Abstract
Background.
Trauma exposure is associated with development of depression and anxiety; yet, some individuals are resilient to these trauma-associated effects. Differentiating mechanisms underlying development of negative affect and resilience following trauma is critical for developing effective interventions. One pathway through which trauma could exert its effects on negative affect is reward-learning networks. In this study, we examined relationships among lifetime trauma, reward-learning network function, and emotional states in young adults.
Methods.
One hundred eleven young adults self-reported trauma and emotional states and underwent functional magnetic resonance imaging during a monetary reward task. Trauma-associated neural activation and functional connectivity were analyzed during reward prediction error (RPE). Relationships between trauma-associated neural functioning and affective and anxiety symptoms were examined.
Results.
Number of traumatic events was associated with greater ventral anterior cingulate cortex (vACC) activation, and lower vACC connectivity with the right insula, frontopolar, inferior parietal, and temporoparietal regions, during RPE. Lower trauma-associated vACC connectivity with frontoparietal regions implicated in regulatory and decision-making processes was associated with heightened affective and anxiety symptoms; lower vACC connectivity with insular regions implicated in interoception was associated with lower affective and anxiety symptoms.
Conclusions.
In a young adult sample, two pathways linked the impact of trauma on reward-learning networks with higher v. lower negative affective and anxiety symptoms. The disconnection between vACC and regions implicated in decision-making and self-referential processes may reflect aberrant regulatory but appropriate self-focused mechanisms, respectively, conferring risk for v. resilience against negative affective and anxiety symptoms.
Keywords: Anxiety, depression, resilience, reward learning, reward prediction, trauma
Introduction
Exposure to trauma in childhood and adolescence is common and associated with heightened risk for negative affective and anxiety symptoms (Chapman et al., 2004; Anda et al., 2006; Nanni et al., 2012; Kristjansson et al., 2016; Li et al., 2016). Trauma exposure in childhood is associated with 2.0 greater odds of developing depression, 2.7 greater odds of developing anxiety (Li et al., 2016), and a more chronic, severe, and treatment resistant course of psychiatric illness (Nanni et al., 2012). Despite this risk, not all individuals exposed to trauma develop negative affective and anxiety symptoms and some adapt positively following trauma exposure (Merikangas et al., 2009). Differentiating the neural mechanisms underlying the development of negative affective and anxiety symptoms v. resilience in response to trauma is critical for developing effective interventions and treatment.
Aberrant reward processing is a critical mechanism underlying the development of anhedonia and depression (Pizzagalli, 2014) in adolescence and young adulthood, and has been implicated in anxiety (White et al., 2016). Youth with depressive symptoms demonstrate abnormally low activation in the ventral striatum (VS), a key region in reward learning, and blunted VS activation predicts future symptoms of depression and anhedonia (Stringaris et al., 2015). Traumatic experiences may impair reward processing via altered VS reactivity. Stress exacerbates dysfunctional reward processing, which may facilitate development of negative affective symptoms (Pizzagalli, 2014). Individuals exposed to childhood adversity exhibit blunted positive affect (Marusak et al., 2015) and diminished VS response to reward in young adulthood (Guyer et al., 2006; Mehta et al., 2010; Goff et al., 2013; Corral-Frias et al., 2015; Hanson et al., 2015), an already vulnerable time for the development of affective psychopathology (Kessler et al., 2007). Higher VS reactivity may be protective, as individuals with heightened VS reward reactivity are less likely to develop negative affective symptoms following trauma exposure (Nikolova et al., 2012; Dennison et al., 2016).
While the above studies suggest trauma-associated impairments in VS reactivity may underlie development of negative affective symptoms, reward processing is complex. Reward processing is influenced by regulatory/attentional and affective neural networks that may be impacted by trauma. Disruption of these networks can impair reward learning, an important component of reward processing guiding decision-making and behaviors to obtain future rewards. Reward learning can be measured by the reward prediction error (RPE), calculated as the difference between the predicted value of a forthcoming reward based on prior experience and the actual amount of reward that is subsequently received (Schultz, 2016). RPE thus relies on the recognition and contextualization of potentially rewarding stimuli, as well as the ability to experience reward, both of which may be disrupted by traumatic experiences (Marusak et al., 2015). The inability to use rewards to regulate behavior (e.g. failure to recognize or seek reward) has been associated with negative affective symptoms (Vrieze et al., 2013). RPE is signaled through dopamine neurons projecting from the midbrain to frontostriatal regions, including the VS, medial prefrontal cortex (mPFC), and anterior cingulate cortex (ACC) (O’Doherty et al., 2003; Wang et al., 2017). Combined with the inferior parietal lobule (IPL), the cingulo-frontal-parietal network is critical in reward valuation and subsequent decision-making about reward pursuit, and its disruption leads to erroneous value-based decisions (Polanía et al., 2015). Heightened engagement of salience/affective networks, including the ACC and anterior insula (aIns), is observed during reward anticipation and receipt (von Rhein et al., 2017). These two dissociable networks integrate sensory, emotional, and reward-related information to facilitate a goal-directed response to reward.
Little is known about the impact of trauma exposure on these broader networks during reward processing in children and adolescents; however, trauma-associated disruptions of these neural networks occur during emotion processing. Youth exposed to trauma demonstrated altered involvement in frontolimbic fear and salience processing networks during emotional regulation. Specifically, these youth exhibited heightened activation of mPFC, ACC, and aIns during stress (Elsey et al., 2015), error monitoring, and inhibitory control (Lim et al., 2015), and lower ACC connectivity with affective regions during emotion processing (Marusak et al., 2015) and during resting state (Herringa et al., 2013); these alterations may also contribute to the risk for affective disorders (Herringa et al., 2013). Interestingly, and perhaps indicative of resilience (i.e. lower vulnerability to the impact of stress), youth exposed to trauma who engaged in effortful attempts to buffer against negative stimuli showed greater activation in mPFC and ACC compared with peers not exposed to trauma (McLaughlin et al., 2015). Combined, these data highlight that exposure to trauma in childhood and adolescence alters functioning in emotional regulation networks, with concomitant heightened involvement of emotion and salience processing networks.
