Abstract
Background
Rattus rattus is a widely distributed, invasive species that presents an important role in disease transmission, either directly or through vector arthropods such as fleas. These black rats can transmit a wide variety of pathogens, including bacteria of the genus Bartonella, which can cause diseases in humans and animals. In Chile, no data are available identifying fleas from synanthropic rodents as Bartonella vectors. The aim of this study was to investigate the prevalence of Bartonella spp. in the fleas of R. rattus in areas with different climate conditions and featuring different human population densities.
Methods
In all, 174 fleas collected from 261 R. rattus captured from 30 localities with different human densities (cities, villages, and wild areas) across five hydrographic zones of Chile (hyper-arid, arid, semi-arid, sub-humid, and hyper-humid) were examined. Bartonella spp. presence was determined through polymerase chain reaction, using gltA and rpoB genes, which were concatenated to perform a similarity analysis with BLAST and phylogenetic analysis.
Results
Overall, 15 fleas species were identified; Bartonella gltA and rpoB fragments were detected in 21.2% (37/174) and 19.5% (34/174) of fleas, respectively. A total of 10 of the 15 fleas species found were positive for Bartonella DNA. Leptopsylla segnis was the most commonly collected flea species (n = 55), and it also presented a high prevalence of Bartonella DNA (P% = 34.5%). The highest numbers of fleas of this species were collected in villages of the arid zone. There were no seasonal differences in the prevalence of Bartonella DNA. The presence of Bartonella DNA in fleas was recorded in all hydrographic areas, and the arid zone presented the highest prevalence of this species. Regarding areas with different human densities, the highest prevalence was noted in the villages (34.8% gltA and 31.8% rpoB), followed by cities (14.8% gltA and 11.1% rpoB) and wild areas (7.4% gltA and 14.8% rpoB). The BLAST analysis showed a high similitude (>96%) with four uncharacterized Bartonella genotypes and with two species with zoonotic potential: B. mastomydis and B. tribocorum. The phylogenetic analysis showed a close relationship with B. elizabethae and B. tribocorum. This is the first study to provide evidence of the presence of Bartonella in fleas of R. rattus in Chile, indicating that the villages and arid zone correspond to areas with higher infection risk.
Keywords: Infection, Infectious diseases, Molecular epidemiology, Fleas, Rodent, Chile, Anthropogenic effect, Public health, Diseases, Ectoparasites
Introduction
Bartonella spp. are vector-borne bacteria that have been identified in a wide range of mammals (Breitschwerdt, 2017). Among these, rodents are described as important reservoirs of Bartonella (Ying et al., 2002; Favacho et al., 2015; Gonçalves et al., 2016). Of the 45 species named to date, 35 have been registered in rodents and/or fleas, of which 13 have been identified as potentially pathogenic to humans (Chomel et al., 2009; Jiyipong et al., 2014; Alsarraf et al., 2017), and five have been implicated in different infections in humans (Daly et al., 1993; Kosoy et al., 2003; Serratrice et al., 2003; Fenollar, Sire & Raoult, 2005; Buffet, Kosoy & Vayssier-Taussat, 2013).
Although reports of human transmission are not frequent, some recent studies support the possibility that rodent-associated Bartonella species may be responsible for human infections, especially in areas where humans and rats are in close contact; these infections are most prevalent in homeless people and are more likely to be contracted while engaging in outdoor activities (Kosoy et al., 2008, 2010; Ying et al., 2012). In several of these infection cases, fleas were recognized as the vectors or potential vectors of these bacteria (Chomel et al., 2009); as such, fleas are believed to play a key role in maintaining the Bartonella species in rodents (Buffet, Kosoy & Vayssier-Taussat, 2013; Billeter et al., 2014), although the role they could play in human infections is unknown.
Rattus rattus (black rat) has been identified as a Bartonella reservoir in different areas of the world (Ellis et al., 1999; Hsieh et al., 2010; Pangjai et al., 2014; Bai et al., 2009; Gonçalves et al., 2016; Peterson et al., 2017). Rattus rattus is widely distributed in most areas of the world due to human movement (Krystufek et al., 2016) and it has been cataloged as the most harmful invasive species in the world, as it has caused the extinction and displacement of several species of birds and mammals; it is also considered one of the main disease vectors for humans and wild animals (Banks & Hughes, 2012; Harris, 2009; Towns, Atkinson & Daugherty, 2006). The latter fact is due to the rat’s close contact with humans, as they live in cities and rural areas, and have been able to colonize wild environments, interacting with native species (Lobos, Ferres & Palma, 2005). Thus, the Bartonella species in R. rattus may be the result of a host switching between native species (Ellis et al., 1999).
In Chile, only five Bartonella species have been described in domestic animals and humans: B. rochalimae in the human flea Pulex irritans (Pérez-Martínez et al., 2009), B. koehlerae, B. clarridgeiae, and B. henselae in cat blood (Ferrés et al., 2005; Zaror et al., 2002; Müller et al., 2017); these last two species have also been detected in cat fleas (Pérez-Martínez et al., 2009), and no species have been detected in rodents and/or the fleas associated with them. In humans, there are reports of a high prevalence of B. henselae in children, veterinarians, and zookeepers (Ferrés et al., 2005; Troncoso et al., 2016), and reports of infection-related diseases with B. henselae and B. quintana in people (Uribe et al., 2012; Sandoval et al., 2014; Zepeda et al., 2016; Arce, González & Madrid, 2017).
Chile presents a contrasting diversity of climates due to its long extension (between −35.675148 and −71.5429688), with regions ranging from deserts to rainforests (CONAMA, 2008). Rattus rattus has been able to colonize many of these environments (Lobos, Ferres & Palma, 2005; Iriarte, 2007), which present seasonal changes that can affect the presence and density of hosts and vectors, and can impact the prevalence of Bartonella (Telfer et al., 2007; Friggens et al., 2010). Due to the close contact that black rats maintain with humans and wild species; the high number of flea species described for rats in Chile (12 species; Beaucournu, Moreno & González-Acuña, 2014), and how the fleas that parasitize them can act as potential Bartonella vectors; as well as the scarce knowledge that exists about Bartonella in Chile associated with human synanthropic rodents, we investigated the prevalence of Bartonella spp. in R. rattus fleas in areas characterized by different human population densities throughout the different hydrographic zones and seasons in Chile.
Materials and Methods
Sample localities and rodent-trapping procedure
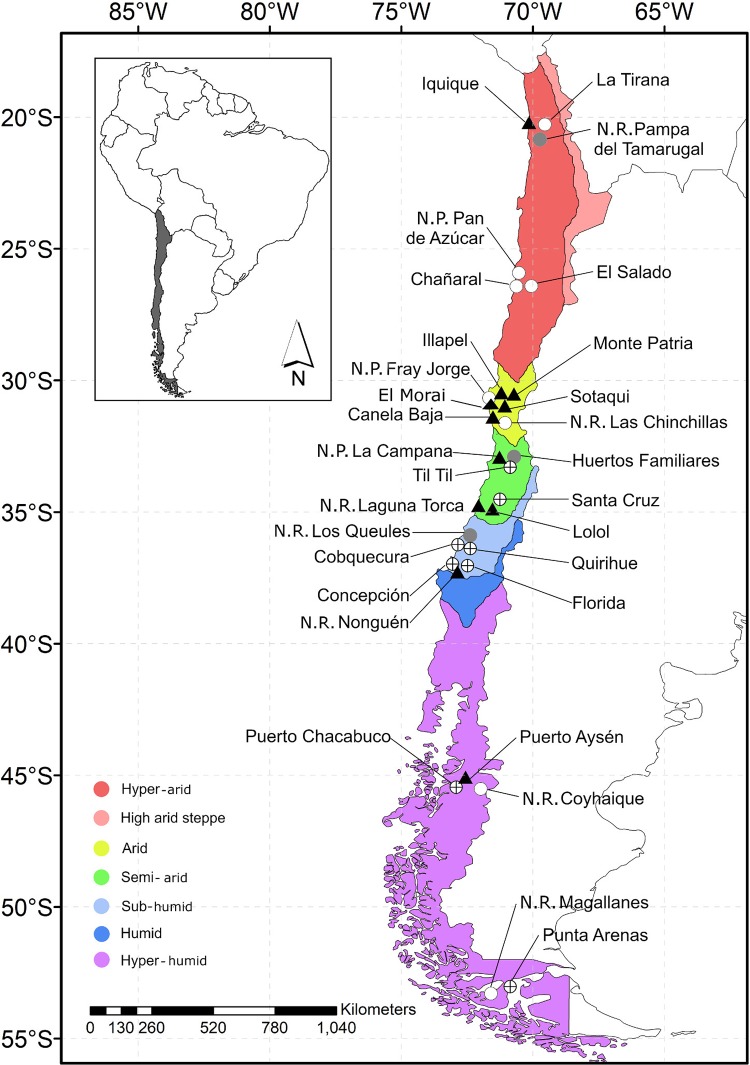
This study surveyed rodents and fleas in 30 localities (cities, n = 10; village, n = 10; wild areas, n = 10) of five hydrographic zones (hyper-arid, arid, semi-arid, sub-humid, and hyper-humid, between −20.2167 and −53.1667 lat.; Fig. 1) of Chile from December 2015 to January 2018. This study took place during austral summer (December to February) and austral winter (July and September), except in the hyper-arid and hyper-humid hydrographic zones, which were visited only in the winter and summer, respectively. The sample localities were chosen based on the following demographic characteristics: (1) City: an urban entity with more than 5,000 inhabitants; (2) Village: an urban entity with a population ranging from 2,001 to 5,000 inhabitants or between 1,001 and 2,000 people and met the economic activity requirement (INE, 2005); and (3) wild area: a protected area without human settlement; for the latter, permission was requested from the Corporación Nacional Forestal (CONAF N°018-2015).
Figure 1. Map of Chile indicating the location of the study sites.
Each data point indicates sample locality. Gray circle: locality featuring rats without fleas; circle with a cross: locality featuring rats with fleas; black triangle: locality featuring fleas that tested positive for Bartonella DNA; white circle: locality without rats.
The rodents were captured using live cage traps baited with oats. Each locality was sampled for two consecutive nights. In each sampling locality, the traps were placed in four parallel lines (approximately 100 m from each other) and each line was equipped with 50 traps (with a distance of 10 m between each other), with a total sampling effort of 12,000 traps per night. The rodents were removed from the traps according to standard techniques (Mills et al., 1995). Each animal was identified using the description by Iriarte (2007), anesthetized with ketamine:xylazine (1:1), and euthanized by cervical dislocation (American Veterinary Medical Association, 2013). The carcasses were placed in individual bags with 95% alcohol and transported to the laboratory. Animal use was conducted in accordance with the protocols for field and laboratory rodent studies (Herbreteau et al., 2011), and the protocols were approved by the Comité de Ética de la Vicerrectoría de Investigación y Desarrollo de la Universidad de Concepción.
