Significance Statement
In the Systolic BP Intervention Trial (SPRINT), intensive targeting of systolic BP (goal <120 mm Hg) versus standard targeting (goal of <140 mm Hg) significantly reduced risks of cardiovascular events and all-cause mortality. However, the intensive intervention also resulted in greater early reduction of eGFR in the first 6 months, raising the question of whether reductions in eGFR resulting from intensive BP control worsen an individual’s health status. In causal mediation analyses of SPRINT data, the authors found no evidence that this early eGFR decline either mediated or modified the beneficial effects of intensive systolic BP lowering on cardiovascular events or all-cause mortality. However, longer-term follow-up studies with causal modeling are needed to better understand the downstream effects of the early reduction in eGFR that results from intensive systolic BP lowering.
Keywords: renal hemodynamics, hypertension, cardiovascular disease, mortality
Visual Abstract
Abstract
Background
The Systolic BP Intervention Trial (SPRINT) found that intensive versus standard systolic BP control (targeting <120 or <140 mm Hg, respectively) reduced the risks of death and major cardiovascular events in persons with elevated cardiovascular disease risk. However, the intensive intervention was associated with an early decline in eGFR, and the clinical implications of this early decline are unclear.
Methods
In a post hoc analysis of SPRINT, we defined change in eGFR as the percentage change in eGFR at 6 months compared with baseline. We performed causal mediation analyses to separate the overall effects of the randomized systolic BP intervention on the SPRINT primary cardiovascular composite and all-cause mortality into indirect effects (mediated by percentage change in eGFR) and direct effects (mediated through pathways other than percentage change in eGFR).
Results
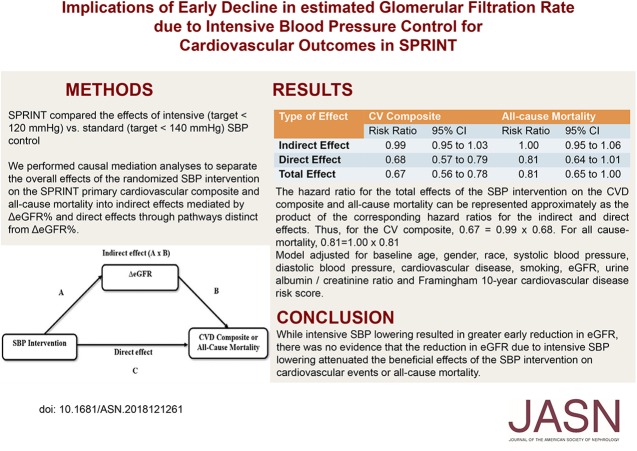
About 10.3% of the 4270 participants in the intensive group had a ≥20% eGFR decline versus 4.4% of the 4256 participants in the standard arm (P<0.001). After the 6-month visit, there were 591 cardiovascular composite events during 27,849 person-years of follow-up. The hazard ratios for total effect, direct effect, and indirect effect of the intervention on the cardiovascular composite were 0.67 (95% confidence interval [95% CI], 0.56 to 0.78), 0.68 (95% CI, 0.57 to 0.79), and 0.99 (95% CI, 0.95 to 1.03), respectively. All-cause mortality results were similar.
Conclusions
Although intensive systolic BP lowering resulted in greater early decline in eGFR, there was no evidence that the reduction in eGFR owing to intensive systolic BP lowering attenuated the beneficial effects of this intervention on cardiovascular events or all-cause mortality.
Hypertension is strongly associated with stroke, heart failure, sudden death, ESRD, and death from all causes.1–4 Recently, the Systolic BP Intervention Trial (SPRINT) demonstrated that intensive systolic BP (SBP) lowering (SBP target <120 mm Hg versus <140 mm Hg) reduced the risks of death and major cardiovascular events in persons with elevated cardiovascular disease (CVD) risk.5,6 However, compared with the standard SBP arm participants, intensive SBP arm participants had a relative mean decline in eGFR of −3.31±0.30 ml/min per 1.73 m2 by 6 months,7 and, a 3.5-fold higher relative risk of incident CKD among participants without CKD at baseline.5 Similarly, an early decline in eGFR with intensive SBP lowering in SPRINT participants with CKD at baseline was also noted.8
Whether the increased incidence of CKD with intensive SBP lowering reflected a hemodynamic effect or resulted from intrinsic kidney injury is controversial. However, the relationship of lower eGFR with increased risks of mortality and CVD events9,10 raises the question of whether reductions in eGFR that result from intensive SBP lowering worsen an individual’s health status. Previous studies have investigated the association between early eGFR decline with BP lowering and clinical outcomes.11–16 However, this association reflects the consequences of all of the variations in the early change in eGFR, some of which reflects variation due to the randomized SBP intervention and some of which reflects other factors, including natural variation in eGFR, measurement error, and disease progression that would have occurred even in the absence of the intensive SBP intervention. To address these limitations, we conducted a causal mediation analysis17–19 to examine the extent to which early eGFR decline that was specifically due to the intensive BP intervention attenuated the overall beneficial effects of the intensive SBP intervention on CVD events and all-cause mortality.
Methods
SPRINT was a randomized, controlled, open-label trial of 9361 participants6 sponsored by the National Institutes of Health to compare the effects of intensive (SBP target <120 mm Hg) versus standard (SBP target <140 mm Hg) BP control on cardiovascular outcomes in participants aged 50 years or older with baseline SBP of 130–180 mm Hg and an increased risk of CVD. Details of the SPRINT protocol including details of inclusion and exclusion criteria, intervention, measurements, and follow-up have been published.20,21 The current analysis is limited to participants with data available to calculate the change in eGFR (∆eGFR) between the baseline and the 6-month visit. Participants who had a CVD composite end point during the first 6 months of follow-up were excluded from our primary analyses (Supplemental Figure 1).
