Abstract
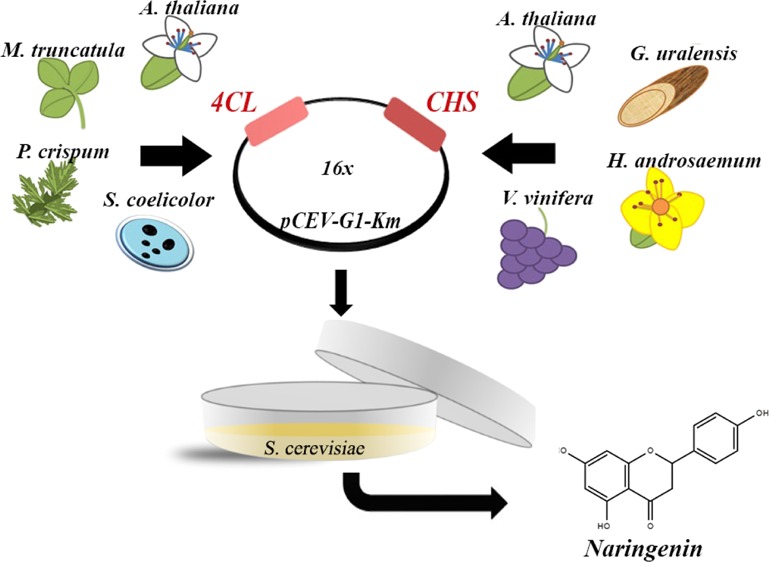
Flavonoids are plant secondary metabolites with great potential in the food industry. Metabolic engineering of Saccharomyces cerevisiae is a sustainable production technique. However, the current naringenin production yield is low because of inefficient enzymatic activity. Hence, this study uses gene source screening as a tool to identify the best gene source for enzymes such as 4-coumarate: coenzyme ligase (4CL) and chalcone synthase (CHS). For the first time, the 4CL gene from Medicago truncatula and the CHS gene from Vitis vinifera were expressed in S. cerevisiae, and this combination provided the highest yield of naringenin, which was 28-fold higher as compared to the reference strain. The combinations obtained similar performance in the Y-28 strains, where the highest production was 28.68 mg/L. Our results demonstrated that the selection and combination of enzymes from the correct gene source could greatly improve naringenin production. For the future, this could help commercialize flavonoid production, which would result in natural food preservatives and additives.
Introduction
Flavonoids are natural compounds with highly advantageous health benefits. Their antioxidant, antiallergenic, anticancer, and anti-inflammatory properties have been shown to help prevent the development of neurodegenerative and cardiovascular diseases, Parkinson’s disease, obesity, diabetes, and aging.1−3 Flavonoids can be found in plants, especially in berries. Consumption of flavonoid-rich foods has been related to improved health. Because of this, flavonoids have the potential as food additives, and they can be used in food or beverages to increase their nutritional value and create a value-added product.4 Furthermore, flavonoids possess highly active antimicrobial properties, which present the opportunity to apply flavonoids as food preservatives. As increasing disadvantages of using synthetic compounds in the food industry are emerging, the use of natural food preservatives and additives is a better solution.5
Even though there is a significant potential for the use of flavonoids in the food industry, the current production, which is mainly based on plant extraction, has many limitations. First, many of these plants are also food sources. Hence, their usage for flavonoid extraction means a decrease in the overall food availability. Second, the amount of available plants is restricted by small plantation sizes, unfavorable climate, long growing time, and cycles. At the same time, the concentration of flavonoids is relatively low in these plants. All these limitations add up to an expensive, high energy demanding preparation process, which leaves place for further improvements.6
An alternative solution is chemical synthesis, but because of the fact that certain flavonoid structures are complex, synthesis is expensive and time and energy demanding.7 Recently, more sustainable options have been investigated, such as the potential use of food waste. The possibility to extract antioxidants from food wastes, residues from fruit and vegetable processing, for use as food additives had been investigated.8 However, the extraction process is not optimal, which is very laborious, and the obtained amount of flavonoids is relatively low.
A potential alternative for sustainable production of flavonoids is the application of metabolic engineering on food-grade microorganisms, such as Saccharomyces cerevisiae. Metabolic engineering alters the function of the microorganism by the insertion of non-native DNA sequences, which produces enzymes that are capable of producing the target compound. This newly established enzymatic system mimics the flavonoid producing pathway in plants and reproduces it, utilizing the already existing precursors in the host microorganism.9 This method saves time, labor, and energy and does not compete with food source. Thus, it could provide a better and more cost-effective alternative sustainable method for the production of flavonoids.
