMed] [Google Scholar]
- 42.Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1614–1617. [DOI] [PubMed] [Google Scholar]
- 43.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood). 2009;28(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288(15):1909–1914. [DOI] [PubMed] [Google Scholar]
- 45.Tangri N, Grams ME, Levey AS, et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA. 2016;315(2): 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medical Education Institute. My Life, My Dialysis Choice 2016; http://mydialysischoice.org/#values. Accessed February 15, 2018.
- 47.Ephraim PL, Powe NR, Rabb H, et al. The providing resources to enhance African American patients' readiness to make decisions about kidney disease (PREPARED) study: protocol of a randomized controlled trial. BMC Nephrol. 2012;13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill-Briggs F Problem solving in diabetes self-management: a model of chronic illness self-management behavior. Ann Behav Med. 2003;25(3):182–193. [DOI] [PubMed] [Google Scholar]
- 49.Legare F, O'Connor AC, Graham I, et al. Supporting patients facing difficult health care decisions: use of the Ottawa Decision Support Framework. Can Fam Physician. 2006;52:476–477. [PMC free article] [PubMed] [Google Scholar]
- 50.The Ottawa Hospital. Ottawa Decision Support Framework (ODSF). 2017; https://decisionaid.ohri.ca/odsf.html. Accessed March 23, 2018.
- 51.National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. 2002;http://www2.kidney.org/professionals/kdoqi/guidelines_ckd/p6_comp_g9.htm. Accessed 2014.
- 52.Kidney Disease Improving Global Outcomes. KDIGO 2012 Clincal Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2013.
- 53.Ephraim PL, Hill-Briggs F, Roter DL, et al. Improving urban African Americans' blood pressure control through multi-level interventions in the Achieving Blood Pressure Control Together (ACT) study: a randomized clinical trial. Contemp Clin Trials. 2014;38(2):370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ameling JM, Auguste P, Ephraim PL, et al. Development of a decision aid to inform patients' and families' renal replacement therapy selection decisions. BMC Med Inform Decis Mak. 2012;12:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elwyn G, O'Connor AM, Bennett C, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLoS One. 2009;4(3):e4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Kidney Foundation. NKF PEERS. http://www.kidney.org/patients/peers Accessed September 19, 2014.
- 57.Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ. 2013;347:f6753. [DOI] [PubMed] [Google Scholar]
- 58.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lev EL, Owen SV. A measure of self-care self-efficacy. Res Nurs Health. 1996; 19(5):421–429. [DOI] [PubMed] [Google Scholar]
- 60.Wild MG, Wallston KA, Green JA, et al. The Perceived Medical Condition Self-Management Scale can be applied to patients with chronic kidney disease. Kidney Int. 2017;92(4):972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prawitz AD, Garman ET, Sorhaindo B, Kim J, O'Neill B, Drentea P. InCharge financial distress/financial well-being scale: development, norming, and score interpretation. Journal of Financial Counseling and Planning. 2006;17(1):34–50. [Google Scholar]
- 62.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Heart L, and Blood Institute,. The Practical Guide Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health;2000. [Google Scholar]
- 64.National Heart L, and Blood Institute,. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). National Institutes of Health;2002. [Google Scholar]
- 65.American Diabetes Association. Standards of medical care in diabetes--2007. Diabetes Care. 2007;30 Suppl 1:S4–s41. [DOI] [PubMed] [Google Scholar]
- 66.Inker LA, Astor bC, Fox CH, et al. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am J Kidney Dis. 2014;63(5):713–735. [DOI] [PubMed] [Google Scholar]
- 67.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8). JAMA. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 68.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- 69.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. [DOI] [PubMed] [Google Scholar]
- 70.Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res. 2012;21(1): 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han HR, Song HJ, Nguyen T, Kim MT. Measuring self-care in patients with hypertension: a systematic review of literature. J Cardiovasc Nurs. 2014;29(1):55–67. [DOI] [PubMed] [Google Scholar]
- 72.Stewart M, Meredith L, Ryan BL, Brown JB. The patient perception of patient centeredness questionannaire (PPPC) #04–1. Ontario, Canada Centre for Studies in Family Medicine; 2004. [Google Scholar]
- 73.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 74.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27(5):663–671. [DOI] [PubMed] [Google Scholar]
- 75.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. [DOI] [PubMed] [Google Scholar]
- 76.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 77.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 78.O'Connor A Decision Self-Efficacy Scale. 1995; http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decision_SelfEfficacy.pdf. Accessed February 11,2011.
- 79.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 Pt 1): 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glasgow rE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lorenzo V, Martn M, Rufino M, Hernandez D, Torres A, Ayus JC. Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: an observational cohort study. Am J Kidney Dis. 2004;43(6):999–1007. [DOI] [PubMed] [Google Scholar]
- 82.Adams RE, Urosevich TG, Hoffman SN, et al. Social Support, Help-Seeking, and Mental Health Outcomes Among Veterans in Non-VA Facilities: Results from the Veterans' Health Study. Military behavioral health. 2017;5(4):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolff JL, Darer JD, Berger A, et al. Inviting patients and care partners to read doctors' notes: OpenNotes and shared access to electronic medical records. J Am Med Inform Assoc. 2017;24(e1):e166–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smeeth L, Ng ES. Intraclass correlation coefficients for cluster randomized trials in primary care: data from the MRC Trial of the Assessment and Management of Older People in the Community. Control Clin Trials. 2002;23(4):409–421. [DOI] [PubMed] [Google Scholar]
- 85.Dixon J, Borden P, Kaneko TM, Schoolwerth AC. Multidisciplinary CKD care enhances outcomes at dialysis initiation. Nephrol Nurs J. 2011;38(2): 165–171. [PubMed] [Google Scholar]
- 86.Hayek S, Nieva R, Corrigan F, et al. End-of-life care planning: improving documentation of advance directives in the outpatient clinic using electronic medical records. J Palliat Med. 2014;17(12):1348–1352. [DOI] [PubMed] [Google Scholar]
- 87.National Quality Forum. NQF-Endorsed Measures for Renal Conditions, 2015: Technical Report 2015