Abstract
This review discusses the personalised dietary approach with respect to inflammatory bowel disease (IBD). It identifies gene–nutrient interactions associated with the nutritional deficiencies that people with IBD commonly experience, and the role of the Western diet in influencing these. It also discusses food intolerances and how particular genotypes can affect these. It is well established that with respect to food there is no “one size fits all” diet for those with IBD. Gene–nutrient interactions may help explain this variability in response to food that is associated with IBD. Nutrigenomic research, which examines the effects of food and its constituents on gene expression, shows that—like a number of pharmaceutical products—food can have beneficial effects or have adverse (side) effects depending on a person’s genotype. Pharmacogenetic research is identifying gene variants with adverse reactions to drugs, and this is modifying clinical practice and allowing individualised treatment. Nutrigenomic research could enable individualised treatment in persons with IBD and enable more accurate tailoring of food intake, to avoid exacerbating malnutrition and to counter some of the adverse effects of the Western diet. It may also help to establish the dietary pattern that is most protective against IBD.
Keywords: inflammatory bowel disease, Westernisation, genotypes, nutrient deficiency, food intolerance, FODMAPs, gluten, fructose, lactose, brassica, mushrooms
1. Introduction
The personalised dietary approach in combination with the knowledge of the genotype and genetic variants of an individual who has nutrient deficiencies, follows the Western diet, or may have food intolerances may be helpful for people who have inflammatory bowel disease (IBD). Gene–nutrient interactions could help explain the variability in response to food that is associated with IBD, and using this knowledge with a personalised dietary approach may offer a way forward. Identification of individuals’ genotypes is a first step to building up data sets which combine this information with data on individuals and their gut microbiome, dietary patterns, exercise routines, blood parameters and anthropometric measurements. This information can be used to build machine-learning algorithms to predict what foods/meals reduce inflammation and abdominal symptoms and maintain a healthy gut flora. Zeevi et al. have pioneered this approach in their work with diabetes [1]. They used this combination of information to successfully predict which foods/meals lowered postprandial blood glucose. Identifying genotypes associated with nutrient deficiencies or low nutrient absorption, the negative effects of the Western diet and the tolerance or intolerance of particular foods may also advise people on how to eat a greater variety of food. A wider range of food available to the gut microbiota enables their greater diversity, and this is associated with a healthy gut [2].
Pharmacogenetic research (the study of the variability in drug response due to genotype) is identifying gene variants with adverse reactions to drugs, and this is modifying clinical practice and allowing individualised treatment. The aim is to provide the right drug, at the right dose, at the right time, to the right patient [3]. Individual responses can be dependent on an individual’s genotype. Genetic variants can alter the metabolism of drugs as well as responsiveness, and this may necessitate changes to standardised drug regimes. The standard dose may not maintain the required therapeutic level if individuals metabolise the drug more quickly; conversely, if they are slow metabolisers the standard dose maybe toxic. Knowledge of pharmacogenetics and its application to precision medicine to enhance treatment responses and minimize drug-related adverse events is one approach that has been suggested as the way forward for IBD [4]. Pharmacogenetics information facilitates the identification of responders and non-responders to drugs and guides the decision about optimal treatment [5]. Similarly, further nutrigenomic research could identify nutrient–gene interactions in people with IBD. This would enable the more accurate tailoring of food intake and could suggest dietary patterns which avoid exacerbating malnutrition, curtail the adverse effects of the Western diet and explain intolerance to particular foods. This could lead to the minimization of abdominal symptoms and disease activity in those who experience IBD.
In an extensive study of 366,351 European individuals in 2016 aiming to identify dietary patterns and risk of IBD, no dietary pattern was identified with the risk of either of the two main expressions of IBD (i.e., ulcerative colitis (UC) or Crohn’s disease (CD)) [6]. However, when cases occurring within the first two years after dietary assessment were excluded, there was a positive association between a “high sugar and soft drinks” pattern and UC risk if they also had a low vegetable intake [6]. This study shows there are many challenges in determining the best dietary pattern for individuals with a particular chronic disease like IBD, given the genetic differences between people and their environments [7]. The current consensus is that no single nutrition recommendation can be made to modify this situation with respect to IBD—that is, there is no “one size fits all” solution for people with IBD [8]. However, using the personalised dietary approach combined with information on people’s genotypes may offer a way to resolve this conundrum.
The aim of this narrative review (based on searching two data bases, PubMed and Google Scholar) is to discuss the personalised dietary approach with respect to IBD. First it will identify nutritional deficiencies which individuals with IBD commonly experience, and give an example of a key nutrient whose absorption is affected by genotype. Secondly it will review the role of the Western diet in contributing to nutrient deficiencies and IBD, and give examples of some of the genes involved. Thirdly it will review the various food intolerances and food avoidances associated with IBD which may also contribute to nutrient deficiencies, and how particular genotypes can contribute to these.
2. Nutrient Deficiencies Associated with IBD
2.1. Nutritional Status of People with Inflammatory Bowel Disease
Three studies which have assessed the nutritional status of people with IBD are illustrated in Table 1. Assessment of people with IBD often shows they are under weight, with specific vitamin and mineral imbalances, and this contributes to their ill health [9,10,11,12,13,14]. The three studies in this table illustrate the common nutritional findings. Vagianos and colleagues collected dietary information by questionnaires (including dietary supplements) and observed multiple and clinically significant vitamin and mineral deficiencies in adults with CD who attended a gastroenterology outpatient clinic [9]. Inadequate reported intake was defined as <66% of the dietary reference intake (RFI) for each micronutrient. These authors reported that when comparisons were made by disease type or disease activity, there were no significant differences in the percentage of subjects consuming inadequate amounts of micronutrients. Stein and Bott (2008) reported on nutrient deficiencies in hospitalised adults with CD [10]. Hartman et al. in 2016 [15] investigated the nutritional status of children and adolescents who were ambulatory outpatients at a gastroenterology clinic. They reported on the percentage median nutrient intake compared with the required daily allowance (RDA) and dietary intake of healthy children. The intake was considered low if it was less than 80% of the RDA. During this study, two thirds of participants had active disease. However, there was no significant difference in nutrient intake between those with active disease and those in remission, and no association of nutrient levels with disease activity.
Table 1.
Nutrient deficiencies in adults and adolescents with inflammatory bowel disease (IBD).
| Reference | Vagianos et al. [9] | Stein and Bott [10] | Hartman et al. [15] |
|---|---|---|---|
| Outpatient | Inpatient | Outpatient | |
| Year of Investigation | 2007 | 2008 | 2016 |
| Number of Participants | 71 | na | 68 |
| Gender (Male, Female) | 32, 52 | na | na |
| Age (years) | 37.6 ± 14.3 | Adult | 13.9 ± 3.2 |
| Country | Canada | Germany | Israel |
| Dietary Measurement | FFQ, 4-day records, Anthropometric | na | 3-day records, Anthropometric |
| Laboratory Measurement | Biomarkers for nutrients (CD) | na | Biomarkers for nutrients |
| Dietary Deficiency | |||
| Measures | % <66% RFI | % Inadequate Intake | % <80% of RDA |
| Vitamin B6 | 4.2 | na | 10 |
| Vitamin B12 | 5.6 | 48 | 12 |
| Folate | 20 | 56–62 | 34 |
| Vitamin C | 9.9 | na | 31 |
| Vitamin A | 30 | 11–50 | 57 |
| Vitamin D | 38 | 23–75 | 25 |
| Vitamin E | 59 | na | 65 |
| Calcium | 25 | 13 | 79 |
| Iron | 14 | 25–50 | 22 |
| Magnesium | na | 14–33 | 69 |
| Zinc | 8.5 | 40 | 28 |
CD: Crohn’s disease; FFQ: food frequency questionnaire; na: not available; RFI: dietary reference intake; RDA: required daily allowance.
All three studies showed low levels of vitamin A and D and folate in people with CD. Stein and Bott [10] and Hartman et al. [15] both found substantial risk for deficiencies of magnesium, zinc and iron (Table 1). Stein and Bott [10] also showed lower B12 concentrations for inpatients and Vagianos et al. commented that in those with CD (compared to those with UC, the other main expression of IBD) had lower B12 concentrations with their serum biomarkers. Also, among men with CD there was a significantly higher proportion who had haemoglobin below the normal range compared to men with UC. Hartman et al. reported that anaemia was the most common sign of nutrient deficiency in their adolescents (51%). These studies illustrate that people with different expressions of IBD (i.e., CD or UC) have different nutrient needs. It also illustrates that it is important to assess nutritional status (through laboratory measurement of nutrient biomarkers) to avoid exacerbating malnutrition if particular foods are removed from the diet.
For people with CD in particular, malnutrition may also occur because of the severity of oral lesions experienced, which results in difficulties with tasting, chewing and swallowing. This means that some foods are avoided (e.g., tomatoes because of their perceived acidity). Oral involvement in CD has been identified in 8%–29% of people, irrespective of disease activity [16]. Examples of oral symptoms include labial swelling (which also may include gingiva and mucosa), mucosal polyps, cracking at the edges of the lips (angular cheilitis), ulcers, fissures and granulomas [17,18]. The mucous membranes in the mouth normally have a rapid cell turnover of 3–7 days, in contrast to the skin which is up to 28 days [19]. Loss of cells without replacement can lead to the mouth showing early signs of systemic disease and early predisposition to nutritional deficiency. This is another reason why optimal nutrition is important for people with IBD.
Many vitamins and minerals are considered necessary for healthy mucous membranes (Table 2) [20]. A number of these essential nutrients are low in people with people with IBD (Table 1 [9,10,15]). These nutrient deficiencies, and a higher presence of bacteria associated with dental caries, undoubtedly contribute to the oral symptoms experienced by people with CD. Sufficient levels of these nutrients would need to be included in any food regime suggested for IBD.
Table 2.
Nutrients associated with healthy mucous membranes [20].
| Water Soluble Vitamins | Fat-Soluble Vitamins | Minerals |
|---|---|---|
| Vitamin B2 (riboflavin) | A | Calcium |
| Vitamin B3 (niacin) | D | Fluoride |
| Vitamin B6 family | E | Zinc |
| Vitamin B12 (cobalamin) | Iron | |
| Folic acid (folate) | ||
| Vitamin C (ascorbic acid) |
2.2. Nutrient Absorption Impacted by Genotype
One example of a nutrient whose absorption can be impacted by genotype is beta-carotene. The gene that affects its absorption is the beta-carotene 15,15′-monooxygenase gene (BCMO1). This gene encodes a protein which is an important enzyme in the metabolism of the nutrient beta-carotene to vitamin A [21]. It is expressed in a number of tissues (e.g., liver, lungs, skin, small intestine). Within the enterocytes of the mucous membranes, the enzyme cleaves beta-carotene into two retinal molecules. These are packaged with other dietary lipids into chylomicrons and then secreted into the lymphatic system. Fat in the diet enables optimal chylomicron formation [21,22]. According to the study by Leung et al., carrying one of the two polymorphisms (R267S: rs12934922 or A379V: rs7501331) of the BCMO1 gene impedes the conversion of beta-carotene to retinol [23]. This study was based on a healthy population from the UK. Up to 45% of people in this study carried one of these two polymorphisms. Having one of the two polymorphisms of the BCMO1 gene in conjunction with IBD could thus have an impact on the health status of the carrier. New Zealand (NZ) is a country with a high incidence of IBD (26/100,000 in 2016) [24], and in their most recent National Nutrition Survey (2008/9) reported that the NZ population sourced 58% of their vitamin A intake from carotenoids. The NZ population as a whole was also recorded as 22.7% and 12.1% deficient in vitamin A for males and females, respectively. For those aged 15-18 years, these percentages increased to 27.4% and 37.5% [25].
Adequate vitamin A intake is necessary for optimal activity of the innate and adaptive immune systems. Vitamin A is needed for the appropriate innate immune defence response. It affects neutrophil reactions to microbes, macrophage phagocytosis, and natural killer cell activity. Cultured human macrophages enriched with retinoic acid have been shown to have increased resistance to M. tuberculosis by down-regulating tryptophan–aspartate-containing coat protein (TACO) [26]. Vitamin A is also needed for the optimal formation of T and B cells in the adaptive immune system and the balanced type 1 and type 2 cytokine responses. Impairment of these functions through vitamin A deficiency leads to epithelial barrier dysfunction and inflammation—hallmarks of IBD [27]. Vitamin A deficiency in children increases the risk of signs and symptoms often associated with CD (i.e., impairment of iron use that aggravates anaemia, respiratory infections and diminished growth rates) [28]. If individuals with IBD also carry one of these at-risk alleles, they are at risk of being vitamin A deficient.
This may be a contributing factor to this nutrient deficiency reported in people with IBD. Using the personalised dietary approach and identifying if a person with IBD carries these variants linked to poor absorption of vitamin A would enable the tailoring of nutrient advice to ameliorate this. The Western diet may also contribute to nutrient deficiencies.
3. The Western Diet
The incidence of gastro-intestinal disorders like CD and UC has been increasing [29,30,31,32]. This has often been related to the current Western diet, which has changed substantially in the past seven decades [33,34,35,36,37,38,39]. This is because of changes in food production and technology, which provides easy access of urban based populations to cheap ultra-processed foods and foods high in refined grains, oil and sugar [40]. In Bangladesh, for example, the incidence of diagnosed IBD is very low. However, when Bangladeshis move to the United Kingdom where they are more highly exposed to Western culture and food choices, the incidence of IBD increases substantially within a generation [41]. This may also be associated with autoimmune disease developing as a result of increasing latitude, decreased exposure to sunlight and lower vitamin D intake [42]. They may also receive better health care which identifies their disease pathology more accurately. However, modern Western food is also becoming more available in the developing world. In the review of the global epidemiology of IBD by M’Koma, it was noted that there has been an increase in the incidence and prevalence of IBD in parts of Asia and Africa—particularly in urban areas and in higher socio-economic classes [43]. This Westernisation of the diet has resulted in lower intakes of dietary fibre, increased consumption of saturated fatty acids, and sugar. Westernisation has also introduced other factors such as changes in birthing methods, increased use of artificial formula instead of breast feeding, and the use of sugar substitutes, food additives and antibiotics. Many of these changes have been associated with a reduction in gut microbial diversity, which is a feature of IBD [44,45].
3.1. Lower Intakes of Dietary Fibre
As the diet becomes more Westernised and processed, “Western” food often contains less fibre. Current intakes of fibre associated with the Western diet are assessed to be about 20 g/day, compared to a fibre content of 70–120 g/day when diets rich in fruit, vegetables and grains are consumed, as is estimated for hunter-gathers and agriculturalists in human history [46]. The low consumption of fruit, vegetables and dietary fibre and the increased intake of animal fat and refined sugars have been observed in people with disorders like IBD [47], as are exclusion diets for IBD that are made popular in the media [48].
