Abstract
Protein supplementation is a major nutritional practice among professional and amateur team-sport athletes representing a market of $5 billion in the USA alone. This practice, however, may not be supported by evidence-based science. Our objective as to present a thorough review of literature investigating the effects of protein supplementation on performance recovery and exercise-induced muscle damage following team-sport activity. PubMed-derived, full English language articles investigating the effects of protein-based supplementation/feeding on skeletal muscle performance, muscle damage and inflammatory status during recovery following team-sport activity were included. Studies investigated professional or amateur team-sport athletes participating in regular training and competition as well as examining the impact of protein supplementation on performance, muscle damage/soreness and inflammatory markers after team-sport activity. Finally, ten articles (150 participants) met the inclusion criteria. Experimental designs were evaluated for confounders. All protocols employing team-sport activity increased systemic muscle damage indicators and inflammatory markers and deteriorated performance during recovery. Protein-based supplementation attenuated the rise in muscle damage markers and enhanced performance recovery in six (60% of the studies included) and three (30% of the studies included) out of 10 studies, respectively. In contrast, immunity and muscle soreness remained unaffected by protein ingestion, independent of dosage and distribution pattern. In conclusion, there are limited and inconsistent data showing that protein supplementation may enhance performance recovery following team-sport activity despite an attenuation of indirect markers of muscle damage. Interpretation of results is limited by small sample sizes, high variability in tested supplements, participants’ training level, length of recovery periods, absence of direct measurement of myofibrillar disruption, protein turnover and protein metabolism, and lack of dietary monitoring during experimentation.
Key points.
Protein supplements facilitate muscle repair and mitigate muscle damage markers during recovery.
Protein supplementation attenuate inflammatory responses, however it is not combined with enhanced performance measures
The lack of direct markers to determine EIMD, makes it clear that future researches should incorporate muscle protein synthesis and breakdown measurements.
Key words: Nutrition, exercise performance, muscle damage, amino acids, anabolism, supplementation
Introduction
Team sports, such as soccer, basketball, rugby, and handball, are intermittent-type activities characterized by a high number of repetitive high-intensity efforts interspersed with low-intensity actions or rest (Mohr et al., 2003; Narazaki et al., 2009; Póvoas et al., 2017). For instance, most team sports matches include 150-400 high-intensity movement patterns mostly consisting of running, sprinting, jumping, acceleration, deceleration, changes of direction and various sport-specific actions such as tackling, shuffling and throwing (Mohr et al., 2003; Narazaki et al., 2009; Póvoas et al., 2017). All these actions though, incorporate a strong eccentric component associated with exercise-induced muscle damage (EIMD) that results in an acute inflammatory response and performance deterioration for as long as 24-72 h (Ispirlidis et al., 2008; Fatouros et al., 2010; Chatzinikolaou et al., 2014a; 2014b; Draganidis et al., 2015; Mohr et al., 2016).
Characteristically, muscle damage induced by match-play results in marked deterioration of strength (concentric and eccentric force of knee flexors and extensors), lower limb muscle power (jumping, speed, agility), and repeated sprint ability (Ispirlidis et al., 2008; Fatouros et al., 2010; Chatzinikolaou et al., 2014a; 2014b; Draganidis et al., 2015; Mohr et al., 2016). This performance deterioration is accompanied by a rise in delayed onset of muscle soreness (DOMS), immune system activation, inflammatory response and EIMD markers such as creatine kinase activity (CK) and C-reactive protein (CRP), pro-inflammatory cytokines, adhesion molecules and oxidative stress markers (Ispirlidis et al., 2008; Fatouros et al., 2010; Chatzinikolaou et al., 2014a; 2014b; Draganidis et al., 2015; Mohr et al., 2016). However, professional athletes in these sports follow a congested match schedule of three matches/week and daily practices (> 50 matches annually) that allows them only a 3- or 4-day recovery period between successive matches. Research indicates that this time may be inadequate to restore normal homeostasis resulting in prolonged performance deterioration and increased likelihood for occurrence of musculoskeletal injuries (Ekstand et al., 2004; Montgomery et al., 2008; Dupont et al., 2010). Therefore, increased attention has been focused on recovery strategies able to treat symptoms of EIMD and to restore muscles’ function. These strategies are applied as either lifestyle (e.g., sleep), exercise (e.g., active recovery), physiological (e.g., cooling, massage, compression), nutritional [e.g. protein supplements, carbohydrate (CHO) feeding], or pharmacological interventions (e.g. anti-inflammatory medications) aiming to blunt the inflammatory response, enhance muscle regeneration and thus overall performance recovery (Minett and Costello, 2015).
Amongst nutritional strategies, protein supplements are widely used by athletes and physically active individuals to increase their muscle mass and enhance post-exercise recovery and performance, representing up to 70% of the sport supplement industry (5 billion dollars) (Petroczi and Naughton, 2008; Pasiakos et al., 2013; Draganidis et al., 2017). These consumers have been convinced that protein supplements will offset EIMD, facilitate skeletal muscle repair and contribute to an upregulated glycogen re-synthesis when co-administered with CHO during recovery (Pasiakos et al., 2014). Indeed, numerous studies have addressed this proof of concept (Cockburn et al., 2008; 2010; 2013; Cooke et al., 2010; Shenoy et al., 2016) providing evidence that protein-based supplementation following acute damaging exercise protocols, attenuates the decrease in muscle performance, mitigates the rise in DOMS and muscle damage markers such as CK, myoglobin (Mb) and lactate dehydrogenase, and enhances muscle regeneration and remodeling process by increasing the proliferation of satellite cells (Farup et al., 2014) during recovery. This protein-mediated effect is primarily attributed to the fact that protein supplementation during recovery increases the rate of muscle protein synthesis (Lunn et al., 2012; Breen et al., 2011) and as such facilitates muscle repair and remodeling (Breen et al., 2011) and accelerates performance recovery (Saunders, 2007). In the absence of protein or insufficient feeding following damaging exercise, EIMD accelerates muscle protein turnover by upregulating both its synthesis and degradation (Draganidis et al., 2017; Pitkanen et al., 2003), however, degradation increases over synthesis thereby resulting in a negative protein balance (Koopman et al., 2005).
An earlier systematic review on the effects of protein supplementation on recovery from endurance exercise concluded that protein supplements may promote protein synthesis acutely but no meaningful improvement in EIMD and performance recovery has been observed (Pasiakos et al., 2014). Team sports, on the other hand, such as soccer, basketball, and handball are probably the most popular sports worldwide attracting the largest number of professional and amateur participants and have a unique activity profile which is associated with a specific pattern of EIMD and recovery distinguishing them from other athletic activities such as running, biking, resistance training etc. (Ispirlidis et al., 2008; Fatouros et al., 2010; Chatzinikolaou et al., 2014a; 2014b; Draganidis et al., 2015; Mohr et al., 2016). However, a systematic review of the available evidence that supports or refutes the use of protein supplements as a post-match recovery strategy in these sports is lacking. Therefore, this paper reviews all available investigations that examined the effects of protein-based supplementation on EIMD markers and performance restoration following team sport activity.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were applied for this review.
Search strategy
To review whether protein supplementation affects recovery following team sport match or training activity, a search was performed with no date restriction up to 2018 in PubMed to identify relevant peer-reviewed articles. Key-terms were grouped and searched within the article title, abstract, and keywords using the conjunctions ‘OR’ and ‘AND’. The terms that were used in the search were: “Protein”, “exercise-induced muscle damage”, “exercise-induced inflammation”, “recovery”, “redox status”, “glutathione”, “casein’, “cysteine”, “whey protein”, “soy protein”, “team sports”, “soccer”, “basketball”, and “team handball”. Reviews previously published were also screened for similar headings and key-words. Search was limited to articles published in the English language and studies that utilized protein supplementation/feeding during recovery following match-play. References of these articles were also searched to find potential relevant articles.
Study selection, inclusion and exclusion
Articles were included if: 1) they involved healthy, non-smoking adults (18-40 years) classified as professional, semi-professional or amateur athletes (training experience ≥2 years) who did not consume performance-enhancing supplements and medications; 2) athletes participated in ≥3 training sessions/week and played at least one match/week; 3) participants had a baseline dietary protein consumption of ≥0.8 g/kg/day; 4) examined the effects of protein supplementation on ≥1 muscle damage, inflammatory, and performance markers following match and/or training activity for ≥2 h of recovery; 5) used a single- or double-blind, repeated measures design; 6) protein supplementation was utilized before and/or immediately after match or training activity and throughout recovery; and 6) examined the effects of either protein supplementation/feeding alone or in combination with CHO supplementation. Articles were excluded if they: 1) involved animals, youth (<18 years) or adults ≥40 years, and non-team sport athletes 2) allowed participation to smokers; 3) used dietary manipulation (unclear supplement or dietary protocol); 5) articles were not accessible (no full text); 6) measurement of study’s outcomes was based on questionnaires; 7) the intervention did not contain acute match-play or organized training or simulated play (training studies were not included); 8) the article did not contain original data (e.g., review). Articles were evaluated in detail, i.e. investigators searched for potential confounders, methodological flaws and issues that could have affected their dependent variables (e.g. supplements’ energy content, dietary monitoring, participants’ conditioning level etc.). Two independent reviewers (AP, DD), screened all abstracts and selected those for full-text evaluation. Discrepancies were resolved through a consensus process with a third independent reviewer (KG). After consensus on the primary selection, two researchers (AP and DD) evaluated the full-text articles to determine if these studies could be included in the review. When there was doubt, a third independent reviewer was involved to discuss discrepancies (KG).
