Abstract
Background:
Air pollution exposure has been shown to increase the risk of obesity and metabolic dysfunction in animal models and human studies. However, the metabolic pathways altered by air pollution exposure are unclear, especially in adolescents and young adults who are at a critical period in the development of cardio-metabolic diseases.
Objectives:
The aim of this study was to examine the associations between air pollution exposure and indices of fatty acid and amino acid metabolism.
Methods:
A total of 173 young adults (18–23 years) from eight Children’s Health Study (CHS) Southern California communities were examined from 2014–2018. Near-roadway air pollution (NRAP) exposure (freeway and non-freeway) and regional air pollution exposure (nitrogen dioxide, ozone and particulate matter) during one year before the study visit were estimated based on participants’ residential addresses. Serum concentrations of 64 targeted metabolites including amino acids, acylcarnitines, non-esterified fatty acid (NEFA) and glycerol were measured in fasting serum samples. Principal component analysis of metabolites was performed to identify metabolite clusters that represent key metabolic pathways. Mixed effects models were used to analyze the associations of air pollution exposure with metabolomic principal component (PC) scores and individual metabolite concentrations adjusting for potential confounders.
Results:
Higher lagged one-year averaged non-freeway NRAP exposure was associated with higher concentrations of NEFA oxidation byproducts and higher NEFA-related PC score (all p’s≤0.038). The effect sizes were larger among obese individuals (interaction p=0.047). Among females, higher freeway NRAP exposure was also associated with a higher NEFA-related PC score (p=0.042). Among all participants, higher freeway NRAP exposure was associated with a lower PC score for lower concentrations of short- and median-chain acylcarnitines (p=0.044).
Conclusions:
Results of this study indicate that NRAP exposure is associated with altered fatty acid metabolism, which could contribute to the metabolic perturbation in obese youth.
Keywords: traffic, air pollution, obesity, metabolic diseases, metabolomics
INTRODUCTION
Childhood obesity rates have steadily risen over the past few decades, contributing to the increased epidemic of cardio-metabolic diseases in children and adults.1–5 Unhealthy diet, inadequate exercise, and genetics are well-known risk factors in the burgeoning obesity epidemic, but recent work suggests an important role for environmental exposures, including air pollution. A growing body of evidence indicates that early-life near-roadway air pollution (NRAP) and regional air pollution exposures are associated with childhood obesity, higher growth trajectory of body mass index (BMI) and higher attained BMI at age 18 years.6,7 In addition, air pollution exposure has been associated with obesity-related metabolic dysfunction, including glucose intolerance and insulin resistance.8–12 However, the mechanism linking air pollution exposure and metabolic dysfunction remains unclear.
Air pollution exposure may increase the risk of obesity and metabolic dysfunction by altering key metabolic pathways. Application of metabolomics technology allows measurement of a broad array of metabolites, including amino acids, fatty acids, and acylcarnitines in biological fluids such as serum.13 A series of metabolomics studies have linked alterations in amino acid, fatty acid, glucose, bile acid, and choline metabolism with increased adiposity,14–18 including visceral and liver fat,19–25 glucose intolerance,26–30 and insulin resistance.31–35 Furthermore, mounting evidence suggests that increased NRAP and regional particulate matter with aerodynamic diameter <2.5 μm (PM2.5) exposures are associated with dysregulated metabolism of fatty acids, amino acids and leukotrienes,36–39 which may contribute to the onset and progression of cardiovascular diseases and asthma. To our knowledge, no human study has used metabolomics to investigate links of NRAP and regional air pollution exposure to obesity and metabolic dysfunction, especially among adolescents and young adults.
In this study, we leveraged an existing cohort of adolescents and young adults from the Southern California Children’s Health Study (CHS)6 to examine the influence of NRAP and regional air pollution exposures on serum concentrations of amino acids, ketones, non-esterified free fatty acids, glycerol and acylcarnitine intermediates that report on mitochondrial metabolism, reflecting key metabolic pathways for three main classes of nutrients – carbohydrates, lipids and protein. We also investigated the relationship between metabolomic profiles and the outcome of obesity and BMI. Finally, increasing evidence suggests that there are potential sex and ethnicity disparities in the metabolic effect of air pollution exposure, possibly due to pollution inequity and physiology differences.40–43 Furthermore, metabolomic profiles can also be sex-, ethnicity- and obesity-dependent.19,44–46 Therefore, we explored whether the associations between air pollution and metabolomic profiles differed by obesity status, ethnicities and sex.
MATERIALS AND METHODS
Participants and Health Measures:
A total of 173 participants from eight Southern California communities were selected from the original CHS cohort6 for targeted metabolomics analysis. Because the main aim of this study was to examine the influence of air pollution exposures on metabolic dysfunction in adolescents, we first identified 1,154 participants who were overweight or obese (age- and sex-specific BMI of ≥85th percentile compared to the CDC 2000 BMI growth curves47) at their last in-school visit of the CHS follow-up (October 2011-June 2012). Among these participants who are at a higher risk for metabolic dysfunction, we further used a probability weighted sampling approach to enroll 137 participants with the selection probability as a logistic function of the quadratic form of percentiles of individual exposures to NRAP within each community during the last visit in the CHS. Therefore, participants with extremely high and low NRAP exposure within each community have the highest probability to be selected. To increase the generalizability to a normal weight population, a similar exposure-weighted sampling approach was used to additionally enroll 36 participants from a total of 1,957 CHS participants who were normal weight at their last CHS visit. Participants were excluded if they had type 1 or type 2 diabetes or if they were using a medication or diagnosed with a condition known to influence insulin and/or glucose metabolism or body composition.
In this project, participants completed the study visit at Diabetes and Obesity Research Institute (DORI) or Clinical Trials Unit (CTU) at the University of Southern California between 2014 and 2018. The 7-hour clinical visit included several questionnaires as well as clinical measures. Questionnaires detailing demographic, health and occupational history, parental health information, updated residential history and smoking history were administered. Individual height and weight were measured by a trained technician where height was measured to the nearest centimeter and weight to the nearest 0.1 kilogram without shoes. These objective measures of height and weight were used to calculate BMI (kg/m2), as the primary obesity outcome. BMI was further categorized into normal weight, overweight and obese groups based on CDC criteria48,49 as a secondary outcome. In a subset of participants who were overweight or obese at their last in-school CHS visit (N=132), self-reported physical activity status was assessed by the questions of “Have you taken any exercise classes, lessons, or special programs (e.g., dance, martial arts, aerobics, gymnastics or tumbling and swimming) during the past 12 months?” and “Please place yourself on the scale (0–100) to rate your usual physical activity”. The self-evaluation of physical activity scale was further categorized into three categories of low- (0–40), moderate- (50–60) and high- (70–100) activity levels. Additionally, two non-consecutive 24-hour diet recalls50 including serving sizes of 168 food items were collected among the subset of 132 participants to estimate total calorie and macronutrient intake, as well as glycemic index. The 24-hour recalls were analyzed with the Nutrition Data System for Research (NDSR) software (Version 2014),51 which is based on Nutrition Coordinating Center (NCC) Food and Nutrient Database. Informed written consent was obtained from all participants for this study. The USC Institutional Review Board (IRB) approved this study.
Air Pollution Exposures:
NRAP exposure was estimated through detailed residential history obtained at the study visit. Residential addresses were standardized at street level and geocoded using the Texas A&M Geocoder (http://geoservices.tamu.edu/Services/Geocode/). Latitude and longitude of residences were given to Sonoma Technology Inc., who then provided monthly NRAP exposure data for up to 12 months prior to each visit for each participant. NRAP exposure from freeway and non-freeway roads was estimated using modelled nitrogen oxides (NOx) at residential addresses by applying the California line-source dispersion (CALINE4) model. CALINE4 dispersion model is an air quality model designed to estimate the incremental ambient concentration contributed by vehicle emissions on local roadways.52 The modeled annual concentration estimates (parts per billion, ppb) were based on roadway geometry, traffic counts, traffic volumes, vehicle NOx emission rates, and meteorological conditions. Traffic counts and road geometry were obtained from Caltrans and TeleAtlas/GDT. Annual average daily traffic volumes were assigned based on calendar year. All road emissions from freeway or non-freeway sources were calculated within 5-kilometer buffer of the residence. It is noted that the modeled NRAP exposures reflect the mixture of multiple pollutants from nearby traffic, and the high correlation of pollutants in the mixture precludes identifying the effect of any specific pollutant in the mixture, as described in previous publications.53,54
Regional air pollution exposure levels of each participant are estimated based on residential histories for three regional air pollutants: nitrogen dioxides (NO2), PM2.5 and ozone (O3).55–57 Hourly air quality data from ambient monitoring stations were downloaded from the U.S. Environmental Protection Agency’s Air Quality System (AQS, http://www.epa.gov/ttn/airs/airsaqs) from year 2011–2018 and averaged to daily level. In California, air monitor stations are spaced 20–30 km apart and provide a monitoring network with good characterization of the pollution gradients across Los Angeles. The gaseous pollutants were measured using Federal Reference Method (FRM) continuous monitors, whereas PM2.5 data was restricted to FRM or Federal Equivalent Method (FEM) monitors. Monthly averages were calculated from the daily data using a 75% completeness criterion, and monthly exposure values were spatially interpolated from the air quality monitoring station’s locations within 50 km of each residential address using an inverse distance-squared weighting (IDW2) algorithm, as previously described.58 Data from up to four monitors were used to estimate exposure for each location. Prior work by our group59 has shown that the IDW2 method in California was robust to a leave one out validation for monthly monitoring AQS site data and performs as well as more sophisticated models that are limited by shorter spatial-temporal coverage.