Serum specimens were obtained at each monthly visit for the first 3 months, then at sixth months and every 6 months thereafter, for measurement of creatinine at the SPRINT central laboratory located at the University of Minnesota an enzymatic assay (Roche, Indianapolis, IN) standardized with calibration traceable to an isotope dilution mass spectrometry reference measurement procedure. The SPRINT protocol prespecified the four-variable Modification of Diet Renal Disease (MDRD) equation to estimate GFR.22 In an earlier report, we observed that the effects of intensive SBP lowering on incident CKD defined either with the MDRD equation or the CKD Epidemiology Collaboration equation were similar.7 Hence, we used MDRD eGFR in the current analysis to define ∆eGFR as 6-month eGFR minus the baseline eGFR, expressed in ml/min per 1.73 m2, and ∆eGFR% as the percent change in eGFR over the same 6-month period.
SPRINT Outcomes
Definitions of SPRINT outcomes are provided in the SPRINT protocol.20,21 A committee blinded to the study group assignments adjudicated the primary outcomes specified in the protocol.20,21 The primary outcome in SPRINT was a composite of nonfatal myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from CVD causes. Death from any cause was a predefined secondary outcome. The main secondary kidney outcome was a composite of a ≥50% decrease in eGFR or development of ESRD in participants with baseline CKD (eGFR<60 ml/min per 1.73 m2). As there were very few kidney events (16 events per 4675 years in the intensive group and 12 events per 4536 years in the standard SBP group), the kidney composite outcome was not included in the current analysis.
Statistical Methods
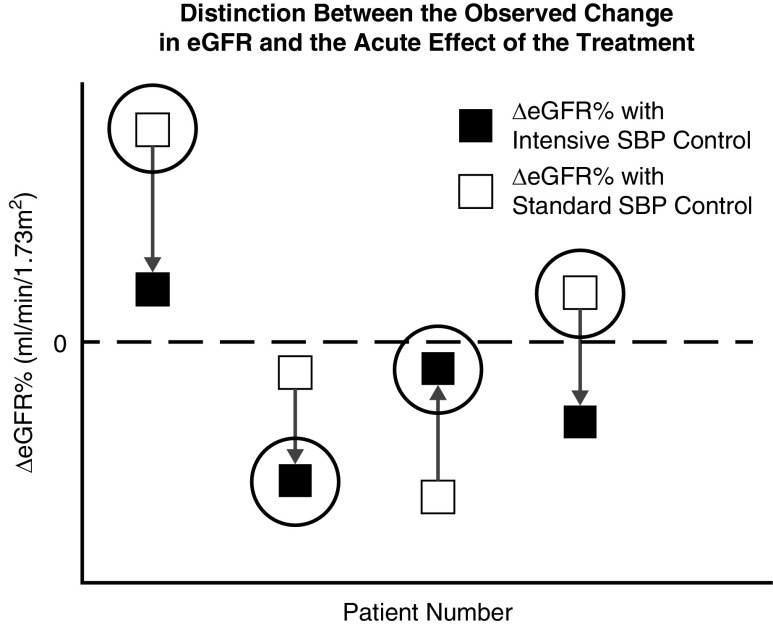
To understand the implications of the acute effect of the intensive SBP control on early eGFR change for subsequent outcome, it is important to distinguish between the observed changes in eGFR that occur after initiating the SBP interventions and the effect of the SBP interventions on those changes. Figure 1 illustrates this distinction for four hypothetical patients. The actual observed changes in eGFR from baseline to 6 months, which reflect the effects of the treatment and numerous other factors, are depicted by the ΔeGFR% values enclosed in large open circles, two assigned to intensive SBP control (participants 2 and 3) and two to standard SBP control (participants 1 and 4). To represent the effect of the treatment on eGFR change, we must also consider the unobserved counterfactual changes that would have occurred on the intervention the participant did not receive; these changes are represented by the ΔeGFR% values not enclosed by open circles. The acute effect of the treatment on eGFR change is defined by the vertical arrows extending from the change in eGFR on standard SBP control to the change in eGFR on intensive SBP control. Note that both the magnitude and the direction of the acute effect may differ from the observed eGFR change; for example, the observed eGFR for participant 3 declined under intensive SBP control, but the acute effect of intensive SPB control increased the eGFR compared with standard SBP control.
Figure 1.
Distinction between the observed change in eGFR and the acute effect of the treatment. Shown are percent changes in eGFR from baseline to 6 months under the standard SBP intervention (open squares) and under the intensive SBP intervention (solid squares) for four hypothetical participants. The difference in these changes between the intensive and standard SBP interventions (represented by the vertical arrows) define the acute effects of the treatment for these four patients. We actually observe the change in eGFR for only one of the two interventions, depending on each patient’s randomly assigned treatment (depicted by the large open circles). We cannot observe the acute effect in any individual participant, but instead can observe the percent change in eGFR only under the participants’ assigned interventions. The direction and magnitude of the acute effects may deviate from the direction and magnitude of the observed percent changes in eGFR. The indirect effect of the treatment which is mediated by change in eGFR is the difference in the outcome that results if the acute change in eGFR is modified by the amounts indicated by the vertical arrows.