Fleas: sample collection
Each rodent was placed on a white plastic basin and the fleas were collected immediately in the field. The rodents’ fur was thoroughly brushed with a toothbrush and the fleas were collected by hand or with forceps, and stored individually in sterile cryovials with 95% ethanol. Later, in the laboratory, the carcasses were checked to verify that all fleas had been collected. For each rodent, the total number of fleas extracted was recorded (abundance) and with this data, the mean intensity of infection (the number of fleas collected from all species/number of infested hosts), the mean abundance (MA) of infection (the number of collected fleas from all species/total number of hosts), and prevalence (the number of infected hosts) were calculated.
DNA extraction
For DNA extraction, every flea was washed and cut between the third and fourth abdominal tergites with a sterile scalpel. The material used to handle the fleas was sterilized between each sample. DNA was extracted using a commercial kit (Qiagen®, Hilden, Germany) according to the manufacturer’s instructions. The incubation time was 5 h, after which point a final elution step was performed using 200 μL of AE buffer and stored at −20 °C.
Following the DNA extraction, the fleas’ exoskeletons were recovered and stored in 96% ethanol; there were subsequently mounted for fleas’ species identification. DNA contamination was monitored by an extraction control using distilled water, every 10 samples.
PCR amplification of gltA and rpoB genes
The presence of Bartonella was screened using the citrate synthase (gltA) and RNA polymerase beta-subunit (rpoB) genes. The primers used for DNA amplification and sequencing in this study were designed from a partial gltA and rpoB gene sequence of B. tribocorum (GenBank code: AM260525.1; Table 1).
Table 1. Primer sequences used for PCR amplifications.
| Name | Primer | Product length (bp) |
|---|---|---|
| BaGlta_F | TCTACGGTACGTCTTGCTGGATCA | 201 |
| BaGlta_R | GCCCATAAGGCGGAAAGGATCATT | 201 |
| BaRpoB_F | CGCGCGATCATGTTGATTTGATGG | 159 |
| BaRpoB_R | ATGGTGCTTCAGCACGTACAAGAG | 159 |
Note:
F, forward; R, reverse.
For the amplification of the gltA gene fragment, the polymerase chain reaction (PCR) program was modified with an initial denaturation for 5 min at 95 °C, followed by 40 cycles (95 °C for 30 s, 56.2 °C for 30 s, and 72 °C for 30 s), and a final extension step at 72 °C for 5 min. For the rpoB gene, the PCR was started by denaturation for 5 min in 95 °C, followed by 40 cycles (95 °C for 30 s, 56.6 °C for 30 s, and 72 °C for 30 s), and a final extension step at 72 °C for 5 min. Reactions were performed in 26 μL of mixture containing GoTaq® Green Master Mix (Promega Corporation, Madison, WI, USA) 2× 12.5 μL + 5.5 μL of free ultrapure water nuclease + two μL of forward primer (10 μM) + two μL of reverse primer (10 μM) + four μL of DNA sample. Negative controls for the PCR consisted of a blank DNA extraction and distilled water was added to the PCR mix instead of DNA. Positive control was the genomic DNA of Bartonella henselae (Vircell Microbiologist, Granada, Spain). PCR products were subjected to electrophoresis on 1% agarose gel at a voltage of 100 V. Then, the PCR products from positive samples were sequenced by Macrogen Company (Seoul, South Korea).
Sequencing, BLAST, and phylogenetic analysis
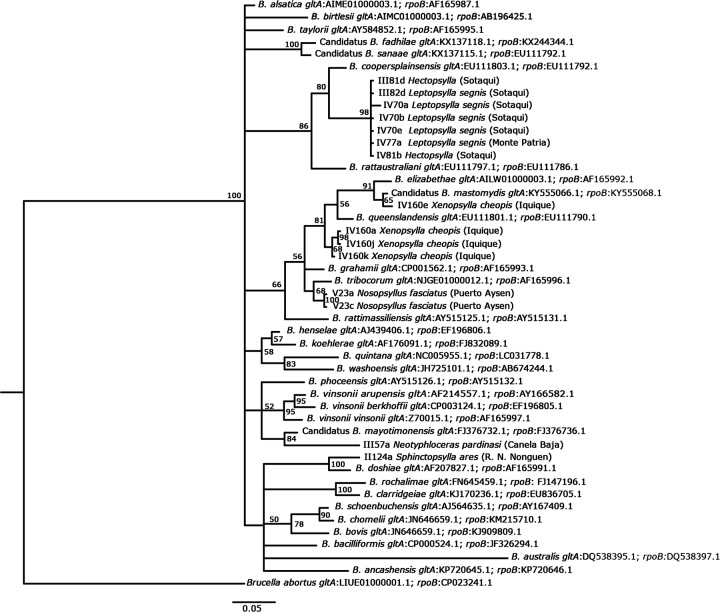
The DNA sequences used in this study and the known Bartonella species retrieved from GenBank were aligned using Codon Code Aligner (Codon Code Corporation; Files S1 and S2). The sequencing data of gltA and rpoB were concatenated and compared with the sequences of Bartonella available in GenBank using the nucleotide–nucleotide BLAST (blastn) program (see http://www.ncbi.nlm.nih.gov/BLAST/). A substitution saturation test with DAMBE (Xia, 2017) was performed, showing that the sequences were not saturated (Xia et al., 2003; Xia & Lemey, 2009). We used MEGA7 (Kumar, Stecher & Tamura, 2016) to calculate the genetic distances between sequences. A tree with Bayesian probabilities was computed using MrBayes 3.2 (Ronquist et al., 2012) based on concatenated gltA (142 bp) and rpoB (95 bp) gene fragments, using Brucella abortus as an outgroup (Accession number, gltA: LIUE01000001.1; rpoB: CP023241.1). The GTR substitution model was used to reconstruct the tree and perform 10,000,000 bootstrap trials. Haplotype diversity (Hd), segregating sites (S), and nucleotide diversity (π) were calculated using DNAsp 6. The accession numbers of the GenBank sequences used to reconstruct the tree are detailed in Fig. 2.
Figure 2. Phylogenetic tree of Bartonella, as based on concatenated gltA and rpoB genes using a GTR substitution model.
The phylogenetic tree was constructed using a Bayesian method. Brucella abortus was included as an outgroup. Bootstrap values were calculated with 10,000,000 replicates. The corresponding accession number for each genotype is indicated below each species of Bartonella. The flea species from which Bartonella DNA was detected is indicated, and the locality and the location from where it was collected is noted in parentheses.
Mounting fleas
Fleas were mounted on glass slides using conventional procedures (Hastriter & Whiting, 2003). Fleas were identified at the species level using the taxonomic keys and the descriptions of Hopkins & Rothschild (1956, 1962, 1966), Smit (1987), Schramm (1987), Beaucournu, Torres Mura & Gallabdo (1988), Beaucournu & Gallardo (1988), Beaucournu & Kelt (1990), Beaucournu, Moreno & González-Acuña (2011), Sánchez et al. (2012), and Sánchez & Lareschi (2014). Voucher specimens of each flea species were deposited in the specimen repository of the Museo de Zoología, Universidad de Concepcion, Concepción, Chile (MZU-CCCC-46329–46336).
Data analysis
The prevalence (P%), MA, and mean infestation intensity (MI) of fleas was calculated according to Rózsa, Reiczigel & Majoros (2000) and were compared between seasons, hydrographic zones, and areas with different population densities using chi-squared and Fisher’s exact tests to compare prevalence, while a Bootstrap t-test with 2,000 replications was used to compare MA and MI. The Clopper–Pearson test was used to calculate the confidence interval (CI) for prevalence, and a Bootstrap test was used to calculate the CI for MI and MA. Bartonella prevalence (percentage of infected fleas) was calculated based on the PCR results. The associations between Bartonella infection and hydrographic zone, human density, and season were evaluated using the chi-squared test, and for small sample sizes, F-Fisher was used. A P-value less than 0.05 was considered to be statistically significant. Statistical analyses were performed using the Quantitative Parasitology software (QP 3.0; Rózsa, Reiczigel & Majoros, 2000).
Nucleotide sequence accession numbers
The sequences of Bartonella gene fragments generated in this study were deposited in the NCBI GenBank database under the following accession numbers: gltA: MK720786–MK720800, and rpoB: MK720801–MK720815.
Results
A total of 261 R. rattus (summer: n = 139; winter: n = 122) were collected in 21 of the 30 localities (city: n = 149; village: n = 53; wild area: n = 59) and 31% (n = 81) of the black rats were positive for fleas. A total of 174 fleas were collected (winter: n = 99; summer: n = 75), representing 15 species from 10 different genera and seven families (Table 2). Ctenoparia jordani Smit, 1955; Neotyphloceras chilensis (Jordan, 1936); Neothphloceras pardinasi Sánchez & Lareschi, 2014; Delostichus smiti Jameson & Fulk, 1977; Tetrapsyllus rhombus Smit, 1955; Plocopsylla wolffsohni (Rothschild, 1909); and Sphinctopsylla ares (Rothschild, 1911) are new records for R. rattus in Chile. The MI was 2.18 (range: 1–15) fleas per black rat. In nine cities, between one and five species of fleas were collected, in eight villages between one and four, and in three wild areas between three and four species of fleas. The hydrographic zones (i.e., hyper-arid and hyper-humid zones) demonstrated the lowest richness of flea species, with one and three species, respectively, while the semi-arid and sub-humid zones presented eight species each. The flea species L. segnis (Schönherr, 1811) (n = 55) and Nosopsyllus fasciatus (Bosc d’Antic, 1800) (n = 45) were the most abundant species in villages and cities, respectively, and were not found in wild area. Two species of fleas were found only in wild areas: D. coxalis (Rothschild, 1909) and C. jordani. Hectopsylla sp. and Neotyphloceras chilensis were collected only in villages. Ctenoparia inopinata Rothschild, 1909, D. smiti, and Xenopsylla cheopis Glinkiewicz, 1907 were exclusive to cities. Neotyphloceras pardinasi and S. ares were found in cities, villages, and wild areas (Table 2).