Based on monthly air pollution exposure data, we further calculated lagged annual average exposures (as representatives of long-term exposures) to freeway, non-freeway and total (combined freeway and non-freeway) NRAP, as well as regional air pollutants (NO2, PM2.5 and O3) as the cumulative averages of monthly exposure concentrations over one year prior to the DORI/CTU study visit and were weighted by time spent at each different address since some subjects began attending college and no longer lived at home. In these instances, one-year average air pollution exposure prior to the study visit accounts for both college and home air pollution exposures. Additionally, short-term air pollution exposures were represented by one-month exposure level prior to the clinical visit.
Metabolomic Signatures:
Concentrations of 64 endogenous metabolites were assayed in fasting serum samples as previously described.31,38,60–62 These 64 targeted metabolites have been successfully used in many studies of obesity and cardiometabolic diseases,13,61,63,64 and have been linked to increased adiposity,31,32 insulin resistance,61,64 as well as air pollution exposures.38 In order to assure the stability of analyzed serum metabolites,65 all unthawed serum samples were stored at −80 °C and were transferred to the laboratory at Duke University on dry ice. Targeted analyses included three analytic modules: 1) 15 amino acids, 2) 45 acylcarnitines, and 3) three conventional metabolites: non-esterified free fatty acids (NEFA), lactate, beta-hydroxybutyrate were assayed across three batches with samples block randomized by sex on each assay plate. Later, we measured glycerol using the leftover samples that had been freeze-thawed once in a separate batch as an additional measurement of lipolysis. Lipolysis is a process of degrading triglycerides into fatty acids and glycerol,66 therefore, serum NEFA and glycerol are expected to increase with enhanced adipocyte lipolysis.
Proteins were first removed by precipitation with methanol. Aliquoted supernatants were dried, and then esterified with hot acidic methanol (acylcarnitines) or n-butanol (amino acids). Acylcarnitines and amino acids were analyzed by flow injection-tandem mass spectrometry (MS/MS).31,67,68 Quantitative and reproducible measurement of metabolites was achieved by inclusion of stable isotope-labeled internal standards for the amino acid and acylcarnitine modules,31,38,60–62 with data expressed in molarities rather than relative units. A Beckman Unicel DxC 600 autoanalyzer was used for analysis of conventional metabolites.31
Based on the concentration data of all metabolites, principal components analysis (PCA) with varimax orthogonal rotation was used for dimension reduction and to classify correlated metabolites into clusters of fewer uncorrelated factors, principal components (PC).69,70 To eliminate the influence of variations across batches and freeze-thaw cycles of serum samples in the final PC classifications, glycerol was not included in the PCA. There were two acylcarnitines (C6 and C7-DC) with >20% zero-value due to their concentrations below the lower limits of quantification for that assay (Supplemental Table 1). To further assess the influence of zero-value data, we applied a Markov chain Monte Carlo-based multiple imputation (MI)71,72 for C6, C7-DC, and calculated averaged concentrations from 1000 imputation samples before the PCA to verify the consistency of PC classifications. The scree plot was used to determine the number of prioritized PCs for the main association analyses. Metabolites with a factor load of an absolute value ≥ 0.4 were reported as the main components of a given PC. Scoring coefficients were used to calculate metabolomic PC scores for each individual.
Statistical Methods:
Details of model forms of the regression models are presented in the supplemental material. Briefly, a generalized additive model with a cubic smoothing spline of the exposure variable was used to assess nonlinear relationships between air pollution exposures and metabolomic factor scores, as well as relationships of exposure variables of metabolomic factor scores with BMI as the outcome. If no nonlinear relationships were found, linear regression models were applied in subsequent analyses. Generally, mixed effects models were used to examine the associations of long-term and short-term NRAP and regional air pollution exposures with the outcome of each metabolomic PC score and individual metabolite concentrations after adjusting for potential confounders including age, sex, race/ethnicity, parental education (proxy for social economic status), BMI, whether or not the participant smoked cigarettes during the last week, ever/never smoked electronic cigarettes, season of the study visit and random intercepts for CHS communities and metabolomics analytical batches. Beyond the analysis of individual pollutants, multi-pollutant models with exposures to multiple air pollutants as independent variables were used to assess the independent effects of each air pollutant on metabolomic outcomes adjusting for exposures to other air pollutants.
For the analyses of individual metabolite concentrations, we focused on the metabolites that were representative of significant PCs (a factor loading of an absolute value ≥ 0.4, Supplemental Table 2) that had significant associations with air pollution exposures. The false discovery rate (FDR) method73 was used to adjust for multiple testing that could inflate type 1 error. Statistical tests were considered significant with an FDR-adjusted p-value less than 0.1.
Next, the mixed effects model was used to assess the joint associations between all prioritized metabolomic PC scores and the primary obesity outcome of BMI as a continuous variable after adjusting for potential confounders including age, sex, ethnicity, parental education levels as a proxy for socioeconomic status, whether or not cigarettes were smoked during the last week, ever/never smoked electronic cigarettes, and random intercepts for CHS communities and metabolomics analytical batches. In addition, BMI categories [normal weight (BMI<25 kg/m2), overweight (25⩽BMI<30 kg/m2) and obese (BMI⩾30 kg/m2)] were used as a secondary outcome for obesity. Multinomial logistic regression with random intercepts for CHS communities and metabolomics analytical batches was used to analyze the associations between metabolomic PC scores and BMI categories.
Additionally, in a subset of 132 participants, we assessed the influence of physical activity (whether or not exercise classes were taken outside of the school in the previous year and self-rated physical activity scale) and diet (total calorie intake, percent calorie intake from fat and protein, and glycemic index estimated from the 24-hr recalls) in the association of metabolomics factor scores with air pollution exposures or the obesity outcomes of BMI as a continuous variable or BMI categories.
Lastly, we investigated whether sex, race/ethnicity and BMI categories could modify the effects of air pollution exposure and metabolomic PC scores by including multiplicative interaction terms in the model. All independent statistical tests were considered significant with a two-sided p-value less than 0.05. SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) was used for data analysis.
RESULTS
This study included 173 participants who had complete metabolomic data, weight and height measures, and at least exposure data for one air pollutant. Sociodemographic characteristics are described in Table 1. The age range of our participants at the study enrollment was 18–23 years old with a mean age (standard deviation, SD) of 19.8 (1.1) years. The mean BMI (SD) was 28.2 (5.2) kg/m2, and 73.4% participants were overweight or obese.
Table 1.
Sociodemographic characteristics of 173 Children’s Health Study (CHS) adolescents and young adults enrolled during year 2014–2018.
| Entire sample (N=173) |
||
|---|---|---|
| N | % | |
| Sex | ||
| Female | 79 | 45.7 |
| Male | 94 | 54.3 |
| Race/Ethnicity | ||
| Non-Hispanic White | 50 | 28.9 |
| Hispanic White | 97 | 56.1 |
| Other1 | 26 | 15.0 |
| Parental education | ||
| Less than high school | 58 | 33.5 |
| Completed high school | 52 | 30.1 |
| Some college or higher | 59 | 34.1 |
| Unknown | 4 | 3.3 |
| Ever used e-cigarette | ||
| Yes | 46 | 26.6 |
| No | 127 | 73.4 |
| Cigarette smoke in the last week | ||
| Yes | 9 | 5. 2 |
| No | 164 | 94.8 |
| Body mass index (BMI) categories | ||
| Normal weight (<25 kg/m2) | 46 | 26.6 |
| Overweight (≥25 and <30 kg/m2) | 77 | 44.5 |
| Obese (≥30 kg/m2) | 50 | 28.9 |
Other race/ethnicity includes African American, Asian/Pacific Islander, mixed three or more races, other Non-White race/ethnicity and unknown race/ethnicity.
Means (SDs) of air pollution exposure concentrations are presented in Table 2. Correlations among long-term and short-term air pollution exposures are presented in Supplemental Table 3. Generally, lagged 1-month (short-term) NRAP and regional air pollution exposures were highly correlated with lagged 1-year averages (long-term) of exposures (all R2≥0.5), except O3. However, the correlations between NRAP exposures and regional air pollution exposures were relatively small (all R2<0.3).
Table 2.
