Abstract
South Africa is home to the largest number of people living with HIV/AIDS in the world. Alongside the HIV/AIDS epidemic, problematic alcohol and other drug (AOD) use is prevalent and associated with poor HIV treatment and secondary HIV prevention outcomes. International guidelines and local policy both support the integration of mental health care and AOD treatment into HIV care, yet barriers exist to implementation. This study aimed to explore patient and provider perspectives on the integration of HIV and AOD treatment services in Cape Town, South Africa. This included barriers and facilitators to task sharing AOD treatment in HIV care and preferences for a task shared approach to integrating AOD treatment in HIV care, including who should deliver the behavioural intervention. We conducted thirty semi-structured qualitative interviews with HIV and AOD treatment staff, providers, and people living with HIV/AIDS (PLWH) with moderate, problematic AOD use and difficulties (personal or structural) adhering to HIV treatment. Findings illustrated several key themes: (1) the separation between AOD and HIV services (a “siloed treatment experience”), even in the context of geographic co-location; (2) low AOD treatment literacy among HIV patients and providers, including a low awareness of existing AOD use services, even when co-located; (3) substance use stigma as a barrier to HIV and AOD treatment integration; (4) a strong patient preference for peer interventionists; and (5) the role of community health workers (CHWs) in detecting AOD use among some PLWH who had not followed up in HIV care. These findings will inform a future type 1 hybrid effectiveness-implementation trial, guided by the RE-AIM framework, to evaluate a task shared, evidence-based intervention to address problematic AOD use and improve HIV medication adherence in this setting.
Keywords: HIV/AIDS, South Africa, global mental health, task sharing, integrated care, problematic substance use
Introduction
Approximately 20% of people living with HIV (PLWH) in the world reside in South Africa (7.1 million) (UNAIDS, 2017). Adequate antiretroviral therapy (ART) medication adherence is imperative for PLWH, as non-adherence and sub-optimal adherence are associated with serious health complications, ART-resistant strains, viral suppression failure (Bangsberg et al., 2001), and death (García de Olalla et al., 2002). The consequences of ART non-adherence (defined as not taking enough medications to prevent viral breakthrough) are particularly significant in the South African setting, where only first and second line ART regimens are readily available (Abaasa et al., 2008; Marconi et al., 2008) and other structural barriers to adherence exist (Kagee et al., 2011). Further, given evidence of the lower likelihood of HIV transmission with undetectable HIV virus (Cohen et al., 2011), nonadherence can also affect HIV transmission in the context of HIV treatment as prevention (Safren et al., 2015).
Problematic alcohol and other drug (AOD) use is prevalent alongside the HIV/AIDS epidemic in South Africa, especially in the Western Cape, where there is a high burden of methamphetamine use (locally known as “tik”) (Plüddemann, Flisher, McKetin, Parry, & Lombard, 2012; Shisana et al., 2014; Watt et al., 2014). South Africa also has one of the highest global rates of alcohol consumption per capita (WHO, 2014). Approximately 13–37% of PLWH in care in peri-urban Cape Town present with problematic AOD use (Kader, Seedat, Govender, Koch, & Parry, 2014). Problematic AOD use among PLWH is associated with worse ART adherence, lower rates of viral suppression, and higher rates of HIV transmission risk (Cook et al., 2001; Kader et al., 2014; Kalichman, Simbayi, Kaufman, Cain, & Jooste, 2007; Morojele, Kekwaletswe, & Nkosi, 2014; Patterson, Semple, Zians, & Strathdee, 2005)
Despite evidence that combining HIV and AOD treatment services can improve HIV treatment adherence and outcomes (Haldane et al., 2017), and emphasis on integrated care models for PLWH in international (WHO, 2016a) and national guidelines (National Department of Health, 2014), there is minimal integration of AOD treatment and HIV care in South African health services (Parry, Ferreira-Borges, Poznyak, Lönnroth, & Rehm, 2013). Integration of services in low- and middle-income countries has been previously defined as efforts to “bring together inputs, delivery, management, and organization of particular service functions” in order to improve the “efficiency and quality” of services (Briggs & Garner, 2006, p. 2). There are a number of models for bringing AOD services into primary health care (WHO, 2008a, 2016b). For instance, in co-location, mental health and other AOD providers may be geographically housed within the same primary health care practice; alternatively, integration may take the form of a collaborative system where different healthcare team members continuously communicate to create a comprehensive healthcare plan for the patient, or a single provider may address all patient issues (Briggs & Garner, 2006). Ideally, in a collaborative, integrated care model for AOD and HIV services, the patient is aware of different services offered and the mental health or AOD provider is truly part of the primary health care team; providers work closely together with ongoing communication through team meetings, shared medical records, and other multidisciplinary communication (Krüsi, Small, Wood, & Kerr, 2009; Mur-Veeman, Hardy, Steenbergen, & Wistow, 2003; Weisberg & Magidson, 2014).
Regardless of the type of integrated care model, a known barrier to integrating an AOD intervention in HIV care in South Africa is the shortage of trained providers for mental health and AOD treatment (Pasche, Kleintjes, Wilson, Stein, & Myers, 2015; Saxena, Thornicroft, Knapp, & Whiteford, 2007). To be feasible and sustainable in this setting, an intervention must incorporate a “task sharing” approach; that is, expanding care delivery models to include lay health care workers – for instance peers, community health workers (CHWs), or lay counsellors – under close supervision and training by specialist providers (Magidson, Gouse, Psaros et al., 2017; Schaefer, 2015; WHO, 2008b). Although lay health workers may have less formal training to deliver mental health and AOD interventions, they bring important lived experience to their work. CHWs typically come from the community they serve and bring an important understanding of cultural practices, local conceptualizations of disease and healing, and community respect. Prior work in South Africa has demonstrated the acceptability to patients of task sharing counselling to lay health workers for mental health and chronic disease care (Myers et al., 2018a). In high income settings, such as the US, peers with their own history of SUD are increasingly being hired in care settings to help individuals, including hard-to-reach individuals, to navigate services, access harm reduction resources, increase motivation for recovery or harm reduction, and reduce barriers to care (Jack, Oller, Kelly, Magidson, & Wakeman, 2017; Marshall, Dechman, Minichiello, Alcock, & Harris, 2015; Needle et al., 2005; Ti & Kerr, 2013). Specifically, through their expertise from lived experience, lay health workers such as CHWs and peers often expand the reach of traditional health care providers by reducing barriers to care, such as decreasing stigma (Marshall et al., 2015; Ti & Kerr, 2013). Yet, it remains unclear who should deliver a behavioural intervention to treat problematic AOD use in HIV care using a task sharing model in South Africa. Further, it is essential to identify barriers and facilitators to implementation prior to introducing a new intervention in a resource-limited context. Prior research has assessed patient preference for alcohol and mental health counselling among PLWH in South Africa (Myers et al., 2018a); however, this work has not included other drug use, and the focus was limited to the current cadres of workers in the South African health care system (i.e., excluding peers). Further, prior literature examining integration of HIV and treatment for problematic AOD has rarely incorporated feedback from both patients and providers to dictate how integration should occur.
This study examined patient and provider feedback to inform a subsequent type 1, hybrid effectiveness-implementation trial (Curran, Bauer, Mittman, Pyne, & Stetler, 2012) to integrate a behavioural AOD intervention into HIV care for individuals with problematic AOD use and ART nonadherence. This formative, qualitative phase was designed to gather information to adapt the intervention approach and implementation strategy to increase the likelihood of the intervention’s feasibility and effectiveness. The current qualitative, implementation science study aimed to elucidate barriers and facilitators to integrating services to treat problematic AOD use in HIV care in Khayelitsha, a peri-urban area of Cape Town, South Africa. Specific aims were to explore: (1) patient and provider perspectives on the integration of HIV and AOD treatment services; (2) patient and provider perspectives on barriers and facilitators to task sharing AOD treatment in HIV care; and (3) patient and provider preferences for a task shared approach to integrating AOD treatment in HIV care, including who should deliver the behavioural intervention.
