Abstract
BACKGROUND
In vitro and experimental animal studies have demonstrated that high levels of omega‐6 (n‐6) polyunsaturated fatty acids (PUFAs) and high ratios of n‐6 to omega‐3 (n‐3) PUFAs are strongly associated with the development and progression of prostate cancer (PCA). However, epidemiological studies in humans have demonstrated inconsistent findings linking dietary PUFAs and PCA risk. We hypothesize that genetic and epigenetic variations within the fatty acid desaturase (FADS) gene cluster produce gene–diet interactions that may explain these disparate findings. This study tested the relationship of the genotype of a single nucleotide polymorphism, rs174537, and the methylation status of a CpG site, cg27386326, with PUFA composition, and markers of PUFA biosynthesis in PCA tissue.
METHODS
Sixty PCA specimens from patients undergoing radical prostatectomy were genotyped, pyrosequenced and quantitated for fatty acids (FAs).
RESULTS
Long‐chain (LC)‐PUFAs, such as arachidonic acid (ARA), were abundant in these specimens, with ARA accounting for 15.8% of total FAs. In addition, there was a positive association of the G allele at rs174537 with concentrations of ARA and adrenic acid and ratios of products to precursors within the n‐6 PUFA pathway such that specimens from homozygous G individuals exhibited increasingly higher values as compared to specimens from heterozygous individuals and homozygous T individuals. Finally, the methylation status of cg27386326 was inversely correlated with tissue concentrations of LC‐PUFAs and markers of LC‐PUFA biosynthesis.
CONCLUSIONS
These data reveal that genetic and epigenetic variations within the FADS cluster are highly associated with LC‐PUFA concentrations and LC‐PUFA biosynthetic capacity in PCA tissue. They also raise the potential that gene–PUFA interactions play an important role in PCA risk and severity. Prostate 76:1182–1191, 2016. © 2016 The Authors. The Prostate published by Wiley Periodicals, Inc.
Keywords: arachidonic acid, PUFA, rs174537, cg27386326, omega‐6
INTRODUCTION
Prostate cancer (PCA) is the most common noncutaneous cancer affecting men worldwide, with over a million new cases diagnosed each year. It is well known that PCA incidence and prevalence vary among racial/ethnic groups and, more recently, have been shown to vary with geographic location. PCA rates are six times higher in Western countries than non‐Western countries 1. Numerous studies suggest that exposure to components of the modern Western diet (MWD) are associated with this elevated risk for PCA 2. Dietary omega‐6 (n‐6) polyunsaturated fatty acids (PUFAs), such as linoleic acid (LA; 18:2, n‐6) and arachidonic acid (ARA; 20:4, n‐6), have been implicated as potential risk factors for PCA. Pro‐inflammatory eicosanoids derived primarily from ARA, including prostaglandins and leukotrienes, have been demonstrated to promote both PCA development and progression in well characterized in vitro studies and in vivo animal models 3, 4, 5, 6, 7. However, despite extensive experimental data linking n‐6 PUFAs to PCA, the impact of dietary n‐6 PUFAs remains controversial, due in large part to inconsistent epidemiologic studies 8 with the majority finding no association between dietary n‐6 PUFAs and PCA in humans 9.
Long chain (LC; >20 carbons) n‐6 PUFAs, such as ARA, can be obtained preformed in the diet or synthesized via the enzymatic conversion of LA, an essential (18 carbon, 18C‐) n‐6 PUFA (Fig. 1). This biosynthetic pathway utilizes a series of alternating desaturation and elongation steps. The desaturation reactions have long been known to be the rate limiting steps in this pathway 10 and the enzymes that catalyze these reactions are encoded by the fatty acid desaturase 1 and 2 (i.e., FADS1 and FADS2) genes in a region of chromosome 11 known as the FADS cluster (11Q12.2‐q13) 11. These same enzymes also serve as regulatory enzymes for the metabolism of the essential omega‐3 (n‐3) 18C‐PUFA, α‐linolenic acid (ALA; 18:3, n‐3), to n‐3 LC‐PUFAs including eicosapentaenoic acid (EPA; 20:5, n‐3), docosapentaenoic acid (DPA; 22:5, n‐3), and docosahexaenoic acid (DHA; 22:6, n‐3) (Fig. 1) 12, 13.
Figure 1.
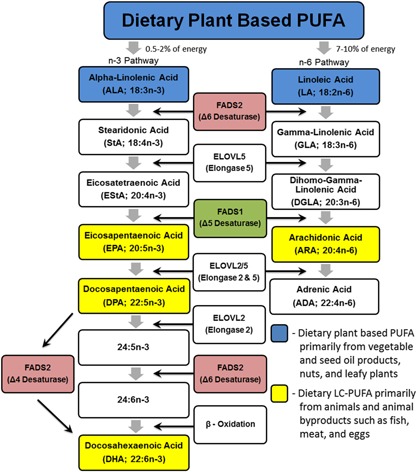
Biochemical pathway for the conversion of essential (18 carbon) n‐6 and n‐3 PUFA to LC‐PUFA.
