Abstract
Red blood cells (RBCs) have been historically regarded as a critical model to investigate cellular and oxidant stress biology. First of all, they are constantly exposed to oxidant stress, as their main function is to transport and deliver oxygen to tissues. Second, they are devoid of de novo protein synthesis capacity, which prevents RBCs from replacing irreversibly oxidised proteins with newly synthesised ones. As such, RBCs have evolved to (i) protect themselves from oxidant stress, in order to prevent oxidant damage from reactive species; (ii) repair oxidatively damaged proteins, through mechanisms that involve glutathione and one-carbon metabolism; (iii) destroy irreversibly oxidised proteins through proteasomal or protease-dependent degradation; and (iv) sacrifice membrane portions through mechanism of vesiculation. In this brief review we will summarize these processes and their relevance to RBC redox biology (within the context of blood storage), with a focus on how polymorphisms in RBC antioxidant responses could contribute to explaining the heterogeneity in the progression and severity of the RBC storage lesion that can be observed across the healthy donor population.
Keywords: metabolomics, mouse models, G6PD, isoaspartyl-damage, proteasome
Introduction
Red Blood Cells (RBCs) are by far the most abundant cell in the human body, since ~83% of human cells are RBCs1,2. Each RBC contains ~250–270 million molecules of haemoglobin (Hb)/cell, allowing the transport of up to 1 billion molecules of oxygen/cell3. To facilitate oxygen transport, haemoglobin tetramers are loaded with four haeme groups and as many molecules of iron. Indeed, ~66% of total iron in the human body (~2.275 g) is in RBCs4. While these molecules are critical for oxygen transport, they also create oxidant stress, fueling radical-generating Fenton and Haber-Weiss reactions. The RBC lifespan is ~120 days, oxidant stress is significant, and no new proteins can be synthesised by the mature RBC owing to the lack of nuclei and organelles. As such, oxidant damage to RBCs is a critical factor in proper erythrocyte function and senescence. Due to a lack of ongoing protein synthesis, RBCs are also an excellent model to investigate mechanisms of redox damage and repair5 without the confounding factor of ongoing gene expression. Recently, we reviewed how oxidant stress to RBCs and their capacity to mitigate it are critical etiological contributors to many diseases5. In the present short review we will focus on the impact of oxidant stress to RBC biology and storage. In particular, this review will focus on RBC responses to oxidant stress by four main means: i) protection from oxidatve stress through anti-oxdiant pathways, such as the glutathione system and the pentose phosphate pathway (PPP); ii) repair of oxidatively damaged macromolecules, such as lipids, metabolites and proteins; iii) to destroy irreversibly damaged components through the activity of the proteasome or other proteolytic cascades; and iv) to expel cytosolic and membrane portions enriched in irreversibly oxidised components through mechanisms of vesiculation (“sacrifice” in Figure 1). Given the vast nature of the subject treated, herein we will mostly focus on some of our recent studies6–8 on the interplay between the PPP, (some) nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzymes and a recently appreciated6 potential bridge between these pathways and damage-repair mechanisms based on non-epigenetic protein methylation. Throughout the text we will refer the interested reader to extensive reviews on the other related topics.
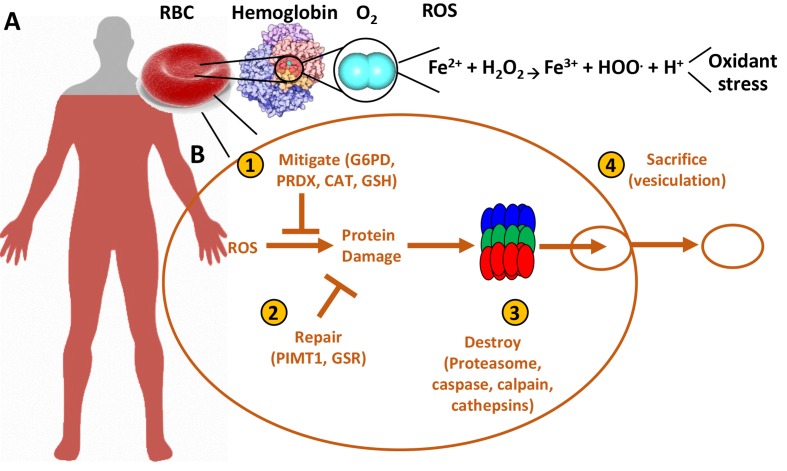
Figure 1.
Oxidant stress in the red blood cell.
Red blood cells are the most abundant cell in the human body (~83% of the total human cells), and are loaded with oxidant stress-generating oxygen and iron (A). They have, therefore, developed mechanisms (B) to prevent oxidant stress-induced damage, repair the damage that is generated despite the strong antioxidant system, and degrade oxidised components through proteolytic activity or vesiculation (sacrifice) of irreversibly damaged biomolecules, including proteins and lipids.
RBC: red blood cell; ROS: reactive oxygen species; G6PD: glucose 6-phosphate dehydrogenase; PRDX: Peroxiredoxin 6; CAT: catalase; GSH: reduced and oxidised glutathione; PIMT1: protein L-isoaspartyl methyltransferase; GSR: glutathione-disulfide reductase.