Table 2. Detection of Bartonella DNA from fleas collected on Rattus rattus from different seasons and locality types.
| Family | Specie of flea | Total of fleas analyzed by seasons | Total of fleas analyzed by type of locality | Number of fleas positive for gene fragment | ||||
|---|---|---|---|---|---|---|---|---|
| Summer | Winter | City | Village | Reserve | gltA | rpoB | ||
| Pulicidae | Xenopsylla cheopis | 0 | 11 | 11 | 0 | 0 | 7 (63.6) | 5 (45.5) |
| Leptopsyllidae | Leptopsylla segnis | 22 | 33 | 19 | 36 | 0 | 19 (34.5) | 15 (27.3) |
| Ceratophyllidae | Nosopsyllus fasciatus | 19 | 26 | 33 | 12 | 0 | 4 (8.9) | 4 (8.9) |
| Hectopsyllidae | Hectopsylla sp. | 3 | 0 | 0 | 3 | 0 | 3 (100) | 2 (66.7) |
| Hystrichopsyllidae | Ctenoparia inopinata | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Ctenoparia jordani | 0 | 1 | 0 | 0 | 1 | 0 | 0 | |
| Neotyphloceras sp. | 4 | 0 | 1 | 3 | 0 | 0 | 1 (25.0) | |
| Neotyphloceras chilensis | 2 | 0 | 0 | 2 | 0 | 1 (50.0) | 1 (50.0) | |
| Neotyphloceras pardinasi | 14 | 9 | 9 | 6 | 8 | 1 (4.3) | 3 (13.0) | |
| Rhopalopsyllidae | Delostichus coxalis | 6 | 0 | 0 | 0 | 6 | 0 | 1 (16.7) |
| Delostichus smiti | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Tetrapsyllus rhombus | 1 | 2 | 0 | 1 | 2 | 1 (33.3) | 0 | |
| Stephanocircidae | Plocopsylla sp. | 0 | 2 | 1 | 0 | 1 | 0 | 0 |
| Plocopsylla wolffsohni | 0 | 1 | 0 | 1 | 0 | 0 | 0 | |
| Sphinctopsylla ares | 3 | 13 | 5 | 2 | 9 | 1 (6.2) | 2 (12.5) | |
| Total | 75 | 99 | 81 | 66 | 27 | 37 (21.2) | 34 (19.5) | |
Although the number of captured rodents (n = 149) and collected fleas (n = 111) was higher in cities than in villages (rodents: n = 53; fleas: n = 96) and wild areas (rodents: n = 59; fleas: n = 38), the prevalence of fleas was significantly higher in the villages (45.3%, chi-squared = 6.679, df = 2, P = 0.035), while wild areas (30.5%) and cities (26.2%) did not show significant differences (chi-squared = 0.399, df = 1, P = 0.528). The MI was higher in villages (MI = 2.83), than in cities (MI = 2.26) and wild areas (MI = 1.89), but no statistically significant differences were found (Bootstrap P-value (two-sided) > 0.05). The MA was also higher in villages (MA = 1.28) than in the wild area (MA = 0.57) and cities (MA = 0.40; Bootstrap t-test P < 0.05), but there were no significant differences between cities and wild areas (Bootstrap t-test P = 0.9360; Table 3).
Table 3. Prevalence, mean abundance, and mean intensity of fleas, as well as the prevalence of Bartonella DNA from black rats captured from five hydrographic zones and 21 localities in Chile.
| Hydrographic zone | Locality | Number rodent | % Prevalence of fleas [95% CI] | Abundance mean of fleas [95% CI] | Intensity mean of fleas [95% CI] | Number of fleas analyzed | Number of fleas positive to gltA (%) | Number of fleas positive rpoB (%) |
|---|---|---|---|---|---|---|---|---|
| Hyper-arid | IquiqueC | 2 | 50.0 [0.01–0.99] | 5.5 [0.00–5.50] | 11.0* | 11 | 7 (63.6) | 5 (71.4) |
| N.P. Pampa del TamarugalW | 10 | 0.0 | 0.0 | – | 0 | 0 (0.0) | 0 (0.0) | |
| Arid | IllapelC | 17 | 76.5 [0.50–0.93] | 3.2 [1.53–4.00] | 4.2 [2.15–5.00] | 33 | 2 (6.1) | 1 (3.0) |
| Monte PatriaC | 14 | 42.9 [0.13–0.65] | 0.9 [0.14–1.14] | 2.0 [1.00–2.20] | 7 | 1 (14.3) | 1 (14.3) | |
| El MoraiV | 8 | 25.0 [0.03–0.65] | 0.5 [0.00–1.50] | 2.0 [1.00–2.00] | 4 | 0 (0.0) | 0 (0.0) | |
| Canela BajaV | 6 | 50.0 [0.12–0.88] | 1.8 [0.17–4.67] | 3.7 [1.00–5.67] | 8 | 2 (25.0) | 7 (87.5) | |
| SotaquíV | 14 | 85.7 [0.57–0.98] | 4.8 [2.57–7.21] | 5.6 [3.25–8.17] | 40 | 21 (52.5) | 13 (32.5) | |
| Semi-arid | Til TilC | 3 | 33.3 [0.01–0.91] | 0.3 [0.00–0.67] | 1.0* | 1 | 0 (0.0) | 0 (0.0) |
| Santa CruzC | 5 | 20.0 [0.01–0.72] | 0.2 [0.00–0.40] | 1.0* | 0 | 0 (0.0) | 0 (0.0) | |
| Huertos FamiliaresV | 2 | 0.0 | 0.0 | – | 0 | 0 (0.0) | 0 (0.0) | |
| LololV | 1 | 100.0 [0.25–1.00] | 4.0* | 4.0* | 4 | 0 (0.0) | 1 (25.0) | |
| N.P. La CampanaW | 22 | 22.7 [0.08–0.45] | 0.6 [0.18–1.14] | 2.6* | 13 | 1 (7.7) | 1 (7.7) | |
| N.R. Laguna TorcaW | 2 | 50.0 [0.01–0.99] | 2.5 [0.00–2.50] | 5.0* | 5 | 0 (0.0) | 2 (40.0) | |
| Sub-humid | QuirihueC | 77 | 13.0 [0.06–0.22] | 0.2 [0.09–0.40] | 1.6 [1.10–2.40] | 15 | 0 (0.0) | 0 (0.0) |
| ConcepciónC | 22 | 22.7 [0.08–0.45] | 0.4 [0.09–0.77] | 1.6 [1.00–2.20] | 8 | 0 (0.0) | 0 (0.0) | |
| CobquecuraV | 1 | 100.0 [0.02–1.00] | 2.0* | 2.0* | 2 | 0 (0.0) | 0 (0.0) | |
| FloridaV | 18 | 22.2 [0.64–0.48] | 0.3 [0.06–0.78] | 1.5 [1.00–2.00] | 6 | 0 (0.0) | 0 (0.0) | |
| N.R. NonguénW | 25 | 48.0 [0.28–0.69] | 0.8 [0.44–1.36] | 1.7 [1.17–2.58] | 9 | 1 (11.1) | 1 (11.1) | |
| Hiper-humid | Puerto AysénC | 4 | 25.0 [0.01–0.80] | 1.5 [0.0–3.0] | 6.0* | 5 | 2 (40.0) | 2 (40.0) |
| Punta ArenasC | 5 | 20.0 [0.01–0.72] | 0.2 [0.0–0.4] | 1.0* | 1 | 0 (0.0) | 0 (0.0) | |
| Puerto ChacabucoV | 3 | 33.3 [0.01–0.91] | 0.7 [0.0–1.33] | 2.0* | 2 | 0 (0.0) | 0 (0.0) |
Notes:
C, city; V, village; W, wild area; N.P., national park; N.R., national reserve.
One specimen of R. rattus was captured or only one individual was positive to fleas, the confidentiality intervals could not be determined.
Seasonally, the prevalence, MA, and MI of fleas were higher in winter (P% = 34.4%, MA = 0.90, MI = 2.62) than in summer (P% = 28.1%, MA = 0.57, MI = 2.05), but no statistically significant differences were found between seasons (P%: chi-squared statistic = 1.231, df = 1, P = 0.267; MA: Bootstrap t-test P = 0.0870; MI: Bootstrap t-test P = 0.1340; Table 4). In winter, only prevalence was significantly higher in the villages (P% = 57.89%, MA = 1.789, MI = 3.091) than in the cities (P% = 28.8%, MA = 0.881, MI = 3.059; P%: chi-squared statistic = 5.282, df = 1, P = 0.022; AM: Bootstrap t-test P = 0.0755; MI: Bootstrap t-test P = 0.9660) and wild areas (P% = 31.8%, MA = 0.545, MI = 1.714; P%: chi-squared statistic = 3.77, df = 1, P = 0.052; MA: Bootstrap t-test P = 0.0155; MI: Bootstrap t-test P = 0.0360). Regarding the hydrographic zones, in winter, the highest prevalence, mean intensity, and MA were found in the arid zone (P% = 64.3%, MA = 2.79, MI = 4.33), and these values were significantly different from those of the sub-humid zone (P% = 27.7%, MA = 2.78, MI = 1.78; P%: chi-squared statistic = 11.045, df = 1, P = 0.001; MA: Bootstrap t-test P = 0.011; MI: Bootstrap t-test P = 0.038), while with the semi-arid zone, the prevalence and MA differed significantly (P%: chi-squared statistic = 5.841, df = 1, P = 0.016; MA: Bootstrap t-test P = 0.0235), but this was not the case for mean intensity (Bootstrap t-test P = 0.22). There was no significant difference between the sub-humid (P% = 27.7%, MA = 0.492, MI = 1.78) and semi-arid zones (P% = 27.8%, MA = 0.788, MI = 2.8; P%: Fisher’s exact test P = 1.00; MA: Bootstrap t-test P = 0.467, MA: Bootstrap t-test P = 0.254).
Table 4. Prevalence of the Bartonella species in fleas collected from different localities and seasons in Chile.
| Season | Type of locality | Number rodent collected | % Prevalence of fleas [95% Cl] | Intensity mean [95% Cl] | Number fleas analyzed | Number of fleas positive to gltA (%) | Number of fleas positive rpoB (%) |
|---|---|---|---|---|---|---|---|
| Winter | City | 59 | 28.81 [0.18–0.42] | 3.06 [2.12–4.13] | 49 | 9 (18.36) | 6 (12.24) |
| Village | 19 | 57.89 [0.33–0.80] | 3.09 [2.27–3.91] | 33 | 15 (45.45) | 6 (18.18) | |
| Wild area | 44 | 31.80 [0.19–0.47] | 1.77 [1.00–2.57] | 17 | 2 (11.76) | 3 (17.65) | |
| Total | 122 | 34.43 [0.26–0.43] | 2.62 [2.10–3.31] | 99 | 26 (26.26) | 15 (15.15) | |
| Summer | City | 90 | 24.44 [0.16–0.35] | 1.64 [1.27–2.23] | 32 | 3 (9.37) | 3 (9.37) |
| Village | 34 | 38.20 [0.22–0.56] | 2.62 [1.77–3.62] | 33 | 8 (24.24) | 15 (44.45) | |
| Wild area | 15 | 26.67 [0.08–0.55] | 2.50 [1.00–3.00] | 10 | 0 | 1 (10) | |
| Total | 139 | 28.06 [0.21–0.36] | 2.05 [1.64–2.49] | 75 | 11 (14.67) | 19 (25.33) | |
In the summer, there were no significant differences in the prevalence (chi-squared statistic = 2.341, df = 2, P = 0.310), MA (Bootstrap t-test P = 0.077), and MI (Bootstrap t-test P = 0.089) of fleas between cities, villages, and wild areas (Table 4). The arid zone showed a higher prevalence, MA, and MI (P% = 54.8%, MA = 1.645, MI = 3.00) than the semi-arid (P% = 23.5%, MA = 0.588, MI = 2.51) and sub-humid zones (P% = 17.9%, MA = 0.256, MI = 1.43), although it only differed significantly in terms of prevalence with the semi-arid zone (P%: chi-squared statistic = 4.373, df = 1, P = 0.037; MA: Bootstrap t-test P = 0.045, MI: Bootstrap t-test P = 0.5635), and in terms of prevalence and MA with the sub-humid zone (P%: chi-squared statistic = 14.833, df = 1, P = 0.000; MA: Bootstrap t-test P = 0.0280, MI: Bootstrap t-test P = 0.0605). No significant differences were observed in these parameters between the semi-arid and sub-humid zones (P%: Fisher’s exact test P = 0.733, MA: Bootstrap t-test P = 0.2545, MI: Bootstrap t-test P = 0.2275).
