Abstract
The tumor suppressor protein P53 is strongly involved in orchestrating cellular defenses in the diverse variety of human tissues. Anomalies to lung endothelium permeability are streaming severe consequences towards human health, often associated with fatal outcomes. Ongoing investigations suggest that P53 exerts a prominent strategic role in crucial signaling cascades, in charge of both the maintenance and defense of pulmonary endothelium against toxic intruders. The current study employs human and bovine microvascular cells, as well as pharmacologic and genetic P53 modulators to reveal the negative regulation of APE/Ref1 by P53. Moreover, it includes real time measurements of endothelial permeability, to reveal the disruptive role of APE1/Ref1 towards endothelial integrity. Those findings supports our efforts to elucidate the highly sophisticated endothelial regulatory network that enact endothelial adaptations under the plethora of challenging environmental factors.
Keywords: Hsp90 inhibitors, inflammation, Acute Lung Injury, Acute Respiratory Distress Syndrome
Introduction
The endothelial cells form a semipermeable barrier, which regulates the blood fluid and proteins migration through the vascular wall. This dynamic structure is subjected to a constant remodeling, in response to the plethora of extra- and intra- cellular stimuli (1). The function of this vascular wall is affected in a plethora of pathophysiological conditions, including inflammation (2), oxidative (3), and endoplasmic reticulum stress(4, 5), sepsis(6), and cancer(7, 8). Indeed, a severe anomaly of the endothelial barrier may lead to serious respiratory abnormalities, such as Acute Lung Injury and Acute Respiratory Distress Syndrome. The latter cardiovascular conditions is the consequence of impaired endothelium function, which in turn results to anomalous transportation of essential respiratory elements across the endothelial and epithelial barriers of the lung (9).
Microvascular permeability is increased by various inflammatory and carcinogenetic stimuli, such as LPS, growth factors (i.e. Growth Hormone Releasing Hormone), cytokines, and reactive oxygen and nitrogen oxygen species (10, 11). These stimuli “trigger’ signaling events that abuse the normal function of barrier integrity, by modulating the properties of junction and adhesion proteins, and inducing cytoskeleton remodeling (12, 13). Multifarious cellular messengers modulate molecular cascades that reform cytoskeletal integrity and contractility, causing fluctuations of the endothelial barrier function (14, 15). Hence, the discovery of the molecular components that regulate the activities of the endothelial barrier function, may lead to new therapeutic strategies against ALI/ARDS.
Our recent efforts on the elucidation of the molecular machinery that operates towards the maintenance of the endothelial barrier, revealed that the tumor suppressor P53 (16) is a key player on the endothelium defense against LPS (17). The guardian of the genome prevented the MLC2–triggered formation of actin stress fibers (17, 18), and sabotaged the actin severing activity of cofilin, via the activation of the Rac1 (6, 18). The latter Rho-GTPase protects the vasculature by preserving cortical actin, which in turn fully integrates junctional elements into the barrier (1, 17). Hsp90 inhibitors, which exert strong anti- inflammatory activities, suppressed the LPS–induced P53 phosphorylation(s). The latter modification has been shown to lead to P53 degradation (19, 20). Moreover, those anti- inflammatory compounds prevented the intracellular P53 loss, by increasing the abundance of the Hsp90/P53 complexes in the intracellular niche (17). Indeed, 17–DMAG, a potent hsp90 inhibitor, opposes the LPS-inflicted vascular leak in mouse lungs, by reducing the IL–2 and IL–10 BALF levels(21).
Apurinic/apyrimidinic endonuclease 1 (APE1) is a multitalented transcription factor, associated with a diverse variety of intracellular activities. APE1 is in charge with DNA repair due to oxidative base damage, and exerts a prominent role towards the redox regulation of transcription factors responsible for driving cell survival pathways. The same protein is also known as redox effector factor 1 (Ref-1)(22). Dysregulation of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref1) is linked to various human pathologies, such as inflammation, cancer, cardiovascular diseases and neurodegeneration(23). The importance of that molecule as a potential therapeutic target, is underlined by the fact that intense efforts are oriented towards the development of different classes of APE1/Ref1 inhibitors that directly affect its nuclease, redox and nucloeoplosmin activity(24). Remarkable, APE1/Ref1 negatively affects the activation of the Rac1 pathway(14), which has been shown previously shown by our group to be induced by P53(18). In light of those associations, we decided to proceed with the present study.
