Abstract
Cytochrome P450s (CYPs) are phase-I metabolic enzymes playing important roles in drug metabolism, dietary chemicals and endogenous molecules. Age is a key factor influencing P450s expression. Thus, age-related changes of CYP 1–4 families and bile acid homeostasis-related CYPs, the corresponding nuclear receptors and a few phase-II genes were examined. Livers from male Sprague-Dawley rats at fetus (−2 d), neonates (1, 7, and 14 d), weanling (21 d), puberty (28 and 35 d), adulthood (60 and 180 d), and aging (540 and 800 d) were collected and subjected to qPCR analysis. Liver proteins from 14, 28, 60, 180, 540 and 800 days of age were also extracted for selected protein analysis by western blot. In general, there were three patterns of their expression: Some of the drug-metabolizing enzymes and related nuclear receptors were low in fetal and neonatal stage, increased with liver maturation and decreased quickly at aging (AhR, Cyp1a1, Cyp2b1, Cyp2b2, Cyp3a1, Cyp3a2, Ugt1a2); the majority of P450s (Cyp1a2, Cyp2c6, Cyp2c11, Cyp2d2, Cyp2e1, CAR, PXR, FXR, Cyp7a1, Cyp7b1. Cyp8b1, Cyp27a1, Ugt1a1, Sult1a1, Sult1a2) maintained relatively high levels throughout the adulthood, and decreased at 800 days of age; and some had an early peak between 7 and 14 days (CAR, PXR, PPARα, Cyp4a1, Ugt1a2). The protein expression of CYP1A2, CYP2B1, CYP2E1, CYP3A1, CYP4A1, and CYP7A1 corresponded the trend of mRNA changes. In summary, this study characterized three expression patterns of 16 CYPs, five nuclear receptors, and four phase-II genes during development and aging in rat liver, adding to our understanding of age-related CYP expression changes and age-related disorders.
Keywords: mRNA/protein expression, Ontogeny, Aging, Cytochrome P450’s, Nuclear receptors, Rat liver
Introduction
Cytochrome P450s (CYPs) are phase-I metabolic enzymes playing important roles in drug metabolism, dietary chemicals, as well as endogenous molecules in the liver (Agrawal & Shapiro, 2003; Donato & Castell, 2003; Hart et al., 2009; Munro et al., 2018). CYP1, 2, 3, and 4 families are responsible for the biotransformation of most foreign substances including 70–80% of all drugs in clinical use, CYP 4 families also participate in lipid metabolism (Cui, Renaud & Klaassen, 2012; Zanger & Schwab, 2013). The CYP7 families, together with CYP8 and CYP27 are important for cholesterol and bile acid metabolism and homeostasis (Cuesta de Juan et al., 2007; Liu et al., 2014).
CYPs are regulated by many physiological, genetic, environmental, and pathological factors. For example, CYPs expression can be affected by hormones (Daskalopoulos et al., 2012), cytokines (Kot & Daujat-Chavanieu, 2018), pregnancy (He et al., 2005), sex (Agrawal & Shapiro, 2003; Das, Banerjee & Shapiro, 2014) and age. CYPs are subjected to age-dependent changes in cell differentiation (Czekaj et al., 2010) and epigenetic regulation (Li et al., 2009), and age-related metabolic syndrome (Bondarenko et al., 2016), kidney diseases (Velenosi et al., 2012), diabetes (Park et al., 2016), nonalcoholic steatohepatitis (Li et al., 2017), virus hepatitis, and cirrhosis (Kirby et al., 1996).
Age of animals greatly affects drug metabolism (Durnas, Loi & Cusack, 1990), alters pharmacokinetics of xenobiotics (Matalova, Urbanek & Anzenbacher, 2016; Shi & Klotz, 2011), and thus alters the sensitivity to drugs and toxicants such as acetaminophen (Mach et al., 2014), isoniazid (Mach et al., 2016), aflatoxin B1 (Kirby et al., 1996; Wang et al., 2018), and thioacetamide (Kang et al., 2008). Age also influences drug-drug interactions (Jia et al., 2014). Age-associated changes in P450 and corresponding nuclear factors are a major determinant in CYP regulation of drug metabolism, especially during development (children) and in senescence (elderly) (Durnas, Loi & Cusack, 1990; Kilanowicz et al., 2015; Shi & Klotz, 2011).
The expression and maturation of CYPs during development is a major topic of research (Kilanowicz et al., 2015), and immature rats have been proposed as a potential model for xenobiotics risk evaluation for children (McPhail et al., 2016). The ontogeny of CYPs greatly affects the drug metabolism especially during the developmental period (De Zwart et al., 2008), and is the major cause of altered susceptibility to drugs and toxicants in children (Li et al., 2017; Yun et al., 2010).
Aging is a physiological process characterized by a progressive functional decline in various organs over time. Aging is an important factor leading to alterations in the biotransformation, either by reduced expression or decreased function. Many cytochrome P450 genes from CYP 1–3 families show decreased expression in the older rats.(Yun et al., 2010) The ability of liver CYPs to metabolize xenobiotics decreases with aging in vitro (Salmin et al., 2017) and in vivo (Wauthier, Verbeeck & Calderon, 2007), and hepatic CYP mRNA expressions are decreased with aging (Mori et al., 2007). The ability of CYPs in response to inducers such as phenobarbital is also decreased in old rats (Agrawal & Shapiro, 2003). Age-associated CYP3A expression changes in the liver are more remarkable as compared to that occurred in the intestine and kidney, and are tissue-specific (Warrington, Greenblatt & Von Moltke, 2004). Since P450 enzymes in humans are regulated in a manner similar to that in animals (Durnas, Loi & Cusack, 1990; Wauthier, Verbeeck & Calderon, 2007). Thus, to examine CYP expressions in the old laboratory animals would help evaluation of drug metabolism, efficacy and toxicity in the elderly.
In children, three patterns of drug metabolizing enzymes are proposed (Hines, 2008). The first pattern (e.g., CYP3A7) is expressed at the highest level during the first trimester and either remains at high concentrations or decreases during gestation, but is silenced or reduced within one to two years after birth; the 2nd pattern (e.g., SULT1A1) is expressed at relatively constant levels throughout gestation and minimal changes are observed postnatally; and the 3rd pattern (e.g., ADH1C) is not expressed or is expressed at low levels in the fetus (Hines, 2008; Hines, 2013). Age-associated changes of drug metabolism in humans, especially during stages before birth and during early development (neonate/infant/child), could be studied in laboratory animals (Hines, 2013; Saghir, Khan & McCoy, 2012).
Considering the variations of P450 in children, adult, and elderly, this study was initiated to characterize age-associated changes in hepatic P450, to extend our prior work on age-associated changes in hepatic uptake Oatp transporters (Hou et al., 2014a; Hou et al., 2014b), efflux MRP transporters. Zhu et al. (2017), the Nrf2 antioxidant pathways (Xu et al., 2018b), glutathione S-transferases (Xu et al., 2018a), and the antioxidant metallothionein gene expression (Hou et al., 2014a; Hou et al., 2014b). The expression of 16 major CYP isoforms, five corresponding nuclear receptors (NRs), and four phase-II conjugation genes were examined. Protein expressions of selected CYPs were also performed to confirm qPCR results. Similar to three patterns of CYP expression during mouse liver development (Hart et al., 2009), the current study identified three patterns of CYP expression in the liver of rats at 11 time points of entire life span to help our understanding the age-associated changes in these important phase-I and phase-II drug metabolism genes, and age-associated disorders.
Materials and Methods
Animals
Adult male and female SD rats (250–300 g, 10 males and 30 females) were purchased from the Experimental Animal Center of Third Military Medical University (Chongqing, China; Certificate No: CXK 2007–0005). Rats were kept in a SPF-grade animal Facilities with controlled environment (22 ± 1 °C, 50 ± 2% humidity and a 12 h: 12 h light: dark cycle) at Key Lab for Basic Pharmacology of Ministry of Education. Rats had free access to purified water and standard laboratory chow (Experimental Animal Center, Chongqing, China). All animal care and experimental protocols were complied with the Animal Management Guidelines of China and approved by the Animal Use and Care Committee of Zunyi Medical University (2012-02).
Sample collection
Rats were acclimatized for one week before timely mating overnight and a positive vaginal plug next morning was considered as gestation day 1. Livers of offspring male rats were collected at gestation day 19 (−2 d), at birth (1 d), at the neonatal stage (7 and 14 d), at weanling (21 d), at puberty (28 and 35 d), at the adulthood (60 and 180 d), and at aging (540 and 800 d). Six samples per time point were collected, however, n = 4–5 was used to for a 96-well qPCR plate to hold all time points. Rats were anesthetized by hloral hydrate (10%, 5 mL/kg, ip), followed by decapitation to minimize potential pain and distress. Liver tissues were stored at −80 °C prior to analysis.
