Abstract
Glioblastoma is a highly aggressive and treatment resistant primary brain tumor. Features of glioblastoma include peritumoral cerebral edema, the major contributor to neurological impairment. Although the current clinical approach to edema management is administration of the synthetic corticoid dexamethasone, increasing evidence indicates numerous adverse effects of dexamethasone on glioblastoma burden at the molecular, cellular and clinical level. The contradictions of dexamethasone for glioblastoma and brain metastasis therapy are discussed in this article. Finally, alternative strategies for cerebrovascular edema therapy with vascular stabilizing, anti-permeability agents that are either approved or in clinical trials for diabetic retinopathy and macula edema, are addressed.
Keywords: Glioblastoma, blood-brain barrier, cerebral edema, dexamethasone, anti-vascular endothelial growth factor, Angiopoietin/Tie2, vascular endothelial protein tyrosine phosphatase
Introduction
Patients with central nervous system (CNS) malignancies such as glioblastoma (GBM) and brain metastasis usually present with peritumoral vasogenic edema, the pivotal contributor to morbidity and mortality in neuro-oncology. Peritumoral edema is caused by breakdown of the blood-brain barrier (BBB) with subsequent leakage of plasma components into the cerebral extracellular space and consequent elevation of intracranial pressure (ICP).1–4 The increase in ICP leads to functional impairment of the BBB associated with neurological deficits and poor penetration of chemotherapeutics such as temozolomide (TMZ), which at least in part contribute to poor clinical outcome in GBM.5 To date, the neurosurgical and neuro-oncological drug of choice in peritumoral edema management is the synthetic corticoid dexamethasone (DEX), an agent with a pleiotropic action profile and 30-fold greater glucocorticoid effect compared to the endogenous hormone cortisol. Although clinically effective in vasogenic edema management, DEX administration is associated with numerous systemic side effects including insomnia, psychiatric alteration, tremor, hyperglycemia, muscle atrophy, cushingoid appearance, hypertension, gastrointestinal perforation and notably, immunosuppression.6 Despite decades of treatment, the mechanism by which DEX reduces peritumoral edema remains poorly understood. The current hypotheses include the DEX-mediated improvement of BBB function via upregulation of tight-junction proteins and inhibition of inflammatory pathways, resulting in reduced vessel permeability.7–10 This brief opinion article highlights the state of knowledge regarding the impact of DEX on GBM burden and discusses alternative therapeutic approaches targeting the vascular endothelial growth factor (VEGF) and Tie2 pathways, shown to reduce edema by improving BBB function.3,11,12 We emphasize the utilization (“repurposing”) of drugs that are approved or in clinical trials for edema management in retinal vascular diseases that show defects in the blood-retina barrier.13,14
BBB dysfunction in GBM
One of the major hallmarks of GBM is the dysfunction of the BBB, a critical barrier that maintains CNS homeostasis. The BBB plays a major role as a transport and metabolic barrier which is constituted by the neurovascular unit (NVU) with microvascular endothelial cells (ECs) as the main cellular component whose function is supported by other cell types such as pericytes, astrocytes, neurons and microglia.15–18 However, the highly specific and regulated nature of the NVU allows for transport of essential nutrients across the BBB. Although the BBB protects the CNS, it presents a challenge for transport of therapeutics in neurological diseases targeting parenchymal cells.19 In GBM and brain metastasis, NVU dysfunction and BBB breakdown is manifested by edema formation that results in increased ICP limiting local perfusion and a transport barrier leading to hypoxia and ensuing pathologic tumor angiogenesis.1,3,4 The disrupted barrier also poses serious challenges for drug delivery due to increased ICP and disorganized and poorly perfused angiogenic vasculature.19 Several studies demonstrated the negative effects of peritumoral edema in GBM progression. Patients with minor edema on preoperative cranial MRI scans were shown to exhibit longer survival compared to major peritumoral edema.5 Several mechanisms were hypothesized for this finding, for example edematous brain-tumor tissue enabling enhanced GBM cell invasion and preservation. GBM stem-cells were also suspected to persist in this edematous area, escaping cytotoxic treatment and promoting tumor progression. Based on these findings, reduction of edema by restoring NVU function represents an important approach to GBM therapy. Dexamethasone, the synthetic, potent version of endogenous cortisol is widely used in this regard; however, the mechanisms by which DEX controls brain edema at the level of endothelial and tumor cells is still unclear. Furthermore, there are numerous side effects of DEX that demand for novel therapeutics to restore NVU function and BBB integrity in GBM.6
Effects of dexamethasone in GBM therapy