The Hsp90 inhibitors 17–AAG, 17–DMAG, AUY–922, where employed to induce P53. They represent three different generations on the development of those compounds. 17–AAG belongs to the earlier generation of those therapeutic agents, and AUY–922 is the most advanced representative. Bovine Pulmonary Aortic Endothelial Cells (BPAEC) and Human Lung Microvascular Cells (HULEC–5a) were exposed to those compounds. Our results indicate that all those inhibitors induced P53 and suppressed APE1/Ref1 in both cell lines. Furthermore, the induction of P53 by both Nutlin and Tunicamycin (P53 inducers) resulted to similar effects. On the other hand, reduction of P53 by siRNA or Pifithrin, exerted the opposite effects, namely the increase of APE1/Ref1 expression. To directly associate APE/Ref1 function with endothelial permeability, we silenced APE1/Ref1 expression in the cells with siRNA, and we measured their barrier function under AUY–922 exposure via transendothelial resistance means. Our results indicated that APE1/Ref1 weakens the endothelial barrier function, and in its absence due to siRNA transfection, the effects of Hsp90 inhibition/P53 induction towards the vascular barrier enhancement were much stronger.
The purpose of the current project is to investigate the hypothesis that P53 exerts its beneficial effects in the vasculature by suppressing APE1/Ref1. Our findings, in support of our hypothesis, enrich our understanding on the complex regulatory network that dictates endothelial function, and expands our knowledge on the molecular mechanisms that support the role of the “Endothelial Defender”(25) in the vasculature. Indeed, they enhance our armamentarium against severe respiratory disorders such as ALI/ARDS, by providing novel insights on the development of advanced and targeted therapeutic approaches against endothelial dysfunction.
Materials and Methods
Reagents:
The Hsp90 inhibitors 17–AAG (cat. no. AAJ66960-EX3), 17–DMAG (cat. no. 102513–662), AUY–922 (cat. no. 101756–820), Nutlin (cat. no. 101761–034), Pifithrin (cat. no. 10187–524), Tunicamycin (cat. no. 89156–900), RIPA buffer (cat. no. AAJ63306–AP), anti-mouse (cat. no. 95017–554) and anti-rabbit IgG HRP linked antibodies (cat. no. 95017–556), as well as the nitrocellulose membranes (cat. no. 10063–173) were obtained from VWR (Radnor, PA). The P53 (cat. no. 9282S) and APE1/Ref1 (cat. no. 4128S) antibodies were obtained from Cell Signaling Technology (Danvers, MA). The β–actin antibody (cat. no. A5441) was purchased from Sigma–Aldrich (St Louis, MO).
Cell Culture:
In-house harvested bovine pulmonary arterial endothelial cells (BPAEC) were subcultured from primary cultures and used at an early passage. Those cells were maintained in DMEM medium supplemented with 10% fetal bovine serum. Human Lung Microvascular Endothelium cells HULEC–5a (CRL–3244) were purchased from ATCC (Manassas, VA) and maintained in PromoCell Endothelial Cell Growth Medium MV. All cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 – 95% air medium supplemented with and 1X penicillin/streptomycin. All reagents were purchased from VWR (Radnor, PA).
Protein isolation, Western Blot Analysis and transfections:
Proteins were isolated from cells or tissues using RIPA buffer. Protein-matched samples were separated by electrophoresis through 12% sodium dodecyl sulfate (SDS–PAGE) Tris-HCl gels. Wet transfer was used to transfer the proteins onto nitrocellulose membranes. The membranes were incubated for 1 h at room temperature in 5% non-fat dry milk in Tris buffered saline (TBS) – 0.1% (v/v) Tween 20. The blots were then incubated at 4°C overnight with the appropriate primary antibody (1:1000). The signal for the immunoreactive proteins was developed by using the corresponding secondary antibody (1:2000) and was visualized in a ChemiDoc™ Touch Imaging System from Bio–Rad (Hercules, CA). β–Actin antibody (1:5000) was used as a loading control. All reagents were purchased from VWR (Radnor, PA). The transfections were conducted as previously described(17, 26)
Measurement of endothelial barrier function:
The barrier function of endothelial cell monolayers was estimated by electric cell‐substrate impedance sensing (ECIS), utilizing ECIS model ZΘ (Applied Biophysics, Troy, NY, USA). All the experiments were conducted on confluent cells which had reached a steady‐state resistance of at least 800 Ω(17).
Densitometry and Statistical Analysis:
Image J software (National Institute of Health) was used to perform densitometry of immunoblots. All data are expressed as mean values ± SEM (standard error of mean). A value of P<0.05 was considered significant. GraphPad Prism 5.01 from GraphPad (CA, USA) was used for data analysis. The letter n represents the number of experimental repeats.
Results
17-DMAG induces P53 and suppresses APE1/Ref1 expression in BPAEC.
