Significance Statement
Evidence suggests that anemia and disordered mineral metabolism (including abnormalities in phosphate and fibroblast growth factor 23 [FGF23]) contribute to adverse outcomes in patients with advanced CKD. To investigate the effects of fixed-dose ferric citrate coordination complex in such patients, the authors randomly assigned 199 patients with eGFR<20 ml/min per 1.73 m2 2:1 to ferric citrate coordination complex or usual care. Treatment with ferric citrate coordination complex significantly increased hemoglobin, ferritin, and transferrin saturation and significantly reduced FGF23, while maintaining serum phosphate in the normal range in the majority of patients. It also significantly reduced use of erythropoiesis-stimulating agents and intravenous iron, hospital admissions, hospital days, and time to the composite end point of death, provision of dialysis, or kidney transplantation. These findings support the conduct of a placebo-controlled trial using ferric citrate coordination complex in advanced CKD.
Keywords: ferric citrate, CKD, anemia, hyperphosphatemia, FGF23, RCT
Abstract
Background
Researchers have yet to determine the optimal care of patients with advanced CKD. Evidence suggests that anemia and CKD–related disordered mineral metabolism (including abnormalities in phosphate and fibroblast growth factor 23 [FGF23]) contribute to adverse outcomes in this population.
Methods
To investigate whether fixed-dose ferric citrate coordination complex favorably affects multiple biochemical parameters in patients with advanced CKD, we randomly assigned 203 patients with eGFR≤20 ml/min per 1.73 m2 2:1 to receive a fixed dose of ferric citrate coordination complex (two tablets per meal, 210 mg ferric iron per tablet) or usual care for 9 months or until 3 months after starting dialysis. No single biochemical end point was designated as primary; sample size was determined empirically.
Results
The two groups had generally similar baseline characteristics, although diabetes and peripheral vascular disease were more common in the usual-care group. Ferric citrate coordination complex significantly increased hemoglobin, transferrin saturation, and serum ferritin, and it significantly reduced serum phosphate and intact FGF23 (P<0.001 for all). Of the 133 patients randomized to ferric citrate coordination complex, 31 (23%) initiated dialysis during the study period, as did 32 of 66 (48%) patients randomized to usual care (P=0.001). Compared with usual care, ferric citrate coordination complex treatment resulted in significantly fewer annualized hospital admissions, fewer days in hospital, and a lower incidence of the composite end point of death, provision of dialysis, or transplantation (P=0.002).
Conclusions
The beneficial effects of fixed-dose ferric citrate coordination complex on biochemical parameters, as well as the exploratory results regarding the composite end point and hospitalization, suggest that fixed-dose ferric citrate coordination complex has an excellent safety profile in an unselected population with advanced CKD and merits further study.
Patients with advanced CKD (aCKD) are at high risk of multiple adverse events including progressive loss of kidney function leading to a need for RRT (dialysis or kidney transplantation), cardiovascular (CV) events, and mortality.1 Although traditional risk factors including diabetes mellitus, hypertension, and atherosclerotic vascular disease contribute substantially to these risks, they do not fully explain event rates 5–10-fold higher relative to age-matched peers without CKD.1
Epidemiologic data suggest that anemia and CKD–mineral bone disorder (CKD-MBD) contribute to adverse outcomes in this population.2,3 Numerous reports have described a direct relation between the severity of anemia and CV events and provided the scientific rationale for randomized clinical trials (RCTs) testing the effects of erythropoiesis-stimulating agents (ESAs) targeting a normal or near-normal hemoglobin concentration. Unexpectedly, multiple RCTs demonstrated that the use of ESAs in this manner does not reduce CV events and may lead to harm, with higher rates of stroke, venous thromboembolic disease, and vascular access thrombosis observed in some studies.4–6 However, there remains clinical equipoise as to whether anemia, per se, increases CV risk and reduces quality of life; it has been suggested that the risk observed in clinical trials using ESAs to normalize hemoglobin may be related to adverse drug effects, possibly related to excessive dosing of ESAs in treatment-resistant patients, rather than to the correction of anemia itself.7 Clinical trials with stabilizers of hypoxia-inducible factor are ongoing and will be the first to test the hypothesis that treatment of CKD-related anemia with agents other than ESAs reduces CV or other clinical event rates.
Disordered mineral metabolism encompasses abnormalities in calcium, phosphate (P), parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23). Preclinical and epidemiologic data suggest that elevations in P and FGF23 are associated with progression of CKD, CV events, and mortality, yet no RCT has tested the ability of P- or FGF23-lowering therapy to reduce these events.8,9
We hypothesized that treatment with ferric citrate coordination complex (FCCC) would favorably affect patients with aCKD irrespective of whether iron deficiency or overt hyperphosphatemia were present. To inform the design of a multicenter, placebo-controlled RCT, we designed and conducted an open-label, pilot, single-center RCT of fixed-dose FCCC in patients with eGFR≤20 ml/min per 1.73 m2 without restriction to patients with or without hyperphosphatemia and/or iron deficiency.
