Abstract
In recent decades, intensive selective breeding programs have allowed the development of disease-resistant and flavorsome apple cultivars while leading to a gradual decline of a large number of ancient varieties in many countries. However, the re-evaluation of such cultivars could lead to the production new apple-based products with health beneficial properties and/or unique flavor qualities. Herein, we report the comprehensive characterization of juices obtained from 86 old, mostly Danish, apple cultivars, by employing traditional analysis (ion chromatography, °Brix, headspace gas chromatography/mass spectrometry (GC–MS), and panel test evaluation) as well as an innovative nuclear magnetic resonance (NMR)-based screening method developed by Bruker for fruit juices, known as Spin Generated Fingerprint (SGF) Profiling™. Principal component analysis showed large differences in aroma components and sensory characteristics, including odd peculiar odors and flavors such as apricot and peach, and very different levels of phenolic compounds, acids and sugars among the analyzed juices. Moreover, we observed a tendency for late-season juices to be characterized by higher °Brix values, sugar content and they were perceived to be sweeter and more flavor intense than early-season juices. Our findings are useful for the production of specialty vintage-cultivar apple juices or mixed juices to obtain final products that are characterized both by healthy properties and peculiar sensory attributes.
Keywords: apple juice, ancient apple cultivars, Bruker-SGF Fruit Juice Screener, NMR, sensory analysis, HS-GC/MS
1. Introduction
Apple (Malus domestica) juice consumption is widely spread worldwide, and it is supposed to grow in coming years [1], probably due to its specific pleasant taste and potential health benefits [2]. In particular, European populations seems to prefer apple juice over peach, pineapple and other fruit juices. This is in line with the juice market in Denmark, where the apple juice represents 29.7% of total juice consumption, being the third most consumed juice after orange juice and flavor mixes. Focusing on Danes’ habits, a very interesting trend has been observed in the past few years: people drink less juice but of better quality, thus the demand for premium products grows [3].
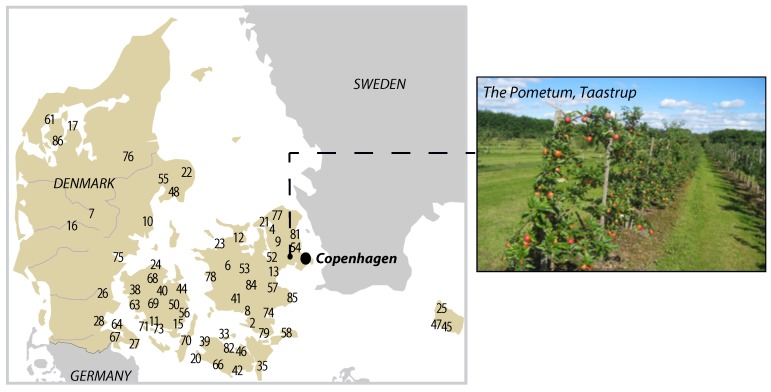
In recent decades, apple cultivars such as Gala, Fuji, Braeburn, Honeycrisp, Pink Lady, and Empire have become more popular among consumers all over the world. These cultivars are often the result of intensive selective breeding programs developed in order to improve disease resistance and shelf life of the fruit together with its sensory attributes. This process has led to a gradual decline in the growing of a large number of ancient varieties. However, in the last few years, a strong tendency to re-evaluate these ancient apple cultivars has appeared, for example in Italy [4,5], the United Kingdom [6], Croatia [7], Poland [8] and Germany [9]. These studies are mainly conducted with the aim of developing new apple-based products with health beneficial properties and/or unique flavor qualities that can be attractive to an emerging segment of consumers. Denmark, and in particular, the University of Copenhagen, has dedicated huge efforts to the study of local apple cultivars. A vast apple orchard and gene bank collection called ‘The Pometum’, located in Taastrup (Denmark) (Figure 1), comprises about 750 apple varieties of which approximately 250 are of Danish origin. Until recently, many of these cultivars were only characterized with respect to phenotypic parameters that are publicly available on the Pometum website [10]. However, lately, also a genotype fingerprinting of 448 of these cultivars was performed by Larsen and co-authors to investigate their ancestral geographical origin [11]. In this frame, we decided to provide, for the first time, an extensive chemical, aroma, and sensory characterization of 86 apple cultivars harvested from the Pometum by employing a combination of analytical techniques such as nuclear magnetic resonance (NMR)-based juice screening, dynamic headspace gas-chromatography-mass spectrometry (GC–MS), ion-chromatography and sensory evaluation. With the help of multivariate data analysis, we searched for correlations and differences among the data of different nature as well as clusters of cultivars having similar chemical and/or unique sensory properties, with the aim to identify cultivars for the production of ‘vintage juices’ for niche markets.
Figure 1.
Geographical location of the University of Copenhagen ‘Pometum’ that hosts the collection of national and international fruit genotypes. The numbers on the map refer to the sample number in Table 1 and show the place of origin of the Danish cultivars.
In Denmark, apples are normally harvested between late August and mid-October depending on cultivar and weather conditions. Late-ripening apple cultivars may have better conditions for development of flavors and other substances; however, whether there is an association between the time of ripening of a cultivar in the season and the chemical and sensory qualities of the juice, has, to the best of our knowledge, not been reported in the literature. Thus, an additional aim was to investigate how juices of early and late cultivars differ with respect to their chemical and sensory parameters.
2. Materials and Methods
2.1. Fruit Material and Juice Preparation
Apples (Malus domestica) of 86 varieties were harvested in 2010 at maturity at the experimental orchard and gene bank ‘The Pometum’ belonging to Copenhagen University. Each apple cultivar was harvested from two apple trees at optimal picking time. Since most of the apple cultivars are not commercially grown, there are no standard values for optimal picking time. However, experienced staff with knowledge of the apple cultivars assessed the optimal picking time of the apples upon kernel color, taste, peel color, and ease of picking. A random sample consisting of 10–20 healthy fruits, from all over the trees, was picked. The apples were stored at 4 °C for 0 to 10 weeks, depending on the post-harvest ripening requirement of the individual cultivars based on continuous evaluation of texture and taste of the apples. The fruits were then washed, cut with a Braun Food processor and pressed in a stainless steel 20 L hydro-press (Speidel, Germany). Juices for chemical analyses were bottled in 25 mL white polyethylene bottles (ApodanNordic PharmaPackaging A/S, Copenhagen, Denmark) and immediately frozen (–18 °C). Juices for sensory evaluation were bottled in 500 mL white polyethylene bottles (ApodanNordic PharmaPackaging A/S, Copenhagen, Denmark), and to ensure microbiological safety, the bottled samples were pasteurized in a micro-wave oven (2.5 min at 900 watt) to a temperature of approximately 80 °C. Samples were rapidly cooled in an ice/water bath and stored at 2 °C. The sensory evaluations were performed after approximately four months of storage.
In order to evaluate differences between juices of early and late cultivars, the harvest day of each apple cultivar was registered. The first apple cultivars were harvested on 23 August, and the last cultivars were harvested 52 days later. Juices were divided into two groups according to harvest day i.e., harvest day 1–30 (23 August–21 September) was assigned to class ‘early’, while harvest day 31–52 (22 September–14 October) was assigned to class ‘late’. The former group contained 39 cultivars, while the latter contained 47 cultivars. All the details about the cultivars’ characteristics are reported in Table 1.
Table 1.
