Abstract
Scorpions, a characteristic group of arthropods, are among the earliest diverging arachnids, dating back almost 440 million years. One of the many interesting aspects of scorpions is that they have venom arsenals for capturing prey and defending against predators, which may play a critical role in their evolutionary success. Unfortunately, however, scorpion envenomation represents a serious health problem in several countries, including Iran. Iran is acknowledged as an area with a high richness of scorpion species and families. The diversity of the scorpion fauna in Iran is the subject of this review, in which we report a total of 78 species and subspecies in 19 genera and four families. We also list some of the toxins or genes studied from five species, including Androctonus crassicauda, Hottentotta zagrosensis, Mesobuthus phillipsi, Odontobuthus doriae, and Hemiscorpius lepturus, in the Buthidae and Hemiscorpiidae families. Lastly, we review the diverse functions of typical toxins from the Iranian scorpion species, including their medical applications.
Keywords: scorpion, fauna, venom, toxin, Iran
1. Introduction
Scorpions, a characteristic group of arthropods, diverged from other arachnids relatively early, at about 440 million years ago [1,2]. Their morphology has stayed constant since they adapted to a terrestrial habitat. Their morphological stasis has not been, however, an impediment to successfully colonizing different ecological ecosystems such as caves, high peaks, and deserts [3,4].
Morphological phylogenetic analyses suggest that scorpions are sister taxa to either the rest of the arachnids or to the Opiliones [5]. However, recent phylogenomic analysis suggests that scorpions are closely related to spiders and allies, forming the clade Arachnopulmonata [6,7].
Among the 18 scorpion families with more than 2200 species described in the world, about thirty species have been identified as potentially deadly toxic to humans [8,9].
Our studies on scorpions are based on two remarkable aspects of this group: Their evolutionary origin and systematic classification, and the diversity and origin of their venom components, with emphasis on the use of these components as potential sources of molecules with therapeutic applications [10].
Scorpions have fascinated scientists and laypersons for their venom, which is a complex mixture of bioactive components secreted in specialized organs [11]. These animals inject venom to subdue prey or to defend against attackers. Their venoms consist of a variety of toxins, which may vary according to species, habitat, or fluctuations in climate [12]. Within scorpion venom components, the peptidic fraction has been considered a great source of lead compounds for drugs to treat various cancers and infectious diseases [13]. Hence, studies on scorpion venom components are important, especially in terms of medical treatments for human diseases.
Here, we review the diversity of scorpions in Iran. We also highlight the importance of the venomic studies of Iranian scorpions. Establishing what is known about of scorpions and scorpion venom in Iran will allow the identification of important gaps to be addressed in the future.
2. Scorpion Species from Iran
Iran is a vast land with diverse climates. There are two main mountain ranges in Iran: (1) Alborz; and (2) Zagros and neighboring mountains. Diverse climates in Iran are the direct result of the presence of the Persian Gulf, the Oman Sea bordering Southeastern Iran, and the Caspian Sea in Northern Iran. According to Safaei-Mahroo et al. [14] 16 terrestrial ecoregions have been reported from Iran including: Arabian Desert and East Saharo-Arabian xeric shrublands (0.1%), Azerbaijan shrub desert and steppe (0.4%), Badkhiz-Karabil semi-desert (0.1%), Caspian lowland desert (0.3%), Central Persian desert basins (34.7%), Kopet Dagh semi-desert (0.4 %), Registan-North Pakistan sandy desert (3%), South Iran Nubo-Sindian desert and semi-desert (17.3%), Mesopotamian shrub desert (0.1%), Tigris-Euphrates alluvial salt marsh (0.4%), Kopet Dagh woodlands and forest steppe (1.6%), Kuh Rud and Eastern Iran Montane woodlands (7.5%), Caspian Hyrcanian mixed forests (3.4%), Zagros Mountains forest steppe (21.8%), Alborz Range forest steppe (4.3%), and Eastern Anatolian montane steppe (4.6%).
Iran is located in a strategic position in the Palearctic region and is a bridge between the Oriental and African zoogeographical regions, suggesting the possibility of endemic arthropod species in this region. Among these arthropods, scorpions stand out as there are diverse species in desert and semi-desert regions of Iran (for more details see appendix: Table S1).
Historically, the fauna of Iran has been studied by many researchers. The earliest descriptions of the first species were made by Olivier (Androctonus crassicauda (Olivier, 1807)) [15]. Later, Alexei Andreevich Byalynitskii-Birulya [16,17,18,19] published a series of scorpion studies from Iran that included well known species and several rare taxa [20,21,22,23,24,25,26]. In addition, pioneering zoologists such as Pocock [27] and Werner [28] described a few more species in the region. In the middle of the 20th century, Max Vachon carried out preliminary studies on the scorpions of Iran, with a later report of two families, nine genera, and 15 species [29]. Later, Habibi [30] reported 24 species belonging to 11 genera and two families. Farzanpay [31,32] reported fewer species (23 species), but increased the number of genera (17), as well as two families. More recently, Kovařík [23] reported a list of three families, 17 genera, and 32 species of scorpions. A more extensive study of Iranian scorpions continued with publications by Navidpour et al. [33,34,35,36,37], who recorded the dispersal of scorpions in all of Iran. Lastly, Mirshamsi et al. [38] reported 51 species belonging to 18 genera in four families. According to Vachon [29] and Mirshamsi et al. [38], there are Androctonus baluchicus, Androctonus crassicauda, and Androctonus finitimus in Iran. However, Yağmur et al. [39] believed that Androctonus crassicauda and Androctonus robustus are present in Iran while rejecting the occurrence of Androctonus baluchicus and Androctonus finitimus in Iran. Compsobuthus kafkai and Compsobuthus sobotniki were synonymized with Sassanidotus gracilis [37,40]. Farzanpay [32] believed that Hottentotta alticola alticola are present in Iran but Mirshamsi et al. [38] believed that records show Hottentotta alticola alticola are in doubt. Mirshamsi [41] believed that Mesobuthus phillipsi includes the Mesobuthus phillipsi pachysoma and Mesobuthus phillipsi mesopotamicus subspecies. Although Vachon [29], Farzanpay [32], Mirshamsi et al. [38], and Nejati et al. [42], reported Odontobuthus odonturus in Iran, Lowe [43] rejected Odontobuthus odonturus in Iran. Based on field work, study collections, literature reviews, and personal communications, the total number of species confirmed within the Iranian border is 78 species and subspecies belonging to 19 genera and four families. The family Buthidae is the most diverse with 68 species and subspecies (87.17%), followed by Hemiscorpiidae with seven species (8.97%), Scorpionidae with two subspecies (2.56%), and Diplocentridae with one species (1.28%) [23,24,25,26,29,32,33,34,35,36,37,38,39,40,41,42,44,45,46,47,48,49] (Table S2). Forty-five out of 78 species and subspecies of the Iranian scorpions are endemic to Iran (57.69%, for more details see appendix: Tables S1 and S2).
3. Previous Studies on Drug Discovery of Scorpion Venoms
Animal venoms are a mixture of different compounds for defense and prey capture. Many peptide toxins from deadly animal venoms have been influenced by ion channel (including sodium, potassium, and calcium channels) functions. The ion channels play important roles in the regulation of the heart beat and neuronal excitability [50,51].
Scorpion venoms are certainly important natural drug resources for medical applications. In scorpions, family Buthidae has always been interesting from the public health perspective in terms of their dangerous venoms. Many studies have concentrated on non-Buthidae families and reported several new venom peptides and proteins which have shown unique primary structures and biological activities [52,53,54,55]. However, the first disulfide-bridged peptide toxin extracted from a non-buthid scorpion was St20 from Scorpiops tibetanus. This peptide has immunosuppressive and anti-inflammatory effects that suggest its potential use as a new peptide medicine for human diseases [56].
Scorpion toxins have been used in variety of fields, including biotechnology (examining the effects on ion channels), identifying cancer mass [57], treating cancer [58], and to treat neuronal [59], autoimmune [60], and cardiovascular diseases [61].
The venoms of Pandinus imperator and Scorpio maurus palmatus have peptides named imperatoxin A (IpTxa) and maurocalcin (MCa), respectively, and these venoms are of interest in many cardiovascular diseases [62,63].
Classification of polypeptide toxins is important for understanding the structure–function relationship of each individual group. The major criteria used for classification are based on receptor/ion channel specificity (e.g., K+, Na+, Ca2+ and Cl_), peptide length (e.g., short- and long-chain), structural scaffold α, αβ and βαββ), disulfide bonds (three or four and pairing pattern), the mechanism of action/binding sites (α- or β-like toxins), their cellular target, and others. [64,65,66]. Ion channels play critical roles in the secretion of hormones, cell proliferation and motility, muscle activity, sense perception, and brain activities of which the functions are applicable for drug development [67,68].
4. Venomic Studies in Iranian Scorpions and Their Potential in Therapeutic
Biologically, venoms of scorpions are diverse and have activity due to their predatory and defensive use in nature [69,70]. In addition, venom of scorpions contains phospholipases A2, serine proteases, metalloproteases, lipolysis activating peptides (LVPs) and hyaluronidases, proteins, and peptides (antimicrobial and toxic peptides performing on ion channels) [71,72,73].
Several peptide toxins in venomous animals are being considered for pharmacological applications, including treating pain, diabetes, multiple sclerosis, and cardiovascular illnesses [12,51,74].
Worldwide, peptides are progressively emerging as a novel class of therapeutics. In total, 438 peptides are represented in the pharmaceutical trade, including 72 in Phase III clinical trials and 48 that have been approved. Four are famous and sold in the pharmaceutical market: Copaxane, Lupron, Byetta, and Forteo [75]. The majority of these peptides act through G protein-coupled receptors or ion channels [74].
5. Scorpion Venom and Cancer Therapy
Several studies have reported that scorpion peptides have antineoplastic activity [76,77]. Some researchers showed that scorpion venoms have potential as a source of drug-like molecules to treat diverse cancers such as human neuroblastoma, leukemia, glioma, brain tumor, breast cancer, melanoma, prostate cancer, and human lung adenocarcinomas [78].
Anti-proliferative, cytotoxic, and apoptogenic properties of scorpion venom peptides on different types of cancers have also been discovered. Scorpion venom peptides with fluorescent labeling have been used to visualize the boundaries of cancerous tissues in cancer patients [78].
For example, the venom of Androctonus crassicauda (100 μg/mL) blocked propagation of MCF-7 cells by suppressing S-phase of the cell cycle [79,80,81]. High doses produce necrosis, killing the cells, while apoptosis diminishes cell growth at lower doses, producing inhibition of growth of breast cancer cells [79].
The venom of Odontobuthus doriae causes apoptosis in breast cancer by depolarizing mitochondria membranes and controlling S-phase proliferation in human breast cancer cells. MCF-7, on the other hand, reduced catalase activity, glutathione production, DNA fragmentation, and apoptosis [80].
6. Scorpion Toxin and Ion Channels
Ion channels include voltage-gated sodium, calcium, and potassium channels which create electrical signals required for action potential generation and conduction, and are the molecular targets for a broad range of potent neurotoxins [82].