The conversion of 18C‐PUFAs to LC‐PUFAs has traditionally been considered to be inefficient and thus contribute little to overall tissue levels of LC‐PUFAs. However, recent studies have revealed that genetic and epigenetic variability within the FADS cluster impacts the expression of FADS genes 14, 15, cellular and circulating levels of LC‐PUFAs 16, 17, and the efficiency by which individuals convert n‐6 18C‐PUFAs to LC‐PUFAs, particularly ARA 18, 19. Importantly, this work has also demonstrated that human ancestry plays a vital role in determining the frequency of these variants, with populations of African ancestry having much higher frequencies of variants associated with efficient LC‐PUFA biosynthesis resulting in increased circulating ARA concentrations 20, 21. With regard to the potential impact of these variations on disease phenotypes, studies to date have primarily focused on coronary artery disease (CAD) and brain function, and have not examined the implications of FADS variations on cancer development and progression.
Considering the large number of in vitro and in vivo animal studies showing a powerful relationship between ARA and PCA development and the marked differences in the prevalence and development of PCA in distinct racial/ethnic populations, we hypothesized that these genetic and epigenetic variants will affect the risk of PCA in humans. To begin to test this hypothesis, the current study has examined the associations between these FADS genetic and epigenetic variants and PUFA metabolism in PCA tissue from patients undergoing radical prostatectomy.
MATERIALS AND METHODS
Prostate Tissue Procurement
The Wake Forest Baptist Health Comprehensive Cancer Center maintains a tumor tissue bank of frozen surgical samples approved for research use. Under a protocol approved by the Wake Forest School of Medicine Institutional Review Board, we queried the tumor tissue bank PCA specimens from patients undergoing radical prostatectomy and identified 60 (N = 60) samples of sufficient quality and quantity to be used for fatty acid (FA), genotype, and pyrosequencing analysis. These samples consisted of 55 European American, 4 African American, and 1 Asian American donor (Table I). The median age was 60 years with a median Gleason score of 6. The Gleason score was not available for four samples. No information was available for these patients’ serum prostate specific antigen concentration, clinical course, or post‐surgical follow‐up.
Table I.
Demographics of the Patient Population
| Total (N = 60) | GG (N = 27) | GT (N = 21) | TT (N = 12) | P | |
|---|---|---|---|---|---|
| Age | 60 (55, 64) | 60 (55, 65) | 59 (53, 63) | 60 (56, 63) | 0.67 |
| Race | |||||
| Caucasian | 55 (91.67%) | 22 (81.5%) | 21 (100%) | 12 (100%) | 0.15 |
| African American | 4 (6.67%) | 4 (14.8%) | – | – | |
| Asian | 1 (1.67%) | 1 (3.7%) | – | – | |
| Gleason Score | |||||
| <6 | 2 (3%) | 2 (7%) | – | – | 0.32 |
| 6 | 35 (58%) | 13 (48%) | 13 (62%) | 9 (75%) | |
| 7 | 19 (32%) | 10 (37%) | 7 (33%) | 2 (17%) | |
| >7 | 0 |
PCA tissue was obtained from patients with the following characteristics. Kruskal–Wallis and chi‐square tests were used to evaluate differences between genotypes. Age is presented as median (interquartile range), race and Gleason score are presented as number (percent of total).
Fatty Acid Analysis
Fatty acids (FAs) were extracted from PCA tissue and measured as fatty acid methyl esters (FAMEs). FAMEs were prepared following a modification of the protocol by Metcalfe et al. 22, as previously described 20, 23. Briefly, prostate tissue samples were thawed and homogenized in distilled water at 100 mg tissue/ml. One hundred microgram (100 μg) of triheptadecanoin (a triglyceride of C17:0; NuChek Prep, Elysian, MN) was added to homogenates as an internal standard. Total fatty acids were extracted by base hydrolysis and then derivatized under alkaline conditions in methanol in the presence of boron trifluoride (5 min, 100°C) to form FAMEs. FAMEs were analyzed on an Agilent J&W DB‐23 column (30 m × 0.25 mm ID, film thickness 0.25 µm) using an HP 5890 gas chromatography (GC) with a flame ionization detector (FID). Individual FAs were identified by their elution times relative to authenticated FA standards. Twenty‐four FAs, which account for 99% of the total FAs within the extract, were routinely identified from these tissue samples. FA quantities were determined by their relative abundance to that of the added C17:0 internal standard. Individual FA concentrations were expressed as the percentage of each FA relative to the total FA concentration within each sample.