Systemic adverse effects of DEX treatment for peritumoral edema in GBM and brain metastasis have been reported in up to 50% of the patients including hyperglycemia, cushingoid appearance and psychiatric symptoms among the top three.6,20 A retrospective multivariate analysis of 73 GBM patients revealed that concurrent DEX administration to radio-chemotherapy was a significant risk factor for poor overall survival (OS) (12.7 vs. 22.6 months, p = 0.004) and progression free survival (PFS) (3.6 vs. 8.4 months, p<0.0001).21 These observations were supported by a retrospective multivariate analysis of more than 2000 GBM patients, providing evidence that DEX administration was an independent negative prognostic factor when adjusting for extent of resection, initial treatment, age and Karnofsky Performance Score (OS 12 vs. 17 months, p = 0.001).22 Wong et al. studied 119 patients with recurrent GBM receiving either tumor-treating alternating electric fields (TTFields) or chemotherapy. In both groups DEX administration of >4.1 mg per day was associated with reduced OS (4.8 vs. 11.0 months, p = 0.0001 in the TTFields cohort and 6.0 vs. 8.9 months in the chemotherapy group, p = 0.0009).23 A single center retrospective analysis showed that patients with dexamethasone-induced leukocytosis (DIL) had reduced OS and PFS (15 vs. 32 months for OS and 7 vs. 15 months for PFS, p = 0.009).24 Furthermore, reduced CD3 lymphocyte and CD15 granulocyte infiltration in the tumor parenchyma of DIL positive patients (p<0.05) was demonstrated. This suggests that DEX might negatively interfere with immunotherapy as also demonstrated by Giles et al. (see below).25 Beneficial effects of DEX-sparing has been assessed in combination with corticorelin acetate (CrA),28 a synthetic formulation identical to the endogenous corticotropin-releasing factor/hormone which led to reduced vascular permeability.26 The study demonstrated a significant reduction in DEX dose in patients that correlated with better neurological scores.27 DEX is also administered in patients with brain metastasis that display severe vasogenic edema and ICP (reviewed in Ryken et al.4), although the benefit has not been clearly demonstrated. In breast cancer patients, it has been reported that the incidence of metastasis increases with administration of DEX mediated by activation of glucocorticoid receptor leading to increased colonization in distant sites.28 Dexamethasone is also used as an adjuvant for edema management in bacterial meningitis; however, its benefit for patient survival or neurological performance has not been unequivocally demonstrated.29–32 Overall, the negative influence of DEX on the therapeutic outcome warrant alternative treatment options for edema management.
Effects of dexamethasone in preclinical studies
Although unfavorable clinical effects of DEX in GBM patients are documented, preclinical studies revealed conflicting and in part opposing results. Beneficial results were observed in a rat permeability model where DEX led to reduced permeability of the brain ECs to tumor-derived VEGF and to reduced VEGF secretion by tumor cells.33,34 In addition, DEX was shown to decrease MMP-2 (matrix metalloprotease) secretion and cell invasiveness via MKP-1 (mitogen activated protein kinase phosphatase) induction in human glioma cells.35 DEX also increased cell–cell adhesion and reorganized actin into stress fibers – resulting in reduced cell motility and invasion, which was also observed in human GBM neurospheres.36,37 Similar results were obtained in brain ECs where hydrocortisone treatment increased actin reorganization and reduced permeability.38 Furthermore, DEX inhibited glioma cell proliferation in a dosage-dependent manner and even induced cell death.39 On the counter side, an unfavorable interplay of DEX and alkylating agents was described. In primary GBM cell lines treated with TMZ, the addition of DEX counteracted TMZ-induced apoptosis and caused resistance to nutrient deprivation.40–42 Furthermore, in vitro DEX administration reduced the bystander effect of the thymidine kinase/ganciclovir system in suicide-gene therapy and reduced the antitumor effect of interleukin-4.43,44 The alkylating impact of TMZ targets the O6-alkylguanin in the carcinogenic lesion, which is reversed by the O6-methylguanine-DNA-methyltransferase (MGMT) repair enzyme. The human MGMT promotor harbors two glucocorticoid response elements and recent findings report the DEX-mediated induction of MGMT promotor as the most important factor of chemo-resistance.45 Furthermore, mesenchymal glioma stem cells showed a significant upregulation of DEX-regulated gene network resulting in increased invasion, proliferation and angiogenesis in human-derived orthotropic tumors.46 In the context of prevailing immunotherapies that are currently under way in GBM, further studies are needed to understand immune modulatory effects of DEX that include T lymphocyte depletion and suppression of co-stimulatory pathways.25,47
Anti-VEGF therapy for vasogenic peritumoral edema in GBM
Since the introduction of bevacizumab (BEV), a recombinant humanized monoclonal antibody against VEGF-A, for anti-angiogenic therapy in GBM, several clinical studies outlined an improved progression-free survival. However, the addition of BEV to standard radio-chemotherapy failed to show benefit in GBM OS.48,49 The chronic vascular hyperpermeability in GBM reflects the growth of structurally abnormal and immature vessels that have defective cell junctions and are deficient in pericytes.50 The improper composition and functioning of the NVU in addition to high VEGF levels contribute to vascular permeability, which may lead to life-threatening edema.1–3 In fact, the permeability-inducing effects of VEGF were described before the effects on EC proliferation.51–53 Increased brain VEGF levels induced a 10-fold breakdown of the BBB.54 In astrocyte-specific VEGF-A conditional knock-out mice, vascular leakage was prevented and brain edema reduced in a model of CNS inflammation.55 Similarly, mechanistically blocking VEGF from reaching its capillary targets or blocking VEGFR2 function reduced capillary leakage and vasogenic edema.56,57