Methods
Study Setting
Patients were recruited from a large nephrology practice (32 physicians, six Advanced Practitioners, Denver Nephrologists, Denver, CO) from March of 2015 through March of 2017 with the last patient follow-up visit in March of 2018. Active drug (ferric citrate coordination complex, 1-g tablets containing 210 mg ferric iron) was supplied by Keryx Biopharmaceuticals, Inc. The study was funded by Keryx Biopharmaceuticals, Inc.; however, the Principal Investigator (GAB) and coinvestigators were responsible for the design, conduct, and analysis of the study with no input from Keryx Biopharmaceuticals, Inc. The study was approved by the Shulman Institutional Review Board (Cincinnati, OH) and registered at ClinicalTrials.gov (NCT 02492620).
Study Population
We obtained written informed, consent from all patients. We considered for participation all patients with eGFR (Modification of Diet in Renal Disease four-variable eGFR)≤20 ml/min per 1.73 m2. We stratified enrollment by eGFR above or below 15 ml/min per 1.73 m2 with a goal of enrolling 50% of participants within the lower eGFR stratum. The only inclusion criteria were age≥18 years, serum P ≥3.0 mg/dl, eGFR≤20 ml/min per 1.73 m2, hemoglobin>8.0 g/dl, transferrin saturation <55%, and anticipated to have at least 8 weeks before the need for dialysis. Exclusion criteria included use of intravenous iron, ESAs, or receipt of blood transfusion within 2 weeks before day 1; lack of evidence for CKD (i.e., only AKI); scheduled kidney transplant within 24 weeks; life expectancy <6 months; a confirmed conviction that the patient would not accept RRT; active drug or alcohol abuse; and a contra-indication to FCCC such as hemochromatosis.
Study Design
An independent statistician performed randomization using SAS software version 9.4 (SAS Institute, Cary, NC). Patients were block randomized into one of two arms, FCCC or usual care, in a 2:1 fashion with varying block sizes. Sealed envelopes stratified by eGFR above or below 15 ml/min per 1.73 m2 were provided by the statistician to the site before study start and opened sequentially. Patients randomized to FCCC were instructed to begin with one tablet per meal for 2 weeks and then increase to a fixed dose of two tablets per meal. On the basis of demonstrated tolerability, the protocol was subsequently amended to start patients on two tablets with each meal up to a maximum of six tablets per day. There was no titration of FCCC unless the patient developed persistent low P (<3.0 mg/dl) or high P (>5.0 mg/dl) or persistently high transferrin saturation (>60%). Patients randomized to usual care could be treated with any medication, including P binders other than FCCC ESAs, red blood cell transfusion, active vitamin D, and oral or intravenous iron were permitted at the discretion of the treating nephrologist.
Study visits were conducted at day 1, week 2, week 4, and monthly thereafter until month 9. If patients initiated dialysis at any time during the 9-month study period, they were instructed to contact the study center before starting dialysis. We assessed adherence with pill counts at each study visit and calculated on the basis of the expected number of pills taken/meal times the number of meals eaten per day. We ascertained data on hospitalization, transplant, and death through direct patient contact, review of a practice-wide electronic medical record, and hospital records. All decisions regarding initiation of dialysis were at the discretion of the treating nephrologist without involvement of the study team.
Study Assessments
Laboratory Assessments
All clinical chemistry analyses were performed by Quest Diagnostics (Denver, CO). Samples were obtained in a nonfasting state, generally at the same time of day. Serum for biomarker analysis was frozen at −70°C and assays were performed in a single batch at study completion. Assays of 25-vitamin D and 1,25 dihydroxy-vitamin D were performed using a commercially available chemiluminescent immunoassay (LIAISON, LIAISON XL; Diasorin Inc., Stillwater, MN). Intact and C-terminal FGF23 concentrations were measured in plasma at the Diabetes Research Institute Biomarker Core Laboratory (University of Miami School of Medicine, Miami, FL) using commercially available second-generation ELISA kits (Quidel, San Diego, CA). For FGF23, approximately 11% of randomly selected samples were analyzed in duplicate to establish the intra-assay variability. Samples with values greater than the highest standard in either assay were quantified after dilution. For intact FGF23, the average intra-assay coefficient of variation was 3.7% and the interassay coefficient of variation (determined using a common control in all runs) was 5.0%. Corresponding values for C-terminal FGF23 were 3.3% and 5.5%.
Laboratory Parameters and Clinical End Points
The primary hypothesis was that treatment with FCCC at fixed doses would result in improved parameters of anemia (higher hemoglobin and transferrin saturation) and bone and mineral metabolism (reduced serum P, FGF23, and PTH) as compared with usual care from baseline to initiation of RRT or end of study (9 months). No single statistical biochemical end point was designated as primary. Safety end points included progression of CKD to RRT, adverse events, and cumulative exposure to ESA and intravenous iron.
Although the sample size was empirically determined, it was anticipated that treatment with FCCC would reduce serum P by 0.6±1.4 mg/dl as compared with an anticipated 0.5±1.4 mg/dl increase in those assigned to usual care; thus, a sample of 200 patients would have >80% power to detect this difference at a two-sided α of 0.05.