Information about the 86 cultivars collected a.
| Sample | Apple Variety | Country of Origin | Usage | Introduction Date | Harvest Season b | Picking Day c |
|---|---|---|---|---|---|---|
| 1 | Louisendal | Unknown | unknown | Unknown | E | 1 |
| 2 | Skovfoged | Denmark | Dessert | 1830 | E | 2 |
| 3 | Ferskenrødt sommeræble | France | Dessert | before 1839 | E | 2 |
| 4 | Ejby æble | Denmark | Dessert | 1840 | E | 5 |
| 5 | Augustæble | Netherlands | dessert, culinary | before 1795 | E | 5 |
| 6 | Uggerløse æble | Unknown | unknown | Unknown | E | 9 |
| 7 | Herschendsgave | Denmark | Dessert | 1850 | E | 16 |
| 8 | Gadeskovæble | Denmark | Dessert | 1924 | E | 19 |
| 9 | Auroravej | Unknown | unknown | Unknown | E | 19 |
| 10 | Ondrup sommeræble | Denmark | Dessert | 1900 | E | 16 |
| 11 | Fåborgæble | Denmark | Culinary | Unknown | E | 29 |
| 12 | Kundbyæble | Denmark | Dessert | 1995 | E | 29 |
| 13 | Ingers æble | Denmark | Dessert | 1870 | E | 29 |
| 14 | Ørdings æble | Denmark | culinary | Unknown | E | 30 |
| 15 | Ulderup æble | Denmark | Dessert | 1890 | E | 29 |
| 16 | Thyregod kalvil | Denmark | Dessert | 1800 | E | 29 |
| 17 | Fuhræble | Norway | unknown | 1660 | E | 29 |
| 18 | Guldspir | Denmark | culinary | 1937 | E | 29 |
| 19 | Gravenfin | Denmark | Dessert | 1932 | E | 29 |
| 20 | Sofie æble | Denmark | Dessert | 1896 | E | 29 |
| 21 | Nina’s æble | Denmark | Dessert | 1835 | E | 29 |
| 22 | Søde æbler fra Alsrode | Denmark | Dessert | Unknown | E | 29 |
| 23 | Vallekilde Sommeræble | Denmark | Dessert | 1913 | E | 29 |
| 24 | Rosenholm | Denmark | culinary | 1870 | E | 19 |
| 25 | Dynnegårdsæble | Denmark | dessert, culinary | before 1924 | E | 29 |
| 26 | Nonnetit Bastard | Denmark | dessert, culinary | 1800 | E | 29 |
| 27 | Miang æble | Denmark | Dessert | before 1913 | L | 51 |
| 28 | Gråsten gul | Denmark | dessert, cider, culinary | 1750 | L | 39 |
| 29 | Pigeon Stribet | Denmark | Dessert | about 1860 | L | 52 |
| 30 | Butteræble | Unknown | unknown | Unknown | E | 29 |
| 31 | Vejløæble | Denmark | unknown | Unknown | E | 29 |
| 32 | Nybøllegård | Denmark | unknown | Unknown | E | 29 |
| 33 | Fejø æble | Denmark | Dessert | 1913 | E | 29 |
| 34 | Hindbæræble | Denmark | culinary | 1876 | E | 29 |
| 35 | Pilehavesæble | Denmark | culinary | 1800 | L | 39 |
| 36 | Langt rødt Hinbæræble | Germany | dessert, culinary | before 1802 | E | 29 |
| 37 | Mosede æble | Unknown | unknown | Unknown | E | 29 |
| 38 | Ingrid Marie | Denmark | dessert, culinary, cider | 1910 | L | 50 |
| 39 | Maglemer rød | Denmark | Dessert | Unknown | E | 29 |
| 40 | Fynsk udvalg V | Denmark | Dessert | 1960’ies | E | 29 |
| 41 | Lundbytorp æble | Denmark | dessert, culinary | 1913 | L | 50 |
| 42 | Bodil Neergård | Denmark | dessert, culinary | 1850 | L | 50 |
| 43 | Filippa Harritslev | Denmark | dessert | Unknown | L | 50 |
| 44 | Tønnes | Denmark | dessert, cider, culinary | 1820 | L | 50 |
| 45 | Flaskehalser | Denmark | dessert, culinary | before 1913 | L | 50 |
| 46 | Flintinge | Denmark | cullinary | before 1889 | L | 50 |
| 47 | Jakober | Denmark | culinary | before 1850 | L | 39 |
| 48 | Æbeltoftæble | Denmark | culinary | Unknown | L | 43 |
| 49 | Skenkelsø æble | Denmark | culinary | 1792 | L | 50 |
| 50 | Broholm Rosenæble | Denmark | dessert | 1866 | L | 50 |
| 51 | Ondrup moseæble | Denmark | dessert, culinary | 1850 | E | 29 |
| 52 | Høje Taastrup æble | Denmark | dessert | Unknown | E | 17 |
| 53 | Knud Lunn | Denmark | culinary | 1865 | L | 50 |
| 54 | Niels Juul | Denmark | dessert, culinary | 1875 | L | 50 |
| 55 | Thyrislund | Denmark | dessert | Unknown | L | 50 |
| 56 | Broholm | Denmark | dessert | 1866 | L | 50 |
| 57 | Skensved æble | Denmark | dessert | 1800 | E | 8 |
| 58 | Pigeon spejlsby | Denmark | dessert | 1850 | L | 52 |
| 59 | Borgherre | Netherlands | culinary | before 1788 | L | 50 |
| 60 | Mathilde æble | Denmark | dessert | 1913 | L | 52 |
| 61 | Jølbyæble | Denmark | culinary | 1938 | L | 40 |
| 62 | Gråsten rød | Germany | dessert, cider, culinary | before 1875 | L | 45 |
| 63 | Holstenhus | Denmark | dessert, culinary | 1875 | L | 40 |
| 64 | Nonnetit fra Als | Denmark | dessert | Unknown | L | 39 |
| 65 | Pigeon Rød Vinter | Denmark | culinary | Unknown | L | 39 |
| 66 | Pigeon fra Maribo | Denmark | dessert | 1930 | L | 39 |
| 67 | Alsisk Citronæble | Denmark | culinary | before 1911 | L | 37 |
| 68 | Antonius | Denmark | dessert, culinary | 1932 | L | 37 |
| 69 | Fynsk udvalg II | Denmark | dessert, culinary | 1960’ies | L | 38 |
| 70 | Annas æble | Denmark | dessert | 1900 | L | 37 |
| 71 | Bedstefars æble | Denmark | dessert | Unknown | L | 37 |
| 72 | Elstar | Netherlands | dessert | 1955 | L | 37 |
| 73 | Arreskov | Denmark | dessert | 1850 | L | 38 |
| 74 | Gråsten Høvdinggård | Denmark | dessert, culinary | 1927 | L | 38 |
| 75 | Barritskov madæble | Denmark | dessert, culinary | 1890 | L | 44 |
| 76 | Gråmølles æble | England | culinary | 1740 | L | 44 |
| 77 | Farum æble | Denmark | culinary | 1900 | L | 44 |
| 78 | Ildrød Pigeon | Denmark | dessert | 1800 | L | 44 |
| 79 | Dronning Louise | Denmark | dessert | 1892 | L | 45 |
| 80 | Risskov Rambour | Denmark | culinary | 1870 | L | 50 |
| 81 | Tagesminde æble | Denmark | culinary | Unknown | L | 45 |
| 82 | Apple 207 Knuthenborg | Denmark | culinary | Unknown | L | 46 |
| 83 | Mormors æble | Denmark | dessert | Unknown | L | 45 |
| 84 | Mølleskov | Denmark | dessert, culinary | 1840 | L | 37 |
| 85 | Herfølge voksæble | Denmark | dessert, culinary | 1825 | L | 45 |
| 86 | Lise Legind | Denmark | dessert | 1880 | L | 45 |
a Data about country of origin, usage and introduction date have been compiled from the ‘Pometum apple key’ database. b Harvest season has been classified in E = early and L = late. c Picking day 1 corresponds to 23 August while day 52 corresponds to 14 October.
2.2. Sugar and Acid Measurements: Brix and Ion Chromatography
Sucrose, glucose, fructose, malic, citric and succinic acid were measured using ion chromatography (IC). After thawing, 1.0 mL juice was filtered through a 25 mm Q-Max syringe filter with a 0.45 µm (pore size) cellulose acetate membrane (Frisenette Aps, Knebel, Denmark) and diluted 50 times with distilled water. The sample was injected on a Compact IC Pro model 881 combined with a Basic IC plus 883 unit equipped with a split port for simultaneous analysis of sugars and acids. For carbohydrate separation, a Metrozep C4-250/4.0 cation column was used, and for organic acids, a Metrozep Organic Acids –250/7.8 column was used. Detectors were an IC amperometric detector and a conductivity detector for carbohydrates and organic acids, respectively. The system was combined with a 919 IC auto sampler plus. All units were from Metrohm AG, Switzerland and controlled with ‘MagIC Net’ professional software. Calibration curves were made for glucose, fructose, sucrose and malic, citric and succinic acid using pure analytical grade solutions. °Brix was measured in one drop of juice using a QUICK°BRIX60 (Mettler Toledo, Glostrup, Denmark) portable °Brix meter. Analyses were made as single determinations, however, a measure of the IC accuracy was obtained by analyzing seven replicates of a standards’ mixture (see ‘Analytical Platforms Reliability’ in Supplementary Material).