6.1. Nav or Gated Sodium Channel Specific Toxins
Voltage-gated sodium channels (Navs) are composed of transmembrane proteins that conduct sodium ion (Na+) into the cytosol upon activation. An Nav contains a main α-subunit (220–260 kDa) and one or two auxiliary β-subunits (30–40 kDa) [83,84]. The Nav α-subunit is composed of four homologous domains (DI-DIV), each containing six transmembrane α-helixes (S1–S6). The α-subunit includes multiple domains, which are involved in pore-forming, voltage-sensing, and Na+ selectivity [85]. Navs play an important role for action potential (AP) generation and proliferation in excitable cells, including cardiac myocytes, skeletal muscle cells, and neurons [86,87]. Navs are also marginally expressed in non-excitable cells involved in noncanonical roles in controlling some pathophysiological activities [87,88].
Many studies have focused on roles of Nav subtypes (Nav1.3, 1.7, 1.8, and 1.9) in nociceptive transduction. These Navs probably show attractive targets for analgesic drug discovery. However, their channels also introduce valuable probes to demonstrate the structures, gating properties, and cellular functions of ion channels (for more details see Wu et al. [89].
Three categories of Nav toxins can be defined: (1) Pore-blocking toxins that inhibit Na+ conductance by interacting with neurotoxin site 1, including tetrodotoxin (TTX), μ-conotoxins, and saxitoxin (STX); (2) toxins that negatively shift the activation voltage and produce a persistent activation by connecting to membrane-embedded neurotoxin site 2 (such as veratridine, grayanotoxin, and batrachotoxin), or 5 (such as ciguatoxin and brevetoxin), and that prefer to interact with the open state of channel; and (3) toxins that delay inactivation by binding to extracellular neurotoxin site 3, such as sea-anemone toxins and α-scorpion toxins. According to recent investigations, β-scorpion toxins shift activation voltage to either a depolarized or hyperpolarized direction through main effects of neurotoxin site 4. Delta-conotoxin prolongs channel inactivation, similarly to that caused by α-scorpion toxins, by binding to neurotoxin site 6. Although the neurotoxin binding sites are topologically distinct, allosteric coupling has been elucidated between sites 3 and 6 and between sites 2 and 5 [90,91]. In addition, four β-subunits (β1–β4; encoded by SCN1B-4B genes) have been reported in mammals. The β-subunits are type I transmembrane proteins, including an extracellular signal peptide in the N-terminus, a transmembrane segment, and an immunoglobulin domain [92,93]. Both excitable and non-excitable cells have considerable amounts of β-subunit expression that plays critical roles in modulating the localization, kinetics, and gating of Nav α-subunits [87,91,93,94].
There are several studies that provide evidence of these mechanisms. As described above, there are several kinds of toxins in scorpion venoms that modulate the activity of ion channels, and these are usually responsible for several signs of envenoming. From the human perspective, the most medically important toxins are those that modulate mammalian NaV channels [64,69].
The first neurotoxin targeting voltage-gated sodium channels extracted from an Iranian scorpion venom (Odontobuthus doriae) was OD1, which blocks the fast inactivation of mammalian channels Nav1.7, Nav1.4, and Nav1.6 [95,96]. Additionally, OD1 inhibits fast inactivation of the para/tipE insect channel (EC50 80 nM), but it scarcely influences mammalian Nav1.3 or Nav1.5 (EC50 > 1 μM) and has no effect on Nav1.2 and Nav1.8. OD1 rapidly induces pain when injected into animals in association with, or without, veratridine, and has been used to test the analgesic effects of Nav1.7 blockers in vivo [96,97]. In fact, dose-dependent increases in the amplitude of Na+ currents resulting in incomplete inactivation in steady-state conditions has been demonstrated by OD1 [98,99]. It is probable that continuous inward Na+ current causes sustained depolarization of the cell membrane, and the remaining Nav1.7 channels that were not affected by OD1 are trapped in the inactivated state, resulting in the loss of electrical excitability of nociceptor neurons [97,98].
Buthotus schach venom is an important source of active peptides, some of which affect voltage-gated sodium channels involved in local pain, inflammation, convulsion, necrosis, respiratory depression, and cardiac arrest in humans [100,101].
6.2. K+ Channel Specific Scorpion Toxins
Potassium channels are the most abundant ion channels and are found in all living organisms [102]. The channels are involved in the resting potential, and shape the action potential, in nerves and muscles [67]. Most potassium channels are composed of tetramers of principal α-subunits (heteromeric assemblies are more common) [103], with auxiliary β-subunits as complementary parts [104]. There are 78 genes encoding α-subunits of potassium channels in the human genome divided into five classes: Kir, K2P, KV, and two groups of KCa [105,106,107,108].
Based on many studies, potassium channel ligands are probably classified into two large groups. These are pore blockers that physically obstruct the channel pore, and gating modifiers that influence channel traits [82]. The potassium channel ligands can be metal ions, low-molecular-mass substances, and polypeptides [109].
Scorpion venom has K+ channel specific toxins (KTx). Thus far, 293 KTxs have been described in UniProt and 174 KTxs have been described in the Kalium database at http://kaliumdb.org/ [110,111]. The toxins interact with different subtypes of channels, such as the Kv1, Kv3, Kv4, Kv7, Kv11, and KCa channels [112].
Venom of scorpions are an important source of K+ channel specific toxins (KTx), and are important tools for the structural and functional characterization of various K+ [113,114]. Kv channel inhibitors have medical applications in the treatment of various specific human diseases, especially autoimmune disorders, inflammatory neuropathies, and cancer [111].
Autoimmune diseases are usually accompanied by tissue injury caused by autoantigen-specific T-cells. KV1.3 channels contribute to the control of calcium signaling to induce T-cell proliferation, immune activation, and cytokine production. In many autoimmune diseases, effector memory T (TEM) cells, which play major roles, are controlled by blocking KV1.3 channels on the membrane. Animal toxins are capable of suppressing the activation and proliferation of TEM cells and may improve TEM cell-mediated autoimmune diseases, for example in multiple sclerosis and type I diabetes mellitus [115].
The first K+ channel toxin isolated from the venom of the Iranian scorpion Odontobuthus doriae (OdK1) was categorized as α-KTx 8.5. The pharmacological effects of OdK1 were investigated on Xenopus laevis oocytes heterologously expressing Kv1.2 channels. OdK1 selectively blocked the currents through Kv1.2 channels, with no effect on the other channels tested [116].
There is a Kv1.3 channel-selective toxin, OdK2, in venom of Odonthobuthus doriae, one of the endemic scorpion species of Iran. OdK2 is composed of 38 amino acid residues, including six conserved half-cystine residues and a C-terminal lysine residue. The toxin was named KTX3.11. Pharmacologically, OdK2 selectively suppresses the currents via Kv1.3 channels. [117].
Hemitoxin (HTx), a K+ channel blocker, was isolated from the venom of Hemiscorpius lepturus, which is classified in subfamily six of the α-KTx family of potassium channel scorpion toxins and has the highest amino acid sequence similarity with maurotoxin (MTX), extracted from the Tunisian scorpion Scorpio maurus palmatus. Additionally, MTX is also a K+ channel inhibitor with 34 (instead of 35) amino acid residues.
HTX reversibly inhibits type Kv1.1, Kv1.2, and Kv1.3 channels. HTX has subtype-selective effects on K+ channels. It is 20 times less potent on Kv1.2 channels, and 90 times more potent on Kv1.3 channels, compared to the α-KTx6 family member MTX [118].
6.3. Ca2+ Release-Channel Specific Peptides (Calcins)
Voltage-gated calcium channels (Cav) play a critical role in electrical signaling, converting depolarization of the cell membrane to an influx of calcium ions that initiates contraction, secretion, neurotransmission, and other intracellular regulatory events [92]. Voltage-gated calcium channels (Cav) facilitate cellular calcium influx in response to membrane depolarization. They control hormone secretion, neurotransmitter release, propagation of cardiac action potential, muscle contraction, and gene expression in different cell types [119]. Similarly to the Nav channels, the α1 subunit of Cav channels is organized in four homologous domains (I–IV), each containing six TM segments (S1–S6). The S1–S4 segments are involved in the voltage sensor, whereas S5–S6 constitutes the pore. Auxiliary subunits usually associate with α1, regulating channel expression and function. Cav channels are classified in the following groups in terms of electrophysiological and pharmacological properties and tissue distribution—L-type (Cav1 subfamily: Cav1.1-Cav1.4); P/Q-, N-, and R-types (Cav2.1, Cav2.2 and Cav2.3, respectively); and T-type (Cav3 subfamily: Cav3.1-Cav3.3) [120].
The various subfamilies have been presented for classification of calcium channels as follows: Voltage-gated channels, voltage independent channels, and ligand-activated channels. Ligand-activated channels include the ryanodine receptors (RyRs), which are high-permeability Ca2+ channels of the sarcoplasmic reticulum in muscle and of the endoplasmic reticulum in other cells. In striated muscle fibers, contraction has been carried out via RyRs that releases Ca2+ rapidly. There are various isoforms of ryanodine-sensitive calcium channels including RyR1, RyR2, and RyR3. In mammals, RyR1 is expressed mainly in skeletal muscle and RyR2 is expressed mainly in cardiac muscle. RyR3 seems to be confined to the brain, smooth muscle, and epithelial cells. RyR1 and RyR2 are expressed in some of these tissues as well. The activity of surface-membrane Ca2+ channels, the dihydropyridine receptors (DHPRs), stimulates the activation of RyRs during the early part of the excitation–contraction linking cascade [121,122].
There is selective activity on ryanodine receptors (RyRs) by the venom of Hadrurus gertschi [123].
In Odontobuthus doriae, one calcium channel toxin was recognized and is referred to as ODCaTx1 (ID: KU365856) [124]. It shares 91% of its identity with the ryanodine receptor toxin isoform 2 isolated from Hottentotta judaicus [125]. Analysis and characterization of this peptide could produce remarkable biological and therapeutic research [124].
Hemicalcin is a new toxin that was extracted from the venom of Hemiscorpius lepturus, it represents 0.6% of the total protein content [126].
6.4. Cl− Channels (CLCs)
CLC chloride channels contain one pore per subunit (a ‘double-barreled’ channel), and also provide clues about gating and permeation [127]. Cl− channels are classifed into three groups: Ligand-gated Cl− channels, the cystic fibrosis transmembrane conductance regulator (CFTR) channel, and the CLC channels [128]. The mammalian CIC family contains nine members, divided into three subgroups. It includes plasma membrane channels and Cl−/H+ antiporters that are thought to contribute to plasma membrane transport, lysosomal acidification, and the maintenance of the cell membrane potential [128,129,130].
Short scorpion toxin chloride channel inhibitors are short-chain neurotoxins (SCNs) that block small-conductance chloride channels. They are 30-40-residue long and contain four intramolecular disulphide bridges, which have been labeled as C1-C4, C2-C6, C3-C7, and C5-C8 [131,132,133].
According to Naderi Soorki et al. [124], one chloride channel-acting toxin, ODClTx1, was identified in Odontobuthus doriae venom (ID: KU-365857); it is composed of four intramolecular disulfide bridges and a putative conserved domain belonging to the toxin-5 superfamily. There are a variety of secreted short scorpion toxins in the superfamily. Such toxins are not associated with the toxin-2 superfamily (pfam00451) that affects potassium channels [124].