Statistical Analysis
We analyzed all data (efficacy) in accordance with a modified intent-to-treat principle wherein we included any randomized participant who attended at least one postrandomization follow-up visit. We summarized data by treatment and by visit. We summarized continuous laboratory parameters using N, mean, SD, or median, and either 25th–75th or 10th–90th percentile ranges. We summarized categoric variables using numbers and percentages. We compared baseline characteristics between the two treatment arms using t test, the Wilcoxon rank-sum test, or chi-squared test, as appropriate. For biochemical parameters we performed mixed-effects models with treatment group as a fixed effect to assess on-study main effect, and examined differences by treatment group over time using a group by time interaction term to determine difference in slope over time. We log-transformed FGF23 due to non-normal distribution. To assess time to death, provision of dialysis or transplantation, and time to first hospitalization, we calculated Kaplan–Meier product limit estimates and compared survival curves with the log-rank test. We performed proportional hazards (Cox) regression, and calculated hazard ratios (HRs) and 95% confidence intervals (95% CIs) with adjustments for age, sex, race/ethnicity, diabetes, congestive heart failure, coronary artery disease, hypertension, coronary revascularization, peripheral vascular disease, hyperlipidemia, and baseline eGFR, P, and bicarbonate. Proportionality was assessed graphically and confirmed by examining the log time by group interaction. Change in slope of eGFR over time between the two treatments was tested using a mixed-effects model with patient as a random effect and baseline eGFR as a covariate. For patients starting dialysis or transplanted before week 36, an eGFR of 10 ml/min per 1.73 m2 was imputed. We only considered biochemical data obtained before RRT; no other missing laboratory data were imputed. Annualized hospital admissions and hospital days were compared using the Wilcoxon rank-sum. No adjustment for multiple comparisons was performed. We considered two-tailed P values <0.05 statistically significant. We performed all statistical analyses using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Enrollment, Baseline Characteristics, and Study Conduct
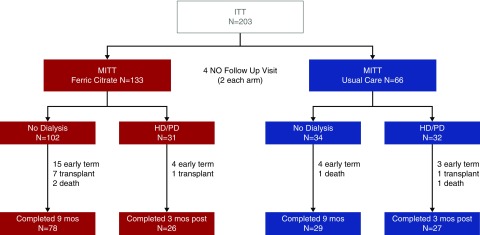
Figure 1 shows patient disposition. Two-hundred twenty patients were screened and 203 were enrolled and randomized. Two patients in each treatment group did not return for any postbaseline visit, yielding 199 patients in the modified intention-to-treat (MITT) data set (133 FCCC and 66 usual care). Baseline characteristics (Table 1) were generally similar, although diabetes and peripheral vascular disease were more common in the group randomized to usual care. There were no differences between treatment arms in baseline albumin-creatinine ratio (P=0.85) or baseline risk of kidney failure using the six-variable Tangri10 risk equation model (P=0.54). In the FCCC arm, the average dose was 5.0±1.4 tablets/d and mean adherence was 90%. Thirty-seven percent of patients in the usual-care arm received P binders during the nondialysis period. In patients randomized to FCCC, six (5%) withdrew early due to adverse events or intolerance of study medication and five (4%) withdrew due to poor or nonadherence with study medication.
Figure 1.
Patient disposition. Two-hundred twenty patients were screened, 203 patients randomized (17 screen failures). All analyses were conducted using MITT population (patients who attended at least one postbaseline follow-up visit). Patients could be pre-emptively transplanted before dialysis or receive transplant after initiating dialysis. HD, hemodialysis; ITT, intent to treat; PD, peritoneal dialysis.
Table 1.
Baseline characteristics
| Characteristic | Ferric Citrate (n=133) | SOC (n=66) | Significance |
|---|---|---|---|
| eGFR<15, % | 55 | 58 | NS |
| eGFR 15–20, % | 45 | 42 | NS |
| Mean age±SD, yr | 63.1±13 | 61.1±12 | NS |
| Race, % | |||
| White | 79 | 70 | |
| Black | 15 | 27 | |
| Male sex, % | 62 | 62 | NS |
| Hispanic ethnicity, % | 14 | 18 | NS |
| Primary cause, % | |||
| Diabetes | 35 | 64 | |
| HTN | 16 | 9 | |
| Comorbidity, % | |||
| Congestive heart failure | 21 | 30 | NS |
| Diabetes mellitus | 47 | 77 | 0.001 |
| Coronary artery disease | 27 | 24 | NS |
| Hypertension | 96 | 92 | NS |
| Myocardial infarction | 15 | 12 | NS |
| Peripheral vascular disease | 9 | 27 | 0.001 |
| Cerebrovascular disease | 13 | 11 | NS |
| Fracture | 8 | 9 | NS |
| Secondary hyperparathyroidism | 88 | 86 | NS |
| Dyslipidemia | 83 | 82 | NS |
| Anemia | 95 | 94 | NS |
| Transplant status, % | NS | ||
| Active on list | 20 | 11 | |
| Referred to transplant | 35 | 44 | |
| Not candidate | 20 | 24 | |
| Laboratory (mean±SD) | |||
| eGFR, ml/min per 1.73 m2 | 14.2±3.9 | 14.5±4.1 | NS |
| Ferritin, ng/ml | 202±173 | 170±169 | 0.04 |
| Hemoglobin, g/dl | 11.3±1.6 | 11±1.6 | NS |
| Transferrin saturation, % | 25±9 | 23±9 | 0.04 |
| P, mg/dl | 4.5±0.9 | 4.4±0.7 | NS |
| 25D, ng/ml | 30±11 | 29±12 | NS |
| 1,25D, pg/ml | 28±13 | 24±9 | NS |
| PTH, pg/ml | 207±152 | 213±178 | NS |
| Median intact FGF23 (25–75), pg/ml | 352 (222–552) | 331 (229–704) | NS |
25–75, 25th–75th percentile; HTN, hypertension.