Means and standard deviations of short-term1 and long-term2 near-roadway and regional air pollution exposure levels among 173 adolescents and young adults enrolled from the Southern California Children’s Health Study during year 2014–2018.
| Mean | Standard Deviation | |
|---|---|---|
| Short-term Exposures | ||
| Near-roadway air pollutants (NRAP) | ||
| Freeway NRAP (ppb)3 | 4.8 | 6. 3 |
| Non-freeway NRAP (ppb)3 | 1.5 | 1.0 |
| Total NRAP (ppb)3 | 6.3 | 6.6 |
| Regional air pollutants | ||
| NO2 (ppb)3 | 15.4 | 5. 9 |
| O3 (ppb)3 | 48.3 | 14.3 |
| PM10 (μg/m3) | 29.5 | 10.2 |
| PM2.5 (μg/m3) | 12.0 | 4.4 |
| Long-term Exposures | ||
| Near-roadway air pollutants (NRAP) | ||
| Freeway NRAP (ppb)3 | 5.1 | 6. 2 |
| Non-freeway NRAP (ppb)3 | 1.5 | 1.0 |
| Total NRAP (ppb)3 | 6.6 | 6.6 |
| Regional air pollutants | ||
| NO2 (ppb)3 | 15.7 | 4. 0 |
| O3 (ppb)3 | 48.2 | 6.4 |
| PM10 (μg/m3) | 30.2 | 7.3 |
| PM2.5 (μg/m3) | 12.0 | 2.6 |
Short-term air pollution exposure was estimated from the individual residential history for lagged 1-month exposure level prior to the study visit.
Long-term air pollution exposure was estimated from the individual residential history for lagged 1-year exposure level prior to the study visit.
ppb: parts per billion, as the unit of NRAP, NO2 and O3 exposure
Classifications of Metabolomic PCs
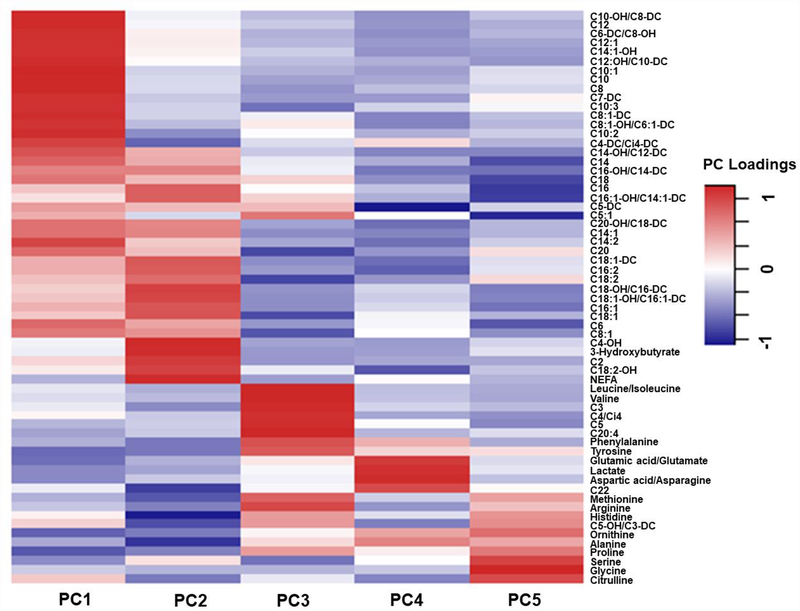
Means (SDs) of serum concentrations of all metabolites analyzed from 173 samples are presented in Supplemental Table 1. Similar PC patterns were identified comparing raw metabolomic data and the MI-imputed data (Supplemental Table 2). Therefore, we used PCs classified from the raw metabolomic data for all the following analyses. Overall, principal component analysis identified 14 orthogonal factors with eigenvalues ≥1.0. In our main analysis, we focused on the top five PCs, which explained a total of 52.6% variance of serum concentrations of 63 metabolites (Figure 1, Supplemental Table 2 and Supplemental Figure 1). Among these five PCs, PC1 mainly represents a group of short- and median-chain acylcarnitines, PC2 is characterized by NEFA and its oxidation by-products, PC3 represents metabolites involved in branched-chain amino-acid (BCAA) catabolism, PC4 represents amino acids involved in general amino acid catabolism and the urea cycle, and PC5 represents metabolites related to glycine metabolism.
Figure1.
Heatmap of loadings for the first five principal components identified from targeted metabolite concentrations among 173 adolescents and young adults enrolled in the Children’s Health Study.
NRAP exposure was associated with lower concentrations of short- and median-chain acylcarnitines and higher concentrations of NEFA oxidation by-products
No significant departure from a linear relationship was observed between short-term or long-term NRAP and regional air pollution exposures with the outcome variables of metabolomic factor PC scores (all p’s for cubic spline terms>0.10). Therefore, results described below are based on the linear relationships between air pollution exposure and PC scores.
Table 3 presents the associations between NRAP exposure and metabolomic PC1-PC5 scores. After adjusting for potential confounders, higher long-term (lagged one-year average) freeway NRAP and total NRAP (a combination of freeway and non-freeway NRAP) exposure was associated with lower PC1 score, which represents lower concentrations of various short- and median-chain acylcarnitines (p=0.044 and 0.049, respectively). Meanwhile, higher exposure to long-term non-freeway NRAP was associated with higher NEFA-related PC2 score (p=0.038). Similar associations were found for short-term (lagged one-month) exposures to freeway, non-freeway and total NRAP with metabolomic PC scores (Supplemental Table 4). However, due to the relatively small sample size, none of the associations were statistically significant after multiple testing adjustment for five PC scores (all FDR’s >0.19).
Table 3.
Associations between long-term1 near-roadway air pollution (NRAP) exposure and metabolomic principal component (PC) scores estimated from 173 adolescents and young adults in the Children’s Health Study.
| Entire sample (N=173) |
Normal weight (N=46)3 |
Overweight (N=77)3 |
Obese (N=50)3 |
Interaction p4 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| β2 | p3 | β2 | p3 | β2 | p3 | β2 | p3 | ||
| Outcome=PC15 | |||||||||
| Freeway NRAP | −0.34 | 0.0 44 | −0. 77 | 0.23 | −0. 36 | 0.18 | −0.48 | 0.09 | 0.71 |
| Non-freeway NRAP | −0.07 | 0.63 | −0.67 | 0.12 | −0.37 | 0.16 | 0.45 | 0.08 | 0.10 |
| Total NRAP | −0.33 | 0.049 | −0.88 | 0.15 | −0.39 | 0.14 | −0.37 | 0.21 | 0.51 |
| Outcome=PC26 | |||||||||
| Freeway NRAP | 0.09 | 0.58 | 0.08 | 0.85 | 0.01 | 0.97 | 0.10 | 0.73 | 0.53 |
| Non-freeway NRAP | 0.30 | 0.038 | −0.21 | 0.51 | 0.51 | 0.039 | 0.68 | 0.007* | 0.047 |
| Total NRAP | 0.14 | 0.42 | 0.003 | 0.99 | 0.07 | 0.78 | 0.23 | 0.44 | 0.48 |
| Outcome=PC37 | |||||||||
| Freeway NRAP | −0.21 | 0.18 | 0.25 | 0.64 | −0.03 | 0.91 | −0.80 | 0.006* | 0.66 |
| Non-freeway NRAP | 0.03 | 0.81 | −0.01 | 0.99 | 0.17 | 0.47 | 0.01 | 0.96 | 0.87 |
| Total NRAP | −0.19 | 0.22 | 0.22 | 0.67 | 0.002 | 0.99 | −0.86 | 0.005* | 0.60 |
| Outcome=PC48 | |||||||||
| Freeway NRAP | −0.01 | 0.92 | −0.07 | 0.82 | −0.10 | 0.66 | 0.16 | 0.57 | 0.49 |
| Non-freeway NRAP | −0.03 | 0.80 | −0.24 | 0.24 | 0.001 | 1.00 | −0.36 | 0.18 | 0.96 |
| Total NRAP | −0.02 | 0.87 | −0.11 | 0.68 | −0.09 | 0.69 | 0.09 | 0.76 | 0.50 |
| Outcome=PC59 | |||||||||
| Freeway NRAP | 0.21 | 0.24 | −0.20 | 0.71 | 0.30 | 0.33 | 0.22 | 0.44 | 0.75 |
| Non-freeway NRAP | 0.21 | 0.19 | 0.25 | 0.49 | 0.38 | 0.22 | −0.27 | 0.30 | 0.17 |
| Total NRAP | 0.24 | 0.18 | −0.10 | 0.85 | 0.34 | 0.27 | 0.17 | 0.56 | 0.66 |
Long-term air pollution exposure was estimated by cumulative averages of monthly exposure concentrations over one year prior to the study visit and were weighted by time spent at each different address.
Mixed effects model was used to estimate regression associations between freeway, nonfreeway and total NRAP exposure with the outcome variables of metabolomic factor scores after adjusting for confounders including age, sex, race/ethnicity, parental education levels as a proxy for social economic status, whether or not smoked cigarettes during the last week, ever/never smoked electronic cigarettes, season of the study visit and random intercepts for CHS communities and metabolomics analytical batches. NRAP exposure was scaled by 2 standard deviations. Association estimates (β) and p-values for significance of statistical tests are presented in the table.
