Abstract
Background:
2S-albumins Ara h 2 and Ara h 6 are the most potent peanut allergens and levels of specific IgE toward these proteins are good predictors of clinical reactivity. Because of structural homologies, Ara h 6 is generally considered to cross-react extensively with Ara h 2.
Objective:
We aimed to quantify the IgE cross-reactivity between Ara h 2 and Ara h 6.
Methods:
Peanut 2S-albumins were purified from raw peanuts. The IgE cross-reactivity between Ara h 2 and Ara h 6 was evaluated with 32 sera from French and US peanut-allergic patients by measuring the residual IgE binding to one 2S-albumin after depletion of IgE antibodies recognizing the other 2S-albumin. The IgE cross-reactivity between Ara h 2 and Ara h 6 was further investigated by competitive inhibition of IgE binding and by a model of mast cell degranulation.
Results:
A highly variable level of IgE cross-reactivity was revealed among the patients. The mean fraction of cross-reactive IgE antibodies represented only 17.1% of 2S-albumins-specific IgE antibodies and was lower than the mean fraction of IgE specific to Ara h 2 (57.4%) or to Ara h 6 (25.5%). The higher level of Ara h 2-specific IgE was principally due to the IgE-binding capacity of an insertion containing the repeated immunodominant linear epitope DPYSPOHS. The impact of IgE cross-reactivity on diagnostic testing was illustrated with a serum displaying an Ara h 6-specific IgE response of 26 UI/mL that was not associated with the capacity of Ara h 6 to trigger mast cell degranulation.
Conclusions & Clinical Relevance:
IgE antibodies specific to peanut 2S-albumins are mainly non-cross-reactive but low-affinity cross-reactivity can affect diagnostic accuracy. Testing IgE binding to a mixture of 2S-albumins rather than to each separately may enhance diagnostic performance.
Keywords: allergy, peanut, 2S-albumin, Ara h 2, Ara h 6, cross-reactivity, IgE, epitope, sensitization
1. INTRODUCTION
IgE-mediated peanut allergy is a potentially life-threatening disease that affects 1 to 3% of the children. While allergy to cow’s milk or hen’s egg are generally outgrown before adulthood, peanut allergy persists in more than 80% of cases.1
The peanut 2S-albumins, Ara h 2 and Ara h 6, are the most potent peanut allergens. While Ara h 2 was immediately recognized as a major allergen, Ara h 6 was first considered as a minor allergen because its IgE reactivity had been evaluated on a denatured recombinant form.2,3 Afterwards, studies with native or correctly refolded recombinant proteins demonstrated that Ara h 2 and Ara h 6 display comparable allergenic potencies.4-10 Accordingly, levels of specific IgE to Ara h 2 and Ara h 6 are equally good predictors of clinical reactivity to peanut.11-14
Because of a sequence identity of 59% and similar tertiary structures between peanut 2S-albumins, Ara h 6 is considered to cross-react extensively with Ara h 2.15-18 IgE binding to Ara h 6 has even been suggested to result essentially from cross-reactivity with other peanut allergens.18 Such cross-reactivity would then question the usefulness of testing both 2S-albumins for the diagnosis of peanut allergy. Nevertheless patients sensitized to only one peanut 2S-albumin have been reported thus demonstrating the presence of non-cross-reactive IgE-binding epitopes on Ara h 2 and Ara h 6.19-21 In this regard, Ara h 2 isoforms differ from Ara h 6 by an insertion of either 14 or 26 residues containing the repeated linear epitope DPYSPOHS (Fig. E1). In a previous work, we showed that proline hydroxylation of DPYSPOHS motifs was required for high affinity IgE-binding to this major linear epitope of Ara h 2.22 In contrast, the presence of such an immunodominant linear epitope in Ara h 6 was not detected, thus confirming the importance of conformational IgE-binding epitopes in peanut 2S-albumins.22-24
Considering the structural homologies between 2S-albumins, but also their sequence dissimilarities, we then aimed to quantify the IgE cross-reactivity between Ara h 2 and Ara h 6 by two different approaches, one using solid-phase coated allergens and the other one using fluid-phase competition.
2. METHODS
2.1. Human sera
Sera were collected from 20 French peanut-allergic children recruited at the Paediatric Allergy Clinic of Hospital Necker-Enfants Malades after informed consent from patient’s parents (Table E1, sera 1 to 20).22,25 All samples were collected during routine clinical practice and were studied in accordance with the purpose of the initial study. Based on their medical history, symptoms of the IgE-mediated peanut allergy involved skin, respiratory tract, gastrointestinal tract and cardiovascular system. Twelve sera from US peanut-allergic patients with a strong history of peanut-induced immediate hypersensitivity and peanut-specific IgE ≥ 13 KAU/L (ImmunoCap, Thermo Fischer Scientific) in serum were also included (Table E1, sera 21 to 32). All adult patients and the parents or guardians of minors signed informed consent. Minors who were >6 years of age, signed an assent. The University of Colorado Denver Institutional Review Board approved this study.