Anemia-Related Parameters
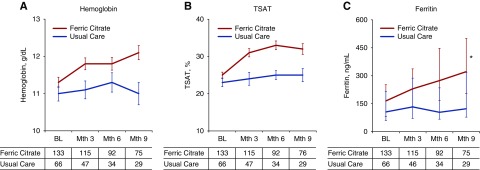
As shown in Figure 2, there were significant effects of FCCC on hemoglobin, transferrin saturation (TSAT), and serum ferritin (between-group difference, P<0.001 for each). Hemoglobin significantly increased with FCCC (within-group difference, P<0.001), whereas it decreased with usual care (within-group difference, P=0.02). TSAT and ferritin increased with FCCC (within-group difference, P<0.001 for each), whereas they remained unchanged with usual care.
Figure 2.
Change in Anemia-related P parameters. Mean values of hemoglobin (A), transferrin saturation (B), and median serum ferritin (C). Statistical inference testing using mixed-effects model with repeated measures, *P<0.001 for between-group differences. BL, baseline.
Use of Erythropoiesis-Stimulating Agents and Intravenous Iron
Eight (6%) patients randomized to FCCC received supplemental epoetin alfa at a mean dose of 4450±4870 U/wk during the study as compared with ten (15%) randomized to usual care at a mean dose of 7521±13,312 U/wk (between-group difference, P=0.03 for any use).
Four (3%) patients randomized to the FCCC arm received intravenous iron at a mean dose of 113±73 mg/wk during the study as compared with 11 (17%) randomized to usual care at a mean dose of 113±171 mg/wk (between-group difference, P=0.001 for any use).
CKD-MBD Parameters
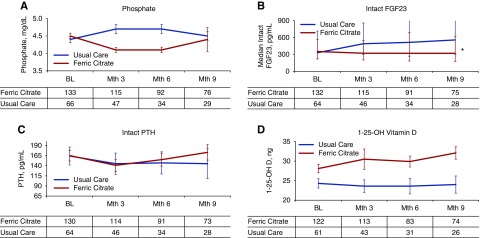
There was no significant effect of FCCC on the slope of change in serum P (Figure 3A); however, patients randomized to FCCC had significantly lower least squares mean serum P (4.17±0.04 mg/dl) as compared with usual care (4.63±0.06 mg/dl, P<0.001). At month 9, 70% of patients randomized to FCCC and 45% of patients randomized to usual care had a serum P within the population reference range (2.7–4.5 mg/dl, P=0.03).
Figure 3.
Change in bone and mineral related parameters. Mean values of serum P (A), median intact FGF23 (B), median intact PTH (C), and mean 1,25 dihydroxy vitamin D (D). Statistical inference testing using mixed-effects model with repeated measures, *P<0.001 for between-group differences. BL, baseline.
Intact FGF23 concentrations were significantly different over the duration of the trial (P<0.001), remaining stable in patients randomized to FCCC while increasing in patients randomized to usual care (Figure 3B). Similar results were observed with serum concentrations of C-terminal FGF23.
Mean serum PTH and 1,25-dihydroxy vitamin D3 concentrations were not significantly altered by treatment with FCCC (Figure 3, C and D).
Safety
As expected for patients with aCKD, adverse events were common in both treatment arms, as shown in Table 2. Gastrointestinal (GI) was the only system organ class in which related adverse events occurred in ≥5% of patients (relatedness of events could not be assessed in the usual-care arm due to the open-label design of the trial). The most common related GI adverse events were discolored feces (28%), constipation (12%), and diarrhea (9%.) No related serious adverse events occurred. Two deaths occurred in patients randomized to FCCC and one death occurred in those randomized to usual care.
Table 2.
