Abstract
Background:
Imaging techniques are increasingly being used to examine the neural correlates of stress and emotion processing; however, relations between the primary stress hormone cortisol, the fMRI environment, and individual differences in response to emotional challenges are not yet well studied. The present study investigated whether cortisol activity prior to, and during, an fMRI scan may be related to neural processing of emotional information.
Methods:
Twenty-six healthy individuals (10 female) completed a facial emotion perception test (FEPT) during 3T fMRI.
Results:
Pre-scan cortisol was significantly correlated with enhanced amygdala, hippocampal, and subgenual cingulate reactivity for facial recognition. Cortisol change from pre- to post-scanning predicted greater activation in precuneus for both fearful and angry faces. A negative effect between overall face accuracy and activation in limbic regions was observed.
Conclusion:
Anticipation of the fMRI environment may serve as a stressful experience that can lead to greater heterogeneity of brain activation in control samples, decreasing power to detect differences between clinical and comparison groups.
Keywords: Emotion, fMRI, amygdala, stress, cortisol
The hypothalamic-pituitary-adrenal (HPA) axis is the primary circuit of the neuroendocrine stress system and is the main route by which the brain influences physical and psychological processes following exposure to threatening stimuli [1]. The HPA axis response to stress is a relatively slow hormonal cascade resulting in the production of glucocorticoids (cortisol in humans and corticosterone in animals). One of the primary functions of the HPA axis is to mobilize resources for defense during stress and/or threat and subsequently for repair and healing [2]. Although fundamentally adaptive, the stress response system can become dysregulated under conditions of chronic or traumatic stress, resulting in increased susceptibility to physical and mental disorder [3]. Illustratively, exaggerated cortisol stress responses and elevated basal levels of cortisol have been implicated in the development and maintenance of depression and other mental disorders [4]. This relation may be partially mediated by enhanced amygdala activation and subsequently reduced dendritic arborization in both the amygdala and hippocampus [5]. However, much remains to be learned about the relations between neurobiological and physiological responses to stress. Few investigations have examined anticipatory cortisol or cortisol change in adults undergoing a functional magnetic resonance imaging (fMRI) scan in the absence of a standardized stressor task prior to scanning. Thus, it is still unknown whether the actual assessment of neural activity in a novel, confined space itself induces a physiological stress response in healthy adults, which could have implications for interpreting neuroimaging data.
Networks involved in cortisol production and modulation overlap with cognitive and affective networks that direct emotion experience and regulation. Experimental and neuroimaging research on the neural correlates of cortisol reactivity has largely focused on the hippocampus, amygdala, and the prefrontal cortex [5,6]. Following stress, increased activity in the hippocampus facilitates down-regulation of the HPA axis via inhibitory connections to the paraventricular nucleus of the hypothalamus [7], revealing an inverse relationship between hippocampal activation and cortisol responses to stress [8–10]. Conversely, increased activation in the amygdala is correlated with increased cortisol levels, suggesting that the amygdala is involved in the recruitment and ongoing excitation of the HPA axis [11,12]. In addition, the insula is involved in interoceptive monitoring [13] and may respond to fluctuations in cortisol. Insula and amygdala involvement in saliency, emotion, and attention is well established [14], and hyperactivity within this network may underlie anxiety-related processes [5,14,15]. Anatomical connections to the hypothalamus, amygdala, nucleus accumbens and additional limbic structures have led to the supposition that this network is involved in autonomic and visceral regulation [16]. Areas of the lateral and medial prefrontal cortex (PFC) participate in both the activation and regulation of the HPA axis [17]. For example, activation of the medial PFC has been associated with decreased cortisol reactivity to stress [8]. Given its functional connectivity with the amygdala, the medial PFC likely participates in the down-regulation of the stress response and might facilitate attenuation of cortisol levels [18–21]. Finally, left and right lateral PFC activation has been associated with increased and decreased cortisol reactivity to psychosocial stress, respectively [8,17,22,23].
Functional magnetic resonance imaging (fMRI) is increasingly being used to examine the neural circuits implicated in the activation and modulation of the stress response system. However, it is unknown whether the fMRI scanner environment itself may serve as a stressor and elicit HPA activation. The anticipation and experience of fMRI procedures can potentially evoke distress, anxiety, claustrophobia and arousal of the sympathetic nervous system [24–27]. Low to moderate levels of stress from fMRI exposure may increase cortisol levels, thereby affecting neural functioning and task performance, even among healthy control subjects [28]. A few investigations have demonstrated that anticipation of, and first-time exposure to, fMRI procedures increases cortisol reactivity and heart rate [25,28–30] whereas others have found that fMRI produces a more nuanced stress effect, impacting only some individuals or dissipating over the course of a fMRI scan [24,31]. The lack of clarity on this important topic is notable given the substantial amount of research conducted using emotion regulation paradigms that operate on existing assumptions about cortisol reactivity for “baseline” conditions. In short, these presumptions maintain that cortisol levels measured over the course of fMRI procedures do not differ from HPA activity as measured over typical daily activities [32,33]. These assumptions may impede the appropriate use of emotion and stress challenge tasks in the fMRI environment and may threaten clear interpretation of results when these challenge tasks are used in concert with assessment of HPA reactivity.
In building a better understanding of the stress response elicited by fMRI procedures, we tested the following hypotheses: 1) cortisol levels immediately prior to the fMRI procedures would be higher compared to baseline day measurements in a time-locked comparison; 2) individual differences in elevated cortisol in anticipation of an fMRI scanning session (pre-scan cortisol) will predict greater subsequent activation in the limbic and regulatory regions of the brain during an emotional perception task; 3) greater cortisol reactivity during the emotional perception task will be related to greater activation in the limbic and regulatory regions of the brain during the emotional perception task; 4) increased brain activation in the limbic and regulatory regions of the brain during the emotional perception task will be associated with poorer performance on the task.