2.2. Allergens and peptide preparation
The 2S-albumins Ara h 2 and Ara h 6 were purified from whole peanut protein extract prepared with raw peanuts of a typical blend consumed in Europe (50% Chinese Hsuji’s and Red Skins varieties, 50% Argentinian Runners variety), as previously described.26,27 Recombinant 2S-albumins were obtained by expression of genes optimized for bacterial expression (Genscript USA Inc., Piscataway, NJ, USA) and inserted into the E.coli expression plasmid pET9c (Novagen-Merck, Damstadt, Germany).22,28 The variant rAra h 2.Δ was obtained by replacing the domain GRDPYSPSQDPYSPSP of rAra h 2.01 by the dipeptide DS occurring naturally in Ara h 6. Expression, purification and refolding of recombinant proteins were performed as previously described.22,29 Correct refolding of the recombinant proteins was verified by circular dichroism spectroscopy.22 The peptide containing three hydroxyprolines (pep 3POH, DPYSPOHSQDPYSPOHSQDPDRRDPYSPOHSPY) corresponds to the major linear epitope found in Ara h 2.02 isoform. The peptide was synthesized using a standard solid phase synthesis by the Fmoc (9-fluorenyl-methoxycarbonyl) continuous-flow method (peptide synthesizer 433A, Applied Biosystems, Foster City, CA). After standard procedure including TFA cleavage and ether precipitation, crude peptides were purified by RP-HPLC and characterized by MALDI-TOF.22
2.3. IgE-binding measurement by solid-phase assay
IgE levels to individual native 2S-albumins were quantified using a direct enzyme allergosorbent test in which purified antigens (2.5 μg/mL) were passively adsorbed on microtiter plates as previously described (Fig. 1A; Ara h 2, red triangle; Ara h 6, blue triangle).25 After overnight incubation with 2 or 3 dilutions of sera (50 μL/well, Fig. 1A, step 1), IgE binding was revealed by the addition of a labeled anti-human IgE monoclonal antibody (clone BS17). Tracer was prepared by covalent linkage of the monoclonal antibody to the tetrameric form of acetylcholinesterase.25 After washing, Ellman’s reagent was used as the enzyme substrate and absorbance was measured at 414 nm.
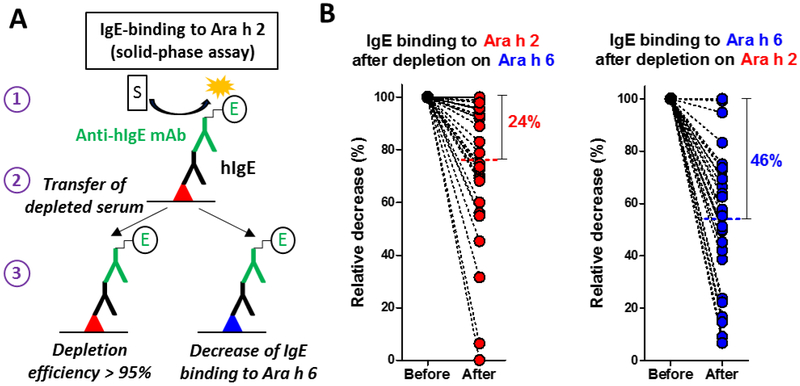
Figure 1.
IgE cross-reactivity between Ara h 2 and Ara h 6 investigated with a solid-phase assay. A, schematic principle of the solid-phase assay (Ara h 2, red triangle; Ara h 6, blue triangle, see Methods); B, relative decrease of IgE binding to one 2S-albumin after depletion of IgE antibodies recognizing the other 2S-albumin (n=32, mean is indicated).
Cross-reactivity between Ara h 2 and Ara h 6 was evaluated by measuring the residual IgE binding to one 2S-albumin after depletion of IgE antibodies recognizing the other 2S-albumin. After overnight incubation of 50 μL of diluted sera in microtiter plate coated with one 2S-albumin (Fig. 1A, step 1), 45 μL of the resulting depleted serum was transferred into another microtiter plate (Fig. 1A, step 2) coated either with the same 2S-albumin (in order to measure the efficacy of IgE depletion) or with the other 2S-albumin (in order to evaluate the decrease of IgE binding to that 2S-albumin, Fig. 1A, step 3). Depletion efficacy of Ara h 2-specific IgE antibodies was 96.5 ± 6.2% and depletion efficacy of Ara h 6-specific IgE antibodies was 98.3 ± 3.8% (n=32, data not shown).
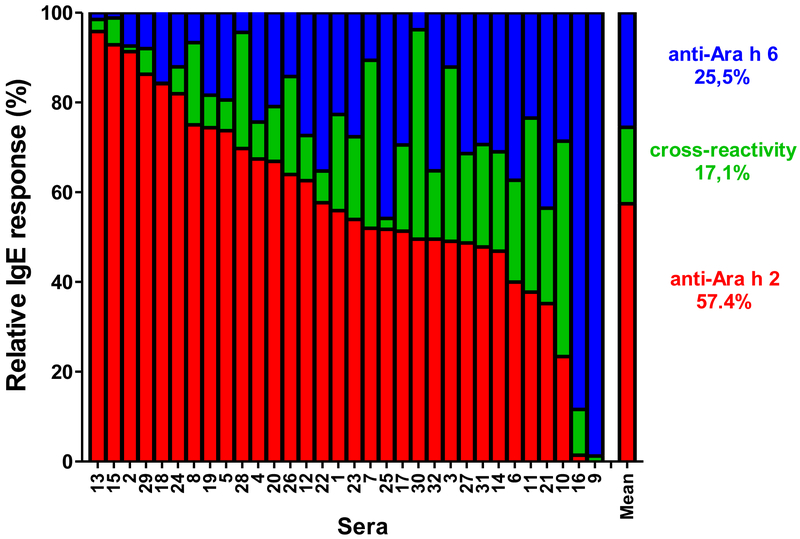
IgE levels to both 2S-albumins (Ara h 2/6Calc) were calculated by adding the level of Ara h 2-specific IgE (after IgE-depletion on Ara h 6, in red, Fig. 2), the level to Ara h 6-specific IgE (after IgE-depletion on Ara h 2, in blue) and the level of cross-reactive IgE (corresponding to the mean decrease of IgE-binding induced by IgE-depletion on Ara h 2 and on Ara h 6, in green).