Adverse events
| Organ System Class | All Adverse Events | Serious Adverse Events | ||
|---|---|---|---|---|
| Ferric Citrate n (%) | Standard of Care n (%) | Ferric Citrate n (%) | Standard of Care n (%) | |
| Blood and lymphatic system | 7 (5) | 2 (3) | ||
| Cardiac disorders | 13 (10) | 8 (12) | 5 (4) | 3 (4) |
| Endocrine disorders | 1 (1) | |||
| Eye disorders | 4 (3) | |||
| GI disorders | 63 (47) | 9 (13) | 1 (1) | 3 (4) |
| General | 13 (10) | 7 (10) | 1 (1) | 1 (2) |
| Hepatobiliary | 3 (4) | 1 (2) | ||
| Immune system | 1 (1) | |||
| Infections and infestations | 40 (30) | 26 (38) | 3 (2) | 6 (9) |
| Injury, poisoning | 9 (7) | 7 (10) | 1 (1) | |
| Investigations | 2 (2) | 2 (3) | ||
| Metabolism | 13 (10) | 6 (9) | 1 (1) | 2 (3) |
| Musculoskeletal | 8 (6) | 8 (12) | 2 (2) | 1 (2) |
| Neoplasms | 7 (5) | 1 (2) | ||
| Nervous system | 7 (5) | 4 (6) | 1 (1) | 3 (4) |
| Psychiatric | 1 (1) | 3 (4) | ||
| Renal and urinary | 10 (7) | 7 (10) | 4 (3) | 6 (9) |
| Reproductive | 2 (2) | |||
| Respiratory | 17 (13) | 7 (10) | 3 (2) | 2 (3) |
| Skin | 4 (3) | 2 (3) | ||
| Vascular | 9 (7) | 6 (9) | 2 (2) | 3 (4) |
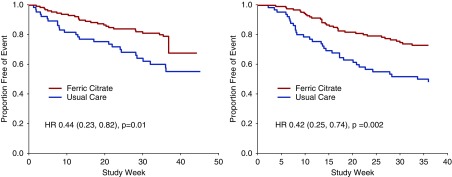
Hospital admission rates were significantly lower in patients randomized to FCCC relative to those randomized to usual care (mean±SD, 0.8±3.2 versus 1.71±3.48; and median, 10%–90% range, 0, 0–1.9 versus 0, 0–5.2 hospitalization events per year; Wilcoxon rank-sum P=0.001). The number of annualized hospital days was significantly lower in patients randomized to FCCC relative to usual care (mean±SD, 3.50±14.3 versus 10.6±21.8; and median, 10%–90% range, 0, 0–10.2 versus 0, 0–26 days; Wilcoxon rank-sum P=0.001). Kaplan–Meier analysis of the MITT population demonstrated a significant difference in time to first hospitalization (log-rank P=0.001) (Figure 4A). Multivariable adjusted proportional hazards regression (Cox) models showed a significant benefit on hospitalization in patients randomized to FCCC relative to patients randomized to usual care (HR, 0.44; 95% CI, 0.23 to 0.82; P=0.01).
Figure 4.
Kaplan–Meier survival function curve for time to first hospitalization (A) and time to the composite end point of death, dialysis, or transplant in all patients (B).
Thirty-one (23%) patients randomized to FCCC and 32 (48%) patients randomized to usual care initiated dialysis during the study period. eGFR declined in both groups although the decline was significantly attenuated in patients randomized to FCCC P=0.03. In a mixed-effects model with imputation of an eGFR of 10 ml/min per 1.73 m2 for patients initiating dialysis, the overall change in slope of eGFR was −0.20 ml/min per month in patients randomized to FCCC and −0.28 ml/min per month in patients randomized to usual care (P<0.001).
Kaplan–Meier analysis of the MITT population demonstrated a significant difference in time to death, dialysis, or transplant with FCCC relative to usual care (log-rank P=0.003) (Figure 4B). Multivariable adjusted Cox models showed that patients randomized to FCCC experienced a significant reduction in the risk of the composite end point of death, provision of dialysis, or kidney transplantation (HR, 0.42; 95% CI, 0.25 to 0.74; P=0.002).
Given the imbalance in the proportion of patients with diabetes randomized to both groups, we conducted companion analyses stratified by baseline diabetes status. Supplemental Figure 1 shows changes in hemoglobin, TSAT, ferritin, P, and FGF23 among patients with and without diabetes. Supplemental Figure 2, A and B, shows the composite end point (time to death, provision of dialysis, or kidney transplantation) in patients with and without diabetes. Events were more frequent in patients with diabetes, as expected.