Despite progress in understanding the impact of trauma on reward-learning networks in youth, the role of these neural networks in the affective sequelae of trauma remains unclear. Furthermore, no studies have examined how reward-learning network function may buffer against development of anhedonia, depressive, and anxiety symptoms. In the current study, we examined the impact of trauma exposure on reward-learning network reactivity and relationships with affective states in a large sample of young adults. We employed a standardized monetary reward task to determine trauma-associated reward-learning network reactivity to RPE. While previous research has focused specifically on depressive symptoms, we focused on a range of affective symptoms, given the high correlations between trauma-associated anhedonia, depressive, and anxiety symptoms (Price and van Stolk-Cooke, 2015). The main objective of this study was to determine the impact of trauma exposure on reward-learning networks, and whether trauma-associated disruption in these networks would be associated with affective symptoms. We also wished to differentiate between pathways linking trauma and reward-learning network functioning with low v. high severity negative affect and anxiety, as a first stage toward identification of neural mechanisms underlying risk for v. resilience against development of negative affective symptoms states after trauma exposure.
We hypothesized that trauma exposure would be associated with dysregulated response to RPE in reward-learning networks, including cingulo-fronto-parietal and aIns-ACC networks, and that these trauma-associated neural network changes would be associated with the severity of negative affective and anxiety symptoms. Additionally, we hypothesized that there would be distinct relationships among trauma exposure and patterns of reward-learning network activation and connectivity associated with lower v. higher negative affect.
Materials and methods
Participants
One hundred thirty-two individuals between the ages of 18 and 25 were recruited to participate in a study examining the development of psychiatric symptoms and disorders in young adults seeking treatment for psychological distress, i.e. emotions negatively impacting level of functioning. The goal of the study was to recruit a young adult community sample, given that young adulthood is the age during which the majority of psychiatric illnesses first manifest (Kessler et al., 2007). Fifty-eight individuals considering or seeking mental healthcare for psychological distress, irrespective of present psychiatric diagnosis, were recruited through community advertisement and student counseling centers in the Pittsburgh area. Seventy-four typically developing individuals without present psychologic distress or previous personal history of psychiatric illness were recruited through a Pittsburgh participant registry and community advertisement. All individuals were right-handed and spoke fluent English. Twelve individuals were excluded due to incomplete data, two individuals were excluded due to excessive motion (>5 mm), one participant was excluded due to excessive task performance errors (20, other participants <12), and six participants were excluded due to excessive signal loss (>30%; see online Supplementary Material for full exclusion criteria). The final sample was comprised of 111 individuals, 50 experiencing psychological distress, and 61 typically developing individuals (see Table 1).
Table 1.
Sample characteristics by psychological distress
| Cohort |
|||||
|---|---|---|---|---|---|
| No distress (N = 61) |
Distress (N = 50) |
||||
| N | Mean ± s.d. | N | Mean ± s.d. | p value | |
| Gender | |||||
| Female | 39 | n/a | 39 | n/a | 0.107 |
| Male | 22 | 11 | |||
| Race | |||||
| White | 44 | n/a | 33 | n/a | 0.604 |
| Black or African American | 4 | 7 | |||
| Asian | 11 | 9 | |||
| More than one race | 2 | 1 | |||
| Age | n/a | 21.55 ± 1.79 | n/a | 21.96 ± 2.13 | 0.266 |
| IQ | n/a | 108.38 ± 7.18 | n/a | 107.91 ± 7.37 | 0.737 |
| Affective symptoms | |||||
| Anxiety (HAMA) | n/a | 0.93 ± 1.79 | n/a | 11.82 ± 7.34 | <0.001 |
| Depression (HRSD) | n/a | 1.28 ± 2.65 | n/a | 14.36 ± 7.73 | <0.001 |
| Anhedonic depression (MASQ-AD) | n/a | 2.34 ± 0.41 | n/a | 3.34 ± 0.78 | <0.001 |
| Anxious arousal (MASQ-AA) | n/a | 1.11 ± 0.15 | n/a | 1.68 ± 0.69 | <0.001 |
| Anhedonia (SHAPS) | n/a | 19.02 ± 4.95 | n/a | 26.74 ± 7.86 | <0.001 |
| Trauma | |||||
| Events (THQ) | n/a | 1.41 ± 1.49 | n/a | 2.70 ± 2.18 | <0.001 |
HAMA, Hamilton Anxiety Rating Scale; HRSD, Hamilton Depression Rating Scale; MASQ-AA, Mood and Anxiety Symptom Questionnaire-Anxious Arousal; MASQ-AD, Mood and Anxiety Symptom Questionnaire-Anhedonic Depression; SHAPS, Snaith-Hamilton Pleasure Scale; THQ, Trauma History Questionnaire.
Trauma exposure and affective measures
The Trauma History Questionnaire (THQ) (Hooper et al., 2011) assessed trauma exposure. Participants reported lifetime traumatic experiences across three categories: crime-related events (e.g. robbery), general disaster and trauma (e.g. serious car accident), and unwanted physical/sexual experiences. Cumulative trauma exposure is associated with affective and anxiety symptom severity (Myers et al., 2015) and previous research has demonstrated linear relationships between trauma exposure and brain activity (Hanson et al., 2015). Thus, trauma exposure was quantified as a continuous variable by summing the number of events endorsed across categories, the most common way of quantifying trauma using the THQ (Hooper et al., 2011).
To assess negative affective and anxiety symptoms, participants completed the following five scales: the Mood and Anxiety Symptom Questionnaire-Anhedonic Depression (MASQ-AD) and clinician-administered Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) assessed depressive symptom severity; the Snaith–Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995) measured anhedonia; and the MASQ-Anxious Arousal (MASQ-AA) and clinician-administered Hamilton Anxiety Rating Scale (HAMA) (Hamilton, 1959) assessed anxiety symptom severity.
