Abstract
Arctigenin (ARC-G) is the main active ingredient extracted from Great Burdock Achene, with extensive pharmacological effects. In addition, ARC-G has been suggested to show excellent efficacy on inflammatory disease. This study aimed to defined that the function of Arctigenin attenuates inflammation in dextran sulfate sodium (DSS)-induced acute colitis, to determine its possible mechanism. Mice was induced by giving 2.0% DSS in the drinking water for DSS-induced acute colitis. Mice of acute colitis were injected intraperitoneally with 20 mg/kg per day of Arctigenin for 7 days. MPO activity levels were measured using MPO activity kits. Western Blot Analysis was used to determine the protein expression. Arctigenin prevents colitis and attenuates inflammation in DSS-induced acute colitis. Arctigenin suppressed NLRP3 inflammasome by SIRT1 in DSS-induced acute colitis. In THP-1 cell by LPS model, Arctigenin suppressed NLRP3, caspase-1 and IL-1β protein expression by SIRT1. Si-NLRP3 increases the effects of Arctigenin on inflammation in THP-1 cell by LPS model. Si-SIRT1 decreases the effects of Arctigenin on inflammation in THP-1 cell by LPS model. INF39, NLRP3 inhibitor also increased the effects of Arctigenin on inflammation in DSS-induced acute colitis. SIRT1 inhibitor also decreases the effects of Arctigenin on inflammation in DSS-induced acute colitis. Taken together our results demonstrated that Arctigenin attenuates inflammation in DSS-induced acute colitis through suppression of NLRP3 inflammasome by SIRT1.
Keywords: Arctigenin, acute colitis, NLRP3, SIRT1
Introduction
Ulcerative colitis (UC) is one of the major types of inflammatory bowel disease (IBD), which belongs to the autoimmune disease [1]. Its clinical manifestations include diarrhea, abdominal pain and mucosanguineous stools [1]. Meanwhile, it is associated with various disease severity and repeated attack. UC is a common disease in European and American countries [2]. In China, no epidemiological data among the ordinary population are available [1]. However, the morbidity of UC in China shows an increasing trend year by year [3]. Few severe UC patients are seen in China, however, UC patients suffer from repeated attack [3]. UC will not only affect the life quality of patients, but can easily induce colon cancer [4]. According to statistics, approximately 5-10% chronic UC patients in China would develop into colon cancer annually [5]. Particularly, the morbidity of colon cancer in UC patients with a history of over 25 years can be as high as 40%. Therefore, it is of great value and urgent to conduct UC research to reduce its morbidity. At present, treatment for UC is still dominated by anti-inflammation and anti-immunity [5]. However, these drugs have severe side effects and UC is likely to relapse after drug withdrawal.
Inflammation is the self-protection response of the body during the pathogenesis and development of inflammatory disease, which will also cause damage of the body. Besides, it is found in clinic that, the protection of most inflammatory responses is far smaller than their damage to the body [6]. Therefore, it is of great significance to study the inflammatory response process and to regulate the inflammatory response to prevent inflammatory disease [7]. The Nod-like receptor protein 3 (NLRP3) inflammasome is an intracellular protein complex, which can regulate the inflammatory response in the body [8]. NLRP3 inflammasome plays a key role in activating cysteine-requiring aspartate protease-1 (caspase-1). The activated caspase-1 can mediate the maturation and differentiation of pro-inflammatory cytokines IL-1β and IL-18 [7]. Subsequently, the IL-1β receptor on target cell surface can bind with the activated IL-1β, thus activating the IL-1β signaling pathway and MyD88-dependent NF-κB pathway. Finally, it will induce the inflammatory response in the body [8]. Existing studies find that, NLRP3, ASC and caspase-1 can regulate intestinal integrity and modulate intestinal inflammatory response [6]. Moreover, recent studies suggest that NLRP3 inflammasome is closely correlated with the pathogenesis of UC [9].
SIRT1, a member of the Sirtuins family (Sirt1-7), is the recently-discovered III type nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase [10]. SIRT1 mainly locates in the cell nucleus in mammal, which exerts its function through the acetylation activity [11]. Plenty of evidence has suggested that SIRT1 shows protection in multiple kidney diseases, such as acute kidney injury [11]. The major mechanism involves oxygen radical reduction, inflammation alleviation, autophagy enhancement, and mitochondrial function stabilization [11]. Meanwhile, it can regulate blood glucose and blood lipid, resist fibrosis, and show protection in diabetic nephropathy and renal fibrosis [12]. Additionally, SIRT1 can regulate NLRP3 to control the genesis of inflammation and delay the occurrence of inflammatory in mouse [12].
Great Burdock Achene is a traditional Chinese medicinal (TCM) material that can effectively alleviate pain and fever in rheumatism. ARC-G (C21H24O6; molecular weight: 372.41) is a phenylalanine dibenzyl butyl lactone lignan extracted from Great Burdock Achene [13]. It has multiple bioactivities, including anti-inflammation, anti-oxidation, anti-cancer, and anti-virus [14-16]. Besides, domestic and foreign research on the anti-inflammatory effect of ARC-G mainly select the animal inflammatory models like colitis, arthritis, acute lung injury and cerebral injury [14]. In fact, many clinically common diseases are accompanying with inflammation, such as diabetes and atherosclerosis [13]. Intensive study on the anti-inflammatory effect and mechanism of ARC-G using diabetes and atherosclerosis models will lay down foundation for exploring the new clinical application pathways for ARC-G and developing new preparations [17]. This study aimed to defined that the function of Arctigenin attenuates inflammation in dextran sulfate sodium (DSS)-induced acute colitis, to determine its possible mechanism.
Materials and methods
Animals and vivo model group
C57BL/6 mice were obtained from Animal testing center of Yangzhou University (Yangzhou, China). C57BL/6 mice were housed at 22-23°C, 55-60% humidity, in a 12-h light/dark cycle. The present study was approved by the Animal Care and Use Committee of Yijishan Hospital of Wannan Medical College. C57BL/6 mice were randomly assigned to four groups: sham (n = 6), only Arctigenin group (n = 6), DSS-induced model (n = 7), and Arctigenin treatment group (n = 7). In sham or only Arctigenin group, mice were injected intraperitoneally with normal saline for 7 days. In DSS-induced model or Arctigenin treatment group, mice was induced by giving 2.0% DSS in the drinking water for 7 d. In Arctigenin treatment group, mice were injected intraperitoneally with 20 mg/kg per day of Arctigenin for 7 days.
Next, C57BL/6 mice were randomly assigned to four groups: sham (n = 6), only DSS-induced model (n = 7), Arctigenin treatment group (n = 7) and SIRT1 inhibitor or NLRP3 inhibitor group. In SIRT1 inhibitor or NLRP3 inhibitor group, mice were induced by giving 2.0% DSS in the drinking water for 7 d and injected intraperitoneally with 20 mg/kg per day of Arctigenin and 20 mg/kg of Cambinol (MedChemExpress, Shanghai, China) or 12.5 mg/kg of INF39 (MedChemExpress, Shanghai, China) for 7 days.
Histomorphological analysis
Colon tissue samples were fixed in 4% paraformaldehyde, paraffin-embedded and then sectioned into 5 μm slices for H&E staining. Colon tissue samples were observed using fluorescence microscope (Zeiss Axio Observer A1, Germany).
MPO activity levels
Colon tissue samples were collected and used to measure MPO activity levels using MPO activity levels kits (A044, Nanjing Jiancheng Biological Engineering Institute, Nanjing, China), following the manufacture’s instructions.