7. Antimicrobial Peptides
These days, antimicrobial components represent one of the important resources for modern medical care [134]. Natural antimicrobial peptides (AMPs) have been extracted from scorpion venom, are broadly expressed, and exert different effects on bacteria, viruses, fungi, and parasites [135]. Recently, 871 peptides/proteins have been discovered from 72 scorpion species [71]. Of these molecules, 638 peptides (73%) are from 47 scorpion species of the Buthidae family [71]. These consist both of disulfide-bridged peptides (DBPs), and of non-disulfide-bridged peptides (NDBPs) [55,136,137]. According to Almaaytah and Albalas [52], DBPs cause neurotoxic effects, and NDBPs reveal diverse structures and activities. There is generally more antimicrobial activity in NDBPs, which contain 13–56 amino acid residues; their amino acid sequences show structural diversity and multifunctional activity. So far, more than 40 peptides have been recognized and functionally categorized from scorpion venoms. Therapeutic and biological applications of NDBPs are related to their antibacterial, antifungal, antiviral, insecticidal, antimalarial, anticancer, cytolytic, anti-inflammatory, immune-modulatory, and bradykinin potentiating activities [52].
Globally, approximately 36.7 million people suffer HIV, but still there is no definite cure to eradicate HIV transmission [138]. The five antimicrobial peptides (AMPs) of scorpion venoms have been separated to evaluate potential anti-HIV effects. Three of them (mocoporin-M1, BmKn2, and Kn2-7) showed powerful anti-HIV activity [78].
Moreover, some distinctive characteristics of scorpion physiology are related to the mixture, and to individual effects. These properties are mostly found in the Buthidae family, whose venom is pharmacologically the most important and prominent compared to non-buthids. Hemocyanin is a protein for transporting oxygen in all scorpions and has the ability to bind with molecular oxygen in reverse. The protein has three parts with an enzymatic role: Pseudo-catalase, peroxidase, and superoxide-dismutase [139,140,141]. Additionally, the hemocyanins probably have an antimicrobial function from multiple oxidative enzymatic activities [142].
Hemiscorpius lepturus scorpion venom contains several components with significant anti-HIV activity, suggesting it as a potential source of novel therapeutic agents against HIV infection [143].
An antimicrobial toxin extracted from Mesobuthus eupeus venom glands was isolated that was encoded by a 213 bp cDNA fragment. The full-length sequence of the coding region was 210 bp and included an open reading frame of 70 amino acid residues with a predicted molecular mass of 7970.48 Da and theoretical pI of 9.10. The precursor (70 amino acid residues) includes a signal peptide of 23 amino acid residues and a mature peptide of 34 amino acid residues with no disulfide bridge. The resulted peptide of Mesobuthus eupeus has been named MeVAMP-2 (98%), MeVAMP-9 (60%). The other AMPs have been reported from Mesobuthus martensii (94%) and Buthus occitanus israelis (82%) [144].
There are six AMPs in venom of Odontobuthus doriae, called ODAMP1-6. Four of them (ID: KU212813, KU212814, KU212815, KU212816) have signal peptides with peptides containing 55, 51, 52, and 51 amino acid residues, respectively. There are no signal peptides in ODAMPs (ODAMP1, 6). ODAMP1 (ID: KU212812) has 78 amino acid residues and resembles the antimicrobial peptide androcin 18-1 from Androctonus bicolor scorpion (78% identity). ODAMP6 (ID: KU365855) is a short chain peptide with only 47 amino acid residues, which is similar to Tx65 with antimicrobial activity from Buthus occitanus israelis (100% identity), and which could be beneficial and applicable in drug and food industries [124].
8. Metalloproteinases
Matrix metalloproteinases (MMPs) affect cellular activities like growth and cell differentiation directly or indirectly [145,146,147]. In addition, MMP plays a role in cellular connection to the matrix using proteolysis of the adhesion places [148]. The enzymes can effect an increase and survival of tumor cells [149]. Metalloproteases of venom component play important role in hemorrhage [150]. Metalloproteinase sequences that have been identified in the transcriptome of Odontobuthus doriae include ODVP4; KU365871 [124] and those in Hemiscorpius lepturus include HLMP1; KX924496, HLMP2; KX924497, HLMP3; KX924498 [151,152,153].
9. Phospholipase A2 (PLA2)
Phospholipases (PLA) (types A1, A2, C, and D) are a type of enzyme with a high disulfide bridge content and a conserved histidine/aspartic catalytic dyad. They that act on phospholipids to produce different products, including lysophospholipids, diacylglycerols (DGs), free fatty acids (FFAs), choline phosphate, and phosphatidates [154,155]. The four main groups of superfamily Phospholipase A2 (PLA2) include secreted (sPLA2), cytosolic (cPLA2), calcium independent (iPLA2), and platelet activating factor acetyl hydrolase (PAF-AH) or lipoprotein PLA2 (LpPLA2). In vertebrates and invertebrates, the most common type of PLA is sPLA2, which is grouped into 15 types [156,157,158,159].
Secretory phospholipase A2 (sPLA2) is the most common type of phospholipase observed in animals including vertebrates and invertebrates. According to primary sequences alignments, disulfide bond patterna, and their biochemical properties, it is divided into 15 groups [157,159]. The low molecular weight of sPLA2 is13–15 kDa, with approximately six to eight disulfide bonds [154].
sPLA2 of scorpion venom are considered as group III and contained a long enzymatic chain and a short covalently fixed C-terminal chain generated after the release of five residues (penta-peptide) during the maturation processes [160]. HfPLA2 has been extracted from a scorpion named Heterometrus fulvipes [161], MtPLA2 from Mesobuthus tamulus [162], Imperatoxin (IpTxi) [163] and Phospholipin from Pandinus imperator [164], Phaiodactylipin from Anuroctonus phaiodactylus [165], Heteromtoxin (HmTx) from Heterometrus laoticus [166], Hemilipin from Hemiscorpius lepturus [167], and Sm-PLVG from Scorpio maurus [168]. Hemiscorpius lepturus has potent phospholipase D activities that have been related to the highly toxic (even lethal) necrosis activity of the venom [169].
In addition, a novel sPLA2 named hemilipin was recently isolated from dangerous scorpion of Iran, Hemiscorpius lepturus [167]. Edman degradation revealed its primary structure, and titration of fatty acids elucidated its enzymatic PLA2 activity on egg yolk phospholipids. Hemilipin widely affects angiogenesis in vitro and in vivo, whereas it doesn’t have any effect on apoptosis. Additionally, the study demonstrated that this new non-toxic sPLA2 could be used as an innovative tool to disrupt human angiogenesis at various points [167]. In a subsequent study, Jridi et al. [170] proposed a second sPLA2: Hemilipin2. This component has a robust calcium-dependent PLA2 activity and influences angiogenesis without any cytotoxic or apoptotic effects both in vitro and in vivo. However, there is a prominent capability in hemilipin2 to prevent blood vessel formation both in vitro and in vivo. The results suggest a beginning point to produce novel molecules that act as specific suppressors of human angiogenesis.
10. Protease and Serine Protease Inhibitors
Proteases are a kind of enzymes which are necessary to preserve homeostasis in cell. So far, 12 protease therapies have been proposed by the U.S. FDA (Food and Drug Administration) [171]. Based on Cao et al. [172], proteases control cellular events by growth factors, cytokines, chemokines, and cellular receptors, both through activation and inactivation leading to downstream intracellular signaling and gene regulation. Upregulation of proteolysis is related to different types of cancer and tumor metastasis, invasion, and growth [173].
Protease peptide inhibitors that occur in scorpion venoms have broad applications in medicine. Accordingly, proteases and protease inhibitors have important affects in pharmacology [171].
Protease inhibitors (PIs) are kinds of proteins or peptides which can be used to inhibit the catalytic activity of proteolytic enzymes [174]. SPIs have been found in scorpions and are classified in two groups—(1) Kunitz-type inhibitors, and (2) Ascaris-type inhibitors [174].
10.1. Kunitz-Type Inhibitors
Kunitz-type inhibitors are a group of serine protease inhibitors that are specified by a conserved spacing between their cysteine residues. There are one or more Kunitz domains in the inhibitor and these possess a typical disulfide bonding pattern [175,176]. Kunitz-type inhibitors are frequently found in arthropod venoms. In the scorpion and spider venoms, these peptides have dual functions [177].
10.2. Primary Sequence of Kunitz-Type Inhibitors
All the Kunitz-type protease inhibitors of scorpions have been recognized by the primary amino acid sequences of some inhibitor peptides that have been found in the NCBI protein data bank [174].
10.3. Ascaris-Type Inhibitors
Ascaris-type peptides usually possess a conserved structure with four short β-strands organized in two approximately vertical β-sheets and stabilized by five disulfide bridges: C1–C7, C2–C6, C3–C5, C4–C10, and C8–C9 [178].
10.4. Primary Sequence of Ascaris-Type Inhibitors
The sequences of all the Ascaris-type protease inhibitors of scorpions which have been found in NCBI protein data bank [174].
10.5. Functional Diversity of Protease Inhibitors
Some of Kunitz-type protease inhibitors of scorpions have been found to inhibit potassium channel KV1.3 [179]. Seven Kunitz-type protease inhibitors (LmKTT-1a, LmKTT-1b, LmKTT-1c, BmKTT-1, BmKTT-2, BmKTT-3, and Hg1) were tested on voltage-gated potassium channel subtype 1.3 (KV1.3 channel) and it was found that six of seven scorpion toxins, excepting rBmKTT-3, which had weak activity, inhibited ~50–80% of Kv1.3 channel currents at a concentration of 1 μM [174].
The serine proteinase inhibitor (serpin) superfamily participates in various necessary biological processes such as blood coagulation, complement activation, fibrinolysis, angiogenesis, inflammation, and tumor suppression. The members of this superfamily are expressed in a cell-specific manner [180]. Because of the abundance of Kunitz-type protease inhibitors in several organisms, they are the best-characterized family of serine protease inhibitors [181]. Two full-length coding sequences of Hemiscorpius lepturus transcriptome were coded as serine proteinase (KX932440 and KX932441 [152].
However, four putative serine protease inhibitors were discovered from the venom of three scorpion species including SjAPI (Scorpiops jendeki Ascaris-type protease inhibitor), SjAPI-2 (Scorpiops jendeki Ascaris-type protease inhibitor 2), CtAPI (Chaerilus tricostatus Ascaris-type protease inhibitor), and BmAPI (Buthus martensii Ascaris-type protease inhibitor) [182].
11. Scorpionism in Iran, a Major Public Health Problem
The risk of scorpion stings in rural areas is higher than in urban regions, and also more common in summer [183]. Much attention has been paid to the mortality rate of scorpion stings, whereas the incidence of scorpion stings is generally underestimated [8].
Scorpions are the most dangerous venomous animals for humans after snakes (venomous snakes) [184].
Climatic conditions, dryness, and heat are factors that increase the threat of scorpion stings [185]. Khuzestan is a province that is located in Southwestern Iran, along with the Persian Gulf region, with a hot and tropical climate. Scorpion stings are a main public health issue in the region especially for children and young adolescents [186]. In the Old World, Iran is acknowledged as one of the world’s hotspots for scorpionism [187].