Methods
The present study is the first phase of a program of research broadly aimed to support the implementation of national and international priorities to integrate HIV, mental health and AOD treatment services. This qualitative, implementation science study is the formative phase designed to gather feedback to inform the implementation of a task shared behavioural AOD intervention in HIV care for individuals with problematic AOD use and ART nonadherence. The RE-AIM framework was used as a conceptual model to guide the overall study design (Glasgow, Vogt, & Boles, 1999). RE-AIM guides the planning and evaluation of evidence-based interventions, specifically to improve the reach and adoption of evidence-based interventions in diverse, underserved settings (King, Glasgow, & Leeman-Castillo, 2010), including in sub-Saharan Africa (Jones, Weiss, & Chitalu, 2014; Weiss, Jones, Lopez, Villar-Loubet, & Chitalu, 2011). RE-AIM proposes a series of sequential steps (reach, effectiveness, adoption, implementation and maintenance) and was selected given its focus on both implementation and effectiveness outcomes and patient and provider-level outcomes.
Setting
This research included in-depth individual interviews with patients and providers purposively sampled from two large primary care clinics in Khayelitsha, a peri-urban area of ape Town, South Africa. Khayelitsha’s population comprises over 400,000 people (officially, although this is likely a significant underestimate), of which, 99% identify as Black African and are isiXhosa speaking, and over half live in informal dwellings or shacks (Statistics South Africa, 2011). HIV prevalence is approximately 33% (Shaikh et al., 2006), unemployment rates are approximately 40% (Strategic Development Information and GIS Department, City of Cape Town, 2013), and nearly 20% of households have no annual income (Western Cape Government, 2016). The primary health facilities where recruitment took place offer HIV/AIDS-related services, including ART provision to local communities. At both of the sites, CHWs are employed by local NGOs to support care engagement and retention for patients by conducting home visits for individuals who have missed their recent clinic visits. One of the two clinics also has a Matrix substance use treatment centre co-located on its premises, which is an evidence-based, 16-week outpatient cognitive-behavioural treatment originally developed in the US as a treatment for stimulant use disorders (Rawson et al., 1995, 2004; Shoptaw, Rawson, McCann, & Obert, 1994) and later implemented in Cape Town for problematic AOD (Gouse et al., 2016; Magidson, Gouse, Burnhams et al., 2017). The Matrix programme is city-funded and offers a free, same day drop-in model to initiate services.
Recruitment
Patients were eligible to participate if they were HIV positive, on ART, English or isiXhosa speaking, between 18 and 65 years of age, reported moderate AOD use (based on the WHO-ASSIST; score 4–26) and either had a detectable viral load, or failure of or re-initiation on first-line treatment. Potentially eligible patients were referred by their providers in primary care, either from a risk of treatment failure programme (available at both HIV clinicsites) or from the Matrix substance use treatment centre (co-located at one of the primary care clinics) and then screened for eligibility by a field worker. The field worker administered an abbreviated WHO-ASSIST measure (WHO ASS ST Working Group, 2002) to indicate severity of problematic AOD use in one’s lifetime and over the past three months. The ASSIST screens for alcohol, cannabis, cocaine, opiates, amphetamines, hallucinogens, and other drugs in primary health care and has been previously used in South frica (Gouse et al., 2016). Participants indicate how often each item applied to them in the past three months (asked separately for each substance). Example items include “How often has your use of [insert substance] led to health, social, legal, or financial problems?” and “How often have you failed to do what was normally expected of you because of your use of [insert substance]?” The ASSIST provides a total score by substance, and a score in the “moderate” range reflects “risk of health and other problems from current pattern of use” (WHO ASSIST Working Group, 2002).
Eligible providers were identified by the medical officer at each site. Providers were recruited if they were involved in delivering HIV or AOD care in one of the medical clinic sites, including the co-located Matrix substance use site. Providers were purposively sampled across role types.
Procedures
Research staff described the study to eligible participants and asked if they would be willing to be interviewed while emphasising that participation was voluntary and confidential. Each interview lasted approximately one hour and was digitally audio recorded, which was included in the informed consent. All patient and provider interviews were conducted between October 2016 and February 2017 and took place in private rooms at the clinics. Participants were compensated with a grocery voucher valued at ZAR 150 (approximately 11 US dollars at the time of the interview). Recruitment sites were approved for research by the City of Cape Town. All study procedures were approved by the University of Maryland Institutional Review Board (IRB), Partners Human Research Committee and the Human Research Ethics Committee at University of Cape Town.
Qualitative Interviews
All interviews followed pre-set, semi-structured interview guides developed based upon theoretically-driven domains (Glasgow et al., 1999; Marshall et al., 2015) that were adapted based on key stakeholder input. The interview guide’s primary domains followed study aims, including: 1) perspectives on integration of HIV and AOD treatment services; 2) barriers and facilitators to task sharing AOD treatment in this setting; and 3) patient and provider preferences for task sharing AOD treatment in HIV care, including with whom an integrated AOD intervention should be task shared. All questions included suggested probes for open ended questions to explore participant responses more deeply. Separate interview guides were developed for the patient and provider interviews.
Patient interviews
Eligible participants who provided informed consent were then invited to participate in a semi-structured individual interview with a trained research assistant. The patient interview guide was translated into isiXhosa and then back-translated into English to ensure consistent translation of key concepts. All patient interviews were conducted by two trained research assistants, bilingual in English and isiXhosa, who had prior experience conducting qualitative interviews with patients in community treatment settings. All participants chose to conduct the interview in isiXhosa. These interviews were translated and transcribed into English by an independent, trained bilingual translator with prior experience with qualitative interview transcription. Research assistants also collected demographics and basic information from all participants that would later be used to inform intervention adaptation and implementation (i.e., one’s prior AOD treatment history, whether someone had to take off work to come to the clinic, whether they hid medications from friends or family, and whether they owned a cell phone). Whether someone had to take off work to come to the clinic was assessed as a potential structural barrier to care (inaccessible hours for employed patients). Whether individuals hid medications from friends or family was used as a measure of HIV non-disclosure and internalized stigma that may guide future intervention adaptation and implementation plans given the relationship between HIV non-disclosure and ART adherence, and stigma as a barrier to integration of services. Finally, we assessed whether they owned a cell phone to determine the feasibility of incorporating technology into the plans for intervention delivery (i.e. delivering intervention components using technology, as well as incorporating cell phone reminders into adherence support), and feasibility of strategies for retaining participants in the future trial.
Provider interviews
The provider interview guide was tailored to whether the provider worked in AOD treatment or HIV care. All but one of the provider interviews were conducted in English by a trained PhD student in clinical psychology. One provider (a CHW) elected to conduct the interview in isiXhosa and was interviewed by one of the trained bilingual research assistants. The interviewer collected demographic information and job-related characteristics from all participants, including role type, years of experience in current role, and years of experience overall.
