Abstract
Nowadays, the bacterial drug resistance leads to serious healthy problem worldwide due to the long-term use and the abuse of traditional antibiotics result in drug resistance of bacteria. Finding a new antibiotic is becoming more and more difficult. Antimicrobial peptides (AMPs) are the host defense peptides with most of them being the cationic (positively charged) and amphiphilic (hydrophilic and hydrophobic) α-helical peptide molecules. The membrane permeability is mostly recognized as the well-accepted mechanism to describe the action of cationic AMPs. These cationic AMPs can bind and interact with the negatively charged bacterial cell membranes, leading to the change of the electrochemical potential on bacterial cell membranes, inducing cell membrane damage and the permeation of larger molecules such as proteins, destroying cell morphology and membranes and eventually resulting in cell death. These AMPs have been demonstrated to have their own advantages over the traditional antibiotics with a broad-spectrum of antimicrobial activities including anti-bacteria, anti-fungi, anti-viruses, and anti-cancers, and even overcome bacterial drug-resistance. The natural AMPs exist in a variety of organisms and are not stable with a short half-life, more or less toxic side effects, and particularly may have severe hemolytic activity. To open the clinical applications, it is necessary and important to develop the synthetic and long-lasting AMP analogs that overcome the disadvantages of their natural peptides and the potential problems for the drug candidates.
Keywords: Antimicrobial peptides, antibiotics, microbes, cationic, amphiphilic, hydrophilic, hydrophobic, membrane permeability
Introduction
Various antibiotics have broadly been applied to treat human infectious diseases since the first antibiotic Penicillin was discovered by Alexander Fleming in 1928 [1,2]. Antibiotics usually are effective on the treatments of pathogenic bacteria [3,4]. However, antibiotics gradually lost their antibacterial ability and drug-resistant bacteria appeared with the use of the large amounts of antibiotics and even the abuse of various antibiotics [5,6]. Thus, the findings of new antibiotics or other new antibacterial resources become an urgent need and are catching more and more attentions of scientists all over the world. Antimicrobial peptides (AMPs) or host defense peptides were firstly discovered in the 1980’s. AMPs display their broad-spectrum and potent antimicrobial efficacy against bacteria, fungi and viruses [2,7]. Antimicrobial peptides (AMPs) are the indispensable components of the innate immune system in various species including human, animals and plants and become the first-line defense against foreign attacks [3,8]. And their antimicrobial mechanisms are different from traditional antibiotics. Thus, they are capable of being applied to treat various microbes and even drug-resistant ones. AMPs exist in various organisms (bacteria, fungi, animals and plants) and thousands of AMPs have been discovered and demonstrated [1,3]. Of them, most are cationic AMPs that play the key antimicrobial roles. Here, we discussed AMPs with the existing problems, the improvement strategies and the prospects of AMPs.
The structures and the characteristics of antimicrobial peptides
Antimicrobial peptides (AMPs) are ubiquitous in nature. They exist in various organisms including bacteria, fungi, animals and plants, and in all other mammalian species [1]. However, LL37 consisting of 37 amino acids with two leucine residues at its N-terminus is the only one of the AMP family discovered in human (Figure 1; Table 1) [3,7]. Antimicrobial peptides (AMPs) are expressed by the specific genes. They are constitutive or inducible by specific external factors. AMPs usually contain a composition rich in cationic and hydrophobic amino acids, and have the cationic (positively charged) and amphiphilic (both hydrophilic and hydrophobic) characteristics, due to these AMPs containing the rich hydrophobic groups, and having both hydrophobic regions and hydrophilic regions [1,2,9]. These cationic AMPs generally are positively charged and helical polypeptides with short amino acid sequences (less than 100 amino acid residues) including excessive amounts of the positively charged amino acids lysine and arginine (Figure 2; Tables 1 and 2) [5].
Figure 1.