Figure 2.
Fractions of 2S-albumin-specific IgE antibodies recognizing nAra h 2 (red), nAra h 6 (blue) or both (green), n=32.
In addition, IgE binding to a mixture of Ara h 2 and Ara h 6 was assessed by binding both proteins (each at 1 μg/mL) on the solid-phase and IgE binding was measured as previously described. The experimental IgE level toward the mixture, Ara h 2/6Meas was compared to the sum of IgE levels measured separately against Ara h 2 and Ara h 6 (Ʃ) and to Ara h 2/6Calc (see above).
2.4. IgE-binding analysis by fluid-phase assay
We performed a second set of experiments by using a reverse enzyme allergo-sorbent test that measures the binding of labeled allergens by patients’ IgE antibodies captured by an anti-human IgE monoclonal antibody immobilized on the solid phase.30 Here, plates were first coated with an anti-human IgE monoclonal antibody (clone LE27). Fifty μL/well of serum from each patient at adequate dilutions were incubated overnight at 4°C. After washing, 25 μL of inhibitors (i.e. increasing concentrations of native 2S-albumins) and 25 μL of biotinylated native 2S-albumins were added and incubated for 4 h at room temperature. After washing, neutravidin labeled with acetylcholinesterase was added for 15 min at room temperature and acethylcholinesterase enzymatic activity was revealed after extensive washing and addition of Ellman’s reagent.30,31 Results were expressed as B/B0: B0 and B represent the amount of labelled 2S-albumin bound to immobilized IgE antibodies in the absence or presence of a known concentration of inhibitor, respectively.
2.6. Mediator release assay
Degranulation assay was performed with rat basophilic leukemia (RBL) SX −38 cells as previously described.8 Cells were passively sensitized with IgE antibodies immunopurified from individual serum.22 Mediator release was induced by incubation with different concentrations of native 2S-albumins and was measured by assaying the β-hexosaminidase activity in cell culture supernatant. Results were expressed as the percentage of the reference release, determined for each serum and induced with anti-human IgE (LE27 clone; 100 ng/mL).
2.7. Statistical analysis
Data were analyzed using the non-parametric Friedman’s test with Dun’s multiple comparison test and correlation was assessed with the non-parametric Spearman correlation test. Statistical analyses were performed with GraphPad Prism 5.04 software. A p<0.05 was considered as significant.
3. RESULTS
3.1. IgE cross-reactivity between Ara h 2 and Ara h 6 investigated with a solid-phase assay
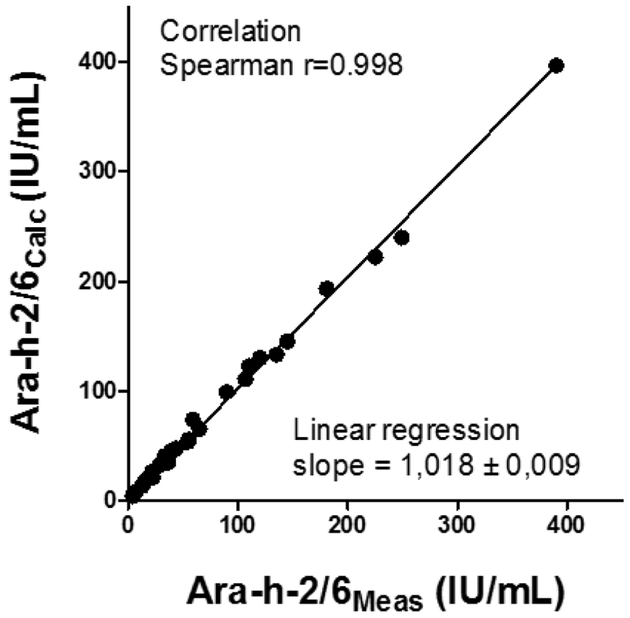
Using allergens purified from raw peanut and coated on the solid-phase (Table E1), the levels of Ara h 2-specific IgE (median 33.7 IU/mL, interquartile range (IQR) 14.1-109.2) were on average higher than the levels of Ara h 6-specific IgE (median 23.2 IU/mL, IQR 7.7-63.4) but IgE responses to the two 2S-albumins remained highly correlated (Spearman r value = 0.89, p<0.0001, data not shown). We then measured the relative decrease of IgE binding to one 2S-albumin after depletion of IgE antibodies recognizing the other 2S-albumin (Fig 1A). As shown in Fig 1B, a highly variable level of IgE cross-reactivity was revealed among patients. Overall, depletion of Ara h 6-specific IgE antibodies induced a mean decrease of IgE binding to Ara h 2 of 24% (median 19%) thus indicating that about 76% of IgE binding to Ara h 2 was mediated by non-cross-reactive antibodies. Depletion of Ara h 2-specific IgE antibodies induced a higher mean decrease of IgE binding to Ara h 6 of 46% (median 47%). Subsequently, IgE levels to both 2S-albumins were calculated for each patient by summing levels of IgE recognizing Ara h 2, Ara h 6 and both (see Methods). As shown in Fig 2, the mean fraction of cross-reactive antibodies represented only 17.1% of 2S-albumins-specific IgE antibodies and was thereby lower than the mean fraction of IgE specific to Ara h 2 (57.4%) or to Ara h 6 (25.5%). The IgE level against a mixture of Ara h 2 and Ara h 6 was also measured in order to validate our calculation of the 2S-albumin total IgE levels (Table 1). As expected, IgE levels to the mixture (Ara h 2/6Meas) were lower than the mere sum of Ara h 2 and Ara h 6-IgE levels since these latter encompassed the binding of common cross-reactive IgE antibodies. In contrast, the calculated IgE levels to 2S-albumins, Ara h 2/6Calc, were in excellent correlation with the experimental IgE levels to Ara h 2/6 mixture (Spearman r value = 0.998, linear regression slope = 1.018, Fig. 3).
