Abstract
Background: The purpose of this guideline is to optimize evaluation and management of patients with obesity hypoventilation syndrome (OHS).
Methods: A multidisciplinary panel identified and prioritized five clinical questions. The panel performed systematic reviews of available studies (up to July 2018) and followed the Grading of Recommendations, Assessment, Development, and Evaluation evidence-to-decision framework to develop recommendations. All panel members discussed and approved the recommendations.
Recommendations: After considering the overall very low quality of the evidence, the panel made five conditional recommendations. We suggest that: 1) clinicians use a serum bicarbonate level <27 mmol/L to exclude the diagnosis of OHS in obese patients with sleep-disordered breathing when suspicion for OHS is not very high (<20%) but to measure arterial blood gases in patients strongly suspected of having OHS, 2) stable ambulatory patients with OHS receive positive airway pressure (PAP), 3) continuous positive airway pressure (CPAP) rather than noninvasive ventilation be offered as the first-line treatment to stable ambulatory patients with OHS and coexistent severe obstructive sleep apnea, 4) patients hospitalized with respiratory failure and suspected of having OHS be discharged with noninvasive ventilation until they undergo outpatient diagnostic procedures and PAP titration in the sleep laboratory (ideally within 2–3 mo), and 5) patients with OHS use weight-loss interventions that produce sustained weight loss of 25% to 30% of body weight to achieve resolution of OHS (which is more likely to be obtained with bariatric surgery).
Conclusions: Clinicians may use these recommendations, on the basis of the best available evidence, to guide management and improve outcomes among patients with OHS.
Keywords: hypercapnia, Pickwickian, sleep-disordered breathing, chronic hypercapnic respiratory failure, bilevel PAP
Contents
Overview
Introduction
Target Audience
Methods
Panel Composition
Conflict-of-Interest Declaration and Management
Meetings and Conference Calls
Formulation of Key Questions and Selection of Outcomes of Interest
Evidence Review and Development of Clinical Recommendations
Manuscript Preparation
Peer Review
How to Use These Guidelines
Results
Question 1: Should Serum Bicarbonate and/or Oxygen Saturation by Pulse Oximetry Rather Than PaCO2 in Arterial Blood Be Used to Screen for OHS in Obese Adults with Sleep-disordered Breathing?
Question 2: Should Adults with OHS Be Treated with PAP—Either CPAP or NIV—or Not Be Treated with PAP?
Question 3: Should Adults with OHS Be Treated with CPAP or with NIV?
Question 4: Should Hospitalized Adults Suspected of Having OHS, in Whom the Diagnosis Has Not Yet Been Made, Be Discharged from the Hospital with or without PAP Treatment Until the Diagnosis of OHS Is Either Confirmed or Ruled Out?
Question 5: Should a Weight-Loss Intervention or No Such Intervention Be Used for Adults with OHS?
Discussion
Plans for Updating These Guidelines
Adapting Recommendations Locally
Overview
The purpose of this guideline is to improve early recognition of obesity hypoventilation syndrome (OHS) and advise clinicians concerning the management of OHS, with the goal of reducing variability in clinical practice. The guideline should empower clinicians to make appropriate clinical decisions about the management of patients with OHS in the context of individual patient values and preferences. For each recommendation, the reader is strongly encouraged to consider both the summary of the evidence reviewed and discussed by the panel as well as the remarks for each specific question, including the values and preferences. Undoubtedly, no guideline can account for all clinical scenarios.
A panel of sleep and pulmonary physicians, intensivists, a hospitalist, a pulmonary hypertension specialist, a respiratory therapist, experts in weight reduction in patients with sleep-disordered breathing (SDB), methodologists, and a patient representative identified priority questions, reviewed the relevant literature, and followed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to assess the evidence and formulate recommendations. The panel considered several questions that might be raised in the acute and chronic settings of primary, secondary, and tertiary levels of health care. In this document, we addressed five questions related to early recognition and management of OHS that the panel identified as most important from the perspective of practicing clinicians and provided recommendations (Table 1).
Table 1.
Summary of Recommendations
| Recommendation | Explanations and Other Considerations |
|---|---|
|
Question 1: Should serum bicarbonate (HCO3−) and/or SpO2 rather than PaCO2 be used to screen for OHS in obese adults with sleep-disordered breathing? | |
| Recommendation 1A: For obese patients with sleep-disordered breathing with a high pretest probability of having OHS, we suggest measuring PaCO2 rather than serum bicarbonate or SpO2 to diagnose OHS (conditional recommendation, very low level of certainty in the evidence). | Patients with a high pretest probability of having OHS are usually severely obese with typical signs and symptoms of OHS and can be mildly hypoxemic during wake and/or significantly hypoxemic during sleep. |
| This is a recommendation for screening for OHS in patients with sleep-disordered breathing, most typically OSA. | |
| Recommendation 1B: For patients with low to moderate probability of having OHS (<20%), we suggest using serum bicarbonate level to decide when to measure PaCO2: in patients with serum bicarbonate <27 mmol/L, clinicians might forego measuring PaCO2, as the diagnosis of OHS in them is very unlikely; in patients with serum bicarbonate ≥27 mmol/L, clinicians might need to measure PaCO2 to confirm or rule out the diagnosis of OHS (conditional recommendation, very low level of certainty in the evidence). | Using a 27-mmol/L threshold in serum bicarbonate in obese patients with OSA and low to moderate clinical suspicion of OHS (initial probability of OHS not more than 20%) would likely permit forgoing further testing, such as arterial blood gases, in those with bicarbonate level <27 mmol/L (64–74% of obese patients with OSA) and performing arterial blood gas analysis only in those with serum bicarbonate ≥27 mmol/L (26–36% of obese patients with OSA). |
| We found insufficient evidence for serum bicarbonate thresholds other than 27 mmol/L. | |
| Recommendation 1C: We suggest that clinicians avoid using SpO2 during wakefulness to decide when to measure PaCO2 in patients suspected of having OHS until more data about the usefulness of SpO2 in this context become available (conditional recommendation, very low level of certainty in the evidence). | We found insufficient data to investigate the clinical usefulness of any threshold of awake SpO2 for screening for OHS in obese patients with OSA. Guideline panel members believed that relevant studies have to be done before the clinical usefulness of awake SpO2 in this context can be assessed. This is a temporary recommendation reflecting lack of evidence about a potentially useful intervention, rather than evidence that it is not useful. Thus, this recommendation should not be used as an argument against additional research and will likely change once additional data are available. |
|
Question 2: Should adults with OHS be treated with PAP—either CPAP or NIV—or not be treated with PAP? | |
| Recommendation 2: For stable ambulatory patients diagnosed with OHS, we suggest treatment with PAP during sleep (conditional recommendation, very low level of certainty in the evidence). | Note: Patients with symptomatic OHS who have significant comorbidities and those with chronic respiratory failure after an episode of acute-on-chronic hypercapnic respiratory failure may particularly benefit from using PAP. |
|
Question 3: Should adults with OHS be treated with CPAP or with NIV? | |
| Recommendation 3: For stable ambulatory patients diagnosed with OHS and concomitant severe OSA (apnea–hypopnea index ≥ 30 events/h), we suggest initiating first-line treatment with CPAP therapy rather than NIV (conditional recommendation, very low level of certainty in the evidence). | More than 70% of patients with OHS also have severe OSA; therefore, this recommendation applies to the majority of patients with OHS who have concomitant severe OSA. However, panel members lacked certainty on the clinical benefits of initiating treatment with CPAP, rather than NIV, in patients with OHS who have sleep hypoventilation without severe OSA. |
|
Question 4: Should hospitalized adults suspected of having OHS, in whom the diagnosis has not yet been made, be discharged from the hospital with or without PAP treatment until the diagnosis of OHS is either confirmed or ruled out? | |
| Recommendation 4: We suggest that hospitalized patients with respiratory failure suspected of having OHS be started on NIV therapy before being discharged from the hospital, until they undergo outpatient workup and titration of PAP therapy in the sleep laboratory, ideally within the first 3 mo after hospital discharge (conditional recommendation, very low level of certainty in the evidence). | Note: Discharging patients from the hospital with NIV should not be a substitute for arranging the outpatient sleep study and PAP titration in the sleep laboratory, as soon as it is feasible. |
|
Question 5: Should a weight-loss intervention or no such intervention be used for adults with OHS? | |
| Recommendation 5: For patients with OHS, we suggest using weight-loss interventions that produce sustained weight loss of 25–30% of actual body weight. This level of weight loss is most likely required to achieve resolution of hypoventilation (conditional recommendation, very low level of certainty in the evidence). | Note: Many patients may not be able to achieve this degree of sustained weight loss despite participating in multifaceted comprehensive weight-loss lifestyle intervention program; those who have no contraindications may benefit from being evaluated for bariatric surgery. |
Definition of abbreviations: CPAP = continuous positive airway pressure; NIV = noninvasive ventilation; OHS = obesity hypoventilation syndrome; OSA = obstructive sleep apnea; PAP = positive airway pressure; SpO2 = oxygen saturation by pulse oximetry.
Introduction
OHS is defined by the combination of obesity (body mass index [BMI] ≥ 30 kg/m2), SDB, and awake daytime hypercapnia (awake resting PaCO2 ≥ 45 mm Hg at sea level), after excluding other causes for hypoventilation (1, 2). OHS is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization due to acute-on-chronic hypercapnic respiratory failure, among others (3, 4).
Severe obesity is a major risk factor for the development of OHS. In recent decades, the prevalence of obesity and severe obesity (class III or BMI ≥ 40 kg/m2) has increased worldwide (5). The CDC estimated that 7.6% of the adult U.S. population has a BMI ≥40 kg/m2 (6). Although the prevalence of OHS in the general population is unknown, it is likely to increase concurrent with the epidemic of obesity. Multiple studies have reported a prevalence of OHS between 8% and 20% in obese patients referred to sleep centers for evaluation of SDB (7–10).
Although the definition of OHS suggests a diurnal pathology, polysomnography or sleep respiratory polygraphy is required to determine the pattern of SDB and hypoventilation (obstructive or nonobstructive), to tailor treatment, and to establish the optimal settings of positive airway pressure (PAP) therapy. Approximately 90% of patients with OHS have coexistent obstructive sleep apnea (OSA) defined by an apnea–hypopnea index (AHI) ≥5 events/h, with nearly 70% having severe OSA (AHI ≥ 30 events/h) (11). The remaining 10% of patients with OHS without OSA (AHI < 5 events/h) have nonobstructive, sleep-dependent hypoventilation (12).
PAP has become the primary management option for controlling SDB and reversing awake hypoventilation in patients with OHS. The most commonly prescribed PAP treatment modalities are noninvasive ventilation (NIV) or continuous PAP (CPAP). NIV consists of the application of positive-pressure ventilation, usually with bilevel PAP settings (with or without a backup respiratory rate) or volume-targeted pressure support, an autotitrating pressure support mode that delivers to a preset target volume and includes backup respiratory rate. Despite the availability of effective therapies, most patients with OHS remain undiagnosed and untreated until late in the course of the disease when they present to high-acuity settings with acute-on-chronic hypercapnic respiratory failure (13, 14) or, alternatively, when ambulatory care is escalated to include evaluation by pulmonary or sleep specialists (12). During this delay, patients with OHS use more healthcare resources than eucapnic patients of comparable obesity (15). Unfortunately, OHS is misdiagnosed even in patients with severe obesity who are hospitalized with hypercapnic respiratory failure (16). Early recognition and effective treatment are important in improving morbidity and mortality.
To address gaps in diagnosis and management and improve patient-centered outcomes, the American Thoracic Society (ATS) commissioned this multidisciplinary panel to generate evidence-based recommendations for evaluation and management of patients with OHS. The panel opted to narrow the focus of the guidelines to clinical issues that are most relevant to clinicians and patients.
Target Audience
Our target audience is obese adults and their family members, pulmonologists, intensivists, emergency medicine specialists, sleep medicine specialists, respiratory therapists, and sleep technologists. Primary care physicians, hospitalists, obesity specialists, bariatric surgeons, nursing home personnel, and other healthcare professionals involved in care for obese adults may also benefit from these guidelines.
Methods
This clinical practice guideline was developed in accordance with ATS policies and procedures.
Panel Composition
The project was proposed by the chair (B.M.) and co-chair (J.F.M.) through the ATS Sleep and Respiratory Neurobiology Assembly and was approved by the ATS Board of Directors. Potential panelists were identified by the chair and co-chair, on the basis of their expertise in pulmonary sleep medicine, critical care medicine, hospital medicine, respiratory therapy, weight management in patients with SDB, and pulmonary hypertension.
The ATS Documents Development and Implementation Committee approved the composition of the final panel, which consisted of 18 members: a chair, a co-chair, 11 additional pulmonary medicine specialists with expertise in SDB, 1 hospitalist, 1 intensivist, 1 pulmonary hypertension specialist, and 1 respiratory therapist. The guideline included one patient who participated on the guideline panel and provided perspective on patient values and preferences. The panel worked with one senior ATS methodologist and three ATS Scholars, who performed the systematic review of the literature to answer the guideline questions and participated in the discussions, but not in formulating recommendations.
