Abstract
Background
Age-related decline in muscle oxidative capacity reduces muscle function and physical performance leading to disability and frailty. Whether age-related decline in oxidative capacity is modified by exercise and other life-style practices is unclear. Therefore, we tested the hypothesis that physical activity is associated with better oxidative capacity independent of age.
Design
Cross-sectional study performed in the Baltimore Longitudinal Study of Aging conducted by the Intramural Research Program (IRP) of the National Institute on Aging (NIA).
Setting
NIA IRP Clinical Research Unit, Baltimore, MD.
Participants
Participants included 384 adults (54.7% women) aged 22 to 92 years seen between 2013 and 2017.
Measurements
Muscle oxidative capacity was measured in vivo using phosphorous magnetic resonance spectroscopy. We determined the post-exercise time constant (τPCr, in seconds) for phosphocreatine (PCr) recovery, with lower values of τPCr, (i.e, more rapid recovery of PCr levels after exercise) reflecting greater oxidative capacity. Time spent in moderate-to-vigorous physical activity (MVPA) was assessed using wearable accelerometers that participants wore 5.9 ± 0.9 consecutive days in the free-living environment.
Results
In linear regression models, higher τPCr was associated with older age (standardized β = 0.39, p-value <.001) after adjusting for sex, race, height and weight. After including MVPA as an independent variable, the standardized regression coefficient of age decreased by 40%, but remained associated with τPCr (βage = 0.22, p-value <.001) and had a smaller standardized regression coefficient than MVPA (βMVPA = −0.33, p-value <.001). After adjusting for health status, education and smoking history, the standardized regression coefficient for age decreased 12% (βage = 0.20, p-value = .003), while the standardized coefficient for MVPA decreased only 3% (βMVPA = −0.32, p-value <.001).
Conclusion
Study findings suggest that MVPA is strongly associated with muscle oxidative capacity independent of age, providing mechanistic insights into the health benefits of exercise in older age.
Keywords: 31P MRS, bioenergetic, in vivo, accelerometer, moderate-to-vigorous physical activity
Introduction:
Aging is accompanied by structural and functional changes of mitochondria which negatively affect mobility in older adults.1,2 Although the notion of age-associated mitochondrial dysfunction remains controversial,3,4 compelling evidence from in vivo phosphorous magnetic resonance spectroscopy (31P MRS) studies of maximum oxidative capacity in skeletal muscle and ex-vivo respirometry in permeabilized muscle fibers demonstrates that impaired mitochondrial function is associated with chronological age.5,6 It has been argued that this impairment may occur as a result of deterioration in health and decreased daily physical activity levels with aging, rather than resulting from the aging process.7 Larsen et al.8 showed that oxidative capacity measured by 31P-MRS in vastus lateralis muscle is higher in older active men (n = 6, age = 69.3 ± 5.3 years) than relatively sedentary older men (n = 8, age = 68.9 ± 3.9 years), suggesting that differences in maximal oxidative capacity between trained and untrained adults is largely associated with regular participation in moderate-to-vigorous physical activity (MVPA).9,10 In a comprehensive study, Distefano et al.11 showed that both ex-vivo mitochondrial respiration and in-vivo maximal mitochondrial capacity measured in the vastus lateralis did not differ between younger active (n = 10, age = 31.2 ± 5.4 years) and older active groups (n = 10, age = 67.5 ± 2.7 years) while both of these markers of mitochondrial function were lower in an older sedentary group (n = 19 men, age = 70.7 ± 4.7 years). In spite of sound design and sophisticated technology used in these studies, the small sample sizes limit the interpretation and generalizability of the results. Therefore, to better determine the association between physical activity and mitochondrial function with aging, we conducted cross-sectional analyses of objectively measured MVPA and maximal muscle mitochondrial oxidative capacity in 384 (54.7% women) adults participating in the Baltimore Longitudinal Study of Aging (BLSA).