The acute effects of the intensive SBP intervention on eGFR change are unobservable for each individual because we are able to observe the eGFR change under one of the intensive or standard interventions, but not both. This constraint, which is particular case of the fundamental limitation of causal inference, highlights the challenge of evaluating the clinical implications of the acute effect. However, the framework of causal mediation analysis can be used to connect relationships involving the observed ΔeGFR% values to relationships involving the unobserved effects of the intensive SBP intervention on ΔeGFR%, and articulate specific assumptions under which the implications of the acute effect for longer-term outcomes can be estimated. Accordingly, we apply mediation analysis to estimate three types of effects of the intensive SBP intervention relative to the standard SBP intervention:
The total effect of the intensive intervention on long-term outcome (either CVD or mortality), expressed as a hazard ratio comparing the intensive and standard interventions. The total effect is decomposed into the following two components.
The indirect effect, measuring the portion of total effect mediated through a pathway that extends through ΔeGFR%. In terms of Figure 1, the indirect effect represents the effect on the long-term outcome of changing ΔeGFR% from its value under standard SPB control (given by the beginnings of the vertical arrows) to its value under intensive SBP control (given by the ends of the vertical arrows).
The direct effect, measuring the portion of total effect occurring through other pathways that do not include early change in eGFR.
Both the indirect and direct effects are expressed as hazard ratios, and provide the approximate decomposition: total effect hazard ratio=indirect effect hazard ratio×direct effect hazard ratio (Figure 2). In addition to the overall direct effect, which is averaged across the study population, we also estimate the controlled direct effects given by hazard ratios comparing the long-term outcome between intensive and standard SBP control when ΔeGFR% is held fixed at specific values. Evaluating the controlled direct effect across a grid of specific values for ΔeGFR% that extends throughout the observed ΔeGFR% range allows us to assess if the direct effect of the SBP intervention differs between patients with greater versus lesser early eGFR decline. A more precise description of the direct and indirect effects is given in Supplemental Appendix 1.
Figure 2.
Mediation of effect of the SBP interventions on the CVD composite or mortality by early eGFR decline (∆eGFR). The overall (or total) effect of the SBP intervention on the CVD composite or all-cause mortality may be decomposed into the indirect effect (A and B) mediated by ∆eGFR and the direct effect (C), which represents the effect of the intervention through pathways unrelated to ∆eGFR. The effect of ∆eGFR on the CVD composite or all-cause mortality (B) reflects the consequences of variation in ∆eGFR resulting from the SBP intervention as well as other factors, including natural variation in eGFR, measurement error, and disease progression that would have occurred in the absence of the intervention. The indirect effect (A×B) represents the consequences of the acute effect of the SBP intervention on ∆eGFR for the CVD composite or all-cause mortality. Although the total effect of the SBP intervention can be estimated using intent-to-treat analysis under the randomized design, estimation of the indirect and direct effects requires control of confounding factors that jointly influence ∆eGFR and the CVD composite or all-cause mortality.
The mediation analyses are on the basis of two regression models: (1) a multiple linear regression relating ΔeGFR% to the randomized intervention group and ten baseline covariates selected using subject matter knowledge (baseline age, sex, race, smoking status, SBP, diastolic BP, history of CVD, Framingham 10-year CVD risk score, eGFR, and urine albumin-to-creatinine ratio); and (2) Cox proportional hazard regressions relating the outcome (either the CVD composite or all-cause mortality) to randomized group, ΔeGFR%, the interaction between randomized group and ΔeGFR%, and the same baseline covariates as the first model. Including the interaction term provides a joint evaluation of whether ΔeGFR% mediates and/or moderates the effect of the SBP intervention on the outcomes. Before the final mediation analyses, we used Cox regressions to relate the CVD composite and all-cause mortality outcomes to a cubic spline in ΔeGFR% with knot points at each ΔeGFR% quintile, as well as the randomized SBP group, the interaction between SBP group and the cubic spline in ΔeGFR%, and the ten baseline covariates. Given no evidence of nonlinear effects of ΔeGFR%, our final mediation analyses assumed linear effects of ΔeGFR%. We used bootstrap resampling with 500 bootstrap samples to compute confidence intervals and P values.
Under the randomized design, the causal interpretation of the direct and indirect effects depends primarily on two key assumptions: (1) the baseline covariates included in the regression models must control for all confounding between ΔeGFR% and the long-term clinical outcomes, and (2) The effect of changing ΔeGFR% on the long-term clinical outcomes must be the same irrespective of whether the changes are caused by the SBP intervention or other causes.
We will return to the second assumption in the Discussion and in Supplemental Appendix 1. To address the first assumption, our primary analyses included the above ten baseline covariates selected using subject matter knowledge. In sensitivity analyses, the mediation analyses were repeated with expanded sets of covariates (Supplemental Table 1) obtained by applying forward stepwise variable selection to 58 additional baseline factors with the ten covariates from the primary analyses forced in the model. The forward stepwise regression led to selection of 22 additional covariates for the CVD composite outcome and 16 additional covariates for the mortality outcome. Finally, we performed an additional sensitivity analysis in which the methods described by Vanderweele19,23 were applied to assess the robustness of our conclusions to the possibility of an uncontrolled binary confounder whose occurrence leads both to greater risk of adverse clinical outcomes and to greater initial eGFR decline. Sensitivity analyses are also presented, in which CVD composite events in the first 6 months were retained.
Results
Of the 9361 SPRINT participants, 8526 (standard arm n=4256 and intensive arm n=4270) with data on early eGFR decline were included in the current analysis (Supplemental Figure 1). Clinical characteristics are summarized by ∆eGFR% groups in Table 1 and intervention groups in Supplemental Table 2. Those with larger percentage declines in eGFR had higher baseline eGFR, SBP, diastolic BP (DBP), change in SBP (6 months minus baseline), change in DBP (6 months minus baseline), Framingham risk score, and urine albumin-to-creatinine ratio. Conversely, the two groups with the greatest 6-month increase in eGFR had a higher baseline prevalence of CKD.