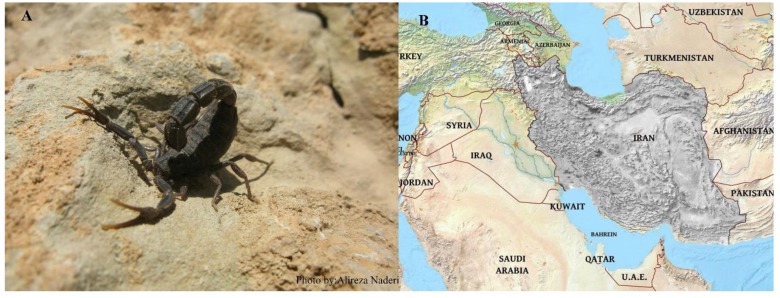
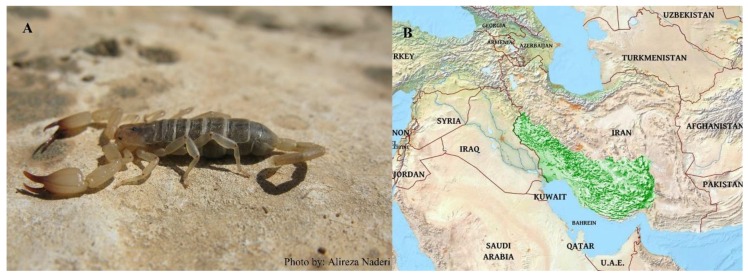
Annually, more than 42,500 scorpion stings from 2001 to 2009 have been reported with about a 19.5% fatality rate. Hemiscorpius lepturus, Androctonus crassicauda, Mesobuthus eupeus, Odontobuthus doriae, Hottentotta saulcyi, Hottentotta schach, Compsobuthus matthiesseni, Olivierus caucasicus, Orthochirus scrobiculosus, and Apistobuthus pterygocercus are significant species in terms of medical and pharmacological relevance. Among Iranian scorpions, Androctonus crassicauda and Hemiscorpius lepturus have the highest risk of envenoming humans [188,189]. Distribution of Androctonus crassicauda (Figure 1) and Hemiscorpius lepturus (Figure 2) in Iran.
Figure 1.
Androctonus crassicauda in its natural habitat (A) and its distribution map in gray (B).
Figure 2.
Image of Hemiscorpius lepturus in its natural habitat (A) and its distribution map in green (B).
According to Shahbazzadeh et al. [190], 12,150 scorpion stings were reported from medical centers in six cities in the Khuzestan province in 2003. The prevalence of human scorpion stings is 3.1/1000 residents. By region, the highest prevalence is in Masjed-Soleiman (27.1%), followed by Ramhormoz (26.6%), Izeh (15.3%), Shush (12%), Baghmalek (11.7%), and Behbahan (7.3%). The most scorpion stings are inflicted by Mesobuthus eupeus, Hottentotta saulcyi, Odontobuthus doriae, and Hemiscorpius lepturus, responsible for 53.3%, whereas 17.4% were related to Androctonus crassicauda and Hottentotta schach, and 29.3% to other species. The maximum and minimum frequencies occur in June and February, respectively.
According to Mirshamsi et al. [48], specimens of Mesobuthus eupeus from Southwestern Iran belong to Mesobuthus phillipsi. Thus, all specimens of Mesobuthus eupeus in previous studies [144,191] should be considered Mesobuthus phillipsi. Based on Kovařík [23], all specimens of Buthotus schach or Hottentotta schach in prior research [100,101,188,189,190] should be considered Hottentotta jayakari, Hottentotta khoozestanus, and Hottentotta zagrosensis. Based on Lourenço [192], Mirshamsi et al. [38], and Navidpour and Lowe [193], there is no Apistobuthus pterygocercus in Iran and all specimens of Apistobuthus pterygocercus belong to Apistobuthus susanae, so all specimens of Apistobuthus pterygocercus in prior research [188,189] should be considered Apistobuthus susanae. The Razi Vaccine and Serum Research Institute, Hesarak, Karaj produces antivenoms of scorpions including six of the most dangerous scorpion species, including Androctonus crassicauda, Hottentotta saulcyi, Hottentotta schach, Mesobuthus eupeus, Odontobuthus doriae, and Hemiscorpius lepturus in Iran.
The Razi Vaccine and Serum Research Institute (Department of Venomous Animals and Antivenin Production) extracts venoms of Hottentotta jayakari, Hottentotta khoozestanus, Hottentotta lorestanus, and Hottentotta zagrosensis. These have previously been considered as Hottentotta schach, but occur in the southern provinces of Iran. Venoms of Mesobuthus brutus, Mesobuthus caucasicus, and, Mesobuthus phillipsi have been considered as Mesobuthus eupeus. According to Mirshamsi et al. [38,48] and Fet et al. [45], specimens of Mesobuthus eupeus should be named Mesobuthus brutus, Mesobuthus caucasicus, and Mesobuthus phillipsi based on geographical distribution. Also, According to Kovařík [23] and Navidpour et al. [33,35], specimens of Hottentotta schach should be named Hottentotta jayakari jayakari, Hottentotta khoozestanus, Hottentotta lorestanus, and Hottentotta zagrosensis. The Department of Venomous Animals and Antivenin Production, Razi Vaccine and Serum Research Institute should recognize the specimens carefully and then produce high quality scorpion antivenoms.
In conclusion, studying toxins of scorpion species from Iran is a way to present patterns and connection between species of scorpion and their venoms, which could be useful for understanding the molecular and functional diversities of scorpion venom sources, their evolutions, and probably the connection between scorpion species and their toxins.
Acknowledgments
This study is dedicated to the memory of the late Iranian scorpionologist and toxinologist, Reza Farzanpay. The first author appreciates Carlos E. Santibáñez-López for helpful comments on preliminary draft of the manuscript. We are grateful to Tina Li, Victor Fet, Hossein Vatanpour and Fan Zhang for guiding us and for providing required literature. We are grateful to Ann V. Paterson, Andrew A. Walker and Steven C. Anderson for editing the English of our manuscript and to Alireza Naderi for his images. We also thank anonymous reviewers for their helpful comments.
Supplementary Materials
The following are available online, Table S1: List of Scorpion species of Iran. Asterisk indicates endemic species, Table S2: Number and percentage of scorpions in Iran.
Author Contributions
S.M.K. conceptualized the idea of the manuscript and wrote it. J.-M.S. participated in writing and submitting the manuscript.
Funding
The authors received no specific funding for this work.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Dunlop J.A., Selden P.A. Scorpion fragments from the Silurian of Powys, Wales. Arachnology. 2013;16:27–32. doi: 10.13156/arac.2013.16.1.27. [DOI] [Google Scholar]
- 2.Waddington J., Rudkin D.M., Dunlop J.A. A new mid-Silurian aquatic scorpion—One step closer to land? Biol. Lett. 2015;11:20140815. doi: 10.1098/rsbl.2014.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polis G.A. Ecology. In: Polis G.A., editor. The Biology of Scorpions. Stanford University Press; Palo Alto, CA, USA: 1990. p. 587. [Google Scholar]
- 4.Santibáñez-López C.E., Francke O.F., Ureta C., Possani L.D. Scorpions from Mexico: From species diversity to venom complexity. Toxins. 2016;8:2. doi: 10.3390/toxins8010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shultz J.W. A phylogenetic analysis of the arachnid orders based on morphological characters. Zool. J. Linn. Soc. 2007;150:221–265. doi: 10.1111/j.1096-3642.2007.00284.x. [DOI] [Google Scholar]
- 6.Sharma P.P., Kaluziak S.T., Pérez-Porro A.R., González V.L., Hormiga G., Wheeler W.C., Giribet G. Phylogenomic interrogation of Arachnida reveals systemic conflicts in phylogenetic signal. Mol. Biol. Evol. 2014;31:2963–2984. doi: 10.1093/molbev/msu235. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P.P., Fernández R., Esposito L., González-Santillán E., Monod L. Phylogenomic resolution of scorpions reveals multilevel discordance with morphological phylogenetic signal. Proc. R. Soc. B. 2015;282:20142953. doi: 10.1098/rspb.2014.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chippaux J.P., Goyffon M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008;107:71–79. doi: 10.1016/j.actatropica.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Lourenço W.R. Scorpion diversity and distribution; past and present patterns. In: Gopalakrishnakone P., Possani L.D., Schwartz E.F., Rodríguez de la Vega R.C., editors. Scorpion Venoms, Toxinology. Springer + Business Media; Dordrecht, The Netherlands: 2015. pp. 3–23. [Google Scholar]
- 10.Smith J.J., Vetter I., Lewis R.J., Peigneur S., Tytgat J., Lam A., Gallant E.M., Beard N.A., Alewood P.F., Dulhunty A.F. Multiple actions of phi-LITX-Lw1a on ryanodine receptors reveal a functional link between scorpion DDH and ICK toxins. Proc. Natl. Acad. Sci. USA. 2013;110:8906–8911. doi: 10.1073/pnas.1214062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King G.F. Venoms to Drugs: Translating Venom Peptides into Therapeutics. Aust. Biochem. 2013;44:13–15. [Google Scholar]
- 12.Rodríguez de la Vega R.C., Schwartz E.F., Possani L.D. Mining on scorpion venom biodiversity. Toxicon. 2010;56:1155–1161. doi: 10.1016/j.toxicon.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz E., Gurrola G.B., Schwartz E.F., Possani L.D. Scorpion venom components as potential candidates for drug development. Toxicon. 2015;93:125–135. doi: 10.1016/j.toxicon.2014.11.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safaei-Mahroo B., Ghaffari H., Fahimi H., Broomand S., Yazdanian M., Najafi-Majd E., Hosseinian Yousefkhani S.S., Rezazadeh E., Hosseinzadeh M.S., Nasrabadi R., et al. The herpetofauna of Iran: Checklist of taxonomy, distribution and conservation status. Asian Herpetol. Res. 2015;6:257–290. [Google Scholar]
- 15.Olivier G.A. In: Voyage dans l’Empire Ottoman, l’Egypte et la Perse, fait par Ordre du Gouvernement, Pendant les Six Premières Années de la République. Agasse C.H., editor. Volume 3. Imprimeur-Libraire; Paris, France: 1807. pp. 96–98. [Google Scholar]
- 16.Birula A.A. Beiträge zur Kenntniss der Scorpionenfauna Ost-Persiens (1. Beitrag) Bull. Acad. Imp. Sci. St.-Pétersbg. Sér. 1900;12:355–375. [Google Scholar]
- 17.Birula A.A. Beitrage zur Kenntniss der Scorpionenfauna Ost-Persiens (2. Beitrag) Bull. Acad. Imp. Sci. St.-Pétersbg. Sér. 1903;19:67–80. [Google Scholar]
- 18.Birula A.A. Beiträge zur Kenntriss der Skorpionenfauna Persien (3. Beiträge) Bull. Imp. Acad. Sci. St.-Pétersbg. Sér. 1905;23:119–148. [Google Scholar]
- 19.Birula A.A. Fauna of Russia and Adjacent Countries. Arachnoidea, Scorpions. Fauna Rossii St. Petersbg. Acad. Sci. Mus. Zool. 1917;1:1–224. English translation by Israel Program for Scientific Translations, Jerusalem 1965, 154p. [Google Scholar]
- 20.Fet V. New for the USSR genus and species of scorpions from Badkhyz, Kraepelinia palpator (Birula, 1903) (Scorpiones, Buthidea) Proc. Acad. Sci. Turkm. 1984;4:37–43. (In Russian) [Google Scholar]
- 21.Fet V. Neohemibuthus zarudnyi (Birula, 1903) from Iran, a senior synonym of N. kinzelbachi Lourenço, 1996 (Scorpiones, Buthidae) Rev. Arachnol. 1997;12:65–68. [Google Scholar]
- 22.Fet V., Capes E.M., Sissom W.D. A new genus and species of psammophilic scorpion from eastern Iran (Scorpiones: Buthidae) In: Gary M., Polis A., Fet V., Selden P.A., editors. Scorpions 2001. British Arachnological Society; Bucks, UK: 2001. pp. 183–189. [Google Scholar]