The Bartonella gltA and rpoB fragment was detected in 21.26% (37/174) and 19.54% (34/174) of the fleas, respectively, collected from 22 different R. rattus individuals. Although a higher prevalence of Bartonella was detected using the gltA fragment, this finding was not statistically significant (chi-squared statistic = 0.159, df = 1, P = 0.690). A total of 10 of the 15 flea species found were positive for Bartonella. We observed the highest prevalence in Hectopsylla sp. (100%) and X. cheopis (63%), although the number of flea samples collected from these species was low (Table 2). While L. segnis was the most commonly collected flea (n = 55), it also presented a high prevalence of Bartonella DNA (gltA = 34.54% and rpoB = 27.3%); the highest numbers of fleas of this species were collected in villages in the arid zone (Table 2).
The presence of Bartonella DNA in fleas was recorded in all hydrographic areas, but not in all localities. Of the hydrographic zones with more than 20 fleas analyzed, the arid zone had a greater prevalence of Bartonella DNA (26.1% gltA and 28.3% rpoB) than in the sub-humid zone (2.5% gltA and rpoB) for both genes (gltA: chi-squared = 10.103, df = 1, P = 0.001; rpoB: chi-squared = 11.371, df = 1, P = 0.001), whereas with the semi-arid area, the only difference was found in the prevalence with the gltA gene (4.3% gltA: chi-squared = 5.111, df = 1, P = 0.024; 17.4% rpoB: chi-squared: 1.127, df = 1, P = 0.288). The prevalence of Bartonella DNA in the semi-arid and sub-humid zones did not differ significantly (gltA: Fisher’s exact test P = 1.00; rpoB: Fisher’s exact test P = 0.055). The highest prevalence was noted in the villages (34.8% gltA and 31.8% rpoB), which differed significantly from that in the city (14.81%; chi-squared = 6.039, df = 1, P = 0.014) and the in the wild area (7.41%; chi-squared = 7.34, df = 1, P = 0.007) for the gltA gene, whereas for the rpoB gene, only the villages (31.82%) and cities (11.11%) were significantly different (chi-squared = 9.6, df = 1, P = 0.002). No significant differences were observed between the cities and wild areas (Fisher’s exact test P = 0.733), and between the wild areas and villages (chi-squared = 2.82, df = 1, P = 0.093). In all, 22 rodents carried fleas that were positive for Bartonella. The fleas positive for Bartonella were extracted from 13 village rodents, five city rodents, and four wild-area rodents.
No statistically significant differences (gltA: chi-squared = 3.427, df = 1, P = 0.064; rpoB: chi-squared = 2.814, df = 1, P = 0.093) were observed in the prevalence of Bartonella DNA in fleas between the winter (gltA: 26.26%; rpoB: 15.15%) and summer (gltA: 14.67%; rpoB: 25.33%; Table 4).
The BLAST analyses showed similar findings for eight Bartonella spp. (Table 5). The genetic distances between the findings from GenBank and the sequences obtained in this study are shown in File S3. The phylogenetic analysis showed that concatenated gltA and rpoB sequences could be related to a known Bartonella species (Fig. 2). It was found that Bartonella present in the flea species (Hectopsylla sp. and L. segnis) collected from cities and villages in arid zones are closely related to B. coopersplainsensis. Bartonella DNA detected in X. cheopis collected only in a city from a hyper-arid zone was closely related to B. mastomydis and B. queenslandensis. Bartonella DNA in Nosopsyllus fasciatus was closely related to B. tribocorum. Finally, Bartonella detected in Neotyphloceas pardinasi was related to B. mayotimonensis, while that detected in S. ares was related to B. doshiae. Eight haplotypes were found (Hd = 0.838, S = 60, π = 0.095).
Table 5. Bartonella species detected with BLAST using concatenated gltA and rpoB genes, in the identified flea species collected in Chile.
| Flea species | Bartonella species isolated | BLAST Sequence similarity (%) | GenBank accession number | Locality/hydrographic zone |
|---|---|---|---|---|
| Xenopsylla cheopis | Bartonella sp. B28297 | 100 | KM233489.1 | Iquique/Hyper-arid |
| Bartonella mastomydis | 100 | KY555066.1 | Iquique/Hyper-arid | |
| Leptopsylla segnis | Bartonella sp. 16/40 | 97 | AY584859.1 | Sotaquí/Arid |
| Nosopsyllus fasciatus | Bartonella sp. 16/40 | 97 | AY584859.1 | Sotaquí/Arid |
| Bartonella tribocorum | 100 | HG969192.1 | Puerto Aysén/Hyper-humid | |
| Hectopsylla sp. | Bartonella sp. 16/40 | 96 | AY584859.1 | Sotaquí/Arid |
| Neotyphloceras chilensis | Bartonella sp. (strain C1phy) | 99 | Z70022.1 | Canela Baja/Arid |
| Neotyphloceras pardinasi | Bartonella sp. (strain C1phy) | 99 | Z70022.1 | Canela Baja/Arid |
| Sphinctopsylla ares | Uncultured Bartonella sp. clone LBCE 10781 | 95 | KX270236.1 | Nonguén/Sub-humid |
Discussion
The presence of Bartonella DNA in R. rattus fleas has not been previously reported in Chile; therefore, this is the first study to report on and document the prevalence of this bacteria in fleas over a large spatial scale (−20° to −53° lat.) covering five hydrographic zones with differences in human density. To our knowledge, this is the first report of the detection of Bartonella spp. in several flea species: Neotyphloceras chilensis, Neotyphloceas pardinasi, Neotyphloceras sp., D. coxalis, T. rhombus, S. ares, and Hectopsylla sp., all of which parasitize native rodents of Chile. This indicates that R. rattus is in contact with wild-rodent populations and can act as a reservoir for and facilitator in the dispersion of these fleas—and in the Bartonella species detected. While the presence of Bartonella in X. cheopis, L. segnis, and Nosopsyllus fasciatus confirms the findings made by other authors in other parts of the world (Parola et al., 2003; Winoto et al., 2005; Loftis et al., 2006; Reeves et al., 2007; Li et al., 2007; Tsai et al., 2010; Hornok et al., 2015), the Bartonella reported in these three flea species is also new to Chile. Although it cannot be stated that these flea species are competent vectors of Bartonella, as they may have consumed Bartonella-infected blood from a host with bacteremia, their role as vectors of these bacteria cannot be ruled out; as such, future laboratory tests to verify their competence are necessary (Billeter et al., 2008).
The prevalence of Bartonella in the rodent fleas in our study is within the ranges documented by other authors (2.1–40.5%), values that vary with respect to the geographical area and flea species analyzed (Loftis et al., 2006; Marie et al., 2006; Li et al., 2007; Bitam et al., 2012; Billeter et al., 2014; Dieme et al., 2015; Lipatova et al., 2015). Bartonella DNA was found in several flea species with variations observed in the infection prevalence of Bartonella detected between flea species (4.2–100%). In five flea species collected from R. rattus, Bartonella DNA was not detected, which could be due to the low number of fleas analyzed in these species (between one and four individuals). However, Bartonella DNA prevalence was high in other species that were not abundant in the sample, such as Hectopsylla sp., X. cheopis, and T. rhombus. Each flea species was collected from a single rodent, which could be infected with Bartonella, which would explain the high prevalence. It is unlikely that finding Bartonella DNA in Hectopsylla sp. would pose a risk to human health, because these fleas are neosomatic and females are semipenetrating (Linardi & De Avelar, 2014), they stay attached to the host for long periods of time, representing little chance that it will infect humans. The high prevalence of Bartonella DNA reported in this study for X. cheopis (63.6%) would be within the ranges reported for other parts of the world (Billeter et al., 2011: 95%; Leulmi et al., 2014: 34.7%; Klangthong et al., 2015: 25.8%; Billeter et al., 2013: 59.1%; Dieme et al., 2015: 6.7%). Xenopsylla cheopis is the most frequently occurring and abundant species to isolate from R. rattus (Loftis et al., 2006; Christou et al., 2010; Guernier et al., 2014), and is associated with the transmission of several pathogens to humans (e.g., the plague, endemic murine typhus, helminth parasites; Farhang-Azad, Traub & Baqar, 1985; Bitam et al., 2006; Gárate et al., 2011). Several species of Bartonella have been detected in X. cheopis (B. elizabethae, B. grahamii, B. tribocorum, B. rochalimae, B. rattimassiliensis, B. queenslandensis, and Bartonella sp. 1.1C; Billeter et al., 2008, 2011; Tsai et al., 2010; Dieme et al., 2015), although its competence as a vector has only been determined experimentally for B. elizabethae, which would be eliminated through the feces (McKee et al., 2018). Tetrapsyllus rhombus was another very rare species, but which had a high prevalence of Bartonella DNA; there is no known history of pathogens that this flea species transmits. However, the finding in R. rattus provides evidence of the exchange of fleas between wild and introduced species. Tetrapsyllus rhombus is widely distributed in central and southern Chile, parasitizing 13 species of wild rodents of the families Cricetidae, Octodontidae, and Ctenomyidae (Beaucournu, Moreno & González-Acuña, 2014).
Conversely, fleas that were abundant in R. rattus, such as L. segnis and Nosopsyllus fasciatus, presented significant differences in the prevalence of Bartonella DNA. Few studies have detected Bartonella in L. segnis, with prevalence rates of 0% (0/174), 3% (1/37), and 10% (1/10; Loftis et al., 2006; Li et al., 2007, Hornok et al., 2015). Leptopsylla segnis is widely distributed, attributed to the dispersal of its hosts (rats and mice), as a result of human activity. This species is distributed in temperate zones, although in our study, it was distributed from the arid zone to the hyper-humid zone, with the greatest abundance observed in the arid zone. In our study, we did not find the species in wild areas, while there are records in Chile that indicate a high prevalence in wild species (68–82% in Octodon degus; Burger et al., 2012). The finding of Bartonella DNA in this flea species is important because it is an abundant species. Although this flea has rarely been reported to feed on humans (Li & Xio, 1993), it has the potential to transmit Bartonella through the skin via contamination of infected feces, as with other Bartonellae. Nosopsyllus fasciatus presents an abundance and geographical distribution similar to L. segnis; however, the prevalence of Bartonella DNA in this species was lower, with only 8% of fleas testing positive for the bacteria, although this value is within the ranges recorded by other authors (Parola et al., 2003: 3% (1/26); Zurita, 2018: from 4% to 13%). Nosopsyllus fasciatus and L. segnis exhibit a cosmopolitan distribution and live in temperate environments; in our study, these species were distributed in all zones, except in the hyper-arid zone, presenting with a greater abundance in the arid zone. Nosopsyllus fasciatus spends more time in the nest of its hosts than actually on them (Bitam et al., 2010) and it is fed fewer times per day (two to three times) compared to L. segnis (three to five times; Kunitskaya et al., 1965). This feature may decrease the likelihood with which these fleas acquire bacteria, as the feeding frequency and mobility of the fleas are important factors that influence pathogen transmission (Laudisoit et al., 2014). Although the prevalence of this flea was low and its transmission potential is unknown, it could be acting as a Bartonella reservoir. In addition, it is considered an important flea in public health because it occasionally infects other mammals, including humans (Pratt & Wiseman, 1962). On the other hand, Neotyphloceas pardinasi was another abundant species in this study; this species was described in Argentina as parasites of Sigmodontinae rodents (Sánchez & Lareschi, 2014). Although the prevalence of Bartonella was low in this species, this is the first report of Bartonella DNA in Neotyphloceas pardinasi, and the first record of this flea species in R. rattus in Chile. The differences in prevalence found among the flea species analyzed may be due to the specificity of Bartonella, although more studies are needed to test this hypothesis.