Conflict-of-Interest Declaration and Management
All candidate panelists disclosed their potential interests according to ATS policy. The ATS staff and representatives of the ATS Conflict of Interest Committee reviewed the declarations, and panelists determined to have no substantial conflicts of interest were approved to participate without limitations. Those with potential conflicts of interest that were considered manageable were allowed to participate in discussions about the evidence but not in the formulation of recommendations related to their conflicts of interest. Two initially invited panelists were disqualified because of conflicts of interest deemed not manageable. At least 50% of the chairs and panel members were free from financial or intellectual conflict of interest. The chair (B.M.) was responsible for monitoring the discussions for ensuring strict adherence to these rules. A conflict-of-interest grid is included in the online supplement.
Meetings and Conference Calls
Face-to-face meetings were held at the ATS conferences in Washington, D.C. (May 2017) and San Diego, California (May 2018). Members who could not attend were invited to participate via teleconference. All panel members also met via teleconference in June, August, and October 2018.
Formulation of Key Questions and Selection of Outcomes of Interest
The chair and co-chair drafted the initial key clinical questions in a PICO (Population, Intervention, Comparator, and Outcome) format, shared them with the panel, and requested additional questions, leading to a total of 19 questions identified as relevant for clinicians. The guideline panel discussed all questions and chose five that were deemed to be of highest priority to be answered (Table 1). The remaining 14 questions were identified as important, but of lower priority in the management of OHS, and were not addressed in this document (Table 2).
Table 2.
Questions Initially Drafted but Not Highly Prioritized by the Panel, and Not Addressed in This Document
| 1. Should screening for OHS vs. no such screening be used in obese patients with suspected or confirmed OSA before an elective noncardiac surgery? |
| 2. Should in-laboratory polysomnography vs. out-of-center sleep testing be used for initial evaluation of patients who are either at risk of OHS or have established OHS? |
| 3. Should PAP treatment in adults with OHS be guided by combined monitoring of SpO2 and noninvasive monitoring of CO2 rather than monitoring of SpO2 alone? |
| 4. Should treatment of OHS be tailored based on severity (e.g., level of PaCO2, serum bicarbonate, degree of nocturnal hypoventilation, hypoxemia, or comorbidities)? |
| 5. Should switch to NIV vs. continued CPAP be used in patients with OHS who remain hypercapnic despite adequate adherence to CPAP therapy for 6 to 8 weeks? |
| 6. Should the long-term efficacy of prescribed PAP settings be monitored by polysomnography vs. simplified home monitoring (pulse oximetry and/or capnography)? |
| 7. Should nocturnal supplemental oxygen be added to PAP therapy vs. PAP therapy alone in patients with OHS experiencing persistent hypoxemia despite PAP optimization? |
| 8. Should ventilation by tracheostomy vs. continued CPAP/NIV be used in patients with OHS who are not adherent to CPAP/NIV and have prior history of acute-on-chronic hypercapnic respiratory failure? |
| 9. Should a respiratory stimulant (e.g., acetazolamide) vs. no respiratory stimulant be used in patients with OHS who have persistent hypercapnia despite adequate NIV therapy? |
| 10. Should PAP vs. no PAP be used in the management of pulmonary hypertension in patients with OHS? |
| 11. Should pulmonary hypertension–specific vasodilator combined with PAP vs. PAP alone be used in patients with OHS and pulmonary hypertension and/or right ventricular dysfunction? |
| 12. Should screening for comorbidities associated with OHS (i.e., metabolic syndrome, pulmonary hypertension, coronary artery disease) vs. no such screening be used in patients with OHS at the time of diagnosis? |
| 13. Should PAP therapy (CPAP or NIV) vs. no PAP therapy be used in the management of pulmonary hypertension in patients with OHS? |
| 14. Should a bariatric procedure vs. no bariatric procedure be used as first-line therapy for OHS? |
For definition of abbreviations, see Table 1.
The panel selected the following patient-important outcomes: death, quality of life, resolution of daytime hypercapnia and hypoxemia, need for supplemental oxygen, resolution of OHS, daytime hypersomnolence, motor vehicle accidents, sleep quality (polysomnographic measured microarousal index), control of SDB (AHI, time spent with nocturnal oxygen saturations <90%), exercise capacity, cardiovascular events, healthcare use (hospitalization, hospital length of stay, emergency department visits), improvement of pulmonary hypertension, and PAP-related adverse effects. A patient advocate was consulted about the appropriateness of the questions and the most relevant clinical outcomes (17).
Evidence Review and Development of Clinical Recommendations
For each question, the methodologists performed a full, systematic review of the literature up to July 2018 using Medline, Embase, and Cochrane CENTRAL electronic databases to identify and summarize the evidence about the effects of interventions on the outcomes of interest. Titles and abstracts, and subsequently full-text articles, were screened in duplicate by the senior ATS methodologist and ATS Scholars to assess eligibility according to prespecified criteria. Co-chairs and panel members were consulted to confirm eligibility criteria and completeness of the body of evidence and whenever clinical expertise was needed to interpret the studies.
To obtain the effect estimates of interventions on each outcome of interest, meta-analyses were performed using the Cochrane Collaboration Review Manager Software, version 5.3.5, when appropriate (18). Results of studies were summarized in a narrative format when their reporting did not allow statistical analysis or when results were judged to be too heterogeneous to permit using a common estimate.
For Questions 2 and 3, the chair and co-chair contacted the authors of randomized controlled trials (RCTs) to obtain additional information on outcomes that were not presented in the published articles (11, 19–22). To answer Question 4, the chair and co-chair contacted authors of 10 published studies to request individual patient data on hospitalized patients with OHS (3, 14, 22–29). Individual patient data were obtained for all except one of these studies (23).
Evidence summaries (online supplement) were prepared for each question following the GRADE approach (30), using the GRADEpro Guideline Development Tool online application (www.gradepro.org).
When continuous outcomes (e.g., symptom scores or quality of life) were measured using different scales, the results were combined in meta-analyses using standardized mean difference (SMD), which is expressed in SD units. To facilitate interpretation of the results when expressed as SMD, effect sizes were used following Cohen’s conventional criteria: an SMD of ∼0.2 is considered a small effect, ∼0.5 a moderate effect, and ∼0.8 or higher, a large effect.
The risk of bias was assessed at the outcome level using the Cochrane Collaboration’s risk-of-bias tool for randomized trials (31) and the Newcastle-Ottawa scale (32) for observational studies with a control group. A high risk of bias was assumed for all single-arm studies (which lacked control group data) and did not assess other risk-of-bias criteria, because doing so would not impact decisions about the certainty of the evidence. Subsequently, the certainty of the evidence (i.e., confidence in the estimated effects, also known as “quality of the evidence”) was assessed for each of the outcomes of interest following the GRADE approach (33, 34). Certainty of the evidence was categorized into four levels: high, moderate, low, and very low.
For each question, all information was summarized in Evidence-to-Decision (EtD) frameworks (online supplement) that included concise description of desirable and undesirable health effects, certainty of the evidence about those effects, evidence and assumptions about patients’ values and preferences, required resources and cost-effectiveness, potential influence on health equity, acceptability of the intervention to various stakeholders, and feasibility of implementation (35, 36). All panel members reviewed and discussed the EtDs for all questions during a face-to-face meeting at the ATS International Conference in San Diego, California in May 2018 and during subsequent conference calls. After the discussion, panel members formulated final recommendations and assessed their strength and approved them by consensus. The final EtD frameworks are available in the online supplement.
Recommendations were labeled as either “strong” or “conditional” according to the GRADE approach. The panel could use the words “we recommend” for strong recommendations and “we suggest” for conditional recommendations. Table 3 provides suggested interpretation of strong and conditional recommendations. All recommendations made in this document were conditional (“weak”) because of the paucity and limitations of the available evidence.
Table 3.
Implications of Strong and Conditional Recommendations
| Strong Recommendation | Conditional Recommendation | |
|---|---|---|
| For patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | Most individuals in this situation would want the suggested course of action, but many would not. |
| For clinicians | Most individuals should receive the recommended course of action. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for different patients and that you must help each patient arrive at a management decision consistent with her or his values and preferences. Decision aids may well be useful in helping individuals making decisions consistent with their values and preferences. Clinicians should expect to spend more time with patients when working toward a decision. |
| For policy makers | The recommendation can be adopted as policy in most situations, including for use as performance indicators. | Policy making will require substantial debate and involvement of many stakeholders. Policies are also more likely to vary between regions. Performance indicators would have to focus on the fact that adequate deliberation about the management options has taken place. |
Manuscript Preparation
The chair and co-chair developed five writing groups, one for each question. The initial draft of the manuscript was written by the chair and co-chair, with major contributions from panel members. The manuscript was then reviewed by the entire panel. The entire committee had the opportunity to correct errors, clarify the presentation of background information or evidence summaries, and suggest changes to the rationale sections to capture face-to-face discussions accurately. The wording of recommendations (including strength and direction) was not altered once the recommendations were finalized. Once the final manuscript was approved by the full panel, it was submitted for external peer review.
Peer Review
External peer review was organized and overseen by the ATS Documents Editor. The guideline underwent anonymous peer review by five content experts and one methodologist. After multiple cycles of review and revision, the guideline was reviewed and approved by a multidisciplinary Board of Directors (Table 4).
Table 4.
Summary of Guideline Development Methods
| Activity | Yes | No |
|---|---|---|
| Panel assembly included experts from various relevant clinical and nonclinical disciplines | ✓ | |
| Management of conflict of interest in the guideline development group and establishing transparency | ✓ | |
| Included a methodologist with appropriate expertise (documented expertise in conducting systematic reviews to identify the evidence base and the development of evidence-based recommendations) | ✓ | |
| Included an individual who represents the views of patients and society at large | ✓ | |
| Literature review performed in collaboration with methodologists | ✓ | |
| Searched multiple electronic databases; reviewed reference lists of retrieved articles | ✓ | |
| Evidence synthesis applied prespecified inclusion and exclusion criteria | ✓ | |
| Evaluated included studies for sources of bias | ✓ | |
| Used GRADE to describe quality of evidence | ✓ | |
| Generation of recommendations used GRADE to rate the strength of recommendations | ✓ | |
| External review | ✓ |
Definition of abbreviation: GRADE = Grading of Recommendations, Assessment, Development, and Evaluation.
How to Use These Guidelines
These guidelines provide a basis for rational, informed decision-making by all healthcare professionals who care for obese adults and patients with OSA. However, stakeholders should not treat the recommendations in these guidelines as binding mandates. No recommendation can take into account all of the variable and often compelling circumstances that might affect the potential benefits, harms, and burdens of an intervention in specific cases and contexts. Thus, no one charged with overseeing or evaluating actions of clinicians or other stakeholders should apply the recommendations from these guidelines in a blanket fashion, without an understanding of the individual patient context. Qualifying remarks accompanying each recommendation should never be omitted when quoting or translating recommendations from these guidelines. These statements are integral to the recommendations and serve to facilitate more accurate interpretation. The guideline will be reviewed by the ATS 3 years after publication and it will be determined if updating is necessary.
Results
Question 1: Should Serum Bicarbonate and/or Oxygen Saturation by Pulse Oximetry Rather Than PaCO2 in Arterial Blood Be Used to Screen for OHS in Obese Adults with Sleep-disordered Breathing?
Background
The two tests required to diagnose OHS are a sleep study (polysomnography or respiratory polygraphy) to establish the presence of SDB and a measurement of arterial blood gases during wakefulness to establish the presence of hypercapnia. The diagnosis of OHS can be delayed because measurement of arterial blood gases is not a standard practice in the management of patients with SDB (37, 38). Moreover, clinicians may misattribute hypercapnia to chronic obstructive pulmonary disease (39). Therefore, in obese patients suspected of having SDB, simple tests to screen for OHS are needed. Commonly used tests to identify patients likely to have OHS assess consequences of hypoventilation, namely elevated serum bicarbonate levels (i.e., total serum CO2, which equals bicarbonate plus dissolved CO2) and hypoxemia. The term “serum bicarbonate” is not strictly accurate because the chemical methods measure all CO2 liberated from the serum. Some laboratories use the more correct term “total serum CO2” to describe measured serum bicarbonate. It is important to note that bicarbonate represents 96% of total serum CO2, and the remainder is mostly dissolved CO2. The kidneys respond to chronic respiratory acidosis by increasing the serum bicarbonate level. Therefore, in the absence of alternative causes (e.g., use of loop diuretics), an increased serum bicarbonate level suggests the presence of an increased PaCO2. Hypoxemia, another consequence of hypercapnia as dictated by the alveolar gas equation, can also serve as a surrogate marker for hypercapnia. Pulse oximetry, which is noninvasive and accessible, is an attractive tool for identifying obese patients who are likely to be hypercapnic. However, hypoxemia can occur in morbidly obese patients without hypercapnia for other reasons.
Hence, the question posed by the panel was whether an increased level of serum bicarbonate and hypoxemia during wakefulness are useful to screen obese patients with SDB for OHS.
Summary of evidence
We identified 31 studies of interest and excluded 18 studies because they did not report the data required to answer the posed question. Of the remaining 13 articles we reviewed, 9 were full manuscripts (40–48) and 4 were in abstract form (49–52). Six were prospective (42, 43, 45, 47, 51, 52), six were retrospective, and one had a retrospective and a prospective design (40).
Bicarbonate level
Six studies evaluated the bicarbonate level and provided enough data for analysis (40–43, 45, 46). We excluded one whose prevalence of OHS was very low (2%), because the sample comprised obese ambulatory patients undergoing blood tests for a variety of reasons (43). The prevalence of OHS in the remaining studies ranged between 17% and 42%. Only two studies specified how serum bicarbonate level was measured (40, 42). In two studies, bicarbonate level was calculated from blood gas measurements (45, 46), and one study did not specify whether the bicarbonate was measured or calculated (41).