Methods
Participants
Twenty-six participants (ten female) were recruited as healthy control comparison subjects for one of two studies, an investigation of emotion processing in Cushing’s Disease [5] and an investigation of emotion processing in Major Depressive Disorder [34]. Participants for both studies were recruited through advertisements at a large Midwestern university and in the surrounding communities. Healthy controls for the study of Cushing’s Disease were scanned from 2003–2008, and healthy controls for the Major Depressive Disorder study were scanned from 2006–2011. In the current study, participants ranged in age from 18–65, with an average age of 37.2 (SD=17.2).
Procedure
Upon arrival at the study laboratory, participants completed informed consent and were interviewed by a psychologist or a clinical psychology trainee at the advanced graduate level in accordance with the Structured Clinical Interview for DSM-IV (SCID-IV) non-patient edition. Only participants who did not endorse any past or current psychiatric or neurologic disorder, including alcohol or other substance abuse or dependence (both for themselves and first degree family members) were included. Participants were also screened using a structured interview to assess for neurological conditions and for fMRI safety. At the time of the screening interview, participants were briefed on the scanning procedure. The fMRI data were collected on a separate day following the screening interview. All participants in the current study were run through an identical screening, diagnostic, neuroimaging and cortisol protocol, including acquisition of images in the same 3T Signa scanner. Upon arrival, participants’ salivary cortisol (pre-scan cortisol) was measured (see procedure timeline, Figure S1). Participants were then guided through a practice run of the study tasks while outside of the scanner in order to limit learning bias in the results. The study tasks, including the Facial Emotion Perception Test, were then administered in the scanner, for a total time of approximately 70 minutes (including placement). After being removed from the scanner, participants were given a brief break before providing a final saliva sample. Finally, all participants were debriefed and compensated for their time.
Salivary Cortisol Collection and Assay
Participants provided saliva samples 15–30 minutes prior to entering the scanner and approximately 20–35 minutes following completion of the FEPT [35,36], which lasted 25 minutes, using Salivette Cortisol tubes (STARSTEDT AG & Co.; Figure S1). These cortisol measurements provide an assessment of participants’ anticipatory stress related to the fMRI tasks/scanner, as indicated by pre-scan cortisol level, and of their response to the FEPT, as indicated by measuring the change between pre-scan and post-scan cortisol levels. Scans were collected between 8 am and 4 pm, though the majority of the scans (62%) were collected in the morning. Time of scan was transformed into a 24-hour variable for use as a covariate in imaging regression analyses to account for circadian profile throughout the day. Due to concerns about pre-scan anticipatory stress with fMRI, we invited the last thirteen subjects (5 female) to provide additional saliva samples throughout a weekday. These subjects consented to collecting saliva samples at 8 am, 12 pm, 4 pm, and 9 pm during the course of their normal weekday activities (Tuesday, Wednesday or Thursday) and on a day different from the fMRI scan. These measurements allowed for a comparison of baseline cortisol to pre- and post-scan levels assessed on the day of fMRI participation.
All cortisol samples were stored at −80C until they were sent to the Clinical Ligand Assay Service Satellite (CLASS) Laboratory at the University of Michigan School of Public Health Department for analysis. The competitive immunoassay was on a Siemen Centaur automated analyzer, using chemiluminescent technology. The inter- and intra-assay coefficients of variation at 0.7 μg/dl were 12.4% and 3.6% respectively [37]. All salivary cortisol values (μg/dL) were log transformed. One subject was excluded from pre-scan cortisol modeling and one subject was excluded from post-scan cortisol modeling for having insufficient quantities of saliva for analysis. To assess cortisol change over the course of the experiment, a standardized residualized change score was computed by regressing the post-scan cortisol sample on the pre-scan sample. A residualized change score was the chosen method because it adjusts for the pre-scan sample and avoids some of the reliability concerns with difference scores. The two subjects missing one cortisol sample were excluded from analyses of cortisol reactivity (pre- to post-scan cortisol change), resulting in 24 subjects for this regression model.
Facial Emotion Perception Task
The Facial Emotion Perception Task (FEPT) [5,34,38] was designed to assess both the accuracy and speed with which participants can identify facial expressions. As part of the task, participants also identify animals, in order to provide a way by which to control for visual processing ability and fine motor speed and isolate the face-specific performance. During the task, participants categorize faces (MACBrain Foundation) [39,40] into one of four categories (fearful, angry, happy, or sad) and animals into one of four categories (dogs, cats, primates, or birds). To limit the bias introduced by learning, participants first complete a trial run with the same timing and instructions, outside the scanner, using the Ekman faces [35,41]. The task was designed based upon prior work to detect biases in emotional identification, so neutral faces are presented for some trials, yet neutral is not a choice available to participants.
Each trial began with a briefly presented orienting cross (500ms) followed by presentation of the stimulus face or animal (300ms), a visual mask (100ms), and a response window (2600ms) during which participants select the category of choice using a five-button response claw. The practice run, which was conducted outside of the scanner, included 12 animal trials and 43 face trials and ran for 7 minutes. The in-scanner version of the task was comprised of 56 animal and 147 face trials and ran for 24 minutes. The emotions portrayed on the faces were counterbalanced to control for any unanticipated effects on subsequent processing speed or accuracy. Trials were scored based on the number of faces correctly categorized and the speed of response time for each emotion. Accuracy for correctly identified facial emotions (Face Accuracy) for this sample was 83%, with an average reaction time of 1296.3ms (SD 142.6ms), which is consistent with previous findings [35].
fMRI Acquisition and Processing
Whole brain imaging was performed using a GE Signa 3T scanner. fMRI series consisted of 30 contiguous oblique-axial sections acquired using a forward-reverse spiral sequence. The image matrix was 64×64 over a 24 cm field of view for a 3.75×3.75×4mm voxel. The 30 slice volume was acquired serially at 1750ms temporal resolution (TR) for a total of 590 time points for the Facial Emotion Perception Task. One hundred six to one hundred twenty-four high-resolution Fast SPGR IR axial anatomic images [TE= 3.4ms; TR (repetition time)=10.5 ms, 27 degree flip angle, NEX (number of excitations)=1, slice thickness=1–1.2 mm, FOV (Field of view)=24cm, matrix size= 256×256] were obtained for each participant for co-registration and normalization purposes.
