Abstract
Background:
Systematic reviews of randomized, controlled trials in patients with influenza suggest a lack of evidence about the effects of antiviral therapy on several patient-important outcomes of influenza.
Purpose:
To systematically review observational studies for benefits and harms of oseltamivir, zanamivir, amantadine, or rimantadine in the treatment of influenza.
Data Sources:
MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, CINAHL, SIGLE, the Chinese Biomedical Literature Database, Panteleimon, and LILACS up to November 2010; contact with pharmaceutical companies; and reference lists.
Study Selection:
Observational studies in any language that compared single antiviral therapy with no therapy or other antiviral therapy, or that had no comparator, for influenza or influenza-like illness.
Data Extraction:
Two independent investigators extracted data. Confidence in the estimates of the obtained effects (quality of evidence) was assessed by using the Grading of Recommendations Assessment, Development, and Evaluation approach.
Data Synthesis:
74 studies fulfilled the inclusion criteria. Meta-analyses of the few studies providing effects with adjustment for confounders suggest that, in high-risk populations, oral oseltamivir may reduce mortality (odds ratio, 0.23 [95% CI, 0.13 to 0.43]; low-quality evidence), hospitalization (odds ratio, 0.75 [CI, 0.66 to0.89]; low-quality evidence), and duration of symptoms (33 hours [CI, 21 to 45 hours]; very low–quality evidence) compared with no treatment. Earlier treatment with oseltamivir was generally associated with better outcomes. Inhaled zanamivir may lead to shorter symptom duration (23 hours [CI, 17 to 28 hours]; moderate-quality evidence) and fewer hospitalizations (odds ratio, 0.66 [CI, 0.37 to1.18]) but more complications than no treatment. Direct comparison of oral oseltamivir and inhaled zanamivir suggests no important differences in key outcomes. Data from 1 study suggest that oral amantadine may reduce mortality and pneumonia associated with influenza A. No included study evaluated rimantadine.
Limitations:
Mortality was assessed in high-risk patients, and generalizability is limited. The overall body of evidence is limited by risk for confounding and selection, reporting, and publication bias.
Conclusion:
Therapy with oral oseltamivir and inhaled zanamivir may provide a net benefit over no treatment of influenza. However, as with the randomized trials, the confidence in the estimates of the effects for decision making is low to very low.
Primary Funding Sources:
World Health Organization and Mc-Master University.
Influenza virus infections result in major health and economic burdens worldwide. The World Health Organization (WHO) estimates that the average global burden of interpandemic influenza is approximately 1 billion cases of influenza, 3 to 5 million cases of severe illness, and 300 000 to 500 000 deaths annually (1). Among the 90 million influenza cases in children younger than 5 years in 2008, an estimated 28 000 to 111 500 children died of influenza-associated lower respiratory tract infection (2). Most cases of influenza are self-limited, and prevention through annual influenza vaccination may be an effective strategy. However, antiviral treatment with neuraminidase inhibitors (oseltamivir or zanamivir) or M2 ion channel blockers (amantadine or rimantadine) is used to reduce signs and symptoms and to prevent hospitalizations or death in patients with severe disease.
In February 2010, WHO updated its guidelines for the treatment of influenza, which are used worldwide (3). However, evidence about the effects and safety of antiviral agents continues to increase and, in 2012, Jefferson and colleagues (4) updated a review of the randomized, controlled trial (RCT) literature to inform treatment decisions. Data from only 25 of 67 RCTs could be used for the analyses. The investigators found that oral oseltamivir reduced the duration of symptoms by around 21 hours from a median of 160 hours in the placebo groups but had no effect on hospitalizations (odds ratio [OR], 0.95 [95% CI,0.57 to 1.61]) on the basis of 7 studies with a median event rate of 0.84% in the placebo group.
In theory, the best evidence for health care decisions comes from RCTs. However, the quality of evidence across these RCTs has raised concerns (4), due in part to the lack of precision in the effect estimates; the lack of evidence for certain patient-important health outcomes, such as death; and poor assessment and reporting of other outcomes, such as adverse events. In addition, questions remain about the effects of antiviral agents to treat influenza A or B and in specific groups, such as hospitalized or immunocompromised patients.
Observational studies may provide important additional information or higher-quality evidence than available RCTs for certain elements of the health care problem, such as specific populations, administration modes, and outcomes (such as mortality). We reviewed the evidence from observational studies to inform WHO guidelines and the WHO essential medicine list about the antiviral treatment of influenza.
METHODS
Our primary objective was to evaluate the effectiveness and safety of neuraminidase inhibitors (oseltamivir and zanamivir) for treatment of influenza (A or B) virus infection, and M2 ion channel blockers or adamantanes (amantadine and rimantadine) to treat influenza A virus infections. The original research questions were coordinated with WHO. We developed a protocol that included the following criteria.
Data Sources and Searches
We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, CINAHL, SIGLE, the Chinese Biomedical Literature Database, Panteleimon, and LILACS for relevant studies up to 16 November 2010. We used no restrictions by language or study design (the Appendix, available at www.annals.org, lists our search strategies). We contacted the WHO, 8 pharmaceutical companies, the U.S. Food and Drug Administration, the European Medicines Agency, the U.S. Centers for Disease Control and Prevention, and the European Centre for Disease Prevention and Control for unpublished observational data. We also reviewed reference lists of relevant studies and other reviews for studies.
Study Selection
Investigators independently screened all citations by title and abstract in pairs. We then retrieved the full text of these studies, and 2 investigators independently screened them for inclusion. Disagreements about inclusion were resolved by discussion or by consulting a third investigator.
We followed a priori study eligibility criteria for study selection. We included any observational study that compared any of the antiviral drugs with no antiviral treatment or with another antiviral drug for the treatment of laboratory-confirmed influenza or influenza-like illness (not confirmed) and any observational study with no independent comparison group if studies with an independent comparison group were not available. The antiviral drugs were oseltamivir, zanamivir, amantadine, and rimantadine in any dose or by any route, with the exception of intravenous administration. We also evaluated studies that compared early antiviral treatment with late antiviral treatment by using 48 hours from the onset of symptoms or treatment as the reference point between early and late treatment. We excluded RCTs, studies with fewer than 25 patients, and studies evaluating antiviral chemopro-phylaxis of influenza. We included studies in all populations with influenza or influenza like-illness.
We determined a priori to report on the following outcomes because they were judged to be important or critical for decision making: death; hospitalization; intensive care unit (ICU) admission, mechanical ventilation and respiratory failure; duration of hospitalization; duration of signs and symptoms; time to return to normal activity; complications; critical adverse events, such as major psychotic disorders, encephalitis, stroke, or seizure; important adverse events, such as pain in extremities, clonic twitching, body weakness, or dermatologic changes (such as urticaria or rash); influenza viral shedding; and emergence of antiviral resistance.
Data Extraction and Quality Assessment
Two independent investigators extracted data from the included studies by using a pretested electronic form. We extracted details of the study design, limitations in study design or execution (risk of bias), the country where the study was conducted, patient characteristics, method of influenza diagnosis, influenza characteristics (including virus strain, outbreak setting, and disease severity), intervention characteristics (including drug type, dose, duration of use, start time, and co-interventions), study funding, and outcomes. When abstracted data differed, the investigators resolved these differences by consensus. We used the Newcastle–Ottawa Scale (5) to assess the risk of bias of the included case–control and cohort studies on the basis of selection of study groups, comparability of groups, and ascertainment of the exposure or outcome of interest.
Two trained investigators with experience in using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach assessed the confidence in the estimates of effect of the body of evidence (quality of evidence) by outcome and produced the draft evidence profiles according to GRADE (6, 7). The completed evidence summaries and GRADE assessments were discussed by several investigators and reviewed by the senior investigator. Factors that affect the confidence in the estimate of effect include risk of bias (also known as detailed design and study limitations), imprecision, indirectness (directness in the GRADE approach includes generalizability and applicability), inconsistency of results (heterogeneity), publication bias, dose–effect responses, magnitude of effect, and issues of residual plausible confounding. The confidence in the estimate of effect is categorized into 4 levels, ranging from very low to high.
Data Synthesis and Analysis
We conducted meta-analyses of dichotomous outcomes with random-effects models by using the odds ratio (OR) and conducted meta-analyses of continuous outcomes in Review Manager, version 5.1 (The Nordic Cochrane Center and the Cochrane Collaboration, Copenhagen, Denmark) by using mean differences or standardized mean differences (SMDs). Standardized mean differences were used to pool effects across studies when outcomes (such as duration of symptoms of expected different length) were measured or reported differently in these studies. To facilitate interpretation of the SMDs, we back-transformed them into common units based on the mean across studies for these outcomes. We pooled the data by using ORs when the number of events were available and pooled the logarithm of the ORs weighted by the inverse variance when events were not available. Analyses were performed separately for studies that provided adjusted as opposed to crude ORs.
For studies that reported events for certain outcomes in only 1 treatment group (for example, adverse events for the treatment group but no comparison group), we combined proportions weighted by the generic inverse variance. We grouped adverse events and complications according to a priori ratings of importance (critical, important, or not important) according to the GRADE approach(8). For these outcomes, we calculated a rate ratio and pooled the logarithm of the rate ratios weighted by the generic inverse variance.
