Abstract
Renal Cell Carcinoma (RCC) associated with Xp11.2 translocation (TFE3-RCC) has been recently defined as a distinct subset of RCC classified by characteristic morphology and clinical presentation. The Xp11 translocations involve the TFE3 transcription factor and produce chimeric TFE3 proteins retaining the basic helix-loop-helix leucine zipper structure for dimerization and DNA binding suggesting that chimeric TFE3 proteins function as oncogenic transcription factors. Diagnostic biomarkers and effective forms of therapy for advanced cases of TFE3-RCC are as yet unavailable. To facilitate the development of molecular-based diagnostic tools and targeted therapies for this aggressive kidney cancer, we generated a translocation RCC mouse model, in which the PRCC-TFE3 transgene is expressed specifically in kidneys leading to the development of RCC with characteristic histology. Expression of the receptor tyrosine kinase Ret was elevated in the kidneys of the TFE3-RCC mice, and treatment with RET inhibitor vandetanib significantly suppressed RCC growth. Moreover, we found that Gpnmb (Glycoprotein nonmetastatic B) expression was notably elevated in the TFE3-RCC mouse kidneys as seen in human TFE3-RCC tumors, and confirmed that GPNMB is the direct transcriptional target of TFE3 fusions. While GPNMB immunohistochemical staining was positive in 9/9 cases of TFE3-RCC, Cathepsin K, a conventional marker for TFE3-RCC, was positive in only 67% of cases. These data support RET as as potential target and GPNMB as a diagnostic marker for TFE3-RCC. The TFE3-RCC mouse provides a preclinical in vivo model for the development of new biomarkers and targeted therapeutics for patients affected with this aggressive form of renal cell carcinoma.
Keywords: TFE3, Xp11.2 translocation RCC, biomarker, diagnosis, GPNMB
1. Introduction
Xp11.2 translocation, t(X;1)(p11.2;q21.2), was first described in a pediatric RCC case in 1986, and the fusion gene was confirmed to be PRCC-TFE3 in 1995 (1,2). TFE3 Xp11.2 translocation RCC (TFE3-RCC) was defined as an independent subtype of RCC by WHO in 2004 and is characterized by distinctive morphological features and Xp11.2 rearrangements that create TFE3 gene fusions with a variety of partner genes (PRCC, SFPQ, ASPSCR, CLTC, NONO, RBM10, PARP14, LUC7L3, KHSRP etc.) (2–15)(16,17). TFE3 encodes a transcription factor that has a basic helix-loop-helix leucine zipper (bHLH-Zip) structure through which TFE3 dimerizes and interacts with M-box DNA sequences (TCAYRTGA) in transcriptional target genes. All TFE3 fusion genes encode inframe chimeric proteins which retain the bHLH-Zip domain of TFE3(16,18). Nuclear accumulation of TFE3 is one of the most significant histopathologic characteristics of TFE3-RCC (19,20). The evidence is strong for TFE3 fusions to be oncogenes with constitutively active transcriptional activity.
TFE3-RCC is more common than was previously thought, comprising from 2 to 5% of adult cases (21,22) and from 25 to 40% of pediatric RCC cases (14,23). TFE3-RCC is known for its aggressive malignant nature with a propensity to metastasize when the primary tumor is small. There is currently no standard or effective form of therapy for patients with advanced disease(4,16). Reduced awareness of TFE3-RCC and the technical complexity of diagnosis including TFE3 staining and TFE3 gene break-apart FISH have led to a decrease in awareness and early diagnosis of this disease (21,24–26). It is, therefore, important to develop novel diagnostic methods for TFE3-RCC. While several diagnostic markers for TFE3-RCC have been reported, such as Cathepsin K, melan A and HMB45, the sensitivity and specificity of these conventional markers are limited and not robust enough to confirm the diagnosis of TFE3-RCC (20,26,27). Transcriptional target genes of TFE3 that are upregulated following TFE3 nuclear localization and activation could potentially be useful markers for the diagnosis of TFE3-RCC.
In this study, we have generated a TFE3-RCC mouse model that expresses PRCC-TFE3, which is the first reported TFE3 fusion partner and frequently observed in human disease, specifically in kidney epithelial cells, and develops a variety of kidney epithelial neoplastic lesions including hyperplastic cysts, adenomas and solid tumors. This mouse model provides a preclinical in vivo system for development of new diagnostic markers and targeted therapeutics. Genes that were upregulated in the kidneys of this mouse model were identified. We determined that GPNMB (glycoprotein nonmetastatic B) is directly transcribed and upregulated by chimeric TFE3 and performed GPNMB immunohistochemical staining in human TFE3-RCCs to investigate its potential in the diagnosis of this form of RCC.
2. Materials and Methods
2.1. Generation of TFE3-RCC Mouse Model
The cDNA of the human PRCC-TFE3 chimeric gene, which is composed of exon 1 of PRCC and exons 4–10 of TFE3, was generated by overlap extension polymerase chain reaction, subcloned into an entry vector of the Gateway Protein Expression system (Invitrogen), and sequence verified. PRCC-TFE3 cDNA was cloned into a targeting vector, pRosa26-DEST (Addgene plasmid # 21189) (28), which has a LoxP-Stop-LoxP (LSL) cassette preceding the gene of interest, using the Gateway Protein Expression System according to manufacturer’s protocol. The targeting vector (pRosa26-DEST- PRCC-TFE3) was electroporated into mouse embryonic stem (ES) cells and selected for G418 resistance as previously described. ES cells (LSL-PRCC-TFE3), in which the Rosa26 locus was correctly targeted, were identified by Southern blot analysis and injected into blastocysts to produce chimeras. Backcrossing to C57BL/6 mice produced heterozygous F1 offspring with germline transmission of the Rosa26-LSL-PRCC-TFE3 knockin (KI) allele. Cadherin 16 (KSP)-Cre transgenic mice, which express Cre recombinase under the cadherin 16 promoter specifically in renal epithelial cells(29), were crossed with Rosa26-LSL-PRCC-TFE3 KI (LSL-PRCC-TFE3) mice to generate PRCC-TFE3;KSP-Cre+ mice. Mice were housed in Frederick National Laboratory for Cancer Research animal facilities and euthanized by CO2 asphyxiation for analyses according to the NCI-Frederick Animal Care and Use Committee guidelines. Animal care procedures followed the NCI-Frederick Animal Care and Use Committee guidelines.
2.2. Drug Treatment
All animal studies were conducted in accordance with the institutional guidelines for animal care and experimental neoplasia and according to a protocol approved by the Animal Care and Use Committee of the Frederick National Laboratory for Cancer Research. PRCC-TFE3;KSPCre+ mice, 6–12 months of age, were randomly assigned to vandetanib treated group (n=4) and vehicle treated group (n=4). Vandetanib treated animals received vandetanib at 100mg/kg by oral gavage 5 days/ week for 16 weeks. Control animals received vehicle (PBS) by oral gavage at the same volume and frequency. Magnetic resonance imaging scans were obtained at 4 week intervals and maximum tumor dimensions were measured from the scans using Image J software and plotted to calculate tumor growth rates.
2.3. Magnetic Resonance Imaging
Magnetic Resonance Imaging (MRI) was implemented for noninvasive detection of kidney lesions as previously described (30,31), monitoring their progression and following the therapeutic response. The images were acquired on a 3.0T clinical scanner (Philips Intera Achieva) using a 40-mm diameter mouse-dedicated solenoid receiver coil (Philips Research). A T2 weighted (T2w) Turbo Spin Echo (TSE) sequence was acquired with an in-plane resolution of 0.180×0.180 mm and 0.5 mm slice thickness. A contrast medium gadolinium chelate, Dotarem, (Guerbet USA) was administrated intravenously at 0.1 mmol/kg and a post-contrast T1weighted (T1w) image was obtained using a gradient echo scan with the same geometry as the T2w sequence. A fat saturation technique (Spectral Presaturation with Inversion Recovery, SPIR) was implemented for both T1w and T2w sequences to suppress the fat and create a dark background around the kidneys to enhance contrast and distinguish fat from cystic and tumor masses in the kidneys.
2.4. DNA Microarray Analysis
Total RNA was isolated from flash frozen mouse kidneys using TRIzol reagent (Invitrogen). cDNA preparation and hybridization of the probe arrays were performed according to the manufacturer’s instructions (Affymetrix). Affymetrix GeneChip Mouse Gene 2.0 ST Arrays were applied. Partek Genomics Suite 6.6 was used for RMA-based data normalization and the subsequent data analysis, including PCA and ANOVA. Data are available at the NCBI GEO database under accession number (GSE130072). Gene set enrichment analysis (GSEA) was performed with GSEA software as previously described (32,33).
2.3. Cell Lines and Biochemical Analysis
HEK293 cell lines which express HA-TFE3 and HA-PRCC-TFE3 in a doxycycline-dependent manner were established using the Flp-In T-Rex System (Invitrogen) as previously described (34) and cultured in DMEM with 10% Tetracycline-Free Fetal Bovine Serum (Clontech) and selection antibiotics, 15μg/ml Blasticidin S and 150μg/ml Hygromicin B (Invitrogen). UOK109, UOK120, UOK124 and UOK146 cell lines were derived from primary tumors of four TFE3-RCC patients treated in the Urologic Oncology Branch (UOB), NCI (Bethesda, MD) and carry the NONO-TFE3 or PRCC-TFE3 gene fusions, as described previously (3,8,15). UOK111, UOK115 and UOK140 are cell lines derived from ccRCC tumors with VHL gene mutations that were established in the UOB, NCI (35,36). All cell lines were maintained in vitro in DMEM media supplemented with L-glutamine (4 mM), sodium pyruvate (110mg/l), glucose (4.5g/l), and 1X essential amino acids, Penicillin-Streptomycin (100U/ml) (Gibco, Gaithersburg, MD), with 10% fetal bovine serum (Sigma Aldrich, St. Luis, MO). Cell lines were authenticated using short tandem repeat DNA profiling (Genetica DNA Laboratories, Burlington, NC) and confirmed to be mycoplasma-free. For gene expression profiling, HEK293 cell lines were cultured with or without 250ng/ml doxycycline. For quantitative real-time PCR, total RNA was isolated using Trizol reagent (Invitrogen) and was reverse transcribed to cDNA using ReverTra Ace qPCR RT Master Mix (Toyobo). Quantitative real-time PCR was performed with a LightCycler 96 Instrument (Roche) using THUNDERBIRD SYBR qPCR Mix (Toyobo) as previously described (37). All reactions were performed with RPS 18 as an internal control. Primer sequences are as follows: Gpnmb forward 5’- GCTACTTCAGAGCCACCATCACAA -3’, Gpnmb reverse 5’- GGAGATGATCGTACAGGCTTCCA -3’, Ret forward 5’- CCACATGTTACCCGTGCAGTTC -3’, Ret reverse 5’- CCAGGCAGTCTGGGTCACAA -3’, Nr4a1 forward 5’-AAACAAGGATTGCCCTGTGG-3’, Nr4a1 reverse 5’-CGCCCTTTTAGGCTGTCTGT-3’, Rps18 forward 5’- TTCTGGCCAACGGTCTAGACAAC -3’, Rps18 reverse 5’- CCAGTGGTCTTGGTGTGCTGA -3’. Western blotting was performed as previously described(34). Primary antibodies were used as follows: GPNMB (R&D systems) 1:2000 dilution, beta actin (Cell Signaling) 1:1000 dilution.