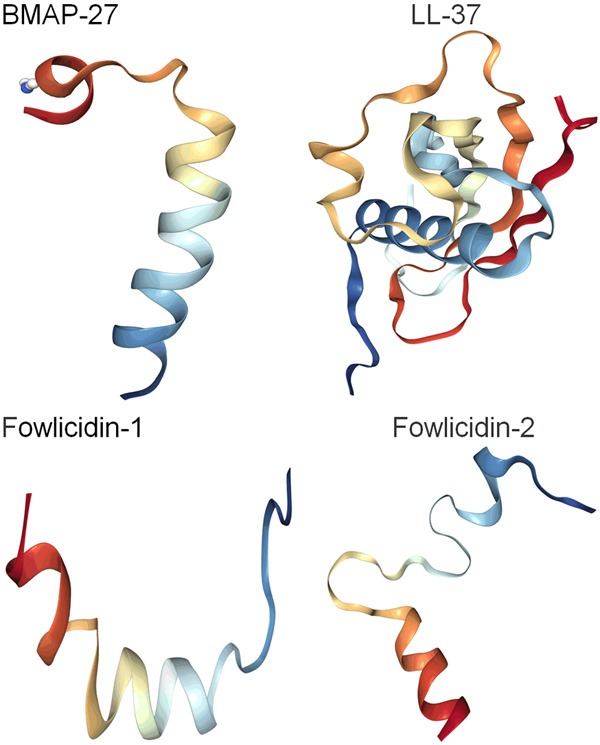
The three-dimensional structures of the cathelicidin subfamily of antimicrobial peptides including BMAP-27, LL-37, fowlicidin-1, fowlicidin-2, cited from RCSB PDB (Research Collaboratory for Structural Bioinformatics Protein Data Bank) (Website: https://www.rcsb.org).
Table 1.
Certain cathelicidin subfamily of antimicrobial peptides containing a highly conserved region (cathelin domain)
| Cathelicidin | Sequence | Secondary Structure | Length (Amino acids) | Organism | PDB ID |
|---|---|---|---|---|---|
| Fowlicidin-1 | RVKRVWPLVI RTVIAGYNLY RAIKKK | 50% helical (2 helices, 13 residues) | 26 | Chicken | 2AMN |
| Fowlicidin-2 | LVQRGRFGRF LRKIRRFRPK VTITIQGSAR F | 41% helical (2 helices, 13 residues) | 31 | N/A | 2GDL |
| Fowlicidin 3 | KRFWPLVPVA INTVAAGINL YKAIRRK | 33% helical (1 helices, 9 residues) | 27 | Gallus gallus | 2HFR |
| LL-37 (hCLD) | GSHMQVLSYK EAVLRAIDGI NQRSSDANLY RLLDLDPRPT MDGDPDTPKP | 16% helical (2 helices, 18 residues) | 107 | Homo sapiens | 4EYC |
| VSFTVKETVC PRTTQQSPED CDFKKDGLVK RCMGTVTLNQ ARGSFDISCD | 34% beta sheet (7 strands/bridges, 37 residues) | ||||
| KDNKRFA | |||||
| BMAP-27 | GRFKRFRKKF KKLFKKLSPV IPLLHLX | 70% helical (2 helices, 19 residues) | 27 | Bos taurus | 2KET |
| Protegrin-3 | RGGGLCYCRR RFCVCVGR | 61% beta sheet (2 strands, 11 residues) | 18 | Sus scrofa | 2MZ6 |
| Protegrin PG-5 | RGGRLCYCRP RFCVCVGR | 55% beta sheet (2 strands, 10 residues) | 18 | Sus scrofa | 2NC7 |
Date were collected and referred from RCSB PDB (Research Collaborative for Structural Bioinformatics Protein Data Bank) (Website: https://www.rcsb.org).
Figure 2.