Table 1.
Serum IgE concentrations to a mixture of Ara h 2 and Ara h 6
| Specific IgE levels to 2S-albumins (IU/mL) | |||||
|---|---|---|---|---|---|
| Patient no. | Ara h 2Meas | Ara h 6Meas | Ʃ (Ara h 2Meas + Ara h 6Meas) |
Ara h 2/6Meas | Ara h 2/6Calc |
| 1 | 77.8 | 45.0 | 122.8 | 89.6 | 98.8 |
| 2 | 3.7 | 0.4 | 4.1 | 3.8 | 4.0 |
| 3 | 114.0 | 66.4 | 180.4 | 120.0 | 129.8 |
| 4 | 90.7 | 43.3 | 134.0 | 109.9 | 122.3 |
| 6 | 30.3 | 25.6 | 55.9 | 39.4 | 45.3 |
| 7 | 94.6 | 81.1 | 175.7 | 106.7 | 110.6 |
| 8 | 52.2 | 26.4 | 78.6 | 64.7 | 65.1 |
| 10 | 29.8 | 29.7 | 59.5 | 33.0 | 40.1 |
| 11 | 35.1 | 23.4 | 58.5 | 36.4 | 41.6 |
| 12 | 32.4 | 20.7 | 53.1 | 43.6 | 47.3 |
| 13 | 12.6 | 0.9 | 13.5 | 12.2 | 12.9 |
| 14 | 18.8 | 15.1 | 33.9 | 21.7 | 26.1 |
| 15 | 15.6 | 2.2 | 17.8 | 15.2 | 17.0 |
| 16 | 4.7 | 19.0 | 23.7 | 16.9 | 18.7 |
| 17 | 122.8 | 88.9 | 211.7 | 180.7 | 193.0 |
| 18 | 122.0 | 23.0 | 145.0 | 144.9 | 144.9 |
| 19 | 293.0 | 124.0 | 417.0 | 390.0 | 396.9 |
| 20 | 24.1 | 14.5 | 38.6 | 28.1 | 31.8 |
| 21 | 40.2 | 54.2 | 94.4 | 58.9 | 73.5 |
| 22 | 5.5 | 3.6 | 9.1 | 6.8 | 7.6 |
| 23 | 5.2 | 3.8 | 9.0 | 7.2 | 7.8 |
| 24 | 3.4 | 1,0 | 4.4 | 4.3 | 4.2 |
| 25 | 19.2 | 17.2 | 36.4 | 31.5 | 35.6 |
| 26 | 48.7 | 18.7 | 67.4 | 55.4 | 55.3 |
| 27 | 140.7 | 146.8 | 287.5 | 249.2 | 239.8 |
| 28 | 213.3 | 66.3 | 279.6 | 225.2 | 222.1 |
| 29 | 30.5 | 6.1 | 36.6 | 36.2 | 34.6 |
| 30 | 119.8 | 75.0 | 194.8 | 135.0 | 132.8 |
| 31 | 13.6 | 12.5 | 26.1 | 22.4 | 21.2 |
| 32 | 35.8 | 25.4 | 61.2 | 53.8 | 53.1 |
Ʃ, sum of IgE levels to Ara h 2 and Ara h 6; Ara h 2/6Meas, measured IgE levels to a mixture of Ara h 2 and Ara h 6; Ara h 2/6Calc, calculated IgE levels to Ara h 2 and Ara h 6 by taking into account IgE cross-reactivity (see supplementary Methods).
Figure 3.
Correlation between the IgE levels Ara h 2/6Calc (calculated as described in Methods) and Ara h 2/6Meas (determined experimentally against a mixture of Ara h 2 and Ara h 6).
3.2. Contribution of the DPYSPOHS motifs to the higher IgE-binding capacity of Ara h 2
The fraction of Ara h 2-specific IgE was strikingly two-fold higher than that of Ara h 6. Since a major structural difference between Ara h 2 and Ara h 6 is the presence of the repeated DPYSPOHS motif inserted on a surface loop of Ara h 2 (Fig. E1), we further investigated the IgE-reactivity of a synthetic peptide containing three DPYSPOHS motifs (pep3POH) that overlapped the natural insert found in the isoform 2.02. The solid-phase assay revealed that IgE-binding to pep3POH accounted for about half of the overall IgE-binding capacity of Ara h 2 (Fig. 4). The IgE level to Ara h 2 was then fully recapitulated by adding the IgE level to pep3POH to that of a complementary construct, rAra h 2.Δ, which was generated by deleting the DPYSPOHS motifs (Fig 4). The difference of IgE reactivity between Ara h 2 and Ara h 6 was thus largely due to the IgE-binding capacity of DPYSPOHS motifs.
Figure 4.