We extracted data and evaluated the diagnostic accuracy of four serum bicarbonate cutoffs: 26, 27, 28, and 29 mmol/L. The corresponding number of studies that reported evaluable data for pooled analysis was two, four, three, and one, respectively. The cutoff value of 27 mmol/L was the only one with sufficient data to perform a pooled analysis. One of the four studies included a retrospective and prospective sample and provided diagnostic accuracy data for both samples at the cutoff of 27 mmol/L (40). Therefore, the pooled analysis includes five samples, with a total of 335 patients with OHS and 1,037 obese patients without OHS. The pooled sensitivity was 0.86 (95% confidence interval [CI], 0.70–0.94), and the pooled specificity was 0.77 (95% CI, 0.60–0.89). The diagnostic performance of the pooled sensitivity and specificity was simulated in three hypothetical populations of 1,000 obese patients with SDB that have different prevalence (pretest probabilities) of OHS. Although it is difficult to estimate the exact prevalence of OHS, we used BMI as a rough estimate to determine the prevalence of OHS in these populations. In general, a prevalence of 5% is typically seen in patients with SDB with BMI of 30 to 34.9 kg/m2, 10% is typically seen in patients with BMI of 35 to 40 kg/m2, and 20% is seen in patients with BMI >40 kg/m2 (7).
A bicarbonate level <27 mmol/L effectively rules out hypercapnia, irrespective of the prevalence of OHS. In a population with an OHS prevalence of 5% (for example, patients with BMI 30–34.9 kg/m2) or 50 cases of OHS per 1,000 patients, bicarbonate level <27 mmol/L would lead to seven false-negative results (95% CI, 5–13). In a patient population with an OHS prevalence of 10% (BMI of 35–40 kg/m2) or 100 cases per 1,000 patients, a bicarbonate level <27 mmol/L would lead to 14 false-negative results out of 1,000 patients tested (95% CI, 6–30). Last, in a patient population with an OHS prevalence of 20% (BMI of >40 kg/m2), a bicarbonate level <27 mmol/L would lead to 28 false-negative results per 1,000 patients tested (95% CI, 12–60). Therefore, a bicarbonate level <27 mmol/L has a very high negative predictive value (99.0%; 95% CI, 97.9–99.6%), making it effective in ruling out OHS in situations where the pretest probability can be as high as 20%. Consequently, a bicarbonate level ≥27 mmol/L was most useful when the likely prevalence of OHS was 20% or higher, resulting in a positive predictive value of 48.3% (95% CI, 34.9–66.1%). The positive predictive value decreased to 29.3% (95% CI, 19.2–46.4%) when the prevalence of OHS was 10% and decreased further to 16.4% (95% CI, 10.1–29.0%) when the prevalence was 5%.
Daytime and nocturnal hypoxemia
Nine studies evaluated hypoxemia by pulse oximetry (41, 42, 44, 45, 47–51) and had prevalence of OHS between 17% and 66%. We identified 14 different measures of hypoxemia in these studies, including: awake oxygen saturation by pulse oximetry (SpO2) below a defined threshold (92–98%), sleep SpO2 below 90% for various percentages of sleep time (10–46%), nadir SpO2 during sleep below a defined threshold (76% or 80%), mean SpO2 during sleep below 90%, or a combination of SpO2 and bicarbonate level. This heterogeneity precluded pooling of the data.
Panel judgement
The panel acknowledged the significant variability in the accuracy of serum bicarbonate to screen obese patients with SDB for OHS, leading to uncertainty regarding the pooled accuracy and the consequences of using bicarbonate level in the management of patients with SDB. Yet, the panel placed high importance on identifying patients with OHS and favored using the bicarbonate level to stratify patients with SDB who should have an arterial blood gas to measure the PaCO2 to confirm the presence of hypercapnia over not measuring PaCO2 at all or measuring it in all obese patients with SDB. This decision was also favored because serum bicarbonate levels are frequently available for clinicians (feasibility), and using them could reduce unnecessary measurements of arterial blood gases (moderate cost savings and a reduction in patient discomfort from arterial punctures). The panel speculated that hypercapnia is probably mild in the very few cases of OHS missed when the bicarbonate level is <27 mmol/L. The panel’s judgements are summarized in the online supplement.
ATS recommendation
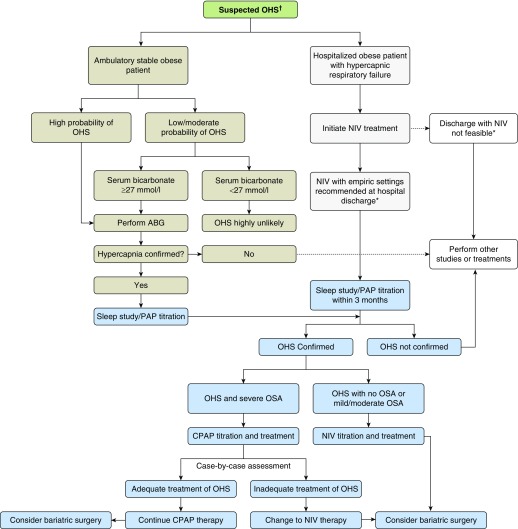
The panel suggests that clinicians should measure PaCO2 in obese patients with SDB who are strongly suspected of having OHS. Clinical features to assess such risk include severe obesity with typical signs and symptoms of OSA/OHS, and mild hypoxemia while awake, significant hypoxemia during sleep, or both. The panel suggests that clinicians use a serum bicarbonate level of 27 mmol/L to decide when to measure PaCO2 in patients with low to moderate probability of having OHS (i.e., <20%, for example patients with a BMI of 30–40 kg/m2). A bicarbonate level <27 mmol/L precludes the need for an arterial blood gas measurement in this population. Consequently, a bicarbonate level ≥27 mmol/L should trigger clinicians to measure PaCO2 as a confirmatory diagnostic test, especially when the pretest probability of OHS is 10 to 20% (generally a BMI >35 kg/m2).
-
1.
Recommendation 1A: For obese patients with SDB who are strongly suspected of having OHS, we suggest measuring PaCO2 rather than serum bicarbonate or SpO2 to diagnose OHS (conditional recommendation, very low level of certainty in the evidence).
-
2.
Recommendation 1B: For patients with low to moderate probability of having OHS (pretest probability < 20%), we suggest using serum bicarbonate level to decide whether to measure PaCO2: in patients with serum bicarbonate <27 mmol/L, clinicians might forego measuring PaCO2, as the diagnosis of OHS is very unlikely; in patients with serum bicarbonate ≥27 mmol/L, clinicians might need to measure PaCO2 to confirm or rule out the diagnosis of OHS (conditional recommendation, very low level of certainty in the evidence).
-
3.
Recommendation 1C: We suggest that clinicians do not use SpO2 to decide when to measure PaCO2 in patients suspected of having OHS until more data about the usefulness of SpO2 in this context become available (conditional recommendation, very low level of certainty in the evidence).
Remarks
-
1.
Remarks for Recommendation 1A: Patients strongly suspected of having OHS are usually severely obese with typical signs and symptoms such as dyspnea, nocturia, lower extremity edema, excessive daytime sleepiness, fatigue, loud disruptive snoring, witnessed apneas, as well as mild hypoxemia during wake and/or significant hypoxemia during sleep. This is a recommendation for screening for OHS in obese patients with SDB, typically OSA.
-
2.
Remarks for Recommendation 1B: Using a serum bicarbonate threshold of 27-mmol/L in obese patients with OSA and low to moderate clinical suspicion of OHS (initial probability of OHS not more than 20%) would likely obviate the need for further testing such as arterial blood gases in those with bicarbonate level <27 mmol/L (64–74% of obese patients with OSA), but arterial blood gas analysis would be useful in those with serum bicarbonate ≥27 mmol/L (26–36% of obese patients with OSA). Evidence to investigate the usefulness of serum bicarbonate thresholds other than 27 mmol/L was insufficient.
-
3.
Remarks for Recommendation 1C: Data were insufficient to investigate the usefulness of any threshold of SpO2 for screening for OHS in obese patients with OSA. This is a temporary recommendation reflecting lack of evidence about a potentially useful intervention, rather than evidence that it is not useful. Thus, this recommendation should not be used as an argument against additional research and will likely change once additional data are available.
Future research opportunities
Randomized trials are necessary to ascertain whether screening obese patients with SDB for OHS using bicarbonate levels or oxygen saturation will improve patient outcomes and reduce healthcare use.
Question 2: Should Adults with OHS Be Treated with PAP—Either CPAP or NIV—or Not Be Treated with PAP?
Background
Effective treatment of OHS with PAP was first reported in a case series in 1983 (53). Since then, multiple observational studies and a few randomized trials assessing the efficacy of PAP in OHS have been performed. However, given PAP’s effectiveness in treating OHS, conducting large, long-term, randomized trials of PAP versus no PAP in patients with symptomatic OHS poses ethical challenges. The formulated question referred to the use of PAP therapy and includes studies using CPAP or NIV, either alone or in combination depending on the OHS phenotype.
Summary of evidence
We identified 32 articles: 27 were single-arm case series (3, 14, 16, 23–26, 28, 54–72), 2 were observational studies with a control group published in abstract form (29, 73), and 3 were RCTs (11, 19, 21).
For the critical outcome of death, data could be pooled from three RCTs (11, 19, 21). However, because of the very low number of events (PAP, 0 of 209; no PAP, 0 of 205) and short follow-up period (1–2 mo), the estimated effect size was of low certainty with a serious risk of bias (estimated mortality, 0 of 1,000; 95% CI, +13 deaths to −13 deaths). Although 21 observational studies reported mortality, because of significant variation in their patient populations (stable chronic or post-acute respiratory failure), duration of follow-up, and use of control groups, pooling of these data was not possible.
The use of PAP improved control of OSA (pooled data from two RCTs, n = 235: mean difference in AHI, −50 events/h; 95% CI, −42 to −58 events/h) (11, 21) and other parameters of SDB (pooled data from three RCTs, n = 319: mean difference in percentage of total sleep time spent with SpO2 <90%, −31%; 95% CI, −25% to −38%) (11, 19, 21). The improved control of SDB was followed by improvements in daytime respiratory failure (pooled data from three RCTs, n = 319: mean difference in awake PaO2, 3.2 mm Hg; 95% CI, 0.8–5.5 mm Hg; mean difference in awake PaCO2, −2.4 mm Hg; 95% CI, −1.0 to −3.8 mm Hg) (11, 19, 21). With PAP, 90% of the patients achieved an awake PaO2 greater than 55 mm Hg compared with 78.3% not treated with PAP. Using this threshold of awake hypoxemia for prescription of supplemental oxygen therapy, 13 fewer patients per 100 treated with PAP (95% CI, 3 fewer to 23 fewer) would require supplemental oxygen during wakefulness (11, 19).
Sleep quality also improved, using both objective and subjective measures. In patients with OHS treated with PAP, the microarousal index, measured on polysomnogram, improved. Data pooled from two RCTs of patients with significant concomitant OSA (n = 235) showed a mean difference in microarousals of −35 events/h (95% CI, −28 to −43 events/h) (11, 21). In patients with OHS but without severe OSA, data from a single RCT (n = 84) showed a mean difference in microarousals of −10 events/h (95% CI, −6 to −47events/h) (19). Using the Functional Outcome of Sleep Questionnaire as a measure of sleep quality, data pooled from two RCTs (n = 284) showed a mean difference of 6.6 (95% CI, 2.5 to 10.7) with PAP therapy (11, 19). Daytime sleepiness improved significantly (data pooled from three RCTs, n = 319: mean difference in Epworth Sleepiness Scale score, −2.5; 95% CI, −1.0 to −4.0) (11, 19, 21). Short-term rates of hospitalization were reduced by PAP, but the event rate was low and data were reported in only one RCT (19). Longer-term data suggest high rates of hospitalization in patients with OHS on PAP but without a comparison group (data from five observational studies: 10% at 3 mo to 49% at 5 yr) (20, 22, 65, 70, 74).
The evidence underpinning this recommendation has several limitations. The majority of the studies are observational rather than RCTs, and the inclusion criteria, length of follow-up and titration of PAP were not standardized. Furthermore, for many of the outcomes reported, only a few articles provided data on relatively few patients. Therefore, some of the reported data are at serious risk of bias, and the level of certainty regarding reported outcomes is low to very low, with the exception of sleep quality, which is reported to moderate certainty. The majority of the RCT data were from a large, well-conducted multicenter study in a developed European nation, which may affect generalizability (11). The RCTs identified by the literature search were limited by the lack of a sham device or placebo and of blinding of patients and clinicians to allocated treatment.
Panel judgement
Notwithstanding the limitations of the available evidence, the panel agreed that the desirable effects of PAP outweigh its undesirable effects, which the panel deemed trivial. The panel surmised that costs of treating patients with OHS with PAP were moderate, with regional differences, but could not determine the cost-effectiveness of this treatment, because an economic analysis has not been published yet. In healthcare systems where the cost of PAP is not reimbursed by a third-party payer, direct out-of-pocket expenses may pose a barrier to obtaining therapy. Moreover, additional intervention to support adherence incurs indirect expenses (i.e., behavioral approaches and frequent follow-up). The panel’s judgements are summarized in the online supplement.
ATS recommendation
For stable ambulatory patients diagnosed with OHS, we suggest treatment with PAP during sleep (conditional recommendation, very low level of certainty in the evidence).