Protection
RBCs modify their cellular metabolism and biology based upon their microenvironment, both as an adaptation to maintain their own integrity, and also to facilitate their function. As RBCs have no mitochondria, glucose is their main source of metabolic energy. Glucose can be metabolised by two pathways: (i) glycolysis that gives rise to a net of 2 adenosine triphosphate (ATP) and 2 nicotinamide adenine dinucleotide (reduced) (NADH), and (ii) the PPP that gives rise to 2 NADPH and a pentose phosphate sugar moiety that can reenter late glycolysis to fuel the generation of high energy phosphate compounds. Both pathways are essential to RBC survival; ATP is required for numerous enzymes and pumps that maintain homeostasis, NADH is required as a cofactor for enzymatic reduction of methaemoglobin to haemoglobin, and NADPH is required to drive multiple anti-oxidant pathways in RBCs. Such pathways include recycling of oxidised glutathione to its reduced form (GSH) by glutathione reductase, and fueling of NADPH-dependent antioxidant enzymes (e.g., glutathione peroxidase, catalase, peroxiredoxins9,10, glutaredoxins, thioredoxin reductase system, biliverdin reductase B7, and the ascorbate11-tocopherol12 axis). All of these pathways have been found to be dysregulated during RBC storage13,14, though it is as of yet unclear how interdonor variability impacts these protective mechanisms. Indeed, as storage progresses under refrigerated conditions, free glutathione is consumed to glutathionylate redox sensitive thiols, especially on Hb (Cys93 of Hb beta15–17). Similarly, peroxiredoxins 2 and 6 have been offered as markers of the redox storage lesion9,13,18,19, as both migrate to the membrane as a function of oxidant stress20, even though the former inactivates upon changing its multimeric status10, while the latter exerts a “moonlighting” function as a phospholipase A221. Genomics studies curently underway promise to shed further light on the specific polymorphisms potentially contributing to the heterogeneity of such mechanisms amongst blood donors. In the meantime, observational data from the Recipient Epidemiology and Donor assessment Study REDS-III clearly indicate a heterogeneous response to the free radical-generating compound 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH) across the donor population, with RBCs from male, younger donors of specific ethnicities (e.g., African Americans) being more susceptible to AAPH-induced haemolysis ex vivo22–30. Interdonor heterogeneity of direct radical scavenging or hydrogen peroxide dismutating enzymes, such as catalase and superoxide dismutase (extensively reviewed elsewhere31), could underlie the biology of stored RBC responses to free radical-generating compounds such as AAPH, and potentially mirror the impact of stored RBC redox biology upon transfusion in the recipient.
Regulating diversion of metabolic fluxes from glycolysis to the PPP is essential for an RBC to respond to its particular metabolic needs, which in the human body are a function of whether the RBCs are exposed to high or low oxygen tensions in the lung and peripheral capillaries32, and the oxidant stress that arises. In response to the high oxygen states as RBCs pass through the lungs, RBCs strengthen the linkage of their cytoskeleton to the lipid membrane, which allows them to survive turbulent flow as they transition back into capillary beds. In contrast, in low oxygen states the cytoskeleton-membrane link is relaxed to allow the contortion required to transverse capillary beds of peripheral tissues33–37. Activation of G6PD, the rate-limiting enzyme of the PPP, is critical to RBC antioxidant homeostasis. G6PD activation and a metabolic shift from glycolysis to the PPP is observed following oxidative insults like methylene blue, xanthine oxidase in presence of hypoxanthine, or oxidant stress arising from iatrogenic interventions (e.g., RBC storage in the blood bank, the most common in hospital medical procedure together with vaccination), as we have extensively reported6,15,16,38,39 and reviewed3,5,14,40,41.
Glucose 6-phosphate dehydrogenase (G6PD) is the rate limiting enzyme of the PPP, which generates a critical reducing equivalent involved in the mitigation of oxidant stress to RBC proteins (Figure 2): NADPH. NADPH drives multiple anti-oxidant pathways in RBCs, including recycling of oxidised glutathione to its reduced form (GSH) by glutathione reductase, glutathione peroxidase, catalase, peroxiredoxins, glutaredoxins, biliverdin reductase B, the ascorbate-tocopherol axis and the ferroreductase six-transmembrane epithelial antigen of the prostate 3 (STEAP3)42. Nonetheless, ~400 million people carry mutations to G6PD that impact its activity to a variable extent, a condition referred to as G6PD deficiency, the most common enzymopathy in humans43. While G6PD-deficient subjects are perfectly healthy, they can have brisk haemolysis following oxidant insults. Of note, even healthy subjects are characterised by progressive decreases in G6PD activity as they get old44. Conversely, transgenic mice overexpressing G6PD have improved lifespans45. G6PD-deficiency is usually associated with decreased G6PD protein stability46, an issue that nucleated cells resolve by up-regulating G6PD expression. However, circulating RBCs cannot synthesize new proteins and are thus particularly susceptible to G6PD deficiency during RBC senescence47, especially in older individuals.