Evaluation of pathway analysis of Arctigenin
The TCMSP server (http://ibts.hkbu.edu.hk/LSP/tcmsp.php) is a systems-level pharmacology database for TCM as literature [18] for interesting, naturally occurring compounds. GEO DataSets is a flexible, user-friendly web interface as literature [18] for generating hypotheses about gene function, analysing gene lists and prioritising genes. Relationships gene targets and diseases, networks were constructed and analyzed using Cytoscape 3.0 for a deeper understanding of the complex.
Western blot analysis
Colon tissue samples or cell was splitted using RIPA assay and protein was quantified using BCA assay. Proteins were electrophoresed on 10% SDS-acrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat milk in TBST for 1 h at 37°C and incubated with IL-1β (BS3506, 1:1000, Bioworld Technology, Inc.), IL-18 (BS6823, 1:1000, Bioworld Technology, Inc.), caspase-1 (sc-1780, 1:1000, Santa Cruz, USA), NLRP3 (sc-66846, 1:1000, Santa Cruz, USA), SIRT1 (sc-135791, 1:1000, Santa Cruz, USA) and GAPDH (sc-293335, 1:5000, Santa Cruz, USA) at 4°C overnight. The membranes were washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibodies (sc-2004 or sc-2005, 1:5000, Santa Cruz, USA) for 1 h at 37°C. Protein was measured using an enhanced chemiluminescence system and analyzed using a Image Lab 3.0 (Bio-Rad Laboratories, Inc.).
Immunofluorescence
Colon tissue samples were fixed in 4% paraformaldehyde, paraffin-embedded and then sectioned into 5 μm slices. Slices samples were incubated with 0.25% Tris-X100 for 10 min at room temperature for permeabilization and repaired using citric acid for 10 min at 95°C. Slices samples were blocked with 5% BSA in TBS for 1 h at room temperature and incubated with NLPR3 (1:100) at 4°C overnight. After washing with TBST for three times, sections were incubated with secondary peroxidase-conjugated goat anti-rabbit IgG (1:100, Santa Cruz Biotechnology) antibody for 2 h at room temperature. After washing with TBST for three times, sections were stained with DAPI for 15 min at darkness. Colon tissue samples were observed using fluorescence microscope (Zeiss Axio Observer A1, Germany).
Vitro model and treatment groups
THP-1 cell was purchased from Shanghai Cell Institute Country Cell Bank and maintained in RPMI 1640 medium (Gibco) with 10% heat-inactivated fetal bovine serum (Gibco) under a humidified 5% (v/v) CO2 atmosphere at 37°C. THP-1 cell was induced with 100 nM of PMA for 24 h, and treated with 100 ng/mL of LPS and for 2 mM of ATP 4 h or treated with 20 μM of arctigenin, 100 ng/mL of LPS and for 2 mM of ATP 4 h.
Si-NLRP3 (Santa Cruz Biotechnology) or si-SIRT1 (Santa Cruz Biotechnology) and control negative mimics were transfected into THP-1 cell using Lipofectamine 2000 Reagent (Invitrogen) for 4 h. Old medium was removed and new RPMI 1640 medium were added into cell for 20 h. Next, THP-1 cell transfection induced using with 100 nM of PMA for 24 h, treated with 100 ng/mL of LPS and for 2 mM of ATP 4 h or treated with 20 μM of arctigenin, 100 ng/mL of LPS and for 2 mM of ATP 4 h.
Statistical analysis
Data were expressed as mean ± SEM using GraphPad Prism 8. Multiple comparisons were performed by one-way ANOVA followed by Tukey’s post-test. P values < 0.05 were considered statistically significant.
Results
Arctigenin prevents colitis and attenuates inflammation in DSS-induced acute colitis
Firstly, we determined that the function of arctigenin in DSS-induced acute colitis. As showed in Figure 1A, the weight of DSS-induced acute colitis mice was reduced, compared with control sham mice group. Next, treatment with arctigenin recovered the weight of DSS-induced acute colitis mice, compared with DSS-induced acute colitis mice model group (Figure 1A). Then, colon length was reduced, and histochemical score was increased in DSS-induced acute colitis mice were reduced, compared with control sham mice group (Figure 1B-E). Treatment with arctigenin increased colon length, and reduced histochemical score in DSS-induced acute colitis mice, compared with DSS-induced acute colitis mice model group (Figure 1B-E). MPO activity levels were enhanced in DSS-induced acute colitis mice were reduced, compared with control sham mice group (Figure 1F). The activation of MPO activity levels was inhibited in DSS-induced acute colitis mice by arctigenin, compared with control sham mice group (Figure 1F). Collectively, these results suggest that arctigenin prevents colitis and attenuates inflammation in DSS-induced acute colitis.
Figure 1.
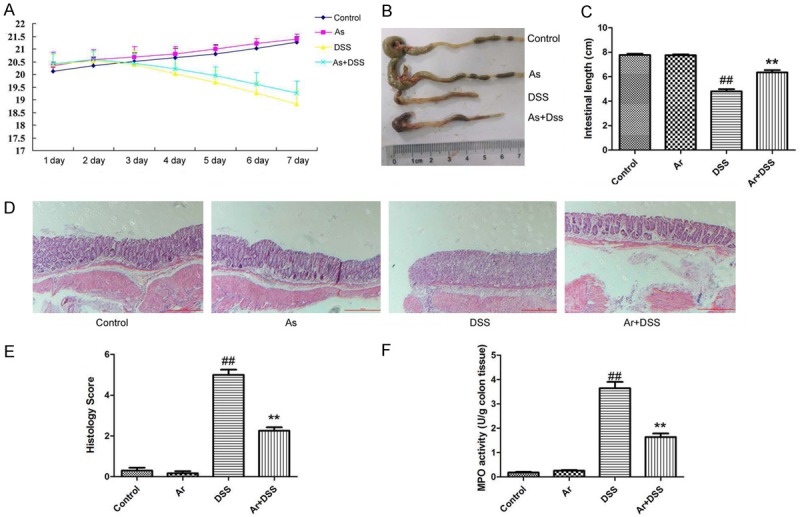
Arctigenin prevents colitis and attenuates inflammation in DSS-induced acute colitis. The weight (A), colon length (B and C), histochemical score (D and E) and MPO activity levels (F). Control, control sham mice group; Ar, 20 mg/kg of arctigenin group; DSS, 2.0% DSS-induced colitis mice group; Ar+DSS, 2.0% DSS-induced colitis mice by treatment with 20 mg/kg of arctigenin group. ##P < 0.01 compared with control sham mice group, **P < 0.01 compared with 2.0% DSS-induced colitis mice group. Data were expressed as mean ± SEM.
Systematic understanding of the mechanism Arctigenin in colitis
The investigated the function targets of Arctigenin in colitis using TCMSP server (http://ibts.hkbu.edu.hk/LSP/tcmsp.php). As shown in Figure 2A, 32.86% displayed similar co-expression characteristics, and 30.34% shared the same protein domain. Physical interactions, pathways and co-localisation are showed at Figure 2A. Based on target fishing and pathway analysis for colitis using GEO DataSets (Figure 2B), and the results of Figure 2C showed that three genes were common target fishing and pathway of colitis and Arctigenin. Then, we analyzed SIRT1 and SIRT1 for target fishing and pathway.
Figure 2.