2.3. Analysis of Aroma Compounds by Dynamic Headspace Gas Chromatography/Mass Spectrometry (GC–MS)
Samples were thawed over night at 5 °C. 15 mL of apple juice was pipetted into a 100 mL glass flask, and 1.00 mL of internal standard (50 µL L−1 4-methyl-1-pentanol (97%), Aldrich, Steinheim, Germany) was added. The use of internal standard allowed us to control sampling and analysis was unproblematic, i.e., the internal standard peak areas were in the same range in all chromatograms. The glass flask containing the sample was equipped with a purge head and transferred to a water bath, where the sample temperature was equilibrated at 30 °C for 10 min. Under magnetic stirring (200 rpm), the sample was then purged with nitrogen (100 mL min−1) for 20 min, while the volatiles were collected in traps containing 250 mg of Tenax TA (mesh size 60/80, Buchem BV, Apeldoorn, The Netherlands). Dynamic headspace collections for gas chromatography/mass spectrometry (GC–MS) were carried out as single determinations. For details about the CG-MS accuracy, please see ‘Analytical Platforms Reliability’ in Supplementary Material.
The collected volatiles were thermally desorbed using an Automated Thermal Desorber (ATD 400, Perkin Elmer, Waltham, MA, USA). Primary desorption was carried out at 250 °C (15 min) to a cold trap (30 mg Tenax TA, 5 °C), with a helium flow of 50 mL min−1. Volatiles were desorbed from the cold trap to the GC-column by heating to 300 °C for 4 min (secondary desorption), using a split ratio of 1:10. The volatiles were transferred through a heated transfer-line (225 °C) to a gas chromatograph/mass spectrometer (GC–MS, 7890A GC-system interfaced with a 5975C VL MSD (Mass Selective Detector) with Triple-Axis detector from Agilent Technologies, Palo Alto, Santa Clara, CA, USA) equipped with a J & W Scientific DB-Wax column (30 m × 0.25 mm × 0.25 µm) using helium as carrier gas (1 mL min−1). The column temperature was kept at 40 °C for 10 min, increased with 8 °C min−1 to 240 °C, and kept isothermal for 5 min. The mass selective detector used the electron ionization mode, and the mass/charge (m/z) range between 15 and 300 was scanned.
The software program, MSD-Chemstation (Version E.02.00, Agilent Technologies, Palo Alto, California), was used for data analysis, and the volatile compounds were identified by probability-based matching with mass spectra in the G1035A Wiley library (Hewlett-Packard, Palo Alto, Santa Clara, CA). Calculations were based on peak areas divided by area of internal standard (arbitrary unit based on single ions selected for each individual compound).
2.4. Sensory Evaluation
Simplified descriptive sensory evaluations were performed by five subjects belonging to the project group. During two training sessions, the panel developed and trained six sensory descriptors, i.e., ‘color’ (in practice degree of yellow/brown color), ‘overall odor’, ‘apple flavor’, ‘overall flavor’, ‘sweet taste’ and ‘sour taste’. A reference juice (cultivar ‘Jonagored’) was used for training the descriptors; and for sweet and sour taste, sucrose (11%) and malic acid (0.5%) solutions in water were also used; 50 mL of juice was served at room temperature in clear wine glasses with glass petri dishes as lids. The attribute intensities of the samples were evaluated on a 15 cm unstructured continuous line-scale, ranging from 0 (none) to 14 (very much), and the scores of each sample were averaged over five assessors. For the assessors to memorize the intensity of the descriptors between sensory evaluation sessions, a reference juice of cultivar Jonagored with its intensities pre-scored at the evaluation score sheets were used as the calibration sample at all sessions. Samples were served in random order, and at each session twenty juices were evaluated, with a short break after every four samples. Crackers, fresh cucumber and water were provided during the sensory evaluation sessions. Due to the large number of samples and a limited sample amount available, each juice was evaluated once by each judge. Moreover, distinct odors (e.g., ‘artichoke’) and flavors (e.g., ‘pear’, ‘peach’, ‘pineapple’ and ‘citrus’) were identified by the judges for some of the tested juices. In particular, the flavors listed in “The World’s first flavor wheel for apples” [12], were used to help the panel group in the juice description.
2.5. Bruker Spin Generated Fingerprint (SGF) Fruit Juice Screener
Bruker Spin Generated Fingerprint (SGF) profiling [13,14] was employed for the NMR analysis. Each sample required minimal preparation consisting of 90% juice with 10% buffer containing 0.1% of TSP (sodium salt of 3-trimethylsilyl-propionate acid-d4) and 0.013% of sodium azide to suppress microbial activity. This NMR-based screening method is based on an Avance 400 NMR spectrometer with a 9.4-T Ultrashield™ Plus magnet and utilizes flow-injection NMR (BEST™ NMR) with a 4-mm flow-cell probe with Z-gradient and a Gilson liquid handler for sample storage, preparation and transfer. Samples were provided in bar-coded cryo-vials placed in a Gilson cooling rack that kept the temperature low (about 4 °C) prior to injection. Then a heated transfer line from the Gilson unit to the probe allowed the pre-equilibration of the sample to the desired temperature (300 K) during the transfer. The overall experimental procedure was fully controlled by Bruker’s SampleTrack™ software including temperature adjustment, tuning and matching, locking, shimming and the optimization of the pulses and presaturation power for each sample. Two NMR experiments were executed: the pulse sequence ‘noesygppr1d’ allowed a quantitative evaluation even close to the water signal while the sequence ‘jresgpprgf’ was employed to obtain a fast 2D J-resolved spectrum [15] which facilitates unambiguous signal identification. The resulting spectra did not need any manual processing step, as they were automatically phase corrected and referenced by the Bruker procedure.
2.5.1. Quantification (Targeted Analysis)
After the acquisition of spectra, the identification and absolute quantification of 26 compounds was performed by comparing the acquired sample spectra with the reference spectra. The compounds included: sugars (sucrose, glucose, fructose, and xylose), acids (malic acid, citric acid, citramalic, succinic acid, chlorogenic acid, quinic acid) and indicators of apple juice quality (proline, alanine, 5-hydroxymethylfurfural, ethanol, methanol, acetaldehyde, benzaldehyde, acetoine, arbutin, and the acids lactic, fumaric, formic, benzoic, pyruvic, sorbic, and galacturonic). Furthermore, two useful relationships between compound concentrations were calculated (glucose/fructose and malic/quinic acid ratios) as well as the total sugar content for a total of 29 parameters. These are very helpful parameters for the detection of frauds and/or not optimal processing and storage conditions in commercial apple juices. For example, an exhaustive enzymatic treatment of the apple juice is detectable from an increase of galacturonic acid and the usage of unripe fruits is revealed by high concentration of quinic acid while 5-hydroxymethylfurfural is formed by acid-heat catalyzed degradation of sugars and often used as an index for heat, storage, and processing abuse [16].
2.5.2. Statistical Analysis (Non-Targeted)
The non-targeted approach consisted of an exhaustive statistical analysis based on a large reference database of 1171 apple juice samples from eight countries (China, Poland, Germany, Turkey, Brazil, Spain, Italy, and Hungary). This analysis allows us to discriminate between direct juice and juice from concentrate and it can detect also the type of fruit and its geographical origin. Then, the sample is verified in two steps. First, a univariate analysis compares each spectral region of interest with the reference data set and detects deviations in compound concentrations. The second approach is a multivariate analysis based on the principal component analysis (PCA)/SIMCA (Soft Independent Modeling of Class Analogy) approach [17] for detecting deviations which are not apparent in a univariate analysis. If both methods give the same inconspicuous result, the sample is consistent with the models.
2.6. Multivariate Data Analysis
Four different datasets containing (i) metabolite quantification from SGF profiling, (ii) headspace GC–MS relative peak areas, (iii) ion chromatography quantifications, and (iv) sensory evaluation scores were submitted to PLS toolbox version 8.1.1 (Eigenvector Research, Manson, USA) in the MATLAB (Version 8.6.0.267246, R2015b, The Mathworks, Inc., Natick, MA, USA) environment. Prior to the principal component analysis (PCA) [18], the variables were mean centered and scaled to unit variance. This kind of pre-process, called autoscaling, let all variables have a standard deviation of one and be analyzed on the basis of correlations instead of covariances, as is the case with mean centering [19].
The four datasets were then combined in a single block of data in order to consider the correlations among the different analytical parameters together with the exploration of similarities and differences among the juices analyzed.
The raw 1D NOESY NMR spectra provided by Bruker were also submitted to multivariate data analysis. However, they were first imported in Matlab using an in-house developed routine, aligned using the interval correlation optimized shifting algorithm ‘icoshift’ [20], and then normalized using an artificially generated signal at 12 ppm. The presence of this artificial standard signal allows the scaling of the signal intensity according to changes in parameters and experimental conditions [21]. The spectra were then mean centered. The assignment of the major and minor components of apple juice was carried out according to literature references [22,23,24,25].
