Abstract
The fall armyworm (FAW), Spodoptera frugiperda, threatens maize production in Africa. A survey was conducted to determine the distribution of FAW and its natural enemies and damage severity in Ethiopia, Kenya and Tanzania in 2017 and 2018. A total of 287 smallholder maize farms (holding smaller than 2 hectares of land) were randomly selected and surveyed. FAW is widely distributed in the three countries and the percent of infested maize fields ranged from 33% to 100% in Ethiopia, 93% to 100% in Tanzania and 100% in Kenya in 2017, whereas they ranged from 80% to 100% and 82.2% to 100% in Ethiopia and Kenya, respectively, in 2018. The percent of FAW infestation of plants in the surveyed fields ranged from 5% to 100%. In 2017, the leaf damage score of the average of the fields ranged from 1.8 to 7 (9 = highest level of damage), while 2018, it ranged from 1.9 to 6.8. In 2017, five different species of parasitoids were recovered from FAW eggs and larvae. Cotesia icipe (Hymenoptera: Braconidae) was the main parasitoid recorded in Ethiopia, with a percent parasitism rate of 37.6%. Chelonus curvimaculatus Cameron (Hymenoptera: Braconidae) was the only egg-larval parasitoid recorded in Kenya and had a 4.8% parasitism rate. In 2018, six species of egg and larval parasitoids were recovered with C. icipe being the dominant larval parasitoid, with percentage parasitism ranging from 16% to 42% in the three surveyed countries. In Kenya, Telenomus remus (Hymenoptera: Scelionidae) was the dominant egg parasitoid, causing up to 69.3% egg parasitism as compared to only 4% by C. curvimaculatus. Although FAW has rapidly spread throughout these three countries, we were encouraged to see a reasonable level of biological control in place. Augmentative biological control can be implemented to suppress FAW in East Africa.
Keywords: fall armyworm, Telenomus remus, Cotesia icipe, local parasitoid, maize
1. Introduction
Maize (Zea mays L.) is the most important staple food crop in Africa [1] and is predominantly grown by smallholder farmers. However, the production of this crop and consequently the livelihood of the growers is threatened by the invasion and widespread infestation of the fall army worm (FAW), Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) which has led to substantial maize yield losses [2,3]. FAW was first reported in late 2016 in West Africa and it rapidly spread to different parts of the continent. Currently, its occurrence has been officially reported in 44 African countries [4,5]. In the Americas, there are two races of FAW, namely the rice strain (R-strain), which is most consistently found in millet and grass species associated with pasture habitats, whereas the corn strain (C-strain) prefers maize and sorghum. The two strains of FAW have also been reported in Africa [2,6,7,8]. FAW causes devastating damage to almost 100 plant species, including maize, sorghum, rice, soybean, cotton, wheat and sugarcane. On the other hand, the recent review by Montezano et al. [9] documented a total of 353 FAW larval host plant species belonging to 76 plant families, with the greatest number of host taxa in the family Poaceae (106 taxa), followed by Asteraceae and Fabaceae (31 taxa each). Due to its ability to rapidly spread and inflict widespread damage across multiple crops, FAW poses a serious threat to the food and nutrition security and livelihoods of millions of farming households in sub-Saharan Africa (SSA) [2,3,4,5].
In maize, FAW attacks all crop stages from seedling emergence through to ear development. They defoliate and can kill young plants, whorl damage can result in yield losses, and ear feeding can result in grain quality and yield reductions [10]. Detecting FAW infestations before it causes economic damage is the key to its management. If infestations are detected too late, the impacts of damage maybe irreversible [4,10]. Recent estimates by CABI in 12 maize-producing countries showed that without control, FAW can cause maize yield losses ranging from 4.1 to 17.7 million tonnes per year, which is equivalent to an estimated loss between US$ 1088 and US$ 4661 million annually [4]. Recently, Baudron et al. [11] reported yield loss of 11.57% due to FAW damage in smallholder maize fields in Zimbabwe, which is relatively lower than the perceived losses reported by smallholder farmers in different countries such as in Ghana and Zambia [2,4].
The common management strategy for the FAW in the Americas has been the use of insecticide sprays and genetically modified crops (Bt maize) [2]. Soon after the occurrence of FAW infestation, a massive spraying programme of chemical insecticides was deployed by governments of African countries [2,5]. However, most smallholder farmers in Africa cannot afford repeated sprays of insecticides and Bt maize is not available in Africa. Furthermore, excessive use of chemical insecticides removes potential natural enemies, negatively impacts human and livestock health, leads to resistance development in target pests and increases crop production costs [2,5,12]. In general, the excessive usage of insecticides and associated risks has raised food safety and sustainability concerns. This highlights the need for development of integrated pest management (IPM) strategies that suit the needs of the African smallholder farmers. Furthermore, FAW being arecent invader in the continent, information on natural enemies associated with this pest is not well-documented for Africa.