Remarks
The panel acknowledged that patients with symptomatic OHS who have comorbidities (defined as stage IV OHS by the European Respiratory Society) (2) and those with chronic respiratory failure after an episode of acute respiratory failure (see also Question 4) were likely to benefit most. In patients with mild OHS (i.e., patients with mildly elevated awake PaCO2 between 45 and 50 mm Hg) or borderline OHS (stage I and II of obesity-associated sleep hypoventilation as defined by the European Respiratory Society) (2), the effect was less clear. Other than the abolition of obstructive events in patients with OSA, insufficient evidence exists to recommend a specific policy regarding PAP titration for OHS. Data on optimal targets for titration of PAP are limited in OHS, and accepted core outcome measures to assess treatment efficacy are lacking. The consensus opinion is that PAP should be titrated based on the individual’s overnight respiratory monitoring. There is insufficient evidence to recommend specific monitoring parameters, but, as a minimum, overnight monitoring should include continuous oximetry. The use of only empiric settings for initial PAP in patients with OHS, without guidance of overnight physiological assessment, is not ideal. Once PAP treatment is established, patients should receive standardized education and training regarding device and interface usage with early (4–8 wk) follow-up to assess clinical and physiological response to PAP.
Follow-up assessment of patients on PAP should include monitoring objective adherence to therapy, as data show that higher rates of adherence to PAP are associated with superior control of respiratory failure in OHS (20, 27, 63). Among patients with eucapnic OSA, telemonitoring and educational support have been shown to improve patient adherence to PAP (75), but no such data exist in patients with OHS. Nevertheless, guidance on the use of ventilator-derived data in patients with eucapnic OSA on PAP can be reasonably extrapolated to patients with OHS (76).
Future research opportunities
The use of PAP for OHS is standard practice, so long-term trials to compare its efficacy against an untreated control population are not ethical in those with significant symptoms or more severe respiratory failure. Currently, the minimum threshold of adherence to PAP to reduce symptoms, cardiovascular risk, or mortality is unknown. In addition, the assessment methods and targets that represent adequate control of SDB remain undefined in this population. Use of telemonitoring and PAP device data in guiding clinical decision-making in the management of patients with OHS requires focused research to assess clinical benefit and cost-effectiveness.
Question 3: Should Adults with OHS Be Treated with CPAP or with NIV?
Background
PAP is the primary management option for controlling SDB and reversing awake hypercapnia in patients diagnosed with OHS. Therapy can be delivered using either CPAP or NIV during sleep. However, the optimal mode of PAP to correct gas exchange and improve long-term outcomes in this population has not been fully elucidated.
Observational studies and RCTs have reported improvements in awake respiratory failure, symptoms, and quality of life to a similar degree in both CPAP- and NIV-treated individuals with OHS (11, 20, 22, 55, 58, 63, 77–80). Although CPAP does not increase alveolar ventilation, it can improve awake respiratory failure by facilitating the unloading of CO2 accumulated during complete or partial airflow obstruction during sleep (81, 82). Because >70% of people with OHS have concomitant severe OSA (11), CPAP may be effective in improving nocturnal and awake gas exchange in at least a subset of these individuals.
Despite the physiologic benefits of CPAP, several surveys of home ventilation practices show that OHS is a major indication for home nocturnal NIV therapy (83–85). In part, NIV prescription in the home setting may follow its initial prescription for acute respiratory failure (26, 27), without consideration of switching to CPAP (86). In patients with stable hypercapnic respiratory failure, the response to CPAP is often monitored over a single night, and the inability to maintain oxyhemoglobin saturation above 85% to 90% within this short time is considered “CPAP failure.” This triggers a switch to long-term NIV (63, 65, 87). A small, prospective study found that the majority of patients with stable OHS may be transferred from NIV to CPAP without worsening gas exchange, sleep quality, or quality of life (86). However, the paucity of robust data affirming long-term clinical benefits of CPAP treatment may dissuade clinicians from treating OHS with CPAP. Conversely, the higher cost and increased complexity and skill required for titration may limit access to NIV. Although CPAP therapy may offer significant reductions in equipment costs compared with NIV (20, 86), benefits arising from these lower initial set-up costs will be quickly outweighed if CPAP is also associated with higher ongoing healthcare use.
Summary of evidence
Our search identified three RCTs comparing NIV to CPAP (11, 20, 22, 80). Data from the large Spanish Pickwick trial was reported by two studies (11, 20). One of the trials enrolled participants presenting with either chronic stable respiratory failure or after a hospital admission with acute respiratory acidosis, after a short period of stabilization and normalization of pH on bilevel PAP therapy, before being randomized (22). Participants in the other three studies presented with chronic stable respiratory failure (11, 20, 80). One study excluded 9 out of 45 participants with severe persisting nocturnal desaturation during CPAP titration (SpO2 < 80% for >10 min or increase in transcutaneous CO2 > 10 mm Hg) (80). The other three studies had no exclusion criteria on the basis of initial PAP titration failure (11, 20, 22). Participants were younger (mean age, ∼52 yr) and heavier (mean BMI, 53 kg/m2) in the Australian studies (22, 80) than in the Spanish study (mean age, 60 yr; BMI, 44 kg/m2) (11). The percentage of women in the two Australian studies was 47% (22) and 36% (80). In contrast, 56% of the participants in the Spanish Pickwick trial were women (11). Participants in all three trials demonstrated concomitant OSA in addition to OHS, with mean AHI >60 events/h. On average, adherence to CPAP and NIV were similar (5 to 6 h/night, with CPAP used only 7 min/night less than NIV; 95% CI, 43 min less to 29 min more). The duration of these RCTs was either short term (for up to 3 mo [11, 22, 80]) or long term (for up to 5 yr [20]).
The resolution of hypercapnia (i.e., awake PaCO2 < 45 mm Hg) occurred to a similar extent in both NIV- and CPAP-treated patients. During short-term follow-up, 46.6% of individuals treated with NIV and 36.3% treated with CPAP had a PaCO2 <45 mm Hg (11 more per 100 NIV-treated patients; 95% CI, 2 fewer to 28 more; relative risk [RR], 1.29; 95% CI, 0.94–1.77) (11, 22, 80). During long-term follow-up, 51.9% treated with NIV and 40.7% treated with CPAP had a PaCO2 <45 mm Hg (11 more per 100 NIV-treated patients; 95% CI, 4 fewer to 32 more; RR, 1.28; 95% CI, 0.91–1.79) (20). There was no difference in the degree of improvement in PaO2 between NIV and CPAP. Moreover, the need for oxygen supplementation during wakefulness, on the basis of an awake PaO2 <55 mm Hg, was not different during both short-term (11, 22, 80) and long-term follow-up (20). Similarly, resolution of daytime sleepiness, as assessed by an Epworth Sleepiness Score <10 after therapy, occurred at similar rates in NIV and CPAP groups, in both short-term (11, 22, 80) (RR, 1.04; 95% CI, 0.87 to 1.23) and longer-term (RR, 1.04; 95% CI, 0.86 to 1.26) (20) follow-up. Changes in quality of life from three short-term trials (11, 22, 80) and one long-term trial (20) were not different.
No deaths were recorded in three short-term studies, which included 311 patients treated with either NIV or CPAP (11, 22, 80). After 5 years of following 204 participants, mortality rates were similar between NIV- and CPAP-treated groups (NIV 11% vs. CPAP 15%; adjusted hazard ratio, 0.82; 95% CI, 0.36–1.87; P = 0.631) (20). Similarly, there was no difference in composite cardiovascular events (RR, 1.17; 95% CI, 0.56–2.44) between NIV and CPAP groups during long-term follow-up. Two studies reported hospitalization rates; in one study of 3 months’ duration (22), 3 of 29 patients allocated to CPAP required hospitalization versus 3 of 31 patients using NIV (RR, 1.07; 95% CI, 0.23–4.88). In the long-term study, over a median of 5.2 years, 48 of 107 patients receiving CPAP were hospitalized compared with 51 of the 97 NIV users (RR, 0.85; 95% CI, 0.64–1.13) (20). No difference between NIV and CPAP was found for other outcomes considered important by the panel, including awake hypoxemia, hypercapnia, percentage of night spent with oxygen saturation <90%, AHI, mood, or emergency department visits. Exercise capacity was reported in one study of 2 months’ duration with an increase in 6-minute-walk distance of 26 m, favoring NIV (11). However, when these patients were followed long term, there was no significant difference in 6-minute-walk distance between NIV and CPAP (20). No study reported on motor vehicle accidents.
Panel judgement
The panel assessed that the difference in outcomes between NIV and CPAP in stable ambulatory patients with OHS with concomitant severe OSA would be trivial. However, the panel acknowledged that NIV may offer greater benefits than CPAP among patients with OHS who did not have concomitant severe OSA (i.e., AHI < 30 events/h). The panel’s judgements are summarized in the online supplement.
ATS recommendation
After reviewing current evidence, we suggest CPAP rather than NIV be used as the initial treatment of stable ambulatory adult patients with OHS and concurrent severe OSA (AHI ≥ 30 events/h) presenting with chronic stable respiratory failure. Importantly, >70% of patients with OHS have severe OSA. Therefore, this recommendation is applicable to the majority of patients with OHS. However, there is less certainty in patients with OHS who do not have concomitant severe OSA (conditional recommendation, very low level of certainty in the evidence).
Remarks
Although the current data do not favor one form of PAP therapy over another in people with stable chronic OHS, further studies are needed to confirm this position. Improvements in awake hypercapnia may be achieved more slowly with CPAP than with NIV during the initial weeks of treatment. Patients presenting with a greater degree of initial ventilatory failure, poorer lung function, advanced age, or less severe OSA may be less likely to respond to CPAP (22, 63, 88). The variation in response to therapy requires close monitoring of the patient, especially during the first 2 months of treatment, to ensure improvement is achieved and sustained, with adjustment of therapy as appropriate. This particularly applies to patients with OHS without severe OSA who are prescribed CPAP.
There are also moderate cost implications around the choice of PAP therapy, with NIV being substantially more expensive than CPAP (20). In addition, NIV may require more resources for titration and equipment training. These considerations may delay access to NIV in comparison to CPAP, particularly in areas where skills necessary for more complex NIV devices are limited or where economic resources are a consideration. Similar levels of adherence are reported with CPAP and NIV, of 5 to 6 h/night. Previous work has suggested adherence is an important modifiable predictor of hypercapnia in OHS (55, 63).
Future research opportunities
In a recent RCT, long-term CPAP therapy was associated with lower cost than NIV therapy (20). Whether this cost difference leads to long-term cost-effectiveness requires further investigation. Similarly, studies evaluating the impact of various PAP modes in patients with OHS without severe OSA are needed. Data are also lacking to guide decisions regarding the timing and benefits of transferring patients with OHS who present with acute respiratory acidosis to CPAP, after they are treated initially with NIV, or whether long-term NIV is superior to CPAP in more severe forms of OHS. The panel identified these as high-priority areas for research.
Question 4: Should Hospitalized Adults Suspected of Having OHS, in Whom the Diagnosis Has Not Yet Been Made, Be Discharged from the Hospital with or without PAP Treatment Until the Diagnosis of OHS Is Either Confirmed or Ruled Out?
Background
Hospitalized patients suspected of having OHS who develop an acute-on-chronic hypercapnic respiratory failure have higher short-term (1–2 yr) mortality than ambulatory patients with OHS (16). Whether prescribing empiric PAP at the time of hospital discharge versus awaiting outpatient workup (i.e., outpatient sleep study and outpatient PAP titration in the sleep laboratory) reduces mortality is unknown. No RCTs have addressed this question, but one observational study reported a mortality of 23% at 18 months in patients discharged from the hospital without PAP (23). In contrast, in another observational study in which all patients were discharged on NIV, the 2-year mortality rate was 8% (24). In lieu of the paucity of data, the panel identified studies that included hospitalized patients with OHS or hospitalized patients suspected of having OHS and requested individual patient data from the authors.
Summary of evidence
We identified 10 studies that included hospitalized patients (3, 14, 22–29). The chair and co-chair requested and obtained limited individual data from the authors regarding patients who survived hospitalization and obtained these data for all but one study (23). The requested data were age, sex, BMI, baseline arterial blood gases (pH, PaCO2, and PaO2), arterial blood gases on discharge if available, FEV1, FVC, FEV1/FVC, whether the patient was discharged on PAP, and all-cause mortality at 3 months. For the critical outcome of death, the panel agreed that 3-month survival is more likely related to the recent hospitalization. Other outcomes were unavailable from most of the studies.
In three studies, the entire cohort consisted of hospitalized patients (14, 24, 29). The remaining six studies had a subgroup of patients who were hospitalized (3, 22, 25–28). In aggregate, there were 1,162 hospitalized patients with OHS or suspected of having OHS who survived hospitalization and were discharged. Of these, 119 (10%) were discharged without PAP and 1,043 (90%) on PAP. In seven studies (n = 955), NIV was prescribed on hospital discharge (3, 24–29). The remaining two studies used either CPAP or NIV on discharge (14, 22). PAP prescription levels were unavailable.
The two groups had important differences. Those discharged without PAP were older (73 ± 10 yr vs. 64 ± 13 yr; P < 0.001), had a higher baseline PaCO2 (78.4 ± 18 mm Hg vs. 62.2 ± 16 mm Hg; P < 0.001), and were more likely to be female (14% vs. 5%; P < 0.001). Data were missing at high rates: pH (50%), FEV1 (37%), PaO2 (23%), and BMI (8%).