3. Results and Discussion
3.1. Sugar and Acid Measurements
The results obtained by ion chromatography and refractometer are presented in Table 2 and in Supplementary Table S1. The average level of °Brix in the apple juices was 11.4 ranging from 8.1–14.2. With an average of 54.4 g L−1, ranging from 30.1 to 78.3 g L−1 fructose was the component found in the highest amount, in agreement with the literature [26,27,28]. Both the glucose and sucrose concentrations were rather variable, ranging from 5.4 to 20.7 (average 12.0) g L−1 and from 8.5 to 63.2 (average 33.5) g L−1, respectively. Sucrose is higher and glucose lower than reported by Eisele and Drake [28], who found averages of 21.6 g L−1 sucrose and 20.1 g L−1 glucose in juices from 175 apple cultivars grown in 12 countries and different regions in the USA. Also the dominant acid in apples, malic acid, was found in concentrations in agreement with literature values. (8.8 g L−1, versus 8.5 g L−1 in juices from apples grown in 12 countries and different regions in the USA [28] and 7.4 g L−1 in juices obtained from Polish/French apple cultivars [29]). However, the range was remarkably wide with values from 2.2–18.9 g L−1. The average citric acid concentration of 0.104 g L−1 was also similar to what has been reported earlier [28,29,30]. In the present study, the average succinic acid level was found to be 6.4 mg L−1, with non-detectable levels in many cultivars. Averages of 15 mg L−1 [31] down to 0.06 mg L−1 succinic acid [32] have earlier been reported in apples. Also, the sugar/acid ratio varied widely, from 5.8 to 33.1 with an average of 12.0. Sugar/acid ratios at 15–16 have been shown to give desired sweet taste-sourness balance of juices of Danish apple cultivars [33]. Thus, as expected in a cool climate as Denmark, the average apple juice is in the slightly sour range, but due to the large range in especially malic acid, unique cultivars were also identified with very high sugar/acid ratios (e.g. ‘Søde æbler fra Alsrode’).
Table 2.
Average concentrations of sugars and acids in the 86 apple juices, determined by ion chromatography.
| Units | Mean | SD a | % CV b | Min | Max | Range | |
|---|---|---|---|---|---|---|---|
| °Brix | % | 11.4 | 1.2 | 10.5 | 8.1 | 14.2 | 6.1 |
| Sucrose | g L−1 | 33.5 | 11.6 | 34.7 | 8.5 | 63.2 | 54.8 |
| Glucose | g L−1 | 12.0 | 3.9 | 32.7 | 5.4 | 20.7 | 15.3 |
| Fructose | g L−1 | 54.4 | 9.3 | 17.1 | 30.1 | 78.3 | 48.3 |
| Total sugar | g L−1 | 100.0 | 13.5 | 13.5 | 63.2 | 134.4 | 71.2 |
| Malic | g L−1 | 8.80 | 2.46 | 28.0 | 2.2 | 18.9 | 16.7 |
| Citric | g L−1 | 0.104 | 0.060 | 57.8 | 0.000 | 0.308 | 0.308 |
| Succinic | g L−1 | 0.006 | 0.006 | 100.8 | 0.000 | 0.033 | 0.033 |
| Sugar/acid ratio | 12.0 | 3.5 | 29.1 | 5.8 | 33.1 | 27.3 |
a SD = standard deviation. b % CV = percent coefficient of variation calculated as (SD/mean) *100.
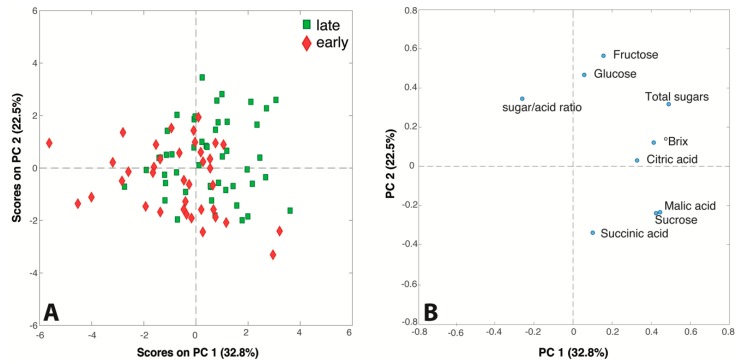
The data matrix obtained by ion chromatography and °Brix analysis was also submitted to PCA to detect trends and correlations among variables and samples. The PC1/PC2 score and loading plots (55.3% of explained variance) generated by the model are shown in Figure 2. The samples in the score plot have been colored according to the harvest season of the respective apple cultivars: a slight trend is detectable along the diagonal of the plot, since all the late-season juices seem to gather in the top right area while the late-season juices lie mostly in the bottom left part.
Figure 2.
(A) Score and (B) loading plots of the PCA model performed on the ion chromatography and °Brix dataset.
The variables responsible for this trend are the sugars (fructose, glucose, total sugar and °Brix value), suggesting, as expected, that juices produced from late season cultivars allow high-content sugar products to be obtained, while early season apples are suitable for juices low in sugar. Interestingly, the loading plot shows the correlations among the measured variables. Malic acid and sucrose, which are very close in the bottom of the plot, are positively correlated, and both are negatively correlated with fructose and glucose that lie in the top part of the plot. The inverse relationship between malate accumulation and sugars (glucose and fructose) formation in apples has been previously reported [34,35]. The reason of this phenomenon has been attributed to the transformation of malate into sugars via gluconeogenesis during fruit development [35]. At the same time, the converse levels of sucrose and glucose may be due to their interconversion while fructose is mainly formed from sorbitol and is much more highly abundant in apples than glucose [23,36].
3.2. Dynamic Headspace Gas Chromatography/Mass Spectrometry (GC–MS)
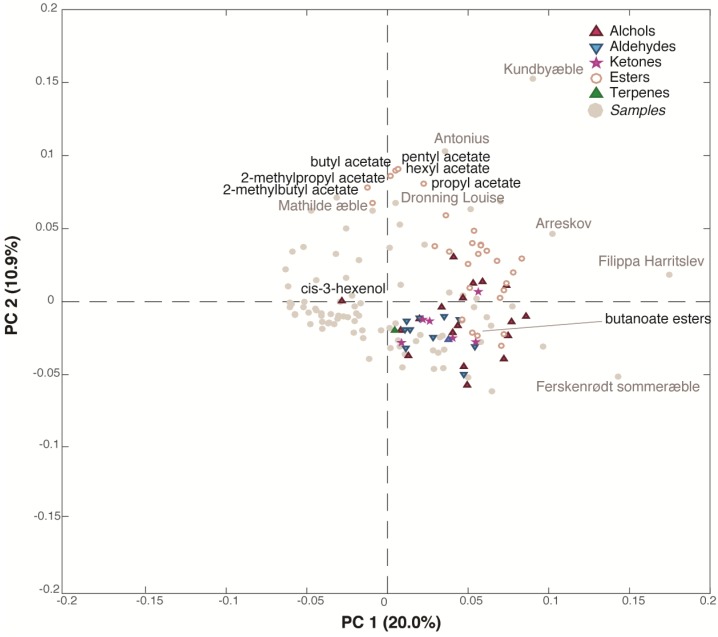
A total of 65 aroma compounds were identified in the juices, the main chemical groups being esters, alcohols, aldehydes and ketones (Supplementary Table S2) as previously reported [37]. This dataset (86 samples x 65 variables) was submitted to PCA, and it showed large differences between aroma profiles of the juices. The PC1/PC2 (30.9% of explained variance) bi-plot (Figure 3), which shows both the samples and the variables distribution, indicates that the majority of the juice samples are situated in the bottom left part of the plot, in the opposite direction of the variables. This means that they are characterized by low values of all the volatile compounds except for cis-3-hexenol. Indeed, all the alcohols (except cis-3-hexenol), esters, ketones, aldehydes lay in the right side of the plot, having high loadings values on PC1, while a small group of acetate esters (pentyl, hexyl, propyl, butyl, 2-methylpropyl and 2-methylbutyl acetate) is grouped at the top of the plot (high loadings values on PC2). The samples were found to be richer in aroma compounds (very right region of the plot) were ‘Arreskov’, ‘Filippa Harritslev’ and ‘Ferskenrødt sommeræble’ while the juices produced from ‘Kundbyæble’, ‘Antonius’, ‘Dronning Louise’ and ‘Mathilde æble’ showed a high content of acetate esters being situated in the top of the plot. Interestingly, the butanoate esters tended to gather with alcohols, aldehydes and ketones along PC1 together with hexanoate esters. For apples, groupings according to acetate and/or butanoate esters have been reported earlier [38,39]. The demonstrated correlation within the different acetate esters and butanoate esters, respectively, could indicate common formation pathways and genetic background.