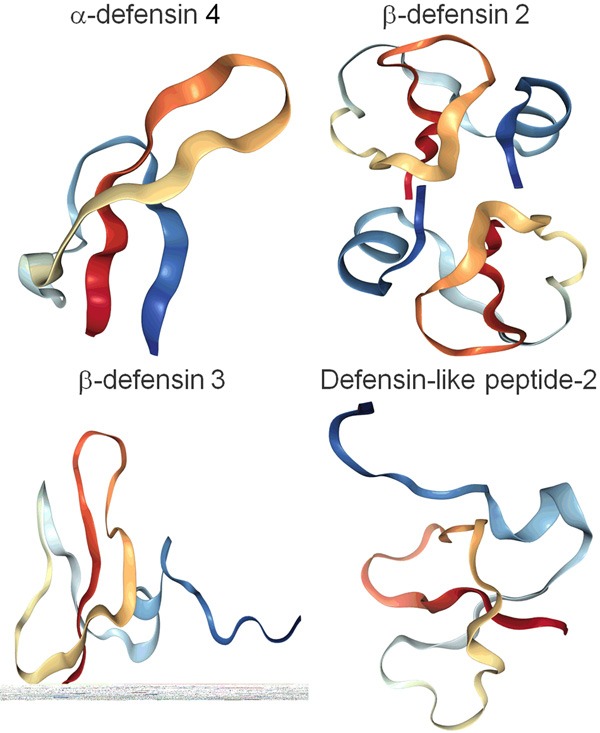
The three-dimensional structures of the defensin subfamily of antimicrobial peptides including α-defensin 4, β-defensin 2, β-defensin 3, defensin-like peptide-2, cited from RCSB PDB (Research Collaboratory for Structural Bioinformatics Protein Data Bank) (Website: https://www.rcsb.org).
Table 2.
Certain defensin subfamily members that are cationic and cysteine-rich antimicrobial peptides
| Defensins | Sequence | Cys Residues | Secondary Structure | Length (Amino acids) | Organism | PDB ID |
|---|---|---|---|---|---|---|
| α-defensin 1 | ACYCRIPACI AGERRYGTCI YQGRLWAFCC | 6 | 53% beta sheet (3 strands; 16 residues) | 30 | Homo sapiens | 3GNY |
| α-defensin 4 | VCSCRLVFCR RTELRVGNCL IGGVSFTYCC TRV | 6 | 57% beta sheet (3 strands; 19 residues) | 33 | Homo sapiens | 1ZMM |
| α-defensin 5 | ATCYCRTGRC ATRESLSGVC EISGRLYRLC CR | 6 | 59% beta sheet (3 strands; 19 residues) | 32 | Homo sapiens | 1ZMP |
| α-defensin 6 | AFTCHCRRSC YSTEYSYGTC TVMGINHRFC CL | 6 | 65% beta sheet (3 strands; 21 residues) | 32 | Homo sapiens | 1ZMQ |
| β-defensin 1 | DHYNCVSSGG QCLYSACPIF TKIQGTCYRG KAKCCK | 6 | 16% helical (1 helices; 6 residues) | 36 | Homo sapiens | 1IJU |
| 33% beta sheet (3 strands; 12 residues) | ||||||
| β-defensin 2 | GIGDPVTCLK SGAICHPVFC PRRYKQIGTC GLPGTKCCKK P | 6 | 14% helical (1 helices; 6 residues) | 41 | Homo sapiens | 1FD3 |
| 31% beta sheet (5 strands; 13 residues) | ||||||
| β-defensin 3 | GIINTLQKYY CRVRGGRCAV LSCLPKEEQI GKCSTRGRKC CRRKK | 6 | 11% helical (1 helices; 5 residues) | 45 | Homo sapiens | 1KJ6 |
| 28% beta sheet (3 strands; 13 residues) | ||||||
| β-defensin 4 | EFELDRICGY GTARCRKKCR SQEYRIGRCP NTYACCLRKW DES | 6 | 6% helical (1 helices; 3 residues) | 43 | Homo sapiens | 5KI9 |
| 39% beta sheet (4 strands; 17 residues) | ||||||
| Antifungal heliomicin | DKLIGSCVWG AVNYTSDCNG ECKRRGYKGG HCGSFANVNC WCET | 6 | 15% helical (1 helices; 7 residues) | 44 | Heliothis virescens | 1I2U |
| 29% beta sheet (4 strands; 13 residues) | ||||||
| Defensin-like peptide-2 | IMFFEMQACW SHSGVCRDKS ERNCKPMAWT YCENRNQKCC EY | 6 | 9% helical (1 helices; 4 residues) | 42 | Ornithorhynchus anatinus | 1D6B |
| 21% beta sheet (3 strands; 9 residues) | ||||||
| Sugarcane defensin 5 | HTPTPTPICK SRSHEYKGRC IQDMDCNAAC VKESESYTGG FCNGRPPFKQ CFCTKPCKRE RAAATLRWPG L | 8 | 15% helical (1 helices; 11 residues) | 71 | Saccarum officinarum | 2KSK |
| 21% beta sheet (3 strands; 15 residues) |
Date were collected and referred from RCSB PDB (Research Collaborative for Structural Bioinformatics Protein Data Bank) (Website: https://www.rcsb.org).
