Abstract
Leptospira interrogans genome is predicted to encode multiple isoforms of caseinolytic proteases (ClpP1 and ClpP2). The ClpP proteins with the aid of its ATPase chaperone are known to be involved in establishing cellular proteostasis and have emerged as a target for developing new antibiotics. We report the molecular characterization of recombinant ClpP1 (rClpP1) and rClpP2 of Leptospira along with its ATPase chaperone rClpX. The two isoforms of rClpPs when coupled together in an equivalent concentration exhibit optimum activity on small fluorogenic peptide substrates, whereas the pure rClpP isoforms are enzymatically inactive. Isothermal titration calorimetry analysis suggests that the two rClpP isoforms bind each other moderately in a 1:1 stoichiometry with a dissociation constant of 2.02 ± 0.1 μM at 37 °C and is thermodynamically favored. Size exclusion chromatography fractionates the majority of pure rClpP1 at ≥308 kDa (14–21-mer) and the pure rClpP2 at 308 kDa (tetradecamer), whereas the functionally active rClpP isoform mixture fractionates as a tetradecamer. The distinct and unprecedented oligomeric form of rClpP1 was also evident through native-gel and dynamic light scattering. Moreover, the rClpP isoform mixture formed after the site-directed mutation of either or both the isoforms at one of the catalytic triad residues (Ser 98/97 to Ala 98/97) resulted in the complete loss of protease activity. The rClpP isoform mixture gets stimulated to degrade the casein substrate in the presence of rClpX and in an energy-dependent manner. On the contrary, pure rClpP1 or the rClpP2 isoform in association with rClpX are incapable of forming operative protease. The reported finding suggests that in Leptospira, the enzymatic activity of the rClpP protease complex in the presence or absence of cochaperone is performed solely by the tetradecamer structure which is hypothesized to be composed of 2-stacked ClpP heptameric rings, wherein each ring is a homo-oligomer of ClpP1 and ClpP2 subunits. Understanding the activities and regulation principle of multi-isoforms of ClpP in pathogenic bacteria may aid in intervening disease outcomes particularly to the co-evolving antibiotic resistance strains.
Introduction
Leptospira interrogans is a spirochete of zoonotic importance responsible for causing leptospirosis disease in animals and humans worldwide.1 It is estimated that over a million human cases of severe leptospirosis are reported each year globally, with approximately 60 000 deaths from this disease.2 It is worth noting that leptospirosis is a serious economic problem in developing tropical and subtropical countries.3,4 Each year, there are significant economic losses due to reproductive disorders in cattle, sheep, pigs, and horses that are suffering from leptospirosis.5 Despite the severity of leptospirosis and its global importance, the molecular mechanisms of the Leptospira pathogenesis are not well understood, possibly due to the difficulty in studying the causal agent by the reverse genetics approach.6 Identification of the Leptospira virulence factors and characterization of their activity is particularly important for understanding the mechanisms of the disease. In the last two decades, there is a growing list of pathogens shown to be impaired in their ability to infect or cause disease when lacking the components of the Clp protease system,7−15 including the chaperone–peptidase complex (ClpY-Q) in pathogenic spirochete L. interrogans.16 The caseinolytic protease system is composed of the core catalytic components, regulatory chaperones (ATPases), and the adaptor proteins.17 It has been observed that Escherichia coli ClpP forms complexes with AAA+ (ATPases associated with various cellular activities) chaperones, ClpX and ClpA.18,19 The Clp adaptors20 and anti-adaptors21 provide other means for the extensive regulation of ClpP-dependent protein degradation. Clp adaptor protein binds to the N-terminal domain of ClpA altering its substrate specificities.22
The core catalytic components of the Clp protease system in leptospires are encoded by clpP1, clpP2, and clpQ gene. The protease complex of ClpP and its cochaperone constitutes a fascinating Clp protease system that is still required to be explored for designing suitable drugs either by targeting protein activities or protein interactions during cellular proteostasis. One of the Leptospira caseinolytic protease complex ClpQ–ClpY (HslUV)23 has been demonstrated to have a critical role in leptospiral survival in hosts and transmission of leptospirosis.16 However, there is a lack of biochemical and functional information regarding multi-ClpP isoforms present in the Leptospira.
Caseinolytic protease P (ClpP) is a well-conserved cylindrical/barrel-shaped serine protease in most bacterial species.24 A bacterium needs such protease to maintain protein homeostasis. Bacterial ClpP has an important role in the degradation of misfolded or damaged proteins,25 virulence,26 numerous regulatory processes27−29 such as cell division, stress tolerance, morphological differentiation, and antibiotic resistance. Unlike other serine proteases, ClpP proteases have evolved as self-compartmentalized barrel-shaped enzymes to establish cellular proteostasis.30 Such a scaffold of ClpP prevents the bacterial cytosol matrix from breaching their catalytic site (Ser-His-Asp) harbored inside the barrel-shaped enzyme. In recent years, with the advent of ClpP-targeting antibacterial compounds, the major emerging focus of such a study is to develop an alternative therapy for antibiotic-resistant and persistent microbes.31 While ClpPs from single isoform-expressing bacteria have been studied in detail, the function and regulation of species including L. interrogans with more than one clpP gene are still poorly understood. Several pathogenic bacteria like Mycobacterium tuberculosis, Listeria monocytogenes, Chlamydia trachomatis, and Pseudomonas aeruginosa that harbor two isoforms of ClpP have been demonstrated to play an indispensable role in virulence.11,32−34 These multiple ClpP isoforms tend to reveal structural and functional disparity in an organism-specific manner. It has been established that in E. coli(35) and Mycobacterium,36 the ClpP protomers must undergo proteolytic preprocessing at the N-terminal for achieving the functional activity. Nevertheless, in many other pathogenic bacterial ClpP protomers32,34,37 with multiple isoforms, the peptidase activity have been demonstrated even without such preprocessing. The barrel-shaped ClpP protease with axial pores strictly gives access to the unfolded polypeptide generated by ATP-dependent regulatory chaperones. Probing the behavior of ClpP systems in multi-isoform-containing organisms enables additional understanding of the function and regulation of ClpP systems leading to its clinical exploitation in intervening disease outcomes. In the present study, comprehensive molecular characterization of the multi-ClpP isoforms of Leptospira along with its co-chaperone ClpX was executed.
Results and Discussion
Caseinolytic Protease Genes in the Leptospira Genome
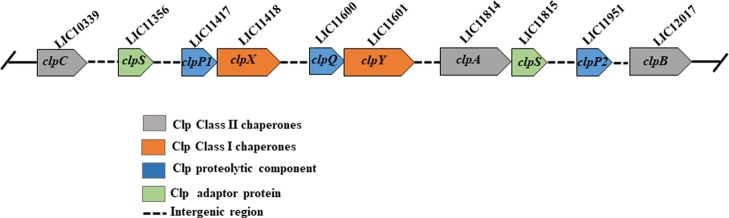
The genome analysis of the sequenced spirochete, L. interrogans serovar Copenhageni strain Fiocruz L1-130 shows that it harbors various genes of the caseinolytic protease (clp) system. The caseinolytic protease system in Leptospira is composed of genes encoding catalytic, regulatory, and adaptor proteins. The set of genes encoding catalytic components of the clp system in Leptospira are clpP1 (LIC11417), clpP2 (LIC11951), and clpQ (LIC11600). The Clp AAA+ (ATPases associated with various cellular activities) are encoded by clpX (LIC11418), clpA (LIC11814), clpB (LIC12017), clpC (LIC10339), and clpY (LIC11601) genes, whereas the adaptor proteins are encoded by clpS (LIC11356 and LIC11815) (Figure 1 and Table 1). It has been previously reported that the genes encoding caseinolytic proteases are highly conserved in both saprophytic and pathogenic strains of Leptospira and comprise a part of the core group of genes in the genus Leptospira.38 The two core catalytic components ClpP1 and ClpP2 are the paralogs of peptidase ClpP, a serine protease, which is predicted to perform the proteolysis activity mainly in association with the regulatory proteins. The components ClpA, ClpB, ClpC, ClpX, and ClpY are the chaperone proteins belonging to the Clp/Heat shock protein 100 (Hsp100) family that carry AAA+ domains, a characteristic of the ATPase superfamily. These Clp ATPase subunits have been clustered as the class-I and class-II chaperone based on the number of the AAA+ domain it carries. The class-I chaperone (ClpX and ClpY) in Leptospira carries one AAA+ domain, whereas class-II chaperone (ClpA, ClpB, and ClpC) carries two AAA+ domains (Figure 1). Among the class-II chaperone, ClpB lacks a binding motif (P-loop motif) at the C-terminal that is essential for forming the Clp proteolytic complex. Therefore, the Leptospira ClpB mainly mediates protein disaggregation with or without the co-operation of the DnaK chaperone system and is established to be a virulence factor in pathogenic Leptospira.23,39 In addition, deletion of the ClpB chaperone in Leptospira exhibited impaired growth under stress conditions.23 In the Leptospira genome, the genes clpP1 and clpX are located in close proximity to each other with an intergenic gap of 10 base pairs (bps), whereas clpP1 and clpP2 are separated by 34 different genes (Table 1). Based on the location of the genes encoding ClpP isoforms in the Leptospira genome, these clpP genes may be independently transcribed and regulated. Using a quantitative real-time polymerase chain reaction (qRT-PCR), the analysis of mRNA transcripts of clpP isoforms in Leptospira demonstrated each gene to be differentially transcribed (Figure S1 and Table S1). In Leptospira spp., the existence of two paralogs of the clpS gene were also predicted, where one (LIC11815) of the clpS genes lies adjacent to clpA (LIC11814), whereas the other (LIC11356) clpS is located distant apart on the chromosome (Figure 1 and Table 1). The catalytic unit ClpQ, a threonine protease of Leptospira has been established previously to interact with the chaperone ClpY to form the functional ClpYQ protease (also known as HslUV).16 Moreover, in a previous study, it is proposed that regulatory proteins ClpX, ClpA, and ClpC of Leptospira have a binding motif (P-loop motif) at the C-terminal that may interact with the N-terminal loop of ClpP and form the Clp proteolytic complex.23 Therefore, it was interesting to study how multi-isoforms of ClpP in Leptospira interact with its chaperone to form the operative proteases.
Figure 1.
Schematic arrangement of genes encoding a caseinolytic protease (clp) system in L. interrogans genome. The arrangement of Clp genes and its annotation in the Leptospira genome are represented by an arrow diagram (not scaled) based on bioinformatics analysis and previous report.23 The caseinolytic protease system in Leptospira is composed of genes encoding catalytic subunits (clpP1, clpP2, clpQ; blue color), Clp AAA+ subunits (clpA, clpB, clpC, clpX, and clpY), and adaptor proteins (clpS; green color). The intergenic regions are depicted as interrupted lines. The Clp AAA+ proteins have been grouped as class-I (ClpX, ClpY; orange color) and as the class-II chaperone (ClpA, ClpB, ClpC; grey color) based on the number of ATPase domain(s).