Processing of images was conducted using SPM8, including slice timing, realignment, motion correction, co-registration, DARTEL warping (using VBM8 toolbox), normalization to the MNI world space, and smoothing with a 5 FWHM filter. Contrast images were derived by subtracting Blood Oxygenation Level Dependent (BOLD) signal during the animal processing blocks from BOLD signal during the face processing blocks (Faces - Animals). Images from the event-related (ER) models were created by subtracting the BOLD signal from emotional faces events from neutral face events (e.g., Fear - Neutral). Fearful and angry faces in particular were chosen for analysis given the ability of threatening stimuli to influence HPA axis and amygdala reactivity in both human and animal studies [6,23,42]. Sad and Happy, in comparison to Neutral, faces are included as supplementary material for purposes of completeness. These individual emotions were counterbalanced in order to the second degree.
Data Analysis
The SPM8 hemodynamic response function (hrf) model was used to model the BOLD response. Multivariate linear regression analyses were conducted using whole brain analyses from the individual group contrasts in SPM8. All coordinates for activation foci were translated from MNI to Talairach space. Statistical significance for regression analyses in SPM8 was set at p <.005, with cluster minimum of 440 mm3 (55 2mm cubic voxels). Based upon 1000 Monte Carlo simulations with AlphaSim inside the whole brain search region, a whole brain corrected alpha of .05 is achieved with this combined height by extent threshold strategy [43]. Based upon a priori hypotheses, the hypothalamus, amygdala and hippocampus were used as regions of interest (ROI), with uncorrected p<.05 and extent threshold of 15 mm3. Post-hoc analyses used extracted data from regions identified in analyses to better probe underlying performance correlates.
All regression analyses were completed with gender, age, percent of correctly identified faces (Face accuracy), and time of scan (converted to 24-hour time variable) as covariates. Multivariate linear regression was used to determine if pre-scan cortisol levels were predictive of activation in the limbic and regulatory regions of the brain during the task and to determine if activation in the limbic and regulatory regions of the brain during the task were predictive of cortisol change across the task in those with pre and post measurements. Multiple regression was also used to examine whether brain activation in the limbic and regulatory regions of the brain predicted poorer task performance. The dependent variables of interest were brain activation for Faces - Animals (block), Fear - Neutral (ER), Anger - Neutral (ER). Sad - Neutral and Happy - Neutral were included for completeness, although there is a weaker link between these emotions and stress reactivity. These data are available in the supplementary section.
Results
fMRI Cortisol and Baseline Day Cortisol
To evaluate whether the anticipation and completion of fMRI alters cortisol reactivity from baseline (Hypothesis 1), weekday log-transformed cortisol values were used to create an interpolated trend line representing average cortisol across the day for the subsample (n = 13). The trend line is depicted in Figure 1 and includes standard error bars to evaluate fit. Pre- and post-scan cortisol values were plotted for each subject (n = 26) against the interpolated trend line. Average log pre-scan cortisol was −0.7μL (SD −0.86) and log post-scan cortisol was −0.81μL (SD 0.76). There was an average of 16% increase in cortisol for the pre-scan measurement compared to baseline assessments on a non-scanning day, a small effect size (d = .28). Sixteen individuals had values above the interpolated baseline and ten had values at or below the baseline, adjusting for time of day. For a more specific analysis to make certain that there were not undue influence of cortisol measurements from those who contributed baseline values, the analyses were repeated for only the 13 participants who contributed values at baseline and during scanning day. There was a 20% increase of cortisol at baseline relative to the baseline day. Nine subjects exhibited an increase in cortisol, three subjects showed a decrease, and one subject had no change relative to the interpolated baseline from the same subjects.
Figure 1.
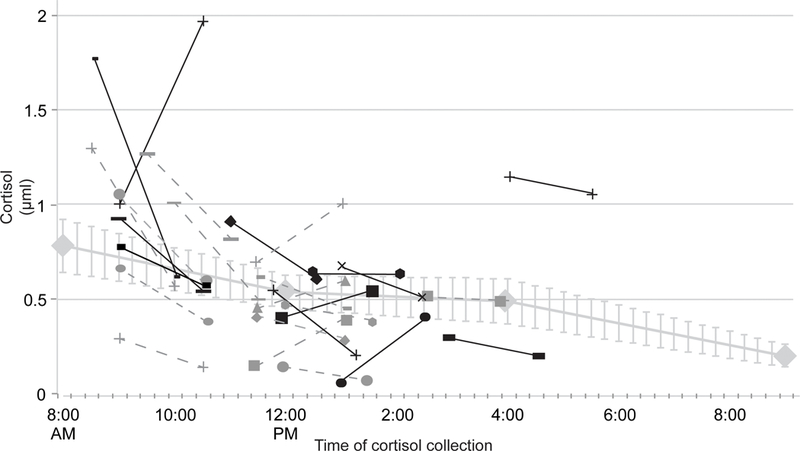
Pre- to post-fMRI cortisol by individual subject compared to baseline average. Blackened markers indicate subjects who contributed to baseline values (N=13).