Participants
Patient participants (n=19) were 58% female, had a median age of 41 years old (IQR=33–46), and 100% identified as Black African. Most commonly reported substances were alcohol and cannabis; of those who reported alcohol use (n=18), 50% were in the moderate severity range (i.e., indicating problematic use; WHO ASSIST ≥ 11). Of those who reported cannabis use (n=8), 37.5% were in the moderate severity range (i.e., indicating problematic use; WHO ASSIST ≥ 4). Fifteen patients were recruited from primary HIV care (79%), and four (21%) were recruited from the co-located Matrix programme. None of the participants recruited from HIV care reported prior experience in AOD treatment. Of the patients interviewed, 63% reported owning a cell phone, 32% reported having to take off work to come to the clinic, and 26% reported hiding their HIV medications from friends or family.
Providers (n=11) were 82% female, with a median age of 44 years (IQR=36–52). Seventy-three percent identified as Black African and the remainder identified as white. They represented a variety of treatment roles in HIV care, including HIV adherence counsellor (n=1), HIV and TB nurse (n=3), physician/clinical medical officer (n=2), and CHW (n=2). Participant providers from the co-located AOD treatment centre included a treatment director (n=1) and addictions counsellors (n=2). Providers reported a median of four years of experience in their current role, and a median of 11 years of overall career experience.
Data Analysis
All transcripts were analysed with thematic analysis, and NVivo Version 11 was used for data management. Two trained, independent coders coded all interviews. A codebook was developed following open coding of the first five interviews. The codebook included higher order codes, sub-codes, and definitions for each that were arrived at through consensus across coders. The codebook was modified iteratively as new concepts which did not adequately fit into the coding scheme arose. If a new code emerged, all prior interviews were re-coded using the modified codebook. Coders met weekly to review coding. Discrepancies in coding or interpretation of codes were resolved by discussion. third person was not needed to break coding ties. Inter-coder reliability checks were conducted; the coders obtained a Kappa score >0.80 in the final analysis.
Results
This study identified five intersecting themes related to our primary aims, which were to explore patient and provider perspectives on: (1) the integration of HIV and AOD services; (2) barriers and facilitators to task sharing AOD treatment in HIV care; and (3) a task sharing model for integrating AOD treatment in HIV care, including who should deliver the behavioural intervention. The five main themes included: 1) Siloed treatment experience, which describes a lack of integration between HIV and AOD treatment services; 2) Low treatment literacy—lack of awareness of AOD treatment services available; 3) Stigma towards AOD use as a barrier to service integration; 4) Preference for peer delivery to integrate AOD treatment into HIV care; and 5) Role of CHWs in detecting AOD use among patients who had not followed up in HIV care. Figure 1 shows how these themes intersect.
Figure 1.
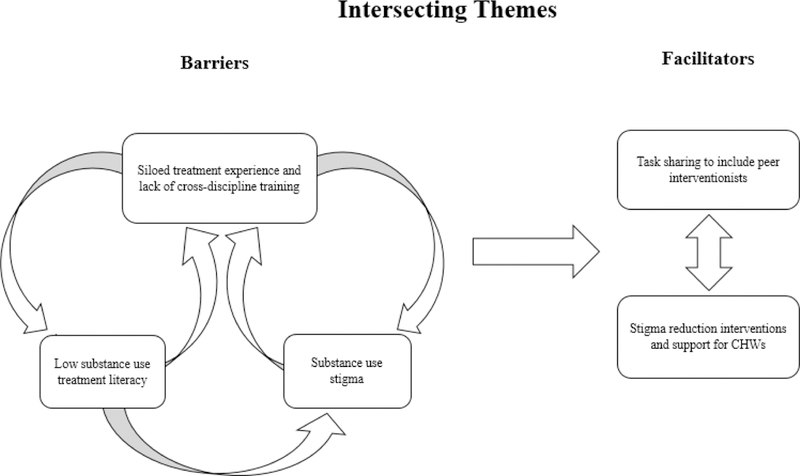
Intersecting themes depicting barriers and faciliators to task sharing an intervention for problematic substance use in HIV care in South Africa. Figure 1 depicts how these themes intersect. HIV and substance use care were isolated, even in the context of co-located services. This “siloed” treatment experience contributes to low substance use treatment literacy and awareness of existing services, particularly among patients in HIV care. The siloed treatment experience and low substance use treatment literacy may perpetuate substance use stigma. In the context of substance use stigma, patients voiced a preference to connect with peers who could share their lived experience. Finally, CHWs were identified as playing an important role in re-engaging patients who may be lost to follow up and are using substances, yet may benefit from stigma reduction interventions.
Siloed treatment experience: A lack of integration of HIV and AOD services
Almost all patients and providers described a lack of integration between HIV and AOD services. Even when HIV and AOD services were co-located, most patients and providers described infrequent interaction across services. While providers voiced an openness to greater communication between services, providers explained that a lack of communication, cross-training, and collaboration contributed to the segregation of services. One HIV provider shared that:
“Ideally it should be a one-stop shop… But there needs to be two services talking to each other.”
-- [HIV Provider (H1)]
Even where services were co-located, almost all providers noted that services remained siloed. This siloed treatment experience was evident in the physical space; although the co-located AOD centre was adjacent to the HIV clinic, one AOD treatment provider mentioned that individuals had to exit one side to enter the other. This divide in the physical space contributed to the siloed nature of services, the lack of collaboration between providers, and likely to the low awareness of existing AOD services among patients living with HIV (detailed more in Theme 2).
In addition to greater communication, providers expressed the need for more formal training across disciplines. Without sufficient cross-training, communication, or collaboration, HIV providers felt “powerless” treating patients using AODs. When asked what came to mind when they thought of these patients, one HIV provider shared:
“I feel completely powerless… I feel I am not really making a dent, that I cannot be the person to help them… what do you do to address the problem when a patient is using drugs or alcohol?” – [HIV Provider (H2)]
This feeling of powerlessness, together with high caseloads and patient burden, contributed to some HIV providers acknowledging that they did not even want to ask about AOD use.
Low AOD treatment literacy and awareness
Most patient participants lacked awareness of available AOD treatment services; the majority of patient participants recruited from HIV care did not know that there was a co-located AOD treatment centre (Matrix), nor had they previously heard of the Matrix model. This was despite the fact that HIV providers reported frequently referring patients to Matrix. Patients recruited from HIV care had low awareness of where to seek services, often describing that there was “no place they could go” for help:
“There isn’t something that I think [is keeping me from] making a decision. It’s just that– What’s [hindering] me is: where would go? … Where will I go if I want assistance? Where to go. Yes, that’s [right].” – [HIV Clinic Patient (P1)]
Almost all HIV care providers expressed an awareness of AOD use problems among PLWH and made referrals to Matrix. Providers also shared their difficulties actually getting patients to attend Matrix.
AOD use stigma a barrier to service integration
Some providers demonstrated stigma toward patients who use AODs, particularly CHWs working in HIV care. In some cases, providers asserted that they did not have judgmental attitudes toward patients who use AODs and acknowledged the role of judgment and stigma in patient initiation and retention in care. However, the same providers would then share opinions reflecting stigma toward AOD use, for instance, stating that “drug users” would automatically “become violent” if pushed too hard. Stigma toward patients who use AODs seemed greatest among providers with the least training (i.e., among CHWs). When asked their opinion toward people who use AODs, one CHW shared:
“Those ones that use drugs are naughty… they steal… Because of their addiction to drugs they don’t have control over themselves … A person that is using drugs has no hope about life, his health and he doesn’t even care about the medication” -- [CHW (H3)]
Most patients stated that they often felt stigmatised. They shared examples of times they felt judged or “scolded” by HIV providers when they were late or “caught” using, for instance:
“What they do is to shout at you when you come to collect your medication that you are not taking the treatment well... They would just shout at you...they scold at you and give you your treatment and you go home... they would ask what is my problem and I would tell them that I drink alcohol on weekends then they will scold at me for that and that will be all” - [HIV Clinic Patient (P2)]
These stigmatizing interactions perpetuate the assumption that AOD may be contra-indicated for ART, which is not accurate (ACON, n.d.-a; Schneider, Chersich, Temmerman, & Parry, 2016; Schneider, Neuman, Chersich, & Parry, 2012). These findings suggest the need for greater efforts in HIV care settings to distinguish problematic AOD use from any AOD use, especially to ascertain when AOD use directly interferes with ART adherence and care engagement. Additionally, numerous structural barriers may interfere with HIV care engagement, including overburdened providers, space challenges, and long wait times, which must be considered in this context (Kagee et al., 2011).
