Abstract
A unified and common intermediate strategy for syntheses of juglomycins and their derivatives is reported. The use of a 1,4-dimethoxynaphthalene derivative as a key intermediate enabled easy access to various juglomycin derivatives. In this study, juglomycins A–D, juglomycin C amide, khatmiamycin and its 4-epimer, and the structure proposed for juglomycin Z were synthesized from this intermediate. The absolute configuration of natural khatmiamycin has been established to be 3R,4R through our synthesis. Unfortunately, the spectroscopic data for synthetic juglomycin Z were not consistent with the data reported for the natural one, strongly suggesting a structural misassignment.
Introduction
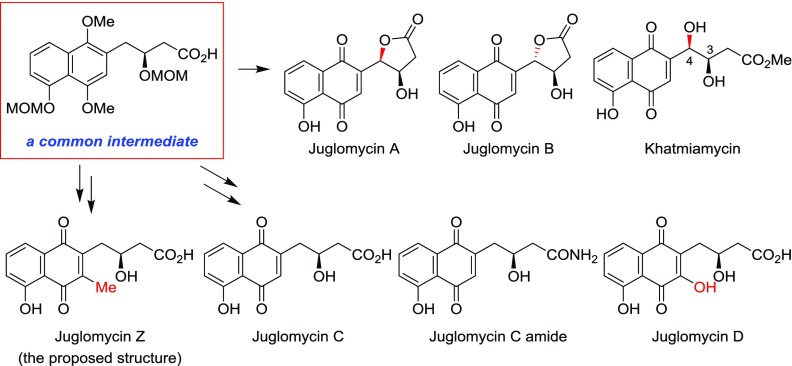
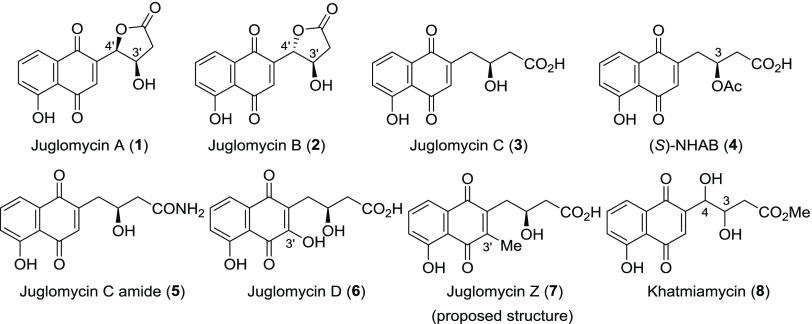
Juglomycins A (1) and B (2) were isolated from Streptomyces sp. 190-2 (Figure 1).1,2 They are composed of 1,4-naphthoquinone with a lactone at the side chain and are diastereomers possessing different stereochemistries at the 4′-position of each structure. The absolute configuration of 1 was determined to be 3′R,4′R with X-ray crystallography.3 The absolute configuration of 2 was subsequently determined to be 3′R,4′S.3 Juglomycin C (3) was isolated from Streptomyces sp. 815 and 3094.4 This compound, which possesses a carboxyl group at the side chain, is a reduced form of 1 and 2 at the 4′-position. Naphthoquinone-8-hydroxy-3-[(3S)-acetoxybutyric acid] [(S)-NHAB, 4], a 3-O-acetylated derivative of 3, was isolated from a disruptant of the actVI-ORFA gene for the biosynthesis of actinorhodin in Streptomyces coelicolor A3(2).5,6 Compound 4 is considered to be a key intermediate in the biosynthesis of juglomycins A–C.7 During the course of the identification of the gene clusters for these natural 1,4-naphthoquinones, juglomycin C amide (5) was isolated from S. coelicolor A3(2) M145.7 Juglomycin D (6), isolated from Streptomyces sp. 815 and 3094, is an oxidized form of 3 at the 3′-position.4 Juglomycin Z (7), isolated from the culture filtrate of Streptomyces tendae Tü 901/8c, has a methyl group at the 3′-position.8 Khatmiamycin (8) was isolated from the culture broth of Streptomyces sp. ANK313. This compound is an ester derivative of 1 or 2, but the absolute configurations at the 3- and 4-positions have not been determined.9
Figure 1.
Structures for juglomycins and their derivatives. Selected carbon atoms have been labeled using the IUPAC numbering system.
Natural 1,4-naphthoquinones have attractive biological activities.10 Juglomycins A and B show antibacterial activity against Gram-positive bacteria such as Bacillus subtilis, Staphylococcus aureus, and Streptococcus pneumoniae, and Gram-negative bacteria such as Escherichia coli, and Mycobacterium tuberculosis.1,2 Juglomycin C and the methyl ester of juglomycin D exhibit moderate antibacterial activity against B. subtilis and E. coli.4 Juglomycin Z shows antibacterial activity against Gram-positive and Gram-negative bacteria and yeast. The antibacterial activity of this compound against Bacillus brevis is a 10-fold potent than that of juglomycin A.8 Khatmiamycin exhibits potent motility inhibitory and lytic activities against zoospores as well as potent antibacterial activity against S. aureus and Streptomyces viridochromogenes.9
Several synthetic studies on juglomycins and their derivatives have been reported.10 The synthesis of (±)-1 and (±)-2 was reported by Giles and co-workers.11 Brimble and co-workers achieved the formal synthesis of (±)-1 and (±)-2 via oxidative fragmentation of furo[3,2-b]naphtho[2,1-d]furans.12,13 The racemic and asymmetric synthesis of 1 using a reaction of a naphthol anion with a chiral aldehyde was reported by Kraus and co-workers.14,15 Min and co-workers synthesized (±)-1 from 1-hydroxy-5-methoxynaphthalene.16 The Dötz benzannulation route to the enantioselective synthesis of (−)- and (+)-1 has been reported by Fernandes and co-workers.17−19 Both enantiomers of 3 and 4 were synthesized by the stereoselective aldol reaction of chiral sulfoxides with an aldehyde by our group.20 During the course of our synthesis on juglorubin, 6 was obtained as a byproduct by treatment of 3 with a phosphate buffer (pH 8.5) under aerobic conditions.21
In this paper, a unified approach toward the syntheses of juglomycins A–D, juglomycin C amide, khatmiamycin, and the structure proposed for juglomycin Z is reported. Through the synthesis of khatmiamycin and its 4-epimer, determination of the relative and absolute configuration of natural khatmiamycin has been achieved. The spectroscopic data for synthetic juglomycin Z and its methyl ester do not match those reported, suggesting that the structure assigned to juglomycin Z is incorrect.
Results and Discussion
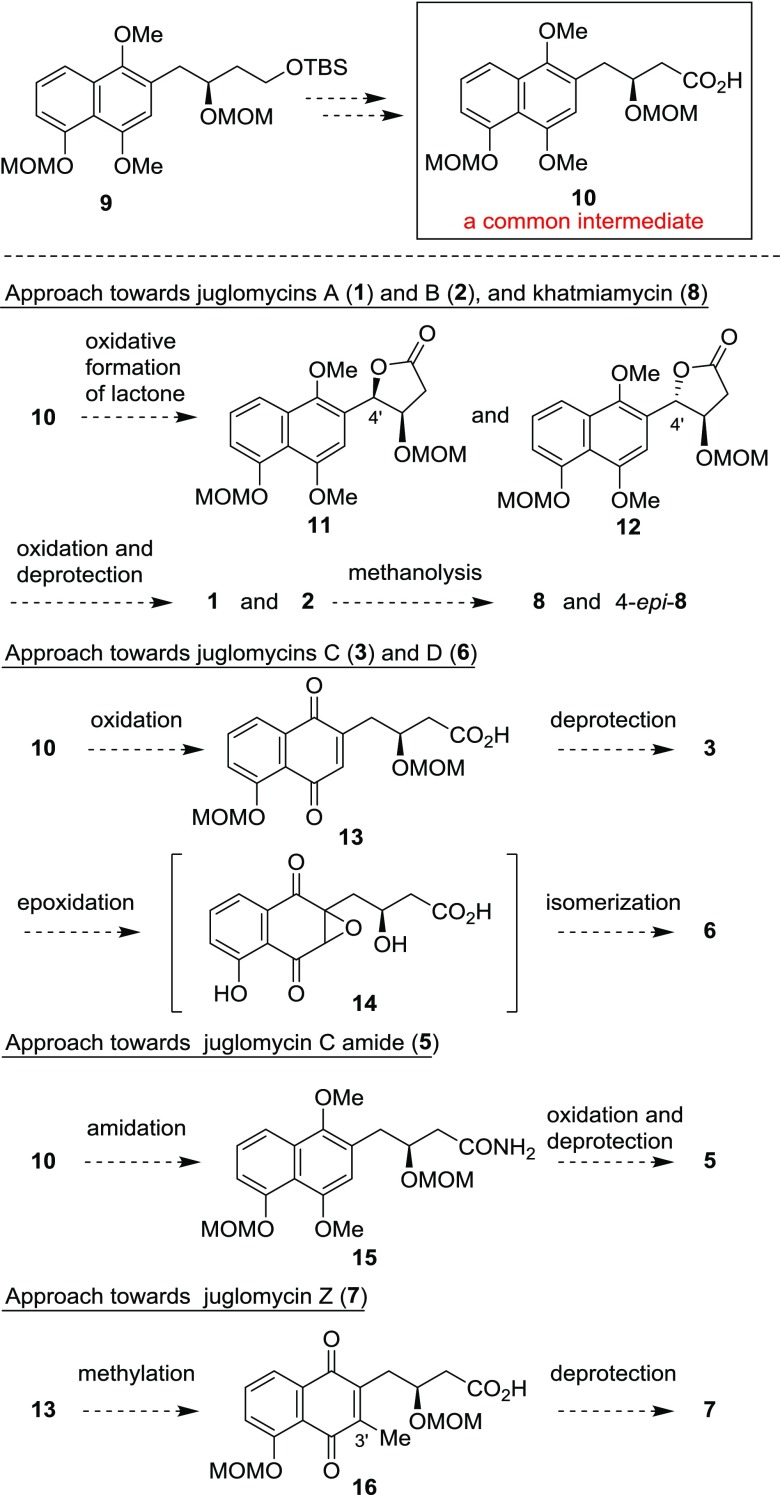
Our synthetic approach toward the syntheses of juglomycins A–D, Z, juglomycin C amide, and khatmiamycin is outlined in Scheme 1. The optically active compound 9(22) can be easily converted into the corresponding carboxylic acid (10). We envisioned that 10 would serve as a potential common intermediate to access to the member of juglomycins and their related derivatives. Juglomycins A (1) and B (2) will be synthesized by formation of a lactone through benzylic oxidation of 10, followed by oxidation of naphthalene and removal of protective groups. Khatmiamycin (8) can be prepared by methanolysis of 1 or 2. Oxidation of the naphthalene in 10 and removal of the protective groups in 13 will afford juglomycin C (3). Epoxidation of 3 and isomerization of the corresponding epoxide 14 will give juglomycin D (6). Amidation of 10, oxidation, and removal of the protective groups in 15 will afford juglomycin C amide (5). Juglomycin Z (7) will be synthesized by introduction of the methyl group into the 3′-position of 13, followed by deprotection of the protective groups in 16.
Scheme 1. Synthetic Approach toward Juglomycins and Their Derivatives.
Selected carbon atoms have been labeled using the IUPAC numbering system.