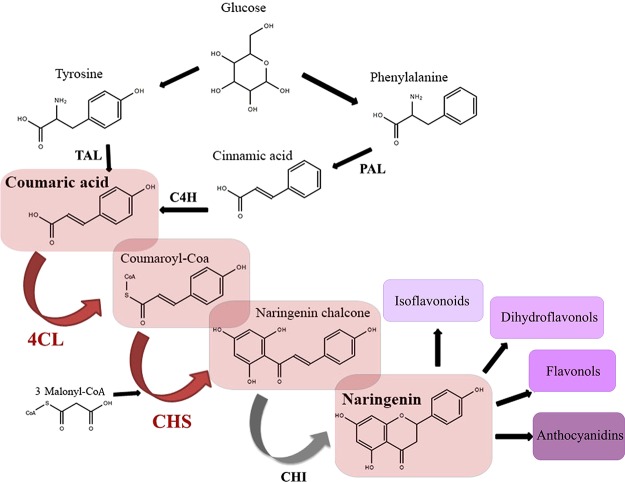
Naringenin is the central precursor of the flavonoid pathway. It is produced through the phenylpropanoid metabolic pathway. As it is a central compound in this pathway, it is essential for the production of other flavonoids such as isoflavonoids, flavonols, dihydroflavonols, and anthocyanidins.10 The pathway for naringenin production (Figure 1) consists of five enzymes, which are phenylalanine ammonia lyase (PAL), cinnamate 4-hydroxylase (C4H), 4-coumarate: coenzyme (CoA) ligase (4CL), chalcone synthase (CHS), and chalcone isomerase (CHI). Alternatively, tyrosine ammonia lyase (TAL) is able to convert tyrosine to p-coumaric acid (Figure 1). In this way, the pathway can bypass C4H. Additionally, naringenin chalcone is able to convert to naringenin without CHI under certain conditions. This opens up the possibility to bypass CHI and shorten the necessary changes in the host microorganism.11
Figure 1.
Biosynthetic pathway of naringenin.
Naringenin was first produced with the aid of metabolic engineering tools in Escherichia coli by Hwang et al.12 Then, a fine pathway was introduced into S. cerevisiae by Jiang et al.13 In order to boost the naringenin production, p-coumaric acid-overexpressing strains were applied, and gene overexpression, deletion, and replacement were performed.6,14−16 Further engineering steps made it possible to use glucose as the feedstock, which resulted in the yield of 108 mg/L naringenin.17,18 However, the current enzymatic activity was generally low, which leaves room for further improvements. While the genetic changes were well-explored, the enzyme sources were unexplored, even though it is known that the origin of the enzyme greatly influences its enzymatic activities and could affect the production rate. Hence, gene screening should be carried out. This research focused on identifying the source of the 4CL and CHS enzymes, which lead to the highest naringenin production.
Results and Discussion
Gene Source Selection
4CL and CHS gene sequences were screened in the NCBI database based on their origin, which was compared by their evolutionary background, gene sequences, and protein tertiary structures. On the basis of the results, four 4CL and four CHS gene sources were selected and expressed in S. cerevisiae. The 4CL gene sources were Arabidopsis thaliana, Medicago truncatula, Petroselinum crispum, and Streptomyces coelicolor. The CHS gene sources were A. thaliana, Glycyrrhiza uralensis, Hypericum androsaemum, and Vitis vinifera. A. thaliana is a suitable reference as it is the most frequently used gene source for the expression of 4CL and CHS genes by research groups. Most of the other enzymes have been expressed before, but this is the first time that 4CL from M. truncatula and CHS from V. vinifera are used for naringenin production. Additionally, many unique combinations have been expressed for the first time.
On the basis of the gene sequence blast results (Tables 1 and 2), the 4CL sources were very diverse. It was found that S. coelicolor showed the least similarities, as compared with the other gene sequences. This was reasonable as S. coelicolor is a bacterium, while the others are plants. For CHS, there was less diversity. The gene sequence from G. uralensis was more distinguished, with a slightly less similarity percentage.
Table 1. Sequence Blast Results for the 4CL Gene from Different Sources.
| similarity |
||||
|---|---|---|---|---|
| 4CL | amount of nucleotides | A. thaliana | M. truncatula | P. crispum |
| A. thaliana | 1671 | |||
| M. truncatula | 1764 | 62.5% | ||
| P. crispum | 1635 | 63% | 57.1% | |
| S. coelicolor | 1569 | 51.4% | 46.8% | 54.7% |
Table 2. Sequence Blast Results for the CHS Gene from Different Sources.
| similarity |
||||
|---|---|---|---|---|
| CHS | amount of nucleotides | A. thaliana | G. uralensis | H. androsaemum |
| A. thaliana | 1188 | |||
| G. uralensis | 1170 | 68% | ||
| H. androsaemum | 1173 | 73.9% | 72.8% | |
| V. vinifera | 1182 | 74.7% | 69.7% | 74.7% |
The percentage identity of the protein tertiary structures was shown to be similarly aligned with the gene sequence results (Tables 3 and 4). The 4CL structures were more diverse compared to the CHS structures. In particular, the results from S. coelicolor were shown to be the most noticeably different. However, the tertiary structure of M. truncatula 4CL was unlike the others, as the percentage identity was only around 50%, which was lower than the DNA sequence similarity value. An aligned tertiary structure comparison is shown in Figure 2a.
Table 3. Percentage Identity for 4CL Protein Tertiary Structures.
| percentage
identity |
|||
|---|---|---|---|
| 4CL | A. thaliana | M. truncatula | P. crispum |
| A. thaliana | |||
| M. truncatula | 51.4% | ||
| P. crispum | 68.75% | 48.8% | |
| S. coelicolor | 37.92% | 42.16% | 37.92% |
Table 4. Percentage Identity for CHS Protein Tertiary Structures.
| percentage
identity |
|||
|---|---|---|---|
| CHS | A. thaliana | G. uralensis | H. androsaemum |
| A. thaliana | |||
| G. uralensis | 83.12% | ||
| H. androsaemum | 86.49% | 87.53% | |
| V. vinifera | 86.75% | 84.94% | 87.92% |
Figure 2.