Patient preference for a peer interventionist
When asked from whom patients would prefer to receive an integrated intervention for HIV and substance use treatment, almost all patients responded that they preferred a peer—that is, someone with their own prior lived experience with problematic AOD use. Many patients described that the interventionist who would be most acceptable to them would be “someone who is in this thing that I am suffering from” and a “survivor of drugs/alcohol.”
Given the high rates of experienced stigma noted above, patients would likely feel less judged by someone who had gone through similar experiences or with whom they could relate to on a more personal level:
“The one that will be talking based on own experiences, because she/he’s been there and knows what he/she is talking about… The person who is receiving the counselling will feel comfortable talking to a peer because he/she will be talking to someone who can relate to his/her own experiences.” -- [HIV Clinic Patient (P4)]
Patients wanted to be able to relate to their interventionist and felt that peers would be able to tie in their own experiences and stories, creating a welcoming, bidirectional, and therapeutic relationship. Patients felt that a peer with lived experience could guide patients while simultaneously providing a tangible example of the intended results, offering patients the opportunity to see that change is possible,and acting as a role model for change. One patient elaborated:
“You see, the one who had been addicted and then quit, is the [best] person. Because he will be telling me that, ‘Me too, dude, I used to do that; in this way and this way -’ ‘Here am today -’ You see? So, that would encourage me too - That, ‘No, man. I may be able to change from this. Because this person is telling me about his own [story].’” -- [HIV Clinic Patient (P1)]
Providers also acknowledged the value of embedding peers in the health care system to support the management of patients with problematic AOD use:
“It is going to be a person that doesn’t got any sort of already stigmatised or prejudiced toward this, and somebody that maybe have a little bit of experience, like either had a family member or himself used drugs ... I can’t tell a drug addict know what you are going through… I have no idea, whereas if you tell him, “I have been on this drug, and I knew how to get off; I knew how to get past the weak points...”“ – [HIV Provider (H2)]
Likewise, a CHW also acknowledged the importance of a peer who could relate to patients’ experiences:
“A peer, is actually the best person because he’s well knowledgeable than a person who will just talk without relating to the situation of the patient. It is not the same when you talk about something you never experienced than the person that did. So, those people are highly needed.” – [CHW (H3)]
Role of CHWs in detecting problematic AOD use
When HIV providers were asked how they became aware of problematic AOD use in their patients, several described how CHWs, when conducting home visits for patients who had missed recent HIV clinic appointments, often detected problematic AOD use:
“When [the CHW] visited the place… when she comes with her report, she will tell you ‘no, this person is staying at the shebeen or this person is using drugs.’” – [HIV Provider (H4)]
Their role in detecting substance use also was reflected in the interviews with CHWs:
“And sometimes we went into their house, and we knock, and we ask at the next door, they say ‘they are inside.’ When we open, you see that they are using. You know there are substances.” – [HIV Provider (H5)]
Although the CHWs described regularly interacting with patients with active AOD use who were poorly engaged in HIV care, they also described little formal training in screening or intervention for problematic AOD use. CHWs typically receive basic health department training in HIV testing and counselling, which often includes adherence counselling, but does not emphasize screening for unhealthy AOD. Further, in the interviews, CHWs exhibited stigmatizing responses towards patients who were using AODs as quoted above, describing them as “naughty”, without control over themselves, nor hope for their lives, health, or medication adherence. In addition to exhibiting substance use stigma towards PLWH who use substances, CHWs also demonstrated low AOD treatment literacy, including low awareness of Matrix as a resource for AOD treatment. Additionally, there also was some evidence that the CHWs may perpetuate inaccurate messaging to patients about not mixing alcohol and ARVs, when in fact that is not contra-indicated (ACON, n.d.-a; Schneider et al., 2016; Schneider et al., 2012). Numerous areas for additional training were identified for CHWs, including the need for efforts to reduce AOD use stigma, improve awareness, confidence, and knowledge for screening for problematic AOD use, and ensuring the provision of accurate psychoeducation as to not contribute to misconceptions about the risk of mixing AOD and ART that may fuel ART nonadherence.
Discussion
Despite international guidelines promoting integrated care (WHO, 2016) and South Africa’s national guidelines that similarly propose improving clinical outcomes of PLWH through integration (National Department of Health, 2014), implementation has been suboptimal, in part because barriers and facilitators to an integrated approach are largely unknown. Prior research in this area has focused on alcohol use exclusively as opposed to AOD use, and only on the current cadres of workers in the South African health care system (i.e., not peers; Myers et al., 2018a). Further, prior work in this area has rarely integrated both patient and provider perspectives on integration. This study explored patient and provider perspectives on the integration of HIV and AOD treatment services in South Africa, including perceived barriers and facilitators, and preferences for a task sharing model to inform the future adaptation and delivery of an integrated behavioural intervention for problematic AOD use and ART nonadherence in HIV care. Findings illustrated that despite their close proximity, AOD use and HIV services were separated from one another, with few examples of coordination, and a lack of cross-training opportunities for providers. Low AOD treatment literacy among some HIV providers, and thereby their patients, contributed to limited patient awareness of available AOD services (even when co-located) and poor utilisation of AOD treatment services among PLWH. Patients and providers voiced the need for incorporating peer interventionists in task sharing models, particularly in the context of pervasive stigma towards AOD use. However, future work is needed to understand and address barriers to peer-delivered, evidence-based interventions for problematic AOD use and ART adherence. Findings pointed to the important role of HWs in re-engaging patients with problematic AOD use, yet substance use stigma and limited AOD literacy may be barriers that require addressing for CHWs to intervene effectively with this population.
Regarding patient and provider perspectives on the integration of HIV and AOD services, providers and patients both described a “siloed treatment experience”—HIV and AOD care were isolated, even when co-located. These findings build on prior research that has identified barriers to service use among PLWH or other viruses and AOD use who have multiple service needs (Myers, Carney, & Wechsberg, 2016; Treloar & Rance, 2014; Wolfe, Carrieri, & Shepard, 2010), and research that has pointed to the need for alcohol and HIV integration in South Africa (Kalichman et al., 2008). Unfortunately, there are many barriers to successfully integrating these two services. For instance, there are numerous structural barriers to AOD care for disadvantaged communities in Cape Town, including poor capacity and infrastructure barriers at local clinic sites, lack of communication, consultation and collaboration with other providers, fragmented service delivery, and lack of funding for evidence-based AOD treatment (Howard et al., 2017; Myers et al., 2016; Myers, Louw, & Fakier, 2008). Similarly, a qualitative study of HIV service providers in South Africa demonstrated that without sufficient cross-training and support, providers lack the confidence to administer mental health and AOD screenings (Mall, Sorsdahl, Swartz, & Joska, 2012). Yet, patients have indicated acceptability and openness to mental health and AOD services in primary health care (Myers et al., 2018a). Cross team meetings may foster true integrated, collaborative care, which has been successful in this setting in TB/HIV care integration. Further, mobile technology platforms are promising in other settings to improve care coordination between HIV and AOD treatment providers (Claborn, Becker, Ramsey, Rich, & Friedmann, 2017); additional work is needed to evaluate feasibility and acceptability in this setting. Mobile technology platforms may also be useful to address some structural barriers to care for some patients, as 32% reported having to take off work to come to the clinic and 63% of our sample reported owning a cell phone. Future work is needed to understand how to use technology to extend the reach of existing evidence-based interventions to support ART adherence and treatment of problematic AOD use outside the clinic setting. Further, mobile technology platforms have also been shown to be useful in this setting to support the fidelity of lay health worker delivered adherence interventions (Gouse et al., 2018; Remien et al., 2013; Robbins et al., 2015), although future work is needed to evaluate this approach to support integration of HIV and AOD treatment services.
