Summary
Background:
Childhood BMI predicts adult obesity. How growth trajectories during childhood relate to adult obesity risk is not well defined.
Objective:
We aimed to characterize BMI growth trajectories from childhood to midlife and to examine the associations between BMI growth rates at childhood age points and adult obesity risk.
Methods:
The longitudinal study included 2,732 participants with repeated BMI measurements from childhood (4–19 years) to adulthood (20–51 years). A random-effects model was used to construct BMI growth curves by race and sex. Model-estimated levels and linear growth rates of BMI were linked to adult obesity in separate multivariable logistic regression models at individual childhood age points.
Results:
BMI followed cubic growth curves. Childhood BMI linear slope estimates were higher in adults with obesity than in adults without obesity (p<0.001). The association between childhood BMI growth rate and adult obesity was significantly higher in puberty and post puberty (12–19 years) than in early childhood (4–11 years) with a peak at age 14 (odds ratio=3.1 and 95% confidence interval=2.7–3.5).
Conclusions:
Rates of change in BMI at different childhood ages are differentially associated with adult obesity. Puberty and post puberty are crucial periods for the development of obesity in later life.
Keywords: Body mass index, Childhood, Obesity, Growth curve, Longitudinal study
Introduction
Obesity is a major public health challenge due to its high prevalence1 and associated increased comorbidity2 and mortality3. Numerous studies have documented that obesity in adult life has its origins in childhood4–6. Body mass index (BMI), the most commonly used measure to diagnose obesity, in childhood is highly correlated with BMI in later life, with correlations varying from 0.3 to 0.9, depending on the time interval and age period4–10. In addition to absolute BMI values, rapid BMI increase is also a predictor of obesity in adult life11,12. We have shown that the overall rate of change in BMI during childhood (age range: 5 to 19 years) is significantly associated with risk of obesity in adult life, independent of absolute BMI values12. Recently, Aris et al. reported that infant peak BMI and pre-peak BMI velocity (rate of change) were associated with overweight at age 48 months, suggesting an important impact of early BMI growth patterns13.
BMI levels at different ages in childhood indicate the body weight status at a particular time point, and the rate of increase in BMI at specific childhood ages reflects the velocity at which weight changes relative to height. A steeper slope of BMI at a particular age point is indicative of the potential to gain weight later. Previous studies have examined different characteristics of BMI growth patterns in early life, such as early rebound14–16, period growth velocity17, and group membership of modeled trajectories18,19, for their relevance to adult obesity and cardiometabolic health. Yet the relevance of rate of change at different ages during childhood for adult obesity risk has not been reported. Information in this regard is important to understanding how childhood BMI trajectories relate to risk of obesity in adulthood and will facilitate developing informed strategies for early intervention and prevention. We hypothesized that rate of change in BMI at different time points during childhood may be predictive of adult obesity risk, independent of the absolute BMI value at the same time point.
Using BMI data from childhood to adult life from the Bogalusa Heart Study cohort20, we aimed to characterize BMI growth trajectories from early school age to midlife and to examine the associations of BMI growth curve parameters, including BMI levels and rates of change at different ages during childhood, with adult obesity risk.
Methods
Study cohort
The Bogalusa Heart Study, a series of long-term observations in a semi-rural community with about 65% Whites and 35% Blacks in Bogalusa, Louisiana, has been continuously in operation since 1973. It is focused on understanding the early natural history of atherosclerosis beginning in childhood20. Nine cross-sectional surveys of children and adolescents aged 4–19 years and eleven cross-sectional surveys of adults aged 20–51 years who were previously examined as children were conducted between 1973 and 2010 in the Bogalusa community. Linking data from these repeated cross-sectional examinations conducted every 2–3 years provided 40,365 serial observations from childhood to adulthood on 14,164 individuals. In the current study, 2,732 adult subjects (1,772 Whites and 960 Blacks; 44.9% males) who had been examined for BMI 4–15 times (7.0 times on average, at least 2 times in childhood and at least 2 times in adulthood) formed the longitudinal study cohort, with 19,164 observations. The average follow-up time was 28.4 years.
All participants or their legal guardians at each examination provided written informed consent. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
Examinations
All measurements were obtained by trained study staff members who followed a standard protocol20. At each study visit, weight was measured with participants in light clothing without shoes to the nearest 0.1 kg on a study-designated scale that was routinely calibrated, and height was measured to the nearest 0.1 cm with a free-standing stadiometer. Height and weight were measured at least twice and the mean value of each of these was used to calculate BMI (calculated as weight in kilograms divided by height in meters squared). Intraclass correlation based on rescreening of about 10% randomly selected participants was 0.99 for both height and weight. Information on smoking and alcohol use was obtained by means of a staff-administered standardized questionnaire21,22. Current smoking and drinking in adulthood were defined as smoking at least one cigarette per day and consuming alcohol every day, respectively, during the prior 12 months.