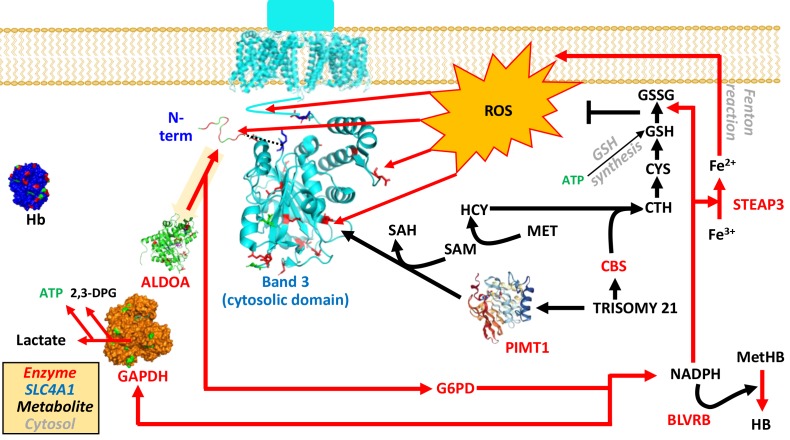
Figure 2.
Overview of the main antioxidant pathways in red blood cells and how they relate to energy metabolism and structural homeostasis, with band 3 as the key molecular lynchpin.
Metabolites are represented in black, enzymes in red (Uniprot abbreviations).
2,3-DPG: 2,3-diphosphoglycerate; ALDOA: aldolase; BLVRB: biliverdin reductase B; CBS: cystathionine beta-synthase; CTH: cystathioneine; CYS: cysteine; G6PD: glucose 6-phosphate dehydrogenase; GSH and GSSG: reduced and oxidised glutathione; Hb and MetHb: haemoglobin and methaemoglobin; HCY: homocysteine; MET: methionine; NAPDH: nicotinamide adenine dinucleotide phosphate; PIMT1: protein L-isoaspartyl methyltransferase; ROS: reactive oxygen species; SAM and SAH: S-adenosylmethionine and homocysteine; STEAP3: six-transmembrane epithelial antigen of the prostate 3.
Owing to its central role in RBC biology, G6PD activity in erythrocytes has been extensively investigated over the past decades in observational studies. Results clearly indicate that declines in RBC antioxidant capacity are observed in senescent RBCs (e.g., decreases in G6PD activity48 and reduced glutathione49), as they age in the bloodstream, as confirmed by our recent preliminary metabolomics work47. Similarly, in keeping with the “free radical theory of aging”50, RBCs from older subjects are characterised by increased oxidant stress and decreased antioxidant capacity, as gleaned from the literature51. Conversely, Serrano’s group recently reported that 2-fold up-regulation of G6PD transcript (resulting in modest increases in G6PD activity) in transgenic mice was sufficient to improve the lifespan without an increasing rate of neoplasia45. These considerations are relevant in that G6PD polymorphisms or other environmental/biological factors impacting G6PD activity may underlie the heterogeneity of antioxidant responses observed in stored units. Interestingly, previous studies measuring G6PD activity as a function of storage duration are contradictory; some have detected decreases52,53 whereas others found no significant43 changes in the activity of this enzyme as a function of storage duration. Hetereogenity of such measurement may be attributable to blood processing or donor biology (European studies vs American studies). An alternative explanation lies in the appreciation of the biochemistry behind the different methodologies used to determine G6PD activity in those studies, especially when noting that spectrophotemetric measurements of NADP+ to NADPH conversion could be susceptible to confounding factors, such as the activity of other NADPH-generating/consuming enzymes54.
Indeed, we and others recently used tracing experiments to reveal alternative metabolic pathways in RBCs that rely on the activity of the cytosolic isoforms of Krebs cycle enzymes such as isocitrate and malate dehydrogenase 1 - IDH1 and MDH1. These enzymes catalyze reactions that are involved in NADH and NADPH homeostasis39,41,55. In parallel, we also recently commented on the important roles in RBC redox homeostasis of the NADPH-dependent enzyme bilverdin reductase B (BLVRB), which is extremely abundant in RBCs owing to its role as a flavin reductase involved in the reduction of methaemoglobin7. Of note, a more direct measurement based on tracing experiments with 13C1,2,3-glucose under refrigerated storage conditions revealed increases in PPP activity in RBCs from leukofiltered units as a function of storage-induced oxidation of rate-limiting enzymes of glycolysis, such as glyceraldehyde 3-phosphate dehydrogenase16 (GAPDH in Figure 2). However, these tracing experiments were performed in stored, unstimulated RBCs. Therefore, it is unclear whether a stored RBC response to pro-oxidant stimuli upon transfusion (e.g., in the context of transfusion to a septic patient) would be comparable to that of a fresh RBC with respect to G6PD activation and PPP fluxes, nor it is clear whether a stored RBC from a G6PD deficient patient is less capable of coping with storage-induced oxidant stress and, as such, perform poorly upon transfusion (i.e., decreased post-transfusion recovery and/or capacity to carry and deliver oxygen in comparison to stored RBCs from G6PD sufficient donors).