Monetary reward functional magnetic resonance imaging (fMRI) task
An adapted event-related card-guessing task was used to evaluate neural activation during reward processing (Delgado et al., 2000; Forbes et al., 2009; Chase et al., 2017). There were four trials: win, loss, mixed, and neutral (see online Supplementary Fig. S1). Win trials included trials with the expectation of a win followed by a win outcome or no change; loss trials included the expectation of a loss followed by a loss or no change; mixed trials included the expectation of either win or loss, followed by win or loss; and neutral trials had no expectation of either win or loss, followed by no change. For each trial, a visually presented card was displayed and participants were asked to guess via button press for each trial whether the value of a presented card was higher or lower than the number ‘5’ (4 s). Participants were then shown an expectancy cue (jittered 2–6 s), where they awaited feedback regarding their guess and whether monetary reward was received. The outcome appeared for 1 s, followed by a second inter-trial interval of 0.5–1.5 s. The paradigm was presented in two 8-min blocks comprised of 48 trials per block (12 per trial type). Trials were randomized with predetermined outcomes. All participants practiced the task prior to scanning and were informed their performance would result in a monetary reward after the scan: $1 per win and $0.75 deduction per loss. The total possible reward received was $6. Participants expected that monetary outcome was due to performance; however, a fixed amount was given (Chase et al., 2017).
RPE was the regressor of interest for subsequent first-level imaging analyses (Chase et al., 2017). RPE was calculated as the difference between the expected and outcome reward values for each trial type: +$0.50 for a win (i.e. the positive difference between $0 and 1) and −$0.50 for no win (i.e. the negative difference between $0 and 1) in the possible win condition; +$0.375 for a no loss (i.e. the positive difference between $0 and 0.75) and −$0.375 for a loss in the possible loss condition (i.e. the negative difference between $0 and 0.75); +$0.875 for a win (i.e. the positive difference between $1 and 0.75) and −$0.875 for a loss in the mixed condition (i.e. the negative difference between $1 and 0.75); and zero in the neutral condition. Reward expectancy (RE) and outcome expectancy (OE) were also calculated (see online Supplementary Material).
fMRI preprocessing
Imaging data were processed using SPM, FSL, and AFNI using Nipype (Gorgolewski et al., 2011) as previously described (Chase et al., 2017). For each participant, BOLD images were realigned to the first volume in the time series and co-registered with the participant’s structural image. Field maps were used to correct for image distortion using FSL FUGUE. Structural images were normalized using a non-linear transformation to the standard MNI/ICBM 152 template and segmented into gray matter, white matter, and cerebrospinal fluid (CSF). Using DARTEL, BOLD images were transformed into the same space using the structural image and resampled at 2 mm3 isotropic voxel size. Activation spikes in the BOLD images were corrected using AFNI 3dDespike. BOLD images were then normalized for intensity and spatially smoothed [full-width at half-maximum (FWHM) 6 mm] using an adaptive smoothing method implemented in FSL SUSAN.
For each participant, Statistical Parametric Mapping (SPM8) software was used to build a fixed-effect general linear model (GLM) using RPE, OE, and RE as regressors. RE was included as a parametric modulator coupled to the arrow cards (anticipation phase; 2–6 s) reflecting the expected value of the arrow; OE was included as a regressor coupled to the arrow cards (anticipation phase; 2–6 s) reflecting the unsigned value range of possible future outcomes; RPE was coupled to the outcome and defined as the difference between the outcome and expected value. Another regressor modeled any omission errors. Gram-Schmidt orthogonalization was applied to GLM regressors, as is standard in SPM. The GLM was fit to each of the two blocks separately and the parameter estimates for a given effect type were combined across each. In addition to field map correction and removal of high-motion volumes with AFNI 3dDespike, noise was also reduced by adding determining physiologic fluctuations with the mean signal in CSF, white matter, and high-deviation voxels using CompCor (Behzadi et al., 2007; Fournier et al., 2014) and entered as a covariate. Motion parameters during scanning were also entered as covariates to control for head movement. A 60 s high-pass filter and autoregressive modeling were implemented during fitting.
Based on the initial activation analyses (see Results section), functional connectivity maps were generated using generalized psychophysiological interaction (gPPI) (O’Reilly et al., 2012) with Brodmann area (BA) 32, the ACC, as the seed region. The BOLD signal in BA32 was deconvolved to estimate neuronal activity during each regressor condition. This estimated activity was then multiplied by each column in the GLM, including each regressor condition (e.g. RPE, RE, and OE), and convolved with a hemodynamic response function (HRF). These three PPI regressors were then included in the GLM alongside the three task regressors, motion parameters, and mean time course in the seed region. Whole-brain PPI contrast images were generated by regressing the BOLD signal across brain regions onto (1) the task main effect, (2) the BOLD signal from the seed region, and (3) each of the three convolved PPI interaction regressors. Functional connectivity for each participant was thus estimated as the magnitude difference between the β coefficient between the seed and each PPI regressor.
Data analyses
To evaluate the impact of trauma on RPE-associated neural activity, two second-level regression models were run in SPM: one model to examine individual activation and another model to examine functional connectivity. For each model, the neural activation and functional connectivity contrast images were respectively entered as the dependent variables with the total THQ score as the independent regressor. Age, gender, race, intelligence quotient (IQ), and presence/absence of distress were included as covariates in both activation and connectivity models. Using a mask defined by the Wake Forest University (WFU) PickAtlas, activation analyses were constrained to reward-learning regions critical for reward learning: bilateral VS, insula, BAs 10/11/47 comprising pre- and orbito-frontal cortices, and BAs 24/32 comprising dorsal and ventral ACC (vACC). To examine trauma-associated reward-learning network connectivity (using regions from trauma-related activation as seeds; see activation Results), gPPI analyses were performed across the whole brain. As recommended by Eklund et al., (2016) and Woo et al., (2014), a voxelwise inference of punc < 0.001 was utilized (see online Supplementary Material).
After determining regions of trauma-associated neural activation and connectivity using regression models in SPM, separate multivariate linear regression models were used to test associations between these measures of trauma-associated neural activation and connectivity and affective and anxiety symptoms. Individual parameter estimates of BOLD response for significant group-level trauma-associated neural activation and connectivity clusters (during RPE) in second-level analyses were extracted using Marsbar (http://marsbar.sourceforge.net/). Two multivariate linear regression models were run: one for neural activation and one for connectivity. For each model, the five negative affective and anxiety symptom scores were entered as dependent variables, and all neural regions with significant trauma-associated activation/connectivity were entered as independent variables. Two participants were removed from the analyses due to residuals of >2 s.d. Because trauma-associated neural activation and connectivity measures had already been corrected for demographic variables and distress, these variables were excluded from the multivariate regression models. Supplementary analyses were run to examine the effects of gender.