Data extraction and management
The following data were extracted from the selected studies: study design, participants’ characteristics, the type of exercise or training in which the participants were submitted (match, simulated match, training), the type of protein supplement, performance indicators, and EIMD, oxidative stress, inflammatory and metabolic markers.
Assessment of methodological quality
The reviewers used the Cochrane Collaboration Tool (CCT) to characterize the quality of the selected studies as low, high or unclear risk of bias or applicability (Tables 1 and 2) (Higgins et al., 2011). A study that satisfied all criteria for low risk it was rated with an A. A study that satisfied all criteria for high risk it was rated with a C. A study that satisfied all criteria for unclear it was rated with a D. Studies with mixed criteria were rated with a B.
Table 1.
The Cochrane risk of bias tool for quality assessment as it was implemented in the selected studies.
| Study | Sequence generation |
Allocation concealment |
Blinding of participants, personnel and outcome assessors | Incomplete outcome data |
Selective outcome reporting |
Other sources of bias |
|---|---|---|---|---|---|---|
| Arent et al., 2010 | ? | + | + | ? | ? | + |
| Betts et al., 2009 | ? | ? | ? | + | + | ? |
| Cockburn et al., 2013 | ? | ? | ? | + | + | ? |
| Gentle et al., 2014 | ? | + | + | - | ? | ? |
| Gilson et al., 2010 | + | + | - | + | ? | + |
| Gunnarson et al., 2013 | - | - | ? | ? | - | + |
| Highton et al., 2013 | + | ? | + | + | + | ? |
| Naclerio et al., 2015 | ? | ? | ? | + | ? | - |
| Naclerio et al., 2014 | - | ? | ? | + | ? | ? |
| Poulios et al. 2018 | ? | ? | + | + | + | ? |
+: Low risk of bias, - : High risk of bias, ? : unclear risk of bias.
Table 2.
Summary of risk of bias of the selected studies.
| Risk of Bias Summarized (%) | |||
|---|---|---|---|
| Low Risk | High Risk | Unclear Risk | |
| Sequence Generation | 22,2 | 22,2 | 55,6 |
| Allocation Concealment | 33,3 | 0 | 66,7 |
| Blinding of participants, personnel & outcome assessors | 33,3 | 11,1 | 55,6 |
| Incomplete outcome data | 66,7 | 11,1 | 22,2 |
| Selective outcome reporting | 33,3 | 11,1 | 55,6 |
| Other sources of bias | 22,2 | 11,1 | 66,7 |
Results
The flowchart of the search is presented in Figure 1. In total, 69 abstracts were screened for eligibility. A total of 44 abstracts were excluded (63.7%), mostly because the study population did not participate in team sports or the exercise protocol did not resemble a team sport (88.6%). Furthermore, five abstracts did not contain original data (11.4%). The full-text examination of the 25 remaining articles excluded 15 (60%) more studies (three studies were not accessible, three studies examined another type of exercise, two studies did not use protein supplementation for recovery, three studies did not include team sports, one study did not contain original data, one investigation utilized questionnaires, and four studies did not use an acute exercise protocol). Consequently, ten studies were analyzed for this systematic review (Arent et al., 2010; Betts et al., 2009; Cockburn et al., 2013; Gentle et al., 2014; Gilson et al., 2010; Gunnarsson et al., 2013; Highton et al., 2013; Naclerio et al., 2015; 2014; Poulios et al., 2018).
Figure 1.
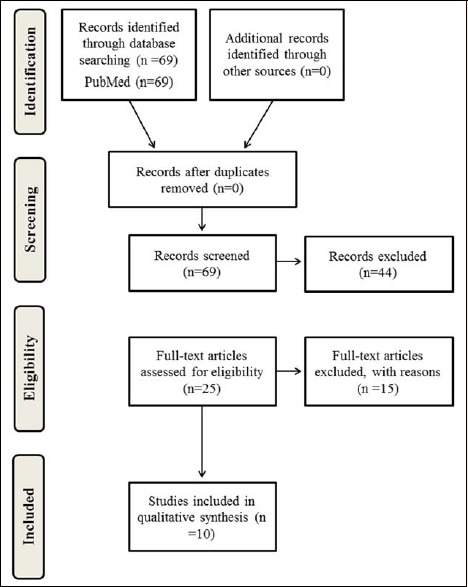
The PRISMA flow chart for systematic reviews.
Quality of the studies
The individual risk of bias in each domain for the reviewed studies are presented in Tables 1 and 2. As indicated, none of the studies was characterized as a “low risk of bias” in all key domains. In contrary, five studies (50%) were classified as at “high risk of bias’’ and five studies (50%) were classified as at “unclear risk of bias” in at least one key domain.
Intervention characteristics
Ten studies were identified in total after a thorough literature search of which seven (70%) utilized a randomized, repeated-measures crossover design and three (30%) used a blinded, two-arm parallel design (Table 3). A placebo-control was included only in four out of the ten studies while five studies utilized CHO as a comparator supplement and one study compared semi-skimmed milk with water. Doses and administration schemes varied considerably among the reviewed investigations (Table 4). Six out of ten studies (60%) examined the efficacy of protein intake on team-sport related performance as well as on muscle damage and inflammatory markers by applying repeated doses of protein during exercise and the subsequent recovery period. Two studies (20%) employed a more prolonged protein supplementation protocol. The effectiveness of a single-dose, protein-based supplementation either before or immediately after acute exercise bouts was investigated by two studies (20%) (Table 3). Supplementation strategies included exclusively oral administration; four studies dealt with concurrent administration of protein and CHO, either in the form of a supplement or as a daily diet, two dealt with protein-CHO multi-ingredients, two with milk supplementation (semi-skimmed milk and low-fat chocolate milk), one with a protein-enriched nutraceutical blend and one study included high protein versus high carbohydrate. The reported amount of protein ingested per intervention ranged from 0.7 g to 1 g/kg body mass, 29 g to 164 g and 28 g/day to 236 g/day when single dose, repeated doses (during exercise and the subsequent recovery period) and long-term supplementation was applied, respectively. Dairy proteins were the predominant source administered in these studies (90%). Another study provided 1,750 mg of branched-chain amino acids (BCAA) as a nutraceutical blend (Table 4).
Table 3.
Intervention characteristics of the selected studies.
| Study | Design | Type of exercise test |
Supplementation | Diet control |
Measurement Time-points |
Performance markers |
Muscle damage markers |
Inflammatory & oxidative stress markers |
|---|---|---|---|---|---|---|---|---|
| Arent et al., 2010 | Blinded, placebo-controlled | Graded maximal treadmill test to exhaustion | Resurgex Plus® vs Isocaloric placebo | 3-day dietary recall, prior to trials | Before & after treadmill test, at the beginning & end of preseason | Lactate threshold, VO2max, time to exhaustion | CK | 8-isoprostane, lipid hydroperoxide |
| Betts et al., 2009 | Single-blind, repeated measures crossover | 90min of high-intensity intermittent shuttle-running | CHO vs CHO+PRO | 2-day dietary recall, prior to trials & 1-day dietary recall post exercise | At baseline and 4h, 24h, 48h & 168h after exercise | Peak isometric torque-flexors & extensors | Myoglobin, CK, LDH, cortisol | IL-6, IL10, IL-1 receptor antagonist, CRP, WBC |
| Cockburn et al., 2013 | Blinded, repeated measures parallel | Unilateral knee flexions (6 sets x 10 reps, 1.05 rad/s-1, 90-s rest) on isokinetic dynamometer | Semi-skimmed milk vs water | Diet recorded throughout the study | At baseline and 24h, 48h and 72h post-exercise | CMV jump height, reactive strength index, 15-m sprint, agility, LIST | Passive & active DOMS, CK, myoglobin | N/A |
| Gentle et al., 2014 | Randomized cross-over | 87-min basketball game stimulation | CHO meal vs CHO+PRO meal | Diet recorded 24 hours prior to the first trial | At baseline, during as well as 30 min and 24h post-exercise | Free throw success, sprint time, jump height | Passive & active DOMS, CK | N/A |
| Gilson et al., 2010 | Double-blind, randomized cross-over | 4-day soccer training period, with increased training duration | CHO vs Low-fat chocolate milk | Diet recorded during the 4-day training period | At baseline, day 2 and day 4 post-ITD training period | MVC, T-drill test, vertical jump | Muscle soreness, CK, myoglobin, | N/A |
| Gunnarsson et al., 2013 | Two-group repeated measures | A competitive soccer match | Normal diet vs high carbohydrate-high protein diet | Recorded over 48h post-match | At baseline and 15 min, 24h and 48h after the match | N/A | CK, myoglobin, muscle glycogen resynthesis | N/A |
| Highton et al., 2013 | Double-blind, randomized cross-over | Modified LIST | CHO vs CHO+PRO | 2-day dietary recall, prior to trials | At baseline, during and immediately after LIST | Distance covered, maximal speed & average running speed during LIST | N/A | N/A |
| Naclerio et al., 2014 | Double-blind, single group repeated measures | 90-min intermittent repeated sprint test | PLA vs CHO vs CHO+PRO multi-ingredient | 3-day dietary recall, prior to trials | At baseline, immediately post, 1h and 24h post-exercise | Total sprint time, 15-m sprint time | CK, myoglobin | IL-6 |
| Naclerio et al., 2015 | Double-blind, repeated measures, counter-balanced, crossover | 90-min intermittent repeated sprint (IRS) test | PLA vs CHO vs CHO+PRO multi-ingredient | 3-day dietary recall, prior to trials | At baseline, immediately post, 1h and 24h post-exercise | Time for 90-min IRS, 15-m sprint test | Myoglobin, CK, | IL-6, Neutrophil, Lymphocytes, Monocyte |
| Poulios et al., 2018 | Randomized, repeated-measures, crossover, double-blind | Two-trial (2 weeks), 2 soccer matches & 4 practices /week | Milk Protein Smooth (PRO) vs isoenergetic placebo (maltodextrin) | Diet recorded Prior to each trial (RMR, REE) 7-day dietary recall during both trials | At baseline, immediately post, 24h, 48h, 72h post -1st match, 24h, 48h, 72h post-2nd match for blood, At baseline 24h, 48h post -1st match, 24h, 48h, 72h post-2nd match for performance testing | 10-m sprint(s), 30-m sprint (s), CMJ (cm), KE strength, KF strength GPS: Average HR, Total distance, Distance at >14 km/h, Peak speed (km/h), Accelerations, Decelerations | CK, DOMS | WBC, Granulocyte, TBARS, PC, GSH, TAC |
CK: Creatine kinase, LDH: Lactate dehydrogenase, CHO: Carbohydrate, PRO: Protein, DOMS: Delayed onset of muscle soreness, LIST: Loughborough Intermittent Shuttle Test, HR: Heart rate, RPE: Ratings of perceived exertion, MVC: Maximal voluntary contraction, PLA: Placebo, RMR: Resting Metabolic Rate, TDEE: Total Daily Energy Expenditure, KE: Knee extensors, KF: Knee Flexors, WBC: White blood, cells, GSH: Reduced glutathione, TBARS: Thiobarbituric acid reactive substances, PC: Protein carbonyls, TAC: Total antioxidant capacity, GPS: global positioning system.