The risk of IBD has been negatively associated with the intake of fibre [34,49,50]. Fibre can be defined in a number of ways, as illustrated by the different definitions by the American Association of Cereal Chemists (AACC) [51] and The Codex Alimentarius Commission [52]. A recent AACC definition describes fibre as being a component of “edible plants or analogous carbohydrates that are resistant to digestion and absorption in the human small intestine, with complete or partial fermentation in the large intestine” [53]. They include in their definition polysaccharides, oligosaccharides, lignin and associated plant substances. Fibre has been further subdivided into rapidly digested starch, slowly digested starch and resistant starch (RS), and the latter is classified into five categories (RS1–RS5). High levels of resistant starch are found in unripe bananas, raw potatoes, maize, pulse grains and legumes. However, depending on their processing, the RS content can vary, and in legumes this can range from a few percent to 80% [54]. The RS3 category (retrograded starch) has been shown in wild type interleukin-10-deficient IBD murine studies to decrease ileal and colonic inflammatory lesions compared to a low-fat diet [55,56]. The authors suggested that soluble fibre and resistant starch suppress gut inflammation. The inclusion of soluble fibre and resistant starch in the diet of individuals with IBD may be helpful for this reason.
Fibre can also be described as soluble and insoluble [57]. The removal of insoluble fibre, a component of the skins of fruit, vegetables, whole grains, and seeds, is a current recommendation by the Crohn’s and Colitis Foundation of America [58]. When people are experiencing disease activity (sometimes described as a flare or relapse), insoluble fibre may intensify bloating, pain, diarrhoea and gas. However, the consumption of soluble fibre in vegetables (e.g., the brassica family, dried peas and beans, parsnips, carrots, potatoes, spinach, squash, zucchini) and fruit (e.g., pip and stone fruit, berries, bananas, dates, cantaloupe, grapes and pineapple), oat cereals and barley is recommended. Soluble fibre promotes water absorption, enabling the gut contents to form a gel-like consistency, which delays emptying of the gut and reduces diarrhoea [58]. Soluble fibre also provides a substrate for bacterial fermentation in the colon [59]. The soluble plant fibres in plantain and broccoli reduce the movement of E. coli across M cells in Peyer’s patches in CD patients [60]. In CD, E. coli is found in increased numbers in the mucosa [61,62]. This means it is important that appropriate fibre types are chosen if people with IBD are removing food or food groups (e.g., selecting gluten-free diets or foods low in FODMAPs (fermentable oligo-, di- and mono-saccharides and polyols)) so they do not inadvertently contribute to an increase in disease activity later in their health journey [63].
There are also identified gene–nutrient interactions associated with the role of fibre. One of these is the free fatty acid receptor 2 gene (FFAR2; previously known as G protein-coupled receptor 43—GPR43). This gene is a member of the largest gene family—the seven-transmembrane receptor group known as G-protein-coupled receptors (GPCRs) [64]. These are involved as mediators of cellular responses to many neurotransmitters and hormones [65]. FFAR2 is highly expressed in neutrophils [66]. One pathological marker of CD is the migration of neutrophilic granulocytes into the mucosa of the intestine [67]. FFAR2 also activates pathways involved in intracellular Ca2+ release and inhibits the build-up of cAMP [68].
The FFAR2 receptors are present in fat tissues, inflammatory cells and the large intestine [69]. In the large intestine, the receptors are stimulated by short-chain fatty acids (SCFAs), which are produced by bacteria fermenting dietary fibre [21,70]. Lower intakes of dietary fibre decrease the production of SCFAs, which stimulates FFAR2 signalling. The study by Sivaprakasam et al. showed the critical role of FFAR2 in the maintenance of healthy gut microbiota, leading to the suppression of intestinal carcinogenesis [71].
The interaction of SCFAs and FFAR2 greatly affects inflammatory processes. SCFAs play an important role in colon homeostasis, as they promote pathways involved in cell differentiation, growth regulation, immune regulation and apoptosis [72]. SCFAs and butyrate in particular seem to be the favoured fuel source of the bacteria in the colon as well as of colonocytes, which are required for the functional integrity of the epithelium [73,74,75,76]. The source of SCFAs is the intake of highly fermentable fibres, the residues of which are converted by anaerobic bacteria into SCFAs upon reaching the large bowel [77]. SCFAs have been observed to increase the beneficial populations of Bifidobacterium and Lactobacillus, which have a role in anti-inflammatory outcomes and immune functions [38,78,79]. SCFAs appear to be important in epithelial barrier maintenance and immune system regulation. Maslowski and Mackay consider the SCFAs produced in the colon to be very important for immunoregulation. They propose that the lower dietary fibre intake associated with the modern Western diet is the driver behind the increased incidence of inflammatory diseases associated with this lifestyle factor [70].
However, there is currently no published literature about the significance of this gene with respect to people with IBD and their intake of fibre. People with IBD (whether with disease activity or in remission) have been observed to have a low intake of fruit, vegetables and dietary fibre [49,80]. Perhaps particular variants in the FFAR2 gene may affect a person’s response to fibre, and this in turn contributes to changes in microbiota composition. This may explain how those who follow a strict FODMAP diet experience a reduction in beneficial bacteria [81,82].
3.2. Increased Consumption of Saturated Fatty Acids
Some authors believe that the Western diet has also become pro-inflammatory as a result of the increased consumption of saturated fatty acids and decreased consumption of polyunsaturated fatty acids (PUFAs), especially long-chain PUFAs (LC-PUFAs) [83,84,85,86,87]. There has also been a decrease in the ratio of the omega-3 and omega-6 PUFAs ingested, partly because of a lower consumption of fish. Fish intolerance and allergy is well-recognised in childhood, being the third most frequent allergen after cow’s milk and egg in European children [88,89,90]. There is less evidence implicating fish intolerance or allergy in CD. Untersmayr et al. observed that antacid medication plus fish consumption impeded the digestion of dietary proteins in their BALB/c mouse fish allergy model [91].
In earlier times, people consumed only small amounts of these PUFAs, and in about the same proportion. Today it is estimated that this ratio is now about 1:10–1:20–25 omega-3:omega-6. This has occurred from the increased intake of vegetable oils (e.g., soy, safflower, corn and sunflower) [83,92,93]. The genes and their variants linked to differences in fatty acid absorption include fatty acid desaturase genes (FADS1, FADS2), the peroxisome proliferator-activated receptor genes (PPARA, PPARG), the X-ray repair cross-complementing protein 1 gene (XRCC1) and stearoyl-CoA desaturase (SCD1). A number of studies have discussed the role that these genes play in the serum levels of LC-PUFA-omega-3 and omega-6 fatty acids, as well as the effects of various variants on metabolic pathways and how this impacts inflammation and cancer risk [94,95,96,97,98,99,100,101,102,103,104]. These studies show that the impact of these fatty acids—especially on inflammation—is also influenced by the person’s genotype. This may have deleterious effects on individuals with IBD who exclude fish and fish oils from their diet and have a high omega-3:omega-6 PUFA ratio.
3.3. Fructose and Sugar Consumption
There is much debate in the literature about the effects of fructose consumption [105,106,107,108,109,110,111,112,113,114,115,116]. The rise in obesity, diabetes, metabolic syndrome, IBD, coronary heart disease, and whether the increased consumption of fructose or fructans has contributed to these disorders, has been widely discussed [105,106,107,108,109,110,111,112,113,114,115,116]. Over recent decades, increasing amounts of fructose have been consumed as part of the Western diet. This has been particularly associated with the addition of sugars to processed foods, particularly high-fructose corn syrup (HFCS, comprising ~60% fructose) in the USA or with cane sugar (50% fructose) in NZ. Both are cheap and ubiquitous sweeteners, especially in soft drinks and cereal products [117]. The effect of fructose in infant formulas on vascular inflammation has also been investigated in full-term babies. The study found that the fructose formula had a higher inflammatory response than the fructose-free formula, but the difference was not significant [118]. The authors commented that the result may become significant in a preterm infant [118]. According to recent USDA figures, the estimated calories of HFCS consumed daily has risen from 0.4 g/day in 1970, peaked at 44.3 g/day in 1997, and has gradually declined (31.0 g/day in 2015) [25,119,120]. However, total “sweetener” consumption has increased compared to the 1970 figures (from 87.3 g/day in 1970 to 94.1 g/day in 2015) [119]. In NZ, the Adult Nutrition surveys of 1997 and 2008–2009 report that the mean fructose intake and the mean total sugar consumption for European males and females has declined from 1997 (26 g/day and 139 g/day, respectively), to 2008 (23.4 g/day and 100.3 g/day respectively) [121]. Unfortunately, there are no recent national consumption figures to see whether that national trend has continued. A small regional study (n = 227) in 2014 on a Canterbury (NZ) population aged 50 years reported that the mean intake for fructose for men was 25.5 g and 20.6 g for females [122]. This suggests that the downward trend has not continued.
Once ingested, fructose is transported into the blood stream through sugar-transporting proteins GLUT2 and GLUT5, via the small intestine in limited amounts to the liver. The liver removes some of the fructose from circulation to maintain the correct proportion of glucose to fructose (10 times lower) [123,124,125,126], and converts fructose into other metabolites including triglycerides and uric acid. This latter function is thought to be a contributor to the rise in obesity, metabolic syndrome and diabetes [106,110,127,128]. Fructose intake also stimulates the thioredoxin-interacting protein gene (TXNIP), which can lead to higher fructose intake [129]. The TXNIP gene has been associated with promoting inflammation in endothelial cells, mediating hepatic inflammation, and regulating NF-κB [130,131,132,133,134]. TXNIP has been shown to be involved in the differentiation of epithelial cells in the intestine [135]. TXNIP mRNA, which negatively regulates the TXNIP gene, shows decreased expression in the inflamed mucosa of the colon in people with UC [136]. Interestingly, thioredoxin-1 produced by the gene TXNIP is also inhibited by the production of vitamin D3 up-regulated protein 1 (VDUP1) when vitamin D3 is given [137,138].
The relationship between fructose uptake and the TXNIP gene may explain why fructose intake has been increasingly linked to intestinal symptoms of bloating, abdominal pain, diarrhoea and fructose intolerance in individuals with IBD [126,139,140,141,142]. This underlies the need to consider fructose intake when managing these symptoms. A recent NZ study by Spencer et al. on people with irritable bowel syndrome (IBS) (n = 277) based in Canterbury, NZ found that the participants’ IBS score, constipation and diarrhoea were not associated with fructose intake. However, they found that greater mean daily fructose intakes (p = 0.05) were associated with a lower prevalence of IBS pain [122].
In adults, fructose intolerance is most commonly assessed by breath testing after swallowing 25 g of fructose dissolved in water. Breath measurements are made on a 30-min schedule for up to three hours. A value of ≥20 ppm H2, accompanied by symptoms (e.g., bloating, diarrhoea), is an affirmation of fructose intolerance [139,143]. This is because the hydrogen produced by gut bacteria is reacting with the fructose which has not been absorbed in the small intestine. Breath testing is a common method of testing for fructose, and there are a variety of methodologies in its application, as well as questions about its reliability [144]. A North American consensus statement has been drawn up for hydrogen and methane-based breath testing in gastrointestinal disorders to address these issues [145].
When it is suggested that high-fructose-containing foods be eliminated in the diet, care must be taken in deciding which foods are excluded, as the assessment of what constitutes a high-fructose food varies [146]. If people stay on a low-fructose diet for too long, they may be in danger of nutrient deficiencies due to lack of fruit and vegetables in the diet. It also needs to be remembered that fructose taken as a bolus (e.g., after swallowing 25 g of fructose in a fructose intolerance test) is metabolized differently to when it is ingested in the presence of glucose. Many people absorb fructose easily along with sucrose or with glucose that is at similar levels [147].
3.4. Other Western Diet Factors
Westernisation has also introduced other factors into the diet, such as changes in birthing methods, using artificial infant formula instead of breast feeding, and the use of sugar substitutes, food additives and antibiotics (Table 3). Many of these changes have been associated with a reduction in gut microbial diversity as well as dysbiosis (i.e., microbial imbalances in the gut)—a feature of IBD [44,45]. This path to dysbiosis may start early, with lack of exposure to appropriate gut microbiota as an infant. The composition of an infant’s gut bacteria is affected by the delivery method (i.e., whether infants are born by caesarean (C-section) or vaginally, and whether breast-fed or given infant formula. C-section babies have microbiota that resembles that on the mother’s skin, and those born vaginally are seeded by the microbiota in the mother’s vagina [148,149]. The microbiota seeded from the mother’s vagina is thought to contribute to a normal infant microbiome, whereas having a C-section and all the factors associated with the reason for having a C-section does not [150].
Table 3.
Western diet food factors, effects related to IBD and associated genes. LC-PUFA: long-chain polyunsaturated fatty acid; SCFA: short-chain fatty acid.
| Western Diet Food Factors | Effects Related to IBD | Associated Genes |
|---|---|---|
| Foods low in resistant starch and soluble dietary fibre [47] |
FFAR2 (The interaction of SCFAs and FFAR2 greatly affects inflammatory processes) [21,70] |
|
| Increased consumption of saturated fatty acids with decreased consumption of LC-PUFAs from the increased intake of soy, safflower, corn and sunflower oils and reduced consumption of fish [83,92,93] |
FADS1 and FADS2 PPARA, PPARG XRCC1, SCD1 [94,95,96,97,98,99,100,101,102,103,104] |
|
| Increased fructose consumption [25,119,120] |
|
TXNIP [128] (associated with promoting inflammation in endothelial cells, mediating hepatic inflammation, and regulating NF-κB) [129,130,131,132,133] |
| Increased use of infant formula, artificial sweeteners, food emulsifiers and antibiotics [167,168] | Genes in the microbiome [168] |
The type of first foods also contributes to the composition of the microbiota. Breast feeding promotes immune development and protects against many diseases. In a small study on 3-month-old babies 1214 probe sets for epithelial cells were significantly differently expressed in exclusively breast-fed (n = 12) and formula-fed babies (n = 10) [151]. This may explain some of the differences that have been noted in clinical and epidemiological observations of these two groups. The two groups have different gut development, with the breast-fed babies being described as being “less leaky”. Bacteroidetes species, which are important players in the commensal bacteria, were not found in the stools of the formula-fed infants [151]. Breast milk also contains human milk oligosaccharides (HMOs) which stimulate the growth of the intestinal flora, acting as prebiotics for beneficial bacteria [152]. Human bioactive milk proteins modulate the immune system, and this too would contribute to the enhancement of gut barrier function [153,154]. Formula feeding is becoming an increasingly common pattern of infant feeding in Western culture.