Stratified analysis was conducted in each subgroups of body mass index (BMI) categories: normal weight (BMI<25 kg/m2), overweight (BMI≥25 and <30 kg/m2) and obese (BMI≥30 kg/m2) groups.
Interaction p-values<0.05 represent statistically significant differences in effect sizes for the associations between NRAP exposure and metabolomic PC scores across three BMI categories.
Metabolomic principal component (PC) 1 represents a variety of short- and median-chain acylcarnitines and explains 26.3% variance of concentrations of all metabolites analyzed in the study samples. More details of the loadings of specific metabolites for each PC are described in Supplemental Table 4.
PC2 represents non-esterified fatty acids (NEFA) and NEFA oxidation by-products including long-chain acylcarnitines, acetylcarnitine (C2) and 3-Hydroxybutyrylcarnitine (C4-OH), which explains 10.2% variance of concentrations of all metabolites analyzed in the study samples.
PC3 represents metabolites involved in branched-chain amino-acid (BCAA) catabolism, which explains 7.2% variance of concentrations of all metabolites analyzed in the study samples.
PC4 represents amino acids involved in general amino acid catabolism and the urea cycle, which explains 4.9% variance of concentrations of all metabolites analyzed in the study samples.
PC5 represents amino acids involved in glycine metabolism, which explains 4.1% variance of concentrations of all metabolites analyzed in the study samples.
Association tests are significant after FDR adjustment for multiple testing of five PC scores (FDR<0.1).
Findings from single pollutant models were further supported by the multi-pollutant regression analyses, which included both freeway and non-freeway NRAP exposure in one model for each PC (Supplemental Table 5). Among all study participants, long-term freeway NRAP exposure was negatively associated with PC1 score and non-freeway NRAP exposure was positively associated with PC2 score (p=0.05 and 0.025, respectively). No significant associations were found between NRAP exposure and other PC scores.
Next, we explored long-term NRAP exposure association with concentrations of individual metabolites among 32 metabolites that have loadings>0.4 in either PC1 or PC2 (Supplemental Table 6). Glycerol was also included in the single metabolite analyses as an additional measurement of lipolysis.66 Higher long-term and short-term non-freeway NRAP exposures were significantly associated with increased circulating glycerol and higher concentrations of NEFA-oxidation products: two long-chain acylcarnitines (C18:1-DC and C20-OH/C18-DC), 3-Hydroxybutyrylcarnitine (C4-OH) (FDR-adjusted p≤0.1). No other NRAP exposures were found to be significantly associated with concentrations of individual metabolites after adjusting for multiple testing (data not shown).
Furthermore, multi-pollutant analysis including exposures to three regional and near-roadway air pollutants in one model for each PC showed that higher lagged one-month exposure to regional PM2.5 was associated with lower PC1 score, while higher lagged one-month exposure to regional NO2 was associated with higher PC1 score (Supplemental Table 7, p=0.041 and 0.002, respectively). We further confirmed that the previously observed associations between freeway NRAP exposure with PC1 score were not significantly influenced by adjustment for regional air pollution exposures. However, adjustment for regional air pollution exposure attenuates the association between nonfreeway NRAP exposure and metabolomic PC2 score. No other significant associations were observed for long-term or short-term exposures to regional air pollutants with metabolomic PCs in this study. It was not possible to disentangle the relative importance of short- versus long- term exposures due to the high collinearity between these two exposure metrics (Supplemental Table 3).
Lastly, in a subset of 132 participants who had data from 24-hr diet recalls and self-reported physical activity status in the previous year (detailed characteristics are presented in Supplemental Table 8), we confirmed that the associations between nonfreeway NRAP exposure and PC2 score remained significant after additionally adjusting for total calorie intake, percent calorie intake from fat and protein, glycemic index in the daily diet, whether or not participating in exercise classes in the previous year and self-rated physical activity scale (Supplemental Table 9). The associations between freeway and total NRAP exposures with PC1 score were still negative but not statistically significant among this subset of participants. It is noted that sociodemographic characteristics among this subset of participants were not significantly different from the entire study sample, though there was a 10% higher frequency of overweight and obese participants in the subset of participants compared to the entire study sample.
Associations between NRAP exposure and metabolomic PCs are differentiated by BMI categories and sex
The associations of long-term non-freeway NRAP exposure with NEFA oxidation-related PC2 score were different across BMI categories (interaction p=0.047) (Table 3). Non-freeway NRAP exposure had the strongest positive association with PC2 score among overweight and obese participants. Two standard deviations (2.02 ppb) increase in lagged one-year average of non-freeway NRAP exposure was associated with 0.5 and 0.7 unit increase in PC2 score among overweight and obese participants [β (p) for overweight: 0.51(0.039) and obese 0.68 (0.007)]. While associations between non-freeway NRAP exposure and PC2 score had smaller effect size and was not statistically significant among normal-weight participants (β=−0.21, p=0.51). Similar effect modifications by BMI categories were also found for the associations of short-term non-freeway NRAP exposure with PC2 score (Supplemental Table 4). In addition, although there was no significant association between NRAP exposure and the BCAA-related PC3 score, higher freeway and total NRAP exposure was associated with lower BCAA-related PC3 score in obese participants (p=0.006 and 0.005, respectively).
Associations of long-term freeway NRAP exposure with NEFA-related PC2 score were more evident in females than in males (Table 4, interaction p=0.049). Two standard deviations (12.4 ppb) increase in lagged one-year average of freeway NRAP exposure was associated with 0.5 unit increase in PC2 score among females (β=0.52, p=0.042), but there was no significant association between freeway NRAP exposure and PC2 score among males (β=−0.13, p=0.55). Similar effect modification was found for short-term freeway NRAP exposure and PC2 score [β (p) for females: 0.71 (0.008) and for males: −0.15 (0.51)]. Along with these results, the mean concentration of NEFA was 0.39 mmol/L higher in females than males (p=0.011). However, there was no significant difference in mean BMI between males and females [mean BMI (SD) for males: 28.1 (5.2) kg/m2 and for females: 28.5 (5.3) kg/m2]. Lastly, no significant difference was found for associations between NRAP exposure and metabolomic PC2 score between non-Hispanic white and Hispanic white participants.
Table 4.
Associations between near-roadway air pollution (NRAP) exposure and NEFA-related metabolomic principal component (PC)1 scores stratified by sex and race/ethnicity among adolescents and young adults in the Children’s Health Study.
| NRAP Exposure | Males (N=94) |
Females (N=79) |
Interaction p3 |
Non-Hispanic Whites (N=50) |
Hispanic Whites (N=97) |
Interaction p4 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| β2 | p | β2 | p | β2 | p | β2 | p | |||
| Long-term exposure5 | ||||||||||
| Freeway NRAP | −0.13 | 0.55 | 0.52 | 0.0 42 | 0.049 | −0.30 | 0.63 | 0.17 | 0.35 | 0.27 |
| Non-freeway NRAP | 0.31 | 0.08 | 0.50 | 0.032 | 0.21 | 0.24 | 0.39 | 0.39 | 0.044 | 0.62 |
| Total NRAP | −0.05 | 0.83 | 0.65 | 0.008 | 0.049 | −0.05 | 0.93 | 0.23 | 0.21 | 0.29 |
| Short-term exposure6 | ||||||||||
| Freeway NRAP | −0.15 | 0.51 | 0.71 | 0.008 | 0.033 | −0.40 | 0.55 | 0.24 | 0.16 | 0.49 |
| Non-freeway NRAP | 0.32 | 0.09 | 0.45 | 0.06 | 0.44 | 0.31 | 0.30 | 0.32 | 0.11 | 0.75 |
| Total NRAP | −0.07 | 0.76 | 0.74 | 0.005 | 0.036 | −0.06 | 0.92 | 0.27 | 0.12 | 0.52 |
NEFA-related metabolomic PC (also known as PC2 in this paper) represents non-esterified fatty acids (NEFA) and NEFA oxidation by-products including long-chain acylcarnitines, acetylcarnitine (C2) and 3-Hydroxybutyrylcarnitine (C4-OH), which explains 10.2% variance of concentrations of all 63 metabolites analyzed in the study samples.
Mixed effects model was used to estimate regression associations between freeway, non-freeway and total NRAP exposure with the outcome variables of metabolomic factor scores after adjusting for confounders including age, sex, race/ethnicity, parental education levels as a proxy for social economic status, whether or not smoked cigarettes during the last week, ever/never smoked electronic cigarettes, season of the study visit and random intercepts for CHS communities and metabolomics analytical batches. NRAP exposure was scaled by two standard deviations. Association estimates (β) and p-values for significance of statistical tests are presented in the table.
Interaction p-values <0.05 represent statistically significant differences in effect sizes for the associations between NRAP exposure and PC2 score comparing males to females.
Interaction p-values <0.05 represent statistically significant differences in effect sizes for the associations between NRAP exposure and PC2 score comparing Non-Hispanic Whites to Hispanic Whites.