2.5. TCGA Data Analysis
The Cancer Genome Atlas (TCGA) (12,38) dataset was used to validate candidate transcriptional target genes of chimeric TFE3, which were identified by RNAseq analysis.
The data set of four TFE3-RCC samples [TCGA-AK-3456–01, TCGA-BP-4756–01, TCGA-B8-5546-01 (all SFPQ-TFE3), TCGA-CJ-5681–01 (KHSRP-TFE3)] was compared to the dataset of 465 ccRCC for gene expression levels of candidate genes using cBioPortal (39) and six TFE3-RCC samples [TCGA-BQ-5882–01, TCGA-BQ-5887–01, TCGA-BQ-7050–01 (all PRCC-TFE3), TCGA-DZ-6131–01 (RBM10-TFE3), TCGA-G7-7501-01 (SFPQ-TFE3), TCGA-J7-8537-01 (DVL2-TFE3)] were compared to the remaining 283 samples with gene expression data within the papillary RCC dataset. The papillary RCC dataset was further subdivided into papillary type 1 (161 samples) and papillary type 2 RCC (79 samples).
2.6. Luciferase Reporter Gene Assay
A 527bp fragment of the 5’ region of the human GPNMB gene was amplified by PCR using KOD-Plus-Neo from the human BAC clone (RP11–469O17) (Advanced Geno Techs Co.). The PCR product amplified with primers containing restriction enzyme site (Forward: ATGTACATGCTAGCACATAGTGAAACCTGCCTCTACT, Reverse: AACTTGATAAGCTTTGAATTCTCACGGACGCAGG) was digested by NheI and HindIII, and ligated into a pGL3-Basic Vector (Promega). Two M-box sequences in the GPNMB promoter construct were mutated using PrimeSTAR Mutagenesis Basal Kit (Takara) following the manufacturer’s protocol. Primers for mutagenesis were as follows: M-box Mt1 Forward : TAAGCTCGAGAGTTGTAAGAGGTTGAA, M-box Mt1 Reverse : CAACTCTCGAGCTTATGACTCACTCCTT; M-box Mt2 Forward : CCATCTCGAGATCCTCCCCGAGGCCCT, M-box Mt2 Reverse : AGGATCTCGAGATGGTATTAAGCGGCAC. Reporter plasmids were cotransfected with phRL vector as an internal control into a HEK293-Dx-PRCC-TFE3 cell line which expresses PRCC-TFE3 in a doxycycline dependent manner. Luciferase activity was measured using Dual-Luciferase® Reporter Assay System according to manufacturer’s protocols (Promega).
2.7. Chromatin Immunoprecipitation (ChIP) Assay
HEK293-Dx-HA-PRCC-TFE3 cell lines were cultured with or without doxycycline for 24 hr, and cross-linked with 1% formaldehyde at room temperature for 5 minutes followed by incubation with 125mM glycine. Nuclear lysates were sonicated with a Bioruptor USD-200 (Diagenode) for 10 minutes twice. To purify HA-PRCC-TFE3 bound chromatin, 50 μg of chromatin was subjected to immunoprecipitation with Anti-HA Affinity Matrix (Roche) at 4 degree overnight. Immunoprecipitates were washed five times, eluted, and reverse-crosslinked. DNA was purified with NucleoSpin® Gel and PCR Clean-up (Takara) following the manufacturer’s protocol. DNA enrichment in the ChIP samples was determined by quantitative real-time PCR with THUNDERBIRD SYBR qPCR Mix (Toyobo) and a LightCycler 96 Instrument (Roche) following the manufacturer’s protocol. Primers for the GPNMB promoter region containing two M-box motifs are as follows: Forward : GATGCCAAGAAGGAGTGAGTCATA, Reverse : ATCTGTGGTGCCTCCCTCTC. As an internal negative control, a 142bp region, which is 2kbp upstream from the aforementioned GPNMB promoter region was selected. Primers for this negative control region are as follows: Forward : TCACTGGGACTTCAGGTACACATC, Reverse : AGGGCCATTTTGGGTAAAGAA. Data are expressed as percentage of input DNA.
2.8. Case Selection
TFE3-immunoreactive RCCs were collected through consultation systems by The Japanese Society of Pathology (http://pathology.or.jp/). Histological features were evaluated by two pathologists with expertise in renal tumors (N. Kuroda and Y. Nagashima). Clinicopathological findings including immunostaining were summarized in Table 1. Sporadic clear cell RCCs (n = 56) and papillary RCCs (n=20), among which 20 ccRCC and 11 papillary RCC were previously reported by us (40), were used for comparison of GPNMB staining. The possibility that these cases represented patients with von Hippel-Lindau disease, Birt-Hogg-Dubé (BHD) syndrome, tuberous sclerosis complex, hereditary papillary RCC, or hereditary leiomyomatosis RCC was carefully examined and excluded in all patients by thorough medical examination and family history. This study was approved by the Institutional Review Board of the Kumamoto University (#1245), Yokohama City University, Saitama Medical University International Medical Center (#16–226), and Kochi Red Cross Hospital.
Table 1.
Summary of clinicopathologic information of patients with TFE3-RCC.
| Case # | Sex | Age | Size(cm) | Stage | Fuhrman Grade | Histological finding | FISH | fusion | TFE3 | GPNMB | Cathepsin K | RET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 11 | 2.2 | pT1a-Nx-Mx | 3 | Clear & papillary | + | ASPL-TFE3 | 2 | 2 | - | 1 |
| 2 | F | 16 | 9.0 | pT2apN1M1 | 2 | Clear & tubular | + | ASPL-TFE3 | 2 | 1 | 1 | 2 |
| 3 | F | 19 | 2.5 | pT1a-Nx-Mx | 2 | Clear & papillary, calcification | + | SFPQ-TFE3 | 2 | 2 | 1 | 2 |
| 4 | F | 20 | 6.0 | pT1b-Nx-Mx | 3 | Eosinophilic, alveolar, tubular | + | PRCC-TFE3 | 2 | 2 | - | 1 |
| 5 | M | 26 | 3.3 | pT1a-Nx-Mx | 2 | Eosinophilic, alveolar, tubular | + | PRCC-TFE3 | 2 | 2 | 2 | - |
| 6 | F | 33 | 1.0 | pT1a-Nx-Mx | 2 | Clear & alveolar | + | SFPQ-TFE3 | 2 | 1 | 1 | - |
| 7 | M | 52 | 2.7 | pT1a-N2-M0 | 2 | Clear & papillary | + | PRCC-TFE3 | 2 | 2 | - | 1 |
| 8 | F | 66 | 5.5 | pT1b-Nx-Mx | 1 | Clear & multicystic, calcification | + | SFPQ-TFE3 | 1 | 2 | 2 | 2 |
| 9 | F | 73 | 2.5 | pT3-Nx-Mx | 3 | Alveolar, papillary, Mixed clear and eosinophlic, psammoma body | + | ASPL-TFE3 | 2 | 2 | 1 | 2 |
2.9. Fluorescence In Situ Hybridization (FISH)
To assess the status of TFE3 in the TFE3-RCCs, FISH analysis was performed by using a dual-color TFE3 break-apart probe (GSP Laboratory), which was labeled with FITC at 5’ side (550 kb) and with Texas Red at 3’ side (570 kb) of TFE3. In each case, at least 60 non-overlapping nuclei in the tumor area were counted. The signal pattern of a normal cell depends on sex, i.e., one fused signal in men, and two fused signals in women. Typical TFE3 rearrangement pattern of a TFE3-RCC cell is one split signal in men, and one split signal and one fused signal in women. The split signal was defined according to the evaluation method described in previous studies (25,27). When the case exhibited a TFE3 split but did not show a chimeric band of known partners in RT-PCR, a dual-color SFPQ-TFE3 fusion FISH probe (Cyto Test Inc) was used. If a yellow signal was observed in 30% or more of 100 counted cells, the tumor was defined as harboring SFPQ-TFE3.
2.10. RT-PCR
RNAs were extracted from the 9 TFE3-RCCs using QIAGEN RNeasy FFPE kit (QIAGEN) according to the manufacturer’s instructions. Gene fusion products were amplified by RT-PCR using the following primers: ASPSCR1 (exon 7) forward 5’-CCAAGCCAAAGAAGTCCAAG-3’, SFPQ (exon 9) forward 5’-AGGTGGTGGTGGCAT AGGTT-3’, PRCC (exon 3) forward 5’-ATGCCTAAGCCTGGGGACGACTA-3’, TFE3 reverse (exon 4) 5’-TGGACAGGTACTGTTTCACCTG-3’, and TFE3 reverse (exon 6) 5’-CCTTGACTACTGTACACATC-3’.
2.11. Immunohistochemistry
The resected tissues were fixed with 10% formalin and embedded in paraffin. Four μm-thick paraffin sections were subjected to immunohistochemistry. Sections were autoclaved at 121°C for 15 min. The sections were treated with the diluted antibodies at 4°C overnight. Working dilutions were 1:200 for GPNMB (goat polyclonal antibody AF-2550 from R&D Systems) and Cathepsin K (Abcam), and 1:500 for TFE3 (Sigma,). The GPNMB immunostaining was scored as (-), (1+) and (2+) according to the method previously described(40).