The increasing use of artificial sweeteners (ASs) may also contribute to dysbiosis [155]. A number of studies have been conducted on the effects of ASs on the gut. These have looked at their effects on secretion, absorption, gut motility, the microbiome and gastrointestinal symptoms [156,157,158,159,160]. Some studies imply that ASs have measurable effects on the oral/gut microbiota [155,161,162]. In one example, male rats were randomised into control and AS groups and were exposed to doses ranging from 1.1 to 11 mg/kg/day for 12 weeks (the USA Federal Drug Administration (FDA) acceptable daily intake for humans is 5 mg/kg/day). At the low dose of 1.1 mg/kg, the researchers reported that total anaerobes were reduced by approximately 50% in all sucralose plus maltodextrin groups. However, when this study was reviewed by an expert panel [163], it was criticised for not having an isocaloric carbohydrate control, and not correcting for the water content of the stools. Both these factors can affect the interpretation of the bacterial concentrations. This report also commented that the difference between control and treatment groups for bacterial counts given were also less than 10-fold. They concluded that this meant that the results could not be interpreted as indicative of adverse change, but reflected the normal range of variation in bacteria over this period of time. However, further research in healthy human subjects continues to suggest that microbiota are adversely affected by ASs. Suez and colleagues showed that with 11 weeks of exposure to three common ASs, saccharin altered gut microbiota and induced glucose intolerance [161]. This indicates the need for more studies—particularly in humans—to elucidate the effects of ASs on microbiota [164,165,166].
Two other recent additions to the Western diet are emulsifiers in food to improve mouth feel in low-fat products, and antibiotics in animal feed [167,168]. Emulsifiers have been shown in murine studies to have an impact on the mouse gut microbiota that promotes colitis and decrease in (alpha) diversity [167]. Antibiotics are used to reduce disease in livestock kept in confined quarters. Repeated courses of antibiotics can lead to the development of what is termed “the resistome”—the collection of genes in bacteria that confers resistance to antibiotics [169]. Increased antibiotic exposure in humans through food sourced from animals that have had antibiotics may affect the human biome. When these products from these animals are consumed, they contribute to antibiotic resistance. The US Food and Drug Administration (2009) indicated that 80% of antimicrobial products in the USA were used in animal feed as growth stimulants, not for veterinary use [170]. In the highly pastoral country New Zealand it is reported that about 60% of antibiotics are administered to animals [171]. The Ministry for Primary Industries (NZ) states the use of antibiotics in cattle and sheep is low compared to the intensive rearing pig and poultry industries [172].
The issue for people with IBD is its effect on the genetics of their microbiome when they incorporate these products into their diet. For example, with antibiotics, antibiotic resistance genes are built up in the microbial community both in pathogenic and non-pathogenic bacteria, and this builds the resistome [169]. Genes in the resistome are readily transferred not only from one bacterial cell to another, but also by cassettes of multiple genes [173,174]. This has been shown to result in a build-up of multidrug resistance to E. coli in domestic animals [175,176]. These bacteria have also been identified as being in increased numbers in those with CD [177,178,179]. Collectively, all these Western diet factors impinge on the genetics of the biome and reduce microbiota diversity. Personalising the dietary approach for people with IBD and identifying the effects of genes associated with these changes (Table 3) would help reduce this adverse impact on the genes of the microbiome. It would help maintain microbiotal diversity and positively modulate disease activity.
4. Food Intolerances
IBD has been linked with a number of food intolerances, especially with foods containing gluten, dairy products/lactose or fructose (discussed earlier), or food high in FODMAPs. Many of these intolerances can also be linked to specific genes or genotypes. Other food intolerances linked with genotypes and IBD are brassica vegetables, mustard, wasabi, raw and cooked tomatoes, sweet potatoes, mushrooms, sulphur dioxide, sulphites and sulphur compounds. The specific genes or genotypes associated with these intolerances will be discussed further. This section begins with a discussion of the FODMAP approach, as this is the newest dietary strategy suggested as being helpful for IBD. It also links to a number of food intolerances associated with particular genotypes and/or gene variants.
4.1. FODMAPs
FODMAPs are fermentable oligo-, di- and mono-saccharides and polyols which, if lowered in the diet, have been shown to be effective in decreasing the abdominal symptoms associated with IBS. This approach is being proposed as being helpful also for people with IBD [2]. Foods that are identified as FODMAPs have been discussed extensively in the literature [111,180,181,182,183,184]. Table 4 gives examples of food sources of FODMAPs [185,186,187,188,189,190,191] and genes that are associated with them.
Table 4.
Sources of foods high in fermentable oligo-, di- and mono-saccharides and polyols (FODMAPs) [185,186,187,188,189,190,191].
| FODMAPs | Examples of Food Sources | Examples of Associated Genes |
|---|---|---|
| Fructose | Honey, mangoes, watermelon, grapes, fruit juices, high-fructose corn syrup | TXNIP with fructose that is linked to intestinal symptoms of bloating, abdominal pain, diarrhoea in IBD [125,138,139,140,141] |
| Lactose | Dairy products (e.g., milk, ice-cream, yoghurt, soft cheeses) | Lactose intolerance associated with variants of the LCT gene [191,192] |
| Fermentable oligosaccharides | Brussels sprouts, broccoli, cabbage, peas, beetroot, garlic, leeks, onions, wheat or rye bread, pasta, couscous, chickpeas, lentils | GSTMl and GSTTl, and DIO1 variants, with tolerance of brassica vegetables [193,194,195,196]; HLA-DQA1 and HLA-DQB1 variants with gluten intolerance [197] |
| Polyols | Stone fruits, apples, pears, prunes, avocados, mushrooms; sweeteners: mannitol, sorbitol, xylitol, erythritol and isomalt. | OCTN1 with mushrooms [198] |
Foods high in FODMAPs have been characterized in a healthy population (i.e., those who do not experience inflammation) as not being well absorbed in the small intestine, having a laxative effect if taken in higher doses and fermented rapidly by bacteria [2]. Inflammation of the gut wall—a characteristic of IBD—also interrupts enzyme production by the brush border cells, and this also contributes to the sugars not being absorbed. Unabsorbed sugars go to the large intestine, where bacteria interact with them. When some metabolites (e.g., butan 2,3 diol, ketones, acids, aldehydes) are formed, they can result in an alteration of the signalling mechanisms of the bacteria and this causes gas, pain and diarrhoea [192]. Diarrhoea has a negative impact on water and electrolyte balance, and may lead to dehydration. Gas, pain and diarrhoea commonly occur in non-IBD gastro-intestinal tracts (i.e., healthy populations) if doses of oral fructose or polyols over 50 g are taken [113,193,194].
This physiological reaction is the rationale for using the FODMAP dietary regime in gastrointestinal disorders like IBD [111,195]. This is based on studies with irritable bowel syndrome (IBS), where the decreased consumption of these fermentable carbohydrates has decreased abdominal symptoms (i.e., pain, flatulence, bloating, constipation, diarrhoea). There is no question that the by-products of FODMAP digestion and absorption may be especially unpleasant, exacerbating the symptoms of individuals with gastrointestinal disorders. Thus, it might appear as self-evident that avoidance of this group of compounds could be therapeutic for individuals with IBD—especially if they also have IBS [111,195]. A number of trials with IBS have suggested that this approach shows potential for improving symptoms in IBD. However, to avoid compromising people’s nutritional status, care must be taken so that any changes instigated in sources of fruits and vegetables do not reduce the total recommended intake of fruits, vegetables or fibres [196].
How does the FODMAP approach relate to genotypes? As will be discussed further on, intolerance to some vegetables (e.g., brassica, mushrooms, cooked tomatoes) whole grains and dairy products may be linked to specific genotypes and gene variants (Table 5). Recent research has also indicated that following a strict FODMAP diet can also have an effect on the genome of the microbiome and encourage harmful gut microbiota and a decrease in Bifidobacterium [183,184]. In people with disorders like IBD, their microbiotal composition is different from those found in healthy people [197,198,199]. Individuals with IBD have reduced numbers of microbiota, fewer divergent species with lower numbers of Firmicutes, and increased number of Proteobacteria species compared to healthy people [200,201,202,203]. This means that strict adherence to a low-FODMAP diet could contribute to this dysbiosis.
Table 5.
Differences in glucosinolate structures.
| Glucobrassicin (in bok choy) | Glucoraphanin (in broccoli) |
|---|---|
| Indole-3- | Sulforaphane |
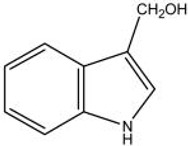
|
|
In addition, people with IBD may have a loss of function of the fucosyltransferase 2 (FUT2) gene, which identifies them as a “non-secretor”. A non-secretor has a significant reduction in microbiotal diversity, richness and abundance [204]. The FUT2 gene has been identified as increasing the susceptibility to CD [205,206,207]. Non-secretor individuals are also infrequently colonised by Bifidobacterium bifidum (important in the infant flora), B. adolescentis and B. catenulatum/pseudocatenulatum (important in adult intestinal flora) [204]. Bifidobacteria are an important part of the human intestinal microbiome. They are crucial to the development of a healthy infant microbiota, encouraging appropriate gut maturity and gut integrity, modulating immunity and negating the effects of pathogens [208,209]. This would suggest that people with IBD who are non-secretors have an increased risk of having a less-effective defence against a number of pathogens and are consequently more vulnerable to having an inflamed gut. Following a low-FODMAP diet may exacerbate this. Further research is needed on those who are a non-secretors, using the low-FODMAP approach to see how this affects their microbiota.
4.2. Gluten
Intolerance to foods containing gluten is associated with the autoimmune disease coeliac disease and with non-coeliac gluten sensitivity. These two have symptoms in common (e.g., bloating, diarrhoea, constipation, abdominal pain and weight loss). Unlike coeliac disease, gluten sensitivity is not associated with antibody formation and damage to the epithelium of the gut. Coeliac disease has genetic links. Gluten intolerance is associated with gene variants of the major histocompatibility complex, class II, DQ alpha 1 (HLA-DQA1) gene and the major histocompatibility complex, class II, DQ beta 1 (HLA-DQB1) gene [210]. Festen and colleagues in their meta-analysis of two genome-wide association studies GWAS also identified the genes interleukin 18 receptor accessory protein (IL18RAP); phosphatase, non-receptor type 2 (PTPN2); T cell activation RhoGTPase activating protein (TAGAP) and pseudouridine synthase 10 (PUS10) as having shared risk loci for CD and coeliac disease [211]. The gene PTPN2 is involved in the regulation of the epithelial barrier function and is modulated by the vitamin D receptor gene (VDR) [212]. Current nutrition advice for people with IBD includes checking whether they have the autoimmune coeliac disease or if they have non-coeliac gluten sensitivity. The blood test to detect the presence of anti-tissue transglutaminase (tTG) antibodies can be taken. These antibodies are produced in the small intestine when inflammation occurs in response to gluten. Another test that has a high specificity for coeliac disease is the anti-endomysial antibody (EMA) test which detects villus atrophy—a feature of coeliac disease [213]. If IBD patients prove to have an adverse response to gluten from these tests, then avoiding these foods and products containing them is advised. These foods include wheat, oats, barley (which includes malt and malt vinegar) and rye. There is no definitive test for non-coeliac gluten sensitivity except for eliminating gluten from the diet to see if symptoms subside. The role of non-coeliac gluten or wheat sensitivity in the general population as well as in IBD has been debated extensively. A review of the topic suggests that it may be linked in IBD with those who also have IBS.
4.3. Dairy Products and Lactose
The sensitivity to dairy foods in people with IBD is about 10%–20% [214,215,216]. Some studies have also linked increased incidence of IBD with the increased intake of dairy foods [217], particularly cheese [218]. This may be because of the fat content of cheese [219]. Nolan-Clark and associates noted that in their study on CD, sensitivity to dairy products was not related to disease activity status [219]. In a year-long trial on UC conducted by Wright et al. it was noted that patients had fewer relapses on a milk-free diet [216].
This sensitivity to dairy products is often associated with the reduction of lactase enzyme in adulthood. Lactase is an enzyme located in the villus enterocytes that is required for the digestion of lactose in milk. Lactase catalyses lactose, the disaccharide in milk, to galactose and glucose (i.e., monosaccharides which can be absorbed into the blood stream). In most mammals, its activity declines shortly after weaning. In humans, the enzyme may persist with its activity into adulthood. This is known as lactase persistence, and it is a genetically determined trait. In Europe, a single allele—T-13910 of the lactase phlorizin hydrolase (LCT) gene—is the main one. However, in Africa and the Middle East many more mutations are associated with lactase persistence [220,221].
This lactase persistence has associations with CD. In an NZ-based study [222], lactase persistence in the Caucasian population was associated with an increased risk of CD for those with the lactase (LCT) gene who were homozygous for the T allele of rs4988235 as compared with those homozygous for the C allele (OR = 1.61, 95% CI: 1.03–2.51). Increasing lactase non-persistence in populations has also been linked to a decreased risk of CD [223]. In a study encompassing populations from 26 countries around the world, CD decreased as lactase non-persistence increased (p < 0.01), [223].
Not consuming dairy products raises the risk of having insufficient intakes of calcium in the diet, especially if few other sources of calcium are consumed. Calcium is not only essential for bone health, but Ca2+ is extensively used in biological messenger systems and cell functions [224]. Low calcium intake of people with Crohn’s disease increases their risk of bone demineralisation and osteoporosis. These are well-known complications of this condition. Bernstein reported for people with IBD that “The overall relative risk of fractures is 40% greater than that in the general population and increases with age” [225]. Osteoporosis can also be exacerbated by the use of steroid medications (a common treatment in IBD), as they have a detrimental effect on calcium and phosphate metabolism [226]. Calcium intake is important with respect to IBD because, as discussed earlier, Vagianos et al. observed that 25% of their adult outpatients consumed less than the recommended intake of calcium. Further, the study of Hartman et al. showed that 79% of the adolescent outpatients consumed less than 80% of RDA [9,15]. If people are not tolerant to dairy foods, non-dairy sources of calcium are available, such as fortified soy, almond and rice milks, tofu, sardines, almonds, sesame seeds, broccoli and fortified breakfast cereals and juices [227].
4.4. Brassica
Vegetables such as broccoli, cabbage, Brussels sprouts and cauliflower are members of the brassica family of vegetables. These are known to be a good source of various micronutrients (e.g., selenium, iodine) [228] and various phytochemicals (e.g., glucosinolates and quercetin) [229]. Common members from this group of plants are recommended to be excluded from the low-FODMAP diet (Brussels sprouts, broccoli and cabbage) [195]. However, two randomized controlled diet studies conducted by Brauer et al. found different responses in individuals depending on their glutathione S-transferase Mu 1 (GSTMl) genotype with supplements of specific brassica vegetables (broccoli, cabbage, cauliflower and radish sprouts) [230]. Their study found that this combination of brassicas altered the circulating peptides transthyretin (TTR) and zinc alpha 2-glycoprotein (ZAG) fragments in a sensitive and identifiable manner. TTR declines in serum with early signs of inflammation. ZAG (an adipokine) is mainly involved in lipid mobilisation. In GSTMl+ individuals, ZAG and TTR declined with brassica intake. However, this did not occur in those with the GSTMl-nul1 genotype.