A wide range of natural enemies, including parasitoids, arthropod predators and entomopathogens attack FAW in its native region. For example, Molina-Ochoa et al. [13] listed about 150 species of parasitoids of FAW in the Americas and Caribbean. Some species of egg and larval parasitoids have been reported in East and West Africa [4]. For the development of IPM programs for FAW, it is imperative to determine its current distribution and the magnitude of damage it causes in maize-growing areas, and to develop an inventory of indigenous natural enemies that have made new associations with the pest. Therefore, the objectives of the present study were to assess the level of maize damage caused by FAW in Ethiopia, Kenya and Tanzania, and to determine the association of indigenous natural enemies with FAW.
2. Materials and Methods
2.1. Study Area Description
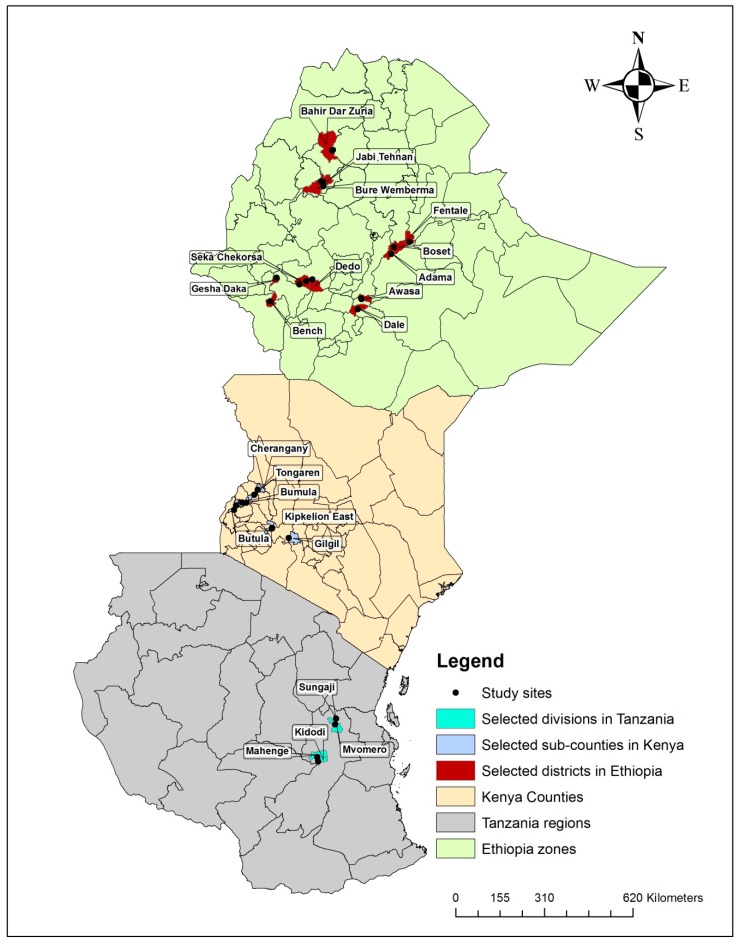
Surveys of FAW were conducted in major maize-growing districts of Ethiopia, Kenya and Tanzania (Figure 1 and Table 1).
Figure 1.
Map showing study districts in Ethiopia, Kenya and Tanzania.
Table 1.
Survey districts in Ethiopia, Kenya and Tanzania.