Figure 3.
Bi-plot of the PCA model performed on the dynamic headspace GC-MS dataset.
3.3. Sensory Evaluation
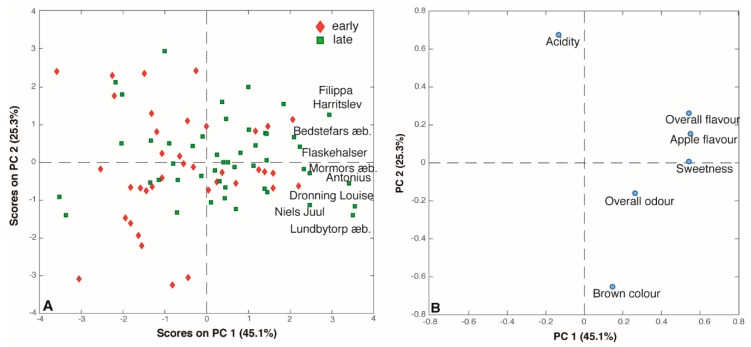
Six sensory attributes were evaluated by the assessors: sweet taste, sour taste, brown color, apple flavor, overall flavor and overall odor. Thus, the scores obtained by the panel test generated an 86 x 6 matrix that was submitted to PCA. The PC1/PC2 (70.4% of total variance) score plot reported in Figure 4A shows that, as seen for the aroma compounds, there is no clear grouping according the harvest period of the apples. Interestingly, the loading plot (Figure 4B) helps to understand the correlations among the six sensory descriptors. Indeed, ‘overall flavor’, ‘apple flavor’ and ‘sweet taste’ appear to be strictly correlated, being well clustered on the right part of the plot (high PC1 loadings), while sour taste and brown color seem to be inversely correlated given the fact that they are situated in the opposite direction of the PC2. Thus, the most appreciated juices, according to the scores, were the ones obtained from ‘Filippa Harritslev’, Bedstefars æble’, ‘Flaskehalser’, ‘Mormors æble’, ‘Antonius’, ‘Dronning Louise’, ‘Niels Juul’, and ‘Lundbytorp æble’. Seven of these eight samples were also given distinct odors and flavor. Indeed, fresh green odor and fresh apple flavor were assigned to ‘Filippa Harritslev’ and ‘Bedstefars æble’ juices while the odor and flavor of the ‘Mormors æble’s juice resemble apricots. Interestingly, the juice obtained from the ‘Antonius’ cultivar turned out to have a peach-like odor and a flavor reminiscent of tropical fruits. Overall, the sensory panel noted distinct odor notes for 31 out of 86 of the juices, while 56 juices were characterized by peculiar flavors. Most of the odors resembled other fruits like ‘pear’, ‘peach’, ‘pineapple’ and ‘berries’, but also ‘Rhubarb’ was identified and in one juice (‘Auroravej’) turned out to recall ‘artichoke’ flavor (Supplementary Table S3).
Figure 4.
(A) Score and (B) loading plots of the PCA performed on the sensory analysis dataset.
3.4. Bruker-SGF Fruit Juice Screener
3.4.1. Metabolite Quantification
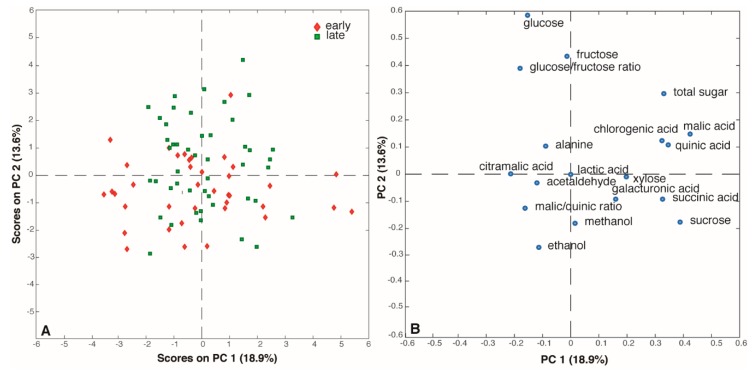
The output of the quantification analysis performed by the Bruker-SGF profiling is reported in Supplementary Table S4. Sugar and acid concentrations were in agreement with the ion chromatography measurements reported in Table 2. Interestingly, average sucrose levels turned out to be slightly above the maximum value established by the European Fruit Juice Association (AIJN) [40], while average glucose concentration is just below the minimum suggested by the association. Among the 17 indicators of apple juice quality, 12 had, as expected, levels below the limit of quantification (LOQ) of the instrument in all the analyzed samples. However, acetaldehyde, alanine, ethanol, galacturonic acid and methanol could be measured in a few juice samples. In particular, one sample (juice from cultivar ‘Louisendal’) was characterized by acetaldehyde values higher than LOQ (5 mg L−1). Alanine was quantified in 69 out of 86 samples and its average concentration was within the range established by AIJN (from 1 to 50 mg L−1), except in four samples (juices from the cultivars ‘Skovfoged æble’, ‘Ejby æble’, ‘Gråsten Rød’, and ‘Alsisk Citronæble’) that showed an alanine content above 50 mg L−1. A concentration of galacturonic acid higher than 100 mg L−1 (LOQ) was detected in ‘Gadeskovæble’ and ‘Mormors æble’ juice samples, and methanol levels above 10 mg L−1 (LOQ) was were found in 21 juice samples; this is probably due to its release from methyl esterified apple pectin during the postharvest storage of the apples, as reported in literature [41,42]. Finally, an average ethanol level of 88 mg L−1 was detected in 34 samples, in agreement with AIJN recommendations. In order to better explore this dataset, a PCA was performed after filtering out the 12 variables with levels below the LOQ in all samples, as well as the acetaldehyde; thus, only 16 parameters (variables) were included. The PC1/PC2 (32.5% of total variance) score and loading plots are reported in Figure 5. Interestingly, the loading plot indicates a strong correlation among malic acid and two of the minor acids, chlorogenic and quinic acid, that appear very close to each other in the right side of the loading plot (Figure 5B). By contrast, glucose is situated in the opposite direction of sucrose in agreement with the results obtained by ion chromatography.
Figure 5.
(A) Score and (B) loading plots of the PCA performed on the Bruker SGF profiling dataset.
3.4.2. Raw Nuclear Magnetic Resonance (NMR) Spectra Analysis
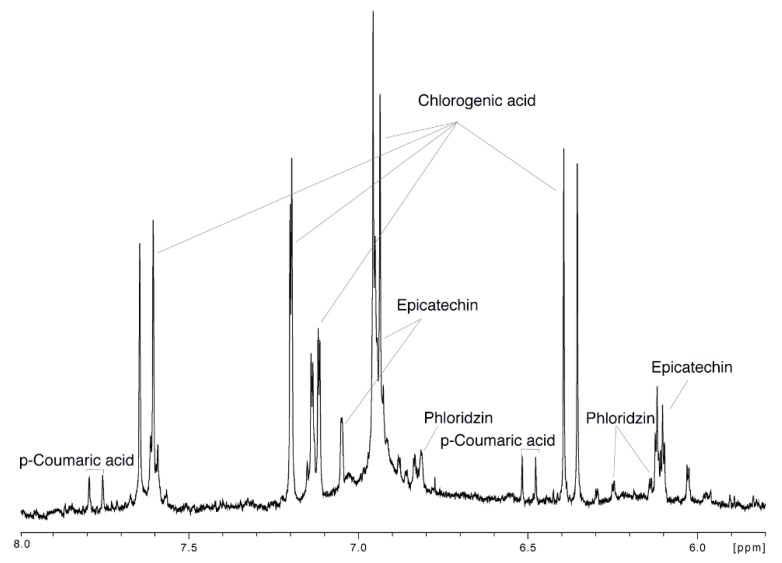
Only the region between 5.8 and 8.0 ppm of the 1H-NMR spectra of the 86 apple juices was considered during the analysis in order to i) remove the spectral region containing the most dominant signals of sugars and acids ii) explore the polyphenols-related variation in the analyzed apple juices. The signal assignment of the aromatic region is reported in Figure 6, while the remaining part of the spectrum is showed in Supplementary Figure S1.
Figure 6.
Aromatic region and relative signal assignment of a representative 1H-NMR (nuclear magnetic resonance ) spectrum of apple juice.