Table 1. Caseinolytic Protease (clp) Genes Location in Leptospira Genome.
| gene (locus tag) | gene coordinates (NCBI accession no. NC_005823.1) | UniProt accession no. | gene size (bp) | intergenic region (bp) (between successive clp genes) |
|---|---|---|---|---|
| clpC (LIC10339) | 384 088–386 628 | Q72VF8 | 2541 | 1 283 538 (clpC & clpS) |
| clpS (LIC11356) | 1 670 167–1 670 487 | Q72SM3 | 321 | 72 860 (clpS & clpP1) |
| clpP1 (LIC11417) | 1 743 348–1 743 944 | Q72SG6 | 597 | 10 (clpP1 & clpX) |
| clpX (LIC11418) | 1 743 955–1 745 217 | Q72SG5 | 1263 | 214 309 (clpX & clpQ) |
| clpQ (LIC11600) | 1 959 527–1 960 069 | Q72RY8 | 543 | 9 (clpQ & clpY) |
| clpY (LIC11601) | 1 960 079–1 961 518 | Q72RY7 | 1440 | 245 082 (clpY & clpA) |
| clpA (LIC11814) | 2 206 601–2 208 841 | Q72RD2 | 2241 | 4 bp overlap (clpA & clpS) |
| clpS (LIC11815) | 2 208 838–2 209 173 | Q72RD1 | 336 | 150 158 (clpS & clpP2) |
| clpP2 (LIC11951) | 2 359 332–2 359 925 | Q72R01 | 594 | 68 240 (clpP2 & clpB) |
| clpB (LIC12017) | 2 428 166–2 430 748 | Q72QU2 | 2583 | not applicable |
Molecular Characterization of Core Catalytic Component ClpP
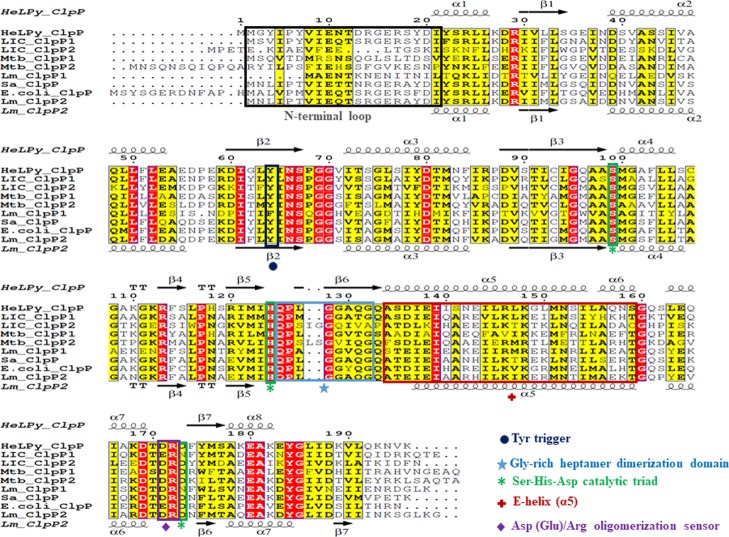
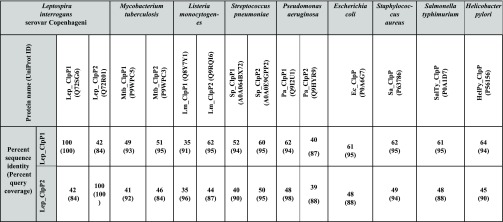
The open reading frames of full-length clpP1 (597 bp), clpP2 (594 bp), and clpX (1263 bp) were amplified by PCR using genomic DNA as a template of L. interrogans. The PCR amplicons of clpP1, clpP2, and clpX were cloned separately in pET23a expression vector, and the recombinant proteins were overexpressed in E. coli BL21 (DE3) cells (Figure 2A). The overexpressed recombinant proteins (rClpP1, 23 kDa; rClpP2, 22 kDa; and rClpX, 47 kDa) of Leptospira were purified using Ni-NTA affinity column chromatography (Figure 2B). The purified rClpP1 and rClpP2 were used to generate polyclonal antibodies in rabbit and BALB/c mice, respectively. In silico analysis of the available amino acid (aa) sequence of clpP1 and clpP2 in L. interrogans Copenhageni exhibits 100% identity to its orthologs in another pathogenic serovar, L. interrogans Lai (clpP1-LA2559; clpP2-LA1953), whereas nonpathogenic serovar Leptospira biflexa Patoc, possesses only 44.1% (clpP1-LEPBI_I1760) and 42.5% (clpP2-LEPBI_I0969) identities. The ClpP isoforms in the Leptospira (serovars Lai and Copenhageni) lysates were detected at a comparable molecular size to that of the recombinant protein on immunoblotting and no remarkable detection was observed in the serovar Patoc (Figure 2C,D). The immunoblot result of the rClpP isoforms in Leptospira serovars is in agreement with the in silico findings. We also validated the cross-reactivity of the generated polyclonal antibodies towards each rClpP isoforms of Leptospira as they show 42% identities within themselves. To our surprise, a cross-reactivity of anti-ClpP1 antibodies with rClpP2 using immunoblots was detected; however, anti-ClpP2 antibodies were very specific (data not shown). In recent years, well-designed biophysical studies have established the ClpP function to be governed by inter and intramolecular conformational switches that conduct regulation signals through the complex.26,37 Therefore, to determine if critical motifs for conducting regulation signals are conserved, we aligned the primary sequences of Leptospira ClpP1 and ClpP2 to other organisms having well-characterized ClpP orthologs. Multiple sequence alignment (MSA) of ClpPs in Leptospira and its orthologs in Helicobacter, Listeria, Mycobacterium, Staphylococcus, and Escherichia was demonstrated to have a conserved catalytic triad (Ser-His-Asp), an essential motif for the charge-relay in the serine peptidase family (Figure 3). The catalytic triad residues in ClpP1 and ClpP2 of Leptospira achieved alignment at Ser98-His123-Asn172 and Ser97-His122-Asp173, respectively. Interestingly, ClpP1 isoforms of Leptospira has an Asn172 residue instead of the commonly observed Asp residue in the catalytic triad, which is similar to that reported ClpP1 catalytic triad in Listeria(37) (Figure 3). Other key hot spots of ClpP, like the Tyr activation trigger, Asp (Glu)/Arg oligomerization sensor domains, and the Gly-rich heptamer dimerization domain, are highly conserved in both ClpP isoforms of L. interrogans. In contrast, significant variations are observed in the motif E-helix (α5) and the N-terminal loop of ClpP isoforms that is known to intercalate with the cognate opposite heptameric ring and its hexameric ATPase chaperone, respectively. Such amino acid sequence variations at these hotspots encouraged us to characterize the ClpP isoform function and its regulation in Leptospira. The sequence identity of ClpP1 and ClpP2 amino acid of Leptospira with the orthologs in selected pathogenic bacteria is in the range of 35–64 and 35–50%, respectively (Table 2). The highest sequence identity of ClpP1 and ClpP2 amino acids of Leptospira was found with its orthologs in Helicobacter pylori (64%) and Streptococcus pneumoniae (50%), respectively.
Figure 2.

Molecular characterization of L. interrogans ClpP1, ClpP2, and ClpX. (A) Cloning and overexpression of ClpPs and ClpX in E. coli. The Coomassie-blue stained 12% SDS-polyacrylamide gel showing the resolved protein lysates of E. coli cells overexpressing LeptospiraclpP1 (pET23a-clpP1), clpP2 (pET23a-clpP2), and clpX (pET23a-clpX), after induction with (+) or without (−) 1 mM IPTG. (B) The affinity-purified Leptospira recombinant proteins. The Ni-NTA affinity-purified rClpP1 (∼23 kDa), rClpP2 (∼22 kDa), and rClpX (∼47 kDa) proteins were resolved on 12% SDS-polyacrylamide gel and stained with Coomassie-blue. In image (A,B), M denotes the standard protein molecular size marker (in kDa). (C,D) Western blot of native ClpP1 and ClpP2 of Leptospira, respectively. The native ClpP1 and ClpP2 in Leptospira lysates were detected in pathogenic strains (serovar Lai and Copenhageni) but not in the nonpathogenic strain (serovar Patoc) using the primary antibodies generated against rClpP1 and rClpP2, respectively. Native and recombinant ClpP isoforms were detected at a similar molecular size.
Figure 3.
MSA and secondary structure assignment of ClpP proteases. MSA of ClpP orthologs from various pathogenic bacteria were performed using Clustal Omega software. The ClpP orthologs used for amino acid alignment are HelPy_ClpP: H. pylori (P56156), LIC_ClpP1: L. interrogans Copenhageni (Q72SG6), LIC_ClpP2: L. interrogans Copenhageni (Q72R01), Mtb_ClpP1: M. tuberculosis (P9WPC5), Mtb_ClpP2: M. tuberculosis (P9WPC3), Lm_ClpP1: L. monocytogenes (Q8Y7Y1), Sa_ClpP: Staphylococcus aureus (P63786); E.coli_ClpP: E. coli (P0A6G7), Lm_ClpP2: L. monocytogenes (Q9RQI6). The UniProt accession numbers are provided in the parenthesis. Secondary structural elements present in the HelPy_ClpP (PDB ID: 2ZL0) and Lm_ClpP2 (PDB ID: 4YRF) structures are shown on the top and bottom of the sequence alignment, respectively. Identical and semi-identical residues in ClpP proteases are highlighted in red and yellow colors, respectively, with residue numbers at the top of the alignment after HelPy_ClpP. The conserved key structural motifs in the ClpP isoforms including the Tyr63 activation trigger, catalytic triad (Ser-His-Asp), Asp(Glu)/Arg oligomerization sensor domains, E-helix, and the Gly-rich heptamer dimerization domain are outlined in different colored boxes and marked with specific symbols at the bottom of the box.
Table 2. Comparative Analyses of Leptospira ClpP Orthologs in Selective Pathogenic Bacteria.
ClpP1 and ClpP2 Coupling are Essential for Cleaving Small Peptide Substrates
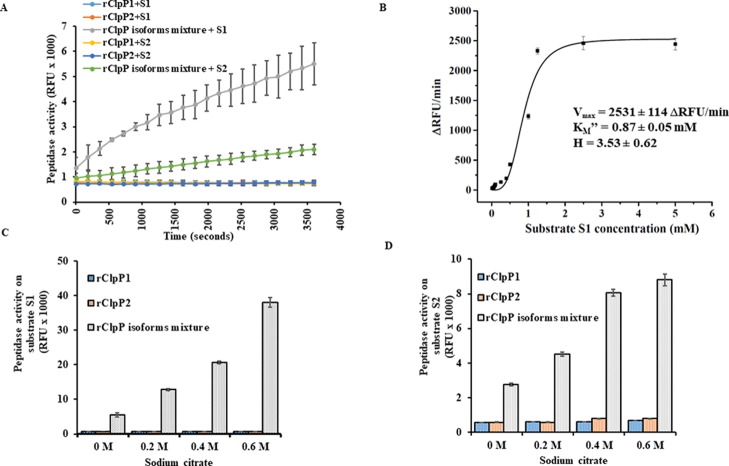
The MSA of ClpP isoforms of Leptospira shows that both ClpPs retain their key structural motifs, although only 42% percent identity exists within themselves (Table 2). Such an observation incites queries whether single or both ClpP isoform(s) would form active tetradecameric complexes capable of peptide/protein degradation. The catalytic triad Ser-His-Asp alignment of ClpP in the extended conformation is capable of degrading small peptides (<5–6 amino acids) without the requirement of its chaperone or ATP.40 Therefore, the capability of pure rClpP isoforms to hydrolyze fluorogenic di- and tetra-peptides such as Suc-LY-AMC40 (S1) and Suc-LLVY-AMC40 (S2), respectively, and a fluorescein isothiocyanate-labeled full-length casein protein substrate41 (FITC-casein, S3) were evaluated. Unexpectedly, the pure rClpP isoforms of Leptospira was enzymatically inactive on the fluorogenic small peptide substrates (S1 and S2) till 1 h of the reaction (Figure 4A). For further validation, the peptidase reaction time was extended till 48 h but no peptidolytic activity was measured using the pure rClpP isoforms (data not shown). The possibility of N-terminal preprocessing of ClpP isoforms of Leptospira to produce mature ClpP peptidase was not determined as there are other reported pathogenic bacterial species like Listeria,37Clostridium,42Pseudomonas,32 and Chlamydia(34) whose pure ClpP isoforms showed functional activity without any preprocessing. However, when pure rClpP isoforms of Leptospira were incubated together in an equimolar concentration for 10 min at 37 °C, the resulting rClpP isoform mixture was able to hydrolyze the fluorogenic small peptide substrates (S1 and S2) at variable rates (Figure 4A). The substrate S1 was selected to determine the enzyme kinetic parameters of the rClpP isoform mixture due to its faster rate of hydrolysis and accepted model peptide substrate for ClpP protease study. Unlike other reported enzyme kinetics of the functional ClpP16,42 which show Michaelis–Menten kinetics, Leptospira rClpP isoform mixture exhibit Hill kinetics on fluorogenic substrate S1. The substrate S1 cleavage saturation curve by rClpP isoform mixture was sigmoidal with a Vmax of 2531 ± 114 ΔRFU/min and apparent KM (KM″) of 0.87 ± 0.05 mM. The dependency on the substrate (S1) concentration for increased peptidase activity of rClpP isoforms reflected a slightly cooperative mechanism with a Hill coefficient (H) of 3.53 ± 0.62 (Figure 4B). This suggests that the multiple molecules of the substrate (S1) bind to the rClpP1P2 mixture of Leptospira and stimulate its activity, in addition to being its substrate. Previously, activation of such an inactive ClpP1P2 complex of M. tuberculosis by peptide activators (Z-Leu-Leu and Z-Leu-leucinal) in the hydrolysis of Z-Gly-Gly-Leu-AMC (0.1 mM) and Ac-Nle-Pro-Nle-Asp-AMC (0.1 mM) substrates followed a highly cooperative mechanism (H = 5–7).36 Additionally, in agreement with the earlier evidence of the ClpP biochemical function in other pathogens,22 no protease activity was recorded on the FITC-casein substrate (S3) by the rClpP isoform mixture of Leptospira (data not shown). These multiple ClpP isoforms tend to exhibit the functional disparity in an organism-specific manner.11,32−34 In a recent study on Clostridium difficile, both ClpP isoforms are capable of forming functional peptidase independently.42 Our results of ClpP isoform peptidase activity are in agreement with M. tuberculosis, where both isoforms are also inactive towards the di-peptide substrate.36 It is also possible that Leptospira pure rClpP isoforms may show activity independently on other types of small peptides that are yet to be evaluated. In L. interrogans coupling of each rClpP isoforms ensures achieving peptidase activity on small model peptide substrates (S1 and S2), whereas activity on the large protein substrate (S3) may require additional unfolding by its cochaperone. In addition, although gene encoding each ClpP isoforms of Leptospira are chromosomally far apart and show lower sequence identity, this assay provided us a clue that pure rClpP1 and rClpP2 of Leptospira may interact and self-assemble into a functional heteromeric peptidase complex.