Long-term air pollution exposure was estimated by cumulative averages of monthly exposure concentrations over one year prior to the study visit and were weighted by time spent at each different address.
Short-term air pollution exposure was estimated by one-month exposure concentrations prior to the study visit.
Lower concentrations of short- and median-chain acylcarnitines and higher concentrations of BCAA-related metabolites were associated with higher BMI and an increased odd of obesity.
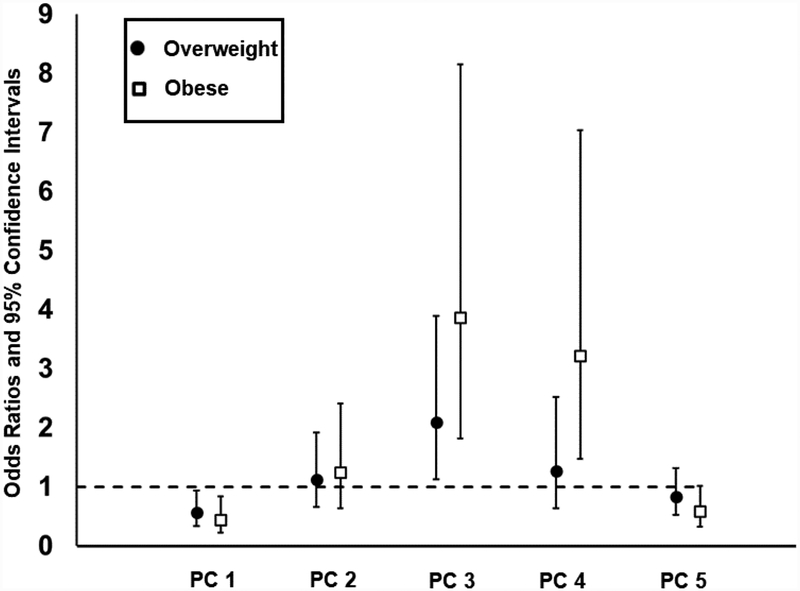
We found that lower metabolomic PC1 score (i.e., lower concentrations of short- and median-chain acylcarnitines), higher PC3 score (i.e., higher concentrations of BCAA and related metabolites), higher PC4 score (i.e., higher concentrations of intermediates involved in catabolism of multiple amino acids), and lower PC5 scores (i.e., lower concentrations of glycine and other related amino acids) were all significantly associated with higher BMI after adjusting for potential confounders (Table 5, all p’s≤0.001). A one-unit increases in the PC1 score was associated with 44% and 57% lower odds for an individual to be overweight and obese rather than normal weight [Figure 2, odds ratios (ORs) and 95% confidence intervals (CIs)=0.56 (0.34, 0.94) and 0.43 (0.23, 0.84), respectively]. Meanwhile, individuals with one-unit increases in the PC3 score were 2–4 fold more likely to be overweight and obese compared to normal weight [Figure 2, ORs (95% CIs) = 2.09 (1.12, 3.89) and 3.86 (1.82, 8.15), respectively].
Table 5.
Associations between metabolomic principal component (PC) scores and body mass index (BMI) among 173 adolescents and young adults.
| Independent Variables | β6 | p |
|---|---|---|
| Metabolomic PCs | ||
| PC11 | −1.21 | <0.001 |
| PC22 | 0.30 | 0.42 |
| PC33 | 1.51 | <0.001 |
| PC44 | 2.34 | <0.001 |
| PC55 | −1.01 | 0.001 |
| Race/Ethnicity | ||
| Non-Hispanic White | ref | |
| Hispanic White | 0.44 | 0.56 |
| Other | 0.95 | 0.34 |
| Sex | ||
| Male | ref | |
| Female | −0.42 | 0.55 |
| Age | −0.01 | 0.96 |
| Parental education | ||
| Less than high school | ref | |
| Completed high school | −0.21 | 0.79 |
| Some college or higher | −0.87 | 0.30 |
| Unknown | −2.04 | 0.35 |
| Ever used e-cigarette | ||
| No | ref | |
| Yes | 0.87 | 0.25 |
| Cigarette smoke in the last week | ||
| No | ref | |
| Yes | −1.15 | 0.40 |
Metabolomic principal component (PC) 1 represents a variety of short- and median-chain acylcarnitines and explains 26.3% variance of concentrations of all metabolites analyzed in the study samples. More details of the loadings of specific metabolites for each PC are described in Supplemental Table 4.
PC2 represents non-esterified fatty acids (NEFA) and NEFA oxidation by-products including long-chain acylcarnitines, acetylcarnitine (C2) and 3-Hydroxybutyrylcarnitine (C4-OH), which explains 10.2% variance of concentrations of all metabolites analyzed in the study samples.
PC3 represents metabolites involved in branched-chain amino-acid (BCAA) catabolism, which explains 7.2% variance of concentrations of all metabolites analyzed in the study samples.
PC4 represents amino acids involved in general amino acid catabolism and the urea cycle, which explains 4.9% variance of concentrations of all metabolites analyzed in the study samples.
PC5 represents amino acids involved in glycine metabolism, which explains 4.1% variance of concentrations of all metabolites analyzed in the study samples.
Mixed effects model with all five PCs in one regression model and adjustment for socio-demographic confounders was used to estimate independent association of each metabolomic PC with BMI as a continuous outcome variable. Association estimates (β) and p-values are presented for each independent variable in the analysis model.
Figure 2.
Associations between metabolomic principal component (PC) scores and relative odds to be overweight or obese compared to normal weight. Odds ratios and 95% confidence intervals are presented for comparing odds to be overweight and obese compared to normal weight with one unit increase in metabolomic PC scores (PC1-PC5) among 173 adolescents and young adults.
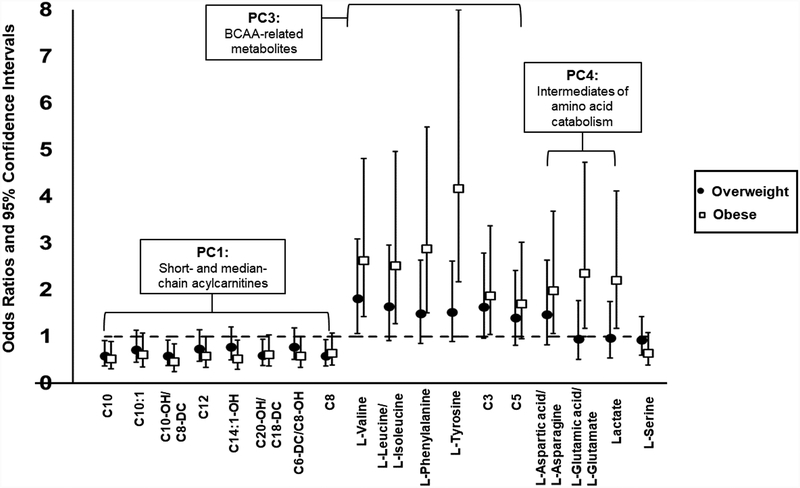
We further explored the associations between individual metabolite concentrations and odds of being overweight and obese (Figure 3). After adjusting for multiple testing across 33 metabolites that had the loadings in PC1, PC3, PC4 and PC5 greater than 0.4, as well as glycerol, we found that increased concentrations of eight acylcarnitines had significant associations with reduced odds of being overweight or obese (all FDR-adjusted p’s<0.1). Conversely, increased concentrations of six amino acids (including valine, leucine/isoleucine) and byproducts of BCAA metabolism, C3 and C5 acylcarnitines, were associated with increased odds of being overweight or obese (all FDR-adjusted p’s <0.1).
Figure 3.
Significant associations of concentrations of individual metabolites that represent principal components (PC) - PC1, PC3 and PC5 with relative odds of being overweight or obese compared to normal weight. Odds ratios and 95% confidence intervals are presented for comparing odds to be overweight and obese compared to normal weight with one standard deviation increase in each metabolite among 173 adolescents and young adults. Significant associations are selected based on the criterion of FDR-adjusted p-values<0.1.
In the subset of 132 participants who had complete dietary and physical activity data collected, we verified that the association of lower PC1 and PC5 scores, as well as higher PC3 and PC4 scores with higher BMI and increased odds of obesity remained statistically significant after adjustment for total calorie intake, percent calorie intake from fat and protein, glycemic index in the daily diet, whether or not participating exercise classes in the previous year and self-rated physical activity scale (Supplemental Table 10 and Supplemental Figure 2).
CONCLUSIONS
A main finding of this study is that higher NRAP exposure, especially non-freeway NRAP, was associated with higher concentrations of glycerol and metabolites related to NEFA oxidation. This finding provides parallel evidence for our previous observations from the longitudinal CHS cohort that increased NRAP exposure during early life and childhood periods was associated with increased risk of obesity in later life.6,54,74 It is well-known that plasma levels of NEFA and its oxidation byproducts such as C4-OH are associated with increased adiposity and insulin resistance.75–78 Expanding fat mass releases correlate with increases in circulating NEFA and glycerol, which serve to induce insulin resistance and inflammation.75–77 Accordingly, we found that higher NRAP exposure was associated with an increased PC score related to increased concentrations of a mixture of metabolites including NEFA and its oxidation byproducts (C2, C4-OH and several long-chain acylcarnitines). Higher NRAP exposure was also associated with increased serum glycerol. These results suggest that NRAP exposure could contribute to increased demand for mitochondrial NEFA oxidation. However, the association between air pollution exposure and serum NEFA concentration was not statistically significant in this study, which suggests that adolescents and young adults may still have adequate mitochondrial capacity to compensate for environmental stressors such as air pollution exposures.