| Country | District | GPS Record |
|---|---|---|
| Ethiopia | Shebe Senbo | 7.464N, 36.4219E |
| Dedo | 7.613533333N, 36.83481667E | |
| Seka Chekorsa | 7.56465N, 36.64643333E | |
| Debub Bench | 6.925316667N, 35.50806667E–6.926083333N, 35.51278333E | |
| Semen Bench | 7.673166667N, 35.7101E | |
| Lock Abaya | 6.6757N, 38.26178333E | |
| Hawassa | 7.000421667N, 38.38775E–7.019233333N, 38.37613333E | |
| Bahir-Dar | 11.6815N, 37.4575E–11.69873333N, 37.48608333E | |
| Jabitenan | 10.56158333N, 37.1779E–10.69175N, 37.17113333E | |
| Bure | 10.70236667N, 37.10995E–10.70781667N, 37.11526667E | |
| Adama | 8.414033333N, 39.32258333E–8.420366667N, 39.32061667E | |
| Metehara | 8.637966667N, 39.41063333E–8.8016N, 39.89383333E | |
| Kenya | Webuye East | 0.5877N, 34.75556667E |
| Tongaren | 0.84182N, 35.00448333E | |
| Mt. Elgon | 0.50456N, 34.4345E | |
| Kabuchai | 0.36023N, 34.37336667E | |
| Kipkelion East | −0.206816667N, 35.56015E | |
| Gilgil | −0.521383333N, 36.09068333E | |
| Tanzania | Kilombero | −7.42583N, 36.98886667E– −7.557N, 37.01718333E |
| Morogoro | −6.2149N, 37.57918333E– −6.39344N, 37.5582E |
2.2. Damage Assessment
In the three countries surveyed, districts and farms were purposely selected based on reported occurrence of FAW. A total of 287 randomly selected maize farms were surveyed, with 188 farms in Ethiopia, 81 farms in Kenya and 18 farms in Tanzania. Surveys were conducted from March to October 2017 and June to August 2018 in Ethiopia, from April to August 2017 and June to August 2018 in Kenya and from July to November 2017 in Tanzania. In all districts, the surveys covered the growing period of maize one month after planting to harvest. In each surveyed farm, three quadrants measuring 3 m × 3 m were randomly selected and total number of plants and damaged plants were counted. Percent infested fields were calculated as follows:
| %FAW infested fields = (Number of FAW infested fields)/(Total number of fields surveyed) × 100 |
Percent infested plants per quadrant was calculated using the formula:
| %FAW infestation = (Number of FAW infested plants)/(Total number of plants observed) × 100 |
Leaf damage was scored by visual observation using the scoring scale of 0–9 reported by Davis and Williams [13] (Table 2). Leaf damage scores of individual plants in quadrants of each surveyed farm were averaged to determine the leaf damage ratings of a district.
Table 2.
Visual rating scales for leaf damage assessment [14].
| Scale | Description |
|---|---|
| 0 | No visible leaf damage |
| 1 | Only pinhole damage on leaves |
| 2 | Pinhole and shot hole damage to leaf |
| 3 | Small elongated lesions (5–10 mm) on 1–3 leaves |
| 4 | Midsized lesions (10–30 mm) on 4–7 leaves |
| 5 | Large elongated lesions (>30 mm) or small portions eaten on 3–5 leaves |
| 6 | Elongated lesions (>30 mm) and large portions eaten on 3–5 leaves |
| 7 | Elongated lesions (>30 cm) and 50% of leaf eaten |
| 8 | Elongated lesions (30 cm) and large portions eaten on 70% of leaves |
| 9 | Most leaves with long lesions and complete defoliation observed |
2.3. Assessment of Natural Enemies of FAW
Surveys of natural enemies were conducted in Ethiopia from March to October in 2017, and in Kenya and Tanzania from July 2017 to November 2017. In Ethiopia and Kenya, surveys of FAW natural enemies were also conducted from June to August 2018. A total of 101 farms in Ethiopia, 21 farms in Kenya and 13 in Tanzania were sampled. Location details such as latitude, altitude and longitude were taken using GPS. In each surveyed farm, three quadrants measuring 3 m × 3 m were randomly selected. The number of egg masses and larvae were counted on the damaged maize plants. The egg masses were placed in plastic cups with about 5 g of natural diet (fresh maize leaf). Upon hatching, the larvae were placed in rectangular plastic cages (4 cm height × 15 cm width × 21 cm length), covered on top with a fine screen to prevent the escape of parasitoids. The larvae were fed with around 60 g of maize shoot, replaced every 48 h, until pupation.The eggs and larvae were kept in the laboratory at room temperature of 24–26 °C, 50–70% RH and a photoperiod of 12:12 (L:D) hour until parasitoids emerged. The parasitoids that emerged from the eggs or larvae were recorded every 24 h until pupation. No dissections of dead eggs or larvae were made to examine for dead parasitoids [13,15]. Parasitoids were preserved in 70% ethanol and sent for identification to the Natural History Museum, UK. We did not find any occurrences of multiple parasitism in this study. Percent parasitism was calculated according to Pair et al. [16].
| % Parasitism = (Number of parasitoids)/(Number of larvae collected) × 100 |
2.4. Data Analysis
Percent fall armyworm-infested fields, percent fall armyworm infestation, leaf damage score (scale 1–9) and percent parasitism of natural enemies were summarised and descriptive statistics (means and percentages) were calculated. All statistical analysis was done using MINITAB 16 statistical software.