Although the prevalence of Bartonella in fleas detected with the gltA gene was higher than in fleas with rpoB, this finding was not statistically significant. These genes were shown to have high discriminatory power inter-species; however, to be able to validate this species, long fragments are needed. La Scola et al. (2003) propose that newly encountered Bartonella isolates should be considered a new species if a 327-bp gltA fragment shares <96.0% sequence similarity with those of validated species, and if an 825-bp rpoB fragment shares <95.4% sequence similarity with those of validated species. Fragments shorter than those recommended by La Scola et al. were obtained (gltA = 142 bp and rpoB = 95 bp); therefore, we could not determine with certainty if Bartonella corresponds to a new species. Although the gltA and rpoB segments used for this analysis were short, these represent a reliable taxonomic tool for distinguishing between differences among closely related organisms (Birtles & Raoult, 1996).
Phylogenetic analysis based on the concatenated gltA and rpoB gene sequences identified groups close to well-known rat-associated Bartonellae: B. coopersplainsensis, B. mastomydis, B. tribocorum, B. mayotimonensis, and B. doshiae. BLAST analysis of concatenated sequences obtained from X. cheopis revealed 100% similarity with B. mastomydis and Bartonella sp. B28297. Both Bartonella species are found within the B. elizabethae complex (Halliday et al., 2015), but it is unknown whether they are pathogenic for humans. Nosopsyllus fasciatus from the hyper-humid zone harbored a bacterium that was 100% identical to B. tribocorum and the phylogenetic analysis also showed a close relationship with this bacterium. Although this bacterium is associated with rodents and their ectoparasites, it has recently been described as a bacterium with pathogenic potential for humans, since it was isolated in human patients from Thailand (Kosoy et al., 2010) and France, causing acute febrile illnesses and nonspecific symptoms (Vayssier-Taussat et al., 2016). Hectopsylla sp., L. segnis, and Nosopsyllus fasciatus presented between 96% and 97% similarity with Bartonella sp. 16/40 detected in the rodent Apodemus peninsulae in Russia by Mediannikov et al. (2005), although these authors indicated that this species could be new, as it is in an independent and well-isolated clade. In our study, the species was forming a well-differentiated clade, but it was related to B. coopersplainsensis (Fig. 2). Bartonella DNA detected in Neotyphloceras chilensis and Neotyphloceas pardinasi showed 99% similarity with Bartonella sp. C1phy detected in the blood of Phyllotis sp. in Peru. Similar results were found by Cicuttin et al. (2019), who detected Bartonella in Neotyphloceras crackensis Sánchez & Lareschi 2014 in the province of Santa Cruz, Argentina, and which shared 100% similarity with Bartonella C1phy. In the phylogenetic analysis, this Bartonella constitutes a clade with B. mayotimonensis. This bacterium has been recognized as a pathogen for humans and was isolated from the resected aortic valve tissue of a person with infective endocarditis in the US (Lin et al., 2010). S. ares showed a 95% similarity with uncultured Bartonella sp. clone LBCE 10781 detected in Oxymycterus dasytrichus in Brazil (Rozental et al., 2017), and was found to form a monophyletic clade with B. doshiae. This species holds pathogenic potential in humans, since it has been detected in human patients in France who had a history of being bitten by ticks (Vayssier-Taussat et al., 2016).
It is important to know the distribution of pathogens among the different biotic communities, since it implies that certain areas pose a higher risk of infection for humans (Mills & Childs, 1998). In our study, 10 of the 30 sampled locations (four cities, four villages, two wild areas), had fleas that were positive for Bartonella DNA; it was found that villages had a higher prevalence than cities and wild areas, which coincides with the greater MA and prevalence of fleas registered in villages, in addition to the differences in the dominant flea species in the different areas studied. For example, L. segnis, which had the highest prevalence of Bartonella DNA, was present in cities and villages but not in wild areas, and its MA and prevalence was greater in villages. According to our results, this species would constitute an important potential vector of Bartonella, as it is abundant in R. rattus and has a wide distribution in Chile, concentrating its greater abundance in cities and villages of the arid zone. In addition, parasitizing native rodent species were found (Beaucournu, Moreno & González-Acuña, 2014; Burger et al., 2012). Although the prevalence of Bartonella DNA was lower in wild areas, and only parasitic flea species of native Chilean rodents were collected, this result is important because it means that R. rattus could disperse Bartonella species present in fleas from wild areas to rural areas. It also highlights the presence of Bartonella in wild areas, which are used as recreational spaces for people, who may then become exposed to Bartonella infection. These findings suggest that the probability of coming into contact with fleas infected with Bartonella is higher in rural areas than in cities and wild areas.
Our study shows variations in the prevalence of Bartonella in the different hydrographic zones analyzed, and the differences could be associated to both the distribution of flea species and environmental factors. A study conducted on fleas of domestic animals from Tunisia (Zouari et al., 2017) found a higher prevalence of Bartonella in fleas from humid areas, followed by semi-arid, sub-humid, and arid regions; this bacterium was not found in the dry zone, contrary to what was found in our study. During our investigation, we found a higher prevalence of the bacterium in arid, semi-arid, and sub-humid zones. These differences could be explained by the differences in humidity and temperature in these areas, which determine the presence of certain flea species in some areas, affecting the dynamics of the vectors and their survival (Chinga-Alayo et al., 2004). Although in the hyper-arid zone the prevalence of fleas in rodents was low, we found only one species (X. cheopis), and noted that the prevalence of Bartonella DNA was high (6/11; 54%). On the other hand, in our study, a significantly higher prevalence of Bartonella DNA observed in the arid zone, as compared with the other zones, could be linked with the higher prevalence of L. segnis and may also be responsible for the transmission of this pathogen.
Bartonella prevalence did not change between seasons. Although other studies have found seasonal differences in the prevalence of Bartonella in fleas, the authors attributed these differences to changes in the population dynamics of different flea species, as well as to changes in community composition, where the dominant species changes (Telfer et al., 2007). In our study, we did not find significant changes in the composition of flea species, abundance, or prevalence among the seasons analyzed, where the most abundant and prevalent species (L. segnis and Nosopsyllus fasciatus) remained stable. We highlight the high richness of flea species detected in our study compared to other studies (one to five species; Loftis et al., 2006; Marie et al., 2006; Li et al., 2007; Reeves et al., 2007; Tsai et al., 2010), which could be explained due to the wide geographical range considered in our study (20°–53° lat.) and also to the inclusion of wild areas, in contrast to other studies that only include rural areas or cities.
Conclusions
This is the first report on Bartonella DNA among a large number of flea species in rodents that explored a gradient of urbanization across a wide geographic distribution in Chile. This paper adds seven new species to the list of fleas already reported to carry Bartonella. Although the prevalence of Bartonella DNA detected in this study was low, it is important to note that the villages and arid zone were the areas with the highest prevalence. In addition, the flea species that showed the highest Bartonella infection (L. segnis and X. cheopis) are fleas that have a wide distribution worldwide and are abundant in R. rattus. The other flea species collected in R. rattus corresponded to fleas that parasitize native rodents, which would indicate the degree of contact that these synanthropic rodents have with wild rodents, either directly or indirectly through the use of burrows, as they transmit parasites. This indicates that there are several species of Bartonella circulating in wild species. This finding is relevant, as parasite transmission could amplify bacterial infection among wild rodents, also increasing the probability with which infected fleas come into contact with humans in rural and wild areas. The results suggest the need to conduct further studies to verify whether these fleas might be transmitted to humans and cause disease.
Supplemental Information
The first column corresponds to the reference sequences of GenBank and the first row, the samples analyzed from our study.
Acknowledgments
We thank to Nicole Inostroza, Elaine Monalize, and Maria Ignacia Najgle for their collaboration in collecting fleas. English-language editing of this manuscript was provided by Journal Prep Services.
Funding Statement
This work was supported by the National Fund for Scientific and Technological Development (FONDECYT) N° 11150875. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Lucila Moreno Salas conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, approved the final draft.
Mario Espinoza-Carniglia performed the experiments, analyzed the data, prepared figures and/or tables, approved the final draft.
Nicol Lizama Schmeisser performed the experiments, approved the final draft.
L. Gonzalo Torres contributed reagents/materials/analysis tools, approved the final draft, field work.
María Carolina Silva-de la Fuente contributed reagents/materials/analysis tools, approved the final draft.
Marcela Lareschi contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Daniel González-Acuña contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Comité de Ética de la Vicerrectoría de Investigación y Desarrollo de la Universidad de Concepción provided full approval for this research.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field experiments were approved by Corporación Nacional Forestal (CONAF N°018-2015).
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The sequences described here are available at GenBank: MK720786–MK720815. The sequences are also available as a Supplemental File.
Data Availability
The following information was supplied regarding data availability:
Voucher specimens of each new association flea-host were deposited in the specimen repository of the Museo de Zoología, Universidad de Concepcion, Concepción, Chile (accession numbers: MZU-CCCC-46329–46336).