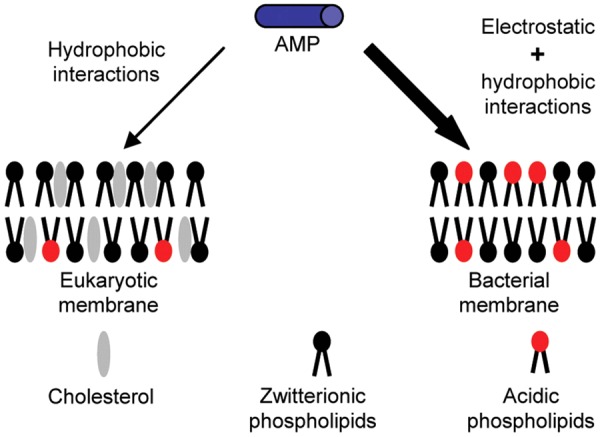
The amphiphilic peptide molecules are α-helices with hydrophobic and hydrophilic halves and display their amphiphilicity while interacting with bacterial cell membranes. These peptides fold into amphipathic α-helices with both hydrophilic and hydrophobic sides when adsorbed to the bilayer lipid membranes [2,10]. These positively charged AMPs interact with negatively charged cell membranes through electrostatic interactions and undergo membrane adsorption and conformational change. Peptides bind to the membrane surfaces with their hydrophobic sides anchored in the hydrophobic lipid core of the bilayer (Figures 3, 4) [10,11]. These peptides at their N-terminal ends are rich in basic amino acids with strong alkaline, and they at their C-terminal ends are amidated with C-terminal neutral hydrophobicity. The number of the cationic net charges of these peptides is related to the antibacterial activity, and their hydrophobicity is consistent with the hemolytic activity. There are multiple mechanism models to explain the action of these peptides, including the toroidal pore model, the barrel-stave model, the carpet model and so on (discussion below) [11,12]. Nowadays, there are several thousands of various AMPs that are natural or synthetic. These AMPs are small peptides and are water-soluble with a net positive charge and membrane activity. They are stable with extension of half-life once chemically modified. Some synthetic peptides can stabilize and keep biological activity at high temperature, and some can resist the hydrolysis of trypsin and pepsin [13]. AMPs are the important components of innate immunity. They can resist the invasion of foreign microorganisms and have broader spectrum antibacterial properties compared to the traditional antibiotics [5,11].
Figure 3.
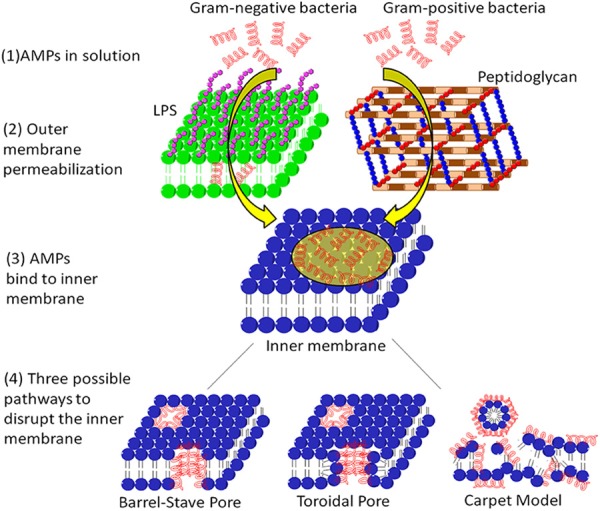
The schematic structures of the eukaryotic membrane and bacterial membrane, cited from Dr. Schmidt-wolf [12].