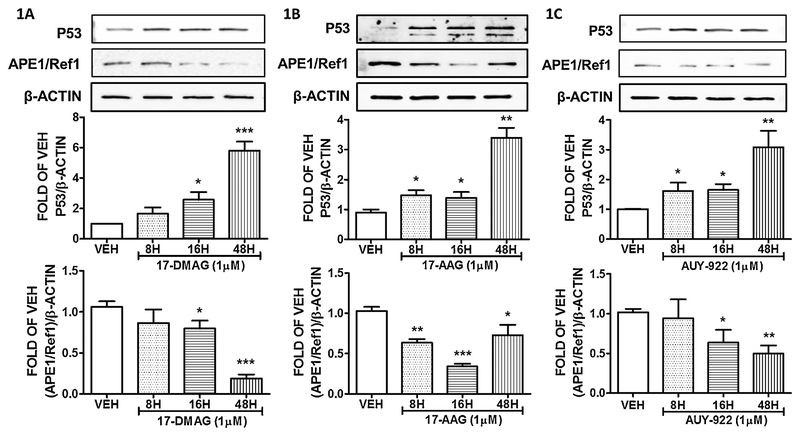
BPAEC were treated with either vehicle (DMSO) or 1μM 17–DMAG for 8, 16 and 48 hours. This Hsp90 inhibitor significantly induced p53, and suppressed APE1/Ref1 expression after 16 and 48 hours of treatment. Treatment of BPAECs for a shorter period, did not significantly affected both proteins. The results appear in Figure 1A.
Figure 1: Hsp90 inhibition induces P53 and suppresses APE1/Ref1 expression in BPAEC.
Western Blot analysis of P53, APE1/Ref1 and β actin after treatment of BPAEC with vehicle (DMSO), 17-DMAG (1μM) (A), 17-AAG (1μM) (B), and AUY-922 (C) for 8, 16 and 48 hours. The blots shown are representative of 3 independent experiments. The signal intensity of the P53 and APE1/Ref1 bands were analyzed by densitometry. Protein levels were normalized to β actin. *P<0.05, **P<0.01, ***P<0.001 vs vehicle. Means ± SEM.
17-AAG induces P53 and suppresses APE1/Ref1 expression in BPAEC.
The endothelial cells were treated with the Hsp90 inhibitor 17–AAG or vehicle (DMSO) for 8, 16 and 48 hours. 17–AAG elevated P53 expression levels in all treatments. The most prominent effect occurred after only 48 hours of incubation. On the other hand, APE1/Ref1 levels were lessened due to that treatment in all time points (8, 16 and 48 hours). The results are shown in Figure 1B.
AUY-922 induces P53 and suppresses APE1/Ref1 expression in BPAEC.
BPAEC were treated with either vehicle (DMSO) or 1μM of AUY–922 for 8, 16 and 48 hours. The results indicate that this Hsp90 inhibitor significantly induced p53 expression levels after 16 and 48 hours of treatment. Indeed, APE1/Ref1 expression was reduced after 16 and 48 hours of AUY–922 exposure (Fig. 1C).
17-DMAG induces P53 and suppresses APE1/Ref1 in HULEC-5a.
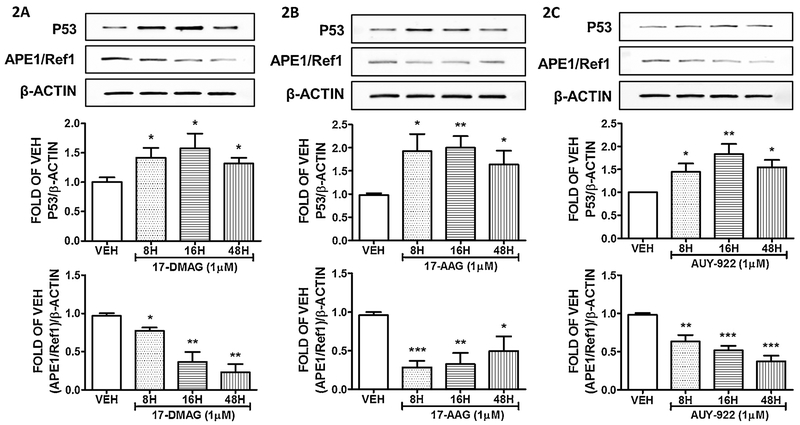
The endothelial cells were subjected to treatment with either vehicle (DMSO), or 1μM of the Hsp90 inhibitor 17–DMAG for 8, 16 and 48 hours. Our observations (Fig. 2A) reveal that this compound reduced the expression of APE1/Ref1 in a time dependent manner, while it induced P53 abundance.
Figure 2: Hsp90 inhibition induces P53 and suppresses APE1/Ref1 expression in HuLEC-5a.
Western Blot analysis of P53, APE1/Ref1 and β actin after treatment of HULEC-5a with vehicle (DMSO), (A) 17-DMAG (1μM), (B) 17-AAG (1μM) and (C) AUY-922(1μM) for 8, 16 and 48 hours. The blots shown are representative of 3 independent experiments. The signal intensity of the P53 and APE1/Ref1 bands were analyzed by densitometry. Protein levels were normalized to β actin. *P<0.05, **P<0.01, ***P<0.001 vs vehicle. Means ± SEM.
17-AAG induces P53 and suppresses APE1/Ref1 in HULEC-5a.
Human Lung Microvascular Endothelial Cells were treated with vehicle (DMSO) or 17–AAG for 8, 16 and 48 hours. This Hsp90 inhibitor significantly induced P53, and suppressed APE1/Ref1 expression levels in all treatments. The results are shown in Figure 2B.