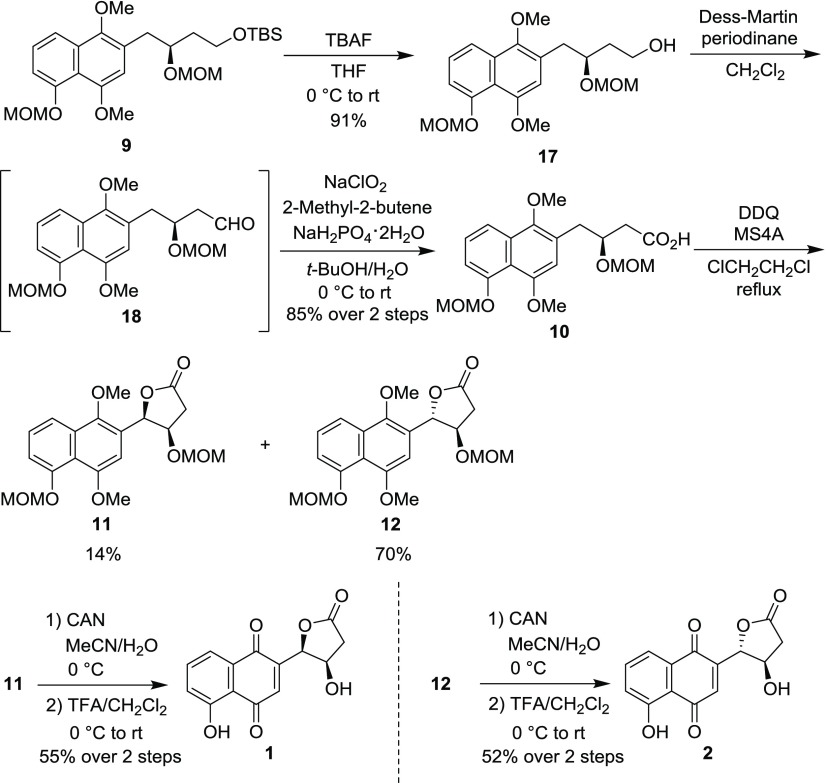
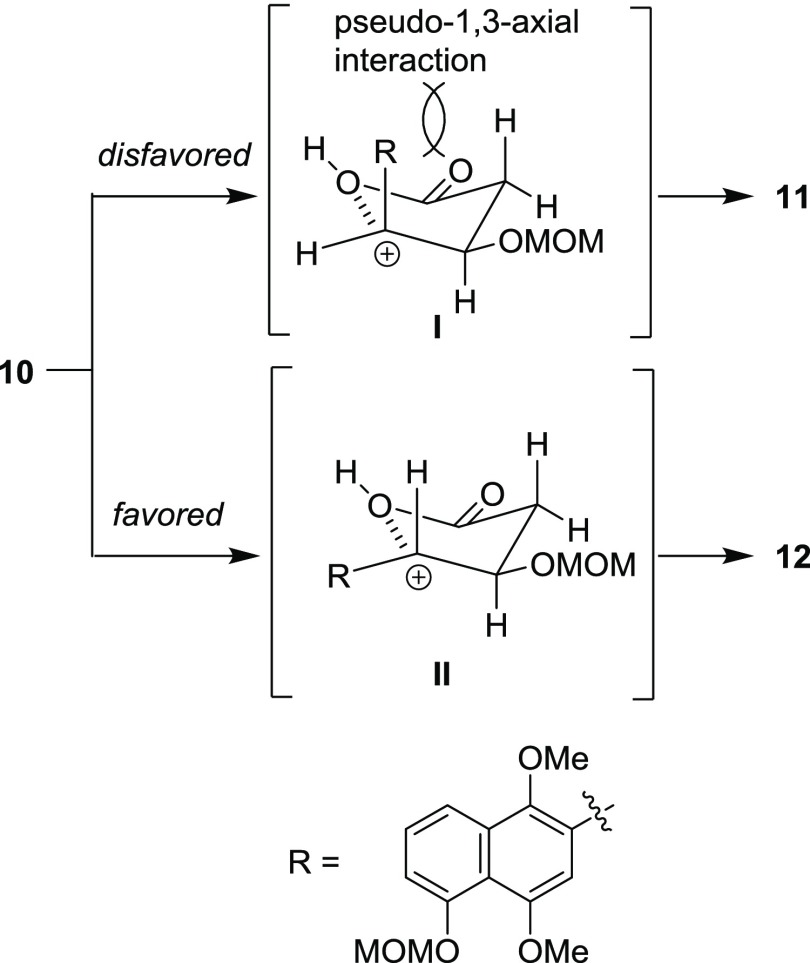
The synthesis of juglomycins A (1) and B (2) is depicted in Scheme 2. Deprotection of the tert-butyldimethylsilyl (TBS) group in 9(22) with tetra-n-butylammonium fluoride (TBAF) gave the corresponding alcohol 17. Oxidation of the primary alcohol in 17 through Dess–Martin oxidation23 and Pinnick oxidation24 gave carboxylic acid 10. The desired intramolecular oxidative cyclization proceeded by treatment of 10 with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) in the presence of molecular sieves 4A (MS4A) in dichloroethane to give 11 and 12 in 14 and 70% yields, respectively. The selectivity of this reaction can be rationalized by the following hypothesis: the formation of 11 would be disfavored by the pseudo-1,3-diaxial interaction between the naphthyl group and the hydrogen at the pseudo-axial position in intermediate I (Scheme 3). Oxidation of the 1,4-dimethoxynaphthalene moiety in 11 and 12 with ceric ammonium nitrate (CAN) followed by removal of two methoxymethyl (MOM) groups afforded 1 and 2, respectively. The spectroscopic data of synthetic 1 and 2 are identical with those of natural ones.3
Scheme 2. Synthesis of Juglomycins A (1) and B (2).
Scheme 3. Proposed Mechanism for the Stereoselectivity of the Intramolecular Oxidative Cyclization of 10.
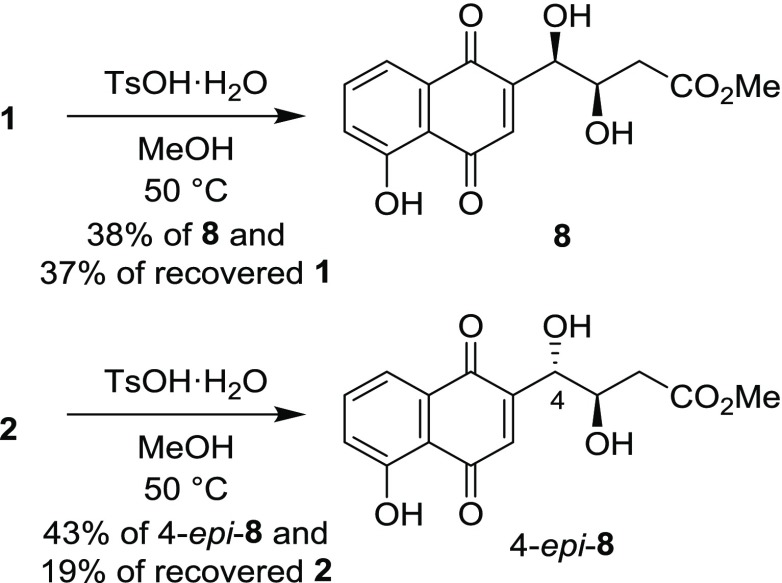
The synthesis and determination of the absolute configuration of khatmiamycin (8) were achieved (Scheme 4). Treatment of 1 with p-toluenesulfonic acid monohydrate (TsOH·H2O) in methanol at 50 °C for 2.5 h gave 8 in 38% yield with 37% of recovered 1. Although several conditions were investigated to improve the yield of 8, no significant improvement was achieved. Increased reaction time and reaction temperature caused decomposition of 1 and 8. Methanolysis of 1 under basic conditions gave a complex mixture. We presume that the equilibrium between 1 and 8 exists. The 1H and 13C NMR spectra of synthetic 8 agreed with those reported for natural 8.9 The specific rotation of synthetic 8 was determined to be [α]D25 −103.3 (c 0.10, MeOH). The specific rotation for natural 8 was reported to be [α]D −21.0 (c 0.10, MeOH). Although the specific rotation value of synthetic 8 was different from that of natural 8, the sign of synthetic 8 was identical to that of natural 8. The differences in the values should be due to the presence of impurities in natural 8. On the basis of these results, the absolute configuration of natural khatmiamycin was determined to be 3R,4R. Methanolysis of 2 under the same conditions as 1 gave 4-epi-8 in 43% yield with 19% of recovered 2. The 1H and 13C NMR spectra of 4-epi-8 were different from those of natural khatmiamycin. The specific rotation of 4-epi-8 was [α]D23 +81.6 (c 0.10, MeOH).
Scheme 4. Synthesis of Khatmiamycin (8) and Its 4-Epimer (4-epi-8).
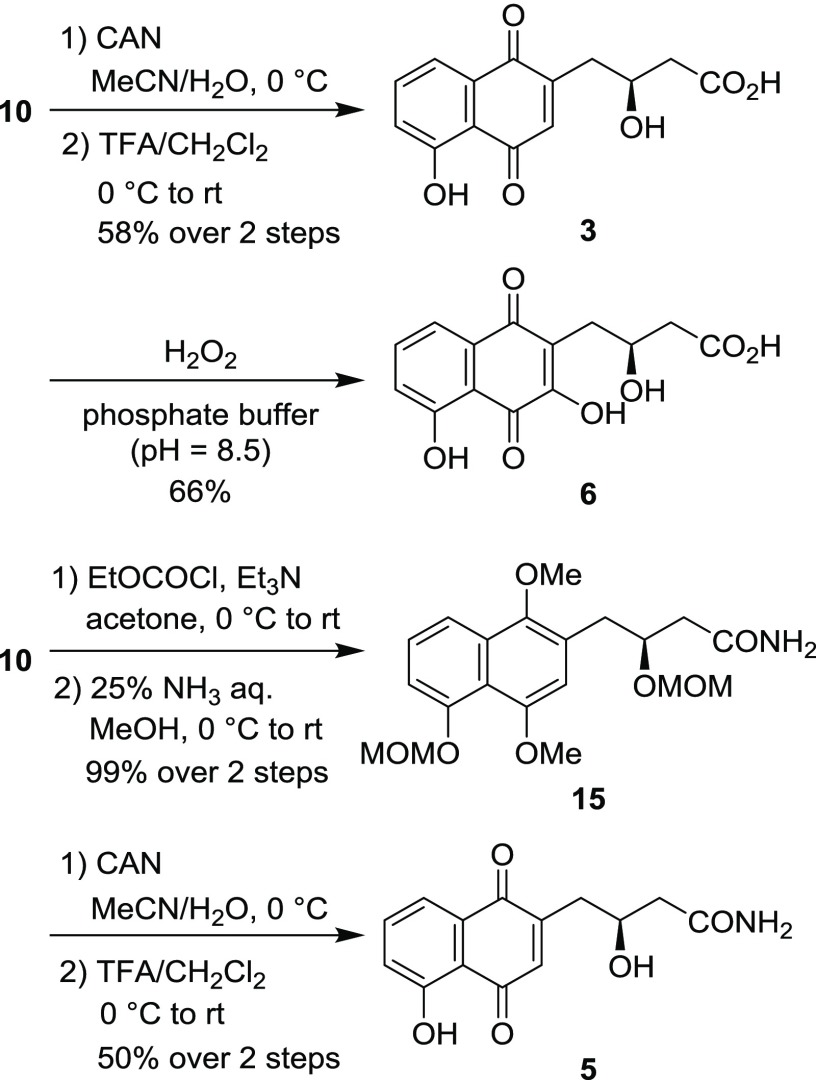
The synthesis of juglomycins C (3), D (6), and juglomycin C amide (5) is shown in Scheme 5. Oxidation of the naphthalene ring in 10 with CAN followed by removal of the MOM groups gave juglomycin C (3). Oxidation of 3 with hydrogen peroxide in a phosphate buffer (pH = 8.5) afforded juglomycin D (6). Treatment of 10 with ethyl chloroformate gave the corresponding mixed anhydride. Without further purification, the mixed anhydride was reacted with ammonia to afford amide 15. Oxidation of 15 with CAN followed by removal of the MOM groups gave juglomycin C amide (5). The spectroscopic data for synthetic 3, 5, and 6 are identical with the reported data.4,7,21
Scheme 5. Synthesis of Juglomycins C (3) and D (6) and Juglomycin C Amide (5).