Figure 4.
Peptide degradation assays using recombinant ClpP (rClpP) isoforms of Leptospira. (A) Peptidase activity of rClpP isoforms on the small fluorogenic peptide substrates S1 and S2. Peptide degradation was measured fluorometrically as a relative fluorescent unit (RFU × 1000) using the fluorogenic substrates, Suc-LY-AMC (S1) and Suc-LLVY-AMC (S2) for 1 h. The pure rClpP isoforms are inactive on small peptide substrates (S1 and S2), whereas the rClpP isoform mixture is enzymatically active. (B) Peptidase kinetics of ClpP isoforms on fluorogenic substrate S1 determined using the Hill function. The apparent KM″ or the substrate concentration corresponding to the half-maximal velocity of the reaction (KM = 0.87 ± 0.05 mM), maximal reaction velocity (Vmax = 2531 ± 114 ΔRFU/min), and the Hill coefficient (H = 3.53 ± 0.62) of rClpP isoform mixture during fluorogenic substrate S1 hydrolysis was determined by the nonlinear curve fitting function of Origin Software. (C,D) Effect of sodium citrate on the peptidase activity of rClpP isoform mixture on fluorogenic substrate S1 and S2, respectively. The gradient increase in the sodium citrate concentration stimulates the peptidase activity of rClpP isoform mixture but not of the pure rClpP isoforms. The error bars represent the standard deviations (SDs) from the two independent experiments performed in duplicates.
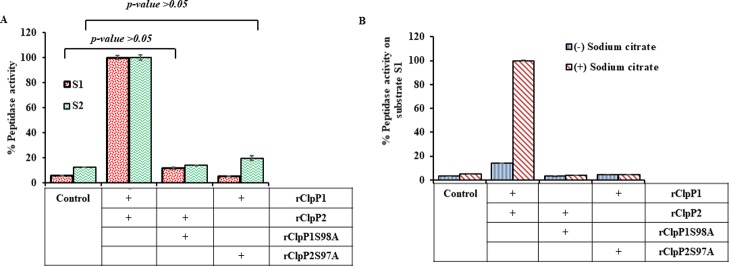
We next assessed whether the activity of rClpP isoform mixture could be enhanced in the presence of sodium citrate, a salt that promotes oligomeric complex stabilization and has been reported to increase ClpP peptidase activity.30,32 It is to be noted that salts like sodium citrate have high Hofmeister strength causing a “salting out” effect and thereby stabilizing functional heteromeric complexes. Interestingly, with the gradient increase in the sodium citrate concentration (0.2–0.6 M), there was an increase in peptidase activity of rClpP isoform mixture on the model peptide substrates (S1 and S2), whereas the pure rClpP1 and rClpP2 did not show any gain in peptidase activity (Figure 4C,D). The effect of sodium citrate on ClpP peptidase activity was in agreement to other reported ClpP multi-isoforms.30,34
Leptospira rClpP1 and rClpP2 Display Optimum Peptidase Activity at 1:1 Stoichiometry
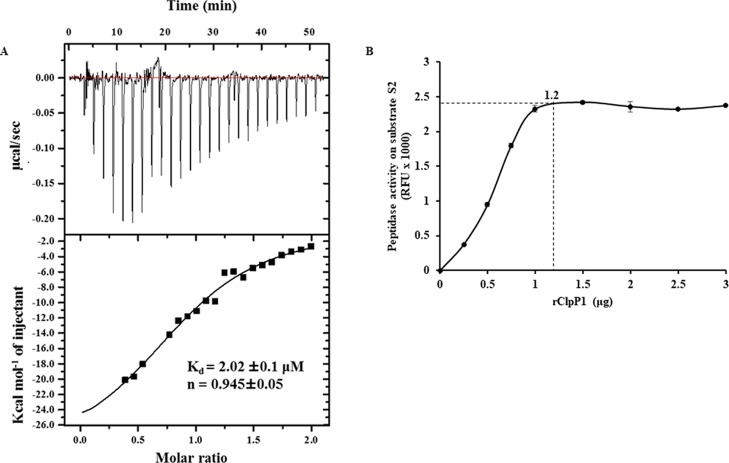
As we showed that in Leptospira each rClpP isoform in combination is essential for peptidase activity on smaller peptide substrates (S1 and S2), it captured our interest to identify the optimum molar-ratio of pure ClpP isoforms required for heterocomplex formation. Isothermal titration calorimetry (ITC) is a well-established method employed to study protein–protein interactions and estimate the stoichiometry of the heterocomplex.43−46 Therefore, the molar ratio required for forming the heterocomplex between the pure rClpP isoforms of Leptospira was determined using ITC measurements. The ITC data demonstrate that 1 molecule (n = 0.945) of rClpP1 was bound to 1 molecule of pure rClpP2 with a dissociation constant (Kd) of 2.02 ± 0.1 μM at 37 °C and is thermodynamically favored (Figure 5A). Moreover, the measured Kd value demonstrates a moderate binding affinity between the two rClpP isoforms and therefore, the coupling of rClpP isoforms and its stabilization may be time dependent. In support to ITC data, an additional peptidase assay was performed with varying ratios of the pure rClpP isoform mixture required to exhibit maximal activity. Upon increasing the amounts of pure rClpP1 (0–2.5 μg) with a constant amount of pure rClpP2 (1.0 μg) in the mixture, the peptidase activity gradually increased on substrate S2. The peptidase activity on fluorogenic substrate S2 (tetrapeptide) reached its optimum when the equimolar ratio (1.1–1.2) of rClpP1 and rClpP2 was mixed (Figure 5B). Such a finding was in agreement with the reported optimum peptidase activity of multi-isoforms of ClpP in Mycobacterium.36 These results suggest that the maximum peptidase activity on substrate S2 is achieved when the mixture of pure rClpP1 and rClpP2 isoforms of Leptospira acquires 1:1 stoichiometry under the given in vitro conditions.
Figure 5.
Leptospira rClpP isoform mixture interacts in an equimolar ratio for optimal peptidase activity. (A) ITC of pure rClpP1 and rClpP2. One molecule of rClpP1 binds with one molecule of rClpP2 at 37 °C. Parameters of the ITC experiment obtained includes the stoichiometry factor (n) = 0.945 ± 0.05; dissociation constant (Kd) = 2.02 ± 0.1 μM; enthalpy (ΔH) = −30 220 ± 2636 cal–1 mol–1; entropy (ΔS) = −71.4 cal–1 mol–1 K–1. (B) Optimum peptidase activity of the rClpP mixture on the fluorogenic substrate Suc-LLVY-AMC (S2). Constant amounts of pure rClpP2 (1 μg) were mixed with increasing amounts of pure rClpP1 and hydrolysis of fluorogenic substrate S2 was measured as a relative fluorescence unit (RFU × 1000). The maximal activity of the rClpP isoform mixture is indicated by the dotted lines in the graph. The error bars represent the SDs from the two independent experiments performed in duplicates.
rClpP1 and rClpP2 of Leptospira Undergo Subunit Rearrangement for Composing Functional ClpP Protease
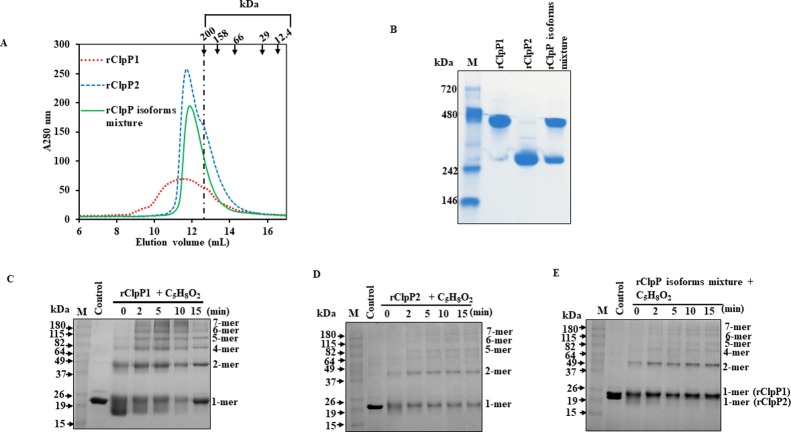
The size-exclusion chromatography (SEC) of the pure rClpP isoforms or its mixture were performed to know if these have the tendency to oligomerize as reported in other ClpP of bacteria.32,36 The SEC analysis demonstrated that the rClpP isoform mixture of Leptospira tends to elute as a tetradecamer exactly like the pure rClpP2 (22 kDa × 14 subunits ∼308 kDa), whereas the pure rClpP1 displays a broad elution profile indicating higher oligomeric species in addition to the tetradecamer (Figure 6A). The pure rClpP isoforms also demonstrated a possible heptameric species (∼200 kDa) as a small shoulder peak during SEC (Figure 6A). Such an oligomerization property was also observed when the pure rClpP isoforms or its mixture were resolved on native polyacrylamide gel electrophoresis (native-PAGE) and stained with Coomassie blue (Figure 6B). However, the oligomeric forms of ClpP1 and the heterocomplex mixture on native-PAGE showed discrepancy in size with respect to the SEC elution profile. The Leptospira pure rClpP1 on native-PAGE got resolved in majority at a higher order of oligomeric species (∼480 kDa; 21-mer) and the pure rClpP2 resolved at the tetradecameric form (∼308 kDa). In SEC and native-PAGE gel, the proteins migrate not only according to their size but also to their shape and hydrodynamic properties. Such a difference in migration behavior of proteins might explain the inconsistency between the sizes of the ClpP oligomers determined. To further validate, the pure rClpP isoforms and its mixture were incubated for a short period (1 h) and an extended period (48 h) at 4 °C to allow time for subunit re-arrangement, and thereafter, resolved on the native-PAGE. A shift in the mobility of rClpP1 to a functional tetradecamer size from 21-mer species was detected on native-PAGE in the rClpP isoform mixture after 48 h of incubation but not in the short period-incubated rClpP isoform mixture (Figure S2). In contrast, the pure rClpP1 resolved at 21-mer species even after 48 h of incubation, an observation that is in agreement with the finding under SEC.