Genotype at Rs174537
DNA was isolated from archived tissues as previously described 20. The genotype at rs174537 was determined for each individual as part of a larger panel of 77 SNPs from six fatty acid candidate genes (FADS1, FADS2, ELOVL2, ELOVL5, ACAD11, and ACOX1). Rs174537 was chosen because it has been shown in a large genome‐wide association study (GWAS) to be the strongest genetic determinant associated circulating plasma ARA levels (P = 5.95 × 10−46) 24. Additionally, frequency differences in genotypes at rs174537 between African‐Americans and European‐Americans have been shown to be highly associated with differences in LC‐PUFA levels in circulating (serum and plasma) lipids between the two populations 20, 21. SNP genotyping was performed with the Sequenom iPlex genotyping system, using the manufacturer's instructions (Sequenom, Inc., San Diego, CA). The primers for this assay were 5′ Capture: 5′‐ ACGTTGGATGAGCACCATGTCTGCTGTGTG‐3′; 3′ Capture: 5′‐ ACGTTGGATGAGCCCTGTCGCCCTGCAGAA‐3′; Extend: 5′‐ ACGTCGCCCTGCAGAAGAGACAG‐3′.
Methylation Status of Cg2736326
Cg27386326 is a CpG site located within the FADS cluster, between the proximal promoters of FADS1 and FADS2, in a region with an “enhancer” signature. We recently demonstrated that the methylation status of cg27386326 is highly associated with surrogate markers of FADS metabolic activity in liver tissue 18. Genomic DNA (1 µg) from each individual was treated with sodium bisulfite using the EZ 96‐DNA methylation kit (Zymo Research, CA), following the manufacturer's standard protocol. To assay the cg27386326 CpG site, pyrosequencing with the cg27386326_04_PM assay was used with a PyroMark Q96 MD (Qiagen, Inc.). Methylation was then quantitated with Pyro Q‐CpG (version 1.0.9; Biotage, Inc.).
Statistical Analysis
Analysis of variance (ANOVA) and nonparametric (Kruskal–Wallis) statistical tests were used to compare the means and medians between genotypes, respectively. Chi‐square tests were used to evaluate proportions between groups. Post hoc pairwise comparisons were made using Tukey or Bonferroni tests. The correlation between the degree of methylation at cg27386326 and FAs (expressed as a percentage of total FAs) and FA ratios were analyzed using Pearson's correlation. All statistical analyses were conducted in STATA (IC12.1, College Station, TX) with significance set at the 0.05 level.
RESULTS
Table I shows the distribution of genotypes among the 60 PCA tissue samples. The distribution in Caucasian donors revealed more individuals in the TT genotype than anticipated from previous Caucasian cohorts (22% vs. ∼11% anticipated). However, this genotypic distribution was not significantly different from expected values (P = 0.162) and the observed difference is likely due to the limited sample size.
Examination of the overall distribution of FAs in the PCA samples (Table II) showed palmitic acid, stearic acid, oleic acid, LA, and ARA to be the primary FAs within the tissue. ARA was the primary n‐6 LC‐PUFA and comprised 15.8% of the total FAs within the tissue. DHA and DPA were the primary n‐3 LC‐PUFAs in the tissue representing 2.9% and 1.2% of the total FAs, respectively. There was a high ratio of n‐6 LC‐PUFA products, ARA and its elongation product adrenic acid (ADA; 22:4, n‐6), to the n‐6 18C‐PUFA precursor, LA, (1.7) and a similarly high ratio of n‐3 LC‐PUFA products, DHA and DPA, to the n‐3 18C‐PUFA precursor, ALA (51.3). The distribution of PUFAs and product to precursor ratios suggest that PCA tissue efficiently synthesizes and accumulates biologically active LC‐PUFAs such as ARA and DHA.
Table II.
Impact of Rs174537 Genotype on the Fatty Acid Composition of PCA Tissue
| % Fatty acid | Total (N = 60) | GG (N = 27) | GT (N = 21) | TT (N = 12) | P |
|---|---|---|---|---|---|
| C14:0 [myristolate] | 0.42 (0.25, 0.49) | 0.42 (0.23, 0.51) | 0.44 (0.35, 0.47) | 0.34 (0.11, 0.46) | 0.47 |
| C14:1 [myristoleic] | – | – | – | – | – |