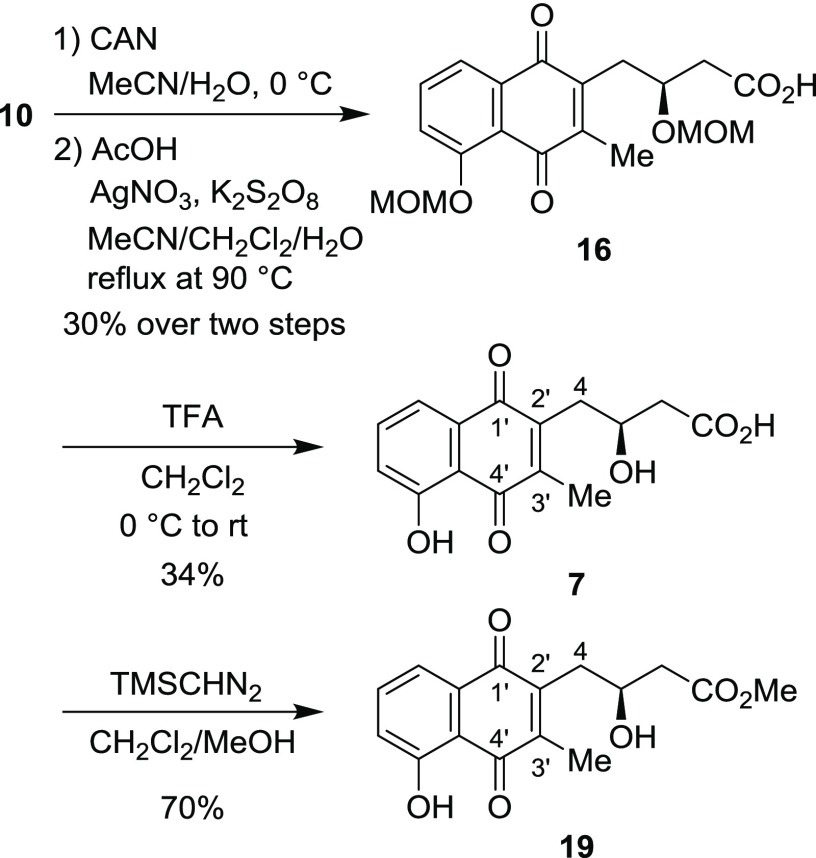
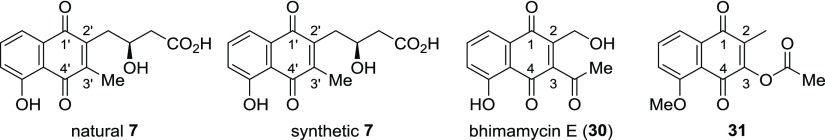
The synthesis of the structure proposed for juglomycin Z (7) is depicted in Scheme 6. Oxidation of the naphthalene ring in 10 with CAN gave the corresponding naphthoquinone derivative. Radical methylation25 of the naphthoquinone by treatment with silver nitrate and potassium persulfate in the presence of acetic acid gave 16 in 30% yield over two steps. Removal of the MOM groups with TFA gave the proposed structure for juglomycin Z (7) in 34% yield. Unfortunately, the 1H and 13C NMR spectroscopic data for synthetic 7 do not match those reported for the natural one (Table S1 in the Supporting Information).8 The specific rotation value of synthetic 7 {[α]D28 −44.3 (c 0.10, MeOH)} is completely different from that of the natural one {[α]D +144 (c 0.025, MeOH)}. Synthetic 7 was converted into its methyl ester 19. The 1H and 13C NMR spectroscopic data for synthetic 19 are not identical with those reported for the same compound derived from natural juglomycin Z (Table S2 in the Supporting Information).8 The specific rotation value of synthetic 19 {[α]D26 −40.9 (c 0.32, MeOH)} is different from that of the same compound derived from natural 7 {[α]D +37.3 (c 0.01, MeOH)}.
Scheme 6. Synthesis of the Proposed Structure for Juglomycin Z (7) and Its Methyl Ester 19.
Selected carbon atoms have been labeled using the IUPAC numbering system.
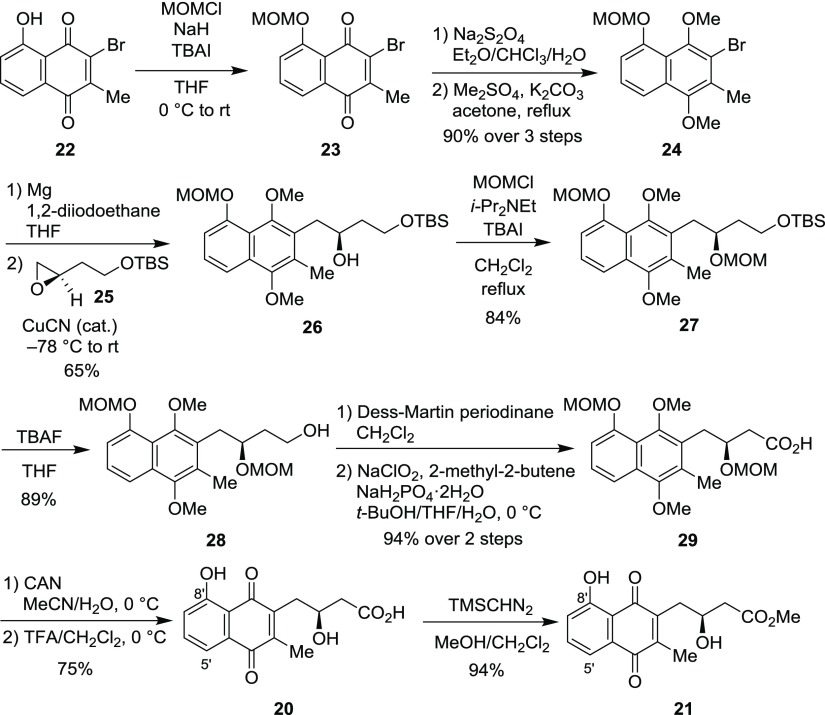
A juglomycin Z isomer 20 and its methyl ester 21 were also synthesized (Scheme 7). Both 20 and 21 have a hydroxyl group at the 8′-position instead of the 5′-position. The hydroxyl group of 3-bromoplumbagin (22)26 was protected as a MOM ether to give 23. Reduction of the 1,4-naphthoquinone in 23 with sodium hydrosulfite followed by methylation of two hydroxyl groups in the resultant hydroquinone gave 24. A Grignard reagent, which was prepared from 24, was treated with optically active epoxide 25(22) (>97% ee) in the presence of a catalytic amount of CuCN to give 26 in 65% yield. After protection of the hydroxyl group in 26 as a MOM ether, deprotection of the TBS group in 27 with TBAF afforded 28. Oxidation of the primary alcohol in 28 through Dess–Martin oxidation and Pinnick oxidation gave carboxylic acid 29. Oxidation of 29 with CAN and deprotection of the MOM group gave 20. Because of the low solubility of 20 in CDCl3, this compound was converted into its methyl ester 21 by treatment with trimethylsilyldiazomethane. The 1H and 13C NMR spectroscopic data for synthetic 21 do not match those reported for juglomycin Z methyl ester 19 (Table S3 in the Supporting Information).8
Scheme 7. Synthesis of Juglomycin Z Isomer (20) and Its Methyl Ester 21.
Selected carbon atoms have been labeled using the IUPAC numbering system.
The results obtained in Schemes 6 and 7 indicate that the structure proposed for natural juglomycin Z is incorrect. The 1H and 13C NMR signals at the 4,1′,2′,3′,4′-positions as well as the methyl group at the 3′-position in synthetic 7 are quite different from those reported for natural juglomycin Z (Table 1). In particular, the 1H and 13C NMR signals of the methyl group at the 3′-position in synthetic 7 (δH = 2.26 ppm; δC = 12.7 ppm) are shifted upfield in comparison with those reported for 7 (δH = 2.67 ppm; δC = 18.5 ppm). These observations indicate that natural juglomycin Z does not have a methyl group at the 3′-positon. The 1H and 13C NMR signals at the 1′,2′,3′,4′-positions in natural 7 are quite different from those at the 1,2,3,4-positions reported for bhimamycin E (30)27 and 2-methyl-3-acetoxy-5-methoxy-1,4-naphthoquinone (31).28 Furthermore, the chemical shifts of the methyl group in natural 7 are quite different from those derived from the methyl group in the acetyl group in 30 and 31. These spectral comparisons suggest that natural juglomycin Z does not possess an acetyl or acetoxy group at the 3′-position. Therefore, the structure for natural juglomycin Z still remains unclear.
Table 1. Comparison of NMR Spectroscopic Data for Natural and Synthetic Juglomycin Z (7), Bhimamycin E (30), and 2-Methyl-3-acetoxy-5-methoxy-1,4-naphthoquinone (31).
| natural 7b |
synthetic 7c |
30d |
31e |
||||||
|---|---|---|---|---|---|---|---|---|---|
| positiona | δC | δH | δC | δH | positiona | δC | δH | δC | δH |
| 1′ | 182.2 | 184.8 | 1 | 183.1 | 185.0 | ||||
| 2′ or 3′ | 148.4 | 145.9 | 2 | 153.7 | 133.5 | ||||
| 3′ or 2′ | 149.6 | 144.2 | 3 | 119.9 | 151.9 | ||||
| 3′-Me | 18.5 | 2.67 | 12.7 | 2.26 | COMe | 30.1 | 2.34 | 20.4 | 2.06 |
| 4′ | 186.6 | 190.6 | 4 | 184.3 | 176.6 | ||||
Carbon atoms have been labeled using the IUPAC numbering system.
1H NMR (200 MHz, CDCl3/CD3OD) and 13C NMR (50.3 MHz, CDCl3).8
1H NMR (400 MHz, CDCl3/CD3OD = 9/1, TMS) and 13C NMR (100 MHz, CDCl3).
1H NMR (300 MHz, CDCl3) and 13C NMR (75.5 MHz, CDCl3).27
1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3, TMS).28
Conclusions
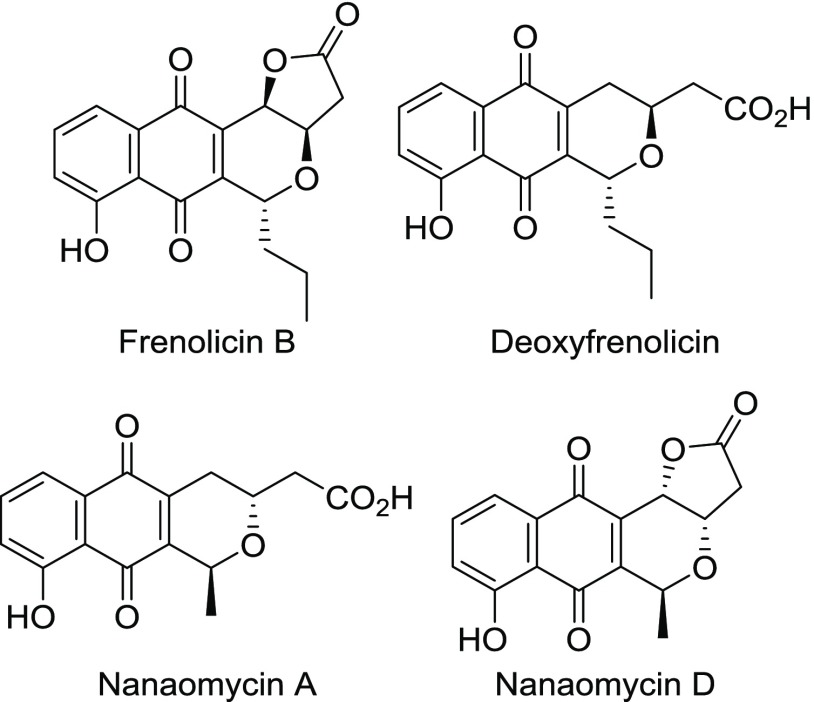
2-Alkyl-1,4-naphthoquinones generally act as electrophiles at their electron-deficient α,β-unsaturated ketone moieties. On the other hand, their tautomers, o-quinone methides, react as both nucleophiles and electrophiles. Natural 1,4-naphthoquinones undergo several biotransformations by both enzymatic and nonenzymatic modifications because of their high reactivities. Juglomycins have remarkable structural diversity by available modifications.7 To date, 11 juglomycins (A–J and Z) have been isolated and characterized.1,2,4,8,29 Related natural naphthoquinones such as frenolicins30−32 and nanaomycins33−36 have also been isolated (Figure 2).37 Thus, a divergent synthesis from a common intermediate will provide easy access to these natural products.38
Figure 2.