Figure 6.
Leptospira pure rClpP isoforms and its mixture oligomerization. (A) Size exclusion chromatography of pure rClpP isoforms and its mixture. The oligomeric states of pure rClpP isoforms and its mixture were monitored by size exclusion chromatography using an Enrich 650 High-Resolution column. Elution positions of the standard proteins with its molecular weight are indicated in arrows pointing downward. The molecular weight standard protein markers in kiloDalton (kDa) include β-amylase (200), alcohol dehydrogenase (158), albumin (66), carbonic anhydrase (29), and cytochrome C (12.4). The pure rClpP2 and the rClpP isoform mixture were eluted as a tetradecamer (308 kDa), whereas the pure rClpP1 display a broad elution profile (≥308 kDa). A small shoulder peak corresponding to 200 kDa was also seen for pure rClpP1 and rClpP2. (B) Pure rClpP isoforms or its mixture resolved on the native-polyacrylamide gel. The purified rClpP isoforms or its mixture (2 μg) were resolved on a 4–20% native-PAGE and stained with Coomassie-blue. The standard molecular weight marker was run in lane 1 (M). The purified rClpP1 and rClpP2 resolved at around 480 kDa (23 kDa × 21 = 483 kDa) and 308 kDa (22 kDa × 14 = 308 kDa), respectively, whereas the rClpP isoform mixture was resolved at 480 and 308 kDa. (C) Denaturing polyacrylamide gel electrophoresis of pure rClpP1 after glutaraldehyde (C5H8O2) cross-linking. Chemical cross-linking of pure rClpP1 with glutaraldehyde shows distinct higher molecular weight bands that get intensified with time (0–15 min) (D) Denaturing polyacrylamide gel electrophoresis of pure rClpP2 oligomeric subunits after glutaraldehyde cross-linking. Chemical cross-linking of pure rClpP2 with glutaraldehyde (C5H8O2) show distinct higher molecular weight bands in comparison to control (without C5H8O2). (E) Denaturing gel electrophoresis of the rClpP isoform mixture after glutaraldehyde cross-linking. The cross-linked subunits pattern of rClpP isoform mixture is similar to that of pure rClpP isoforms. The denaturing polyacrylamide gels were stained with Coomassie-blue and for clarity; the image obtained is represented after inversion.
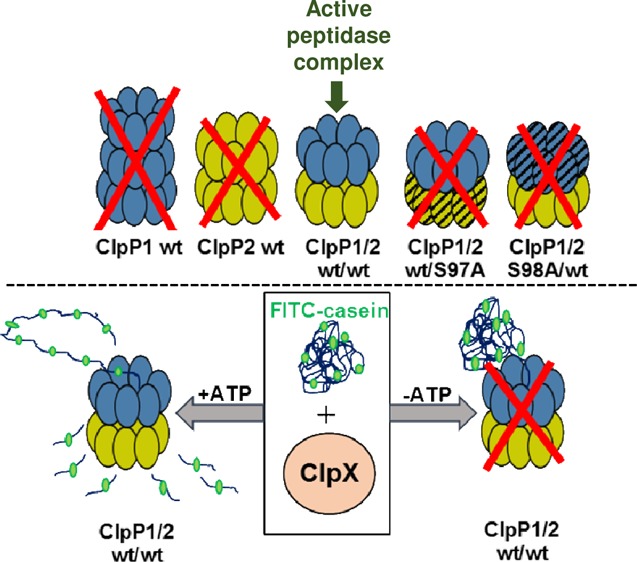
It is to be noted that the pure rClpP1 and rClpP2 of Leptospira are functionally inactive and are fractionating by SEC empirically at ≥308 kDa (14–21-mer) and 308 kDa (tetradecamer), respectively, whereas the functionally active rClpP isoform mixture fractionate as a tetradecamer (Figures 6A and S2). The functional activity in Leptospira rClpP protease is attained only when both the isoforms are contributing together to frame the tetradecameric structure where possibly the catalytic triad are aligned (i.e., active) in an extended conformation. Moreover, because the ITC result demonstrates that the rClpP1 and rClpP2 bind in a 1:1 stoichiometry, the active rClpP protease (14-mer) macromolecule may be composed of 2-stacked heptameric rings (7 + 7-mer) formed by homogenous subunits of rClpP1 and rClpP2. It is also conceivable that each heptameric ring contains a mixture of rClpP1 and rClpP2 subunits, as has been reported in the Synecchococcus ClpP complexes.47,48 To determine the composition of the rClpP heptameric ring, the subunits of pure rClpP isoforms or its mixture was chemically cross-linked using glutaraldehyde. After 15 min of the cross-linking reaction, distinct bands of cross-linked rClpP1 intermediates corresponding to 1-, 2-, 4-, 5-, 6-, and 7-mers on molecular weight were evident on SDS-PAGE stained with Coomassie blue (Figure 6C). Similar patterns of oligomeric bands of rClpP2 corresponding to 1-, 2-, 5-, 6-, and 7-mers were also evident after 15 min of the chemical cross-linking reaction (Figure 6D). With the increasing time of chemical cross-linking, the intensity of transient forms of pure rClpP1 and rClpP2 cross-linked bands were observed with reduced intensity of protomers after gel electrophoresis. Moreover, when the rClpP isoform mixture were chemically cross-linked, bands of cross-linked products identical to pure rClpP isoforms were observed, suggesting each heptameric forms of pure rClpP isoforms constitute the active tetradecameric structure of the ClpP protease of Leptospira (Figure 6E).
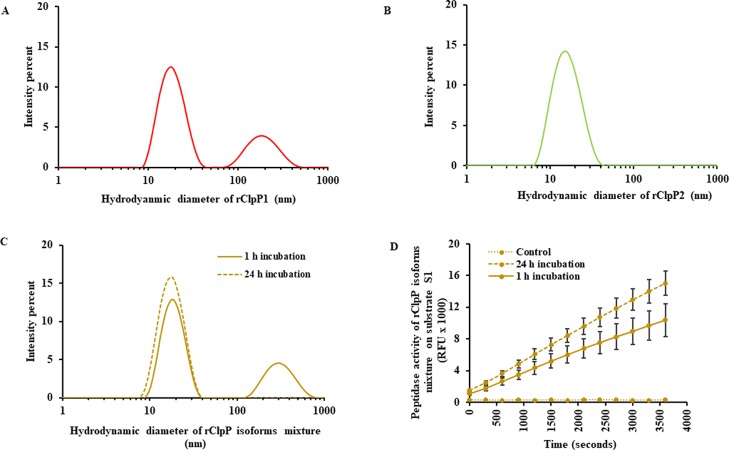
To address the size distribution (hydrodynamic diameter) of the oligomeric species of rClpP in solution, dynamic light scattering (DLS) was performed on pure rClpP and its isoform mixture. The pure rClpP1 incubated either for 1 or 24 h demonstrated two major peaks with a similar hydrodynamic diameter by DLS. The representative two major peaks for pure rClpP1 (24 h incubation) correspond to mean hydrodynamic diameters of 19.06 ± 5.76 and 197.8 ± 73.2 nm (Figure 7A). On the contrary, only one population of oligomeric species of a lower particle size (16.57 ± 6.03 nm) was detected in pure rClpP2 incubated either for 1 or 24 h (Figure 7B). The rClpP isoform mixture after a short incubation period of 1 h for self-assembly showed two major peaks with mean hydrodynamic diameters of 19.06 ± 5.29 and 312.5 ± 113.7 nm (Figure 7C). The DLS results demonstrated the existence of multiple populations of self-assembled oligomeric species for both pure rClpP1 and the rClpP isoform mixture. Interestingly, the rClpP isoform mixture after a longer period of incubation (24 h) showed a shift in the oligomeric species from multiple to single populations with an average hydrodynamic diameter of 18.21 ± 5.54 nm (Figure 7C). For further substantiation, the obtained values of polydispersity, polydispersity index, count rate, and estimated molecular weight of pure rClpP isoforms or its mixture (1 or 24 h incubation) during DLS analyses are listed (Table S2) along with their correlation coefficient and size distribution by mass (Figures S3 and S4). Such a shift in the population of oligomeric species to a single population was accompanied with higher peptidase activity on fluorogenic substrate S1 in comparison to the rClpP isoform mixture displaying multiple populations of oligomeric species formed during the short re-arrangement/self-assembly period (1 h) (Figure 7D).
Figure 7.
Size distribution in rClpP isoforms by dynamic light scattering (DLS). (A) The size distribution profile of oligomeric subunits in pure rClpP1. The pure rClpP1 after 24 h of incubation shows two major peaks of oligomeric species with a particle diameter of 19.06 ± 5.76 and 197.8 ± 73.2 nm. (B) The size distribution profile of oligomeric subunits in pure rClpP2. The representative size distribution data in pure rClpP2 after 24 h of incubation showed a single oligomeric species with an average hydrodynamic diameter of 16.57 ± 6.03 nm. Consistent size distribution profile was obtained for pure rClpP isoforms during short incubation (1 h) too. (C) The size distribution profile of oligomeric subunits in rClpP isoform mixture. The rClpP isoform mixture, when incubated for a longer duration (24 h) (dashed line), showed a shift in the size distribution of the oligomeric species to the single population compared to the equivalent amount of rClpP isoform mixture incubated for a shorter duration (1 h) (solid line). The representative size distribution data of the 10 scans performed in duplicates are presented in (A–C). (D) Effect on peptidase activity of the rClpP isoform mixture subsequent to a longer duration of a rClpP self-assembly. Incubation of rClpP isoform mixture for 24 h to self-assemble resulted in higher (1.4 times) and statistically significant (p-value <0.05) peptidase activity on fluorogenic substrate S1 than the rClpP isoform mixture incubated for 1 h. Control denotes the background fluorescence of substrate S1 in the reaction.
The Leptospira ClpP isoforms being classified in the serine peptidase family, the effect of mutation of rClpP isoforms (rClpP1S98A and rClpP2S97A) at one of the residues (Ser) of the catalytic triad was investigated. The complex of the mutant rClpP isoform mixture (rClpP1S98A-rClpP2 and rClpP1-rClpP2S97A) did not demonstrate any peptidase activity on S1 and S2 substrates (Figure 8A), despite the fact that the mutant retained its ability to oligomerize, like wild-type ClpP isoforms (Figure S5). In contrast, the complex of Listeria LmClpP2-S98A and LmClpP1 has been previously demonstrated to have a 75-fold increase in peptidase activity compared with pure LmClpP1.37 The addition of sodium-citrate (0.6 M) to such a complex of mutant rClpP isoform mixture failed to display any gain of peptidase activity on fluorogenic substrate S1 (Figure 8B) and S2 substrates (data not shown). Thus, the analysis of the pure rClpP isoforms or its mixture macromolecule using various techniques suggests that the functional rClpP protease in Leptospira is a tetradecamer and hypothesized to be composed of 2-heptameric rings of homogenous subunits of rClpP1 and rClpP2 where the proper alignment of all the 14-catalytic triad is essential.