Materials and methods
Participants
The BLSA is a study of normative human aging, established in 1958 and currently conducted by the Intramural Research Program (IRP) of National Institute on Aging (NIA).12 Certified technicians conducted measurements, following standardized protocols,12–14 and participants gave written informed consent at the time of testing. Enrollment into the BLSA is ongoing, and once enrolled, participants are followed at intervals of 1 to 4 years, becoming more frequent at older ages. The sample for the present study consists of the first visit of 384 individual adults (210 women) aged 22 to 92 years, who underwent a 3-day comprehensive examination (between 2013 and 2017) including a complete physical, health history assessment, and ³¹P-MRS measurements at the NIA IRP Clinical Research Unit. To quantify habitual physical activity, on the last day of the visit, participants were fitted with a wearable accelerometer and asked to wear it continuously for the next 7 days. From 2013 – 2015, the BLSA used the Actiheart (CamNtech, Cambridge, United Kingdom) and from 2015 – 2017, the Actigraph GT9X (Actigraph, Pensacola, FL, USA). All data were collected from the same visit and those participants who could not participate in 31P MRS scan or successfully wear the accelerometer were eliminated from this study.
Muscle oxidative capacity measured by 31P-MRS
31P-MRS data were acquired using a 3T Achieva MR scanner (Philips, Best, The Netherlands) with a 10-cm 31P-tuned surface coil (PulseTeq, Surrey, UK), fastened above the middle of the left thigh over the vastus lateralis muscle. Data were collected using a standardized protocol described previously.14,15 Briefly, participants were instructed to perform an in-magnet rapid knee extension exercise of an average duration of 30 seconds while a total of 75 pulse-acquired 31P-MRS acquisitions with 6 second time-resolution, were collected for 60 seconds before, for the 30 seconds during, and for 360 seconds after exercise. To standardize the measure of oxidative function within different subjects, the duration of exercise was carefully optimized by considering two criteria: (i) achieving a reduction in phosphocreatine (PCr) peak height of 30–70% compared with initial baseline values and (ii) avoidance of intracellular muscle acidification, defined as pH < 6.8.16 A mono-exponential function was used to fit the time-dependent post-exercise PCr recovery, to determine the recovery time constant of the PCr (τPCr, measured in seconds). This time constant is inversly proportional to the maximum in-vivo muscle oxidative capacity, with higher τPCr reflecting slower recovery and hence lower oxidative capacity.17 There are minimal energy demands during post-exercise PCr resynthesis and thus τPCr approximates maximum mitochondrial adenosine triphosphate production.18–20
Moderate-to-vigorous physical activity
Minute-level activity counts were downloaded from wearable accelerometers and averaged across the number of days worn to arrive at an average activity count per minute (CPM) for every minute of the day. For Actigraph, non-wear times were identified as intervals of 90 consecutive minutes where activity counts were equal to zero.21 For Actiheart, the presence of heart rate was used to determine wear-time compliance. Days with more than 8% of the day missing (more than 120 minutes per day) were excluded, and a minimum of four days of valid wear was required for inclusion in the analysis.13 For Actiheart, time spent in MVPA was defined using the heart rate data and the Karvonen formula for heart rate reserve to define intensity of movement.22 For Actigraph, population-specific cut-points for MVPA were estimated by: (1) calculating the correlation between Actiheart-derived MVPA and measured maximal aerobic fitness VO2max (r = 0.3), (2) testing Actigraph data cut-points ranging from 3890 CPM (80th percentile) to 9120 CPM (99th percentile) for correlation with VO2max, and (3) identifying a cut-point of 4500 CPM to most closely correspond with the correlation between Actiheart and VO2max (r = 0.3).
Other covariates
Covariates include sex, self-reported black or non-black race, never smoked history, education, height, and weight. An index of multimorbidity was defined as a count of the 15 most prevalent chronic diseases known to be correlated with frailty and mortality in older adults.23,24
Statistical analyses
The association of τPCr with age and MVPA were separately and then jointly assessed using linear regression analyses. An indicator variable to distinguish Actiheart from Actigraph was added as an independent variable in multilinear regression analysis as a type of empirical calibration between devices to capture potential methodologic differences in the measurement of MVPA; MVPA-by-device type interactions were also assessed. The interaction terms between the level of MVPA with sex; and age were also tested, separately. Differences in participant characteristics based on subgroups defined by wearable accelerometer type were also tested. Further analyses were performed to test the association of τPCr with age and MVPA after adjusting for education, smoking and multimorbidity. All analyses were performed using R statistical software (Version 3, R Foundation for Statistical Computing, Vienna, Austria), and p-value <.05 was considered statistically significant.