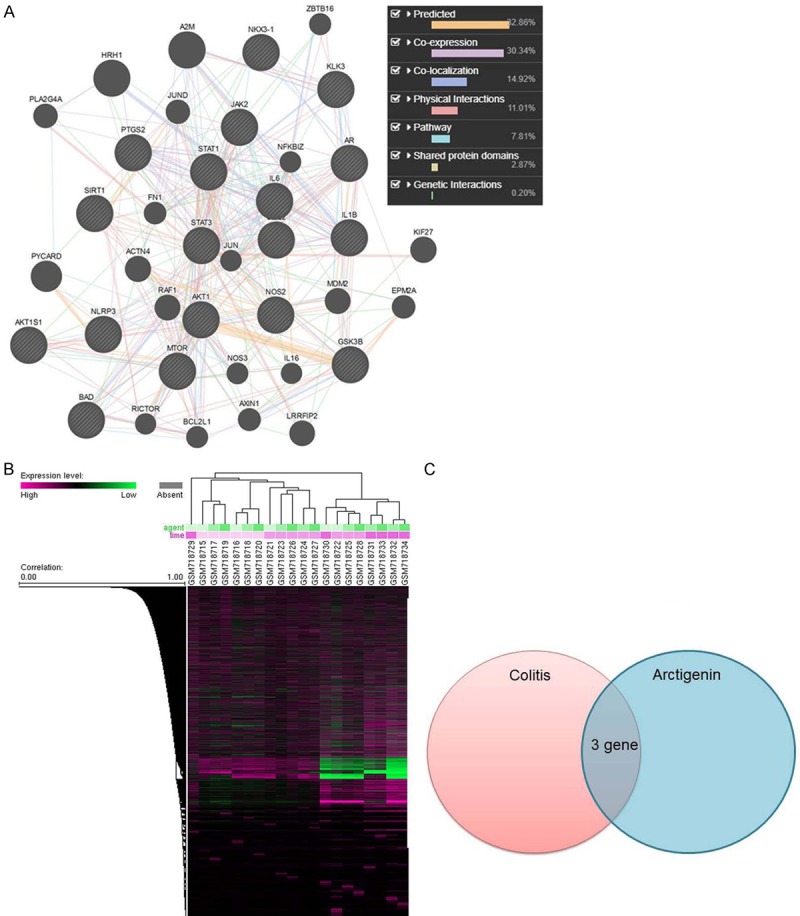
Systematic Understanding of the Mechanism Arctigenin in colitis. Network of potential Arctigenin targets (A), Target fishing and pathway analysis for colitis using GEO DataSets (B), common target fishing and pathway (C). Data were expressed as mean ± SEM.
Arctigenin suppressed NLRP3 inflammasome by SIRT1 in DSS-induced acute colitis
To explore the mechanism of arctigenin in DSS-induced acute colitis, western blot analysis was used to analyze the protein expression. As showed in Figure 3A-F, the protein expression of IL-1β, IL-18, caspase-1 and NLRP3 were induced, SIRT1 protein expression was suppressed in DSS-induced acute colitis, compared with control sham mice group. Treatment with arctigenin suppressed IL-1β, IL-18, caspase-1 and NLRP3 protein expression, and induced SIRT1 protein expression in DSS-induced acute colitis, compared with DSS-induced acute colitis model group (Figure 3A-F). IF showed SIRT1 protein expression was induced in colon tissue of DSS-induced acute colitis mice by arctigenin, compared with DSS-induced acute colitis model group (Figure 3G). So, these results showed that arctigenin may regulates SIRT1 to adjust NLRP3 inflammasome in DSS-induced acute colitis.
Figure 3.
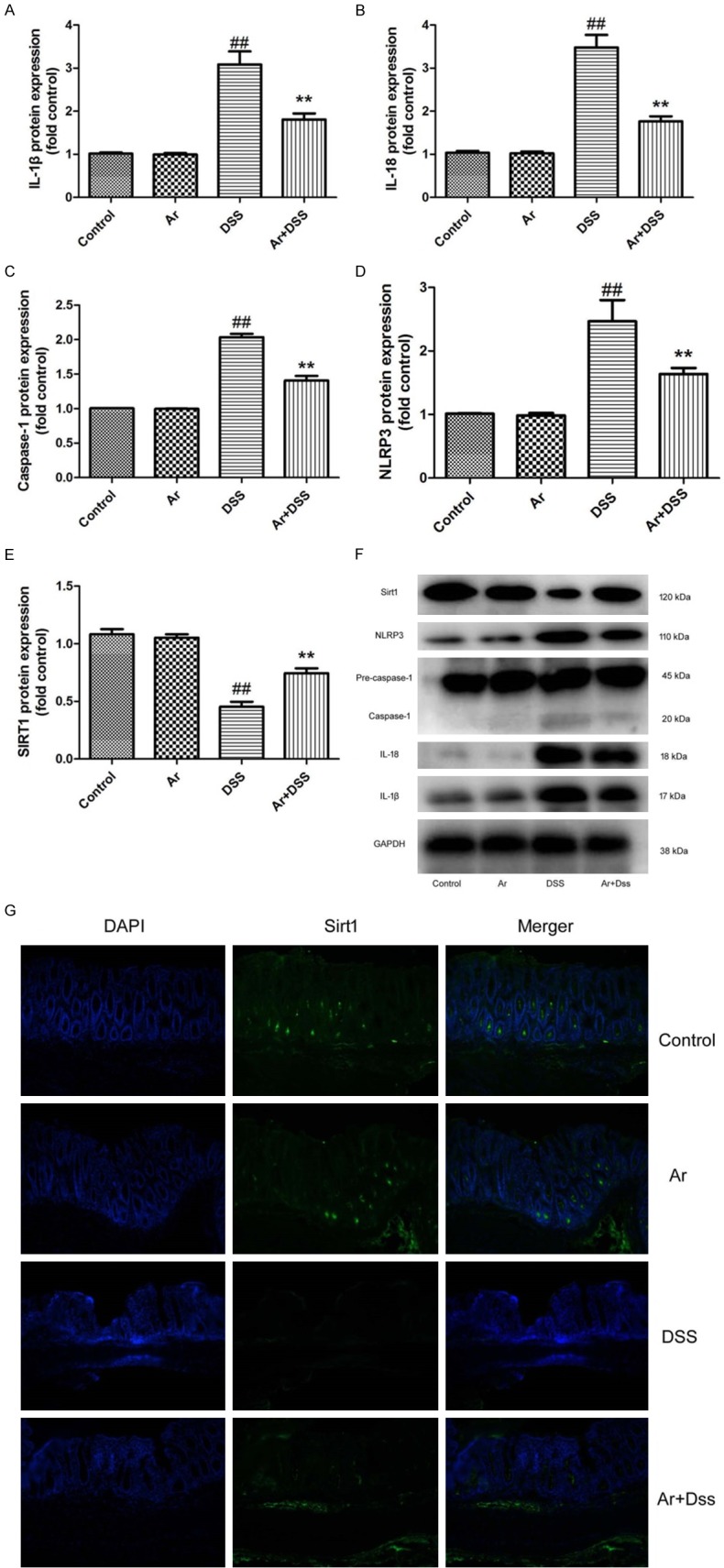
Arctigenin suppressed NLRP3 inflammasome by SIRT1 in DSS-induced acute colitis. IL-1β, IL-18, caspase-1, NLRP3 and SIRT1 protein expressions (A-E) and western blotting analysis for IL-1β, IL-18, caspase-1, NLRP3 and SIRT1 protein expressions (F), IF for SIRT1 protein expression (G). Control, control sham mice group; Ar, 20 mg/kg of arctigenin group; DSS, 2.0% DSS-induced colitis mice group; Ar+DSS, 2.0% DSS-induced colitis mice by treatment with 20 mg/kg of arctigenin group. ##P < 0.01 compared with control sham mice group, **P < 0.01 compared with 2.0% DSS-induced colitis mice group. Data were expressed as mean ± SEM.