Figure 8.
Peptidase activity of the mutant rClpP isoform mixture. Peptidase activity of the rClpP mixture is presented as a percentage (%), wherein, after 1 h of enzymatic reaction end-point fluorescence was measured. The measured end-point value of the wild-type rClpP isoform mixture was considered as 100% for measuring relative peptidase activity. The control shows the background fluorescence of the substrates in the reaction. (A) Comparison of peptidase activity of wild-type rClpP isoform mixture with mutant rClpP isoform mixtures on fluorogenic peptide substrates. Mutation of rClpP isoforms (rClpP1S98A and rClpP2S97A) was performed by substituting the Ser98/97 to Ala98/97 residue in the catalytic triad. The rClpP isoform mixture generated in various combinations with the mutant and wild-type ClpP isoforms showed a complete loss of peptidase activity in the mutant rClpP isoform mixture. (B) Effect of the presence of sodium citrate on peptidase activity of the wild-type rClpP isoform mixture and mutant rClpP isoform mixtures. The presence or absence of sodium citrate (0.6 M) does not lead to a gain in the peptidase activity in mutant rClpP isoform mixtures on fluorogenic peptide substrate S1. The error bars represent the SDs from the two independent experiments performed in duplicates. Statistical analysis was performed by the Student’s t-test for comparing the measured fluorescence value obtained for control and mutant rClpP isoform mixtures after 1 h of the assay (p-value >0.05).
Leptospira rClpX (LIC11418) Stimulates rClpP Heterocomplex for Protein Degradation in an Energy-dependent Process
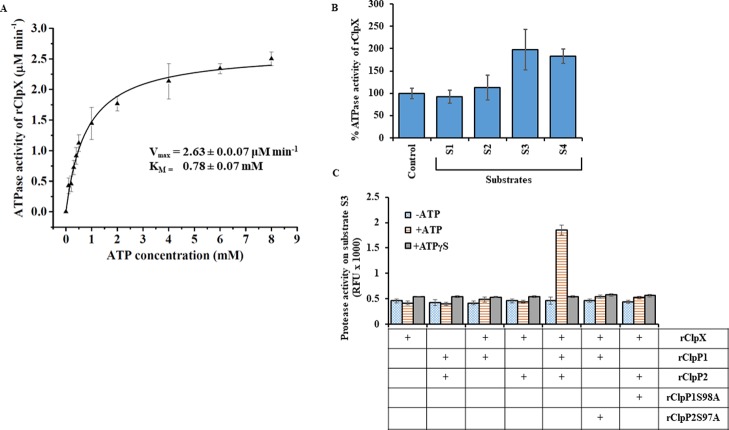
The overexpressed and purified rClpX of Leptospira was assessed for its ATPase activity, an activity essentially required for unfolding and permitting the unfolded larger protein substrate to pass through the axial pores of ClpP protease.22 Moreover, ClpX oligomerization is stabilized by ATP; therefore, the assembly can be followed indirectly by monitoring the changes in the rate of ATP hydrolysis.32,49 The free inorganic phosphate generated during the enzymatic hydrolysis of ATP by rClpX of Leptospira was quantified as described before for ClpC in M. tuberculosis(36) (Figure S6A,B). The rClpX exhibited surge in ATPase activity with increasing ATP concentration (0.25–4.0 mM) and thereafter, the activity reached saturation at the tested ATP concentration (4.0–8.0 mM) (Figure 9A). The ATPase reaction follows Michaelis–Menten kinetics and is in agreement to earlier study on ATP-dependent protease (Ti and ClpX) of E. coli.49,50 The calculated half-maximal ATP concentration for rClpX (KM) of Leptospira was 0.78 ± 0.07 mM (Figure 9A). In addition, we carried out a peptidase/protease assay using the substrates S1, S2, and S3 to investigate if pure rClpX has any peptide hydrolysis activity. As expected, rClpX did not exhibit any peptidase/protease activity on substrates S1, S2, and S3 (data not shown). These observations also advocate that there are no contaminants of E. coli ClpP that might have got associated during purification of rClpX of Leptospira. We next addressed whether the presence of protein substrates like casein could enhance the rClpX ATPase activity of Leptospira in a fashion similar to that reported in other chaperones like HslU16 and ClpA.50 To our surprise, the presence of FITC-casein (S3) and β-casein (S4) substrates stimulated the ATPase activity of Leptospira rClpX by 1.8-fold compared to the control (no substrate), whereas the small peptide substrates (S1 and S2) failed to significantly stimulate the ATP hydrolysis by rClpX (Figure 9B). This shows that the energy is utilized by rClpX for unfolding the protein substrate (casein) as increasing the concentration of casein leads to an increase in the reaction rate of ATP hydrolysis. Moreover, no such increase in ATP hydrolysis was observed in rClpX on the addition of the small peptide substrate.
Figure 9.
Biochemical activity of purified recombinant ClpX. (A) Effect on ATPase activity of pure rClpX at increasing concentration of ATP. The rate of ATP hydrolysis by rClpX increases as a function of ATP concentration and gets saturated at 8 mM. The kinetic constants of the rClpX enzymatic reaction on ATP were determined using Michaelis–Menten kinetics. The KM or the substrate concentration corresponding to the half-maximal velocity of the reaction and maximal reaction velocity during ATP hydrolysis was determined by the nonlinear curve fitting function of Origin software. The calculated Vmax and KM of the rClpX was 2.63 ± 0.07 μM min–1 and 0.78 ± 0.07 μM, respectively. (B) The ATPase activity of pure rClpX is increased in the presence of protein substrates. Increase in ATPase activity of pure rClpX was observed in the presence of bigger protein substrates (S3 and S4) and was unaltered in the presence of small peptide substrates (S1 and S2). Substrates S3 and S4 stimulate ATPase activity of rClpX in comparison to the control where no peptide/protein substrate was present. The measured ATPase activity of rClpX for 1 h at 37 °C (control) was considered as 100%. (C) Comparison of protease activity of wild-type and mutant rClpP isoform mixture in the presence of rClpX. The wild-type rClpP isoform mixture is stimulated by rClpX to degrade fluorogenic substrate S3 only in the presence of ATP. Substitution of ATP with ATPγS, a nonhydrolysable ATP analogue, impairs substrate S3 degradation. The pure rClpP isoforms and the mutant rClpP isoform mixture fails to degrade substrate S3 in the presence of rClpX and ATP. The error bars represent the SDs from the two independent experiments performed in duplicates. Statistical analysis was performed by the Student’s t-test for comparing the rClpX ATPase activity obtained for control and in the presence of different substrates after 1.5 h of the assay (p-value <0.05).
Previous studies on C. difficile(42) and P. aeruginosa(32) have described ClpP1–ClpX together form an active protease complex and the genes encoding these components are regulated under a single operon. In a similar fashion, the CDS of clpP1 and clpX in L. interrogans are separated by only 10 bps and apparently is regulated under a single operon. Thus, it was interesting to address if the association of Leptospira rClpX with pure rClpP isoforms or its mixture could stimulate protease activity on fluorogenic substrate S3. Unexpectedly, rClpX of L. interrogans failed to stimulate the pure rClpP isoform enzymatic activity in the presence or absence of ATP. However, in agreement with our peptidase assay of rClpP isoforms (Figure 4A), rClpX was able to stimulate rClpP isoform mixture for protease activity on fluorogenic substrate S3 in the presence of ATP (Figure 9C). Moreover, rClpX in the presence of a nonhydrolysable ATP-analogue (ATPγS) failed to stimulate the rClpP isoform mixture for protease activity (Figure 9C). These biochemical behaviors of rClpX are in consensus with the previous findings where ATP hydrolysis is required for unfolding the protein substrate and permit its passage in the axial-pore of the ClpP catalytic chamber.51,52 In addition, it was interesting to address if a mutation in the catalytic-triad of ClpP isoforms could affect its proteolytic activity. Therefore, the protease activity of the mutant ClpP protease macromolecule in association with rClpX was evaluated. The chaperone rClpX, however, could not stimulate the mutant ClpP protease macromolecule (rClpP1S98A-rClpP2 and rClpP1-rClpP2S97A) in degrading the fluorogenic substrate S3 (Figure 9C). Thus, the conformational orientation of all the 14-catalytic triad of ClpP protease is the prerequisite of controlled degradation processes in addition to the concerted action of energy-dependent chaperone ClpX.
Conclusions
Exploration of genomic and proteomic data of L. interrogans specified that the ClpP system in Leptospira might exhibit a divergent behavior from that of other reported multi-ClpP organisms studied to date. We have unraveled an unconventional oligomeric self-assembly of Leptospira ClpP1 and ClpP2 mixtures that is intrinsically active and distinct from those of other ClpPs, especially the well-characterized Mycobacterium ClpP isoforms. Earlier reported ClpP orthologs in pathogenic bacteria demonstrated that one or both of the pure isoforms of ClpP could self-assemble into a functional tetradecamer form, whereas in Leptospira both the pure isoforms despite self-assembling were found to be functionally inactive. Exploitation of this unprecedented regulation of proteolytic complexes may enable the development of an effective therapeutic target because of large diversity between bacterial and mammalian proteolytic systems.53 However, to precisely understand the existing evolutionary invented ClpP diversity, elucidation of conformation of the catalytic triad in the protease complex of Leptospira by high-resolution X-ray structure is further warranted. Likewise, genetic manipulation of the ClpP system in recalcitrant L. interrogans may shed light on the biological significance of harboring multi-isoform of ClpP and chaperone proteins. Substrate specificity has been demonstrated by the ClpP1P2 complex of M. tuberculosis,36,54 however, the substrate cleavage preference for leptospiral pure ClpP isoforms or its mixture demands further exploration. The role of the other class of chaperones and adaptor proteins in regulating ClpP biochemical behavior in Leptospira still remains to be answered.
Materials and Methods
Bacterial Strains, Primers, and Plasmids
Bacterial strains, primers, and plasmids used in the work are listed in Table 3. L. interrogans serovar Copenhageni strain Fiocruz L1-130, L. interrogans serovar Lai, and L. biflexa serovar Patoc were obtained from the Indian Council of Medical Research (ICMR), Regional Medical Research Centre (RMRC), Port Blair, Andaman and Nicobar Island, India. Spirochetes were cultured in Ellinghausen–McCullough–Johnson–Harris media at 28–30 °C at an interval of 5–7 days. Luria–Bertani (LB) medium was used for culturing E. coli DH5α and BL21 (DE3) (Novagen) required for cloning and expression of recombinant proteins.