2.12. Statistical Analysis
Experimental data are summarized as the mean values with standard deviation SD. Statistical analyses were performed using a two-tailed unpaired t-test with or without Welch’s correction using GraphPad Prism 6. When the p-value of the F test was less than 0.05, Welch’s correction was applied. Differences were considered to be statistically significant at a value of p less than 0.05. Survival data were estimated and plotted with the Kaplan-Meier method; differences between survival groups were assessed with the log-rank test. Nonlinear regression analysis for % tumor growth was performed with GraphPad Prism 6.
3. Results
3.1. PRCC-TFE3 expression in mouse kidney epithelial cells produces RCC
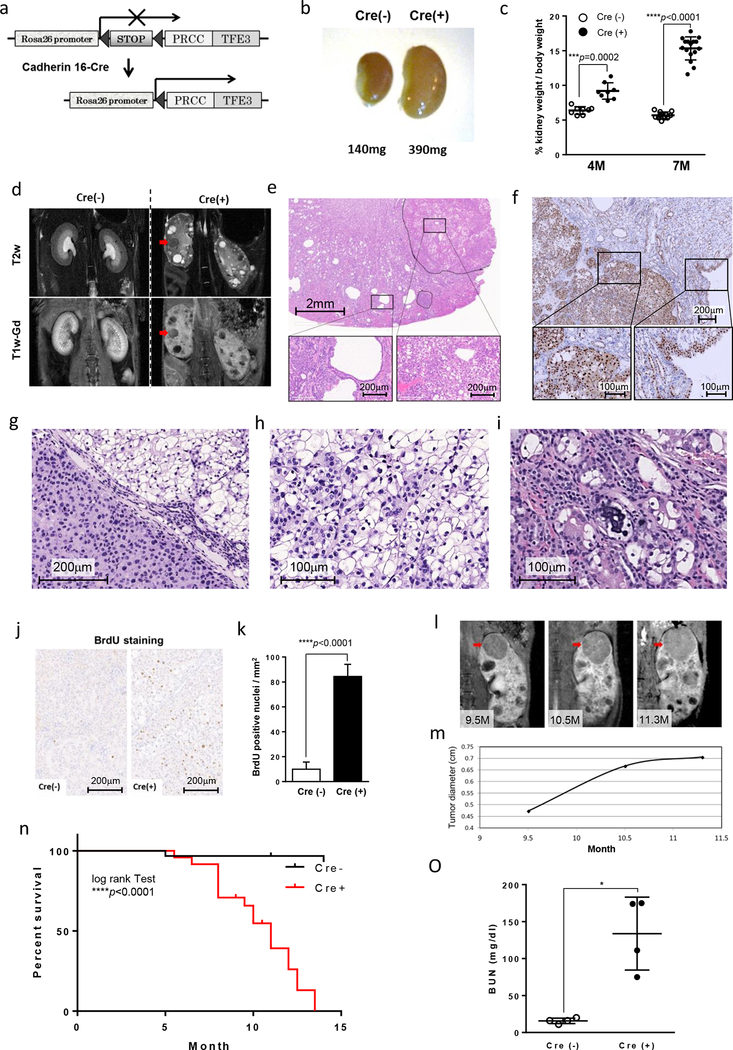
PRCC-TFE3 knockin mice, generated by inserting human PRCC-TFE3 cDNA preceded by a loxP-flanked neomycin cassette into the ROSA26 locus, were crossed with cadherin 16-Cre (KSP-Cre) transgenic mice to express PRCC-TFE3 specifically in mouse kidney epithelial cells (Fig.1a). PRCC-TFE3;KSP-Cre+ mice and PRCC-TFE3;KSP-Cre- littermate control mice were evaluated for their phenotype. PRCC-TFE3;KSP-Cre+ mice showed significantly larger and heavier kidneys at 4 and 7 months of age (Fig.1b, c). MRI imaging revealed a disorganized kidney structure, multiple cysts and renal tumors in PRCC-TFE3;KSP-Cre+ mice (Fig.1d). Histopathological analysis revealed a variety of proliferating morphologies, dilation of tubules, cystic lesions, neoplastic regions protruding into the cystic lumen, and solid tumors of various sizes (Fig. 1e). Complete necropsy with histopathology revealed no metastasis or primary tumors in organs other than kidney. All pathological lesions in kidneys showed strong nuclear staining of TFE3 (Fig.1f). TFE3 nuclear staining was observed without obvious phenotype in renal papillae, pelvis and ureter, but not in bladder, consistent with the CDH16-Cre (KSP-Cre) expression pattern (Supplemental Fig. S1a–f) (29). The cyst lumens were lined by monolayers of cuboidal and/or hyperplastic cells with occasional adenomatous lesions proliferating in multiple layers (Fig.1e). Solid tumors displayed characteristics of RCC with eosinophilic cytoplasm, clear cytoplasm and occasional mixture of both populations (Fig.1g, h). The eosinophilic cells tended to be arranged in tubules, trabeculae, and lobules, and were supported by a fine fibrovascular stroma. On the other hand, clear cells were arranged in lobules, and frequently exhibited an alveolar and/or multicystic pattern. Psammoma bodies, calcified lesions which are characteristic of TFE3-RCC (41), were occasionally observed (Fig.1i). An increased cell proliferation rate in the kidneys of PRCC-TFE3;KSP-Cre+ mice was indicated by a significantly increased BrdU incorporation (Fig.1j,k) as well as higher number of Ki67 positive cells (Supplemental Fig. S1g, h). Increased BrdU incorporation and Ki67 positive cells in cyst-lining cells indicated that the cystic regions were produced by uncontrolled hyperproliferation of tubular epithelial cells. Intriguingly, multiple stages of RCC development from cystic regions with abnormally proliferating monolayers to solid tumors were observed in a single kidney of PRCC-TFE3;KSP-Cre+ mice. The renal tumor growth rate was calculated from magnetic resonance imaging (MRI) measurements (Fig1l,m). Renal neoplastic lesions in this mouse model display heterogeneous histological features, which are also seen in human TFE3-RCC. As expected, growth rates of individual tumors measured by MRI imaging were variable, probably because of their heterogeneous features. As indicated from the histology and MRI imaging, kidney structures were disorganized because of abnormal epithelial cell proliferation and enlarged tumors in aged mice, resulting in renal failure and early death compared to control PRCC-TFE3;KSP-Cre- mice. The mean survival of PRCC-TFE3;KSP-Cre+ mice was 11 months (Fig.1n). The blood urea nitrogen (BUN) levels were significantly higher in PRCC-TFE3;KSP-Cre(+) mice when moribund over the age of 10 months, compared to PRCC-TFE3;KSP-Cre(-) control mice (Fig.1o). This newly established PRCC-TFE3;KSP-Cre+ mouse model will be useful for development of novel therapeutics, identification of potential diagnostic biomarkers, and as a preclinical model for testing new therapies for TFE3-RCC.
Figure 1. Generation and characterization of TFE3-RCC mouse model.
(a)A schematic diagram showing the generation of kidney specific PRCC-TFE3 expressing mice. A floxed Neomycin resistance cassette with stop codon, followed by a human PRCC-TFE3 cDNA, was inserted into the Rosa26 locus (PRCC-TFE3). Kidney-specific PRCC-TFE3 expression was obtained by crossing PRCC-TFE3 mice with Cadherin 16-Cre (KSP-Cre) mice. (b) Macroscopic appearance of PRCC-TFE3;KSP-Cre- [Cre(-)] and PRCC-TFE3;KSP-Cre+ [Cre(+)] mouse kidneys at the age of 7 months. (c) Relative ratio of kidney to body weight (100 X kidney weight/BW) of 4-month-old mice (n=8 for PRCC-TFE3;KSP-Cre- [Cre(-)]; n=8 for PRCC-TFE3;KSP-Cre+ [Cre(+)], unpaired t-test: ***p =0.0002) and 7-month-old mice (n=12 for [Cre(-)]; n=16 for [Cre(+)], unpaired t-test: ****p <0.0001) was calculated. Data are presented as mean with SD. (d) T2 weighted coronal magnetic resonance imaging (MRI) image (upper panel) and corresponding Gadolinium-enhanced T1 weighted MRI image (lower panel) of 7-month-old PRCC-TFE3;KSP-Cre- and PRCC-TFE3;KSP-Cre+ mice. The cysts appear bright on the T2w image and dark on the T1w. The tumor (red arrow) is well distinguished on both type of images. (e) A representative histology of hematoxylin and eosin (H&E) stained kidney from a 7-month-old PRCC-TFE3;KSP-Cre+ mouse. Lower panels are higher magnified images of the rectangular areas in upper panel. (f) Representative images of TFE3 immunohistochemistry of a kidney from a 7-month-old PRCC-TFE3;KSP-Cre+ mouse. Lower panels are higher magnified images of the rectangular areas in upper panel. (g, h, i) Representative H&E staining of solid tumors in 7-month-old PRCC-TFE3;KSP-Cre+ mice. Solid tumors are composed of two types of cells with eosinophilic cytoplasm (g) and large clear cytoplasm (h). (i) Psammoma body, a characteristic calcified lesion seen in human TFE3-RCC, was occasionally seen. (j) 5´-Bromo-2´-deoxyuridine (BrdU) (100 μg/g body wt) was injected intraperitoneally into 7-month-old PRCC-TFE3;KSP-Cre- mice and PRCC-TFE3;KSP-Cre+ mice 2 hr before euthanization. BrdU staining detects few proliferating cells in PRCC-TFE3;KSP-Cre- kidneys (left) and many proliferating cells in PRCC-TFE3;KSP-Cre+ kidneys (right). (k) Greater than 8-fold more BrdU incorporated cells were detected in PRCC-TFE3;KSP-Cre+ mouse kidneys compared with PRCC-TFE3;KSP-Cre- mouse kidneys (n=4 for each group, mean=10.0 per mm2 versus 84.4 per mm2, unpaired t-test: ****p<0.0001). BrdU positive cells per field were counted in 4 randomly selected fields from 4 mice for each group. Data are represented as means and SD. (l, m) Representative MRI images of and tumor (red arrow) diameters in PRCC-TFE3;KSP-Cre+ mouse kidneys, which were chronologically examined at 9.5, 10.5, and 11.3 months of age. (n) Kaplan-Meier survival analysis shows a statistically significant difference between PRCC-TFE3;KSP-Cre- and PRCC-TFE3;KSP-Cre+ mice (n=31 for [Cre(-)]; n=24 for [Cre(+)], log-rank test, ****p<0.0001). Median survival time of PRCC-TFE3;KSP-Cre+ mice is 11 months. (o) PRCC-TFE3;KSP-Cre+ mice die of renal failure. Blood urea nitrogen (BUN) levels were determined for PRCC-TFE3;KSP-Cre+ mice when moribund at ages ranging from 10 month to 12 month. Statistically significant elevation of BUN levels was observed in PRCC-TFE3;KSP-Cre+ mice compared with PRCC-TFE3;KSP-Cre- mice (n=4 for each group, mean=133.8 mg/dL versus mean=15.8 mg/dL, unpaired t-test: *p<0.017). Data are presented as mean with SD.
