Abstract
Background.
In absence of definitive molecular risk markers, clinical management of patients diagnosed with ductal carcinoma in situ (DCIS) remains largely guided by patient and tumor characteristics. In this study, we analyzed recent trends in DCIS incidence and compared them against trends in mammography use.
Methods.
The Surveillance, Epidemiology and End Results (SEER) registry was queried for patients diagnosed with DCIS from 2000 to 2014 (18 registries). Joinpoint regression analyses were used to compute age- and race-stratified trends in age-adjusted incidence of DCIS. The patterns of DCIS incidence were compared against mammography utilization data from the National Health Interview Survey.
Results.
Between 2000 and 2014, overall DCIS incidence in the US population was stable (P=0.24). Among age groups 20–44 years and 45–55 years DCIS incidence increased by 1.3% (P=0.001) and 0.6% (P=0.02) per year, respectively. While stable among white women, DCIS incidence increased among black women and women of other races by 1.6% (P<0.001) and 1.0% (P=0.002) per year, respectively. Mammography uptake correlated well with DCIS incidence, with the exception of women aged 40–49 years and black women who experienced an increase in DCIS incidence despite stagnating and decreasing mammography uptake, respectively.
Conclusions.
Overall DCIS incidence rates have remained stable between 2000 and 2014. However, subgroup analyses revealed an increase in incidence among both younger women and black women.
Impact.
DCIS incidence trends did not correlate with the mammography uptake patterns, suggesting that etiologic factors other than screening may be leading to an increased DCIS incidence in these groups.
INTRODUCTION
The introduction of mammographic screening in the early 1980s has led to a dramatic increase in the detection of ductal carcinoma in situ (DCIS) over the past three decades (1,2). In the United States, more than 50,000 women are diagnosed with DCIS each year and DCIS accounts for an estimated 18–25% of the total number of newly diagnosed breast tumors (3).
Consisting of a heterogeneous group of pre-invasive neoplastic lesions, DCIS is primarily characterized by clonal proliferation of malignant cells that are confined to the lumen of breast ducts and lobules (4). The phenotypic spectrum of DCIS ranges from indolent and slowly growing disease to fast growing, aggressive subtypes that can quickly invade the surrounding stroma and metastasize to distant organs (5,6). However, due to a lack of reliable prognostic markers that delineate the different risk groups, the vast majority of patients undergo invasive treatment in the form of surgery, either alone or in combination with radiation and hormone therapy (7). To enable effective and personalized management of DCIS patients, there is an ongoing effort to identify clinical and biologic markers that predict the propensity to progress to invasive cancer (8). As a more concise picture of patient-specific risk profiles is bound to emerge, it becomes necessary to characterize the potential impact of risk-stratified management strategies at the population level. In particular, a better understanding of subtype-specific incidence patterns by age and race will inform cost-effectiveness analyses and the estimation of overdiagnosis rates in the population.
In this study, we analyzed data from the US population-based cancer registry SEER to characterize recent trends in (i) DCIS incidence by age and race, and (ii) the distribution of disease subtypes among patients diagnosed with DCIS. Because DCIS is a primarily screen-detected disease, we further compared the incidence trends against uptake of mammography screening.
METHODS
SEER data
Women diagnosed with DCIS in the United States between 2000 and 2014 were identified through the population-based cancer registries that participate in the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER), which represents approximately 28% of the U.S. population (9). Women aged 20 years or older were included if diagnosed with behavior code ICD-O-3 “in situ” and one of following histology codes for DCIS: 8050, 8140, 8200, 8201, 8211, 8230, 8246, 8260, 8343, 8401, 8480, 8481, 8490, 8500, 8501, 8503, 8504, 8507, 8508, 8521, 8522, 8523, 8543, or 8550. Patients without microscopic confirmation of the diagnosis, those identified at autopsy or on death certificate only, and patients for whom DCIS was not the first cancer diagnosis were excluded from analyses. Ethics approval for this study was obtained from Duke University.
Patient-specific variables included age at diagnosis (20–44 years, 45–54 years, 55–69 years and 70 years or older), race (white, black, other, unknown), and year of diagnosis (2000–03, 2004–07, 2009–11, and 2012–14). Tumor-specific variables included nuclear grade (low, intermediate, high, unknown), tumor size (≤10mm, 11–30mm, >30mm, unknown), estrogen receptor (ER) and progesterone receptor (PR) status (positive, negative, unknown). In addition to race- and age-specific subgroups, we further defined a subgroup of “low-risk” DCIS patients based on the eligibility criteria of the COMET active surveillance trial (10). Patients were included in this subgroup if they were 40 years or older at diagnosis and had an ER- or PR-positive lesion of low or intermediate nuclear grade.