Discussion
Our results demonstrate several important findings that relate to the care of patients with aCKD. When compared with usual care, the prescription of fixed-dose FCCC to patients with eGFR<20 ml/min per 1.73 m2 results in significant improvements in anemia-related end points (increases in hemoglobin, TSAT, and ferritin) and significant reductions in serum P and FGF23. These beneficial effects on biochemical end points were achieved despite enrolling patients without regard to any threshold definition for either serum hemoglobin or P and in patients who were generally not iron deficient or hyperphosphatemic. Additionally, these effects were achieved while reducing the need for exogenous ESAs and intravenous iron. Ferric citrate coordination complex was rarely discontinued due to intolerability and adverse events were essentially limited to the GI organ system, as has been previously reported.11
Globally, nearly 70% of patients with eGFR<20 ml/min per 1.73 m2 are anemic, largely the result of relative erythropoietin deficiency, inflammation, and iron deficiency.12 The optimal management of anemia in patients with CKD is unclear and the role of oral versus intravenous iron remains controversial. The FIND-CKD investigators randomized 626 patients with non–dialysis-dependent CKD (eGFR<60 ml/min per 1.73 m2) and presumed iron deficiency (TSAT<20%, ferritin<100 ng/ml) to two different intravenous iron strategies (high or low ferritin) versus oral iron (ferrous sulfate 100 mg twice daily) and showed that the low-dose intravenous iron and oral iron strategies were equivalent in raising hemoglobin (mean increase 0.9–1.0 g/dl).13 These same investigators recently addressed safety concerns with intravenous iron, reporting that after 1 year of follow-up there were no significant differences in serious adverse events, cardiac disorders, or infections between groups.14 The effects on anemia and mineral metabolism that we observed here among patients with late stage 4 and stage 5 CKD (eGFR≤20 ml/min per 1.73 m2) are consistent with previously published results from placebo- or active-controlled RCTs in patients with generally higher eGFR, presumed iron deficiency, and/or elevated serum P concentrations. In aggregate, these results suggest that use of FCCC would be consistent with the recommendation of the Kidney Disease: Improving Global Outcomes Clinical Practice Guideline for Anemia in CKD to use iron therapy in patients with a serum ferritin <500 μg/L or a TSAT≤30% if it is desired to increase hemoglobin or reduce the use of ESAs.2 Perhaps as a result of concerns about the safety of ESAs, recent observations report a progressive decline in hemoglobin in the 12 months before renal replacement and that this decline is associated with an increased risk of hospitalization.15,16 Our results in this high-risk population suggest that a simple, fixed-dose intervention may attenuate the decline in hemoglobin before initiation of dialysis and may reduce the need for “rescue” with ESAs, intravenous iron, or blood transfusion.
The effects of FCCC on serum P and FGF23 are also encouraging. Advances in our understanding of P physiology suggest that determinants of serum P concentrations are complex and are likely related to alterations in tissue-specific concentration of nicotinamide phosphoribosyltransferase (Nampt)/NAD+ effecting expression of sodium P transporters and transcellular P flux in active/nonactive periods.17,18 Nonetheless, serum P concentrations above or near the upper end of the population reference range have been associated with mortality, CV disease, vascular calcification, and incidence and progression of CKD in diverse populations.19–22 A meta-analysis of 12 cohort studies including 25,546 patients with non–dialysis-dependent CKD observed a 20% (95% CI, 5% to 37%) increase in mortality and a 36% (95% CI, 20% to 55%) increase in the risk of progressive CKD, defined as a doubling of serum creatinine, 50% decline in eGFR, or ESRD with each 1 mg/dl increase in serum P.23 Serum P was identified in the Chronic Renal Insufficiency Cohort (CRIC) study as the strongest CKD-specific risk factor for the development of coronary artery calcification in patients without coronary artery calcification at baseline.24 Dietary P loading has recently been implicated in the development of hypertension, acting via increased metanephrine and normetanephrine, an effect that resolved with discontinuation of P loading.25 There are no approved therapeutic agents for reducing P load in patients with CKD. Clinical trials examining the effects of P binders in non–dialysis-dependent CKD have yielded mixed results. Most studies, including those using FCCC have shown very modest effects on serum P11,26–29; effects on PTH and FGF23 have been mixed and agent-dependent, although FCCC has consistently resulted in relative reductions in FGF23. Our results demonstrate that fixed-dose FCCC maintained serum P concentrations within, or just above, the population reference range and abrogated the rise in FGF23 in the large majority of patients, despite very low eGFR.
Serum FGF23 concentration, independent of serum P, contributes to cardiomyocyte hypertrophy and systemic inflammation in patients with CKD and has been consistently associated with CV events (primarily congestive heart failure) and mortality.8,30–32 Data from CRIC suggest that the trajectory of change in FGF23 over time tracks with outcomes.33 Compared with patients with stable FGF23, those with rapidly rising FGF23 were at a 15-fold increased risk of death in fully adjusted analyses. In our pilot RCT, intact FGF23 rose sharply over a 9-month period in patients randomized to usual care, whereas fixed-dose FCCC abrogated this rise. FGF23 synthesis and secretion are stimulated by iron deficiency and it is likely that our findings vis-à-vis FGF23 may be due to the combined result of effects of ferric citrate on P load, serum P concentration, and correction of iron deficiency.34
We designed the trial to assess the feasibility and acceptance of an open-label intervention in a diverse population of patients nearing the need for dialysis; adverse events were consistent with previously published reports from placebo- and active-controlled trials of FCCC. Adherence to the prescribed dose was excellent overall, suggesting that GI tolerability was not a treatment-limiting event—a relevant consideration if such a strategy were to be adopted more broadly.
In light of the imbalance in baseline characteristics, we note with caution our exploratory finding that treatment with FCCC resulted in lower rates of death or the provision of dialysis, lower rates of hospitalization, and fewer hospital days. These effects strongly suggest that a therapeutic strategy including FCCC in patients with aCKD is likely to be safe. Observational studies have consistently linked hemoglobin, P, and FGF23 with clinically relevant outcomes. This pilot trial was not designed to determine the mechanism or mediators of a therapeutic effect of FCCC, but, rather, to determine whether the aggregate effects of FCCC might effectuate improved health in patients with aCKD.