Results
Trauma exposure
There were no differences in age, race, gender, and IQ between distressed and non-distressed participants (Table 1). Eighty participants (72.0%) reported traumatic events (M = 2.0 ± 1.93 events; range 0–10 events). This is less than the general adult population (89.7%) (Kilpatrick et al., 2013), which may reflect the younger adult sample. Of individuals experiencing traumatic events, 38.8% (n = 31; range 0–2 events) reported crime-related events, 91.3% (n = 73; range 0–6 events) reported general disasters, and 32.5% (n = 26; range 0–5 events) reported physical and/or sexual trauma.
Distressed participants (41/50; 82.0%) reported more traumatic events than non-distressed participants (39/61, 63.9%) (χ2 = 4.45, p = 0.04) and had more severe affective symptoms (Table 1). Female participants reported more traumatic experiences [t(109) = 2.04, p = 0.04] and higher negative affective symptoms with the exception of anhedonia [range: t(109) =2.48–3.91, p = <0.001–0.02] (online Supplementary Table S1). Race and IQ were not significantly associated with trauma exposure (race: F3,107 = 2.23, p = 0.09; IQ: F1,109 = 1.81, p = 0.18) or affective symptoms (race: F3,107 = 0.39–1.31, p = 0.27–0.76; IQ: F1,109 = <0.01–0.30, p = 0.58–0.98) with the exception of an association between IQ and anxious arousal (F1,109 = 3.88, p = 0.05).
Trauma exposure associations with reward learning network functioning
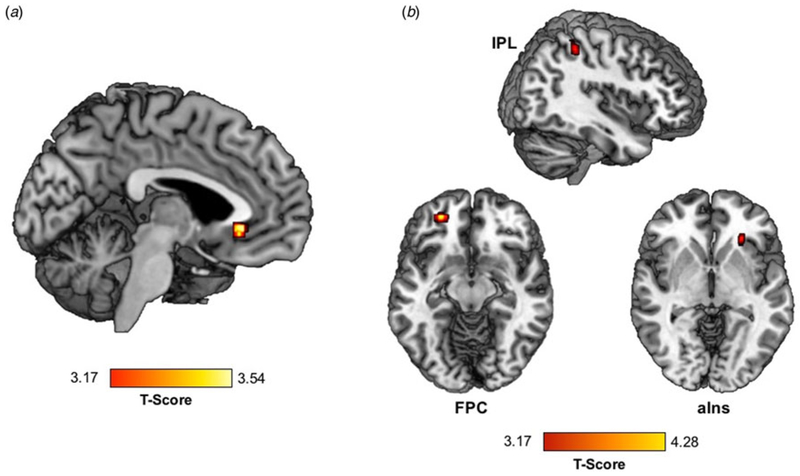
Greater trauma was significantly associated with greater activation of the vACC) (within BA32) during RPE (Table 2, Fig. 1a).
Table 2.
Trauma-associated activation and vACC connectivity during RPE in a standardized monetary reward task
| Region | Hemisphere | Voxel punc value | Cluster size | T-score | X | Y | Z |
|---|---|---|---|---|---|---|---|
| Functional activation during RPE | |||||||
| Positive regression with traumatic events | |||||||
| vACC | R/L | <0.001 | 22 | 3.54 | −4 | 30 | −6 |
| ACC functional connectivity during RPE | |||||||
| Negative regression with traumatic events | |||||||
| FG | L | <0.001 | 191 | 4.28 | −28 | −56 | −18 |
| FPC | L | <0.001 | 35 | 4.09 | −26 | 48 | −12 |
| R | <0.001 | 30 | 3.9 | 36 | 44 | 28 | |
| STG | L | <0.001 | 39 | 3.52 | −50 | −28 | 8 |
| MTG | R | <0.001 | 28 | 3.54 | 62 | −38 | 4 |
| SMG | R | <0.001 | 23 | 3.59 | 60 | −16 | 26 |
| Lingual gyrus | R | <0.001 | 76 | 3.53 | 24 | −80 | −6 |
| IPL | R | <0.001 | 25 | 3.45 | 40 | −40 | 50 |
| aIns | R | <0.001 | 23 | 3.36 | 46 | 2 | 12 |
Fig. 1.
Trauma exposure is associated with (a) greater activation in the vACC and (b) lower vACC connectivity with the right aIns, left FPC, and right IPL during RPE in a standardized monetary reward task.
Greater trauma exposure was associated with lower connectivity between vACC (BA32 anatomical mask) and nine regions during RPE: bilateral frontopolar cortex (FPC); left fusiform gyrus (FG) and superior temporal gyrus (STG); and right middle temporal gyrus (MTG), supramarginal gyrus (SMG), IPL, aIns, and lingual gyrus (Table 2, Fig. 1b).
Trauma exposure was not associated with neural activation during RE and OE conditions. Trauma exposure was also not associated with changes in VS activation or connectivity.
Trauma-associated reward-learning network functioning associations with negative affective and anxiety measures
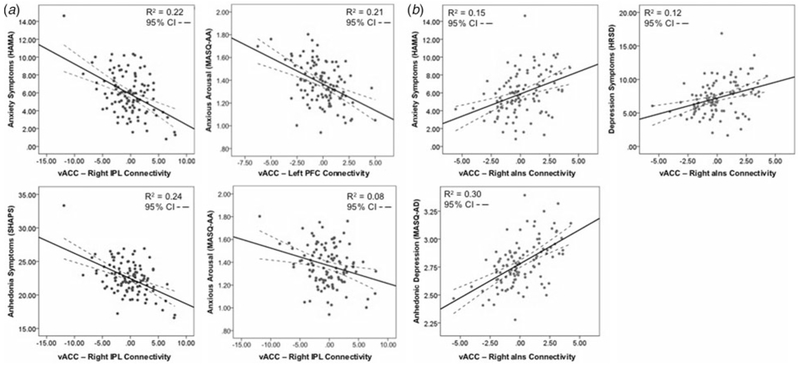
Trauma-associated vACC activation was not associated with negative affect and anxiety symptoms. After including all nine regions of significant vACC connectivity in the multiple linear regression model, several regions with significant trauma-associated vACC connectivity were associated with the severity of negative affective and anxiety symptom measures (Table 3). Lower vACC-right IPL connectivity was associated with greater anxiety symptom severity (HAMA: β −0.58, t = −1.97, p = 0.05), greater anxious arousal (MASQ-AA: β = −0.04, t = −2.03, p = 0.05), and greater anhedonia (SHAPS: β −0.60, t = −2.06, p = 0.04; Fig. 2a). Lower vACC-left FPC connectivity was associated with greater anxious arousal (β = −0.06, t = −1.98, p = 0.05; Fig. 2a). Interestingly, lower vACC connectivity with the right aIns was associated with lower anxiety symptom severity (HAMA: β = 1.21, t = 2.41, p = 0.02), lower depression symptom severity (HRSD: β = 1.29, t = 2.20, p = 0.03), and lower anhedonic depression severity (MASQ-AD: β = 0.11, t = 2.07, p = 0.04) (Fig. 2b).