3. Results
3.1. Distribution and Damage by FAW
FAW is widely distributed across maize-growing districts of Ethiopia, Kenya and Tanzania (Table 3 and Table 4) and was present in most fields surveyed. In 2017, the percentage of infested fields ranged from 33% to 100% in Ethiopia, 93% to 100% in Tanzania and was 100% for the farms observed in Kenya (Table 3). In Ethiopia, the highest infestation was found in Shebe Senbo (62.3%), whilst the lowest infestation was recorded in Bahir Dar (5.7%). In Kenya, relatively high percentages of FAW infestation were observed, ranging from 77% in Mt Elgon to 100% in Webuye East and Tongaren in 2017. In Tanzania, Morogoro and Kilombero showed 72.7% and 95.7% infestation, respectively. Leaf damage score ranged from 1.8 to 7 in Ethiopia, 3.2 to 5.3 in Kenya and 3.7 to 5.2 in Tanzania in 2017 (Table 3).
Table 3.
Mean percent of fields infested by fall armyworm (FAW) and level of infestation in different survey districts of Ethiopia, Kenya and Tanzania in 2017.
| Country | District | % Infested Fields | % FAW Infestation | Leaf Damage Ratings (Scale 1–9) |
Number of Fields |
|---|---|---|---|---|---|
| Ethiopia | Dedo | 81 | 33.8 ± 5.8 | 3.5 ± 0.524 | 27 |
| Seka Chekorsa | 69 | 15.8 ± 5.66 | 2.1 ± 0.515 | 16 | |
| Shebe Senbo | 100 | 62.3 ± 6.88 | 5.7 ± 0.518 | 13 | |
| Debub bench | 33 | 44.4 ± 23.4 | 4.3 ± 2.33 | 3 | |
| Semen bench | 100 | 78.7± 0.0 | 7.0 ± 0 | 1 | |
| Lock Abaya | 100 | 30.0 ± 11.9 | 4.0 ± 1.15 | 3 | |
| Hawassa | 100 | 43.7 ± 10.7 | 4.8 ± 0.701 | 8 | |
| Bahir Dar | 100 | 5.3 ±1.09 | 1.8 ± 0.200 | 6 | |
| Jabitenan | 100 | 13.9 ± 6.49 | 2.5 ± 0.50 | 4 | |
| Bure | 100 | 6.7 ± 0.98 | 2.0 ± 0.00 | 2 | |
| Adama | 100 | 60.2 ± 7.03 | 5.0 ± 0.408 | 4 | |
| Kenya | Webuye East | 100 | 100.0 ± 9.51 | 3.5 ± 0.130 | 20 |
| Tongaren | 100 | 100.0 ± 0.0 | 3.2 ± 0.519 | 4 | |
| Kabuchai | 100 | 91.0 ± 1.83 | 3.9 ±0.246 | 16 | |
| Mt Elgon | 100 | 77.0 ± 3.04 | 3.8 ±0.300 | 9 | |
| Kipkelion East | 100 | 86.0 ± 2.09 | 4.6 ±0.399 | 8 | |
| Gilgil | 100 | 91.0 ± 3.66 | 5.3 ± 0.894 | 3 | |
| Tanzania | Morogoro | 93 | 72.7 ± 4.71 | 3.7 ± 0.384 | 10 |
| Kilombero | 100 | 95.7± 95.1 | 5.2 ± 0.648 | 8 |
Table 4.
Mean percent of fields infested by FAW and level of infestation in different survey districts of Ethiopia and Kenya in 2018.
| Country | District | % Infested Fields | % FAW Infestation | Leaf Damage Ratings (Scale 1–9) |
Number of Fields |
|---|---|---|---|---|---|
| Ethiopia | Dedo | 86.7 | 28.6 ± 6.55 | 3.7 ± 0.25 | 12 |
| SekaChekorsa | 93.3 | 20.2 ± 6.95 | 3.5 ± 0.50 | 15 | |
| ShebeSenbo | 80.0 | 9.9 ± 1.64 | 2.5 ± 0.56 | 15 | |
| Lock Abaya | 90.0 | 4.9 ±1.32 | 1.9 ± 0.15 | 10 | |
| Hawassa | 92.0 | 26.5 ± 5.98 | 4.1 ± 0.70 | 26 | |
| Adama | 100.0 | 11.0 ± 2.17 | 2.7 ± 0.56 | 7 | |
| Metehara | 100.0 | 49.3 ± 10.96 | 5.4 ± 0.40 | 16 | |
| Kenya | Tongaren | 100.0 | 74.0 ± 2.67 | 6.4 ± 0.125 | 3 |
| Kabuchai | 100.0 | 69.3 ± 3.25 | 5.2 ± 0.15 | 2 | |
| Webuye | 100.0 | 71.0 ± 2.5 | 6.5 ± 0.25 | 4 | |
| Elgon | 100.0 | 66.2 ± 2.57 | 5.0 ± 0.3 | 3 | |
| Kepkelion Masheni |
94.0 82.2 |
50.4 ± 1.9 96.2 ± 3.78 |
4.8 ± 0.05 6.8 ± 0.17 |
3 6 |
In 2018, the percentage of FAW-infested fields ranged from 80% to 100% in Ethiopia and Kenya. In Ethiopia, the highest percentage infestation was recorded in Metehara (49.3%), while the lowest was in Lock Abaya (4.9%). In Kenya, the highest percentage infestation was recorded in Masheni (96.2%), while the lowest was in Kepkelion (50.4%). In Ethiopia, maize leaf damage score ranged from 1.9 to 5.4, whereas in Kenya, it ranged from 5.0 to 6.8 (Table 4).