Results
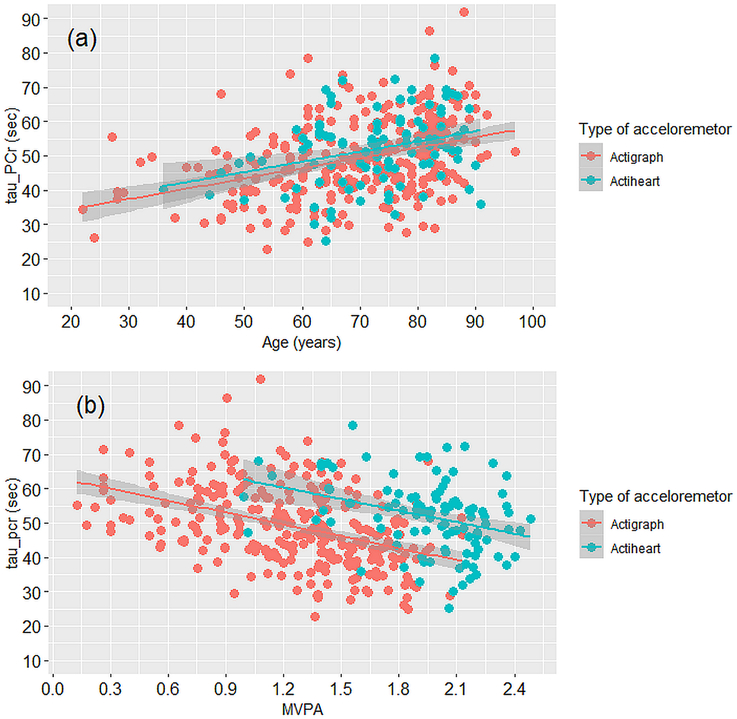
Table 1 compares participant characteristics by type of wearable accelerometer. Participants measured with Actiheart were older and had higher τPCr than participants measured with Actigraph (p<.05). Figure 1(a) and Table 2 (Model 1) demonstrate that τPCr was greater with older age (Figure 1(a)), after adjusting for sex, race, height and weight (Table 2, Model 1). τPCr was also greater in adults with lower MVPA after adjusting for sex, race, height and weight, irrespective of type of accelerometer (Table 2, Model 2). An interaction term between MVPA and sex was not significant. An additional model that included a MVPA-by-device interaction found no significant interaction (beta = 0.007, p-value = .91; not shown) with similar findings in both subgroups. Figure 1(b) reflects this finding, showing that the slope of the linear relationship between τPCr and MVPA is similar in both accelerometer subgroups; although the intercept differs, which primarily reflects the different scaling between devices.
Table 1.
Participant characteristics by type of wearable accelerometer.
| Actiheart (n=104) |
Actigraph (n= 280) |
p-value | |
|---|---|---|---|
| Age (years) | 72.7 ± 11.1 | 69.2 ± 14.1 | .01 |
| Weight (kg) | 76.6 ± 15.7 | 75.4 ± 16.4 | .51 |
| Height (cm) | 168.1 ± 9.1 | 167.3 ± 9.0 | .45 |
| Sex, women (%) | 51.9 | 55.7 | .58¶ |
| Race, black (%) | 25.9 | 25.4 | 1¶ |
| Index-morbidity | 3.5 ± 1.8 | 3.3 ± 1.7 | .30 |
| Education (years) | 17.1 ± 2.6 | 17.5 ± 2.5 | .11 |
| Never-Smoked (%) | 64.4 | 65.0 | .87¶ |
| τPCr (sec) | 51.9 ± 10.4 | 49.1 ± 11.5 | .02 |
| †MVPA | 1.9 ± 0.3 | 1.2 ± 0.4 | |
| ‡Valid days | 5.6 ± 0.7 | 6.1 ± 1 |
Moderate-to-vigorous physical activity (MVPA) measured as minutes per day in both devices (MVPAactigraph = 25.2 ± 23.4 min, MVPAactiheart = 107.8 ± 62.7 min) and because of the skewed distribution of MVPA, it is reported as log10(MAPA+1) in the table.