Almost two decades ago a correlation between the VEGF mRNA expression and the tissue plasma vascular volume and capillary permeability was described in human gliomas in vivo.58 These findings supported the hypothesis that VEGF may play a pivotal role in peritumoral edema formation in addition to tumor angiogenesis in human gliomas. From those studies, it was concluded that inhibition of VEGF might be a useful approach to prevent edema formation in addition to tumor vascularization in vivo.58,59 Surprisingly, a clinical trial comparing DEX vs. VEGF inhibitors for edema treatment is still lacking, although several reports described a beneficial steroid-sparing effect in BEV-treated patients.6,60,61 In a murine GBM model, Pitter et al. reported benefit of anti-VEGF therapy compared to DEX and relate this finding to DEX-induced deceleration of cell cycle progression, via the induction of the cell cycle inhibitor p21, and thus elevated radio-resistance.22 However, several adverse effects of anti-VEGF therapy limit its utility in perifocal edema therapy for GBM such as thromboembolic events, hemorrhage, surgical wound dehiscence, gastrointestinal perforation, hypertension and diarrhea.62,63 Besides these, the development of resistance mechanisms has been a major obstacle in anti-VEGF therapy.64 VEGF/VEGFR inhibition failed to achieve durable responses in solid cancers due to acquired or tumor intrinsic resistance mechanisms which are not yet completely understood.65 Although first-line BEV-treatment in GBM patients failed to show a survival benefit, it improved PFS and decreased the usage of steroids.48,49,64 Currently, BEV is commonly applied for edema management in recurrent GBM.64 Resistance to anti-VEGF therapy in GBM includes the upregulation of additional growth factors such as Ang-2 as demonstrated in Scholz et al.66 Furthermore, resistance is confined to innate immune cells which secrete a plethora of growth factors and cytokines that drive angiogenesis in GBM65,67 and negatively affect pre-clinical and clinical outcomes following anti-VEGF therapy.64,66,68,69 Consequently, alternative therapeutic options to treat peri-tumoral edema are warranted.
Modifying Tie2 signaling to treat vascular leakage
Tie2 is a receptor tyrosine kinase predominantly expressed in vascular ECs that modulates vascular permeability via interaction with the Angiopoietin family of protein ligands12,70 (Figure 1). In the normal brain, Ang-1/Tie2 signaling promotes vascular stability via its effects on EC junctions. In the GBM microenvironment, increased Ang-2 limits Tie2 receptor tyrosine kinase phosphorylation leading to NVU dysfunction and vascular leakage.3 VEGF promotes neovessel formation and BBB breakdown in conjunction with the Ang-2/Tie2 axis.3,12 It was previously shown that the combinatorial blockade of VEGF and Ang-2, an endogenous EC-derived antagonist for Tie2, leads to vascular normalization and decreased vascular permeability in mouse GBM models.66,68,69 In addition to Tie2 ligands, vascular endothelial protein tyrosine phosphatase (VE-PTP) is a modulator of Tie2 phosphorylation and vascular permeability, whose therapeutic targeting has been shown to improve EC barrier function in preclinical models for breast cancer/metastasis, brain ischemia, inflammation, renal and ocular diseases.71–74 The expression of VE-PTP is induced by hypoxia73,75 and upregulated within the hypoxic tumor vasculature.76 The recent development of small-molecule VE-PTP inhibitors that restore Tie2 phosphorylation independent of ligand binding, and their efficacy in preclinical models of ischemic and VEGF-induced retinopathy14,75,77 resulted in Phase I/II clinical trials with AKB-9778, the lead VE-PTP inhibitor. AKB-9778 showed increased efficacy in combination with anti-VEGF therapy in the treatment of diabetic macular edema, a disease in which vascular leakage, similar to GBM, is driven by hypoxia (ClinicalTrials.gov: NCT02050828). The safety and efficacy of subcutaneously administered AKB-9778 in patients with moderate to severe non-proliferative diabetic retinopathy is currently being evaluated in a Phase IIb study (NCT03197870). Further clinical trials are similarly targeting the Angiopoietin/Tie2 pathway in conjunction with VEGF in diabetic macula edema using anti-Ang-2 and anti-VEGF (NCT02712008) or bi-specific Ang-2/VEGF antibody approaches (NCT02699450). In a mouse model of breast cancer metastasis, Tie2 activation by VE-PTP inhibition prevented vascular leakage through a normalized tumor vasculature, delayed tumor growth, metastatic progression, and enhanced response to chemo and radiation therapy.74 Recent evidence from a preclinical brain ischemia model showed that neurovascular permeability can be successfully counteracted by restoring Tie2 phosphorylation via VE-PTP inhibition.71 Together, these results provide a rationale in restoration of Tie2 signaling for edema management in GBM and brain metastases.