Structures for frenolicin B, deoxyfrenolicin, and nanaomycins A and D.
In this study, we demonstrated that compound 10 is a common intermediate for the divergent synthesis of juglomycins and their derivatives. The most significant feature of the approach described in this paper is that these natural products can be synthesized through only two to four steps from the common intermediate 10. Further synthetic studies toward related natural naphthoquinones and evaluation of the biological activities of synthetic compounds are currently ongoing in our laboratory.
Experimental Section
General Information
All solvents and reagents were used without further purification unless otherwise noted. Analytical TLC was performed using Silica gel 60 F254 plates (0.25 mm, normal phase) and Silica gel 60RP-18 F254S plates (0.25 mm, reverse phase). Normal phase flash column chromatography was performed using Silica gel 60 (particle size 40–63 μm; 230–400 mesh ASTM). Reverse phase flash column chromatography was performed using an octadecyl (C18) silica gel (particle size 20–30 μm). Melting point (mp) data were uncorrected. Specific rotations were recorded on a polarimeter and recorded as [α]D values (concentration in g/100 mL). IR spectra were recorded on an IR spectrometer using NaCl (neat) or KBr pellets (solid). 1H and proton-decoupled 13C (13C{1H}) NMR spectra were recorded on an NMR spectrometer (400 and 100 MHz, respectively) using chloroform-d (CDCl3), acetone-d6, dichloromethane-d2 (CD2Cl2), and methanol-d4 (CD3OD) as solvents. Chemical shift values are expressed in δ (ppm) relative to tetramethylsilane (TMS, δ 0.00 ppm) or the solvent resonance (CDCl3, δ 7.26 ppm for 1H NMR and δ 77.0 ppm for 13C NMR; acetone-d6, δ 2.04 ppm for 1H NMR and δ 29.8 ppm for 13C NMR; CD2Cl2, δ 5.32 ppm for 1H NMR and δ 53.7 ppm for 13C NMR; CD3OD, δ 49.0 ppm for 13C NMR). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, br = broad, dd = double doublet, m = multiplet), coupling constants (J; Hz), and integration. Mass spectra were obtained by Fourier transformation–ion cyclotron resonance–mass spectrometry (FT–ICR–MS) using a spectrometer with electrospray ionization (ESI) or on a high-resolution double-focusing mass spectrometer using fast atom bombardment (FAB). Preparative reverse phase high-performance liquid chromatography (HPLC) was performed by the LC-2000 Plus system (pump: PU-2086; UV detector: UV-2075) with a COSMOSIL 5C18-MS-II Packed Column (20 mm i.d. × 250 mm). Preparative gel-permeation chromatography (GPC) was carried out using a LC-2000 Plus system equipped with GPC H-2001 and GPC H-2002 (20 × 500 mm) columns using CHCl3 as the eluent.
(S)-4-[1′,4′-Dimethoxy-5′-(methoxymethoxy)naphthalen-2′-yl]-3-(methoxymethoxy)butan-1-ol (17)
A 1 M solution of TBAF in tetrahydrofuran (THF, 8.60 mL, 8.60 mmol) was added to a solution of 9(22) (2.12 g, 4.29 mmol) in THF (200 mL) at 0 °C. The mixture was stirred at room temperature (rt) for 2 h. The reaction was quenched by the addition of water. The resultant mixture was diluted with EtOAc. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 5/1, then CHCl3/MeOH = 95/5) to give 17 (1.49 g, 91%) as yellow oil. [α]D21 +14.8 (c 1.00, EtOAc); IR (neat) νmax: 3473, 2937, 2898, 2842, 1619, 1598, 1583, 1508 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.74 (dd, J = 8.4, 0.8 Hz, 1H), 7.40 (dd, J = 8.4, 8.0 Hz, 1H), 7.07 (dd, J = 8.0, 1.2 Hz, 1H), 6.68 (s, 1H), 5.25 (s, 2H), 4.59 (d, J = 6.8 Hz, 1H), 4.53 (d, J = 6.8 Hz, 1H), 4.17 (m, 1H), 3.93 (s, 3H), 3.86 (s, 3H), 3.86 (overlapped, 1H), 3.75 (m, 1H), 3.60 (s, 3H), 3.31 (s, 3H), 3.10 (dd, J = 13.2, 6.8 Hz, 1H), 2.92 (dd, J = 13.2, 6.4 Hz, 1H), 2.49 (br s, 1H), 1.86 (m, 1H), 1.74 (m, 1H); 13C{1H} NMR (100 MHz, CDCl3): δ 154.2, 152.8, 147.8, 131.4, 126.8, 126.6, 118.7, 116.7, 113.4, 108.9, 96.8, 96.4, 76.9, 61.7, 59.7, 56.8, 56.4, 55.8, 37.0, 35.9; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C20H28O7Na, 403.1727; found, 403.1721.
(S)-4-[1′,4′-Dimethoxy-5′-(methoxymethoxy)naphthalen-2′-yl]-3-(methoxymethoxy)butanoic Acid (10)
Dess–Martin periodinane (3.32 g, 7.83 mmol) was added to a solution of 17 (1.49 g, 3.92 mmol) in CH2Cl2 (170 mL). The mixture was stirred at rt for 40 min. The mixture was filtrated through a pad of Celite, and the filtrate was concentrated to give a crude aldehyde 18.
NaClO2 (80%, 1.33 g, 11.8 mmol) was added to a solution of the crude aldehyde 18, 2-methyl-2-butene (4.16 mL, 39.3 mmol), and NaH2PO4·2H2O (3.06 g, 19.6 mmol) in tert-butyl alcohol/H2O (1:1, 240 mL) at 0 °C. The mixture was stirred at rt for 1 h. The reaction was quenched by the addition of saturated aqueous NH4Cl solution. The resultant mixture was diluted with CHCl3. The organic layer was separated, washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 1/1 with 1% AcOH) to give 10 (1.31 g, 85% over two steps) as orange oil. [α]D22 −1.6 (c 1.00, EtOAc); IR (neat) νmax: 3163, 3070, 3010, 2995, 2937, 2844, 1734, 1712, 1660, 1620, 1601, 1583, 1460 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.74 (dd, J = 8.4, 1.2 Hz, 1H), 7.39 (dd, J = 8.4, 7.6 Hz, 1H), 7.07 (dd, J = 7.6, 1.2 Hz, 1H), 6.70 (s, 1H), 5.25 (s, 2H), 4.69 (d, J = 6.8 Hz, 1H), 4.63 (d, J = 6.8 Hz, 1H), 4.39 (m, 1H), 3.93 (s, 3H), 3.85 (s, 3H), 3.59 (s, 3H), 3.25 (s, 3H), 3.16, (dd, J = 13.6, 6.8 Hz, 1H), 2.98, (dd, J = 13.6, 6.8 Hz, 1H), 2.63, (dd, J = 16.0, 7.2 Hz, 1H), 2.56, (dd, J = 16.0, 5.2 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3): δ 176.9, 154.1, 152.8, 147.9, 131.4, 126.6, 126.1, 118.7, 116.8, 113.5, 108.7, 96.8, 96.1, 74.6, 61.7, 56.7, 56.4, 55.5, 39.7, 35.5; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C20H26O8Na, 417.1512; found, 417.1512.
(4R,5R)-5-[1′,4′-Dimethoxy-5′-(methoxymethoxy)naphthalen-2′-yl]-4-(methoxymethoxy)dihydrofuran-2(3H)-one (11) and (4R,5S)-5-[1′,4′-Dimethoxy-5′-(methoxymethoxy)naphthalen-2′-yl]-4-(methoxymethoxy)dihydrofuran-2(3H)-one (12)
DDQ (1.38 g, 6.08 mmol) was added to a suspension of 10 (800 mg, 2.03 mmol) and MS4A (1 g) in ClCH2CH2Cl (150 mL) at rt. The mixture was refluxed by heating in an oil bath under a nitrogen atmosphere for 4.5 h. The mixture was cooled to rt and filtrated through a pad of Celite. The filtrate was diluted with CHCl3 and water. The organic layer was separated, washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 2/1) to give 11 (107.8 mg, 14%) as orange oil and 12 (555.4 mg, 70%) as orange needles. 11: [α]D24 −17.2 (c 0.50, CHCl3); IR (neat) νmax: 3570, 3014, 2937, 2898, 1784, 1601, 1585, 1460 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.73 (dd, J = 8.0, 1.2 Hz, 1H), 7.43 (dd, J = 8.0, 8.0 Hz, 1H), 7.13 (dd, J = 8.0, 1.2 Hz, 1H), 6.93 (s, 1H), 5.96 (d, J = 4.0 Hz, 1H), 5.28 (d, J = 6.4 Hz, 1H), 5.26 (d, J = 6.4 Hz, 1H), 4.78 (dd, J = 5.2, 4.0 Hz, 1H), 4.26 (d, J = 6.8 Hz, 1H), 4.17 (d, J = 6.8 Hz, 1H), 3.96 (s, 3H), 3.89 (s, 3H), 3.61 (s, 3H), 2.98 (dd, J = 17.6, 5.2 Hz, 1H), 2.97 (s, 3H), 2.79 (d, J = 17.6 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3): δ 175.3, 154.4, 153.1, 146.3, 130.6, 126.8, 123.1, 119.5, 116.5, 114.2, 104.9, 96.8, 95.3, 80.6, 73.9, 62.1, 56.7, 56.4, 55.4, 37.6; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C20H24O8Na, 415.1363; found, 415.1362. 12: mp 57–59 °C; [α]D −11.3 (c 1.00, CHCl3); IR (neat) νmax: 3564, 2997, 2943, 2898, 2846, 2827, 1790, 1599, 1460 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.75 (dd, J = 8.4, 1.0 Hz, 1H), 7.45 (dd, J = 8.4, 7.7 Hz, 1H), 7.14 (dd, J = 7.7, 1.1 Hz, 1H), 6.62 (s, 1H), 5.86 (d, J = 2.8 Hz, 1H), 5.26 (s, 2H), 4.76 (m, 2H), 4.51 (m, 1H), 3.93 (s, 3H), 3.91 (s, 3H), 3.60 (s, 3H), 3.37 (s, 3H), 2.91 (dd, J = 17.9, 6.6 Hz, 1H), 2.66 (dd, J = 17.9, 3.3 Hz, 1H); 13C{1H} NMR (100 MHz, CDCl3): δ 175.5, 154.4, 153.8, 146.9, 131.3, 127.2, 125.2, 119.6, 116.6, 114.2, 102.5, 96.7, 95.8, 83.0, 78.6, 62.4, 56.7, 56.4, 55.8, 35.3; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C20H24O8Na, 415.1363; found, 415.1362.