Repair
Storage-induced oxidant damage to RBC purines, lipids and proteins ultimately results in the accumulation of hypoxanthine38,56, oxylipins8,57 and oxidation of asparagine (N) and aspartate (D) residues into L-isoaspartyl groups6. Tracing experiments suggest that purine oxidation is triggered by the activity of RBC-specific and redox/calcium sensitive AMP deaminase 3, while salvage is minimally active in mature RBCs38. Similarly, while phospholipase activation (including peroxiredoxin 6 [PRDX6]) can release and oxidize free fatty acids21, oxylipin detoxification or export pathways exist in mature RBCs that rely on glutathione and NADPH-dependent enzymes (e.g. aldehyde dehydrogenase and the glyoxalase pathway, D’Alessandro et al. AABB 2018 and in preparation). Similarly, a repair pathway for isoaspartyl damage exists in humans58–63. By altering the orientation of the protein backbone, this modification has structural and functional implications critical to RBC protein function.
L-isoaspartyl methyltransferase (PIMT1)64 is a natural repair pathway by which isoaspartyl groups are methylated to form an isoaspartyl methyl ester. Spontaneous decomposition of the methylated intermediate results in the release of a molecule of methanol and reformation of a succinimide intermediate, which again undergoes spontaneous decay either back into a normal aspartyl group (returning the amino acid to its initial state in the case of aspartate) or into a “damaged” L-isoaspartyl group. Due to the stochastic nature of this reversion, to complete repair of each of the residues in a given protein, multiple and ongoing cycles of PIMT1 methylation are required - this requires both sufficient PIMT1 activity and a sufficient pool of methyl donors (e.g., methionine) as co-factors (Figure 2). Because mature RBCs are incapable of synthesizing new proteins, PIMT1 is critical to their capacity to repair damaged proteins. PIMT1 methylates L-isoaspartyl moieties to form a L-isoaspartyl methyl ester, which (through a succinimide intermediate) can then spontaneously hydrolyze to aspartate (30% of the time), but can also revert back into a L-isoaspartyl group (70% of the time), requiring another round of methylation for repair. Pioneering work by Clarke, Ingrosso and colleagues identified methylation of L-isoaspartyl groups on RBC membrane proteins in response to aging or pathological oxidative stress, such as in G6PD deficiency or Down syndrome60,62,63,65–68. More recently, we mapped the methylated residues in critical structural and functional RBC proteins; methylation was found to preferentially target aspartates and deamidated asparagines in proximity to functional sites of haemoglobins, the N-term of band 3 (involved in the mechanism of oxygen-dependent metabolic modulation35,69,70) and rate-limiting glycolytic enzymes (including GAPDH, aldolase and lactate dehydrogenase)6.
While a direct role of PIMT1 in maintaining RBC viability as a function of blood storage has not been formally tested - PIMT1-KO mice have been reported by two different groups, both of which had the same phenotype - they are born viable, but die at ~6–8 weeks from a seizure disorder due to accumulated protein damage in brain tissue59,71. To allow longer-term analysis of such animals through adult and older age, an additional transgenic mouse was made in which PIMT1 was selectively expressed in brain tissue, and was then back-crossed with PIMT1-KO mice. These “rescued animals” lived longer, but still had a significantly shortened life span (approximately 50% mortality by 210 days)58. Overall, the rescued animals had increased accumulation of damaged aspartyl residues, but it was self-limited with an unexpected plateau by 100 days of age58. This plateau was concluded to occur due to proteolysis of damaged proteins, as evidenced by a sufficient increase in peptides with damaged residues in the urine to account for lack of protein accumulation58. Of note, no analysis of RBC lifespan, senescence, or biology was carried out in PIMT1-KO mice to date.
Destroy/sacrifice
As storage progresses, oxidant stress to RBC proteins becomes irreversible, such as in the case of beta elimination of thiol groups from cysteines that generates dehydroalanine (e.g. Cys152 of GAPDH16 and Cys93 of HBB15,17). Similarly, protein carbonylation increases over storage20,72–76, a phenomenon that is counteracted by (i) increased protein degradation through protease activity (destroy) or (ii) sub-compartmentalisation and release via vesiculation of oxidised components, a process that also sacrifices RBC membrane portions and increases surface-to-volume ratios, ultimately priming RBCs for lysis following mechanical insults. Several different mechanisms contribute to the process of “destroying” oxidised proteins, including metalloproteases (e.g., cathepsins), some of which calcium-dependent (e.g., calpains) or ATP-dependent/independent (the ubiquitinylation-proteasome system). Metalloproteases have been shown to be present and active in RBCs. This is especially relevant during storage, when calpains77 and cathepsin have been shown to target critical residues on the N-terminus of band 3 - the most abundant protein in RBC membrane and a docking site for critical structural proteins (e.g., ankyrin) and enzymes14. However, the same N-term (at different residue) is susceptible to direct cleavage by oxidant stress20,78 (Figure 2).
Indeed, though historically thought to be devoid of organelles, mature RBCs still retain some macromolecular machineries such as ubiquitin transferases and the proteasome79, that are critical under physiological conditions (e.g., in response to high-altitude hypoxia to promote the degradation of the adenosine transporter ENT180) or under pathological conditions to promote protein degradation (e.g., sickle cell disease81). However, during storage the proteasome is progressively inactivated, in part because of ATP depletion as a function of storage duration, and in part because of its release in the supernatants82.