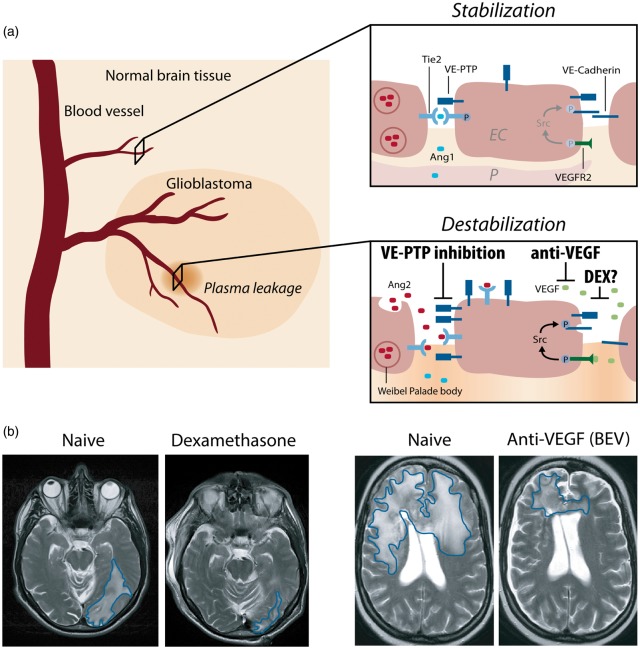
Figure 1.
Interference of angiogenic growth factor signaling with junctional proteins and vascular leakage in GBM. (a) In the normal brain vasculature, paracrine Ang-1 signaling leads to the phosphorylation and subsequent translocation of Tie2 to endothelial cell junctions (EC junctions; upper right panel). VEGF receptor 2 (VEGFR2) is activated due to low amounts of the activating ligand VEGF, but a stable vasculature is maintained by concomitant Tie2 activation. In GBM, several mechanisms lead to the induction of vascular leakage: (1) Ang-2 is secreted from Weibel–Palade bodies and acts in an autocrine manner on Tie2, thereby blocking the Ang1-induced Tie2 phosphorylation and vessel stabilization (lower right panel). (2) Tie2 phosphorylation is further reduced by the activity of VE-PTP that dephosphorylates Tie2 at EC junctions, thereby promoting vascular permeability. VE-PTP expression is increased in hypoxic endothelial cells and in neovascular endothelial cells in mice with ischemic retinopathy suggesting increased expression in tumor vessels.76 (3) Local VEGF levels are increased leading to vascular endothelial cadherin (VE-cadherin) phosphorylation and internalization in an SRC kinase-dependent manner. Dexamethasone interferes with and prevents vascular leakage by a yet unknown mechanism that may include inhibition/downregulation of VEGF, whereas anti-VEGF treatment (BEV) neutralizes excess VEGF, and VE-PTP inhibition (AKB-9778) reduces the catalytic activity of VE-PTP to restore Tie2 activation to prevent vascular leakage. Illustration: Visual Science Communication. (b) Illustration of brain edema in GBM patients. Upon DEX treatment (left), the T2 weighted cMRI signal shows the anti-edema effect of DEX when compared with the DEX naive scenario in a GBM patient with perifocal edema (encircled in blue) in the left parietooccipital lobe. Upon BEV (anti-VEGF) therapy of another GBM patient (right), a weighted cMRI axial section shows decreased T2 signal enhancement demonstrating the anti-edema effect of BEV compared to progressive disease and perifocal edema (encircled in blue) of the corpus calosum before treatment.