AUY-922 induces P53 and suppresses APE1/Ref1 in HULEC-5a.
Commercially available human lung microvascular endothelial cells were exposed to vehicle (DMSO) or AUY–922 (1μM) for 8, 16 and 48 hours. Those treatments significantly upregulated P53 expression, while suppressed APE1/Ref1 levels. The results are shown in Figure 2C.
Suppression of P53 by Pifithrin induces APE1/Ref1 in both BPAEC and HuLEC-5a.
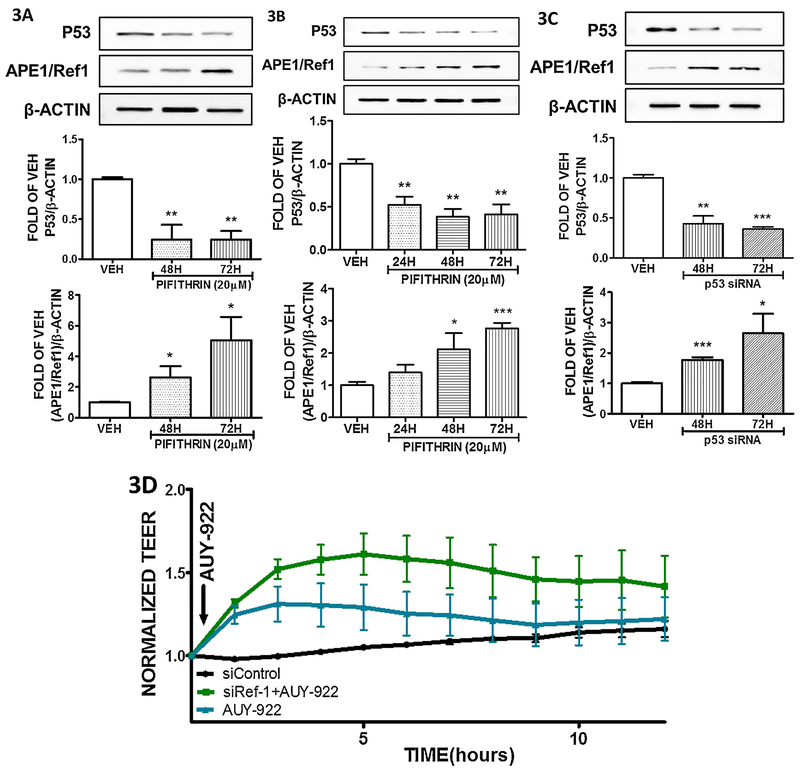
Bovine Pulmonary Aortic Endothelial Cells and Human Lung Microvascular Endothelial Cells were treated with vehicle (DMSO), or Pifithrin (20 μM) to reduce the P53 expression levels. The results depicted in Figure 3A and 3B shows the significant efficiency of this inhibitor towards the P53 reduction. Figure 3A demonstrates the elevation of APE1/Ref1 expression due to P53 suppression in bovine cells, and similar effects are shown in the case of the human cells (Figure 3B).
Figure 3: Effects of APE1/Ref1 modulation on endothelial permeability.
(A) Western blot analysis of P53, APE1/Ref1 and β actin levels in BPAEC treated with vehicle (DMSO) or Pifithrin (20 μM) for 48 and 72 hours. (B) Western blot analysis of P53, APE1/Ref1 and β actin levels in HULEC-5a treated with vehicle (DMSO) or Pifithrin (20 μM) for 24, 48 and 72 hours. (C) Western blot analysis of P53, APE1/Ref1 and β actin levels in HULEC-5a treated with si-CTR or si-P53 for 48 and 72 hours. The blots shown are representative of 3 independent experiments. The signal intensity of the P53 and APE1/Ref1 bands were analyzed by densitometry. Protein levels were normalized to β actin. *P<0.05, **P<0.01, ***P<0.001 vs vehicle. Means ± SEM. (D) AUY-922 was added to the media of the si-APE1/Ref1 transfected HULEC-5 cells. A gradual decrease in endothelial permeability (increased TEER) was observed in all the AUY-922 - treated cells. However, this Hsp90 inhibitor exerted a stronger effect in the cells exposed to si- APE1/Ref1 than those treated with siCTR. n = 3 per group. Means ± S.E.
Silencing of P53 by siRNA induces APE1/Ref1 in HULEC-5.
Human Lung Endothelial Cells were exposed to either irrelevant siRNA (siCTR) or siRNA that specifically targets P53 (siP53) for 48 and 72 hours. The results shown in Figure 3C, are in line with those observations shown in Figures 3A and 3B, and support our hypothesis that APE1/Ref1 is negatively regulated by P53.
Suppression of APE1/Ref1 by siRNA potentiates the barrier enhancement effect of AUY-922 in HULEC-5a.