Figure 4.
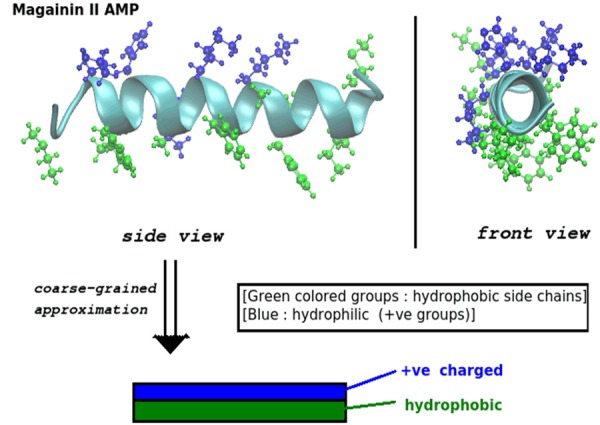
The schematic helical structures of antimicrobial peptides (AMPs) that are helical and amphiphilic with hydrophobic side (green) and hydrophilic side (positive charged groups) (blue), cited from Dr. Tew [11].
The advantages of antimicrobial peptides
The conventional antibiotics mainly target bacteria with their antibacterial activity. The long-term and frequent use of them can lead to bacterial mutation and bacterial drug resistance that has already been reported to result in serious healthy problem all over the world [2,14]. Finding a new antibiotic become much more difficult than before. The cationic AMPs are a type of peptides. Different from traditional antibiotics, these AMPs interact with the bacterial cell membranes (Figure 5) via neutralizing the charge, and further penetrate through bacterial membranes and cause bacterial death, reducing the possibility of bacterial drug resistance [11,13]. Moreover, these peptides are more efficient than traditional antibiotics. They display their advantages over the conventional antibiotics with the broad-spectrum antibacterial, antifungal and antivirus activities [13,15]. They are also potent with rapid germ-killing ability and low bactericidal concentration, even effective on traditional antibiotic-resistant strains, and even have synergistic effects with typical antibiotics to neutralize endotoxin [2,13]. Furthermore, these AMPs are safe with no toxic side effects or less, and hard to induce bacterial drug resistance compared to the conventional antibiotics [14].
Figure 5.
The schematic modes and processes of antimicrobial peptides interacting with Gram-positive and Gram-negative bacterial membrane, including barrel stave pore, toroidal pore and carpet model, cited from Dr. Beuerman [30].
Besides their broad spectrum of antimicrobial activities, they have good thermal stability and good water solubility [16-18]. They are small molecules with low synthetic cost, simple structure-activity relationship, and weak or low sensitization [18,19]. They can be widely used in medicine development. For instance, daptomycin, one of AMPs, has been approved and marketed in 2003 as an anionic antibacterial peptide to treat skin infections caused by Gram-positive bacteria (Table 3). This peptide even showed their inhibitory effects on high drug-resistant Typhoid bacillus and Staphylococcus aureus. Moreover, these peptides displayed their inhibitory ability to cancer cells [13,20]. Many studies have demonstrated that cancer cells are more sensitive to AMPs than normal cells. The cytoskeleton of cancer cells is not well developed in comparison with that of normal cells. The cationic AMPs are associated with the high acidic phospholipids on the outer surfaces of these cancer cells. The high metabolism in cancer cells causes the potential changes in membrane, cytoskeleton or extracellular matrix of cancer cells [13,20-22]. These peptides easily insert into the lipid membranes and form ion channels or pores to eventually destroy the cancer cells or result in leakage of cell contents (Figure 3) [12,13,21]. These membrane-permeabilizing AMPs represent a potential new therapy against drug-resistant microbes that result in more morbidity and mortality, and may be clinically applied as a strategy to overcome the frequent resistance of many common microbes to conventional antibiotics.