At 3 months, 20 out of 119 patients (16.8%; 95% CI, 10.6–24.8%) who were discharged without PAP had died, as opposed to 24 out of 1,043 patients (2.3%; 95% CI, 1.5–3.3%) discharged with PAP (P < 0.0001). After adjusting for age, sex, and baseline PaCO2, the odds ratios (ORs) for mortality were significantly lower in the group discharged on PAP (adjusted OR, 0.16; 95% CI, 0.08–0.33; P < 0.0001; estimated risk difference: 136 fewer deaths per 1,000 patients, with 95% CI from 105 fewer to 152 fewer deaths). We also explored 3-month mortality in the subgroup of 328 patients for whom arterial blood gases were available at baseline and on discharge from the hospital (n = 100 discharged without PAP and n = 228 discharged on PAP). In this subgroup, there were no differences in age (73 ± 10 yr vs. 71 ± 13 yr; P = 0.313), baseline pH (7.25 ± 0.08 vs. 7.26 ± 0.08; P = 0.146), baseline PaCO2 (78.8 ± 18 mm Hg vs. 79.5 ± 17 mm Hg; P = 0.748), pH before discharge (7.38 ± 0.03 vs. 7.39 ± 0.03; P = 0.108), and PaCO2 before discharge (55.4 ± 7.3 mm Hg vs. 54.5 ± 7.2 mm Hg; P = 0.304) in patients discharged without PAP versus on PAP, respectively. In this subgroup, mortality remained higher in those discharged without PAP. At 3 months, 9 out of 100 patients (9.0%; 95% CI, 4.2–16.4%) discharged without PAP had died. In contrast, 10 out of 228 patients (4.4%; 95% CI, 2.1–7.9%) discharged with PAP had died at 3 months (P = 0.085). After adjusting for age and sex, the OR for mortality was lower in the group discharged on PAP (adjusted OR, 0.48; 95% CI, 0.19–1.24; estimated risk difference, 44 fewer deaths per 1,000 patients, with 95% CI from 72 fewer to 19 more).
The evidence behind this recommendation has key limitations. The data are observational, because no randomized trials exist to answer this question. The studies had variable inclusion and exclusion criteria, did not specify the decision-making process to discharge with or without PAP, and did not report other relevant outcomes. Therefore, the reported data are at serious risk of bias, and the level of certainty regarding reported outcomes is very low.
Panel judgment
The desirable effect assessed by the panel was mortality at 3 months after hospital discharge. The panel was certain that this outcome was important to patients and that the difference in mortality was large between the two groups. Despite the limitations of the available evidence, the panel agreed that the desirable effects of PAP outweigh its trivial, undesirable effects. Although cost-effectiveness data are lacking, panel members surmised that a majority of patients discharged from the hospital without PAP would likely require PAP after an outpatient sleep study. The panel also acknowledged the regional variations in the cost and availability of PAP. Healthcare settings where significant effort is required from healthcare providers to prescribe PAP at discharge, third party payers do not cover PAP, or patients pay out of pocket for PAP may have low rates of adherence to this recommendation, leading to health inequity. In certain situations, patients discharged on NIV may be able to be switched to CPAP after the sleep study. Switching to CPAP, if appropriate, may lead to additional cost savings in some settings.
Notwithstanding the above-mentioned limitations, the panel agreed that discharging patients on PAP will probably be acceptable to key stakeholders, and this intervention is probably feasible to implement. Importantly, the great majority of patients (92%) from the analyzed studies were discharged on NIV, as opposed to CPAP. Moreover, these patients had not undergone sleep studies and PAP titration studies, and, as such, the panel was uncertain whether OHS will be as responsive to CPAP or not (i.e., OHS phenotype without severe OSA). Therefore, NIV should be considered the treatment of choice until further evaluation is performed with subsequent sleep studies. The panel’s judgements are summarized in the online supplement.
ATS recommendation
Hospitalized patients suspected of having OHS should be started on NIV therapy before being discharged from the hospital and continued on NIV therapy until they undergo outpatient workup and titration of PAP therapy in the sleep laboratory, ideally during the first 3 months after hospital discharge (conditional recommendation, very low level of certainty in the evidence).
Remarks
When discharging patients on NIV, clinicians may choose to discharge them on the same NIV settings that were used during hospitalization. The panel could not recommend empiric settings for NIV. In settings where NIV is not available because of limited resources, discharging patients on auto-PAP is preferable to no PAP. Ultimately, discharging patients from the hospital on NIV or auto-PAP therapy should not be a substitute for arranging an outpatient sleep study to appropriately titrate PAP therapy.
Future research opportunities
There is a need for well-designed RCTs to assess whether patients should be discharged on PAP (NIV or CPAP) or not. In addition to mortality, future clinical trials should assess the impact of PAP on discharge on important patient-centered outcomes, such as readmission to the hospital, healthcare resource use, quality of life, resolution of symptoms, and cost-effectiveness. More research is needed on the optimal timing of the sleep study after a hospitalization for acute-on-chronic hypercapnic respiratory failure. Last, the role of autotitrating NIV devices in lieu of in-laboratory PAP titration requires further investigation.
Question 5: Should a Weight-Loss Intervention or No Such Intervention Be Used for Adults with OHS?
Background
Obesity is a major risk factor for the development of OHS. The current treatment of patients with OHS focuses mainly on treating SDB with PAP and not on addressing the underlying obesity or reducing overall cardiovascular risk profile. Despite adequate adherence to PAP, however, important cardiovascular and metabolic risk factors related to severe obesity persist (11, 21, 22), and cardiovascular morbidity and mortality rates remain high (3, 20, 55).
Weight-loss interventions can improve OHS and OSA, as well as cardiovascular and metabolic outcomes. Many strategies are available to achieve weight loss. However, losing weight and maintaining weight loss using commercially available programs is challenging (89). Very intensive lifestyle intervention has been proven to be successful in achieving weight loss in obese patients with type 2 diabetes or prediabetes but does not improve long-term cardiovascular outcomes, as patients often regain the lost weight (90, 91). Unsurprisingly, bariatric interventions are effective in achieving significant, sustainable weight loss that can improve cardiovascular and metabolic outcomes. Moreover, the safety of bariatric procedures has improved over time (92). Most recent clinical trials have reported improvements in metabolic (93–95) and cardiovascular morbidities (96) and reductions in all-cause and cardiovascular mortality (97, 98) in patients undergoing laparoscopic sleeve gastrectomy or gastric bypass surgery. Unfortunately, the vast majority of studies either did not assess for OHS or excluded patients with OHS entirely.
Summary of evidence
Two randomized trials (61, 99) and seven case series (100–106) were identified for inclusion. No observational studies with control groups were identified.
Summary of randomized trials
The first trial randomly assigned 63 patients with BMI >35 kg/m2 and OSA (AHI > 30) or OHS (PaCO2 > 6.5 kPa or 48.7 mm Hg) on PAP to either laparoscopic adjustable gastric banding (LAGB, n = 30) or to intensive nutritional care (n = 33) and assessed outcomes over a 3-year period (99). Of these patients, 25 had evidence of hypercapnia (PaCO2 > 48.7 mm Hg) and were labeled as OHS or mixed syndrome (i.e., OSA and OHS). Eleven of the hypercapnic patients were in the LAGB group and 14 were in the intensive nutritional care group. Resolution of OHS was arbitrarily defined as being able to discontinue PAP with sufficient improvement in PaCO2. At 1 year, more patients having LAGB were able to discontinue PAP (35% vs. 13%, with an RR of 2.48; 95% CI, 0.85–7.21). However, the proportion of patients with OHS or eucapnic OSA was unknown. At 1 year, both groups experienced reduction in AHI; the reduction was 22 events/h greater in the LAGB group (95% CI, 6 fewer to 39 fewer events per hour). This effect persisted at 3 years, with the difference in reduction in AHI being 13 events/h (95% CI, 32 fewer to 6 more), again favoring the LAGB group. Follow-up at 1 year indicated that the reduction in weight was greater, by an average of 12.9 kg (95% CI, 30.2 kg lower to 4.4 kg higher), in the LAGB versus intensive nutritional care group. At 3 years, weight loss continued to be greater in the LAGB group, which lost an average of 15.7 kg (range, 36.5 kg lower to 5.1 kg higher) more than the intensive nutritional care group. Relative risk for adverse effects could not be estimated, because none were reported in the lifestyle group. The panel’s confidence in the estimated effects was low to very low (for weight loss and resolution of OHS) to moderate (for reduction in AHI), because the trial carried a serious risk of bias due to the small sample size (only 25 patients with OHS), and the authors did not report outcomes separately for the 25 patients with OHS.
In contrast, the second trial included only patients with OHS (BMI > 30 kg/m2 and PaCO2 > 6 kPa or 45 mm Hg) (61). All 37 patients were treated with NIV and randomized to either an intensive lifestyle intervention for weight loss (baseline weight, 139 ± 29 kg and baseline PaCO2, 51 mm Hg; 95% CI, 49–55 mm Hg) or to usual care (baseline weight, 141 ± 31 kg and baseline PaCO2, 53 mm Hg; 95% CI, 49–55 mm Hg), with outcomes reassessed at 3 months. The lifestyle intervention consisted of a motivational session, a personalized exercise and dietary plan, monthly review, and weekly phone calls/reminders. The lifestyle intervention led to 9.6 ± 6.7-kg weight loss. The control group was given only nutritional and exercise advice, which led to 3.0 ± 6.2 kg weight loss. The open-label study was terminated early because of low patient accrual, large loss to follow-up, and unavailability of personnel to provide the intervention. A single death was observed among 20 patients randomized to the control group. Only 30 patients had available data regarding gas exchange values at 3 months, which showed similar reductions in daytime PaCO2 and PaO2, as well as nocturnal hypoxemia. In the intensive lifestyle intervention group, the PaCO2 decreased by −6.8 mm Hg (95% CI, −1 to −12 mm Hg), and in the control group it decreased by −5.5 mm Hg (95% CI, −1.5 to −6.9 mm Hg). The improvements in gas exchange (PaCO2, PaO2, sleep hypoxemia, sleep transcutaneous CO2) from baseline to 3 months were statistically similar between the two treatment approaches at 3 months. No significant differences were seen between groups at 3 months in daytime sleepiness, exercise or functional capacity, dyspnea scores, or mood.
This second trial conducted a cost-effectiveness analysis and showed that although the intensive lifestyle intervention was more expensive than nutritional and exercise advice (£385.63 more; 95% CI, £343.59–425.93 more), it was also more effective (61). The study found a difference in quality-adjusted life years (QALYs) in favor of the intervention (0.018 more; 95% CI, 0.011–0.026). The incremental cost-effectiveness ratio on the basis of EQ-5D was on average £21,730 (95% CI, £14,353.0–35,238.7) per additional QALY gained. For reference, they assumed that the usual threshold for potential cost-effectiveness in the United Kingdom is ∼£30,000 per QALY gained.
The panel’s confidence in the estimated effects was low to very low because both trials were small, with a serious risk of bias due to the absence of blinding and high drop-out rates.
Summary of case series
We found data from seven observational studies (100–106), in which patients with OHS underwent bariatric surgery. Three of these studies (103–105) evaluated patients with OHS who had gastric bypass surgery, and a fourth (101) evaluated those who had biliopancreatic diversion with duodenal switch (BPD/DS). One of the studies used an outdated procedure, comparing vertical banded gastroplasty against Roux-en-Y gastric bypass (100), and two reported no relevant outcomes of interest (102, 106). We excluded case series that contained fewer than 20 patients (107), excluded (108) or lacked information about presence of OHS (102, 109–111), did not include a comparison group (112), or reported no usable data (113).
In the earliest observational study (104), a total of 25 of 29 (86.2%) had resolution of OHS, and 2 of 29 (6.9%) participants died during a 2-year follow-up period. The mean weight loss was −50 kg (95% CI, −39 kg to −60 kg) or 32% (95% CI, 25–39%) weight loss from a mean baseline weight of 155 kg in one study (104), and −44 kg (95% CI, −33 kg to −55 kg) or 27% (95% CI, 20–34%) weight loss from a mean baseline weight of 163 kg in 38 patients from another study (105). Improvements in gas exchange were seen at 2 years, with a mean increase in PaO2 of 15 mm Hg (95% CI, 9–21 mm Hg) (104) and 19 mm Hg (95% CI, 11–27 mm Hg) (103) in the two series. PaCO2 decreased in both studies, by 10 mm Hg (95% CI, −7 to −13 mm Hg) (104), and 10 mm Hg (95% CI, −6 to −14 mm Hg) (103). In one study with longer-term follow-up in 38 patients, the improvements in PaO2 and PaCO2 observed at 1 year were sustained at 5 years (105). Self-reported daytime hypersomnolence resolved. Only one observational study assessed pulmonary artery pressure using right heart catheterization in 18 patients with OHS, 3 to 9 months after surgery (103). The mean pulmonary artery pressure decreased on average by 13 mm Hg (95% CI, −5.8 to −20.2 mm Hg) from a baseline of 36 mm Hg. A fourth study of BPD/DS had longer follow-up of 5 to 7 years, included 16 patients with OHS, and reported resolution of OHS in all patients (101).
All of these studies had serious risk of bias due to small numbers and the absence of a control group, which yielded very low certainty in estimates of the outcomes.