Table 1.
Clinical characteristics by early eGFR decline groups (n=8526)
| Characteristic | <− 20% | −20% to <−10% | −10% to <10% | 10% to <20% | ≥20% | P Value |
|---|---|---|---|---|---|---|
| n=623 (7.3%) | n=1103 (13.0%) | n=4547 (53.3%) | n=1393 (16.3%) | n=860 (10.1%) | ||
| ∆eGFR (%) | −28.3±8.3 | −14.3±2.8 | 0.1±5.5 | 14.5±2.9 | 30.7±12.8 | |
| ∆eGFR (ml/min per 1.73 m2) | −21.1±10.4 | −10.7±3.9 | 0.0±4.1 | 10.0±3.4 | 18.9±7.8 | |
| Baseline MDRD eGFR (ml/min per 1.73 m2) | 74±25 | 75±22 | 73±19 | 69±19 | 63±18 | <0.001 |
| Baseline age (year) | 68.4±9.7 | 67.8±9.4 | 67.8±9.3 | 68.0±9.2 | 67.1±9.3 | 0.095 |
| Female (%) | 35 | 33 | 33 | 37 | 42 | <0.001 |
| Black (%) | 34 | 32 | 31 | 28 | 32 | 0.025 |
| Never smoked (%) | 42 | 44 | 44 | 45 | 44 | 0.60 |
| Intensive SBP group (%) | 70 | 60 | 49 | 42 | 41 | <0.001 |
| Baseline SBP, mm Hg | 146±17 | 143±15 | 139±15 | 137±15 | 135±15 | <0.001 |
| Baseline DBP, mm Hg | 79±13 | 79±12 | 78±12 | 77±11 | 76±12 | <0.001 |
| ∆SBP (6 mo–baseline) (mm Hg) | −24±20 | −18±18 | −11±18 | −6±18 | −3±20 | <0.001 |
| ∆DBP (6 mo–baseline) (mm Hg) | −12±12 | −9±11 | −6±11 | −4±10 | −2±12 | <0.001 |
| Baseline CKD (%) | 30 | 26 | 25 | 31 | 43 | <0.001 |
| Baseline CVD (%) | 20 | 20 | 20 | 18 | 19 | 0.54 |
| Baseline Framingham 10-yr risk score | 25 (17–36) | 24 (17–34) | 22 (15–32) | 21 (15–30) | 20 (14–28) | <0.001 |
| Baseline urine ACR, mg/g | 14 (7–42) | 11 (6–26) | 9 (6–20) | 8 (5–17) | 9 (5–18) | <0.001 |
Data are presented as mean±SD or median (interquartile range) for continuous measures and percent for categorical measures. ∆SBP, change in SBP; ∆DBP, change in DBP; ACR, albumin-to-creatinine ratio.
Effect of the Randomized SBP Intervention on Early Change in eGFR
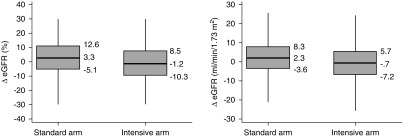
The median (25th and 75th percentiles) of ∆eGFR and ∆eGFR% in the intensive SBP arm were −0.7 (−7.2, 5.7) ml/min per 1.73 m2 and −1.2% (−10.3%, 8.5%), respectively (Figure 3). Corresponding values in the standard SBP arm were 2.3 (−3.6, 8.3) ml/min per 1.73 m2 and 3.3% (−5.1%, 12.6%), respectively. A ≥10% decline in eGFR occurred in 25.6% in the intensive arm and 14.9% in the standard arm (P<0.001) and a ≥20% decline occurred in 10.3% in the intensive arm and 4.4% in the standard arm (P<0.001). After adjustment for the ten baseline covariates, the adjusted mean ∆eGFR% was 5.07% (95% confidence interval [95% CI], 4.41% to 5.73%) lower in the intensive SBP group than the standard SBP group, which corresponded to an effect size (defined as the ratio of the estimated mean difference to the SD of ∆eGFR%) of 0.31. The squared correlation (R2) between ∆eGFR% and the randomized group was 0.023 indicating that randomization to the intensive group explained 2.3% of the variance in 6-month change in eGFR.
Figure 3.
Box plots for ∆eGFR% and for ∆eGFR in the standard and intensive SBP arms. Shown are the first percentile, 25th percentile, median, 75th percentile, and 99th percentile.