3.2. Identification of GPNMB and RET as upregulated genes in PRCC-TFE3;KSP-Cre+ kidneys
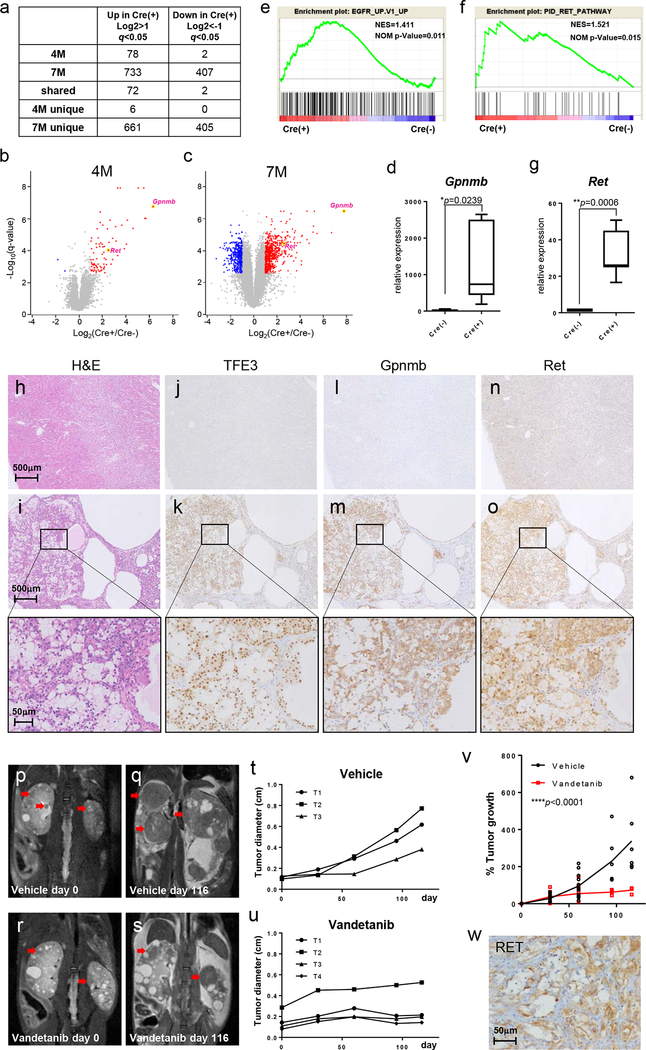
To identify candidate genes for biomarkers or therapeutic target molecules that could be useful for the diagnosis or molecular based therapy of TFE3-RCC, we performed microarray analysis of kidneys from 4 month old and 7 month old PRCC-TFE3;KSP-Cre+ mice and control PRCC-TFE3;KSP-Cre- mice (Fig.2a). Most of 78 significantly upregulated genes in kidneys from 4 month old PRCC-TFE3;KSP-Cre+ mice were also upregulated in kidneys from 7 month old PRCC-TFE3;KSP-Cre+ mice (Fig.2a, Supplemental Table S1a, Table S2a). A previous report of a TFEB driven RCC mouse model demonstrated activation of the Wnt-β catenin and ErbB signaling pathways (42). However, GSEA analysis did not demonstrate either a Wnt-β catenin or ErbB activation signature in PRCC-TFE3;KSP-Cre+ mouse kidneys, indicating that TFEB and PRCC-TFE3 have distinct oncogenic functions (Supplemental Fig. S2). One of the most significantly elevated genes in kidneys from both 4 month and 7 month old PRCC-TFE3;KSP-Cre+ mice was Gpnmb that encodes a type 1 transmembrane glycoprotein (Fig.2b, c, d, Supplemental Table S1a, Table S2a). GSEA analysis demonstrated significant enrichment of genes associated with EGFR signaling activation and RET signaling activation among those genes upregulated in kidneys from PRCC-TFE3;KSP-Cre+ mice (Fig.2e, f). In addition, a Kras activated signature was seen in PRCC-TFE3;KSP-Cre+ mouse kidneys (Supplemental Fig. S3). Although Egfr was not significantly upregulated in PRCC-TFE3 expressing kidneys, expression of the Ret proto-oncogene, a receptor tyrosine kinase that shares downstream signaling with EGFR, including the Kras pathway, was significantly higher in PRCC-TFE3;KSP-Cre+ kidneys than in control kidneys (Fig.2b, c, g). Immunohistochemical staining revealed robust Gpnmb staining that overlapped with strong TFE3 nuclear staining in kidneys from PRCC-TFE3;KSP-Cre+ mice, while control kidneys demonstrated negative staining for both TFE3 and Gpnmb (Fig.2h–m, Supplemental Fig. S4). In addition, immunohistochemical staining demonstrated strong Ret expression in kidney tumors of PRCC-TFE3;KSP-Cre+ mice and negative staining in control kidneys (Fig.2n, o, Supplemental Fig. S4), which motivated us to test therapeutic agents that target Ret in this TFE3-RCC mouse model. Vandetanib is a multiple tyrosine kinase inhibitor that efficiently inhibits EGFR, VEGFR, and RET. We administered vandetanib to PRCC-TFE3;KSP-Cre+ mice. Vandetanib treatment (100mg/kg) demonstrated a therapeutic effect on tumor growth compared to vehicle treatment with no evidence of toxicity (Fig.2p–u). Nonlinear regression analysis demonstrated statistically significantly reduced tumor growth in the vandetanib treated group compared to the control treated group (Fig.2v). These data underscore the usefulness of this TFE3-RCC mouse model not only for the development of biomarkers and targeted therapeutics but also as a preclinical model to test drugs or imaging methods. Furthermore, we have performed immunohistochemical staining of RET on human TFE3-RCC samples and found that 7 of 9 cases (77.8%) were positive for RET staining (Fig.2w, Table1), potentially supporting further evaluation of vandetanib treatment in advanced human TFE3-RCC cases.
Figure 2. Overexpression of Gpnmb and Ret in TFE3-RCC mouse model.
(a) Numbers of genes differentially expressed between PRCC-TFE3;KSP-Cre+ kidneys and PRCC-TFE3;KSP-Cre- kidneys. (b, c) Volcano plot of gene expression changes for 4 month-old (b) and 7 month-old (c) PRCC-TFE3;KSP-Cre- kidneys and PRCC-TFE3;KSP-Cre+ kidneys. The x-axis specifies the fold-changes [Log2(Cre(+)/Cre(-))] and the y-axis specifies the negative log to the base 10 of the t-test q-values. Red and blue dots represent genes expressed at significantly higher or lower levels (>2 fold vs. <-2 fold) in PRCC-TFE3;KSP-Cre+ kidneys (q<0.05). Gpnmb was one of the most significant genes showing increased expression in both 4 month-old and 7 month-old PRCC-TFE3;KSP-Cre+ kidneys. Ret was identified as one of the highly expressed and druggable receptor tyrosine kinases in both 4 month-old and 7 month-old PRCC-TFE3;KSP-Cre+ kidneys. (d) Expression of Gpnmb was quantified by qRT-PCR analysis of 8 month-old PRCC-TFE3;KSP-Cre- mouse kidneys (n=6) and PRCC-TFE3;KSP-Cre+ mouse kidneys (n=7). Data are represented as box-and-whisker plot. (unpaired t-test) (e, f) Gene Set Enrichment Analysis (GSEA) of microarray data from 4 month old PRCC-TFE3; KSP-Cre+ mouse kidneys (n=4) vs 4 month old PRCC-TFE3; KSP-Cre- mouse kidneys (n=4) (e) and 7 month old PRCC-TFE3; KSP-Cre+ mouse kidneys (n=4) vs 7 month old PRCC-TFE3; KSP-Cre- mouse kidneys (n=3) (f). Significant enrichment of genes associated with EGFR activation (e) and RET activation (f) was seen in kidneys from PRCC-TFE3; KSP-Cre+ mice. NES: normalized enrichment score, NOM p-Value: Nominal p value. (g) Expression of Ret was quantified by qRT-PCR analysis of 8 month-old PRCC-TFE3;KSP-Cre- mouse kidneys (n=6) and PRCC-TFE3;KSP-Cre+ mouse kidneys (n=7). Data are represented as box-and-whisker plot. (unpaired t-test) (h) H&E staining of 7 month-old PRCC-TFE3;KSP-Cre- and (i) PRCC-TFE3;KSP-Cre+ mouse kidneys. (j-o) Representative immunostaining for TFE3 (j, k), Gpnmb (l, m), and Ret (n, o) on serial sections of 7 month-old PRCC-TFE3;KSP-Cre- (j, l, n) and PRCC-TFE3;KSP-Cre+ (k, m, o) kidneys. Lower panels are higher magnified images of the rectangular areas in upper panels. (p-v) Treatment of PRCC-TFE3;KSP-Cre+ mice with vandetanib, an inhibitor of RET. Representative coronal T2 weighted MRI images of vehicle treated (p, q) and vandetanib treated (100mg/kg) (r, s) PRCC-TFE3;KSP-Cre+ mice chronologically taken on day 0 and day 116 of the study. Red arrows indicate the tumors which were tracked for growth. (t, u) Representative tumor growth curves of vehicle treated (t) and vandetanib treated (u) PRCC-TFE3;KSP-Cre+ mice shown in (p, q) and (r, s), respectively. The largest dimension of each tumor was measured from sequential MRI images taken on day 0, 30, 60, 95, and 116 of treatment. (v) Nonlinear regression analysis of % tumor growth in the vehicle treated group (black dots) and vandetanib treated group (red square). (****p<0.0001) (w) Representative immunostaining for RET on human TFE3-RCC demonstrates significant cytoplasmic staining.