Statistical methods
Parameters of nonlinear growth curves of BMI from childhood to adulthood were estimated using a random-effects mixed model by SAS proc MIXED as previously reported23,24. The mixed model incorporates fixed and random effects and allows the intercept, linear and nonlinear parameters to vary from individual to individual. The random effect coefficients represent deviations of BMI for individuals from the fixed effect parameters. In addition, the mixed model allows for repeated measurements and different numbers of unequally spaced observations across individuals. The model computes maximum likelihood estimates of growth curve parameters, including fixed effect parameters (for a group) and random-effect parameters (for each individual within the group). By combining these two types of parameters, 2,732 different sets of growth curve parameters were generated for each of 2,732 participants in the study cohort. The model selection was based on the Akaike’s information criterion (AIC) and p-values of the independent variable (age) at the significance level of 0.05. Age and its higher-order terms were included one by one for model building. The higher-order terms of age were not included in the model if they were not significant, or made lower-order terms not significant, or did not improve the goodness-of-fit of the model based on AIC values. Cubic curves were fitted for BMI in the four race and sex groups:
where β = (β0, β1, β2, β3)’ is a vector of fixed effect parameters, b = (b0, b1, b2, b3)’ is a vector of random effect parameters, and ɛ is an unknown error term. Age was centered to the mean age (20.1 years) to reduce the collinearity of age with its higher-order terms. The term age2 was divided by 10 and age3 by 20 to improve model fitting. We modeled the growth curves for the four race and sex group separately. To illustrate differences in BMI growth curves from childhood to adulthood between adults with and without obesity, we also modeled the growth curves further by adult obesity status.
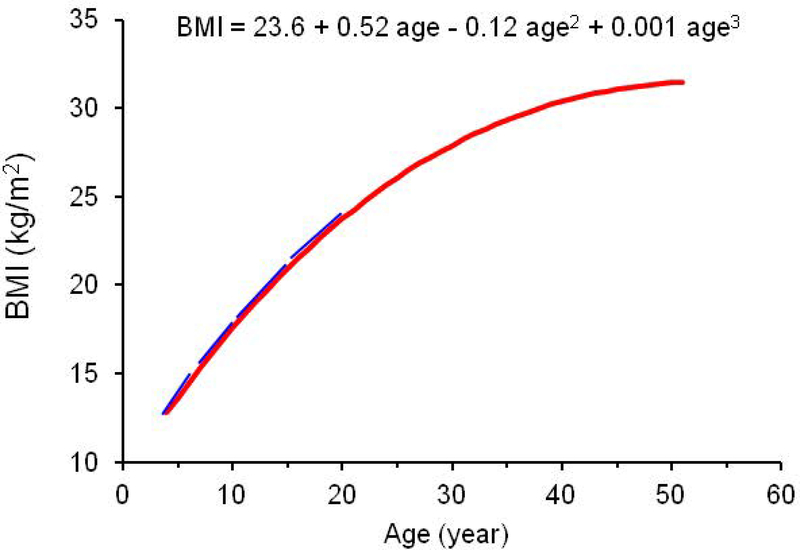
Figure 1 shows the BMI growth curve of a white male participant as an example to describe the calculation of model-estimated rate of change (slope) in BMI at different age points during childhood. The rate of change (blue tangent lines in Figure 1) in BMI at each childhood age point was estimated during the 4- to 19-year age period for each individual. The levels and rates of changes of BMI were calculated using individual-specific curve parameters and their first derivatives, respectively.
Figure 1.
Illustration of model-estimated linear slopes of BMI during childhood in a white male participant. Age was centered to 20.1 years for model fitting.
Obesity, the outcome variable, was defined as BMI ≥ 30 kg/m2 in the last adult survey. The significance of differences in mean levels of study variables and curve parameters between race-sex groups was tested using analysis of covariance in generalized linear models. Logistic regression models were used to estimate the association of rate of change in BMI at each childhood integer age point with adult obesity, adjusting for age, race, sex, smoking and alcohol drinking in the last adult survey. The odds ratios (ORs) of levels and level-adjusted slopes of BMI at individual childhood age points were estimated in separate models. Prior to logistic regression, rate of change in BMI at each childhood age point was adjusted for the BMI level at the same age point by regression residual analyses by race and sex to avoid the collinearity between BMI levels and rates of change in the same model, and then multiplied by 5 such that ORs represent increased odds of having obesity associated with each 0.2 kg/m2/year change in the slope. Because the associations of rates of change with adult obesity largely followed similar patterns across the race and sex groups, we presented the results with the four groups combined (with additional adjustment for race and sex) in the main text and the results by race and sex in the supplementary materials.