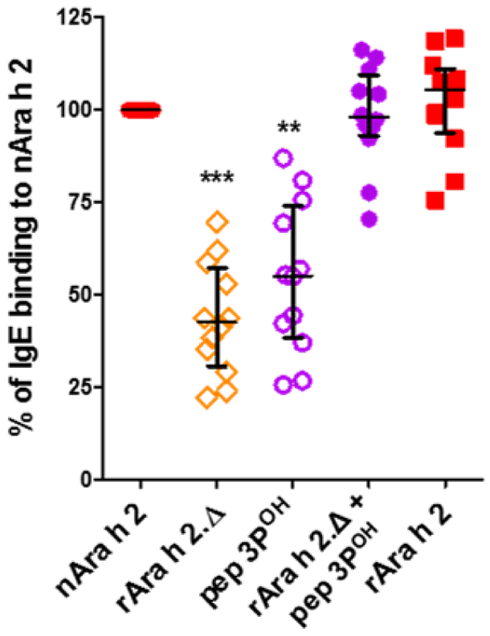
IgE-binding capacity of rAra h 2, rAra h 2Δ and pep 3POH relatively to that of nAra h 2. The sum of IgE binding to rAra h 2.Δ and pep 3POH is shown (n=12, median with interquartile range). Data were analyzed using the non-parametric Friedman test with Dunn’s multiple comparison test.
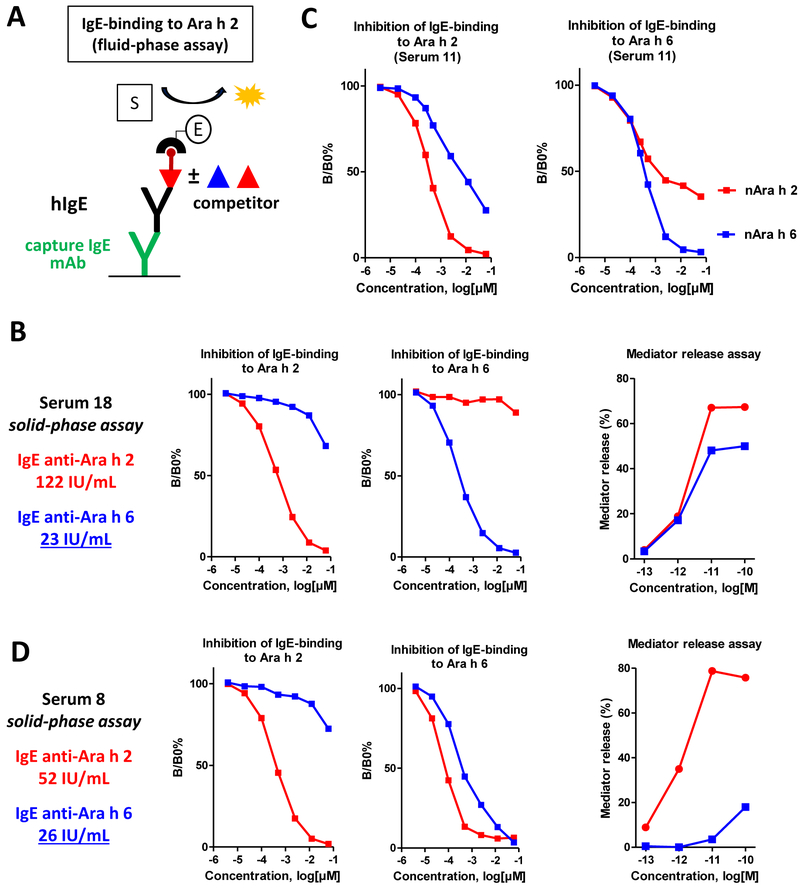
3.3. IgE cross-reactivity between Ara h 2 and Ara h 6 investigated with a competitive fluid-phase assay
Solid-phase assays are performed in excess of coated allergens, which allows maximum binding of IgE (and IgG) antibodies but without distinction between low- and high-affinity bindings. This absence of distinction is illustrated in Fig 4 with nAra h 2 and rAra h 2 displaying similar IgE-binding capacities although the absence of hydroxyproline in rAra h 2 has been shown to reduce the binding affinity of IgE recognizing the DPYSPOHS motifs in nAra h 2. In contrast, assays such as IgE-binding competitive inhibition or in vitro mast cell degranulation can evidence differences of binding affinity and could thus demonstrate the higher allergenic activity of nAra h 2 compared to rAra h 2.22,32 We therefore investigated cross-reactivity between 2S-albumins with a competitive fluid-phase assay (Fig 5A). This assay confirmed that IgE reactivity of Ara h 2 and Ara h 6 was mainly mediated by non-cross-reactive antibodies. Indeed, some sera, such as serum 18, exhibited a very low cross-reactivity between the two 2S-albumins (Fig 5B) and in most cases, half-inhibition of IgE binding to one 2S-albumin was not achieved with the other 2S-albumin at the concentration sufficient for complete self-inhibition (Figs E2 and E3). Otherwise, about 10- to 100-fold higher concentrations of Ara h 6 than of Ara h 2 were required for half-inhibition of IgE binding to Ara h 2. Nevertheless, we observed that for some sera, such as sera 11, 17 or 26 (Fig 5C and Fig. E3), Ara h 2 and Ara h 6 inhibition curves of IgE-binding to Ara h 6 were partially overlapping, probably because of a fraction of cross-reactive IgE antibodies primarily directed toward Ara h 2. However the presence of non-cross-reactive epitopes was almost always established for both allergens since one 2S-albumin could not totally inhibit IgE binding to the other one (Figs E2 and E3). Complete inhibition of IgE binding to Ara h 6 by Ara h 2 was observed only for serum 8 (Fig 5D). In this case, Ara h 2 was even a better inhibitor of IgE binding to Ara h 6 than Ara h 6 itself, thus suggesting that IgE binding to Ara h 6 was only due to cross-reactive IgE antibodies. Indeed, conversely to Ara h 2, Ara h 6 was unable to induce an efficient degranulation of mast cells passively sensitized with IgE antibodies immunopurified from serum 8 (Fig 5D). The same difference of potency between Ara h 2 and Ara h 6 was also observed with serum 15 (Fig E4), in which nearly 99% of the 2S-albumins-specific IgE antibodies recognized Ara h 2 (Fig 2). Comparatively, both 2S-albumins triggered an efficient degranulation of mast cells sensitized with IgE antibodies from serum 18 (Fig 5B) or from other sera exhibiting various levels of IgE cross-reactivity between Ara h 2 and Ara h 6 (Fig E4). Therefore, although an IgE response higher than 20 IU/mL pointed to Ara h 6 as a harmful allergen for patients 18 and 8, the mast cell assay suggested that patient 8 was sensitized to Ara h 6 only because of low-affinity cross-reactivity of anti-Ara h 2 specific IgE.