3.2. Recruitment of Local Parasitoids by FAW
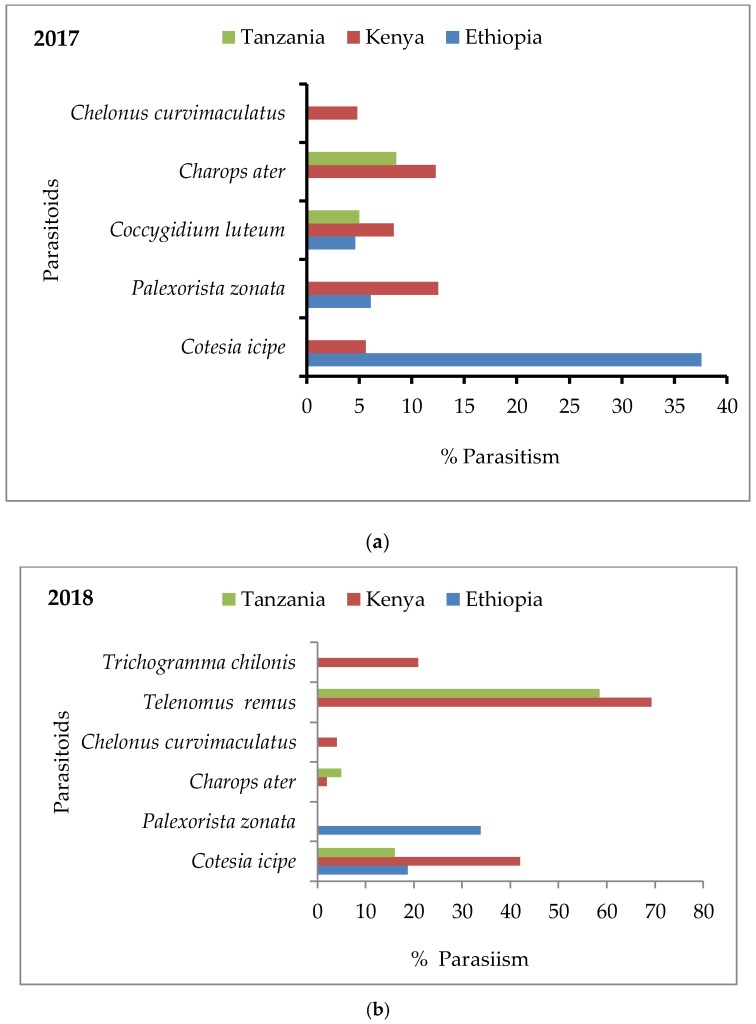
In 2017, in Ethiopia, Kenya and Tanzania, five different species of parasitoids belonging to Hymenoptera and Diptera were recovered from FAW eggs and larvae (Figure 2). In Ethiopia, Cotesia icipe Fernández-Triana & Fiaboe (Hymenoptera: Braconidae) was the main larval parasitoid, with 37.6% parasitism, followed by Palexorista zonata (Curran) (Diptera: Tachinidae) and Coccygidium luteum (Brullé) (Hymenoptera: Braconidae), with 6.1% and 4.6% parasitism, respectively. In Kenya, one species of egg-larval parasitoid and four species of larval parasitoids were recorded. Palexorista zonata was the dominant parasitoid, with 12.5% parasitism, followed by Charops ater Szépligeti (Hymenoptera: Ichneumonidae) and C. luteum, with 12.3% and 8.3% parasitism, respectively. In addition, Chelonus curvimaculatus Cameron (Hymenoptera: Braconidae) was the only egg-larval parasitoid recorded in Kenya and had low levels of parasitism (4.8%). In Tanzania, only two species of larval parasitoids were recovered, namely C. ater and C. luteum, causing 8.5% and 5% parasitism, respectively.
Figure 2.