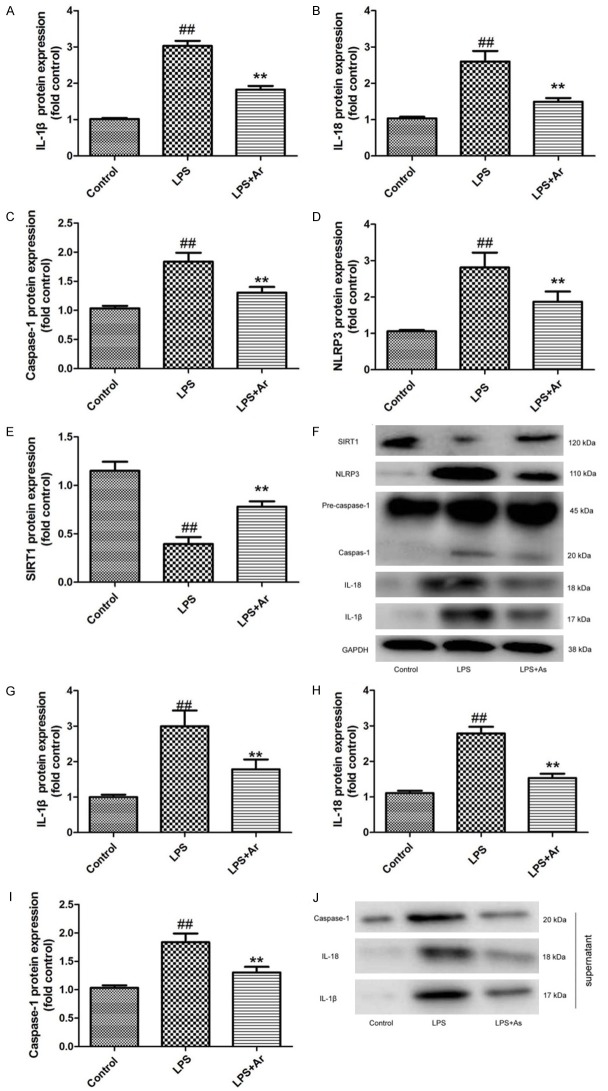
Arctigenin suppressed NLRP3, caspase-1 and IL-1β protein expression by SIRT1 in THP-1 cell by LPS model
In vitro model, we analyze the anti-inflammaiton function of arctigenin in THP-1 cell by LPS. As showed in Figure 3A-F, the protein expression of IL-1β, IL-18, caspase-1 and NLRP3 were induced, SIRT1 protein expression was suppressed in THP-1 cell by LPS model, compared with control THP-1 cell group. Treatment with arctigenin suppressed IL-1β, IL-18, caspase-1 and NLRP3 protein expression, and induced SIRT1 protein expression in THP-1 cell by LPS model, compared with THP-1 cell by LPS model group (Figure 4A-F). Next, we analyzed the supernatant of IL-1β, IL-18 and caspase-1 protein expressions in THP-1 cell by LPS model. The supernatant of IL-1β, IL-18 and caspase-1 protein expressions were increased in THP-1 cell by LPS model, compared with control THP-1 cell group (Figure 4G-J). The induction of IL-1β, IL-18 and caspase-1 protein expressions in supernatant of THP-1 cell by LPS model, compared with THP-1 cell by LPS model group (Figure 4G-J). These findings indicated that arctigenin suppressed NLRP3 inflammasome by SIRT1 in THP-1 cell by LPS model.
Figure 4.
Arctigenin suppressed NLRP3, caspase-1 and IL-1β protein expression by SIRT1 in THP-1 cell by LPS model IL-1β, IL-18, caspase-1, NLRP3 and SIRT1 protein expressions (A-E) and western blotting analysis for IL-1β, IL-18, caspase-1, NLRP3 and SIRT1 protein expressions (F); the supernatant of IL-1β, IL-18 and caspase-1 protein expressions (G-I), and western blotting analysis for IL-1β, IL-18 and caspase-1 protein expressions (J). Control, control THP-1 cell group; LPS, 100 ng of LPS and 2 mM of ATP in THP-1 cell for 4 h group; LPS+Ar, 20 μM of arctigenin, 100 ng of LPS and 2 mM of ATP in THP-1 cell for 4 h group. ##P < 0.01 compared with control THP-1 cell group, **P < 0.01 compared with LPS group. Data were expressed as mean ± SEM.
Si-SIRT1 decreases the effects of Arctigenin on inflammation in THP-1 cell by LPS model
The study ascertained the role of SIRT1 in ischemic stroke in the effects of arctigenin on inflammation in THP-1 cell by LPS model. As showed in Figure 5A-F, si-SIRT1 suppressed the expression of SIRT1 protein, and induced IL-1β, IL-18, caspase-1 and NLRP3 protein expression in THP-1 cell by LPS model and arctigenin, compared with LPS model and arctigenin group. Meanwhile, si-SIRT1 also induced the supernatant of IL-1β, IL-18 and caspase-1 protein expression in THP-1 cell by LPS model and arctigenin, compared with LPS model and arctigenin group (Figure 5G-J). Taken together, these results indicated that arctigenin may regulates SIRT1 to adjust NLRP3 inflammasome in THP-1 cell by LPS model.
Figure 5.
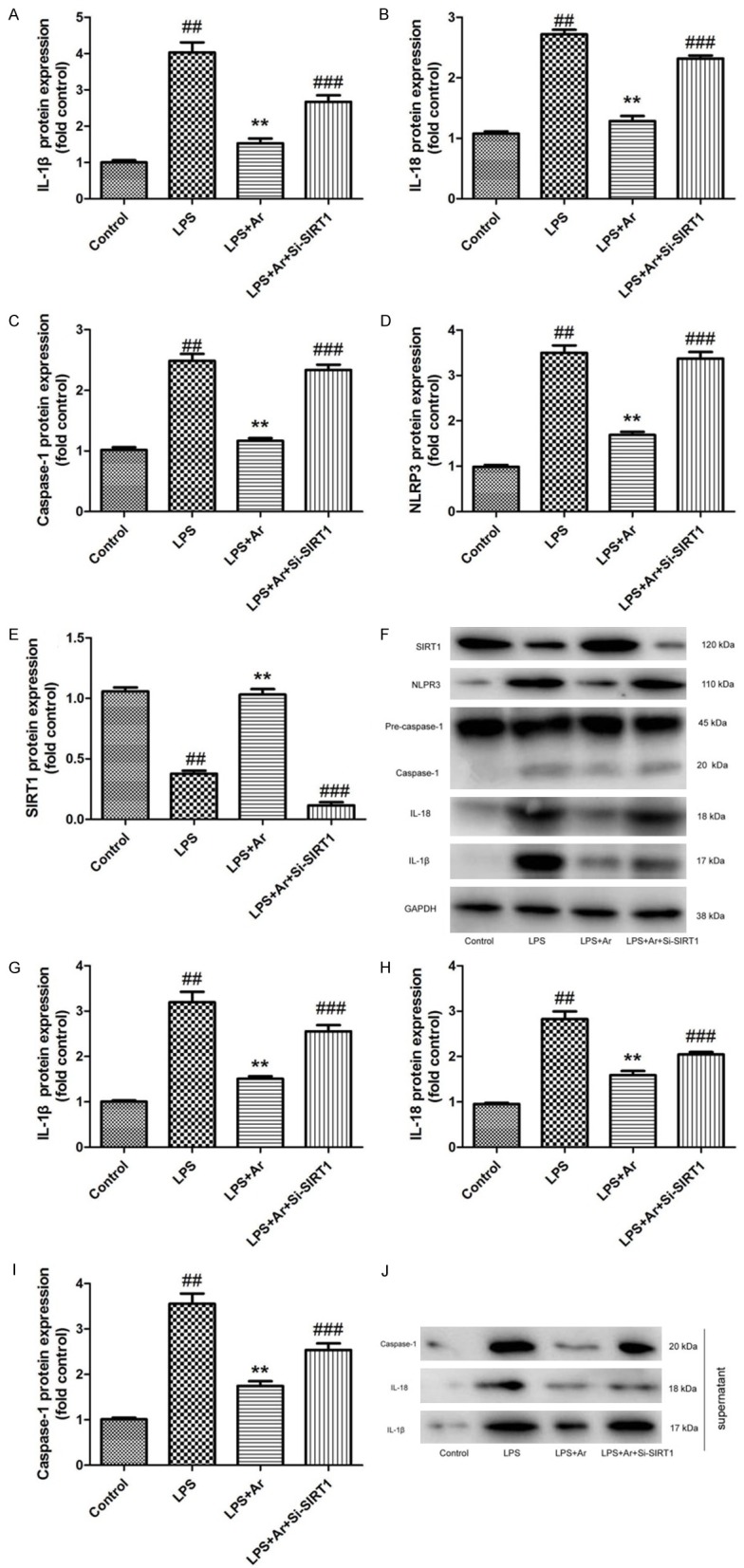
Si-SIRT1 decreases the effects of Arctigenin on inflammation in THP-1 cell by LPS model. IL-1β, IL-18, caspase-1, NLRP3 and SIRT1 protein expressions (A-E) and western blotting analysis for IL-1β, IL-18, caspase-1, NLRP3 and SIRT1 protein expressions (F); the supernatant of IL-1β, IL-18 and caspase-1 protein expressions (G-I), and western blotting analysis for IL-1β, IL-18 and caspase-1 protein expressions (J). Control, control THP-1 cell group; LPS, 100 ng of LPS and 2 mM of ATP in THP-1 cell for 4 h group; LPS+Ar, 20 μM of arctigenin, 100 ng of LPS and 2 mM of ATP in THP-1 cell for 4 h group; LPS+Ar+Si-SIRT1, 100 ng of Si-SIRT1, 20 μM of arctigenin, 100 ng of LPS and 2 mM of ATP in THP-1 cell for 4 h group. ##P < 0.01 compared with control THP-1 cell group, **P < 0.01 compared with LPS group, ###P < 0.01 compared with LPS+Ar group. Data were expressed as mean ± SEM.