Regarding barriers and facilitators to task sharing AOD treatment in HIV care, there was an apparent lack of AOD treatment literacy among patients in HIV care. Findings illustrated a disconnect between providers and patients, as providers reported making referrals to Matrix, while patients lacked awareness of referrals and services available. Patients in HIV care, including those attending care at the co-located site, were unaware of the co-located Matrix treatment centre. Given that Matrix is a free, same-day drop in service, these findings illustrate an under-utilisation of this evidence-based and readily accessible service in the community that currently has capacity for greater use. Lack of awareness and low utilization of AOD treatment have been identified in other research in Cape Town (Meade et al., 2015; Myers, Louw, & Pasche, 2010). In resource-constrained environments, low awareness of services points to the need to promote utilisation of existing resources. Future efforts are needed to increase community-level awareness of Matrix, including bolstering existing outreach efforts by the Matrix programme in the community, and potentially in alternative community venues (Hankerson & Weissman, 2012). Findings also suggest strengthening the referral process from HIV care to Matrix, including examining patient comprehension of the referral process, and expectations of the treatment process.
Another factor perpetuating separation of HIV and AOD treatment services was stigma. HIV stigma is a known barrier to engaging in HIV care (Katz et al., 2013). Indeed in our sample, over a quarter of the patients reported hiding their HIV medications from friends or family, suggesting HIV non-disclosure and internalized HIV stigma may be crucial barriers to consider in future adaptation and implementation of an integrated HIV and AOD intervention. Concurrently, research has consistently evidenced both external and internalised stigma around AOD use among PLWH (Sorsdahl, Mall, Stein, & Joska, 2011; Sorsdahl, Stein, & Myers, 2012). Other studies have shown that if patients feel stigmatised by providers for their AOD use, they are less likely to disclose problematic use and accept the offer of an AOD intervention (Myers et al., 2018a). For example, if HIV-positive patients felt stigma from providers, they may have been less likely to attend AOD treatment on-site, ask for a referral, or disclose other personal concerns around their AOD. The findings from the present research indicate the need to consider stigma reduction interventions in task sharing models.
Indeed, participants voiced a preference for peer interventionists. Although task sharing alcohol interventions with lay health workers providing adherence counselling is being explored in the South African context (Myers et al., 2018b), there has been little effort to include peers in these tasks. Expanding health services to include peers may yield many benefits to patients. Peers may offer hope about the possibility of change and serve as an example of that change, an influence that is not readily accessible in this community, although has been shown to be successful with other HIV patient populations (m2m, 2018). Peers also may help increase patients’ trust of providers (Treloar & Rance, 2014) and give patients added treatment support and information (Treloar et al., 2015). Further, if peers with lived experience guide treatment, patients may be able to find common ground with their providers, thus bolstering the counsellor-client alliance and potentially improving retention (Pasche, Myers, & Adam, 2010). This solution of adding peers might also relieve some burden for HIV providers who report feeling “powerless” with patients who use substances, and have limited time to take on additional duties such as AOD treatment (Bogart et al., 2013). More research is needed to identify potential barriers to implementing peer-delivered interventions, especially for structured approaches such as cognitive behavioural therapy. Additional research is also needed to evaluate what type of supervision and training models are necessary and how resources should be most efficiently allocated with peers integrated into the care model. Future work may test a “stepped care” approach, in which only more complex, severe cases are seen by specialized providers when possible, as trained health care workers may be more equipped to address the needs of complex cases.
Further, future work is needed to define and evaluate additional roles for peers, including helping patients navigate medical and social services, and providing harm reduction resources. If policy in South Africa shifts towards being more accepting of harm reduction approaches, we believe peers are an ideal health worker to deliver these interventions and should be further studied as an important cadre. In the US, peer recovery coaches have offered a promising solution for better integrating AOD treatment into busy primary care clinics in the US that are facing high prevalence of alcohol and opioid use disorders (Magidson et al., 2018), especially when their role is clearly defined and well-supported (Jack et al., 2017; Marshall et al., 2015). The primary barriers identified in this work have been insufficient peer preparation, stigmatized policies and attitudes both from the care team and the public (Marshall et al., 2015; Ti & Kerr, 2013), and tension within the care team when incorporating the peer role, especially when the peer role is not sufficiently delineated or defined (Jack et al., 2017). It will be important to define a core set of competencies for peer providers and a clear scope of practice, including personal and professional boundaries, and establish an infrastructure for appropriate supervision, self-care, and monitoring. Further, integrating peers into the health care system may also serve to shift AOD stigma in health care workers through exposure to individuals who have successfully shifted problematic patterns of AOD (Jack et al., 2017). However, integrating diverse cultural and philosophical perspectives on approaches to care, such as whether the goal should be recovery or harm reduction, may also present tensions within care teams and peer-patient relationships (Guise et al., 2017; Jack et al., 2017; Sylla, Bruce, Kamarulzaman, & Altice, 2007). Future work is needed to understand how to effectively integrate different orientations regarding the provision of AOD treatment. This issue may be especially important when individuals with lived experience are the providers of this care, and therefore, inherently bring their own preferences and biases for how change may occur.
Finally, HIV providers identified CHWs as playing an important role in detecting problematic AOD use among patients who are poorly engaged in care. CHWs are often the front-line workforce in interacting with the most vulnerable PLWH who are most in needed of support to re-engage in HIV care and other services. Findings suggest the need for greater support and training to reduce substance use stigma among CHWs when they encounter problematic AOD use among their patients and to encourage a more flexible, empathetic stance with patients with problematic AOD use. Future work must consider how CHWs may facilitate overcoming other structural barriers to HIV care, including long wait times, clinic overcrowding and inaccessible hours, and transportation barriers (Kagee et al., 2011), which may be heightened for individuals with problematic AOD use (Myers et al., 2016).
The results of this study must be interpreted in the context of existing limitations. First, the results are limited by the small sample size of providers; with only a few providers in each role, these findings might not be representative of other areas of the workforce. However, we felt it was important to represent a breadth of provider perspectives, and only a limited number of each provider type was available at the sites. Second, patient recruitment was clinic-based. Thus, responses might not demonstrate views of patients not currently engaged in HIV care. Third, while key stakeholders in the government and at NGOs were consulted throughout the study, they were not included as participants in the formal interviews. Finally, treatment non-adherence was conceptualized as taking enough medication to prevent viral failure. In this case, the study examined adherence in the context of existing structural and biomedical limitations. Future biomedical and potential larger social structural changes could affect the degree to which PLWH could benefit from treatment; however the present study was focused on behavioural intervention delivery given the existing contextual factors that currently exist for HIV treatment.