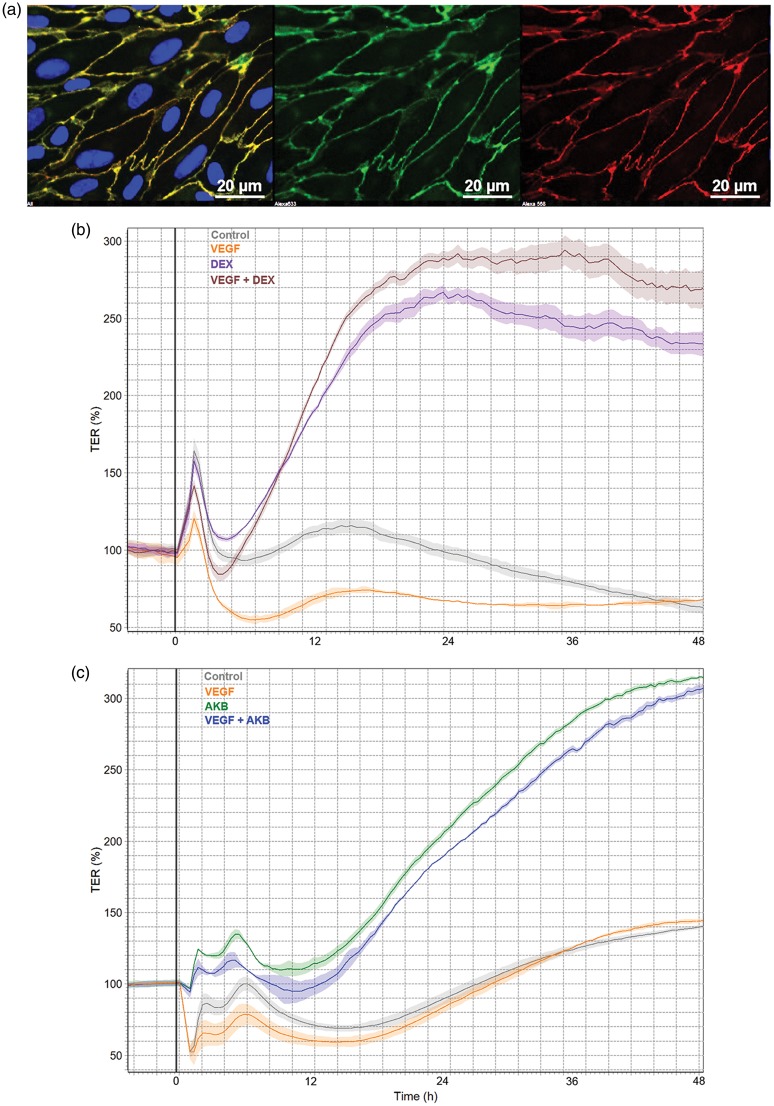
In Figure 2, a BBB-tightening effect of DEX is illustrated in transendothelial electrical resistance (TEER) measurements in vitro using primary porcine brain microvascular ECs as an in vitro BBB model similar to previous reports.9,78 DEX is able to rescue the VEGF-mediated endothelial permeability in this BBB model (Figure 2). However, the mechanisms responsible for reduced endothelial permeability by DEX are poorly understood and given the unfavorable immunosuppressive effects of DEX administration, there is a need for novel candidates to improve NVU function and BBB integrity in tumors. In this regard, targeting the Tie2 pathway via VE-PTP inhibition represents an attractive approach. Underscoring this possibility, a potent VE-PTP inhibitor is able to reduce endothelial permeability in the in vitro BBB model and is able to rescue VEGF-mediated permeability to a level comparable to DEX (Figure 2).
Figure 2.
Rescue of VEGF-mediated permeability by activating Tie2 pathway comparable to dexamethasone in porcine brain endothelial cells in vitro. (a) Primary porcine brain endothelial cells isolated and cultured as described previously71 were subjected to TEER measurements in cellZscope device (Nanoanalytics).80 Expression of EC marker (VE-Cadherin/green, Claudin-5/red, DAPI/blue) indicate purity. VEGF (10 ng/ml), DEX (100 nM) and AKB 9785 (1 µm) treatments were performed in basal serum-free MCDB-131 medium upon reaching a plateau in TEER indicating confluency and barrier maturation. (b) Dexamethasone treatment resulted in high TEER values almost three-fold compared to controls and furthermore was able to rescue VEGF-mediated BBB breakdown. (c) AKB 9785 treatment was also able to tighten the BBB to similar levels as DEX and rescued VEGF-mediated permeability in this model.