Table 4.
Description of protein and their comparator supplements - dosages and administration schemes of the selected studies.
| Study | Protein Supplement | Comparator Supplement | Administration scheme |
|---|---|---|---|
| Arent et al., 2010 |
Resurgex Plus® 75 mg CoQ10, 500 U SOD/Gliadin, 1,750 mg ornithine ketoglutarate, 300 mg L-Carnitine, 100 mg nucleotides, 750 mg d-ribose, 500 mg L-glutamine, 100 mg beta glucans, 12.5 mg fruit polyphenols, and 1,750 mg BCAA. |
Isocaloric equivalent | Twice daily (morning: 10:00 – 11:00 and evening: 19:00 – 20:00) over a 20-day period |
| Betts et al., 2009 |
Protein – Carbohydrate mixture Total volume: 5.5 ± 0.5 L (relative to BM) Total energy: 10 975 ± 972 kJ (CHO: 492 ± 44g, PRO: 164 ± 15g); Concentration: 9% sucrose (1.2 g/kg-1 BM/h-1) & 3% whey protein isolate (0.4 g/kg-1 BM/h-1). Amino acid profile: 20% glutamine, 11% leucine, 10% asparagine, 9% lysine, 7% proline, 6% threonine, 6% isoleucine, 5% valine, 5% alanine, 21% other amino acids. |
Carbohydrate Total volume: 5.5 ± 0.5 L (relative to BM) Total energy: 8231 ± 729 kJ (CHO: 492 ± 44g) Concentration: 9% sucrose (1.2 g/kg-1 BM/h-1) |
Before exercise (1 x 7.0 ml/kg-1 BM), during exercise (5 x 2.6 ml/ kg-1 BM) and post-exercise (8 x 6.7 ml/ kg-1 BM, every 30min over a 4-hour recovery period). |
| Cockburn et al., 2013 |
Semi-skimmed milk Total volume: 500 ml; Composition: Whey protein, casein carbohydrate (lactose) & fat (1.7%). |
Water Total volume: 500 ml |
A single dose immediately post exercise. |
| Gentle et al., 2014 |
Protein – Carbohydrate meal Meal composition (for a 75 kg individual): Energy: 611 kcals, CHO: 75g (1g/kg-1 BM), Protein: 75g (1g/kg-1 BM), Fat: 2.6g, Fibre: 1g. Food items: 2 slices white bread, 40g Jam, 76g whey protein drink, 190 ml sports drink (Powerade isotonic), 1100ml water. |
Carbohydrate meal Meal composition (for a 75 kg individual): Energy: 635 kcals, CHO: 150g (2g/kg-1 BM), Protein: 6g, Fat: 2g, Fibre: 1g. Food items: 2 slices white bread, 60g Jam, 1290 ml sports drink (Powerade isotonic). |
90 minutes before the exercise protocol. |
| Gilson et al., 2010 |
Low-fat chocolate milk Total volume/serving: 672 ml Macronutrient content/serving: CHO: 84g, PRO: 28g, Fat: 7g – 504 kcal in total. |
Carbohydrate Total volume/serving: 672 ml; Macronutrient content/serving: CHO: 122g, PRO: 0g, Fat: 2g – 504 kcal in total. |
A single dose (672 ml), once a day (following training session) over a 4-day period. |
| Gunnarsson et al., 2013 |
High protein-carbohydrate diet Diet composition over the first 24h: 775 ± 26 g CHO, 229 ± 8 g PRO (whey) and 39 ± 1 g fat. Diet composition over the subsequent 24h: 797 ± 23 g CHO, 236 ± 7 g PRO (whey) and 40 ± 1 g fat. |
Normal diet Diet composition over the first 24h: 392 ± 48 g CHO, 123 ± 12 g PRO and 72 ± 8 g fat. Diet composition over the subsequent 24h: 378 ± 57 g CHO, 120 ± 17 g PRO and 91 ± 16 g fat. |
Diets consisted of breakfast, lunch and dinner supplemented with snacks over a 48-hour period following a soccer match. |
| Highton et al., 2013 |
Protein – Carbohydrate mixture Mean ingestion rate: 52.7 ± 8.35 g/h-1 CHO (maltodextrin & dextrose) and 17.6 ± 2.8 g/h-1 PRO (whey protein isolate). |
Carbohydrate Mean ingestion rate: 70.2 ± 11.1 g/h-1 CHO (maltodextrin & dextrose) |
Before (1 x 5ml/kg-1 at -15min) and during exercise (5 x 2.5ml/kg-1 every 15 min) |
| Naclerio et al., 2014 |
Protein-carbohydrate-based multi-ingredient Total volume: 1 L Macronutrient content/1L: CHO: 106 g (maltodextrin & dextrose), PRO: 29 g (whey protein), Fat: 2.4 g, Glutamine: 10g, L-carnitine-L-tartrate: 3g – 560 kcals in total. |
Carbohydrate Total volume: 1 L; Macronutrient content/500 ml: CHO: 139 g (maltodextrin) – 530 kcals in total. Placebo Total volume: 1 L; A low kcal beverage (20.97 kcal/500ml) with identical color and flavor. |
Before (1x125ml), during exercise (3x125ml) and 20 min post-exercise (1x500ml) |
| Naclerio et al., 2015 |
Protein-carbohydrate-based multi-ingredient Total volume: 1 L Macronutrient content/1L: CHO: 106 g (maltodextrin & dextrose), PRO: 29 g (whey protein), Fat: 2.4 g, Glutamine: 10g, L-carnitine-L-tartrate: 3g – 560 kcals in total. |
Carbohydrate Total volume: 1 L; Macronutrient content/500 ml: CHO: 139 g (maltodextrin) – 530 kcals in total. Placebo Total volume: 1 L; A low kcal beverage (20.97 kcal/500ml) with identical color and flavor. |
Before (1x125ml), during exercise (3x125ml) and 20 min post-exercise (1x500ml) |
| Poulios et al., 2018 |
Milk Protein Smooth (80% casein, 20% whey) Match days: Total amount PRO: 80g/day80% casein & 20% whey); Composition of 25 g Supplement (80% casein & 20% whey)/4.7 g carbohydrate/1.6 g fat/~133 kcals), 30 g (80% casein & 20% whey)/5.6 g carbohydrate/1.9 g fat/~160 kcals), and 25g Training days: Total amount PRO: Composition of 20 g Supplement (80% casein & 20% whey)/3.75 g carbohydrate/0.25 g fat/~97 kcals) |
Isoenergetic placebo (maltodextrin) Match days: Total amount: 1.37g carbohydrate/kg BM Training Days: Total amount: 0.31g carbohydrate/kg BM |
Match day: 25 g after game, 30 g at +3h post game, 25 g at +6 h post game (PRO or PLA) Training day: 20g (PRO or PLA) with breakfast |
BCAA: Branched chain amino acids, BM: Body mass, CHO: Carbohydrate, PRO: Protein.
The effectiveness of protein-based supplementation on performance recovery was examined following intermittent running protocols in four studies (40%). The remaining six studies applied a variety of sports events and exercise testing protocols. The exercise intensity is adequately described in most studies. Continuous heart rate monitoring was applied in four studies, two studies utilized global positioning system (GPS) to record activity profile whereas three studies measured metabolites such as blood lactate and cortisol, before, during and after the event (Table 3).
The reported time-frame in which dependent variables were measured includes mainly baseline (pre-intervention) and repeated post-exercise time-points up to 168 h of recovery (immediately to 168 h post-exercise) when short-term interventions were applied (80%). However, three of these studies also reported measurements during the exercise protocol.