- 23.Kovařík F. Results of the Czech Biological Expedition to Iran. Part 2. Arachnida: Scorpiones, with descriptions of Iranobuthus krali gen. n. et sp. n. and Hottentotta zagrosensis sp. n. (Buthidae) Acta Soc. Zool. Bohem. 1997;61:39–52. [Google Scholar]
- 24.Kovařík F., Navidpour S., Soleglad M.E. Hemiscorpius shahii sp. n. from Iran (Scorpiones: Hemiscorpiidae) Euscorpius. 2017;249:1–9. doi: 10.18590/euscorpius.2017.vol2017.iss249.1. [DOI] [Google Scholar]
- 25.Kovařík F., Yağmur E.A., Fet V., Hussen F.S. A review of Orthochirus from Turkey, Iraq, and Iran (Khoozestan, Ilam, and Lorestan Provinces), with descriptions of three new species (Scorpiones: Buthidae) Euscorpius. 2019;278:1–31. doi: 10.18590/euscorpius.2019.vol2019.iss278.1. [DOI] [Google Scholar]
- 26.Kovařík F., Yağmur E.A., Moradi M. Two new Hottentotta species from Iran, with a review of Hottentotta saulcyi (Scorpiones: Buthidae) Euscorpius. 2018;265:1–14. doi: 10.18590/euscorpius.2018.vol2018.iss265.1. [DOI] [Google Scholar]
- 27.Pocock R.I. Arachnida. In: Blanford W.T., editor. Fauna of British India Including Ceylon and Burma. Taylor and Francis; London, UK: 1900. p. 279. [Google Scholar]
- 28.Werner F. Reptilien und Gliedertiere aus Persien. Festsch Embrik Strand Riga. 1936;2:193–204. [Google Scholar]
- 29.Vachon M. Liste des scorpions connus en Egypte, Arabie, Israël, Liban, Syrie, Jordanie, Turquie, Irak, Iran. Toxicon. 1966;4:209–218. doi: 10.1016/0041-0101(66)90052-3. [DOI] [PubMed] [Google Scholar]
- 30.Habibi T. Liste de scorpions de l’Iran. Bull. Fac. Sci. Teheran Univ. 1971;2:42–47. (In French and Farsi) [Google Scholar]
- 31.Farzanpay R. Knowing Scorpions. Central University Publications; Tehran, Iran: 1987. p. 231. No. 312, Biology, 4, in Farsi, with Latin Index. [Google Scholar]
- 32.Farzanpay R. A catalogue of the scorpions occurring in Iran, up to January 1986. Rev. Arachnol. 1988;8:33–44. [Google Scholar]
- 33.Navidpour S., Kovařík F., Soleglad M.E., Fet V. Scorpions of Iran (Arachnida, Scorpiones). Part I. Khuzestan Province. Euscorpius. 2008;65:1–41. [Google Scholar]
- 34.Navidpour S., Kovařík F., Soleglad M.E., Fet V. Scorpions of Iran (Arachnida, Scorpiones). Part X. Alborz, Markazi and Tehran Provinces with a description of Orthochirus carinatus sp. n. (Buthidae) Euscorpius. 2019;276:1–20. doi: 10.18590/euscorpius.2019.vol2019.iss276.1. [DOI] [Google Scholar]
- 35.Navidpour S., Nayebzadeh H.H., Soleglad M.E., Fet V., Kovařík F., Kayedi M.H. Scorpions of Iran (Arachnida, Scorpiones). Part VI. Lorestan Province. Euscorpius. 2010;99:1–23. doi: 10.18590/euscorpius.2010.vol2010.iss104.2. [DOI] [Google Scholar]
- 36.Navidpour S., Soleglad M.E., Fet V., Kovařík F. Scorpions of Iran (Arachnida, Scorpiones). Part II. Bushehr Province. Euscorpius. 2008;67:1–33. doi: 10.18590/euscorpius.2008.vol2008.iss67.1. [DOI] [Google Scholar]
- 37.Navidpour S., Soleglad M.E., Fet V., Kovařík F. Scorpions of Iran (Arachnida, Scorpiones). Part IX. Hormozgan Province, with a description of Odontobuthus tavighiae sp. n. (Buthidae) Euscorpius. 2013;170:1–29. doi: 10.18590/euscorpius.2013.vol2013.iss170.1. [DOI] [Google Scholar]
- 38.Mirshamsi O., Sari A., Hosseinie S. History of study and checklist of the scorpion fauna (Arachnida: Scorpiones) of Iran. Prog. Biol. Sci. 2011;1:16–28. [Google Scholar]
- 39.Yağmur E.A., Moradi M., Larti M., Lashkari S. First record of Androctonus robustus Kovařík & Ahmed, 2013 (Scorpiones: Buthidae) for Iran. Zool. Middle East. 2016;62:370–372. [Google Scholar]
- 40.Kovařík F., Ojanguren Affilastro A.A. Illustrated catalog of scorpions Part II. Bothriuridae; Chaerilidae; Buthidae I., genera Compsobuthus, Hottentotta, Isometrus, Lychas, and Sassanidotus. Clairon Production; Prague, Czech Republic: 2013. 400p [Google Scholar]
- 41.Mirshamsi O. Ph.D. Thesis. University of Tehran; Tehran, Iran: 2010. A Biosystematic Approach to Mesobuthus eupeus in Iran.200p [Google Scholar]
- 42.Nejati J., Mozafari E., Saghafipour A., Kiyani M. Scorpion fauna and epidemiological aspects of scorpionism in southeastern Iran. Asian Pac. J. Trop. Biomed. 2014;4:S217–S221. doi: 10.12980/APJTB.4.2014C1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe G. A New Species of Odontobuthus (Scorpiones: Buthidae) from Northern Oman. Euscorpius. 2010;96:1–22. doi: 10.18590/euscorpius.2010.vol2010.iss96.1. [DOI] [Google Scholar]
- 44.Kovařík F., Yağmur E.A., Fet V. Review of Hottentotta described by A. A. Birula, with descriptions of two new species and comments on Birula’s collection (Scorpiones: Buthidae) Euscorpius. 2019;282:1–30. [Google Scholar]
- 45.Fet V., Kovařík F., Gantenbein B., Kaiser R.C., Stewart A.K., Graham M.R. Revision of the Mesobuthus caucasicus complex from Central Asia, with descriptions of six new species (Scorpiones: Buthidae) Euscorpius. 2018;255:1–77. doi: 10.18590/euscorpius.2018.vol2018.iss255.1. [DOI] [Google Scholar]
- 46.Karataş¸ A., Mouradi-Gharkheloo M. A new Hemiscorpius Peters, 1861 (Scorpiones: Hemiscorpiidae) from southwestern Iran. Turk. J. Zool. 2013;37:15–23. [Google Scholar]
- 47.Mirshamsi O., Azghadi S., Navidpour S., Aliabadian M., Kovařík F. Odontobuthus tirgari sp. nov. (Scorpiones, Buthidae) from the eastern region of the Iranian Plateau. Zootaxa. 2013;3731:153–170. doi: 10.11646/zootaxa.3731.1.7. [DOI] [PubMed] [Google Scholar]
- 48.Mirshamsi O., Sari A., Elahi E., Hosseinie S. Mesobuthus eupeus (Scorpiones: Buthidae) from Iran: A polytypic species complex. Zootaxa. 2011;2929:1–21. [Google Scholar]
- 49.Teruel R., Kovařík F., Navidpour S., Fet V. The first record of the genus Anomalobuthus Kraepelin, 1900 from Iran, with description of a new species (Scorpiones: Buthidae) Euscorpius. 2014;192:1–10. doi: 10.18590/euscorpius.2014.vol2014.iss192.1. [DOI] [Google Scholar]
- 50.Catterall W.A. Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 51.Lewis R.J., Garcia M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003;2:790–802. doi: 10.1038/nrd1197. [DOI] [PubMed] [Google Scholar]
- 52.Almaaytah A., Albalas Q. Scorpion venom peptides with no disulfide bridges: A review. Peptides. 2014;51:35–45. doi: 10.1016/j.peptides.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Bhavya J., Francois N.N., More V.S., More S.S. Scorpion toxin polyptides as therapeutic agents: An overview. Protein Pept. Lett. 2016;23:848–859. doi: 10.2174/0929866523666160630184635. [DOI] [PubMed] [Google Scholar]
- 54.He Y.W., Zhao R.M., di Z.Y., Li Z.J., Xu X.B., Hong W., Wu Y.L., Zhao H.B., Li W.X., Cao Z.J. Molecular diversity of Chaerilidae venom peptides reveals the dynamic evolution of scorpion venom components from Buthidae to non-Buthidae. J. Proteom. 2013;89:1–14. doi: 10.1016/j.jprot.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz E.F., Diego-Garcia E., Rodríguez de la Vega R.C., Possani L.D. Transcriptome analysis of the venom gland of the Mexican scorpion Hadrurus gertschi(Arachnida: Scorpiones) BMC Genom. 2007;8:119. doi: 10.1186/1471-2164-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao M., Ding L., Yang W., Chai L., Sun Y., Yang X., Li D., Zhang H., Li W., Cao Z., et al. St20, a new venomous animal derived natural peptide with immunosuppressive and anti-inflammatory activities. Toxicon. 2017;127:37–43. doi: 10.1016/j.toxicon.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Veiseh M., Gabikian P., Bahrami S.B., Veiseh O., Zhang M., Hackman R.C., Ravanpay A.C., Stroud M.R., Kusuma Y., Hansen S.J., et al. Tumor paint: A chlorotoxin: cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 58.Sarfo-Poku C., Eshun O., Lee K.H. Medical application of scorpion venom to breast cancer: A mini-review. Toxicon. 2016;122:109–112. doi: 10.1016/j.toxicon.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 59.De Souza J.M., Goncalves B.D.C., Gomez M.V., Vieira L.B., Ribeiro F.M. Animal toxins as therapeutic tools to treat neurodegenerative diseases. Front. Pharmacol. 2018;9:145. doi: 10.3389/fphar.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen B., Cao Z., Li W., Sabatier J.M., Wu Y. Treating autoimmune disorders with venom-derived peptides. Expert Opin. Biol. Ther. 2017;17:1065–1075. doi: 10.1080/14712598.2017.1346606. [DOI] [PubMed] [Google Scholar]
- 61.Chan Y.S., Cheung R.C., Xia L., Wong J.H., Ng T.B., Chan W.Y. Snake venom toxins: Toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 2016;100:6165–6181. doi: 10.1007/s00253-016-7610-9. [DOI] [PubMed] [Google Scholar]
- 62.Estève E., Smida-Rezgui S., Sarkozi S., Szegedi C., Regaya I., Chen L., Altafaj X., Rochat H., Allen P., Pessah I.N., et al. Critical amino acid residues determine the binding affinity and the Ca2+ release efficacy of maurocalcine in skeletal muscle cells. J. Biol. Chem. 2003;278:37822–37831. doi: 10.1074/jbc.M305798200. [DOI] [PubMed] [Google Scholar]
- 63.Gurrola G.B., Capes E.M., Zamudio F.Z., Possani L.D., Valdivia H.H. Imperatoxin A, a Cell-Penetrating Peptide from Scorpion Venom, as a Probe of Ca2+Release Channels/Ryanodine Receptors. Pharmaceuticals. 2010;3:1093–1107. doi: 10.3390/ph3041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Possani L.D., Becerril B., Delepierre M., Tytgat J. Scorpion toxins specific for Na+channels. Eur. J. Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 65.Goudet C., Chi C.W., Tytgat J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon. 2002;40:1239–1258. doi: 10.1016/S0041-0101(02)00142-3. [DOI] [PubMed] [Google Scholar]
- 66.Possani L.D., Merino E., Corona M., Bolivar F., Becerril B. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie. 2000;82:861–868. doi: 10.1016/S0300-9084(00)01167-6. [DOI] [PubMed] [Google Scholar]
- 67.Hille B. Ion Channels of Excitable Membranes. 3rd ed. Sinauer Associates, Inc.; Sunderland, MA, USA: 2001. [Google Scholar]
- 68.Wickenden A., Priest B., Erdemli G. Ion channel drug discovery: Challenges and future directions. Future Med. Chem. 2012;4:661–679. doi: 10.4155/fmc.12.4. [DOI] [PubMed] [Google Scholar]