Percent occurrence of FAW parasitoids collected from Ethiopia, Kenya and Tanzania in (a) 2017 and (b) 2018.
In 2018, in Ethiopia, Kenya and Tanzania, six different species of parasitoids were found to be associated with eggs and larvae of FAW (Figure 2). In Ethiopia, two species of larval parasitoids were recovered, namely P. zonata and C. icipe, which accounted for 33.9% and 18.7% of parasitism, respectively. In Kenya, Telenomus remus (Hymenoptera: Scelionidae) was the dominant egg parasitoid and accounted for 69.3% of egg parasitism, followed by Trichogramma chilonis, which accounted for 20.9% of egg parasitism, whereas Chelonus curvimaculatus caused low levels of egg parasitism (4%). Cotesia icipe was the main larval parasitoid, which caused 42% parasitism, whilst C. ater caused low levels of parasitism (2%). In Tanzania, Telenomus remus was the dominant egg parasitoid, causing 58.5% parasitism. Cotesia icipe caused 16% parasitism, whereas C. ater caused low levels of parasitism (5%) (Figure 2).
4. Discussion
Fall armyworm has rapidly spread throughout most of the maize-producing areas of Ethiopia, Kenya and Tanzania, as suggested by the moderate to high levels of infestation of FAW in almost all maize fields surveyed. Following the first report of FAW in West Africa in January 2016, the pest was soon found in East Africa in 2017 [2,3]. Its modality of introduction, along with its ecological adaptation and spread across Africa, is still speculative. However, wind-assisted flight, hidden infestations in trade commodities and human-assisted transport have been implicated as likely mechanisms for facilitating the rapid spread of the pest [3]. Percent FAW infestation varied considerably among the farms we surveyed in the three countries, with mean percent FAW infestation ranging from 5.3% to 100%. Relatively high percentages of FAW infestation (>73%) were recorded in Kenya and Tanzania. In addition, most of the farms surveyed had low to moderate leaf damage scores. In Ethiopia, high mean percent FAW infestation was recorded in Semen Bench (78.7%), Shebe Senbo (62.2%) and Adama (60.2%) in 2017. High mean percent FAW mortality (49.25%) was recorded in Dedo in 2018. Although larvae feed on both vegetative and reproductive maize [2,10], feeding on leaf tissue only may not cause yield loss as the plant can tolerate such damage. Subsequent yield loss also depends on the growth stage of maize and level of infestation [2].
In this study, a total of five species of parasitoids were recorded from FAW eggs and larvae collected in Ethiopia, Kenya and Tanzania. Results of this study are consistent with recent findings of Sisay et al. [17], who reported that C. icipe was the dominant larval parasitoid, with parasitism ranging from 33.8% to 45.3% in Ethiopia. However, in the present study, percent of parasitism by C. icipe was lower in Ethiopia, but higher in Kenya as compared to the previous year’s results reported by Sisay et al. [17]. In Kenya, the Tachinid fly, P. zonata, was the primary parasitoid, with 12.5% parasitism. Charops ater and C. luteum were the most common parasitoids in Kenya and Tanzania, with parasitism ranging from 6% to 12% and 4% to 8.3%, respectively [17]. Furthermore, two additional egg parasitoids, namely Telenomus remus and Trichogramma chilonis, were recovered in 2018, with high levels of egg parasitism found in Telenomus remus. Those parasitoids found in the present study have been also recovered from FAW eggs and larvae in East and West Africa [18]. Recently, Kenis et al. [19] reported the presence of T. remus in different countries in Africa. Furthermore, some of the parasitoids recovered in this study have been reported as parasitising other insect species; for example, C. icipe, which is a new species from eastern Africa, was reared in Kenya as a solitary parasitoid from Spodoptera littoralis (Boisduval, 1833) and S. exigua (Hübner, 1808) [20]; further, Charops ater was reported to parasitise African bollworm and other species in Kenya [21]. In addition, P. zonata was recorded from the African armyworm, Spodoptera exempta, by Rose et al. [22] indicating the recruitment of native parasitoids to FAW in eastern Africa. Variations in parasitoid species composition and the level of parasitism may be due to differences in geographical locations, agronomic practices and crop type and stage [16,23]. In North and South America, which are native regions of FAW, various species of natural enemies attacking FAW have been documented. For example, Molina-Ochoa et al. [13] reported a total of 150 species of parasitoids of FAW. This may indicate that while emphasis should be given to local surveys of native natural enemies, introduction of effective natural enemies from the Americas through classical biocontrol can be an option when there is a gap [5,20].