In conclusion, despite a supportive policy environment at the national level to integrate HIV and AOD use services, this qualitative study found little evidence of integration at the service-level. The introduction of peers to this system, along with stigma reduction efforts and AOD treatment literacy programmes for providers and patients, may help overcome integration barriers to ensure that national and local policies are translated into improvements to services and coordination efforts. This work can build off of numerous prior examples in other settings – such as the United States (Sylla et al., 2007; Weiss et al., 2011), Australia (ACON, n.d.-b; cohealth, 2014.; North Richmond Community Health, n.d.; Uniting, 2016) and Tanzania (Bruce et al., 2014) – of integrating HIV and AOD services, with an eye towards understanding how to provide a range of AOD services including harm reduction approaches (ADF - Alcohol & Drug Foundation, n.d.). In the current formative work, patients and providers shared rich perceptions around barriers and facilitators to better integrating care that will guide a subsequent hybrid effectiveness-implementation trial (Curran et al., 2012) to evaluate the effectiveness and implementation of integrating AOD treatment into HIV care in this setting.
Acknowledgments
Role of funding source
This research was supported by National Institute of Drug Abuse (NIDA) grant K23DA041901, awarded to Dr. Jessica Magidson. Dr. Bronwyn Myers is supported by the South African Medical Research Council and National Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None.
References
- Abaasa AM, Todd J, Ekoru K, Kalyango JN, Levin J, Odeke E, & Karamagi CA (2008). Good adherence to HAART and improved survival in a community HIV/AIDS treatment and care programme: The experience of The AIDS Support Organization (TASO), Kampala, Uganda. BMC Health Services Research, 8(1), 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACON. (n.d.-a). All about HIV treatment Retrieved September 12,2018,from https://endinghiv.org.au/treat-early/all-about-treatment/
- ACON. (n.d.-b). What we’re here for: Alcohol & drugs Retrieved September 13, 2018, from https://www.acon.org.au/what-we-are-here-for/alcohol-drugs/
- ADF - Alcohol & Drug Foundation. (n.d.). Medically supervised injecting centres Retrieved September 14, 2018, from https://adf.org.au/insights/medically-supervised-injecting-centres/
- Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, & Moss A (2001). Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS, 15(9), 1181–1183. [DOI] [PubMed] [Google Scholar]
- Bogart LM, Chetty S, Giddy J, Sypek A, Sticklor L, Walensky RP, … Bassett IV (2013). Barriers to care among people living with HIV in South Africa: Contrasts between patient and healthcare provider perspectives. AIDS Care, 25(7), 843–853. 10.1080/09540121.2012.729808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CJ,&Garner P(2006).Strategies for integrating primary health services in middle-and low-income countries at the point of delivery. The Cochrane Database of Systematic Reviews, (2), CD003318 10.1002/14651858.CD003318.pub2 [DOI] [PubMed] [Google Scholar]
- Bruce RD, Lambdin B, Chang O, Masao F, Mbwambo J, Mteza I, … Dunbar MS (2014). Lessons from Tanzania on the integration of HIV and tuberculosis treatments into methadone assisted treatment. International Journal of Drug Policy, 25(1), 22–25. [DOI] [PubMed] [Google Scholar]
- Claborn K, Becker S, Ramsey S, Rich J, & Friedmann PD (2017). Mobile technology intervention to improve care coordination between HIV and substance use treatment providers: Development, training, and evaluation protocol. Addiction Science & Clinical Practice, 12, 8 10.1186/s13722-017-0073-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- cohealth. (2014). Cohealth model of care summary: Victorian nurse practioner project round 4.13 (open round). Melbourne, Australia: Retrieved from http://www.health.vic.gov.au/__data/assets/pdf_file/0006/918069/CoHealth_Alcohol-and-Other-Drugs.pdf [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N,..Fleming TR (2011). Prevention of HIV-1 Infection with early antiretroviral therapy. New England Journal of Medicine, 365(6), 493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R, Sereika S, Hunt S, Woodward W, Erlen J, & Conigliaro J (2001). Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med,16(2), 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran GM, Bauer M, Mittman B, Pyne JM,&Stetler C(2012).Effectiveness-implementation Hybrid Designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical Care, 50(3), 217–226. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García de Olalla P, Knobel H, Carmona A, Guelar A, López-Colomés JL, & Caylà JA(2002). Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes, 30(1), 105–110. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Vogt TM, & Boles SM (1999). Evaluating the public health impact of health promotion interventions: The RE-AIM framework. American Journal of PublicHealth, 89(9), 1322–1327. 10.2105/AJPH.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouse H, Robbins RN, Mellins CA, Kingon A, Rowe J, Henry M, … Joska JA(2018). Empowering lay-counsellors with technology: Masivukeni, a standardized multimedia counselling support tool to deliver ART counselling. AIDS and Behavior. 10.1007/s10461-018-2145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouse Hetta, Magidson JF, Burnhams W, Remmert JE, Myers B, Joska JA, & Carrico AW (2016). Implementation of cognitive-behavioral substance abuse treatment in sub-Saharan Africa: Treatment engagement and abstinence at treatment exit. PLOS ONE, 11(1), e0147900 10.1371/journal.pone.0147900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise A, Seguin M, Mburu G, McLean S, Grenfell P, Islam Z, … Vickerman P (2017). Integrated opioid substitution therapy and HIV care: A qualitative systematic review and synthesis of client and provider experiences. AIDS Care, 29(9), 1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane V, ervero-Liceras F, Chuah FL, Ong SE, Murphy G, Sigfrid L, … Legido-Quigley H (2017). Integrating HIV and substance use services: A systematic review. Journal of the International AIDS Society, 20(1). 10.7448/IAS.20.1.21585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankerson SH, & Weissman MM (2012). Church-based health programs for mental disorders among African Americans: A review. Psychiatric Services, 63(3), 243–249. 10.1176/appi.ps.201100216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BN, Van Dorn R, Myers BJ, Zule WA, Browne FA, Carney T, & Wechsberg WM (2017). Barriers and facilitators to implementing an evidence-based woman-focused intervention in South African health services. BMC Health Services Research, 17, 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack HE, Oller D, Kelly J, Magidson JF, & Wakeman SE (2017). Addressing substance use disorder in primary care: The role, integration, and impact of recovery coaches. Substance Abuse, 1–8. [DOI] [PubMed] [Google Scholar]
- Jones D, Weiss S, & Chitalu N (2014). HIV prevention in resource limited settings: A case study of challenges and opportunities for implementation. Int J Behav Med , 22(3), 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader R, Seedat S, Govender R, Koch J,&Parry C(2014).Hazardousandharmfuluseof alcohol and/or other drugs and health status among South African patients attending HIV clinics. AIDS Behav, 18(3), 525–534. [DOI] [PubMed] [Google Scholar]
- Kagee A, Remien RH, Berkman A, Hoffman S, Campos L, & Swartz L (2011). Structural barriers to ART adherence in Southern Africa: Challenges and potential ways forward. Global Public Health, 6(1), 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Kaufman M, Cain D, & Jooste S (2007). Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: Systematic review of empirical findings. Prevention Science, 8(2), 141–151. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Vermaak R, Cain D, Smith G, Mthebu J, & Jooste S (2008). Randomized trial of a community-based alcohol-related HIV risk-reduction intervention for men and women in Cape Town South Africa. Annals of Behavioral Medicine, 36(3), 270–279. [DOI] [PubMed] [Google Scholar]
- Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, & Tsai AC (2013). Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. JournaloftheInternational AIDS Society, 16(3S2), 18640. 10.7448/IAS.16.3.18640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DK, Glasgow RE, & Leeman-Castillo B (2010). Reaiming RE-AIM: Using the model to plan, implement, and evaluate the effects of environmental change approaches to enhancing population health. American Journal of Public Health, 100(11), 2076–2084. 10.2105/AJPH.2009.190959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüsi A, Small W, Wood E, & Kerr T (2009). An integrated supervised injecting program within a care facility for HIV-positive individuals: A qualitative evaluation. AIDS Care, 21(5), 638–644. [DOI] [PubMed] [Google Scholar]
- m2m. (2018). What we do and why [m2m.org]. Retrieved May 24, 2018, from https://www.m2m.org/what-we-do-and-why/
- Magidson JF, Gouse H, Burnhams W, Wu CYY, Myers B, Joska JA, & Carrico AW (2017). Beyond methamphetamine: Documenting the implementation of the Matrix model of substance use treatment for opioid users in a South African setting. Addictive Behaviors, 66, 132–137. 10.1016/j.addbeh.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson JF, Gouse H, Psaros C, Remmert JE, O’Cleirigh C, & Safren SA (2017). Task Shifting and delivery of behavioral medicine interventions in resource-poor global settings: HIV/AIDS treatment in sub-Saharan Africa. In Vranceanu A-M, Greer JA, & Safren SA (Eds.), The Massachusetts General Hospital Handbook of Behavioral Medicine: A clinician’s guide to evidence-based psychosocial interventions for individuals with medical illness (pp. 297–320). New York, NY: Humana Press. [Google Scholar]
- Magidson JF, Regan S, Jack H, Wakeman S. Reduced hospitalizations and increased abstinence six months after recovery coach contact. Poster Presentation Presented at the American Society of Addiction Medicine (ASAM) 2018 [Google Scholar]
- Mall S, Sorsdahl K, Swartz L, & Joska J (2012). “I understand just a little…” Perspectives of HIV/AIDS service providers in South Africa of providing mental health care for people living with HIV/AIDS. AIDS Care, 24(3), 319–323. 10.1080/09540121.2011.608790 [DOI] [PubMed] [Google Scholar]
- Marconi VC, Sunpath H, Lu Z, Gordon M, Koranteng‐Apeagyei K, Hampton J, …South Africa Resistance Cohort Study Team. (2008). Prevalence of HIV‐1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clinical Infectious Diseases, 46(10), 1589–1597. 10.1086/587109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall Z, Dechman MK, Minichiello A,Alcock L, & Harris GE (2015). Peering into the literature: A systematic review of the roles of people who inject drugs in harm reduction initiatives. Drug and Alcohol Dependence, 151, 1–14. [DOI] [PubMed] [Google Scholar]
- Meade CS, Towe SL, Watt MH, Lion RR, Myers B, Skinner D, … Pieterse D (2015).Addiction and treatment experiences among active methamphetamine users recruited from a township community in Cape Town, South Africa: A mixed-methods study. Drug & Alcohol Dependence, 152, 79–86. 10.1016/j.drugalcdep.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morojele NK, Kekwaletswe CT, & Nkosi S (2014). Associations between alcohol use, other psychosocial factors, structural factors and antiretroviral therapy (ART) adherence among South African ART recipients. AIDS and Behavior, 18(3), 519–524. 10.1007/s10461-013-0583-0 [DOI] [PubMed] [Google Scholar]
- Mur-Veeman I, Hardy B, Steenbergen M, & Wistow G (2003). Development of integrated care in England and the Netherlands: Managing across public–private boundaries. Health Policy, 65(3), 227–241. [DOI] [PubMed] [Google Scholar]
- Myers B, Carney T, & Wechsberg WM (2016). “Not on the agenda”: A qualitative study of influences on health services use among poor young women who use drugs in Cape Town, South Africa. International Journal of Drug Policy, 30, 52–58. 10.1016/j.drugpo.2015.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers BJ, Louw J, & Pasche SC (2010). Inequitable access to substance abuse treatment services in Cape Town, South Africa. Substance Abuse Treatment, Prevention, and Policy, 5, 28 10.1186/1747-597X-5-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Joska JA, Lund C, Levitt NS, Butler CC, Naledi T, … Sorsdahl K (2018a). Patient preferences for the integration of mental health counseling and chronic disease care in South Africa. Patient Preference and Adherence, 12, 1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Louw J, & Fakier N (2008). Alcohol and drug abuse: Removing structural barriers to treatment for historically disadvantaged communities in Cape Town. International Journal of Social Welfare, 17(2), 156–165. [Google Scholar]
- Myers B, Lund C, Lombard C, Joska J, Levitt N, Butler C, … Sorsdahl K (2018b). Comparing dedicated and designated models of integrating mental health into chronic disease care: Study protocol for a cluster randomized controlled trial. Trials, 19(1), 185 10.1186/s13063-018-2568-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Department of Health. (2014). National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults Pretoria, South Africa: National Department of Health, Republic of South Africa; Retrieved from http://www.sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf [Google Scholar]
- Needle RH, Burrows D, Friedman SR, Dorabjee J, Touzé G, Badrieva L, … Manning G (2005). Effectiveness of community-based outreach in preventing HIV/AIDS among injecting drug users. International Journal of Drug Policy, 16, 45–57. [Google Scholar]
- North RichmondCommunityHealth.(n.d.). Medically supervised injecting room Retrieved September 13, 2018, from https://nrch.com.au/services/alcohol-and-other-drugs/medically-supervised-injecting-room/
- Parry C, Ferreira-Borges C, Poznyak V, Lönnroth K, & Rehm J (2013). The international study on alcohol and infectious diseases: Three priorities for research. Addict Abingdon Engl, 108(1), 1–2. [DOI] [PubMed] [Google Scholar]
- Pasche S, Kleintjes S, Wilson D, Stein DJ, & Myers B (2015). Improving addiction care in South Africa: Development and challenges to implementing training in addictions care at the University of Cape Town. International Journal of Mental Health and Addiction, 13(3), 322–332. 10.1007/s11469-014-9537-7 [DOI] [Google Scholar]
- Pasche S, Myers B, & Adam M (2010). Factors associated with retention in alcohol and other drug treatment among disadvantaged communities in Cape Town, South Africa. Journal of Studies on Alcohol and Drugs, 71(3), 395–399. 10.15288/jsad.2010.71.395 [DOI] [PubMed] [Google Scholar]
- Patterson T, Semple S, Zians J, & Strathdee S (2005). Methamphetamine-using HIV-positive men who have sex with men: Correlates of polydrug use. J Urban Health Bull N Y Acad Med, 82(1 Suppl 1), i120–i126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plüddemann A, Flisher AJ, McKetin R, Parry CD, & Lombard CJ (2012). Methamphetamine use and sexual risk behavior among high school students in Cape Town, South Africa. Journal of Child & Adolescent Substance Abuse, 21(2), 181–191. 10.1080/1067828X.2012.662437 [DOI] [Google Scholar]
- Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, … McCann MJ (2004). A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction, 99(6), 708–717. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Shoptaw SJ, Obert JL, McCann MJ,Hasson AL,Marinelli-Casey PJ, … Ling W (1995). An intensive outpatient approach for cocaine abuse treatment: The Matrix model. Journal of Substance Abuse Treatment, 12(2), 117–127. [DOI] [PubMed] [Google Scholar]