HULEC–5a cells were transfected with either irrelevant siRNA or siRNA for APE1/Ref1 and consequently transfected with AUY–922 inhibitor. The results shown in Figure 3D suggest that the suppression of APE1/Ref1 enhanced the beneficial effects of AUY–922 towards the enhancement of the vascular barrier structure.
Induction of P53 suppresses APE1/Ref1 in BPAECs and HuLEC-5a.
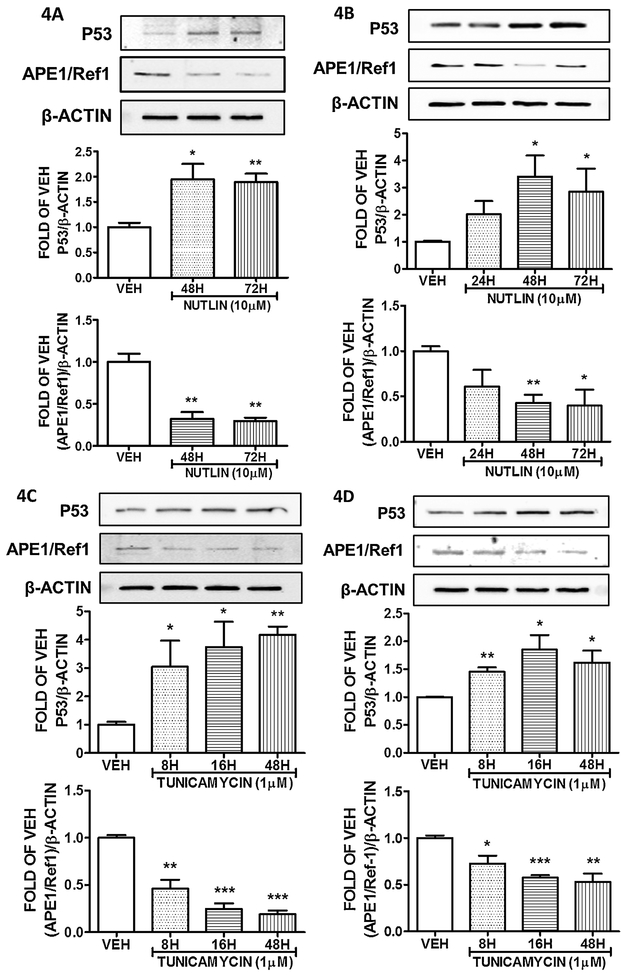
Bovine Pulmonary Aortic Endothelium cells were exposed to 10μM of Nutlin, to induce p53 expression. The treatment was efficient, since P53 levels were elevated after 48 and 72 hours of treatment. That P53 upregulation resulted to APE1/Ref1 suppression (Figure 4A). A similar effect was exerted in HULEC–5a cells, as it appears on figure 4B. When those cells were treated with 10 μM of Nutlin for 24, 48 and 72 hours, elevated their P53 levels and reduced APE1/Ref1 expression. When BPAECs (Fig. 4C) and HULEC–5a (Fig. 4D) were treated with the unfolded protein response element inductor Tunicamycin, they induced their P53 levels and suppressed their APE1/Ref1 levels; in a similar manner to Nutlin and Hsp90 inhibitors.
Figure 4: Induction of P53 suppresses APE1/Ref1 in lung endothelium cells.
(A) Western Blot analysis of P53, APE1/Ref1 and β actin after treatment of BPAEC with vehicle (DMSO) or Nutlin (10μM) for 48 and 72 hours. (B) Western Blot analysis of P53, APE1/Ref1 and β actin after treatment of HULEC-5a with vehicle (DMSO) or Nutlin (10μM) for 24, 48 and 72 hours. (C) Western Blot analysis of P53, APE1/Ref1 and β actin after treatment of BPAEC with vehicle (DMSO) or Tunicamycin (1μM) for 8, 16 and 48 hours. (D) Western Blot analysis of P53, APE1/Ref1 and β actin after treatment of HULEC-5a with vehicle (DMSO) or Tunicamycin (1μM) for 8, 16 and 48 hours. The blots shown are representative of 3 independent experiments. The signal intensity of the P53 and APE1/Ref1 bands were analyzed by densitometry. Protein levels were normalized to β actin. *P<0.05, **P<0.01, ***P<0.001 vs vehicle. Means ± SEM.
DISCUSSION
The “Endothelium Defender” P53, has long been associated with anti-cancer activities(25). When it was first discovered, the investigators concluded that it was a cancer promoter, since they had isolated and focused their studies to its mutated form. However, it was soon became an undisputable fact that the “Guardian of the genome” was counteracting malignancies (27). Those P53 activities occur in conjunction with anti- inflammatory responses, since cancer and inflammation are highly interrelated processes. Sites of chronic inflammation exert the highest potential to develop cancers(11).
Hsp90 inhibitors block the maturation of proteins strongly involved in both metastatic and inflammatory phenomena. These Hsp90 clients are elements crucial for cell survival, proliferation and adaption to intra- and extra- cellular stimuli(28). However, when those carefully orchestrated cellular processes are compromised due to severe inflammatory and carcinogenetic stimuli, they convert to “Trojan horses” of severe pathological manifestations. In those instances, Hsp90 inhibition blocks the progression of those anomalies, and virtually assist the cells to counteract those challenges and recover to the prior non–pathological state(29).