FAW is the most difficult pest to control due to its multiple generations, ability to migrate and ability to feed on a wide range of host plants. To tackle the menace of the fall armyworm pest to avoid economic adversity for smallholder farmers in Africa, rapid and coordinated action, enormous awareness creation, technological innovation and national, regional and international collaborations are required. Biological control can play a significant role in an integrated management of FAW to provide sustainable solutions to effectively tackle the adverse effects of this pest. The current study, therefore, contributes to the management of the FAW in identifying effective natural enemies that could be used in the integrated management of FAW. The new associations of various species of natural enemies with FAW in Africa across countries and season in the current study shows the paramount importance of designing biological controls of FAW both through the conservation of native natural enemies and augmentative release. The present massive application and indiscriminate use of pesticides in Africa against FAW might have negative impact on the natural enemies; hence, it is vital to protect natural enemies from adverse effects of pesticide and design IPM strategies for FAW management in the region.
5. Conclusions
In conclusion, the present study confirms the rapid and substantial expansion of the FAW range in eastern Africa. Native species of parasitoids recovered from eggs and larvae of FAW are crucial for implementing biological control programmes of this pest, which are essential components in developing integrated pest management approaches for FAW in eastern Africa.
Acknowledgments
We gratefully acknowledge the financial support of the UK’s Department for International Development (DFID), the Swedish International Development Cooperation Agency (SIDA), the Swiss Agency for Development and Cooperation (SDC), the Kenyan Government and the Government of Ethiopia. The views expressed herein do not necessarily reflect the official opinion of the donors. We acknowledge Abdel-Rahman Elfatih and Kerubo Vivian for producing a map showing the study areas, and Peter Malusi and Nsami Elibariki for assisting during the survey in Kenya and Tanzania, respectively.
Author Contributions
Conceptualisation, B.S., T.T. and E.M.; methodology, B.S., J.S., T.T., E.M. and G.A.; formal analysis, E.M..; investigation, B.S., J.S., E.M. and T.T.; writing—original draft preparation, B.S., T.T., P.L., G.A. and E.M.; writing—review and editing, E.M., T.T., S.M. and S.S.; funding acquisition, T.T., B.S. and J.S. are graduate students supported by USAID Feed the Future IPM Innovation Lab.
Funding
This research was funded by the USAID Feed the Future IPM Innovation Lab, Virginia Tech, Cooperative Agreement No. AID-OAA-L-15-00001, implemented by ICIPE.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Food and Agricultural Organization of the United Nations (FAO) World Crop Production Data. [(accessed on 10 April 2019)];2017 Available online: http://www.fao.org/faostat/en/
- 2.Abrahams P., Bateman M., Beale T., Clottey V., Cock M., Colmenarez Y., Corniani N., Day R., Early R., Godwin J.L., et al. Fall armyworm: Impacts and Implications for Africa. CABI; Oxfordshire, UK: 2017. Evidence Note (2) [Google Scholar]
- 3.Goergen G., Lava K.P., Sankung S.B., Togola A., Tamò M. First Report of Outbreaks of the Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera, Noctuidae): A New Alien Invasive Pest in West and Central Africa. PLoS ONE. 2016;11:e0165632. doi: 10.1371/journal.pone.0165632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rwomushana I., Bateman M., Beale T., Beseh P., Cameron K., Chiluba M., Clottey V., Davis T., Day R., Early R., et al. Fall Armyworm: Impacts and Implications for Africa. CABI; Oxfordshire, UK: 2018. Evidence Note Update. [Google Scholar]
- 5.Prasanna B.M., Huesing J.E., Eddy R., Peschke V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management. 1st ed. CIMMYT; Edo Mex, Mexico: 2018. [Google Scholar]
- 6.Nagoshi R.N., Koffi D., Agboka K., Tounou K.A., Banerjee R., Jurat-Fuentes J.L., Meagher R.L. Comparative molecular analyses of invasive fall armyworm in Togo reveal strong similarities to populations from the eastern United States and the Greater Antilles. PLoS ONE. 2017;12:e0181982. doi: 10.1371/journal.pone.0181982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cock M.J.W., Beseh P.K., Buddie A.G., Cafá G., Crozier J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci. Rep. 2017;7:4013. doi: 10.1038/s41598-017-04238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gichuhi J., Subramanian S., Khamis F.M., van den Berg J., du Plessis H., Ekesi S., & Herren J. Diversity of fall armyworm, Spodoptera frugiperda and their gut bacterial community in Kenya. BioRxiv. 2019 doi: 10.1101/664987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montezano D.G., Specht A., Sosa-Gómez D.R., Roque-Specht V.F., Sousa-Silva J.C., Paula-Moraes S.V., Peterson J.A., Hunt T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018;26:286–300. doi: 10.4001/003.026.0286. [DOI] [Google Scholar]
- 10.Capinera J.L. Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae) [(accessed on 10 October 2017)];2017 Available online: http://edis.ifas.ufl.edu/in255.