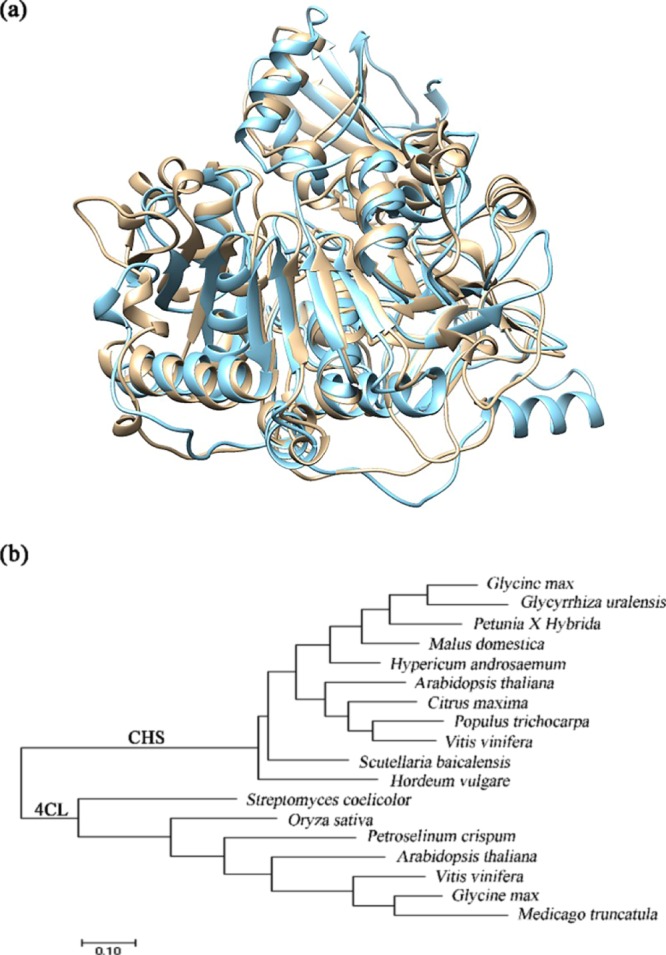
Gene screening by tertiary structures and phylogenetic tree. (a) Alignment of A. thaliana and M. truncatula 4CL tertiary structure. (b) Phylogenetic tree of known 4CL and CHS gene sources.
A phylogenetic tree, which displayed the evolutionary relationship between the enzymes, was created for 4CL and CHS. As shown in Figure 2b, the 4CL gene from A. thaliana and P. crispum was more similar as they were closer, whereas M. truncatula and S. coelicolor were at two branches on different ends of the tree. Similarly, the CHS gene from A. thaliana and H. androsaemum was found in the middle of the branch, while G. uralensis and V. viniferawere found toward two different ends. These findings were found to be in alignment with the DNA sequence and protein structure similarities.
Expression of New Combinations in S. cerevisiae
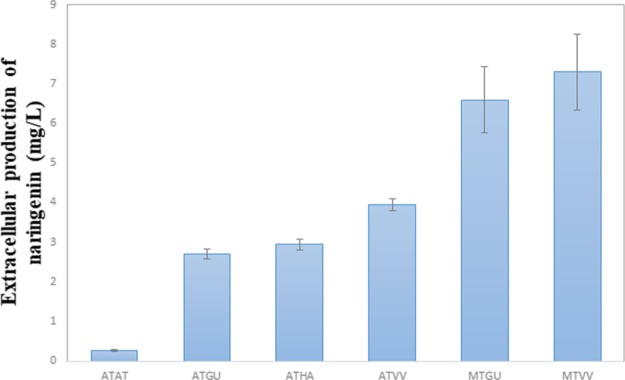
The pCEV-G1-Km plasmids containing the different 4CL–CHS combinations were expressed in S. cerevisiae, and the naringenin production was quantified by high-performance liquid chromatography (HPLC) (Table 5 and Figure 3). The highest yield was reached by expressing 4CL from M. truncatula and CHS from V. vinifera. This is a novel combination as neither of these enzymes has been expressed in S. cerevisae. Their naringenin production was 28-fold higher as compared to the reference strain which expressed the 4CL and CHS genes from A. thaliana.
Table 5. Effect of Expressing Six Different Gene Combinations on Naringenin Production in the S. cerevisiae BY4741 Strain.
| BY4741 strain | naringenin amount (mg/L) | p-coumaric acid amount (mg/L) |
|---|---|---|
| pCEV-G1-Km-mt4CL-vvCHS | 7.3 | 3.32 |
| pCEV-G1-Km-mt4CL-guCHS | 6.6 | 3.16 |
| pCEV-G1-Km-at4CL-vvCHS | 3.95 | 4.67 |
| pCEV-G1-Km-at4CL-haCHS | 2.95 | 5.09 |
| pCEV-G1-Km-at4CL-guCHS | 2.7 | 3.15 |
| pCEV-G1-Km-at4CL-atCHS | 0.26 | 7.65 |
Figure 3.
Naringenin production in BY4741 S. cerevisiae.
Interestingly, even when paired up with different enzyme sources, the 4CL gene from M. truncatula provided the highest naringenin production rate. In comparison, the CHS gene from V. vinifera was inferior as compared to the other CHS sources (Figure 3). A Western blot analysis confirmed that the 4CL gene from P. crispum and S. coelicolor could not be expressed in the S. cerevisiae BY4741 strain. It was also discovered that the 4CL gene from M. truncatula was not compatible with the CHS gene from A. thaliana and H. androsaemum.