3.3. GPNMB is a direct transcriptional target gene of chimeric TFE3
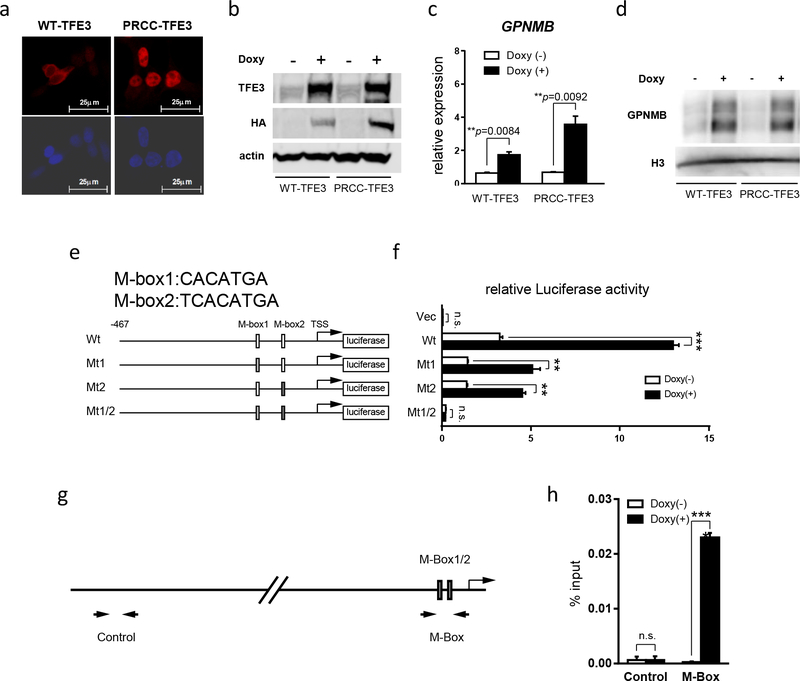
In order to investigate the molecular mechanisms of Gpnmb overexpression in PRCC-TFE3;KSP-Cre+ kidneys, we established stable cell lines derived from HEK293 cells which express wild type TFE3 or PRCC-TFE3 in a doxycycline-dependent manner (Fig.3a, b). Induced wild type TFE3 localized predominantly in the cytoplasm. On the other hand, PRCC-TFE3 localized predominantly in the nucleus (Fig.3a). These results support the concept that the PRCC-TFE3 chimeric protein works as an oncoprotein with constitutively active transcriptional activity. Induction of PRCC-TFE3 and, to a lesser extent, wild type TFE3, resulted in upregulated GPNMB expression at both mRNA and protein levels (Fig.3c, d). To determine if GPNMB is the direct transcriptional target of chimeric TFE3, we inserted the promoter region of human GPNMB, from -467 to the Transcription Start Site (TSS), into a luciferase reporter plasmid. Two M-box motifs (M-box1 and M-box2), which are putative TFE3 binding sequences, were included in this construct. We transfected reporter plasmids carrying wild type or mutant M-box motifs into doxycycline-inducible PRCC-TFE3 expressing HEK293 cells (Fig.3e). As shown in Fig.3f, the promoter activity of GPNMB was upregulated by the induced expression of PRCC-TFE3. Importantly, this PRCC-TFE3 dependent GPNMB promoter activity was attenuated in cells transfected with mutated M-box1 or M-box2 reporter plasmids. Furthermore, complete absence of PRCC-TFE3 dependent promoter activity was seen in cells transfected with a reporter plasmid in which both M-box1 and M-box2 were mutated (Fig.3f). These findings indicate that GPNMB is transcriptionally upregulated by PRCC-TFE3 through both M-box motifs in its promoter. In addition, we have confirmed the direct binding of PRCC-TFE3 to the minimal GPNMB promoter region containing these two M-box motifs by chromatin immunoprecipitation followed by quantitative PCR (Fig.3g,h). These experimental data clearly indicate that GPNMB is a direct transcriptional target gene of PRCC-TFE3.
Figure 3. Gpnmb is a direct transcriptional target of PRCC-TFE3.
(a) Immunocytochemistry using anti-HA antibody on HEK293 derived stable cell lines which express HA tagged wild type TFE3 and PRCC-TFE3 in a doxycycline- dependent manner. (b) Western blotting with anti-TFE3, anti-HA, and anti-beta actin on HEK293 derived doxycycline-inducible cell lines cultured without and with doxycycline.(c) GPNMB expression was quantified by qRT-PCR on HEK293 derived doxycycline-inducible cell lines which were cultured without (open bar) and with doxycycline (solid bar). Data represent means ± SD (triplicate, unpaired t-test: WT-TFE3 **p=0.0084, PRCC-TFE3 **p=0.0092). Representative data from at least three independent experiments are shown. (d) Western blotting with anti-GPNMB and anti-histone H3 on HEK293 derived doxycycline-inducible cell lines cultured without and with doxycycline. (e) Scheme of Luciferase reporter constructs with human GPNMB promoter. Putative TFE3 consensus sequences are listed as M-box1 (CACATGA) and M-box2 (TCACATGA). Wt: wild type GPNMB promoter construct; Mt1: M-box1 is mutated to CTCGAGA; Mt2: M-box2 is mutated to TCTCGAGA; M-box1/2: Both M-box1 and M-box2 are mutated to CTCGAGA and TCTCGAGA, respectively. (f) Each Luciferase reporter construct and pGL4 as a negative control were transfected into PRCC-TFE3 doxycycline- inducible HEK 293 cell line with phRL internal control. 12hr after transfection, medium was changed to new medium with or without doxycycline, followed by additional 24hr incubation and harvest. The x-axis displays relative Luciferase activity. GPNMB promoter activity is upregulated by PRCC-TFE3 induction in an M-Box- dependent manner. Data represent means ± SD (triplicate, unpaired t-test: n.s. not significant, **p <.01, ***p <.001). Representative data from at least three independent experiments are shown. (g, h) ChIP was performed using anti-HA antibody on PRCC-TFE3 doxycycline-inducible HEK 293 cells cultured with or without doxycycline, followed by qPCR on ChIP samples. (g) Scheme indicating the primer sets used for qPCR. (h) ChIP-qPCR results demonstrate PRCC-TFE3 specifically binds to M-Box containing sequence in GPNMB promoter. Y-axis indicates % of input. Data represent means ± SD (triplicate, unpaired t-test: n.s. not significant, ***p <.001).
3.4. Specific expression of GPNMB in TFE3-RCC
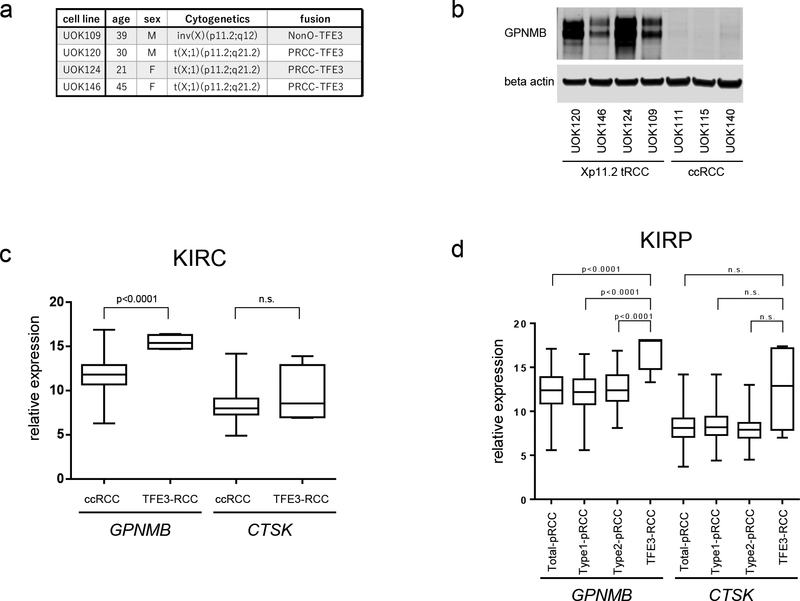
Since GPNMB was proven to be a direct transcriptional target of chimeric TFE3, we evaluated the utility of GPNMB as a diagnostic marker for TFE3-RCC. To determine whether GPNMB expression was specific for TFE3-RCC, we compared GPNMB expression in RCC cell lines established from human TFE3-RCCs, and in cell lines from sporadic clear cell RCCs (ccRCC). The TFE3 RCC cell lines UOK109, which we developed from tumor material removed from a 39 year old female (15), UOK120, developed from tumor material from a 30 year old male(3), UOK124, developed from a 21 year old female (3), and UOK146, developed from tumor material from a 45 year old male (3,35), expressed significantly higher amounts of GPNMB protein, while ccRCC cell lines UOK111, UOK115, and UOK140 (15) expressed no detectable GPNMB (Fig.4a, b). To further confirm the specificity of GPNMB expression in TFE3-RCC, we utilized the gene expression database for ccRCC (KIRC) from the Cancer Genome Atlas project (12,38) from which there were 4 cases of TFE3-RCC. We compared the GPNMB mRNA expression levels in 465 cases of ccRCC with 4 cases of TFE3-RCC. GPNMB expression levels were statistically significantly higher (p<0.0001) in TFE3-RCC than ccRCC (Fig. 4c). We also compared the expression levels of Cathepsin K, which has been used as a diagnostic marker for TFE3-RCC, but were unable to see statistically significant Cathepsin K expression differences between ccRCC and TFE3-RCC (Fig. 4c), thereby underscoring the utility of GPNMB as a diagnostic marker. Although TFE3-RCC can present with a variety of histologies, the most frequently observed histological characteristics are papillary, tubular, or alveolar structures composed of clear cells, which may lead to misdiagnosis of TFE3-RCC as ccRCC or papillary RCC. Hence, we compared GPNMB mRNA expression in TFE3-RCC and papillary RCC using the TCGA gene expression database for papillary RCC (KIRP) (14) comprised of 6 cases of TFE3-RCC, 161 cases of papillary type 1 RCC, and 79 cases of papillary type 2 RCC. As shown in Fig. 4d, while GPNMB expression levels were statistically significantly higher (p<0.0001) in TFE3-RCC than in either papillary type 1 RCC, papillary type 2 RCC (p<0.0001) or total papillary RCC (p<0.0001), there were no statistically significant differences in Cathepsin K expression between TFE3-RCC and either papillary type 1 RCC, papillary type 2 RCC or total papillary RCC. (Fig. 4d). These data show that GPNMB expression is a useful biomarker for distinguishing TFE3-RCC from other types of RCCs.