Association between ∆eGFR% and the CVD Composite and Mortality
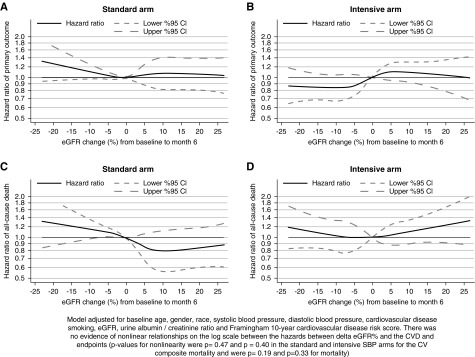
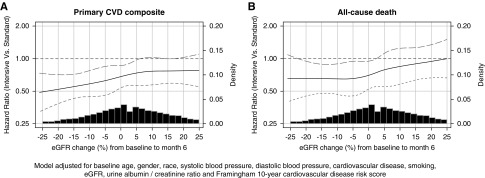
There were 591 primary CVD composite events over 27,849 years of follow-up and 382 all-cause deaths over 28,733 years of follow-up after (n=8526). Figure 4 displays the relationship between ∆eGFR% and the hazards for subsequent CVD composite and all-cause mortality events after adjustment for the ten baseline covariates within each randomized SBP group under a cubic spline model. Tests for the presence of nonlinear relationships in ∆eGFR% were not statistically significant for the CVD composite (P=0.40 and 0.47 in the intensive and standard SBP arm, respectively) or mortality (P=0.33 and 0.19 in the intensive and standard SBP arms, respectively). In linear models relating the log transformed hazards for the clinical outcomes to ∆eGFR% and its interaction with randomized SBP group, each 10% decrement in ∆eGFR% (representing greater initial eGFR decline) was associated with adjusted hazard ratios of 1.04 (95% CI, 0.97 to 1.12) for the CVD composite outcome and 1.08 (95% CI, 0.98 to 1.19) for 2 the all-cause mortality in the intensive SBP arm and 0.97 (95% CI, 0.89 to 1.05) for the CVD composite outcome and 0.98 (95% CI, 0.89 to 1.09) for all-cause mortality in the standard SBP arm; the hazard ratios for the two intervention arms did not differ significantly from each other for either outcome (interaction P=0.12 for the CVD composite and interaction P=0.20 for all-cause mortality).
Figure 4.
Association of ∆eGFR% with the CVD composite and all-cause mortality by randomized treatment arm. There was no evidence of nonlinear relationships on the log scale between the hazards between ∆GFR% and the CVD and all-cause mortality endpoints (P values for nonlinearity in the standard and intensive SBP arms were P=0.40 and P=0.47 for the CVD composite and P=0.33 and P=0.19 for all-cause mortality, respectively).
Controlled Direct Effects of the Intensive SBP Group on the CVD Composite and All-Cause Mortality Outcomes at Fixed ∆eGFR%
Figure 5 presents estimates of the controlled direct effects that evaluate the effect of the intensive versus the standard SBP intervention on the CVD composite and all-cause mortality outcomes when ∆eGFR% is held fixed at the values indicated in the horizontal axis, on the basis of the same cubic spline model used in the previous section. The hazard ratios defining the controlled direct effects of the intensive SBP interventions were significantly <1 throughout much of the ∆eGFR% range for both clinical outcomes, but did not differ significantly between different ∆eGFR% levels for either the CVD composite (interaction P=0.12) or all-cause mortality (P=0.20). This suggests that the benefits of the intensive SBP intervention through mechanisms other than through ∆eGFR% occur irrespective of the level of ∆eGFR%.
Figure 5.
Controlled direct effects of SBP intervention at different levels of ΔeGFR%. The figure displays the estimated controlled direct effects of the intensive SBP intervention on the CVD composite (left) and all-cause mortality (right) when ΔeGFR% is held fixed at the values indicated on the horizontal axis. The interaction P values between ΔeGFR% and the randomized SBP group are 0.12 for the CVD composite and 0.20 for all-cause mortality, indicating that controlled direct effects do not differ significantly between different levels of early change in ΔeGFR%.
Mediation Analysis for the CVD Composite Outcome
The estimated hazard ratio for the CVD composite outcome corresponding to the total effect of the intensive SBP intervention was 0.67 (95% CI, 0.56 to 0.78) in the analytic sample (Table 2). In mediation analysis, the hazard ratio for the direct effect (not mediated through ∆eGFR) of the intervention on the primary CVD composite end point was 0.68 (95% CI, 0.57 to 0.79) and the hazard ratio corresponding to the indirect effect (mediated through ∆eGFR%) was 0.99 (95% CI, 0.95 to 1.03) (Table 2). Thus, the indirect effect mediated through ∆eGFR% had a negligible contribution to the total effect of the intervention on the CVD composite outcome.
Table 2.
Mediation analysis of the effect of the SBP intervention on the CVD composite and all-cause mortality
| Type of Effect | CVD Composite | All-Cause Mortality | ||
|---|---|---|---|---|
| Risk Ratio | 95% CI | Risk Ratio | 95% CI | |
| Indirect effect | 0.99 | 0.95 to 1.03 | 1.00 | 0.95 to 1.06 |
| Direct effect | 0.68 | 0.57 to 0.79 | 0.81 | 0.64 to 1.01 |
| Total effect | 0.67 | 0.56 to 0.78 | 0.81 | 0.65 to 1.00 |
The hazard ratio for the total effects of the SBP intervention on the CVD composite and all-cause mortality can be represented approximately as the product of the corresponding hazard ratios for the indirect and direct effects. Thus, for the CVD composite, 0.67=0.99×0.68. For all cause-mortality, 0.81=1.00×0.81. Model adjusted for baseline age, sex, race, SBP, diastolic BP, CVD, smoking, eGFR, urine albumin-to-creatinine ratio, and Framingham 10-year CVD risk score.
Mediation Analysis for All-Cause Mortality
The hazard ratio corresponding to the total effect of the intervention was 0.81 (95% CI, 0.65 to 1.00) in the analytic sample. In mediation analysis, the hazard ratio for the direct effect of the intervention on all-cause mortality was 0.81 (95% CI, 0.64 to 1.01) and the hazard ratio corresponding to the indirect effect mediated through ∆eGFR% was 1.00 (95% CI, 0.95 to 1.06) (Table 2), indicating no evidence of mediation of the intervention effect on the all-cause mortality by ∆eGFR%.