To evaluate the long-term relationship between mammography screening and DCIS incidence, we compared crude mammogram rates (see below) to the corresponding crude DCIS incidence rates over the extended interval from 1992 to 2014 (SEER, 13 registries).
Mammography data
Mammography utilization data for women aged 40 years or older was obtained from the National Health Interview Survey (NHIS) (11). More precisely, we used the fraction of women who self-reported having had a mammogram in the two years prior to the survey round as a proxy for mammogram uptake. To include a historical perspective on mammography uptake data we included survey rounds from 1987, 1993, 1994, 2000, 2005, 2008, 2010, 2013 and 2015. The age-unadjusted (crude) rates were further stratified by age category (40–49, 50–64 and ≥65 years) and race (black and white).
Statistical Analyses
SEER*Stat software version 8.34 (National Cancer Institute, Bethesda, MD, released March 22, 2017) was used to calculate age-adjusted DCIS incidence rates from 2000 to 2014 using the SEER 18 database. Rates were age-adjusted to the 2000 U.S. standard population. The magnitude and direction of DCIS incidence trends by grade, ER status, PR status and tumor size were evaluated using the Joinpoint Regression Program version 4.5.0.1 (National Cancer Institute, Bethesda, MD, released June 2017). This program uses permutation analysis to fit a series of joined straight lines on a logarithmic scale to observed rates and estimate the annual percent change (APC). The study period was divided into intervals 2000–2003, 2004–2007, 2008–2011, and 2012–2014, and associations between time intervals and patient and tumor characteristics were evaluated using χ2 tests. Trends in the proportion of specific tumor subtypes between 2000 and 2014 were evaluated using Cochrane-Armitage tests.
Crude screening rates from the NHIS survey (1987–2015) were plotted alongside crude DCIS incidence rates (SEER 13, 1992–2014), stratified by race and age groups, and trends in crude DCIS incidence and mammography uptake rates were evaluated using Kendall’s tau correlation test, separately for the time periods 1992–1999 and 2000–2014.
All P-values were calculated as two-sided, with statistical significance declared for P-values below 0.05. Unless otherwise stated, statistical analyses were performed in statistical software SAS version 9.4 (SAS Institute Inc, Cary, NC).
Interactive Visualizations
Trends in age-adjusted DCIS incidence for different combinations of age, race and tumor features (nuclear grade, tumor size, ER and PR status) are presented in an online supplementary in the form of an interactive web-page. The visualizations were created using Tableau software (version 9.1, Tableau Software Inc, Seattle, WA): https://public.tableau.com/profile/yiling.liu#!/vizhome/tableau_new_0/Story1?publish=yes.
Incidence and mortality of colorectal, cervical and female breast cancer
To provide a broader context for our findings, we compared the US incidence and mortality rates of female breast, colorectal and cervical cancers between 1999 and 2015. Annual incidence and mortality rates were obtained from the U.S. Cancer Statistics Data Visualizations Tool (12). Relative rates were obtained by rescaling the absolute rates with respect to the corresponding baseline rates from 1999.
RESULTS
Between 2000 and 2014, 145,670 women with DCIS met the inclusion criteria. Demographic and clinicopathologic characteristics of the study population are summarized in Table 1. The majority of diagnoses were recorded in white women (n=113,396, 77.8%), followed by black women (15,712, 10.8%) and women of other races (15,112, 10.4%).
Table 1:
Trends in patient and tumor features.