Participants with less than 4 valid days were excluded from the study.
Chi-Square test p-value
Figure 1.
Scatterplot of relationships between muscle oxidative capacity-τPCr (a) and age, (b) and the log transfer of moderate-to-vigorous physical activity (MVPA) calculated as (log10(MVPA+1)), categorized by type of wearable accelerometer. Actigraph data points are shown in coral pink and actiheart data points shown in caribbean green. Simple linear regression lines for each category are shown with shaded areas indicating confidence intervals.
Table 2:
Multiple linear regression models test the association between τPCr and age (model. 1), and moderate-to-vigorous physical activity (MVPA) (model. 2), and both age and MVPA as independent variables (model. 3) and adjusted by other possible predictors (model. 4).
| Actiheart | Actigraph | Either Actiheart or Actigraph |
||||
|---|---|---|---|---|---|---|
| β (95% CI) |
p-value | β (95% CI) |
p-value | β (95% CI) |
p-value | |
| Model. 1 | ||||||
| Age | 0.34 (0.14, 0.55) |
.001 | 0.39 (0.28, 0.51) |
<.001 | 0.39 (0.30, 0.49) |
<.001 |
| Model. 2 | ||||||
| MVPA | −0.33 (−0.51, −0.14) |
<.001 | −0.41 (−0.52, −0.30) |
<.001 | −0.49 (−0.61, −0.37) |
<.001 |
| †Non-reference accelerometer | — | — | 0.42 (0.30, 0.54) |
<.001 | ||
| Model. 3 | ||||||
| Age | 0.22 (−0.01, 0.45) |
.062 | 0.23 (0.09, 0.37) |
.002 | 0.22 (0.11, 0.34) |
<.001 |
| MVPA | −0.22 (−0.44, −0.01) |
.039 | −0.27 (−0.41, −0.13) |
<.001 | −0.33 (−0.48, −0.19) |
<.001 |
| †Non-reference accelerometer | — | — | 0.29 (0.16, 0.43) |
<.001 | ||
| Model. 4 | ||||||
| Age | 0.19 (−0.06, 0.45) |
.130 | 0.20 (0.05, 0.36) |
.012 | 0.20 (0.07, 0.33) |
.003 |
| MVPA | −0.22 (−0.44, 0.01) |
.055 | −0.26 (−0.40, −0.12) |
<.001 | −0.32 (−0.47, −0.17) |
<.001 |
| †Non-reference accelerometer | — | — | 0.28 (0.15, 0.42) |
<.001 | ||
| Index (morbidity) | 0.03 (−0.21, 0.26) |
.824 | 0.06 (−0.07, 0.18) |
.389 | 0.05 (−0.06, 0.16) |
.351 |
| Education | −0.08 (−0.26, 0.11) |
.392 | −0.08 (−0.19, 0.03) |
.139 | −0.08 (−0.17, −0.01) |
.074 |
| Smoking | −0.03 (−0.16, 0.22) |
.759 | 0.01 (−0.11, 0.12) |
.920 | 0.01 (−0.09, 0.10) |
.905 |
All models were additionally adjusted for sex, race, weight and height. All regression coefficients are standardized.
Indicator variable to distinguish Actiheart from Actigraph acetometers.
To examine whether the association between age and oxidative capacity is explained by MVPA, we added MVPA to model 1 as an independent variable (Table 2, Model 3). After accounting for MVPA (see Table2, Model 3), the regression coefficient for age was reduced from 36% to 43% in all groups. There was no interaction between age and MVPA for either device, suggesting that the independent association of MVPA with τPCr is similar across all ages examined. Finally, Model 3 was adjusted for additional covariates: the multimorbidity index, education and smoking as shown in Model 4. After adjustment, the regression coefficient of age decreased between 10% to 12% for both devices, while the coefficient of MVPA decreased by only about 3%. The magnitude of the standardized regression coefficient for MVPA was greater than age in both Models 3 and 4 demonstrating the higher impact of MVPA on τPC compared with age.