Consistent with the foregoing constructs, we also found that the positive association between NRAP exposure and NEFA-related PC was strongest among obese participants. The effect modification by obesity status indicates that increased NRAP exposure could exacerbate obesity-induced inflammation and metabolic dysfunction. NRAP exposure can stimulate lung and systemic inflammation through the activation of immunomodulatory pattern-recognition receptors such as Toll-like receptors (TLRs).79–82 Meanwhile, NEFA plays an important role in obesity-induced inflammation.75–78 One pathway for NEFA to activate systemic inflammation is through its activation of proinflammatory nuclear factor (NF)-κB pathway, which was partially mediated by the TLR-4.83–85 Therefore, NRAP exposure could share similar inflammatory pathways as NEFA and have far-reaching impact on systemic inflammation and insulin resistance in obese individuals.
Another interesting finding about the NEFA oxidation-related PC is that even though freeway NRAP exposure was not significantly associated with NEFA-related PC score in the entire sample, there was a statistically significant positive association between freeway NRAP exposure and NEFA-related PC score among females. Previous studies showed that sex differences in metabolomic patterns were observed in childhood and may persist in adolescents and adults.86,87 In this study, we also observed that female participants had higher mean serum NEFA concentrations than males. Although the overall BMI was not significantly different between males and females, differences in body fat distribution19,88,89 and sex steroids90 may contribute to differential impacts of NRAP exposure on NEFA-related metabolomic profiles.
Meanwhile, we also found that lower concentrations of a variety of short- and medium-chain acylcarnitines were associated with an increased risk of obesity. The lower levels of acylcarnitines in obese subjects observed here are consistent with observations from another metabolomics study.91 One possible interpretation of these findings is that with increased demand for mitochondrial NEFA oxidation, early oxidative intermediates (embodied by the long-chain acylcarnitines) begin to accumulate, while levels of downstream metabolites decrease, possibly suggesting saturation of early steps in the β-oxidative pathway. This signature in adolescence may eventually give way to a more complete dysregulation of fatty acid metabolism as has been observed in adults, involving accumulation of essentially all lipid-derived acylcarnitine intermediates, suggested to be a signal of mitochondrial substrate overload.92
Our study also confirms the association of BCAAs and metabolites generated by their oxidation, C3, C5 acylcarnitines, with BMI. Similar associations between dysregulated metabolism of BCAAs, obesity and insulin resistance have been widely observed in adult animal31,64 and human studies.13–16,31–35,93 We also found freeway NRAP exposure was associated with lower BCAA and related metabolites only among obese participants, suggesting that NRAP exposure could interact with obesity in the BCAA profile. The inverse relationship between NRAP and BCAA may be confounded by diet and other uncontrolled socio-behavior factors.94–97 Therefore, more studies are needed to explore the associations between NRAP exposure and BCAA-related metabolism.
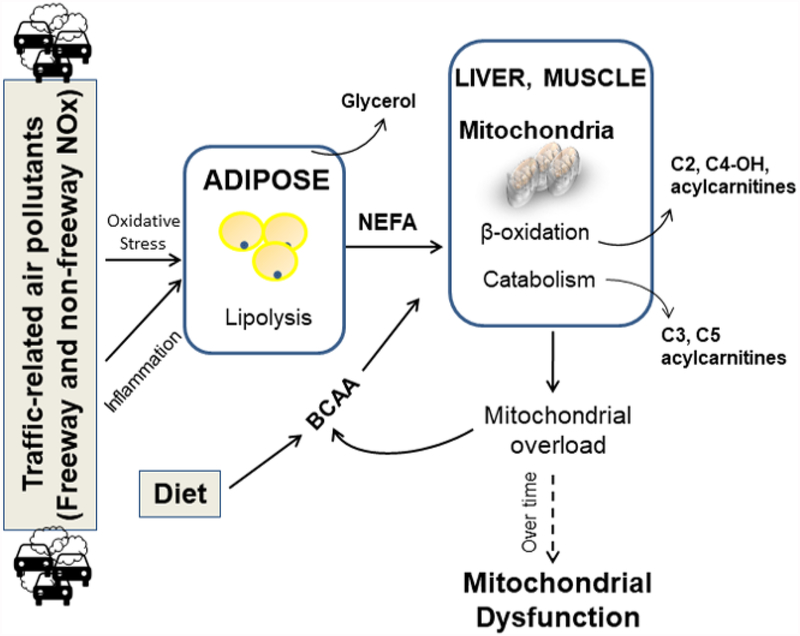
Based on the above findings, we summarized the potential pathways for NRAP exposure to influence lipid and amino acid metabolism in Figure 4. NRAP exposure might increase adipose lipolysis and release of NEFA and glycerol, contributing to activation of oxidative stress and inflammation pathways.98–102 Increased circulating NEFA increases demand for fatty acid oxidation. Even though adolescents and young adults have generally normal mitochondrial function to compensate for the increased demand of NEFA oxidation, this early adaptive metabolic plasticity is very likely to be diminished over time from youth to adulthood, especially for obese individuals. Finally, it is expected that persistent NRAP exposure and obesity from childhood to adulthood will eventually cause mitochondrial dysfunction and the development of metabolic diseases.
Figure 4.
The potential pathways for near-roadway air pollution (NRAP) exposure to influence lipid and amino acid metabolism. NEFA: Non-esterified fatty acids; BCAA: branched-chain amino acids. Increased NRAP exposure might increase adipose lipolysis and release of NEFA and glycerol. Increased circulating NEFA requires increased mitochondrial function for NEFA oxidation, which competes the mitochondrial capacity of amino acid metabolism. Therefore, serum concentrations of BCAAs and other amino acids can be elevated at the same time.
To our knowledge, this is the first targeted-metabolomics study in adolescents and young adults that investigates the role of metabolomic profiles in the impact of air pollution exposure on metabolic dysfunction. Several advantages of this study include 1) robust measures of targeted metabolites that participate in metabolic pathways of the three major classes of macronutrients—lipids, carbohydrates, and protein, 2) unique study population of adolescents and young adults who are at the initial stage in developing metabolic diseases, and 3) state-of-the-art air pollution exposure assessment and detailed sociodemographic and health behavior covariates. Overall, results of this study support the link between air pollution exposure and dysregulated lipid metabolism, which was observed in several other non-targeted and targeted metabolomics studies.36–39 More importantly, results of this study further provide evidence that dysregulated fatty acid metabolism could be an essential pathway in obese youth for NRAP exposure to influence metabolism and trigger the development of cardio-metabolic diseases in later life.
We also acknowledge that the current study has several limitations. First, the cross-sectional and observational design of our study precludes us from conclusions inferring any causal relationship between air pollution exposures, metabolomic changes, and the development of metabolic dysfunction. Directionality cannot be discerned - i.e., whether the metabolites are leading to obesity or obesity is modifying metabolism cannot be distinguished from this study. Future longitudinal studies and animal models are needed to verify the mediation role of metabolomic changes in the relationship between air pollution exposure and the development of obesity and metabolic diseases. Also, the sample size of this study is relatively small. Findings of this study will need to be verified by other large replication cohorts. Second, we were not able to discriminate the impact of short-term and long-term air pollution exposures on metabolomic outcomes because the short-term and long-term air pollution exposures were highly correlated in this study. Furthermore, future longitudinal studies with dynamic assessment of life-time air pollution exposure and health outcomes are warranted to identify critical exposure window during life time for the adverse effect of air pollution exposures on metabolomic dysfunction in children and youth. Third, there could be residual confounders not included in the analysis such as geospatial clustering and early-life socio-behavioral factors, which may explain our observation of the opposing effect of NO2 on the PC1 score of short- and median-chain acylcarnitines compared to the effect of PM2.5 and freeway NRAP exposures. Finally, most of our significant findings of NRAP associations with altering fatty acid metabolism were related to non-freeway NRAP exposure. It has been shown that chemical composition of NARP exposure from freeway versus non-freeway roads are different due to differences in vehicle types and vehicle volume.103 Non-freeway NRAP exposure also contains more non-exhaust particles (e.g. brake wear and tire wear). It is also possible that non-freeway NRAP exposures capture other neighborhood characteristics (e.g. housing and built environment) that introduce some residual confounding. Future studies are needed to identify which specific components or chemicals of NRAP are detrimental to metabolic dysfunction.