- 69.Rodríguez de la Vega R.C., Possani L.D. Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure function relationships and evolution. Toxicon. 2005;46:831–844. doi: 10.1016/j.toxicon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Martin-Eauclaire M.F., Couraud F. Scorpion neurotoxins: Effects and mechanisms. In: Chang L.W., Dyer R.S., editors. Handbook of Neurotoxicity. Marcel Drekker; New York, NY, USA: 1995. pp. 683–716. [Google Scholar]
- 71.Abdel-Rahman M.A., Quintero-Hernández V., Possani L.D. Venom Genomics Proteomics. Springer Science+Business Media Dordrecht; Berlin/Heidelberg, Germany: 2016. Scorpion venom gland transcriptomics and proteomics: An overview; pp. 105–124. [Google Scholar]
- 72.Morey S.S., Kiran K.M., Gadag J.R. Purification and properties of hyaluronidase from Palamneus gravimanus (Indian black scorpion) venom. Toxicon. 2006;47:188–195. doi: 10.1016/j.toxicon.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 73.Pessini A.C., Takao T.T., Cavalheiro E.C., Vichnewski W., Sampaio S.V., Giglio J.R., Arantes E.C. A hyaluronidase from Tityus serrulatus scorpion venom: Isolation, characterization and inhibition by flavonoids. Toxicon. 2001;39:1495–1504. doi: 10.1016/S0041-0101(01)00122-2. [DOI] [PubMed] [Google Scholar]
- 74.Dardevet L., Rani D., Aziz T.A., Bazin I., Sabatier J.M., Fadl M., Brambilla E., De Waard M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins. 2015;7:1079–1101. doi: 10.3390/toxins7041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kastin A. Handbook of Biologically Active Peptides. 2nd ed. Academic Press; San Diego, CA, USA: 2013. p. 408. [Google Scholar]
- 76.Fu Y.J., Yin L.T., Liang A.H., Zhang C.F., Wang W., Chai B.F., Fan X.J. Therapeutic potential of chlorotoxin-like neurotoxin from the Chinese scorpion for human gliomas. Neurosci. Lett. 2007;412:62–67. doi: 10.1016/j.neulet.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 77.Mamelak A.N., Jacoby D.B. Targeted Delivery of Antitumoral Therapy to Glioma and Other Malignancies with Synthetic Chlorotoxin (TM 601) Expert Opin. Drug Deliv. 2007;4:175–186. doi: 10.1517/17425247.4.2.175. [DOI] [PubMed] [Google Scholar]
- 78.Mishal R., Tahir H.M., Zafar K., Arshad M. Anti-cancerous applications of scorpion venom. Int. J. Biol. Pharm. Res. 2013;4:356–360. [Google Scholar]
- 79.Zargan J., Sajad M., Umar S., Naime M., Ali S., Khan H.A. Scorpion (Androctonus crassicauda) venom limits growth of transformed cells (SH-SY5Y and MCF-7) by cytotoxicity and cell cycle arrest. Exp. Mol. Pathol. 2011;91:447–454. doi: 10.1016/j.yexmp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Zargan J., Sajad M., Umar S., Naime M., Ali S., Khan H.A. Scorpion (Odontobuthus doriae) venom induces apoptosis and inhibits DNA synthesis in human neuroblastoma cells. Mol. Cell. Biochem. 2011;348:173–181. doi: 10.1007/s11010-010-0652-x. [DOI] [PubMed] [Google Scholar]
- 81.Zargan J., Umar S., Sajad M., Naime M., Ali S., Khan H.A. Scorpion venom (Odontobuthus doriae) induces apoptosis by depolarization of mitochondria and reduces S-phase population in human breast cancer cells (MCF-7) Toxicol. In Vitro. 2011;25:1748–1756. doi: 10.1016/j.tiv.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Catterall W.A., Cestèle S., Yarov-Yarovoy V., Yu F.H., Konoki K., Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 83.Beneski D.A., Catterall W.A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc. Natl. Acad. Sci. USA. 1980;77:639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Payandeh J., Scheuer T., Zheng N., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang J.T., Yarov-Yarovoy V., Kahn R., Gordon D., Gurevitz M., Scheuer T., Catterall W.A. Mapping the receptor site for α-scorpion toxins on a Na+ channel voltage sensor. Proc. Natl. Acad. Sci. USA. 2011;108:15426–15431. doi: 10.1073/pnas.1112320108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Catterall W.A. Voltage-gated sodium channels at 60: Structure, function and pathophysiology. J. Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Catterall W.A., Goldin A.L., Waxman S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 88.Black J.A., Waxman S.G. Noncanonical roles of voltage-gated sodium channels. Neuron. 2013;80:280–291. doi: 10.1016/j.neuron.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Wu Y., Ma H., Zhang F., Zhang C., Zou X., Cao Z. Selective Voltage-Gated Sodium Channel Peptide Toxins from Animal Venom: Pharmacological Probes and Analgesic Drug Development. ACS Chem. Neurosci. 2018;9:187–197. doi: 10.1021/acschemneuro.7b00406. [DOI] [PubMed] [Google Scholar]
- 90.Cestele S., Catterall W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/S0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 91.Zhang F., Xu X., Li T., Liu Z. Shellfish toxins targeting voltage-gated sodium channels. Mar. Drugs. 2013;11:4698–4723. doi: 10.3390/md11124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Catterall W.A. Structure and regulation of voltage-gated calcium channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 93.Patino G.A., Isom L.L. Electrophysiology and beyond: Multiple roles of Na+ channel beta subunits in development and disease. Neurosci. Lett. 2010;486:53–59. doi: 10.1016/j.neulet.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Savio-Galimberti E., Gollob M.H., Darbar D. Voltage-gated sodium channels: Biophysics, pharmacology, and related channelopathies. Front. Pharmacol. 2012;3:124. doi: 10.3389/fphar.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Durek T., Vetter I., Wang C.I., Motin L., Knapp O., Adams D.J., Lewis R.J., Alewood P.F. Chemical engineering and structural and pharmacological characterization of the α-scorpion toxin OD1. ACS Chem. Biol. 2013;8:1215–1222. doi: 10.1021/cb400012k. [DOI] [PubMed] [Google Scholar]
- 96.Jalali A., Bosmans F., Amininasab M., Clynen E., Cuypers E., Zaremirakabadi A., Sarbolouki M.N., Schoofs L., Vatanpour H., Tytgat J. OD1, the first toxin isolated from the venom of the scorpion Odonthobuthus doriae active on voltage-gated Na+ channels. FEBS Lett. 2005;579:4181–4186. doi: 10.1016/j.febslet.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 97.Maertens C., Cuypers E., Amininasab M., Jalali A., Vatanpour H., Tytgat J. Potent modulation of the voltage-gated sodium channel Nav1.7 by OD1, a toxin from the scorpion Odonthobuthus doriae. Mol. Pharmacol. 2006;70:405–414. doi: 10.1124/mol.106.022970. [DOI] [PubMed] [Google Scholar]
- 98.Bosmans F., Maertens C., Verdonck F., Tytgat J. The poison dart frog’s batrachotoxin modulates Nav1.8. FEBS Lett. 2004;577:245–248. doi: 10.1016/j.febslet.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 99.Gold M.S., Reichling D.B., Shuster M.J., Levine J.D. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc. Natl. Acad. Sci. USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aboutorabi A., Naderi N., Gholami Pourbadie H., Zolfagharian H., Vatanpour H. Voltage-gated sodium channel modulation by Bothutous Schach venom scorpion. Iran. J. Pharm. Sci. 2016;12:55–64. [Google Scholar]
- 101.Vatanpour H., Ahmadi F., Zare Mirakabadi A., Jalali A. Two Biological Active Fractions Isolated from Buthotus schach (BS) Scorpion Venom Examined on Striated Muscle Preparation, In-vitro. Iran. J. Pharm. Res. 2012;11:905–911. [PMC free article] [PubMed] [Google Scholar]
- 102.González C., Baez-Nieto D., Valencia I., Oyarzún I., Rojas P., Naranjo D., Latorre R. K (+) channels: Function-structural overview. Compr. Physiol. 2012;2:2087–2149. doi: 10.1002/cphy.c110047. [DOI] [PubMed] [Google Scholar]
- 103.Doyle D.A., Morais Cabral J., Pfuetzner R.A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 104.Heinemann S.H., Rettig J., Graack H.R., Pongs O. Functional characterization of Kv channel beta-subunits from rat brain. J. Physiol. 1996;493:625–633. doi: 10.1113/jphysiol.1996.sp021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kubo Y., Adelman J.P., Clapham D.E., Jan L.Y., Karschin A., Kurachi Y., Lazdunski M., Nichols C.G., Seino S., Vandenberg C.A. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol. Rev. 2005;57:509–526. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- 106.Goldstein S.A.N., Bayliss D.A., Kim D., Lesage F., Plant L.D., Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol. Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- 107.Gutman G.A., Chandy K.G., Grissmer S., Lazdunski M., MacKinnon L., Pardo L.A., Robertson G.A., Rudy B., Sanguinetti M.C., Stühmer W., et al. International union of pharmacology. Liii. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 108.Wei A.D., Gutman G.A., Aldrich R., Chandy K.G., Grissmer S., Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol. Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 109.Wulff H., Zhorov B.S. K + channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem. Rev. 2008;108:1744–1773. doi: 10.1021/cr078234p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuzmenkov A.I., Krylov N.A., Chugunov A.O., Grishin E.V., Vassilevski A.A. Kalium: A Database of Potassium Channel Toxins from Scorpion Venom. Database. 2016 doi: 10.1093/database/baw056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mouhat S., Andreotti N., Jouirou B., Sabatier J.M. Animal toxins acting on voltage-gated potassium channels. Curr. Pharm. Des. 2008;14:2503–2518. doi: 10.2174/138161208785777441. [DOI] [PubMed] [Google Scholar]
- 112.Bergeron Z.L., Bingham J.P. Scorpion toxins specific for potassium (K) channels: A historical overview of peptide bioengineering. Toxins. 2012;4:1082–1119. doi: 10.3390/toxins4111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodríguez de la Vega R.C., Possani L.D. Current views on scorpion toxins specific for K+-channels. Toxicon. 2004;43:865–875. doi: 10.1016/j.toxicon.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 114.Rodríguez de la Vega R.C., Vidal N., Possani L.D. In: Handbook of Biologically Active Peptides. 2nd ed. Kastin A., editor. Academic Press; San Diego, CA, USA: 2013. p. 423. [Google Scholar]