References
- Alsarraf et al. (2017).Alsarraf M, Mohallal E, Mierzejewska E, Behnke-Borowczyk J, Welc-Falęciak R, Bednarska M, Dziewit L, Zalat S, Gilbert F, Behnke J, Bajer A. Description of Candidatus Bartonella fadhilae n. sp. and Candidatus Bartonella sanaae n. sp. (Bartonellaceae) from Dipodillus dasyurus and Sekeetamys calurus (Gerbillinae) from the Sinai Massif (Egypt) Vector-Borne and Zoonotic Diseases. 2017;17(7):483–494. doi: 10.1089/vbz.2016.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Veterinary Medical Association (2013).American Veterinary Medical Association AVMA guidelines for the Euthanasia of animals: 2013 Editions. 2013. https://www.avma.org/KB/Policies/Documents/euthanasia.pdf https://www.avma.org/KB/Policies/Documents/euthanasia.pdf
- Arce, González & Madrid (2017).Arce N, González E, Madrid V. Neurorretinitis asociada a infección por Bartonella henselae: reporte de un caso clínico y revisión de la literatura. Revista Hospital Clínico Universidad de Chile. 2017;28:12–17. [Google Scholar]
- Bai et al. (2009).Bai Y, Kosoy MY, Lerdthusnee K, Peruski LF, Richardson JH. Prevalence and genetic heterogeneity of Bartonella strains cultured from rodents from 17 provinces in Thailand. American Journal of Tropical Medicine and Hygiene. 2009;81(5):811–816. doi: 10.4269/ajtmh.2009.09-0294. [DOI] [PubMed] [Google Scholar]
- Banks & Hughes (2012).Banks PB, Hughes NK. A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildlife Research. 2012;39(1):78. doi: 10.1071/WR11086. [DOI] [Google Scholar]
- Beaucournu, Torres Mura & Gallabdo (1988).Beaucournu J-C, Torres Mura JC, Gallabdo MN. Description de la femelle de Ctenoparia topali Smit, 1963 et clef dichotomique du genre Ctenoparia Rothschild, 1909 (Siphonaptera, Hystrichopsyllidae) Annales de Parasitologie Humaine et Comparée. 1988;63(5):380–383. doi: 10.1051/parasite/1988635380. [DOI] [Google Scholar]
- Beaucournu & Gallardo (1988).Beaucournu J-C, Gallardo MN. Puces nouvelles d’Argentine (Insecta, Siphonaptera) Revue suisse de Zoologie. 1988;95(1):99–112. doi: 10.5962/bhl.part.79641. [DOI] [Google Scholar]
- Beaucournu & Kelt (1990).Beaucournu JC, Kelt DA. Contribution à la faune du Chili: puces nouvelles ou peu connues de la partie sud (Insecta, Siphonaptera) Revue suisse de Zoologie. 1990;97(3):647–668. doi: 10.5962/bhl.part.79755. [DOI] [Google Scholar]
- Beaucournu, Moreno & González-Acuña (2011).Beaucournu J-C, Moreno L, González-Acuña D. Deux espèces nouvelles de puces (Siphonaptera: Ctenophthalmidae & Rhopalopsyllidae) du Chili. Parasite. 2011;18(3):241–246. doi: 10.1051/parasite/2011183241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucournu, Moreno & González-Acuña (2014).Beaucournu J-C, Moreno L, González-Acuña D. Fleas (Insecta Siphonaptera) of Chile: a review. Zootaxa. 2014;3900(2):151–203. doi: 10.11646/zootaxa.3900.2.1. [DOI] [PubMed] [Google Scholar]
- Billeter et al. (2014).Billeter SA, Borchert JN, Atiku LA, Mpanga JT, Gage KL, Kosoy MY. Bartonella species in invasive rats and indigenous rodents from Uganda. Vector-Borne and Zoonotic Diseases. 2014;14(3):182–188. doi: 10.1089/vbz.2013.1375. [DOI] [PubMed] [Google Scholar]
- Billeter et al. (2013).Billeter SA, Colton L, Sangmaneedet S, Suksawat F, Evans BP, Kosoy MY. Short report: molecular detection and identification of Bartonella species in rat fleas from Northeastern Thailand. American Journal of Tropical Medicine and Hygiene. 2013;89(3):462–465. doi: 10.4269/ajtmh.12-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter et al. (2011).Billeter SA, Gundi VAKB, Rood MP, Kosoy MY. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvegicus rats in Los Angeles. Applied and Environmental Microbiology. 2011;77(21):7850–7852. doi: 10.1128/AEM.06012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter et al. (2008).Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Medical and Veterinary Entomology. 2008;22(1):1–15. doi: 10.1111/j.1365-2915.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- Birtles & Raoult (1996).Birtles RJ, Raoult D. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. International Journal of Systematic Bacteriology. 1996;46(4):891–897. doi: 10.1099/00207713-46-4-891. [DOI] [PubMed] [Google Scholar]
- Bitam et al. (2006).Bitam I, Baziz B, Rolain J-M, Belkaid M, Raoult D. Zoonotic focus of Plague, Algeria. Emerging Infectious Diseases. 2006;12(12):1975–1977. doi: 10.3201/eid1212.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitam et al. (2010).Bitam I, Dittmar K, Parola P, Whiting MF, Raoult D. Fleas and flea-borne diseases. International Journal of Infectious Diseases. 2010;14(8):e667–e676. doi: 10.1016/j.ijid.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Bitam et al. (2012).Bitam I, Rolain JM, Nicolas V, Tsai Y-L, Parola P, Gundi VAKB, Raoult D. A multi-gene analysis of diversity of Bartonella detected in fleas from Algeria. Comparative Immunology, Microbiology and Infectious Diseases. 2012;35(1):71–76. doi: 10.1016/j.cimid.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bosc d’Antic (1800).Bosc d’Antic LAG. Description d’une nouvelle espéce de puce (Pulex fasciatus) Bulletín des scíences, par la Société Philomatique, Paris. 1800;2:156. [Google Scholar]
- Breitschwerdt (2017).Breitschwerdt EB. Bartonellosis, one health and all creatures great and small. Veterinary Dermatology. 2017;28(1):96-e21. doi: 10.1111/vde.12413. [DOI] [PubMed] [Google Scholar]
- Buffet, Kosoy & Vayssier-Taussat (2013).Buffet J-P, Kosoy M, Vayssier-Taussat M. Natural history of Bartonella-infecting rodents in light of new knowledge on genomics, diversity and evolution. Future Microbiology. 2013;8(9):1117–1128. doi: 10.2217/fmb.13.77. [DOI] [PubMed] [Google Scholar]
- Burger et al. (2012).Burger JR, Chesh AS, Muñoz P, Fredes F, Ebensperger LA, Hayes LD. Sociality, exotic ectoparasites, and fitness in the plural breeding rodent Octodon degus. Behavioral Ecology and Sociobiology. 2012;66(1):57–66. doi: 10.1007/s00265-011-1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinga-Alayo et al. (2004).Chinga-Alayo E, Huarcaya E, Nasarre C, Del Aguila R, Llanos-Cuentas A. The influence of climate on the epidemiology of bartonellosis in Ancash, Peru. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2004;98(2):116–124. doi: 10.1016/S0035-9203(03)00017-8. [DOI] [PubMed] [Google Scholar]
- Chomel et al. (2009).Chomel BB, Kasten RW, Williams C, Wey AC, Henn JB, Maggi R, Carrasco S, Mazet J, Boulouis HJ, Maillard R, Breitschwerdt EB. Bartonella endocarditis: a pathology shared by animal reservoirs and patients. Annals of the New York Academy of Science. 2009;1166(1):120–126. doi: 10.1111/j.1749-6632.2009.04523.x. [DOI] [PubMed] [Google Scholar]
- Christou et al. (2010).Christou C, Chochlakis D, Toumazos P, Mazeris A, Antoniou M, Ioannou I, Tselentis Y, Psaroulaki A. Rickettsia typhi and Rickettsia felis in Xenopsylla cheopis and Leptopsylla segnis parasitizing rats in Cyprus. American Journal of Tropical Medicine and Hygiene. 2010;83(6):1301–1304. doi: 10.4269/ajtmh.2010.10-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicuttin et al. (2019).Cicuttin G, De Salvo MN, Sanchez J, Cañón C, Lareschi M. Molecular detection of Bartonella in fleas (Hexapoda, Siphonaptera) collected from wild rodents (Cricetidae, Sigmodontinae) from Argentina. Medical and Veterinary Entomology. 2019;8:1117. doi: 10.1111/mve.12370. [DOI] [PubMed] [Google Scholar]
- CONAMA (2008).CONAMA Biodiversidad de Chile, Patrimonio y desafíos. Comisión Nacional del Medio Ambiente, Ocho Libros editors, Santiago de Chile. 2008.
- Daly et al. (1993).Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, Weyant RS, Steigerwalt AG, Weaver RE, Daneshvar MI, O’Connor SP. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. Journal of Clinical Microbiology. 1993;31(4):872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieme et al. (2015).Dieme C, Pagès F, Lagadec E, Raoult D, Tortosa P, Balleydier E, Guernier V, Socolovschi C, Dellagi K, Parola P, Le Minter G. Rickettsia and Bartonella species in fleas from Reunion Island. American Journal of Tropical Medicine and Hygiene. 2015;92(3):617–619. doi: 10.4269/ajtmh.14-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis et al. (1999).Ellis BA, Regnery RL, Beati L, Bacellar F, Rood M, Glass GG, Marston E, Ksiazek TG, Jones D, Childs JE. Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: an old world origin for a new world disease? Journal of Infectious Diseases. 1999;180(1):220–224. doi: 10.1086/314824. [DOI] [PubMed] [Google Scholar]
- Farhang-Azad, Traub & Baqar (1985).Farhang-Azad A, Traub R, Baqar S. Transovarial transmission of murine typhus rickettsiae in Xenopsylla cheopis fleas. Science. 1985;227(4686):543–545. doi: 10.1126/science.3966162. [DOI] [PubMed] [Google Scholar]
- Favacho et al. (2015).Favacho AR, Andrade MN, De Oliveira RC, Bonvicino CR, D’Andrea PS, De Lemos ER. Zoonotic Bartonella species in wild rodents in the state of Mato Grosso do Sul. Brazil Microbes and Infection. 2015;17(11–12):889–892. doi: 10.1016/j.micinf.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Fenollar, Sire & Raoult (2005).Fenollar F, Sire S, Raoult D. Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. Journal of Clinical Microbiology. 2005;43(2):945–947. doi: 10.1128/JCM.43.2.945-947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrés et al. (2005).Ferrés M, Abarca K, Godoy P, García P, Palavecino E, Méndez G, Valdés A, Ernst S, Thibaut J, Koberg J, Chanqueo L, Vial P. Presencia de Bartonella henselae en gatos: cuantificación del reservorio natural y riesgo de exposición humana de esta zoonosis en Chile. Revista Médica de Chile. 2005;133(12):1465–1471. doi: 10.4067/S0034-98872005001200008. [DOI] [PubMed] [Google Scholar]
- Friggens et al. (2010).Friggens MM, Parmenter RR, Boyden M, Ford PL, Gage K, Keim P. Flea abundance, diversity, and plague in gunnison’s prairie dogs (Cynomys gunnisoni) and their burrows in montane grasslands in northern New Mexico. Journal of Wildlife Diseases. 2010;46(2):356–367. doi: 10.7589/0090-3558-46.2.356. [DOI] [PubMed] [Google Scholar]
- Gárate et al. (2011).Gárate I, Jiménez P, Flores K, Espinoza B. Registro de Xenopsylla cheopis como hospedero intermediario natural de Hymenolepis diminuta en Lima, Perú. Revista Peruana de Biología. 2011;18(2):249–252. [Google Scholar]
- Gonçalves et al. (2016).Gonçalves LR, Favacho ARM, Roque ALR, Mendes NS, Junior OLF, Benevenute JL, Herrera EM, D’Andrea PS, De Lemos ERS, Machado RZ, André MR. Association of Bartonella species with wild and synanthropic rodents in different brazilian biomes. Applied and Environmental Microbiology. 2016;82(24):7154–7164. doi: 10.1128/AEM.02447-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guernier et al. (2014).Guernier V, Lagadec E, LeMinter G, Licciardi S, Balleydier E, Pagès F, Laudisoit A, Dellagi K, Tortosa P. Fleas of small mammals on reunion island: diversity, distribution and epidemiological consequences. PLOS Neglected Tropical Diseases. 2014;8(9):e3129. doi: 10.1371/journal.pntd.0003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday et al. (2015).Halliday JEB, Knobel DL, Agwanda B, Bai Y, Breiman RF, Cleaveland S, Kariuki Njenga M, Kosoy M. Prevalence and diversity of small mammal associated Bartonella species in rural and urban Kenya. PLoS Neglected Tropical Diseases. 2015;9(3):e0003608. doi: 10.1371/journal.pntd.0003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris (2009).Harris DB. Review of negative effects of introduced rodents on small mammals on islands. Biological Invasions. 2009;11(7):1611–1630. doi: 10.1007/s10530-008-9393-0. [DOI] [Google Scholar]
- Hastriter & Whiting (2003).Hastriter MW, Whiting MF. Siphonaptera (fleas) In: Resh VH, Carde R, editors. Encyclopedia of Insects. Orlando: Elsevier Science; 2003. pp. 1039–1045. [Google Scholar]
- Herbreteau et al. (2011).Herbreteau V, Jittapalapong S, Rerkamnuaychoke W, Chaval Y, Cosson J-F, Morand S, editors. Protocols for field and laboratory rodent studies. 2011. http://www.ceropath.org/FichiersComplementaires/Herbreteau_Rodents_protocols_2011.pdf http://www.ceropath.org/FichiersComplementaires/Herbreteau_Rodents_protocols_2011.pdf CERoPath project.