| Total (n=145,670) | % | 2000–03 (n=34,554) | % | 2004–07 (n=37,188) | % | 2008–11 (n=41,783) | % | 2012–2014 (n=32,145) | % | p-value* | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | <0.001 | ||||||||||
| 20–44 years | 17,860 | 12.3 | 4,503 | 13 | 4,725 | 12.7 | 4,999 | 12 | 3,633 | 11.3 | |
| 45–54 years | 40,512 | 27.8 | 9,534 | 27.6 | 10,710 | 28.8 | 11,767 | 28.2 | 8,501 | 26.4 | |
| 55–69 years | 56,512 | 38.8 | 12,510 | 36.2 | 13,782 | 37.1 | 16,554 | 39.6 | 13,666 | 42.5 | |
| >=70 years | 30,786 | 21.1 | 8,007 | 23.2 | 7,971 | 21.4 | 8,463 | 20.3 | 6,345 | 19.7 | |
| Race | <0.001 | ||||||||||
| White | 113,396 | 77.8 | 28,125 | 81.4 | 29,440 | 79.2 | 32,084 | 76.8 | 23,747 | 73.9 | |
| Black | 15,712 | 10.8 | 3,222 | 9.3 | 3,897 | 10.5 | 4,647 | 11.1 | 3,946 | 12.3 | |
| Other | 15,112 | 10.4 | 2,978 | 8.6 | 3,561 | 9.5 | 4,609 | 11.0 | 3,964 | 12.0 | |
| Unknown | 1,450 | 1.0 | 229 | 0.7 | 290 | 0.8 | 443 | 1.1 | 488 | 1.5 | |
| ER status | <0.001 | ||||||||||
| Positive | 83,168 | 57.1 | 4,857 | 14.1 | 21,276 | 57.2 | 30,865 | 73.9 | 26,170 | 81.4 | |
| Negative | 14,592 | 10.0 | 1,198 | 3.5 | 4,521 | 12.2 | 5,125 | 12.3 | 3,748 | 11.7 | |
| Unknown | 47,910 | 32.9 | 28,499 | 82.5 | 11,391 | 30.6 | 5,793 | 13.9 | 2,227 | 6.9 | |
| PR status | <0.001 | ||||||||||
| Positive | 68,961 | 47.3 | 3,909 | 11.3 | 17,314 | 46.6 | 25,676 | 61.5 | 22,062 | 68.6 | |
| Negative | 22,783 | 15.6 | 1,731 | 5 | 6,732 | 18.1 | 8,212 | 19.7 | 6,108 | 19 | |
| Unknown | 53,926 | 37.0 | 28,914 | 83.7 | 13,142 | 35.3 | 7,895 | 18.9 | 3,975 | 12.4 | |
| Tumor grade | <0.001 | ||||||||||
| Grade I | 17,359 | 11.9 | 3,854 | 11.2 | 4,282 | 11.5 | 5,081 | 12.2 | 4,142 | 12.9 | |
| Grade II | 50,862 | 34.9 | 10,301 | 29.8 | 12,553 | 33.8 | 15,450 | 37 | 12,558 | 39.1 | |
| Grade III/IV | 55,060 | 37.8 | 11,848 | 34.3 | 14,803 | 39.8 | 16,177 | 38.7 | 12,232 | 38.1 | |
| Unknown | 22,389 | 15.4 | 8,551 | 24.7 | 5,550 | 14.9 | 5,075 | 12.1 | 3,213 | 10 | |
| Tumor Size | <0.001 | ||||||||||
| 1–10 mm | 49,926 | 34.3 | 9,865 | 28.6 | 12,612 | 33.9 | 15,305 | 36.6 | 12,144 | 37.8 | |
| 11–30 mm | 37,351 | 25.6 | 7,510 | 21.8 | 9,027 | 24.3 | 11,038 | 26.4 | 9,776 | 30.4 | |
| >30 mm | 13,162 | 9.0 | 2,204 | 6.4 | 3,055 | 8.2 | 4,156 | 9.9 | 3,747 | 11.7 | |
| Unknown | 45,231 | 31.1 | 14,891 | 43.2 | 12,494 | 33.6 | 11,284 | 27 | 6,478 | 20.2 | |
Chi-square test
DCIS incidence trends
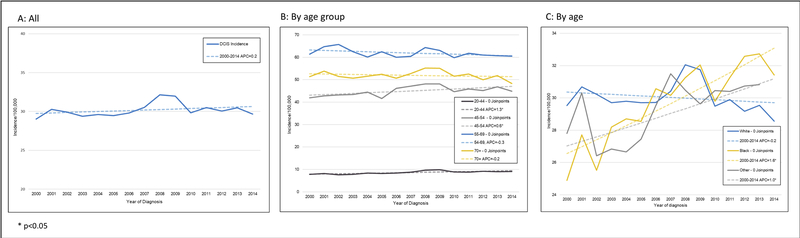
While the overall DCIS incidence rate remained stable between 2000 and 2014 (P=0.24, Figure 1A), significant changes were observed for specific subgroups. When stratified by race (Figure 1B), DCIS incidence rates increased among black women and women of other races by 1.6% (P<0.001) and 1.0% (P=0.002) per year, respectively. In an age-stratified analysis (Figure 1C), incidence rates increased among women of ages 20–44 years and 45–54 years by 1.3% (P=0.001) and 0.6% (P=0.02) per year, respectively. Among women of ages 55–69 years, the incidence rates trended downward by 0.3% per year (P=0.08).