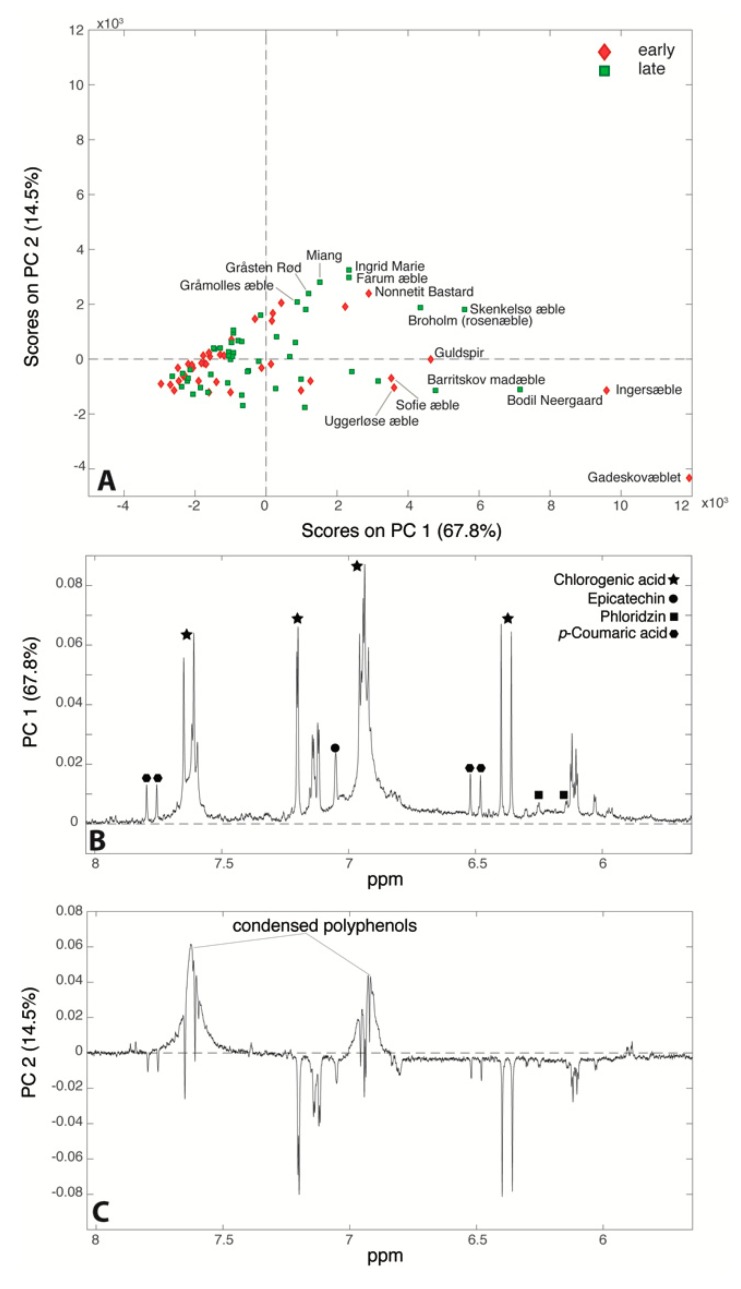
Besides the chlorogenic acid that was already quantified by Bruker, three more polyphenols were identified: epicatechin, phloridzin and p-coumaric acid. A PCA (82.3% of total variance) was performed on the 86 spectra, and the relative score and loading plots are reported in Figure 7. Figure 7A clearly shows that juices from cultivars ‘Gadeskovæble’, ‘Ingersæble’, ‘Bodil Neergaard’, and ‘Barritskov Madæble’ (far right region of the plot) are characterized by intense signals of all the polyphenols identified in the NMR spectrum, while the majority of the samples lie in the left part suggesting that their polyphenol content is too low to be detectable by NMR. The PC2 loading plot (Figure 7C) indicates that the samples that are situated in the top of the score plot (‘Ingrid Marie’, ‘Farum æble’, ‘Miang’, ‘Gråsten rød’ and ‘Nonnetit Bastard’) are characterized by higher intensities of condensed polyphenols signals (around 6.9 and 7.7 ppm): the aggregation of monomeric phenolic compounds (epicatechin, chlorogenic acid, phloridzin and p-coumaric acid) is probably mainly due to non-enzymatic oxygenation as reported elsewhere [22,23]. At the same time, the samples that lie at the bottom of the score plot only present the sharp signals of the monomeric polyphenols (chlorogenic acid, epicatechin, phloridzin and p-coumaric acid), which also suggests a strong correlation among these compounds. Vermathen et al. also found that chlorogenic acid and epicatechin were positively correlated [23]. This correlation may be explained by the shared initial biosynthetic pathway steps of these phenolic compounds both having coumaric acid as common precursor [43]. In a study conducted recently by Kschonsek et al., it was highlighted that apple cultivars such as ‘Braeburn’, ‘Elstar’, and ‘Jonagold’ have a lower content of polyphenols compared to the old cultivars. This is obtained on purpose during the breeding, because of the astringent taste and rapid enzymatic browning given by the polyphenols [9]. Indeed, apple juices obtained from ‘Broholm rosenæble’, ‘Guldspir’, ‘Sofie æble’ (right part of the score plot—high polyphenol content) were assigned a bitter taste as reported in Supplementary Table S3.
Figure 7.
Score plot (A) and PC1 (B) and PC2 (C) loading plots of the principal component analysis (PCA) performed on the 1H-NMR spectra of the 86 apple juices under study.
3.5. Multivariate Analysis on Datasets Combined (Ion Chromatography, Headspace GC–MS, Sensory Evaluation, Bruker-SGF Profiling)
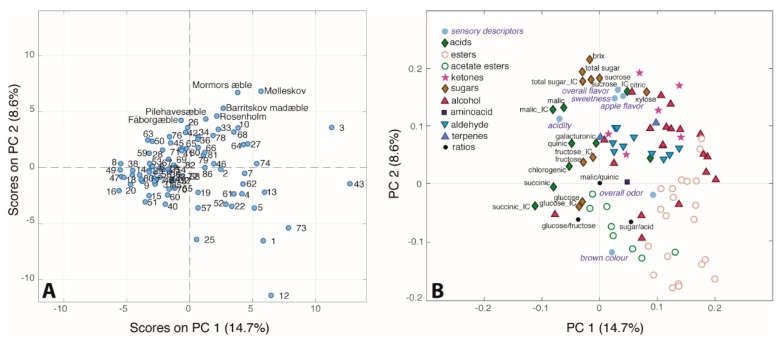
The unsupervised chemometric approach PCA was employed to analyse the four different datasets (ion chromatography, headspace GC–MS, sensory evaluation and Bruker-SGF profiling) combined giving a final data matrix of 86 samples and 96 variables. The PC1/PC2 (23.3% of total variance) score plot reported in Figure 8A shows large differences between the juices of different apple cultivars. Many samples lie on the left side of the score plot indicating that juices of these cultivars are characterized by low levels of most of the measured variables. Fewer cultivars are characterized by high levels of many of the variables. Indeed, the loading plot (Figure 8B), shows that the majority of the variables lie on the right side of the plot. Interestingly, the loading plot shows the clustering of all the esters in the right bottom region of the plot, while the alchols and the aldehydes are grouped in the top right part. Finally acids and sugars are situated in the left part of the plot. In particular, sucrose is situated at the top of the score plot together with the °Brix value and total sugar content, all highly correlated with sweet taste, apple flavor and overall flavor. In the opposite direction, we can find glucose, the glucose/fructose ratio, the sugar/acid ratio, the brown colour as well as some esters. The inverse relation between the sucrose and glucose content is already known from literature, and it is likely due to their interconversion [23]. As already reported in a previous work by our group, the sucrose turned out to be the main factor responsible for the sweet taste, even though the fructose is known to be the main sugar and thus sweetener in apple juice [44]. This confirms, once again, the complexity in assigning the sweet taste to a specific chemical compound when it should be considered the result of the synergy of several components [45]. Indeed, the loading plot (Figure 8B) shows that also citric acid, xylose and some ketones and alcohols are correlated with sweet taste and apple flavor. Interestingly, the acetate esters seem to be positively correlated with the brown colour of the apple juice, in agreement with the literature [46]. In turn, brown colour is negatively correlated with the acid content, as shown also in the correlation map (Supplementary Figure S2). The overall odor is mostly correlated with butanoate esters. Moreover, the sour taste of the juices seem as one would expect mainly due to the content of the dominant malic acid.
Figure 8.
(A) Score and (B) loading plots of the PCA performed on the 86 × 96 dataset.
Finally, the supervised chemometric tool PLS-DA was also employed, in order to detect the variables responsible for the differences between juices obtained from early and late season cultivars. The intensity of the sensory descriptors ‘apple flavor’, ‘overall flavor’ and ‘sweet taste’ were higher in juices of apples harvested late in the season compared to early in the season, as well as glucose, total sugar content and °Brix values (Supplementary Figure S3). By contrast, 2-hexen-1-ol, cis-3-hexenol, hexanal and 3-methyl-3-butenol were present at the highest levels in the early cultivars juices. Most aldehydes also had highest levels in early-season juices.