Metabolite Profiling
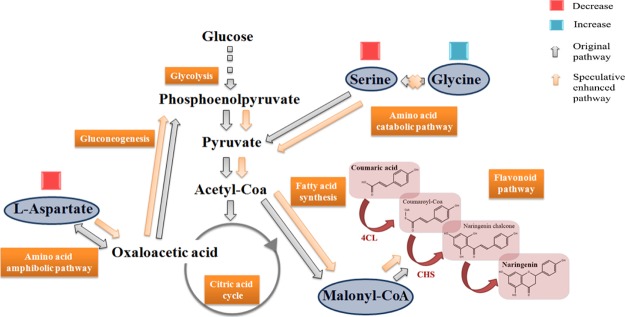
In order to understand the key mechanisms for naringenin production and how this mechanism influenced cell behavior, metabolite profiling was performed using gas chromatography–mass spectrometry (GC–MS). Three key metabolites were found, such as l-aspartate, glycine, and serine. These three metabolites were shown to be present in very different amounts in the naringenin producing and nonproducing strains (Table 6). In the naringenin producing strain, the amount of l-aspartate and serine was found to be only half, while the glycine amount was almost doubled.
Table 6. Comparison of the Three Key Metabolites in the Naringenin Producing and Nonproducing Strains.
| abundance | l-aspartate | glycine | serine |
|---|---|---|---|
| pCEV-G1-Km-at4CL-atCHS | 1 515 237 | 9 851 703 | |
| pCEV-G1-Km-at4CL-guCHS | 1 071 890 | 9 833 160 | 1 682 530 |
| pCEV-G1-Km-at4CL-haCHS | 1 475 277 | 9 840 346 | 1 278 530 |
| pCEV-G1-Km-at4CL-vvCHS | 1 178 761 | 4 187 608 | 1 453 023 |
| pCEV-G1-Km-mt4CL-guCHS | 1 214 060 | 10 096 217 | 1 223 493 |
| pCEV-G1-Km-mt4CL-vvCHS | 1 698 566 | 11 824 813 | 1 629 505 |
| Average Abundance: | |||
| naringenin producing strains | 1 358 465 | 9 272 308 | 1 211 172 |
| naringenin nonproducing strains | 2 757 179 | 5 007 451 | 2 417 988 |
The decrease in the l-aspartate amount indicated that more oxaloacetic acid was produced, which then enhanced gluconeogenesis.19,20 Through the fatty acid synthesis pathway, it could lead to higher malonyl-CoA amount,21 which could then have been consumed by the CHS enzyme during naringenin production. Similarly, the decrease in the serine amount might be due to higher conversion toward malonyl-CoA, which has been consumed by pyruvate, acetyl-CoA, and fatty acid synthesis.22 On the other hand, the increased glycine amount showed that it was not converted into serine, even though the amount of serine was depleted.23 This indicated that the amino acid catabolic pathway was inhibited (Figure 4). However, more research is required to further understand the connection between these pathways and the naringenin producing pathway.
Figure 4.
Metabolic changes induced by naringenin production in S. cerevisiae. The speculative enhanced pathway by naringenin producing strains (shown by orange arrows) mapped onto metabolic pathways (shown by gray arrows). Blue and red boxes demonstrate the decrease and increase of the targeted amino acid amount.
Combination Expression in the Y-28 S. cerevisiae Strain
In order to increase the naringenin production yield, six pCEV-G1-Km plasmids were expressed in a genetically engineered S. cerevisiae Y-28 strain, along with the pCEV-G1-Ph-fjTAL-msCHI plasmid. The Y-28 strain was engineered to use a modified GAL system, which helped in gene overexpression. Gene IDH1 was silenced for the overexpression of ACL from Yarrowia lipolytica and native ACS1 to increase the malonyl-CoA supply. Additionally, the tyrosine supply was also increased with the downregulation of PHA2 and deletion of PDC5 and ARO10.6 The six combinations expressed in the Y-28 strain used glucose as the carbon source, and the naringenin production was then analyzed by HPLC (Table 7).
Table 7. Six Gene Combinations Expressed in the S. cerevisiae Y-28 Strain and Its Effect on Naringenin Production When Grown on Glucose as the Carbon Source.
| Y-28 strain | naringenin amount (mg/L) |
|---|---|
| pCEV-G1-Km-mt4CL-vvCHS | 24.41 |
| pCEV-G1-Km-mt4CL-guCHS | 24.51 |
| pCEV-G1-Km-at4CL-vvCHS | 22.31 |
| pCEV-G1-Km-at4CL-haCHS | 20.46 |
| pCEV-G1-Km-at4CL-guCHS | 20.72 |
| pCEV-G1-Km-at4CL-atCHS | 19.15 |
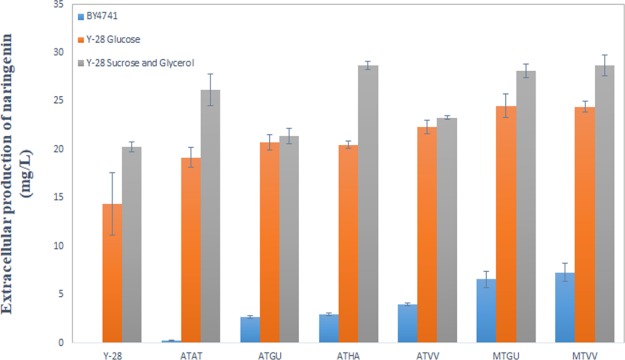
The results showed a similar pattern as compared to the wild-type BY4741 strain, as the expected increased yield was reached (Figure 5). The lowest naringenin production was achieved by the 4CL and CHS genes from A. thaliana. However, the production value significantly increased from 0.26 mg/L in BY4741 to 19.15 mg/L in Y-28. The highest production was 24 mg/L of naringenin, which was three times higher than the highest production in the BY4741 strain. This was reached by two strains expressing the 4CL gene from M. truncatula and the CHS gene from V. vinifera and G. uralensis.