Results
The overall prevalence of adult obesity was 34.0%, with a significant sex difference in Blacks (44.7% in women versus 30.0% in men, P<0.001) and a significant race difference in women (44.7% in Blacks versus 30.3% in Whites). BMI showed sex differences from puberty to middle-age adulthood in Blacks (males<females, P<0.01) and Whites (males>females, P<0.01) and race differences in females only (Blacks>Whites, P<0.001) (Table 1).
Table 1.
Characteristics of the study cohort by race and sex
| Study variable | Whites |
Blacks |
P for Race Difference |
|||
|---|---|---|---|---|---|---|
| Males (n=803) | Females (n=969) | Males (n=423) | Females (n=537) | Males | Females | |
| Pre-Puberty (4–11 years) | ||||||
| Age (years) | 8.1 (2.0) | 7.9 (2.1) | 8.2 (2.1)* | 7.9 (2.0) | 0.200 | 0.819 |
| BMI (kg/m2) | 16.5 (2.3) | 16.6 (2.9) | 16.5 (2.5) | 16.5 (2.6) | 0.986 | 0.662 |
| Puberty (12–19 years) | ||||||
| Age (years) | 16.7 (2.0) | 16.7 (2.0) | 17.0 (1.8) | 16.9 (1.8) | 0.012 | 0.016 |
| BMI (kg/m2) | 22.3 (4.4)** | 21.6 (4.4) | 22.4 (4.7)** | 23.5 (5.3) | 0.591 | <0.001 |
| Adulthood (20–51 years) | ||||||
| Age (years) | 35.0 (8.9) | 34.7 (9.0) | 32.3 (9.4) | 32.9 (9.2) | <0.001 | <0.001 |
| BMI (kg/m2) | 28.5 (6.2)** | 27.6 (7.5) | 27.7 (7.0)** | 30.3 (8.8) | 0.041 | <0.001 |
| Obesity (%) | 33.5 | 30.3 | 30.0** | 44.7 | 0.241 | <0.001 |
| Smoker (%) | 33.0** | 32.2 | 36.2** | 25.5 | 0.294 | 0.008 |
| Drinker (%) | 32.1** | 15.8 | 42.1** | 17.3 | 0.001 | 0.486 |
Sex difference within racial groups:
p<0.05
p<0.01
Data are presented in means (standard deviations) are presented unless otherwise indicated.
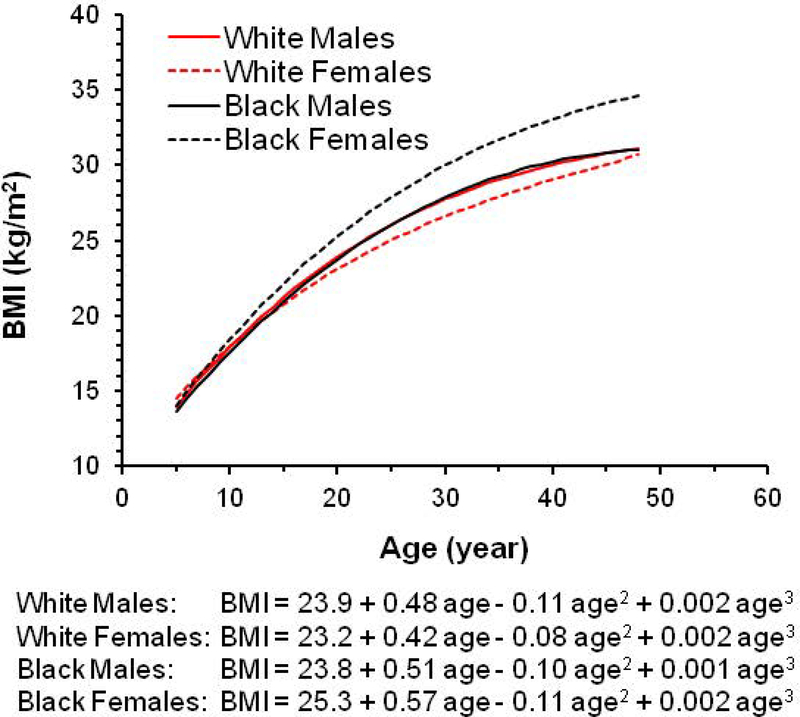
The longitudinal trajectories of BMI from childhood to adulthood differed by race and sex (Figure 2). The growth curves fitted well with the observed BMI values except in early childhood (age 4–6 years) (Supplementary Figure 1). Growth curves in White males and Black males had similar trajectories. BMI increased faster in Black females than the remaining race-sex groups, and this difference was discernible from around 10 years of age; White females tended to have a lower BMI than the remaining race-sex groups after 15 years of age. All growth curve parameters were significant (p<0.001) except the cubic terms in Blacks (p=0.353 for males and p=0.054 for females) (Supplementary Table 1). Model-estimated BMI levels and rates of change by race and sex, calculated according to the growth curves at different ages during childhood, are presented in Supplementary Tables 2 and 3, respectively. BMI levels were significantly correlated with rates of change in BMI at different ages during childhood (P<0.001 for all).