Real-time RT-PCR analysis
Liver total RNA was extracted by using RNAiso Plus kit (TaKaRa Biotechnology Co., Ltd., Dalian, China). The quality and quantity of total RNA were determined by nanodrop and the 260/280 nm ratio >1.8. The total RNA was reverse transcribed to cDNA (Applied Biosystems, Foster City, CA, USA) and real-time RT-PCR analysis (Bio-Rad Laboratories, Hercules, CA, USA) was conducted as described (Xu et al., 2018b). Relative expression of genes was calculated by the 2−ΔΔCt method and normalized to the house-keeping gene β-actin or GAPDH (results were similar, data not shown), and expressed as relative transcript levels, setting controls as 100%. The primer sequences used in this study were shown in the Table S1 including CYP1 family gene Cyp1a1, Cyp1a2 and corresponding nuclear receptor AhR; CYP2 family gene Cyp2b1, Cyp2b2, Cyp2c6, Cyp2c11, Cyp2d2, Cyp2e1 and corresponding nuclear receptor CAR; CYP3 family genes Cyp3a1,Cyp3a2 and corresponding nuclear receptor PXR; CYP4 family Cyp4a1 and nuclear receptor PPARα; as well as CYPs for bile acid metabolism Cyp7a1, Cyp7b1, Cyp8b1,Cyp27a1 and corresponding nuclear receptor FXR. In addition, the phase 2 genes for glucuronidation Ugt1a1 and Ugt1a2, sulfation (Sult1a1 and Sult1a2) were also examined.
Western blot analysis
Liver tissues (50–100 mg) were homogenized in RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing 1 mM phenylmethanesulfonyl fluoride (PMSF) and freshly prepared proteinase inhibitors. Protein concentrations were quantified by the BCA assay (Beyotime Institute of Biotechnology, Shanghai, China) and denatured (90 °C, 10 min with Nupage Loading buffer). Aliquoted proteins (30 µg) were separated on NUPAGE 10% BT gels (Thermo Fisher Scientific, Waltham, MA, USA) and transferred to PVDF membranes. After blocking with 5% nonfat milk at room temperature for two hours, membranes were incubated with primary mouse antibody against β-actin, rabbit polyclonal antibodies against rat CYP1A2 (bs-2589R), CYP2B1 (bs-14177R), CYP2E1 (bs-4562R), CYP3A1 (bs-20586R), CYP4A1 (bs-5054R) and CYP7A1 (bs-21429R) (1:1000) (Biosynthesis Biotechnology Co., LTD. Beijing, China) overnight at 4 °C. After washes with TBST, membranes were incubated with horseradish peroxidase conjugated anti-rabbit, anti-mouse IgG secondary antibodies (1:5000) for 1 h at room temperature. Protein antibody complexes were visualized using an Enhanced Chemiluminescent reagent and a ChemiDoc XRS system (Bio Rad Laboratories, Inc., Hercules, CA, USA). Band intensities were semi-quantified by densitometry using Quantity One® software (version 4.6.2; Bio Rad Laboratories, Inc., Hercules, CA, USA) (Xu et al., 2018a).
Statistical analysis
The software SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Data were expressed as the mean ± SEM (n = 4–5 per time point). Age associated differences were analyzed by one-way analysis of variance, followed by the least significant difference post hoc test, p < 0.05 was considered to indicate a statistically significant difference from the levels of birth.
Results
Age-related expression of CYP-1 family
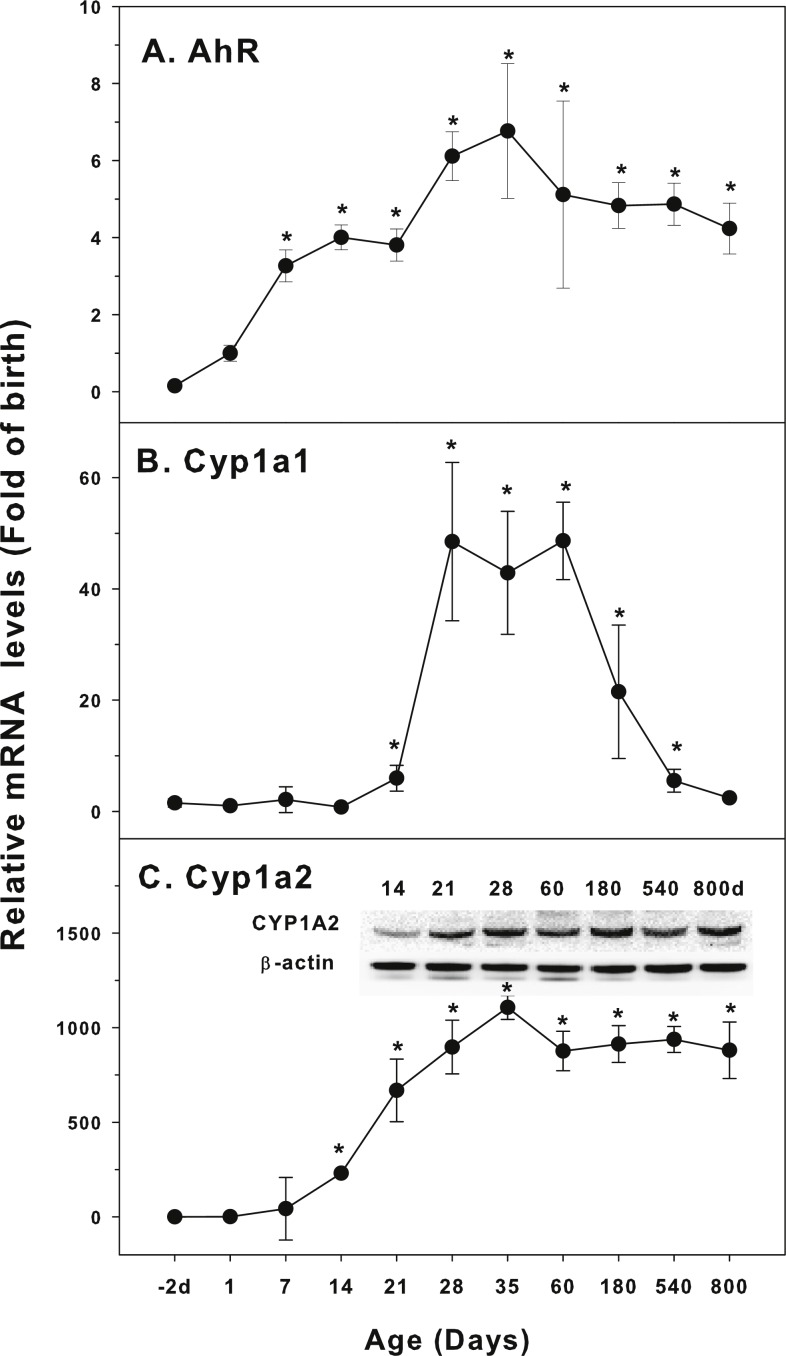
The expression of CYP-1 family (Cyp1a1, Cyp1a2, and corresponding nuclear receptor AhR) is shown in Fig. 1. Aryl hydrocarbon receptor (AhR) mainly mediates the expression of CYP1A1 and CYP1A2 proteins. 2,3,7,8-Tetrachlorodibenzodioxin (TCDD) is a typical CYP1A1 inducer. When TCDD is combined with AhR, AhR is dissociated from the complex and transferred to the nucleus. It forms heteromeric dimers with AhR nuclear transport protein, and then induces the expression of target genes. In this way, TCDD and other AhR activators significantly induce the expression of CYP1A genes (Aleksunes & Klaassen, 2012). AhR was low in fetal livers (−2 d), and gradually increase after birth, and reached the highest levels at 35 days of age (6.7-fold of birth), and gradually declined, and at 800 days of age, it remained 4.2-fold higher than at birth (Fig. 1A). Cyp1a1 was low at −2 days of age through 14 days of age, and begin to increase at 21 days of age, rapidly increased 48-fold at 28–60 days of age, and decline rapidly after 60 days of age, and returned to 2.4-fold of the birth level at 800 days of age (Fig. 1B). In contrast, Cyp1a2 followed the similar pattern as AhR. Cyp1a2 increased dramatically after birth, reached 250-fold at weanling (21 day), and peaked on 35 days of age (1,100 fold). It was declined to 850 fold of birth at 60 days of age and remained till 800 days of age. CYP1A2 protein expression followed the similar pattern (Fig. 1C).
Figure 1. Age-related expression of CYP-1 family gene/proteins in livers of male rats.