Figure 4. GPNMB overexpression differentiates TFE3-RCC from clear cell RCC and papillary RCC.
(a,) Detailed information of four TFE3-RCC cell lines. (b) Western blotting demonstrates significantly higher GPNMB expression in TFE3-RCC cell lines compared to ccRCC cell lines. (c) Comparison of GPNMB and Cathepsin K (CTSK) expression in TFE3-RCC (n=4) and ccRCC (n=465). The gene expression database for ccRCC (KIRC) from the Cancer Genome Atlas project which contained 4 cases of TFE3-RCC was utilized for analysis. (d) The gene expression database for papillary RCC (KIRP) from the Cancer Genome Atlas containing 6 cases of TFE3-RCC was utilized to compare GPNMB and Cathepsin K (CTSK) expression in TFE3-RCC and papillary RCC. Total papillary RCC (n=283 without TFE3-RCC) includes 161 cases of type1 papillary RCC and 79 cases of type2 papillary RCC. Data are represented as a box-and-whisker plot. (unpaired t-test with or without Welch’s correction, based on p-value of the F test: n.s. not significant)
3.5. GPNMB immunostaining as potential diagnostic marker for TFE3-RCC
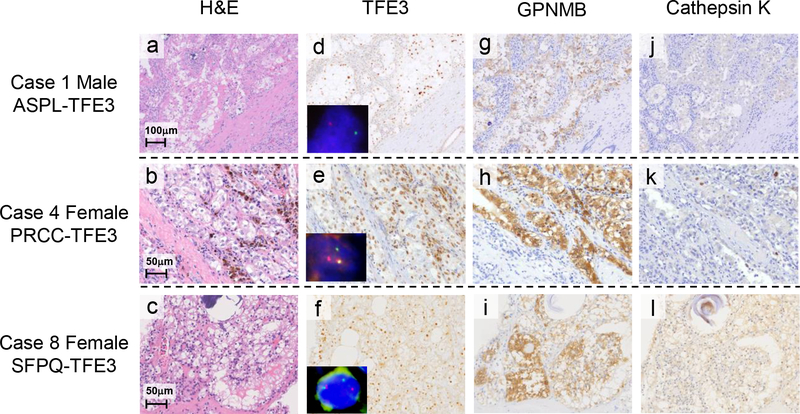
To evaluate the immunohistochemical expression pattern of GPNMB in formalin-fixed paraffin-embedded (FFPE) TFE3-RCCs, we collected 9 cases with characteristic histologies of TFE3 translocation RCC. They displayed papillary, tubular, or alveolar histology with frequent calcification and were composed of clear cells or mixtures of clear cells and eosinophilic cells (Fig. 5a, b, c). All cases demonstrated nuclear TFE3 immunoreactivity, and were confirmed to be TFE3-RCC by FISH and RT-PCR analyses (Fig. 5d, e, f). Clinicopathological findings are summarized in Table 1. All cases of TFE3-RCC were confirmed to have nuclear TFE3 staining (Table 2). All TFE3-RCCs showed positive immunoreactivity for GPNMB (Fig.5g, h, i; Table 2), two cases with 1+ staining, and 7 cases with 2+ staining. Cathepsin-K was positively stained in only 6 of 9 TFE3-RCC cases (Fig. 5j, k, l; Table 2). There was no obvious correlation between Fuhrman grade and immunoreactivity of GPNMB or Cathepsin-K. (Table 1) To further confirm the specificity of GPNMB immunostaining as a potential diagnostic marker for TFE3-RCC, we have stained 56 ccRCCs and 20 papillary RCCs for GPNMB [(40) and this report]. Comparisons between TFE3-RCC and ccRCC and between TFE3-RCC and papillary RCC were statistically significant (p<0.0001) for GPNMB staining (Table 3). Interestingly, the 4 cases from a total of 76 clear cell and papillary RCC that were positive for GPNMB were also positive for nuclear TFE3 staining, which suggests that GPNMB positivity is the consequence of TFE3 activation by an unknown mechanism other than Xp11.2 translocation. Overall, these data demonstrate that GPNMB immunostaining can be a useful diagnostic marker for TFE3-RCC.
Figure 5. Immunostaining of TFE3, GPNMB and Cathepsin K in human TFE3-RCCs.
(a-l) Histology, immunohistochemistry (TFE3, GPNMB, and Cathepsin K), and TFE3 gene break-apart FISH on representative cases of human TFE3-RCC with different TFE3 fusion genes (Cases #1, 4, 8 in Table 1). (a, b, c) H&E staining displays characteristic histology resembling clear cell RCC and papillary RCCs composed of epithelioid clear cells and eosinophilic cells. Occasional psammoma bodies are seen. (d, e, f) Immunohistochemistry shows nuclear staining for TFE3. Representative images of TFE3 gene break-apart FISH are inserted. All cases show TFE3 translocations represented by separation of green and red probes. The male case has a split (d) and the female cases have split and fused signals (e, f). (g, h, i) Immunostaining for GPNMB demonstrates significant cytoplasmic staining. (j, k, l) Immunohistochemistry of Cathepsin K demonstrates either negative staining (j, k) or positive staining (l).
Table 2.
Summary of immunohistochemical findings in patients with TFE3-RCC.
| Negative | Positive | 1+ | 2+ | |
|---|---|---|---|---|
| TFE3 | 0%(0/9) | 100%(9/9) | 1 | 8 |
| GPNMB | 0%(0/9) | 100%(9/9) | 2 | 7 |
| Cathepsin K | 33%(3/9) | 67%(6/9) | 4 | 2 |
Table 3.
Summary of GPNMB staining on human RCC samples.
| Negative | Positive | 1+ | 2+ | |
|---|---|---|---|---|
| TFE3-RCC | 0%(0/9) | 100%(9/9) | 2 | 7 |
| clear cell RCC | 98.2%(55/56) | 1.8%(1/56) | 1 | 0 |
| papillary RCC | 85.0%(17/20) | 15.0%(3/20) | 3 | 0 |
4. Discussion
TFE3 Xp11.2 translocation RCC (TFE3-RCC) was initially described in 1996 (3,43) and established as an independent subtype of RCC in 2004 by the World Health Organization (44). Due to the relatively low incidence of TFE3-RCC (14), the biological characteristics of TFE3-RCC have not been fully clarified and effective forms of therapy for patients with advanced disease have yet to be established (16). Although many cases of TFE3-RCC display the characteristic histology, other cases of TFE3-RCC are misdiagnosed as ccRCC, papillary RCC or unclassified RCC (12,25,26). Given the aggressive nature of this disease, it is of great importance to develop a concise, sensitive, and specific method for diagnosis and treatment of TFE3-RCC. Here we have generated the first TFE3-RCC mouse model, which we have utilized to further characterize the chimeric PRCC-TFE3 protein and identify a robust and reliable diagnostic marker for TFE3-RCC, and as a model for the development of targeted therapeutic approaches for this disease.
Results from the evaluation of our PRCC-TFE3-expressing mouse model have confirmed that chimeric PRCC-TFE3 is an oncogene which is responsible for RCC development in vivo. Since all the chimeric genes reported to date in TFE3-RCC encode the carboxy-terminal half of TFE3, which retains the basic-helix-loop-helix leucine-zipper structures through which TFE3 dimerizes and binds to DNA (16,18), it is predicted that these chimeric genes function as oncogenic transcription factors (45) (46). Indeed, overexpressed PRCC-TFE3 and SFPQ-TFE3 (data not shown) demonstrated predominant nuclear localization, while overexpressed wild-type TFE3 localized in the cytoplasm of HEK 293 cells. This finding suggests that chimeric TFE3 proteins acquire the ability to localize in the nucleus and function as constitutively active transcription factors. Aberrant upregulation of PRCC-TFE3 transcriptional target genes, followed by perturbation of the transcriptional network is most likely responsible for TFE3-RCC development. Further analysis of the transcriptional network alterations caused by PRCC-TFE3 expression may provide clues to understanding the molecular mechanisms of TFE3-RCC development. Indeed, our TFE3-RCC mouse model demonstrated Ret overexpression and therapeutic responses to vandetanib treatment. We utilized publicly available databases and searched for putative transcription factors which may regulate Ret gene expression (data not shown). There was no clear evidence for TFE3 mediated Ret regulation. However, among the many putative transcription factors which may regulate Ret transcription, Nr4a1 expression was dramatically elevated in PRCC-TFE3 expressing kidneys (Supplemental Fig. S5). Nr4a1 expression was significantly higher in TFE3-RCC than ccRCC and papillary RCC (Supplemental Fig. S5). It will be of great importance to clarify the details of the transcriptional network, including Nr4a1, perturbed by chimeric TFE3.
Another type of translocation RCC has been described involving chromosome 6p21 translocation, in which a second MIT family transcription factor, TFEB, is fused to and transcribed by a strong promoter of the MALAT1 gene (47,48). In chromosome 6p21 translocation RCC, overexpression of wild type TFEB drives RCC development. Indeed, kidney-specific overexpression of wild type TFEB in a transgenic mouse model produced clear cell and papillary renal cell carcinomas (42). This mouse model displays aberrant activation of the Wnt signaling and ErbB signaling pathways, which was not observed in our PRCC-TFE3 mouse model by GSEA analysis (Supplemental Fig. S2). The difference between these two models might reflect differences between wild type TFEB and chimeric PRCC-TFE3. As we have shown, PRCC-TFE3 localizes in the nucleus in most of the cells, whereas wild type TFE3 and TFEB localize predominantly in the cytoplasm under normal conditions (34,37,49–51). In addition, PRCC which displaces the N-terminal half of TFE3 may contribute to altered transcriptional activity affecting different transcriptional targets compared to wild type TFE3. Indeed, when we compared significantly upregulated genes in PRCC-TFE3 expressing kidneys and TFEB expressing kidneys (42), less than 10% of PRCC-TFE3 upregulated genes were commonly upregulated in TFEB expressing kidneys (Supplemental Table S3). Our unique PRCC-TFE3 mouse model will provide a powerful tool for further clarification of molecular mechanisms responsible for TFE3-RCC development.