Juglomycin A, 5-Hydroxy-2-[(2′R,3′R)-3′-hydroxy-5′-oxotetrahydrofuran-2′-yl]naphthalene-1,4-dione (1)
CAN (140 mg, 0.26 mmol) was added to a solution of 11 (40.0 mg, 0.102 mmol) in a 1:1 mixture of MeCN and water (5 mL) at 0 °C. The mixture was stirred at 0 °C for 45 min. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated to give a crude naphthoquinone.
TFA (2 mL) was added to a solution of the crude naphthoquinone in CH2Cl2 (6 mL) at 0 °C. The mixture was stirred at rt for 5 h and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3/MeOH = 10/1) and preparative reverse phase HPLC (MeCN/H2O = 2/3) to give 1 (15.4 mg, 55% over two steps) as yellow solids. mp 172–174 °C (decomp.), lit.2 172 °C (decomp.); [α]D27 −74.1 (c 1.00, MeOH), lit.3 [α]D −74.1 (c 0.1, MeOH); IR (KBr) νmax: 3529, 3394, 2924, 1780, 1647, 1614, 1462 cm–1; 1H NMR (400 MHz, acetone-d6): δ 11.92 (s, 1H), 7.78 (dd, J = 8.4, 7.6 Hz, 1H), 7.62 (dd, J = 7.6, 1.0 Hz, 1H), 7.34 (dd, J = 8.4, 1.0 Hz, 1H), 6.94 (d, J = 1.7 Hz, 1H), 5.70 (dd, J = 3.6, 1.7 Hz, 1H), 4.92 (dd, J = 4.5, 3.9 Hz, 1H), 4.77 (dd, J = 4.3, 0.9 Hz, 1H), 3.16 (dd, J = 17.4, 5.4 Hz, 1H), 2.50 (d, J = 17.4 Hz, 1H); 13C{1H} NMR (100 MHz, acetone-d6): δ 190.8, 183.8, 175.1, 162.1, 147.1, 137.6, 134.9, 133.1, 125.0, 119.5, 115.7, 81.4, 70.2, 39.5; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C14H10O6Na, 297.0370; found, 297.0371.
Juglomycin B, 5-Hydroxy-2-[(2′S,3′R)-3′-hydroxy-5′-oxotetrahydrofuran-2′-yl]naphthalen-1,4-dione (2)
CAN (297 mg, 0.542 mmol) was added to a solution of 12 (85.0 mg, 0.217 mmol) in a 1:1 mixture of MeCN and water (9 mL) at 0 °C. The mixture was stirred at 0 °C for 40 min. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated to give a crude naphthoquinone.
TFA (3 mL) was added to a solution of the crude naphthoquinone in CH2Cl2 (9 mL) at 0 °C. The mixture was stirred at rt for 4 h and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 2/1) and preparative reverse phase HPLC (MeCN/H2O = 3/2) to give 2 (31.1 mg, 52% over two steps) as yellow solids. mp 199–202 °C (decomp.), lit.2 202 °C (decomp.); [α]D15 +103.1 (c 0.16, MeOH), lit3 [α]D +102 (c 0.23, MeOH); IR (KBr) νmax: 3435, 2927, 1784, 1651, 1614, 1460 cm–1; 1H NMR (400 MHz, acetone-d6, TMS): δ 11.87 (s, 1H), 7.80 (dd, J = 8.4, 7.6 Hz, 1H), 7.64 (dd, J = 7.6, 1.2 Hz, 1H), 7.35 (dd, J = 8.4, 1.2 Hz, 1H), 6.82 (d, J = 1.2 Hz, 1H), 5.50 (s, 1H), 5.23 (d, J = 4.0 Hz, 1H), 4.67 (m, 1H), 3.00 (dd, J = 18.0, 6.0 Hz, 1H), 2.43 (dd, J = 18.0, 1.6 Hz, 1H); 13C{1H} NMR (100 MHz, acetone-d6): δ 190.8, 184.1, 175.9, 162.1, 148.1, 137.7, 134.0, 133.2, 125.1, 119.7, 115.8, 84.8, 71.9, 36.6; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C14H10O6Na, 297.0370; found, 297.0370.
Khatmiamycin, Methyl(3R,4R)-3,4-dihydroxy-4-(5′-hydroxy-1′,4′-dioxo-1′,4′-dihydronaphthalen-2′-yl)butanoate (8)
TsOH·H2O (13.9 mg, 73.1 μmol) was added to a solution of 1 (20.0 mg, 73.0 μmol) in distilled MeOH (9 mL) at rt. The mixture was stirred at 50 °C by heating in an oil bath for 2.5 h. The reaction was quenched by the addition of water. The resultant mixture was diluted with EtOAc. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (toluene/EtOAc = 3/2) and preparative GPC (CHCl3) to give 8 (8.4 mg, 38%) as yellow solids and recovered 1 (7.4 mg, 37%). mp 152–155 °C (decomp.); [α]D19 −103.3 (c 0.10, MeOH), lit.9 [α]D −21 (c 0.1, MeOH); IR (KBr) νmax: 3458, 3378, 2956, 2856, 1722, 1674, 1647, 1621, 1452 cm–1; 1H NMR (400 MHz, CD2Cl2): δ 11.92 (s, 1H), 7.64 (dd, J = 8.0, 7.6 Hz, 1H), 7.60 (dd, J = 7.6, 1.4 Hz, 1H), 7.28 (dd, J = 8.0, 1.4 Hz, 1H), 7.04 (d, J = 1.2 Hz, 1H), 4.79 (br s, 1H), 4.22 (br d, J = 5.7 Hz, 1H), 3.71 (s, 3H), 3.30 (br s, 1H), 3.13 (br d, J = 7.2 Hz, 1H), 2.78 (dd, J = 16.6, 8.8 Hz, 1H), 2.67 (dd, J = 16.6, 3.8 Hz, 1H); 13C{1H} NMR (100 MHz, CD2Cl2): δ 190.4, 184.7, 173.3, 161.6, 151.0, 136.7, 135.4, 132.4, 124.7, 119.4, 115.2, 70.5, 69.5, 52.3, 38.5; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C15H14O7Na, 329.0632; found, 329.0635.
4-epi-Khatmiamycin, Methyl(3R,4S)-3,4-dihydroxy-4-(5′-hydroxy-1′,4′-dioxo-1′,4′-dihydronaphthalen-2′-yl)butanoate (4-epi-8)
TsOH·H2O (24.3 mg, 0.128 mmol) was added to a solution of 2 (35.0 mg, 0.128 mmol) in MeOH (12 mL) at rt. The mixture was stirred at 50 °C by heating in an oil bath for 2.5 h. The reaction was quenched by the addition of water. The resultant mixture was diluted with EtOAc. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (toluene/EtOAc = 2/1) and preparative GPC (CHCl3) to give 4-epi-8 (16.7 mg, 43%) as yellow solids and recovered 2 (6.6 mg, 19%). mp 112 °C; [α]D23 +81.6 (c 0.10, MeOH); IR (KBr) νmax: 3381, 2954, 2852, 1730, 1691, 1639, 1608, 1482, 1454 cm–1; 1H NMR (400 MHz, CD2Cl2): δ 11.90 (s, 1H), 7.64 (dd, J = 8.0, 7.6 Hz, 1H), 7.60 (dd, J = 7.6, 1.6 Hz, 1H), 7.27 (dd, J = 8.0, 1.6 Hz, 1H), 7.07 (d, J = 1.2 Hz, 1H), 4.97 (d, J = 3.2 Hz, 1H), 4.41 (m, 1H), 3.63 (s, 3H), 3.54 (br s, 1H), 3.25 (br s, 1H), 2.55 (dd, J = 16.8, 8.8 Hz, 1H), 2.47 (dd, J = 16.8, 3.6 Hz, 1H); 13C{1H} NMR (100 MHz, CD2Cl2): δ 190.4, 184.5, 173.5, 161.6, 149.9, 136.8, 135.8, 132.4, 124.8, 119.5, 115.2, 71.3, 70.0, 52.2, 35.5; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C15H14O7Na, 329.0632; found, 329.0637.
Juglomycin C, (S)-3-Hydroxy-4-(5′-hydroxy-1′,4′-dioxo-1′,4′-dihydronaphthalen-2′-yl)butanoic Acid (3)
CAN (417 mg, 0.761 mmol) was added to a solution of 10 (120 mg, 0.305 mmol) in a 1:1 mixture of MeCN and water (12 mL) at 0 °C. The mixture was stirred at 0 °C for 15 min. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated to give a crude naphthoquinone.
TFA (4 mL) was added to a solution of the crude naphthoquinone in CH2Cl2 (12 mL) at 0 °C. The mixture was stirred at rt for 2 h and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 2/1) to give 3 (48.7 mg, 58% over two steps) as yellow solids. The 1H and 13C NMR spectra were identical with those of our authentic sample.20
Juglomycin D, (S)-3-Hydroxy-4-(3′,5′-dihydroxy-1′,4′-dioxo-1′,4′-dihydronaphthalen-2′-yl)butanoic Acid (6)
A 30% aqueous H2O2 solution (48 μL, 0.423 mmol) was added in portions to a solution of 3 (100 mg, 0.362 mmol) in 1 M sodium phosphate buffer (pH 8.5, 18.9 mL). The mixture was stirred at rt for 5 h. The mixture was diluted with EtOAc. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3/MeOH = 10/1 with 1% AcOH) to give 6 (69.3 mg, 66%) as yellow solids. The 1H and 13C NMR spectra were identical with those of our authentic sample.21
(S)-4-[1′,4′-Dimethoxy-5′-(methoxymethoxy)naphthalen-2′-yl]-3-(methoxymethoxy)butanamide (15)
Ethyl chloroformate (94 μL, 0.99 mmol) was added to a solution of 10 (130 mg, 0.330 mmol) and Et3N (138 μL, 0.99 mmol) in acetone (20 mL) at 0 °C. The mixture was stirred at rt for 1 h and filtered. The filtrate was concentrated to a crude mixed anhydride.
A 25% aqueous NH3 solution (12 mL) was added to a solution of the crude mixed anhydride in MeOH (18 mL) at 0 °C. The mixture was stirred at rt for 1.5 h. The mixture was diluted with CHCl3 and water. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 1/2, then CHCl3/MeOH = 10/1) to give 15 (128.6 mg, 99%) as orange oil. [α]D22 +4.5 (c 1.00, EtOAc); IR (neat) νmax: 3431, 3352, 3197, 3008, 2935, 2904, 2844, 2827, 1678, 1620, 1601, 1583, 1460 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.73 (dd, J = 8.0, 0.8 Hz, 1H), 7.39 (dd, J = 8.0, 8.0 Hz, 1H), 7.07 (dd, J = 8.0, Hz, 1H), 6.71 (s, 1H), 6.25 (br s, 1H), 6.00 (br s, 1H), 5.25 (s, 2H), 4.68 (d, J = 6.8 Hz, 1H), 4.60 (d, J = 6.8 Hz, 1H), 4.36 (m, 1H), 3.93 (s, 3H), 3.84 (s, 3H), 3.59 (s, 3H), 3.24 (s, 3H), 3.15 (dd, J = 13.6, 6.4 Hz, 1H), 2.96 (dd, J = 13.6, 6.4 Hz, 1H), 2.46 (m, 2H); 13C{1H} NMR (100 MHz, CDCl3): δ 173.4, 154.1, 152.8, 147.7, 131.3, 126.5, 126.2, 118.7, 116.6, 113.5, 108.7, 96.7, 95.9, 74.9, 61.6, 56.6, 56.3, 55.5, 41.1, 35.2; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C20H27NO7Na, 416.1680; found, 416.1674.