Figure 5.
Comparison of naringenin production in BY4741, Y-28 utilizing glucose, and Y-28 utilizing sucrose and glycerol as the carbon source.
Sucrose and Glycerol as a Carbon Source
It was previously shown that the use of 1% sucrose and 1% glycerol as the carbon source instead of 2% glucose could increase the naringenin yield in the Y-28 strains.6 In order to investigate if the different carbon sources had an effect on the amount of naringenin produced, the six plasmids, which were expressed in the Y-28 strains, were also grown on sucrose and glycerol solution as a carbon source (Table 8). A general increase had been detected in the naringenin yields when sucrose and glycerol were used as the carbon source. It was due to the different breakdown processes of glucose, sucrose, and glycerol. Glycerol converts to glycerol 3-phosphate and then to dihydroxyacetone phosphate by glycerol kinase and glycerol 3-phosphate dehydrogenase, which allows easy access to the carbon source.24 On the other hand, sucrose takes a longer time to break down into glucose through the conversion to sucrose-6-phosphate and then to glucose-6-phosphate and fructose by a sucrose hydrolyzing enzyme. Furthermore, fructose can be converted to glucose-6-phosphate by glucose-6-phosphate isomerase.25 When the cultures were grown on glucose, the carbon source would be immediately consumed at the initial growth stage. However, the use of glycerol and sucrose would provide carbon source both at the initial stage by glycerol breakdown and in later stages by sucrose breakdown.
Table 8. Amount of Naringenin Produced in the S. cerevisiae Y-28 Strains Expressing Six Combinations When Grown on Sucrose and Glycerol Solution as a Carbon Source.
| Y-28 strain | naringenin amount (mg/L) |
|---|---|
| pCEV-G1-Km-mt4CL-vvCHS | 28.68 |
| pCEV-G1-Km-mt4CL-guCHS | 28.10 |
| pCEV-G1-Km-at4CL-vvCHS | 23.29 |
| pCEV-G1-Km-at4CL-haCHS | 28.66 |
| pCEV-G1-Km-at4CL-guCHS | 19.42 |
| pCEV-G1-Km-at4CL-atCHS | 26.17 |
The results displayed a similar pattern as before, where the use of the 4CL gene from M. truncatula resulted in the highest naringenin production (Figure 5). However, it was surprising to note that the strain expressing the pCEV-G1-Km-at4CL-haCHS recombinant plasmid had the same naringenin production as the strain expressing the 4CL gene from M. truncatula. In this study, the highest obtained naringenin yield from a Y-28 strain was 28.68 mg/L, which was lower as compared to the 90 mg/L naringenin production obtained by Lyu et al.6 After looking into the utilized plasmids, it was clear that pCEV-G1-Km was a less satisfying choice for naringenin production compared to the pks plasmid. The main reason for this might be that the antibiotic used for pCEV-G1-Km was G418. G418 had a negative effect on the growth rate of S. cerevisiae.26 As the growth rate decreased, less naringenin was produced, which might explain that the lower yields are obtained. The results had clearly demonstrated that gene source selection has a high impact on naringenin production. However, as the naringenin production increased, the gene source selection was shown to have less impact on the final amount of naringenin.
In summary, several 4CL and CHS gene source combinations were analyzed and expressed in S. cerevisiae BY4741 and Y-28 strains. For the first time, the 4CL gene from M. truncatula and the CHS gene from V. vinifera were expressed in S. cerevisiae, and their combination resulted in the highest naringenin yield. The production was 28-fold higher as compared to that of the reference BY4741 strain expressing the 4CL gene and CHS gene from A. thaliana. In addition, the similarity pattern of the combination of production yields between the BY4741 and Y-28 strains was matching. These results had shown that gene source selection indeed had an effect on naringenin production in S. cerevisiae. With regard to the cellular mechanism behind naringenin production, metabolic profiling concluded that l-aspartate, glycine, and serine amounts were significantly altered. These three metabolites are related to the consumption of malonyl-CoA. However, further studies are needed to unravel the exact mechanism of these metabolites on naringenin production.
Overall, gene screening was shown to be a necessary tool for optimal flavonoid production. The correct selection and combination of gene sources could improve enzyme efficiency, leading to more efficient and sustainable production platform for flavonoid production. This could help increase the possibility to commercialize flavonoid production for future works. The flavonoids produced could also be used as a natural, healthier food preservative and could be incorporated into food and beverages products to add value.
Experimental Section
Culture Media and Microbial Growth Conditions
E. coli cell cultures were grown in Luria-Bertani (LB) broth at 37 °C and supplemented with 100 μg/mL of ampicillin for recombinants harboring pUC57 and pCEV-G1-Km-derived plasmids. Yeast was cultured in YPD (2% peptone, 1% yeast extract, and 2% glucose) or YPSg (1% yeast extract, 2% peptone, and 1% sucrose–1% glycerol) media supplemented with either 200 μg/mL of geneticin (G418) or additional 40 μg/mL of phleomycin antibiotics at 30 °C. The chemicals and antibiotics were purchased from Sigma (Sigma-Aldrich, USA).