In this TFE3-RCC mouse model, PRCC-TFE3 expression was induced by cadherin 16 promoter driven Cre recombinase with broad expression in the distal nephron and some regions of the proximal tubules (29,30). Notably, PRCC-TFE3 expressing kidneys demonstrated different histologic features, dilated monolayer tubules with hyperproliferation, adenomatous epithelial cells growing as multiple layers into the lumen of dilated tubules, and solid tumors with a variety of sizes. The diversity of histologic features resulting from PRCC-TFE3 expression in mouse kidney may represent different stages of RCC development, which have acquired further genetic and/or epigenetic changes in addition to PRCC-TFE3 expression. Of note, recent advances in next generation sequencing technology have revealed the heterogeneity of cancer. Clonal heterogeneity with different combinations of driver gene mutations has been well characterized in clear cell RCC (52,53). We hypothesize that TFE3-RCC may also display clonal heterogeneity with a variety of driver gene mutations. In fact, our TFE3-RCC mice developed variable histologies, tumor doubling times, and responses to targeted therapeutics. This diverse phenotype may result from a variety of driver gene mutations. Identification and characterization of additional TFE3-RCC driver gene mutations will contribute to a better understanding of the causes of TFE3-RCC heterogeneity and facilitate the development of effective targeted therapeutics. This PRCC-TFE3 mouse model can be utilized in future studies to evaluate potential driver gene mutations by crossing with genetically engineered mice for selective gene deletion.
Since the chimeric TFE3 proteins responsible for TFE3-RCC development act as oncogenic transcription factors, transcriptionally upregulated direct targets of chimeric TFE3 could be promising candidates for TFE3-RCC diagnostic markers. Gpnmb was one of the most significantly upregulated genes in PRCC-TFE3 expressing kidneys, and, with luciferase reporter assays and ChIP-qPCR, we have shown that GPNMB is a direct transcriptional target of PRCC-TFE3. GPNMB expression was significantly higher in human TFE3-RCCs than in clear cell or papillary RCCs, thereby demonstrating high sensitivity and specificity as a biomarker for TFE3-RCC.
In pathologic diagnosis of TFE3-RCCs using FFPE tissues, strong TFE3 nuclear immunostaining is considered the diagnostic gold standard (19), but TFE3 immunohistochemistry can be problematic because the antigenicity of TFE3 can be easily altered by improper fixation and sample storage conditions. Therefore, pathologists often face difficulties to evaluate TFE3 staining (24,26). The break-apart FISH assay is recommended to augment the histopathologic diagnosis of TFE3-RCC (25,27). However, the FISH result may be judged as negative in cases in which the partner localizes to the vicinity of TFE3 on the short arm of chromosome X (54). Hence, a surrogate marker which can be used for routine pathological diagnosis in FFPE tissues is desired. We found that GPNMB immunostaining using FFPE tissues was positive in all 9 human TFE3-RCCs in the current study. Furthermore, our study showed that 55 of 56 (98.2%) sporadic ccRCCs and 17 of 20 (85.0%) sporadic papillary RCCs were negatively stained for GPNMB (Table 3). Although sporadic chromophobe RCCs and hybrid oncocytic tumors associated with Birt-Hogg-Dubé syndrome also stain for GPNMB (40), these tumors are histologically distinctive and rarely need to be distinguished from TFE3-RCCs. Pathologists may find GPNMB immunostaining useful in diagnosing TFE3-RCC when tumors have papillary and/or clear cell morphologies with indefinite TFE3 staining. The current histopathologic study was based on a limited number of human TFE3-RCC cases. Amassing additional clinicopathologic data will be necessary for a better understanding of the clinicopathological signature of TFE3-RCC, which will contribute to the design of potential therapeutic agents for treating advanced cases of TFE3-RCC.
Our study provides a valuable model for the development of targeted therapies for advanced TFE3-RCC. PRCC-TFE3 mouse model kidneys and human TFE3-RCC samples demonstrate significant elevation of RET expression. Moreover, the RET inhibitor vandetanib significantly suppressed TFE3-RCC growth in mice. The receptor tyrosine kinase inhibitor vandetanib is FDA approved for the treatment of advanced cases of medullary thyroid carcinoma (55). Since there is no established treatment for advanced TFE3-RCC with poor prognosis, vandetanib or other multiple tyrosine kinase inhibitors that target RET could be promising candidates as effective therapeutic agents for advanced TFE3-RCC. In addition, GPNMB itself could be a therapeutic target for advanced TFE3-RCC. An antibody-drug conjugate (ADC) targeting GPNMB, glembatumumab vedotin, comprised of an anti-GPNMB antibody conjugated to a cellular toxin, monomethylauristatin E (MMAE), causes death of GPNMB positive cells. Indeed, glembatumumab vedotin is being evaluated in clinical trials for several cancers, including advanced melanoma and breast cancer (56). It would be of interest to test this ADC in a preclinical study in the PRCC-TFE3 mouse model. GPNMB is also known to promote tumor growth and invasion through integrin signaling, VEGFR activation, or EGFR activation (45,57). Targeting GPNMB signaling may also be a promising strategy for treatment of advanced TFE3-RCC. Our current work has provided the basis for the development of effective therapies against advanced TFE3-RCC that eventually could lead to clinical trials that may benefit patients with this very aggressive form of renal cell carcinoma.
Supplementary Material
Implications: Key findings from studies with this preclinical mouse model of TFE-RCC underscore the potential for RET as a therapeutic target for treatment of patients with TFE3-RCC, and suggest that GPNMB may serve as diagnostic biomarker for TFE3 fusion renal cell carcinoma.
Acknowledgements
We thank Maria Merino for informative in-depth discussions of comparative human/mouse normal and abnormal histopathology, Nobuko Irie for excellent technical support and acknowledge the support provided to us by the University of Texas Southwestern O’Brien Kidney Research Core Center and Peter Igarashi for Cadherin 16 (KSP)-Cre transgenic mice. pRosa26-DEST was a gift from Nick Hastie and Peter Hohenstein (Addgene plasmid # 21189). M. Baba was supported in part by a JSPS KAKENHI Grant-in-Aid for Scientific Research (S), (#18H05284, #26221309), Grant-in-Aid for Scientific Research (B) (# 15H04975, # 18H02938), Grant-in-Aid for Challenging Research (Exploratory) (# 18K19619, #18K19553), Grant-in -Aid for Scientific Research on Innovative Areas (#16H06276), Grant-in-Aid for Scientific Research (C) (#18K09140), Novartis Research Grant, a Research grant from Ono Pharmaceutical Co. Ltd and Bristol-Myers Squibb K.K. Grant, the Joint Usage/Research Center Program of the Advanced Medical Research Center, Yokohama City University, and the program of the Joint Usage/Research Center for Developmental Medicine, Institute of Molecular Embryology and Genetics, Kumamoto University. M. Furuya was supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) (#17K08745). T. Motoshima was supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) (#16K11013) and AKUA (Asahi Kasei pharma Urological Academy) Research Grant. T. Kadomatsu was supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) (#18K07236). H. Hasumi was supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) (#16K11020). Y. Nagashima was supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) (#17K11162). T. Kamba was supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) (#18K09140). T. Suda was supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (S), (#18H05284, #26221309) and the National Medical Research Council grant of Singapore Translational Research Investigator Award (NMRC/STaR/0019/2014)., Y. Oike was supported in part by a JSPS KAKENHI Grant-in-Aid for Challenging Research (Exploratory) (# 18K19519). This research was supported in part by the Intramural Research Program of NIH, Frederick National Laboratory, Center for Cancer Research. This project has been funded in part with Federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Heath and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
NCI-Frederick at the Frederick National Laboratory for Cancer Research is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals (National Research Council; 1996; National Academy Press; Washington DC).
Funding Sources: All the funding sources had no involvement in study design, data collection, data interpretation, report writing, or decision to submit the paper for publication.
Footnotes
Disclosure of Potential Conflicts of Interest: Masaya Baba received a research grant from Ono Pharmaceutical Co. Ltd and Bristol-Myers Squibb K.K. Other authors declare no conflict of interest.