Juglomycin C Amide, (S)-3-Hydroxy-4-(5′-hydroxy-1′,4′-dioxo-1′,4′-dihydronaphthalen-2′-yl)butanamide (5)
CAN (348 mg, 0.635 mmol) was added to a solution of 15 (100 mg, 0.254 mmol) in a 1:1 mixture of MeCN and water (12 mL) at 0 °C. The mixture was stirred at 0 °C for 20 min. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated to give a crude naphthoquinone.
TFA (5 mL) was added to a solution of the crude naphthoquinone in CH2Cl2 (10 mL) at 0 °C. The mixture was stirred at rt for 1.5 h and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3/MeOH = 10/1) to give 5 (35.0 mg, 50% over two steps) as yellow solids. mp 161 °C (decomp.); [α]D28 −117 (c 0.01, MeOH); IR (KBr) νmax: 3338, 3167, 2972, 2926, 1670, 1641, 1614, 1454 cm–1. 1H NMR (400 MHz, CD3OD, TMS): δ 7.68 (dd, J = 8.0, 7.6 Hz, 1H), 7.62 (dd, J = 7.6, 0.8 Hz, 1H), 7.27 (dd, J = 8.0, 1.2 Hz, 1H), 6.93 (s, 1H), 4.27 (m, 1H), 2.82 (ddd, J = 13.6, 4.4, 1.2 Hz, 1H), 2.64 (dd, J = 13.6, 8.4 Hz, 1H), 2.43 (m, 2H); 13C{1H} NMR (100 MHz, CD3OD): δ 191.7, 185.6, 176.4, 162.3, 150.6, 138.0, 137.4, 133.7, 124.8, 120.1, 116.3, 68.1, 44.2, 38.4; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C14H13NO5Na, 298.0686; found, 298.0687.
(S)-3-(Methoxymethoxy)-4-{5′-(methoxymethoxy)-3′-methyl-1′,4′-dioxo-1′,4′-dihydronaphthalen-2′-yl}butanoic Acid (16)
CAN (139 mg, 0.254 mmol) was added to a solution of 10 (40.0 mg, 0.102 mmol) in a 1:1 mixture of MeCN and water (8 mL) at 0 °C. The mixture was stirred at 0 °C for 30 min. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated to give a crude naphthoquinone.
A solution of the crude naphthoquinone and AgNO3 (8.6 mg, 51 μmol) in a 1:1:1:1 mixture of AcOH, MeCN, CH2Cl2, and water (4 mL) was degassed. A solution of K2S2O8 (82.2 mg, 0.304 mmol) in degassed water (6 mL) was added to the mixture at rt. The mixture was refluxed at 90 °C by heating in an oil bath under a nitrogen atmosphere for 2.5 h. The resultant mixture was diluted with CHCl3 and water. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (toluene/EtOAc = 3/2 with 1% AcOH) to give 16 (11.5 mg, 30% over two steps) as brown oil. [α]D21 −5.9 (c 0.50, CHCl3); IR (KBr) νmax: 3213, 3072, 2956, 2931, 1732, 1712, 1657, 1627, 1587, 1468 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.79 (d, J = 8.4 Hz, 1H), 7.60 (t, J = 8.4 Hz, 1H), 7.48 (d, J = 8.4 Hz, 1H), 5.34 (s, 2H), 4.63 (m, 2H), 4.25 (m, 1H), 3.55 (s, 3H), 3.24 (s, 3H), 3.01 (dd, J = 13.1, 7.5 Hz, 1H), 2.91 (dd, J = 13.1, 6.0 Hz, 1H), 2.67 (dd, J = 15.7, 7.2 Hz, 1H), 2.56 (dd, J = 15.7, 5.0 Hz, 1H), 2.23 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 184.6, 184.3, 176.3, 156.8, 147.5, 140.8, 134.3, 134.1, 121.8, 121.0, 120.5, 96.3, 95.1, 73.9, 56.6, 55.7, 40.4, 32.5, 13.6; HRMS (FAB/double-focusing MS) m/z: [M + Na]+ calcd for C19H22O8Na, 401.1214; found, 401.1212.
Proposed Structure for Juglomycin Z, (S)-3-Hydroxy-4-(5′-hydroxy-3′-methyl-1′,4′-dioxo-1′,4′-dihydronaphthalen-2′-yl)butanoic Acid (7)
TFA (2 mL) was added to a solution of 16 (11.5 mg, 0.030 mmol) in CH2Cl2 (6 mL) at 0 °C. The mixture was stirred at rt for 1 h and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3/MeOH = 10/1) and preparative thin-layer chromatography (CHCl3/MeOH = 20/1 with 1% AcOH) to give 7 (3.0 mg, 34%) as yellow solids. mp 88–90 °C, lit.8 >300 °C; [α]D28 −44.3 (c 0.10, MeOH), lit.8 [α]D +144 (c 0.025, MeOH); IR (KBr) νmax: 3402, 3184, 2925, 1728, 1654, 1633, 1606, 1454 cm–1; 1H NMR (400 MHz, CDCl3/CD3OD = 9/1, TMS): δ 7.61 (m, 1H), 7.60 (m, 1H), 7.24 (dd, J = 7.0, 2.6 Hz, 1H), 4.23 (m, 1H), 2.89 (d, J = 6.6 Hz, 2H), 2.57 (m, 2H), 2.26 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 190.1, 184.8, 176.2, 161.2, 145.9, 144.2, 136.1, 131.9, 124.1, 119.2, 114.9, 67.5, 41.2, 34.0, 12.8 HRMS (ESI/FT–ICR–MS) m/z: [M – H]− calcd for C15H13O6, 289.0707; found, 289.0720.
Methyl (S)-3-Hydroxy-4-(5′-hydroxy-3′-methyl-1′,4′-dioxo-1′,4′-dihydronaphthalen-2′-yl)butanoate (19)
A 0.6 M solution of trimethylsilyldiazomethane in hexane (1.2 mL, 0.72 mmol) was added to a solution of 7 (3.0 mg, 10.3 μmol) in a 1:2 mixture of CH2Cl2 and MeOH (3 mL). The mixture was stirred at rt for 35 min. The reaction was quenched by the addition of AcOH (2 mL). The resultant mixture was diluted with CHCl3 and water. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 3/1) to give 19 (2.2 mg, 70%) as orange solids. mp 52–53 °C, lit.8 85 °C; [α]D22 −40.9 (c 0.32, MeOH), lit.8 [α]D +37.3 (c 0.01, MeOH); IR (KBr) νmax: 3525, 3020, 2953, 2854, 1731, 1657, 1635, 1610, 1579, 1458 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 12.14 (s, 1H), 7.61 (dd, J = 7.4, 1.8 Hz, 1H), 7.57 (dd, J = 7.8, 7.4 Hz, 1H), 7.23 (dd, J = 7.8, 1.8 Hz, 1H), 4.24 (m, 1H), 3.72 (s, 3H), 3.16 (br s, 1H), 2.87 (m, 2H), 2.65 (dd, J = 16.6, 4.2 Hz, 1H), 2.58 (dd, J = 16.6, 7.8 Hz, 1H), 2.25 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 190.2, 184.6, 172.8, 161.2, 145.7, 144.4, 136.0, 132.0, 124.0, 119.1, 115.0, 67.7, 51.9, 41.3, 34.0, 12.7; HRMS (ESI/FT–ICR–MS) m/z: [M + Na]+ calcd for C16H16O6Na, 327.0839; found, 327.0842.
3-Bromo-1,4-dimethoxy-5-(methoxymethoxy)-2-methylnaphthalene (24)
NaH (60% dispersion in mineral oil, 593.3 mg, 14.8 mmol) was added to a solution of 22(26) (3.29 g, 12.3 mmol), tetra-n-butylammonium iodide (TBAI, 226 mg, 0.61 mmol), and chlorodimethyl ether (MOMCl, 1.6 mL, 20.9 mmol) in THF (200 mL) at 0 °C. The mixture was stirred under an argon atmosphere at 0 °C for 15 min and at rt for 15 min. The reaction was quenched by the addition of water. The resultant mixture was diluted with EtOAc. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated. The residue was washed with hexane to remove mineral oil to afford crude 23. This compound was used for the next reaction.
A solution of Na2S2O4 (10.7 g, 61.6 mmol) in water (200 mL) was added to a solution of crude 23 in ether (200 mL) and CHCl3 (40 mL). The biphasic solution was stirred vigorously at rt for 10 min. The organic layer was collected and washed with water and brine, dried over Na2SO4, and concentrated. The residue was used for the next reaction without further purification.
Dimethyl sulfate (9.4 mL, 98.5 mmol) and K2CO3 (13.6 g, 98.5 mmol) were added to a solution of the crude hydroquinone in acetone (250 mL) under an argon atmosphere. The mixture was refluxed for 13.5 h and was cooled to rt. The reaction was quenched by the addition of water. The resultant mixture was diluted with EtOAc. The organic layer was separated, washed with 28% aqueous NH3 solution, water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was passed through silica gel column (toluene) to give 24 (3.98 g, 90% over three steps) as pale yellow solids. mp 57–59 °C; IR (KBr) νmax: 2999, 2954, 2930, 2841, 1614, 1580, 1571, 1491 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.76 (dd, J = 8.4, 0.7 Hz, 1H), 7.41 (dd, J = 8.4, 7.7 Hz, 1H), 7.14 (dd, J = 7.7, 0.7 Hz, 1H), 5.31 (s, 2H), 3.89 (s, 3H), 3.85 (s, 3H), 3.60 (s, 3H), 2.53 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 152.8, 150.2, 149.3, 130.2, 128.0, 126.6, 120.3, 119.6, 116.5, 112.4, 96.3, 61.47, 61.45, 56.5, 16.9; HRMS (FAB/double-focusing MS) m/z: [M]+ calcd for C15H1779BrO4, 340.0310; found, 340.0310.
(S)-4-((tert-Butyldimethylsilyl)oxy)-1-(1,4-dimethoxy-8-(methoxymethoxy)-3-methylnaphthalen-2-yl)butan-2-ol (26)
Magnesium powder (128 mg, 5.27 mmol) was activated by the addition of 1,2-diiodoethane (6.4 mg, 227 μmol) with vigorous stirring for 10 min under an argon atmosphere. A solution of 24 (719 mg, 2.11 mmol) in THF (3.5 mL) was added, and the mixture was stirred at rt for 30 min. The Grignard reagent was added to a solution of 25 (512 mg, 2.53 mmol) and CuCN (9.5 mg, 106 μmol) in THF (20 mL) at −78 °C under an argon atmosphere. The mixture was warmed up to rt and stirred for 1 h. The reaction was quenched by the addition of saturated aqueous NH4Cl solution. The resultant mixture was diluted with EtOAc. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 6/1) to give 26 (639 mg, 65%) as pale yellow oil. [α]D17 −13.7 (c 1.00, CHCl3); IR (neat) νmax: 3502, 2954, 2931, 2892, 2856, 1617, 1594, 1572, 1496 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.76 (dd, J = 8.4, 0.8 Hz, 1H), 7.35 (dd, J = 8.4, 7.7 Hz, 1H), 7.11 (dd, J = 7.6, 0.8 Hz, 1H), 5.30 (s, 2H), 4.10 (1H, m), 3.89 (m, 1H), 3.83 (s, 3H), 3.81 (s, 3H), 3.77 (m, 1H), 3.70 (d, J = 2.2 Hz, 1H), 3.59 (s, 3H), 3.03 (d, J = 6.4 Hz, 2H), 2.44 (s, 3H), 1.81 (m, 1H), 1.70 (m, 1H), 0.90 (s, 9H), 0.07 (s, 3H), 0.06 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 152.9, 150.2, 150.0, 130.2, 129.1, 127.5, 125.7, 119.7, 116.5, 112.0, 96.3, 72.1, 62.4, 61.8, 61.0, 56.4, 38.5, 34.9, 25.8 (3C), 18.1, 13.0, −5.56, −5.58; HRMS (FAB/double-focusing MS) m/z: [M]+ calcd for C25H40O6Si, 464.2594; found, 464.2594.