- Remien RH, Mellins CA, Robbins RN, Kelsey R, Rowe J, Warne P, … Abrams EJ (2013). Masivukeni: Development of a multimedia based antiretroviral therapy adherence intervention for counselors and patients in South Africa. AIDS and Behavior, 17(6), 1979– 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RN, Mellins CA, Leu C-S, Rowe J, Warne P, Abrams EJ, … Remien RH (2015). Enhancing lay counselor capacity to improve patient outcomes with multimedia technology. AIDS and Behavior, 19(2), 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Mayer KH, Ou S-S, McCauley M, Grinsztejn B, Hosseinipour MC, … Cohen MS (2015). Adherence to early antiretroviral therapy: Results from HPTN 052, a Phase III, multinational randomized trial of ART to prevent HIV-1 sexual transmission in serodiscordant couples. Journal of Acquired Immune Deficiency Syndromes (1999), 69(2), 234 10.1097/QAI.0000000000000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Thornicroft G, Knapp M, & Whiteford H (2007). Resources for mental health: Scarcity, inequity, and inefficiency. Lancet, 370(9590), 878–889. 10.1016/S0140-6736(07)61239-2 [DOI] [PubMed] [Google Scholar]
- Schaefer L (2015). Task sharing implant insertion by community health workers: Not just can it work, but how it might work practically with impact in the real world. Global Health: Science and Practice, 3(3), 327–329. 10.9745/GHSP-D-15-00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, hersich M, Temmerman M, & Parry CD (2016). Addressing the intersection between alcohol consumption and antiretroviral treatment: Needs assessment and design of interventions for primary healthcare workers, the Western Cape, South Africa. Globalization and Health, 12(1), 65 10.1186/s12992-016-0201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Michelle, Neuman M, Chersich M, & Parry C (2012). Alcohol and antiretroviral therapy - a lethal cocktail. Journal of AIDS and Clinical Research, (Suppl. 1), 1–8. 10.4172/2155-6113.S1-005 [DOI] [Google Scholar]
- Shaikh N, Abdullah F, Lombard CJ, Smit L, Bradshaw D, & Makubalo L (2006). Masking through averages-intraprovincial heterogeneity in HIV prevalence within the Western Cape. South African Medical Journal, 96(6), 538–543. [PubMed] [Google Scholar]
- Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, … Onoya D (2014). South African national HIV prevalence, incidence and behaviour survey, 2012 HSRC Press. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rawson RA, McCann MJ, & Obert JL (1994). The Matrix model of outpatient stimulant abuse treatment: Evidence of efficacy. Journal of Addictive Diseases, 13(4), 129–141. [DOI] [PubMed] [Google Scholar]
- Sorsdahl KR, Mall S, Stein DJ, & Joska JA(2011).Theprevalenceandpredictorsof stigma amongst people living with HIV/AIDS in the Western Province. AIDS Care, 23(6),680–685. 10.1080/09540121.2010.525621 [DOI] [PubMed] [Google Scholar]
- Sorsdahl K, Stein DJ, & Myers B (2012). Negative attributions towards people with substance use disorders in South Africa: Variation across substances and by gender. BMC Psychiatry, 12, 101 10.1186/1471-244X-12-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics South Africa. (2011). Khayelitsha (Statistics by place) Pretoria, South Africa:Statistics South Africa; Retrieved from http://www.statssa.gov.za/?page_id=4286&id=328 [Google Scholar]
- Strategic Development Information and GIS Department, City of Cape own. (2013). City of Cape Town - 2011 census suburb Khayelitsha Cape Town, South Africa: Retrieved from http://resource.capetown.gov.za/documentcentre/Documents/Maps%20and%20statistics/2011_Census_CT_Suburb_Khayelitsha_Profile.pdf [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, & Altice FL (2007). Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. The International Journal on Drug Policy, 18(4), 306–312. 10.1016/j.drugpo.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ti L,&Kerr T(2013).Taskshiftingredefined: Removing social and structural barriers to improve delivery of HIV services for people who inject drugs. Harm Reduction Journal, 10(1), 20 10.1186/1477-7517-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar C, & Rance J (2014). How to build trustworthy hepatitis C services in an opioid treatment clinic? A qualitative study of clients and health workers in a co-located setting. International Journal of Drug Policy, 25(5), 865–870. [DOI] [PubMed] [Google Scholar]
- Treloar C, Rance J, Bath N, Everingham H, Micallef M, Day C, … Dore GJ (2015). Evaluation of two community-controlled peer support services for assessment and treatment of hepatitis virus infection in opioid substitution treatment clinics: The ETHOS study, Australia. International Journal of Drug Policy, 26(10), 992–998. [DOI] [PubMed] [Google Scholar]
- UNAIDS. (2017). UNAIDS Data 2017. Joint United Nations Programme on HIV/AIDS(UNAIDS) Retrieved from http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf [PubMed]
- Uniting. (2016, April 7). Supervised injecting centre Kings Cross Retrieved September 14, 2018, from https://uniting.org/services/services/uniting-medically-supervised-injecting-centre/uniting-medically-supervised-injecting-centre-kings-cross
- Watt MH, Meade CS, Kimani S, MacFarlane JC, Choi KW, Skinner D, … Sikkema KJ (2014). The impact of methamphetamine (“tik”) on a peri-urban community in Cape Town, South Africa. International Journal of Drug Policy, 25(2), 219–225. 10.1016/j.drugpo.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg RB, & Magidson JF (2014). Integrating cognitive behavioral therapy into primary care settings. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Netherland J, Egan JE, Flanigan TP,Fiellin DA,Finkelstein R,… Collaborative, for the B. (2011). Integration of Buprenorphine/Naloxone treatment into HIV clinical care: Lessons from the BHIVES collaborative. JAIDS Journal of Acquired Immune Deficiency Syndromes, 56, S68 10.1097/QAI.0b013e31820a8226 [DOI] [PubMed] [Google Scholar]
- Weiss S, Jones D, Lopez M, Villar-Loubet O, & Chitalu N (2011). The many faces of translational research: A tale of two studies. Transl Behav Med, 1(2), 327–330. 10.1007/s13142-011-0044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western Cape Government. (2016). 2016 socio-economic profile: city of Cape Town (p. 36).Cape Town, South Africa: Retrieved from https://www.westerncape.gov.za/assets/departments/treasury/Documents/Socio-economic-profiles/2016/City-of-Cape-Town/city_of_cape_town_2016_socio-economic_profile_sep-lg.pdf [Google Scholar]
- WHO ASSIST Working Group. (2002). The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): dDvelopment, reliability and feasibility. Addiction, 97(9), 1183– 1194. 10.1046/j.1360-0443.2002.00185.x [DOI] [PubMed] [Google Scholar]
- Wolfe D,Carrieri MP,&Shepard D (2010). Treatment and care for injecting drug users with HIV infection: A review of barriers and ways forward. The Lancet, 376(9738), 355–366. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2008a). Technical brief No 1: Integrated health services -what and why Geneva: WHO Press. [Google Scholar]
- World Health Organization (WHO). (2008b). Task shifting: Rational redistribution of tasks among health workforce teams: Global recommendations and guidelines Geneva,Switzerland: WHO Press. [Google Scholar]
- World Health Organization (WHO). (2014). Global status report on alcohol and health 2014 Geneva, Switzerland: WHO Press. [Google Scholar]
- World Health Organization (WHO). (2016a). Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations - 2016 update Geneva, Switzerland: WHO Press; Retrieved from http://www.deslibris.ca/ID/10063272 [PubMed] [Google Scholar]
- World Health Organization (WHO). (2016b). Integrated care models: An overview: Working document. World Health Organization, Regional Office for Europe; Retrieved from http://www.euro.who.int/__data/assets/pdf_file/0005/322475/Integrated-care-models-overview.pdf [Google Scholar]