Figure 5.
IgE cross-reactivity between Ara h 2 and Ara h 6 investigated with a competitive fluid-phase assay. A, schematic principle of fluid-phase assay (see Methods); B, an example of serum displaying co-sensitization with very low IgE-cross-reactivity to Ara h 2 and Ara h 6 and the corresponding capacity of 2S-albumins to trigger mast cell degranulation; C, an example of serum displaying high IgE-cross-reactivity between Ara h 2 and Ara h 6; D, an example of serum displaying IgE reactivity to Ara h 6 (as detected by solid-phase assay) only due to cross-reactive IgE specific to Ara h 2 and the corresponding capacity of 2S-albumins to trigger mast cell degranulation.
4. DISCUSSION
Diagnostic testing of food allergies have been significantly improved by the use of purified or recombinant allergenic components for identification of the offending food allergens.33 However, diagnostics based on serum IgE measurements are still hampered by their inability to discriminate clinically relevant IgE binding from clinically irrelevant sensitization.34 Evaluation of IgE cross-reactivity between two homologous proteins also required to consider the IgE epitope specificity, critical epitopes and affinity relevant for the capacity of a cross-reactive allergen to induce or not mast cell degranulation.24,34,35 In this regard, peanut allergens have been extensively studied and structural homologies between Ara h 2 and Ara h 6 seemed to ensure a high level of IgE-cross-reactivity between these two proteins. Nevertheless, the report of peanut-allergic patients sensitized only to Ara h 6 or with highly variable ratio between Ara h 2-specific IgE and Ara h 6-specific IgE suggested that cross-reactivity between peanut 2S-albumins was not as consistent as previously considered.12,36 We thus aimed to provide a quantification of the IgE-cross-reactivity between these two homologous allergens.
For this purpose, we used two different assays to characterize the IgE-cross-reactivity between Ara h 2 and Ara h 6. Solid-phase and fluid-phase assays showed both that the IgE-cross-reactivity was not as exhaustive as initially expected and that the IgE-reactivity of Ara h 2 and Ara h 6 was mainly due to the binding of non-cross-reactive antibodies. Moreover, when a significant cross-reactivity was evidenced, the 2S-albumin that was not primarily targeted by the cross-reactive IgE antibodies generally exhibited a 10- to 100-fold lower inhibitory capacity than the other 2S-albumin. A strong correlation between IgE levels to Ara h 6 and to Ara h 2 has been nevertheless consistently observed (here and 11-13,23). This was usually attributed to an exhaustive cross-reactivity between 2S-albumins, with Ara h 2 generally considered as the most allergenic protein. However, our results suggest that this strong correlation was rather due to the similar intrinsic allergenic properties of Ara h 2 and Ara h 6 that resulted in comparable levels of co-sensitization. In this regard, the two-fold higher fraction of Ara h 2-specific IgE was unexpected. However, as previously reported, around half of the Ara h 2 IgE-reactivity corresponded to the IgE-binding to DPYSPOHS motifs which was not associated or correlated with that to a corresponding epitope in Ara h 6.18,22,23 Thereby, when considering only the IgE-binding to the remaining folded parts of Ara h 2 that also contains the potentially cross-reactive epitopes, the IgE-reactivity of Ara h 2 was then comparable to that of Ara h 6. Why the natural insertions containing the DPYSPOHS motifs are such an immunodominant linear epitope in Ara h 2 remains an important question whose answer would certainly provide critical insights in predicting IgE-binding epitopes in food allergens. It would be also interesting to determine whether the level of sensitization to this particular epitope could be associated with the severity of the allergic reaction or with a pattern of peanut processing and/or consumption.
We also found an example of sensitization to Ara h 6 only due to cross-reactive IgE antibodies primarily directed against Ara h 2. In this case, Ara h 6 was unable to induce an efficient mast cell degranulation. This result sheds some light on the sensitization to a food allergen without clinical reactivity. Since even an IgE response higher than 20 UI/mL could be clinically irrelevant, it confirmed the difficulty to establish an accurate risk estimation of the IgE-cross-reactivity between allergenic proteins. This question is particularly critical for the assessment of potential cross-reactivity between “novel” food proteins and already known allergenic food.