Sensitivity Analyses
Results were similar when the analyses were repeated after adjustment for the extended sets of covariates that resulted from stepwise selection (Supplemental Figure 2), and when CVD composite events were incorporated throughout the full follow-up period, including the first 6 months (Supplemental Tables 3–5), during which 656 CVD composite outcomes and 404 all-cause deaths were noted. We performed an additional sensitivity analysis to assess the possibility that failure to control for an unmeasured confounder could have led us to underestimate the indirect effect by an amount large enough to cause our estimated indirect effect hazard ratios to fall close to the null effect of 1 (0.99 and 1.00 for the CVD composite and mortality, respectively) in the presence of true indirect effects large enough to matter clinically. We considered the hazard ratio for the indirect effect to be clinically relevant if it was 1.10 or higher. In principle, such a bias could occur if, among subsets of patients with the same ∆eGFR%, the unmeasured confounder was more prevalent in the standard SBP arm compared with the intensive SBP arm (which might account for equal ∆eGFR% values in spite of the greater mean acute eGFR decline in the intensive SBP arm), and was also associated with greater risk of the clinical outcomes. We allowed for up to a 4% greater prevalence of the uncontrolled confounder in the standard SBP arm given the same ∆eGFR%, a difference much larger than the maximum difference 2.61% in prevalence for any of the 68 baseline variables we considered as possible covariates (Supplemental Table 6). The hazard ratios for such a confounder would have to be very high (>3.78 for the CVD composite outcome and 3.50 for all-cause mortality) to be consistent with a hazard ratio for the true indirect effect as large as 1.10. It appears unlikely that a confounder with such high hazard ratios could have been missed from the SPRINT trial baseline data, but this possibility cannot be ruled out completely.
Discussion
The main findings of this study are that, although intensive SBP lowering resulted in a higher proportion of individuals with early decline in eGFR, there was no evidence that this early decline in eGFR either mediated or modified the effects of intensive SBP lowering on the primary CVD end point or all-cause mortality in the SPRINT trial among hypertensive individuals randomized to intensive versus standard SBP intervention.
The primary results of SPRINT showed that intensive SBP lowering reduced the risk of the primary CVD composite outcome by 25% and all-cause mortality by 27%.5,6 Indeed, the SPRINT intervention was stopped early because of beneficial effects of the intervention. However, intensive SBP lowering resulted in an increased risk of incident CKD in the SPRINT,7 Action to Control Cardiovascular Risk in Diabetes BP,24 and Secondary Prevention of Small Subcortical Strokes25 trials and an increased risk of AKI in the SPRINT trial.26 We have reported that intensive SBP lowering resulted in an acute early decline in eGFR in both the SPRINT trial non-CKD7 and CKD8 subgroups.
To understand the clinical implications of the effects of intensive SBP lowering and other interventions on early change in GFR (∆eGFR), previous reports have investigated the association of longer-term end points with ∆eGFR after initiation of the intervention.11–16,27 Although the intensive SBP intervention did have a highly statistically significant effect on ∆eGFR%, and led to a more than two-fold increase in the proportion of patients with at least a 20% acute eGFR decline, the intervention accounted for a small percentage (2.3%, on the basis of the R2 value) of the overall variation in ∆eGFR% between participants. Thus, analyses relating ∆eGFR to the clinical end points primarily reflect consequences of variation in ∆eGFR due to factors besides the intervention. To address the implications of the ∆eGFR that were specifically due to the intervention, it is necessary to adopt the framework of mediation analysis as presented herein. In this framework, the indirect effect of the intervention that was mediated by ∆eGFR describes the implications of the response of eGFR to the intervention on subsequent clinical events, and the direct effect describes intervention effects that operate through mechanisms distinct from ∆eGFR.
Using this mediation analysis framework, we found that the hazard ratios for the indirect effects of the intervention on the CVD composite outcome and all-cause mortality were close to 1, and that the direct effects of the intervention closely approximated the total effects, indicating that almost all of the effects of the intervention on the clinical end points occurred through mechanisms distinct from ∆eGFR.
In our setting, where the intervention assignment was randomized, the primary assumption required for a meaningful interpretation of the indirect and direct effects is that the covariates included in the analysis are sufficient to control for confounding factors that jointly influenced ∆eGFR and the clinical end points. The risk that this assumption may be violated is the core threat to the validity of our mediation analyses, and is analogous to similar risks of uncontrolled confounding that can occur whenever postrandomization covariates are related to outcomes in randomized, controlled trials. To limit this risk, we capitalized on the extensive set of baseline variables in the SPRINT trial database to apply a comprehensive strategy to covariate adjustment. We adjusted for ten potential confounders in our primary analyses, and in sensitivity analyses confirmed that the primary results were unaltered after adjusting for additional factors selected by forward stepwise variable selection from among 58 additional covariates identified as possible confounders by the study investigators. We also performed a sensitivity analysis to assess the likelihood that failure to control for an unmeasured confounder might have masked the true adverse indirect effect of the intervention on the clinical outcomes. This sensitivity analysis indicated that, under plausible scenarios, an uncontrolled confounder must have extremely strong relationships with the CVD composite and all-cause mortality to influence our results sufficiently to mask an adverse indirect effect that is large enough to be clinically meaningful. The likelihood of such a powerful unmeasured confounder appears small, but cannot be ruled out entirely.
In Supplemental Appendix 1, we provide additional arguments that demonstrate that measurement error in eGFR and variation between patients in the 6-month change in SBP within the intensive and standard SBP groups are not likely to have affected our conclusion that the indirect effect of the SBP intervention mediated by ΔeGFR% is clinically negligible.
The robustness of the conclusion of negligible indirect effects mediated by ΔeGFR% may explained in part by the relatively small effect size of the intervention on the mean ΔeGFR% level when considered in relation to the full variation in ΔeGFR% across the SPRINT study population. Because of this relatively small effect size (corresponding to a standardized mean difference of 0.31 and a squared multiple correlation of 0.02), indirect effects mediated through ΔeGFR% are consistently small across the range of estimated effects of ΔeGFR% on the clinical end points in our various sensitivity analyses.