| C15:0 | 0 (0, 0.12) | 0 (0, 0.19) | 0 (0,0) | 0 (0, 0.13) | 0.09 |
| C16:0 [palmitic] | 21.1 (20.6, 21.7) | 20.9 (20.1, 21.3) | 21.4 (21, 22.6) | 21.6 (20.5, 22.1) | 0.09 |
| C16:1 [palmitoleic] | 0.59 (0.46, 0.72) | 0.58 (0.47, 0.73) | 0.59 (0.41, 0.71) | 0.63 (0.47, 0.90) | 0.68 |
| C18:0 [stearic] | 16.1 (15.3, 17.1) | 16.6 (15.5, 17.8) | 15.4 (14.9, 16.4) | 15.8 (15.3, 16.0) | 0.03 |
| C18:1n‐9t [elaidic] | 0 (0, 0.3) | 0 (0, 0.3) | 0 (0, 0.27) | 0 (0, 0.31) | 0.98 |
| C18:1n‐9c [oleic] | 19.1 (17.3, 20.8) | 18.2 (15.6, 20.1) | 18.4 (17.7, 20.6) | 21 (20.05, 21.6) | 0.01 |
| C18:1n‐11c | 3 (2.64, 3.25) | 2.91 (2.52, 3.12) | 3.08 (2.81, 3.32) | 3.14 (2.56, 3.94) | 0.31 |
| C18:2n‐6c [linoleic] | 10.6 (9.77, 11.4) | 10.5 (9.86, 11.2) | 9.87 (8.86, 11.3) | 11.3 (10.8, 12.7) | 0.02 |
| C18:3n‐6 [GLA] | – | – | – | – | – |
| C18:3n‐3 [ALA] | 0.08 (0,0) | 0.07 (0,0) | 0.11 (0,0) | 0.12 (0,0) | 0.62 |
| C18:4n‐3 [SDA] | – | – | – | – | – |
| C20:0 [arachidic] | 0.32 (0, 0.41) | 0.33 (0, 0.43) | 0.31 (0.13, 0.40) | 0.31 (0, 0.41) | 0.84 |
| C20:1n‐9 | 0.89 (0.57, 1.17 | 0.71 (0.44, 1.14) | 0.91 (0.76, 1.18) | 1.09 (0.74, 1.36) | 0.09 |
| C20:2n‐6 | 0.95 (0.72, 1.14) | 0.83 (0.7, 1.12) | 0.95 (0.72, 1.14) | 1 (0.91, 1.24) | 0.27 |
| C20:3n‐6 [DGLA] | 2.82 (2.4, 3.34) | 2.52 (2.19, 2.82) | 3 (2.7, 3.56) | 3.27 (3.12, 3.89) | 0.0001 |
| C20:4n‐6 [ARA] | 15.8 (14, 17.6) | 17.4 (15.1, 18.5) | 15.7 (13.9, 17.1) | 13.08 (12.4, 14.2) | 0.0002 |
| C20:5n‐3 [EPA] | 0.03 (0, 0.22) | 0.064 (0, 0.22) | 0.11 (0, 0.25) | 0.24 (0, 0.18) | 0.56 |
| C22:0 [behenic] | 0 (0, 0.19) | 0 (0, 0.21) | 0 (0, 0.19) | 0 (0, 0.14) | 0.96 |
| C22:1n‐9 [euricic] | 0.24 (0.04, 0.29) | 0.24 (0, 0.28) | 0.23 (0, 0.29) | 0.28 (0.18, 0.38) | 0.23 |
| C22:4n‐6 [adrenic] | 2.14 (1.78, 2.55) | 2.32 (2.16, 2.63) | 2.06 (1.74, 2.28) | 1.77 (1.43, 1.91) | 0.0016 |
| C22:5n‐6 | 0.52 (0.33, 0.77) | 0.45 (0.24, 0.65) | 0.56 (0.44, 0.89) | 0.54 (0.26, 0.77) | 0.44 |
| C22:5n‐3 [DPA] | 1.22 (0.93, 1.41) | 1.32 (1.09, 1.62) | 1.19 (0.99, 1.45) | 0.89 (0.69, 1.07) | 0.006 |
| C22:6n‐3 [DHA] | 2.89 (2.27, 3.66) | 3.05 (2.68, 3.89) | 3.05 (2.04, 3.52) | 2.11 (1.79, 3) | 0.03 |
| Total n‐6/Total n‐3 | 7.84 (6.19, 9.32) | 7.45 (6.04, 8.78) | 7.33 (6.23, 8.28) | 10.24 (7.83, 11.74) | 0.0281 |
| Total LC n‐6/LC n‐3 | 5.32 | 5.01 | 5.34 | 5.99 | 0.2494 |
Fatty acids were expressed as percentage of a given fatty acid relative to total fatty acids within PCA tissue. Levels of individual fatty acids were tested for their potential association with genotype at rs174537. Median and interquartile range are presented and P‐values determined using Kruskal–Wallis test. Fatty acids showing a statistically significant relationship with the genotype at rs174537 are highlighted in bold.
Table II also illustrates the distribution of FA as a function of the genotype at rs174537. The presence of the G allele was significantly associated with higher levels of both n‐6 and n‐3 LC‐PUFAs including ARA, ADA, DPA, and DHA and lower levels of dihomo‐γ‐linolenic acid (DGLA; 20:3, n‐6). Twenty‐seven percent of the variations in ARA levels within PCA tissues were found to be attributable to differences in genotype at rs174537. Post hoc pairwise comparisons between genotypes revealed that the mean difference in ARA levels between genotypes GT and TT was −2.35 (P = 0.019) and genotypes GG and TT was −3.85 (P < 0.0001). Similarly, the mean difference in DHA levels between GG and TT genotypes was −0.75 (P = 0.03). Figure 2 shows the relationship between rs174537 genotypes and the levels of the most abundant PUFAs in PCA tissue. When focusing on the n‐6 PUFAs, the G allele was positively associated with ARA and ADA levels and negatively associated with levels of LA and DGLA. The G allele was also positively related to the n‐3 LC‐PUFAs, DPA, and DHA. While there were significant associations between average LC‐PUFA concentrations and genotypes at rs174537, substantial variation remained between individuals within each genotype (Fig. 2).