Figure 1: Age-adjusted DCIS incidence trends, overall and by age and race.
Annual percentage change (APC) in the DCIS incidence rate in the United States, 2000–2014, overall (A), by race (B) and age group (C). Carat (^) indicates that the APC is statistically significantly different from zero (P<0.05). In order to highlight trends, the scales of the y-axes vary.
DCIS tumor characteristics at diagnosis
The proportions of incident DCIS cases with unknown grade, unknown ER and PR status and unknown size decreased drastically between 2000 and 2005, and then continued to decrease at a slower rate until 2014 (Figure S1). During the period 2000–2003, 82.5% of women had unknown ER status, 83.7% had unknown PR status, 24.7% had unknown grade, and 38% had unknown tumor size (Table 1). By 2012–2014, these proportions had decreased to 6.9%, 12.4%, 10.0%, and 19.0% respectively.
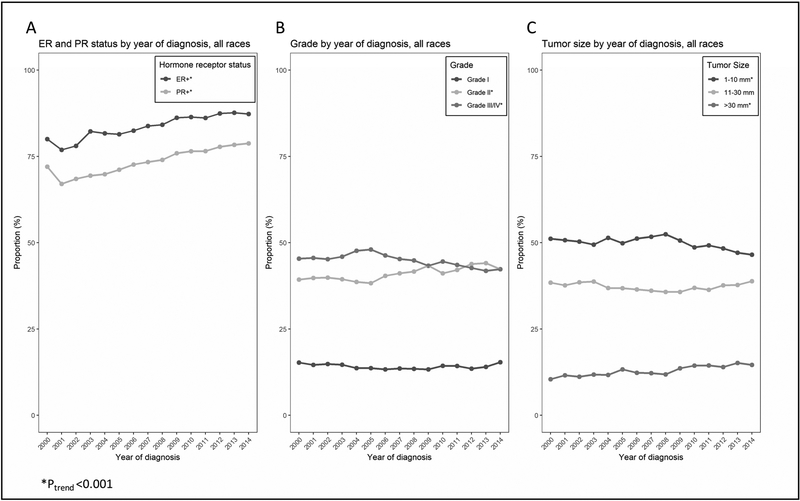
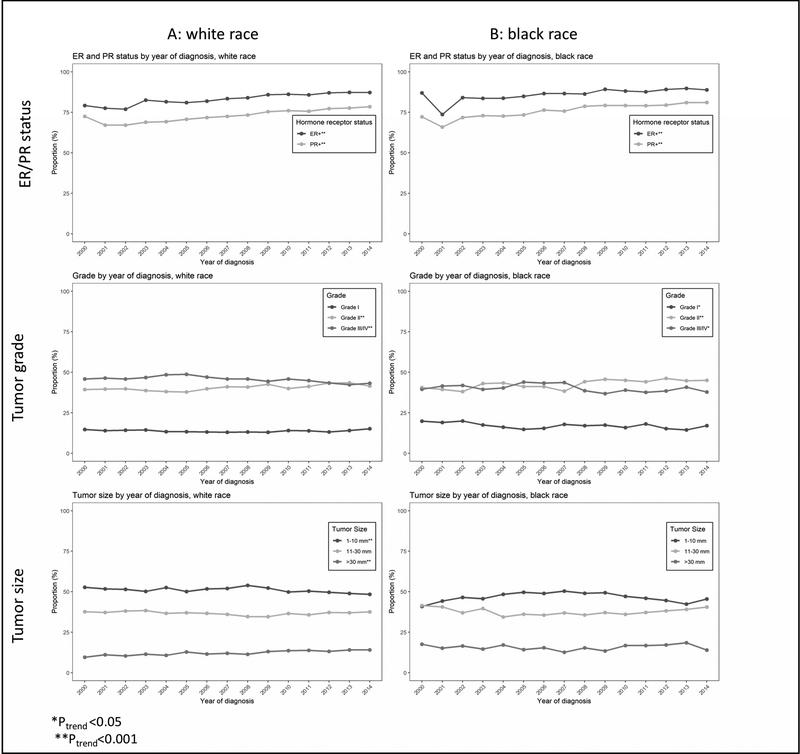
Among newly diagnosed cases with known tumor characteristics, the distributions of nuclear grade, tumor size and hormone receptor status underwent significant changes (Figure 2 and online materials). With respect to hormone receptor status, the proportions of both ER and PR positive tumors increased significantly (P<0.001 for both, Figure 2A). While there was no measurable trend among grade I tumors, the proportions of grade II and grade III tumors increased and decreased, respectively (P<0.001, Figure 2B). Finally, the proportion of small tumors (1–10mm) decreased (P<0.001) while the proportion of tumors larger than 30mm increased (P<0.001); there was no measurable trend among tumors of size 11–20 mm (Figure 2C). Changes in tumor characteristics for different age- and race-groups are found in Figure 3 and online materials. While the trends among white women (Figure 3A) mirrored those of the overall population (Figure 2), differential trends were found in black women (Figure 3B). First, the proportion of grade I tumors decreased among black women (P<0.05) while being stable among white women. Second, there were no noticeable trends in the tumor size distributions among black women, in contrast to the observed shift from smaller (≤1cm) to larger (>3cm) tumors in white women.