Figure 2.
BMI growth curve from childhood to adulthood by race and sex. Age was centered to 20.1 years for model fitting.
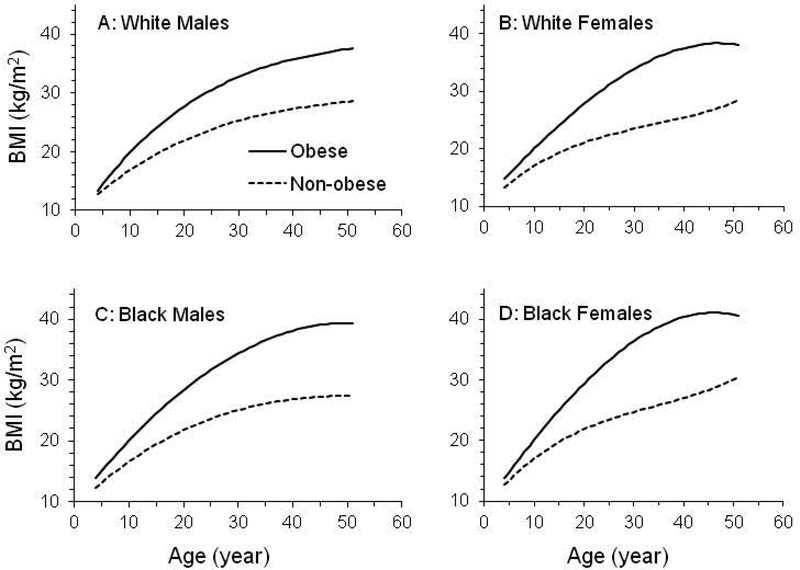
BMI growth curves for adults with and without obesity separated in early childhood (Figure 3). Intercepts (β0+b0) and linear growth rates (β1+b1) during childhood were greater for adults with obesity than for adults without obesity (Table 2); model-estimated BMI levels and rates of change at childhood ages also were higher for adults with obesity than for adults without obesity (Table 3).
Figure 3.
BMI growth curve from childhood to adulthood by race, sex, and adult obesity status
Table 2.
Fixed effect parameters of BMI in means (SDs) between adults with and without obesity by race and sex
| Curve parameters | Whites (n=1,772) |
Blacks (n=960) |
||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| N (Obese/Non-obese) | 190/613 | 178/791 | 121/302 | 153/384 |
| β0+b0 (kg/m2) | ||||
| Obese | 27.8 (4.6) | 27.9 (5.6) | 28.4 (5.8) | 29.5 (6.2) |
| Non-obese | 22.0 (2.7) | 21.1 (2.9) | 21.8 (2.4) | 21.9 (2.9) |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
| β1+b1 (kg/m2/year) | ||||
| Obese | 0.63 (0.22) | 0.70 (0.30) | 0.72 (0.26) | 0.82 (0.34) |
| Non-obese | 0.41 (0.14) | 0.30 (0.17) | 0.42 (0.18) | 0.36 (0.18) |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
| β2+b2 (kg/m2/year2) | ||||
| Obese | −0.143 (0.112) | −0.088 (0.121) | −0.115 (0.130) | −0.116 (0.141) |
| Non-obese | −0.087 (0.069) | −0.082 (0.073) | −0.096 (0.071) | −0.097 (0.082) |
| P-value | <0.001 | 0.183 | 0.106 | 0.018 |
| β3+b3 (kg/m2/year3) | ||||
| Obese | 0.0027 (0.0096) | −0.0021 (0.0097) | 0.0002 (0.0150) | −0.0022 (0.0120) |
| Non-obese | 0.0015 (0.0060) | 0.0040 (0.0063) | 0.0012 (0.0085) | 0.0045 (0.0070) |
| P-value | 0.109 | <0.001 | 0.086 | <0.001 |
P-values for the differences between the two groups were adjusted for average age.
Table 3.