When data were available, we performed a priori subgroup analyses by age (1 to 15 years, 16 to 65 years, and ≥65 years), risk for complications (patients at low risk, patients admitted to the ICU, and immunocompromised patients), influenza type (A or B), laboratory-confirmed influenza versus influenza-like illness (not confirmed), pandemic or interpandemic influenza, dose of antiviral agent, and potential for funding conflict. Heterogeneity was assessed by using the chi-square test and quantified by using the I2 statistic (9). If results showed substantial heterogeneity I2 >60%), we explored heterogeneity on the basis of the a priori hypotheses. Evidence summaries were prepared for each research question by using the GRADE Profiler (GRADEpro), version 3.6 (McMaster University, Hamilton, Ontario, Canada).
Role of the Funding Source
This review was commissioned and partially funded by WHO as an independent review. It was conducted as a collaboration of researchers from the McMaster Healthcare Grading and Recommendations Center as part of the activity of the McMaster WHO collaborating center for evidence-informed policy and the Norwegian Knowledge Center for the Health Services. The protocol for the review was discussed with WHO staff, and WHO staff provided references to studies that were assessed for inclusion.
RESULTS
Literature Flow
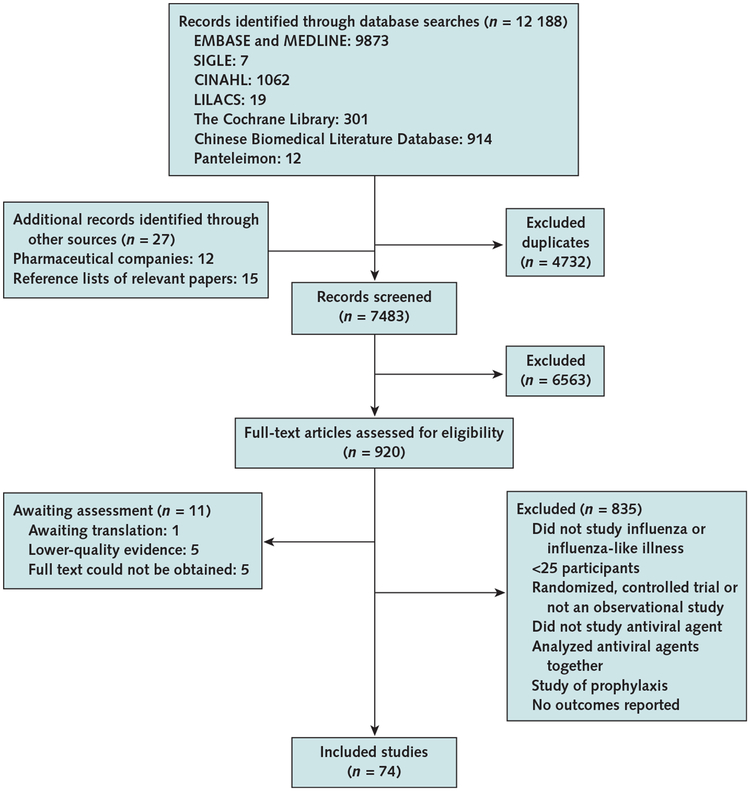
We identified 12 188 citations from the electronic search of the databases and 27 articles by reviewing reference lists of relevant papers and studies sent to us by pharmaceutical companies (Hoffman-La Roche, Basel, Switzerland, and GlaxoSmithKline, Brentford, Middlesex, United Kingdom) in response to our request for data (Appendix Figure, available at www.annals.org). The U.S. Food and Drug Administration provided us with the same postmarketing adverse event data described by Jefferson and colleagues in 2010 (10), which lists adverse event reports generated worldwide. However, these data were not suitable for our analyses because no denominator was provided for the population. We obtained the full text of 920 articles and included 74 articles after full-text review. We translated articles from Chinese, French, Japanese, Russian, and Spanish.
Oseltamivir
We found 51 observational studies that compared the effects of oral oseltamivir with those of no antiviral therapy (11–61). Only a few studies (13, 14, 20, 24, 37, 41, 45,48) adjusted for confounders, such as age and comorbid conditions, when reporting mortality, hospitalization, and complications, and we rated these studies as having low risk for observational study bias. Many of the other studies measuring complications drew patient data from health insurance administrative databases that listed unconfirmed diagnoses. Substantial reporting and publication bias may exist for several of the evaluated outcomes (in particular, complications) because the studies were funded by for-profit organizations. Table 1 summarizes the findings and the quality of the evidence for oral oseltamivir compared with no antiviral therapy, and the Figure shows the summary results of the meta-analyses. The Supplement Figures (available at www.annals.org) show the related forest plots and those of all other analyses.
Table 1.
GRADE Evidence Profile for Oral Oseltamivir Versus No Antiviral Therapy
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Participants (Studies), n* | Overall Quality of Evidence | Study Event Rates, n/N (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | |||
| No Antiviral Treatment | Oseltamivir | Risk With No Antiviral Treatment | Absolute Effect With Oseltamivir (95% CI) | ||||
| Mortality | 681 (3) | Low† | 59/242 (24.4) | 31/439 (7.1) | Adjusted OR, 0.23 (0.13–0.43) | 240 deaths per 1000 patients | 172 fewer deaths (120 to 201 fewer deaths) per 1000 patients |
| 1557 (9) | Very low due to risk of bias†‡ | 61/320 (19.1) | 228/1237 (18.4) | OR, 0.51 (0.23–1.14)§ | 240 deaths per 1000 patients | 101 fewer deaths (172 fewer to 25 more deaths) per 1000 patients | |
| Hospitalization | 150 710 (4) | Low‖ | 1238/100 585 (1.2) | 431/50125 (0.86) | Adjusted OR, 0.75 (0.66–0.89) | 12 hospitalizations per 1000 patients | 3 fewer hospitalizations (1 to 4 fewer hospitalizations) per 1000 patients |
| 242 762 (6) | Very low due to risk of bias‡‖ | 1738/146 410(1.2) | 1086/96 352 (1.1) | OR, 0.75 (0.66–0.86) | 12 hospitalizations per 1000 patients | 3 fewer hospitalizations (2 to 4 fewer hospitalizations) per 1000 patients | |
| ICU admissions, mechanical ventilation, or respiratory failure | 1032 (6)¶ | Very low due to inconsistency and risk of bias†** | - | 200/1032 (19.4) Pooled risk, 13.0% (95% CI, 11%−15%) |
- | - | |
| Days hospitalized | 832 (5) | Very low due to inconsistency and risk of bias†¶** | - | 832 | - | - | Mean duration of hospital stay, 5.16 d (5.02 to 5.29 d) |
| Duration of signs and symptoms†† | 5842 (6) | Very low due to inconsistency†** | 449 | 5393 | - | - | Mean time was 0.91 SD lower (1.25 to 0.57 lower SD)‡‡ |
| Complications | |||||||
| Pneumonia | 150 466(3) | Very low due to inconsistency‖** | 2111/100 449 (2.1) | 647/50 017 (1.3) | Adjusted OR, 0.83 (0.59–1.16) | 21 cases per 1000 patients | 4 fewer cases (9 fewer to 3 more cases) per 1000 patients |
| 265 276 (6) | Very low due to inconsistency and risk of bias‡‖** | 3244/166 256 (2) | 1273/99 020 (1.3) | OR, 0.64 (0.46–0.88) | 20 cases per 1000 patients | 7 fewer cases (2 to 10 fewer cases) per 1000 patients | |
| Otitis media | 78 407 (2) | Low‖ | 546/40 022 (1.4) | 285/38 385 (0.74) | Adjusted OR, 0.75 (0.64–0.87) | 14 cases per 1000 patients | 3 fewer cases (2 to 5 cases) per 1000 patients |
| 193 105 (4) | Very low due to inconsistency and risk of bias‡‖** | 2053/105 758 (1.9) | 1381/87 347 (1.6%) | OR, 0.77 (0.63–0.94) | 19 cases per 1000 patients | 4 fewer cases (1 to 7 fewer cases) per 1000 patients | |
| Cardiovascular outcomes | 100 830 (2) | Low‖ | 62 385 | 38 445 | Adjusted OR, 0.58 (0.31–1.10) | 200 cardiac events per 1000 patients | 73 fewer cardiac events (128 fewer to 16 more events) per 1000 patients |
| 60 678 (2) | Very low due to inconsistency and risk of bias‡‖** | 6814/50 696 (13.4) | 606/9982 (6.1) | OR, 0.45 (0.25–0.81) | 110 cardiac events per 1000 patients | 57 fewer cardiac events (19 to 80 fewer events) per 1000 patients | |
| Critical adverse events | 104 930(5) | Low‖ | 60 817 | 44 113 | Rate ratio, 0.76 (0.70–0.81) | 420 events per 1000 patient-years | 101 fewer events (80 to 126 fewer events) per 1000 patient-years |
GRADE = Grading of Recommendations Assessment, Development, and Evaluation; ICU = intensive care unit; OR = odds ratio.