Figure 2: Trends in tumor characteristics among women diagnosed with DCIS.
Proportions of women diagnosed with ER-positive and PR-positive DCIS (A), grade I, II and III DCIS (B), and small (1mm-10mm), intermediate (11mm-30mm) and large (>30mm) DCIS (C) in the United States, 2000–2014. Only cases with known tumor features are included in the analyses.
Figure 3: Trends in tumor characteristics among women diagnosed with ductal carcinoma in situ (DCIS); subgroup analyses.
Proportions of women diagnosed with estrogen receptor (ER) positive and progesterone (PR) positive DCIS, grade I, II and III DCIS, and small (1mm-10mm), intermediate (11mm-30mm), large (>30mm) DCIS among white women (A) and black women (B), in the United States, 2000–2014. Only cases with known tumor features are included in the analyses.
During the most recent time period from 2012 to 2014 (Table 2, Supplementary Table S1), 14.3% of DCIS patients with known tumor grade had a grade I lesion, 43.4% a grade II lesion, and 42.3% a grade III lesion. Among women with known ER status, 87.5% had an ER-positive lesion and 12.5% an ER-negative lesion. Among lesions of known size, 46.6% were 1–10 mm, 37.5% were 11–30 mm, 14.4% were larger than 30 mm, and 1.5% were micro-invasive. Among patients with known tumor grade and ER or PR status, the proportion of women who satisfied the criteria of the low-risk subgroup (age ≥40 years with grade I/II and ER- or PR-positive tumor) increased from 47.2% (2000–2004) to 52.5% (2012–2014) (P<0.001).
Table 2:
Tumor features in patients diagnosed between 2012–2014.
| n | % | |
|---|---|---|
| Known tumor grade (n=28,932) | ||
| Grade I | 4,142 | 14.3% |
| Grade II | 12,558 | 43.4% |
| Grade III/IV | 12,232 | 42.3% |
| Known ER status (n=29,918) | ||
| ER-positive | 26,170 | 87.5% |
| ER-negative | 3,748 | 12.5% |
| Known PR status (n=28,170) | ||
| PR-positive | 22,062 | 78.3% |
| PR-negative | 6,108 | 21.7% |
| Known tumor size (n=25,667) | ||
| 1–10 mm | 12,144 | 47.3% |
| 11–30 mm | 9,776 | 38.1% |
| >30 mm | 3,747 | 14.6% |
| Known tumor grade, ER and/or PR status* (n=26,552) | ||
| Low risk** | 14,762 | 55.6% |
| Non-low risk | 11,790 | 44.4% |
Restricted to age>=40 years.