Our results have implications for the design of a subsequent placebo-controlled trial by providing event rates for clinical end points in a population of patients selected solely by eGFR≤20 ml/min per 1.73 m2 demonstrating feasibility of enrollment, adherence, and safety. However, there are several important limitations to this pilot trial. First, the trial was single-center, of modest size, of relatively short duration, and was not designed or powered to detect effects on hard clinical end points. Because of the challenges of creating matching placebo with iron-based therapy, we did not address the feasibility of maintaining a placebo control over an extended duration. We would not have anticipated a 60% reduction in the occurrence of death, provision of dialysis, or kidney transplantation. Therefore, taking a Bayesian perspective, there is a strong possibility that the observed effect on the composite end point was overestimated, and possibly a “false positive.” As such, the results of our exploratory, event-driven analyses should be interpreted with caution. Because the decision to initiate dialysis was not protocol-driven, the lack of blinding may have influenced these events, further prompting caution in the interpretation of the results. Because decisions regarding hospitalization were generally made by emergency medicine or hospital medicine staff, it is unlikely that the difference in hospitalization could be attributed to lack of blinding (of treating physician or patient). Moreover, lack of blinding would be unlikely to influence results of objective laboratory tests including hemoglobin, serum P, and FGF23, which were well balanced at baseline.
In summary, treatment with fixed-dose FCCC in patients with eGFR≤20 ml/min per 1.73 m2 corrects anemia (without the routine use of intravenous iron or ESA) and favorably affects parameters of CKD-MBD. Effects of FCCC on death, provision of dialysis, and hospitalization suggest that this strategy is safe and potentially beneficial. These findings strongly support the conduct of an event-driven, multicenter, placebo-controlled RCT examining the effects of ferric citrate coordination complex on death, the provision of dialysis, or kidney transplantation in patients with aCKD.
Disclosures
Dr. G.A. Block reports grants from Keryx Biopharmaceuticals, Inc., during the conduct of the study; and personal fees and nonfinancial support from Akebia; personal fees and nonfinancial support from Amgen; personal fees and nonfinancial support from Astra-Zeneca; grants, personal fees, and nonfinancial support from Keryx Biopharmaceuticals, Inc.; personal fees and nonfinancial support from Ardelyx; personal fees and other from Reata; personal fees from Tricida; personal fees from OPKO; personal fees from Vifor Pharma; personal fees from Medici, Inc.; and personal fees from KHK, outside the submitted work. Dr. M.S. Block reports grants from Keryx Biopharmaceuticals, Inc., during the conduct of the study. Dr. Mehta reports other from Abbott Laboratories, other from Abbvie, Inc., other from Teva Pharmaceuticals Industries, Ltd, outside the submitted work. Dr. Isakova reports other from Shire, personal fees from Bayer, and personal fees from Eli Lilly, outside the submitted work. Dr. Wolf reports personal fees from Keryx Biopharmaceuticals, Inc., during the conduct of the study; and personal fees from AMGEN, personal fees from DIASORIN, personal fees from AKEBIA, personal fees from AMAG, personal fees from ARDELYX, personal fees from LUTIPOLD, personal fees from SANOFI, and grants from SHIRE, outside the submitted work. Dr. Chertow reports personal fees from Keryx Biopharmaceuticals, Inc., during the conduct of the study; and personal fees from Akebia, personal fees from AMAG, personal fees from Amgen, personal fees and other from Ardelyx, personal fees from Astra Zeneca, personal fees from Baxter, personal fees from Bayer, other from Durect, personal fees from Gilead, other from Outset, personal fees from Reata, personal fees from ReCor, personal fees from Sanifit, other from Cricket Health, and other from DxNow, outside the submitted work.
Funding
Funding for this trial and active study medication were provided by Keryx Biopharmaceuticals, Inc., through an investigator-initiated grant. Keryx Biopharmaceuticals, Inc. did not direct the design, conduct, or analysis of the trial.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Katrin Uhlig from Keryx Biopharmaceuticals, Inc. for her assistance in analysis and manuscript review.
Dr. Block: concept, design, conduct, analysis, and manuscript. M.S. Block: concept, design, conduct, and manuscript. Dr. Smits: conduct, analysis, and manuscript. Dr. Mehta: analysis and manuscript. Dr. Isakova: analysis and manuscript. Dr. Wolf: analysis and manuscript. Dr. Chertow: analysis and manuscript. Dr. Block had full ownership and responsibility for all data with no restrictions on publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018101016/-/DCSupplemental.
Supplemental Figure 1. Biochemical parameters in patients with diabetes (A–E).
Supplemental Figure 1. Biochemical parameters in patients without diabetes (F–J).
Supplemental Figure 2. KM curve for composite outcome of death, dialysis, or kidney transplantation in patients with (A) and without (B) diabetes.
Laboratory parameters by treatment arm over time for all patients.