GSTMl and genotypes of GSTTl are also significant with respect to individuals and their intakes of brassica. There have been over 500 studies on GST enzymes showing that genotypes of these GSTMl and GSTTl have an influence on cancer outcomes, contingent on the expression or non-expression of these genes [231]. GSTT1 and GSTMl belong to the GST gene super family, which is an extensive group of enzymes. The GST family contains several classes (e.g., alpha, kappa, mu, omega, pi, sigma, theta and zeta) [232,233]. GST enzymes have significant roles in the metabolism of electrophilic compounds (e.g., drugs and carcinogens) [234].
At the GSTMl locus, four allelic variants have been discovered in humans, and the null allele is present in about 50% of individuals [235]. Where the GSTMl gene deletions or the null genotype exist, there is a total lack of the protein encoded by the gene [236]. People with deletions are thought to be more vulnerable to toxic xenobiotics (e.g., antibiotics and drugs) [237]. There is also a variation between different cultural groups in the frequency of the GSTMl deletion. In Caucasian and Asian populations, the frequency of the GSTMl null genotype genetic polymorphism is estimated to be about 53%. However, the frequency is lower in African- American populations, at 27% [231].
GST enzymes can also be acted on by isothiocyanates (found in brassicas) either as inducers or as substrates in liver detoxification pathways, so a deletion or lowered activity of a GST gene can result in a persistent or elevated level of isothiocyanates in the body [238]. Studies based in Asia suggest that the null or less-active genotypes for GSTT1 and GSTM1 are protective and lower the risk of colorectal cancer [239]. However, the opposite occurred in the USA-based studies. The active or expressed genotypes, not the null genotypes, were associated with higher risk reduction [240]. The disparity of these results may reflect the different glucosinolate content of the brassica consumed. In Asia, Chinese cabbage (bok choy) is the predominately eaten brassica, and its main glucosinolate is glucobrassicin, whereas in the USA where raw broccoli is the more favoured brassica, the glucosinolate contents differ [241]. The main glucosinolate in this vegetable is glucoraphanin [241]. Glucobrassicin is an indole, whereas glucoraphanin is a sulforaphane. Their distinct chemical structures as shown in Table 5 (adapted from Higdon et al.) may mean that they are metabolised differently as they pass through the intestine, and this would suggest that their influence on the GST genes could also be dissimilar.
A pilot study by Campbell et al. [242] was conducted on the GSTT1 (-/-) deletion in people with CD, in a New Zealand cohort from the Auckland IBD study [243]. This also showed those consuming broccoli, cauliflower and Chinese greens were more likely to report experiencing a beneficial effect if they had the GSTT1 deletion. This may well apply to people with other gastrointestinal disorders such as IBS [244]. Responses to another brassica, rocket (arugula), have also been found to be dependent on a variant in the single-nucleotide polymorphism (SNP) rs9469220 of the human leucocyte antigen gene (HLA) in people with CD [245]. An inflammatory colonic phenotype has also been associated with variants in the HLA gene region [246,247,248]. This polymorphism has been linked to total IgE levels, which have a key role in sensitivity type 1 reactions in allergies linked with rhinitis, dermatitis, asthma and urticaria [249,250,251]. This adverse reaction to rocket in people with CD suggests that consumption of this food initiates the immune response in a type I sensitivity reaction. Another variant in the SNP rs7515322 in the iodothyronine deiodinase 1 gene (DIO1) has also been associated with an adverse response to broccoli. DIO1 is part of the selenoenzyme family, which are important as signalling molecules for thyroid hormones and pivotal to thyroid metabolism. Both selenium and iodine deficiencies exist in NZ, and this may heighten individuals’ adverse response to broccoli if they have this variant [245]. This influence of micronutrients may also explain inter-country variation in responses to broccoli.
4.5. Mustard, Wasabi, Raw and Cooked Tomatoes and Sweet Potatoes
In another study by Marlow et al. [252], tolerance of mustard, wasabi, raw and cooked tomatoes and sweet potatoes was dependent on the G variant of the SNP rs12212067 in the FOX03 gene. Seventeen foods were identified with beneficial effects, and four foods with detrimental effects. Sweet potatoes had the highest reported frequency of beneficial effects, and mustard, wasabi, raw and cooked tomatoes were detrimental for over 25% of the study population with the G allele (SNP rs12212067) associated with the FOX03 gene. FODMAP guidelines suggest that tomato products such as canned tomatoes are not appropriate in the low-FODMAP diet, although plum tomatoes are [139]. This guidance could perhaps be tailored more for people using the low-FODMAP diet if their gene variants in the FOX03 gene were identified.
4.6. Mushrooms
In a study on CD in an NZ population, a statistically significant association with a variant (the T allele) of the SNP rs1050152 of the organic cation transporter gene OCTN1 was found. This variant is related to mushroom intolerance in those with CD, when compared with the general population. This indicates that NZ people with CD who have this variant have an increased risk of adverse symptoms associated with the intake of mushrooms [244].
4.7. Sulphur Dioxide, Sulphites and Sulphur Compounds
Sulphur dioxide and sulphites (at concentrations greater than 10 mg/kg or 10 mg/L) are now listed by the EU as a common cause of food intolerances and allergens [253]. Additive sulphites are often used in the food industry, as they have multiple functions. They are used as food preservatives, as they stop microbial growth and reduce the spoilage of food. They can act as antioxidants, inhibiting browning actions. They can modify texture in doughs. They are found in wine, soft drinks, sausages and burgers [254]. There is some evidence that they may aggravate IBD symptoms. Magee et al. report in their novel method of diet analysis that in UC, sulphites adversely affect symptoms through their anti-thiamine activity. Thiamine has a critical role in energy metabolism and cell function [255]. Low levels have been implicated in fatigue and IBD in those in remission (UC) or quiescent (CD) [256]. Those with very active disease who ate large quantities of food containing sulphites also had high food sigmoidoscopy scores [257].
Earlier studies linking sulphur compounds with IBD have described hydrogen sulphide as a bacterial toxin in people with UC [258]. This description was based on the experimental work where sulphated polysaccharides were ingested by guinea pigs and rabbits [259,260]. These sulphur products impaired butyrate oxidation—a characteristic of UC [261]. Butyrate, as a SCFA (as mentioned earlier), plays an important part in colon homeostasis by promoting the development of the intestinal barrier through enhancing tight-junction formation, which is impaired in people with IBD [262]. More recent work by Carbonero et al. has summarised the significance of microbiota in colonic sulphur metabolism and identified a number of gut microbiota taxa that participate in it [263]. These authors suggest that common variants in host genes involved in the sulphide oxidation pathways may be implicated in ineffective epithelial sulphide detoxification. This makes people with these variants predisposed to sulphide intolerance and consequent inflammation. The literature suggests that sulphur dioxide sulphites, and other ingested sulphur-containing products, are another example of where nutrient–gene interaction occurs. Particular variants are associated with their intolerance. Using the personalised nutrition approach and identifying these gene variants in people with IBD would identify those who are intolerant to sulphur dioxide, sulphites and sulphur compounds, and so remove foods containing these substances and reduce inflammation.
5. Ethical Issues
The willingness of individuals to engage in genetic testing that would enable a personalised diet/nutrition approach will depend on how they understand the benefits this would bring compared to their perceived privacy risk [264]. Genetic testing for individuals can now be readily accessed with a number of commercial applications available (e.g., Fitgenes, 23andMe) [265,266]. The availability of genetic testing also raises a number of questions: Who should hold and have access to this information? How safely is the information kept? How informed is the consent for consumers? [266]. The protection of an individual’s genetic information and the validation of genetic tests needs to be guaranteed to ensure consumer safety. Health practitioners also need to be able to understand the role of genetic testing in their clinical practice so that they can integrate this information appropriately and offer a personalised nutrition approach.
6. Conclusions
This review gives examples of genotypes and genetic variants that have been associated with nutrient deficiencies, the Western diet, and food intolerances. Variation in genotypes could help explain the variability in response to food that is associated with IBD. We need to identify the variants associated with adverse reactions to food, as is currently being done in pharmacogenetic research with respect to drugs. Identification of gene variants would allow more precise tailoring of diets to avoid exacerbating malnutrition in vulnerable IBD groups. Use of the personalised nutrition approach can also more accurately identify individual food intolerances, help ameliorate abdominal symptoms and improve the quality of life for people with IBD. Further nutrigenomic research can continue to inform and improve the personalised nutrition approach in IBD. In the future, this information could be combined with data on individuals such as their gut microbiome, dietary patterns, exercise routines, blood parameters and anthropometric measurements. This combination of information could be used to build machine-learning algorithms to predict which foods/meals reduce inflammation and abdominal symptoms. This may also help to establish the dietary patterns and lifestyles that are most protective against IBD in those genetically susceptible to IBD. This combination of data may also help individuals with established IBD to tailor their diet and lifestyle to avoid exacerbating their signs and symptoms, and promote longer periods of remission. The protection of an individual’s genetic information and the validation of genetic tests needs to be guaranteed to ensure consumers’ safety when using this information with a personalised nutrition approach.
Author Contributions
Writing—original draft preparation, review and editing, B.B.L.; draft preparation, review and editing, A.G.L.; conceptualization, draft preparation, review and editing, L.R.F.
Conflicts of Interest
There are no conflicts of interest to be declared.
References
- 1.Zeevi D., Korem T., Zmora N., Israeli D., Rothschild D., Weinberger A., Ben-Yacov O., Lador D., Avnit-Sagi T., Lotan-Pompan M. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Ercolini D., Fogliano V. Food design to feed the human gut microbiota. J. Agric. Food Chem. 2018;66:3754–3758. doi: 10.1021/acs.jafc.8b00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grissinger M. The five rights: A destination without a map. Pharm. Ther. 2010;35:542. [Google Scholar]
- 4.Halawi H., Camilleri M. Pharmacogenetics and the treatment of functional gastrointestinal disorders. Pharmacogenomics. 2017;18:1085–1094. doi: 10.2217/pgs-2017-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J., Shen J., Seifer B.J., Jiang S., Wang J., Xiong H., Xie L., Wang L., Sui X. Approaches and genetic determinants in predicting response to neoadjuvant chemotherapy in locally advanced gastric cancer. Oncotarget. 2017;8:30477–30494. doi: 10.18632/oncotarget.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Racine A., Carbonnel F., Chan S.S., Hart A.R., Bueno-de-Mesquita H.B., Oldenburg B., van Schaik F.D., Tjonneland A., Olsen A., Dahm C.C., et al. Dietary patterns and risk of inflammatory bowel disease in europe: Results from the EPIC Study. Inflamm. Bowel Dis. 2016;22:345–354. doi: 10.1097/MIB.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 7.Frazier-Wood A.C. Dietary patterns, genes, and health: Challenges and obstacles to be overcome. Curr. Nutr. Rep. 2015;4:82–87. doi: 10.1007/s13668-014-0110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson L.R. Nutrigenetics, nutrigenomics and inflammatory bowel diseases. Expert Rev. Clin. Immunol. 2013;9:717–726. doi: 10.1586/1744666X.2013.824245. [DOI] [PubMed] [Google Scholar]
- 9.Vagianos K., Bector S., McConnell J., Bernstein C.N. Nutrition assessment of patients with inflammatory bowel disease. J. Parenter. Enter. Nutr. 2007;31:311–319. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 10.Stein J., Bott C. Diet and Nutrition in Crohn’s Disease and Ulcerative Colitis 20 Questions 20 Answers. Falk Foundation e.V.; Freiburg, Germany: 2008. [Google Scholar]
- 11.Basson A. Nutrition management in the adult patient with Crohn’s disease. S. Afr. J. Clin. Nutr. 2012;25:164–172. doi: 10.1080/16070658.2012.11734423. [DOI] [Google Scholar]
- 12.Harries A., Rhodes J. Undernutrition in Crohn’s disease: An anthropometric assessment. Clin. Nutr. 1985;4:87–89. doi: 10.1016/0261-5614(85)90048-2. [DOI] [PubMed] [Google Scholar]