Grade I/II, ER-positive and/or PR-positive
Mammography utilization and DCIS incidence
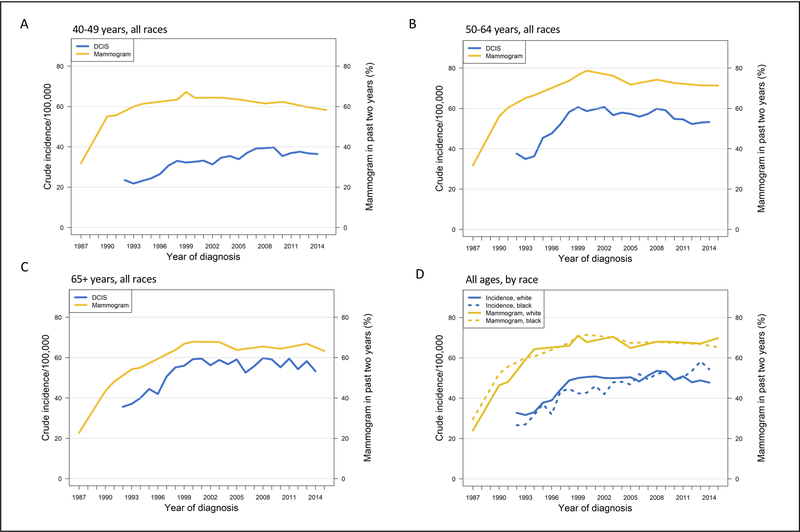
To provide historical context, we performed a comparison of mammography utilization and DCIS incidence over an extended time period. Prior to 2000, both DCIS incidence and self-reported mammography uptake increased among all age groups and races (Figure 4, P<0.01 for all subgroups). After 2000, more complex patterns emerged. Among women aged 40–49 years (Figure 4A), DCIS incidence continued to increase (P=0.015) while mammography uptake decreased (P=0.01). Among women aged 50–64 (Figure 4B), both mammography uptake and DCIS incidence decreased (P=0.01 and P=0.004). Screening uptake and DCIS incidence were stable among women of age 65 years and older (Figure 4C). Among black women of all ages (Figure 4D), we found that the DCIS incidence continued to increase during the period 2000–2014 (P<0.0001) despite unchanged mammogram use (P=0.9). Among white women, mammogram use decreased (P=0.006) yet there was no accompanying downward trend in DCIS incidence (P=0.2).
Figure 4: Mammogram utilization (1987–2015) and DCIS incidence (1992–2014), by age groups and race.
Crude mammogram utilization (NIHS data) and DCIS incidence (SEER 13) rates among women of all races aged 40–49 years (A), 50–64 years (B), and greater than 65 years (C), and for all ages by race (D). Scales for DCIS incidence and mammogram utilization on the left and right sides of the graphs, respectively.
Incidence and mortality of colorectal, cervical and female breast cancers
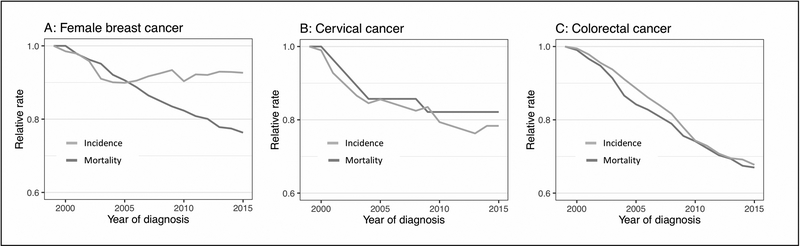
The relative US incidence and mortality rates of colorectal, cervical and female breast cancers between 1999 and 2015 are shown in Figure 5. While mortality rates consistently decreased in all three cancer sites, a concurrent decrease in incidence was only observed in colorectal and cervical cancers; the incidence rate of female breast cancers remained unchanged after 2005.
Figure 5: Incidence and mortality rates of female breast, cervical and colorectal (male and female) cancers in the US, 1999–2015.
Relative rates are obtained by rescaling the actual rates with respect to the baseline rates in 1999. Data extracted from the U.S. Cancer Statistics Data Visualizations Tool (12).
DISCUSSION
It is well established that widespread mammographic screening has resulted in a marked increase in DCIS detection since the mid-1980s (13–15). In this study we found that the overall incidence rate of DCIS has now stabilized between 2000 and 2014. However, we noted an unanticipated discrepancy between breast cancer screening and incidence rates of DCIS in some groups. NHIS survey data have shown that between 2000 and 2008, the age-adjusted rate of breast cancer screening among women aged 40–49 and 50–64 years fell by 2.7% and 4.4%, respectively (16). Paradoxically, our analysis shows that the incidence rates of DCIS have in fact increased among women of ages 20–44 years and 45–54 years (1.3% and 0.6% per year respectively; P<0.05 for both). The increase in DCIS incidence among women 20–44 is especially notable, as most of these women are younger than the age at which screening is recommended. Moreover, although screening rates have not markedly changed among blacks, the DCIS incidence rate is disproportionately increasing in this group as well (1.6% annually, P<0.05). This divergence between DCIS incidence rate and breast cancer screening rate has not been previously observed and suggests an effect in some groups of either endogenous or environmental risk factors for DCIS that have not yet been recognized or identified (17,18).