Table 3. Bacterial Strains, Plasmids, and Primers Used in This Study.
| bacterial strains, plasmids, or primers | characteristics or sequence | source or reference | |
|---|---|---|---|
| bacterial strains | L. interrogans serovar Copenhageni strain Fiocruz L1-130 | wild-type | ICMR, Port Blair |
| L. interrogans serovar Lai | |||
| L. biflexa serovar Patoc | |||
| E. coli DH5α | supE44 ΔlacU169 (ϕ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96thi-1relA1 | Novagen | |
| E. coli BL21 (DE3) | F–ompT hsdSb (rBmB–) gal(λ c I 857 ind 1 Sam7 nin5 lacUVt7gene 1)dcm (DE3) | ||
| plasmids | pTZ57R/T | linearized TA vector with 3′-ddT overhangs for TA cloning of PCR products | Thermo Scientific |
| pET23a | bacterial vector for expression of C-terminally His6-tagged proteins | Novagen | |
| primers | clpP1F | 5′CTAGCTAGCATGGCGTAATCCCGTATGTG3′(NheI) | this work |
| clpP1R | 5′CCGCTCGAGTTCAGTTTGTTTACGATCGATCT3′(XhoI) | ||
| clpP2F | 5′CTAGCTAGCATGCCAGAAACAGAAAAATCG3′(NheI) | ||
| clpP2R | 5′CCGCTCGAGATTAAAATCGATTTTAGTAGCGAG3′(XhoI) | ||
| clpXF | 5′CTAGCTAGCTTGGCTAAGAAAACACCGG3′(NheI) | ||
| clpXR | 5′CCGCTCGAGAGCAATCTTAGATTCTTTTTTGAG3′(XhoI) | ||
| clpP1(S98A)F | 5′TCAGGCTTCTgctCTATGGCGGC3′ | ||
| clpP1(S98A)R | 5′CCTAAACAAAGAGTTCTTACATC3′ | ||
| clpP2(S97A)F | 5′AATGGCTGCTgctCTATGGGTTCTG3′ | ||
| clpP2(S97A)R | 5′CCCATACAAACCGTGTGAAC3′ | ||
Cloning, Expression, and Purification of Recombinant Proteins
The full-length genes clpP1 (LIC11417), clpP2 (LIC11951), and clpX (LIC11418) of L. interrogans serovar Copenhageni strain Fiocruz L1-130 were PCR amplified using its genomic DNA as a template. The oligonucleotides for PCR were designed using the genomic sequence of L. interrogans Copenhageni strain Fiocruz L1-130 available on the National Centre for Biotechnology Information (NCBI). The clpP1, clpX, and clpP2 genes were cloned individually into the pET23a vector at the NheI and XhoI multiple cloning sites that can express a C-terminal (His)6-tagged recombinant protein. To rule out any mutation in the generated recombinant pET23a plasmids, sequencing was performed by outsourcing (Eurofins, India) the DNA. The recombinant plasmids were transformed in BL21 (DE3) cells and the transformed cells were cultivated at 37 °C in LB medium supplemented with 100 μg mL–1 of ampicillin. Protein expression in the transformed BL21 (DE3) cells was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After cultivation of the bacterial cells for another 6 h at 37 °C, the cells were harvested and washed with 1× phosphate buffer saline (PBS; 10 mM sodium phosphate, 137 mM NaCl and 2.7 mM KCl; pH 7.4) by centrifugation at 3000×g for 5 min to remove cellular debris. The recombinant proteins were purified by affinity column chromatography using nickel-nitrilotriacetic acid (Ni-NTA) resins (Invitrogen). The recombinant ClpP1 and ClpP2 protein purification was carried out in native conditions as described before in our laboratory.55 The recombinant proteins were eluted in buffer-A (50 mM Tris-Cl, 300 mM NaCl, 250 mM imidazole; pH 8.0) containing 10% glycerol. For recombinant ClpX purification, a hybrid method was followed as described previously.56 Briefly, E. coli BL21-pET23a-clpX cells expressing ClpX were lysed in denaturing lysis buffer-B (200 mM sodium phosphate, 500 mM NaCl, 8 M urea; pH 7.8). The soluble lysate was allowed to bind to the Ni-NTA beads and washed with native wash buffer-C (50 mM Tris-Cl, 300 mM NaCl; pH 8.0). The bound recombinant protein to beads was eluted in native elution buffer-D (50 mM Tris-Cl, 150 mM NaCl, 250 mM imidazole; pH 8.0) containing 10% glycerol. Elutes of purified ClpP1, ClpP2, or ClpX were concentrated using 10 kDa centrifugal filter units (Amicon, catalog no. UFC901024) and dialyzed against buffer-E (50 mM Tris-Cl, 100 mM NaCl; pH 8.0) or 1× PBS, both containing 10% glycerol. The purified proteins were visualized on 12% sodium dodecyl sulfate-polyacrylamide gel by Coomassie staining. Protein concentrations were estimated by the Bradford method with bovine serum albumin as the standard or by measuring the absorption at 280 nm using the extinction coefficients of ClpP1 (ε0.1% = 0.741 [mg/mL]−1 cm–1), ClpP2 (ε0.1% = 0.856 [mg/mL]−1 cm–1), or ClpX (ε0.1% = 0.261 [mg/mL]−1 cm–1) calculated from the amino acid composition using program ProtParam.57
MSA and Site-Directed Mutagenesis of Leptospira rClpP Isoforms
Amino acid sequences of ClpP orthologs from different pathogenic bacteria were retrieved from UniProtKB database58 and MSA was performed using Clustal Omega software59 to find out conserved sequence motifs in Leptospira ClpP isoforms. MSA is represented using online tool ESPript (Easy Sequencing in PostScript) for better clarity.60 The secondary structures used in the study were obtained from the Protein Data Bank (PDB).61 Based on the findings of MSA, a site-directed single amino acid mutation was introduced in the generated recombinant plasmids pET23a-clpP1 and pET23a-clpP2 using a Q5 site-directed mutagenesis kit (NEB, catalog no. E0554S). Using a NEBaseChanger tool, the primers were designed for site-directed mutation at 98th and 97th Ser to the Ala residue in rClpP1 and rClpP2, respectively, resulting in the generation of rClpP1S98A and rClpP2S97A (Table 3). The rClpP1S98A and rClpP2S97A variants were overexpressed and purified from E. coli cells as described for the rClpP isoforms.
Generation of Polyclonal Antibodies Against Purified rClpP1 and rClpP2
Antibodies against rClpP1 were generated in rabbits by outsourcing the purified protein to Abgenex, Bhubaneswar, India. To generate anti-rClpP2 antibody, 4–6 weeks old BALB/c mice were immunized with purified rClpP2 subcutaneously. About 10 μg per mouse of the rClpP2 protein emulsified in the Freund’s complete adjuvant (catalog no. sc-3727; Santa Cruz Biotechnology) was used for primary immunization (4 mice per group). A negative control group was injected with an equal volume of PBS along with the adjuvant. Immunized mice were further given two booster injections of rClpP2 antigen emulsified in Freund’s incomplete adjuvant (catalog no. 3726; Santa Cruz Biotechnology) at 14 and 24 days of primary immunization. At 10 days after the second booster, blood was collected from each mouse by retro-orbital bleeding and then the mouse was sacrificed using the atlanto-occipital dislocation method as described previously.56 Sera obtained were pooled for the antibody titer analysis by the enzyme-linked immunosorbent assay (ELISA) before experimental use. Immunization experiments with mice were performed at the Department of Veterinary Microbiology, College of Veterinary Science, Assam Agriculture University Guwahati, India, after approval from the Institutional Animal Ethics Committee.
Western Blotting of Leptospira Whole-Cell Lysates
To detect ClpP1 and ClpP2 expressions in Leptospira, whole-cell lysates of 109 spirochetes were re-suspended in SDS loading dye. The resulting lysates of Leptospira were resolved in 12% SDS-PAGE and transferred to a nitrocellulose membrane (Santa Cruz Biotechnology). The membranes were blocked with 5% non-fat dried milk prepared in Tris-buffered saline (TBS; pH 8.0) containing 0.05% Tween 20 (TBS-T) and separately probed with anti-rClpP1 (1:1000) or anti-rClpP2 (1:250) antibodies for 2 h at room temperature. After being washed, the membranes were incubated with horse radish peroxidase (HRP)-conjugated goat antirabbit or antimouse IgG (1:5000; Sigma) for 1 h and immunoblots were developed by adding the chemiluminescence substrate (Thermo Scientific, catalog no. 32209). All dilutions of antibodies were prepared using 2% non-fat dried milk in 0.1% TBS-T.
Peptide Hydrolysis
Peptidase activity was monitored by the rate of production of fluorescent AMC (7-amino-4-methylcoumarin) after cleavage from fluorogenic peptide substrates. The peptide substrates used in the hydrolysis assay were N-succinyl-Leu-Tyr-AMC (Suc-LY-AMC) as substrate 1 (S1) and Suc-LLVY-AMC as substrate 2 (S2) (Sigma). Peptidase assays were performed in black flat-bottom 96-well plates (Invitrogen) at 37 °C. Each 96-well contained a fluorogenic peptide substrate (0.1 mM) and pure rClpP isoforms or its mixture (0.0125 μg μL–1) in 80 μL of buffer-F (50 mM phosphate buffer, 100 mM KCl, 5% glycerol; pH 7.6). Fluorescence was measured in the Infinite M200Pro plate reader (Tecan) at 380 and 460 nm wavelength of excitation and emission, respectively. The peptide substrate S1 (0.01–5.0 mM) was incubated with rClpP isoform mixture (0.025 μg μL–1) in 80 μL of buffer-F to determine the kinetic parameters of leptospiral rClpP isoform mixture. The subsequent experimental procedure to detect the hydrolysis of substrate S1 was the same as described above. Initial and final readings were taken at 0 and 1 h, respectively (excitation: 380 nm/emission: 460 nm). The measurements obtained were processed in Microsoft Excel and then data were transferred to Origin9.0 for Hill kinetics and statistical analysis. Similar peptidase assays were carried out on substrates S1 and S2 using pure rClpP mutant isoforms (rClpP1S98A and rClpP2S97A) or the rClpP isoform mixture constituted by either of the mutant isoforms. All the experiments were performed twice independently and in duplicates.
ATPase Assay of Leptospira Recombinant ClpX Chaperone
The ATPase assays were carried out using an ATPase/GTPase activity assay kit (Sigma, catalog no. MAK113). The amount of free phosphate determined from the standard graph was used to calculate the ATPase activity of rClpX on ATP in μmole min–1 μL–1 or μM min–1. The ATP hydrolysis rates of rClpX (0.05 μg μL–1) were monitored with increasing concentrations of ATP (0–8 mM) in 40 μL of buffer-G (50 mM Tris-Cl, 50 mM KCl, 1 mM DTT, and 8 mM MgCl2; pH 7.8) at 37 °C for 1.5 h. The measurements obtained were processed in Microsoft Excel and then data were transferred to Origin9.0 for Michaelis–Menten kinetics and statistical analysis. In addition, the ATPase activity of rClpX (0.08 μg μL–1) was measured in the presence of various substrates (0.1 μg μL–1) including small peptides (S1 and S2) and large protein substrates (Fluorescein isothiocyanate tagged-casein; FITC-casein [S3] and β-casein [S4]) in a total reaction volume of 25 μL in buffer-G at 37 °C for 1.5 h. In every ATPase assay, the reaction mix was prepared with the activity buffer, rClpX, and the substrate; and pre-incubated at 37 °C for 10 min prior to the addition of ATP. The measurements of absorbance at 620 nm were performed using a Multiskan GO Microplate UV–vis spectrophotometer (Thermo Scientific). All the experiments were performed twice independently and in duplicates.
Protease Assay of Leptospira Recombinant ClpP Isoforms in Association with Its ATPase Chaperone
All protease assays were performed using a protease fluorescent detection kit (Sigma, catalog no. PF0100) as per the manufacturers’ instructions. The pure rClpP isoforms or its mixture along with the chaperone rClpX were assayed for protease activity using fluorescent protein substrate S3 provided in the kit. Briefly, 20 μL of substrate S3 (1.5 μg μL–1) was prewarmed at 37 °C and incubated with pure rClpP isoforms or its mixture (0.02 μg μL–1) and rClpX (0.04 μg μL–1) in buffer-G for 10 min prior to addition of 2 mM ATP/ATPγS or without ATP in a total volume of 100 μL of the reaction. The protease reactions were performed in the dark at 37 °C for 1.5 h, followed by termination of the reaction with trichloroacetic acid (0.6 N). The resulting fluorescence was recorded at 492 and 519 nm wavelength of excitation and emission, respectively. A similar proteolysis assay was carried out using the rClpP isoform mixture constituted of wild-type and mutant isoforms (rClpP1S98A or rClpP2S97A). All the experiments were performed twice independently and in duplicates.