Changes in performance, muscle damage and inflammatory markers were predominantly investigated in these studies while one study also examined muscle glycogen resynthesis (Table 3). CK activity, Mb and DOMS were most often measured (90%) to assess skeletal muscle damage. Inflammatory markers were assessed in four studies. Various oxidative stress markers were investigated in two studies (Table 3).
Participant characteristics
In the ten studies included for review, a total number of 150 male participants (range 9-22) were recruited with mean age and body mass index (BMI) of 22.8 years (range 19.5-26) and 24.02 kg·m-2 (range 23.5–24.4), respectively (Table 5). Seven studies (70%) recruited highly trained athletes and most participants were athletes (121 soccer players, 10 basketball players, 2 rugby players, 11 not defined). Data on VO2max and daily dietary intake are provided only in five studies and therefore only indicative means can be extracted. Accordingly, the mean (range) of VO2max level was 55.8 ml·kg-1·min-1 (49.8 – 61). Participants’ daily dietary intake was recorded in all studies through dietary recalls over a 2-7 day period prior to their first trial, according to which the dietary plan prior to subsequent trials was designed. However, only five studies provided data regarding the participants’ daily macronutrient intake. Based on that, the average daily consumption of CHO, protein, fat and energy was 340.0 ± 61.8 g, 108.0 ± 13.1 g, 78 ± 15.4 g and 2,387.7 ± 218.4 kcals, respectively, for participants involved in five studies. The main results of the studies were summarized in Table 6.
Table 5.
Population characteristics of the selected studies.
| Study | n | Age (years) |
Sex | Training age | Training level | Type of sport | BMI (kg/m2) |
Body fat (%) |
VO2max (ml/kg/min) | Daily dietary intake |
|---|---|---|---|---|---|---|---|---|---|---|
| Arent et al., 2010 | 22 | 19.5±1.5 | Male | N/A | Division I college team |
Soccer players | 24.4 | N/A | 49.8±4.1 | N/A |
| Betts et al., 2009 | 17 | 26 ± 5 | Male | N/A | Highly trained | Cyclists (n=8), Team sport players(n=9) |
N/A | N/A | 61 ± 5 | 9992±3077 kJ 53±13% CHO 32±10% Fat 15±5% PRO |
| Cockburn et al., 2013 | 14 | 24 ± 4 | Male | N/A | Semi-professional | Soccer players | 23.9 | N/A | N/A | N/A |
| Gentle et al., 2014 | 10 | 22 ± 2 | Male | N/A | Well-trained | Basketball players | 24.4 | 9.5±2.7 | N/A | N/A |
| Gilson et al., 2010 | 13 | 19.5±0.3 | Male | N/A | NCAA Division I |
Soccer players | 23.5 | N/A | N/A | N/A |
| Gunnarsson et al., 2013 | 19 | 24 ± 1 | Male | N/A | First & second division in Denmark |
Soccer players | 24.3 | N/A | 58.4±1.4 | N/A |
| Highton et al., 2013 | 9 | 23.4±1.8 | Male | N/A | University-Standard athletes |
Soccer players (n=7) rugby players (n=2) | 24.0 | N/A | 52.5±3.8 | 2086±279 kcal 282.5±67 g CHO 55.3±17 g Fat 114.8±32 g PRO |
| Naclerio et al., 2014 | 10 | 25 ± 3.8 | Male | N/A | Recreationally active | Team sports | 24.0 | N/A | N/A | 31.2±1.6 kcal/kg 4.2±0.18 g CHO/kg 1. ±0.20 g PRO/kg 1.04±0.15 g Fat/kg |
| Naclerio et al. 2015 | 16 | 24 ± 3.7 | Male | N/A | Amateur | Soccer players | 23.7 | N/A | N/A | 33.5±1.3 kcal/kg 5.51±0.21 g CHO/kg 1.4 0.26 g PRO/kg 1.15±0.16 g Fat/kg |
| Poulios ets al. 2018 | 20 | 20.6±1.1 | Male | N/A | Semi-professional | Soccer players | 24.0 | 9.9±2.2 | 57.7±3.4 |
Game days6.7 g CHO/kg 1.3 g PRO/kg Training days 5.1 g CHO/kg 1.2 g PRO/kg |
BMI, body mass index; VO2max, maximal oxygen consumption; N/A, not applicable; CHO, carbohydrates; PRO, protein.
Table 6.
The main results of the studies that examined the effects of protein supplementation on recovery kinetics in team sports.
| Study | Type of exercise test |
Supplementation | Muscle damage markers |
Inflammatory markers /oxidative markers |
Muscle soreness |
Performance markers |
|---|---|---|---|---|---|---|
| Arent et al., 2010 | Graded maximal treadmill test to exhaustion |
Resurgex Plus® vs isocaloric placebo |
Resurgex < PLA: ↑ CK |
Resurgex < PLA: ↑ 8-isoprostane & LPO |
------ |
Resurgex = PLA VO2max, VLT & time-to-exhaustion |
| Betts et al., 2009 | 90 min of high-intensity intermittent shuttle-running |
CHO vs CHO+PRO |
CHO + WP = CHO: ↑ CK CHO + WP = CHO: ↑ Myoglobin CHO + WP = CHO: ↑ LDH |
CHO + WP = CHO: ↑ IL-6 CHO + WP = CHO: ↑ IL-10 CHO + WP = CHO: ↑ IL-1 receptor antagonist CHO + WP = CHO: ↑ CRP |
CHO+WP = CHO |
CHO + WP = CHO: Peak isometric force |
| Cockburn et al., 2013 | Unilateral knee flexions (6 sets x 10 reps, 1.05 rad/s-1, 90-s rest) on isokinetic dynamometer |
Semi-skimmed milk vs water |
SSM = Water: CK SSM = Water: Myoglobin |
------ |
SSM = Water |
SSM = Water: CM jump height SSM = Water: Reactive strength SSM < Water (tendency): ↑10-m & 15-m sprint time |
| Gentle et al., 2014 | 87-min basketball game stimulation test |
CHO meal vs CHO+PRO meal | CHO+PRO < CHO: ↑ CK | ------ | CHO+PRO =CHO |
CHO+PRO > CHO: Free throw success CHO+PRO < CHO (tendency): Mean sprint time CHO+PRO = CHO: Jump height |
| Gilson et al., 2010 | 4-day soccer training period, with increased training duration | CHO vs Low-fat chocolate milk | LCM = CHO : Myoglobin, LCM < CHO: ↑ CK (at 4d) | ------ |
LCM = CHO |
LCM = CHO T-drill, Vertical jump, isometric quadriceps force (MVC) & fatigue ratings |
| Gunnarsson et al., 2013 | A competitive soccer match | Normal diet vs high carbohydrate-high protein diet |
WP + CHO > NDiet: ↑ CK (at 24h) WP + CHO < NDiet: ↑ Myoglobin |
------ | ------ | ------ |
| Highton et al., 2013 | Modified LIST | CHO vs CHO+PRO | ------ | ------ | ------ |
WP + CHO = CHO: Distance covered WP + CHO = CHO: Maximal speed WP + CHO < CHO: ↑ Average speed |
| Naclerio et al., 2014 | 90-min Intermittent repeated sprint test |
PLA vs CHO vs CHO+PRO multi-ingredient |
WPCM < CHO & PL: ↑ CK (at 24h) WPCM (tendency) & CHO < PL: ↑ myoglobin (at 1h) |
WPCM = CHO = PL: ↑ IL-6 | ------ |
WPCM = CHO = PL: Total sprint time WPCM = CHO = PL: 15-m sprint time |
| Naclerio et al., 2015 | 90-min IRS test |
PLA vs CHO vs CHO+PRO multi-ingredient |
WPCM = CHO = PL: ↑ CK (at 24h) WPCM & CHO < PL: ↑ myoglobin (at 1h) |
WPCM = CHO = PL: ↑ IL-6 CHO < WPCM & PL: ↑ Neutrophil CHO < WPCM & PL: ↑ Monocyte WPCM = CHO = PL: Lymphocytes |
------ |
WPCM = CHO = PL: Total sprint time WPCM = CHO = PL: Time IRS+15-m sprint WPCM < CHO & PL: Perception of fatigue |
| Poulios et al., 2018 | Two-trial (2 weeks), 2 soccer matches & 4 practices/week |
Milk Protein Smooth (PRO) vs isoenergetic placebo (maltodextrin) |
PRO=PL: ↑ CK PRO=PLA: ↑WBC(Post G1-Post G2) PRO=PL: ↑Granulocyte (Post G1-Post G2) |
PRO=PL: ↑TBARS (G1-D2,G2-D1), PL: ↑TBARS(G1-D1, G1-D3,G2-D2), PRO=PL: ↑PC(G1-D1, G1-D2, G1-D3, G2-D1), PL: ↑PC(G2-D3) PRO=PL ↓GSH(G1-D1, G1-D2, G1-D3), PRO>PL :↑GSH(G2-D1), PRO=PL: ↑TAC |
PRO=PL: ↑DOMS (G1-D1, G1-D2, G1-D3, G2-D1, G2-D2, G2-D3) |
PRO=PL: ↑10-m sprint, ↑30-m sprint(G1-D1, G1-D2, G1-D3, G2-D1, G2-D2, G2-D3), PRO=PLA:↓CMJ(G1-D1, G1-D2, G1-D3, G2-D1, G2-D2, G2-D3) PRO<PL: ↓KF eccentric strength(4h post G2), PRO<PL: ↓KE concentric strength, PRO<PL: ↓High-intensity running(G2 75–90 min vs. 0–15 min), PL: ↓HR, ↓TD |
PL, placebo; LPO, lipid hydroperoxides; VO2max, maximal oxygen consumption; VLT, velocity at lactate threshold; CHO, carbohydrates; PRO, protein; WP, whey protein; CK, creatine kinase activity; LDH, lactate dehydrogenase activity; IL, interleukin; CRP, C reactive protein; s, seconds; d, days; SSM, semi-skimmed milk; LCM, low-fat chocolate milk; CM, counter-movement; MVC, maximal voluntary contraction; NDiet, normal diet; ↑, increase; WPCM, whey protein carbohydrate multi-ingredient supplement; IRS, intermittent repeated sprint, G, Game; WBC, White blood cells; TBARS, thiobarbituric acid; PC, Protein carbonyls; GSH: Glutathione; KF, Knee Flexors; KE, Knee Extensors; TD, Total Distance.