Model-estimated BMI levels and linear slopes (SDs) at childhood ages between adults with obesity (n=930) and adults without obesity (n=1,802)
| Age (years) | BMI Level (kg/m2) |
BMI Linear Slope (kg/m2/year) |
||||
|---|---|---|---|---|---|---|
| Obese | Non-Obese | Δa | Obese | Non-Obese | Δb | |
| 4 | 14.0 (1.7) | 12.9 (1.1) | 1.1** | 1.07 (0.68) | 0.76 (0.38) | 0.31** |
| 5 | 15.1 (1.8) | 13.7 (1.1) | 1.4** | 1.05 (0.62) | 0.72 (0.35) | 0.33** |
| 6 | 16.1 (2.0) | 14.4 (1.2) | 1.7** | 1.02 (0.56) | 0.69 (0.31) | 0.33* |
| 7 | 17.1 (2.4) | 15.1 (1.4) | 2.0** | 1.00 (0.51) | 0.66 (0.28) | 0.34* |
| 8 | 18.1 (2.7) | 15.7 (1.5) | 2.4** | 0.98 (0.46) | 0.64 (0.25) | 0.35* |
| 9 | 19.1 (3.1) | 16.3 (1.7) | 2.7** | 0.96 (0.42) | 0.61 (0.22) | 0.35** |
| 10 | 20.0 (3.4) | 16.9 (1.9) | 3.1** | 0.94 (0.39) | 0.58 (0.20) | 0.36** |
| 11 | 20.9 (3.7) | 17.5 (2.0) | 3.5** | 0.92 (0.36) | 0.56 (0.18) | 0.36** |
| 12 | 21.9 (4.0) | 18.0 (2.1) | 3.8** | 0.89 (0.33) | 0.53 (0.17) | 0.36** |
| 13 | 22.7 (4.3) | 18.5 (2.3) | 4.2** | 0.87 (0.31) | 0.51 (0.16) | 0.36** |
| 14 | 23.6 (4.5) | 19.0 (2.4) | 4.6** | 0.85 (0.30) | 0.48 (0.15) | 0.37** |
| 15 | 24.4 (4.7) | 19.5 (2.4) | 4.9** | 0.83 (0.29) | 0.46 (0.15) | 0.37** |
| 16 | 25.3 (4.9) | 20.0 (2.5) | 5.3** | 0.81 (0.29) | 0.44 (0.15) | 0.37** |
| 17 | 26.0 (5.1) | 20.4 (2.6) | 5.6** | 0.78 (0.29) | 0.42 (0.16) | 0.36** |
| 18 | 26.8 (5.3) | 20.8 (2.6) | 6.0** | 0.76 (0.29) | 0.40 (0.16) | 0.36** |
| 19 | 27.6 (5.4) | 21.2 (2.7) | 6.4** | 0.74 (0.29) | 0.38 (0.17) | 0.36** |
P-values for the difference (Δ) adjusted for race and sex
P-values for the difference (Δ) adjusted for race, sex, and BMI levels
p<0.05
p<0.01
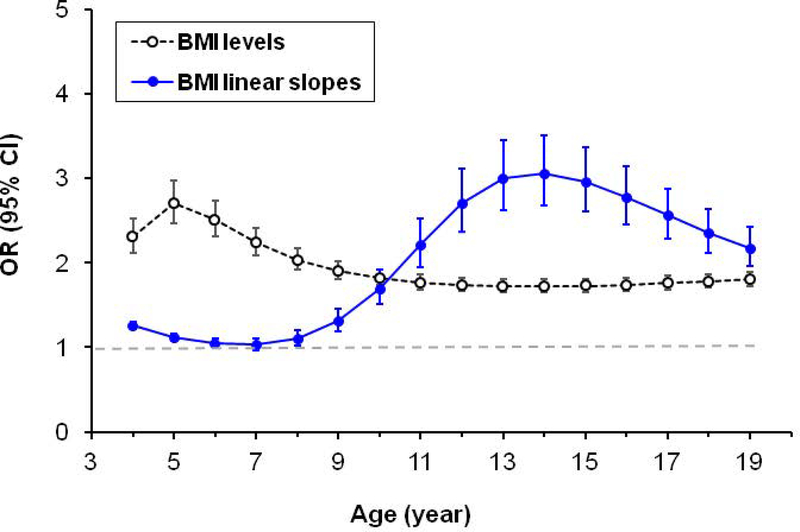
In multiple regression analyses, adjusting for adult age, sex, race, adult smoking, and alcohol drinking, higher rate of change in BMI during childhood (4–19 years) tended to be associated with increased odds of having obesity in adult life. The association weakened in ages 6–8 years, strengthened thereafter, peaked at age 14 (OR=3.1 and 95% CI=2.7–3.5, p<0.001), and gradually decreased but remained above 2.0 at age 19 (Figure 4). In contrast, the BMI levels-adult obesity associations did not show a similar trend although higher BMI levels at childhood ages were all significantly (p<0.001) associated with adult obesity; the strength of the association decreased from 5 to 10 years of age and remained relatively stable after age 11. The associations of model-estimated linear slopes of BMI in childhood ages with adult obesity by race and sex are presented in Supplementary Figure 2. Although the overall association patterns were similar across the four race and sex groups, there were potential race and sex differences: stronger associations during puberty period among Black males than the other three groups and inverse associations in early childhood in Black females (Supplementary Figure 2).