Hsp90 inhibitors have been shown to protect the endothelium against inflammatory insults, by employing a variety of mechanisms. Our laboratory has shown that those effects are exerted by suppressing the src-mediated tyrosine phosphorylation of Hsp90(30), sabotaging the LPS–induced P53 phosphorylation(21), and corrupting the MLCII activation(17), which in turn forms the F actin fibers. Moreover, Hsp90 inhibitors induced P53 to deactivate the actin - severing activity of cofilin(18). Indeed, Hsp90 inhibition induced the abundance of the Hsp90/P53 complexes in the intracellular niche, preventing the proteasomal degradation and elimination of that molecule by LPS(17). Both in vivo and in vitro studies in advanced models of ALI, indicate that Hsp90 inhibitors are holding a great potential to fight lung inflammation (1).
P53 suppress both cancer and inflammation, by eliminating Reactive Oxygen and Nitrogen species. In response to low levels of oxidative stresses, p53 plays primarily antioxidant roles(31). P53 affected proteins, such as sestrin, glutathione peroxidase (GPX), and aldehyde dehydrogenase (ALDH), are involved in reducing oxidative stresses(32). In particular, sestrin protects the cells from hydrogen peroxide–induced damage by generation of peroxiredoxins. GPX scavenges hydrogen peroxide or organic hydroperoxides. ALDH has also shown to be a contributor in the antioxidant function of p53(33). Moreover, P53 has been associated with the anti-inflammatory activities of the anti-cancer compounds Growth Hormone Releasing Hormone antagonists(34–36)
Hsp90 inhibitors have been shown to exert similar anti–oxidant effects in a diverse variety of tissues. HSP90 inhibition by 17-DMAG reduced oxidative stress in experimental atherosclerosis. Treatment of ApoE(−/−) mice with 17–DMAG reduced the generation of ROS in the aortic plaques, as well as the activation of the extracellular signal-regulated kinase (ERK). Vascular smooth muscle cells treated with that compound exerted increased levels of HSP27 and HSP70 (marker of Hsp90 inhibition) and inhibited ERK activation. A significant reduction in NADPH oxidase dependent ROS production was observed when Hsp90 was silenced by transfection of specific siRNA. On the other hand, the suppression of the HSP70 exerted the opposite effects(37). However, in cancer cells, a strong Hsp90 inhibition in combination with combined glutaminase triggers cellular death. The severe blocking of Hsp90 function, activated the unfolded protein response that extent, which in turn triggered cellular death due to elevated endoplasmic reticulum stress(38). Indeed, another study reported that Hsp90 inhibitors selectively decrease superoxide production, thus are predominantly involved in the regulation of reactive oxygen species in COS–7 and HEK293 cells(39).
In light of those events, we decided to investigate whether P53 expression, which is protective against LPS-induced violations of endothelial integrity, is associated with APE1/Ref1. The latter molecule is an upstream effector of VEGF, and it is strongly involved in the pathogenesis of a plethora of inflammatory conditions(22, 40). The activation of APE1/Ref1 may be due to radiation, ROS and RNS, hypoxia, ischemia, as well as intracellular increases of Ca2 (41). It activates TFs including c-Jun, activator protein–1 (AP–1), nuclear factor kappa B (NF–κB), and hypoxia-inducible factor 1α (HIF- 1α). All those proteins are involved in various cellular processes such as cell survival, growth, and inflammation (42–45). APE1/Ref–1 reduces the cysteine (Cys) residues of these TFs by activating their DNA-binding activity(46). Currently there are intense efforts to develop molecules targeting the redox function of the DNA repair/redox protein APE1/Ref-1 in various states of human pathology(24).
We employed bovine pulmonary aortic endothelial cells and human lung microvascular endothelial cells to investigate whether the induction of P53 due to Hsp90 inhibition, would influence the expression levels of APE1/Ref1. Figures 1–3 indicate that 17–DAG, 17–AAG and AUY–922 Hsp90 inhibitors induced P53 expression, in line with previous observations in human lung microvascular endothelial cells (17, 18, 21). Furthermore, the APE1/Ref1 levels were reduced. Similar effects were observed upon Nutlin-induced P53 augmentation (Figure 4A, 4B), as well as after treatment of the cells with the P53 inductor Tunicamycin (Figure 4C, 4D). On the other hand, when the P53 levels were suppressed by Pifithrin (Figure 3A, 3B) and siP53 (Figure 3C), the expression of APE1/Ref1 was elevated.