In conclusion, since each 2S-albumins display non-cross-reactive IgE-binding epitopes, Ara h 6 should be considered as allergenic as Ara h 2 and thereby, using both allergens remains the optimal strategy for diagnostic testing. Furthermore, we suggest that IgE responses to peanut 2S-albumins could be also considered collectively instead of individually and that measurement of IgE specific to a mixture of peanut 2S-albumins could offer significant advantages. First, as shown in Table 1, the sensitivity of IgE-binding detection would be improved since the IgE level to a mixture of Ara h 2 and Ara h 6 was higher than those against only one 2S-albumin. Even if a positive IgE response to Ara h 2 is usually conclusive11,13, further research should also evaluate whether the level of sensitization to the mixture of 2S-albumins can be more insightful than that to Ara h 2 only, in predicting the severity of the allergic symptoms. Accordingly, van Erp et al. proposed to assess the clinical relevance of the sensitization to peanut 2S-albumins in a stepwise approach including a basophil activation test to a mixture of Ara h 2 and Ara h 6.13 Secondly, patients sensitized to only one 2S-albumin could be all identified with one test, which should then limit the production of false negative. Finally, IgE cross-reactivity would not affect IgE measurement since cross-reactive IgE antibodies would be detected regardless of their primary specificity. Moreover, Ara h 7, another peanut 2S-albumin which is present in about 10- to 30-fold lower amounts than Ara h 2 and Ara h 6, has been reported to exhibit some non-cross-reactive IgE-binding epitopes and could then be also included in the mixture of 2S-albumins.37 Thus, in addition to being less expensive by reducing the number of tests, using a mixture of peanut 2S-albumins may result in improved diagnostic performance. Future clinical studies should then assess whether increasing the sensitivity of IgE measurements by using a mixture of 2S-albumins, in detriment of the specificity of the IgE response toward only one representative member of the 2S-albumin family, could improve the accuracy of peanut allergy diagnosis.
Supplementary Material
Acknowledgments
Funding: Supported by AlimH department of INRA and grants R01-AI099029 and R21-AI135397 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, Bethesda MD, USA to Dr. Dreskin
Abbreviations used:
- IgE
Immunoglobulin E
- RBL
Rat Basophilic leukemia
- POH
Hydroxyproline
Footnotes
Disclosure of potential conflict of interest: No conflict of interest in relation to this study.
References
- 1.Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58. [DOI] [PubMed] [Google Scholar]
- 2.Burks AW, Williams LW, Connaughton C, Cockrell G, O’Brien TJ, Helm RM. Identification and characterization of a second major peanut allergen, Ara h II, with use of the sera of patients with atopic dermatitis and positive peanut challenge. J Allergy Clin Immunol. 1992;90(6 Pt 1):962–969. [DOI] [PubMed] [Google Scholar]
- 3.Kleber-Janke T, Crameri R, Appenzeller U, Schlaak M, Becker WM. Selective cloning of peanut allergens, including profilin and 2S albumins, by phage display technology. Int Arch Allergy Immunol. 1999;119(4):265–274. [DOI] [PubMed] [Google Scholar]
- 4.Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. 2004;34(4):583–590. [DOI] [PubMed] [Google Scholar]
- 5.McDermott RA, Porterfield HS, El Mezayen R, et al. Contribution of Ara h 2 to peanut-specific, immunoglobulin E-mediated, cell activation. Clin Exp Allergy. 2007;37(5):752–763. [DOI] [PubMed] [Google Scholar]
- 6.Flinterman AE, van Hoffen E, den Hartog Jager CF, et al. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin Exp Allergy. 2007;37(8):1221–1228. [DOI] [PubMed] [Google Scholar]
- 7.Porterfield HS, Murray KS, Schlichting DG, et al. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009;39(7):1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009;39(8):1277–1285. [DOI] [PubMed] [Google Scholar]
- 9.Kulis M, Chen X, Lew J, et al. The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice. Clin Exp Allergy. 2012;42(2):326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Wang Q, El-Mezayen R, Zhuang Y, Dreskin SC. Ara h 2 and Ara h 6 have similar allergenic activity and are substantially redundant. Int Arch Allergy Immunol. 2013;160(3):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klemans RJ, Knol EF, Bruijnzeel-Koomen CA, Knulst AC. The diagnostic accuracy of specific IgE to Ara h 6 in adults is as good as Ara h 2. Allergy. 2014;69(8):1112–1114. [DOI] [PubMed] [Google Scholar]
- 12.Koid AE, Chapman MD, Hamilton RG, et al. Ara h 6 complements Ara h 2 as an important marker for IgE reactivity to peanut. J Agric Food Chem. 2014;62(1):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Erp FC, Knol EF, Pontoppidan B, Meijer Y, van der Ent CK, Knulst AC. The IgE and basophil responses to Ara h 2 and Ara h 6 are good predictors of peanut allergy in children. J Allergy Clin Immunol. 2017;139(1):358–360 e358. [DOI] [PubMed] [Google Scholar]
- 14.Kukkonen AK, Pelkonen AS, Makinen-Kiljunen S, Voutilainen H, Makela MJ. Ara h 2 and Ara 6 are the best predictors of severe peanut allergy: a double-blind placebo-controlled study. Allergy. 2015;70(10):1239–1245. [DOI] [PubMed] [Google Scholar]