Pre-Scan Cortisol and Activation Responses
Relations between pre-scan cortisol level and activation for fearful faces (Fear - Neutral contrast) were regressed on pre-scan cortisol (Table 1; Figure 2). Higher pre-scan cortisol predicted activation in cingulate gyrus, anterior cingulate and postcentral regions for the Fear - Neutral contrast (Fig. 2A). ROI analyses also revealed a significant positive association between pre-scan cortisol and activation in parahippocampal gyrus. There were no significant foci of activation in the Anger—Neutral contrast in the regression with pre-scan cortisol.
Table 1.
Foci of Significant Activation for Pre-scan Cortisol in Fear Minus Neutral Event Related Contrasts
| Talaraich coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast/ lobe | BA | x | y | z | Z | mm3 |
| Fear minus Neutral | ||||||
| Positive regressor | ||||||
| Parietal | ||||||
| Postcentral | 3, 7 | 32 | −29 | 44 | 4.25 | 4040 |
| Limbic | ||||||
| Cingulate gyrus | 6, 24,32 | −3 | 1 | 44 | 3.97 | 8936 |
| 31 | 8 | −38 | 33 | 3.64 | 528 | |
| Anterior Cingulate | 33 | −3 | 6 | 18 | 4.48 | 2528 |
| 24 | −3 | 22 | 11 | 3.20 | 480 | |
| Temporal | ||||||
| Middle Temporal gyrus | 21 | 47 | 3 | −21 | 4.27 | 696 |
| Subcortical | ||||||
| Insula | 13 | −41 | −17 | −6 | 3.57 | 632 |
| ROI Positive regressor | ||||||
| Limbic | ||||||
| Parahippocampal gyrus | −17 | −3 | −18 | 2.64 | 456 | |
Figure 2.
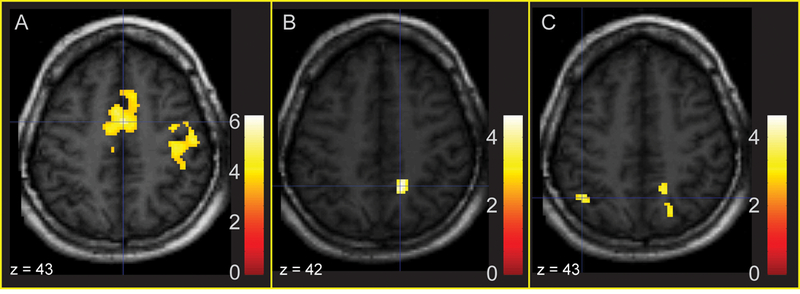
Areas of significant activation regressing Fear - Neutral and Anger - Neutral on prescan cortisol (whole-brain corrected). 2A: Activation in mid-cingulate gyrus and anterior cingulate in response to fearful faces is predicted by increased pre-scan cortisol. 2B: Activation in right precuneus in response to fearful faces is associated with a positive residualized change in cortisol. 2C: Activation in right precuneus and left superior parietal lobule in response to angry faces is associated with a positive residualized change in cortisol.
Activation for contrasts of Sad and Happy are also reported (Supplemental Table 1). Activation for Sad - Neutral contrast was not associated with log pre-scan cortisol. For Happy - Neutral contrast, there was a positive association between pre-scan cortisol and activation in the right rostral cingulate and mid cingulate.
Differences in activation for the Faces - Animals contrast were also examined, illustrated in Figure 3 and reported in Table 2. Whole-brain corrected analyses showed activation in the subgenual cingulate gyrus (Fig. 3A, 3B), which was significantly predicted by higher levels of pre-scan cortisol. ROIs indicated that higher pre-scan cortisol was also significantly related to greater activation in right amygdala (Fig. 3D) and left parahippocampal gyrus. Activation from the subgenual cingulate was significantly positively correlated with right amygdala and hippocampus (Extracted mean activation, Fig. 3C). Posthoc regression analysis was computed for each emotion as a predictor of activation in the subgenual anterior cingulate and right amygdala. Fear (B = .18, t = 2.49, p = .02) and Happy (B = .77, t = 7.51, p < .0001 significantly predicted subgenual anterior cingulate activation for Faces - Animals. Fear (B = .41, t = 2.64, p = .02) and Anger (B = .43, t = 3.34, p = .004) predicted significant overall Faces - Animals activation in the right amygdala.
Figure 3.
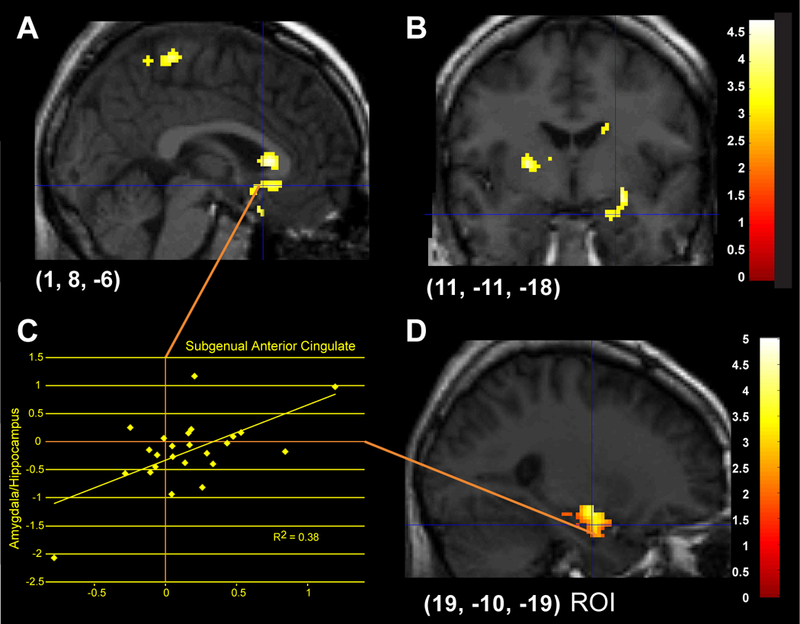
Areas of significant activation regressing Faces — Animals on pre-scan cortisol. 3A, B: Activation in subgenual anterior cingulate in response to emotional faces is predicted by increased pre-scan cortisol (whole-brain corrected). 3C: Extracted mean activations between right subgenual anterior cingulate and amygdala are positively correlated. 3D: ROI analyses show that activation in right amygdala (and hippocampus) is also predicted by higher levels of pre-scan cortisol.