Figure 2.
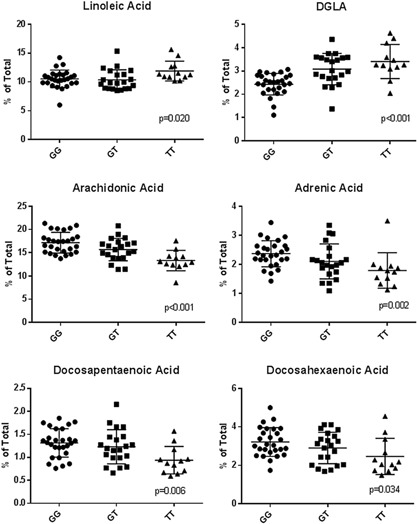
Relationship between the major PUFA and the genotype at rs174537 in PCA tissue. The concentration of individual PUFA, expressed as a percent of total tissue fatty acids, are analyzed as a function of the rs174537 genotype. P values were calculated by Kruskal–Wallis test and presented below.
An estimate of the overall efficiency of the n‐6 PUFA metabolic pathway was determined by calculating the ratio of measurable terminal products (ARA and ADA) to that of the initial precursor (LA). In addition, DGLA can be used with ARA as a product precursor pair to estimate the efficiency of FADS1 activity. Figure 3 reveals that there is a strong rs174537 genotype‐dependent relationship between products and precursors for the efficiency of the overall pathway as well as for FADS1 activity.
Figure 3.
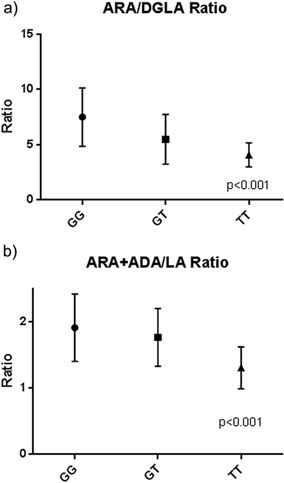
Ratio of products to precursors in the n‐6 PUFA pathway. P values were calculated by the Kruskal–Wallis test and are presented below.
To further examine the variation of PUFA levels within PCA tissue, we evaluated the association between the methylation status of the CpG site, cg27386326, and PUFA concentrations. The dynamic range for methylation at this site was found to be from 75% to 100% in PCA tissue samples. Figure 4 illustrates that the methylation status at cg27386326 was negatively correlated with ARA concentrations. Additionally, the methylation status of this site was negatively correlated with the ratio of ARA + ADA to LA (a marker for the efficiency of the n‐6 biosynthetic pathway) and the ratio of ARA to DGLA (a marker for the efficiency of FADS1). These data indicate that like liver tissue, the methylation status of cg27386326 is highly associated with capacity of PCA tissue to synthesize LC‐PUFAs.
Figure 4.
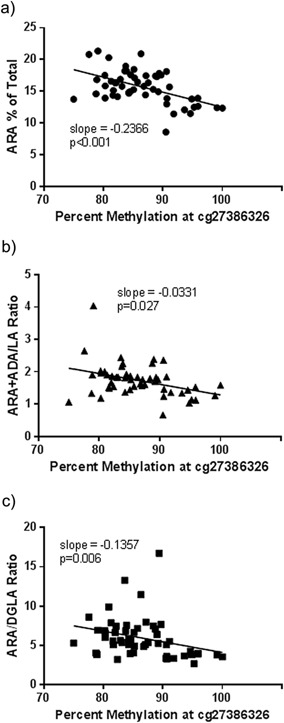
Relationship between the DNA methylation status at cg27386326 and ARA (A) and product to precursor (ARA + Adrenic Acid/LA [B]; and ARA/DGLA [C]) ratios. Statistical significance was tested using Pearson's correlation with P values shown below.
DISCUSSION
The current study examined whether known genetic and epigenetic variants in the FADS cluster impact PUFA levels and the capacity of PCA to produce LC‐PUFAs. PCA tissues were found to have high levels of ARA (∼16% of total FAs) regardless of genotype or methylation status. For comparison, tissues such as brain, cardiac, and inflammatory cells (neutrophils and macrophage), which are conventionally considered to have some of the highest ARA concentrations in humans, have ARA concentrations that range from 5% to 15% of total FAs 25, 26, 27, 28. This together with the high ratio of ARA/LA suggests that PCA tissue has a large capacity to synthesize ARA from LA. Importantly, there were strong associations between the status of genotype at rs174537 as well as methylation at cg27386326 and levels of LC‐PUFAs and PUFA product to precursor ratios. Together, this suggests that the capacity of individuals to synthesize LC‐PUFAs, such as ARA, in a tissue such as the prostate is both more efficient and variable than previously believed.