(S)-1-tert-Butyldimethylsilyloxy-4-[1,4-dimethoxy-8-(methoxymethoxy)-3-methylnaphthalen-2-yl]-3-methoxymethoxybutane (27)
MOMCl (370 μL, 4.81 mmol) was added to a solution of 26 (639 mg, 1.37 mmol), N,N-diisopropylethylamine (850 μL, 4.95 mmol), and TBAI (5.8 mg, 15.7 μmol) in CH2Cl2 (20 mL). The mixture was refluxed for 18 h under an argon atmosphere. The mixture was cooled to rt. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 5/1) to give 27 (588 mg, 84%) as colorless oil. [α]D19 +1.4 (c 1.00, CHCl3); IR (neat) νmax: 2953, 2930, 2887, 2856, 1617, 1593, 1572, 1496, 1471 cm–1; 1H NMR (400 MHz, CDCl3): δ 7.76 (dd, J = 8.4, 0.9 Hz, 1H), 7.35 (dd, J = 8.4, 7.7 Hz, 1H), 7.12 (dd, J = 7.6, 0.9 Hz, 1H), 5.31 (d, J = 6.8 Hz, 1H), 5.29 (d, J = 6.8 Hz, 1H), 4.56 (d, J = 6.8 Hz, 1H), 4.44 (d, J = 6.8 Hz, 1H), 4.09 (m, 1H), 3.82 (s, 3H), 3.80 (s, 3H), 3.73 (m, 2H), 3.59 (s, 3H), 3.13 (dd, J = 13.4, 7.6 Hz, 1H), 3.11 (s, 3H), 3.01 (dd, J = 13.4, 6.4 Hz, 1H), 2.46 (s, 3H), 1.82 (m, 1H), 1.72 (m, 1H), 0.84 (s, 9H), 0.01 (s, 3H), −0.02 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 153.0, 150.5, 149.9, 130.2, 129.3, 127.6, 125.6, 119.8, 116.6, 112.2, 96.6, 95.9, 75.0, 61.9, 61.0, 59.7, 56.4, 55.2, 38.2, 33.1, 25.8 (3C), 18.1, 13.1, −5.37, −5.42; HRMS (FAB/double-focusing MS) m/z: [M]+ calcd for C27H44O7Si, 508.2856; found, 508.2856.
(S)-4-(1,4-Dimethoxy-8-(methoxymethoxy)-3-methylnaphthalen-2-yl)-3-(methoxymethoxy)butan-1-ol (28)
A 1 M solution of TBAF in THF (1.7 mL, 1.70 mmol) was added to a solution of 27 (588 mg, 1.16 mmol) in THF (10 mL) at rt. The mixture was stirred at rt for 16 h. The reaction was quenched by the addition of water. The resultant mixture was diluted with EtOAc. The organic layer was separated, washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane/EtOAc = 2/1) to give 28 (405 mg, 89%) as colorless oil. [α]D20 +16.5 (c 1.00, CHCl3); IR (neat) νmax: 3472, 2949, 2892, 2833, 1616, 1593, 1572, 1496 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.76 (d, J = 8.4 Hz, 1H), 7.36 (dd, J = 8.4, 7.6 Hz, 1H), 7.12 (d, J = 7.6 Hz, 1H), 5.31 (s, 2H), 4.52 (d, J = 6.8 Hz, 1H), 4.42 (d, J = 6.8 Hz, 1H), 4.13 (m, 1H), 3.82 (m, 1H, overlapped), 3.82 (s, 3H), 3.80 (s, 3H), 3.74 (m, 1H), 3.59 (s, 3H), 3.25 (s, 3H), 3.13 (dd, J = 13.3, 7.3 Hz, 1H), 3.00 (dd, J = 13.3, 6.4 Hz, 1H), 2.62 (br s, 1H), 2.45 (s, 3H), 1.83 (m, 2H); 13C{1H} NMR (100 MHz, CDCl3): δ 153.0, 150.4, 149.9, 130.2, 128.8, 127.4, 125.8, 119.7, 116.5, 111.9, 96.4, 96.3, 77.1, 61.9, 61.1, 59.9, 56.5, 55.6, 37.0, 32.8, 13.1; HRMS (FAB/double-focusing MS) m/z: [M]+ calcd for C21H30O7, 394.1992; found, 394.1995.
(S)-4-(1,4-Dimethoxy-8-(methoxymethoxy)-3-methylnaphthalen-2-yl)-3-hydroxybutanoic Acid (29)
Dess–Martin periodinane (740 mg, 1.74 mmol) was added to a solution of 28 (405 g, 1.03 mmol) in CH2Cl2 (15 mL). The mixture was stirred at rt for 40 min. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The aqueous layer was extracted with CHCl3 three times. The combined organic layer was dried over Na2SO4 and concentrated to give a crude aldehyde.
A solution of NaClO2 (80%, 348.5 mg, 3.08 mmol) in H2O (8 mL) was added to a solution of the crude aldehyde, 2-methyl-2-butene (1.1 mL, 10.3 mmol), and NaH2PO4·2H2O (802 mg, 5.14 mmol) in tert-butyl alcohol/THF (1:1, 16 mL) at 0 °C. The mixture was stirred at 0 °C for 1.5 h. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The aqueous layer was extracted with CHCl3 four times. The combined organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3/MeOH = 19/1) to give a crude 29. The crude 29 was further purified by silica gel column chromatography (CHCl3/MeOH = 99/1) to give 29 (395 mg, 94% over two steps) as pale yellow oil. [α]D20 +1.01 (c 1.00, CHCl3); IR (neat) νmax: 3164, 3079, 3057, 2987, 2949, 2834, 1734, 1710, 1593, 1572, 1442 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 7.75 (dd, J = 8.4, 0.8 Hz, 1H), 7.34 (dd, J = 8.4, 7.6 Hz, 1H), 7.11 (dd, J = 7.6, 0.8 Hz, 1H), 5.30 (s, 2H), 4.66 (d, J = 7.0 Hz, 1H), 4.57 (d, J = 7.0 Hz, 1H), 4.33 (m, 1H), 3.82 (s, 3H), 3.79 (s, 3H), 3.59 (s, 3H), 3.21 (s, 3H), 3.17, (dd, J = 13.3, 6.5 Hz, 1H), 3.09, (dd, J = 13.3, 7.7 Hz, 1H), 2.67, (dd, J = 15.9, 7.8 Hz, 1H), 2.55 (dd, J = 15.9, 4.6 Hz, 1H), 2.45 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 177.3, 153.0, 150.5, 150.0, 130.4, 127.9, 127.4, 125.9, 119.7, 116.5, 112.1, 96.4, 96.0, 74.4, 61.9, 61.1, 56.5, 55.4, 39.8, 32.5, 12.9; HRMS (FAB/double-focusing MS) m/z: [M – H]− calcd for C21H27O8, 407.1706; found, 407.1705.
(S)-3-Hydroxy-4-(8-hydroxy-3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)butanoic Acid (20)
CAN (311 mg, 0.834 mmol) was added to a solution of 29 (136 mg, 0.333 mmol) in a 1:1 mixture of MeCN and water (5 mL) at 0 °C. The mixture was stirred at 0 °C for 15 min. The reaction was quenched by the addition of water. The resultant mixture was diluted with CHCl3. The aqueous layer was extracted with CHCl3 three times. The combined organic layer was dried over Na2SO4 and concentrated to give a crude naphthoquinone.
TFA (3 mL) was added to a solution of the crude naphthoquinone in CH2Cl2 (12 mL) at 0 °C. The mixture was stirred at rt for 5 h and concentrated under reduced pressure. The residue was purified by octadecyl silica gel column chromatography (MeOH/H2O = 3/2) to give 20 (72.9 mg, 75% over two steps) as brownish yellow solids. mp 143–148 °C; [α]D15 −58.0 (c 0.15, MeOH); IR (KBr) νmax: 3402, 3184, 2925, 1728, 1654, 1633, 1606, 1454 cm–1; 1H NMR (400 MHz, acetone-d6): δ 12.15 (s, 1H), 7.70 (dd, J = 8.4, 7.6 Hz, 1H), 7.56 (dd, J = 7.6, 1.0 Hz, 1H), 7.25 (dd, J = 8.4, 1.0 Hz, 1H), 4.34 (m, 1H), 2.93 (dd, J = 12.8, 5.2 Hz, 1H), 2.89 (dd, J = 12.8, 8.4 Hz, 1H), 2.63 (dd, J = 15.6, 4.6 Hz, 1H), 2.54 (dd, J = 15.6, 8.2 Hz, 1H), 2.23 (s, 3H); 13C{1H} NMR (100 MHz, acetone-d6): δ 191.3, 184.8, 173.0, 161.9, 147.7, 144.3, 137.0, 133.2, 124.2, 119.2, 115.8, 68.3, 42.6, 34.9, 13.6; HRMS (FAB/double-focusing MS) m/z: [M]− calcd for C15H14O6, 290.0790; found, 290.0791.
Methyl (S)-3-hydroxy-4-(8-hydroxy-3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)butanoate (21)
A 2.0 M solution of trimethylsilyldiazomethane in Et2O (120 μL, 240 μmol) was added to a solution of 20 (17.7 mg, 61.0 μmol) in a 1:1 mixture of CH2Cl2 and MeOH (4 mL). The mixture was stirred at rt for 25 min. The reaction was quenched by the addition of AcOH (100 μL). The resultant mixture was diluted with MeOH and concentrated. The residue was purified by silica gel column chromatography (hexane/acetone = 3/2) to give 21 (17.5 mg, 94%) as yellow solids. mp 86 °C; [α]D16 −89.4 (c 0.15, MeOH); IR (KBr) νmax: 3550, 3486, 3028, 3002, 2989, 2978, 2950, 2927, 2873, 1741, 1724, 1657, 1633, 1610, 1570, 1458 cm–1; 1H NMR (400 MHz, CDCl3, TMS): δ 12.11 (s, 1H), 7.62 (dd, J = 7.5, 1.6 Hz, 1H), 7.58 (dd, J = 8.0, 7.5 Hz, 1H), 7.22 (dd, J = 8.0, 1.6 Hz, 1H), 4.27 (m, 1H), 3.73 (s, 3H), 3.16 (br s, m), 2.89 (m, 2H), 2.66 (dd, J = 16.6, 4.0 Hz, 1H), 2.59 (dd, J = 16.6, 8.0 Hz, 1H), 2.25 (s, 3H); 13C{1H} NMR (100 MHz, CDCl3): δ 190.3, 184.2, 172.9, 161.2, 147.2, 142.9, 136.1, 132.2, 123.9, 119.1, 114.8, 67.6, 51.9, 41.1, 33.3, 13.5; HRMS (FAB/double-focusing MS) m/z: [M + H]+ calcd for C16H17O6Na, 305.1025; found, 305.1025.
Acknowledgments
This study was partly supported by a Grant-in Aid from the Japan Society for the Promotion of Science (JSPS) Fellows (no. 16J00542) to S.K. and a Grant-in-Aid for Scientific Research (C) (KAKENHI no. 15K07416) and the Sumitomo Foundation (no. 170183) to K.K.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01376.