Microbial Strains, Plasmid, and Genes
E. coli Top 10 (Novagen, USA) was chosen as the strain for gene manipulation, while S. cerevisiae BY474127 and Y-286 were used for pathway engineering. The plasmid used for the experiment is pCEV-G1-Km,26 where 4CL and CHS were cloned and expressed. While the Y-28 strains contained the pCEV-G1-Ph plasmid expressing TAL from Flavobacterium johnsoniae and CHI from Medicago sativa, which were given by Lyu et al.28
All 4CL and CHS genes used in this study were synthesized by GenScipt, with codon optimization according to S. cerevisiae and cloned in pUC57. The DNA sequences were obtained from the NCBI GenBank with the following reference numbers for 4CL: A. thaliana (NM_113019.3), M. truncatula (XM_003610795.2), P. crispum (KF765780.1), and S. coelicolor (NC_003888.3) and for CHS: A. thaliana (NM_121396.3), G. uralensis (EF026979.1), H. androsaemum (AF315345.1), and V. vinifera (NM_001280950.1).
Phylogenetic Tree and Protein Tertiary Structure Construction
The gene sequences used for the phylogenetic tree and protein tertiary structure were obtained from NCBI Nucleotide database based on the accession code taken from the published literature. The tree was prepared in MEGA729 using the maximum likelihood statistical method and Tamura–Nei model on MUSCLE aligned sequences.30 The tertiary structures were designed with Phyre2,31 visualized, aligned, and analyzed in Chimera.32
Construction of Plasmids, Yeast Transformation, and Cultivation
During the construction of pCEV-G1-Km-4CL-CHS recombinant plasmids, each 4CL was digested from the pUC57 plasmid using NotI and SpeI. The pCEV-G1-Km plasmid was opened up with the same restriction enzymes. 4CL genes were ligated to the open section of the plasmid using the TEF1 promoter, followed by the NotI restriction site. This section also involves an AHD1 terminator, followed by the SpeI restriction site. The ligation results were checked by gel electrophoresis, which confirmed that all the 4CL genes were successfully placed into the plasmid. All four pCEV-G1-Km-4CL recombinant plasmids were further digested with BamHI and SacII. The CHS genes were digested from pUC57 plasmids using the same restriction enzymes. CHS genes were ligated to the open section of the pCEV-G1-Km-4CL recombinant plasmids. This section uses the PGK1 promoter, followed by the BamHI restriction site. This section also involves a CYC1 terminator, followed by the SacII restriction site. The ligation results were checked by gel electrophoresis, which confirmed that all 16 new recombinant plasmids containing 4CL and CHS genes were successfully generated.
The constructed plasmids were transformed into BY4741 or Y-28 using the lithium acetate/single-stranded carrier DNA/polyethylene glycol method33 in order to generate the recombinant strains for naringenin production. The positive strains for BY4741 were screened on YPD plates supplemented with 200 μg/mL of geneticin, while for Y-28 it was either YPD or YPSg plates supplemented with 200 μg/mL of geneticin and 40 μg/mL of phleomycin. Single colony, which contained pCEV-G1-Km-4CL-CHS, was inoculated in 5 mL of YPD/YPSg with either only geneticin or geneticin and phleomycin and shaken at 200 rpm for 20 h at 30 °C. Then 2% of the overnight culture was inoculated into 50 mL of fresh YPD/YPSg media supplemented with antibiotics and incubated for 72 h. For the BY4741 strains, at 12 h of fermentation, 10 mg of p-coumaric acid was added to the culture as a precursor.
Western Blot
Cell extracts were extracted in Y-PER Yeast Protein Extraction Reagent (ThermoFisher) with the addition of an ethylenediaminetetraacetic acid-free protease inhibitor (Roche). Cell lysates (25 μL) were resolved using a 15% SDS-PAGE gel. The resolved proteins were electro-transferred to polyvinylidene difluoride membranes (Bio-Rad), which were then probed with HRP-conjugated anti-6× His tag antibody (Rabbit polyclonal) (abcam) in 1:4000 dilution. The results were visualized using a Pierce 1-step ultra TMB blotting solution (ThermoFisher).
Naringenin Extraction Method
BY4741 (10 mL) or 1 mL of Y-28 culture at the end of the fermentation was centrifuged at 10 000 rpm for 1 min, and then the supernatant was taken for extracellular naringenin analysis. To the supernatant, 1:1 ethyl acetate was added, which was mixed by vortexing for 30 s and rotating for 2 h at room temperature. The sample was centrifuged at 10 000 rpm for 10 min, after which the upper layer was separated and collected. After all liquid has evaporated, 500 μL of ethyl acetate was added, and the sample was filtered before HPLC analysis.
HPLC Analysis
Naringenin was analyzed by the HPLC system (Agilent 1100) equipped with a variable wavelength detector and a C18 column (4.6 mm × 150 mm, RESTEK). The process was performed with a gradient method involving two solvents: methanol and water. The process included several steps: 25% of methanol was used as a solvent, the concentration increased to 75% in 10 min, and then in the following 20 min, the concentration reached 100%, and it was held there for 10 min. Finally, the methanol content returned to 25% in 2 min, which was held there for 13 min. Each sample was run in triplicates. The analysis was performed with a flow rate of 0.5 mL/min, and the signal was detected at 280 nm.
Intracellular Metabolite Extraction for GC–MS
From the cultivated samples, pellets equalling 10 of OD600 were collected as the cultures were centrifuged at 12 000 rpm for 2 min. The pellets were washed three times with water, to which 0.5 mL of water, 0.5 mL of methanol, and 10 μL of 2 mg/mL ribitol dissolved in water were added along with glass beads. The pellet cells were broken and centrifuged at 12 000 rpm for 2 min. The supernatant was collected, and methanol was evaporated at 37 °C overnight. The samples were completely dried in a freeze drier after 12 h.