Discussion
Effects of protein-based supplementation
Nine out of ten studies included in the present systematic analysis investigated supplements that were a mixture of protein with CHO (Betts et al., 2009; Gentle et al., 2014; Gunnarsson et al., 2013; Highton et al., 2013), milk (Cockburn et al., 2013; Gilson et al., 2010) or multi-ingredients enriched with either BCAA (Arent et al., 2010) or protein (Naclerio et al., 2014; 2015), while only in one study it was administered a protein supplement consisted of protein alone (Milk protein smooth, 80% casein – 20% whey protein) (Poulios et al., 2018). Therefore, the interpretation of results and outcomes in the present analysis is based on protein-based supplements rather than pure protein supplementation per se. Furthermore, in all reported studies the participants’ dietary intake the participants’ was recorded for 1-7 days prior to the main experimental procedure and subsequently they were asked to follow the same dietary habits throughout the intervention in an attempt to secure that participants had a similar macronutrient intake during all trials and the wash-out periods between the experimental trials (Arent et al., 2010; Betts et al., 2009; Cockburn et al., 2013; Gentle et al., 2014; Gilson et al., 2010; Highton et al., 2013; Naclerio et al., 2014; 2015; Poulios et al., 2018). Thus, it can be conceptualized that the reported effects are attributable to the protein-based supplements per se.
The exercise protocols applied in the reviewed studies resulted in significantly elevated systemic indices of muscle damage and inflammation, increased muscle soreness and performance deterioration, lasting 48-72 h during recovery, similarly to what previously reported for team sport match plays (Ispirlidis et al., 2008; Fatouros et al., 2010; Chatzinikolaou et al., 2014a; 2014b; Draganidis et al., 2015; Mohr et al., 2016). Protein-based supplementation often, but not always, reduced plasma CK (Arent et al., 2010; Gentle et al., 2014; Gilson et al., 2010; Naclerio et al., 2015) and Mb (Gunnarsson et al., 2013; Naclerio et al., 2014) responses, whereas, no beneficial effect was observed on immunity and muscle soreness. In terms of performance recovery, there is no clear evidence for the effectiveness of protein-based supplements due to mixed results reported by the reviewed studies.
The only notable effects of protein-based supplementation were related to the attenuation of the rise in systemic CK (in 40% of the studies included) and Mb (in 30% of the studies included) concentration after either acute exercise testing or prolonged training periods that do not coincide though with changes in performance or DOMS. Studies that examined changes in EIMD, DOMS and muscle function in response to acute supplementation following exercise testing are highly variable in terms of protein dose and administration scheme, type, duration and intensity of exercise testing, participants’ competitive level as well as time-frame of post-exercise measurements (Tables 3-5). Therefore, there is a discrepancy among findings and no clear evidence to support a relationship between changes in muscle damage, DOMS and recovery of performance with protein-based supplementation.
More specifically, protein consumption has provided inconsistent results regarding changes in plasma CK and exercise performance in team sports athletes of similar training level (Betts et al., 2009; Gentle et al., 2014), even when they received identical supplements (placebo vs CHO vs CHO + Whey protein multi-ingredient) and performed the same exercise protocol (Naclerio et al., 2014; 2015), suggesting that neither the participants’ training level nor the exercise intensity are key determinants of the responses to protein supplementation.
Furthermore, despite previous reports stating that the ergogenic effects induced by protein ingestion may occur only when participants are characterized by negative nitrogen balance (Lunn et al., 2012; Nelson et al., 2012), Cockburn et al. (2013) did not observe different responses of muscle damage blood markers and performance related-measurements (jump height, reactive strength, sprint time) to unilateral knee flexions on isokinetic dynamometer with consumption of protein in the form of semi-skimmed milk compared with water. When extended protein-based supplementation periods were applied during training periods with increased intensity and/or duration, it resulted in profoundly smaller increase in resting plasma CK (by 36% to 114%) (Arent et al., 2010; Gilson et al., 2010), but once again this reduced response did not coincide with either accelerated recovery or attenuated deterioration of skeletal muscle performance and soreness.
Taken together, the attenuated response of plasma CK observed in some studies with protein supplementation do not coincide with attenuation of the rise in Mb, and is independent to participants’ training level and nitrogen balance, the characteristics of the event (i.e. type, duration and intensity of the exercise protocol or sport) and the amount of protein consumed. Moreover, it appears to have minimal effect on team-sport related performance recovery, since it is not combined with reduced DOMS and enhanced performance measures.
It is well-documented that protein ingestion during recovery from damaging exercise mitigates the elevation of muscle damage, inflammatory and oxidative stress markers and attenuates muscle performance deterioration (Cockburn et al., 2013; Howatson et al., 2012; Rowlands et al., 2008; Cooke et al., 2010; Hansen et al., 2015; Shenoy et al., 2016). The increased availability of amino acids, particularly BCAA, provided by protein intake stimulates muscle protein synthesis (Moore et al., 2009; Areta et al., 2013; Holm et al., 2017) through activation of intracellular signaling proteins involved in the mammalian target of rapamycin (mTOR) signaling cascade and its downstream translational regulators and potentially activation of satellite cells (Areta et al., 2013; Kimball et al., 2002). Therefore, the establishment of a more anabolic environment within skeletal muscle concomitant with attenuated protein degradation (Ferguson-Stegall et al., 2011) and accelerated satellite cell proliferation (Farup et al., 2014) is the predominant mechanism through which protein ingestion enhances tissue repair and remodeling following EIMD.
In addition, protein and BCAA supplementation provide sulphur-containing amino acids that act as precursors for GSH synthesis and as such may prevent redox status disturbances, mitigate inflammatory and oxidative stress responses and enhance muscle recovery (Cruzat et al., 2014). In line with this, one of the two studies in this review that examined oxidative stress markers reported a significantly smaller increase of 8-isoprostane and LPO concomitant with attenuated rise in CK, in response to maximal exercise, following a 20-day protein/antioxidant supplementation (Arent et al., 2010). The second study simulated one-week in-season microcycle with two games performed three days apart, where attenuated rise in TBARS and PC, and decline in GSH, throughout recovery after the first game and for one day after the second game, was observed (Poulios et al., 2018).
Three of the ten studies included for review investigated also the effect of combined protein-CHO supplementation on inflammatory markers; however, they failed to observe any interaction. By contrast, previous studies reported reduced inflammatory responses with protein ingestion compared to control treatments after damaging exercise in well-trained athletes (Shenoy et al., 2016; Nelson et al., 2013). Still, studies investigating muscle responses are currently limited in scientific literature.
Evidence statement—Protein supplementation to facilitate recovery in team sports
There are good qualities but limited and inconsistent data to provide evidence that protein supplementation before, during or following team sport events or training promotes performance restoration. Evidence category B. There are limited and inconsistent quality data to support the hypothesis that protein supplementation in team sport athletes may attenuate muscle soreness and reduce markers of muscle damage after events. Evidence category B. There are limited, good quality data to demonstrate that protein supplementation may attenuate inflammatory responses following events in team sport athletes. Evidence category B. Consequently, the limited and inconsistent available data regardless of whether protein supplementation may attenuate EIMD and enhance performance recovery or not, do not allow us to provide guidelines for protein supplementation in team sport athletes.
Limitations of the reviewed studies
Most of the studies reviewed in the present systematic analysis used crossover, repeated-measures experimental design with adequate intervals between treatments to eliminate any trial-order effects (Betts et al., 2009; Gentle et al., 2014; Gilson et al., 2010; Highton et al., 2013; Naclerio et al., 2014; 2015; Poulios et al., 2018). However, the number of participants recruited often (Betts et al., 2009; Gilson et al., 2010; Naclerio et al., 2014; Poulios et al., 2018), but not always (Arent et al., 2010; Cockburn et al., 2013; Gentle et al., 2014; Gunnarsson et al., 2013; Highton et al., 2013; Naclerio et al., 2015) was justified according to a preliminary power analysis. In fact, several studies conducted with small sample sizes, increasing the risk for a statistical type II error. Moreover, participants varied considerably among studies in terms of their training level, including amateur or recreationally active athletes (Highton et al., 2013; Naclerio et al., 2014; 2015) to highly-trained players participating in first or second division (Betts et al., 2009; Gunnarsson et al., 2013). Therefore, caution is required when interpreting the results of these studies and incorporating the conclusions drawn to team sports daily practice.