- 115.Zhao Y., Huang J., Yuan X., Peng B., Liu W., Han S., He X. Toxins Targeting the Kv1.3 Channel: Potential Immunomodulators for Autoimmune Diseases. Toxins. 2015;7:749–764. doi: 10.3390/toxins7051749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abdel-Mottaleb Y., Clynen E., Jalali A., Bosmans F., Vatanpour H., Schoofs L., Tytgat J. The first potassium channel toxin from the venom of the Iranian scorpion Odonthobuthus doriae. FEBS Lett. 2006;580:6254–6258. doi: 10.1016/j.febslet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 117.Abdel-Mottaleb Y., Vandendriessche T., Clynen E., Landuyt B., Jalali A., Vatanpour H., Tytgat J. OdK2, a Kv1.3 channel-selective toxin from the venom of the Iranian scorpion Odonthobuthus doriae. Toxicon. 2008;51:1424–1430. doi: 10.1016/j.toxicon.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 118.Srairi-Abid N., Shahbazzadeh D., Chatti I., Mlayah-Bellalouna S., Mejdoub H., Borchani L., Benkhalifa R., Akbari A., El Ayeb M. Hemitoxin, the first potassium channel toxin from the venom of the Iranian scorpion Hemiscorpius lepturus. FEBS J. 2008;275:4641–4650. doi: 10.1111/j.1742-4658.2008.06607.x. [DOI] [PubMed] [Google Scholar]
- 119.Catterall W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bajaj S., Han J. Venom-Derived Peptide Modulators of Cation-Selective Channels: Friend, Foe or Frenemy. Front. Pharmacol. 2019;10:58. doi: 10.3389/fphar.2019.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Franzini-Armstrong C., Protasi F. Ryanodine receptors of striated muscles: A complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- 122.Van Petegem F. Ryanodine receptors: Structure and function. J. Biol. Chem. 2012;287:31624–31632. doi: 10.1074/jbc.R112.349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwartz E.F., Capes E.M., Diego-García E., Zamudio F.Z., Fuentes O., Possani L.D., Valdivia H.H. Characterization of hadrucalcin, a peptide from Hadrurus gertschi scorpion venom with pharmacological activity on ryanodine receptors. Br. J. Pharmacol. 2009;157:392–403. doi: 10.1111/j.1476-5381.2009.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Naderi Soorki M., Galehdari H., Baradaran M., Jalali A. First venom gland transcriptomic analysis of Iranian yellow scorpion “Odonthobuthus doriae” with some new findings. Toxicon. 2016;120:69–77. doi: 10.1016/j.toxicon.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 125.Morgenstern D., Rohde B.H., King G.F., Tal T., Sher D., Zlotkin E. The tale of a resting gland: Transcriptome of a replete venom gland from the scorpion Hottentotta judaicus. Toxicon. 2011;57:695–703. doi: 10.1016/j.toxicon.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 126.Shahbazzadeh D., Srairi-Abid N., Feng W., Ram N., Borchani L., Ronjat M., Akbari A., Pessah I.N., De Waard M., El Ayeb M. Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem. J. 2007;404:89–96. doi: 10.1042/BJ20061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Estévez R., Jentsch T.J. CLC chloride channels: Correlating structure with function. Curr. Opin. Struct. Biol. 2002;12:531–539. doi: 10.1016/S0959-440X(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 128.Jentsch T.J., Stein V., Weinreich F., Zdebik A.A. Molecular structure and physiological function of chloride channels. Physiol. Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 129.Alexander S.P., Kelly E., Marrion N., Peters J.A., Benson H.E., Faccenda E., Pawson A.J., Sharman J.L., Southan C., Davies J.A., et al. The concise guide to pharmacology 2015/16: Other ion channels. Br. J. Pharmacol. 2015;172:5942–5955. doi: 10.1111/bph.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thompson C.H., Olivetti P.R., Fuller M.D., Freeman C.S., McMaster D., French R.J., Pohl J., Kubanek J., McCarty N.A. Isolation and characterization of a high affinity peptide inhibitor of ClC-2 chloride channels. J. Biol. Chem. 2009;284:26051–26062. doi: 10.1074/jbc.M109.031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lippens G., Najib J., Tartar A., Lippens G., Wodak S.J. NMR sequential assignments and solution structure of chlorotoxin, a small scorpion toxin that blocks chloride channels. Biochemistry. 1995;34:13–21. doi: 10.1021/bi00001a003. [DOI] [PubMed] [Google Scholar]
- 132.Adjadj E., Naudat V., Quiniou E., Wouters D., Sautiére P., Craescu C.T. Solution structure of Lqh-8:6, a toxin-like peptide from scorpion venom. Eur. J. Biochem. 1997;246:218–227. doi: 10.1111/j.1432-1033.1997.00218.x. [DOI] [PubMed] [Google Scholar]
- 133.Ali S.A., Stoeva S., Schütz J., Kayed R., Abbasi A., Zaidi Z.H., Voelter W. Purification and primary structure of low molecular mass peptides from scorpion (Buthus sindicus) venom. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998;121:323–332. doi: 10.1016/S1095-6433(98)10140-X. [DOI] [PubMed] [Google Scholar]
- 134.Harrison P.L., Abdel-Rahman M.A., Miller K., Strong P.N. Antimicrobial peptides from scorpion venoms. Toxicon. 2014;88:115–137. doi: 10.1016/j.toxicon.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boman H.G. Innate immunity and the normal microflora. Immunol. Rev. 2000;173:5–16. doi: 10.1034/j.1600-065X.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- 136.Chen T., Walker B., Zhou M., Shaw C. Molecular cloning of a novel putative potassium channel-blocking neurotoxin from the venom of the North African scorpion, Androctonus amoreuxi. Peptides. 2005;26:731–736. doi: 10.1016/j.peptides.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 137.Chen T., Folan R., Kwok H., O’Kane E.J., Bjourson A.J., Shaw C. Isolation of scorpion (Androctonus amoreuxi) putative alpha neurotoxins and parallel cloning of their respective cDNAs from a single sample of venom. Regul. Pept. 2003;115:115–121. doi: 10.1016/S0167-0115(03)00146-0. [DOI] [PubMed] [Google Scholar]
- 138.Anonymous. United Nations Programme on HIV/AIDS. UNAIDS. 20: UNAIDS Report on the Global AIDS Epidemic 2016. [(accessed on 31 May 2016)];2016 Available online: http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016.
- 139.Huyart N., Calvayrac R., Briand J., Goyffon M., Vuillaume M. Catalatic properties of hemocyanin in helping to account for the scorpion’s radioresistance. Comp. Biochem. Physiol. 1983;76:153–159. doi: 10.1016/0305-0491(83)90187-6. [DOI] [Google Scholar]
- 140.Quéinnec E., Gardes-Albert M., Goyffon M., Ferradini C., Vuillaume M. Antioxidant activity of hemocyanin; a pulse radiolysis study. Biochim. Biophys. Acta. 1990;1041:153–159. doi: 10.1016/0167-4838(90)90059-O. [DOI] [PubMed] [Google Scholar]
- 141.Vuillaume M., Ducancel F., Calvayrac R., Rabilloud T., Hubert M., Goyffon M. Correlations between the catalase-like activity and the H2O2-ATP production of hæmocyanin and its subunits; implications with the radioresistance of the scorpion Androctonus australis. Comp. Biochem. Physiol. 1989;92:17–23. doi: 10.1016/0305-0491(89)90307-6. [DOI] [Google Scholar]
- 142.Goyffon M. Hemocyanin. Venoms. Defensins. In: Stockmann R., Ythier E., editors. Scorpions of the World. NAP; Verrières-le-Buisson, France: 2010. pp. 91–111. [Google Scholar]
- 143.Zabihollahi R., Bagheri K.P., Keshavarz Z., Motevalli F., Bahramali G., Siadat S.D., Momen S.B., Shahbazzadeh D., Aghasadeghi M.R. Venom Components of Iranian Scorpion Hemiscorpius lepturus Inhibit the Growth and Replication of Human Immunodeficiency Virus 1 (HIV-1) Iran. Biomed. J. 2016;20:259–265. doi: 10.22045/ibj.2016.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Farajzadeh-Sheikh A., Jolodar A., Ghaemmaghami S. Sequence Characterization of cDNA Sequence of Encoding of an Antimicrobial Peptide With No Disulfide Bridge from the Iranian Mesobuthus Eupeus Venomous Glands. Iran. Red Crescent Med. J. 2013;15:36–41. doi: 10.5812/ircmj.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Imai K., Hiramatsu A., Fukushima D., Pierschbacher M.D., Okada Y. Degradation of decorin by matrix metalloproteinases: Identification of the cleavage sites, kinetic analyses and transforming growth factor-β1 release. Biochem. J. 1997;322:809–814. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buckley A. Master’s Thesis. Philadelphia College of Osteopathic Medicine; Philadelphia, PA, USA: 2016. Potential Therapeutic Efficacy of a Novel Metalloproteinase Inhibitor, Extracellular Matrix Protection Factor 1, in Human Osteoarthritic Chondrocyte Primary Cultures. [Google Scholar]
- 147.Kim B.J., Hur J.W., Park J.S., Kim J.H., Kwon T.H., Park Y.K., Moon H.J. Expression of matrix metalloproteinase− 2 and− 9 in human ligamentum fl avum cells treated with tumor necrosis factor—α and interleukin-1β. JNS. 2016;24:428–435. doi: 10.3171/2015.6.SPINE141271. [DOI] [PubMed] [Google Scholar]
- 148.Giannelli G., Falk-Marzillier J., Schiraldi O., Stetler-Stevenson W.G., Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 149.Rudolph-Owen L.A., Chan R., Muller W.J., Matrisian L.M. The matrix metalloproteinase matrilysin influences early stage mammary tumorigenesis. Cancer Res. 1998;58:5500–5506. [PubMed] [Google Scholar]
- 150.Xia X., Ma Y., Xue S., Wang A., Tao J., Zhao Y., Zhang Q., Liu R., Lu S. Cloning and molecular characterization of BumaMPs1, a novel metalloproteinases from the venom of scorpion Buthus martensi Karsch. Toxicon. 2013;76:234–238. doi: 10.1016/j.toxicon.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 151.Jahdasani R., Rahimi Jamnani F., Behdani M., Habibi-Anbouhi M., Yardehnavi N., Shahbazzadeh D., Kazemi-Lomedasht F. Identification of the immunogenic epitopes of the whole venom component of the Hemiscorpius lepturus scorpion using the phage display peptide library. Toxicon. 2016;124:83–93. doi: 10.1016/j.toxicon.2016.11.247. [DOI] [PubMed] [Google Scholar]
- 152.Kazemi-Lomedasht F., Khalaj V., Bagheri K.P., Behdani M., Shahbazzadeh D. The first report on transcriptome analysis of the venom gland of Iranian scorpion, Hemiscorpius lepturus. Toxicon. 2017;125:123–130. doi: 10.1016/j.toxicon.2016.11.261. [DOI] [PubMed] [Google Scholar]