Figure 4.
Odds ratio (OR) and 95% confidence interval of model-estimated levels and level-adjusted linear slopes of BMI for adult obesity by childhood age, adjusting for adult age, sex, race, adult smoking and alcohol drinking
Discussion
In this community-based cohort with repeated measurements of BMI from childhood to midlife, we characterized BMI growth trajectories from childhood to midlife and linked BMI growth parameters during childhood to adult obesity. We showed that the rate of change in BMI at different ages during childhood was differentially associated with adult obesity, independent of absolute BMI values at the corresponding ages. This, to our knowledge, is the first time that rate of change and absolute BMI values at different childhood ages are simultaneously considered for their relevance to obesity risk in midlife. This study provides new insights into the development of obesity and emphasizes the importance of BMI trajectories during childhood for adult obesity risk.
As expected, and consistent with previous studies5–7,25, childhood BMI was predictive of adult obesity risk in the current study. Of particular interest, our study also showed that both BMI levels and rate of change (linear slope) at different age points during childhood were consistently and significantly higher in adults with obesity than in those without obesity. The difference in BMI at age 4 (1.1 kg/m2) was already present between the two groups, which was amplified over time and reached 6.4 kg/m2 at age 19 years. Previous studies have shown that BMI levels are already different at around age 3 between adults with and without obesity26,27.
The associations between rate of change in BMI at different ages during childhood and adult obesity varied during early and late childhood periods. Importantly, these associations were independent of absolute BMI values. Limited literature is available for comparison. Guo et al. previously reported that maximum speed in BMI increases in post-puberty was associated with obesity and fat mass index in young adults11, which is consistent with the observation in the current study. Most recently, Balantekin et al. reported that children with rapid weight gain from age 5 to 15 had a higher rate of obesity at age 2428. Geserick et al. reported in a large longitudinal study that rapid BMI increase during preschool years was associated with increased obesity risk in adolescence29. Adopting a life-course approach, we have extended the previous observations by linking childhood characteristics of BMI growth curves to obesity risk in midlife.
Our study also suggests that puberty (11–16 years of age) and post-puberty (17–19 years of age) are critical periods for obesity risk in midlife as the associations of BMI slopes during puberty and after puberty with adult obesity were much stronger compared to those in other periods. Our findings are also consistent with those by Guo et al11 and Wen et al30. Our study supports the belief that there are critical periods in childhood for risk of developing obesity in adult life. These periods cover intrauterine growth (in particular in the third trimester), BMI rebound, puberty and post-puberty11,31–35. Of note, the potential race and sex differences in these critical periods demand further investigation. Sex differences in growth patterns and in the link between growth patterns in childhood and adult obesity risk have been observed in previous studies11,30. Together with our previous observations that childhood BMI variability and the overall linear rate of change during childhood are predictive of adult obesity risk12, it can be concluded that BMI growth patterns during childhood, in particular during critical periods, are closely related to adult obesity risk.
Previous reports have linked BMI growth trajectories during childhood to adult cardiometabolic health36–40. The importance of early prevention for adult cardiometabolic diseases is well recognized41. It is unknown whether the observed associations in our study were causal or merely reflected the influence of a programmed growth pattern that begins much earlier in life, as childhood growth and development undergoes consecutive, programmed periods with hormones playing a major role42. It is not well understood how growth and development programming during childhood influences BMI growth trajectories and adult obesity outcome. As such, public health implications of our findings remain uncertain; further research is needed to determine if targeting critical periods in childhood to prevent obesity is more efficient and effective than targeting the entire childhood period. Nevertheless, our study suggests that new research in this area may lead to opportunities for developing effective and efficient strategies for prevention of adult obesity and associated cardiometabolic disorders.
The current study has several strengths. Body weight and height were measured from childhood to adulthood using a consistent study protocol. Participants were examined at least four times, and growth curves fit reasonably well with the observed data except for the period between 4–6 years of age. The random-effects model allows for simultaneous estimation of both BMI levels and rates of change at the same time points during childhood. In addition, the associations between rate of change in BMI at different childhood ages and adult obesity risk largely followed similar patterns across the four race and sex groups, despite differences in growth trajectories among the four groups. This study also has limitations. Since BMI was not measured annually in childhood in this study cohort, rate of change in BMI at different ages had to be estimated through statistical modeling. The growth curve did not fit well with the observed data between age 4 and 6 years due to the small number of observations and adiposity rebound during this period. This may have caused bias in the model estimation of BMI levels and slopes during this period. As such, the associations of BMI slopes at ages 4–6 with adult obesity should be interpreted with caution. The current study included only Blacks and Whites in one region of the US. Replications in other populations are needed.