(A) AhR, (B) Cy1a1, (C) Cyp1a2. Livers from male SD rats at the fetus (−2 d before birth), the neonatal stage (1, 7, and 14 d), and at weanling (21 d), at puberty (28 and 35 d), at adult (60 and 180 d), and at aging (540 and 800 d), were collected to extract RNA, followed by qPCR analysis (n = 4–5 for each time point). *Significantly different from at birth, p < 0.05. For western blot insert, the neonatal (14 d), at weanling (21 d), at puberty (28 d), at adult (60 and 180 d), and at aging (540 and 800 d) were collected to extract protein. Aliquoted proteins (30 µg) were separated on NUPAGE 10% BT gels and the representative western blot was inserted into the figure (n = 3). The molecular weight for CYP1A1 was 55 kD, and β-actin 43 kD.
Age-related expression of CYP-2 family
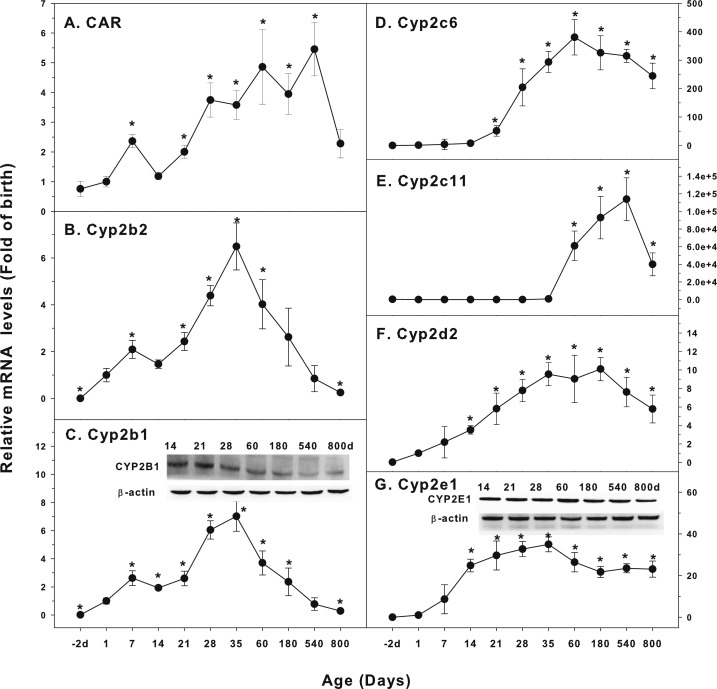
The expression of CYP-2 family is shown in Fig. 2. Constitutive androstane receptor (CAR) is a nuclear receptor of steroid hormones. It regulates the metabolizing enzymes and transporters in liver and small intestine. CAR mediates endogenous hormone or exogenous drug reactions, such as phenobarbital, and transcriptionally regulates CYP2 expression (Aleksunes & Klaassen, 2012). CAR was low in fetal livers (−2 d), and gradually increase after birth, first peaked at 7 days of age, and reached the highest levels at 60 days of age (6-fold of birth), and gradually declined, and at 800 days of age, it remained 2.3-fold higher than at birth (Fig. 2A). Cyp2b2 was low in fetal livers (−2 d), and begin to increase after birth, and rapidly increased at weaning (21 d), reaching the peak at 35 days of age (7-fold of birth) and decreased afterwards, and returned to birth level after 540 days of age (Fig. 2B). The expression of Cyp2b1 followed the similar pattern as Cyp2b2, and the expression of CYP2B1 protein followed the similar pattern (Fig. 2C). Cyp2c6 increased after weanling, reached the peak of liver maturation (350 fold) at 60 days of age, and remained high till 540 days of age, and decreased to 250-fold over birth levels at 800 days of age (Fig. 2D). Cyp2c11 increased 800-fold at puberty (35 days of age), but dramatically increased with liver maturation (60,000-fold at 60 days, 98,000-fold at 180 days, and 110,000-fold at 540 days of age), and rapidly decreased at aging of 800 days of age, but it was still 40,000-fold over the birth level. (Fig. 2E). Cyp2d2 increased gradually after birth, reached the peak (9-fold) of liver maturation at 35–180 days of age, and decreased to 6-fold of birth at 540 and 800 days of age (Fig. 2F). The expression pattern of Cyp2e1 was relatively stable: Cyp2e1 increased rapidly after birth, reached 30-fold of the birth levels at weanling (21 days of age), and peaked on 35 days of age (35 fold). It was gradually declined but remained at the high level at the age of 800 days (22 fold of the birth level). CYP2E1 protein expression followed the similar pattern (Fig. 2G).
Figure 2. Age-related expression of CYP-2 family gene/proteins in livers of male rats.
(A) CAR, (B) Cyp2b2, (C) Cyp2b1, (D) Cyp2c6, (E) Cyp2c11, (F) Cyp2d2, (G) Cyp2e1. Livers from male SD rats at the fetus (−2 d before birth), the neonatal stage (1, 7, and 14 d), and at weanling (21 d), at puberty (28 and 35 d), at adult (60 and 180 d), and at aging (540 and 800 d), were collected to extract RNA, followed by qPCR analysis (n = 4–5 for each time point). *Significantly different from at birth, p < 0.05. For western blot insert, the neonatal (14 d), at weanling (21 d), at puberty (28 d), at adult (60 and 180 d), and at aging (540 and 800 d) were collected to extract protein. Aliquoted proteins (30 µg) were separated on NUPAGE 10% BT gels and the representative western blot was inserted into the figure (n = 3). The molecular weight for CYP2B1 was 56 kD, CYP2E1 57 kD, and β-actin 43 kD.
Age-related mRNA expression of CYP-3 family
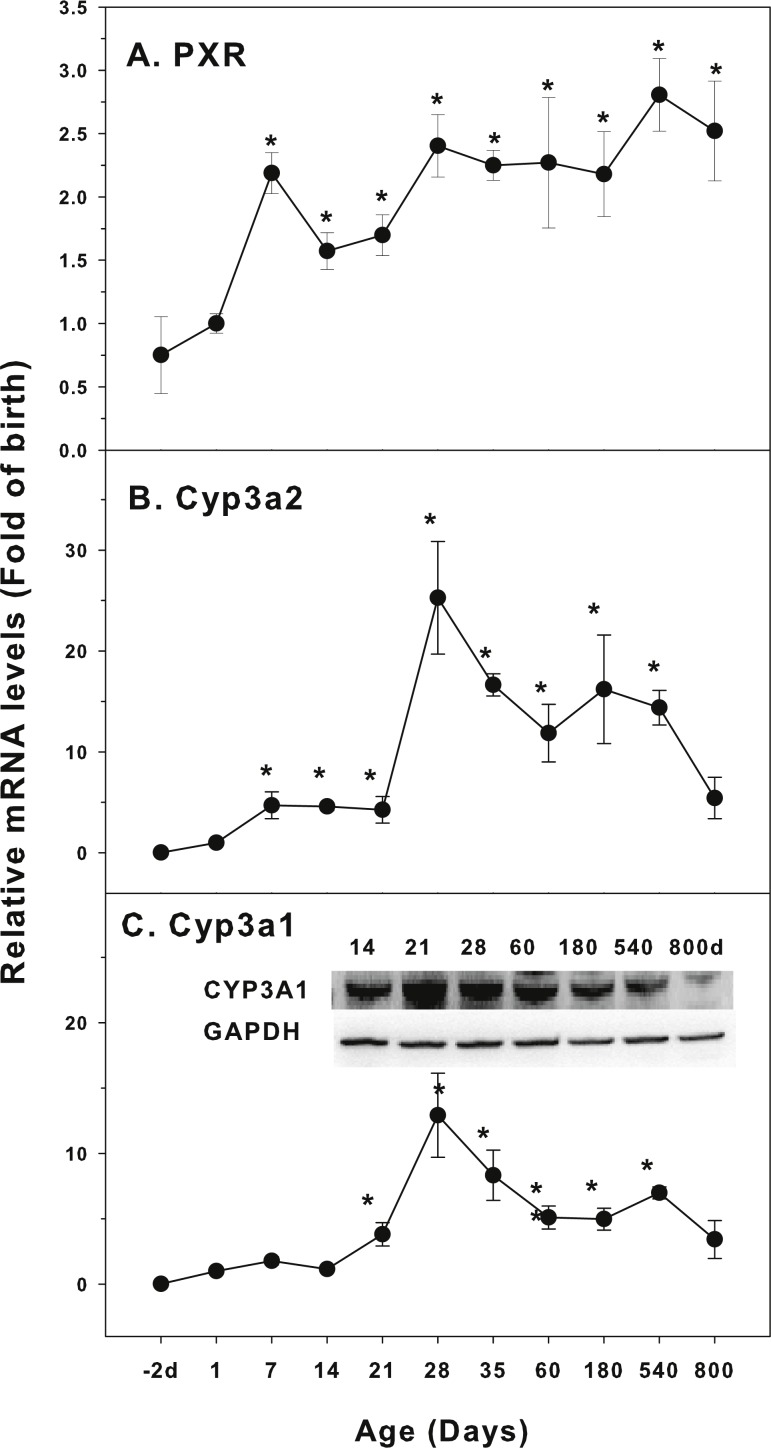
The expression of CYP-3 family is shown in Fig. 3. Pregnane X receptor (PXR) is a highly conserved ligand-dependent transcription factor. It is mainly expressed in the liver and partly expressed in the colon and small intestine. The regulation of CYP3A by PXR-mediated signaling pathway is an important pathway of drug metabolism (Aleksunes & Klaassen, 2012). The expression of PXR was relatively stable throughout the life with approximately 2-fold variations (Fig. 3A). The expression of Cyp3a2 markedly increased 4 fold at 7 days of age, and rapidly increased after weanling (21 days of age), reached the peak at 28 days of age (25-fold of birth) and decreased gradually afterwards, and the level was still 5.4-fold of the birth level at 800 days of age (Fig. 3B). Cyp3a1 follows similar pattern as Cyp3a2. It was markedly increased after weanling, reached the peak at 28 days of age (13-fold of the birth level) and decreased gradually afterwards, and the level was still 3.4-fold of the birth level at 800 days of age. The expression of Cyp3a1 protein followed the similar patter (Fig. 3C).