SIRT1 inhibitor also decreases the effects of Arctigenin on inflammation in DSS-induced acute colitis
In vivo model, we used SIRT1 inhibitor, 20 mg/kg of Cambinol, to reduce SIRT1 protein expression, and induced IL-1β, IL-18, caspase-1 and NLRP3 protein expression in DSS-induced acute colitis by arctigenin, compared with arctigenin treatment group (Figure 6A-F). IF showed SIRT1 protein expression was induced in colon tissue of DSS-induced acute colitis mice by arctigenin and SIRT1 inhibitor, compared with arctigenin treatment group (Figure 6G). Next, we observed SIRT1 inhibitor also decreases the effects of arctigenin on the weight, colon length, histochemical score and MPO activity levels in DSS-induced acute colitis, compared with arctigenin treatment group (Figure 7). So, these results showed that arctigenin prevents colitis and attenuates inflammation in DSS-induced acute colitis by SIRT1.
Figure 6.
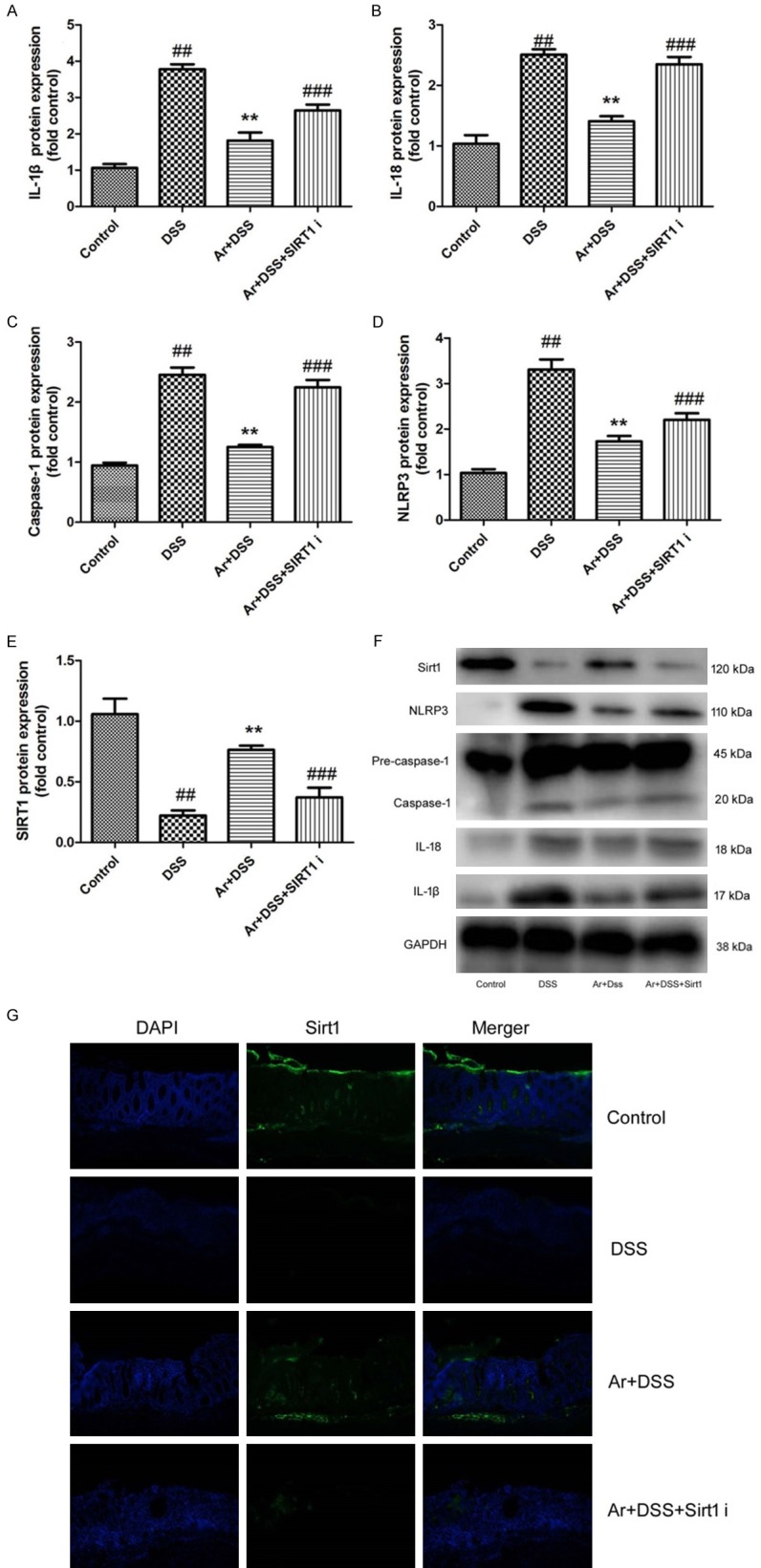
SIRT1 inhibitor also decreases the effects of Arctigenin on NLRP3 inflammasome in DSS-induced acute colitis. IL-1β, IL-18, caspase-1, NLRP3 and SIRT1 protein expressions (A-E) and western blotting analysis for IL-1β, IL-18, caspase-1, NLRP3 and SIRT1 protein expressions (F), IF for SIRT1 protein expression (G). Control, control sham mice group; DSS, 2.0% DSS-induced colitis mice group; Ar+DSS, 2.0% DSS-induced colitis mice by treatment with 20 mg/kg of arctigenin group; Ar+DSS+SIRT1 i, 2.0% DSS-induced colitis mice by treatment with 20 mg/kg of arctigenin and 20 mg/kg of Cambinol group. ##P < 0.01 compared with control sham mice group, **P < 0.01 compared with 2.0% DSS-induced colitis mice group, ###P < 0.01 compared with Ar+DSS group. Data were expressed as mean ± SEM.