Panel judgment
In patients with OHS, weight loss from bariatric surgical procedures is more likely to result in greater and more sustained reductions in weight loss than lifestyle interventions. However, bariatric surgery is also associated with greater cost and greater risk for postoperative morbidity and mortality (102, 114–118). Therefore, the panel advised that bariatric surgery should be offered only when the estimated benefit outweighs the risk. The panel’s judgements are summarized in the online supplement.
ATS recommendation
For patients with OHS we suggest using weight-loss interventions that produce sustained weight loss of 25% to 30% of actual body weight. This level of weight loss is most likely required to achieve resolution or clinically meaningful reduction of hypoventilation (conditional recommendation, very low level of certainty in the evidence).
Remarks
Available data are limited and suggest that greater weight loss results in a greater likelihood of resolution of OHS. Short-term and long-term lifestyle interventions, even intensive ones, produce 2- to 12-kg weight loss (90), an amount that is unlikely to meaningfully impact OHS. To achieve resolution of OHS, a long-term sustained weight loss of ≥25% to 30% of actual body weight is needed. Weight loss of this magnitude is more likely to be achieved with surgical interventions such as laparoscopic sleeve gastrectomy, Roux-en-Y gastric bypass, or BPD/DS and not with laparoscopic gastric banding. The choice of surgical procedure should be based on weighing potential risks of surgery against the maximum possible anticipated weight loss. OSA may persist despite the resolution of OHS after weight reduction surgery (119).
The recommendation places a high value on resolution of OHS with significant weight loss achieved after weight-reduction surgery. Moreover, such weight loss may improve cardiovascular and metabolic comorbidities observed in patients with OHS. However, given that patients with OHS are at higher surgical risk, a balanced, patient-centered, risk–benefit discussion is prudent. Moreover, the degree of weight loss necessary to mitigate cardiovascular and metabolic risk in patients with OHS is unknown.
Future research opportunities
The panel emphasized the need for RCTs to assess the safety and efficacy of various bariatric interventions in patients with OHS.
Discussion
The recommendations in this guideline are the result of our systematic review of the evidence and our interpretation of how the evidence should be applied in clinical practice. They include conditional recommendations for: 1) using serum bicarbonate below 27 mmol/L to exclude the diagnosis of OHS in the appropriate clinical context, 2) treating patients with PAP, 3) favoring CPAP over NIV for the approximately two-thirds of patients who have concomitant severe OSA, 4) discharging hospitalized patients suspected of having OHS on PAP while they await subsequent diagnostic and therapeutic workup, and 5) risk-stratifying patients for bariatric surgery interventions. Although noninvasive monitoring of ventilation (i.e., transcutaneous CO2) can be added to SpO2 monitoring for PAP titration or subsequent PAP adjustment, we found no studies that examined outcomes using monitoring of SpO2 versus combined SpO2 plus noninvasive monitoring of CO2. This area requires further investigation.
Clinicians caring for patients with OHS should view severe obesity as a major, modifiable contributing factor to both the development and severity of OHS and engage in health education and shared decision-making with patients about evidence-based weight-loss strategies. On the basis of indications, patient preferences, and risks, discussion should include bariatric surgery. Our recommendations focus on patient-centered outcomes, such as improving quality of life and quality of sleep, daytime sleepiness, gas exchange, need for supplemental oxygen, hospital resource use, and death. On the basis of our review of available evidence, we propose a diagnostic and therapeutic algorithm for patients with OHS or suspected of having OHS (Figure 1). The panel recognizes, however, that providers must consider options on the basis of resource availability and healthcare policies unique to their geographical regions. We anticipate significant progress in the field with ongoing clinical trials in OHS. New evidence should inform future revisions of these recommendations.
Figure 1.
Flowchart summarizing the panel’s recommendations. Obesity hypoventilation syndrome (OHS) may be suspected when symptoms lead to pulmonary or sleep consultation in stable conditions as an outpatient or during an episode of hospitalization due to acute-on-chronic hypercapnic respiratory failure. In the outpatient setting, the panel recommends performing a measurement of arterial blood gases (ABG) to confirm daytime hypercapnia for patients with high pretest probability of OHS (for example, very symptomatic patients with a body mass index [BMI] >40 kg/m2) or assess serum bicarbonate levels in cases in which there is a moderate or low pretest probability of OHS (for example, less symptomatic patients with a BMI of 30–40 kg/m2). When the bicarbonate level is ≥27 mmol/L, the panel recommends a confirmatory measurement of ABG to confirm the presence of hypercapnia and to carry out a sleep study to ascertain the presence and severity of sleep-disordered breathing. If the serum bicarbonate level is <27 mmol/L, OHS is highly unlikely. For management of hospitalized patients in acute-on-chronic respiratory failure treated with noninvasive ventilation (NIV) treatment, the panel recommends that patients be discharged on empiric NIV settings because of high risk of short-term (3 mo) mortality without therapy. The panel also recommends evaluation with a sleep study and positive airway pressure (PAP) titration in the sleep laboratory as early as possible after discharge from the hospital, ideally within 3 months of discharge. If the sleep evaluation demonstrates OHS and severe obstructive sleep apnea (OSA) (apnea–hypopnea index ≥ 30), the panel recommends continuous positive airway pressure (CPAP) titration and treatment. If, on the other hand, the sleep study demonstrates OHS with no OSA or mild to moderate OSA, the panel recommends NIV titration and treatment. In patients initially treated with CPAP who do not have adequate response to therapy (lack of symptom resolution or insufficient improvement in gas exchange during wakefulness or sleep), the panel recommends changing to NIV therapy. The panel also recommends that patients with OHS should be considered for bariatric surgery. All recommendations are conditional because of the very low level of certainty in the evidence. *In healthcare settings with limited or no access to NIV, discharging patients on auto-PAP would be preferable to no PAP, particularly given that 70% of patients with OHS have coexistent severe OSA. †It is important to note that OHS is a diagnosis of exclusion, and other causes of hypercapnia need to be investigated and excluded.
Plans for Updating These Guidelines
The available evidence to answer most clinical questions is limited. To remain useful, guidelines must be updated regularly with new information. The need for update will be determined by the ATS not later than in 2021.
Adapting Recommendations Locally
We aimed to develop recommendations that are both specific and also generalizable to most settings that provide care for patients with OHS. These ATS guidelines are intended for an international audience. We welcome feedback, which will be considered with future revisions of these guidelines. Local adaptation of the ATS recommendations may be necessary in some circumstances. We suggest that responsible parties use the GRADE process, a combination of direct adoption, adaptation, and de novo development of recommendations (120), preferably in collaboration with the authors of this document.
Supplementary Material
Acknowledgments
This official clinical practice guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Sleep and Respiratory Neurobiology.
Members of the subcommittee are as follows:
Babak Mokhlesi, M.D., M.Sc. (Chair)1
Juan Fernando Masa, M.D., Ph.D. (Co-Chair)2,3,4
Majid Afshar, M.D.5*
Jay S. Balachandran, M.D.6‡
Jan L. Brozek, M.D., Ph.D.7,8§
Raed A. Dweik, M.D.9‡
Ronald R. Grunstein, M.D., Ph.D.10,11‡
Indira Gurubhagavatula, M.D., M.P.H.12‡
Nicholas Hart, M.D., Ph.D.13,14‡
Roop Kaw, M.D.9‡
Geraldo Lorenzi-Filho, M.D., Ph.D.15‡
Patrick B. Murphy, M.D., Ph.D.13,14‡
Sushmita Pamidi, M.D., M.Sc.16‡
Bhakti K. Patel, M.D.1‡
Susheel P. Patil, M.D., Ph.D.17‡
Jean Louis Pépin, M.D., Ph.D.18‡
Amanda J. Piper, Ph.D.10‡
Israa Soghier, M.D.19*
Maximiliano Tamae Kakazu, M.D.20*
Mihaela Teodorescu, M.D.21‡
Aiman Tulaimat, M.D.22‡
1University of Chicago, Chicago, Illinois; 2San Pedro de Alcántara Hospital, Cáceres, Spain; 3Centro de Investigación Biomédica en Red de Enfermedades Respiratoria, Madrid, Spain; 4Instituto Universitario de Investigación Biosanitaria de Extremadura, Cáceres, Spain; 5Loyola University, Maywood, Illinois; 6Columbia–St. Mary’s Hospital, Mequon, Wisconsin; 7McMaster University, Hamilton, Ontario, Canada; 8American Thoracic Society, New York, New York; 9Cleveland Clinic Foundation, Cleveland, Ohio; 10Department of Respiratory and Sleep Medicine, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; 11Woolcock Institute of Medical Research, University of Sydney, Sydney, Australia; 12University of Pennsylvania Perelman School of Medicine, Corporal Michael Crescenz VA Medical Center, Philadelphia, Pennsylvania; 13Guy’s and St Thomas’ National Health Service Foundation Trust, London, United Kingdom; 14King’s College, London, United Kingdom; 15University of Sao Paulo, Sao Paolo, Brazil; 16McGill University, Montreal, Quebec, Canada; 17Johns Hopkins University, Baltimore, Maryland; 18University of Grenoble Alpes, Grenoble, France; 19Albert Einstein College of Medicine, New York, New York; 20Spectrum Health–Michigan State University, Grand Rapids, Michigan; 21University of Wisconsin, Madison, Wisconsin; and 22Cook County Health and Hospitals System, Chicago, Illinois
*ATS Scholars.
‡Guideline panel member.
§Lead methodologist.
Acknowledgment
The guideline panel members are grateful to all of their patients with obesity hypoventilation syndrome for allowing them to participate in their care. The authors thank MySleepApnea.Org and patient representative and advocate Mr. James C. Johnston for participating in the development of these guidelines. The authors thank the following investigators for sharing individual patient data on hospitalized patients with obesity hypoventilation syndrome that enabled us to address question 4: Miquel Ferrer, Andres Carrillo, Pascaline Priou, Frederic Gagnadoux, Andreas Palm, Stephan Budweiser, Olalla Castro-Añon, Luis A. Perez de Llano, Jean-Christian Borel, Jean Louis Pepin, Mark Howard, Amanda J. Piper, Patrick B. Murphy, Jesus Sanchez Gomez, and Virginia Almadana Pacheco. The authors also thank Drs. Esen Kiyan and Züleyha Bingöl for sharing revised data for Table 3 of their original manuscript (42) for question 1.
Footnotes
This official Clinical Practice Guideline of the American Thoracic Society was approved May 2019
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.201905-1071ST.
This guideline was funded by the American Thoracic Society.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: B.M. served as an expert witness for Roetzel and Andress Law Firm. I.G. received research support from BluTech; and served as a consultant for UptoDate. N.H. received research support from Philips and ResMed (managed by Guy’s & St. Thomas’ Foundation Trust); served as a speaker for Philips, ResMed, and Fisher & Paykel; served on an advisory committee for Philips; and has intellectual property rights for the Myotrace technology. G.L.-F. served on an advisory committee for and has ownership or investment interests in Biologix. P.B.M. received research support from Breas Medical, Fisher & Paykel, Philips, and ResMed; and served on an advisory committee for Philips. J.L.P. served as a speaker for AstraZeneca, Fisher & Paykel, Perimetre, Philips, ResMed, and SEFAM; received travel support from Agiradom and Teva; received research support from Agiradom, AstraZeneca, Air Liquide Foundation, Fisher & Paykel, GlaxoSmithKline, Mutualia, Philips, ResMed, and Vitalaire; and received personal fees from Bastide, Boehringer Ingelheim, ELIA Medical, ISIS Medical, Jazz Pharmaceutical, and Night Balance. A.J.P. served as a speaker for Philips and ResMed; served on an advisory committee for Philips; received research support from the ResMed Foundation; and received other transfers of value from Fisher & Paykel. M.T. received research support from Boehringer Ingelheim. J.F.M., M.A., J.S.B., J.LB., R.A.D., R.R.G., R.K., S.P., B.K.P., S.P.P., I.S., M.T.K., and A.T. reported no relevant commercial relationships.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Sleep and Respiratory Neurobiology
References
- 1.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc. 2008;5:218–225. doi: 10.1513/pats.200708-122MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randerath W, Verbraecken J, Andreas S, Arzt M, Bloch KE, Brack T, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49:1600959. doi: 10.1183/13993003.00959-2016. [DOI] [PubMed] [Google Scholar]
- 3.Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, Golpe R, Méndez Marote L, Castro-Castro J, et al. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One. 2015;10:e0117808. doi: 10.1371/journal.pone.0117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masa JF, Corral J, Romero A, Caballero C, Terán-Santos J, Alonso-Álvarez ML, et al. Spanish Sleep Network(*) Protective cardiovascular effect of sleep apnea severity in obesity hypoventilation syndrome. Chest. 2016;150:68–79. doi: 10.1016/j.chest.2016.02.647. [DOI] [PubMed] [Google Scholar]
- 5.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. JAMA. 2018;319:2419–2429. doi: 10.1001/jama.2018.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balachandran JS, Masa JF, Mokhlesi B. Obesity hypoventilation syndrome epidemiology and diagnosis. Sleep Med Clin. 2014;9:341–347. doi: 10.1016/j.jsmc.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harada Y, Chihara Y, Azuma M, Murase K, Toyama Y, Yoshimura C, et al. Japan Respiratory Failure Group. Obesity hypoventilation syndrome in Japan and independent determinants of arterial carbon dioxide levels. Respirology. 2014;19:1233–1240. doi: 10.1111/resp.12367. [DOI] [PubMed] [Google Scholar]
- 9.BaHammam AS. Prevalence, clinical characteristics, and predictors of obesity hypoventilation syndrome in a large sample of Saudi patients with obstructive sleep apnea. Saudi Med J. 2015;36:181–189. doi: 10.15537/smj.2015.2.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest. 2009;136:787–796. doi: 10.1378/chest.09-0615. [DOI] [PubMed] [Google Scholar]
- 11.Masa JF, Corral J, Alonso ML, Ordax E, Troncoso MF, Gonzalez M, et al. Spanish Sleep Network. Efficacy of different treatment alternatives for obesity hypoventilation syndrome: Pickwick study. Am J Respir Crit Care Med. 2015;192:86–95. doi: 10.1164/rccm.201410-1900OC. [DOI] [PubMed] [Google Scholar]
- 12.Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care. 2010;55:1347–1362. [Discussion, pp. 1363–1365.] [PubMed] [Google Scholar]
- 13.Lee WY, Mokhlesi B. Diagnosis and management of obesity hypoventilation syndrome in the ICU. Crit Care Clin. 2008;24:533–549, vii. doi: 10.1016/j.ccc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Carrillo A, Ferrer M, Gonzalez-Diaz G, Lopez-Martinez A, Llamas N, Alcazar M, et al. Noninvasive ventilation in acute hypercapnic respiratory failure caused by obesity hypoventilation syndrome and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1279–1285. doi: 10.1164/rccm.201206-1101OC. [DOI] [PubMed] [Google Scholar]
- 15.Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest. 2001;120:377–383. doi: 10.1378/chest.120.2.377. [DOI] [PubMed] [Google Scholar]
- 16.Marik PE, Chen C. The clinical characteristics and hospital and post-hospital survival of patients with the obesity hypoventilation syndrome: analysis of a large cohort. Obes Sci Pract. 2016;2:40–47. doi: 10.1002/osp4.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selva A, Sanabria AJ, Pequeño S, Zhang Y, Solà I, Pardo-Hernandez H, et al. Incorporating patients’ views in guideline development: a systematic review of guidance documents. J Clin Epidemiol. 2017;88:102–112. doi: 10.1016/j.jclinepi.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. 2014.