Table 3.
Multiple linear regression model demonstrating relationship between trauma-associated vACC connectivity and affective and anxiety symptoms
| Anxiety (HAMA) |
Depression (HRSD) |
Anhedonic depression (MASQ-AD) |
Anxious arousal (MASQ-AA) |
Anhedonia (SHAPS) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (s.e.) | t | p | β (s.e.) | t | p | β (s.e.) | t | p | β (s.e.) | t | p | β (s.e.) | t | p | |
| Left FPC | −0.13 (0.43) | −0.30 | 0.76 | −0.01 (0.50) | −0.03 | 0.98 | 0.02 (0.05) | 0.46 | 0.64 | −0.06 (0.03) | −1.98 | 0.05 | −0.07 (0.43) | −0.15 | 0.88 |
| Left FG | −0.25 (0.46) | −0.54 | 0.59 | −0.08 (0.54) | −0.14 | 0.89 | −0.02 (0.05) | −0.33 | 0.75 | −0.04 (0.03) | −1.14 | 0.26 | 0.08 (0.46) | 0.18 | 0.86 |
| Left STG | 0.10 (0.29) | 0.33 | 0.74 | −0.05 (0.34) | −0.15 | 0.88 | −0.01 (0.03) | −0.34 | 0.73 | 0.00 (0.02) | −0.22 | 0.83 | 0.05 (0.29) | 0.19 | 0.85 |
| Right lingual gyrus | 0.30 (0.35) | 0.85 | 0.40 | 0.22 (0.41) | 0.53 | 0.60 | 0.04 (0.04) | 1.02 | 0.31 | 0.02 (0.03) | 0.73 | 0.47 | 0.53 (0.35) | 1.49 | 0.14 |
| Right FPC | −0.18 (0.23) | −0.81 | 0.42 | −0.16 (0.26) | −0.59 | 0.55 | 0.00 (0.02) | −0.14 | 0.89 | −0.01 (0.02) | −0.63 | 0.53 | −0.30 (0.23) | −1.35 | 0.18 |
| Right IPL | −0.58 (0.29) | −1.97 | 0.05 | −0.47 (0.34) | −1.38 | 0.17 | −0.04 (0.03) | −1.25 | 0.22 | −0.04 (0.02) | −2.03 | 0.04 | −0.60 (0.29) | −2.06 | 0.04 |
| Right insula | 1.21 (0.50) | 2.40 | 0.02 | 1.29 (0.59) | 2.20 | 0.03 | 0.11 (0.05) | 2.07 | 0.04 | 0.06 (0.04) | 1.60 | 0.11 | 0.89 (0.50) | 1.76 | 0.08 |
| Right SMG | −0.22 (0.29) | −0.76 | 0.45 | −0.41 (0.34) | −1.22 | 0.23 | −0.02 (0.03) | −0.55 | 0.58 | 0.03 (0.02) | 1.20 | 0.23 | −0.05 (0.29) | −0.18 | 0.86 |
| Right MTG | 0.14 (0.25) | 0.55 | 0.59 | 0.19 (0.29) | 0.66 | 0.51 | 0.01 (0.03) | 0.29 | 0.78 | 0.03 (0.02) | 1.38 | 0.17 | 0.07 (0.25) | 0.29 | 0.77 |
| Corrected model | R2 = 0.10, p = 0.30 | R2 = 0.08, p = 0.46 | R2 = 0.07, p = 0.62 | R2 = 0.10, p = 0.26 | R2 = 0.11, p = 0.23 | ||||||||||
HAMA, Hamilton Anxiety Rating Scale; HRSD, Hamilton Depression Rating Scale; MASQ-AA, Mood and Anxiety Symptom Questionnaire-Anxious Arousal; MASQ-AD, Mood and Anxiety Symptom Questionnaire-Anhedonic Depression; SHAPS, Snaith–Hamilton Pleasure Scale.
Fig. 2.
Trauma-associated vACC connectivity is associated with negative affective and anxiety symptom severity. (a) Lower trauma-associated connectivity with the right IPL and left FPC is associated with greater severity of anxiety, anxious arousal, and anhedonia symptoms. (b) Lower trauma-associated connectivity with the right aIns is associated with lower severity of anxiety, depression, and anhedonic depression symptoms.
There was a significant interaction with gender in the multivariate model (F5,94 = 3.24, p = 0.01). The effects of gender on the relationships between trauma-associated vACC connectivity and negative affective and anxiety symptoms were examined by running multiple linear regressions separately for males and females. The above associations remained significant for females, although anxious arousal was associated only at trend levels with vACC-left FPC (β = −0.08, t = −1.92, p = 0.06) and vACC-right IPL (β = −0.05, t = −1.74, p = 0.09) connectivity (online Supplementary Table S2). Additionally, among females, lower vACC-right SMG connectivity was associated with lower anxious arousal (β = 0.07, t = 1.97, p = 0.05). The above associations were not significant for males. The slopes of these associations differed between males and females for the vACC-aIns association with HAMA (Fl,109 = 3.29, p = 0.04) and the vACC-SMG association with anxious arousal (F1,109 = 3.02, p = 0.05). For the gender effects, it is important to note that this sample had fewer males (n = 33) than females (n = 78), and males exhibited a narrower range of negative affective and anxiety symptoms (online Supplementary Table S2, Figs S2–S6).