Comparison of NMR spectroscopic data between natural and synthetic juglomycin Z (7), comparison of NMR spectroscopic data between compound 19 derived from natural and synthetic 7, comparison of NMR spectroscopic data between compound 19 derived from natural 7 and compound 21, and copies of 1H and 13C NMR spectra for all synthetic compounds (PDF)
Author Contributions
§ K.Y. and S.K. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Ushiyama K.; Tanaka N.; Ono H.; Ogata H. New antibiotics, juglomycins. I. Biological properties of Streptomyces species 190-2 and its products. Jpn. J. Antibiot. 1971, 24, 197–199. 10.11553/antibiotics1968b.24.197. [DOI] [PubMed] [Google Scholar]
- Tanaka N.; Ogata H.; Ushiyama K.; Ono H. New antibiotics, juglomycins. II. Structures of juglomycins A and B. Jpn. J. Antibiot. 1971, 24, 222–224. 10.11553/antibiotics1968b.24.222.5316819 [DOI] [Google Scholar]
- Krupa J.; Lackner H.; Jones P. G.; Schmidt-Bäse K.; Sheldrick G. M. The Absolute Configuration of the Juglomycins. Z. Naturforsch. 1989, 44, 345–352. 10.1515/znb-1989-0316. [DOI] [Google Scholar]
- Lessmann H.; Krupa J.; Lackner H.; Jones P. G. Neue Juglomycine/New Juglomycins. Z. Naturforsch., B: J. Chem. Sci. 1989, 44, 353–363. 10.1515/znb-1989-0317. [DOI] [Google Scholar]
- Taguchi T.; Ebizuka Y.; Hopwood D. A.; Ichinose K. Identification of a novel shunt product produced by a disruptant of the actVI-ORFA gene involved in the biosynthesis of actinorhodin in Streptomyces coelicolor A3(2). Tetrahedron Lett. 2000, 41, 5253–5256. 10.1016/s0040-4039(00)00824-8. [DOI] [Google Scholar]
- Ozawa M.; Taguchi T.; Itoh T.; Ebizuka Y.; Booker-Milburn K. I.; Stephenson G. R.; Ichinose K. Structure and biosynthetic implication of (S)-NHAB, a novel shunt product, from a disruptant of the actVI-ORFA gene for actinorhodin biosynthesis in Streptomyces coelicolor A3(2). Tetrahedron 2003, 59, 8793–8798. 10.1016/j.tet.2003.09.036. [DOI] [Google Scholar]
- Gubbens J.; Zhu H.; Girard G.; Song L.; Florea B. I.; Aston P.; Ichinose K.; Filippov D. V.; Choi Y. H.; Overkleeft H. S.; Challis G. L.; van Wezel G. P. Natural Product Proteomining, a Quantitative Proteomics Platform, Allows Rapid Discovery of Biosynthetic Gene Clusters for Different Classes of Natural Products. Chem. Biol. 2014, 21, 707–718. 10.1016/j.chembiol.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Fiedler H.-P.; Kulik A.; Schüz T. C.; Volkmann C.; Zeeck A. Biosynthetic capacities of Actinomycetes. 2. Juglomycin Z, a new naphthoquinone antibiotic from Streptomyces tendae. J. Antibiot. 1994, 47, 1116–1122. 10.7164/antibiotics.47.1116. [DOI] [PubMed] [Google Scholar]
- Abdalla M. A.; Win H. Y.; Islam M. T.; von Tiedemann A.; Schüffler A.; Laatsch H. Khatmiamycin, a motility inhibitor and zoosporicide against the grapevine downy mildew pathogen Plasmopara viticola from Streptomyces sp. ANK313. J. Antibiot. 2011, 64, 655–659. 10.1038/ja.2011.68. [DOI] [PubMed] [Google Scholar]
- Thomson R. H.Naturally occurring quinones IV; Chapman and Hall: London, 1997. [Google Scholar]
- Giles R. G. F.; Mitchell P. R. K.; Roos G. H. P.; Strümpfer J. M. M. The Syntheses of (±)-Juglomycin A and (±)-Juglomycin B, Racemates of two Isomeric Naturally Occurring Naphthoquinonoid Antibiotics. J. Chem. Soc., Perkin Trans. 1 1981, 2091–2095. 10.1039/p19810002091. [DOI] [Google Scholar]
- Brimble M. A.; Ireland E.; Phythian S. J. The Synthesis of 5’-Deoxyjuglomycin A and 5’-Methoxyjuglomycin A. Tetrahedron Lett. 1991, 32, 6417–6420. 10.1016/0040-4039(91)80184-8. [DOI] [Google Scholar]
- Brimble M. A.; Ireland E. Formal Synthesis of the Juglomycins. J. Chem. Soc., Perkin Trans. 1 1994, 3019–3114. 10.1039/p19940003109. [DOI] [Google Scholar]
- Maeda H.; Kraus G. A. A Direct Asymmetric Synthesis of Juglomycin A. J. Org. Chem. 1996, 61, 2986–2987. 10.1021/jo952077p. [DOI] [PubMed] [Google Scholar]
- Kraus G. A.; Liu P. A Racemic Synthesis of the Novel Antibacterial Agent Juglomycin A. Synth. Commun. 1996, 26, 4501–4506. 10.1080/00397919608003852. [DOI] [Google Scholar]
- Min J.-P.; Kim J. C.; Park O. S. Efficient Synthesis of (±)-Juglomycin A. Synth. Commun. 2004, 34, 383–390. 10.1081/scc-120027276. [DOI] [Google Scholar]
- Fernandes R. A.; Chavan V. P. A highly enantioselective synthesis of (−)- and (+)-juglomycin A through Dütz annulation and asymmetric dihydroxylation. Tetrahedron Lett. 2008, 49, 3899–3901. 10.1016/j.tetlet.2008.04.059. [DOI] [Google Scholar]
- Fernandes R. A.; Chavan V. P. A Dötz benzannulation route to the enantioselective synthesis of (−)- and (+)-juglomycin A. Tetrahedron: Asymmetry 2011, 22, 1312–1319. 10.1016/j.tetasy.2011.07.018. [DOI] [Google Scholar]
- Fernandes R. A.; Chavan V. P.; Mulay S. V.; Manchoju A. A Chiron Approach to the Total Synthesis of (−)-Juglomycin A, (+)-Kalafungin, (+)-Frenolicin B, and (+)-Deoxyfrenolicin. J. Org. Chem. 2012, 77, 10455–10460. 10.1021/jo3019939. [DOI] [PubMed] [Google Scholar]
- Kamo S.; Maruo S.; Kuramochi K.; Tsubaki K. Synthesis of enantiomerically pure juglomycin C and NHAB. Tetrahedron 2015, 71, 3478–3484. 10.1016/j.tet.2015.03.073. [DOI] [Google Scholar]
- Kamo S.; Kuramochi K.; Tsubaki K. Bioinspired Synthesis of Juglorubin from Juglomycin C. Org. Lett. 2018, 20, 1082–1085. 10.1021/acs.orglett.7b04051. [DOI] [PubMed] [Google Scholar]
- Kamo S.; Yoshioka K.; Kuramochi K.; Tsubaki K. Total Syntheses of Juglorescein and Juglocombins A and B. Angew. Chem. Int. Ed. 2016, 55, 10317–10320. 10.1002/anie.201604765. [DOI] [PubMed] [Google Scholar]
- Dess D. B.; Martin J. C. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983, 48, 4155–4156. 10.1021/jo00170a070. [DOI] [Google Scholar]
- Bal B. S.; Childers W. E. Jr.; Pinnick H. W. Oxidation of α,β-un saturated aldehydes. Tetrahedron 1981, 37, 2091–2096. 10.1016/s0040-4020(01)97963-3. [DOI] [Google Scholar]
- Jacobsen N.; Torssell K. Radikalische Alkylierung von Chinonen: Erzeugung von kalen in Redoxreaktionen. Liebigs Ann. Chem. 1972, 763, 135–147. 10.1002/jlac.19727630115. [DOI] [Google Scholar]
- Ogihara K.; Yamashiro R.; Higa M.; Yogi S. Preparation of Naphthoquinone Derivatives from Plumbagin and Their Ichthyotoxicity. Chem. Pharm. Bull. 1997, 45, 437–445. 10.1248/cpb.45.437. [DOI] [Google Scholar]
- Fotso S.; Maskey R. P.; Grün-wollny I.; Schulz K.-P.; Munk M.; Laatsch H. Bhimamycin A∼ E and Bhimanone: Isolation, Structure Elucidation and Biological Activity of Novel Quinone Antibiotics from a Terrestrial Streptomycete. J. Antibiot. 2003, 56, 931–941. 10.7164/antibiotics.56.931. [DOI] [PubMed] [Google Scholar]
- Likhitwitayawuid K.; Kaewamatawong R.; Ruangrungsi N.; Krungkrai J. Antimalarial Naphthoquinones from Nepenthes thorelii. Planta Med. 1998, 64, 237–241. 10.1055/s-2006-957417. [DOI] [PubMed] [Google Scholar]
- Maskey R. P.; Lessmann H.; Fotso S.; Grün-Wollny I.; Lackner H.; Laatsch H. Juglomycins G-J: Isolation from Streptomycetes and Structure Elucidation. Z. Naturforsch. 2005, 60, 183–188. 10.1515/znb-2005-0210. [DOI] [Google Scholar]
- Ellestad G. A.; Whaley H. A.; Patterson E. L. The Structure of Frenolicin. J. Am. Chem. Soc. 1966, 88, 4109–4110. 10.1021/ja00969a050. [DOI] [PubMed] [Google Scholar]
- Iwai Y.; Kora A.; Takahashi Y.; Hayashi T.; Awaya J.; Masuma R.; Oiwa R.; Omura S. Production of deoxyfrenolicin and a new antibiotic frenolicin B, by Streptomyces roseofulvus strain AM-3867. J. Antibiot. 1978, 31, 959–965. 10.7164/antibiotics.31.959. [DOI] [PubMed] [Google Scholar]
- Wang X.; Shaaban K. A.; Elshahawi S. I.; Ponomareva L. V.; Sunkara M.; Zhang Y.; Copley G. C.; Hower J. C.; Morris A. J.; Kharel M. K.; Thorson J. S.; Frenolicins C.-G. Pyranonaphthoquinones from Streptomyces sp. RM-4-15. J. Nat. Prod. 2013, 76, 1441–1447. 10.1021/np400231r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S.; Tanaka H.; Koyama Y.; O̅iwa R.; Katagiri M.; Awaya J.; Nagai T.; Hata T. Nanaomycins A and B, new antibiotics produced by a strain of Streptomyces. J. Antibiot. 1974, 27, 363–365. 10.7164/antibiotics.27.363. [DOI] [PubMed] [Google Scholar]
- Tanaka H.; Koyama Y.; Awaya J.; Marumo H.; Oiwa R.; Katagiri M.; Nagai T.; Omura S. Nanaomycins, new antibiotics produced by a strain of Streptomyces. I. Taxonomy, isolation, characterization and biological properties. J. Antibiot. 1975, 28, 860–867. 10.7164/antibiotics.28.860. [DOI] [PubMed] [Google Scholar]
- Tanaka H.; Koyama Y.; Nagai T.; Marumo H.; Omura S. Nanaomycins, new antibiotics produced by a strain of Streptomyces. II. Structure and biosynthesis. J. Antibiot. 1975, 28, 868–875. 10.7164/antibiotics.28.868. [DOI] [PubMed] [Google Scholar]
- O̅mura S.; Tanaka H.; Okada Y.; Marumo H. Isolation and Structure of Nanaomycin D, an Enantiomer of the Antibiotic Kalafungin. J. Chem. Soc., Chem. Commun. 1976, 320–321. 10.1039/c39760000320. [DOI] [Google Scholar]
- Brimble M. A.; Nairn M. R.; Duncalf L. J. Pyranonaphthoquinone antibiotics—isolation, structure and biological activity. Nat. Prod. Rep. 1999, 16, 267–281. 10.1039/a804287j. [DOI] [PubMed] [Google Scholar]
- Li L.; Chen Z.; Zhang X.; Jia Y. Divergent Strategy in Natural Product Total Synthesis. Chem. Rev. 2018, 118, 3752–3832. 10.1021/acs.chemrev.7b00653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.