In vivo, vesiculation of RBC membranes is a physiological process (1 vesicle is released per hour by each one of the 25 trillion circulating RBCs), a process that is accelerated by RBC senescence, hypertermia (fever) or inflammation, calcium influx83,84, or, relevant to the field of transfusion medicine, during blood storage74,85. Extensive work by several groups18,74 has characterised the extent of the vesiculation proccess as a funciton of processing strategies and donor biology, within the constraints of relatively small scale studies in comparison to the total blood donor population worldwide. Flow cytometry and omics tools have been used to characterize rheological properties85 and molecular contents of these vesicles18, as well as to determine that vesiculated proteins are indeed less active than the intracellular counterparts as a result of their progressive oxidation14–16,72.
Heterogeneity in blood donors: mice as a model
Large scale clinical studies are currently set out to determine the impact of donors’ genetics on blood storability, haemolytic propensity and transfusion outcomes (e.g., the REDS-III study and the European counterpart - SCANDAT86). However, one major factor impacting the outcome of human studies is the intrinsic heterogeneity of cohorts beyond genetic backgrounds, in that environmental factors such as dietary interventions, physical activity87,88 and smoking habits89 may all confound the results from these studies. As such, with all the limitations acknowledged when adopting animal models90 and their translatability to humans, studies in mice have allowed us to (i) overcome the above-mentioned limitations by feeding mice the same diet in the absence of any environmental confounders; (ii) identify strain-specific heterogeneity in the post-transfusion survival and related metabolic phenotype of stored RBCs from different (mouse) donors; (iii) cross-breed these mice to identify genetic and metabolic markers of the redox storage lesion in mice; (iv) use genetic interventions to establish the mechanistic roles of some of the critical players (e.g., the NADPH-dependent ferroreductase STEAP3) in the etiology of the (lipid peroxidation) storage lesion and poor post-transfusion recovery observed in RBCs from some specific mouse strains (e.g., FVB8,57,91). Of note, some of these enzymes have been reported to be polymorphic in humans, though no study has so far focused on the impact of STEAP3 polymorphisms within the context of storage in the blood bank.
Conclusions
Oxidant stress is a critical player in red blood cell biology and a central factor in the etiology of the red cell storage lesion. While RBCs have historically represented a critical model to investigate antioxidant systems, which are known to be subject to heterogeneity and polymorphisms in humans (e.g., the case of G6PD deficiency), little is known about the impact of donor biology on blood storability from a molecular perspective and whether any of these considerations may impact transfusion outcomes. The establishment of large scale international consortiums such as the REDS and SCANDAT groups will afford the unprecedented opportunity to open a window into the realm of answers to these questions, answers that will likely be extracted through the use of state of the art analytical workflows, such as the omics technologies. In this view, the advent of high-throughput strategies has now made it possible (both technically and cost-affordably) to analyze hundreds to thousands of parameters in thousands of samples in a reasonable amount of time, paving the way for what promises to be another chapter in the fascinating history of RBC biology in transfusion medicine.
Acknowledgements
AD was supported by funds from the Webb-Waring Early Career Award 2017 by the Boettcher Foundation.
Footnotes
Disclosure of conflicts of interest
Though unrelated to the contents of the manuscript, AD’A and KCH are founders of Omix Technologies Inc. AD’A is a consultant for Hemanext Inc. and founder of Altis Biosciences. JCZ is a consultant for Rubius Biotechnologies.
References
- 1.Bianconi E, Piovesan A, Facchin F, et al. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40:463–71. doi: 10.3109/03014460.2013.807878. [DOI] [PubMed] [Google Scholar]
- 2.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemkov T, Reisz JA, Xia Y, et al. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev Proteomics. 2018;15:855–64. doi: 10.1080/14789450.2018.1531710. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz M, Villar I, García-Erce JA. An update on iron physiology. World J Gastroenterol. 2009;15:4617–26. doi: 10.3748/wjg.15.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reisz JA, Barrett AS, Nemkov T, et al. When nature’s robots go rogue: exploring protein homeostasis dysfunction and the implications for understanding human aging disease pathologies. Expert Rev Proteomics. 2018;15:293–309. doi: 10.1080/14789450.2018.1453362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion. 2018;58:2978–91. doi: 10.1111/trf.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paukovich N, Xue M, Elder JR, et al. Biliverdin reductase B dynamics are coupled to coenzyme binding. J Mol Biol. 2018;430:3234–50. doi: 10.1016/j.jmb.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion. 2016;56:2560–70. doi: 10.1111/trf.13748. [DOI] [PubMed] [Google Scholar]