Table 2.
Foci of Significant Activation for Pre-scan Cortisol Regression in Faces – Animals Contrast
| Talaraich coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast/ lobe | BA | x | y | z | Z | mm3 |
| Positive Regressor | ||||||
| Frontal | ||||||
| Paracentral lobule | 5 | 4 | −36 | 54 | 3.18 | 640 |
| Precentral gyrus | 4 | 20 | −19 | 53 | 3.78 | 488 |
| Limbic | ||||||
| Parahippocampal gyrus | 25 | −11 | −10 | 3.41 | 1208 | |
| Midbrain | ||||||
| Red nucleus | −4 | −21 | −8 | 3.67 | 1144 | |
| Subcortical | ||||||
| Caudate/ Subgenual Anterior Cingulate | 25 | −3 | 14 | 4 | 3.75 | 2936 |
| 15 | 1 | 19 | 3.28 | 896 | ||
| Lentiform nucleus | −22 | −15 | 2 | 3.72 | 1504 | |
| ROI Positive Regressor | ||||||
| Anterior | ||||||
| Culmen | −19 | −40 | −6 | 2.26 | 192 | |
| Limbic | ||||||
| Parahippocampal gyrus | 25 | −11 | −10 | 3.41 | 3728 | |
| 28 | −24 | −21 | −14 | 3.90 | 912 | |
| 36 | −29 | −32 | −13 | 2.72 | 176 | |
Cortisol Response During Scan and Activation Responses
We also examined the association between changes in cortisol from pre- to post-scan and activation in response to the Fear - Neutral contrast (Figure 2). Positive associations were observed between the cortisol response and activation for Fear - Neutral in precuneus and lingual gyrus (Fig. 2B). ROIs indicated that activation in parahippocampal gyrus was negatively associated with a change in cortisol during the scan. In the Anger - Neutral contrast, activation in inferior temporal gyrus, superior parietal lobule, precuneus and rectal gyrus was positively related to change in cortisol (Fig. 2C). Activation in the cerebellar tonsil and inferior semi-lunar lobule for whole-brain corrected analyses, as well as the parahippocampal gyrus in ROI analyses, was negatively associated with cortisol change. Results of whole-brain corrected and ROI analyses for the Fear - Neutral and Anger - Neutral contrasts are listed in Table 3.
Table 3.
Residual Cortisol Change (Pre to post) for Fear minus Neutral and Anger Minus Neutral Event-Related Contrasts
| Talaraich coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast/ lobe | BA | x | y | z | Z | mm3 |
| Fear minus Neutral | ||||||
| Positive Residual Regressor | ||||||
| Parietal | ||||||
| Precuneus | 7 | 13 | −52 | 38 | 3.64 | 1376 |
| 7 | 13 | −42 | 57 | 3.2 | 464 | |
| Occipital | ||||||
| Lingual gyrus | 18 | 15 | −77 | 3 | 3.29 | 1680 |
| Negative Residual Regressor | ||||||
| ROI | ||||||
| Limbic | ||||||
| Parahippocampal gyrus | 24 | −11 | −22 | 2.2 | 472 | |
| Anger minus Neutral | ||||||
| Positive Residual Regressor | ||||||
| Frontal | ||||||
| Rectal gyrus | 11 | 3 | 28 | −29 | 3.47 | 592 |
| Parietal | ||||||
| Superior parietal lobule | 7 | −33 | −60 | 44 | 3.35 | 872 |
| Precuneus | 7 | 13 | −54 | 41 | 3.37 | 672 |
| Temporal | ||||||
| Inferior temporal gyrus | 20 | −45 | −15 | −31 | 4.91 | 2200 |
| Negative Residual Regressor | ||||||
| Posterior | ||||||
| Cerebellar tonsil | −27 | −42 | −34 | 3.33 | 520 | |
| Inferior semi-lunar lobule | −1 | −62 | −37 | 3.75 | 504 | |
| Negative Residual Regressor | ||||||
| ROI | ||||||
| Limbic | ||||||
| Parahippocampal gyrus | 25 | −11 | −22 | 2.42 | 928 | |
| 18 | −9 | −11 | 2.02 | 208 | ||
| 36 | −27 | −29 | −11 | 2 | 184 | |
For the Sad - Neutral contrast (Supplemental Table 2) change in cortisol was positively associated with activation in bilateral motor cortex, posterior cingulate, and right precuneus. For the Happy - Neutral contrast, there was also a positive association between change in cortisol and activation in right putamen and rostral cingulate, left posterior middle temporal gyrus and precentral gyrus, and bilateral precuneus, posterior cingulate, middle cingulate, cerebellum, and inferior parietal lobule.
For the Faces - Animals contrast, activation in left superior frontal gyrus (whole-brain) and left parahippocampal gyrus (ROI) was positively associated with cortisol change from pre- to post-scan (Table 4).
Table 4.