We found that the biologic characteristics of DCIS and relationship to screening prevalence could not be easily determined, as many DCIS features were not reported prior to 2000. Between 2000 and 2014, reporting of histopathologic tumor features in the cancer registry SEER increased substantially, at least partly due to evolving coding guidelines and practices of the cancer registry. In particular, the fraction of cases with unknown ER status decreased from 84% (2000–2003) to 7% (2012–2014). This change has been attributed to increased ER testing after FDA approval of Tamoxifen for DCIS in 2000 (19).
However, among cases with known tumor features, we noted changes in the distribution of tumor size, grade and hormone receptor status. As there is little evidence to suggest a reporting bias, these observations likely represent real underlying trends. The most marked change was noted in hormone receptor status, with an increase in the fraction of ER-positive DCIS from 80% in 2000 to 87% in 2014. In contrast, it has been reported that the incidence rate of ER-positive invasive cancers fell during this period, while the incidence of ER-negative invasive cancers did not change significantly (20). This finding has been widely attributed to a lower rate of exogenous hormone use resulting in fewer women being exposed to the proliferative effects of combined estrogen plus progestin on the breast epithelium (21). The disproportionate rise in ER-positive, and not ER-negative DCIS during a time interval when use of exogenous hormones dropped, again suggests that etiologic risk factors for DCIS and invasive cancer may differ.
Comparing screening prevalence and DCIS incidence against invasive breast cancer incidence and mortality provides insights into the respective roles of screening and treatment in prevention. Over the past decade, breast cancer mortality has dropped substantially while invasive cancer incidence remained stable after 2003 (Figure 5A). These trends, in conjunction with relatively stable screening uptake and DCIS incidence patterns (Figure 1A, Figure 4), make it difficult to attribute more than a negligible impact of DCIS detection and treatment on recent breast cancer mortality reductions and suggest a larger effect of treatment improvements on reducing breast cancer mortality (22). This observation stands in contrast to other cancers, such as cervical (Figure 5B) and colorectal cancer (Figure 5C), for which effective screening programs have successfully reduced both incidence and mortality.
A limitation of this study is the use of self-reported mammography utilization data. Indeed, prior studies have suggested that self-reported data overestimate the true utilization rate, and the degree of overestimation varies by age and race (23). However, because we used these data to estimate relative trends only, the impact of potential systematic reporting biases is unlikely to affect our conclusions. Another limitation is that the study populations for cancer incidence (SEER) and mammography utilization (NHIS) are not identical. This limitation is partially addressed by the fact that both the NHIS survey and SEER registry draw from samples that are representative of the US population.
Finally, we note that during the most recent study period (2012–2014), the majority of DCIS lesions diagnosed were of low to intermediate nuclear grade (58%) and ER positive (87%). In particular, 55% of newly diagnosed DCIS patients satisfied the three main criteria for low-risk DCIS as used by the ongoing COMET trial which randomizes patients with DCIS to either usual care or active surveillance (10): age ≥40 years, nuclear grade I/II and hormone receptor-positive. Therefore, should active surveillance become part of usual care, a sizable fraction of DCIS patients may have the option to de-escalate treatment and delay surgery unless there is progression to invasive cancer.
In an era of increasingly tailored treatment options for cancer patients, it is essential to gain a clear understanding of the different disease subtypes and their incidence patterns by age and race groups. For this reason, we developed a web-based visualization tool that enables the user to explore the complex incidence patterns for DCIS during the period from 2000 to 2014 (see Methods section for details). Altogether, the results of this study data provide the basis for future cost-effectiveness studies and other model-based research activities for DCIS.
In conclusion, we observed an emerging divergence between DCIS incidence and mammographic screening patterns in some subgroups. Specifically, we found an increase in ER-positive DCIS and DCIS diagnosed in young patients and among African American individuals. Importantly, these trends did not correlate with the mammography uptake patterns, suggesting that etiologic factors other than screening may be leading to an increased DCIS incidence in these groups.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (K99 CA207872 to MD Ryser; R01 CA185138–01 to ES Hwang; 5P30CA014236 to T Hyslop), the National Science Foundation (DMS-1614838 to MD Ryser), the Patient-Centered Outcomes Research Institute (1505–30497 to ES Hwang and T Hyslop), the Department of Defense (BC132057 to ES Hwang) and the Breast Cancer Research Fund (to ES Hwang). This work was also supported in part by National Institutes of Health grants U01 CA152958 (J. Mandelblatt) and U01 CA157224 (R. Etzioni) to the Cancer Intervention and Surveillance Modeling Network breast and prostate cancer working groups, respectively, for important background discussions relevant to the scientific areas covered in this paper.