- 19.Masa JF, Corral J, Caballero C, Barrot E, Terán-Santos J, Alonso-Álvarez ML, et al. Spanish Sleep Network. Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea. Thorax. 2016;71:899–906. doi: 10.1136/thoraxjnl-2016-208501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masa JF, Mokhlesi B, Benítez I, Gomez de Terreros FJ, Sánchez-Quiroga MA, Romero A, et al. Spanish Sleep Network. Long-term clinical effectiveness of continuous positive airway pressure therapy versus non-invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open-label, randomised controlled trial. Lancet. 2019;393:1721–1732. doi: 10.1016/S0140-6736(18)32978-7. [DOI] [PubMed] [Google Scholar]
- 21.Borel JC, Tamisier R, Gonzalez-Bermejo J, Baguet JP, Monneret D, Arnol N, et al. Noninvasive ventilation in mild obesity hypoventilation syndrome: a randomized controlled trial. Chest. 2012;141:692–702. doi: 10.1378/chest.10-2531. [DOI] [PubMed] [Google Scholar]
- 22.Howard ME, Piper AJ, Stevens B, Holland AE, Yee BJ, Dabscheck E, et al. A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome. Thorax. 2017;72:437–444. doi: 10.1136/thoraxjnl-2016-208559. [DOI] [PubMed] [Google Scholar]
- 23.Nowbar S, Burkart KM, Gonzales R, Fedorowicz A, Gozansky WS, Gaudio JC, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116:1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Budweiser S, Riedl SG, Jörres RA, Heinemann F, Pfeifer M. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med. 2007;261:375–383. doi: 10.1111/j.1365-2796.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 25.Borel JC, Burel B, Tamisier R, Dias-Domingos S, Baguet JP, Levy P, et al. Comorbidities and mortality in hypercapnic obese under domiciliary noninvasive ventilation. PLoS One. 2013;8:e52006. doi: 10.1371/journal.pone.0052006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priou P, Hamel JF, Person C, Meslier N, Racineux JL, Urban T, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest. 2010;138:84–90. doi: 10.1378/chest.09-2472. [DOI] [PubMed] [Google Scholar]
- 27.Murphy PB, Davidson C, Hind MD, Simonds A, Williams AJ, Hopkinson NS, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax. 2012;67:727–734. doi: 10.1136/thoraxjnl-2011-201081. [DOI] [PubMed] [Google Scholar]
- 28.Palm A, Midgren B, Janson C, Lindberg E. Gender differences in patients starting long-term home mechanical ventilation due to obesity hypoventilation syndrome. Respir Med. 2016;110:73–78. doi: 10.1016/j.rmed.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Romero C, Sánchez J, Almadana V, Gómez-Bastero A, Guerrero P, Valido A, et al. Results of noninvasive ventilation in obese patients with acute respiratory failure [abstract] Chest. 2014;145:544A. [Google Scholar]
- 30.Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol. 2016;76:89–98. doi: 10.1016/j.jclinepi.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [accessed 2019 Apr 1]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 33.Zhang Y, Alonso-Coello P, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G, et al. GRADE Guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferences-Risk of bias and indirectness. J Clin Epidemiol. doi: 10.1016/j.jclinepi.2018.01.013. [online ahead of print] 13 Feb 2018; DOI: 10.1016/j.jclinepi.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Coello PA, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G, et al. GRADE guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences-inconsistency, imprecision, and other domains. J Clin Epidemiol. doi: 10.1016/j.jclinepi.2018.05.011. [online ahead of print] 22 May 2018; DOI: 10.1016/j.jclinepi.2018.05.01. [DOI] [PubMed] [Google Scholar]
- 35.Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. doi: 10.1136/bmj.i2089. [DOI] [PubMed] [Google Scholar]
- 36.Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]
- 37.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 38.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manthous CA, Mokhlesi B. Avoiding management errors in patients with obesity hypoventilation syndrome. Ann Am Thorac Soc. 2016;13:109–114. doi: 10.1513/AnnalsATS.201508-562OT. [DOI] [PubMed] [Google Scholar]
- 40.Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11:117–124. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 41.Basoglu OK, Tasbakan MS. Comparison of clinical characteristics in patients with obesity hypoventilation syndrome and obese obstructive sleep apnea syndrome: a case-control study. Clin Respir J. 2014;8:167–174. doi: 10.1111/crj.12054. [DOI] [PubMed] [Google Scholar]
- 42.Bingol Z, Pıhtılı A, Cagatay P, Okumus G, Kıyan E. Clinical predictors of obesity hypoventilation syndrome in obese subjects with obstructive sleep apnea. Respir Care. 2015;60:666–672. doi: 10.4187/respcare.03733. [DOI] [PubMed] [Google Scholar]
- 43.Borel JC, Guerber F, Jullian-Desayes I, Joyeux-Faure M, Arnol N, Taleux N, et al. Prevalence of obesity hypoventilation syndrome in ambulatory obese patients attending pathology laboratories. Respirology. 2017;22:1190–1198. doi: 10.1111/resp.13051. [DOI] [PubMed] [Google Scholar]
- 44.Chung Y, Garden FL, Jee AS, Srikantha S, Gupta S, Buchanan PR, et al. Supine awake oximetry as a screening tool for daytime hypercapnia in super-obese patients. Intern Med J. 2017;47:1136–1141. doi: 10.1111/imj.13496. [DOI] [PubMed] [Google Scholar]
- 45.Elsayed AY, El-Shafey MM, Abdelgawad TT, Abdelhady Ali R. Predictors of early diagnosis of obesity hypoventilation syndrome among patients with sleep disordered breathing. Egypt J Chest Dis Tuberc. 2017;66:453–458. [Google Scholar]
- 46.Macavei VM, Spurling KJ, Loft J, Makker HK. Diagnostic predictors of obesity-hypoventilation syndrome in patients suspected of having sleep disordered breathing. J Clin Sleep Med. 2013;9:879–884. doi: 10.5664/jcsm.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandal S, Suh ES, Boleat E, Asher W, Kamalanathan M, Lee K, et al. A cohort study to identify simple clinical tests for chronic respiratory failure in obese patients with sleep-disordered breathing. BMJ Open Respir Res. 2014;1:e000022. doi: 10.1136/bmjresp-2014-000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pıhtılı A, Bingöl Z, Kıyan E. The predictors of obesity hypoventilation syndrome in obstructive sleep apnea. Balkan Med J. 2017;34:41–46. doi: 10.4274/balkanmedj.2015.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury A, Sargsyan L, Mathew R, Majid R, Castriotta RJ. Role of oxygen saturation during sleep in identifying obesity hypoventilation syndrome and its correlation with supine wake end-tidal pco2 [abstract] Sleep (Basel) 2017;40:A167–A168. [Google Scholar]
- 50.Jee AS, Srikantha S, Gupta S, Buchanan PR, Collett PW, Garden FL, et al. Awake supine oximetry as a screening tool for severe sleep disordered breathing and hypoventilation in the super-obese. Sleep Biol Rhythms. 2015;13:83. [Google Scholar]
- 51.Probert GM, Prudon B, West SD. Is the “time spent with saturations below 90%” on sleep study helpful in identifying obesity hypoventilation syndrome in the sleep clinic [abstract]? Thorax. 2015;70:A19. [Google Scholar]
- 52.Ryan S, Russell A, Nolan G, McNicholas W. Clinical predictors of obesity hypoventilation syndrome in morbidly obese subjects with obstructive sleep apnoea. Eur Respir J. 2015;46:PA2409. [Google Scholar]
- 53.Sullivan CE, Berthon-Jones M, Issa FG. Remission of severe obesity-hypoventilation syndrome after short-term treatment during sleep with nasal continuous positive airway pressure. Am Rev Respir Dis. 1983;128:177–181. doi: 10.1164/arrd.1983.128.1.177. [DOI] [PubMed] [Google Scholar]
- 54.Blankenburg T, Benthin C, Pohl S, Bramer A, Kalbitz F, Lautenschläger C, et al. Survival of hypercapnic patients with COPD and obesity hypoventilation syndrome treated with high intensity non invasive ventilation in the daily routine care. Open Respir Med J. 2017;11:31–40. doi: 10.2174/1874306401711010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouloukaki I, Mermigkis C, Michelakis S, Moniaki V, Mauroudi E, Tzanakis N, et al. The association between adherence to positive airway pressure therapy and long-term outcomes in patients with obesity hypoventilation syndrome: a prospective observational study. J Clin Sleep Med. 2018;14:1539–1550. doi: 10.5664/jcsm.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Damiani MF, Falcone VA, Carratù P, Scoditti C, Bega E, Dragonieri S, et al. Using PaCO2 values to grade obesity-hypoventilation syndrome severity: a retrospective study. Multidiscip Respir Med. 2017;12:14. doi: 10.1186/s40248-017-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinemann F, Budweiser S, Dobroschke J, Pfeifer M. Non-invasive positive pressure ventilation improves lung volumes in the obesity hypoventilation syndrome. Respir Med. 2007;101:1229–1235. doi: 10.1016/j.rmed.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 58.Hida W, Okabe S, Tatsumi K, Kimura H, Akasiba T, Chin K, et al. Nasal continuous positive airway pressure improves quality of life in obesity hypoventilation syndrome. Sleep Breath. 2003;7:3–12. doi: 10.1007/s11325-003-0003-1. [DOI] [PubMed] [Google Scholar]
- 59.Janssens JP, Derivaz S, Breitenstein E, De Muralt B, Fitting JW, Chevrolet JC, et al. Changing patterns in long-term noninvasive ventilation: a 7-year prospective study in the Geneva Lake area. Chest. 2003;123:67–79. doi: 10.1378/chest.123.1.67. [DOI] [PubMed] [Google Scholar]
- 60.Jothieswaran A, Mascareno M, Bokhari S, Chaudhry N, Felton TW, Bentley AM. P191 survival in patients with chronic type 2 respiratory failure: a comparison of obesity hypoventilation syndrome, COPD and overlap syndrome [abstract] Thorax. 2015;70:A172.2–A173. [Google Scholar]
- 61.Mandal S, Suh ES, Harding R, Vaughan-France A, Ramsay M, Connolly B, et al. Nutrition and Exercise Rehabilitation in Obesity hypoventilation syndrome (NERO): a pilot randomised controlled trial. Thorax. 2018;73:62–69. doi: 10.1136/thoraxjnl-2016-209826. [DOI] [PubMed] [Google Scholar]
- 62.Masa JF, Celli BR, Riesco JA, Hernández M, Sánchez De Cos J, Disdier C. The obesity hypoventilation syndrome can be treated with noninvasive mechanical ventilation. Chest. 2001;119:1102–1107. doi: 10.1378/chest.119.4.1102. [DOI] [PubMed] [Google Scholar]
- 63.Mokhlesi B, Tulaimat A, Evans AT, Wang Y, Itani AA, Hassaballa HA, et al. Impact of adherence with positive airway pressure therapy on hypercapnia in obstructive sleep apnea. J Clin Sleep Med. 2006;2:57–62. [PMC free article] [PubMed] [Google Scholar]
- 64.Ojeda Castillejo E, de Lucas Ramos P, López Martin S, Resano Barrios P, Rodríguez Rodríguez P, Morán Caicedo L, et al. Noninvasive mechanical ventilation in patients with obesity hypoventilation syndrome: long-term outcome and prognostic factors. Arch Bronconeumol. 2015;51:61–68. doi: 10.1016/j.arbres.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 65.Pérez de Llano LA, Golpe R, Ortiz Piquer M, Veres Racamonde A, Vázquez Caruncho M, Caballero Muinelos O, et al. Short-term and long-term effects of nasal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Chest. 2005;128:587–594. doi: 10.1378/chest.128.2.587. [DOI] [PubMed] [Google Scholar]
- 66.Piesiak P, Brzecka A, Kosacka M, Jankowska R. Efficacy of noninvasive mechanical ventilation in obese patients with chronic respiratory failure. Adv Exp Med Biol. 2013;788:167–173. doi: 10.1007/978-94-007-6627-3_25. [DOI] [PubMed] [Google Scholar]
- 67.Ricketts C, Murphie P, Little S. Outcomes in a Scottish district general hospital domiciliary NIV service. Eur Respir J. 2014;44:P2096. [Google Scholar]