Figure 7.
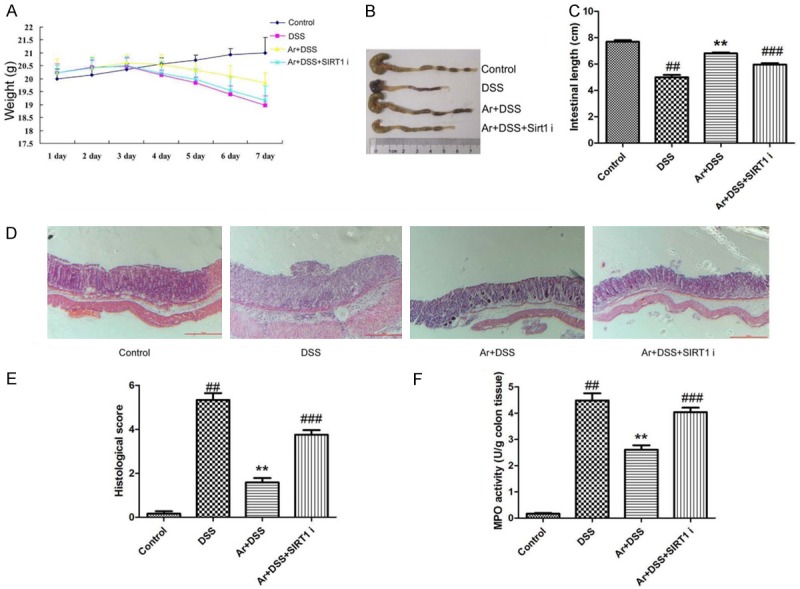
SIRT1 inhibitor also decreases the effects of Arctigenin on inflammation in DSS-induced acute colitis. The weight (A), colon length (B and C), histochemical score (D and E) and MPO activity levels (F). Control, control sham mice group; DSS, 2.0% DSS-induced colitis mice group; Ar+DSS, 2.0% DSS-induced colitis mice by treatment with 20 mg/kg of arctigenin group; Ar+DSS+SIRT1 i, 2.0% DSS-induced colitis mice by treatment with 20 mg/kg of arctigenin and 20 mg/kg of Cambinol group. ##P < 0.01 compared with control sham mice group, **P < 0.01 compared with 2.0% DSS-induced colitis mice group, ###P < 0.01 compared with Ar+DSS group. Data were expressed as mean ± SEM.
INF39, NLRP3 inhibitor also increased the effects of Arctigenin on inflammation in DSS-induced acute colitis
To further explore the role of NLRP3 in the effects of arctigenin on inflammation in DSS-induced acute colitis, NLRP3 inhibitor, 12.5 mg/kg of INF39 was used to DSS-induced acute colitis by arctigenin. As showed in Figure 8A-E, NLRP3 inhibitor suppressed the protein expression of IL-1β, IL-18, caspase-1 and NLRP3 in DSS-induced acute colitis by arctigenin, compared with treatment with arctigenin group. Next, NLRP3 inhibitor also increased the effects of arctigenin on the weight, colon length, histochemical score and MPO activity levels in DSS-induced acute colitis, compared with treatment with arctigenin group (Figure 8F-K).
Figure 8.
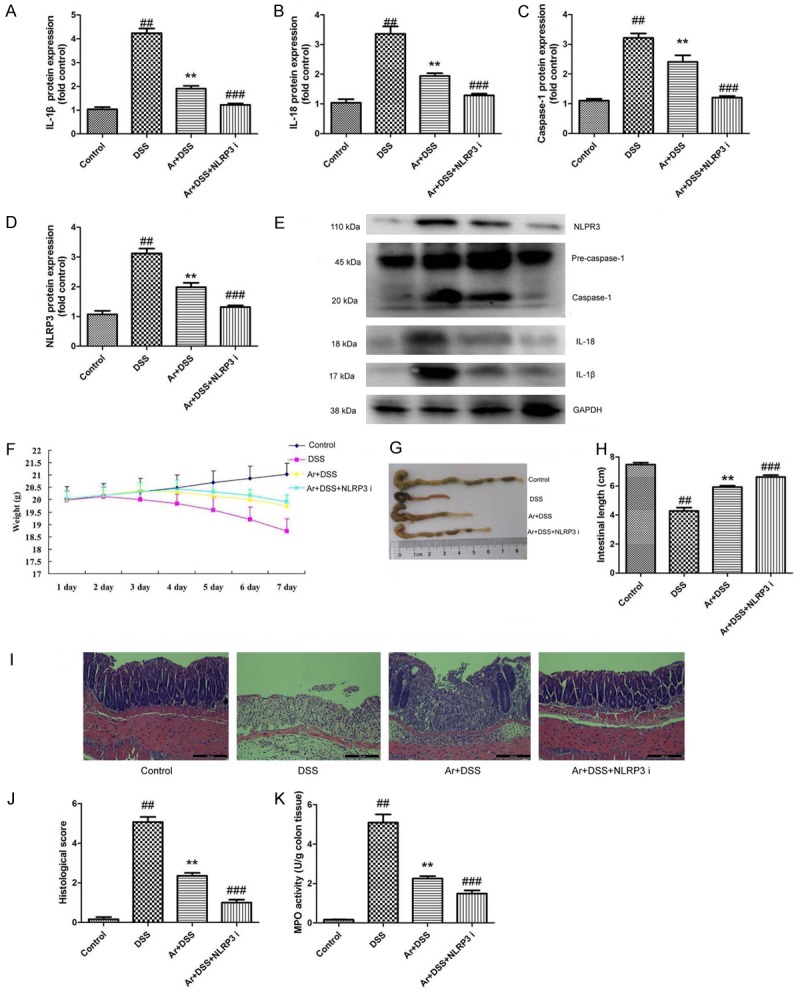
INF39, NLRP3 inhibitor also increased the effects of Arctigenin on inflammation in DSS-induced acute colitis. IL-1β, IL-18, caspase-1 and NLRP3 protein expressions (A-D) and western blotting analysis for IL-1β, IL-18, caspase-1 and NLRP3 protein expressions (E), the weight (F), colon length (G and H), histochemical score (I and J) and MPO activity levels (K). Control, control sham mice group; DSS, 2.0% DSS-induced colitis mice group; Ar+DSS, 2.0% DSS-induced colitis mice by treatment with 20 mg/kg of arctigenin group; Ar+DSS+NLRP3 i, 2.0% DSS-induced colitis mice by treatment with 20 mg/kg of arctigenin and 12.5 mg/kg of INF39 group. ##P < 0.01 compared with control sham mice group, **P < 0.01 compared with 2.0% DSS-induced colitis mice group, ###P < 0.01 compared with Ar+DSS group. Data were expressed as mean ± SEM.