Fifty microliters of 20 mg/mL methoxyamine hydrochloride dissolved in pyridine was added to the dried samples and incubated at 37 °C for 1 h. N-methyl-N-(trimethylsilyl)-trifluoroacetamide (100 μL) with 1% trimethylchlorosilane was added for silylation, and the samples were kept at 70 °C for 30 min. Before GC–MS measurements, the samples were centrifuged at 14 000 rpm for 20 min, and the upper layer was collected for analysis.34
GC–MS Analysis
Metabolite profiling was performed by using the GC–MS system (Agilent Technologies 7890A-5975C) equipped with an HP-5MS, 5% phenyl-methyl-silox capillary column (30 m × 250 μm × 0.25 μm; Agilent J&W Scientific, Folsom, CA, USA) using a Triple-Axis HED-EM detector. The sample (1 μL) was injected by splitless mode. Each sample was run in triplicates. The carrier gas used was helium, with a constant flow rate of 1.1 mL/min. The inlet temperature was set to 250 °C, while the ion source temperature was 230 °C. The oven temperature was set to start at 75 °C for 4 min, and with an increase in 4 °C/min, it reached 280 °C at the end of the run, where it was kept for 2 min. The obtained compounds were analyzed using Agilent MSD Chemstation Data Analysis and identified with the NIST08 mass spectral library. The selected compound autointegrated peaks had been aligned and compared based on their abundance.
Glossary
Abbreviations
- 4CL
4-coumarate:coenzyme ligase
- C4H
cinnamate 4-hydroxylase
- CHI
chalcone isomerase
- CHS
chalcone synthase
- G418
geneticin
- LB
Luria-Bertani
- MSTFA
N-methyl-N-(trimethylsilyl)-trifluoroacetamide
- PAL
phenylalanine ammonia lyase
- TAL
tyrosine ammonia lyase
- TMCS
trimethylchlorosilane
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00364.
Western blot results and GC–MS chromatography (PDF)
Author Contributions
W.N.C. designed the research. R.M. carried out the experiments and wrote the manuscript. X.L. and K.R.N. commented on the experiments and manuscript. W.N.C. edited the manuscript. All authors read and approved the final manuscript.
This work was supported by Nanyang Technological University and Ministry of Education, Singapore.
The authors declare no competing financial interest.
Notes
This article does not contain any studies with human participants or animals conducted by any of the authors.
Supplementary Material
References
- Harborne J. B.; Williams C. A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Vauzour D.; Rodriguez-Mateos A.; Corona G.; Oruna-Concha M. J.; Spencer J. P. E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106. 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A. PHYTOESTROGENS. Annu. Rev. Plant Biol. 2004, 55, 225–261. 10.1146/annurev.arplant.55.031903.141729. [DOI] [PubMed] [Google Scholar]
- Shahidi F.; Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. J. Funct. Foods 2015, 18, 820–897. 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Ng K. R.; Lyu X.; Mark R.; Chen W. N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. 10.1016/j.foodchem.2018.07.077. [DOI] [PubMed] [Google Scholar]
- Lyu X.; Ng K. R.; Lee J. L.; Mark R.; Chen W. N. Enhancement of Naringenin Biosynthesis from Tyrosine by Metabolic Engineering of Saccharomyces cerevisiae. J. Agric. Food Chem. 2017, 65, 6638–6646. 10.1021/acs.jafc.7b02507. [DOI] [PubMed] [Google Scholar]
- Quideau S.; Denis D.; Céline D. C.; Laurent P. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem., Int. Ed. 2011, 50, 586–621. 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- Pérez-Bonilla M.; Salido S.; van Beek T. A.; Waard P. d.; Linares-Palomino P. J.; Sánchez A.; Altarejos J. Isolation of antioxidative secoiridoids from olive wood (Olea europaea L.) guided by on-line HPLC–DAD–radical scavenging detection. Food Chem. 2011, 124, 36–41. 10.1016/j.foodchem.2010.05.099. [DOI] [Google Scholar]
- Cress B. F.; Linhardt R. J.; Koffas M. A. G.. Isoflavonoid Production by Genetically Engineered Microorganisms. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat K. G., Mérillon J.-M., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp 1647–1681. [Google Scholar]
- Forkmann G.; Martens S. Metabolic engineering and applications of flavonoids. Curr. Opin. Biotechnol. 2001, 12, 155–160. 10.1016/s0958-1669(00)00192-0. [DOI] [PubMed] [Google Scholar]
- Watts K. T.; Lee P. C.; Schmidt-Dannert C. Exploring recombinant flavonoid biosynthesis in metabolically engineered Escherichia coli. ChemBioChem 2004, 5, 500–507. 10.1002/cbic.200300783. [DOI] [PubMed] [Google Scholar]