Chemical Cross-Linking of Recombinant ClpP Isoforms of Leptospira
The pure rClpP isoforms or its mixture (0.2 μg μL–1) were cross-linked with the glutaraldehyde solution (0.02%) in assay buffer-F in a total reaction volume of 100 μL. In the case of rClpP isoform mixture, each isoform (0.1 μg μL–1) was mixed to yield a final concentration of 0.2 μg μL–1. From the total reaction volume, a 20 μL of the cross-linking reaction was terminated at various intervals (2–15 min) by addition of 4× sample loading buffer (200 mM Tris-HCl, pH 6.8; 8% SDS; 0.4% bromophenol blue; 100 mM DTT and 40% glycerol) and boiling at 95 °C for 10 min. A control reaction containing pure rClpP isoforms or its mixture without glutaraldehyde was prepared for comparison. The reaction products at each time point were resolved on 10% SDS-PAGE and visualized by Coomassie staining.
Isothermal Titration Calorimetry
ITC experiments were performed on a MicroCal iTC200 system (GE Healthcare) at 37 °C in buffer-B with constant stirring at 250 rpm. The pure rClpP1 and rClpP2 were concentrated using 3 kDa cut-off centrifugal filter units (Amicon, catalog no. UFC800324) in buffer-B to equilibrate the buffer conditions of ITC syringe and sample solutions. The sample cell and the syringe were washed twice with buffer-E before loading the analytes. The equilibrated pure rClpP1 (60 μL of 90 μM) and the rClpP2 (300 μL of 9 μM) was loaded into the ITC syringe and the sample cell, respectively. The experiment was initiated after equilibration for 300 s with the first injection of rClpP1 (0.4 μL) that was discarded during the analysis. rClpP1 (1.5 μL) was titrated into the sample cell at an interval of 120 s. Power was recorded at “high” gain setting with a reference power of 10 μcal s–1 and a 5 s filter period. Data analysis, including baseline correction and evaluation was carried out using OriginPro 8.5 ITC.
Size Exclusion Chromatography (SEC)
An enrich SEC 650 high-resolution column (10 mm × 300 mm, catalog no. 7801650) was used for SEC on an NGC chromatography system (Bio-Rad). The column was equilibrated with a buffer containing 50 mM Tris-Cl pH 8.0, 100 mM NaCl, and 10% glycerol. The column was calibrated with β-amylase (200 kDa), alcohol dehydrogenase (158 kDa), albumin (66 kDa), carbonic anhydrase, (29 kDa) and cytochrome C (12.4 kDa) (Sigma, catalog no. MWGF-200). The rClpP isoforms were used at a final concentration of 0.5 mg mL–1 and were incubated overnight at 4 °C. The rClpP isoform samples (250 μL) equilibrated in the same buffer were loaded onto the column, and gel-filtration experiments were carried out with a flow rate of 0.3 mL min–1 at room temperature.
Native Polyacrylamide Gel Electrophoresis
Pure rClpP isoforms (2 μg) and its mixture in buffer-B were incubated at 37 °C for 10 min followed by mixing with 3× native sample buffer (240 mM Tris-HCl pH 6.8, 30% glycerol, 0.03% bromophenol blue).62 The rClpP isoform subunit interaction in solution was analyzed on a 4–20% gradient gel (Bio-Rad MiniProtean, catalog no. 456–1096) after resolving for 2 h at 120 V. The resolved proteins in gradient gel were visualized using Coomassie stain and compared with known molecular weight standard protein markers (Invitrogen, cata1og no. 928387).
Dynamic Light Scattering
DLS experiments were performed on a Zetasizer Nano ZS (Malvern Instruments) at 25 °C. Pure rClpP isoforms or their mixture (0.5 mg mL–1) in buffer-B were incubated for 1 or 24 h at 4 °C and were added to polystyrene cuvettes. The scattering was recorded at 173° angle; a 633 nm He-Ne laser was used as the light source. A total of ten autocorrelation functions were recorded for each of the protein samples, and intensity-weighted hydrodynamic diameters determined. The profile corresponding to the average particle sizes were generated as reported and discussed elsewhere.63
Acknowledgments
The authors gratefully acknowledge ICMR, Port Blair India for providing the Leptospira strains and Dr. Nitin Chaudhary, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati Guwahati (IIT Guwahati) for providing help in recording and analyzing the DLS experiments. We acknowledge the Central Instruments Facility, IIT Guwahati for ITC measurements. We would also like to thank the Department of Microbiology, College of Veterinary Science, Guwahati, for generating polyclonal antibodies.
Glossary
Abbreviations
- Clp
caseinolytic protease
- AAA+
ATPases associated with various cellular activities
- qRT-PCR
quantitative real time polymerase chain reaction
- AMC
7-amino-4-methylcoumarin
- Suc-LY-AMC
N-succinyl-Leu-Tyr-AMC
- Suc-LLVY-AMC
N-succinyl-Leu-Leu-Val-Tyr-AMC
- FITC
fluorescein isothiocyanate
- ITC
isothermal titration calorimetry
- Kd
dissociation constant
- SEC
size exclusion chromatography
- PAGE
polyacrylamide gel electrophoresis
- SDS
sodium dodecyl sulphate
- DLS
dynamic light scattering
- ATP
adenosine 5′-triphosphate
- ATPγS
adenosine 5’-(3-thiotriphosphate)
- ELISA
enzyme-linked immunosorbent assay
- HRP
horse radish peroxidase
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00399.
Details of RNA isolation and qRT-PCR for differential transcription analysis of clpP1 and clpP2 in Leptospira under in vitro conditions, details of calculation for estimation of rClpX ATPase activity, DLS analysis data of pure rClpP isoforms and its mixture, and native PAGE analysis of pure rClpP isoforms, its mixture, and rClpP serine mutants (PDF)
Author Contributions
M.K. conceived and supervised the study; M.K. and A.D. designed experiments and analyzed data; A.D. performed experiments; M.S.H. performed SEC analysis; D.D. performed and analyzed DLS experiment, M.K., A.D., and M.S.H. wrote the manuscript.
The present work was financially supported by the Department of Science and Technology (DST), Science and Engineering Research Board (SERB), Government of India, bearing project number SERB/EMR/2015/000255.
The authors declare no competing financial interest.
Supplementary Material
References
- Bharti A. R.; Nally J. E.; Ricaldi J. N.; Matthias M. A.; Diaz M. M.; Lovett M. A.; Levett P. N.; Gilman R. H.; Willig M. R.; Gotuzzo E.; Vinetz J. M. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- Costa F.; Hagan J. E.; Calcagno J.; Kane M.; Torgerson P.; Martinez-Silveira M. S.; Stein C.; Abela-Ridder B.; Ko A. I. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Neglected Trop. Dis. 2015, 9, e0003898 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoriano A. F. B.; Smythe L. D.; Gloriani-Barzaga N.; Cavinta L. L.; Kasai T.; Limpakarnjanarat K.; Ong B. L.; Gongal G.; Hall J.; Coulombe C. A. Leptospirosis in the Asia Pacific region. BMC Infect. Dis. 2009, 9, 147. 10.1186/1471-2334-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson P. R.; Hagan J. E.; Costa F.; Calcagno J.; Kane M.; Martinez-Silveira M. S.; Goris M. G. A.; Stein C.; Ko A. I.; Abela-Ridder B. Global burden of leptospirosis: estimated in terms of disability adjusted life years. PLoS Neglected Trop. Dis. 2015, 9, e0004122 10.1371/journal.pntd.0004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis W. A.Animal leptospirosis. In Leptospira and Leptospirosis; Springer, 2015; Vol. 387, pp 99-137. [DOI] [PubMed] [Google Scholar]
- Picardeau M.Genomics, proteomics, and genetics of leptospira. In Leptospira and Leptospirosis, Springer, 2015; pp 43–63. [DOI] [PubMed] [Google Scholar]
- Hensel M.; Shea J.; Gleeson C.; Jones M.; Dalton E.; Holden D. Simultaneous identification of bacterial virulence genes by negative selection. Science 1995, 269, 400–403. 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- Mei J.-M.; Nourbakhsh F.; Ford C. W.; Holden D. W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 1997, 26, 399–407. 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- Frees D.; Qazi S. N. A.; Hill P. J.; Ingmer H. Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol. Microbiol. 2003, 48, 1565–1578. 10.1046/j.1365-2958.2003.03524.x. [DOI] [PubMed] [Google Scholar]
- Zhao B.-b.; Li X.-h.; Zeng Y.-l.; Lu Y.-j. ClpP-deletion impairs the virulence of Legionella pneumophila and the optimal translocation of effector proteins. BMC Microbiol. 2016, 16, 174. 10.1186/s12866-016-0790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillot O.; Pellegrini E.; Bregenholt S.; Nair S.; Berche P. The ClpP serine protease is essential for the intracellular parasitism and virulence of Listeria monocytogenes. Mol. Microbiol. 2000, 35, 1286–1294. 10.1046/j.1365-2958.2000.01773.x. [DOI] [PubMed] [Google Scholar]
- Kwon H.-Y.; Ogunniyi A. D.; Choi M.-H.; Pyo S.-N.; Rhee D.-K.; Paton J. C. The ClpP protease of Streptococcus pneumoniae modulates virulence gene expression and protects against fatal pneumococcal challenge. Infect. Immun. 2004, 72, 5646–5653. 10.1128/iai.72.10.5646-5653.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti C. M.; Boyd D. H.; Rubin E. J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003, 48, 77–84. 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- Bhandari V.; Wong K. S.; Zhou J. L.; Mabanglo M. F.; Batey R. A.; Houry W. A. The Role of ClpP Protease in Bacterial Pathogenesis and Human Diseases. ACS Chem. Biol. 2018, 13, 1413. 10.1021/acschembio.8b00124. [DOI] [PubMed] [Google Scholar]
- Frees D.; Qazi S. N. A.; Hill P. J.; Ingmer H. Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol. Microbiol. 2003, 48, 1565–1578. 10.1046/j.1365-2958.2003.03524.x. [DOI] [PubMed] [Google Scholar]
- Dong S.-L.; Hu W.-L.; Ge Y.-M.; Ojcius D. M.; Lin X.; Yan J. A leptospiral AAA+ chaperone–Ntn peptidase complex, HslUV, contributes to the intracellular survival of Leptospira interrogans in hosts and the transmission of leptospirosis. Emerging Microbes Infect. 2017, 6, e105 10.1038/emi.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Olivares A. O.; Baker T. A.; Sauer R. T. Mechanical Protein Unfolding and Degradation. Annu. Rev. Physiol. 2018, 80, 413–429. 10.1146/annurev-physiol-021317-121303. [DOI] [PubMed] [Google Scholar]
- Gottesman S.; Clark W. P.; de Crecy-Lagard V.; Maurizi M. R. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. Sequence and in vivo activities. J. Biol. Chem. 1993, 268, 22618–22626. [PubMed] [Google Scholar]
- Maurizi M. R.; Singh S. K.; Thompson M. W.; Kessel M.; Ginsburg A. Molecular Properties of ClpAP Protease ofEscherichia coli: ATP-Dependent Association of ClpA and ClpP. Biochemistry 1998, 37, 7778–7786. 10.1021/bi973093e. [DOI] [PubMed] [Google Scholar]