To access the direct effect of APE1/Ref1 silencing to human cells, we employed the ECIS system, in order to monitor the transendothelial resistance of the confluent endothelium monolayers. After the cells were transfected with si RNA that specifically targets this transcription factor, we treated the cells with the P53 inductor and Hsp90 inhibitor AUY922. The results indicate that the suppression of APE1/Ref1 potentiated the effects of Hsp90 inhibition (Figure 3D), suggesting the negative impact of that molecule/redox factor on the vascular barrier integrity.
Conclusions:
The current study supports our ongoing investigations on the crucial role of P53 towards the vascular endothelium, and suggests that it exerts its effects via the downregulation of the APE1/Ref1 protein. Further studies will further substantiate our findings, by employing genetically modified rodents and barrier disruptive elements that cause lethal pulmonary pathologies, such as ALI/ARDS.
Highlights:
Hsp90 inhibition induces P53 expression
P53 negatively regulate APE1/Ref1 expression
APE1/Ref1 increased endothelial permeability
Suppression of Ape1/Ref1 potentiates P53-mediated vascular barrier enhancement.
Funding:
This work was supported by 1) Start – up funds, College of Pharmacy, University of Louisiana Monroe, Monroe LA 71201 (P.I: N.B) 2) Faculty Research Support Program from Dean’s Office, College of Pharmacy, University of Louisiana Monroe, Monroe LA 71201 (P.I: N.B) (3) Malcolm Feist Partners Across Campuses Seed Program, Center for Cardiovascular Diseases and Sciences, Louisiana State University Health Shreveport, Shreveport, LA 71103 (co–PI: N.B) 4) The Institutional Development Award (IDeA), National Institute of General Medical Sciences of the National Institutes of Health (5 P20 GM103424–15 and 3 P20 GM103424–15S1)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interests: None
REFERENCES
- 1.Barabutis N, Verin A, Catravas JD. Regulation of pulmonary endothelial barrier function by kinases. Am J Physiol Lung Cell Mol Physiol. 2016;311(5):L832–L45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breton-Romero R, Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han J, Weisbrod RM, Shao D, Watanabe Y, Yin X, Bachschmid MM, et al. The redox mechanism for vascular barrier dysfunction associated with metabolic disorders: Glutathionylation of Rac1 in endothelial cells. Redox Biol. 2016;9:306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luchetti F, Crinelli R, Cesarini E, Canonico B, Guidi L, Zerbinati C, et al. Endothelial cells, endoplasmic reticulum stress and oxysterols. Redox Biol. 2017;13:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alam S, Abdullah CS, Aishwarya R, Orr AW, Traylor J, Miriyala S, et al. Sigmar1 regulates endoplasmic reticulum stress-induced C/EBP-homologous protein expression in cardiomyocytes. Biosci Rep. 2017;37(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and Ascorbic Acid Synergistically Prevent and Repair Lipopolysaccharide-Induced Pulmonary Endothelial Barrier Dysfunction. Chest. 2017;152(5):954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung YY, Son DJ, Lee HL, Kim DH, Song MJ, Ham YW, et al. Loss of Parkin reduces inflammatory arthritis by inhibiting p53 degradation. Redox Biol. 2017;12:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Feng J, Zhang Y, Feng J, Wang Q, Zhao S, et al. Mst1 deletion attenuates renal ischaemia-reperfusion injury: The role of microtubule cytoskeleton dynamics, mitochondrial fission and the GSK3beta-p53 signalling pathway. Redox Biol. 2019;20:261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrero R, Sanchez G, Lorente JA. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann Transl Med. 2018;6(2):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer A, Braga VMM. Vascular Permeability: Flow-Mediated, Non-canonical Notch Signalling Promotes Barrier Integrity. Curr Biol. 2018;28(3):R119–R21. [DOI] [PubMed] [Google Scholar]
- 11.Barabutis N, Schally AV, Siejka A. P53, GHRH, inflammation and cancer. EBioMedicine.2018;37:557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Yang G, Zhu Y, Peng X, Li T, Liu L. Relationship of Cx43 regulation of vascular permeability to osteopontin-tight junction protein pathway after sepsis in rats. Am J Physiol Regul Integr Comp Physiol. 2018;314(1):R1–R11. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Murata T. Regulation of vascular permeability in anaphylaxis. Br J Pharmacol. 2018;175(13):2538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons S, Erfinanda L, Bartz C, Kuebler WM. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yurdagul A Jr., Finney AC, Woolard MD, Orr AW. The arterial microenvironment: the where and why of atherosclerosis. Biochem J. 2016;473(10):1281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Case AJ, Domann FE. Absence of manganese superoxide dismutase delays p53-induced tumor formation. Redox Biol. 2014;2:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barabutis N, Dimitropoulou C, Birmpas C, Joshi A, Thangjam G, Catravas JD. p53 protects against LPS-induced lung endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2015;308(8):L776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barabutis N, Dimitropoulou C, Gregory B, Catravas JD. Wild-type p53 enhances endothelial barrier function by mediating RAC1 signalling and RhoA inhibition. J Cell Mol Med. 2018;22(3):1792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci U S A. 2009;106(8):2629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tergaonkar V p53 and NFkappaB: fresh breath in the cross talk. Cell Res. 2009;19(12):1313–5. [DOI] [PubMed] [Google Scholar]