Table 3.
The antimicrobial peptide drugs approved by Food and Drug Administration (FDA)
| Drugs | Trade names | Antimicrobial activities | Administrations | In use |
|---|---|---|---|---|
| Bacitracin | Baciim | Gram-positive bacteria | Topical | Localized skin and eye infections, wound infections |
| Dalbavancin | Dalvance, Xydalba | Gram-positive bacteria | Intravenous | Acute bacterial skin infections |
| Daptomycin | Cubicin | Gram-positive bacteria | Intravenous | Bacterial skin infections |
| Enfuvirtide | Fuzeon | Virus | Subcutaneous | HIV-1 infection |
| Oritavancin | Orbactiv | Gram-positive bacteria | Intravenous | Bacterial skin infections |
| Teicoplanin | Targocid | Gram-positive bacteria | Intravenous & intramuscular | Bacterial infections |
| Telaprevir | Incivo, Incivek | Virus | Oral | Hepatitis C |
| Telavancin | Vibativ | Gram-positive bacteria | Intravenous | Bacterial skin infections |
| Vancomycin | Vancocin | Gram-positive bacteria | Oral & intravenous | Bacterial infections |
The classification of antimicrobial peptides
There are thousands of AMPs discovered until now. These peptides can be classified to different groups based on the different amino acid components, structures and biological functions of these peptides. Reportedly, AMPs are classified into two major antimicrobial types according to the amino acid composition and peptide structures [2,3]. One subfamily consists of linear molecules, with a α-helical structure and no cysteine, such as cecropin, magainin, or rich in certain amino acids, such as proline, glycine, arginine, histidine and tryptophan. Another subfamily consists of cysteine-containing polypeptides that form disulfide bridge(s) such as insect defensin. These AMPs can then be separated into subfamilies with single disulfide structure or multiple disulfide structures [2,4,23]. To some extents, a disulfide bridge is necessary for antimicrobial activity of these peptides.
As for the AMPs derived from mammals, they are classified into two subfamilies cathelicidins and defensins according to their structure and biological characteristics (Tables 1, 2) [3,7]. The cathelicidin family such as indolicidin share similar cathelin functional domains. They are an important subfamily of mammal AMPs and have biological activities against bacteria, viruses and fungi. These peptides are potential drugs in the research and development of novel peptide drugs. The peptide LL37, one of the mammal AMPs, is an endogenous antibiotic and the only one of the cathelicidin family discovered in human beings (Figure 1) [3,7,24]. The defensin family such as α-defensin 1 and β-defensin 2 are small cationic peptides rich in conserved cysteine residues. On the other hand, according to structural characteristics, the cationic AMPs can be divided into α-helical peptides and β-sheet peptides (Tables 1, 2) [2,23]. There are a number of α-helical peptides that can bind to lipopolysaccharides, such as the lipopolysaccharide-binding protein CAP18. Compared with the α-helical peptides, the β-sheet peptides have more complicated structures. The β-sheet degrees are extremely different among the different peptides. Moreover, many peptides contain both α-helix and β-sheet structures. The β-sheet peptides mainly include plant defensins, mammal α defensins and β defensins, insect defensins, proline-rich antibacterial peptides, protegrin and tachyplins [2,23]. Besides the peptides containing α-helix and β-sheet, there are many more other AMPs that are poorly investigated (Figure 2). Although most of AMPs are cationic peptides, some of them are anionic peptides or non-cationic peptides such as enkephalin A [25].