- 9.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 10.Rinalducci S, D’Amici GM, Blasi B, Zolla L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie. 2011;93:845–53. doi: 10.1016/j.biochi.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Pallotta V, Gevi F, D’Alessandro A, Zolla L. Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: a metabolomics overview. Blood Transfus. 2014;12:376–87. doi: 10.2450/2014.0266-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva CAL, Azevedo Filho CA, Pereira G, et al. Vitamin E nanoemulsion activity on stored red blood cells. Transfus Med. 2017;27:213–7. doi: 10.1111/tme.12394. [DOI] [PubMed] [Google Scholar]
- 13.D’Alessandro A, Nemkov T, Reisz J, et al. Omics markers of the red cell storage lesion and metabolic linkage. Blood Transfus. 2017;15:137–44. doi: 10.2450/2017.0341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–19. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 15.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion. 2016;56:421–6. doi: 10.1111/trf.13363. [DOI] [PubMed] [Google Scholar]
- 16.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128:e32–42. doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 17.Harper VM, Oh JY, Stapley R, et al. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxid Redox Signal. 2015;22:294–307. doi: 10.1089/ars.2014.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alessandro A, Dzieciatkowska M, Hill RC, Hansen KC. Supernatant protein biomarkers of red blood cell storage hemolysis as determined through an absolute quantification proteomics technology. Transfusion. 2016;56:1329–39. doi: 10.1111/trf.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzieciatkowska M, Silliman CC, Moore EE, et al. Proteomic analysis of the supernatant of red blood cell units: the effects of storage and leucoreduction. Vox Sang. 2013;105:210–8. doi: 10.1111/vox.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher AB, Vasquez-Medina JP, Dodia C, et al. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018;14:41–6. doi: 10.1016/j.redox.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endres-Dighe SM, Guo Y, Kanias T, et al. Blood, sweat, and tears: red blood cell-omics study objectives, design, and recruitment activities. Transfusion. 2019;59:46–56. doi: 10.1111/trf.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS-III-Omics. Transfusion. 2019;59:89–100. doi: 10.1111/trf.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanteri MC, Kanias T, Keating S, et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: results of recall phase of the REDS-III RBC-Omics study. Transfusion. 2019;59:79–88. doi: 10.1111/trf.14987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Busch MP, Seielstad M, et al. Development and evaluation of a transfusion medicine genome wide genotyping array. Transfusion. 2019;59:101–11. doi: 10.1111/trf.15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanias T, Stone M, Page GP, et al. Frequent blood donations alter susceptibility of red blood cells to storage- and stress-induced hemolysis. Transfusion. 2019;59:67–78. doi: 10.1111/trf.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone M, Keating SM, Kanias T, et al. Piloting and implementation of quality assessment and quality control procedures in RBC-Omics: a large multi-center study of red blood cell hemolysis during storage. Transfusion. 2019;59:57–66. doi: 10.1111/trf.15099. [DOI] [PubMed] [Google Scholar]
- 28.Spencer BR, Bialkowski W, Creel DV, et al. Elevated risk for iron depletion in high-school age blood donors. Transfusion. 2019;59:1706–16. doi: 10.1111/trf.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karafin MS, Bruhn R, Westlake M, et al. Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS-III recipient database. Transfusion. 2017;57:2903–13. doi: 10.1111/trf.14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1:1132–41. doi: 10.1182/bloodadvances.2017004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Alessandro A, Zolla L. The SODyssey: superoxide dismutases from biochemistry, through proteomics, to oxidative stress, aging and nutraceuticals. Expert Rev Proteomics. 2011;8:405–21. doi: 10.1586/epr.11.13. [DOI] [PubMed] [Google Scholar]
- 32.Messana I, Orlando M, Cassiano L, et al. Human erythrocyte metabolism is modulated by the O2-linked transition of hemoglobin. FEBS Lett. 1996;390:25–8. doi: 10.1016/0014-5793(96)00624-2. [DOI] [PubMed] [Google Scholar]
- 33.Caro CG, Parker KH, Doorly DJ. Essentials of blood flow. Perfusion. 1995;10:131–4. doi: 10.1177/026765919501000302. [DOI] [PubMed] [Google Scholar]
- 34.Jones SA. A relationship between Reynolds stresses and viscous dissipation: implications to red cell damage. Ann Biomed Eng. 1995;23:21–8. doi: 10.1007/BF02368297. [DOI] [PubMed] [Google Scholar]
- 35.Stefanovic M, Puchulu-Campanella E, Kodippili G, Low PS. Oxygen regulates the band 3-ankyrin bridge in the human erythrocyte membrane. Biochem J. 2013;449:143–50. doi: 10.1042/BJ20120869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- 37.Sprague RS, Ellsworth ML. Erythrocyte-derived ATP and perfusion distribution: role of intracellular and intercellular communication. Microcirculation. 2012;19:430–9. doi: 10.1111/j.1549-8719.2011.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103:361–72. doi: 10.3324/haematol.2017.178608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57:325–36. doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 40.D’Alessandro A, Zolla L. Proteomic analysis of red blood cells and the potential for the clinic: what have we learned so far? Expert Rev Proteomics. 2017;14:243–52. doi: 10.1080/14789450.2017.1291347. [DOI] [PubMed] [Google Scholar]