In addition to the issue of mediation, investigators have also questioned whether ∆eGFR might modify, or moderate, the effect of intensive BP interventions.12,16 This is a complex issue because of the challenges of defining effect modification by a factor that is itself affected by the intervention. However, the analysis of Figure 4 shows that there is no evidence that the direct effect of SBP lowering varies between different levels of ∆eGFR. This result, combined with the absence of an overall indirect effect of the intervention, suggests that early eGFR decline not only does not mediate but also does not modify the effect of intensive SBP intervention on these clinical end points, or at least any mediation or moderation that does occur is not large enough to be clinically relevant.
Major strengths of this study include the rigorous application of the framework of casual mediation analysis to investigate the consequences of ∆eGFR that were specifically due to the intervention; the large sample size of the SPRINT trial, which supported adequate precision in estimates of the direct and indirect effects; the presence of an acute effect on early eGFR change, which was relatively large compared with several other studies that have investigated intensive SBP interventions11–16; and the extensive baseline characterization of the SPRINT cohort, which allowed us to provide a more comprehensive adjustment for confounding factors that would otherwise be possible.
Limitations include the relatively short duration of follow-up in the SPRINT trial. If the early eGFR decline with intensive SBP reduction increases the future risk of CVD events or all-cause mortality, the association of the intervention and of ∆eGFR on those outcomes could follow a nonproportional hazard function (e.g., a decreased risk early, which attenuates or reverses later); hence, it is possible that a nonzero indirect effect could emerge with longer follow-up. The present analyses were post hoc. As we have emphasized, our estimates of direct and indirect effects may be biased because of unmeasured confounding, although our sensitivity analyses suggest that it is fairly unlikely that such a bias would be large enough to substantially alter the clinical interpretation of our findings. Our application of the framework of causal inference should not be interpreted as a claim that our analyses can support causal inferences without qualification, but rather as an approach for applying methods that limit bias to the extent possible, and to clearly define the assumptions that are required to address causal issues on the basis of the data. Furthermore, as there were very few hard kidney end point events in the SPRINT trial, we were unable to extend our mediation analyses to a kidney event outcome. Finally, as there were very few SPRINT trial participants with stage 4 CKD, these results cannot be extrapolated to those with more advanced kidney disease.
In summary, during the SPRINT trial follow-up, there was no evidence that early reductions in eGFR mediated or adversely moderated the CVD and all-cause mortality benefits that resulted from intensive SBP lowering. Longer-term follow-up studies with causal modeling are needed to better understand the downstream effects of early eGFR decline with intensive SBP lowering.
Disclosures
Dr. Beddhu reports other from the National Heart, Lung, and Blood Institute (NHLBI), grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), during the conduct of the study; grants from Bayer, grants from AbbVie, grants from Boehringer Ingelheim, other from Reata, outside the submitted work. Dr. Kimmel reports Editor, Chronic Renal Disease, Elsevier. Dr. Chonchol reports grants from the National Institutes of Health (NIH), during the conduct of the study. Dr. Chertow reports personal fees from Akebia, personal fees from AMAG, personal fees from Amgen, personal fees and other from Ardelyx, personal fees from Astra Zeneca, personal fees from Baxter, other from Cricket Health, other from Durect, other from DxNow. Dr. Rocco reports grants from NIH, during the conduct of the study; grants from Bayer, grants from Boehringer Ingelheim, grants from GSK, personal fees from Abbvie, personal fees from George Clinical, personal fees from Beacon Bioscience, personal fees from Baxter, outside the submitted work; personal fees from Gilead, other from Outset, personal fees from Reata, personal fees from Sanifit, personal fees from Bayer, personal fees from ReCor, personal fees and other from Vertex, outside the submitted work. Dr. Dwyer reports grants from NIH/NIDDK, during the conduct of the study. Dr. Freedman reports grants from NIDDK, outside the submitted work. Dr. Ix reports grants from NIH (NHLBI, NIDDK), grants from American Heart Association, during the conduct of the study. Dr. Ix reports grants from NIH (NHLBI, NIDDK), grants from American Heart Association, during the conduct of the study. Dr. Pisoni reports personal fees from Otsuka, outside the submitted work. Prof. Greene reports grants from NIH, during the conduct of the study; personal fees from Janssen Pharmaceuticals, personal fees from DURECT Corporation, personal fees from Pfizer Inc., grants from Astrazeneca, outside the submitted work. All of the remaining authors have nothing to disclose.
Funding
The Systolic BP Intervention Trial (SPRINT) is funded with federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C, and interagency agreement number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. Statistical analysis and preparation of this manuscript were supported by grants from the NIDDK (RO1DK115814 and R21DK106574) and the University of Utah Study Design and Biostatistics Center (funded in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources).
The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International and the support from the following Clinical and Translational Science Awards, funded by National Center for Advancing Translational Sciences: Case Western Reserve University: UL1TR000439; Ohio State University: UL1RR025755; U Penn: UL1RR024134 and UL1TR000003; Boston: UL1RR025771; Stanford: UL1TR000093; Tufts: UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; University of Texas Southwestern: 9U54TR000017-06; University of Utah: UL1TR000105-05; Vanderbilt University: UL1TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1TR000002; University of Florida: UL1TR000064; University of Michigan: UL1TR000433; and Tulane University: P30GM103337 Centers of Biomedical Research Excellence Award National Institute of General Medical Sciences.