- 13.Gassull M.A., Cabré E. Nutrition in inflammatory Bowel disease. Curr. Opin. Clin. Nutr. Metab. Care. 2001;4:561–569. doi: 10.1097/00075197-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Lomer M.C., Kodjabashia K., Hutchinson C., Greenfield S.M., Thompson R.P., Powell J.J. Intake of dietary iron is low in patients with Crohn’s disease: A case–control study. Br. J. Nutr. 2004;91:141–148. doi: 10.1079/BJN20041022. [DOI] [PubMed] [Google Scholar]
- 15.Hartman C., Marderfeld L., Davidson K., Mozer-Glassberg Y., Poraz I., Silbermintz A., Zevit N., Shamir R. Food intake adequacy in children and adolescents with inflammatory Bowel disease. J. Pediatr. Gastroenterol. Nutr. 2016;63:437–444. doi: 10.1097/MPG.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 16.Lourenço S., Hussein T., Bologna S., Sipahi A., Nico M. Oral manifestations of inflammatory Bowel Disease: A review based on the observation of six cases. J. Eur. Acad. Dermatol. Venereol. 2010;24:204–207. doi: 10.1111/j.1468-3083.2009.03304.x. [DOI] [PubMed] [Google Scholar]
- 17.Malins T., Wilson A., Ward-Booth R. Recurrent buccal space abscesses: A complication of Crohn’s disease. Oral Surg. Oral Med. Oral Pathol. 1991;72:19–21. doi: 10.1016/0030-4220(91)90182-C. [DOI] [PubMed] [Google Scholar]
- 18.Plauth M., Jenss H., Meyle J. Oral manifestations of Crohn’s Disease: An analysis of 79 cases. J. Clin. Gastroenterol. 1991;13:29–37. doi: 10.1097/00004836-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Schlosser B.J., Pirigyi M., Mirowski G.W. Oral manifestations of hematologic and nutritional diseases. Otolaryngol. Clin. N. Am. 2011;44:183–203. doi: 10.1016/j.otc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Thomas D.M., Mirowski G.W. Nutrition and oral mucosal diseases. Clin. Dermatol. 2010;28:426–431. doi: 10.1016/j.clindermatol.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Rebhan M., Chalifa-Caspi V., Prilusky J., Lancet D. GeneCards: A novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics. 1998;14:656–664. doi: 10.1093/bioinformatics/14.8.656. [DOI] [PubMed] [Google Scholar]
- 22.D’Ambrosio D.N., Clugston R.D., Blaner W.S. Vitamin A metabolism: An update. Nutrients. 2011;3:63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung W., Hessel S., Meplan C., Flint J., Oberhauser V., Tourniaire F., Hesketh J., Von Lintig J., Lietz G. Two common single nucleotide polymorphisms in the gene encoding Β-Carotene 15, 15′-monoxygenase alter Β-carotene metabolism in female volunteers. FASEB J. 2009;23:1041–1053. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 24.Su H.Y., Gupta V., Day A.S., Gearry R.B. Rising incidence of inflammatory Bowel Disease in Canterbury, New Zealand. Inflamm. Bowel Dis. 2016;22:2238–2244. doi: 10.1097/MIB.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 25.University of Otago. Ministry of health New Zealand . A Focus on Nutrition: Key Findings of the 2008/09 New Zealand Adult Nutrition Survey. Ministry of Health; Wellington, New Zealand: 2011. [Google Scholar]
- 26.Anand P.K., Kaul D. Downregulation of TACO gene transcription restricts mycobacterial entry/survival within human macrophages. FEMS Microbiol. Lett. 2005;250:137–144. doi: 10.1016/j.femsle.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 27.Stephensen C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 28.Sommer A. Vitamin A Deficiency. Wiley Online Library; Hoboken, NJ, USA: 2001. [Google Scholar]
- 29.Thia K.T., Loftus E.V., Sandborn W.J., Yang S. An update on the epidemiology of inflammatory Bowel Disease in Asia. Am. J. Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 30.Baumgart D.C., Bernstein C.N., Abbas Z., Colombel J.F., Day A.S., D’Haens G., Dotan I., Goh K.L., Hibi T., Kozarek R.A. IBD around the world: Comparing the epidemiology, diagnosis, and treatment: Proceedings of the World Digestive Health Day 2010–Inflammatory bowel disease task force meeting. Inflamm. Bowel Dis. 2011;17:639–644. doi: 10.1002/ibd.21409. [DOI] [PubMed] [Google Scholar]
- 31.Gismera C.S., Aladren B.S. Inflammatory Bowel diseases: A disease (s) of modern times? is incidence still increasing? World J. Gastroenterol. 2008;14:5491–5498. doi: 10.3748/wjg.14.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logan I., Bowlus C.L. The geoepidemiology of autoimmune intestinal diseases. Autoimmun. Rev. 2010;9:A372–A378. doi: 10.1016/j.autrev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Lomer M.C., Thompson R.P., Powell J.J. Proceedings-Nutrition Society of London. Cambridge University Press; Cambridge, UK: 2002. Fine and ultrafine particles of the diet: Influence on the mucosal immune response and association with Crohn’s disease. [DOI] [PubMed] [Google Scholar]
- 34.Persson P., Ahlbom A., Hellers G. Diet and inflammatory bowel disease: A case-control study. Epidemiology. 1992;3:47–52. doi: 10.1097/00001648-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hou J.K., Abraham B., El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 36.Chapman-Kiddell C.A., Davies P.S., Gillen L., Radford-Smith G.L. Role of diet in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 2010;16:137–151. doi: 10.1002/ibd.20968. [DOI] [PubMed] [Google Scholar]
- 37.Sartor R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Rev. Gastroenterol. Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 38.Conterno L., Fava F., Viola R., Tuohy K.M. Obesity and the gut microbiota: Does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011;6:241–260. doi: 10.1007/s12263-011-0230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myles I.A. Fast food fever: Reviewing the impacts of the western diet on immunity. Nutr. J. 2014;13:1. doi: 10.1186/1475-2891-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O’Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 41.Tsironi E., Feakins R.M., Roberts C.S., Rampton D.S. Incidence of inflammatory bowel disease is rising and abdominal tuberculosis is falling in bangladeshis in East London, United Kingdom. Am. J. Gastroenterol. 2004;99:1749–1755. doi: 10.1111/j.1572-0241.2004.30445.x. [DOI] [PubMed] [Google Scholar]
- 42.Dankers W., Colin E.M., Van Hamburg J.P., Lubberts E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2017;7:697. doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.M’koma A.E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013;6:S12731. doi: 10.4137/CGast.S12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segata N. Gut microbiome: Westernization and the disappearance of intestinal diversity. Curr. Biolog. 2015;25:R611–R613. doi: 10.1016/j.cub.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 45.Broussard J.L., Devkota S. The changing microbial landscape of Western society: Diet, dwellings and discordance. Mol. Metab. 2016;5:737–742. doi: 10.1016/j.molmet.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuohy K.M., Conterno L., Gasperotti M., Viola R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J. Agric. Food Chem. 2012;60:8776–8782. doi: 10.1021/jf2053959. [DOI] [PubMed] [Google Scholar]
- 47.Amre D.K., D’Souza S., Morgan K., Seidman G., Lambrette P., Grimard G., Israel D., Mack D., Ghadirian P., Deslandres C. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s Disease in children. Am. J. Gastroenterol. 2007;102:2016–2025. doi: 10.1111/j.1572-0241.2007.01411.x. [DOI] [PubMed] [Google Scholar]
- 48.Hou J.K., Lee D., Lewis J. Diet and inflammatory bowel disease: Review of patient-targeted recommendations. Clin. Gastroenterol. Hepatol. 2014;12:1592–1600. doi: 10.1016/j.cgh.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly D.G., Fleming C.R. Nutritional considerations in inflammatory bowel diseases. Gastroenterol. Clin. N. Am. 1995;24:597–611. [PubMed] [Google Scholar]
- 50.Reif S., Klein I., Lubin F., Farbstein M., Hallak A., Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40:754–760. doi: 10.1136/gut.40.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeVries J.W. On defining dietary fibre. Proc. Nutr. Soc. 2003;62:37–43. doi: 10.1079/PNS2002234. [DOI] [PubMed] [Google Scholar]
- 52.Jones J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014;13:34. doi: 10.1186/1475-2891-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ACCC International Cereals & Grains association. [(accessed on 16 March 2019)];2019 Dietary Fiber. Available online: https://www.aaccnet.org/initiatives/definitions/Pages/DietaryFiber.aspx.
- 54.Ashwar B.A., Gani A., Shah A., Wani I.A., Masoodi F.A. Preparation, health benefits and applications of resistant starch—A review. Starch-Stärke. 2016;68:287–301. doi: 10.1002/star.201500064. [DOI] [Google Scholar]
- 55.Higgins J.A., Brown I.L. Resistant starch: A promising dietary agent for the prevention/treatment of inflammatory bowel disease and bowel cancer. Curr. Opin. Gastroenterol. 2013;29:190–194. doi: 10.1097/MOG.0b013e32835b9aa3. [DOI] [PubMed] [Google Scholar]
- 56.Bassaganya-Riera J., DiGuardo M., Viladomiu M., De Horna A., Sanchez S., Einerhand A.W., Sanders L., Hontecillas R. Soluble fibers and resistant starch ameliorate disease activity in interleukin-10–deficient mice with inflammatory bowel disease. J. Nutr. 2011;141:1318–1325. doi: 10.3945/jn.111.139022. [DOI] [PubMed] [Google Scholar]
- 57.Pituch-Zdanowska A., Banaszkiewicz A., Albrecht P. The role of dietary fibre in inflammatory bowel disease. Prz Gastroenterol. 2015;10:135–141. doi: 10.5114/pg.2015.52753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crohn’s & Colitis Foundation of America . Diet, Nutrition, and Inflammatory Bowel Diseases. Crohn’s & Colitis Foundation of America; New York, NY, USA: 2013. [Google Scholar]
- 59.The role of vegetables in the cause remission and regression of Crohn’s disease in an Auckland cohort. [(accessed on 17 September 2018)]; Available online: https://researchspace.auckland.ac.nz/handle/2292/19961.
- 60.Roberts C.L., Keita Å.V., Duncan S.H., O’Kennedy N., Söderholm J.D., Rhodes J.M., Campbell B.J. Translocation of Crohn’s disease Escherichia coli across M-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59:1331–1339. doi: 10.1136/gut.2009.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller H., Zhang J., Kuolee R., Patel G.B., Chen W. Intestinal M cells: The fallible sentinels? World J. Gastroenterol. 2007;13:1477–1486. doi: 10.3748/wjg.v13.i10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siebers A., Finlay B.B. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4:22–29. doi: 10.1016/0966-842X(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 63.Staudacher H.M., Kurien M., Whelan K. Nutritional implications of dietary interventions for managing gastrointestinal disorders. Curr. Opin. Gastroenterol. 2018;34:105–111. doi: 10.1097/MOG.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 64.Bockaert J., Pin J.P. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stoddart L.A., Smith N.J., Milligan G. International union of pharmacology. LXXI. free fatty acid receptors FFA1,-2, and-3: pharmacology and pathophysiological functions. Pharmacol. Rev. 2008;60:405–417. doi: 10.1124/pr.108.00802. [DOI] [PubMed] [Google Scholar]
- 66.Senga T., Iwamoto S., Yoshida T., Yokota T., Adachi K., Azuma E., Hamaguchi M., Iwamoto T. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood. 2003;101:1185–1187. doi: 10.1182/blood-2002-06-1881. [DOI] [PubMed] [Google Scholar]
- 67.Fournier B., Parkos C. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 68.Le Poul E., Loison C., Struyf S., Springael J., Lannoy V., Decobecq M., Brezillon S., Dupriez V., Vassart G., Van Damme J. Functional characterization of human receptors for short chain fatty acids and their Role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 69.Bindels L.B., Dewulf E.M., Delzenne N.M. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends. Pharmacol. Sci. 2013 doi: 10.1016/j.tips.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., Mackay F., Artis D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor. GPR Nat. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sivaprakasam S., Gurav A., Paschall A., Coe G., Chaudhary K., Cai Y., Kolhe R., Martin P., Browning D., Huang L. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis. 2016;5:e238. doi: 10.1038/oncsis.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bordonaro M., Mariadason J.M., Aslam F., Heerdt B.G., Augenlicht L.H. Butyrate-induced apoptotic cascade in colonic carcinoma cells: modulation of the beta-catenin-Tcf pathway and concordance with effects of sulindac and trichostatin A but not curcumin. Cell Growth Differ. 1999;10:713–720. [PubMed] [Google Scholar]
- 73.Evans W. Pharmacogenomics: Marshalling the human genome to individualise drug therapy. Gut. 2003;52:ii10–ii18. doi: 10.1136/gut.52.suppl_2.ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eastwood M., Kritchevsky D. Dietary fiber: How did we get where we are? Annu. Rev. Nutr. 2005;25:1–8. doi: 10.1146/annurev.nutr.25.121304.131658. [DOI] [PubMed] [Google Scholar]
- 75.Maslowski K.M., Mackay C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2010;12:5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 76.Schneeman B.O., Gallaher D. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine; New York, NY, USA: 1985. Effects of dietary fiber on digestive enzyme activity and bile acids in the small intestine; pp. 409–414. [DOI] [PubMed] [Google Scholar]
- 77.Lupton J.R. Microbial degradation products influence colon cancer risk: The butyrate controversy. J. Nutr. 2004;134:479–482. doi: 10.1093/jn/134.2.479. [DOI] [PubMed] [Google Scholar]
- 78.Blaut M., Clavel T. Metabolic diversity of the intestinal microbiota: Implications for health and disease. J. Nutr. 2007;137:751S–755S. doi: 10.1093/jn/137.3.751S. [DOI] [PubMed] [Google Scholar]
- 79.National Academy Press Dietary, Functional and Total Fibre. [(accessed on 9 August 2018)]; Available online: https://www.nap.edu/read/10490/chapter/9Accessed.