In conclusion, our study suggests that NRAP exposure contributes to lipolysis and the altered fatty acid metabolism, manifest by increased circulating glycerol and NEFA oxidation byproducts. This pollution-triggered alteration in fatty acid metabolism may contribute to greater susceptibility of pollutant-exposed adolescents to metabolic diseases associated with obesity. However, these findings warrant future larger cohorts to replicate and explore these associations. Also, future studies are needed to account for sex and racial/ethnic differences in studying the impact of air pollution exposure on metabolic perturbations.
Supplementary Material
Highlights:
Near-roadway air pollution exposure is associated with altered fatty acid metabolism
Near-roadway air pollution exposure interacts with obesity and sex in the association with fatty acid metabolism.
Air pollution-triggered alteration in fatty acid metabolism may contribute to greater susceptibility of pollutant-exposed adolescents to metabolic diseases associated with obesity
Acknowledgments
Z.C. conducted the analyses and wrote the article. Z.C., F.D.G., E.R.H., C.B.N. and C.B. contributed to study design. Z.C., F.D.G., J.K., T.L.A., O.I., E.A., F.L. and M.M. contributed to the data collection. Z.C., E.R.H., D.C.T. and K.B. contributed to the development of statistical methods. E.R.H., D.C.T., K.B., J.K., T.L.A., C.B.N., C.B., L.C., T.M.B., E.A., F.L., R.M., and F.D.G. edited the article and contributed to discussion. All authors reviewed the article. Z.C. is the guarantor of this work, and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors declare that they have no conflict of interest.
Sources of Funding Support
This work was supported by National Institute of Environmental Health Sciences (K99ES027870, 4R00ES027870–03 and R00ES027853); Southern California Children’s Environmental Health Center grant funded by National Institute of Environmental Health Sciences (5P01ES022845–03) and United States Environmental Protection Agency (RD83544101); the Southern California Environmental Health Sciences Center grant (5P30ES007048) funded by the National Institute of Environmental Health Sciences; National Institute of Environmental Health Sciences (5P01ES011627); and the Hastings Foundation.
Abbreviations:
- NRAP
near-roadway air pollution
- BMI
body mass index
- PM2.5
particulate matter with aerodynamic diameter <2.5 μm
- CHS
Children’s Health Study
- DORI
Diabetes and Obesity Research Institute
- CTU
Clinical Trials Unit
- CDC
Centers for Disease Control and Prevention
- NDSR
Nutrition Data System for Research
- NCC
Nutrition Coordinating Center
- IRB
Institutional Review Board
- NOx
nitrogen oxides
- CALINE4
California line-source dispersion
- NO2
nitrogen dioxides
- O3
ozone
- FRM
Federal Reference Method
- FEM
Federal Equivalent Method
- IDW2
inverse distance-squared weighting
- NEFA
non-esterified free fatty acids
- PCA
principal components analysis
- PC
principal components
- MI
multiple imputation
- FDR
false discovery rate
- SD
standard deviation
- BCAA
branched-chain amino-acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA : the journal of the American Medical Association 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Perrin EM, Skelton JA. Prevalence of obesity and severe obesity in US children, 1999–2014. Obesity 2016;24:1116–23. [DOI] [PubMed] [Google Scholar]
- 3.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight and obesity among children and adolescents: United States, 1963–1965 through 2011–2012. URL: https://www.cdc.gov/nchs/data/hestat/obesity_child_11_12/obesity_child_11_12.htm. Accessed February 5, 2019 Health E-Stats2014.
- 4.National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States 2014. (Accessed Feburary 13th, 2016, at http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html.). 2014.
- 5.Centers for Disease Control and Prevention (CDC). Diabetes Report Card, available at https://www.cdc.gov/diabetes/library/reports/congress.html, accessed on Feb. 1st, 2017 2014.
- 6.McConnell R, Shen E, Gilliland FD, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ Health Perspect 2015;123:360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerrett M, McConnell R, Wolch J, et al. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environmental Health 2014;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Salam MT, Toledo-Corral C, et al. Ambient Air Pollutants Have Adverse Effects on Insulin and Glucose Homeostasis in Mexican Americans. Diabetes Care 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alderete TL, Habre R, Toledo-Corral CM, et al. Longitudinal Associations Between Ambient Air Pollution with Insulin Sensitivity, beta-Cell Function, and Adiposity in Los Angeles Latino Children. Diabetes 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toledo-Corral CM, Alderete TL, Habre R, et al. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr Obes 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teichert T, Vossoughi M, Vierkötter A, et al. Association between Traffic-Related Air Pollution, Subclinical Inflammation and Impaired Glucose Metabolism: Results from the SALIA Study. PLoS ONE 2013;8:e83042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiering E, Markevych I, Bruske I, et al. Associations of Residential Long-Term Air Pollution Exposures and Satellite-Derived Greenness with Insulin Resistance in German Adolescents. Environ Health Perspect 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newgard CB. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab 2017;25:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu F, Narasimhan K. Nutritional genomics and metabolomics in obesity and type 2 diabetes. DOI: 10.1186/1471-2164-15-S2-O10. BMC Genomics 2014;15:O10. [DOI] [Google Scholar]
- 15.Perng W, Gillman MW, Fleisch AF, et al. Metabolomic profiles and childhood obesity. Obesity 2014;22:2570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butte NF, Liu Y, Zakeri IF, et al. Global metabolomic profiling targeting childhood obesity in the Hispanic population. The American Journal of Clinical Nutrition 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isganaitis E, Rifas-Shiman SL, Oken E, et al. Associations of cord blood metabolites with early childhood obesity risk. Int J Obes (Lond) 2015;39:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S, Sadanala KC, Kim EK. A Metabolomic Approach to Understanding the Metabolic Link between Obesity and Diabetes. Molecules and cells 2015;38:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szymanska E, Bouwman J, Strassburg K, et al. Gender-dependent associations of metabolite profiles and body fat distribution in a healthy population with central obesity: towards metabolomics diagnostics. Omics : a journal of integrative biology 2012;16:652–67. [DOI] [PubMed] [Google Scholar]
- 20.Martin F-PJ, Montoliu I, Collino S, et al. Topographical Body Fat Distribution Links to Amino Acid and Lipid Metabolism in Healthy Non-Obese Women. PLoS ONE 2013;8:e73445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalhan SC, Guo L, Edmison J, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011;60:404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Schonfels W, Patsenker E, Fahrner R, et al. Metabolomic tissue signature in human non-alcoholic fatty liver disease identifies protective candidate metabolites. Liver international : official journal of the International Association for the Study of the Liver 2015;35:207–14. [DOI] [PubMed] [Google Scholar]
- 23.Xie Z, Li H, Wang K, et al. Analysis of transcriptome and metabolome profiles alterations in fatty liver induced by high-fat diet in rat. Metabolism 2010;59:554–60. [DOI] [PubMed] [Google Scholar]
- 24.Calvo N, Beltran-Debon R, Rodriguez-Gallego E, et al. Liver fat deposition and mitochondrial dysfunction in morbid obesity: An approach combining metabolomics with liver imaging and histology. World journal of gastroenterology 2015;21:7529–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cano A, Alonso C. Deciphering non-alcoholic fatty liver disease through metabolomics. Biochemical Society transactions 2014;42:1447–52. [DOI] [PubMed] [Google Scholar]
- 26.Ho JE, Larson MG, Vasan RS, et al. Metabolite Profiles During Oral Glucose Challenge. Diabetes 2013;62:2689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krug S, Kastenmuller G, Stuckler F, et al. The dynamic range of the human metabolome revealed by challenges. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2012;26:2607–19. [DOI] [PubMed] [Google Scholar]
- 28.Menni C, Fauman E, Erte I, et al. Biomarkers for Type 2 Diabetes and Impaired Fasting Glucose Using a Nontargeted Metabolomics Approach. Diabetes 2013;62:4270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bain JR. Targeted metabolomics finds its mark in diabetes research. Diabetes 2013;62:349–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma Metabolomic Profiles Reflective of Glucose Homeostasis in Non-Diabetic and Type 2 Diabetic Obese African-American Women. PLoS ONE 2010;5:e15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009;32:1678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010;53:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer ND, Stevens RD, Antinozzi PA, et al. Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab 2015;100:E463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glynn EL, Piner LW, Huffman KM, et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 2015;58:2324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong A, Fiorito G, Keski-Rahkonen P, et al. Perturbation of metabolic pathways mediates the association of air pollutants with asthma and cardiovascular diseases. Environment international 2018;119:334–45. [DOI] [PubMed] [Google Scholar]
- 37.Liang D, Moutinho JL, Golan R, et al. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environment international 2018;120:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breitner S, Schneider A, Devlin RB, et al. Associations among plasma metabolite levels and short-term exposure to PM2.5 and ozone in a cardiac catheterization cohort. Environment international 2016;97:76–84. [DOI] [PubMed] [Google Scholar]
- 39.Ward-Caviness CK, Breitner S, Wolf K, et al. Short-term NO2 exposure is associated with long-chain fatty acids in prospective cohorts from Augsburg, Germany: results from an analysis of 138 metabolites and three exposures. Int J Epidemiol 2016;45:1528–38. [DOI] [PubMed] [Google Scholar]
- 40.Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect 2010;118:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakraborty J, Collins TW, Grineski SE, Maldonado A. Racial Differences in Perceptions of Air Pollution Health Risk: Does Environmental Exposure Matter? Int J Environ Res Public Health 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sohn D, Oh H. Gender-dependent Differences in the Relationship between Diabetes Mellitus and Ambient Air Pollution among Adults in South Korean Cities. Iran J Public Health 2017;46:293–300. [PMC free article] [PubMed] [Google Scholar]
- 43.Grineski SE, Collins TW. Geographic and social disparities in exposure to air neurotoxicants at U.S. public schools. Environ Res 2018;161:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Won EY, Yoon MK, Kim SW, et al. Gender-specific metabolomic profiling of obesity in leptin-deficient ob/ob mice by 1H NMR spectroscopy. PLoS One 2013;8:e75998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perng W, Gillman MW, Fleisch AF, et al. Metabolomic profiles and childhood obesity. Obesity (Silver Spring) 2014;22:2570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel MJ, Batch BC, Svetkey LP, et al. Race and sex differences in small-molecule metabolites and metabolic hormones in overweight and obese adults. Omics : a journal of integrative biology 2013;17:627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002:1–190. [PubMed] [Google Scholar]
- 48.Adult BMI. Retrived March 22nd, 2019, from https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html.