Residual Cortisol Change (Pre to post) for Faces – Animals Contrast
| Talaraich coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast/ lobe | BA | x | y | z | Z | mm3 |
| Positive Residual Regressor | ||||||
| Frontal | ||||||
| Superior frontal gyrus | 10 | 32 | 49 | 18 | 2.99 | 488 |
| ROI | ||||||
| Limbic | ||||||
| Parahippocampal gyrus | 10 | 22 | −30 | −7 | 2.12 | 136 |
| Negative Residual Regressor | ||||||
| ROI | ||||||
| Limbic | ||||||
| Uncus | 24 | −3 | −23 | 2.32 | 128 | |
Emotion Perception Activation Responses Related to Task Performance
Figure 4 (Table 5) illustrates the areas of activation that were significantly negatively correlated with face accuracy when evaluated within the pre-scan cortisol regression models. Whole-brain corrected analyses indicated an inverse relationship between activation in the superior temporal gyrus and insula and accuracy for identifying facial emotions. ROI analyses indicated that increased activation in right posterior parahippocampal gyrus was negatively associated with face accuracy. Pre-scan cortisol and change in cortisol were not related to face accuracy.
Figure 4.
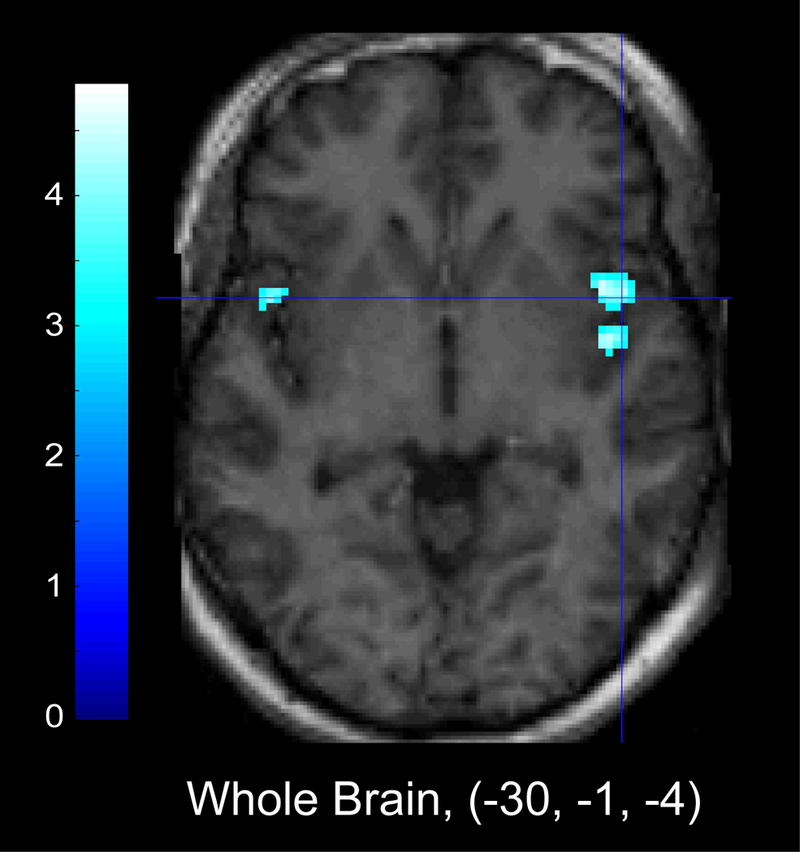
Areas of significant inverse relationship between activation and Face Accuracy, regressed on pre-scan cortisol. Left panel: Whole brain corrected analyses show that increased activation in insula was negatively related to performance on a measure of overall face accuracy when evaluated within the pre-scan cortisol regression model. Right panel: ROI analyses indicate increased activation in parahippocampal gyrus was also negatively related to overall face accuracy.
Table 5.
Foci of Significant Activation Inversely Associated with Face Accuracy in the Faces minus Animals Contrast
| Talaraich coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast/ lobe | BA | x | y | z | Z | mm3 |
| Faces minus Animals | ||||||
| Temporal | ||||||
| Superior temporal gyrus | 39 | 47 | −54 | 25 | 3.16 | 448 |
| Anterior | ||||||
| Cerebellum | 22 | −40 | −23 | 3.82 | 1688 | |
| Sub-cortical | ||||||
| Extra-nuclear | 13 | 40 | 4 | −7 | 3.72 | 1336 |
| Insula | 13 | −41 | 3 | −4 | 3.16 | 648 |
| ROI | ||||||
| Limbic | ||||||
| Parahippocampal gyrus | 27 | 24 | −32 | −9 | 2.01 | 328 |
Discussion
The current study evaluated differences between baseline cortisol and fMRI-related cortisol (pre- and post-scan) and examined whether anticipatory cortisol levels and change in cortisol level in the fMRI environment were related to brain activation and task performance in an emotional faces paradigm. We observed key relationships between anticipatory cortisol prior to an fMRI scan and activation in the parahippocampal gyrus and several other limbic regions during both facial and emotion-face recognition. Individual differences in response to the anticipatory stress of fMRI may produce some of the variability in findings with emotion paradigms when comparing healthy and patient groups [8–10].
Our results support the hypothesis that cortisol reactivity is associated with greater activation in limbic and regulatory regions during an emotion-processing task. These increases in limbic activation, along with heightened cortisol levels on the day of fMRI procedures compared to baseline, can be conceptualized as an index of change in reactivity with exposure to salient emotional stimuli and the stress of the fMRI scanning environment. Interestingly, activation in the anterior parahippocampal gyrus during facial recognition was positively associated with change in cortisol, while activation in a more posterior region during specific aspects of emotional facial expression was negatively associated with cortisol change. This finding is consistent with recent studies suggesting that the function of anterior and posterior hippocampus may be more distinct than previously thought [41,44,45].