Follow-up to 30 d.
Although we did not downgrade, publication bias cannot be excluded.
Not adjusted for potential confounding factors.
Significant differences in effect for pandemic vs. seasonal influenza (Supplement Table 1, available at www.annals.org).
Publication bias was a concern because large studies had for-profit funding and were weighted heavily in analyses.
No independent comparison group.
High heterogeneity among studies.
Measured from onset of symptoms or treatment. Time to return to normal activity was not measured.
Translates to a reduced symptom duration of approximately 33 h (95% CI, 21–45 h). Despite the large effect, we did not upgrade because of important inconsistency across studies.
Figure.
Random-effects meta-analysis of oral oseltamivir versus no antiviral therapy based on studies that provided adjusted effect measures.
Three studies reporting on mortality in hospitalized patients (24, 37, 41) adjusted for age, comorbid conditions, or other prognostic factors, but no study described the reasons for administering oseltamivir to selected patients. The pooled OR (0.23 [CI, 0.13 to 0.43]) suggests that oral oseltamivir may reduce mortality compared with no antiviral therapy, translating to an absolute risk reduction of 17.2% in this high-risk population. The overall grade for the quality of evidence was low. A pooled estimate of unadjusted effects from 9 studies (16, 22, 27, 29, 36, 37, 42, 52, 60) enrolling 1557 patients resulted in a more modest reduction in mortality (OR, 0.51 [CI, 0.23 to 1.14]). Similar results were found in studies that could not be pooled: One study (33) reported an adjusted hazard ratio of 0.27 (CI, 0.13 to 0.55) in 754 patients, and another (47) reported a statistically nonsignificant difference in mortality in approximately 75 000 patients recruited primarily in outpatient settings.
Meta-analysis of data from 4 studies (13, 20, 45, 48) enrolling 150 710 patients showed that oral oseltamivir may reduce hospitalization in outpatients (OR, 0.75 [CI,0.66 to 0.89]). Although all 4 studies adjusted for age, only 1 study (13), with 40 704 patients, adjusted for other important prognostic factors, such as comorbid conditions and geographic region. In absolute terms, approximately 12 of every 1000 patients require hospitalization and oral oseltamivir can reduce this by 3 to 9 per 1000 patients. Meta-analysis of data from 6 studies (30–32, 50, 55, 56) suggests that oral oseltamivir reduces the duration of fever by approximately 33 hours (CI, 21 to 45 hours) from onset of symptoms compared with no antiviral therapy (SMD, −0.91 [CI, −1.25 to −0.57]). Studies that could not be pooled also showed a reduction in the duration of signs and symptoms with oral oseltamivir (43, 58). The results of 5 studies (12, 21, 29, 36, 58) suggest that oral oseltamivir may result in fewer adverse events, such as neuropsychiatric events, than no antiviral therapy (rate ratio, 0.76 [CI, 0.70 to 0.81]). At 6 months, 1 study (40) found a reduction in risk for stroke and transient ischemic attacks in patients younger than 65 years who received oral oseltamivir (adjusted hazard ratio, 0.66 [CI, 0.56 to 0.77]), but no statistically significant difference in patients aged 65 years or older. Evidence also suggested that oral oseltamivir had fewer complications, such as pneumonia (adjusted OR, 0.83 [CI, 0.59 to 1.16]) (13, 45, 48), otitis media (adjusted OR, 0.75 [CI, 0.64 to 0.87]) (13, 48), or any recurrent cardiovascular outcome (adjusted OR, 0.58 [CI,0.31 to 1.10]) (14, 47).
The pooled incidence of resistance to oseltamivir across 5 studies (25, 26, 28, 54, 57) was 30 per 1000 patients receiving oral oseltamivir (CI, 10 to 60 per 1000 patients), and influenza virus was detectable in 330 per 1000 patients (CI, 280 to 370 per 1000 patients) approximately 5 days after treatment with oral oseltamivir (32, 35, 38, 39, 55, 56). No study compared the persistence of influenza virus between patients who received oseltamivir and those who did not.
A subgroup analysis of 9 studies showed differences for seasonal versus pandemic influenza (OR, 0.29 [CI, 0.17 to0.52] vs. 0.93 [CI, 0.47 to 1.84]; P for the difference = 0.011) but not for disease severity or age (Supplement Table 1, available at www.annals.org). Subgroup analyses also showed statistically significant effects in children compared with adults for pneumonia and otitis media. Only for pneumonia did we observe a significantly larger effect for oral oseltamivir in laboratory-confirmed influenza versus influenza-like illness.
We found 16 observational studies (17, 28, 34, 38, 43, 52, 60, 62–70) that evaluated the effects of starting treatment of influenza with oral oseltamivir within 48 hours of symptom onset versus after 48 hours (Table 2). However, none of these studies, including 8 studies that assessed mortality, appropriately adjusted for such confounders as age or disease severity. The results were also imprecise for the outcomes of mortality, duration of hospitalization, and signs and symptoms.
Table 2.
GRADE Evidence Profile for Oral Oseltamivir Received Within or After 48 Hours
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Patients (Studies), n* | Overall Quality of Evidence | Study Event Rates, n/N (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | |||
| Oseltamivir Received >48 h | Oseltamivir Received ≤48 h | Risk With Oseltamivir Received >48 h | Absolute Effect With Oseltamivir Received ≤48 h (95% CI) | ||||
| Mortality | 2141 (8) | Very low due to imprecision and risk of bias†‡§ | 1163 | 978 | OR, 0.33 (0.12–0.86) | 200 deaths per 1000 patients‖ | 124 fewer deaths (23 to 169 fewer deaths) per 1000 patients |
| Hospitalization | 597 (2) | Very low due to risk of bias†§¶ | 144/316 (45.6) | 151/281 (53.7) | OR, 0.52 (0.33–0.81) | 456 hospitalizations per 1000 patients | 152 fewer hospitalizations (52 to 239 fewer hospitalizations) per 1000 patients |
| ICU admission, mechanical ventilation, or respiratory failure | 1102 (4) | Very low due to risk of bias†§¶ | 120/474 (25.3) | 44/628 (7) | OR, 0.22 (0.15–0.33) | 253 admissions per 1000 patients | 184 fewer admissions (153 to 205 fewer admissions) per 1000 patients |
| Duration of hospitalization** | 79 (1)†† | Very low due to imprecision and risk of bias†§‡‡ | 43 | 36 | - | - | Mean duration of hospitalization was 24 h shorter (0 to 48 h shorter)†† |
| Duration of signs and symptoms§§ | 267 (1)‖‖ | Very low due to imprecision and risk of bias†‡§‖ | 178 | 89 | - | - | Mean time was 6.16 more hours (5.76 fewer to 18.08 more hours)‖‖ |
| Critical complication | 899 (2) | Very low due to risk of bias†§ | 38/557 (6.8) | 29/342 (8.5) | OR, 1.22 (0.44–3.36) | 68 complications per 1000 patients | 14 more complications (37 fewer to 129 more complications) per 1000 patients |
| Viral shedding¶¶ | 70 (1) | Very low due to imprecision and risk of bias†§‡‡ | 18/34 (52.9) | 8/36 (22.2) | OR, 0.25 (0.09–0.71) | 529 cases of persistent virus per 1000 patients | 310 fewer cases of persistent virus (85 to 437 fewer cases) per 1000 patients*** |
GRADE = Grading of Recommendations Assessment, Development, and Evaluation; ICU = intensive care unit; OR = odds ratio.
Follow-up to 30 d.
Not adjusted for potential confounding factors.
Effect includes possible important benefit or harm.
Although we did not downgrade, publication bias cannot be excluded.
Calculated from studies with event rates.
Not downgraded, but one half of patients were pregnant women.
Lower values indicate better outcome
Another study reported increased duration with late treatment (adjusted hazards ratio, 1.28 [95% CI, 1.04–1.57]).
This study had few events and patients.
Lower values indicate better outcome. Time to return to normal activity was not measured.
Two other studies reported greater time to alleviation of symptoms when oseltamivir was received after 48 h.
Measured at 7 d
Two other studies found fewer patients with detectable viral RNA in the early therapy group and longer duration of viral shedding in the late therapy group.
Mortality, hospitalizations, ICU admission, and respiratory failure were reduced when oral oseltamivir was received within 48 hours compared with later treatment (Table 2). Two studies (33, 69), which could not be included in the meta-analysis, reported little or no difference in mortality when therapy with oral oseltamivir began early. Two other studies showed positive effects on the duration of hospitalization with early treatment: A Centers for Disease Control and Prevention report (62) showed a reduction of 24 hours (CI, 0 to 48 hours), and another study(33) showed an increased duration with late treatment (adjusted hazard ratio, 1.28 [CI, 1.04 to 1.57]).