- 80.Silva A.F.D., Schieferdecker M.E.M., Amarante H.M.B.d.S. Food intake in patients with inflammatory bowel disease. ABCD Arq. Bras. Cir. Dig. 2011;24:204–209. doi: 10.1590/S0102-67202011000300005. [DOI] [Google Scholar]
- 81.Staudacher H.M., Irving P.M., Lomer M.C., Whelan K. Mechanisms and efficacy of dietary FODMAP Restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014;11:256. doi: 10.1038/nrgastro.2013.259. [DOI] [PubMed] [Google Scholar]
- 82.Halmos E.P., Christophersen C.T., Bird A.R., Shepherd S.J., Gibson P.R., Muir J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 83.Simopoulos A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 84.Razack R., Seidner D.L. Nutrition in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2007;23:400–405. doi: 10.1097/MOG.0b013e3281ddb2a3. [DOI] [PubMed] [Google Scholar]
- 85.Patterson E., Wall R., Fitzgerald G., Ross R., Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012;2012:1–16. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park J., Kwon S., Han Y., Hahm K., Kim E. Omega-3 polyunsaturated fatty acids as potential chemopreventive agent for gastrointestinal cancer. J. Cancer. Prev. 2013;18:201. doi: 10.15430/JCP.2013.18.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomas J., Thomas C., Radcliffe J., Itsiopoulos C. Omega-3 fatty acids in early prevention of inflammatory neurodegenerative disease: A focus on Alzheimer’s disease. BioMed Res. Int. 2015;2015:1–13. doi: 10.1155/2015/172801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pascual C.Y., Reche M., Fiandor A., Valbuena T., Cuevas T., Esteban M.M. Fish allergy in childhood. Pediatr. Allergy. Immunol. 2008;19:573–579. doi: 10.1111/j.1399-3038.2008.00822.x. [DOI] [PubMed] [Google Scholar]
- 89.Vitoria J.C., Camarero C., Sojo A., Ruiz A., Rodriguez-Soriano J. Enteropathy related to fish, rice, and chicken. Arch. Dis. Child. 1982;57:44–48. [PMC free article] [PubMed] [Google Scholar]
- 90.Dannaeus A., Johansson S., Foucard T., Öhman S. Clinical and immunological aspects of food allergy in childhood I. Estimation of IgG, IgA and IgE antibodies to food antigens in children with food allergy and atopic dermatitis. Acta Paediatrica. 1977;66:31–37. doi: 10.1111/j.1651-2227.1977.tb07803.x. [DOI] [PubMed] [Google Scholar]
- 91.Untersmayr E., Schöll I., Swoboda I., Beil W.J., Förster-Waldl E., Walter F., Riemer A., Kraml G., Kinaciyan T., Spitzauer S. Antacid medication inhibits digestion of dietary proteins and causes food allergy: A fish allergy model in BALB/C mice. J. Allergy Clin. Immunol. 2003;112:616–623. doi: 10.1016/S0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 92.Leaf A., Weber P.C. A new era for science in nutrition. Am. J. Clin. Nutr. 1987 doi: 10.1093/ajcn/45.5.1048. [DOI] [PubMed] [Google Scholar]
- 93.Eaton S.B., Konner M. Paleolithic nutrition: A consideration of its nature and current implications. N. Engl. J. Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 94.Schaeffer L., Gohlke H., Muller M., Heid I.M., Palmer L.J., Kompauer I., Demmelmair H., Illig T., Koletzko B., Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 95.Fajas L., Auboeuf D., Raspé E., Schoonjans K., Lefebvre A., Saladin R., Najib J., Laville M., Fruchart J., Deeb S. The organization, promoter analysis, and expression of the human PPARγ gene. J. Biol. Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 96.Tai E.S., Corella D., Demissie S., Cupples L.A., Coltell O., Schaefer E.J., Tucker K.L., Ordovas J.M. Polyunsaturated fatty acids interact with the PPARA-L162V polymorphism to affect plasma triglyceride and apolipoprotein C-III concentrations in the Framingham Heart Study. J. Nutr. 2005;135:397–403. doi: 10.1093/jn/135.3.397. [DOI] [PubMed] [Google Scholar]
- 97.De Keyser C.E., Becker M.L., Uitterlinden A.G., Hofman A., Lous J.J., Elens L., Visser L.E., Van Schaik R.H., Stricker B.H. Genetic variation in the PPARA gene is associated with simvastatin-mediated cholesterol reduction in the Rotterdam Study. Pharmacogenomics. 2013;14:1295–1304. doi: 10.2217/pgs.13.112. [DOI] [PubMed] [Google Scholar]
- 98.Chen M., Wang Y., Zhang W., Han X. Abstract PR428: Association of PparA Rs4253728 G> A gene polymorphisms with Cyp3A4 enzyme activity and fentanyl post-operative intravenous analgesic effect. Anesth. Analg. 2016;123:109–110. doi: 10.1213/01.ane.0000492815.84565.fd. [DOI] [Google Scholar]
- 99.Ferreira P., Cravo M., Guerreiro C.S., Tavares L., Santos P.M., Brito M. Fat intake interacts with polymorphisms of Caspase9, FasLigand and PPARgamma apoptotic genes in modulating Crohn’s Disease activity. Clin. Nutr. 2010;29:819–823. doi: 10.1016/j.clnu.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 100.Jiang Z., Li C., Xu Y., Cai S. A meta-analysis on XRCC1 and XRCC3 polymorphisms and colorectal cancer risk. Int. J. Colorectal Dis. 2010;25:169–180. doi: 10.1007/s00384-009-0817-9. [DOI] [PubMed] [Google Scholar]
- 101.Stern M.C., Butler L.M., Corral R., Joshi A.D., Yuan J.M., Koh W.P., Yu M.C. Polyunsaturated fatty acids, DNA repair single nucleotide polymorphisms and colorectal cancer in the Singapore Chinese Health Study. J. Nutrigenet. Nutrigenomics. 2009;2:273–279. doi: 10.1159/000308467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stryjecki C., Roke K., Clarke S., Nielsen D., Badawi A., El-Sohemy A., Ma D.W., Mutch D.M. Enzymatic activity and genetic variation in SCD1 modulate the relationship between fatty acids and inflammation. Mol. Genet. Metab. 2012;105:421–427. doi: 10.1016/j.ymgme.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Larsson S.C., Kumlin M., Ingelman-Sundberg M., Wolk A. dietary long-chain N-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 104.Nicholson M.L., Neoptolemos J.P., Clayton H.A., Talbot I.C., Bell P.R. Inhibition of experimental colorectal carcinogenesis by dietary N-6 polyunsaturated fats. Carcinogenesis. 1990;11:2191–2197. doi: 10.1093/carcin/11.12.2191. [DOI] [PubMed] [Google Scholar]
- 105.Bray G., Popkin B.M. Calorie-sweetened beverages and fructose: What have we learned 10 years later. Pediatr. Obes. 2013;8:242–248. doi: 10.1111/j.2047-6310.2013.00171.x. [DOI] [PubMed] [Google Scholar]
- 106.Elliott S.S., Keim N.L., Stern J.S., Teff K., Havel P.J. Fructose, weight gain, and the insulin resistance syndrome1—3. Am. J. Clin. Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 107.Basu S., Yoffe P., Hills N., Lustig R.H. The relationship of sugar to population-level diabetes prevalence: An econometric analysis of repeated cross-sectional data. PloS ONE. 2013;8:e57873. doi: 10.1371/journal.pone.0057873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goran M.I., Ulijaszek S.J., Ventura E.E. High fructose corn syrup and diabetes prevalence: A global perspective. Glob. Public Health. 2013;8:55–64. doi: 10.1080/17441692.2012.736257. [DOI] [PubMed] [Google Scholar]
- 109.DiNicolantonio J.J., O’Keefe J.H., Lucan S.C. Added fructose: A principal driver of type 2 diabetes mellitus and its consequences. Mayo Clin. Proc. 2015;90:372–381. doi: 10.1016/j.mayocp.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 110.Malik V.S., Schulze M.B., Hu F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shepherd S.J., Gibson P.R. Fructose malabsorption and symptoms of irritable bowel syndrome: Guidelines for effective dietary management. J. Am. Diet. Assoc. 2006;106:1631–1639. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 112.Gibson P., Shepherd S. Personal view: Food for thought–western lifestyle and susceptibility to Crohn’s Disease. the FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005;21:1399–1409. doi: 10.1111/j.1365-2036.2005.02506.x. [DOI] [PubMed] [Google Scholar]
- 113.Rumessen J.J. Fructose and related food carbohydrates: Sources, intake, absorption, and clinical implications. Scand. J. Gastroenterol. 1992;27:819–828. doi: 10.3109/00365529209000148. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen S., Choi H.K., Lustig R.H., Hsu C. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J. Pediatr. 2009;154:807–813. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rippe J.M., Angelopoulos T.J. Fructose-containing sugars and cardiovascular disease. Adv. Nutr. 2015;6:430–439. doi: 10.3945/an.114.008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DiNicolantonio J.J., Berger A. Added sugars drive nutrient and energy deficit in obesity: A new paradigm. Open Heart. 2016;3:e000469. doi: 10.1136/openhrt-2016-000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brinton E.A. The time has come to flag and reduce excess fructose intake. Atherosclerosis. 2016;253:262–264. doi: 10.1016/j.atherosclerosis.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 118.Schroeder V.A., Mattioli L.F., Kilkenny T.A., Belmont J.M. Effects of lactose-containing vs lactose-free infant formula on postprandial superior mesenteric artery flow in term infants. JPEN J. Parenter. Enteral Nutr. 2014;38:236–242. doi: 10.1177/0148607113478442. [DOI] [PubMed] [Google Scholar]
- 119.USDA ERS . Table High Fructose Corn Syrup: Estimated Number of Per Capita Calories Consumed Daily by Calendar Year. USDA ERS; Washington, DC, USA: 2016. [Google Scholar]
- 120.Russell D., Parnell W., Wilson N., Faed J., Ferguson E., Herbison P., Horwath C., Nye T., Reid P., Walker R. NZ Food: NZ People. Key Results of the 1997 National Nutrition Survey. Ministry of Health; Wellington, New Zealand: 1999. p. 71. [Google Scholar]
- 121.Spencer R., Gearry R., Pearson J., Skidmore P. Relationship between fructose and lactose intakes and functional gastrointestinal symptoms in a sample of 50-year-old cantabrians in New Zealand. NZ Med. J. 2014;127:39. [PubMed] [Google Scholar]
- 122.Gould G.W., Thomas H.M., Jess T.J., Bell G.I. Expression of human glucose transporters in xenopus oocytes: Kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry. 1991;30:5139–5145. doi: 10.1021/bi00235a004. [DOI] [PubMed] [Google Scholar]
- 123.Burant C.F., Takeda J., Brot-Laroche E., Bell G.I., Davidson N.O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 1992;267:14523–14526. [PubMed] [Google Scholar]
- 124.Douard V., Ferraris R.P. Regulation of the Fructose Transporter GLUT5 in Health and Disease. Am. J. Physiol. Endocrinol. Metab. 2008;295:E227–E237. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berni Canani R., Pezzella V., Amoroso A., Cozzolino T., Di Scala C., Passariello A. Diagnosing and treating intolerance to carbohydrates in children. Nutrients. 2016;8:157. doi: 10.3390/nu8030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kolderup A., Svihus B. Fructose metabolism and relation to atherosclerosis, type 2 diabetes, and obesity. J. Nutr. Metab. 2015;2015:823081. doi: 10.1155/2015/823081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L., Hatcher B., Cox C.L., Dyachenko A., Zhang W., et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dotimas J.R., Lee A.W., Schmider A.B., Carroll S.H., Shah A., Bilen J., Elliott K.R., Myers R.B., Soberman R.J., Yoshioka J. Diabetes Regulates Fructose Absorption through Thioredoxin-Interacting Protein. eLife. 2016;5:e18313. doi: 10.7554/eLife.18313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X.Q., Nigro P., World C., Fujiwara K., Yan C., Berk B.C. Thioredoxin Interacting Protein Promotes Endothelial Cell Inflammation in Response to Disturbed Flow by Increasing Leukocyte Adhesion and Repressing Kruppel-Like Factor. Circ. Res. 2012;110:560–568. doi: 10.1161/CIRCRESAHA.111.256362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Y., Lian K., Zhang L., Wang R., Yi F., Gao C., Xin C., Zhu D., Li Y., Yan W. TXNIP Mediates NLRP3 Inflammasome Activation in Cardiac Microvascular Endothelial Cells as a Novel Mechanism in Myocardial Ischemia/Reperfusion Injury. Basic Res. Cardiol. 2014;109:1–14. doi: 10.1007/s00395-014-0415-z. [DOI] [PubMed] [Google Scholar]
- 131.Park M., Kim D., Lim S., Choi J., Kim J., Yoon K., Lee J., Lee J., Han H., Choi I. Thioredoxin-Interacting Protein Mediates Hepatic Lipogenesis and Inflammation Via PRMT1 and PGC-1α Regulation in Vitro and in Vivo. J. Hepatol. 2014;61:1151–1157. doi: 10.1016/j.jhep.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 132.Zhang X., Zhang J., Chen X., Hu Q., Wang M., Jin R., Zhang Q., Wang W., Wang R., Kang L. Reactive Oxygen Species-Induced TXNIP Drives Fructose-Mediated Hepatic Inflammation and Lipid Accumulation through NLRP3 Inflammasome Activation. Antioxid. Redox Signal. 2015;22:848–870. doi: 10.1089/ars.2014.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kelleher Z.T., Sha Y., Foster M.W., Foster W.M., Forrester M.T., Marshall H.E. Thioredoxin-Mediated Denitrosylation Regulates Cytokine-Induced Nuclear Factor kappaB (NF-kappaB) Activation. J. Biol. Chem. 2014;289:3066–3072. doi: 10.1074/jbc.M113.503938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takahashi Y., Ishii Y., Murata A., Nagata T., Asai S. Localization of Thioredoxin-Interacting Protein (TXNIP) mRNA in Epithelium of Human Gastrointestinal Tract. J. Histochem. Cytochem. 2003;51:973–976. doi: 10.1177/002215540305100713. [DOI] [PubMed] [Google Scholar]
- 135.Takahashi Y., Masuda H., Ishii Y., Nishida Y., Kobayashi M., Asai S. Decreased Expression of Thioredoxin Interacting Protein mRNA in Inflamed Colonic Mucosa in Patients with Ulcerative Colitis. Oncol. Rep. 2007;18:531–536. doi: 10.3892/or.18.3.531. [DOI] [PubMed] [Google Scholar]
- 136.Billiet L., Furman C., Cuaz-Pérolin C., Paumelle R., Raymondjean M., Simmet T., Rouis M. Thioredoxin-1 and its Natural Inhibitor, Vitamin D 3 Up-Regulated Protein 1, are Differentially Regulated by PPARα in Human Macrophages. J. Mol. Biol. 2008;384:564–576. doi: 10.1016/j.jmb.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 137.Chung J.W., JEON J., YOON S., Choi I. Vitamin D3 Upregulated Protein 1 (VDUP1) is a Regulator for Redox Signaling and Stress-mediated Diseases. J. Dermatol. 2006;33:662–669. doi: 10.1111/j.1346-8138.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 138.Fedewa A., Rao S.S. Dietary Fructose Intolerance, Fructan Intolerance and FODMAPs. Curr. Gastroenterol. Rep. 2014;16:1–8. doi: 10.1007/s11894-013-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Choi Y.K., Johlin F.C., Summers R.W., Jackson M., Rao S.S. Fructose Intolerance: An Under-Recognized Problem. Am. J. Gastroenterol. 2003;98:1348–1353. doi: 10.1111/j.1572-0241.2003.07476.x. [DOI] [PubMed] [Google Scholar]
- 140.Choi Y.K., Kraft N., Zimmerman B., Jackson M., Rao S.S. Fructose Intolerance in IBS and Utility of Fructose-Restricted Diet. J. Clin. Gastroenterol. 2008;42:233–238. doi: 10.1097/MCG.0b013e31802cbc2f. [DOI] [PubMed] [Google Scholar]