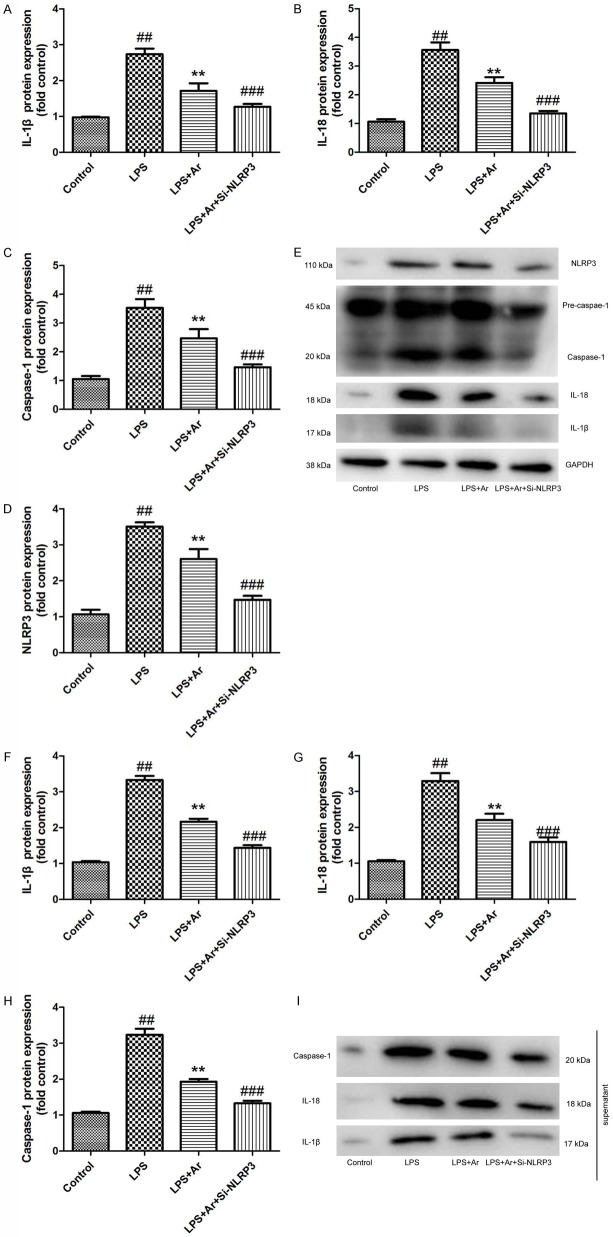
Si-NLRP3 increases the effects of Arctigenin on inflammation in THP-1 cell by LPS model
In vitro model, we further analyzed the role of NLRP3 in the effects of arctigenin on inflammation in THP-1 cell by LPS model. As showed in Figure 9A-E, IL-1β, IL-18, caspase-1 and NLRP3 protein expression were suppressed by si-NLRP3 in THP-1 cell by LPS model following arctigenin, compared with arctigenin treatment group. In supernatant, IL-1β, IL-18 and caspase-1 protein expression were suppressed by si-NLRP3 in THP-1 cell by LPS model following arctigenin, compared with arctigenin treatment group (Figure 9F-I). Taken together, these results indicated that Arctigenin attenuates inflammation in DSS-induced acute colitis through suppression of NLRP3 inflammasome by SIRT1.
Figure 9.
Si-NLRP3 increases the effects of Arctigenin on inflammation in THP-1 cell by LPS model. IL-1β, IL-18, caspase-1 and NLRP3 protein expressions (A-D) and western blotting analysis for IL-1β, IL-18, caspase-1 and NLRP3 protein expressions (E); the supernatant of IL-1β, IL-18 and caspase-1 protein expressions (F-H), and western blotting analysis for IL-1β, IL-18 and caspase-1 protein expressions (I). Control, control THP-1 cell group; LPS, 100 ng of LPS and 2 mM of ATP in THP-1 cell for 4 h group; LPS+Ar, 20 μM of arctigenin, 100 ng of LPS and 2 mM of ATP in THP-1 cell for 4 h group; LPS+Ar+Si-NLRP3, 200 ng of Si-NLRP3, 20 μM of arctigenin, 100 ng of LPS and 2 mM of ATP in THP-1 cell for 4 h group. ##P < 0.01 compared with control THP-1 cell group, **P < 0.01 compared with LPS group, ###P < 0.01 compared with LPS+Ar group. Data were expressed as mean ± SEM.
Discussion
UC is an easily relapsing and refractory autoimmune disease [1]. It is found to be related to alterations of intestinal flora, inflammatory factors, gene and protein expression, and mucous layer. The current clinical treatments for UC are mainly symptomatic treatments [1]. They include western medicine treatment (mesalazine and sulfasalazine), TCM treatment, combined treatment of TCM and western medicine, TCM differentiation and retention enemas [4]. But most of these treatments can only delay the symptoms, rather than thoroughly cure UC. Therefore, UC is a refractory and recurrent disease [5]. In the current study, we demonstrate that treatment with arctigenin increased the weight and colon length, and reduced histochemical score and MPO activity levels in DSS-induced acute colitis mice. Cheng et al. suggested that arctigenin protects against inflammation in liver injury by suppressing immune cells in mice [13]. So, these results showed that arctigenin possessed anti-inflammation effects in acute colitis mice.
A series of experimental studies find that, SIRT1 can remarkably suppress the expression of inflammatory genes [10]. In human mononuclear macrophage, over-expression of SIRT1 can markedly inhibit the secretion of IL-18 stimulated by tobacco extract. On the contrary, interfering with the SIRT1 expression can up-regulate the IL-18 level [10]. Evidently more macrophages can be seen in the adipose tissue of SIRT1 knockout mouse than the wild type mouse. Meanwhile, high-fat diet can notably induce the intrahepatic inflammatory response [10]. Besides, the SIRT1 knockout mouse is highly sensitive to LPS stimulation, and the expression of pro-inflammatory genes, such as IL-6, IL-1β, IL-18 and TNF-α, is markedly increased [10]. By contrast, the autoimmune encephalomyelitis is distinctly alleviated in SIRT1 transgenic mouse [19]. The present studies indicate that, SIRT1 exerts the anti-inflammatory effect mainly through down-regulating NLRP3, p65 and Ap-1, as well as the histone acetylation level [19,20]. Furthermore, we reported that the inhibition of SIRT1 decreases the effects of arctigenin on inflammation in DSS-induced acute colitis through NLRP3 inflammasome. Zhang et al. demonstrated that arctigenin reduced ischemic stroke via SIRT1-dependent inhibition of NLRP3 inflammasome [19]. Therefore, arctigenin activated SIRT1 to suppress NLRP3 inflammasome in acute colitis.
NLRP3 inflammasome is a compound protein, which is constituted by NLRP3 protein, Caspase-1 and apoptosis-associated speck-like protein (ASC) [8]. Its abnormal activation and regulation is related to the pathogenesis and development of multiple inflammatory diseases [8]. Studies have shown that NLRP3 and its downstream inflammatory factors IL-1β and IL-18 can markedly promote inflammation in diseases like UC, allergic rhinitis, asthma, parasitic disease, ischemic reperfusion and tumor [8,21]. Some studied the progress in recent NLRP3 study, so as to provide studying directions for the role of NLRP3 inflammasome in the pathological process of inflammatory disease [22]. In this study, we found that the inhibition of NLRP3 also increased the effects of arctigenin on inflammation in DSS-induced acute colitis. Zhang et al. demonstrated that arctigenin reduced ischemic stroke via SIRT1-dependent inhibition of NLRP3 inflammasome [19]. Accordingly, arctigenin attenuates inflammation in DSS-induced acute colitis through suppression of NLRP3 inflammasome by SIRT1.
In summary, the present study suggested that arctigenin protected against DSS-induced acute colitis, suppressed NLRP3 inflammasome and induced activated SIRT1 signaling (Figure 10). Taken together, these findings suggest that arctigenin regulated NLRP3 inflammasome by SIRT1 in DSS-induced acute colitis. Therefore, arctigenin may be served as a new therapeutic approach in acute colitis.
Figure 10.