Three studies (28, 43, 70) reported inconsistent effects of early versus late treatment on the duration of signs and symptoms. One study (28) reported an increase of 6 hours (CI, 6 fewer to 18 more hours), another (43) found a reduction (median, 5 days with early vs. 9 days with late treatment; P= 0.01), and the third (70) found that the duration of fever may be 1.4 times longer. Critical complications may not differ between early and late treatment with oral oseltamivir (OR, 1.2 [CI, 0.44 to 3.36]) (63, 70). Three studies (38, 67, 70) suggested that early treatment reduces the duration of viral shedding. Overall, the effects may vary across specific populations. For example, the pooled unadjusted OR for mortality across all populations with early treatment compared with later treatment was0.39 (CI, 0.12 to 1.30) (43, 52, 60, 63–66, 68). The corresponding pooled unadjusted ORs were 1.47 (CI, 0.87 to 2.50) in low-risk patients and 0.03 (CI, 0 to 0.21) in pregnant women (Supplement Table 2, available at www.annals.org, provides subgroup analyses) (43, 52, 60, 64, 65, 68, 69). Patients with confirmed influenza seemed to benefit less from earlier treatment than did patients with unconfirmed influenza (OR, 0.67 [CI, 0.25 to 1.76] vs.0.03 [CI, 0 to 0.21]; P for test of interaction = 0.005) (Supplement Table 2).
Zanamivir
We found 5 observational studies (31, 49, 52, 56, 71) and 2 surveys (72, 73) that compared inhaled zanamivir with no antiviral therapy among persons treated as outpatients. One study (52) provided data for mortality, hospitalization, and ICU admissions in pregnant women who did not all have confirmed influenza. Complications from presumed viral infection were measured in patients with influenza-like illness. Only the duration of symptoms was judged as moderate-quality evidence on the basis of a meta-analysis of 3 studies enrolling 770 patients. However, the overall confidence in the estimates of effect was very low for all other outcomes because none of the studies adjusted for potential confounders and results for most outcomes were imprecise. Table 3 summarizes the findings of the studies and the quality of the evidence.
Table 3.
GRADE Evidence Profile for Inhaled Zanamivir Versus No Antiviral Therapy
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Patients (Studies), n* | Overall Quality of Evidence | Study Event Rates, n/N (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | |||
| No Antiviral Treatment | Zanamivir | Risk With No Antiviral Treatment | Absolute Effect With Zanamivir (95% CI) | ||||
| Mortality | 87 (1) | Very low due to indirectness, imprecision, and risk of bias†‡§‖ | 5/74 (6.8) | 0/13 (0) | OR, 0.47 (0.02–8.97) | 68 deaths per 1000 patients | 35 fewer deaths (66 fewer to 326 more deaths) per 1000 patients |
| Hospitalization | 4761 (2) | Very low due to imprecision and risk of bias†§‖ | 69/2411 (2.9) | 22/2350 (0.94) | OR, 0.66 (0.37–1.18) | 10 hospitalizations per 1000 patients | 3 fewer hospitalizations (6 fewer to 2 more hospitalizations) per 1000 patients |
| ICU admission | 87 (1) | Very low due to indirectness, imprecision, and risk of bias†‡§‖ | 15/74 (20.3) | 3/13 (23.1) | OR, 1.18 (0.29–4.83) | 203 admissions per 1000 patients | 28 more admissions (134 fewer to 348 more admissions) per 1000 patients |
| Duration of signs and symptoms | 770 (3) | Moderate; upgraded because of large effect†‖ | 210 | 560 | - | - | Mean time was 0.94 SD lower (0.66 to 1.21 SD lower)¶ |
| Complications | |||||||
| All outpatient complications | 4674 (1) | Very low due to risk of bias†** | 339/2337 (14.5) | 394/2337 (16.9) | OR, 1.2 (1.02–1.40) | 145 complications per 1000 patients | 24 more complications (2 to 47 more complications) per 1000 patients |
| Otitis media | 4674 (1) | Very low due to risk of bias†** | 21/2337 (0.9) | 25/2337 (1.1) | OR, 1.19 (0.67–2.14) | 9 cases per 1000 patients | 2 more cases (3 fewer to 10 more cases) per 1000 patients |
| Respiratory disease | 2600 (1) | Very low due to risk of bias†** | 263/2337 (11) | 301/2337 (12.9) | OR, 1.17 (0.98–1.39) | 113 respiratory complications per 1000 patients | 17 more respiratory complications (2 fewer to 37 more complications) per 1000 patients |
| Viral shedding†† | 136(2) | Very low due to imprecision and risk of bias‖‡‡§§ | - | 44/136 (32.4) Pooled risk, 31% (95% CI, 23%−39%) | - | - | - |
| Time to return to normal activity | Not measured | ||||||
| Resistance | Not measured | ||||||
GRADE = Grading of Recommendations Assessment, Development, and Evaluation; ICU = intensive care unit; OR = odds ratio.
Follow-up to 30 d.
Not adjusted for potential confounding factors.
Study in pregnant women only. Downgraded for possible lack of generalizability to typical population.
Effect includes important benefit or harm with zanamivir and few patients and events.
Although we did not downgrade, publication bias cannot be excluded.
The standardized mean difference represents a large effect and translates into an approximate reduction of 23 h (95% CI, 17 to 28 h).
Publication bias was a concern because the study had for-profit funding.
Assessed with persistent virus.
Studies had no comparison group.
Trial measured select patients and had few events and patients.
Two studies (52, 71) found that patients with laboratory-confirmed influenza or influenza-like illness who receive inhaled zanamivir may be less likely to be hospitalized than those who receive no antiviral therapy (OR, 0.66 [CI, 0.37 to 1.18]). Pooled results from 3 studies (39, 49, 56) indicate that inhaled zanamivir reduced the duration of symptoms by approximately 23 hours (CI, 17 to 28 hours) on the basis of a large SMD (−0.94 [CI, −1.21 to −0.66]). One study in outpatients (71), which could not be included in the meta-analysis, also showed a 45% reduction in the duration of illness and a 40% reduction in the severity of symptoms with inhaled zanamivir. Data from 2 surveys about symptom relief and duration of symptoms (72, 73) reported that most patients had fewer symptoms after 2 days. In patients with influenza-like illness, more may experience complications, such as otitis media (OR, 1.19 [CI, 0.67 to 2.14]), respiratory disease (OR, 1.17 [CI, 0.98 to 1.39]), or all outpatient complications (OR, 1.2 [CI, 1.02 to 1.40]) with inhaled zanamivir(71). A study in pregnant women (52) suffered from imprecision and showed no effect of inhaled zanamivir on ICU admission (OR, 1.18 [CI, 0.29 to 4.83]). In addition, no study clearly reported on adverse events. We found no observational studies that compared early versus late treatment of influenza with inhaled zanamivir and did not identify statistically significant subgroup effects (Supplement Table 3, available at www.annals.org).
Oseltamivir Versus Zanamivir
We found 8 observational studies (31, 49, 52, 56, 74–77) that directly compared oral oseltamivir with inhaled zanamivir. No study adjusted for important potential confounders (age or comorbid conditions). All outcomes were graded as very low quality because of risk of bias or imprecision. Table 4 shows the GRADE evidence profile, and Supplement Table 4 (available at www.annals.org) shows the results of preplanned subgroup analyses.
Table 4.
GRADE Evidence Profile for Oral Oseltamivir Versus Inhaled Zanamivir
| Outcome | Quality Assessment | Summary of Findings | |||||
|---|---|---|---|---|---|---|---|
| Patients (Studies), n* | Overall Quality of Evidence | Study Event Rates, n/N (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | |||
| Zanamivir | Oseltamivir | Risk With Zanamivir | Absolute Effect With Oseltamivir (95% CI) | ||||
| Mortality | 489 (1) | Very low†‡§‖ | 0/13 (0) | 21/476 (4.4) | OR, 1.27 (0.07–22.16)¶ | 5 deaths per 1000 patients** | 1 more death (5 fewer to 95 more deaths) per 1000 patients |
| Hospitalization | 489 (1) | Very low†‡§‖ | 8/13 (61.5) | 329/476 (69.1) | OR, 1.4 (0.45–4.35) | 615 hospitalizations per 1000 patients | 76 more hospitalizations (197 fewer to 259 more hospitalizations) per 1000 patients |
| ICU admissions, mechanical ventilation, or respiratory failure | 489 (1) | Very low†‡‖ | 3/13 (23.1) | 71/476 (14.9) | OR, 0.58 (0.16–2.18) | 231 admissions per 1000 patients | 83 fewer admissions (185 fewer to 165 more admissions) per 1000 patients |
| Duration of signs and symptoms | 1932 (5) | Very low due to risk of bias†‖ | 760 | 1172 | - | - | Mean time was 0.26 SD higher (0.07 to 0.45 SD higher)†† |
| Complications | Not measured | ||||||
| Critical adverse events | 1045 (1) | Very low due to risk of bias†‖ | 402 | 643 | Rate ratio, 2.9 (0.37–23.05) | 7 adverse events per 1000 patient-years | 14 more adverse events (5 fewer to 165 more events) per 1000 patient-years |
| Viral shedding (persistent virus) | 46(1) | Very low due to imprecision and risk of bias§‖ | 4/23 (17.4) | 9/23 (39.1) | OR, 3.05 (0.78–11.96) | 174 cases of persistent virus per 1000 patients | 217 more cases of persistent virus (33 fewer to 542 more cases) per 1000 patients |
GRADE = Grading of Recommendations Assessment, Development, and Evaluation; ICU = intensive care unit; OR = odds ratio.