Figure 3. Age-related expression of CYP-3 family gene/proteins in livers of male rats.
(A) PXR, (B) Cyp3a2, (C) Cyp3a1. Livers from male SD rats at the fetus (−2 d before birth), the neonatal stage (1, 7, and 14 d), and at weanling (21 d), at puberty (28 and 35 d), at adult (60 and 180 d), and at aging (540 and 800 d), were collected to extract RNA, followed by qPCR analysis (n = 4–5 for each time point). *Significantly different from at birth, p < 0.05. For western blot insert, the neonatal (14 d), at weanling (21 d), at puberty (28 d), at adult (60 and 180 d), and at aging (540 and 800 d) were collected to extract protein. Aliquoted proteins (30 µg) were separated on NUPAGE 10% BT gels and the representative western blot was inserted into the figure (n = 3). The molecular weight for CYP3A1 was 57 kD, and β-actin 43 kD.
Age-related expression of CYP-4 family
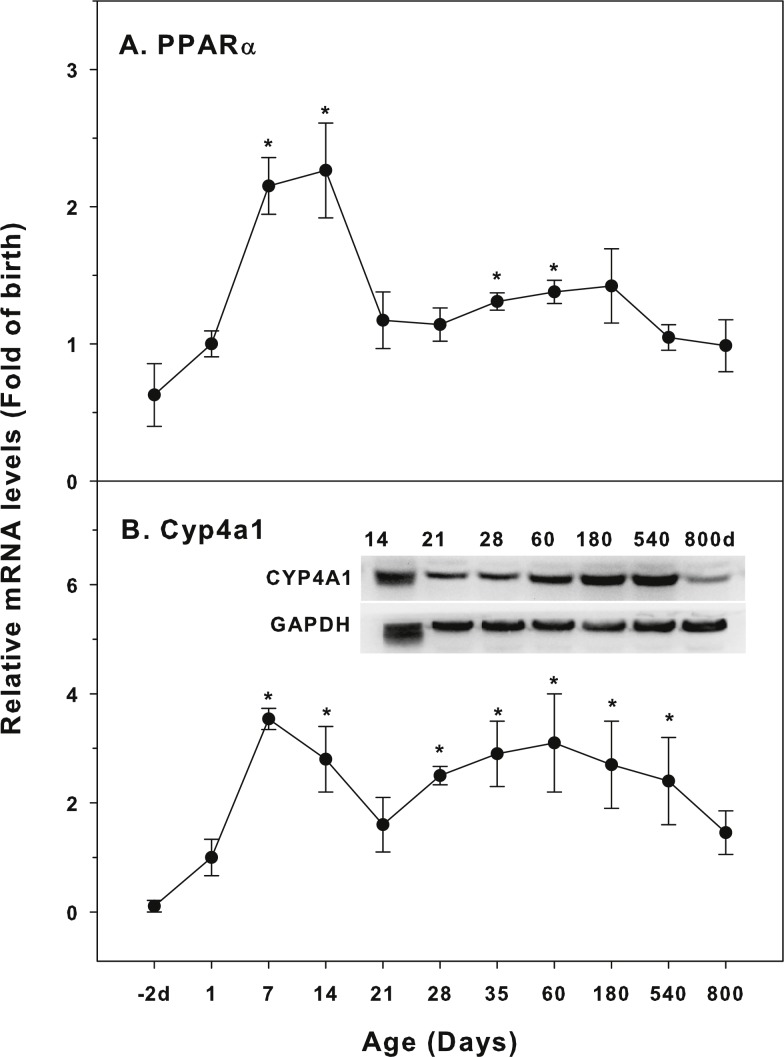
The expression of CYP-4 family is shown in Fig. 4. Peroxisome proliferator-activated receptors (PPARs) nuclear receptor family regulates the expression of genes that control fatty acid synthesis, storage, and catabolism. PPARs mainly include PPARα, PPARβ and PPARγ. The activation of PPAR can improve insulin resistance, slow down atherosclerosis, and promote the metabolism of cholesterol. PPARα regulates induction of CYP4A gene (Aleksunes & Klaassen, 2012). The expression of PPARα was relatively stable throughout the life, except for 7 and 14 days of age (2-fold of the birth level), and at 800 days of age, its levels returned to the birth level (Fig. 4A). Cyp4a1 started to increase after birth, with the first peak at 7 days (3.5-fold) and decreased after 14 days of age, and again gradually increased after weanling and remained high throughout 540 days of age. At 800 days of age, it was still 1.8-fold of the birth level. The expression of CYP4A1 protein followed the similar pattern (Fig. 4B).
Figure 4. Age-related expression of CYP-4 family gene/proteins in livers of male rats.
(A) PPARα, (B) Cyp4a1. Livers from male SD rats at the fetus (−2 d before birth), the neonatal stage (1, 7, and 14 d), and at weanling (21 d), at puberty (28 and 35 d), at adult (60 and 180 d), and at aging (540 and 800 d), were collected to extract RNA, followed by qPCR analysis (n = 4–5 for each time point). For western blot insert, the neonatal (14 d), at weanling (21 d), at puberty (28 d), at adult (60 and 180 d), and at aging (540 and 800 d) were collected to extract protein. Aliquoted proteins (30 µg) were separated on NUPAGE 10% BT gels and the representative western blot was inserted into the figure (n = 3). The molecular weight for CYP4A1 was 59 kD, and β-actin 43 kD.
Age-related mRNA expression of CYPs involved in bile acids homeostasis
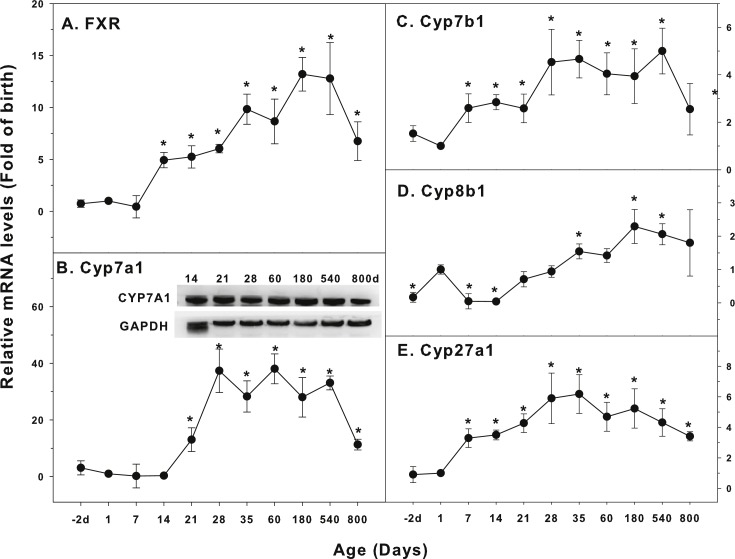
The expression of CYPs involved in cholesterol and bile acids homeostasis is shown in Fig. 5. Bile acids (BAs) are the endogenous ligands of farnesoid X receptor (FXR), so FXR is also called the BA receptor. Cholesterol 7α hydroxylase (Cyp7a1) is important for BA synthesis. When BA overloads in the liver, toxicity to liver cells occurs, including oxidative stress, inflammation, necrosis and even cirrhosis (Cuesta de Juan et al., 2007; Liu et al., 2014). The expression of FXR increased at 14 days of age (5 fold), and gradually increased afterwards with age, reached peak at 180 days (13 fold), and still high at 800 days (6.8 fold) (Fig. 5A). Cyp7a1 started to increase at 14 days of age, reached the peak at 28 days (37 fold of birth), and remained high throughout the adulthood, and was still 11-fold higher than the birth level at 800 days of age. The expression of CYP7A1 protein followed similar pattern (Fig. 5B). The expression of Cyp7b1 started to increase after birth, reached the peak at 28 days of age, and decreased at 800 days of age (Fig. 5C). Cyp8b1 increased at birth and decreased at 7 days of age. Cyp8b1 started to increase again after weanling, reached the peak at adulthoods at 180 days of age, and gradually decreased thereafter. It was still higher at 800 days of age (Fig. 5D). The expression of Cyp27a1 held a similar pattern, reached the peak on 35 days of age (5.8-fold over the birth level), and remained the high levels throughout 540 days of age, and decreased at 800 days of age with 3-fold higher over the birth level (Fig. 5E).