Conclusions
The current study demonstrates that the absolute values of BMI during childhood are associated with adult obesity and that rate of change in BMI, particularly during and after puberty periods, predicts obesity risk, independent of childhood BMI levels. Our observations support early prevention and intervention of childhood obesity. It remains to be determined, however, whether specifically targeting critical periods during childhood is more efficient and effective in reducing adult obesity burden. Further studies are needed to explore the underlying mechanisms, such as hormonal changes during puberty, for the observed differential associations between rate of change in BMI during childhood and adult obesity risk.
Supplementary Material
Acknowledgements
The Bogalusa Heart Study is a joint effort of many investigators and staff members whose contribution is gratefully acknowledged. We especially thank the Bogalusa, LA school system, and most importantly, the children and adults who have participated in this study over many years. We thank Kristin Griffin, MA, MPH (Children’s Minnesota Research Institute, Children’s Hospitals and Clinics of Minnesota, Minneapolis, MN 55404), for her help in editing. This study was supported by grants R01HL121230 from the National Heart, Lung and Blood Institute, R03AG060619 from National Institute of Aging, and P20GM109036 from the National Institute of General Medical Sciences of the National Institutes of Health. T.Z. is supported by grant 81673271 from the National Natural Science Foundation of China.
All authors had played a role in study concept and design (T. Z., S. L. and W. C.); acquisition of data (W. C.); statistical analysis (T. Z., S. L. and W. C.); analysis and interpretation of data (T. Z., S. L. and W. C.); drafting of the manuscript (T. Z., S. L. and W. C); critical revision of the manuscript for important intellectual content (P. K. W., B. X., M. K-W., L. B., and J. H.) and study supervision (S. L. and W. C.). Drs. Li and Chen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest Statement
No conflict of interest was declared.
REFERENCES
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caterson ID, Hubbard V, Bray GA, et al. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group III: worldwide comorbidities of obesity. Circulation. 2004;110(18):e476–483. [DOI] [PubMed] [Google Scholar]
- 3.Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo SS, Chumlea WC. Tracking of body mass index in children in relation to overweight in adulthood. Am J Clin Nutr. 1999;70(1 Part 2):145S–148S. [DOI] [PubMed] [Google Scholar]
- 5.Lake JK, Power C, Cole TJ. Child to adult body mass index in the 1958 British birth cohort: associations with parental obesity. Arch Dis Child. 1997;77(5):376–381. [DOI] [PubMed] [Google Scholar]
- 6.Casey VA, Dwyer JT, Coleman KA, Valadian I. Body mass index from childhood to middle age: a 50-y follow-up. Am J Clin Nutr. 1992;56(1):14–18. [DOI] [PubMed] [Google Scholar]
- 7.Evensen E, Wilsgaard T, Furberg AS, Skeie G. Tracking of overweight and obesity from early childhood to adolescence in a population-based cohort - the Tromso Study, Fit Futures. BMC Pediatr. 2016;16:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aarestrup J, Bjerregaard LG, Gamborg M, et al. Tracking of body mass index from 7 to 69 years of age. Int J Obes (Lond). 2016;40(9):1376–1383. [DOI] [PubMed] [Google Scholar]
- 9.Deshmukh-Taskar P, Nicklas TA, Morales M, Yang SJ, Zakeri I, Berenson GS. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60(1):48–57. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Chen W, Srinivasan SR, Xu J, Berenson GS. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol. 2012;176(Suppl 7):S142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo SS, Huang C, Maynard LM, et al. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. Int J Obes Relat Metab Disord. 2000;24(12):1628–1635. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Chen W, Sun D, et al. Variability and rapid increase in body mass index during childhood are associated with adult obesity. Int J Epidemiol. 2015;44(6):1943–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aris IM, Bernard JY, Chen LW, et al. Infant body mass index peak and early childhood cardio-metabolic risk markers in a multi-ethnic Asian birth cohort. Int J Epidemiol. 2017;46(2):513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes (Lond). 2006;30 Suppl 4:S11–17. [DOI] [PubMed] [Google Scholar]
- 15.Williams SM, Goulding A. Early adiposity rebound is an important predictor of later obesity. Obesity (Silver Spring). 2009;17(7):1310. [DOI] [PubMed] [Google Scholar]
- 16.Koyama S, Ichikawa G, Kojima M, Shimura N, Sairenchi T, Arisaka O. Adiposity rebound and the development of metabolic syndrome. Pediatrics. 2014;133(1):e114–119. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson JG, Osmond C, Kajantie E, Forsen TJ, Barker DJ. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia. 2006;49(12):2853–2858. [DOI] [PubMed] [Google Scholar]