Supplementary Material
Acknowledgments
Dr. Beddhu, Prof. Shen, and Prof. Greene contributed to the idea for and design of the paper. Statistical analysis was led by Prof. Greene and Prof. Shen with contributions from Mr. Wei and Mr. Boucher. Dr. Beddhu, Prof. Greene, Prof. Shen, Mr. Wei, Mr. Boucher, Dr. Cheung, Dr. Chonchol, Dr. Ix, Dr. Freedman, Dr. Whelton, Dr. Kimmel, and Dr. Chertow contributed to writing of the report. Dr. Beddhu, Dr. Cheung, Dr. Chertow, Dr. Chonchol, Dr. Arman, Dr. Campbell, Dr. Contreras, Dr. Dwyer, Dr. Freedman, Dr. Ix, Dr. Kirchner, Dr. Papademetriou, Dr. Pisoni, and Dr. Rocco contributed to patient’s recruitment or provided study materials. All authors contributed to data interpretation, critical revision of the report, and final approval. Dr. Beddhu, Prof. Greene, Dr. Cheung, and Dr. Chertow obtained funding. Dr. Beddhu and Prof. Greene provided administrative, technical, or logistical support, and take responsibility for all aspects of the report and all authors take responsibility for their contributions.
All components of the Systolic BP Intervention Trial (SPRINT) study protocol were designed and implemented by the SPRINT investigators. The authors analyzed and interpreted the data for this manuscript. All aspects of manuscript writing and revision were carried out by the authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the US Government.
For a full list of contributors to SPRINT, please see the supplementary acknowledgment list: https://www.sprinttrial.org/public/dspScience.cfm.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018121261/-/DCSupplemental.
Supplemental Appendix 1. Statistical supplemental text.
Supplemental Table 1. Covariates selected by stepwise regression for CVD composite and all-cause death.
Supplemental Table 2. Clinical characteristics by randomized SBP arm (N=8526).
Supplemental Table 3. Sensitivity mediation analysis with covariates chosen by stepwise regression for the effect of the SBP intervention on the CVD composite and all-cause mortality.
Supplemental Table 4. Sensitivity mediation analysis of CVD composite including patients with CVD composite events before 6 months (N=8611).
Supplemental Table 5. Sensitivity mediation analysis of all-cause mortality including patients with CVD composite events before 6 months (N=8611).
Supplemental Table 6. Adjusted risk difference (%) between the standard and intensive SBP arms controlling for ΔeGFR%.
Supplemental Figure 1. CONSORT flowchart.
Supplemental Figure 2. Controlled direct effects of SBP intervention at different levels of ΔeGFR% with covariates chosen by stepwise regression.
References
- 1.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al.: Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation 134: 441–450, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al.: Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 383: 1899–1911, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration : Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C: Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 165: 923–928, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al.: SPRINT Research Group : A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al.: SPRINT Research Group : Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 Years: A randomized clinical trial. JAMA 315: 2673–2682, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beddhu S, Rocco MV, Toto R, Craven TE, Greene T, Bhatt U, et al.: SPRINT Research Group : Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: A secondary analysis of a randomized trial. Ann Intern Med 167: 375–383, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al.: SPRINT Research Group : Effects of intensive BP control in CKD. J Am Soc Nephrol 28: 2812–2823, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al.: Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al.: An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Ku E, Bakris G, Johansen KL, Lin F, Sarnak MJ, Campese VM, et al.: Acute declines in renal function during intensive BP lowering: Implications for future ESRD risk. J Am Soc Nephrol 28: 2794–2801, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apperloo AJ, de Zeeuw D, de Jong PE: A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int 51: 793–797, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Clase CM, Barzilay J, Gao P, Smyth A, Schmieder RE, Tobe S, et al.: Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int 91: 683–690, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Kiernan MS, Gregory D, Sarnak MJ, Rossignol P, Massaro J, Kociol R, et al.: Early and late effects of high- versus low-dose angiotensin receptor blockade on renal function and outcomes in patients with chronic heart failure. JACC Heart Fail 3: 214–223, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Ku E, Gassman J, Appel LJ, Smogorzewski M, Sarnak MJ, Glidden DV, et al.: BP control and long-term risk of ESRD and mortality. J Am Soc Nephrol 28: 671–677, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robins JM, Greenland S: Identifiability and exchangeability for direct and indirect effects. Epidemiology 3: 143–155, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Pearl J: Direct and indirect effects. Proceedings of the seventeenth conference on uncertainty in artificial intelligence, Seattle, Washington, 2001 [Google Scholar]
- 19.VanderWeele T: Explanation in Causal Inference: Methods for Mediation and Interaction, New York, NY, Oxford University Press, 2015 [Google Scholar]
- 20.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al.: SPRINT Study Research Group : The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 11: 532–546, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Heart, Lung, and Blood Institute : Systolic blood pressure intervention protocol version 4.0. BioLINCC. Available at: https://www.sprinttrial.org/public/Protocol_Current.pdf. Accessed August 2 2018
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 23.VanderWeele TJ: Concerning the consistency assumption in causal inference. Epidemiology 20: 880–883, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Beddhu S, Greene T, Boucher R, Cushman WC, Wei G, Stoddard G, et al.: Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: Secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol 6: 555–563, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peralta CA, McClure LA, Scherzer R, Odden MC, White CL, Shlipak M, et al.: Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: A post hoc analysis of the Secondary Prevention of Small Subcortical Strokes (SPS3) randomized trial. Circulation 133: 584–591, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, et al.: SPRINT Research Group : Effects of intensive blood pressure treatment on acute kidney injury events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis 71: 352–361, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al.: Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.