- 141.Buzas G.M. Fructose and Fructose Intolerance. Orv. Hetil. 2016;157:1708–1716. doi: 10.1556/650.2016.30567. [DOI] [PubMed] [Google Scholar]
- 142.Rao S.S., Attaluri A., Anderson L., Stumbo P. Ability of the Normal Human Small Intestine to Absorb Fructose: Evaluation by Breath Testing. Clin. Gastroenterol. Hepatol. 2007;5:959–963. doi: 10.1016/j.cgh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yao C.K., Tuck C.J., Barrett J.S., Canale K.E., Philpott H.L., Gibson P.R. Poor Reproducibility of Breath Hydrogen Testing: Implications for its Application in Functional Bowel Disorders. United Eur. Gastroenterol. J. 2017;5:284–292. doi: 10.1177/2050640616657978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rezaie A., Buresi M., Lembo A., Lin H., McCallum R., Rao S., Schmulson M., Valdovinos M., Zakko S., Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017;112:775. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Campbell B., Han D.Y., Triggs C.M., Fraser A.G., Ferguson L.R. Brassicaceae: Nutrient Analysis and Investigation of Tolerability in People with Crohn’s Disease in a New Zealand Study. Funct. Foods Health Dis. 2012;2:460–486. doi: 10.31989/ffhd.v2i11.70. [DOI] [Google Scholar]
- 146.Laughlin M. Normal Roles for Dietary Fructose in Carbohydrate Metabolism. Nutrients. 2014;6:3117–3129. doi: 10.3390/nu6083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neu J., Rushing J. Cesarean Versus Vaginal Delivery: Long-Term Infant Outcomes and the Hygiene Hypothesis. Clin. Perinatol. 2011;38:321–331. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota Across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stinson L.F., Payne M.S., Keelan J.A. A Critical Review of the Bacterial Baptism Hypothesis and the Impact of Caesarean Delivery on the Infant Microbiome. Front. Med. 2018;5:135. doi: 10.3389/fmed.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Donovan S.M., Wang M., Li M., Friedberg I., Schwartz S.L., Chapkin R.S. Host-Microbe Interactions in the Neonatal Intestine: Role of Human Milk Oligosaccharides. Adv. Nutr. 2012;3:450S–455S. doi: 10.3945/an.112.001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu S., Tao N., German J.B., Grimm R., Lebrilla C.B. Development of an Annotated Library of Neutral Human Milk Oligosaccharides. J. Proteome Res. 2010;9:4138–4151. doi: 10.1021/pr100362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lönnerdal B. Bioactive Proteins in Breast Milk. J. Paediatr. Child Health. 2013;49:1–7. doi: 10.1111/jpc.12104. [DOI] [PubMed] [Google Scholar]
- 153.Andreas N.J., Kampmann B., Le-Doare K.M. Human Breast Milk: A Review on its Composition and Bioactivity. Early Hum. Dev. 2015;91:629–635. doi: 10.1016/j.earlhumdev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 154.Abou-Donia M.B., El-Masry E.M., Abdel-Rahman A.A., McLendon R.E., Schiffman S.S. Splenda Alters Gut Microflora and Increases Intestinal P-Glycoprotein and Cytochrome P-450 in Male Rats. J. Toxicol. Environ. Health A. 2008;71:1415–1429. doi: 10.1080/15287390802328630. [DOI] [PubMed] [Google Scholar]
- 155.Mattes R.D., Popkin B.M. Nonnutritive Sweetener Consumption in Humans: Effects on Appetite and Food Intake and their Putative Mechanisms. Am. J. Clin. Nutr. 2009;89:1–14. doi: 10.3945/ajcn.2008.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sylvetsky A., Rother K.I., Brown R. Artificial Sweetener use among Children: Epidemiology, Recommendations, Metabolic Outcomes, and Future Directions. Pediatr. Clin. North Am. 2011;58:1467–1480. doi: 10.1016/j.pcl.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sylvetsky A.C., Welsh J.A., Brown R.J., Vos M.B. Low-Calorie Sweetener Consumption is Increasing in the United States. Am. J. Clin. Nutr. 2012;96:640–646. doi: 10.3945/ajcn.112.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bianchi R.G., Muir E.T., Cook D.L., Nutting E.F. The Biological Properties of Aspartame. II. Actions Involving the Gastrointestinal System. J. Environ. Pathol. Toxicol. 1980;3:355–362. [PubMed] [Google Scholar]
- 159.Spencer M., Gupta A., Dam L.V., Shannon C., Menees S., Chey W.D. Artificial Sweeteners: A Systematic Review and Primer for Gastroenterologists. J. Neurogastroenterol. Motil. 2016;22:168–180. doi: 10.5056/jnm15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 161.Prashant G., Patil R.B., Nagaraj T., Patel V.B. The Antimicrobial Activity of the Three Commercially Available Intense Sweeteners Against Common Periodontal Pathogens: An in Vitro Study. J. Contemp. Dent. Pract. 2012;13:749–752. doi: 10.5005/jp-journals-10024-1222. [DOI] [PubMed] [Google Scholar]
- 162.Brusick D., Borzelleca J.F., Gallo M., Williams G., Kille J., Hayes A.W., Pi-Sunyer F.X., Williams C., Burks W. Expert Panel Report on a Study of Splenda in Male Rats. Regul. Toxicol. Pharmacol. 2009;55:6–12. doi: 10.1016/j.yrtph.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 163.Schiffman S.S. Rationale for further Medical and Health Research on High-Potency Sweeteners. Chem. Senses. 2012;37:671–679. doi: 10.1093/chemse/bjs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Suez J., Korem T., Zilberman-Schapira G., Segal E., Elinav E. Non-Caloric Artificial Sweeteners and the Microbiome: Findings and Challenges. Gut Microbes. 2015;6:149–155. doi: 10.1080/19490976.2015.1017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bian X., Chi L., Gao B., Tu P., Ru H., Lu K. The Artificial Sweetener Acesulfame Potassium Affects the Gut Microbiome and Body Weight Gain in CD-1 Mice. PLoS ONE. 2017;12:e0178426. doi: 10.1371/journal.pone.0178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chassaing B., Koren O., Goodrich J.K., Poole A.C., Srinivasan S., Ley R.E., Gewirtz A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature. 2015;519:92. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zinöcker M., Lindseth I. The Western Diet–microbiome-Host Interaction and its Role in Metabolic Disease. Nutrients. 2018;10:365. doi: 10.3390/nu10030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wright G.D. The Antibiotic Resistome: The Nexus of Chemical and Genetic Diversity. Nat. Rev. Microbiol. 2007;5:175–186. doi: 10.1038/nrmicro1614. [DOI] [PubMed] [Google Scholar]
- 169.United States Food and Drug Administration . Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. United States Food and Drug Administration; Silver Spring, MD, USA: 2009. [Google Scholar]
- 170.Kedgley S. Parliament, press release. Scoop Independent News. Apr 11, 2011.
- 171.Ministry for Primary Industries NZ . Antbiotic Resistance and Food. Ministry for Primary Industries NZ; Wellington, New Zealand: 2013. [Google Scholar]
- 172.Ruiz-Garbajosa P., Bonten M.J., Robinson D.A., Top J., Nallapareddy S.R., Torres C., Coque T.M., Cantón R., Baquero F., Murray B.E. Multilocus Sequence Typing Scheme for Enterococcus Faecalis Reveals Hospital-Adapted Genetic Complexes in a Background of High Rates of Recombination. J. Clin. Microbiol. 2006;44:2220–2228. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pray L., Pillsbury L., Tomayko E. The Human Microbiome, Diet and Health Workshop Summary. The National Academies Press; Washington, DC, USA: 2012. pp. 5–7. [Google Scholar]
- 174.Silbergeld E.K., Graham J., Price L.B. Industrial Food Animal Production, Antimicrobial Resistance, and Human Health. Annu. Rev. Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- 175.Lindsey R.L., Frye J.G., Thitaram S.N., Meinersmann R.J., Fedorka-Cray P.J., Englen M.D. Characterization of Multidrug-Resistant Escherichia Coli by Antimicrobial Resistance Profiles, Plasmid Replicon Typing, and Pulsed-Field Gel Electrophoresis. Microb. Drug Resist. 2011;17:157–163. doi: 10.1089/mdr.2010.0148. [DOI] [PubMed] [Google Scholar]
- 176.Knight P., Campbell B.J., Rhodes J.M. Host-Bacteria Interaction in Inflammatory Bowel Disease. Br. Med. Bull. 2008;88:95–113. doi: 10.1093/bmb/ldn038. [DOI] [PubMed] [Google Scholar]
- 177.Martin H.M., Campbell B.J., Hart C.A., Mpofu C., Nayar M., Singh R., Englyst H., Williams H.F., Rhodes J.M. Enhanced Escherichia Coli Adherence and Invasion in Crohn’s Disease and Colon Cancer. Gastroenterology. 2004;127:80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 178.Darfeuille-Michaud A., Boudeau J., Bulois P., Neut C., Glasser A., Barnich N., Bringer M., Swidsinski A., Beaugerie L., Colombel J. High Prevalence of Adherent-Invasive Escherichia Coli Associated with Ileal Mucosa in Crohn’s Disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 179.Gearry R.B., Irving P.M., Barrett J.S., Nathan D.M., Shepherd S.J., Gibson P.R. Reduction of Dietary Poorly Absorbed Short-Chain Carbohydrates (FODMAPs) Improves Abdominal Symptoms in Patients with Inflammatory Bowel Disease—A Pilot Study. J. Crohns Colitis. 2009;3:8–14. doi: 10.1016/j.crohns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 180.Barbalho S.M., Goulart R.d.A. Aranão, Ana Luíza de Carvalho; de Oliveira, Pamela Grazielle Correa. Inflammatory Bowel Diseases and Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols: An Overview. J. Med. Food. 2018;21:633–640. doi: 10.1089/jmf.2017.0120. [DOI] [PubMed] [Google Scholar]
- 181.Smith M.A., Smith T., Trebble T.M. Nutritional Management of Adults with Inflammatory Bowel Disease: Practical Lessons from the Available Evidence. Frontline Gastroenterol. 2012;3:172–179. doi: 10.1136/flgastro-2011-100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hill P., Muir J.G., Gibson P.R. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol. Hepatol. 2017;13:36–45. [PMC free article] [PubMed] [Google Scholar]
- 183.Sloan T.J., Jalanka J., Major G.A., Krishnasamy S., Pritchard S., Abdelrazig S., Korpela K., Singh G., Mulvenna C., Hoad C.L. A Low FODMAP Diet is Associated with Changes in the Microbiota and Reduction in Breath Hydrogen but Not Colonic Volume in Healthy Subjects. PloS ONE. 2018;13:e0201410. doi: 10.1371/journal.pone.0201410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mital B., Steinkraus K. Utilization of Oligosaccharides by Lactic Acid Bacteria during Fermentation of Soy Milk. J. Food Sci. 1975;40:114–118. doi: 10.1111/j.1365-2621.1975.tb03749.x. [DOI] [Google Scholar]
- 185.Naessens M., Cerdobbel A., Soetaert W., Vandamme E.J. Leuconostoc Dextransucrase and Dextran: Production, Properties and Applications. J. Chem. Technol. Biotechnol. 2005;80:845–860. doi: 10.1002/jctb.1322. [DOI] [Google Scholar]
- 186.Van den Ende W. Multifunctional Fructans and Raffinose Family Oligosaccharides. Front. Plant Sci. 2013;4:247. doi: 10.3389/fpls.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kuo T.M., VanMiddlesworth J.F., Wolf W.J. Content of Raffinose Oligosaccharides and Sucrose in various Plant Seeds. J. Agric. Food Chem. 1988;36:32–36. doi: 10.1021/jf00079a008. [DOI] [Google Scholar]
- 188.Wang Y., Wang L. Structures and Properties of Commercial Maltodextrins from Corn, Potato, and Rice Starches. Starch-Stärke. 2000;52:296–304. doi: 10.1002/1521-379X(20009)52:8/9<296::AID-STAR296>3.0.CO;2-A. [DOI] [Google Scholar]
- 189.Plaza-Díaz J., Martinez Augustin O., Gil Hernández A. Foods as Sources of Mono and Disaccharides: Biochemical and Metabolic Aspects. Nutr. Hosp. 2013;28:5–16. doi: 10.3305/nh.2013.28.sup4.6792. [DOI] [PubMed] [Google Scholar]
- 190.Calorie Control Council. [(accessed on 5 November 2018)];2018 Polyols. Available online: https://caloriecontrol.org/category/polyols/
- 191.Ingram C.J., Mulcare C.A., Itan Y., Thomas M.G., Swallow D.M. Lactose Digestion and the Evolutionary Genetics of Lactase Persistence. Hum. Genet. 2009;124:579–591. doi: 10.1007/s00439-008-0593-6. [DOI] [PubMed] [Google Scholar]
- 192.Gerbault P., Liebert A., Itan Y., Powell A., Currat M., Burger J., Swallow D.M., Thomas M.G. Evolution of Lactase Persistence: An Example of Human Niche Construction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:863–877. doi: 10.1098/rstb.2010.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brauer H.A., Libby T.E., Mitchell B.L., Li L., Chen C., Randolph T.W., Yasui Y.Y., Lampe J.W., Lampe P.D. Cruciferous Vegetable Supplementation in a Controlled Diet Study Alters the Serum Peptidome in a GSTM1-Genotype Dependent Manner. Nutr. J. 2011;10:11. doi: 10.1186/1475-2891-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kristal A.R., Lampe J.W. Brassica Vegetables and Prostate Cancer Risk: A Review of the Epidemiological Evidence. Nutr. Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 195.Laing B., Han D.Y., Ferguson L.R. Candidate Genes Involved in Beneficial or Adverse Responses to Commonly Eaten Brassica Vegetables in a New Zealand Crohn’s Disease Cohort. Nutrients. 2013;5:5046–5064. doi: 10.3390/nu5125046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Higdon J.V., Delage B., Williams D.E., Dashwood R.H. Cruciferous Vegetables and Human Cancer Risk: Epidemiologic Evidence and Mechanistic Basis. Pharmacol. Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.NIH . Genetics Home Reference. NIH; Stapleton, NY, USA: 2013. Celiac Disease. [Google Scholar]
- 198.Petermann I., Triggs C.M., Huebner C., Han D.Y., Gearry R.B., Barclay M.L., Demmers P.S., McCulloch A., Ferguson L.R. Mushroom Intolerance: A Novel Diet-Gene Interaction in Crohn’s Disease. Br. J. Nutr. 2009;102:506. doi: 10.1017/S0007114509276446. [DOI] [PubMed] [Google Scholar]
- 199.Campbell A.K., Matthews S.B., Vassel N., Cox C.D., Naseem R., Chaich i.J., Holland I.B., Green J., Wann K.T. Bacterial Metabolic ‘Toxins’: A New Mechanism for Lactose and Food Intolerance, and Irritable Bowel Syndrome. Toxicology. 2010;278:268–276. doi: 10.1016/j.tox.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 200.FAO/WHO . Carbohydrates in Human Nutrition. FAO; Rome, Italy: 1998. Report of a Joint FAO/WHO Expert Consultation; FAO Food and Nutrition Paper. [Google Scholar]
- 201.Hyams J.S. Sorbitol Intolerance: An Unappreciated Cause of Functional Gastrointestinal Complaints. Gastroenterology. 1983;84:30–33. [PubMed] [Google Scholar]