Our results support the hypothesis that pre-fMRI cortisol levels were increased in subjects relative to baseline averages derived from a subsample of participants: over 50% of subjects showed pre-scan cortisol levels above the group, time-corrected average. This relationship suggests that anticipation of the fMRI environment may act as a mild stressor, regardless of scan time, even among adults with no personal or family history of mental illness. It is important to consider the impact of individual differences on stress and how stress responses may change over the course of an fMRI experiment. Capitalizing on these individual differences in elevation and temporal pattern of cortisol activity could have strong predictive capabilities for future illness (i.e., major depressive disorder, anxiety disorders) and may help to reduce heterogeneity in studies of those with active illness. Alternatively, these patterns in healthy subjects may be unrelated to illness, yet still result in increased heterogeneity in control samples that weakens control and patient comparisons.
There is also evidence that stress responses may diminish performance when processing emotional stimuli [5]. Activation in regions such as the superior temporal gyrus and insula were related to poorer performance on face accuracy. It is possible that anticipatory stress prior to entering the fMRI environment affects the ability for subjects to perform at an optimal level during an emotion challenge [46]. However, consistent with other studies [47], cortisol was not found to relate to task performance; future investigations can more explicitly challenge specific emotions (i.e., fear, anger) to assess the potential effects of cortisol change from pre- to post-scan on performance in regions associated with emotion face processing.
We observed an interesting dissociation of links between emotion processing of specific emotions and change in cortisol across the scan. Although there were areas of robust correlation between fear-related activation and cortisol measurements, this was not the case for anger. Historically, fear has a stronger link to HPA function, and the present results are consistent with this area of work [48,49]. It is notable that the activation foci with the largest relationship to fear are also important for inhibitory control and have been implicated in studies of cognitive control in major depressive disorder [5]. Further investigation of the effect of the fMRI environment on cortisol reactivity and emotional task performance should include clinical populations, including those with higher levels of baseline cortisol, such as those diagnosed with major depressive disorder or anxiety disorders. Future research should also continue to address the effectiveness of techniques for reducing stressors related to fMRI procedures, such as mock scans or relaxation techniques, to determine the degree to which these strategies reduce HPA reactivity.
Several limitations to the current study should be considered when interpreting the results. Although the comparison between cortisol levels at baseline (i.e. non-scanning day) and cortisol pre and post scan is a significant contribution to the growing research on this topic, the use of a small subgroup for the collection of baseline cortisol data and the known individuals differences in diurnal cortisol and cortisol reactivity may have influenced results. In the future, researchers should attempt to overcome inherent difficulties in cortisol collection to obtain data that are reflective of an entire sample. Furthermore, we did not evaluate subjective levels of stress prior to or after fMRI. Scores on self-reports such as the PANAS-X [50] or subscales of the Brief Symptom Inventory [51] related to anxiety or stress could be used to corroborate the findings of imaging results and pre/post-scan cortisol measurements.
In conclusion, this study provides important insights into the relationship between individual differences in cortisol activity and increased emotional activation during an emotional face challenge. It provides a context for understanding group and individual differences in activation contrasts with emotional content. This study also extends previous findings regarding the effects of fMRI-related stressors on brain activation and subjective task performance.
Supplementary Material
Figure depicting the timeline of the experimental task. Cortisol is collected just prior to entry into the fMRI scanner and within 10 minutes after exiting the scan.
Acknowledgments
Funding and Disclosure
This project was supported by a National Alliance for Research in Schizophrenia and Depression Award (SAL), General Clinical Research Center pilot grant to Monica Starkman and Scott A Langenecker (for some control fMRI scans, from # MO1 RR00042), KL2 Career Development Award (RR024987, SAL), K23 Award (MH074459, SAL), a Rachel Upjohn Clinical Scholars Award for screening of control subjects (SAL), and some pilot (control) fMRI scans from the University of Michigan Functional MRI lab (SAL, SLW).
Footnotes
The authors declare no conflict of interest and no authors received compensation for professional services during the time the data for this manuscript were collected, nor during the writing and editing of this manuscript.
References
- 1.Tsigos C, Chrousos GP: Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002. October;53:865–71. [DOI] [PubMed] [Google Scholar]
- 2.Susman EJ: Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev 2006. January;30:376–89. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Wingfield JC: The concept of allostasis in biology and biomedicine. Horm Behav 2003. January;43:2–15. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Duran NL, Kovacs M, George CJ: Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology 2009. October;34:1272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langenecker S, Weisenbach S, Giordani B, Briceno E, Guidotti Breting L, Schallmo M, et al. : Impact of chronic hypercortisolemia on affective processing. Neuropharmacology 2012. January;62:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC: The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage 2009. September;47:864–71. [DOI] [PubMed] [Google Scholar]
- 7.Jankord R, Herman JP: Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci 2008;1148:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ: Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology 2008. May;33:517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Chaplin TM, Wang F, Sinha R, Mayes LC, Blumberg HP: Stress reactivity and corticolimbic response to emotional faces in adolescents. J Am Acad Child Adolesc Psychiatry 2012. March;51:304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. : Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 2008. January 15;63:234–40. [DOI] [PubMed] [Google Scholar]
- 11.Root JC, Tuescher O, Cunningham-Bussel A, Pan H, Epstein J, Altemus M, et al. : Frontolimbic function and cortisol reactivity in response to emotional stimuli. Neuroreport 2009. March 4;20:429–34. [DOI] [PubMed] [Google Scholar]