The mechanisms of action of antimicrobial peptides
Generally, natural antimicrobial peptides (AMPs) are not stable with a short half-life. Thus, it is necessary and important to modify and synthesize the long-acting peptide analogs for potential clinical applications. To design new antimicrobial peptides need to be considered for the bilayer lipid membranes and how to destabilize the permeability barriers. Understanding AMPs and their mechanisms of action will gain insight into the strategy to design the new-generation synthetic and efficacious antimicrobial peptides (Figure 5) [26,27]. Some major functions of antimicrobial peptides are dependent on their interfacial activity, not on their specific amino acid components or their three-dimensional structures [13]. The interfacial properties and the physical-chemical interactions are the critical factors to determine the biological activities of these peptides with the membrane-destabilizing and membrane-permeabilizing abilities [11,15]. The interfacial activities of these peptides are up to the balance of physical-chemical interactions among peptides, bilayer lipid membranes and water microenvironment [2,27]. The interfacial activities allow these peptides to partition into the membrane-water interfaces and further to change the lipid structures. AMPs can exert antimicrobial effects without harming normal cells likely due to the positive charge(s) on the α-helix surface of AMPs can interact with negatively charged membranes of microbes, while the membranes of eukaryotic cells are composed of uncharged neutral phospholipids, sphingomyelins and cholesterol (Figures 4, 5). Thus, the interactions of AMPs with anions on the surface of microbial membranes play critical roles in destroying microbes. The amino acid composition of AMPs determines their positive/negative charges, amphiphilic and hydrophobic properties. And these properties show their important effects on the selective action to microbes [2,7].
Although many studies have reported the putative mechanisms of action of cationic AMPs, there is currently no one theory or mode that can be applied to explain the mechanism of all cationic AMPs. Moreover, most of the current studies are based on artificial lipid membranes. The composition and structure of these artificial membranes are different from bacterial cell membranes, the growth environment of microbes are also different from the experimental conditions [11]. Thus, the results from these experiments cannot truly explain the action of AMPs in microbes. The mechanisms of action of AMPs are different from antibiotics. Presently, there are various hypothetical mechanisms of action of these peptides, including the cell membrane damage, intracellular bactericidal mechanism, the inhibition of the synthesis of macromolecules, the damage of the organelles to cause DNA fragmentation, the inhibition of enzyme activity, and antimicrobial effect via participating in immune regulation (Figure 3) [2,11]. Among them, the interaction between cationic AMPs and cell membranes, or membrane permeability, is believed to be one of the potential and recognized mechanisms.
Most AMPs act on microbes via increasing cytoplasmic membrane permeability. In contrast to mammalian cells, these peptides preferentially target microbes, mainly resulting from the difference of membrane composition [28]. The membranes of many microorganisms contain negatively charged lipid groups such as phosphatidylglycerol, cardiolipin, whereas mammalian cell membranes are neutral at net charge and generally rich in phosphatidylethanolamine, phosphatidylcholine (Figure 5) [2,29]. To explain the action of AMPs, scientists have proposed several hypothesial models of pore formation, such as the concave barrel model, the circular model, the wormhole model, and the blanket model. The first step in the mechanism of membrane permeability is the electrostatic interaction of the positively charged AMPs with the surfaces of the negatively charged microbial membranes. Subsequent membrane damage caused by the formation of pores within the microbial membranes ultimately leads to the death of microbes caused by the leakage of ion, metabolites, and biosynthesis, and the blockage of membrane-coupled respiration (Figure 3) [2,13,24].
The mechanism of cell membrane damage
The membrane permeability is mostly recognized as the well-accepted mechanism to describe the action of cationic AMPs. These cationic AMPs generally have membrane-binding activity. They destroy membrane structures of bacteria or cancer cells, resulting in the massive exudation of cell contents and ultimately leading to the death of bacteria or cancer cells [2,22,28,30]. The cationic AMPs can bind to the outer structures of the cell membranes by the interaction among positive charges and negative charges. The extracellular membrane of Gram-negative bacteria contains a negatively charged lipopolysaccharide (LPS). The cationic AMPs can replace the divalent cations such as Mg2+ and Ca2+ bound to LPS, cause a breakage or a cavity on the outer membranes of bacteria and eventually go through extracellular membranes. The cationic AMPs pass through the outer membranes and bind to the negatively charged phospholipids on the inner membranes of the cells combined by electrostatic attraction, causing the formation of a cavity or a temporary passage on the cell membranes, thereby resulting in the disintegration or permeability of cell membranes, and eventually causing the contents of the bacteria to overflow, microbial body lysis and death (Figure 3) [7,11,24].