- Hopkins & Rothschild (1956).Hopkins G, Rothschild M. An illustrated catalogue of the Rothschild collection of fleas (Siphonaptera) in the British Museum (Natural History). Vol II, Coptopsyllidae, Vermipsyllidea, Stephanocircidae, Ischnopsyllidae, Hypsophthalmidae and Xiphiopsyllidae. London: British Museum (Natural History); 1956. [Google Scholar]
- Hopkins & Rothschild (1962).Hopkins G, Rothschild M. An illustrated catalogue of the Rothschild collection of fleas (Siphonaptera) in the British Museum (Natural History). Vol III, Hystrichopsyllidae (Acedestiinae, Anomiosyllinae, Hystrichopsyllinae, Neopsyllinae, Rhadinopsyllinae and Stenoponiinae) London: British Museum (Natural History); 1962. [Google Scholar]
- Hopkins & Rothschild (1966).Hopkins G, Rothschild M. An illustrated catalogue of the Rothschild collection of fleas (Siphonaptera) in the British Museum (Natural History). Vol IV, Hystrichopsyllidae (Ctenophthalminae, Dinopsyllinae, Doratopsyllinae and Listropsyllinae) London: British Museum (Natural History); 1966. [Google Scholar]
- Hornok et al. (2015).Hornok S, Földvári G, Rigó K, Meli ML, Gönczi E, Répási A, Farkas R, Papp I, Kontschán J, Hofmann-Lehmann R. Synanthropic rodents and their ectoparasites as carriers of a novel haemoplasma and vector-borne, zoonotic pathogens indoors. Parasites & Vectors. 2015;8(1):27. doi: 10.1186/s13071-014-0630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh et al. (2010).Hsieh J-W, Tung K-C, Chen W-C, Lin J-W, Chien L-J, Hsu Y-M, Wang H-C, Chomel BB, Chang C-C. Epidemiology of Bartonella infection in rodents and shrews in Taiwan. Zoonoses and Public Health. 2010;57(6):439–446. doi: 10.1111/j.1863-2378.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- INE (2005).INE . Chile: Ciudades, Pueblos, Aldeas y Caserios. Chile: Instituto Nacional de Estadísticas; 2005. [Google Scholar]
- Iriarte (2007).Iriarte A. Mamíferos de Chile. Santiago: Lynx edicions; 2007. [Google Scholar]
- Jiyipong et al. (2014).Jiyipong T, Jittapalapong S, Morand S, Rolain J-M. Bartonella species in small mammals and their potential vectors in Asia. Asian Pacific Journal of Tropical Biomedicine. 2014;4(10):757–767. doi: 10.12980/APJTB.4.2014C742. [DOI] [Google Scholar]
- Jordan (1936).Jordan K. Some Siphonaptera from South America. Novitates Zoologicae. 1936;39:305–310. [Google Scholar]
- Klangthong et al. (2015).Klangthong K, Promsthaporn S, Leepitakrat S, Schuster AL, McCardle PW, Kosoy M, Takhampunya R. The distribution and diversity of Bartonella species in rodents and their ectoparasites across Thailand. PLOS ONE. 2015;10(10):e0140856. doi: 10.1371/journal.pone.0140856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy et al. (2008).Kosoy M, Morway C, Sheff KW, Bai Y, Colborn J, Chalcraft L, Dowell SF, Peruski LF, Maloney SA, Baggett H, Sutthirattana S, Sidhirat A, Maruyama S, Kabeya H, Chomel BB, Kasten R, Popov V, Robinson J, Kruglov A, Petersen LR. Bartonella tamiae sp. nov., a newly recognized pathogen isolated from three human patients from Thailand. Journal of Clinical Microbiology. 2008;46(2):772–775. doi: 10.1128/JCM.02120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy et al. (2003).Kosoy M, Murray M, Gilmore RD, Bai Y, Gage KL. Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. Journal of Clinical Microbiology. 2003;41(2):645–650. doi: 10.1128/JCM.41.2.645-650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy et al. (2010).Kosoy M, Peruski LF, Maloney SA, Boonmar S, Sitdhirasdr A, Lerdthusnee K, Baggett H, Morway C, Bai Y, Sheff K, Dowell SF, Bhengsri S, Richardson J. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. American Journal of Tropical Medicine and Hygiene. 2010;82(6):1140–1145. doi: 10.4269/ajtmh.2010.09-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystufek et al. (2016).Krystufek B, Palomo L, Hutterer R, Mitsain G, Yigit N. https://www.iucnredlist.org/species/19360/115148682. [13 March 2018]. 2016. https://www.iucnredlist.org/species/19360/115148682 Rattus rattus (errata version published in 2017). The IUCN Red List of Threatened Species 2016: e.T19360A115148682, DOI 10.2305/IUCN.UK.2016-3.RLTS.T19360A15137085.en.
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Molecular Biology and Evolution. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitskaya et al. (1965).Kunitskaya NT, Gauzshtein DM, Kunitsky VN, Rodionov IA, Filimonov VI. Feeding activity of fleas parasitic on the great gerbil in experiments. In: Anonymous, editor. Proceedings of the 4th Scientific Conference on Natural Focality and Prophylaxis of Plague. Alma-Ata, USSR: Kainar; 1965. pp. 135–137. [Google Scholar]
- La Scola et al. (2003).La Scola B, Zeaiter Z, Khamis A, Raoult D. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends in Microbiology. 2003;11(7):318–321. doi: 10.1016/S0966-842X(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Laudisoit et al. (2014).Laudisoit A, Van Houtte N, De Bellocq JG, Akaibe D, Wilschut L, Verheyen E, Amundala N, Socolovschi C, Falay D, Raoult D, Parola P, Breno M. High prevalence of Rickettsia typhi and Bartonella species in rats and fleas, Kisangani, Democratic Republic of the Congo. American Journal of Tropical Medicine and Hygiene. 2014;90(3):463–468. doi: 10.4269/ajtmh.13-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulmi et al. (2014).Leulmi H, Socolovschi C, Laudisoit A, Houemenou G, Davoust B, Bitam I, Raoult D, Parola P. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in Fleas (Siphonaptera) from Africa. PLOS Neglected Tropical Diseases. 2014;8(10):e3152. doi: 10.1371/journal.pntd.0003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2007).Li DM, Liu QY, Yu DZ, Zhang JZ, Gong ZD, Song XP. Phylogenetic analysis of bartonella detected in Rodent fleas in Yunnan, China. Journal of Wildlife Diseases. 2007;43(4):609–617. doi: 10.7589/0090-3558-43.4.609. [DOI] [PubMed] [Google Scholar]
- Li & Xio (1993).Li Z-L, Xio B-L. Observations on the breeding and biological characteristics of Leptopsylla segnis. Endemic Disease Bulletin. 1993;8:26–28. [Google Scholar]
- Lin et al. (2010).Lin EY, Tsigrelis C, Baddour LM, Lepidi H, Rolain JM, Patel R, Roult D. Candidatus Bartonella mayotimonensis and endocarditis. Emerging Infectious Diseases. 2010;16(3):500–503. doi: 10.3201/eid1603.081673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardi & De Avelar (2014).Linardi PM, De Avelar DM. Neosomes of tungid fleas on wild and domestic animals. Parasitology Research. 2014;113(10):3517–3533. doi: 10.1007/s00436-014-4081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova et al. (2015).Lipatova I, Paulauskas A, Puraite I, Radzijevskaja J, Balciauskas L, Gedminas V. Bartonella infection in small mammals and their ectoparasites in Lithuania. Microbes and Infection. 2015;17(11–12):884–888. doi: 10.1016/j.micinf.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Lobos, Ferres & Palma (2005).Lobos G, Ferres M, Palma R. Presencia de los géneros invasores Mus y Rattus en áreas naturales de Chile: un riesgo ambiental y epidemiológico. Revista Chilena de Historia Natural. 2005;78(1):113–124. doi: 10.4067/S0716-078X2005000100008. [DOI] [Google Scholar]
- Loftis et al. (2006).Loftis AD, Reeves WK, Szumlas DE, Abbassy MM, Helmy IM, Moriarity JR, Dasch GA. Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis. American Journal of Tropical Medicine and Hygiene. 2006;75(1):41–48. doi: 10.4269/ajtmh.2006.75.41. [DOI] [PubMed] [Google Scholar]
- Marie et al. (2006).Marie J-L, Fournier P-E, Rolain J-M, Briolant S, Davoust B, Raoult D. Molecular detection of Bartonella quintana, B. elizabethae, B. koehlerae, B. doshiae, B. taylorii, and Rickettsia felis in rodent fleas collected in Kabul, Afghanistan. American Journal of Tropical Medicine and Hygiene. 2006;74(3):436–439. doi: 10.4269/ajtmh.2006.74.436. [DOI] [PubMed] [Google Scholar]
- McKee et al. (2018).McKee CD, Osikowicz LM, Schwedhelm TR, Maes SE, Enscore RE, Gage KL, Kosoy MY. Acquisition of Bartonella elizabethae by experimentally exposed oriental rat fleas (Xenopsylla cheopis; Siphonaptera, Pulicidae) and excretion of Bartonella DNA in flea feces. Journal of Medical Entomology. 2018;55(5):1292–1298. doi: 10.1093/jme/tjy085. [DOI] [PubMed] [Google Scholar]
- Mediannikov et al. (2005).Mediannikov O, Ivanov L, Zdanovskaya N, Vysochina N, Fournier PE, Tarasevich I, Raoult D. Molecular screening of Bartonella species in rodents from the Russian Far East. Annals of the New York Academy of Sciences. 2005;1063(1):308–311. doi: 10.1196/annals.1355.049. [DOI] [PubMed] [Google Scholar]
- Mills & Childs (1998).Mills JN, Childs JE. Ecologic studies of rodent reservoirs: their relevance for human health. Emerging Infectious Diseases. 1998;4(4):529–537. doi: 10.3201/eid0404.980403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills et al. (1995).Mills JN, Yates TL, Childs JE, Parmenter RR, Ksiazek TG, Rollin PE, Peters CJ. Guidelines for working with rodents potentially infected with Hantavirus. Journal of Mammalogy. 1995;76(3):716–722. doi: 10.2307/1382742. [DOI] [Google Scholar]
- Müller et al. (2017).Müller A, Walker R, Bittencourt P, Machado RZ, Benevenute JL, DO Amaral RB, Gonçalves LR, André MR. Prevalence, hematological findings and genetic diversity of Bartonella spp. in domestic cats from Valdivia, Southern Chile. Parasitology. 2017;144(6):773–782. doi: 10.1017/S003118201600247X. [DOI] [PubMed] [Google Scholar]
- Pangjai et al. (2014).Pangjai D, Maruyama S, Boonmar S, Kabeya H, Sato S, Nimsuphan B, Petkanchanapong W, Wootta W, Wangroongsarb P, Boonyareth M, Preedakoon P, Saisongkorh W, Sawanpanyalert P. Prevalence of zoonotic Bartonella species among rodents and shrews in Thailand. Comparative Immunology, Microbiology and Infectious Diseases. 2014;37(2):109–114. doi: 10.1016/j.cimid.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Parola et al. (2003).Parola P, Sanogo OY, Lerdthusnee K, Zeaiter Z, Chauvancy G, Gonzalez JP, Miller RS, Telford SR, III, Wongsrichanalai C, Raoult D. Identification of Rickettsia spp. and Bartonella spp. in from the Thai-Myanmar border. Annals of the New York Academy of Sciences. 2003;990(1):173–181. doi: 10.1111/j.1749-6632.2003.tb07359.x. [DOI] [PubMed] [Google Scholar]
- Peterson et al. (2017).Peterson AC, Ghersi BM, Alda F, Firth C, Frye MJ, Bai Y, Osikowicz LM, Riegel C, Lipkin WI, Kosoy MY, Blum MJ. Rodent-Borne Bartonella infection varies according to host species within and among cities. EcoHealth. 2017;14(4):771–782. doi: 10.1007/s10393-017-1291-4. [DOI] [PubMed] [Google Scholar]
- Pérez-Martínez et al. (2009).Pérez-Martínez L, Venzal JM, González-Acuña D, Portillo A, Blanco JR, Oteo JA. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerging Infectious Diseases. 2009;15(7):1150–1152. doi: 10.3201/eid1507.081570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt & Wiseman (1962).Pratt HD, Wiseman JS. Fleas of public health importance and their control. 1962. Training guide–Insect control series, U. S. Department of Health, Education, and Welfare Public Health Service Communicable Disease. Center Atlanta, Georgia, PHS Publication No. 772.