- 68.Russo V, Di Meo F, Rago A, Mosella M, Molino A, Russo MG, et al. Impact of continuous positive airway pressure therapy on atrial electromechanical delay in obesity-hypoventilation syndrome patients. J Cardiovasc Electrophysiol. 2016;27:327–334. doi: 10.1111/jce.12879. [DOI] [PubMed] [Google Scholar]
- 69.Salord N, Mayos M, Miralda RM, Farré A, Carreras M, Sust R, et al. Continuous positive airway pressure in clinically stable patients with mild-to-moderate obesity hypoventilation syndrome and obstructive sleep apnoea. Respirology. 2013;18:1135–1142. doi: 10.1111/resp.12131. [DOI] [PubMed] [Google Scholar]
- 70.Salturk C, Karakurt Z, Takir HB, Balci M, Kargin F, Mocin OY, et al. Comparison of exercise capacity in COPD and other etiologies of chronic respiratory failure requiring non-invasive mechanical ventilation at home: retrospective analysis of 1-year follow-up. Int J Chron Obstruct Pulmon Dis. 2015;10:2559–2569. doi: 10.2147/COPD.S91950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabernero Huguet E, Gil Alaña P, Arana-Arri E, Citores Martín L, Alkiza Basañez R, Hernandez Gil A, et al. Non-invasive ventilation in ‘do-not-intubate’ patients in a chronic disease hospital: one year follow-up study [in Spanish] Rev Esp Geriatr Gerontol. 2016;51:221–224. doi: 10.1016/j.regg.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Tsolaki V, Pastaka C, Kostikas K, Karetsi E, Dimoulis A, Zikiri A, et al. Noninvasive ventilation in chronic respiratory failure: effects on quality of life. Respiration. 2011;81:402–410. doi: 10.1159/000317138. [DOI] [PubMed] [Google Scholar]
- 73.Wong D, Jones S. Obesity hypoventilation syndrome (OHS): the presentations and outcomes of 79 inpatients. Sleep Biol Rhythm. 2013;11:2059. [Google Scholar]
- 74.Sanchez Gomez JF, Pacheco A, Zamora G, Benitez Moya JM, Valido Morales A, et al. Results of non-invasive ventilation in patients with acute hypercapnic ventilatory failure and obesity hypoventilation syndrome (OHS) Eur Respir J. 2012;40:2042. [Google Scholar]
- 75.Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence: the Tele-OSA randomized trial. Am J Respir Crit Care Med. 2018;197:117–126. doi: 10.1164/rccm.201703-0582OC. [DOI] [PubMed] [Google Scholar]
- 76.Schwab RJ, Badr SM, Epstein LJ, Gay PC, Gozal D, Kohler M, et al. ATS Subcommittee on CPAP Adherence Tracking Systems. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188:613–620. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borel JC, Borel AL, Monneret D, Tamisier R, Levy P, Pepin JL. Obesity hypoventilation syndrome: from sleep-disordered breathing to systemic comorbidities and the need to offer combined treatment strategies. Respirology. 2012;17:601–610. doi: 10.1111/j.1440-1843.2011.02106.x. [DOI] [PubMed] [Google Scholar]
- 78.Chouri-Pontarollo N, Borel JC, Tamisier R, Wuyam B, Levy P, Pépin JL. Impaired objective daytime vigilance in obesity-hypoventilation syndrome: impact of noninvasive ventilation. Chest. 2007;131:148–155. doi: 10.1378/chest.06-1159. [DOI] [PubMed] [Google Scholar]
- 79.Kawata N, Tatsumi K, Terada J, Tada Y, Tanabe N, Takiguchi Y, et al. Daytime hypercapnia in obstructive sleep apnea syndrome. Chest. 2007;132:1832–1838. doi: 10.1378/chest.07-0673. [DOI] [PubMed] [Google Scholar]
- 80.Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63:395–401. doi: 10.1136/thx.2007.081315. [DOI] [PubMed] [Google Scholar]
- 81.Berger KI, Ayappa I, Sorkin IB, Norman RG, Rapoport DM, Goldring RM. CO(2) homeostasis during periodic breathing in obstructive sleep apnea. J Appl Physiol (1985) 2000;88:257–264. doi: 10.1152/jappl.2000.88.1.257. [DOI] [PubMed] [Google Scholar]
- 82.Berger KI, Ayappa I, Sorkin IB, Norman RG, Rapoport DM, Goldring RM. Postevent ventilation as a function of CO(2) load during respiratory events in obstructive sleep apnea. J Appl Physiol (1985) 2002;93:917–924. doi: 10.1152/japplphysiol.01082.2001. [DOI] [PubMed] [Google Scholar]
- 83.Lloyd-Owen SJ, Donaldson GC, Ambrosino N, Escarabill J, Farre R, Fauroux B, et al. Patterns of home mechanical ventilation use in Europe: results from the Eurovent survey. Eur Respir J. 2005;25:1025–1031. doi: 10.1183/09031936.05.00066704. [DOI] [PubMed] [Google Scholar]
- 84.Garner DJ, Berlowitz DJ, Douglas J, Harkness N, Howard M, McArdle N, et al. Home mechanical ventilation in Australia and New Zealand. Eur Respir J. 2013;41:39–45. doi: 10.1183/09031936.00206311. [DOI] [PubMed] [Google Scholar]
- 85.Melloni B, Mounier L, Laaban JP, Chambellan A, Foret D, Muir JF. Home-based care evolution in chronic respiratory failure between 2001 and 2015 (Antadir Federation Observatory) Respiration. 2018;96:446–454. doi: 10.1159/000490549. [DOI] [PubMed] [Google Scholar]
- 86.Orfanos S, Jaffuel D, Perrin C, Molinari N, Chanez P, Palot A. Switch of noninvasive ventilation (NIV) to continuous positive airway pressure (CPAP) in patients with obesity hypoventilation syndrome: a pilot study. BMC Pulm Med. 2017;17:50. doi: 10.1186/s12890-017-0391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Storre JH, Seuthe B, Fiechter R, Milioglou S, Dreher M, Sorichter S, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest. 2006;130:815–821. doi: 10.1378/chest.130.3.815. [DOI] [PubMed] [Google Scholar]
- 88.Pérez de Llano LA, Golpe R, Piquer MO, Racamonde AV, Caruncho MV, López MJ, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration. 2008;75:34–39. doi: 10.1159/000105460. [DOI] [PubMed] [Google Scholar]
- 89.Gudzune KA, Doshi RS, Mehta AK, Chaudhry ZW, Jacobs DK, Vakil RM, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. 2015;162:501–512. doi: 10.7326/M14-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orchard TJ, Temprosa M, Barrett-Connor E, Fowler SE, Goldberg RB, Mather KJ, et al. Diabetes Prevention Program Outcomes Study Research Group. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med. 2013;30:46–55. doi: 10.1111/j.1464-5491.2012.03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carlsson LM, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 94.Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–2304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 95.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 97.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 98.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 99.Feigel-Guiller B, Drui D, Dimet J, Zair Y, Le Bras M, Fuertes-Zamorano N, et al. Laparoscopic gastric banding in obese patients with sleep apnea: a 3-year controlled study and follow-up after 10 years. Obes Surg. 2015;25:1886–1892. doi: 10.1007/s11695-015-1627-5. [DOI] [PubMed] [Google Scholar]
- 100.Boone KA, Cullen JJ, Mason EE, Scott DH, Doherty C, Maher JW. Impact of vertical banded gastroplasty on respiratory insufficiency of severe obesity. Obes Surg. 1996;6:454–458. doi: 10.1381/096089296765556322. [DOI] [PubMed] [Google Scholar]
- 101.De Cesare A, Cangemi B, Fiori E, Bononi M, Cangemi R, Basso L. Early and long-term clinical outcomes of bilio-intestinal diversion in morbidly obese patients. Surg Today. 2014;44:1424–1433. doi: 10.1007/s00595-014-0856-x. [DOI] [PubMed] [Google Scholar]
- 102.DeMaria EJ, Murr M, Byrne TK, Blackstone R, Grant JP, Budak A, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg. 2007;246:578–582. doi: 10.1097/SLA.0b013e318157206e. [Discussion, pp. 583–584.] [DOI] [PubMed] [Google Scholar]
- 103.Sugerman HJ, Baron PL, Fairman RP, Evans CR, Vetrovec GW. Hemodynamic dysfunction in obesity hypoventilation syndrome and the effects of treatment with surgically induced weight loss. Ann Surg. 1988;207:604–613. doi: 10.1097/00000658-198805000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sugerman HJ, Fairman RP, Baron PL, Kwentus JA. Gastric surgery for respiratory insufficiency of obesity. Chest. 1986;90:81–86. doi: 10.1378/chest.90.1.81. [DOI] [PubMed] [Google Scholar]
- 105.Sugerman HJ, Fairman RP, Sood RK, Engle K, Wolfe L, Kellum JM. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr. 1992;55:597S–601S. doi: 10.1093/ajcn/55.2.597s. [DOI] [PubMed] [Google Scholar]
- 106.Sugerman HJ, Sugerman EL, Wolfe L, Kellum JM, Jr, Schweitzer MA, DeMaria EJ. Risks and benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234:41–46. doi: 10.1097/00000658-200107000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sugerman HJ, Fairman RP, Lindeman AK, Mathers JA, Greenfield LJ. Gastroplasty for respiratory insufficiency of obesity. Ann Surg. 1981;193:677–685. doi: 10.1097/00000658-198106000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dixon JB, Schachter LM, O’Brien PE, Jones K, Grima M, Lambert G, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142–1149. doi: 10.1001/2012.jama.11580. [DOI] [PubMed] [Google Scholar]
- 109.Dávila-Cervantes A, Domínguez-Cherit G, Borunda D, Gamino R, Vargas-Vorackova F, González-Barranco J, et al. Impact of surgically-induced weight loss on respiratory function: a prospective analysis. Obes Surg. 2004;14:1389–1392. doi: 10.1381/0960892042583996. [DOI] [PubMed] [Google Scholar]
- 110.Nogués X, Goday A, Peña MJ, Benaiges D, de Ramón M, Crous X, et al. Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass [in Spanish] Cir Esp. 2010;88:103–109. doi: 10.1016/j.ciresp.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 111.Ramón JM, Salvans S, Crous X, Puig S, Goday A, Benaiges D, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–1122. doi: 10.1007/s11605-012-1855-0. [DOI] [PubMed] [Google Scholar]
- 112.Cascon JA, Fernandez R, Rubinios G, Vazquez MJ, Jerez FR, Alvarez L, et al. Significant weight loss contribute to the withdrawal of home mechanical ventilation in patients with obesity hypoventilation syndrome [abstract] Am J Respir Crit Care Med. 2014;189:A3595. [Google Scholar]
- 113.Fernandez AZ, Jr, Demaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, et al. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Ann Surg. 2004;239:698–702. doi: 10.1097/01.sla.0000124295.41578.ab. [Discussion, pp. 702–703.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaw R, Bhateja P, Paz Y Mar H, Hernandez AV, Ramaswamy A, Deshpande A, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest. 2016;149:84–91. doi: 10.1378/chest.14-3216. [DOI] [PubMed] [Google Scholar]
- 115.Cooksey J, Mokhlesi B. Postoperative complications in obesity hypoventilation syndrome and hypercapnic OSA: CO2 levels matter! Chest. 2016;149:11–13. doi: 10.1016/j.chest.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 116.Chau EH, Mokhlesi B, Chung F. Obesity hypoventilation syndrome and anesthesia. Sleep Med Clin. 2013;8:135–147. doi: 10.1016/j.jsmc.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ayas NT, Laratta CR, Coleman JM, Doufas AG, Eikermann M, Gay PC, et al. ATS Assembly on Sleep and Respiratory Neurobiology. Knowledge gaps in the perioperative management of adults with obstructive sleep apnea and obesity hypoventilation syndrome: an official American Thoracic Society workshop report. Ann Am Thorac Soc. 2018;15:117–126. doi: 10.1513/AnnalsATS.201711-888WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chau EH, Lam D, Wong J, Mokhlesi B, Chung F. Obesity hypoventilation syndrome: a review of epidemiology, pathophysiology, and perioperative considerations. Anesthesiology. 2012;117:188–205. doi: 10.1097/ALN.0b013e31825add60. [DOI] [PubMed] [Google Scholar]
- 119.Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, et al. American Thoracic Society Assembly on Sleep and Respiratory Neurobiology. The role of weight management in the treatment of adult obstructive sleep apnea: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e70–e87. doi: 10.1164/rccm.201807-1326ST. [DOI] [PubMed] [Google Scholar]
- 120.Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–110. doi: 10.1016/j.jclinepi.2016.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.