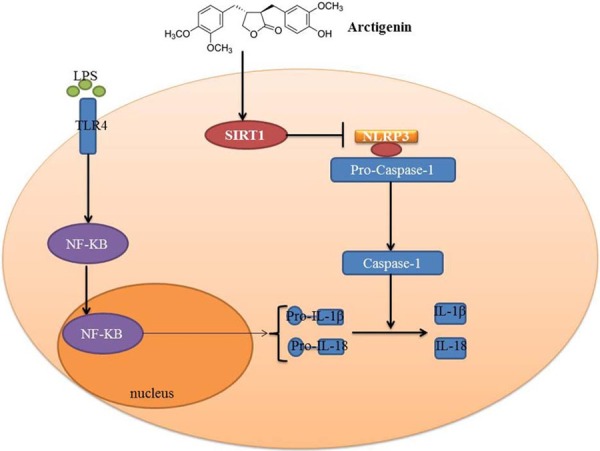
Arctigenin attenuates inflammation in DSS-induced acute colitis through suppression of NLRP3 inflammasome by SIRT1.
Acknowledgements
The study was supported by National Natural Science Foundation of China (81173134).
Disclosure of conflict of interest
None.
References
- 1.Nikolaus S, Schreiber S, Siegmund B, Bokemeyer B, Bastlein E, Bachmann O, Gorlich D, Hofmann U, Schwab M, Kruis W. Patient education in a 14-month randomised trial fails to improve adherence in ulcerative colitis: influence of demographic and clinical parameters on non-adherence. J Crohns Colitis. 2017;11:1052–1062. doi: 10.1093/ecco-jcc/jjx062. [DOI] [PubMed] [Google Scholar]
- 2.Shohdy KS, Rashad W, Elmeligui A. Alopecia universalis associated with ulcerative colitis and the role of azathioprine. Middle East J Dig Dis. 2018;10:50–54. doi: 10.15171/mejdd.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samples J, Evans K, Chaumont N, Strassle P, Sadiq T, Koruda M. Variant two-stage ileal pouch-anal anastomosis: an innovative and effective alternative to standard resection in ulcerative colitis. J Am Coll Surg. 2017;224:557–563. doi: 10.1016/j.jamcollsurg.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Zheng K, Shen H, Jia J, Lu Y, Zhu L, Zhang L, Shen Z. Traditional Chinese medicine combination therapy for patients with steroid-dependent ulcerative colitis: study protocol for a randomized controlled trial. Trials. 2017;18:8. doi: 10.1186/s13063-016-1763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombel JF, Jharap B, Sandborn WJ, Feagan B, Peyrin-Biroulet L, Eichner SF, Robinson AM, Mostafa NM, Zhou Q, Thakkar RB. Effects of concomitant immunomodulators on the pharmacokinetics, efficacy and safety of adalimumab in patients with Crohn’s disease or ulcerative colitis who had failed conventional therapy. Aliment Pharmacol Ther. 2017;45:50–62. doi: 10.1111/apt.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itani S, Watanabe T, Nadatani Y, Sugimura N, Shimada S, Takeda S, Otani K, Hosomi S, Nagami Y, Tanaka F, Kamata N, Yamagami H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Arakawa T. NLRP3 inflammasome has a protective effect against oxazolone-induced colitis: a possible role in ulcerative colitis. Sci Rep. 2016;6:39075. doi: 10.1038/srep39075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazaridis LD, Pistiki A, Giamarellos-Bourboulis EJ, Georgitsi M, Damoraki G, Polymeros D, Dimitriadis GD, Triantafyllou K. Activation of NLRP3 inflammasome in inflammatory bowel disease: differences between crohn’s disease and ulcerative colitis. Dig Dis Sci. 2017;62:2348–2356. doi: 10.1007/s10620-017-4609-8. [DOI] [PubMed] [Google Scholar]
- 8.Guo W, Hu S, Elgehama A, Shao F, Ren R, Liu W, Zhang W, Wang X, Tan R, Xu Q, Sun Y, Jiao R. Fumigaclavine C ameliorates dextran sulfate sodium-induced murine experimental colitis via NLRP3 inflammasome inhibition. J Pharmacol Sci. 2015;129:101–106. doi: 10.1016/j.jphs.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Hanaei S, Sadr M, Rezaei A, Shahkarami S, Ebrahimi Daryani N, Bidoki AZ, Rezaei N. Association of NLRP3 single nucleotide polymorphisms with ulcerative colitis: a case-control study. Clin Res Hepatol Gastroenterol. 2018;42:269–275. doi: 10.1016/j.clinre.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Lv Q, Wang K, Qiao S, Yang L, Xin Y, Dai Y, Wei Z. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 2018;9:258. doi: 10.1038/s41419-018-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sands BE, Joshi S, Haddad J, Freudenberg JM, Oommen DE, Hoffmann E, McCallum SW, Jacobson E. Assessing colonic exposure, safety, and clinical activity of SRT2104, a novel oral SIRT1 activator, in patients with mild to moderate ulcerative colitis. Inflamm Bowel Dis. 2016;22:607–614. doi: 10.1097/MIB.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen A, Chen Z, Xia Y, Lu D, Yang X, Sun A, Zou Y, Qian J, Ge J. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem Biophys Res Commun. 2018;499:267–272. doi: 10.1016/j.bbrc.2018.03.142. [DOI] [PubMed] [Google Scholar]
- 13.Cheng X, Wang H, Yang J, Cheng Y, Wang D, Yang F, Li Y, Zhou D, Wang Y, Xue Z, Zhang L, Zhang Q, Yang L, Zhang R, Da Y. Arctigenin protects against liver injury from acute hepatitis by suppressing immune cells in mice. Biomed Pharmacother. 2018;102:464–471. doi: 10.1016/j.biopha.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Lou Z, Lee SH. Arctigenin represses TGF-beta-induced epithelial mesenchymal transition in human lung cancer cells. Biochem Biophys Res Commun. 2017;493:934–939. doi: 10.1016/j.bbrc.2017.09.117. [DOI] [PubMed] [Google Scholar]
- 15.Daci A, Neziri B, Krasniqi S, Cavolli R, Alaj R, Norata GD, Beretta G. Arctigenin improves vascular tone and decreases inflammation in human saphenous vein. Eur J Pharmacol. 2017;810:51–56. doi: 10.1016/j.ejphar.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Cui Q, Hou Y, Wang Y, Li X, Liu Y, Ma X, Wang Z, Wang W, Tao J, Wang Q, Jiang M, Chen D, Feng X, Bai G. Biodistribution of arctigenin-loaded nanoparticles designed for multimodal imaging. J Nanobiotechnology. 2017;15:27. doi: 10.1186/s12951-017-0263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baba Y, Shigemi Z, Hara N, Moriguchi M, Ikeda M, Watanabe T, Fujimuro M. Arctigenin induces the apoptosis of primary effusion lymphoma cells under conditions of glucose deprivation. Int J Oncol. 2018;52:505–517. doi: 10.3892/ijo.2017.4215. [DOI] [PubMed] [Google Scholar]
- 18.Chen SJ, Cui MC. Systematic understanding of the mechanism of salvianolic acid a via computational target fishing. Molecules. 2017;22 doi: 10.3390/molecules22040644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Jiang L, Che F, Lu Y, Xie Z, Wang H. Arctigenin attenuates ischemic stroke via SIRT1-dependent inhibition of NLRP3 inflammasome. Biochem Biophys Res Commun. 2017;493:821–826. doi: 10.1016/j.bbrc.2017.08.062. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Yang X, He Y, Wang W, Zhang J, Zhang W, Jing T, Wang B, Lin R. Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology. 2017;222:552–561. doi: 10.1016/j.imbio.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhang HX, Wang ZT, Lu XX, Wang YG, Zhong J, Liu J. NLRP3 gene is associated with ulcerative colitis (UC), but not Crohn’s disease (CD), in Chinese Han population. Inflamm Res. 2014;63:979–985. doi: 10.1007/s00011-014-0774-9. [DOI] [PubMed] [Google Scholar]
- 22.Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]