- 11.Baudron F., Zaman-Allah M.A., Chaipa I., Chari N., Chinwada P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Prot. 2019;120:141–150. doi: 10.1016/j.cropro.2019.01.028. [DOI] [Google Scholar]
- 12.Yu S.J. Insecticide resistance in the fall armyworm, Spodoptera frugiperda (J.E. Smith) Pestic. Biochem. Physiol. 1991;39:84–91. doi: 10.1016/0048-3575(91)90216-9. [DOI] [Google Scholar]
- 13.Molina-Ochoa J., Carpenter J.E., Heinrichs E.A., Foster J.E. Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean basin: An inventory. Fla. Entomol. 2003;86:254–289. doi: 10.1653/0015-4040(2003)086[0254:PAPOSF]2.0.CO;2. [DOI] [Google Scholar]
- 14.Davis F.M., Williams W.P. Visual Rating Scales for Screening Whorl-Stage Corn for Resistance to Fall Armyworm. Mississippi Agricultural and Forestry Research Experiment Station; Mississippi State, MS, USA: 1992. [(accessed on 1 October 2017)]. Technical Bulletin 186. Available online: http://www.nal.usda.gov/ [Google Scholar]
- 15.Ruíz-Nájera R.E., Molina-O J., Carpenter J.E., Espinosa M.J.A., Ruíz N.J.A., Lezama G.R., Foster J.E. Survey for hymenopteran and dipteran parasitoids of the fall armyworm (Lepidoptera: Noctuidae) in Chiapas, México. J. Agric. Urban Entomol. 2007;24:35–42. doi: 10.3954/1523-5475-24.1.35. [DOI] [Google Scholar]
- 16.Pair S.D., Raulston J.R., Sparks A.N., Martin P.B. Fall armyworm (Lepidoptera: Noctuidae) parasitoids: Differential spring distribution and incidence on corn and sorghum in the Southeastern United States and Northeastern Mexico. Environ. Entomol. 1986;15:342–348. doi: 10.1093/ee/15.2.342. [DOI] [Google Scholar]
- 17.Sisay B., Simiyu J., Malusi P., Likhay P., Mendesil E., Elibariki N., Wakgari M., Ayalew G., Tefera T. First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J. Appl. Entomol. 2018;142:800–804. doi: 10.1111/jen.12534. [DOI] [Google Scholar]
- 18.Cruz I., Bruce A., Sevgan S., Akutse K., Mohamed F., Niassy S., Rangaswamy M., Sidhu J., Goergen G., Rwomushana I., et al. Biological Control and Biorational Pesticides for Fall Armyworm Management. In: Prasanna B.M., Huesing J.E., Eddy R., Peschke V.M., editors. Fall Armyworm in Africa: A Guide for Integrated Pest Management. 1st ed. CIMMYT; Edo Mex, Mexico: 2018. [Google Scholar]
- 19.Kenis M., du Plessis H., Van den Berg J., Ba M.N., Goergen G., Kwadjo K.E., Baoua I., Tefera T., Buddie A., Cafà G., et al. Telenomus remus, a candidate parasitoid for the biological control of Spodoptera frugiperda in Africa, is already present on the continent. Insects. 2019;10:92. doi: 10.3390/insects10040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiaboe K.K.M., Fernández-Triana J., Nyamu F.W., Agbodzavu K.M. Cotesiaicipe sp. n., a new Microgastrinae wasp (Hymenoptera, Braconidae) of importance in the biological control of Lepidopteran pests in Africa. J. Hymenopt. Res. 2017;61:49–64. [Google Scholar]
- 21.Van den Berg H., Cock M.J.W. African Bollworm and Its Natural Enemies in Kenya. 2nd ed. CABI Bioscience; Wallingford, UK: 2000. [Google Scholar]
- 22.Rose D.J.W., Dewhurst C.F., Page W.W. The African armyworm Handbook: The Status, Biology, Ecology, Epidemiology and Management of Spodoptera exempta (Lepidoptera: Noctuidae) 2nd ed. Natural Resources Institute; Chatham, UK: 2000. [Google Scholar]
- 23.Hall D.G., Meagher R., Nagoshi R., Irey M. Monitoring populations of adult fall armyworm, Spodoptera fruigiperda Smith (Lepidoptera: Noctuidae), in Florida sugarcane using pheromone traps, with special reference to genetic strains of the pest. Proc. ISSCT. 2005;25:784–787. [Google Scholar]