Abbreviation list:
- DCIS
ductal carcinoma in situ
- SEER
Surveillance, Epidemiology and End Results registry
- ER
estrogen receptor
- PR
progesterone receptor
- NIHS
National Health Interview Survey
Footnotes
Conflicts of interest.
None to disclose.
REFERENCES
- 1.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012;367:1998–2005 [DOI] [PubMed] [Google Scholar]
- 2.Ozanne EM, Shieh Y, Barnes J, Bouzan C, Hwang ES, Esserman LJ. Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat 2011;129:165–73 [DOI] [PubMed] [Google Scholar]
- 3.Ward EM, DeSantis CE, Lin CC, Kramer JL, Jemal A, Kohler B, et al. Cancer statistics: Breast cancer in situ. CA Cancer J Clin 2015;65:481–95 [DOI] [PubMed] [Google Scholar]
- 4.Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. J Natl Cancer Inst Monogr 2010;2010:134–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roses RE, Arun BK, Lari SA, Mittendorf EA, Lucci A, Hunt KK, et al. Ductal carcinoma-in-situ of the breast with subsequent distant metastasis and death. Annals of surgical oncology 2011;18:2873–8 [DOI] [PubMed] [Google Scholar]
- 6.Ryser MD, Weaver DL, Zhao F, Worni M, Grimm LJ, Gulati R, et al. Cancer Outcomes in DCIS Patients Without Locoregional Treatment. JNCI: Journal of the National Cancer Institute 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worni M, Akushevich I, Greenup R, Sarma D, Ryser MD, Myers ER, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst 2015;107:djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groen EJ, Elshof LE, Visser LL, Rutgers EJT, Winter-Warnars HAO, Lips EH, et al. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast 2017;31:274–83 [DOI] [PubMed] [Google Scholar]
- 9.Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: SEER 13 Regs Research Data, Nov 2016 Sub (1992–2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.
- 10.Youngwirth LM, Boughey JC, Hwang ES. Surgery versus monitoring and endocrine therapy for low-risk DCIS: The COMET Trial. Bull Am Coll Surg 2017;102:62–3 [PubMed] [Google Scholar]
- 11.Statistics NCfH. Health, United States, 2016: with chartbook on long-term trends in health. 2017 [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services; Centers for Disease Control; and Prevention and National Cancer Institute. 10/20/2018. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999–2015).
- 13.Feuer EJ, Wun LM. How much of the recent rise in breast cancer incidence can be explained by increases in mammography utilization? A dynamic population model approach. Am J Epidemiol 1992;136:1423–36 [DOI] [PubMed] [Google Scholar]
- 14.Wun LM, Feuer EJ, Miller BA. Are increases in mammographic screening still a valid explanation for trends in breast cancer incidence in the United States? Cancer Causes Control 1995;6:135–44 [DOI] [PubMed] [Google Scholar]
- 15.Ernster VL, Barclay J. Increases in ductal carcinoma in situ (DCIS) of the breast in relation to mammography: a dilemma. J Natl Cancer Inst Monogr 1997:151–6 [DOI] [PubMed] [Google Scholar]
- 16.Breen N, Gentleman JF, Schiller JS. Update on mammography trends: comparisons of rates in 2000, 2005, and 2008. Cancer 2011;117:2209–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K, Breast Cancer Surveillance C. Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol 2017;3:1228–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerlikowske K Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr 2010;2010:139–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols HB, Bowles EJ, Islam J, Madziwa L, Sturmer T, Tran DT, et al. Tamoxifen Initiation After Ductal Carcinoma In Situ. Oncologist 2016;21:134–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. New England Journal of Medicine 2016;375:717–29 [DOI] [PubMed] [Google Scholar]
- 21.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA 2003;289:3243–53 [DOI] [PubMed] [Google Scholar]
- 22.Plevritis SK, Munoz D, Kurian AW, Stout NK, Alagoz O, Near AM, et al. Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000–2012. JAMA 2018;319:154–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cronin KA, Miglioretti DL, Krapcho M, Yu B, Geller BM, Carney PA, et al. Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomarkers Prev 2009;18:1699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.