Discussion
This study sought to examine the impact of trauma exposure on reward-learning networks, and whether trauma-associated changes in reward learning were associated with negative affective and anxiety symptoms. In a large sample of young adults with vaiying degrees of negative affective symptoms, greater trauma exposure was associated with greater activation of vACC and lower vACC connectivity with several regions in cingulo-fronto-parietal and aIns-vACC networks, during RPE. In multiple linear regression models, distinct patterns of vACC connectivity were associated with higher and lower anhedonia, depression, and anxiety symptom severity. Lower vACC connectivity with the left FPC and right IPL during RPE were associated with greater anxiety, anxious arousal, and anhedonia, whereas lower connectivity between vACC and the right aIns during RPE was associated with lower depression and anxiety. These findings were specific to reward learning, as trauma was not associated with RE and OE. Together, these results indicate that dissociable patterns of reward-learning network connectivity may be associated with vulnerability to, v. resilience against, development of negative affective and anxiety symptoms following trauma exposure.
Heightened ACC activation during RPE with greater trauma occurred in a ventrally located region, which is notable because the dorsal ACC is more frequently implicated in reward valuation and learning (Niki and Watanabe, 1979; Williams and Goldman-Rakic, 1998; Amiez et al., 2005; Walton et al., 2006; Rushworth and Behrens, 2008). The vACC is anatomically connected with the amygdala, VS, and orbitofrontal cortex (Beckmann et al., 2009; Peters et al., 2009; Etkin et al., 2011; Barker et al., 2014), however, and is implicated in emotion regulation, experiencing negative affect, and encoding reward expectation (Rushworth and Behrens, 2008; Beckmann et al., 2009). Neurons in this region are also involved in decision-making during reward valuation, and may be associated with decision-making influenced by negative emotion (e.g. anxious avoidance) (Amemori and Graybiel, 2012). Greater recruitment of vACC with greater trauma exposure in the current study may thus represent a greater need for emotion regulation and decision-making during unexpected reward-related outcomes (i.e. greater prediction error). While speculative, this may be due to a negative emotional bias that may blunt the perception of reward or alter it such that rewards may be misperceived as aversive. vACC activation, unlike vACC connectivity, did not explain trauma-associated affective and anxiety symptoms, however, suggesting that disruption of distributed reward-learning networks, rather than altered activation in a given neural region, may underlie the development of negative affective and anxiety symptoms following trauma exposure. Indeed, one recent study found that RPE signals in VS were intact in major depression and suggested that altered RPE in affective disorders may result from downstream effects rather than striatal dopaminergic projections themselves (Rutledge et al., 2017).
Our findings suggest two different patterns of vACC connectivity underlying relationships between trauma exposure and negative affective and anxiety symptoms. White matter connections between vACC and FPC facilitate value calculations, motivation, and affect regulation (Boschin et al., 2015), while connectivity between vACC and IPL is implicated in sustained attention and future goal-directed planning (Vincent et al., 2008). Our findings of trauma exposure-related reductions in vACC-IPL and vACC-FPC connectivity thus suggest that trauma disrupts cingulo-frontal-parietal network functioning, including attention to reward and engaging in flexible feedback-based decisionmaking (Ptak, 2012). One study found that youth exposed to trauma exhibited lower ACC-PFC during social rejection (Puetz et al., 2014), indicating aberrant affective processing of negative emotional stimuli. This latter finding suggests trauma-associated disruptions in vACC-FPC and vACC-IPL connectivity during RPE may represent impaired ability to process reward value and/or regulate affective responses to reward. Furthermore, trauma-associated patterns of lower connectivity between the vACC and these regions in the cingulo-frontal-parietal network were associated with more severe anxiety and anhedonia. Similar patterns of disconnection within cingulo-frontal-parietal networks have been associated with depressive and anxiety symptoms (Sylvester et al., 2012; Demirtaş et al., 2016). Together, these findings suggest that without the active ability to focus attention and engage in flexible decision-making in response to reward, trauma-exposed individuals may be more likely to remain in ruminative and/or emotionally distractible states associated with depression and anxiety.
vACC connectivity with the aIns, a region implicated in self-awareness (Pollatos et al., 2007), was also inversely associated with greater trauma exposure. vACC-aIns connectivity is important for interoceptive processing and response to emotional stimuli (Cox et al., 2011). Furthermore, greater vACC-aIns connectivity has been associated with hopelessness (Yao et al., 2009), and may facilitate translation of emotional stimuli into heightened arousal processes (Pollatos et al., 2007). These findings suggest that greater connectivity between these regions may facilitate negative affective and anxiety states, perhaps through maladaptive, heightened self-awareness, and difficulty responding to the external environment to guide behavior. Interestingly, in the current study, lower vACC connectivity with aIns was associated with lower depressive symptom severity and anxious arousal. This finding suggests that weaker connections between regions implicated in interoception-related arousal and self-related processing may diminish arousal and depressogenic processes and confer resilience against the development of negative affective and anxiety symptoms following trauma exposure.
Few studies examined the neural underpinnings of resilience following trauma exposure; examining such pathways is critical for developing effective interventions. Particular focus on neural networks underlying negative affective states is important, as youth exposed to trauma exhibit blunted positive affect (Marusak et al., 2015), and negative emotions predict ongoing trauma-related symptoms (Sadeh et al., 2015). One study demonstrated that lower ACC connectivity with the default mode network, including self-related processing regions, was associated with lower depressive and anxiety symptom severity among youth exposed to trauma, although only in individuals with greater behavioral activation system (BAS) sensitivity (Iadipaolo et al., 2017). Greater BAS sensitivity, which is implicated in reward seeking and positive affect, is associated with decreased risk for negative affect following stress, as it may enhance the ability to disengage from ruminative thought patterns (Heponiemi et al., 2003). Future research should focus on neural factors underlying resilience, and whether intervention strategies support positive adaptation in these neural networks.
While our findings differed between males and females, where trauma-associated vACC connectivity was associated with affective and anxiety outcomes only in females, this should be interpreted with caution. In this sample, female participants experienced a greater number of traumatic events and exhibited a greater range of negative affective and anxiety symptoms compared with males, which may have led to the lack of association in males. Women are more likely than men to develop trauma-related disorders (Tolin and Foa, 2006), however, owing to effects of genetic, epigenetic, and neuroendocrine factors on stress circuitry (Tolin and Foa, 2006), and gender differences in development of affective and anxiety symptoms (Kaczkurkin et al., 2016). Further research, including among males sampled across a greater symptom range, will be necessary to elucidate gender differences in relationships between trauma exposure, reward-learning networks, and affective and anxiety symptoms.