Follow-up to 30 d.
Not adjusted for potential confounding factors.
Quality was downgraded because results were calculated from pregnant women and the effect may therefore not be applicable to typical patients.
Effect includes benefit and harm and few patients and events.
Although we did not downgrade, publication bias cannot be excluded.
No deaths were reported in 2 studies (0 of 84 with oseltamivir and 0 of 76 with zanamivir).
Based on calculations from event rate.
The standardized mean difference represents a small increase in effect.
A small study in pregnant women (52) reported an OR of 1.27 (CI, 0.07 to 22.16) for death with oseltamivir. Two other small studies (75, 76) were conducted in outpatient populations with mild uncomplicated influenza but did not report on deaths. The combined results of 5 Japanese studies in patients with confirmed influenza (31, 49, 55, 74, 75) suggest that inhaled zanamivir may be associated with a slightly shorter symptom duration than oral oseltamivir (7 hours [CI, 2 to 12 hours]; SMD, 0.26 [CI,0.07 to 0.45]). However, data from another study (76), which could not be pooled, reported no statistically significant difference in duration of symptoms. The 2 treatments did not differ for hospitalization (OR, 1.4 [CI, 0.45 to4.35]) or ICU admissions (OR, 0.58 [CI, 0.16 to 2.18]) in a study enrolling pregnant women (52) or for critical adverse events in 1 study of outpatients with confirmed influenza (rate ratio, 2.90 [CI, 0.37 to 23.05]) (31). Another study (56) showed no statistically significant difference in influenza viral RNA detection after 5 days of treatment (OR, 3.05 [CI, 0.78 to 11.96]).
Amantadine and Rimantadine for Influenza A
We found 6 observational studies evaluating influenza seasons from 1988 to 2006: Three studies (30, 78, 79) compared oral amantadine with no antiviral therapy for influenza A, and 3 (80–82) evaluated oral amantadine only (no independent comparison group), providing incidence data for time to alleviation of symptoms and adverse events. One of these studies (82) measured amantadine resistance after treatment in a sample of 111 children in an outpatient setting. Taken together, the quality of this body of evidence is very low because of the serious risk of bias and imprecise results. Supplement Table 5 (available at www.annals.org) summarizes our findings and the quality of the evidence.
One study (78) found that receiving oral amantadine may reduce mortality (OR, 0.04 [CI, 0 to 0.73]) and pneumonia (OR, 0.76 [CI, 0.38 to 1.53]), but time to alleviation of symptoms did not significantly differ between oral amantadine and no antiviral therapy. Another study (30) showed a shorter duration of hospitalization with oral amantadine than with no antiviral therapy. Pooled incidence rates from 3 studies (30, 80, 81) showed a time to alleviation of symptoms of 64 hours (CI, 62 to 65 hours). One study (79) reported that 15 of 45 hospitalized adults (33%) with confirmed influenza A developed pneumonia and 21 of 55 adults (38%) were admitted to the ICU because of respiratory failure while receiving oral amantadine. Pooled incidence rates for adverse events in patients receiving oral amantadine suggested that adverse events were nearly absent (30, 81). For resistance, 1 study found that 28% (CI, 20% to 36%) of children were infected with influenza A virus that was resistant to amantadine after treatment (82).
We found 1 observational study (80) comparing early versus late treatment with oral amantadine. The study included 676 patients with influenza A who received oral amantadine, 50 mg twice daily, for 5 days as outpatients. The mean duration of fever was 52.5 hours (SD, 26.6) after onset of symptoms when amantadine was given between 0 and 12 hours; 63.6 hours (SD, 24) when given between 13 and 24 hours; and 76 hours (SD, 25.9) when given between 25 and 48 hours. Duration of fever was significantly shorter in the 0- to 12-hour group than in the 13-to 24-hour or 25- to 48-hour groups and also shorter in the 13- to 24-hour than in the 25- to 48-hour group.
We found no studies that compared oral rimantadine with no antiviral therapy. However, 2 studies (83, 84) evaluated the use of oral rimantadine within 24 hours versus after 24 to 48 hours in patients with influenza A(H1N1) virus infection in outpatient settings during the 1979 to 1981 seasons and outpatients with influenza-like illness during the reemergence of influenza A(H1N1) virus in 1977. Meta-analysis suggested a reduced risk for complications with oral rimantadine provided within 24 hours (OR,0.05 [CI, 0.01 to 0.38]). We found no studies comparing oral amantadine with oral rimantadine to treat influenza A.
DISCUSSION
Our systematic review summarizes the evidence from 74 observational studies about the pharmacologic treatment of influenza with antivirals. Despite low to very low confidence in the estimates of effect, this review must be viewed in the context of the information available from RCTs and the substantial burden of influenza worldwide. Many of the outcomes for which we summarized the evidence have not been assessed in RCTs; in addition, the body of evidence from RCTs suffers from even greater imprecision than we found in these observational studies. For example, Jefferson and colleagues (4) did not report on mortality because no events were reported in the RCTs. Furthermore, the studies we identified reported on more than 1600 hospitalizations for more than 150 000 patients, compared with the 62 events in 4693 patients in Jefferson and colleagues’ systematic review of RCTs (4).
Our findings indicate that the use of oral oseltamivir to treat influenza may provide net benefit by reducing mortality and the duration of symptoms and complications of influenza. Jefferson and colleagues (4) also showed that oseltamivir shortened the duration of symptoms and complications of influenza, such as asthma. The pooled rate of hospitalization in patients not receiving oseltamivir was similar in the 2 reviews (0.8% vs. 1.2%), but we observed a large, precise effect of oseltamivir on hospitalization (OR,0.75 [CI, 0.66 to 0.86]). This effect is still compatible with the imprecise estimate from the RCTs (OR, 0.95 [CI, 0.57 to 1.61]) (4). In our review, inhaled zanamivir reduces signs and symptoms, but we judged the overall confidence in the estimates of effect to be very low because of the imprecise and possibly biased data on mortality and hospitalization. A direct comparison between oral oseltamivir and inhaled zanamivir in 8 studies showed that zanamivir may have a slight advantage in shortening the duration of signs and symptoms. The evidence about the use of oral amantadine is sparse but may suggest a benefit from using this agent to treat drug-sensitive influenza A virus infection. The evidence also suggests that earlier treatment with antivirals (within 48 hours) may be of greater benefit than later treatment.
The strengths of our review include the comprehensive search, attempts to identify unpublished data, inclusion of studies reported in languages other than English, and detailed assessment of the factors that influence the confidence in the results across questions and studies. It adds data on interventions (such as early vs. late treatment), outcomes (such as mortality, hospitalizations, and complications), and subgroups (such as immunocompromised patients and pregnant women) that are not available from RCTs.
Our review has limitations, relating to the evidence itself, that require attention for both interpreting the results and conducting future research. Potential bias reduces the confidence in the estimates of effect. Many of the identified studies had a high risk for observational study bias due to the lack of control for confounders and covariates (such as the lack of adjustment for age or comorbid conditions). For example, of the entire body of evidence on inhaled zanamivir, only the estimate for the “duration of signs and symptoms” is based on study results that were adjusted for these potential confounders. Confounding by indication (a greater likelihood that sicker patients will be treated) could therefore reduce effects based on analyses that are not adjusted or are insufficiently adjusted; however, investigators or clinicians may also select healthier patients for treatment to reduce potential adverse effects of antivirals, which could bias the results in favor of treatment. Greater emphasis should be placed on data from adjusted meta-analyses. However, to provide a comprehensive view of the available evidence, we present pooled results from the adjusted and the unadjusted studies separately.
Even when adjusted analyses were available, we could not always assess whether the authors considered all pertinent variables or whether even optimal adjustment would permit valid comparisons between treated versus untreated patients in these studies. In addition, for some outcomes, such as death in the oseltamivir studies, the results may apply only to hospitalized patients because the data were derived in this patient group. Reporting and publication bias remain of particular concern in systematic reviews of observational studies (85). Another limitation of our study is that we performed our literature search more than 1 year ago and did not assess several recent published observational studies of neuraminidase inhibitor treatment (86–88). Two additional recently published Cochrane reviews evaluated neuraminidase inhibitors in children and adamantanes for influenza A in children and elderly patients, but they do not address most of the patient-important outcomes we describe (89, 90).
The included studies focused on antiviral treatment of drug-sensitive influenza virus infections; therefore, caution should be used when applying these results to the current treatment of circulating influenza viruses, which are generally resistant to amantadine and rimantadine, or in the future, when the prevalence of antiviral-resistant viruses could increase substantially and unpredictably. Concerns have been raised about the increasing prevalence of oseltamivir resistance among circulating influenza A(H1N1)pdm09 virus strains (91, 92). The development of guidelines based on our review will require judgments about the applicability of the results to populations that may be infected with resistant influenza virus.