- 12.Van Stegeren A, Roozendaal B, Kindt M, Wolf O, Joels M: Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem 2010. January;93:56–65. [DOI] [PubMed] [Google Scholar]
- 13.Damasio A, Carvalho GB: The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci 2013;14:143–52. [DOI] [PubMed] [Google Scholar]
- 14.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. : Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etkin A, Wager TD: Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007;164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ongur D, Ferry AT, Price JL: Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 2003;460:425–449. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan RM, Gratton A: Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology 2002;27:99–114. [DOI] [PubMed] [Google Scholar]
- 18.Amodio DM, Frith CD: Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 2006. April;7:268–77. [DOI] [PubMed] [Google Scholar]
- 19.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. : Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 2006. April 19;26:4415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veer IM, Oei NYL, Spinhoven P, van Buchem M a Elzinga BM, Rombouts S a RB: Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology 2012. July;37:1039–47. [DOI] [PubMed] [Google Scholar]
- 21.Phelps EA: Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 2004;14:198–202. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. : Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A 2005;102:17804–17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD: Neural bases of moderation of cortisol stress responses by psychosocial resources. J Pers Soc Psychol 2008. July;95:197–211. [DOI] [PubMed] [Google Scholar]
- 24.Lueken U, Muehlhan M, Evens R, Wittchen H-U, Kirschbaum C: Within and between session changes in subjective and neuroendocrine stress parameters during magnetic resonance imaging: A controlled scanner training study. Psychoneuroendocrinology 2012. August;37:1299–308. [DOI] [PubMed] [Google Scholar]
- 25.Chapman H, Bernier D, Rusak B: MRI-related anxiety levels change within and between repeated scanning sessions. Psychiatry Res 2010. May 30;182:160–4. [DOI] [PubMed] [Google Scholar]
- 26.Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD: Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology 2009. September;34:1242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf OT: The influence of stress hormones on emotional memory: relevance for psychopathology. Acta Psychol (Amst) 2008;127:513–531. [DOI] [PubMed] [Google Scholar]
- 28.Tessner KD, Walker EF, Hochman K, Hamann S: Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp 2006;27:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lueken U, Muehlhan M, Wittchen H-U, Kellermann T, Reinhardt I, Konrad C, et al. : (Don’t) panic in the scanner! How panic patients with agoraphobia experience a functional magnetic resonance imaging session. Eur Neuropsychopharmacol 2011. July;21:516–25. [DOI] [PubMed] [Google Scholar]
- 30.Peters S, Cleare AJ, Papadopoulos A, Fu CHY: Cortisol responses to serial MRI scans in healthy adults and in depression. Psychoneuroendocrinology 2011. June;36:737–41. [DOI] [PubMed] [Google Scholar]
- 31.Muehlhan M, Lueken U, Wittchen H-U, Kirschbaum C: The scanner as a stressor: evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. Int J Psychophysiol 2011. February;79:118–26. [DOI] [PubMed] [Google Scholar]
- 32.Lovallo W, Farag N, Vincent A: Use of a resting control day in measuring the cortisol response to mental stress: diurnal patterns, time of day, and gender effects. Psychoneuroendocrinology 2010. September;35:1253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone A, Schwartz J, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, et al. : Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology 2001. April;26:295–306. [DOI] [PubMed] [Google Scholar]
- 34.Briceno E, Weisenbach S, Rapport L, Hazlett K, Bieliauskas L, Haase B, et al. : Shifted inferior frontal laterality in women with major depressive disorder is related to emotionprocessing deficits. Psychol Med 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S: Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol 2005;27:320–333. [DOI] [PubMed] [Google Scholar]
- 36.Rapport LJ, Friedman SR, Tzelepis A, Van Voorhis A: Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology 2002;16:102–110. [DOI] [PubMed] [Google Scholar]
- 37.Kamali M, Saunders EFH, Prossin AR, Brucksch CB, Harrington GJ, Langenecker SA, et al. : Associations between suicide attempts and elevated bedtime salivary cortisol levels in bipolar disorder. J Affect Disord 2012;136:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC: Facial Emotion Depression Discrimination : II. Behavioral Findings in. Psychiatry Res 1992;42:241–251. [DOI] [PubMed] [Google Scholar]
- 39.Tottenham N, Borscheid A, Ellersten K, Markus D, Nelson C: Categorization of facial expressions in children and adults: Establishing a larger stimulus set; in : Annual Meeting of the Cognitive Neuroscience Society San Francisco, CA, 2002. [Google Scholar]
- 40.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. : The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009;168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ekman P, Friesen W V: Pictures of Facial Affect. Consulting Psychologists Press, 1976. [Google Scholar]
- 42.Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry 2003. September;54:504–514. [DOI] [PubMed] [Google Scholar]
- 43.Ward B: Simultaneous Inference for FMRI Data [Internet] 2000;: 1–16. [Google Scholar]
- 44.Regestein QR, Jackson WJ, Peterson HF: Effects of various hippocampal lesions on monkey plasma cortisol levels in two experimental conditions. Behav Neural Biol 1986;45:329–341. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe Y, Gould E, McEwen BS: Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 1992;588:341–345. [DOI] [PubMed] [Google Scholar]
- 46.Nagai M, Kishi K, Kato S: Insular cortex and neuropsychiatric disorders: A review of recent literature. Eur Psychiatry 2007;22:387–394. [DOI] [PubMed] [Google Scholar]
- 47.Li S, Weerda R, Milde C, Wolf OT, Thiel CM: Effects of acute psychosocial stress on neural activity to emotional and neutral faces in a face recognition memory paradigm. Brain Imaging Behav 2014; DOI: 10.1007/s11682-013-9287-3 [DOI] [PubMed] [Google Scholar]
- 48.Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, et al. : Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 2010. January;35:33–46. [DOI] [PubMed] [Google Scholar]
- 49.Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, et al. : Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson D, Clark LA: Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J Pers Soc Psychol 1988;54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 51.Derogatis LR, Melisaratos N: The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13:595–605. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure depicting the timeline of the experimental task. Cortisol is collected just prior to entry into the fMRI scanner and within 10 minutes after exiting the scan.