Discussion
To our knowledge, this is the largest cross-sectional study of the independent associations of age and MVPA with muscle oxidative capacity. Initially, we showed that muscle oxidative capacity, as measured by 31P-MRS in vastus lateralis muscle was lower with older age, indicated by the longer recovery times with older age. This result is consistent with previous work, including ours, that used both biopsy and in-vivo 31P-MRS.2,14,25,26 In contrast, several investigations mainly performed using small samples did not observe this association, postulating that the association between age and muscle oxidative capacity is more likely due to the decrease of habitual physical activity level with aging.8,11,27 In the current study, although we demonstrated that physically active adults have shorter post-exercise recovery time independent of age, reflecting greater oxidative capacity, the level of MVPA does not completely explain the age association in both devices. In the Actiheart subgroup; older age did not significantly associate with lower oxidative capacity after accounting for MVPA, most likely because of small sample size, since the standardized beta coefficients for the two accelerometers were of similar magnitude. However, in both the Actigraph and combined sample; both lower MVPA and older age remained significantly associated with lower muscle oxidative capacity. These findings confirm the importance of large sample size for having precise conclusion. Indeed, this result remains important because it demonstrates that, although MVPA is strongly associated with muscle oxidative capacity, some other unknown effects of age negatively affect mitochondrial function via mechanisms that are not fully offset by higher levels of MVPA.3,28,29 These findings contrast with previously published results suggesting that the decline in mitochondrial function with aging is explained by an age-associated reduction of physical activity.8,11 This discrepancy may be due to the small sample size of fewer than 40 participants in these previous studies. In a more extensive analysis, we investigated the impact of adjusting for morbidity, education and smoking on the association of mitochondrial dysfunction with age. Our findings demonstrate that these predictors strongly attenuate the effect of age compared to the level of MVPA, suggesting that the effect of age may be mediated by the emergence of clinical and subclinical pathology. Further studies in more clinical populations are needed to fully address the effect of specific diseases on mitochondrial dysfunction.
The major strength of this study is the sample size compared with previous work. Furthermore, this study includes a diverse population in terms of race, age, sex, MVPA level, and further accounts for the health status of older adults and their lifestyle. Nevertheless, longitudinal data are needed to truly understand the contribution of MVPA to changes in mitochondrial function with advancing age. Further, a more in-depth analysis of the types and patterns of physical activity (such as sedentary time in older population), that may contribute to a greater understanding of the contribution of physical activity to preserving mitochondrial function with aging are required. Given rapid advances in wearable technology, more extensive efforts to harmonize physical activity data across different devices are ongoing and should be available for future studies.30
In conclusion, the results of this study indicate the equivalence of both Actiheart and Actigraph for establishing the relationship between τPCr and MVPA. These results advance our understanding of mitochondrial dysfunction with aging demonstrating that MVPA may attenuate the observed age-associated decline. This finding suggests that moderate-to-vigorous exercise intervention may potentially improve muscle oxidative capacity decline with aging. Additionally, this study warrants further investigation to determine other unknown effects of age that adversely affect mitochondrial function with aging and do not completely explain by the level of MVPA and health status.
Acknowledgment
This work was funded by the Intramural Research Program of the National Institute on Aging, National Institute of Health, Baltimore, MD. JAS is supported by R21AG053198 and U01AG057545 grants.
Funding sources: This work was funded by the Intramural Research Program of the National Institute on Aging, National Institute of Health, Baltimore, MD. JAS is supported by R21AG053198 and U01AG057545 grants.
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
Sponsor’s Role: None.