- Reeves et al. (2007).Reeves WK, Rogers TE, Durden LA, Dasch GA. Association of Bartonella with the fleas (Siphonaptera) of rodents and bats using molecular techniques. Journal of Vector Ecology. 2007;32(1):118–122. doi: 10.3376/1081-1710(2007)32[118:AOBWTF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard M, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild (1909).Rothschild NC. A new flea from Chili. Revista Chilena de Historia Natural. 1909;13(1):104–106. [Google Scholar]
- Rothschild (1911).Rothschild NC. Some new genera and species of Siphonaptera. Novitates Zoologicae. 1911;18:117–122. [Google Scholar]
- Rozental et al. (2017).Rozental T, Ferreira MS, Gutierres A, Mares-Guia MA, Teixeira BR, Goncalves J, Bonvicino CR, D’Andrea PS, De Lemos ER. Zoonotic pathogens in Atlantic Forest wild rodents in Brazil: Bartonella and Coxiella infections. Acta Tropica. 2017;168:64–73. doi: 10.1016/j.actatropica.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Rózsa, Reiczigel & Majoros (2000).Rózsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of hosts. Journal of Parasitology. 2000;86(2):228–232. doi: 10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sánchez et al. (2012).Sánchez J, Amor V, Bazán-León E, Vásquez R, Lareschi M. Redescription of Neotyphloceras chilensis Jordan, new status (Siphonaptera: Ctenophthalmidae: Neotyphloceratini) Zootaxa. 2012;3259:51–57. doi: 10.5281/zenodo.280702. [DOI] [Google Scholar]
- Sánchez & Lareschi (2014).Sánchez J, Lareschi M. Two new species of Neotyphloceras (Siphonaptera: Ctenophthalmidae) from Argentinean Patagonia. Zootaxa. 2014;3784(2):159–170. doi: 10.11646/zootaxa.3784.2.5. [DOI] [PubMed] [Google Scholar]
- Sandoval et al. (2014).Sandoval C, Pinochet C, Peña A, Rabello M, Prado A, Viviani T. Síndrome febril prolongado: un desafío para el infectólogo pediatra. Revista Chilena de Infectología. 2014;31(1):87–91. doi: 10.4067/S0716-10182014000100013. [DOI] [PubMed] [Google Scholar]
- Schönherr (1811).Schönherr CJ. Pulex segnis ny Svensk species. Svenska vetenskaps akadamien Nay handlingar. Kongliga Series. 1811;32:98–101. [Google Scholar]
- Schramm (1987).Schramm BA. A taxonomic revision of the genus Plocopsylla Jordan, 1931 (Siphonaptera: Stephanocircidae) 1987. p. 8591. PhD retrospective theses and dissertations. Iowa State University, Ames, Iowa.
- Serratrice et al. (2003).Serratrice J, Rolain J-M, Granel B, Ene N, Conrath J, Avierinos J-F, Disdier P, Raoult D, Weiller P-J. Bilateral retinal artery branch occlusions revealing Bartonella grahamii infection. La Revue de Medecine Interne. 2003;24(9):629–630. doi: 10.1016/S0248-8663(03)00224-8. [DOI] [PubMed] [Google Scholar]
- Smit (1987).Smit FGAM. An illustrated catalogue of the Rothschild Collection of fleas (Siphonaptera) in the British Museum (Natural History) 7: Malacopsylloidea (Malacopsyllidae and Rhopalopsyllidae) UK: Oxford University; 1987. p. 380. [Google Scholar]
- Telfer et al. (2007).Telfer S, Begon M, Bennett M, Bown KJ, Burthe S, Lambin X, Telford G, Birtle R. Contrasting dynamics of Bartonella spp.in cyclic field vole populations: the impact of vector and host dynamics. Parasitology. 2007;134(Pt3):413–425. doi: 10.1017/S0031182006001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towns, Atkinson & Daugherty (2006).Towns DR, Atkinson IAE, Daugherty CH. Have the harmful effects of introduced rats on islands been exaggerated? Biological Invasions. 2006;8(4):863–891. doi: 10.1007/s10530-005-0421-z. [DOI] [Google Scholar]
- Troncoso et al. (2016).Troncoso I, Fischer C, Arteaga F, Espinoza C, Azócar T, Abarca K. Seroprevalencia de Bartonella henselae en personas con riesgo ocupacional. Revista Chilena de Infectología. 2016;33(3):355–357. doi: 10.4067/S0716-10182016000300019. [DOI] [PubMed] [Google Scholar]
- Tsai et al. (2010).Tsai YL, Chuang ST, Chang CC, Kass PH, Chomel BB. Bartonella species in small mammals and their ectoparasites in Taiwan. American Journal of Tropical Medicine and Hygiene. 2010;83(4):917–923. doi: 10.4269/ajtmh.2010.10-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe et al. (2012).Uribe P, Balcells ME, Giesen L, Cárdenas C, García P, González S. Angiomatosis bacilar por Bartonella quintana como primera manifestación de infección por VIH: Report of one case. Revista Médica de Chile. 2012;140(7):910–914. doi: 10.4067/S0034-98872012000700013. [DOI] [PubMed] [Google Scholar]
- Vayssier-Taussat et al. (2016).Vayssier-Taussat M, Moutailler S, Féménia F, Raymond P, Croce O, La Scola B, Fournier P-E, Raoult D. Identification of novel zoonotic activity of Bartonella spp., France. Emerging Infectious Diseases. 2016;22(3):457–462. doi: 10.3201/eid2203.150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto et al. (2005).Winoto IL, Goethert H, Ibrahim IN, Yuniherlina I, Stoops C, Susanti I, Kania W, Maguire JD, Bangs MJ, Telford SR, Wongsrichanalai C. Bartonella species in rodents and shrews in the greater Jakarta area. Southeast Asian Journal of Tropical Medicine and Public Health. 2005;36(6):1523–1529. [PubMed] [Google Scholar]
- Xia (2017).Xia X. DAMBE6: new tools for microbial genomics, Phylogenetics, and molecular evolution. Journal of Heredity. 2017;108(4):431–437. doi: 10.1093/jhered/esx033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia & Lemey (2009).Xia X, Lemey P. Assessing substitution saturation with DAMBE. In: Lemey P, Vandamme AM, editors. The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny. Second Edition. Cambridge: Cambridge University Press; 2009. pp. 615–630. [Google Scholar]
- Xia et al. (2003).Xia X, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Molecular Phylogenetics and Evolution. 2003;26(1):1–7. doi: 10.1016/S1055-7903(02)00326-3. [DOI] [PubMed] [Google Scholar]
- Ying et al. (2012).Ying B, Kosoy MY, Diaz MH, Winchell J, Baggett H, Maloney SA, Boonmar S, Bhengsri S, Sawatwong P, Peruski LF. Bartonella vinsonii subsp. arupensis in humans. Thailand Emerging Infectious Diseases. 2012;18(6):989–991. doi: 10.3201/eid1806.111750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying et al. (2002).Ying B, Kosoy MY, Maupin GO, Tsuchiya KR, Gage KL. Genetic and ecologic characteristics of Bartonella communities in rodents in southern China. American Journal of Tropical Medicine and Hygiene. 2002;66(5):622–627. doi: 10.4269/ajtmh.2002.66.622. [DOI] [PubMed] [Google Scholar]
- Zaror et al. (2002).Zaror L, Ernst S, Navarrete M, Ballesteros A, Boroschek D, Ferres M, Thibaut J. Detección serológica de Bartonella henselae en gatos en la ciudad de Valdivia, Chile. Archivos de Medicina Veterinaria. 2002;1(1):103–110. doi: 10.4067/S0301-732X2002000100011. [DOI] [Google Scholar]
- Zepeda et al. (2016).Zepeda T, Jorge Morales S, Hugo Letelier A, Luis Delpiano M. Osteomielitis vertebral por Bartonella henselae: a propósito de un caso. Revista Chilena de Pediatría. 2016;87(1):53–58. doi: 10.1016/j.rchipe.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Zouari et al. (2017).Zouari S, Khrouf F, M’ghirbi Y, Bouattour A. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasites & Vectors. 2017;10(1):436. doi: 10.1186/s13071-017-2372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita (2018).Zurita A. Taxonomía, filogenia y papel vectorial de especies del orden Siphonaptera. 2018. Doctoral thesis, Universidad de Sevilla.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The first column corresponds to the reference sequences of GenBank and the first row, the samples analyzed from our study.
Data Availability Statement
The following information was supplied regarding data availability:
Voucher specimens of each new association flea-host were deposited in the specimen repository of the Museo de Zoología, Universidad de Concepcion, Concepción, Chile (accession numbers: MZU-CCCC-46329–46336).