Over the past decade, numerous studies have demonstrated that FADS variants have an important impact on PUFA concentrations (especially ARA), ratios of PUFA products to precursors, and a number of important molecular and clinical phenotypes 29. FADS haplotypes that favor LC‐PUFA biosynthesis have been associated with oxidative products of ARA and elevated markers for inflammation and CAD 30, 31, 32. To date, the impact of FADS variation on cancer has not been examined in humans. Pre‐clinical animal and some clinical studies provide evidence that altering n‐3/n‐6 ratios and ARA metabolism to eicosanoids have the potential to mediate the development and progression of several cancers including those of colon, prostate, and breast 8, 33, 34. Additionally, chronic inflammation is considered an initiating step in the development of many cancers, including PCA 35. However, results from the majority of epidemiologic studies for each of these cancers fail to detect associations between dietary PUFAs and cancer risk 9, 36, 37. We postulate that genetic and epigenetic variations in the FADS cluster may be significant confounders to the results and interpretations of these epidemiologic studies.
It is important to note that the current study did not have access to dietary PUFA information from the PCA tissue donors and thus, a limitation of the study is that it is not possible to model the dietary component of potential gene–diet interactions. However, it is well established that LA found in vegetable oil products (soybean, corn, palm, and canola oils as well as margarine and shortenings) is by far the most abundant PUFA in the MWD, contributing more than 90% of ingested PUFAs and 7–9% of food energy consumed 38. According to Blasbalg and colleagues, the “most striking modification of the US food supply during the 20th century was the 1,000‐fold increase in the estimated per capita consumption of soybean oil from 0.006% to 7.38% of energy” 38. Greater than 50% of the total FAs in most soybean oils is LA. It has been shown that this dramatic increase in dietary LA has led to marked increases in circulating LA that can serve as substrate for conversion, in tissues that have the capacity, to LC‐PUFAs such as ARA 38. Additionally, it has been speculated that this increase in LA consumption is a key component in the MWD that has the capacity to elevate the risk for PCA 39, 40, 41.
Numerous factors can complicate studies that examine the association of dietary PUFA intake with disease risk. Dietary PUFAs, once consumed, reside in multiple pools of circulating lipids and are metabolized to LC‐PUFAs by target tissues such as the liver and fat. Until recently, the bioconversion of dietary 18C‐PUFAs to LC‐PUFAs was thought to be minimal (<1% efficient) and uniform across all human populations. However, as demonstrated in this study, there are genetic and epigenetic variants that impact tissue LC‐PUFA concentrations and the efficiency at which circulating PUFAs are converted to LC‐PUFAs. If FADS variants and cellular levels ARA impact the capacity of PCA tissue to make eicosanoids, as has been shown in whole blood 42, then altered ARA levels associated with these variants could play an important role in eicosanoid‐mediated PCA development and progression.
Additionally, it is now recognized that certain populations (e.g., African ancestry) have much higher frequencies of FADS variants associated with increased LC‐PUFA biosynthesis. A GWAS that included five cohorts with ∼8,000 African Americans confirmed the importance of the FADS cluster in affecting circulating PUFA and CAD biomarker concentrations across populations 43. These factors can confound studies that attempt to study the relationship between dietary FAs and disease outcomes. They may also provide a genetic basis for gene–diet interactions that result in PCA and other health disparities in certain racial/ethnic populations.
AUTHORS' CONTRIBUTIONS
AH, SS, TDH, and FHC conceived and designed the experiments. AH, SS, and TDH performed the experiments. TC, MCS, AH, ER, TDH, and FHC analyzed the data. TC, AH, ER, SS, TDH, and FHC contributed reagents/materials/analysis tools. TC, MCS, SS, ER, and FHC wrote the paper.
ACKNOWLEDGMENTS
We would like to acknowledge the contributions of Dr. Danial B. Rukstalis, Leslie Miller, and Priscilla Ivester as well as the Wake Forest Comprehensive Cancer Center, without whom, this work would not have been possible. Funding source: National Institutes of Health (NIH P50 AT002782 to FHC). Thank you.
Conflicts of interest: FHC is a paid consultant for Eagle Wellness, LLC. This has been revealed to Wake Forest Baptist Medical Center and is institutionally managed. All other authors have no conflicts to disclose.