- Neher S. B.; Sauer R. T.; Baker T. A. Distinct peptide signals in the UmuD and UmuD’ subunits of UmuD/D’ mediate tethering and substrate processing by the ClpXP protease. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 13219–13224. 10.1073/pnas.2235804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battesti A.; Hoskins J. R.; Tong S.; Milanesio P.; Mann J. M.; Kravats A.; Tsegaye Y. M.; Bougdour A.; Wickner S.; Gottesman S. Anti-adaptors provide multiple modes for regulation of the RssB adaptor protein. Genes Dev. 2013, 27, 2722–2735. 10.1101/gad.229617.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress W.; Maglica Ž.; Weber-Ban E. Clp chaperone-proteases: structure and function. Res. Microbiol. 2009, 160, 618–628. 10.1016/j.resmic.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Lourdault K.; Cerqueira G. M.; Wunder E. A.; Picardeau M. Inactivation of clpB in the pathogen Leptospira interrogans reduces virulence and resistance to stress conditions. Infect. Immun. 2011, 79, 3711–3717. 10.1128/iai.05168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Zhang H.; Lv M.; Hu M.; Li Z.; Gao C.; Xu P.; Ma C. Enzymatic production of 5-aminovalerate from L-lysine using L-lysine monooxygenase and 5-aminovaleramide amidohydrolase. Sci. Rep. 2014, 4, 5657. 10.1038/srep05657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S.; Maurizi M. R.; Gottesman S. Posttranslational quality control: folding, refolding, and degrading proteins. Science 1999, 286, 1888–1893. 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- Gersch M.; List A.; Groll M.; Sieber S. A. Insights into the structural network responsible for oligomerization and activity Of the bacterial virulence regulator caseinolytic protease P (ClpP). J. Biol. Chem. 2012, 287, 9484–9494. 10.1074/jbc.m111.336222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp E.; Wright G. D. Bacterial proteases, untapped antimicrobial drug targets. J. Antibiot. 2017, 70, 366. 10.1038/ja.2016.138. [DOI] [PubMed] [Google Scholar]
- Frees D.; Gerth U.; Ingmer H. Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of Staphylococcus aureus. Int. J. Med. Microbiol. 2014, 304, 142–149. 10.1016/j.ijmm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Viala J.; Mazodier P. The ATPase ClpX is conditionally involved in the morphological differentiation of Streptomyces lividans. Mol. Genet. Genomics 2003, 268, 563–569. 10.1007/s00438-002-0783-1. [DOI] [PubMed] [Google Scholar]
- Gersch M.; Stahl M.; Poreba M.; Dahmen M.; Dziedzic A.; Drag M.; Sieber S. A. Barrel-shaped ClpP Proteases Display Attenuated Cleavage Specificities. ACS Chem. Biol. 2016, 11, 389–399. 10.1021/acschembio.5b00757. [DOI] [PubMed] [Google Scholar]
- Malik I. T.; Brötz-Oesterhelt H. Conformational control of the bacterial Clp protease by natural product antibiotics. Nat. Prod. Rep. 2017, 34, 815–831. 10.1039/c6np00125d. [DOI] [PubMed] [Google Scholar]
- Hall B. M.; Breidenstein E. B. M.; de la Fuente-Núñez C.; Reffuveille F.; Mawla G. D.; Hancock R. E. W.; Baker T. A., Two isoforms of Clp peptidase in Pseudomonas aeruginosa control distinct aspects of cellular physiology. J. Bacteriol. 2016, 199, 10.1128/jb.00568-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R. M.; Unnikrishnan M.; Rubin D. H. F.; Krishnamoorthy V.; Kandror O.; Akopian T. N.; Goldberg A. L.; Rubin E. J. Mycobacterium tuberculosis ClpP1 and ClpP2 Function Together in Protein Degradation and Are Required for Viability in vitro and During Infection. PLoS Pathog. 2012, 8, e1002511 10.1371/journal.ppat.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N. A.; Chung K.; Rodrigues de Almeida N.; Conda-Sheridan M.; Fisher D. J.; Ouellette S. P., Initial Characterization of the Two ClpP Paralogs of Chlamydia trachomatis Suggests Unique Functionality for Each. J Bacteriol. 2018, 201 (), 10.1128/jb.00635-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M. R.; Clark W. P.; Katayama Y.; Rudikoff S.; Pumphrey J.; Bowers B.; Gottesman S. Sequence and structure of Clp P, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J. Biol. Chem. 1990, 265, 12536–12545. [PubMed] [Google Scholar]
- Akopian T.; Kandror O.; Raju R. M.; Unnikrishnan M.; Rubin E. J.; Goldberg A. L. The active ClpP protease fromM. tuberculosisis a complex composed of a heptameric ClpP1 and a ClpP2 ring. EMBO J. 2012, 31, 1529–1541. 10.1038/emboj.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler E.; List A.; Alte F.; Gersch M.; Wachtel R.; Poreba M.; Drag M.; Groll M.; Sieber S. A. Structural and functional insights into caseinolytic proteases reveal an unprecedented regulation principle of their catalytic triad. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 11302–11307. 10.1073/pnas.1219125110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardeau M.; Bulach D. M.; Bouchier C.; Zuerner R. L.; Zidane N.; Wilson P. J.; Creno S.; Kuczek E. S.; Bommezzadri S.; Davis J. C.; McGrath A.; Johnson M. J.; Boursaux-Eude C.; Seemann T.; Rouy Z.; Coppel R. L.; Rood J. I.; Lajus A.; Davies J. K.; Médigue C.; Adler B. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One 2008, 3, e1607 10.1371/journal.pone.0001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska J.; Modrak-Wójcik A.; Arent Z. J.; Więckowski D.; Zolkiewski M.; Bzowska A.; Kędzierska-Mieszkowska S. Characterization of the molecular chaperone ClpB from the pathogenic spirochaete Leptospira interrogans. PLoS One 2017, 12, e0181118 10.1371/journal.pone.0181118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. M.; Chung W. J.; Ha D. B.; Goldberg A. L.; Chung C. H. Protease Ti from Escherichia coli requires ATP hydrolysis for protein breakdown but not for hydrolysis of small peptides. J. Biol. Chem. 1989, 264, 2088–2091. [PubMed] [Google Scholar]
- Li M.; Kandror O.; Akopian T.; Dharkar P.; Wlodawer A.; Maurizi M. R.; Goldberg A. L. Structure and Functional Properties of the Active Form of the Proteolytic Complex, ClpP1P2, fromMycobacterium tuberculosis. J. Biol. Chem. 2016, 291, 7465–7476. 10.1074/jbc.m115.700344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavey N. P.; Shadid T.; Ballard J.; Duerfeldt A. S. Clostridium difficile ClpP Homologs are Capable of Uncoupled Activity and Exhibit Different Levels of Susceptibility to Acyldepsipeptide Modulation. ACS Infect. Dis. 2018, 5, 79–89. 10.1021/acsinfecdis.8b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M. M.; Raman C. S.; Nall B. T. Isothermal titration calorimetry of protein-protein interactions. Methods 1999, 19, 213–221. 10.1006/meth.1999.0852. [DOI] [PubMed] [Google Scholar]
- Velazquez-Campoy A.; Leavitt S. A.; Freire E. Characterization of protein-protein interactions by isothermal titration calorimetry. Methods Mol. Biol. 2015, 1278, 183–204. 10.1007/978-1-4939-2425-7_11. [DOI] [PubMed] [Google Scholar]
- Ka D.; Lee H.; Jung Y.-D.; Kim K.; Seok C.; Suh N.; Bae E. Crystal Structure of Streptococcus pyogenes Cas1 and Its Interaction with Csn2 in the Type II CRISPR-Cas System. Structure 2016, 24, 70–79. 10.1016/j.str.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Nuñez J. K.; Kranzusch P. J.; Noeske J.; Wright A. V.; Davies C. W.; Doudna J. A. Cas1-Cas2 complex formation mediates spacer acquisition during CRISPR-Cas adaptive immunity. Nat. Struct. Mol. Biol. 2014, 21, 528. 10.1038/nsmb.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanne T. M.; Pojidaeva E.; Andersson F. I.; Clarke A. K. Distinctive Types of ATP-dependent Clp Proteases in Cyanobacteria. J. Biol. Chem. 2007, 282, 14394–14402. 10.1074/jbc.m700275200. [DOI] [PubMed] [Google Scholar]
- Andersson F. I.; Tryggvesson A.; Sharon M.; Diemand A. V.; Classen M.; Best C.; Schmidt R.; Schelin J.; Stanne T. M.; Bukau B.; Robinson C. V.; Witt S.; Mogk A.; Clarke A. K. Structure and Function of a Novel Type of ATP-dependent Clp Protease. J. Biol. Chem. 2009, 284, 13519–13532. 10.1074/jbc.m809588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R. E.; Baker T. A.; Sauer R. T. Energy-dependent degradation: Linkage between ClpX-catalyzed nucleotide hydrolysis and protein-substrate processing. Protein Sci. 2003, 12, 893–902. 10.1110/ps.0237603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B. J.; Woo K. M.; Goldberg A. L.; Chung C. H. Protease Ti, a new ATP-dependent protease in Escherichia coli, contains protein-activated ATPase and proteolytic functions in distinct subunits. J. Biol. Chem. 1988, 263, 8727–8734. [PubMed] [Google Scholar]
- Martin A.; Baker T. A.; Sauer R. T. Protein unfolding by a AAA+ protease is dependent on ATP-hydrolysis rates and substrate energy landscapes. Nat. Struct. Mol. Biol. 2008, 15, 139. 10.1038/nsmb.1380. [DOI] [PubMed] [Google Scholar]
- Baker T. A.; Sauer R. T. ClpXP, an ATP-powered unfolding and protein-degradation machine. Biochim. Biophys. Acta, Mol. Cell Res. 2012, 1823, 15–28. 10.1016/j.bbamcr.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R. M.; Goldberg A. L.; Rubin E. J. Bacterial proteolytic complexes as therapeutic targets. Nat. Rev. Drug Discovery 2012, 11, 777. 10.1038/nrd3846. [DOI] [PubMed] [Google Scholar]
- Akopian T.; Kandror O.; Tsu C.; Lai J. H.; Wu W.; Liu Y.; Zhao P.; Park A.; Wolf L.; Dick L. R.; Rubin E. J.; Bachovchin W.; Goldberg A. L. Cleavage Specificity ofMycobacterium tuberculosisClpP1P2 Protease and Identification of Novel Peptide Substrates and Boronate Inhibitors with Anti-bacterial Activity. J. Biol. Chem. 2015, 290, 11008–11020. 10.1074/jbc.m114.625640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit B.; Ghosh K. K.; Fernandes G.; Kumar P.; Gogoi P.; Kumar M. Dual nuclease activity of a Cas2 protein in CRISPR-Cas subtype I-B ofLeptospira interrogans. FEBS Lett. 2016, 590, 1002–1016. 10.1002/1873-3468.12124. [DOI] [PubMed] [Google Scholar]
- Ghosh K. K.; Prakash A.; Balamurugan V.; Kumar M. Catecholamine-Modulated Novel Surface-Exposed Adhesin LIC20035 of Leptospira spp. Binds Host Extracellular Matrix Components and Is Recognized by the Host during Infection. Appl. Environ. Microbiol. 2018, 84, e02360 10.1128/aem.02360-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. M.; Jess T. J.; Price N. C. How to study proteins by circular dichroism. Biochim. Biophys. Acta, Proteins Proteomics 2005, 1751, 119–139. 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Consortium U. UniProt: a hub for protein information. Nucleic Acids Res. 2014, 43, D204–D212. 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F.; Wilm A.; Dineen D.; Gibson T. J.; Karplus K.; Li W.; Lopez R.; McWilliam H.; Remmert M.; Söding J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert X.; Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H. M.; Westbrook J.; Feng Z.; Gilliland G.; Bhat T. N.; Weissig H.; Shindyalov I. N.; Bourne P. E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissler S.; Chambers J. E.; Crespillo-Casado A.; Avezov E.; Miranda E.; Perez J.; Hendershot L. M.; Harding H. P.; Ron D. Physiological modulation of BiP activity by trans-protomer engagement of the interdomain linker. Elife 2015, 4, e08961 10.7554/elife.08961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBreck C. J.; May S.; Viola M. G.; Conti J.; Camberg J. L. The Protein Chaperone ClpX Targets Native and Non-native Aggregated Substrates for Remodeling, Disassembly, and Degradation with ClpP. Front. Mol. Biosci. 2017, 4, 26. 10.3389/fmolb.2017.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.