It has to be noted that many factors in the growing conditions can influence the fruit quality, and it seems to be a general tendency to obtain better development of aroma and colour when ripening is happening in a cool climate with cool nights [47,48]. Moreover, both juice constituents and sensory characteristics can be influenced by other parameters than cultivars, such as climate, soil, and processing methods [46,49]. However, we kept these variables constant here, since all the cultivars were harvested in the same year, from the same orchard and processed in the exact same way. Also fruit/leaf ratio strongly influences fruit development and quality, and all trees were handthinned in early season to ensure a good fruit/leaf ratio and thus facilitate optimal fruit development [50]. Also fruit maturity and ripeness are fundamental for the juice characteristics [47,51], for this reason, a team of experts decided the optimal picking and post-harvest storage time for each cultivar.
4. Conclusions
In this work, juices obtained from 86 apple cultivars, of which most are ancient Danish cultivars, have been extensively analyzed for the first time by employing traditional (ion chromatography, dynamic headspace, sensory evaluation) and cutting-edge NMR technology (Bruker-SGF profiling). Large variations with respect to sugars, acids, aroma compounds and sensory attributes were observed among the different samples. The chemical composition of all the analyzed juices were within the general reported ranges of apple juice, however, four cultivars (‘Gadeskovæble’, ‘Ingersæble’, ‘Bodil Neergaard’, and ‘Barritskov Madæble’) yielded juices particularly rich in polyphenols, while ‘Mormors æble’ and ‘Antonius’ juices were characterized by very peculiar odors and flavors such as apricot and peach, respectively. Moreover, we observed the tendency for late-season juices to be characterized by higher °Brix values and sugar content, which is linked to a sweeter and more flavor intense profile than early-season juices. The data reported here could be used for the production of specialty single cultivar ‘vintage’ apple juices (i.e., to be used as juice menus at restaurants), or mixed juices to obtain a final product that is characterized both by healthy properties and peculiar odors and flavors.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-1989/9/7/139/s1, ‘Analytical platforms reliability’. Figure S1: Signal assignment of a representative 1H-NMR spectrum of apple juice (0–5.5 ppm). Figure S2: Correlation map generated from the 86 x 96 matrix. The variables are grouped by similarity. Figure S3: (A) LV1/LV2 scores plot of the PLS-DA model developed to discriminate early-season juices (class 1) from late (class 2) harvest cultivars. Most discriminative markers are shown in the regression vector plot (C) and VIP score plot (D). Area Under the Curve (AUC) of Receiver Operating Characteristic (ROC) (E) and sensitivity and specificity (F) plot of the PLS-DA model. Table S1: °Brix values of the 86 juices., Table S2: List of 65 compounds identified by GC-MS analysis. Table S3: Distinct odors and flavors of the 86 juices and numerical evaluation of their sensory attributes. Table S4: Average concentrations of the compounds and parameters identified from Bruker SGF profiling.
Author Contributions
N.I. and S.B.E. were responsible for the data analysis and the writing of the manuscript. C.V. performed the experimental sensory work. M.A.P. and C.V. performed the dynamic headspace work. B.S. performed the SGF profiling experiments at Bruker. N.V. assisted the NMR analysis. S.B.E., T.B.T.-A. and M.A.P. contributed to the conception and design of the study. T.B.T.-A. supported the work at the Pometum, and performed the juice making and IC analysis. A.R. assisted the data analysis. All authors critically commented on and approved the final submitted version of the paper.
Funding
The project is collaboration with the Nordic Genetic Resource Center (NordGen), and was financially supported by The Ministry of Food, Agriculture and Fisheries through the Danish food Industry (Grant No 3304-FVFP-08-K-15-01).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Apple Juice Market—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2018–2026. [(accessed on 10 July 2019)]; Available online: https://www.transparencymarketresearch.com/apple-juice-market.html.
- 2.Ravn-Haren G., Dragsted L.O., Buch-Andersen T., Jensen E.N., Jensen R.I., Németh-Balogh M., Paulovicsová B., Bergström A., Wilcks A., Licht T.R., et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013;52:1875–1889. doi: 10.1007/s00394-012-0489-z. [DOI] [PubMed] [Google Scholar]
- 3.European Fruit Juice Association Liquid Fruit Market Report. [(accessed on 10 July 2019)];2018 Available online: https://aijn.eu/en/publications/market-reports-1/publication-2.
- 4.Donno D., Beccaro G.L., Mellano M.G., Torello Marinoni D., Cerutti A.K., Canterino S., Bounous G. Application of sensory, nutraceutical and genetic techniques to create a quality profile of ancient apple cultivars. J. Food Qual. 2012;35:169–181. doi: 10.1111/j.1745-4557.2012.00442.x. [DOI] [Google Scholar]
- 5.Cerutti A.K., Bruun S., Donno D., Beccaro G.L., Bounous G. Environmental sustainability of traditional foods: The case of ancient apple cultivars in Northern Italy assessed by multifunctional LCA. J. Clean. Prod. 2013;52:245–252. doi: 10.1016/j.jclepro.2013.03.029. [DOI] [Google Scholar]
- 6.Anastasiadi M., Mohareb F., Redfern S.P., Berry M., Simmonds M.S.J., Terry L.A. Biochemical Profile of Heritage and Modern Apple Cultivars and Application of Machine Learning Methods to Predict Usage, Age, and Harvest Season. J. Agric. Food Chem. 2017;65:5339–5356. doi: 10.1021/acs.jafc.7b00500. [DOI] [PubMed] [Google Scholar]
- 7.Jakobek L., Barron A.R. Ancient apple varieties from Croatia as a source of bioactive polyphenolic compounds. J. Food Compos. Anal. 2016;45:9–15. doi: 10.1016/j.jfca.2015.09.007. [DOI] [Google Scholar]
- 8.Oszmiański J., Lachowicz S., Gławdel E., Cebulak T., Ochmian I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018;244:647–662. doi: 10.1007/s00217-017-2989-9. [DOI] [Google Scholar]
- 9.Kschonsek J., Wolfram T. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the In Vitro Antioxidant Capacity. Antioxidants. 2018;7:20. doi: 10.3390/antiox7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsgaard M., Toldam-Andersen T.B., Nordling J. The Pometum Apple Key. [(accessed on 10 July 2019)]; Available online: https://www.nordgen.org/nak/?chglang=ENG.
- 11.Larsen B., Toldam-Andersen T.B., Pedersen C., Ørgaard M. Unravelling genetic diversity and cultivar parentage in the Danish apple gene bank collection. Tree Genet. Genomes. 2017;13:13. doi: 10.1007/s11295-016-1087-7. [DOI] [Google Scholar]
- 12.Inderbitzin J. The World’s First Flavour Wheel for Apples. [(accessed on 10 July 2019)];2016 Available online: https://www.agroscope.admin.ch/agroscope/en/home/topics/food/sensory-analysis/sensory-analysis-of-plant-products/aromarad-aepfel.html.