Conclusion
In conclusion, DEX, although highly effective in edema resolution in GBM, has a profound profile of adverse effects including T-cell-mediated immunosuppression. DEX application may even be related to reduced OS, although the mechanism of action remains to be elucidated. Increasing evidence supports the notion that DEX-sparing therapies might be warranted, particularly in light of upcoming immunotherapy trials (NCT02287428; J Clin Oncol 36, 2018, suppl; abstr 2020). Of note, DEX has recently been shown to negatively impact the clinical outcome after PD-1 checkpoint blockade in patients with lung cancer,79 whereas GBM patients requiring DEX exceeding physiological replacement dosing have been excluded from immunotherapy trials (NCT02311920). In patients suffering from macula edema, VEGF blockade by BEV, ranibizumab or other inhibitors of VEGF signaling has been highly successful.13 However, although effective in vasogenic edema management, anti-VEGF therapeutics have their limitations and contraindications in GBM patients. The successful inhibition of vascular leakage by inhibiting VE-PTP function in mouse models of macula edema, sepsis, stroke, inflammation and cancer12,14,71,72,74,75 resulted in promising clinical trials for ocular disease (currently Phase IIb, NCT03197870). In light of these recent developments, clinical trials evaluating DEX-sparing substances such as VEGF or VE-PTP inhibitors are highly warranted, in particular in GBM patients receiving immunotherapy.
Acknowledgements
DD is a recipient of an Else-Kröner Foundation Research College fellowship. KHP and YR are supported by the Clinical Translation Platform of the Frankfurt Cancer Institute and the German Consortium for Translational Cancer Research (DKTK), partner site Frankfurt/Mainz, Germany. KD, YR and KHP are supported by the LOEWE Center for Personalized Translational Epilepsy Research (CePTER).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: DD is a recipient of an Else-Kröner Foundation Research College fellowship. KHP and YR are supported by the Clinical Translation Platform of the Frankfurt Cancer Institute and the German Consortium for Translational Cancer Research (DKTK), partner site Frankfurt/Mainz, Germany. KD, YR and KHP are supported by the LOEWE Center for Personalized Translational Epilepsy Research (CePTER).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KGP is an employee of Aerpio Pharmaceuticals.
References
- 1.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci 2007; 8: 610–622. [DOI] [PubMed] [Google Scholar]
- 2.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell 2017; 31: 326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liebner S, Dijkhuizen RM, Reiss Y, et al. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 2018; 135: 311–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010; 96: 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C-X, Lin G-S, Lin Z-X, et al. Peritumoral edema shown by MRI predicts poor clinical outcome in glioblastoma. World J Surg Oncol 2015; 13: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvold ND, Armstrong TS, Warren KE, et al. Corticosteroid use endpoints in neuro-oncology: response Assessment in Neuro-Oncology Working Group. Neuro Oncol 2018; 20: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser C, Wittwer M, Grandgirard D, et al. Adjunctive dexamethasone affects the expression of genes related to inflammation, neurogenesis and apoptosis in infant rat pneumococcal meningitis. PLoS One 2011; 6: e17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidenfeller C, Schrot S, Zozulya A, et al. Murine brain capillary endothelial cells exhibit improved barrier properties under the influence of hydrocortisone. Brain Res 2005; 1053: 162–174. [DOI] [PubMed] [Google Scholar]
- 9.Hoheisel D, Nitz T, Franke H, et al. Hydrocortisone reinforces the blood-brain properties in a serum free cell culture system. Biochem Biophys Res Commun 1998; 247: 312–315. [PubMed] [Google Scholar]
- 10.Förster C, Burek M, Romero IA, et al. Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol 2008; 586: 1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov 2016; 15: 385–403. [DOI] [PubMed] [Google Scholar]
- 12.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat Rev Drug Discov 2017; 16: 635–661. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P, Liew G, Gopinath B, et al. Age-related macular degeneration. Lancet 2018; 392: 1147–1159. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Peters KG. Targeting Tie2 for treatment of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 2016; 16: 126. [DOI] [PubMed] [Google Scholar]
- 15.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 16.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013; 19: 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daneman R. The blood-brain barrier in health and disease. Ann Neurol 2012; 72: 648–672. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Z, Nelson AR, Betsholtz C, et al. Establishment and dysfunction of the blood-brain barrier. Cell 2015; 163: 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banks WA. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 2016; 15: 275. [DOI] [PubMed] [Google Scholar]