Despite these limitations, our summary of the evidence provides information that is not available in systematic reviews of RCTs. The potential positive effect of earlier rather than later administration of oseltamivir on death in hospitalized patients and the suggestion that pregnant women, children, and immunocompromised patients may also benefit from treatment are among the key contributions of our study. We also found moderate-quality evidence that inhaled zanamivir reduces signs and symptoms more than no treatment.
In summary, although we have identified important evidence supporting a role for antivirals in the treatment of influenza, attention must be paid when this evidence is applied because of the various sources of bias. We need high-quality evidence from randomized trials that address patient-important outcomes and include hospitalized patients with influenza. This requires trial preparedness and collaboration among large organizations to implement large RCTs during influenza epidemics, but given the burden caused by influenza, this should be achievable and desirable. Observational studies can supplement this evidence by contributing data about special populations, adverse effects, and rare harms. However, such studies should minimize selection bias; be prospectively designed, permitting data collection for all relevant prognostic factors; and include standardized and validated assessments of adverse events that can be summarized in systematic reviews. Studies should also be prospectively registered to reduce reporting and publication bias (93). Ideally, RCTs and observational studies should also perform serial virologic sampling to correlate with and complement clinical data collection. In the meantime, the available data will inform guidelines and reimbursement decisions. These data suggest that oral oseltamivir could provide a net benefit in the treatment of patients with influenza, including a sizable reduction in mortality in hospitalized patients, although our confidence in these effects is low.
Supplementary Material
Context
Antiviral therapy may reduce complications and mortality associated with influenza.
Contribution
This review of 74 observational studies found that oral oseltamivir may reduce mortality in high-risk populations compared with no treatment. Either oral oseltamivir or inhaled zanamivir might reduce hospitalizations and symptom duration.
Caution
The studies were probably biased because of confounding. Neither costs nor targeting strategies were evaluated. The studies focused on drug-sensitive infections, so the results may not be applicable if resistant viruses are prevalent.
Implication
Antivirals might improve outcomes in some situations, but more evidence is needed to guide decision making about when and in whom to use particular agents.
—The Editors
Acknowledgment:
The authors thank Rasha Khatib and Meg Fukuzawa for Japanese translations of articles, Wojtek Wiercioch for help with reference management, Diane Heels-Ansdell for statistical support, GlaxoSmithKline and James Smith (Roche) for responding to our requests to identify studies, and Dr. Tom Jefferson for his help in interpreting the recent Cochrane reviews that he authored summarizing the RCTs on this topic.
Grant Support: By agreement I2-APW-223 from the World Health Organization and by the Faculty of Health Sciences of McMaster University (internal grant to McMaster Healthcare Grading and Recommendations Center).
Potential Conflicts of Interest: Ms. Santesso; Drs. Mustafa, Brozek, Hopkins, Flottorp, and Schünemann; Mr. Chen; Ms. Cheung; Mr. Wong; and Mr. Tian report the following: Grant (money to institution): World Health Organization. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M11-3115.
APPENDIX: SEARCH STRATEGIES
EMBASE (1980 to Week 452 010), MEDLINE In-Process and Other Nonindexed Citations, MEDLINE (1950 to 16 November 2010), the Cochrane Library databases, Chinese Biomedical Literature Database, and Panteleimon (to November 2010)
influenza$.mp.
amantadine.mp.
oseltamivir.mp.
zanamivir.mp.
rimantadine.mp.
(aminoadamantane or adamantane or symmetrel or flumadine or tamiflu or relenza).tw.
neuraminidase inhibitor$.tw.
(m2 and (inhibitor$ or ion)).tw.
or/2–8
1 and 9
SIGLE (to November 2010)
(influenza*) AND (amantadine or oseltamivir or zanamivir or rimantadine or aminoadamantane or adamantane or symmetrel or flumadine or tamiflu or relenza or neuraminidase inhibitor*)
CINAHL (1981 to November 2010)
S3. S1 and S2
S2. TX (amantadine or oseltamivir or zanamivir or rimantadine) or MW antiviral agents or TX (aminoadamantane or adamantane or symmetrel or flumadine or tamiflu or relenza) or TX neuraminidase inhibitor* or TX (m2 and (inhibitor$ or ion))
S1. TX influenza*
LILACS (to November 2010)
“INFLUENZA HUMANA/DT” or “INFLUENZA HUMANA/PC” or “INFLUENZA HUMANA/TH” and amantadine or oseltamivir or zanamivir or rimantadine or aminoadamantane or adamantane or symmetrel or flumadine or tamiflu or relenza [Words]
Appendix Figure.
Summary of evidence search and selection.
References
- 1.World Health Organization. State of the Art of Vaccine Research and Development. Geneva: World Health Organization; 2005. Accessed at www.who.int/vaccine_research/documents/Dip%20814.pdf on 17 February 2012 [Google Scholar]
- 2.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378:1917–30. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses. Geneva: World Health Organization; 2010. Accessed at www.who.int/csr/resources/publications/swineflu/h1n1_use_antivirals_20090820/en/ on 17 February 2012. [PubMed] [Google Scholar]
- 4.Jefferson T, Jones MA, Doshi P, Del Mar CB, Heneghan CJ, Hama R, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2012;1:CD008965. [DOI] [PubMed] [Google Scholar]
- 5.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Ontario, Canada: Ottawa Hospital Research Institute; 2011. Accessed at www.ohri.ca/programs/clinical_epidemiology/oxford.htm on 17 February 2012. [Google Scholar]
- 6.Schünemann H, Brozek J, Guyatt G, Oxman A, eds. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.6. Hamilton, Ontario, Canada: McMaster University, GRADE Working Group; 2011. Accessed at http://ims.cochrane.org/revman/gradepro on 17 February 2012. [Google Scholar]
- 7.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schünemann HJ, Hill SR, Kakad M, Bellamy R, Uyeki TM, Hayden FG, et al. ; WHO Rapid Advice Guideline Panel on Avian Influenza. WHO Rapid Advice Guidelines for pharmacological management of sporadic human infection with avian influenza A (H5N1) virus. Lancet Infect Dis. 2007;7:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Accessed at www.cochrane-handbook.org on 17 February 2012. [Google Scholar]
- 10.Jefferson T, Jones M, Doshi P, Del Mar C, Dooley L, Foxlee R. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev. 2010:CD001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr CE, Schulman K, Iacuzio D, Bradley JS. Effect of oseltamivir on the risk of pneumonia and use of health care services in children with clinically diagnosed influenza. Curr Med Res Opin. 2007;23:523–31. [DOI] [PubMed] [Google Scholar]
- 12.Blumentals WA, Schulman KL. Impact of oseltamivir on the incidence of secondary complications of influenza in adolescent and adult patients: results from a retrospective population-based study. Curr Med Res Opin. 2007;23: 2961–70. [DOI] [PubMed] [Google Scholar]
- 13.Blumentals WA, Song X. The safety of oseltamivir in patients with influenza: analysis of healthcare claims data from six influenza seasons. MedGenMed. 2007; 9:23. [PMC free article] [PubMed] [Google Scholar]
- 14.Casscells SW, Granger E, Kress AM, Linton A, Madjid M, Cottrell L. Use of oseltamivir after influenza infection is associated with reduced incidence of recurrent adverse cardiovascular outcomes among military health system beneficiaries with prior cardiovascular diseases. Circ Cardiovasc Qual Outcomes. 2009; 2:108–15. [DOI] [PubMed] [Google Scholar]
- 15.Chemaly RF, Ghosh S, Bodey GP, Rohatgi N, Safdar A, Keating MJ, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore). 2006;85:278–87. [DOI] [PubMed] [Google Scholar]
- 16.Chemaly RF, Torres HA, Aguilera EA, Mattiuzzi G, Cabanillas M, Kan-tarjian H, et al. Neuraminidase inhibitors improve outcome of patients with leukemia and influenza: an observational study. Clin Infect Dis. 2007;44:964–7. [DOI] [PubMed] [Google Scholar]
- 17.Chien YS, Su CP, Tsai HT, Huang AS, Lien CE, Hung MN, et al. Predictors and outcomes of respiratory failure among hospitalized pneumonia patients with 2009 H1N1 influenza in Taiwan. J Infect. 2010;60:168–74. [DOI] [PubMed] [Google Scholar]