References
- 1.de Jong B, Molenaar IM, Leeuw JA, Idenberg VJ, Oosterhuis JW. Cytogenetics of a renal adenocarcinoma in a 2-year-old child. Cancer genetics and cytogenetics 1986;21(2):165–9. [DOI] [PubMed] [Google Scholar]
- 2.Shipley JM, Birdsall S, Clark J, Crew J, Gill S, Linehan WM, et al. Mapping the X chromosome breakpoint in two papillary renal cell carcinoma cell lines with a t(X;1)(p11.2;q21.2) and the first report of a female case. Cytogenet Cell Genet 1995;71:280–4. [DOI] [PubMed] [Google Scholar]
- 3.Sidhar SK, Clark J, Gill S, Hamoudi R, Crew J, Gwilliam R, et al. The t(X;1)(p11.2;q21.2) translocation in papillary renal cell carcinoma fuses anovel gene PRCC to the TFE3 transcription factor gene. Hum Mol Genet 1996;5(9):1333–8. [DOI] [PubMed] [Google Scholar]
- 4.Ross H, Argani P. Xp11 translocation renal cell carcinoma. Pathology 2010;42(4):369–73 [DOI] [PubMed] [Google Scholar]
- 5.Weterman MA, Wilbrink M, Dijkhuizen T, van den Berg E, Geurts van Kessel A. Fine mapping of the 1q21 breakpoint of the papillary renal cell carcinoma-associated (X;1) translocation. Human genetics 1996;98(1):16–21. [DOI] [PubMed] [Google Scholar]
- 6.Ladanyi M, Lui MY, Antonescu CR, Krause-Boehm A, Meindl A, Argani P, et al. The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 2001;20(1):48–57 [DOI] [PubMed] [Google Scholar]
- 7.Argani P, Antonescu CR, Couturier J, Fournet JC, Sciot R, Debiec-Rychter M, et al. PRCC-TFE3 renal carcinomas: morphologic, immunohistochemical, ultrastructural, and molecular analysis of an entity associated with the t(X;1)(p11.2;q21). Am J Surg Pathol 2002;26(12):1553–66. [DOI] [PubMed] [Google Scholar]
- 8.Weterman MA, Wilbrink M, Geurts van Kessel A. Fusion of the transcription factor TFE3 gene to a novel gene, PRCC, in t(X;1)(p11;q21)-positive papillary renal cell carcinomas. Proc Natl Acad Sci U S A 1996;93(26):15294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argani P, Antonescu CR, Illei PB, Lui MY, Timmons CF, Newbury R, et al. Primary Renal Neoplasms with the ASPL-TFE3 Gene Fusion of Alveolar Soft Part Sarcoma. The American Journal of Pathology 2001;159(1):179–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang W, Goldfischer M, Babayeva S, Mao Y, Volyanskyy K, Dimitrova N, et al. Identification of a novel PARP14-TFE3 gene fusion from 10-year-old FFPE tissue by RNA-seq. Genes Chromosomes Cancer 2015;54(8):500–5 [DOI] [PubMed] [Google Scholar]
- 11.Argani P, Lui MY, Couturier J, Bouvier R, Fournet JC, Ladanyi M. A novel CLTC-TFE3 gene fusion in pediatric renal adenocarcinoma with t(X;17)(p11.2;q23). Oncogene 2003;22(34):5374–8 [DOI] [PubMed] [Google Scholar]
- 12.Creighton CJMM, Gunaratne PH, Wheeler DA, Gibbs RA, Robertson A, et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499(7456):43–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malouf GG, Su X, Yao H, Gao J, Xiong L, He Q, et al. Next-generation sequencing of translocation renal cell carcinoma reveals novel RNA splicing partners and frequent mutations of chromatin-remodeling genes. Clin Cancer Res 2014;20(15):4129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linehan WMSP, Ricketts CJ, Creighton CJ, Fei SS, Davis C, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med 2016;374(2):135–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark J, Lu YJ, Sidhar SK, Parker C, Gill S, Smedley D, et al. Fusion of splicing factor genes PSF and NonO (p54nrb) to the TFE3 gene in papillary renal cell carcinoma. Oncogene 1997;15(18):2233–9 [DOI] [PubMed] [Google Scholar]
- 16.Kauffman EC, Ricketts CJ, Rais-Bahrami S, Yang Y, Merino MJ, Bottaro DP, et al. Molecular genetics and cellular features of TFE3 and TFEB fusion kidney cancers. Nat Rev Urol 2014;11(8):465–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argani P, Olgac S, Tickoo SK, Goldfischer M, Moch H, Chan DY, et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Am J Surg Pathol 2007;31(8):1149–60 [DOI] [PubMed] [Google Scholar]
- 18.Hemesath TJ, Steingrimsson E, McGill G, Hansen MJ, Vaught J, Hodgkinson CA, et al. microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev 1994;8(22):2770–80. [DOI] [PubMed] [Google Scholar]
- 19.Argani P, Lal P, Hutchinson B, Lui MY, Reuter VE, Ladanyi M. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol 2003;27(6):750–61. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda N, Mikami S, Pan CC, Cohen RJ, Hes O, Michal M, et al. Review of renal carcinoma associated with Xp11.2 translocations/TFE3 gene fusions with focus on pathobiological aspect. Histol Histopathol 2012;27(2):133–40. [DOI] [PubMed] [Google Scholar]
- 21.Komai Y, Fujiwara M, Fujii Y, Mukai H, Yonese J, Kawakami S, et al. Adult Xp11 translocation renal cell carcinoma diagnosed by cytogenetics and immunohistochemistry. Clin Cancer Res 2009;15(4):1170–6 [DOI] [PubMed] [Google Scholar]
- 22.Zhong M, De Angelo P, Osborne L, Paniz-Mondolfi AE, Geller M, Yang Y, et al. Translocation renal cell carcinomas in adults: a single-institution experience. Am J Surg Pathol 2012;36(5):654–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cajaiba MM, Dyer LM, Geller JI, Jennings LJ, George D, Kirschmann D, et al. The classification of pediatric and young adult renal cell carcinomas registered on the children’s oncology group (COG) protocol AREN03B2 after focused genetic testing. Cancer 2018;124(16):3381–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol 2013;37(10):1469–89 [DOI] [PubMed] [Google Scholar]
- 25.Green WM, Yonescu R, Morsberger L, Morris K, Netto GJ, Epstein JI, et al. Utilization of a TFE3 break-apart FISH assay in a renal tumor consultation service. Am J Surg Pathol 2013;37(8):1150–63 [DOI] [PubMed] [Google Scholar]
- 26.Kuroda N, Tanaka A, Ohe C, Nagashima Y. Recent advances of immunohistochemistry for diagnosis of renal tumors. Pathol Int 2013;63(8):381–90 [DOI] [PubMed] [Google Scholar]
- 27.Rao QWS, Zhang S, Eble JN, Grignon DJ, Wang M, Zhou XJ, Huang W, Tan PH, Maclennan GT, Cheng L. TFE3 break-apart FISH has a higher sensitivity for Xp11.2 translocation-associated renal cell carcinoma compared with TFE3 or cathepsin K immunohistochemical staining alone: expanding the morphologic spectrum. The American Journal of Pathology 2013;37(6):804–15. [DOI] [PubMed] [Google Scholar]
- 28.Hohenstein P, Slight J, Ozdemir DD, Burn SF, Berry R, Hastie ND. High-efficiency Rosa26 knock-in vector construction for Cre-regulated overexpression and RNAi. Pathogenetics 2008;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. Journal of the American Society of Nephrology : JASN 2002;13(7):1837–46. [DOI] [PubMed] [Google Scholar]
- 30.Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, et al. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst 2008;100(2):140–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasumi H, Baba M, Hasumi Y, Lang M, Huang Y, Oh HF, et al. Folliculin-interacting proteins Fnip1 and Fnip2 play critical roles in kidney tumor suppression in cooperation with Flcn. Proc Natl Acad Sci U S A 2015;112(13):E1624–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genetics 2003;34(3):267–73. [DOI] [PubMed] [Google Scholar]
- 34.Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A 2006;103(42):15552–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anglard P, Trahan E, Liu S, Latif F, Merino MJ, Lerman M, et al. Molecular and cellular characterization of human renal cell carcinoma cell lines. Cancer Res 1992;52:348–56. [PubMed] [Google Scholar]
- 36.Sourbier C, Srivastava G, Ghosh MC, Ghosh S, Yang Y, Gupta G, Degraff W, Krishna MC, Mitchell JB, Rouault TA, Linehan WM. Targeting HIF2alpha translation with Tempol in VHL-deficient clear cell renal cell carcinoma. Oncotarget 2012;3(11):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baba M, Endoh M, Ma W, Toyama H, Hirayama A, Nishikawa K, et al. Folliculin Regulates Osteoclastogenesis Through Metabolic Regulation. J Bone Miner Res 2018;33(10):1785–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep 2018;23(1):313–26 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2(5):401–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuya M, Hong SB, Tanaka R, Kuroda N, Nagashima Y, Nagahama K, et al. Distinctive expression patterns of glycoprotein non-metastatic B and folliculin in renal tumors in patients with Birt-Hogg-Dube syndrome. Cancer Sci 2015;106(3):315–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuroda N, Katto K, Tanaka Y, Yamaguchi T, Inoue K, Ohara M, et al. Diagnostic pitfall on the histological spectrum of adult-onset renal carcinoma associated with Xp11.2 translocations/TFE3 gene fusions. Med Mol Morphol 2010;43(2):86–90 [DOI] [PubMed] [Google Scholar]
- 42.Calcagni A, Kors L, Verschuren E, De Cegli R, Zampelli N, Nusco E, et al. Modelling TFE renal cell carcinoma in mice reveals a critical role of WNT signaling. Elife 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weterman MA, Wilbrink M, Janssen I, Janssen HA, van den Berg E, Fisher SE, et al. Molecular cloning of the papillary renal cell carcinoma-associated translocation (X;1)(p11;q21) breakpoint. Cytogenet Cell Genet 1996;75(1):2–6 [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol 2006;49(5):798–805 [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M, Homme M, Yamazaki Y, Shimizu R, Takazawa Y, Nakamura T. Modeling Alveolar Soft Part Sarcoma Unveils Novel Mechanisms of Metastasis. Cancer Res 2017;77(4):897–907 [DOI] [PubMed] [Google Scholar]
- 46.Kobos R, Nagai M, Tsuda M, Merl MY, Saito T, Lae M, et al. Combining integrated genomics and functional genomics to dissect the biology of a cancer-associated, aberrant transcription factor, the ASPSCR1-TFE3 fusion oncoprotein. J Pathol 2013;229(5):743–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis IJ, Hsi BL, Arroyo JD, Vargas SO, Yeh YA, Motyckova G, et al. Cloning of an Alpha-TFEB fusion in renal tumors harboring the t(6;11)(p21;q13) chromosome translocation. Proc Natl Acad Sci U S A 2003;100(10):6051–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuiper RP, Schepens M, Thijssen J, van Asseldonk M, van den Berg E, Bridge J, et al. Upregulation of the transcription factor TFEB in t(6;11)(p21;q13)-positive renal cell carcinomas due to promoter substitution. Hum Mol Genet 2003;12(14):1661–9. [DOI] [PubMed] [Google Scholar]
- 49.Hong SB, Oh H, Valera VA, Baba M, Schmidt LS, Linehan WM. Inactivation of the FLCN tumor suppressor gene induces TFE3 transcriptional activity by increasing its nuclear localization. PLoS One 2010;5(12):e15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science 2009;325(5939):473–7 [DOI] [PubMed] [Google Scholar]
- 51.Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Science Signaling;5(228). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet 2014;46(3):225–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turajlic S, Xu H, Litchfield K, Rowan A, Horswell S, Chambers T, et al. Deterministic Evolutionary Trajectories Influence Primary Tumor Growth: TRACERx Renal. Cell 2018;173(3):595–610 e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Argani P, Zhang L, Reuter VE, Tickoo SK, Antonescu CR. RBM10-TFE3 Renal Cell Carcinoma A Potential Diagnostic Pitfall Due to Cryptic Intrachromosomal Xp11.2 Inversion Resulting in False-negative TFE3 FISH. American Journal of Surgical Pathology 2017;41(5):655–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valerio L, Pieruzzi L, Giani C, Agate L, Bottici V, Lorusso L, et al. Targeted Therapy in Thyroid Cancer: State of the Art. Clinical oncology (Royal College of Radiologists (Great Britain)) 2017;29(5):316–24 [DOI] [PubMed] [Google Scholar]
- 56.Rose AAN, Biondini M, Curiel R, Siegel PM. Targeting GPNMB with glembatumumab vedotin: Current developments and future opportunities for the treatment of cancer. Pharmacology & therapeutics 2017;179:127–41 [DOI] [PubMed] [Google Scholar]
- 57.Taya M, Hammes SR. Glycoprotein Non-Metastatic Melanoma Protein B (GPNMB) and Cancer: A Novel Potential Therapeutic Target. Steroids 2018;133:102–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.