- 20.Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects?. Support Care Cancer 2002; 10: 322–328. [DOI] [PubMed] [Google Scholar]
- 21.Shields LBE, Shelton BJ, Shearer AJ, et al. Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol 2015; 10: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain 2016; 139: 1458–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong ET, Lok E, Gautam S, et al. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer 2015; 113: 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubinski D, Won S-Y, Gessler F, et al. Dexamethasone-induced leukocytosis is associated with poor survival in newly diagnosed glioblastoma. J Neurooncol 2018; 137: 503–510. [DOI] [PubMed] [Google Scholar]
- 25.Giles AJ, Hutchinson M-KND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer 2018; 6: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjuvajev J, Uehara H, Desai R, et al. Corticotropin-releasing factor decreases vasogenic brain edema. Cancer Res 1996; 56: 1352–1360. [PubMed] [Google Scholar]
- 27.Recht L, Mechtler L, Wong ET, et al. Steroid-sparing effect of corticorelin acetate in peritumoral cerebral edema is associated with improvement in steroid-induced myopathy. J Clin Oncol 2013; 31: 1182–1187. [DOI] [PubMed] [Google Scholar]
- 28.Obradović MMS, Hamelin B, Manevski N, et al. Glucocorticoids promote breast cancer metastasis. Nature 2019; 567: 540–544. [DOI] [PubMed] [Google Scholar]
- 29.Mai NTH, Chau TTH, Thwaites G, et al. Dexamethasone in vietnamese adolescents and adults with bacterial meningitis. N Engl J Med 2007; 357: 2431–2440. [DOI] [PubMed] [Google Scholar]
- 30.Scarborough M, Gordon SB, Whitty CJM, et al. Corticosteroids for bacterial meningitis in adults in Sub-Saharan Africa. N Engl J Med 2007; 357: 2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Beek D, Farrar JJ, de Gans J, et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol 2010; 9: 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 2016; 374: 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heiss JD, Papavassiliou E, Merrill MJ, et al. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats: involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest 1996; 98: 1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machein MR, Kullmer J, Rönicke V, et al. Differential downregulation of vascular endothelial growth factor by dexamethasone in normoxic and hypoxic rat glioma cells. Neuropathol Appl Neurobiol 1999; 25: 104–112. [DOI] [PubMed] [Google Scholar]
- 35.Lin YM, Jan HJ, Lee CC, et al. Dexamethasone reduced invasiveness of human malignant glioblastoma cells through a MAPK phosphatase-1 (MKP-1) dependent mechanism. Eur J Pharmacol 2008; 593: 1–9. [DOI] [PubMed] [Google Scholar]
- 36.Shannon S, Vaca C, Jia D, et al. Dexamethasone-mediated activation of fibronectin matrix assembly reduces dispersal of primary human glioblastoma cells. PLoS One 2015; 10: e0135951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meleis AM, Mahtabfar A, Danish S, et al. Dexamethasone-mediated inhibition of Glioblastoma neurosphere dispersal in an ex vivo organotypic neural assay. PLoS One 2017; 12: e0186483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrot S, Weidenfeller C, Schärfer TE, et al. Influence of hydrocortisone on the mechanical properties of the cerebral endothelium in vitro. Biophys J 2005; 89: 3904–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan Z, Sehm T, Rauh M, et al. Dexamethasone alleviates tumor-associated brain damage and angiogenesis. PLoS One 2014; 9: e93264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das A, Banik NL, Patel SJ, et al. Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and preventing proteolytic activities. Mol Cancer 2004; 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sur P, Sribnick EA, Patel SJ, et al. Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia 2005; 50: 160–167. [DOI] [PubMed] [Google Scholar]
- 42.Kostopoulou ON, Mohammad AA, Bartek J, et al. Glucocorticoids promote a glioma stem cell-like phenotype and resistance to chemotherapy in human glioblastoma primary cells: biological and prognostic significance. Int J Cancer 2018; 142: 1266–1276. [DOI] [PubMed] [Google Scholar]
- 43.Robe PA, Nguyen-Khac M, Jolois O, et al. Dexamethasone inhibits the HSV-tk/ ganciclovir bystander effect in malignant glioma cells. BMC Cancer 2005; 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedetti S, Pirola B, Poliani PL, et al. Dexamethasone inhibits the anti-tumor effect of interleukin 4 on rat experimental gliomas. Gene Ther 2003; 10: 188–192. [DOI] [PubMed] [Google Scholar]
- 45.Aasland D, Reich TR, Tomicic MT, et al. Repair gene O6-methylguanine-DNA methyltransferase is controlled by SP1 and up-regulated by glucocorticoids, but not by temozolomide and radiation. J Neurochem 2018; 144: 139–151. [DOI] [PubMed] [Google Scholar]
- 46.Luedi MM, Singh SK, Mosley JC, et al. Dexamethasone-mediated oncogenicity in vitro and in an animal model of glioblastoma. J Neurosurg 2018; 129: 1446–1455. [DOI] [PubMed] [Google Scholar]