Figure 5. Age-related expression of CYP-7 family gene/proteins in livers of male rats.
(A) FXR, (B) Cyp7a1, (C) Cyp7b1, (D) Cyp8b1, (E) Cyp27a1. Livers from male SD rats at the fetus (−2 d before birth), the neonatal stage (1, 7, and 14 d), and at weanling (21 d), at puberty (28 and 35 d), at adult (60 and 180 d), and at aging (540 and 800 d), were collected to extract RNA, followed by qPCR analysis (n = 4–5 for each time point). *Significantly different from at birth, p < 0.05. For western blot insert, the neonatal (14 d), at weanling (21 d), at puberty (28 d), at adult (60 and 180 d), and at aging (540 and 800 d) were collected to extract protein. Aliquoted proteins (30 µg) were separated on NUPAGE 10% BT gels and the representative western blot was inserted into the figure (n = 3). The molecular weight for CYP7A1 was 55 kD, and β-actin 43 kD.
Age-related mRNA expression of UGT and SULT families
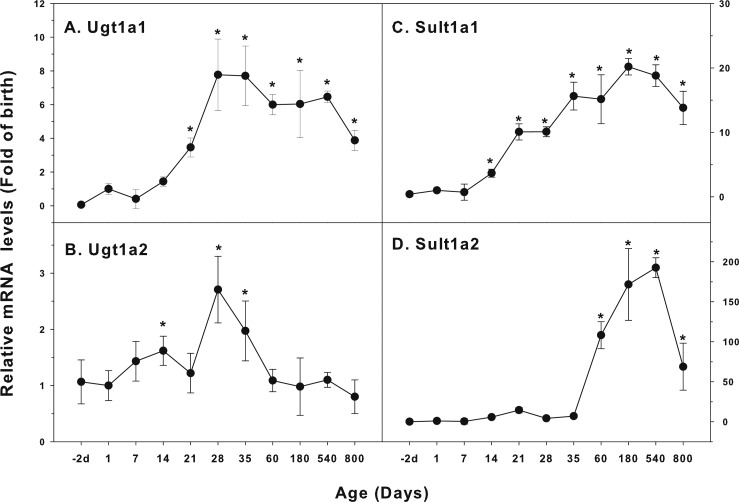
The expression of UGT and SULT families is shown in Fig. 6. UDP-glucuronosyltransferases (UGTs) and sulfotransferases (SULTs) are the two most important phase-2 conjugation enzymes to conjugate the CYP catalyzed metabolites and drugs contain functional groups such as hydroxyls and carboxylic acids (Coughtrie, 2015). Glucuronidation involves the reaction of uridine 5′-diphosphoglucuronic acid with a number of functional groups generated from CYP metabolism and is a major mechanism for the formation of water-soluble substrates for their elimination in bile or in urine, especially for the clearance of a number of drugs in children (Krekels et al., 2012). SULTs transfer the sulfuryl moiety from the universal donor PAPS (3′-phosphoadenosine 5′-phosphosulfate) to a wide variety of substrates with hydroxyl- or amino-groups after CYP metabolism (Coughtrie, 2016). Ugt1a1 was low in fetal livers (−2 d), and gradually increase after birth, marked increase after weanling (21 days), and reached the highest levels at 28 days of age (7.8-fold of birth), and gradually declined, and at 800 days of age, it remained 4-fold higher than the level of birth (Fig. 6A). The expression of Ugt1a2 was relatively stable throughout the life, except for a small peak at day 28 and 35 days of age (2.5-fold higher than the birth level) (Fig. 6B). The expression of Sult1a1 started to increase at 14 days of age, reached the peak at 180 days of age (20-fold), remained 14-fold higher than the birth levels (Fig. 6C). The expression of Sult1a2 started to increase at 14 days of age, reached the first peak at weanling (15 fold), but dramatically increased after 35 days of age, reached 110-fold at 60 days of age, and 190-fold at 540 days of age. It remained 70-fold higher than the levels of birth (Fig. 6D). The expression pattern of Sult1a1 was quite similar to the expression pattern of Cyp2c11.
Figure 6. Age-related mRNA expression of UGT and SULT family genes in livers of male rats.
(A) Ugt1a1, (B) Ugt1a2, (C) Sult1a1, (D) Sult1a2. Livers from male SD rats at the fetus (−2 d before birth), the neonatal stage (1, 7, and 14 d), and at weanling (21 d), at puberty (28 and 35 d), at adult (60 and 180 d), and at aging (540 and 800 d), were collected to extract RNA, followed by real-time RT-PCR analysis (n = 4–5 for each time point). *Significantly different from at birth, p < 0.05.
Discussion
The present study characterized age-related expression of 16 CYPs, 5 NRs, and 4 phase-II genes in livers of rats across 11 time points from fetus (−2 d), neonates (1, 7, 14 and 21 d), puberty (28 and 35 d), adulthood (60 and 180 d), to aging (540 and 800 d). In general, there are three patterns of their expression: (1) The expressions of AhR, Cyp1a1, Cyp2b1, Cyp2b2, Cyp3a1, Cyp3a2, and Ugt1a2 were low in fetal and neonatal stage, increased with liver maturation and decreased extensively after 180 days; (2) the majority of CYPs and other genes maintained relatively high levels throughout the adulthood and decreased at aging of 540 and/or 800 days; (3) the expression of CAR PXR, PPARα, Cyp4a1, Ugt1a2 had a first peak between 7–14 days of age. The protein expression of CYP1A2, CYP2B1, CYP2E1, CYP3A1, CYP4A1, and CYP7A1 followed the trend of mRNA changes. Characterization of CYPs in rat entire life span provides fundamental information for drug metabolism and pharmacology studies in children and elderly.
There are three unique features of the current study: (1) Most of the studies on age-related changes in hepatic CYPs are performed in mice (Cui, Renaud & Klaassen, 2012; Hart et al., 2009; Li et al., 2009), this study characterized CYPs and NRs in rats, another commonly-used laboratory animals; (2) Most of the age-related changes in hepatic P450 are focused on the developmental stages to maturation (Asaoka et al., 2010; Bondarenko et al., 2016; Cui, Renaud & Klaassen, 2012; De Zwart et al., 2008; Kilanowicz et al., 2015; Park et al., 2016), and this study covered the whole life span, and (3) this study extended our efforts to characterize age- and sex-related changes in hepatic drug transporters (Hou et al., 2014a; Hou et al., 2014b; Zhu et al., 2017) and defense mechanisms (Hou et al., 2014a; Hou et al., 2014b; Xu et al., 2018a; Xu et al., 2018b).
P450-1 family
CYP1 family is responsible for activation of toxicants and drugs. Cyp1a1 is expressed very early in rodents and involved in developmental toxicity of hexachloronaphthalene (Kilanowicz et al., 2015), and immature rats has been proposed as a potential model for chemical risks in children (McPhail et al., 2016). Ontogeny of hepatic CYP1A2 showed it rapidly increased after weanling, followed by significant decrease during adulthood (Elbarbry, McNamara & Alcorn, 2007), However, CYP1A1/2 activity did not change when fed Zuker diabetic fatty rats with high fat diet at 5 week (insulin resistant stage) and 11-week (diabetic stage) (Park et al., 2016). Glycyrrhetinic acid potentiation of clozapine hepatotoxicity is associated with CYP1A2 induction and suppression of CYP2C11 and 2C13 (Jia et al., 2014). CYP1A1 and CYP1A2 are regulated differently, CYP1A1 decreased rapidly after maturation, while CYP1A2 remained the high expression levels till 104 weeks (Yun et al., 2010). The present results agreed with the literature.