There were limitations to the current study. The study was cross-sectional and therefore was not designed to identify neural predictors of future negative affective and anxiety symptom severity. Similarly, participants retrospectively reported trauma exposure. Prospective studies of trauma exposure in youth are challenging, but future studies should examine the impact of current trauma exposure on future worsening of negative affective and anxiety symptoms, and mechanisms underlying resilience to the development of these symptoms. Trauma exposure was quantified as the cumulative experience with types of traumatic events and/or neglect; trauma duration, age of trauma exposure, time since trauma exposure, and classes of trauma (e.g. general disaster v. crime) are also important and may be influencing the current results. Studies with larger samples, detailed trauma exposure histories, and ranges of psychopathology severity alongside neuroimaging data will be helpful in differentiating the effects of type and timing of exposure on reward-learning networks. While the study included individuals with one or more psychiatric diagnoses, the transdiagnostic approach to recruitment of healthy and distressed individuals allowed inclusion of individuals across a wide range of trauma exposure and negative affective and anxiety symptom severity, reflecting the fact that many individuals exposed to trauma exhibit negative affective features that are common to multiple diagnostic categories. Finally, while the presented activation and connectivity results do meet uncorrected statistical thresholds, the likelihood of false positives is higher than more rigorous corrected statistical thresholds; however, the large sample and strict control for potential confounding variables in the imaging analyses combined with the control for multiple comparisons during multiple linear regression analyses decrease the likelihood of type I error.
We identified specific patterns of connectivity in reward-learning networks associated with greater v. lower severity negative affective and anxiety symptoms in young adults. Specifically, the combined pattern of trauma-associated greater vACC activation and lower vACC connectivity with FPC and IPL may reflect aberrant regulatory mechanisms in the context of unexpected reward, where greater trauma results in abnormal recruitment of a prefrontal cortical region, the vACC, implicated in emotion regulation, and lower connectivity of this region with neural regions important for decision-making. Yet, the relationship between lower trauma-associated connectivity between vACC and interoceptive and self-related processing regions and lower depression and anxiety symptom severity suggests a neural mechanism underlying a more adaptive, less self-focused response to trauma exposure. These findings highlight dissociable neural mechanisms underlying vulnerability to, v. resilience against, negative affective and anxiety states following trauma exposure, and provide neural targets for future novel, e.g. neuromodulation, interventions.
Supplementary Material
Acknowledgments.
The present work was supported by 1R01MH100041 to MLP.
Financial support. This work was supported by the National Institute of Mental Health (R01MH100041).
Footnotes
Ethical standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718002520
Conflict of interest. The authors declare no conflicts of interest.
References
- Amemori K-i and Graybiel AM (2012) Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nature Neuroscience 15, 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Joseph J-P and Procyk E (2005) Reward encoding in the monkey anterior cingulate cortex. Cerebral Cortex 16, 1040–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR and Giles WH (2006) The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neuro-biology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience 256, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR and Chandler LJ (2014) A unifying model of the role of the infralimbic cortex in extinction and habits. Learning & Memory 21, 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H and Rushworth MF (2009) Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience 29, 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J and Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschin EA, Piekema C and Buckley MJ (2015) Essential functions of primate frontopolar cortex in cognition. Proceedings of the National Academy of Sciences of the United States of America 112, E1020–E1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ and Anda RF (2004) Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders 82, 217–225. [DOI] [PubMed] [Google Scholar]
- Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R, Lockovich J, Graur S, Bebko G, Forbes EE and Phillips ML (2017) A pathway linking reward circuitry impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Translational Psychiatry 7, e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Frias NS, Nikolova YS, Michalski LJ, Baranger DA, Hariri AR and Bogdan R (2015) Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychological Medicine 45, 2605–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP and Kelly C (2011) The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Social Cognitive and Affective Neuroscience 7, 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC and Fiez JA (2000) Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology 84, 3072–3077. [DOI] [PubMed] [Google Scholar]
- Demirtaş M, Tornador C, Falcon C, López-Solà M, Hernández-Ribas R, Pujol J, Menchon JM, Ritter P, Cardoner N and Soriano-Mas C (2016) Dynamic functional connectivity reveals altered variability in functional connectivity among patients with major depressive disorder. Human Brain Mapping 37, 2918–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison MJ, Sheridan MA, Busso DS, Jenness JL, Peverill M, Rosen ML and McLaughlin KA (2016) Neurobehavioral markers of resilience to depression amongst adolescents exposed to child abuse. Journal of Abnormal Psychology 125, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE and Knutsson H (2016) Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsey J, Coates A, Lacadie CM, McCrary EJ, Sinha R, Mayes LC and Potenza MN (2015) Childhood trauma and neural responses to personalized stress, favorite-food and neutral-relaxing cues in adolescents. Neuropsychopharmacology 40, 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T and Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B and Axelson DA (2009) Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 166, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JC, Chase HW, Almeida J and Phillips ML (2014) Model specification and the reliability of fMRI results: implications for longitudinal neuroimaging studies in psychiatry. PLoS One 9, el05169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J and Tottenham N (2013) Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience 249, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML and Ghosh SS (2011) Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Frontiers in Neuroinformatics 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS and Fox NA (2006) Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience 26, 6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1959) The assessment of anxiety states by rating. British Journal of Medical Psychology 32, 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960) A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Albert D, Iselin AR, Carre JM, Dodge KA and Hariri AR (2015) Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Social Cognitive and Affective Neuroscience 11, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heponiemi T, Keltikangas-Järvinen L, Puttonen S and Ravaja N (2003) BIS/BAS sensitivity and self-rated affects during experimentally induced stress. Personality and Individual Differences 34, 943–957. [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ and Essex MJ (2013) Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the United States of America 110, 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LM, Stockton P, Krupnick JL and Green BL (2011) Development, use, and psychometric properties of the Trauma History Questionnaire. Journal of Loss and Trauma 16, 258–283. [Google Scholar]