- 41.Nemkov T, Sun K, Reisz JA, et al. Metabolism of citrate and other carboxylic acids in erythrocytes as a function of oxygen saturation and refrigerated storage. Front Med (Lausanne) 2017;4:175. doi: 10.3389/fmed.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D, Yi S, Zhang X, et al. Human STEAP3 mutations with no phenotypic red cell changes. Blood. 2016;127:1067–71. doi: 10.1182/blood-2015-09-670174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francis RO, Jhang J, Hendrickson JE, et al. Frequency of glucose-6-phosphate dehydrogenase-deficient red blood cell units in a metropolitan transfusion service. Transfusion. 2013;53:606–11. doi: 10.1111/j.1537-2995.2012.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maurya PK, Kumar P, Chandra P. Age-dependent detection of erythrocytes glucose-6-phosphate dehydrogenase and its correlation with oxidative stress. Arch Physiol Biochem. 2016;122:61–6. doi: 10.3109/13813455.2015.1136648. [DOI] [PubMed] [Google Scholar]
- 45.Nobrega-Pereira S, Fernandez-Marcos PJ, Brioche T, et al. G6PD protects from oxidative damage and improves healthspan in mice. Nat Commun. 2016;7:10894. doi: 10.1038/ncomms10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Flora A, Morelli A, Benatti U. Structural variants of human glucose 6-phosphate dehydrogenase (G6PD): role of intracellular decay in the expression of deficiency. Biomed Biochim Acta. 1983;42:S247–52. [PubMed] [Google Scholar]
- 47.D’Alessandro A, Blasi B, D’Amici GM, et al. Red blood cell subpopulations in freshly drawn blood: application of proteomics and metabolomics to a decades-long biological issue. Blood Transfus. 2013;11:75–87. doi: 10.2450/2012.0164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansen G, Koenderman L, Rijksen G, et al. Characteristics of hexokinase, pyruvate kinase, and glucose-6-phosphate dehydrogenase during adult and neonatal reticulocyte maturation. Am J Hematol. 1985;20:203–15. doi: 10.1002/ajh.2830200302. [DOI] [PubMed] [Google Scholar]
- 49.Sass MD, Caruso CJ, O’Connell DJ. Decreased glutathione in aging red cells. Clin Chim Acta. 1965;11:334–40. doi: 10.1016/0009-8981(65)90223-8. [DOI] [PubMed] [Google Scholar]
- 50.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Rodgers GP, Lichtman HC, Sheff MF. Red blood cell glucose-6-phosphate dehydrogenase activity in aged humans. J Am Geriatr Soc. 1983;31:8–11. doi: 10.1111/j.1532-5415.1983.tb06281.x. [DOI] [PubMed] [Google Scholar]
- 52.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152–65. doi: 10.1016/j.freeradbiomed.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Peters AL, van Bruggen R, de Korte D, et al. Glucose-6-phosphate dehydrogenase activity decreases during storage of leukoreduced red blood cells. Transfusion. 2016;56:427–32. doi: 10.1111/trf.13378. [DOI] [PubMed] [Google Scholar]
- 54.Peters AL, Veldthuis M, van Leeuwen K, et al. Comparison of spectrophotometry, chromate inhibition, and cytofluorometry versus gene sequencing for detection of heterozygously glucose-6-phosphate dehydrogenase-deficient females. J Histochem Cytochem. 2017;65:627–36. doi: 10.1369/0022155417730021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolfsson O, Sigurjonsson OE, Magnusdottir M, et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang. 2017;112:326–35. doi: 10.1111/vox.12506. [DOI] [PubMed] [Google Scholar]
- 56.Casali E, Berni P, Spisni A, et al. Hypoxanthine: a new paradigm to interpret the origin of transfusion toxicity. Blood Transfus. 2016;14:555–6. doi: 10.2450/2015.0177-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica. 2016;101:578–86. doi: 10.3324/haematol.2015.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowenson JD, Kim E, Young SG, Clarke S. Limited accumulation of damaged proteins in l-isoaspartyl (D-aspartyl) O-methyltransferase-deficient mice. J Biol Chem. 2001;276:20695–702. doi: 10.1074/jbc.M100987200. [DOI] [PubMed] [Google Scholar]
- 59.Kim E, Lowenson JD, MacLaren DC, et al. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc Natl Acad Sci U S A. 1997;94:6132–7. doi: 10.1073/pnas.94.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Connor CM, Clarke S. Analysis of erythrocyte protein methyl esters by two-dimensional gel electrophoresis under acidic separating conditions. Anal Biochem. 1985;148:79–86. doi: 10.1016/0003-2697(85)90630-x. [DOI] [PubMed] [Google Scholar]
- 61.O’Connor CM, Clarke S. Methylation of erythrocyte membrane proteins at extracellular and intracellular D-aspartyl sites in vitro. Saturation of intracellular sites in vivo. J Biol Chem. 1983;258:8485–92. [PubMed] [Google Scholar]
- 62.McFadden PN, Clarke S. Methylation at D-aspartyl residues in erythrocytes: possible step in the repair of aged membrane proteins. Proc Natl Acad Sci U S A. 1982;79:2460–4. doi: 10.1073/pnas.79.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janson CA, Clarke S. Identification of aspartic acid as a site of methylation in human erythrocyte membrane proteins. J Biol Chem. 1980;255:11640–3. [PubMed] [Google Scholar]
- 64.Desrosiers RR, Fanelus I. Damaged proteins bearing L-isoaspartyl residues and aging: a dynamic equilibrium between generation of isomerized forms and repair by PIMT. Curr Aging Sci. 2011;4:8–18. [PubMed] [Google Scholar]
- 65.Galletti P, De Bonis ML, Sorrentino A, et al. Accumulation of altered aspartyl residues in erythrocyte proteins from patients with Down’s syndrome. FEBS J. 2007;274:5263–77. doi: 10.1111/j.1742-4658.2007.06048.x. [DOI] [PubMed] [Google Scholar]