- 18.Huang RC, de Klerk NH, Smith A, et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care. 2011;34(4):1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peneau S, Giudici KV, Gusto G, et al. Growth trajectories of body mass index during childhood: Associated factors and health outcome at adulthood. J Pediatr. 2017;186:64–71.e61. [DOI] [PubMed] [Google Scholar]
- 20.Berenson GS, Wattigney WA, Bao W, Srinivasan SR, Radhakrishnamurthy B. Rationale to study the early natural history of heart disease: the Bogalusa Heart Study. Am J Med Sci. 1995;310(Suppl 1):S22–28. [DOI] [PubMed] [Google Scholar]
- 21.Hunter SM, Bao W, Berenson GS. Understanding the development of behavior risk factors for cardiovascular disease in youth: the Bogalusa Heart Study. Am J Med Sci. 1995;310(Suppl 1):S114–118. [DOI] [PubMed] [Google Scholar]
- 22.Deshmukh-Taskar PR, O’Neil CE, Nicklas TA, et al. Dietary patterns associated with metabolic syndrome, sociodemographic and lifestyle factors in young adults: the Bogalusa Heart Study. Public Health Nutr. 2009;12(12):2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Li S, Cook NR, et al. An autosomal genome scan for loci influencing longitudinal burden of body mass index from childhood to young adulthood in white sibships: The Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28(4):462–469. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290(17):2271–2276. [DOI] [PubMed] [Google Scholar]
- 25.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Racial differences in the tracking of childhood BMI to adulthood. Obes Res. 2005;13(5):928–935. [DOI] [PubMed] [Google Scholar]
- 26.Stuart B, Panico L. Early-childhood BMI trajectories: evidence from a prospective, nationally representative British cohort study. Nutr Diabetes. 2016;6:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pryor LE, Tremblay RE, Boivin M, et al. Developmental trajectories of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med. 2011;165(10):906–912. [DOI] [PubMed] [Google Scholar]
- 28.Balantekin KN, Hohman EE, Adams EL, et al. More rapid increase in BMI from age 5–15 is associated with elevated weight status at age 24 among non-Hispanic white females. Eating Behaviors. 2018;31:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379(14):1303–1312. [DOI] [PubMed] [Google Scholar]
- 30.Wen X, Kleinman K, Gillman MW, Rifas-Shiman SL, Taveras EM. Childhood body mass index trajectories: modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC Med Res Methodol. 2012;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59(5):955–959. [DOI] [PubMed] [Google Scholar]
- 32.Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101(3):E5. [DOI] [PubMed] [Google Scholar]
- 33.Williams S, Davie G, Lam F. Predicting BMI in young adults from childhood data using two approaches to modelling adiposity rebound. Int J Obes Relat Metab Disord. 1999;23(4):348–354. [DOI] [PubMed] [Google Scholar]
- 34.Rolland-Cachera MF, Peneau S. Growth trajectories associated with adult obesity. World Rev Nutr Diet. 2013;106:127–134. [DOI] [PubMed] [Google Scholar]
- 35.Hughes AR, Sherriff A, Ness AR, Reilly JJ. Timing of adiposity rebound and adiposity in adolescence. Pediatrics. 2014;134(5):e1354–1361. [DOI] [PubMed] [Google Scholar]
- 36.Wadsworth M, Butterworth S, Marmot M, Ecob R, Hardy R. Early growth and type 2 diabetes: evidence from the 1946 British birth cohort. Diabetologia. 2005;48(12):2505–2510. [DOI] [PubMed] [Google Scholar]
- 37.Lammi N, Moltchanova E, Blomstedt PA, Tuomilehto J, Eriksson JG, Karvonen M. Childhood BMI trajectories and the risk of developing young adult-onset diabetes. Diabetologia. 2009;52(3):408–414. [DOI] [PubMed] [Google Scholar]
- 38.Hughes AR, Sherriff A, Ness AR, Reilly JJ. Childhood body mass index trajectories predicting cardiovascular risk in adolescence. Pediatrics. 2014;134(5):e1354–1361.25311600 [Google Scholar]
- 39.Sovio U, Kaakinen M, Tzoulaki I, et al. Adiposity rebound and the development of metabolic syndrome. Int J Obes (Lond). 2014;38(1):53–59. [DOI] [PubMed] [Google Scholar]
- 40.Boyer BP, Nelson JA, Holub SC. Relationships between childhood growth parameters and adult blood pressure: the Fels Longitudinal Study. J Adolesc Health. 2015;56(6):599–605.25746172 [Google Scholar]
- 41.Kotsis V, Tsioufis K, Antza C, et al. Obesity and cardiovascular risk: a call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the High-risk Patient and European Association for the Study of Obesity: part B: obesity-induced cardiovascular disease, early prevention strategies and future research directions. J Hypertens. 2018;36(7):1441–1455. [DOI] [PubMed] [Google Scholar]
- 42.Tanner JM. Human growth hormone. Nature. 1972;237(5356):433–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.