- 202.Gibson P.R., Shepherd S.J. Evidence-based Dietary Management of Functional Gastrointestinal Symptoms: The FODMAP Approach. J. Gastroenterol. Hepatol. 2010;25:252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 203.Catassi G., Lionetti E., Gatti S., Catassi C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients. 2017;9:292. doi: 10.3390/nu9030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ley R.E., Peterson D.A., Gordon J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 206.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature. 2006;444:1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 207.Frank D.N., Robertson C.E., Hamm C.M., Kpadeh Z., Zhang T., Chen H., Zhu W., Sartor R.B., Boedeker E.C., Harpaz N. Disease Phenotype and Genotype are Associated with Shifts in Intestinal-Associated Microbiota in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2010;17:179–184. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Walker A.W., Sanderson J.D., Churcher C., Parkes G.C., Hudspith B.N., Rayment N., Brostoff J., Parkhill J., Dougan G., Petrovska L. High-Throughput Clone Library Analysis of the Mucosa-Associated Microbiota Reveals Dysbiosis and Differences between Inflamed and Non-Inflamed Regions of the Intestine in Inflammatory Bowel Disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nagalingam N.A., Lynch S.V. Role of the Microbiota in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2012;18:968–984. doi: 10.1002/ibd.21866. [DOI] [PubMed] [Google Scholar]
- 211.Wacklin P., Mäkivuokko H., Alakulppi N., Nikkilä J., Tenkanen H., Räbinä J., Partanen J., Aranko K., Mättö J. Secretor Genotype (FUT2 Gene) is Strongly Associated with the Composition of Bifidobacteria in the Human Intestine. PLoS ONE. 2011;6:e20113. doi: 10.1371/journal.pone.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McGovern D.P., Jones M.R., Taylor K.D., Marciante K., Yan X., Dubinsky M., Ippoliti A., Vasiliauskas E., Berel D., Derkowski C. Fucosyltransferase 2 (FUT2) Non-Secretor Status is Associated with Crohn’s Disease. Hum. Mol. Genet. 2010;19:3468–3476. doi: 10.1093/hmg/ddq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miyoshi J., Yajima T., Okamoto S., Matsuoka K., Inoue N., Hisamatsu T., Shimamura K., Nakazawa A., Kanai T., Ogata H. Ectopic Expression of Blood Type Antigens in Inflamed Mucosa with Higher Incidence of FUT2 Secretor Status in Colonic Crohn’s Disease. J. Gastroenterol. 2011;46:1056–1063. doi: 10.1007/s00535-011-0425-7. [DOI] [PubMed] [Google Scholar]
- 214.Rausch P., Rehman A., Künzel S., Häsler R., Ott S.J., Schreiber S., Rosenstiel P., Franke A., Baines J.F. Colonic Mucosa-Associated Microbiota is Influenced by an Interaction of Crohn Disease and FUT2 (Secretor) Genotype. Proc. Natl. Acad. Sci. USA. 2011;108:19030–19035. doi: 10.1073/pnas.1106408108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Salminen S., Gueimonde M. Gut Microbiota in Infants between 6 and 24 Months of Age. Nestle Nutr. Workshop Ser. Pediatr. Program. 2005;56:43–56. doi: 10.1159/000086235. [DOI] [PubMed] [Google Scholar]
- 216.Ebaid H., Hassanein K.A. Comparative Immunomudulating Effects of Five Orally Administrated Bifidobacteria Species in Male Albino Rats. Egypt. J. Biol. 2007;9:14–23. [Google Scholar]
- 217.Festen E.A., Goyette P., Green T., Boucher G., Beauchamp C., Trynka G., Dubois P.C., Lagacé C., Stokkers P.C., Hommes D.W. A Meta-Analysis of Genome-Wide Association Scans Identifies IL18RAP, PTPN2, TAGAP, and PUS10 as Shared Risk Loci for Crohn’s Disease and Celiac Disease. PLoS Genet. 2011;7:e1001283. doi: 10.1371/journal.pgen.1001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Visser R., Barrett K., McCole D. Regulation of Epithelial Barrier Function by Crohn’s Disease Associated Gene PTPN. BMC Proc. 2012;6:O41. doi: 10.1186/1753-6561-6-S4-O41. [DOI] [Google Scholar]
- 219.Armstrong D., Don-Wauchope A.C., Verdu E.F. Testing for Gluten-Related Disorders in Clinical Practice: The Role of Serology in Managing the Spectrum of Gluten Sensitivity. Can. J. Gastroenterol. 2011;25:193–197. doi: 10.1155/2011/642452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mishkin S. Controversies regarding the Role of Dairy-Products in Inflammatory Bowel Disease. Can. J. Gastroenterol. 1994;8:205–212. doi: 10.1155/1994/135687. [DOI] [Google Scholar]
- 221.Truelove S.C. Ulcerative Colitis Provoked by Milk. Br. Med. J. 1961;1:154–160. doi: 10.1136/bmj.1.5220.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wright R., Truelove S.C. A Controlled Therapeutic Trial of various Diets in Ulcerative Colitis. Br. Med. J. 1965;2:138–141. doi: 10.1136/bmj.2.5454.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mishkin S. Dairy Sensitivity, Lactose Malabsorption, and Elimination Diets in Inflammatory Bowel Disease. Am. J. Clin. Nutr. 1997;65:564–567. doi: 10.1093/ajcn/65.2.564. [DOI] [PubMed] [Google Scholar]
- 224.Maconi G., Ardizzone S., Cucino C., Bezzio C., Russo A.G., Bianchi Porro G. Pre-Illness Changes in Dietary Habits and Diet as a Risk Factor for Inflammatory Bowel Disease: A Case-Control Study. World J. Gastroenterol. 2010;16:4297–4304. doi: 10.3748/wjg.v16.i34.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nolan-Clark D., Tapsell L.C., Hu R., Han D.Y., Ferguson L.R. Effects of Dairy Products on Crohn’s Disease Symptoms are Influenced by Fat Content and Disease Location but Not Lactose Content or Disease Activity Status in a New Zealand Population. J. Am. Diet. Assoc. 2011;111:1165–1172. doi: 10.1016/j.jada.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 226.Nolan D., Han D., Lam W., Morgan A., Fraser A., Tapsell L., Ferguson L. Genetic Adult Lactase Persistence is Associated with Risk of Crohn’s Disease in a New Zealand Population. BMC Res. Notes. 2010;3:339. doi: 10.1186/1756-0500-3-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shrier I., Szilagyi A., Correa J.A. Impact of Lactose Containing Foods and the Genetics of Lactase on Diseases: An Analytical Review of Population Data. Nutr. Cancer. 2008;60:292–300. doi: 10.1080/01635580701745301. [DOI] [PubMed] [Google Scholar]
- 228.Barbado M., Fablet K., Ronjat M., De Waard M. Gene Regulation by Voltage-Dependent Calcium Channels. Biochim. Biophys. Acta. 2009;1793:1096–1104. doi: 10.1016/j.bbamcr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 229.Bernstein C.N., Leslie W.D., Leboff M.S. AGA Technical Review on Osteoporosis in Gastrointestinal Diseases. Gastroenterology. 2003;124:795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 230.Caniggia A., Nuti R., Lore F., Vattimo A. Pathophysiology of the Adverse Effects of Glucoactive Corticosteroids on Calcium Metabolism in Man. J. Steroid Biochem. 1981;15:153–161. doi: 10.1016/0022-4731(81)90270-3. [DOI] [PubMed] [Google Scholar]
- 231.New Zealand Nutrition Foundation. [(accessed on 15 June 2019)];2018 Calcium. Available online: https://nutritionfoundation.org.nz/nutrients-vitamins-and-minerals/calcium.
- 232.Burlingame B.A., Milligan G., Apimerika D., Arthur J. The Concise New Zealand Food Composition Tables. New Zealand Institute for Crop & Food Research; Auckland, New Zealand: 2009. [Google Scholar]
- 233.Duke J., Beckstrom-Sternberg S. Phytochemical Database USDA-ARS-NGRL. Beltsbille Agricultural Research Center; Beltsbille, MD, USA: 1998. [Google Scholar]
- 234.Parl F.F. Glutathione S-Transferase Genotypes and Cancer Risk. Cancer Lett. 2005;221:123–129. doi: 10.1016/j.canlet.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 235.Beckett G.J., Hayes J.D. Glutathione S-Transferases: Biomedical Applications. Adv. Clin. Chem. 1993;30:282. doi: 10.1016/s0065-2423(08)60198-5. [DOI] [PubMed] [Google Scholar]
- 236.Wilce M.C., Parker M.W. Structure and Function of Glutathione S-Transferases. Biochim. Biophys. Acta. 1994;1205:1–18. doi: 10.1016/0167-4838(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 237.Webb G., Vaska V., Coggan M., Board P. Chromosomal Localization of the Gene for the Human Theta Class Glutathione Transferase (GSTT1) Genomics. 1996;33:121–123. doi: 10.1006/geno.1996.0167. [DOI] [PubMed] [Google Scholar]
- 238.Sheehan D., Meade G., Foley V., Dowd C. Structure, Function and Evolution of Glutathione Transferases: Implications for Classification of Non-Mammalian Members of an Ancient Enzyme Superfamily. Biochem. J. 2001;360:1–16. doi: 10.1042/bj3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hayes J.D., Strange R.C. Glutathione S-Transferase Polymorphisms and their Biological Consequences. Pharmacology. 2000;61:154–166. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- 240.Inskip A., Elexperu-Camiruaga J., Buxton N., Dias P., MacIntosh J., Campbell D., Jones P., Yengi L., Talbot J., Strange R. Identification of Polymorphism at the Glutathione S-Transferase, GSTM3 Locus: Evidence for Linkage with GSTM1* A. Biochem. J. 1995;312:713. doi: 10.1042/bj3120713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Seow A., Yuan J., Sun C., Van Den Berg D., Lee H., Mimi C.Y. Dietary Isothiocyanates, Glutathione S-Transferase Polymorphisms and Colorectal Cancer Risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23:2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 242.Steck S.E., Hebert J.R. GST Polymorphism and Excretion of Heterocyclic Aromatic Amine and Isothiocyanate Metabolites After Brassica Consumption. Environ. Mol. Mutagen. 2009;50:238–246. doi: 10.1002/em.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Campbell B., Han D., Jiun W., Morgan A., Triggs C., Fraser A.C., Ferguson L.R. Deletion of the GSTT1 Genotype Linked to Tolerance of Brassicaceae in People with Crohn’s Disease in a New Zealand Cohort; Proceedings of the Annual Scientific Meeting of the Nutrition Society of New Zealand Auckland; Auckland, New Zealand. 21–22 November 2012. [Google Scholar]
- 244.Gentschew L., Bishop K.S., Han D.Y., Morgan A.R., Fraser A.G., Lam W.J., Karunasinghe N., Campbell B., Ferguson L.R. Selenium, Selenoprotein Genes and Crohn’s Disease in a Case-Control Population from Auckland, New Zealand. Nutrients. 2012;4:1247–1259. doi: 10.3390/nu4091247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ahmad T., Marshall S.E., Jewell D. Genetics of Inflammatory Bowel Disease: The Role of the HLA Complex. World J. Gastroenterol. 2006;12:3628. doi: 10.3748/wjg.v12.i23.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lees C.W., Satsangi J. Genetics of Inflammatory Bowel Disease: Implications for Disease Pathogenesis and Natural History. Expert Rev. Gastroenterol. Hepatol. 2009;3:513–534. doi: 10.1586/egh.09.45. [DOI] [PubMed] [Google Scholar]
- 247.Annese V., Piepoli A., Latiano A., Lombardi G., Napolitano G., Caruso N., Cocchiara E., Accadia L., Perri F., Andriulli A. HLA-DRB1 Alleles may Influence Disease Phenotype in Patients with Inflammatory Bowel Disease: A Critical Reappraisal with Review of the Literature. Dis. Colon. Rectum. 2005;48:57–65. doi: 10.1007/s10350-004-0747-0. [DOI] [PubMed] [Google Scholar]
- 248.Moffatt M.F., Gut I.G., Demenais F., Strachan D.P., Bouzigon E., Heath S., Von Mutius E., Farrall M., Lathrop M., Cookson W.O. A Large-Scale, Consortium-Based Genomewide Association Study of Asthma. N. Engl. J. Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Levin A., Mathias R., Huang L.R., Roth L.A., Daley D., Myers R.A., Himes B.E., Romieu I., Yang M., Eng C., et al. A Meta-Analysis of Genome Wide Association Studies for Serum Total IgE in Diverse Populations Groups. American Academy of Allergy, Asthma and Immunology; Milwaukee, WI, USA: 2013. p. 1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gould H.J., Sutton B.J., Beavil A.J., Beavil R.L., McCloskey N., Coker H.A., Fear D., Smurthwaite L. The Biology of IgE and the Basis of Allergic Disease. Annu. Rev. Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 251.Marlow G., Han D.Y., Triggs C.M., Ferguson L.R. Food Intolerance: Associations with the rs12212067 Polymorphism of FOXO3 in Crohn’s Disease Patients in New Zealand. J. Nutrigen. Nutrigen. 2015;8:70–80. doi: 10.1159/000435783. [DOI] [PubMed] [Google Scholar]
- 252.Food Safety Authority of Ireland Food Ingredients that must be Declared as Allergens in the EU. [(accessed on 2 February 2019)]; Available online: https://www. fsai. ie/legislation/food_legislation/food_information/14_allergens.html.
- 253.Wedzicha B.L. Chemistry of Sulphur Dioxide in Foods. Elsevier Applied Science Publishers Ltd.; Amsterdam, The Netherlands: 1984. [Google Scholar]
- 254.Coates P.M., Blackman M., Betz J., Cragg G.M., Levine M., Moss J., White J.D. Encyclopedia of Dietary Supplements. Informa Healthcare; London, UK: 2010. [Google Scholar]
- 255.Costantini A., Pala M.I. Thiamine and Fatigue in Inflammatory Bowel Diseases: An Open-Label Pilot Study. J. Altern. Complement. Med. 2013;19:704–708. doi: 10.1089/acm.2011.0840. [DOI] [PubMed] [Google Scholar]
- 256.Magee E.A., Edmond L.M., Tasker S.M., Kong S.C., Curno R., Cummings J.H. Associations between Diet and Disease Activity in Ulcerative Colitis Patients using a Novel Method of Data Analysis. Nutr. J. 2005;4:7. doi: 10.1186/1475-2891-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pitcher M.C., Cummings J.H. Hydrogen Sulphide: A Bacterial Toxin in Ulcerative Colitis? Gut. 1996;39:1–4. doi: 10.1136/gut.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Marcus R., Watt J. Seaweeds and Ulcerative Colitis in Laboratory Animals. Lancet. 1969;2:489–490. doi: 10.1016/S0140-6736(69)90187-1. [DOI] [PubMed] [Google Scholar]
- 259.Marcus R., Watt J. Ulcerative Disease of the Colon in Laboratory Animals Induced by Pepsin Inhibitors. Gastroenterology. 1974;67:473–483. doi: 10.1016/S0016-5085(19)32849-5. [DOI] [PubMed] [Google Scholar]
- 260.Roediger W. The Colonic Epithelium in Ulcerative Colitis: An Energy-Deficiency Disease? Lancet. 1980;316:712–715. doi: 10.1016/S0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- 261.Peng L., Li Z., Green R.S., Holzman I.R., Lin J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly Via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Carbonero F., Benefiel A.C., Alizadeh-Ghamsari A.H., Gaskins H.R. Microbial Pathways in Colonic Sulfur Metabolism and Links with Health and Disease. Front. Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Berezowska A., Fischer A.R., Ronteltap A., Lans I.A., Trijp H.C. Consumer Adoption of Personalised Nutrition Services from the Perspective of a Risk–benefit Trade-Off. Genes Nutr. 2015;10:42. doi: 10.1007/s12263-015-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nutrisearch Fitgenes (DNA Testing) [(accessed on 13 June 2018)];2018 Available online: https://www.nutrisearch.co.nz/lab-diagnostics/fitgenes-dna-testing/
- 265.Zettler P.J., Sherkow J.S., Greely H.T. 23 and Me, the Food and Drug Administration, and the Future of Genetic Testing. JAMA Intern. Med. 2014;174:493–494. doi: 10.1001/jamainternmed.2013.14706. [DOI] [PubMed] [Google Scholar]
- 266.Annas G.J., Elias S. 23andMe and the FDA. N. Engl. J. Med. 2014;370:985–988. doi: 10.1056/NEJMp1316367. [DOI] [PubMed] [Google Scholar]