REFERENCES
- 1. Center MM, Jemal A, Lortet‐Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012; 61(6):1079–1092. [DOI] [PubMed] [Google Scholar]
- 2. Muir CS, Nectoux J, Staszewski J. The epidemiology of prostatic cancer. Geographical distribution and time‐trends. Acta Oncol 1991; 30(2):133–140. [DOI] [PubMed] [Google Scholar]
- 3. Rose DP, Connolly JM. Effects of fatty acids and eicosanoid synthesis inhibitors on the growth of two human prostate cancer cell lines. Prostate 1991; 18(3):243–254. [DOI] [PubMed] [Google Scholar]
- 4. Locke JA, Guns ES, Lehman ML, Ettinger S, Zoubeidi A, Lubik A, Margiotti K, Fazli L, Adomat H, Wasan KM, Gleave ME, Nelson CC. Arachidonic acid activation of intratumoral steroid synthesis during prostate cancer progression to castration resistance. Prostate 2010; 70(3):239–251. [DOI] [PubMed] [Google Scholar]
- 5. Tawadros T, Brown MD, Hart CA, Clarke NW. Ligand‐independent activation of EphA2 by arachidonic acid induces metastasis‐like behaviour in prostate cancer cells. Br J Cancer 2012; 107(10):1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N. Influence of omega‐6 PUFA arachidonic acid and bone marrow adipocytes on metastatic spread from prostate cancer. Br J Cancer 2010; 102(2):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Angelucci A, Garofalo S, Speca S, Bovadilla A, Gravina GL, Muzi P, Vicentini C, Bologna M. Arachidonic acid modulates the crosstalk between prostate carcinoma and bone stromal cells. Endocr Relat Cancer 2008; 15(1):91–100. [DOI] [PubMed] [Google Scholar]
- 8. Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ. A high ratio of dietary n‐6/n‐3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res 2011; 31(1):1–8. [DOI] [PubMed] [Google Scholar]
- 9. Sakai M, Kakutani S, Horikawa C, Tokuda H, Kawashima H, Shibata H, Okubo H, Sasaki S. Arachidonic acid and cancer risk: A systematic review of observational studies. BMC Cancer 2012; 12:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernert JT, Jr. , Sprecher H. Studies to determine the role rates of chain elongation and desaturation play in regulating the unsaturated fatty acid composition of rat liver lipids. Biochim Biophys Acta 1975; 398(3):354–363. [DOI] [PubMed] [Google Scholar]
- 11. Glaser C, Lattka E, Rzehak P, Steer C, Koletzko B. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern Child Nutr 2011; 7(Suppl 2):27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spector AA. Essentiality of fatty acids. Lipids 1999;(34 Suppl):S1–S3. [DOI] [PubMed] [Google Scholar]
- 13. Sprecher H. The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 2002; 67(2–3):79–83. [DOI] [PubMed] [Google Scholar]
- 14. Hoile SP, Clarke‐Harris R, Huang RC, Calder PC, Mori TA, Beilin LJ, Lillycrop KA, Burdge GC. Supplementation with N‐3 long‐chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS ONE 2014; 9(10):e109896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lattka E, Eggers S, Moeller G, Heim K, Weber M, Mehta D, Prokisch H, Illig T, Adamski J. A common FADS2 promoter polymorphism increases promoter activity and facilitates binding of transcription factor ELK1. J Lipid Res 2010; 51(1):182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koletzko B, Lattka E, Zeilinger S, Illig T, Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: Findings from the Avon Longitudinal Study of Parents and Children. Am J Clin Nutr 2011; 93(1):211–219. [DOI] [PubMed] [Google Scholar]
- 17. Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, Tsai MY, Ferrucci L. Genome‐wide association study of plasma polyunsaturated fatty acids in the InCHIANTI study. PLoS Genet 2009; 5(1):e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howard TD, Mathias RA, Seeds MC, Herrington DM, Hixson JE, Shimmin LC, Hawkins GA, Sellers M, Ainsworth HC, Sergeant S, Miller LR, Chilton FH. DNA methylation in an enhancer region of the FADS cluster is associated with FADS activity in human liver. PLoS ONE 2014; 9(5):e97510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lattka E, Illig T, Heinrich J, Koletzko B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clin Nutr 2010; 29(3):277–287. [DOI] [PubMed] [Google Scholar]
- 20. Sergeant S, Hugenschmidt CE, Rudock ME, Ziegler JT, Ivester P, Ainsworth HC, Vaidya D, Case LD, Langefeld CD, Freedman BI, Bowden DW, Mathias RA, Chilton FH. Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br J Nutr 2012; 107(4):547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathias RA, Sergeant S, Ruczinski I, Torgerson DG, Hugenschmidt CE, Kubala M, Vaidya D, Suktitipat B, Ziegler JT, Ivester P, Case D, Yanek LR, Freedman BI, Rudock ME, Barnes KC, Langefeld CD, Becker LC, Bowden DW, Becker DM, Chilton FH. The impact of FADS genetic variants on ω6 polyunsaturated fatty acid metabolism in African Americans. BMC Genet 2011; 12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem 1966; 38(3):514–515. [Google Scholar]