- Iadipaolo AS, Marusak HA, Sala-Hamrick K, Crespo LM, Thomason ME and Rabinak CA (2017) Behavioral activation sensitivity and default mode network-subgenual cingulate cortex connectivity in youth. Behavioural Brain Research 333, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Moore TM, Ruparel K, Ciric R, Calkins ME, Shinohara RT, Elliott MA, Hopson R, Roalf DR and Vandekar SN (2016) Elevated amygdala perfusion mediates developmental sex differences in trait anxiety. Biological Psychiatry 80, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S and Ustun TB (2007) Age of onset of mental disorders: a review of recent literature. Current Opinion in Psychiatry 20, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM and Friedman MJ (2013) National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress 26, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson S, McCutcheon VV, Agrawal A, Lynskey MT, Conroy E, Statham DJ, Madden PA, Henders AK, Todorov AA, Bucholz KK, Degenhardt L, Martin NG, Heath AC and Nelson EC (2016) The variance shared across forms of childhood trauma is strongly associated with liability for psychiatric and substance use disorders. Brain and Behavior 6, e00432. doi: 10.1002/brb3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, D’arcy C and Meng X (2016) Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychological Medicine 46, 717–730. [DOI] [PubMed] [Google Scholar]
- Lim L, Hart H, Mehta MA, Simmons A, Mirza K and Rubia K (2015) Neural correlates of error processing in young people with a history of severe childhood abuse: an fMRI study. American Journal of Psychiatry 172, 892–900. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A and Thomason ME (2015) Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology 40, 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S and Sheridan MA (2015) Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatiy 54, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC and Sonuga-Barke E (2010) Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience 22, 2316–2325. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Nakamura EF and Kessler RC (2009) Epidemiology of mental disorders in children and adolescents. Dialogues in Clinical Neuroscience 11, 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers HF, Wyatt GE, Ullman JB, Loeb TB, Chin D, Prause N, Zhang M, Williams JK, Slavich GM and Liu H (2015) Cumulative burden of lifetime adversities: trauma and mental health in low-SES African Americans and Latino/as. Psychological Trauma 7, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V, Uher R and Danese A (2012) Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. American Journal of Psychiatry 169, 141–151. [DOI] [PubMed] [Google Scholar]
- Niki H and Watanabe M (1979) Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Research 171, 213–224. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Bogdan R, Brigidi BD and Hariri AR (2012) Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biological Psychiatry 72, 157–163. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H and Dolan RJ (2003) Temporal difference models and reward-related learning in the human brain. Neuron 38, 329–337. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM and Johansen-Berg H (2012) Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience 7, 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW and Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & Memory 16, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA (2014) Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R, Moisa M, Opitz A, Grueschow M and Ruff CC (2015) The precision of value-based choices depends causally on fronto-parietal phase coupling. Nature Communications 6, 8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Gramann K and Schandry R (2007) Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping 28, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M and van Stolk-Cooke K (2015) Examination of the interrelations between the factors of PTSD, major depression, and generalized anxiety disorder in a heterogeneous trauma-exposed sample using DSM 5 criteria. Journal of Affective Disorders 186, 149–155. [DOI] [PubMed] [Google Scholar]
- Ptak R (2012) The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. The Neuroscientist 18, 502–515. [DOI] [PubMed] [Google Scholar]
- Puetz VB, Kohn N, Dahmen B, Zvyagintsev M, Schüppen A, Schultz RT, Heim CM, Fink GR, Herpertz-Dahlmann B and Konrad K (2014) Neural response to social rejection in children with early separation experiences. Journal of the American Academy of Child & Adolescent Psychiatry 53, 1328–1337, e8. [DOI] [PubMed] [Google Scholar]
- Rushworth MF and Behrens TE (2008) Choice, uncertainty and value in pre-frontal and cingulate cortex. Nature Neuroscience 11, 389–397. [DOI] [PubMed] [Google Scholar]
- Rutledge RB, Moutoussis M, Smittenaar P, Zeidman P, Taylor T, Hrynkiewicz L, Lam J, Skandali N, Siegel JZ, Ousdal OT, Prabhu G, Dayan P, Fonagy P and Dolan RJ (2017) Association of neural and emotional impacts of reward prediction errors with major depression. JAMA Psychiatry 74, 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Miller MW, Wolf EJ and Harkness KL (2015) Negative emotionality and disconstraint influence PTSD symptom course via exposure to new major adverse life events. Journal of Anxiety Disorders 31, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2016) Dopamine reward prediction error coding. Dialogues in Clinical Neuroscience 18, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D and Trigwell P (1995) A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry 167, 99–103. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, Vulser H, Miranda R, Penttilä J and Struve M (2015) The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. American Journal of Psychiatry 172, 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C, Corbetta M, Raichle M, Rodebaugh T, Schlaggar B, Sheline Y, Zorumski C and Lenze E (2012) Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences 35, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF and Foa EB (2006) Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological Bulletin 132, 959–992. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME and Buckner RL (2008) Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology 100, 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rhein D, Beckmann CF, Franke B, Oosterlaan J, Heslenfeld DJ, Hoekstra PJ, Hartman CA, Luman M, Faraone SV and Cools R (2017) Network-level assessment of reward-related activation in patients with ADHD and healthy individuals. Human Brain Mapping 38, 2359–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M and Claes S (2013) Reduced reward learning predicts outcome in major depressive disorder. Biological Psychiatry 73, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M, Kennerley S, Bannerman D, Phillips P and Rushworth MF (2006) Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Networks 19, 1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ma N, He X, Li N, Wei Z, Yang L, Zha R, Han L, Li X and Zhang D (2017) Neural substrates of updating the prediction through prediction error during decision making. Neuroimage 157, 1–12. [DOI] [PubMed] [Google Scholar]
- White SF, Geraci M, Lewis E, Leshin J, Teng C, Averbeck B, Meffert H, Ernst M, Blair JR and Grillon C (2016) Prediction error representation in individuals with generalized anxiety disorder during passive avoidance. American Journal of Psychiatry 174, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM and Goldman-Rakic P (1998) Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex 8, 321–345. [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A and Wager TD (2014) Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Wang L, Lu Q, Liu H and Teng G (2009) Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting-state fMRI study. Journal of Affective Disorders 115, 430–438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.