P450-2 family
Hepatic CYP2C2 and CYP2C11 decreased with age, along with CYP3A enzyme genes (Mori et al., 2007). In diethyl nitrosamine-induced liver insufficiency, the altered expression of cytokines might contribute to CYP2C and CYP3A isoform regulation (Kot & Daujat-Chavanieu, 2018). The expression of CYP2C11 is under the regulation of growth hormones (Das, Banerjee & Shapiro, 2014), as well as the dopaminergic receptors (Daskalopoulos et al., 2012). The expressions of CYP2C, CYP2E1, and CYP3A are influenced by metabolic syndrome (Bondarenko et al., 2016). CYP2C11 is a male-specific CYP, its expression began to increase after weanling, and at puberty reached 830-fold over the birth, and further dramatically increased over 100,000-fold over the birth level throughout the adulthood, but rapidly decreased at aging, consistent with the literature (Agrawal & Shapiro, 2003; Das, Banerjee & Shapiro, 2014; Yun et al., 2010).
Age-associated CYP2E1 expression has important implications in pharmacology and toxicology. Old rats have decreased CYP2E1, altered acetaminophen pharmacokinetics, resulting in less sensitive to acetaminophen toxicity (Mach et al., 2014). On the other hand, higher CYP2E1 in young rats might be a reason of increased sensitivity to isoniazid toxicity (Mach et al., 2016). The observed expression pattern for CYP2B1 (marked decreases at 800 days of age) and CYP2E1 (slight decreases at 800 days of age) are in agreement of the literature (Yun et al., 2010). CYP2E1 plays roles in thioacetamide hepatotoxicity, as Cyp2e1-/-mice are less sensitive to liver injury (Kang et al., 2008). Age-related differences in CYP2E1 levels can have important implications for the toxic and carcinogenic actions of some hydrocarbons (e.g., benzene, hexane) and short-chain halocarbons, such as carbon tetrachloride (McPhail et al., 2016).
P450-3 family
CYP3 families are responsible for most drug metabolism (Zanger & Schwab, 2013). In rats with chronic kidney diseases, CYP3A and CYP2C mediated metabolism are decreased (Velenosi et al., 2012). Using liver microsomes (S9) from young and old rats, severe metabolism impairment with aging for CYP3A and CYP2D substrates are observed (Salmin et al., 2017). CYP3A1 is higher in cells from young rats than in old rats (Czekaj et al., 2010). CYP3A in the liver are sensitive to aging with 50–70% decreases, while CYP3A in the intestine is unchanged and in the kidney increased (Warrington, Greenblatt & Von Moltke, 2004). Generally speaking, hepatic CYP3A enzymes are decreased with age (Mori et al., 2007). In the present study, age-associated changes of CYP3A1 and CYP3A2 followed “Pattern 1”, while the expression of PXR, Cyp3a11 (mouse), and CYP3A4 protein followed “Pattern 2”, a phenomenon in agreement of the literature (Mori et al., 2007; Yun et al., 2010).
P450-4 family
PPARα activation induces CYP4A1, together with acyl-CoA oxidase and SREBPs, that play important roles in regulating lipid metabolism, especially in rats fed with high-fat-diet (Chang et al., 2011). The herbicide propaquizafop dose-dependently activates PPARα and CYP4A, leading to increased liver weight and hypertrophy as a mode of action in hepato-carcinogenesis (Strupp et al., 2018). In addition to PPARα activation, the induction of CYP4A by K+PFOS also involves CAR and PXR activation (Elcombe et al., 2012), leading to hepatomegaly. In the present study, the expression of PPARα, CYP4A1, CAR, and PXR followed “Pattern 3”. That is the first expression peak appeared at 7–14 days of age, similar to that observed in mice (Hart et al., 2009).
P450-7 family and BA homeostasis
Bile acid (BA) homeostasis is tightly regulated via a feedback loop operated by the nuclear receptors FXR and small heterodimer partner (SHP). Loss of either FXR or SHP alone, or Fxr-/-Shp-/- double knock out mice resulted in cholestasis and liver injury as early as 3 weeks of age, and this dysfunction is linked to the dysregulation of bile acid homeostatic key genes, particularly Cyp7a1 (Anakk et al., 2011). Hepatic CYP7A1 is a rate-limiting enzyme that catabolizes cholesterol to bile acids, together with CYP8B1 as the classic BA synthesis pathway, while CYP27A1 and CYP7B1 contribute to the alternative pathway of BA biosynthesis (Cuesta de Juan et al., 2007; Liu et al., 2014). CYP27A1 is sensitive to inhibition by many xenobiotics (Lam, Mast & Pikuleva, 2018). In the present study, the CYPs responsible for cholesterol metabolism and bile acid homeostasis followed “Pattern 2”, that was low at the neonatal stage, remained relative high levels throughout the adulthood, and decreased at 800 days of age.
Taken together, CYPs are crucial enzymes in drug metabolism and disposition (Zanger & Schwab, 2013). Induction or inhibition of CYPs have been implicated in therapeutic efficacy and toxicity (Jia et al., 2014; Mach et al., 2014; Mach et al., 2016), and age-associated diseases (Bondarenko et al., 2016; Velenosi et al., 2012) especially in children (Kilanowicz et al., 2015; Li et al., 2017; McPhail et al., 2016) and in elderly (McPhail et al., 2016; Salmin et al., 2017; Wauthier, Verbeeck & Calderon, 2007). Thus, a better understanding of age-associated changes of CYPs is of significance for pharmacology, toxicology, and therapeutics.
UGT and SULT
Glucuronidation and sulfation are two most important phase-II reactions to conjugate the CYP-catalyzed metabolites for biliary or urinary elimination. Age has significant impact on hepatic activities of glucuronidation and sulfation. For example, porcine hepatic glucuronidation and sulfation activities were low at birth, peaked at 5–10 weeks, and then declined at 20 weeks (Hu, 2017), similar to the observations in the present study.
In childhood and adolescence, UGT expression can be affected by hormones and is a reason of individual variation to medication (Neumann et al., 2016). Compared to adults, glucuronidation is reduced in children (Krekels et al., 2012). UGT1A1 and UGT1A6 are subjected to CAR and PPARα regulation (Osabe et al., 2008), and age-associated UGT1A1 changes are paralleled with CYP3A expression alterations in rats fed high-fat diet (Kawase et al., 2015; Osabe et al., 2008).
Sulfation is the most highly developed pathway during fetal development where glucuronidation in particular is lacking (Coughtrie, 2015). Sulfation is normally a detoxification reaction to facilitate the elimination of xenobiotics, although for some molecules sulfation could be bioactivation (Coughtrie, 2016). The decreased Sult1a1 paralleled with major CYP metabolism genes in 600-day old rats (Mori et al., 2007), similar to current observations.
Conclusions
Overall, the present study characterized age-related changes in a total of 25 CYP isoforms and relevant genes in rat livers from development to aging. In general, these genes are low in neonatal stages, increase with age, but decreased in aged animals, and three expression patterns are characterized. These data could help our better understanding of the effects of CYPs on drug metabolism, pharmacology, and toxicology in the context of maturation and aging.
Supplemental Information
Funding Statement
This study is supported by the National Natural Science Foundation of China (81560592, 81560682). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Jie J Liu is an Academic Editor for PeerJ.
Author Contributions
Shangfu Xu, Qin Wu and Jie Liu conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper.
Anling Hu performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper.
Lu Xie and Jia-Jia Liu performed the experiments.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All animal care and experimental protocols were complied with the Animal Management Guidelines of China and approved by the Animal Use and Care Committee of Zunyi Medical University (2012−02).
Data Availability
The following information was supplied regarding data availability:
Raw data is available in the Supplemental Files.