- 23. Weaver KL, Ivester P. Seeds MC, Case LD, Arm J, Chilton FH. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J Biol Chem 2009; 284:15400–15407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, Tsai MY, Ferrucci L. Genome‐wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet 2009; 5(1):e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kew S, Banerjee T, Minihane AM, Finnegan YE, Williams CM, Calder PC. Relation between the fatty acid composition of peripheral blood mononuclear cells and measures of immune cell function in healthy, free‐living subjects aged 25–72 y. Am J Clin Nutr 2003; 77(5):1278–1286. [DOI] [PubMed] [Google Scholar]
- 26. Christophersen BO, Hagve TA, Christensen E, Johansen Y, Tverdal S. Eicosapentaenoic‐ and arachidonic acid metabolism in isolated liver cells. Scand J Clin Lab Invest Suppl 1986; 184:55–60. [PubMed] [Google Scholar]
- 27. Freemantle E, Lalovic A, Mechawar N, Turecki G. Age and haplotype variations within FADS1 interact and associate with alterations in fatty acid composition in human male cortical brain tissue. PLoS ONE 2012; 7(8):e42696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizzi TS, van der Sluis S, Derom C, Thiery E, van Kesteren RE, Jacobs N, Van Gestel S, Vlietinck R, Verhage M, Heutink P, Posthuma D. FADS2 genetic variance in combination with fatty acid intake might alter composition of the fatty acids in brain. PLoS ONE 2013; 8(6):e68000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chilton FH, Murphy RC, Wilson BA, Sergeant S, Ainsworth H, Seeds MC, Mathias RA. Diet–gene interactions and PUFA metabolism: A potential contributor to health disparities and human diseases. Nutrients 2014; 6(5):1993–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malerba G, Schaeffer L, Xumerle L, Klopp N, Trabetti E, Biscuola M, Cavallari U, Galavotti R, Martinelli N, Guarini P, Girelli D, Olivieri O, Corrocher R, Heinrich J, Pignatti PF, Illig T. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids 2008; 43(4):289–299. [DOI] [PubMed] [Google Scholar]
- 31. Baylin A, Ruiz‐Narvaez E, Kraft P, Campos H. Alpha‐linolenic acid, Delta6‐desaturase gene polymorphism, and the risk of nonfatal myocardial infarction. Am J Clin Nutr 2007; 85(2):554–560. [DOI] [PubMed] [Google Scholar]
- 32. Li SW, Lin K, Ma P, Zhang ZL, Zhou YD, Lu SY, Zhou X, Liu SM. FADS gene polymorphisms confer the risk of coronary artery disease in a Chinese Han population through the altered desaturase activities: Based on high‐resolution melting analysis. PLoS ONE 2013; 8(1):e55869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang B, Ren XL, Fu YQ, Gao JL, Li D. Ratio of n‐3/n‐6 PUFAs and risk of breast cancer: A meta‐analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer 2014; 14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer 2010; 10(3):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med 2003; 349(4):366–381. [DOI] [PubMed] [Google Scholar]
- 36. Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. Dietary intake of n‐3 and n‐6 fatty acids and the risk of prostate cancer. Am J Clin Nutr 2004; 80(1):204–216. [DOI] [PubMed] [Google Scholar]
- 37. Chavarro JE, Stampfer MJ, Li H, Campos H, Kurth T, Ma J. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2007; 16(7):1364–1370. [DOI] [PubMed] [Google Scholar]
- 38. Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega‐3 and omega‐6 fatty acids in the United States during the 20th century. Am J Clin Nutr 2011; 93(5):950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rose DP. Effects of dietary fatty acids on breast and prostate cancers: Evidence from in vitro experiments and animal studies. Am J Clin Nutr 1997; 66(6 Suppl):1513S–1522S. [DOI] [PubMed] [Google Scholar]
- 40. Wynder EL, Rose DP, Cohen LA. Nutrition and prostate cancer: A proposal for dietary intervention. Nutr Cancer 1994; 22(1):1–10. [DOI] [PubMed] [Google Scholar]
- 41. Willett WC. Specific fatty acids and risks of breast and prostate cancer: Dietary intake. Am J Clin Nutr 1997; 66(6 Suppl):1557S–1563S. [DOI] [PubMed] [Google Scholar]
- 42. Hester AG, Murphy RC, Uhlson CJ, Ivester P, Lee TC, Sergeant S, Miller LR, Howard TD, Mathias RA, Chilton FH. Relationship between a common variant in the fatty acid desaturase (FADS) cluster and eicosanoid generation in humans. J Biol Chem 2014; 289(32):22482–22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lettre G, Palmer CD, Young T, Ejebe KG, Allayee H, Benjamin EJ, Bennett F, Bowden DW, Chakravarti A, Dreisbach A, Farlow DN, Folsom AR, Fornage M, Forrester T, Fox E, Haiman CA, Hartiala J, Harris TB, Hazen SL, Heckbert SR, Henderson BE, Hirschhorn JN, Keating BJ, Kritchevsky SB, Larkin E, Li M, Rudock ME, McKenzie CA, Meigs JB, Meng YA, Mosley TH, Newman AB, Newton‐Cheh CH, Paltoo DN, Papanicolaou GJ, Patterson N, Post WS, Psaty BM, Qasim AN, Qu L, Rader DJ, Redline S, Reilly MP, Reiner AP, Rich SS, Rotter JI, Liu Y, Shrader P, Siscovick DS, Tang WH, Taylor HA, Tracy RP, Vasan RS, Waters KM, Wilks R, Wilson JG, Fabsitz RR, Gabriel SB, Kathiresan S, Boerwinkle E. Genome‐wide association study of coronary heart disease and its risk factors in 8,090 African Americans: The NHLBI CARe Project. PLoS Genet 2011; 7(2):e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]