The control of daily macronutrient intake, particularly protein and CHO ingestion, is crucial in clinical studies aimed at examining differences between nutritional treatments/interventions. Almost all reviewed studies asked participants to record their habitual diet over a 2-4 day period; however, only five studies provide data regarding the participants’ average daily energy and macronutrient consumption (Betts et al., 2009; Highton et al., 2013; Naclerio et al., 2014; 2015; Poulios et al., 2018). In addition, many studies in which recovery periods lasted 24 to 168 h, reported that diet was not controlled during recovery, though it was recorded before intervention (Betts et al., 2009; Gentle et al., 2014; Highton et al., 2013; Naclerio et al., 2014; 2015). Inter-individual variability in daily nutrient intake throughout recovery is critical when comparisons across different nutritional interventions are made.
Nine out of ten protein supplements under investigation were a combination of CHO-protein (Betts et al., 2009; Gentle et al., 2014; Gunnarsson et al., 2013; Highton et al., 2013), milk (Cockburn et al., 2013; Gilson et al., 2010) or multi-ingredients enriched with BCAA (Arent et al., 2010) or protein (Naclerio et al., 2014; 2015) rather than protein alone. Obviously, it is unknown whether the effects observed after treatment with these supplements, are attributed to the protein fraction per se, to other ingredients or to the combination of the included fractions. Milk protein concentrate was used only in one study (Poulios et al., 2018). Furthermore, many studies used only CHO as the control treatment (Betts et al., 2009; Gentle et al., 2014; Gilson et al., 2010; Gunnarsson et al., 2013; Highton et al., 2013; Poulios et al., 2018) instead of including also a placebo supplement, so that we would be able to determine if the effects are due to the protein content per se, the addition of CHO or just due to increased energy availability.
Another limitation is that most of the reviewed studies in this systematic analysis used either a short recovery period (up to 24 h) (Gentle et al., 2014; Naclerio et al., 2014; 2015) or performed measurements only immediately after the exercise testing (Arent et al., 2010; Highton et al., 2013). Considering that following team-sport events muscle damage and inflammatory responses as well as performance deterioration are peaked between 24 and 48 h and may remain significantly increased or decreased compared to baseline for as long as 72 h (Ispirlidis et al., 2008; Fatouros et al., 2010; Chatzinikolaou et al., 2014a; 2014b; Draganidis et al., 2015; Mohr et al., 2016), these studies may have failed to detect significant changes due to very short recovery periods applied.
Moreover, methodological approaches in these studies are based on indirect markers of skeletal muscle damage (i.e. plasma CK, Mb, LDH, DOMS) and performance evaluation while direct measurements of protein metabolism within skeletal muscle (i.e. intracellular protein signaling, gene expression, stable isotope labelling, degree of structural damage) is lacking. Although exercise-induced skeletal muscle damage is characterized by increased leakage of muscle proteins, such as CK, Mb and LDH into the circulation (Clarkson and Hubal, 2002; Proske and Morgan, 2001; Fatouros and Jamurtas, 2016), it has been reported that systemic levels of these enzymes are poorly correlated with muscle function impairments following damaging exercise (Pasiakos et al., 2014; Margaritis et al., 1999). Thus, muscle recovery cannot be evaluated by only measuring levels of indirect markers of the damage to contractile proteins. Studies providing direct evidence at muscular level, potential by EM-analysis are highly warranted in relation to recovery and protein supplementation.
Another limitation of the reviewed studies is that only two of them tested muscle function through isokinetic measures of peak torque and maximal voluntary contraction. In contrast to other performance measurements such as sprint time, jump height or agility time, evaluation of maximal voluntary contraction and isometric torque as well as testing joint range of motion have been characterized as the most valid markers of muscle trauma (Warren et al., 1999). Given that recovery kinetics of knee flexor and extensor strength following a team-sport event demonstrate strength, limb and velocity specificity (Draganidis et al., 2015), isokinetic evaluation of extensor and flexor at multiple velocities in both dominant and non-dominant limb is required to perform a more reliable and quantitative evaluation of muscle function.
Finally, as in all supplementation studies, there is a risk of publication bias in the literature, since studies with negative finding may be published to a lesser degree than studies with positive finding. However, we have no data to support this statement.
Future research
The small number of available investigations provides inconsistent data regarding the effectiveness of protein-based supplements or diets on performance, EIMD and inflammatory markers during recovery in team sport athletes, despite a tendency noted for reduced systemic CK or Mb responses. This inconsistency may be primary attributed to the variability observed among studies in terms of protein supplements/diets tested, the control supplements utilized, the exercise testing protocols applied and participants’ training level. The markers used to evaluate EIMD in these investigations, though traditional and reliable measurement tools (Warren et al., 1999), represent indirect markers of the damage to contractile muscle fractions and may not be correlated with changes in muscle function (Clarkson et al., 1992). Also, the lack of advanced measurements, such as intracellular signaling, muscle damage rating, gene expression and isotopic labelling, to assess changes in muscle protein synthesis and breakdown is a major limitation of the reviewed studies. Therefore, it is evident that future research designs should incorporate measurements of muscle protein synthesis and breakdown to provide evidence on the interaction between changes in muscle protein turnover and skeletal muscle healing process following EIMD and protein ingestion in team sport athletes. Ideally, protein turnover measurements should be combined with intracellular signaling and mRNA assessment to define the molecular mechanisms mediating this interaction.
Moreover, considering that different protein sources (plant vs dairy vs blends) that differentially affect anabolic and catabolic processes in skeletal muscle due to variations in digestion and absorption kinetics and in amino acid profile (Tang et al., 2009; Burd et al., 2012), studies that compare the effectiveness of plant versus animal-based protein supplements should be conducted. Other studies, based on the work by Moore et al. (2012) and Areta et al. (2013) might focus on the timing and distribution pattern of protein intake during recovery from team sport events, especially during in-season microcycles in which athletes must participate in two or three matches and daily practices in-between. The restrictive recovery period (only 3-4 days) between successive matches along with large physiological stress and fatigue appearing during and after matches, predispose athletes to increased inflammatory responses and performance deterioration (Mohr et al., 2016) as well as to increased risk of musculoskeletal injuries (Ekstand et al., 2004; Montgomery et al., 2008; Dupont et al., 2010). Thus, protein-based supplementation during periods characterized by consecutive matches should be further investigated as a possible strategy to alleviate muscle damage and inflammation and to enhance recovery of performance. Finally, adding other nutrients with possible anti-inflammatory properties such as omega-3 fatty acids to protein-based supplementation may decrease the inflammatory response to exercise and attenuate muscle soreness (Black et al., 2018).
Conclusion
This systematic review explores the available evidence that underline the effectiveness of protein-based supplementation in enhancing performance recovery and attenuating muscle damage and inflammatory responses in team sport athletes. Although protein ingestion resulted in reduced systemic CK and Mb responses in some studies, these changes were not accompanied by performance enhancement and therefore are of limited importance. The relative small number of studies that fulfilled the criteria and were included for review in combination with high variability in protein supplements/diets, control supplements, exercise testing protocols, participants’ training levels and length of recovery periods studied, do not allow us to draw a clear conclusion that either supports or refutes the use of protein as a nutritional strategy to enhance performance recovery in team sports. Clearly, this topic warrants further investigation and future research exploring whether alteration in muscle protein turnover induced by protein ingestion is able to affect recovery of muscle function, is required.
Acknowledgements
Authors wish to thank Mr. Theofanis Tzatzakis for his technical assistance. This article was supported by departmental funding only. The study comply with the current laws of the country in which were performed. The authors report no conflict of interest.
Biographies

Athanasios POULIOS
Employment
Research associate, Exercise Physiology & Exercise Biochemistry at the Department of Physical Education and Sport Science of University of Thessaly, Greece
Degree
MSc
Research interests
The effects of nutrition supplements in inflammatory response and in performance indicators after soccer matches in adults
E-mail: athanpoul@gmail.com

Kalliopi GEORGAKOULI
Employment
Postdoctoral researcher, Department of Physical Education and Sport Science of University of Thessaly, Greece.
Degree
PhD
Research interests
The effects of functional foods, food supplements and exercise on various health aspects in both healthy and clinical populations, with a particular interest in alcohol use disorders.
E-mail: kgeorgakouli@gmail.com

Dimitrios DRAGANIDIS
Employment
Research associate, Department of Physical Education and Sport Science of University of Thessaly, Greece.
Degree
PhD
Research interests
The interaction between inflammation, protein intake and skeletal muscle health, with particular emphasis on the effects of inflammation on performance and functional status of the aged as well as on molecular regulators of skeletal muscle protein synthesis and proteolysis.
E-mail: dimidraganidis@gmail.com

Chariklia K. DELI
Employment
Ass. Prof., Exercise Physiology at the Department of Physical Education and Sport Science of University of Thessaly, Greece.
Degree
PhD
Research interests
The effects of exercise-induced inflammation and various supplements on redox responses and performance in adults and the pediatric population in healthy and clinical conditions.
E-mail: delixar@pe.uth.gr

Panagiotis D. TSIMEAS
Employment
Ass. Prof., Exercise Physiology at the Department of Physical Education and Sport Science of University of Thessaly, Greece.
Degree
PhD
Research interests
The field of children’s exercise and physical activity.
E-mail: ptsimeas@gmail.com

Athanasios CHATZINIKOLAOU
Employment
Ass. Prof., Physical Conditioning development with emphasis in strength training at the Department of Physical Education and Sport Sciences, Democritus University of Thrace, Greece.