References:
- 1.Santanasto AJ, Coen PM, Glynn NW, et al. The relationship between mitochondrial function and walking performance in older adults with a wide range of physical function. Exp Gerontol. 2016;81:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zane AC, Reiter DA, Shardell M, et al. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell. 2017;16(3):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent JA, Fitzgerald LF. In vivo mitochondrial function in aging skeletal muscle: capacity, flux, and patterns of use. J Appl Physiol (1985). 2016;121(4):996–1003. [DOI] [PubMed] [Google Scholar]
- 4.Hart CR, Layec G, Trinity JD, et al. Evidence of Preserved Oxidative Capacity and Oxygen Delivery in the Plantar Flexor Muscles With Age. J Gerontol A Biol Sci Med Sci. 2015;70(9):1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Freire M, Adelnia F, Moaddel R, Ferrucci L. Searching for a mitochondrial root to the decline in muscle function with ageing. J Cachexia Sarcopenia Muscle. 2018;9(3):435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Freire M, Scalzo P, D’Agostino J, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell. 2018;17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russ DW, Kent-Braun JA. Is skeletal muscle oxidative capacity decreased in old age? Sports Med. 2004;34(4):221–9. [DOI] [PubMed] [Google Scholar]
- 8.Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab. 2012;37(1):88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. In vivo oxidative capacity varies with muscle and training status in young adults. J Appl Physiol (1985). 2009;107(3):873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F, Wills K, Laslett LL, et al. Moderate-to-Vigorous Physical Activity But Not Sedentary Time Is Associated With Musculoskeletal Health Outcomes in a Cohort of Australian Middle-Aged Women. J Bone Miner Res. 2017;32(4):708–15. [DOI] [PubMed] [Google Scholar]
- 11.Distefano G, Standley RA, Zhang X, et al. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle. 2018;9(2):279–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shock NW, Gerontology Research Center (U.S.). Normal human aging : the Baltimore longitudinal study of aging. Baltimore, Md. Washington, D.C.: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Aging; For sale by the Supt. of Docs., U.S. G.P.O.; 1984. xix, 399, 34 p. p. [Google Scholar]
- 13.Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69(8):973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi S, Reiter DA, Shardell M, et al. 31P Magnetic Resonance Spectroscopy Assessment of Muscle Bioenergetics as a Predictor of Gait Speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71(12):1638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(4):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997;272(2 Pt 1):C501–10. [DOI] [PubMed] [Google Scholar]
- 17.Prompers JJ, Wessels B, Kemp GJ, Nicolay K. MITOCHONDRIA: investigation of in vivo muscle mitochondrial function by 31P magnetic resonance spectroscopy. Int J Biochem Cell Biol. 2014;50:67–72. [DOI] [PubMed] [Google Scholar]
- 18.Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. 1984;1(3):307–15. [DOI] [PubMed] [Google Scholar]
- 19.Edwards LM, Tyler DJ, Kemp GJ, et al. The reproducibility of 31-phosphorus MRS measures of muscle energetics at 3 Tesla in trained men. PLoS One. 2012;7(6):e37237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCully KK, Fielding RA, Evans WJ, Leigh JS Jr., Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol (1985). 1993;75(2):813–9. [DOI] [PubMed] [Google Scholar]
- 21.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrack JA, Leroux A, Fleg JL, et al. Using Heart Rate and Accelerometry to Define Quantity and Intensity of Physical Activity in Older Adults. J Gerontol A Biol Sci Med Sci. 2018;73(5):668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women’s health and aging study. Circulation. 2000;101(9):1007–12. [DOI] [PubMed] [Google Scholar]
- 25.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526 Pt 1:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102(15):5618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimbert V, Boirie Y, Bedu M, et al. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J. 2004;18(6):737–9. [DOI] [PubMed] [Google Scholar]
- 28.Adelnia F, Shardell M, Bergeron CM, et al. Diffusion-weighted MRI with intravoxel incoherent motion modeling for assessment of muscle perfusion in the thigh during post-exercise hyperemia in younger and older adults. NMR Biomed. 2019:e4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adelnia F, Cameron D, Bergeron CM, et al. The Role of Muscle Perfusion in the Age-Associated Decline of Mitochondrial Function in Healthy Individuals. Front Physiol. 2019;10:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrack JA, Cooper R, Koster A, et al. Assessing Daily Physical Activity in Older Adults: Unraveling the Complexity of Monitors, Measures, and Methods. J Gerontol A Biol Sci Med Sci. 2016;71(8):1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]