KM product-limit survival estimates for time to death, dialysis, or kidney transplantation in patients randomized to ferric citrate.
KM product-limit survival estimates for time to death, dialysis, or kidney transplantation in patients randomized to usual care.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group : KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 76: 279–335, 2012 [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2(4): S1–S140, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al.: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al.: CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al.: TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 7.McCullough PA, Barnhart HX, Inrig JK, Reddan D, Sapp S, Patel UD, et al.: Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol 37: 549–558, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Scialla J, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al.: Fibroblast growth factor 23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellasi A, Mandreoli M, Baldrati L, Corradini M, Di Nicolò P, Malmusi G, et al.: Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol 6: 883–891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, et al. : Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-Analysis. JAMA 315(2): 1–11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chertow GM, Block GA, Neylan JF, Pergola PE, Uhlig K, Fishbane S: Safety and efficacy of ferric citrate in patients with nondialysis-dependent chronic kidney disease. PLoS One 12: e0188712, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong MMY, Tu C, Zepel L, Combe C, Lopes AA, pecoits-Filho R, et al. : Anemia prevalence and treatment among patients with chronic kidney disease stage 3-5: Data from the Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDOPPS). Nephrol Dial Transplant 31: i16–i17, 2016 [Google Scholar]
- 13.Macdougall IC, Bock AH, Carrera F, Eckardt KU, Gaillard C, Van Wyck D, et al.: FIND-CKD Study Investigators : FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 29: 2075–2084, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roger SD, Gaillard CA, Bock AH, Carrera F, Eckardt KU, Van Wyck DB, et al.: FIND-CKD Study Investigators : Safety of intravenous ferric carboxymaltose versus oral iron in patients with nondialysis-dependent CKD: An analysis of the 1-year FIND-CKD trial. Nephrol Dial Transplant 32: 1530–1539, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Renal Data System : 2016 USRDS annual data report: epidemology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, national Institue of Diabetes and Digestive and Kidney Diseases, 2016. [Google Scholar]
- 16.Kleine CE, Soohoo M, Ranasinghe ON, Park C, Marroquin MV, Obi Y, et al.: Association of pre-end-stage renal disease hemoglobin with early dialysis outcomes. Am J Nephrol 47: 333–342, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Nomura K, Tatsumi S, Miyagawa A, Shiozaki Y, Sasaki S, Kaneko I, et al. : Hepatectomy-related hypophosphatemia: A novel phosphaturic factor in the liver-kidney axis. J Am Soc Nephrol 25: 761–772, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyagawa A, Tatsumi S, Takahama W, Fujii O, Nagamoto K, Kinoshita E, et al.: The sodium phosphate cotransporter family and nicotinamide phosphoribosyltransferase contribute to the daily oscillation of plasma inorganic phosphate concentration. Kidney Int 93: 1073–1085, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Zoccali C, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, et al.: REIN Study Group : Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 22: 1923–1930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGovern AP, de Lusignan S, van Vlymen J, Liyanage H, Tomson CR, Gallagher H, et al.: Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: A large community based cohort study. PLoS One 8: e74996, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, et al. : Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 2251–2257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Seaghdha CM, Hwang SJ, Muntner P, Melamed ML, Fox CS: Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant 26: 2885–2890, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Da J, Xie X, Wolf M, Disthabanchong S, Wang J, Zha Y, et al.: Serum phosphorus and progression of CKD and mortality: A meta-analysis of cohort studies. Am J Kidney Dis 66: 258–265, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Bundy JD, Chen J, Yang W, Budoff M, Go AS, Grunwald JE, et al.: CRIC Study Investigators : Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis 271: 53–60, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammad J, Scanni R, Bestmann L, Hulter HN, Krapf R: A controlled increase in dietary phosphate elevates BP in healthy human subjects. J Am Soc Nephrol 29: 2089–2098, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block GA, Ix JH, Ketteler M, Martin KJ, Thadhani RI, Tonelli M, et al.: Phosphate homeostasis in CKD: Report of a scientific symposium sponsored by the National Kidney Foundation. Am J Kidney Dis 62: 457–473, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, et al.: A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3-5. Am J Kidney Dis 65: 728–736, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Block GA, Persky MS, Ketteler M, Kestenbaum B, Thadhani R, Kooienga L, et al.: A randomized double-blind pilot study of serum phosphorus normalization in chronic kidney disease: A new paradigm for clinical outcomes studies in nephrology. Hemodial Int 13: 360–362, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al.: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al.: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, et al.: MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al. : Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isakova T, Cai X, Lee J, Xie D, Wang X, Mehta R, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Longitudinal FGF23 trajectories and mortality in patients with CKD. J Am Soc Nephrol 79(12): 579–590, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Block GA, Pergola PE, Fishbane S, Martins JG, LeWinter RD, Uhlig K, et al. : Effect of ferric citrate on serum phosphate and fibroblast growth factor 23 among patients with nondialysis-dependent chronic kidney disease: Path analyses [published online ahead of print October 30, 2018]. Nephrol Dial Transplant 10.1093/ndt/gfy318 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.