- 21.Barabutis N, Uddin MA, Catravas JD. Hsp90 inhibitors suppress P53 phosphorylation in LPS - induced endothelial inflammation. Cytokine. 2019;113:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley MR, Georgiadis MM, Fishel ML. APE1/Ref-1 role in redox signaling: translational applications of targeting the redox function of the DNA repair/redox protein APE1/Ref-1. Curr Mol Pharmacol. 2012;5(1):36–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thakur S, Dhiman M, Tell G, Mantha AK. A review on protein-protein interaction network of APE1/Ref-1 and its associated biological functions. Cell Biochem Funct. 2015;33(3):101–12. [DOI] [PubMed] [Google Scholar]
- 24.Laev SS, Salakhutdinov NF, Lavrik OI. Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1). Bioorg Med Chem. 2017;25(9):2531–44. [DOI] [PubMed] [Google Scholar]
- 25.Uddin MA, Barabutis N. P53: The endothelium defender. J Cell Biochem. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barabutis N, Schally AV. Knocking down gene expression for growth hormone-releasing hormone inhibits proliferation of human cancer cell lines. Br J Cancer. 2008;98(11):1790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine AJ. Reviewing the future of the P53 field. Cell Death Differ. 2018;25(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuehlke AD, Moses MA, Neckers L. Heat shock protein 90: its inhibition and function. Philos Trans R Soc Lond B Biol Sci. 2018;373(1738). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Echeverria PC, Bhattacharya K, Joshi A, Wang T, Picard D. The sensitivity to Hsp90 inhibitors of both normal and oncogenically transformed cells is determined by the equilibrium between cellular quiescence and activity. PLoS One. 2019;14(2):e0208287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barabutis N, Handa V, Dimitropoulou C, Rafikov R, Snead C, Kumar S, et al. LPS induces pp60c-src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung. Am J Physiol Lung Cell Mol Physiol. 2013;304(12):L883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safdar A, Annis S, Kraytsberg Y, Laverack C, Saleem A, Popadin K, et al. Amelioration of premature aging in mtDNA mutator mouse by exercise: the interplay of oxidative stress, PGC- 1alpha, p53, and DNA damage. A hypothesis. Curr Opin Genet Dev. 2016;38:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gambino V, De Michele G, Venezia O, Migliaccio P, Dall’Olio V, Bernard L, et al. Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging. Aging Cell. 2013;12(3):435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D, Xu Y. p53, oxidative stress, and aging. Antioxid Redox Signal. 2011;15(6):1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barabutis N, Schally AV. Antioxidant activity of growth hormone-releasing hormone antagonists in LNCaP human prostate cancer line. Proc Natl Acad Sci U S A. 2008;105(51):20470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barabutis N, Siejka A, Schally AV. Growth hormone releasing hormone induces the expression of nitric oxide synthase. J Cell Mol Med. 2011;15(5):1148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barabutis N, Schally AV. Growth hormone-releasing hormone: extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9(20):4110–6. [DOI] [PubMed] [Google Scholar]
- 37.Madrigal-Matute J, Fernandez-Garcia CE, Gomez-Guerrero C, Lopez-Franco O, Munoz- Garcia B, Egido J, et al. HSP90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovasc Res. 2012;95(1):116–23. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Csibi A, Yang S, Hoffman GR, Li C, Zhang E, et al. Synthetic lethality of combined glutaminase and Hsp90 inhibition in mTORC1-driven tumor cells. Proc Natl Acad Sci U S A. 2015;112(1):E21–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Chen F, Haigh S, Yu Y, Benson T, Wang Y, Li X, et al. Nox5 stability and superoxide production is regulated by C-terminal binding of Hsp90 and CO-chaperones. Free Radic Biol Med. 2015;89:793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi S, Joo HK, Jeon BH. Dynamic Regulation of APE1/Ref-1 as a Therapeutic Target Protein. Chonnam Med J. 2016;52(2):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thakur S, Sarkar B, Cholia RP, Gautam N, Dhiman M, Mantha AK. APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions. Exp Mol Med. 2014;46:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bapat A, Fishel ML, Kelley MR. Going ape as an approach to cancer therapeutics. Antioxid Redox Signal. 2009;11(3):651–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, et al. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18(7):1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bearz A, Tell G, Colombatti A, Formisano S, Pucillo C. Fibronectin binding promotes a PKC-dependent modulation of NF-kappa B in human T cells. Biochem Biophys Res Commun. 1998;243(3):732–7. [DOI] [PubMed] [Google Scholar]
- 45.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;11(2):653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhakat KK, Mantha AK, Mitra S. Transcriptional regulatory functions of mammalian AP- endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid Redox Signal. 2009;11(3):621–38. [DOI] [PMC free article] [PubMed] [Google Scholar]