- Hwang E. I.; Kaneko M.; Ohnishi Y.; Horinouchi S. Production of Plant-Specific Flavanones by Escherichia coli Containing an Artificial Gene Cluster. Appl. Environ. Microbiol. 2003, 69, 2699–2706. 10.1128/aem.69.5.2699-2706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.; Wood K. V.; Morgan J. A. Metabolic Engineering of the Phenylpropanoid Pathway in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005, 71, 2962–2969. 10.1128/aem.71.6.2962-2969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.; Kohli A.; Koffas M. A. G. Biosynthesis of Natural Flavanones in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005, 71, 5610–5613. 10.1128/aem.71.9.5610-5613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantas E.; Panopoulos N.; Ververidis F. Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab. Eng. 2009, 11, 355–366. 10.1016/j.ymben.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Rodriguez A.; Strucko T.; Stahlhut S. G.; Kristensen M.; Svenssen D. K.; Forster J.; Nielsen J.; Borodina I. Metabolic engineering of yeast for fermentative production of flavonoids. Bioresour. Technol. 2017, 245, 1645–1654. 10.1016/j.biortech.2017.06.043. [DOI] [PubMed] [Google Scholar]
- Koopman F.; Beekwilder J.; Crimi B.; van Houwelingen A.; Hall R. D.; Bosch D.; van Maris A. J.; Pronk J. T.; Daran J.-M. De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Microb. Cell Fact. 2012, 11, 155. 10.1186/1475-2859-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehka B. J.; Eichenberger M.; Bjørn-Yoshimoto W. E.; Vanegas K. G.; Buijs N.; Jensen N. B.; Dyekjær J. D.; Jenssen H.; Simon E.; Naesby M. Improving heterologous production of phenylpropanoids in Saccharomyces cerevisiae by tackling an unwanted side reaction of Tsc13, an endogenous double-bond reductase. FEMS Yeast Res. 2017, 17, fox004. 10.1093/femsyr/fox004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest J. C.; Wightman F. Amino Acid Metabolism in Plants. III. Purification and Some Properties of a Multispecific Aminotransferase Isolated from Bushbean Seedlings (Phaseolus vulgaris L.). Can. J. Biochem. 1972, 50, 813–829. 10.1139/o72-113. [DOI] [PubMed] [Google Scholar]
- Izui K.; Matsumura H.; Furumoto T.; Kai Y. PHOSPHOENOLPYRUVATE CARBOXYLASE: A New Era of Structural Biology. Annu. Rev. Plant Biol. 2004, 55, 69–84. 10.1146/annurev.arplant.55.031903.141619. [DOI] [PubMed] [Google Scholar]
- Zu X.; Zhong J.; Luo D.; Tan J.; Zhang Q.; Wu Y.; Liu J.; Cao R.; Wen G.; Cao D. Chemical Genetics of Acetyl-CoA Carboxylases. Molecules 2013, 18, 1704–1719. 10.3390/molecules18021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornaes C.; Petersen J. G.; Holmberg S. Serine and threonine catabolism in Saccharomyces cerevisiae: the CHA1 polypeptide is homologous with other serine and threonine dehydratases. Genetics 1992, 131, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M.; El-Obeid H. A. Inactivation of serine transhydroxymethylase and threonine aldolase activities. Biochim. Biophys. Acta, Enzymol. 1972, 258, 791–799. 10.1016/0005-2744(72)90180-5. [DOI] [PubMed] [Google Scholar]
- Jung J.-Y.; Yun H. S.; Lee J.; Oh M.-K. Production of 1,2-propanediol from glycerol in Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2011, 21, 846–853. 10.4014/jmb.1103.03009. [DOI] [PubMed] [Google Scholar]
- Koschwanez J. H.; Foster K. R.; Murray A. W. Correction: Sucrose Utilization in Budding Yeast as a Model for the Origin of Undifferentiated Multicellularity. PLoS Biol. 2011, 9, e1001122 10.1371/annotation/0b9bab0d-1d20-46ad-b318-d2229cde0f6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C. E.; Bydder S. F.; Zhou Y.; Nielsen L. K. Dual gene expression cassette vectors with antibiotic selection markers for engineering in Saccharomyces cerevisiae. Microb. Cell Fact. 2013, 12, 96. 10.1186/1475-2859-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler E. A.; Shoemaker D. D.; Astromoff A.; Liang H.; Anderson K.; Andre B.; Bangham R.; Benito R.; Boeke J. D.; Bussey H.; Chu A. M.; Connelly C.; Davis K.; Dietrich F.; Dow S. W.; El Bakkoury M.; Foury F.; Friend S. H.; Gentalen E.; Giaever G.; Hegemann J. H.; Jones T.; Laub M.; Liao H.; Liebundguth N.; Lockhart D. J.; Lucau-Danila A.; Lussier M.; M’Rabet N.; Menard P.; Mittmann M.; Pai C.; Rebischung C.; Revuelta J. L.; Riles L.; Roberts C. J.; Ross-MacDonald P.; Scherens B.; Snyder M.; Sookhai-Mahadeo S.; Storms R. K.; Véronneau S.; Voet M.; Volckaert G.; Ward T. R.; Wysocki R.; Yen G. S.; Yu K.; Zimmermann K.; Philippsen P.; Johnston M.; Davis R. W. Functional Characterization of the S. cerevisiae Genome by Gene Deletion and Parallel Analysis. Science 1999, 285, 901–906. 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Lyu X.; Ng K. R.; Mark R.; Lee J. L.; Chen W. N. Comparative metabolic profiling of engineered Saccharomyces cerevisiae with enhanced flavonoids production. J. Funct. Foods 2018, 44, 274–282. 10.1016/j.jff.2018.03.012. [DOI] [Google Scholar]
- Kumar S.; Stecher G.; Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley L. A.; Mezulis S.; Yates C. M.; Wass M. N.; Sternberg M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845. 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Ferrin T. E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Gietz R. D.; Woods R. A.. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods in Enzymology; Elsevier, 2002; Vol. 350, pp 87–96. [DOI] [PubMed] [Google Scholar]
- Wang M.; Bai J.; Chen W. N.; Ching C. B. Metabolomic Profiling of Cellular Responses to Carvedilol Enantiomers in Vascular Smooth Muscle Cells. PLoS One 2010, 5, e15441 10.1371/journal.pone.0015441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.