- 47.Cook AM, McDonnell AM, Lake RA, et al. Dexamethasone co-medication in cancer patients undergoing chemotherapy causes substantial immunomodulatory effects with implications for chemo-immunotherapy strategies. Oncoimmunology 2016; 5: e1066062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014; 370: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014; 370: 709–722. [DOI] [PubMed] [Google Scholar]
- 50.Blanchette M, Daneman R. Formation and maintenance of the BBB. Mech Dev 2015; 138: 8–16. [DOI] [PubMed] [Google Scholar]
- 51.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983; 219: 983–985. [DOI] [PubMed] [Google Scholar]
- 52.Senger DR, Perruzzi CA, Feder J, et al. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res 1986; 46: 5629–5632. [PubMed] [Google Scholar]
- 53.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246: 1306–1309. [DOI] [PubMed] [Google Scholar]
- 54.Proescholdt MA, Heiss JD, Walbridge S, et al. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J Neuropathol Exp Neurol 1999; 58: 613–627. [DOI] [PubMed] [Google Scholar]
- 55.Argaw AT, Asp L, Zhang J, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 2012; 122: 2454–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamoun WS, Ley CD, Farrar CT, et al. Edema control by cediranib, a vascular endothelial growth factor receptor – targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol 2009; 27: 2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 2011; 79: 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machein MR, Kullmer J, Fiebich BL, et al. Vascular endothelial growth factor expression, vascular volume, and capillary permeability in human brain tumors. Neurosurgery 1999; 44: 732–740. [DOI] [PubMed] [Google Scholar]
- 59.Plate KH, Warnke PC. Vascular endothelial growth factor. J Neurooncol 1997; 35: 365–372. [DOI] [PubMed] [Google Scholar]
- 60.Rinne ML, Lee EQ, Nayak L, et al. Update on bevacizumab and other angiogenesis inhibitors for brain cancer. Expert Opin Emerg Drugs 2013; 18: 137–153. [DOI] [PubMed] [Google Scholar]
- 61.Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2012; 82: 2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ueda S, Mineta T, Nakahara Y, et al. Induction of the DNA repair gene O6-methylguanine – DNA methyltransferase by dexamethasone in glioblastomas. J Neurosurg 2004; 101: 659–663. [DOI] [PubMed] [Google Scholar]
- 63.Verheul HMW, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer 2007; 7: 475–485. [DOI] [PubMed] [Google Scholar]
- 64.Wang N, Jain RK, Batchelor TT. New directions in anti-angiogenic therapy for glioblastoma. Neurotherapeutics 2017; 14: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008; 8: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scholz A, Harter PN, Cremer S, et al. Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma. EMBO Mol Med 2015; 8: 39–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Missiaen R, Mazzone M, Bergers G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin Cancer Biol 2018; 52: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peterson TE, Kirkpatrick ND, Huang Y, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci 2016; 113: 4470–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kloepper J, Riedemann L, Amoozgar Z, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci 2016; 113: 4476–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scholz A, Plate KH, Reiss Y. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci 2015; 1347: 45–51. [DOI] [PubMed] [Google Scholar]
- 71.Gurnik S, Devraj K, Macas J, et al. Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol 2016; 131: 753–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frye M, Dierkes M, Küppers V, et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med 2015; 212: 2267–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carota IA, Kenig-Kozlovsky Y, Onay T, et al. Targeting VE-PTP phosphatase protects the kidney from diabetic injury. J Exp Med 2019; 216: 936–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goel S, Gupta N, Walcott BP, et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst 2013; 105: 1188–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen J, Frye M, Lee BL, et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest 2014; 124: 4564–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dominguez MG, Hughes VC, Pan L, et al. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc Natl Acad Sci 2007; 104: 3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campochiaro PA, Sophie R, Tolentino M, et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology 2015; 122: 545–554. [DOI] [PubMed] [Google Scholar]
- 78.Antonetti DA, Wolpert EB, DeMaio L, et al. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem 2002; 80: 667–677. [DOI] [PubMed] [Google Scholar]
- 79.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018; 36: 2872–2878. [DOI] [PubMed] [Google Scholar]
- 80.Czupalla CJ, Liebner S, Devraj K. In vitro models of the blood-brain barrier. Methods Mol Biol 2014; 1135: 415–443. [DOI] [PubMed] [Google Scholar]