Degree
PhD
Research interests
The development of muscle strength and the effect of exercise on the process of lipolysis in obese individuals.
E-mail: achatzin@phyed.duth.gr

Konstantinos PAPANIKOLAOU
Employment
Ph.D. candidate, Department of Physical Education and Sport Science of University of Thessaly, Greece.
Degree
MSc
Research interests
Dietary strategies, particularly on antioxidants and protein, to enhance skeletal muscle performance and recovery following aseptic inflammation induced by exercise and various types of sports in health and disease.
E-mail: guspapa93@gmail.com

Alexios BATRAKOULIS
Ph.D. candidate, Department of Physical Education and Sport Science of University of Thessaly, Greece.
Degree
MSc
Research interests
The effects of exercise training on body composition, health, performance and quality of life in sedentary overweight or obese adults.
E-mail: alexis_batrakoulis_75@hotmail.com

Magni MOHR
Employment
Assoc. Prof., Health Science,University of the Faroe Islands, Faroe Islands.
Degree
PhD
Research interests
Exercise physiology and sports medicine.
E-mail: magnim@setur.fo

Athanasios Z. JAMURTAS
Employment
Prof., Biochemistry of Exercise, Department of Physical Education and Sport Science of University of Thessaly, Greece.
Degree
PhD
Research interests
Exercise-induced oxidative stress, exercise-induced muscle damage, and the effects of exercise on inflammation and on aspects that affect metabolism.
E-mail: ajamurt@pe.uth.gr

Ioannis G. FATOUROS
Employment
Prof., Biochemistry of Exercise, Department of Physical Education and Sport Science of University of Thessaly, Greece
Degree
PhD
Research interests
The study of exercise-induced inflammation, overtraining, developmental exercise physiology, strength and conditioning (mainly in football and basketball) and sports nutrition.
E-mail: ifatouros@pe.uth.gr
References
- Arent S.M., Pellegrino J.K., Williams C.A., Difabio D.A., Greenwood J.C. (2010) Nutritional supplementation, performance, and oxidative stress in college soccer players. Journal of Strength and Conditioning Research 24, 1117-1124. [DOI] [PubMed] [Google Scholar]
- Areta J.L., Burke L.M., Ross M.L., Camera D.M., West D.W., Broad E.M., Jeacocke N.A., Moore D.R., Stellingwerff T., Phillips S.M., Hawley J.A., Coffey V.G. (2013) Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. Journal of Physiology 591, 2319-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts J.A., Toone R.J., Stokes K.A., Thompson D. (2009) Systemic indices of skeletal muscle damage and recovery of muscle function after exercise: effect of combined carbohydrate-protein ingestion. Applied Physiology, Nutrition and Metabolism 34, 773-784. [DOI] [PubMed] [Google Scholar]
- Black K.E., Witard O.C., Baker D., Healey P., Lewis V., Tavares F., Christensen S., Pease T., Smith B. (2018) Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. European Journal of Sport Science 18, 1357-1367. [DOI] [PubMed] [Google Scholar]
- Breen L., Philip A., Witard O.C., Jackman S.R., Selby A., Smith K., Baar K., Tipton K.D. (2011) The influence of carbohydrate–protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. Journal of Physiology 589, 4011-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd N.A., Yang Y., Moore D.R., Tang J.E., Tarnopolsky M.A., Phillips S.M. (2012) Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. British Journal of Nutrition 108, 958-962. [DOI] [PubMed] [Google Scholar]
- Chatzinikolaou A., Christoforidis C., Avloniti A., Draganidis D., Jamurtas A.Z., Stampoulis T., Ermidis G., Sovatzidis A., Papassotiriou I., Kambas A., Fatouros I.G. (2014a) A microcycle of inflammation following a team-handball game. Journal of Strength Conditioning and Research 28, 1981-1994. [DOI] [PubMed] [Google Scholar]
- Chatzinikolaou A., Draganidis D., Avloniti A., Karipidis A., Jamurtas A.Z., Skevaki C.L., Tsoukas D., Sovatzidis A., Theodorou A., Kambas A., Papassotiriou I., Taxildaris K., Fatouros I. (2014b) The microcycle of inflammation and performance changes after a basketball match. Journal of Sports Science 32, 870-882. [DOI] [PubMed] [Google Scholar]
- Clarkson P.M., Hubal M.J. (2002) Exercise-induced muscle damage in humans. American Journal of Physical Medicine and Rehabilitation 81, S52-S69. [DOI] [PubMed] [Google Scholar]
- Clarkson P.M., Nosaka K., Braun B. (1992) Muscle function after exercise- induced muscle damage and rapid adaptation. Medicine and Science in Sports and Exercise 5, 512-520. [PubMed] [Google Scholar]
- Cockburn E., Bell P.G., Stevenson E. (2013) Effect of milk on team sport performance after exercise-induced muscle damage. Medicine and Science in Sports and Exercise 45, 1585-1592. [DOI] [PubMed] [Google Scholar]
- Cockburn E., Hayes P.R., French D.N., Stevenson E., Gibson A.S.C. (2008) Acute milk-based protein-CHO supplementation attenuates exercise-induced muscle damage. Applied Physiology, Nutrition, and Metabolism 33, 775-783. [DOI] [PubMed] [Google Scholar]
- Cockburn E., Stevenson E., Hayes P.R., Robson-Ansley P., Howatson G. (2010) Effect of milk-based carbohydrate-protein supplement timing on the attenuation of exercise-induced muscle damage. Applied Physiology, Nutrition, and Metabolism 35, 270-277. [DOI] [PubMed] [Google Scholar]
- Cooke M.B., Rybalka E., Stathis C.G., Cribb P.J., Hayes A. (2010) Whey protein isolate attenuates strength decline after eccentrically-induced muscle damage in healthy individuals. Journal of the International Society of Sports Nutrition 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat V.F., Krause M., Newsholme P. (2014) Amino acid supplementation and impact on immune function in the context of exercise. Journal of the International Society of Sports Nutrition 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganidis D., Chatzinikolaou A., Avloniti A., Barbero-Álvarez J.C., Mohr M., Malliou P., Gourgoulis V., Deli C.K., Douroudos I.I., Margonis K., Gioftsidou A., Flouris A.D., Jamurtas A.Z., Koutedakis Y., Fatouros I.G. (2015) Recovery kinetics of knee Flexor and extensor strength after a football match. PLoS One 10, e0128072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganidis D., Chondrogianni N., Chatzinikolaou A., Terzis G., Karagounis L.G., Sovatzidis A., Avloniti A., Lefaki M., Protopapa M., Deli C.K., Papanikolaou K., Jamurtas A.Z., Fatouros I.G. (2017) Protein ingestion preserves proteasome activity during intense aseptic inflammation and facilitates skeletal muscle recovery in humans. British Journal of Nutrition 118, 189-200. [DOI] [PubMed] [Google Scholar]
- Dupont G., Nedelec M., McCall A., McCormack D., Berthoin S., Wisløff U. (2010) Effect of 2 soccer matches in a week on physical performance and injury rate. American Journal of Sports Medicine 38, 1752-1758. [DOI] [PubMed] [Google Scholar]
- Ekstand J., Walden M., Haqqlund M. (2004) A congested football calendar and the wellbeing of players: correlation between match exposure of European footballers before the World Cup 2002 and their injuries and performances during that World Cup. British Journal of Sports Medicine 38, 493-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farup J., Rahbek S.K., Knudsen I.S., de Paoli F., Mackey A.L., Vissing K. (2014) Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids 46, 2503-2516. [DOI] [PubMed] [Google Scholar]
- Fatouros I.G., Jamurtas A.Z. (2016) Insights into the molecular etiology of exercise-induced inflammation: opportunities for optimizing sport performance. Journal of Inflammation Research 9, 175-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros I.G., Chatzinikolaou A., Douroudos I.I., Nikolaidis M.G., Kyparos A., Margonis K., Michailidis Y., Vantarakis A., Taxildaris K., Katrabasas I., Mandalidis D., Kouretas D., Jamurtas A.Z. (2010) Time-course of changes in oxidative stress and antioxidant status responses following a soccer game. Journal of Strength Conditioning and Research 24, 3278-3286. [DOI] [PubMed] [Google Scholar]
- Ferguson-Stegall L., McCleave E.L., Ding Z., Doerner P.G., 3rd, Wang B., Liao Y.H., Kammer L., Liu Y., Hwang J., Dessard B.M., Ivy J.L. (2011) Postexercise carbohydrate-protein supplementation improves subsequent exercise performance and intracellular signaling for protein synthesis. Journal of Strength Conditioning and Research 25, 1210-1224. [DOI] [PubMed] [Google Scholar]
- Gentle H., Love T.D., Howe A.S., Black K.E. (2014) A randomised trial of pre-exercise meal composition on performance and muscle damage in well-trained basketball players. Journal of the International Society of Sports Nutrition 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson S.F., Saunders M.J., Moran C.W., Moore R.W., Womack C.J., Todd M.K. (2010) Effects of chocolate milk consumption on markers of muscle recovery following soccer training: a randomized cross-over study. Journal of the International Society of Sports Nutrition 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson T.P., Bendiksen M., Bischoff R., Christensen P.M., Lesivig B., Madsen K., Stephens F., Greenhaff P., Krustrup P., Bangsbo J. (2013) Effect of whey protein- and carbohydrate-enriched diet on glycogen resynthesis during the first 48 h after a soccer game. Scandinavian Journal of Medicine and Science in Sports 23, 508-515. [DOI] [PubMed] [Google Scholar]