- 13.Spraul M., Schütz B., Rinke P., Koswig S., Humpfer E., Schäfer H., Mörtter M., Fang F., Marx U.C., Minoja A. NMR-based multi parametric quality control of fruit juices: SGF profiling. Nutrients. 2009;1:148–155. doi: 10.3390/nu1020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monakhova Y.B., Schütz B., Schäfer H., Spraul M., Kuballa T., Hahn H., Lachenmeier D.W. Validation studies for multicomponent quantitative NMR analysis: The example of apple fruit juice. Accredit. Qual. Assur. 2014;19:17–29. doi: 10.1007/s00769-013-1026-3. [DOI] [Google Scholar]
- 15.Braun S., Kalinowski H.-O., Berger S. 150 and More Basic NMR Experiments. Wiley-VCH; Berlin, Germany: 1998. [Google Scholar]
- 16.Jovanov M.J. Determination of 5-hydroxymethylfurfural in apple juice. Food Process. Qual. Saf. 2003;2:35–39. [Google Scholar]
- 17.Mardi K., Kent J., Bibby J. Multivariate Analysis. 1st ed. Academic Press; Cambridge, MA, USA: 1979. [Google Scholar]
- 18.Bro R., Smilde A.K. Principal component analysis. Anal. Methods. 2014;6:2812–2831. doi: 10.1039/C3AY41907J. [DOI] [Google Scholar]
- 19.Van Den Berg R.A., Hoefsloot H.C.J., Westerhuis J.A., Smilde A.K., Van Der Werf M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006;7:142. doi: 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savorani F., Tomasi G., Engelsen S.B. Icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010;202:190–202. doi: 10.1016/j.jmr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Farrant R.D., Hollerton J.C., Lynn S.M., Provera S., Sidebottom P.J., Upton R.J. NMR quantification using an artificial signal. Magn. Reson. Chem. 2010;48:753–762. doi: 10.1002/mrc.2647. [DOI] [PubMed] [Google Scholar]
- 22.Belton P.S., Delgadillo I., Gil A.M., Roma P., Casuscelli F., Colquhoun I.J., Dennis M.J., Spraul M. High-field proton NMR studies of apple juices. Magn. Reson. Chem. 1997;35:S52–S60. doi: 10.1002/(SICI)1097-458X(199712)35:13<S52::AID-OMR212>3.0.CO;2-D. [DOI] [Google Scholar]
- 23.Vermathen M. Investigation of Different Apple Cultivars by High Resolution Magic Angle Spinning NMR. A Feasibility Study. J. Agric. Food Chem. 2011;59:12784–12793. doi: 10.1021/jf203733u. [DOI] [PubMed] [Google Scholar]
- 24.Berregi I., Del Campo G., Caracena R., Miranda J.I. Quantitative determination of formic acid in apple juices by 1H NMR spectrometry. Talanta. 2007;72:1049–1053. doi: 10.1016/j.talanta.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Del Campo G., Santos J.I., Iturriza N., Berregi I., Munduate A. Use of the 1H nuclear magnetic resonance spectra signals from polyphenols and acids for chemometric characterization of cider apple juices. J. Agric. Food Chem. 2006;54:3095–3100. doi: 10.1021/jf051818c. [DOI] [PubMed] [Google Scholar]
- 26.Jaros D., Thamke I., Raddatz H., Rohm H. Single-cultivar cloudy juice made from table apples: An attempt to identify the driving force for sensory preference. Eur. Food Res. Technol. 2009;229:51–61. doi: 10.1007/s00217-009-1025-0. [DOI] [Google Scholar]
- 27.Thielen C., Ludwig M., Patz C.D., Will F., Dietrich H., Netzel G., Netzel M., Bitsch R., Bitsch I. Characterization of juices of different apple cultivars. Dtsch. Leb. 2006;102:426–435. [Google Scholar]
- 28.Eisele T.A., Drake S.R. The partial compositional characteristics of apple juice from 175 apple varieties. J. Food Compos. Anal. 2005;18:213–221. doi: 10.1016/j.jfca.2004.01.002. [DOI] [Google Scholar]
- 29.Markowski J., Baron A., Mieszczakowska M., Płocharski W. Chemical composition of French and Polish cloudy apple juices. J. Hortic. Sci. Biotechnol. 2009;84:68–74. doi: 10.1080/14620316.2009.11512598. [DOI] [Google Scholar]
- 30.Lee H.S., Wrolstad R.E. Apple Juice Composition: Sugar, Nonvolatile Acid, and Phenolic Profiles. J. Assoc. Off. Anal. Chem. 1988;71:789–794. [PubMed] [Google Scholar]
- 31.Fuleki T., Pelayo E., Palabay R.B. Carboxylic Acid Composition of Varietal Juices Produced from Fresh and Stored Apples. J. Agric. Food Chem. 1995;43:598–607. doi: 10.1021/jf00051a009. [DOI] [Google Scholar]
- 32.Wu J., Gao H., Zhao L., Liao X., Chen F., Wang Z., Hu X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007;103:88–93. doi: 10.1016/j.foodchem.2006.07.030. [DOI] [Google Scholar]
- 33.Poll L. Evaluation of 18 apple varieties for their suitability for juice production. J. Sci. Food Agric. 1981;32:1081–1090. doi: 10.1002/jsfa.2740321107. [DOI] [Google Scholar]
- 34.Blanco D., Morán M.J., Gutiérrez M.D., Moreno J., Dapena E., Mangas J. Biochemical study of the ripening of cider apple varieties. Eur. Food Res. Technol. 1992;194:33–37. doi: 10.1007/BF01191037. [DOI] [Google Scholar]
- 35.Yao Y.X., Li M., Zhai H., You C.X., Hao Y.J. Isolation and characterization of an apple cytosolic malate dehydrogenase gene reveal its function in malate synthesis. J. Plant Physiol. 2011;168:474–480. doi: 10.1016/j.jplph.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Berüter J. Carbohydrate metabolism in two apple genotypes that differ in malate accumulation. J. Plant Physiol. 2004;161:1011–1029. doi: 10.1016/j.jplph.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Espino-Díaz M., Sepúlveda D.R., González-Aguilar G., Olivas G.I. Biochemistry of apple aroma: A review. Food Technol. Biotechnol. 2016;54:375–394. doi: 10.17113/ftb.54.04.16.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karlsen A.M., Aaby K., Sivertsen H., Baardseth P., Ellekjær M.R. Instrumental and sensory analysis of fresh Norwegian and imported apples. Food Qual. Prefer. 1999;10:305–314. doi: 10.1016/S0950-3293(99)00030-0. [DOI] [Google Scholar]
- 39.Thybo A.K., Sørensen L., Christensen L.P., Kühn B.F. Quality of apples grown in a Scandinavian high-density orchard and chemical composition in relation to sensory quality. J. Hortic. Sci. Biotechnol. 2005;80:727–735. doi: 10.1080/14620316.2005.11512006. [DOI] [Google Scholar]
- 40.A.I.J.N. European Fruit Juice Association. [(accessed on 10 July 2019)]; Available online: https://aijn.eu/en.
- 41.Johnston J.W., Hewett E.W., Hertog M.L.A.T.M. Postharvest softening of apple ( Malus domestica ) fruit: A review. N. Z. J. Crop Hortic. Sci. 2002;30:145–160. doi: 10.1080/01140671.2002.9514210. [DOI] [Google Scholar]
- 42.Micheli F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6:414–419. doi: 10.1016/S1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- 43.Treutter D. Biosynthesis of phenolic compounds and its regulation in apple. Plant Growth Regul. 2001;34:71–89. doi: 10.1023/A:1013378702940. [DOI] [Google Scholar]
- 44.Iaccarino N., Varming C., Petersen M.A., Savorani F., Randazzo A., Schütz B., Toldam-Andersen T.B., Engelsen S.B. Application of Recursive Partial Least Square Regression for Prediction of Apple Juice Sensory Attributes from NMR Spectra; Proceedings of the The XIII International Conference on the Applications of Magnetic Resonance in Food Science; Karlsruhe, Germany. 7–10 June 2016; pp. 7–12. [Google Scholar]
- 45.Harker F.R., Marsh K.B., Young H., Murray S.H., Gunson F.A., Walker S.B. Sensory interpretation of instrumental measurements 2: Sweet and acid taste of apple fruit. Postharvest Biol. Technol. 2002;24:241–250. doi: 10.1016/S0925-5214(01)00157-0. [DOI] [Google Scholar]
- 46.Komthong P., Katoh T., Igura N., Shimoda M. Changes in the odours of apple juice during enzymatic browning. Food Qual. Prefer. 2006;17:497–504. doi: 10.1016/j.foodqual.2005.06.003. [DOI] [Google Scholar]
- 47.Dever M.C., Cliff M., Lau O.L. Maturity and ripening effects on chemical and sensory properties of apple juice. J. Sci. Food Agric. 1992;60:355–360. doi: 10.1002/jsfa.2740600313. [DOI] [Google Scholar]
- 48.Toldam-Andersen T.B., Hansen P. Priority of fruit quality parameters based on differences in growing conditions. Acta Hortic. 1998;466:71–76. doi: 10.17660/ActaHortic.1998.466.12. [DOI] [Google Scholar]
- 49.Cliff M., Dever M.C., Gayton R. Juice Extraction Process and Apple Cultivar Influences on Juice Properties. J. Food Sci. 1991;56:1614–1617. doi: 10.1111/j.1365-2621.1991.tb08654.x. [DOI] [Google Scholar]
- 50.Poll L., Rindom A., Toldam-Andersen T.B., Hansen P. Availability of assimilates and formation of aroma compounds in apples as affected by the fruit/leaf ratio. Physiol. Plant. 1996;97:223–227. doi: 10.1034/j.1399-3054.1996.970203.x. [DOI] [Google Scholar]
- 51.Poll L. The influence of apple ripeness and juice storage temperature on the sensory evaluation and composition (volatile and non-volatile components) of apple juice. LWT Food Sci. Technol. 1985;18:205–211. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.