- 15.Koppelman SJ, de Jong GA, Laaper-Ertmann M, et al. Purification and immunoglobulin E-binding properties of peanut allergen Ara h 6: evidence for cross-reactivity with Ara h 2. Clin Exp Allergy. 2005;35(4):490–497. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann K, Schweimer K, Reese G, et al. Structure and stability of 2S albumin-type peanut allergens: implications for the severity of peanut allergic reactions. Biochem J. 2006;395(3):463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller GA, Gosavi RA, Pomes A, et al. Ara h 2: crystal structure and IgE binding distinguish two subpopulations of peanut allergic patients by epitope diversity. Allergy. 2011;66(7):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bublin M, Kostadinova M, Radauer C, et al. IgE cross-reactivity between the major peanut allergen Ara h 2 and the nonhomologous allergens Ara h 1 and Ara h 3. J Allergy Clin Immunol. 2013;132(1):118–124. [DOI] [PubMed] [Google Scholar]
- 19.Asarnoj A, Glaumann S, Elfstrom L, et al. Anaphylaxis to peanut in a patient predominantly sensitized to Ara h 6. Int Arch Allergy Immunol. 2012;159(2):209–212. [DOI] [PubMed] [Google Scholar]
- 20.van der Valk JPM, Schreurs MWJ, El Bouch R, Arends NJT, de Jong NW. Mono-sensitisation to peanut component Ara h 6: a case series of five children and literature review. Eur J Pediatr. 2016;175(9):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballmer-Weber BK, Lidholm J, Fernandez-Rivas M, et al. IgE recognition patterns in peanut allergy are age dependent: perspectives of the EuroPrevall study. Allergy. 2015;70(4):391–407. [DOI] [PubMed] [Google Scholar]
- 22.Bernard H, Guillon B, Drumare MF, et al. Allergenicity of peanut component Ara h 2: Contribution of conformational versus linear hydroxyproline-containing epitopes. J Allergy Clin Immunol. 2015;135(5):1267–1274 e1261-1268. [DOI] [PubMed] [Google Scholar]
- 23.Otsu K, Guo R, Dreskin SC. Epitope analysis of Ara h 2 and Ara h 6: characteristic patterns of IgE-binding fingerprints among individuals with similar clinical histories. Clin Exp Allergy. 2015;45(2):471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Negi SS, Liao S, Gao V, Braun W, Dreskin SC. Conformational IgE epitopes of peanut allergens Ara h 2 and Ara h 6. Clin Exp Allergy. 2016;46(8):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard H, Paty E, Mondoulet L, et al. Serological characteristics of peanut allergy in children. Allergy. 2003;58(12):1285–1292. [DOI] [PubMed] [Google Scholar]
- 26.Bernard H, Mondoulet L, Drumare MF, et al. Identification of a new natural Ara h 6 isoform and of its proteolytic product as major allergens in peanut. J Agric Food Chem. 2007;55(23):9663–9669. [DOI] [PubMed] [Google Scholar]
- 27.Wavrin S, Bernard H, Wal JM, Adel-Patient K. Cutaneous or respiratory exposures to peanut allergens in mice and their impacts on subsequent oral exposure. Int Arch Allergy Immunol. 2014;164(3):189–199. [DOI] [PubMed] [Google Scholar]
- 28.Clement G, Boquet D, Mondoulet L, Lamourette P, Bernard H, Wal JM. Expression in Escherichia coli and disulfide bridge mapping of PSC33, an allergenic 2S albumin from peanut. Protein Expr Purif. 2005;44(2):110–120. [DOI] [PubMed] [Google Scholar]
- 29.Hazebrouck S, Guillon B, Drumare MF, Paty E, Wal JM, Bernard H. Trypsin resistance of the major peanut allergen Ara h 6 and allergenicity of the digestion products are abolished after selective disruption of disulfide bonds. Mol Nutr Food Res. 2012;56(4):548–557. [DOI] [PubMed] [Google Scholar]
- 30.Bernard H, Drumare MF, Guillon B, Paty E, Scheinmann P, Wal JM. Immunochemical characterisation of structure and allergenicity of peanut 2S albumins using different formats of immunoassays. Anal Bioanal Chem. 2009;395(1):139–146. [DOI] [PubMed] [Google Scholar]
- 31.Guillon B, Bernard H, Drumare MF, Hazebrouck S, Adel-Patient K. Heat processing of peanut seed enhances the sensitization potential of the major peanut allergen Ara h 6. Mol Nutr Food Res. 2016;60(12):2722–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deak PE, Vrabel MR, Kiziltepe T, Bilgicer B. Determination of Crucial Immunogenic Epitopes in Major Peanut Allergy Protein, Ara h2, via Novel Nanoallergen Platform. Sci Rep. 2017;7(1):3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flores Kim J, McCleary N, Nwaru BI, Stoddart A, Sheikh A. Diagnostic accuracy, risk assessment, and cost-effectiveness of component-resolved diagnostics for food allergy: A systematic review. Allergy. 2018;73(8):1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehlers AM, Blankestijn MA, Knulst AC, Klinge M, Otten HG. Can alternative epitope mapping approaches increase the impact of B-cell epitopes in food allergy diagnostics? Clin Exp Allergy. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazebrouck S, Ah-Leung S, Bidat E, et al. Goat’s milk allergy without cow’s milk allergy: suppression of non-cross-reactive epitopes on caprine beta-casein. Clin Exp Allergy. 2014;44(4):602–610. [DOI] [PubMed] [Google Scholar]
- 36.Magnusdottir H, Vidarsdottir AG, Ludviksson BR, et al. Ara h 1 and Ara h 6 Sensitization Causes Clinical Peanut Allergy in Ara h 2-Negative Individuals. Int Arch Allergy Immunol. 2018:1–10. [DOI] [PubMed] [Google Scholar]
- 37.Blankestijn MA, Otten HG, Suer W, Weimann A, Knol EF, Knulst AC. Specific IgE to peanut 2S albumin Ara h 7 has a discriminative ability comparable to Ara h 2 and 6. Clin Exp Allergy. 2018;48(1):60–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.