- Hansen M., Bangsbo J., Jensen J., Bibby B.M., Madsen K. (2015) Effect of whey protein hydrolysate on performance and recovery of top-class orienteering runners. International Journal of Sport Nutrition and Exercise Metabolism 25, 97-109. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., Cochrane Bias Methods Group; Cochrane Statistical Methods Group (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton J., Twist C., Lamb K., Nicholas C. (2013) Carbohydrate-protein coingestion improves multiple-sprint running performance. Journal of Sports Science 31, 361-369. [DOI] [PubMed] [Google Scholar]
- Holm L., Rahbek S.K., Farup J., Vendelbo M.H., Vissing K. (2017) Contraction mode and whey protein intake affect the synthesis rate of intramuscular connective tissue. Muscle Nerve 55, 128-130. [DOI] [PubMed] [Google Scholar]
- Howatson G., Hoad M., Goodall S., Tallent J., Bell P.G., French D.N. (2012) Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. Journal of the International Society of Sports Nutrition 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ispirlidis I., Fatouros I.G., Jamurtas A.Z., Nikolaidis M.G., Michailidis I., Douroudos I., Margonis K., Chatzinikolaou A., Kalistratos E., Katrabasas I., Alexiou V., Taxildaris K. (2008) Time course of changes in inflammatory and performance responses following a soccer game. Clinical Journal Sport Medicine 18, 428-431. [DOI] [PubMed] [Google Scholar]
- Kimball S.R., Farrell P.A., Jefferson L.S. (2002) Invited review: role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. Journal of Applied Physiology 93, 1168-1180. [DOI] [PubMed] [Google Scholar]
- Koopman R., Wagenmakers A.J., Manders R.J., Zorenc A.H., Senden J.M., Gorselink M., Keizer H.A., van Loon L.J. (2005) Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. American Journal of Physiology 288, E645-E653. [DOI] [PubMed] [Google Scholar]
- Lunn W.R., Pasiakos S.M., Colletto M.R., Karfonta K.E., Carbone J.W., Anderson J.M., Rodriguez N.R. (2012) Chocolate milk and endurance exercise recovery: protein balance, glycogen, and performance. Medicine and Science in Sports and Exercise 44, 682-691. [DOI] [PubMed] [Google Scholar]
- Margaritis I., Tessier F., Verdera F., Bermon S., Marconnet P. (1999) Muscle enzyme release does not predict muscle function impairment after triathlon. Journal of Sports Medicine and Physical Fitness 39, 133-139. [PubMed] [Google Scholar]
- Minett G.M., Costello J.T. (2015) Specificity and context in post-exercise recovery: it is not a one-size-fits-all approach. Frontiers in Physiology 3, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr M., Draganidis D., Chatzinikolaou A., Barbero-Álvarez J.C., Castagna C., Douroudos I., Avloniti A., Margeli A., Papassotiriou I., Flouris A.D., Jamurtas A.Z., Krustrup P., Fatouros I.G. (2016) Muscle damage, inflammatory, immune and performance responses to three football games in one week in competitive male players. European Journal of Applied Physiology 116, 179-93. [DOI] [PubMed] [Google Scholar]
- Mohr M., Krustrup P., Bangsbo J. (2003) Match performance of high-standard soccer players with special reference to development of fatigue. Journal of Sports Science 21, 519-528. [DOI] [PubMed] [Google Scholar]
- Montgomery P.G., Pyne D.B., Cox A.J., Hopkins W.G., Minahan C.L., Hunt P.H. (2008) Muscle damage, inflammation, and recovery interventions during a 3-day basketball tournament. European Journal of Sport Science 8, 241-250. [Google Scholar]
- Moore D.R., Areta J., Coffey V.G., Stellingwerff T., Phillips S.M., Burke L.M., Cléroux M., Godin J.P., Hawley J.A. (2012) Daytime pattern of post-exercise protein intake affects whole-body protein turnover in resistance-trained males. Nutrition and Metabolism 9, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.R., Robinson M.J., Fry J.L., Tang J.E., Glover E.I., Wilkinson S.B., Prior T., Tarnopolsky M.A., Phillips S.M. (2009) Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. American Journal of Clinical Nutrition 89, 161-168. [DOI] [PubMed] [Google Scholar]
- Naclerio F., Larumbe-Zabala E., Cooper R., Allgrove J., Earnest C.P. (2015) A multi-ingredient containing carbohydrate, proteins L-glutamine and L-carnitine attenuates fatigue perception with no effect on performance, muscle damage or immunity in soccer players. PLoS One 10, e0125188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naclerio F., Larumbe-Zabala E., Cooper R., Jimenez A., Goss-Sampson M. (2014) Effect of a carbohydrate-protein multi-ingredient supplement on intermittent sprint performance and muscle damage in recreational athletes. Applied Physiology, Nutrition, and Metabolism 39, 1151-1158. [DOI] [PubMed] [Google Scholar]
- Narazaki K., Berg K., Stergiou N., Chen B. (2009) Physiological demands of competitive basketball. Scandinavian Journal of Medicine and Science in Sports 19, 425-432. [DOI] [PubMed] [Google Scholar]
- Nelson A.R., Phillips S.M., Stellingwerff T., Rezzi S., Bruce S.J., Breton I., Thorimbert A., Guy P.A., Clarke J., Broadbent S., Rowlands D.S. (2012) A protein-leucine supplement increases branched-chain amino acid and nitrogen turnover but not performance. Medicine and Science in Sports and Exercise 44, 57-68. [DOI] [PubMed] [Google Scholar]
- Nelson A.R., Jackson L., Clarke J., Stellingwerff T., Broadbent S., Rowlands D.S. (2013) Effect of post-exercise protein-leucine feeding on neutrophil function, immunomodulatory plasma metabolites and cortisol during a 6-day block of intense cycling. European Journal of Applied Physiology 113, 2211-2222. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Krustrup P., Nybo L., Gunnarsson T.P., Madsen K., Schrøder H.D., Bangsbo J., Ortenblad N. (2012) Skeletal muscle glycogen content and particle size of distinct subcellular localizations in the recovery period after a high-level soccer match. European Journal of Applied Physiology 112, 3559-3567. [DOI] [PubMed] [Google Scholar]
- Pasiakos S.M., Lieberman H.R., McLellan T.M. (2014) Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: a systematic review. Sports Medicine 44, 655-670. [DOI] [PubMed] [Google Scholar]
- Pasiakos S.M., Montain S.J., Young A.J. (2013) Protein supplementation in U.S. Military personnel. Journal of Nutrition 143, 1815S-1819S. [DOI] [PubMed] [Google Scholar]
- Petroczi A., Naughton C.P. (2008) The age-gender-status profile of high performing athletes in the UK taking nutritional supplements: lessons for the future. Journal of the International Society of Sports Nutrition 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen H.T., Knuutinen J., Lahti K., Keinanen O., Alen M., Komi P.V., Mero A.A. (2003) Free amino acid pool and muscle protein balance after resistance exercise. Medicine and Science in Sports and Exercise 35, 784-792. [DOI] [PubMed] [Google Scholar]
- Poulios A., Fatouros I.G., Mohr M., Draganidis D.K., Deli C., Papanikolaou K., Sovatzidis A., Nakopoulou T., Ermidis G., Tzatzakis T., Laschou V.C., Georgakouli K., Koulouris A., Tsimeas P., Chatzinikolaou A., Karagounis L.G., Batsilas D., Krustrup P., Jamurtas A.Z. (2018) Post-Game High Protein Intake May Improve Recovery of Football-Specific Performance during a Congested Game Fixture: Results from the PRO-FOOTBALL Study. Nutrients 10, pii: E494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Póvoas S.C.A., Castagna C. Resende C. Coelho E.F. Silva P. Santos R. Seabra A. Tamames J. Lopes M. Randers M.B. Krustrup P. (2017) Physical and physiological demands of recreational team handball for adult untrained men. BioMed Research International 2017, 6204603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U., Morgan D.L. (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. Journal of Physiology 537, 333-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands D.S., Rössler K., Thorp R.M., Graham D.F., Timmons B.W., Stannard S.R., Tarnopolsky M.A. (2008) Effect of dietary protein content during recovery from high-intensity cycling on subsequent performance and markers of stress, inflammation, and muscle damage in well-trained men. Applied Physiology, Nutrition, and Metabolism 33, 39-51. [DOI] [PubMed] [Google Scholar]
- Saunders M.J. (2007) Coingestion of carbohydrate–protein during endurance exercise: influence on performance and recovery. International Journal of Sport Nutrition and Exercise Metabolism 17, S87-S103. [DOI] [PubMed] [Google Scholar]
- Shenoy S., Dhawan M., Singh Sandhu J. (2016) Four weeks of supplementation with isolated soy protein attenuates exercise-induced muscle damage and enhances muscle recovery in well trained athletes: a randomized trial. Asian Journal of Sports Medicine 7, e33528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.E., Moore D.R., Kujbida G.W., Tarnopolsky M.A., Phillips S.M. (2009) Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Journal of Applied Physiology 107, 987-992. [DOI] [PubMed] [Google Scholar]
- Warren G.L., Lowe D.A., Armstrong R.B. (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Medicine 27, 43-59. [DOI] [PubMed] [Google Scholar]