- 49.Centers for Disease Control and Prevition (2009b). BMI for children and teens Retrieved March 22, 2009, from https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
- 50.Hoffmann K, Boeing H, Dufour A, et al. Estimating the distribution of usual dietary intake by short-term measurements. Eur J Clin Nutr 2002;56 Suppl 2:S53–62. [DOI] [PubMed] [Google Scholar]
- 51.NDSR. Minneapolis, MN: Nutrition Coordinating Center; URL: http://www.ncc.umn.edu/products/ 2014 [Google Scholar]
- 52.Benson P CALINE4 – A Dispersion Model for Predicting Air Pollutant Concentrations Near Roadways. 1989;Report No. FHWA/CA/TL-84/15.
- 53.McConnell R, Islam T, Shankardass K, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect 2010;118:1021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JS, Alderete TL, Chen Z, et al. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environmental health : a global access science source 2018;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gauderman WJ, Urman R, Avol E, et al. Association of Improved Air Quality with Lung Development in Children. New England Journal of Medicine 2015;372:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters J, Avol E, Navidi W, et al. A Study of Twelve Southern California Communities with Differing Levels and Types of Air Pollution. American Journal of Respiratory and Critical Care Medicine 1999;159:760–7. [DOI] [PubMed] [Google Scholar]
- 57.Peters JM, Avol E, Gauderman WJ, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med 1999;159:768–75. [DOI] [PubMed] [Google Scholar]
- 58.Wong DW, Yuan L, Perlin SA. Comparison of spatial interpolation methods for the estimation of air quality data. J Expo Anal Environ Epidemiol 2004;14:404–15. [DOI] [PubMed] [Google Scholar]
- 59.Eckel SP, Cockburn M, Shu YH, et al. Air pollution affects lung cancer survival. Thorax 2016;71:891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah SH, Bain JR, Muehlbauer MJ, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010;3:207–14. [DOI] [PubMed] [Google Scholar]
- 61.Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012;55:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah SH, Hauser ER, Bain JR, et al. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol 2009;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes 2009;58:2429–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breier M, Wahl S, Prehn C, et al. Targeted metabolomics identifies reliable and stable metabolites in human serum and plasma samples. PLoS One 2014;9:e89728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schweiger M, Eichmann TO, Taschler U, Zimmermann R, Zechner R, Lass A. Measurement of lipolysis. Methods Enzymol 2014;538:171–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrara CT, Wang P, Neto EC, et al. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet 2008;4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nature medicine 2004;10:268–74. [DOI] [PubMed] [Google Scholar]
- 69.Worley B, Powers R. Multivariate Analysis in Metabolomics. Current Metabolomics 2013;1:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bartel J, Krumsiek J, Theis FJ. Statistical methods for the analysis of high-throughput metabolomics data. Comput Struct Biotechnol J 2013;4:e201301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schafer JL. Analysis of imcomplete multivariate data. London: Chapman & Hall; 1997. [Google Scholar]
- 72.Zhang P Multiple Imputation: Theory and Method. International Statistical Review / Revue Internationale de Statistique 2003;71:581–92. [Google Scholar]
- 73.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 74.Jerrett M, McConnell R, Chang CC, et al. Automobile traffic around the home and attained body mass index: a longitudinal cohort study of children aged 10–18 years. Prev Med 2010;50 Suppl 1:S50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boden G Obesity and free fatty acids. Endocrinology and metabolism clinics of North America 2008;37:635–46, viii–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abel ED. Free fatty acid oxidation in insulin resistance and obesity. Heart and metabolism : management of the coronary patient 2010;48:5–10. [PMC free article] [PubMed] [Google Scholar]
- 77.Lopaschuk GD. Fatty Acid Oxidation and Its Relation with Insulin Resistance and Associated Disorders. Annals of nutrition & metabolism 2016;68 Suppl 3:15–20. [DOI] [PubMed] [Google Scholar]
- 78.Turner N, Cooney GJ, Kraegen EW, Bruce CR. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol 2014;220:T61–79. [DOI] [PubMed] [Google Scholar]
- 79.Wei Y, Zhang JJ, Li Z, et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campen MJ, Lund A, Rosenfeld M. Mechanisms linking traffic-related air pollution and atherosclerosis. Current opinion in pulmonary medicine 2012;18:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grunig G, Marsh LM, Esmaeil N, et al. Perspective: ambient air pollution: inflammatory response and effects on the lung’s vasculature. Pulmonary circulation 2014;4:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. The Journal of allergy and clinical immunology 2012;129:14–24; quiz 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghosh AK, O’Brien M, Mau T, Yung R. Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging 2017;9:1971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Konner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab 2011;22:16–23. [DOI] [PubMed] [Google Scholar]
- 86.Newbern D, Gumus Balikcioglu P, Balikcioglu M, et al. Sex differences in biomarkers associated with insulin resistance in obese adolescents: metabolomic profiling and principal components analysis. J Clin Endocrinol Metab 2014;99:4730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng H, Yde CC, Arnberg K, et al. NMR-Based Metabolomic Profiling of Overweight Adolescents: An Elucidation of the Effects of Inter-/Intraindividual Differences, Gender, and Pubertal Development %J BioMed Research International. 2014;2014:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Christen T, Trompet S, Noordam R, et al. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides 2018;107:25–31. [DOI] [PubMed] [Google Scholar]
- 89.Mansour MF, Chan C-WJ, Laforest S, Veilleux A, Tchernof A. Sex Differences in Body Fat Distribution. In: Symonds ME, ed. Adipose Tissue Biology. Cham: Springer International Publishing; 2017:257–300. [Google Scholar]
- 90.Darst BF, Koscik RL, Hogan KJ, Johnson SC, Engelman CD. Longitudinal plasma metabolomics of aging and sex. bioRxiv 2018:436931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mihalik SJ, Michaliszyn SF, de las Heras J, et al. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care 2012;35:605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muoio DM. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell 2014;159:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balikcioglu PG, Newgard CB. Metabolomic Signatures and Metabolic Complications in Childhood Obesity In: Freemark MS, ed. Pediatric Obesity: Etiology, Pathogenesis and Treatment. Cham: Springer International Publishing; 2018:343–61. [Google Scholar]
- 94.Merz B, Frommherz L, Rist MJ, Kulling SE, Bub A, Watzl B. Dietary Pattern and Plasma BCAA-Variations in Healthy Men and Women-Results from the KarMeN Study. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rousseau M, Guenard F, Garneau V, et al. Associations Between Dietary Protein Sources, Plasma BCAA and Short-Chain Acylcarnitine Levels in Adults. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorska-Warsewicz H, Laskowski W, Kulykovets O, Kudlinska-Chylak A, Czeczotko M, Rejman K. Food Products as Sources of Protein and Amino Acids-The Case of Poland. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Z, Herting MM, Chatzi L, et al. Regional and traffic-related air pollutants are associated with higher consumption of fast food and trans fat among adolescents. The American Journal of Clinical Nutrition 2018;109:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chahine T, Baccarelli A, Litonjua A, et al. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect 2007;115:1617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Delfino RJ, Staimer N, Vaziri ND. Air pollution and circulating biomarkers of oxidative stress. Air quality, atmosphere, & health 2011;4:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. Journal of toxicology 2011;2011:487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rich DQ, Kipen HM, Huang W, et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA : the journal of the American Medical Association 2012;307:2068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moller P, Danielsen PH, Karottki DG, et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutation research Reviews in mutation research 2014;762:133–66. [DOI] [PubMed] [Google Scholar]
- 103.Fujita EM, Zielinska B, Campbell DE, et al. Variations in speciated emissions from spark-ignition and compression-ignition motor vehicles in California’s south coast air basin. J Air Waste Manag Assoc 2007;57:705–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.