- 18.Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–26. [DOI] [PubMed] [Google Scholar]
- 19.Cui W, Zhao H, Lu X, Wen Y, Zhou Y, Deng B, et al. Factors associated with death in hospitalized pneumonia patients with 2009 H1N1 influenza in Shenyang, China. BMC Infect Dis. 2010;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharan NJ, Gubareva LV, Klimov AI, Fiore AE, Bresee JS, Fry AM. Antiviral treatment of patients with oseltamivir-resistant and oseltamivir-susceptible seasonal Influenza A (H1N1) infection during the 2007–2008 influenza season in the United States [Letter]. Clin Infect Dis. 2010;50:621–2. [DOI] [PubMed] [Google Scholar]
- 21.Enger C, Nordstrom BL, Thakrar B, Sacks S, Rothman KJ. Health outcomes among patients receiving oseltamivir. Pharmacoepidemiol Drug Saf. 2004; 13:227–37. [DOI] [PubMed] [Google Scholar]
- 22.Estenssoro E, Ríos FG, Apezteguía C, Reina R, Neira J, Ceraso DH, et al. ; Registry of the Argentinian Society of Intensive Care SATI. Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010;182:41–8. [DOI] [PubMed] [Google Scholar]
- 23.Gums JG, Pelletier EM, Blumentals WA. Oseltamivir and influenza-related complications, hospitalization and healthcare expenditure in healthy adults and children. Expert Opin Pharmacother. 2008;9:151–61. [DOI] [PubMed] [Google Scholar]
- 24.Hanshaoworakul W, Simmerman JM, Narueponjirakul U, Sanasuttipun W, Shinde V, Kaewchana S, et al. Severe human influenza infections in Thailand: oseltamivir treatment and risk factors for fatal outcome. PLoS One. 2009; 4:e6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvala H, Gunson R, Simmonds P, Hardie A, Bennett S, Scott F, et al. The emergence of oseltamivir-resistant pandemic influenza A (H1N1) 2009 virus amongst hospitalised immunocompromised patients in Scotland, November-December, 2009. Euro Surveill. 2010;15. [PubMed] [Google Scholar]
- 26.Hatakeyama S, Sugaya N, Ito M, Yamazaki M, Ichikawa M, Kimura K, et al. Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA. 2007;297:1435–42. [DOI] [PubMed] [Google Scholar]
- 27.Hien ND, Ha NH, Van NT, Ha NT, Lien TT, Thai NQ, et al. Human infection with highly pathogenic avian influenza virus (H5N1) in northern Vietnam, 2004–2005. Emerg Infect Dis. 2009;15:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hien TT, Boni MF, Bryant JE, Ngan TT, Wolbers M, Nguyen TD, et al. Early pandemic influenza (2009 H1N1) in Ho Chi Minh City, Vietnam: a clinical virological and epidemiological analysis. PLoS Med. 2010;7:e1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YC, Li WC, Tsao KC, Huang CG, Chiu CH, Lin TY. Influenza-associated central nervous system dysfunction in Taiwanese children: clinical characteristics and outcomes with and without administration of oseltamivir. Pediatr Infect Dis J. 2009;28:647–8. [DOI] [PubMed] [Google Scholar]
- 30.Imamura T, Hosoya M, Oonishi N, Sato K, Katayose M, Kawasaki Y, et al. [The study on efficacy of oseltamivir for influenza A in children]. Kansenshogaku Zasshi. 2003;77:971–6. [DOI] [PubMed] [Google Scholar]
- 31.Kawai N, Ikematsu H, Iwaki N, Maeda T, Kanazawa H, Kawashima T, et al. A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza A and B. J Infect. 2008;56:51–7. [DOI] [PubMed] [Google Scholar]
- 32.Kawai N, Ikematsu H, Iwaki N, Maeda T, Satoh I, Hirotsu N, et al. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clin Infect Dis. 2006;43:439–44. [DOI] [PubMed] [Google Scholar]
- 33.Lee CS, Lee JH. Dynamics of clinical symptoms in patients with pandemic influenza A (H1N1). Clin Microbiol Infect. 2010;16:389–90. [DOI] [PubMed] [Google Scholar]
- 34.Lee N, Chan PK, Hui DS, Rainer TH, Wong E, Choi KW, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee N, Chan PK, Choi KW, Lui G, Wong B, Cockram CS, et al. Factors associated with early hospital discharge of adult influenza patients. Antivir Ther. 2007;12:501–8. [PubMed] [Google Scholar]
- 36.Li IW, Hung IF, To KK, Chan KH, Wong SS, Chan JF, et al. The natural viral load profile of patients with pandemic 2009 influenza A(H1N1) and the effect of oseltamivir treatment. Chest. 2010;137:759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liem NT, Tung CV, Hien ND, Hien TT, Chau NQ, Long HT, et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin Infect Dis. 2009;48:1639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling LM, Chow AL, Lye DC, Tan AS, Krishnan P, Cui L, et al. Effects of early oseltamivir therapy on viral shedding in 2009 pandemic influenza A (H1N1) virus infection. Clin Infect Dis. 2010;50:963–9. [DOI] [PubMed] [Google Scholar]
- 39.Machado CM, Boas LS, Mendes AV, Santos MF, da Rocha IF, Sturaro D, et al. Low mortality rates related to respiratory virus infections after bone marrow transplantation. Bone Marrow Transplant. 2003;31:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madjid M, Curkendall S, Blumentals WA. The influence of oseltamivir treatment on the risk of stroke after influenza infection. Cardiology. 2009;113: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGeer A, Green K, Drews S, Davis I, Downey J, Katz K, et al. Epidemiology of influenza illness requiring intensive care unit admission in Toronto, Canada. Clin Microbiol Infect. 2009;15:S26. [Google Scholar]
- 42.McGeer A, Green KA, Plevneshi A, Shigayeva A, Siddiqi N, Raboud J, et al. ; Toronto Invasive Bacterial Diseases Network. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45:1568–75. [DOI] [PubMed] [Google Scholar]
- 43.McLean E, Pebody RG, Campbell C, Chamberland M, Hawkins C, Nguyen-Van-Tam JS, et al. Pandemic (H1N1) 2009 influenza in the UK: clinical and epidemiological findings from the first few hundred (FF100) cases. Epidemiol Infect. 2010;138:1531–41. [DOI] [PubMed] [Google Scholar]
- 44.Ng S, Cowling BJ, Fang VJ, Chan KH, Ip DK, Cheng CK, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis. 2010;50:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordstrom BL, Sung I, Suter P, Szneke P. Risk of pneumonia and other complications of influenza-like illness in patients treated with oseltamivir. Curr Med Res Opin. 2005;21:761–8. [DOI] [PubMed] [Google Scholar]
- 46.Orzeck EA, Shi N, Blumentals WA. Oseltamivir and the risk of influenzarelated complications and hospitalizations in patients with diabetes. Clin Ther. 2007;29:2246–55. [DOI] [PubMed] [Google Scholar]
- 47.Peters PH, Moscona A, Schulman KL, Barr CE. Study of the impact of oseltamivir on the risk for pneumonia and other outcomes of influenza, 2000–2005. Medscape J Med. 2008;10:131. [PMC free article] [PubMed] [Google Scholar]
- 48.Piedra PA, Schulman KL, Blumentals WA. Effects of oseltamivir on influenza-related complications in children with chronic medical conditions. Pediatrics. 2009;124:170–8. [DOI] [PubMed] [Google Scholar]
- 49.Saito R, Sato I, Suzuki Y, Baranovich T, Matsuda R, Ishitani N, et al. Reduced effectiveness of oseltamivir in children infected with oseltamivir-resistant influenza A (H1N1) viruses with His275Tyr mutation. Pediatr Infect Dis J. 2010;29:898–904. [DOI] [PubMed] [Google Scholar]
- 50.Sato M, Saito R, Sato I, Tanabe N, Shobugawa Y, Sasaki A, et al. Effectiveness of oseltamivir treatment among children with influenza A or B virus infections during four successive winters in Niigata City, Japan. Tohoku J Exp Med. 2008;214:113–20. [DOI] [PubMed] [Google Scholar]
- 51.Shen Y, Lu H. Pandemic (H1N1) 2009, Shanghai, China. Emerg Infect Dis. 2010;16:1011–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. ; Pandemic H1N1 Influenza in Pregnancy Working Group. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith JR, Sacks S. Incidence of neuropsychiatric adverse events in influenza patients treated with oseltamivir or no antiviral treatment. Int J Clin Pract. 2009; 63:596–605. [DOI] [PubMed] [Google Scholar]
- 54.Stephenson I, Democratis J, Lackenby A, McNally T, Smith J, Pareek M, et al. Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children. Clin Infect Dis. 2009;48:389–96. [DOI] [PubMed] [Google Scholar]
- 55.Sugaya N, Mitamura K, Yamazaki M, Tamura D, Ichikawa M, Kimura K, et al. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis. 2007;44:197–202. [DOI] [PubMed] [Google Scholar]
- 56.Sugaya N, Tamura D, Yamazaki M, Ichikawa M, Kawakami C, Kawaoka Y, et al. Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin Infect Dis. 2008;47:339–45. [DOI] [PubMed] [Google Scholar]