- 66.Galletti P, Manna C, Ingrosso D, et al. Hypotheses on the physiological role of enzymatic protein methyl esterification using human erythrocytes as a model system. Adv Exp Med Biol. 1991;307:149–60. doi: 10.1007/978-1-4684-5985-2_14. [DOI] [PubMed] [Google Scholar]
- 67.Ingrosso D, Cimmino A, D’Angelo S, et al. Protein methylation as a marker of aspartate damage in glucose-6-phosphate dehydrogenase-deficient erythrocytes: role of oxidative stress. Eur J Biochem. 2002;269:2032–9. doi: 10.1046/j.1432-1033.2002.02838.x. [DOI] [PubMed] [Google Scholar]
- 68.Ingrosso D, D’Angelo S, di Carlo E, et al. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur J Biochem. 2000;267:4397–405. doi: 10.1046/j.1432-1327.2000.01485.x. [DOI] [PubMed] [Google Scholar]
- 69.Sun K, Zhang Y, D’Alessandro A, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun. 2016;7:12086. doi: 10.1038/ncomms12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H, Zhang Y, Wu H, et al. Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation. 2016;134:405–421. doi: 10.1161/CIRCULATIONAHA.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamoto A, Takagi H, Kitamura D, et al. Deficiency in protein L-isoaspartyl methyltransferase results in a fatal progressive epilepsy. J Neurosci. 1998;18:2063–74. doi: 10.1523/JNEUROSCI.18-06-02063.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delobel J, Prudent M, Tissot JD, Lion N. Proteomics of the red blood cell carbonylome during blood banking of erythrocyte concentrates. Proteomics Clin Appl. 2016;10:257–66. doi: 10.1002/prca.201500074. [DOI] [PubMed] [Google Scholar]
- 73.Delobel J, Prudent M, Rubin O, et al. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J Proteomics. 2012;76(Spec no):181–93. doi: 10.1016/j.jprot.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion. 2010;50:376–89. doi: 10.1111/j.1537-2995.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 75.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J Cell Mol Med. 2007;11:148–55. doi: 10.1111/j.1582-4934.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Membrane protein carbonylation in non-leukodepleted CPDA-preserved red blood cells. Blood Cells Mol Dis. 2006;36:279–82. doi: 10.1016/j.bcmd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 77.Antonelou MH, Kriebardis AG, Papassideri IS. Aging and death signalling in mature red cells: from basic science to transfusion practice. Blood Transfus. 2010;8(Suppl 3):s39–47. doi: 10.2450/2010.007S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rinalducci S, Ferru E, Blasi B, et al. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 2012;10(Suppl 2):s55–62. doi: 10.2450/2012.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Alessandro A, Dzieciatkowska M, Nemkov T, Hansen KC. Red blood cell proteomics update: is there more to discover? Blood Transfus. 2017;15:182–7. doi: 10.2450/2017.0293-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song A, Zhang Y, Han L, et al. Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nat Commun. 2017;8:14108. doi: 10.1038/ncomms14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warang P, Homma T, Pandya R, et al. Potential involvement of ubiquitin-proteasome system dysfunction associated with oxidative stress in the pathogenesis of sickle cell disease. Br J Haematol. 2018;182:559–66. doi: 10.1111/bjh.15437. [DOI] [PubMed] [Google Scholar]
- 82.Neelam S, Kakhniashvili DG, Wilkens S, et al. Functional 20S proteasomes in mature human red blood cells. Exp Biol Med (Maywood) 2011;236:580–91. doi: 10.1258/ebm.2011.010394. [DOI] [PubMed] [Google Scholar]
- 83.Bissinger R, Bhuyan AAM, Qadri SM, Lang F. Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. FEBS J. 2019;286:826–54. doi: 10.1111/febs.14606. [DOI] [PubMed] [Google Scholar]
- 84.Pompeo G, Girasole M, Cricenti A, et al. Erythrocyte death in vitro induced by starvation in the absence of Ca2+ Biochim Biophys Acta. 2010;1798:1047–55. doi: 10.1016/j.bbamem.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 85.Canellini G, Rubin O, Delobel J, et al. Red blood cell microparticles and blood group antigens: an analysis by flow cytometry. Blood Transfus. 2012;10(Suppl 2):s39–45. doi: 10.2450/2012.007S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edgren G, Hjalgrim H. Epidemiology of donors and recipients: lessons from the SCANDAT database. Transfus Med. 2017;29(Suppl 1):6–12. doi: 10.1111/tme.12487. [DOI] [PubMed] [Google Scholar]
- 87.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Apolipoprotein J/clusterin in human erythrocytes is involved in the molecular process of defected material disposal during vesiculation. PLoS One. 2011;6:e26033. doi: 10.1371/journal.pone.0026033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Apolipoprotein J/clusterin is a novel structural component of human erythrocytes and a biomarker of cellular stress and senescence. PLoS One. 2011;6:e26032. doi: 10.1371/journal.pone.0026032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boehm RE, Arbo BD, Leal D, et al. Smoking fewer than 20 cigarettes per day and remaining abstinent for more than 12 hours reduces carboxyhemoglobin levels in packed red blood cells for transfusion. PLoS One. 2018;13:e0204102. doi: 10.1371/journal.pone.0204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimring JC, Spitalnik SL. Scientific advances: fallacy of perfection harms peer review. Nature. 2016;537:34. doi: 10.1038/537034a. [DOI] [PubMed] [Google Scholar]
- 91.Zimring JC, Smith N, Stowell SR, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–48. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]