- 153.Kazemi-Lomedasht F., Shahbazzadeh D., Behdani M. Phylogenetic analysis of metalloprotease from transcriptome of venom gland of Hemiscorpius lepturus. Arch. Biotechnol. Biomed. 2019;3:006–0010. [Google Scholar]
- 154.Schaloske R.H., Dennis E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 155.Shimizu T., Ohto T., Kita Y. Cytosolic phospholipase A2: Biochemical properties and physiological roles. IUBMB Life. 2006;58:328–333. doi: 10.1080/15216540600702289. [DOI] [PubMed] [Google Scholar]
- 156.De Maria L., Vind J., Oxenboll K.M., Svendsen A., Patkar S. Phospholipases and their industrial applications. Appl. Microbiol. Biotechnol. 2007;74:290–300. doi: 10.1007/s00253-006-0775-x. [DOI] [PubMed] [Google Scholar]
- 157.Rouault M., Bollinger J.G., Lazdunski M., Gelb M.H., Lambeau G. Novel mammalian group XII secreted phospholipase A2 lacking enzymatic activity. Biochemistry. 2003;42:11494–11503. doi: 10.1021/bi0349930. [DOI] [PubMed] [Google Scholar]
- 158.Six D.A., Dennis E.A. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta. 2000;1488:1–19. doi: 10.1016/S1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 159.Burke J.E., Dennis E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009;50:S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hariprasad G., Kumar M., Srinivasan A., Kaur P., Singh T.P., Jithesh O. Group III phospholipase A2 from the scorpion, Mesobuthus tamulus: Targeting and reversible inhibition by native peptides. Int. J. Biol. Macromol. 2011;48:423–431. doi: 10.1016/j.ijbiomac.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 161.Ramanaiah M., Parthasarathy P.R., Venkaiah B. Purification and properties of phospholipase A2 from the venom of scorpion, (Heterometrus fulvipes) Biochem Int. 1990;20:931–940. [PubMed] [Google Scholar]
- 162.Hariprasad G., Singh B., Das U., Ethayathulla A.S., Kaur P., Singh T.P., Srinivasan A. Cloning, sequence analysis and homology modeling of a novel phospholipase A2 from Heterometrus fulvipes (Indian black scorpion) DNA Seq. 2007;18:242–246. doi: 10.1080/10425170701243294. [DOI] [PubMed] [Google Scholar]
- 163.Zamudio F.Z., Conde R., Arévalo C., Becerril B., Martin B.M., Valdivia H.H., Possani L.D. The mechanism of inhibition of ryanodine receptor channels by imperatoxin I, a heterodimeric protein from the scorpion Pandinus imperator. J. Biol. Chem. 1997;272:11886–11894. doi: 10.1074/jbc.272.18.11886. [DOI] [PubMed] [Google Scholar]
- 164.Conde R., Zamudio F.Z., Becerril B., Possani L.D. Phospholipin, a novel heterodimeric phospholipase A2 from Pandinus imperator scp6pion venom. FEBS Lett. 1999;460:447–450. doi: 10.1016/S0014-5793(99)01392-7. [DOI] [PubMed] [Google Scholar]
- 165.Valdez-Cruz N.A., Batista C.V., Possani L.D. Phaiodactylipin, a glycosylated heterodimeric phospholipase A from the venom of the scorpion Anuroctonus phaiodactylus. Eur. J. Biochem. 2004;271:1453–1464. doi: 10.1111/j.1432-1033.2004.04047.x. [DOI] [PubMed] [Google Scholar]
- 166.Incamnoi P., Patramanon R., Thammasirirak S., Chaveerach A., Uawonggul N., Sukprasert S., Rungsa P., Daduang J., Daduang S. Heteromtoxin (HmTx), a novel heterodimeric phospholipase A(2) from Heterometrus laoticus scorpion venom. Toxicon. 2013;61:62–71. doi: 10.1016/j.toxicon.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 167.Jridi I., Catacchio I., Majdoub H., Shahbazeddah D., El Ayeb M., Frassanito M.A., Ribatti D., Vacca A., Borchani L. Hemilipin, a novel Hemiscorpius lepturus venom heterodimeric phospholipase A2, which inhibits angiogenesis in vitro and in vivo. Toxicon. 2015;105:34–44. doi: 10.1016/j.toxicon.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 168.Louati H., Krayem N., Fendri A., Aissa I., Sellami M., Bezzine S., Gargouri Y. A thermoactive secreted phospholipase A(2) purified from the venom glands of Scorpio maurus: Relation between the kinetic properties and the hemolytic activity. Toxicon. 2013;72:133–142. doi: 10.1016/j.toxicon.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 169.Borchani L., Sassi A., Shahbazzadeh D., Strub J., Tounsi-Guetteti H., Boubaker M., Akbari A., Dorsselaer A., El Ayeb M. Heminecrolysin, the first hemolytic dermonecrotic toxin purified from scorpion venom. Toxicon. 2011;58:130–139. doi: 10.1016/j.toxicon.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 170.Jridi I., Catacchio I., Majdoub H., Shahbazeddah D., El Ayeb M., Frassanito M.A., Solimando A., Ribatti D., Vacca A., Borchani L. The small subunit of Hemilipin2, a new heterodimeric phospholipase A2 from Hemiscorpius lepturus scorpion venom, mediates the antiangiogenic effect of the whole protein. Toxicon. 2017;126:38–46. doi: 10.1016/j.toxicon.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 171.Craik C.S., Page M.J., Madison E.L. Proteases as therapeutics. Biochem. J. 2011;435:1–16. doi: 10.1042/BJ20100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cao Z.J., Di Z.Y., Wu Y.L., Li W.X. Overview of Scorpion Species from China and Their Toxins. Toxins. 2014;6:796–815. doi: 10.3390/toxins6030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duffy M.J., McGowan P.M., Gallagher W.M. Cancer invasion and metastasis: Changing views. J. Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 174.Hakim M.A., Yang S. Discoveries of Serine Protease Inhibitors from Scorpions. J. Proteom. Bioinform. 2016;9:101–106. doi: 10.4172/jpb.1000395. [DOI] [Google Scholar]
- 175.Laskowski M., Jr., Kato I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- 176.Ranasinghe S., McManus D.P. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev. Comp. Immunol. 2013;39:219–227. doi: 10.1016/j.dci.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 177.Wan H., Lee K.S., Kim B.Y., Zou F.M., Yoon H.J., Je Y.H., Li J., Jin B.R. A Spider-derived Kunitz-Type Serine Protease Inhibitor that acts as a Plasmin Inhibitor and an Elastase Inhibitor. PLoS ONE. 2013;8:e53343. doi: 10.1371/journal.pone.0053343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gronenborn A.M., Nilges M., Peanasky R.J., Clore G.M. Sequential resonance assignment and secondary structure determination of the ascaris trypsin inhibitor, a member of a novel class of proteinase inhibitors. Biochemistry. 1990;29:183–189. doi: 10.1021/bi00453a025. [DOI] [PubMed] [Google Scholar]
- 179.Chen Z.Y., Hu Y.T., Yang W.S., He Y.W., Feng J., Wang B., Zhao R.M., Ding J.P., Cao Z.J., Li W.X., et al. Hg1, novel peptide inhibitor specific for kv1.3 channels from first scorpion kunitz-type potassium channel toxin family. J. Biol. Chem. 2012;287:13813–13821. doi: 10.1074/jbc.M112.343996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Van Gent D., Sharp P., Morgan K., Kalsheker N. Serpins: Structure, function and molecular evolution. Int. J. Biochem. Cell Biol. 2003;35:1536–1547. doi: 10.1016/S1357-2725(03)00134-1. [DOI] [PubMed] [Google Scholar]
- 181.Mourão C.B., Schwartz E.F. Protease inhibitors from marine venomous animals and their counterparts in terrestrial venomous animals. Mar. Drugs. 2013;11:2069–2112. doi: 10.3390/md11062069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Z., Wang B., Hu J., Yang W., Cao Z., Zhuo R., Li W., Wu Y. SjAPI, the first functionally characterized Ascaris-type protease inhibitor from animal venoms. PLoS ONE. 2013;8:e57529. doi: 10.1371/journal.pone.0057529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Celis A., Gaxiola-Robles R., Sevilla-Godinez E., de Orozco Valerio M.J., Armas J. Tendencia de la mortalidad por picaduras de alacran en Mexico, 1979–2003. Rev. Panam. Salud Publ. 2007;6:373–380. doi: 10.1590/S1020-49892007000500005. [DOI] [PubMed] [Google Scholar]
- 184.Mion G., Larreche S., Goyffon M. Aspects Cliniques et Thérapeutiques des Envenimations Graves. Volume 1. Urgence Pratique; Ganges, France: 2010. p. 255. (In French) [Google Scholar]
- 185.Chowell G., Hyman J.M., Diaz-Duenas P., Hengartner N.W. Predicting scorpion sting incidence in an endemic region using climatological variables. Int. J. Environ. Health Res. 2005;15:425–435. doi: 10.1080/09603120500392475. [DOI] [PubMed] [Google Scholar]
- 186.Mirdehghan M.M., Motlagh M.I. Scorpion stings survey (including: Residence, sex and age) and treatment strategy in Abuzar hospital- Ahvaz, Khuzestan during 1994–1999, Iran. J. Trop. Med. Hyg. 2001;14:62–64. (In Persian) [Google Scholar]
- 187.Ward M.J., Ellsworth S.A., Nystrom G.S. A global accounting of medically significant scorpions: Epidemiology, major toxins, and comparative resources in harmless counterparts. Toxicon. 2018;151:137–155. doi: 10.1016/j.toxicon.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 188.Dehghani R., Fathi B. Scorpion sting in Iran: A review. Toxicon. 2012;60:919–933. doi: 10.1016/j.toxicon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 189.Jalali A., Rahim F. Epidemiological review of scorpion envenomation in Iran. Iran. J. Pharm. Res. 2014;13:743–756. [PMC free article] [PubMed] [Google Scholar]
- 190.Shahbazzadeh D., Amirkhani A., Dinparast Djadid N., Bigdeli S., Akbari A., Ahari H., Amini H., Dehghani R. Epidemiological and clinical survey of scorpionism in Khuzestan province, Iran (2003) Toxicon. 2009;53:454–459. doi: 10.1016/j.toxicon.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 191.Baradaran M., Jalali A., Naderi-Soorki M., Jokar M., Galehdari H. First Transcriptome Analysis of Iranian Scorpion, Mesobuthus Eupeus Venom Gland. Iran. J. Pharm. Res. 2018;17:1488–1502. [PMC free article] [PubMed] [Google Scholar]
- 192.Lourenço W.R. A new species of Apistobuthus Finnegan, 1932 (Scorpiones, Buthidae) from Iran. Ent. Mitt. Zool. Mus. Hamburg. 1998;12:237–244. [Google Scholar]
- 193.Navidpour S., Lowe G. Revised diagnosis and redescription of Apistobuthus susanae (Scorpiones, Buthidae) J. Arachnol. 2009;37:45–59. doi: 10.1636/H08-44.1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.