- 57.Tramontana AR, George B, Hurt AC, Doyle JS, Langan K, Reid AB, et al. Oseltamivir resistance in adult oncology and hematology patients infected with pandemic (H1N1) 2009 virus, Australia. Emerg Infect Dis. 2010;16:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsalik EL, Hendershot EF, Sangvai DG, Cunningham HM, Cunningham CK, Lopez-Marti MG, et al. Clinical presentation and response to treatment of novel influenza A H1N1 in a university-based summer camp population. J Clin Virol. 2010;47:286–8. [DOI] [PubMed] [Google Scholar]
- 59.Viasus D, Paño-Pardo JR, Pachón J, Campins A, López-Medrano F, Vil-loslada A, et al. ; Novel Influenza A (H1N1) Study Group of the Spanish Network for Research in Infectious Diseases (REIPI). Factors associated with severe disease in hospitalized adults with pandemic (H1N1) 2009 in Spain. Clin Microbiol Infect. 2011;17:738–46. [DOI] [PubMed] [Google Scholar]
- 60.Xi X, Xu Y, Jiang L, Li A, Duan J, Du B; Chinese Critical Care Clinical Trial Group. Hospitalized adult patients with 2009 influenza A(H1N1) in Beijing, China: risk factors for hospital mortality. BMC Infect Dis. 2010;10:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang P, Deng Y, Pang X, Shi W, Li X, Tian L, et al. Severe, critical and fatal cases of 2009 H1N1 influenza in China. J Infect. 2010;61:277–83. [DOI] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC). Patients hospitalized with 2009 pandemic influenza A (H1N1)—New York City, May 2009. MMWR Morb Mortal Wkly Rep. 2010;58:1436–40. [PubMed] [Google Scholar]
- 63.Chitnis AS, Truelove SA, Druckenmiller JK, Heffernan RT, Davis JP. Epidemiologic and clinical features among patients hospitalized in Wisconsin with 2009 H1N1 influenza A virus infections, April to August 2009. WMJ. 2010;109:201–8. [PubMed] [Google Scholar]
- 64.Dubar G, Azria E, Tesnière A, Dupont H, Le Ray C, Baugnon T, et al. ; French Registry on 2009 A/H1N1v during pregnancy. French experience of 2009 A/H1N1v influenza in pregnant women. PLoS One. 2010;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kandun IN, Tresnaningsih E, Purba WH, Lee V, Samaan G, Harun S, et al. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet. 2008;372:744–9. [DOI] [PubMed] [Google Scholar]
- 66.Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C, et al. ; American Society of Transplantation H1N1 Collaborative Study Group. Out-comes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010;10:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee N, Choi KW, Chan PK, Hui DS, Lui GC, Wong BC, et al. Outcomes of adults hospitalised with severe influenza. Thorax. 2010;65:510–5. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez A, Zaragoza R, Daz E, Daz JJ, Marques A, Figueira JC, et al. Early oseltamivir treatment was associated with improved outcomes in 2009 pandemic influenza A (H1N1)v in Spain. Intensive Care Med. 2010;36:S136. [Google Scholar]
- 69.Subramony H, Lai FY, Ang LW, Cutter JL, Lim PL, James L. An epidemiological study of 1348 cases of pandemic H1N1 influenza admitted to Singapore Hospitals from July to September 2009. Ann Acad Med Singapore. 2010; 39:283–8. [PubMed] [Google Scholar]
- 70.Yu H, Liao Q, Yuan Y, Zhou L, Xiang N, Huai Y, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ. 2010;341:c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cole JA, Loughlin JE, Ajene AN, Rosenberg DM, Cook SE, Walker AM. The effect of zanamivir treatment on influenza complications: a retrospective cohort study. Clin Ther. 2002;24:1824–39. [DOI] [PubMed] [Google Scholar]
- 72.Johnson R, Schweinle JE, Burroughs S. Zanamivir for the treatment of clinically diagnosed influenza in clinical practice: Results of the valuable-insights-from-patients study. Clin Drug Invest. 2000;20:327–36. [Google Scholar]
- 73.Silagy C, Watts R. Zanamivir, a new targeted therapy in the treatment of influenza. A patient perspective assessed by questionnaire. Clin Drug Invest. 2000;19:111–21. [Google Scholar]
- 74.Kawai N, Ikematsu H, Iwaki N, Kondou K, Hirotsu N, Kawashima T, et al. Clinical effectiveness of oseltamivir for influenza A(H1N1) virus with H274Y neuraminidase mutation. J Infect. 2009;59:207–12. [DOI] [PubMed] [Google Scholar]
- 75.Komiya N, Gu Y, Kamiya H, Yahata Y, Matsui T, Yasui Y, et al. Clinical features of cases of influenza A (H1N1)v in Osaka prefecture, Japan, May 2009. Euro Surveill. 2009;14. [DOI] [PubMed] [Google Scholar]
- 76.Yamagishi T, Matsui T, Nakamura N, Oyama T, Taniguchi K, Aoki T, et al. Onset and duration of symptoms and timing of disease transmission of 2009 influenza A (H1N1) in an outbreak in Fukuoka, Japan, June 2009. Jpn J Infect Dis. 2010;63:327–31. [PubMed] [Google Scholar]
- 77.Yates L, Pierce M, Stephens S, Mill AC, Spark P, Kurinczuk JJ, et al. Influenza A/H1N1v in pregnancy: an investigation of the characteristics and management of affected women and the relationship to pregnancy outcomes for mother and infant. Health Technol Assess. 2010;14:109–82. [DOI] [PubMed] [Google Scholar]
- 78.Libow LS, Neufeld RR, Olson E, Breuer B, Starer P. Sequential outbreak of influenza A and B in a nursing home: efficacy of vaccine and amantadine. J Am Geriatr Soc. 1996;44:1153–7. [DOI] [PubMed] [Google Scholar]
- 79.Rabagliati R, Benítez R, Fernández A, Gaete P, Guzmán AM, García P, et al. [Influenza-A as etiology of fever and respiratory insufficiency in adults hospitalized during an outbreak in Chile]. Rev Med Chil. 2004;132:317–24. [PubMed] [Google Scholar]
- 80.Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, Maeda T, et al. Factors influencing the effectiveness of oseltamivir and amantadine for the treatment of influenza: a multicenter study from Japan of the 2002–2003 influenza season. Clin Infect Dis. 2005;40:1309–16. [DOI] [PubMed] [Google Scholar]
- 81.Kawai N, Ikematsu H, Iwaki N, Kawamura K, Kawashima T, Kashiwagi S. A change in the effectiveness of amantadine for the treatment of influenza over the 2003–2004, 2004–2005, and 2005–2006 influenza seasons in Japan. J Infect Chemother. 2007;13:314–9. [DOI] [PubMed] [Google Scholar]
- 82.Saito R, Sakai T, Sato I, Sano Y, Oshitani H, Sato M, et al. Frequency of amantadine-resistant influenza A viruses during two seasons featuring cocirculation of H1N1 and H3N2. J Clin Microbiol. 2003;41:2164–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gagarinova VM, Shadrin AS, Kubar’ OI, Kustikova IuG, Araslanova II. [Organization and evaluation of the effectiveness of emergency prophylaxis and early treatment of influenza with remantadine in Serevodvinsk]. Zh Mikrobiol Epidemiol Immunobiol. 1983:60–3. [PubMed] [Google Scholar]
- 84.Shadrin AS, Araslanova II, Dekterev AN, Gagarinova VM, Tamarkina KN. [Organization and the results of the early ambulatory treatment of influenza patients]. Sov Med. 1980:103–4. [PubMed] [Google Scholar]
- 85.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. [DOI] [PubMed] [Google Scholar]
- 86.Coffin SE, Leckerman K, Keren R, Hall M, Localio R, Zaoutis TE. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J. 2011;30:962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viasus D, Paño-Pardo JR, Pachón J, Riera M, López-Medrano F, Payeras A, et al. ; Novel Influenza A(H1N1) Study Group of the Spanish Network for Research in Infectious Diseases (REIPI). Timing of oseltamivir administration and outcomes in hospitalized adults with pandemic 2009 influenza A(H1N1) virus infection. Chest. 2011;140:1025–32. [DOI] [PubMed] [Google Scholar]
- 88.Yu H, Feng Z, Uyeki TM, Liao Q, Zhou L, Feng L, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011;52:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang K, Shun-Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev. 2012;1:CD002744. [DOI] [PubMed] [Google Scholar]
- 90.Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJ. Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database Syst Rev. 2012;1:CD002745. [DOI] [PubMed] [Google Scholar]
- 91.Hurt AC, Chotpitayasunondh T, Cox NJ, Daniels R, Fry AM, Gubareva LV, et al. ; on behalf of the WHO Consultation on Pandemic Influenza A (H1N1) 2009 Virus Resistance to Antivirals. Antiviral resistance during the 2009 influenza A H1N1 pandemic: public health, laboratory, and clinical perspectives. Lancet Infect Dis. 2011. [DOI] [PubMed] [Google Scholar]
- 92.Hurt AC, Hardie K, Wilson NJ, Deng YM, Osbourn M, Gehrig N, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza [Letter]. N Engl J Med. 2011;365:2541–2. [DOI] [PubMed] [Google Scholar]
- 93.Schünemann HJ, Ghersi D, Kreis J, Antes G, Bousquet J. Reporting research: are we in for better health care by 2020? In: Gigerenzer G, Gray JAM, eds. Better Doctors, Better Patients, Better Decisions: Envisioning Healthcare 2020. Strüngmann Forum Report, vol. 6 Cambridge, MA: MIT Pr; 2011:83–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.