References
- Agrawal & Shapiro (2003).Agrawal AK, Shapiro BH. Constitutive and inducible hepatic cytochrome P450 isoforms in senescent male and female rats and response to low-dose phenobarbital. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2003;31:612–619. doi: 10.1124/dmd.31.5.612. [DOI] [PubMed] [Google Scholar]
- Aleksunes & Klaassen (2012).Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2012;40:1366–1379. doi: 10.1124/dmd.112.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anakk et al. (2011).Anakk S, Watanabe M, Ochsner SA, McKenna NJ, Finegold MJ, Moore DD. Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis. Journal of Clinical Investigation. 2011;121:86–95. doi: 10.1172/jci42846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka et al. (2010).Asaoka Y, Sakai H, Sasaki J, Goryo M, Yanai T, Masegi T, Okada K. Changes in the gene expression and enzyme activity of hepatic cytochrome P450 in juvenile Sprague-Dawley rats. Journal of Veterinary Medical Science. 2010;72:471–479. doi: 10.1292/jvms.09-0397. [DOI] [PubMed] [Google Scholar]
- Bondarenko et al. (2016).Bondarenko LB, Shayakhmetova GM, Voronina AK, Kovalenko VM. Age-dependent features of CYP3A, CYP2C, and CYP2E1 functioning at metabolic syndrome. Journal of Basic and Clinical Physiology and Pharmacology. 2016;27:603–610. doi: 10.1515/jbcpp-2016-0012. [DOI] [PubMed] [Google Scholar]
- Chang et al. (2011).Chang CJ, Tzeng TF, Liou SS, Chang YS, Liu IM. Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPARalpha levels. Planta Medica. 2011;77:1876–1882. doi: 10.1055/s-0031-1279992. [DOI] [PubMed] [Google Scholar]
- Coughtrie (2015).Coughtrie MW. Ontogeny of human conjugating enzymes. Drug Metabolism Letters. 2015;9:99–108. doi: 10.2174/1872312809666150602151213. [DOI] [PubMed] [Google Scholar]
- Coughtrie (2016).Coughtrie MWH. Function and organization of the human cytosolic sulfotransferase (SULT) family. Chemico-Biological Interactions. 2016;259:2–7. doi: 10.1016/j.cbi.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Cuesta de Juan et al. (2007).Cuesta de Juan S, Monte MJ, Macias RI, Wauthier V, Calderon PB, Marin JJ. Ontogenic development-associated changes in the expression of genes involved in rat bile acid homeostasis. Journal of Lipid Research. 2007;48:1362–1370. doi: 10.1194/jlr.M700034-JLR200. [DOI] [PubMed] [Google Scholar]
- Cui, Renaud & Klaassen (2012).Cui JY, Renaud HJ, Klaassen CD. Ontogeny of novel cytochrome P450 gene isoforms during postnatal liver maturation in mice. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2012;40:1226–1237. doi: 10.1124/dmd.111.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekaj et al. (2010).Czekaj P, Bryzek A, Czekaj TM, Koryciak-Komarska H, Wiaderkiewicz A, Plewka D, Sieron AL. Cytochrome P450 mRNA expressions along with in vitro differentiation of hepatocyte precursor cells from fetal, young and old rats. Folia Histochemica et Cytobiologica. 2010;48:46–57. doi: 10.2478/v10042-008-0085-5. [DOI] [PubMed] [Google Scholar]
- Das, Banerjee & Shapiro (2014).Das RK, Banerjee S, Shapiro BH. Irreversible perinatal imprinting of adult expression of the principal sex-dependent drug-metabolizing enzyme CYP2C11. The FASEB Journal. 2014;28:4111–4122. doi: 10.1096/fj.13-248864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalopoulos et al. (2012).Daskalopoulos EP, Lang MA, Marselos M, Malliou F, Konstandi M. D(2)-dopaminergic receptor-linked pathways: critical regulators of CYP3A, CYP2C, and CYP2D. Molecular Pharmacology. 2012;82:668–678. doi: 10.1124/mol.112.078709. [DOI] [PubMed] [Google Scholar]
- De Zwart et al. (2008).De Zwart L, Scholten M, Monbaliu JG, Annaert PP, Van Houdt JM, Van den Wyngaert I, De Schaepdrijver LM, Bailey GP, Coogan TP, Coussement WC, Mannens GS. The ontogeny of drug metabolizing enzymes and transporters in the rat. Reproductive Toxicology. 2008;26:220–230. doi: 10.1016/j.reprotox.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Donato & Castell (2003).Donato MT, Castell JV. Strategies and molecular probes to investigate the role of cytochrome P450 in drug metabolism: focus on in vitro studies. Clinical Pharmacokinetics. 2003;42:153–178. doi: 10.2165/00003088-200342020-00004. [DOI] [PubMed] [Google Scholar]
- Durnas, Loi & Cusack (1990).Durnas C, Loi CM, Cusack BJ. Hepatic drug metabolism and aging. Clinical Pharmacokinetics. 1990;19:359–389. doi: 10.2165/00003088-199019050-00002. [DOI] [PubMed] [Google Scholar]
- Elbarbry, McNamara & Alcorn (2007).Elbarbry FA, McNamara PJ, Alcorn J. Ontogeny of hepatic CYP1A2 and CYP2E1 expression in rat. Journal of Biochemical and Molecular Toxicology. 2007;21:41–50. doi: 10.1002/jbt.20156. [DOI] [PubMed] [Google Scholar]
- Elcombe et al. (2012).Elcombe CR, Elcombe BM, Foster JR, Chang SC, Ehresman DJ, Butenhoff JL. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARalpha and CAR/PXR. Toxicology. 2012;293:16–29. doi: 10.1016/j.tox.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Hart et al. (2009).Hart SN, Cui Y, Klaassen CD, Zhong XB. Three patterns of cytochrome P450 gene expression during liver maturation in mice. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2009;37:116–121. doi: 10.1124/dmd.108.023812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2005).He XJ, Ejiri N, Nakayama H, Doi K. Effects of pregnancy on CYPs protein expression in rat liver. Experimental and Molecular Pathology. 2005;78:64–70. doi: 10.1016/j.yexmp.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Hines (2008).Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacology and Therapeutics. 2008;118:250–267. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hines (2013).Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. International Journal of Phamaceutics. 2013;452:3–7. doi: 10.1016/j.ijpharm.2012.05.079. [DOI] [PubMed] [Google Scholar]
- Hou et al. (2014a).Hou WY, Xu SF, Zhu QN, Lu YF, Cheng XG, Liu J. Age- and sex-related differences of organic anion-transporting polypeptide gene expression in livers of rats. Toxicology and Applied Pharmacology. 2014a;280:370–377. doi: 10.1016/j.taap.2014.08.020. [DOI] [PubMed] [Google Scholar]
- Hou et al. (2014b).Hou WYZD, Zhu QN, Xu SF, Wu Q, Lu YF, Liu J. Age-dependent expression of metallothionein in livers of rats. Journal of Zunyi Medical College. 2014b;37:111–116. [Google Scholar]
- Hu (2017).Hu SX. Age-related change of hepatic uridine diphosphate glucuronosyltransferase and sulfotransferase activities in male chickens and pigs. Journal of Veterinary Pharmacology and Therapeutics. 2017;40:270–278. doi: 10.1111/jvp.12355. [DOI] [PubMed] [Google Scholar]
- Jia et al. (2014).Jia LL, Zhong ZY, Li F, Ling ZL, Chen Y, Zhao WM, Li Y, Jiang SW, Xu P, Yang Y, Hu MY, Liu L, Liu XD. Aggravation of clozapine-induced hepatotoxicity by glycyrrhetinic acid in rats. Journal of Pharmacological Sciences. 2014;124:468–479. doi: 10.1254/jphs.13257FP. [DOI] [PubMed] [Google Scholar]
- Kang et al. (2008).Kang JS, Wanibuchi H, Morimura K, Wongpoomchai R, Chusiri Y, Gonzalez FJ, Fukushima S. Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicology and Applied Pharmacology. 2008;228:295–300. doi: 10.1016/j.taap.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Kawase et al. (2015).Kawase A, Ito A, Yamada A, Iwaki M. Age-related changes in mRNA levels of hepatic transporters, cytochrome P450 and UDP-glucuronosyltransferase in female rats. European Journal of Drug Metabolism and Pharmokinetics. 2015;40:239–244. doi: 10.1007/s13318-014-0208-7. [DOI] [PubMed] [Google Scholar]
- Kilanowicz et al. (2015).Kilanowicz A, Czekaj P, Sapota A, Skrzypinska-Gawrysiak M, Bruchajzer E, Darago A, Czech E, Plewka D, Wiaderkiewicz A, Sitarek K. Developmental toxicity of hexachloronaphthalene in Wistar rats. A role of CYP1A1 expression. Reproductive Toxicology. 2015;58:93–103. doi: 10.1016/j.reprotox.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Kirby et al. (1996).Kirby GM, Batist G, Alpert L, Lamoureux E, Cameron RG, Alaoui-Jamali MA. Overexpression of cytochrome P-450 isoforms involved in aflatoxin B1 bioactivation in human liver with cirrhosis and hepatitis. Toxicologic Pathology. 1996;24:458–467. doi: 10.1177/019262339602400408. [DOI] [PubMed] [Google Scholar]
- Kot & Daujat-Chavanieu (2018).Kot M, Daujat-Chavanieu M. Altered cytokine profile under control of the serotonergic system determines the regulation of CYP2C11 and CYP3A isoforms. Food and Chemical Toxicology. 2018;116:369–378. doi: 10.1016/j.fct.2018.04.051. [DOI] [PubMed] [Google Scholar]
- Krekels et al. (2012).Krekels EH, Danhof M, Tibboel D, Knibbe CA. Ontogeny of hepatic glucuronidation; methods and results. Current Drug Metabolism. 2012;13:728–743. doi: 10.2174/138920012800840455. [DOI] [PubMed] [Google Scholar]
- Lam, Mast & Pikuleva (2018).Lam M, Mast N, Pikuleva IA. Drugs and scaffold that inhibit cytochrome P450 27A1 in vitro and in vivo. Molecular Pharmacology. 2018;93:101–108. doi: 10.1124/mol.117.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2017).Li H, Canet MJ, Clarke JD, Billheimer D, Xanthakos SA, Lavine JE, Erickson RP, Cherrington NJ. Pediatric cytochrome P450 activity alterations in nonalcoholic steatohepatitis. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2017;45:1317–1325. doi: 10.1124/dmd.117.077644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2009).Li Y, Cui Y, Hart SN, Klaassen CD, Zhong XB. Dynamic patterns of histone methylation are associated with ontogenic expression of the Cyp3a genes during mouse liver maturation. Molecular Pharmacology. 2009;75:1171–1179. doi: 10.1124/mol.108.052993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2014).Liu J, Lu H, Lu YF, Lei X, Cui JY, Ellis E, Strom SC, Klaassen CD. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicological Sciences. 2014;141:538–546. doi: 10.1093/toxsci/kfu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach et al. (2014).Mach J, Huizer-Pajkos A, Cogger VC, McKenzie C, Le Couteur DG, Jones BE, De Cabo R, Hilmer SN. The effect of aging on acetaminophen pharmacokinetics, toxicity and Nrf2 in Fischer 344 rats. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2014;69:387–397. doi: 10.1093/gerona/glt095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach et al. (2016).Mach J, Huizer-Pajkos A, Mitchell SJ, McKenzie C, Phillips L, Kane A, Jones B, De Cabo R, Cogger V, Le Couteur DG, Hilmer SN. The effect of ageing on isoniazid pharmacokinetics and hepatotoxicity in Fischer 344 rats. Fundamental and Clinical Pharmacology. 2016;30:23–34. doi: 10.1111/fcp.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalova, Urbanek & Anzenbacher (2016).Matalova P, Urbanek K, Anzenbacher P. Specific features of pharmacokinetics in children. Drug Metabolism Reviews. 2016;48:70–79. doi: 10.3109/03602532.2015.1135941. [DOI] [PubMed] [Google Scholar]
- McPhail et al. (2016).McPhail BT, White CA, Cummings BS, Muralidhara S, Wilson JT, Bruckner JV. The immature rat as a potential model for chemical risks to children: ontogeny of selected hepatic P450s. Chemico-Biological Interactions. 2016;256:167–177. doi: 10.1016/j.cbi.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Mori et al. (2007).Mori K, Blackshear PE, Lobenhofer EK, Parker JS, Orzech DP, Roycroft JH, Walker KL, Johnson KA, Marsh TA, Irwin RD, Boorman GA. Hepatic transcript levels for genes coding for enzymes associated with xenobiotic metabolism are altered with age. Toxicologic Pathology. 2007;35:242–251. doi: 10.1080/01926230601156286. [DOI] [PubMed] [Google Scholar]
- Munro et al. (2018).Munro AW, McLean KJ, Grant JL, Makris TM. Structure and function of the cytochrome P450 peroxygenase enzymes. Biochemical Society Transactions. 2018;46:183–196. doi: 10.1042/bst20170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann et al. (2016).Neumann E, Mehboob H, Ramirez J, Mirkov S, Zhang M, Liu W. Age-dependent hepatic UDP-glucuronosyltransferase gene expression and activity in children. Frontiers in Pharmacology. 2016;7 doi: 10.3389/fphar.2016.00437. Article 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osabe et al. (2008).Osabe M, Sugatani J, Fukuyama T, Ikushiro S, Ikari A, Miwa M. Expression of hepatic UDP-glucuronosyltransferase 1A1 and 1A6 correlated with increased expression of the nuclear constitutive androstane receptor and peroxisome proliferator-activated receptor alpha in male rats fed a high-fat and high-sucrose diet. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2008;36:294–302. doi: 10.1124/dmd.107.017731. [DOI] [PubMed] [Google Scholar]
- Park et al. (2016).Park SY, Kim CH, Lee JY, Jeon JS, Kim MJ, Chae SH, Kim HC, Oh SJ, Kim SK. Hepatic expression of cytochrome P450 in Zucker diabetic fatty rats. Food and Chemical Toxicology. 2016;96:244–253. doi: 10.1016/j.fct.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Saghir, Khan & McCoy (2012).Saghir SA, Khan SA, McCoy AT. Ontogeny of mammalian metabolizing enzymes in humans and animals used in toxicological studies. Critical Reviews in Toxicology. 2012;42:323–357. doi: 10.3109/10408444.2012.674100. [DOI] [PubMed] [Google Scholar]
- Salmin et al. (2017).Salmin SF, Giroux MC, Vachon P, Beaudry F. In vitro metabolism of specific CYP2D and CYP3A opioid substrates using rat liver S9 fractions and mass spectrometry reveal a severe metabolic impairment with increasing age. Biomedical Chromatography. 2017;31:1–11. doi: 10.1002/bmc.3786. [DOI] [PubMed] [Google Scholar]
- Shi & Klotz (2011).Shi S, Klotz U. Age-related changes in pharmacokinetics. Current Drug Metabolism. 2011;12:601–610. doi: 10.2174/138920011796504527. [DOI] [PubMed] [Google Scholar]
- Strupp et al. (2018).Strupp C, Bomann WH, Spezia F, Gervais F, Forster R, Richert L, Singh P. A human relevance investigation of PPARalpha-mediated key events in the hepatocarcinogenic mode of action of propaquizafop in rats. Regulatory Toxicology and Pharmacology. 2018;95:348–361. doi: 10.1016/j.yrtph.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Velenosi et al. (2012).Velenosi TJ, Fu AY, Luo S, Wang H, Urquhart BL. Down-regulation of hepatic CYP3A and CYP2C mediated metabolism in rats with moderate chronic kidney disease. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 2012;40:1508–1514. doi: 10.1124/dmd.112.045245. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang H, Li W, Muhammad I, Sun X, Cui X, Cheng P, Qayum A, Zhang X. Biochemical basis for the age-related sensitivity of broilers to aflatoxin B1. Toxicology Mechanisms and Methods. 2018;28:361–368. doi: 10.1080/15376516.2018.1428258. [DOI] [PubMed] [Google Scholar]
- Warrington, Greenblatt & Von Moltke (2004).Warrington JS, Greenblatt DJ, Von Moltke LL. Age-related differences in CYP3A expression and activity in the rat liver, intestine, and kidney. Journal of Pharmacology and Experimental Therapeutics. 2004;309:720–729. doi: 10.1124/jpet.103.061077. [DOI] [PubMed] [Google Scholar]
- Wauthier, Verbeeck & Calderon (2007).Wauthier V, Verbeeck RK, Calderon PB. The effect of ageing on cytochrome p450 enzymes: consequences for drug biotransformation in the elderly. Current Medicinal Chemistry. 2007;14:745–757. doi: 10.2174/092986707780090981. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2018a).Xu S, Hou D, Liu J, Ji L. Age-associated changes in GSH S-transferase gene/proteins in livers of rats. Redox Report. 2018a;23:213–218. doi: 10.1080/13510002.2018.1546985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2018b).Xu SF, Ji LL, Wu Q, Li J, Liu J. Ontogeny and aging of Nrf2 pathway genes in livers of rats. Life Sciences. 2018b;203:99–104. doi: 10.1016/j.lfs.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Yun et al. (2010).Yun KU, Oh SJ, Oh JM, Kang KW, Myung CS, Song GY, Kim BH, Kim SK. Age-related changes in hepatic expression and activity of cytochrome P450 in male rats. Archives of Toxicology. 2010;84:939–946. doi: 10.1007/s00204-010-0520-1. [DOI] [PubMed] [Google Scholar]
- Zanger & Schwab (2013).Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacology and Therapeutics. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2017).Zhu QN, Hou WY, Xu SF, Lu YF, Liu J. Ontogeny, aging, and gender-related changes in hepatic multidrug resistant protein genes in rats. Life Sciences. 2017;170:108–114. doi: 10.1016/j.lfs.2016.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Raw data is available in the Supplemental Files.