The mechanism of cell membrane damage is generally involved with two steps. First, the positively charged AMPs selectively bind onto the surface of the negatively charged bacterial cell membranes, and then destroy bacterial membranes by the putative perforation or non-perforation mode [28,31]. The membrane perforation mode can be classified into four hypothesial models including the barrel-stave model, the carpet model, the toroidal-pore model and the aggregated channel model [12,32,33]. As for the non-perforation mode, it predicts that AMPs bind to the surface of the bacterial cell membranes to cause the cell death by disrupting the normal physiological functions of the cells, such as DNA replication, RNA transcription, or protein synthesis [7,31].
The action models of cell membrane damage
To give insight into understanding of the action mechanism of antimicrobial peptides, scientists hypothesized different models including carpet model, toroidal pore model, barrel stave model, sinking raft model, molecular electroporation model, induced lateral phase separation and formation of reversed micelles (Figure 3) [12,28]. Most antimicrobial peptides are amphiphilic and positively charged with net charges of +2 to +9, they have both hydrophilic and hydrophobic parts. Peptides permeabilize membranes and result in the formation of pores or ion channels based on their interfacial activity and behave differently than peptides that assemble into water-filled channels across membranes. Positively charged cationic AMPs interact with negatively charged cell membranes through electrostatic interactions and undergo membrane adsorption and conformational change. Following binding of peptides to the cell membrane, these peptides can complete their activity through different mechanisms such as the barrel stave model, the carpet model, the toroidal pore model, the aggregated channel model, and the sinking raft model [12,13,32,33]. Depending on the different mechanisms of action of peptides, the targeted cells will die by apoptosis or necrosis. The barrel-stave model predicts that the AMPs bind to the surface of the cell membrane, their hydrophobic groups are embedded inside the cell membranes to form a pore structure, which result in the bacterial cell contents to overflow and bacteria to die. In the carpet model, AMPs changes the surface tension of the bacterial membranes to deform the membranes, eventually lead to the disintegration of the cell membranes [22,32]. Peptide Aurein belongs to AMPs with this mechanism of action. The third model is the toroidal-pore model, in which the AMPs aggregate, insert inside the cell membranes, and induce change of the bacterial phospholipid monolayer until a ring hole of 1 to 2 nm in diameter is formed, ultimately resulting in bacterial death. The last model is called the aggregated channel model. In this model, AMPs are hypothesized to bind to the phospholipid molecules on the surface of the cell membranes, form the peptide-lipid polymers and eventually get into the cells, thereby resulting in bacterial death (Figure 3). As shown in Figure 3, there are different action models of mechanisms of membrane permeability [12,33].
The intracellular bactericidal mechanism
As well known, many studies have shown that some AMPs can penetrate the bacterial cell membranes into the cytoplasm, affect the biochemical process of the cells, and thereby function in suppressing bacteria and other microbes. Certain AMPs show their inhibitory effects on Gram-negative and -positive bacteria via not only destroying bacterial cell membranes and subsequently leading to the cell death, but also entering the cytoplasm and binding to DNA, disturbing bacterial physiological activity [4,23,26]. These AMPs are more active and potent with these dual mechanisms of action.
The bacteriostatic mechanism via participating in immune regulation
Some AMPs directly target and destroy bacteria. However, certain others may display their antimicrobial activity via participating in immune modulatory effects [34]. The latters are involved in immunomodulation mainly in other different ways, such as reducing endotoxin-induced inflammatory response, inducing synthesis of pro-inflammatory factors, adjusting adaptive immunity, or inducing secretion of cytokines and subsequently recruiting macrophages to exert immune modulatory effects [4,23,34]. These peptides can enhance the body’s anti-infective ability although they do not directly result in bacterial death.
Mitochondrial attack
As reported, some of the cationic AMPs interact with fungal organelles such as mitochondria, eventually leading to fungal death. Certain cationic AMPs rich in such amino acids as histidine display their strong antifungal activities. Peptides enter into the fungal cells via binding to the membrane receptors or trans-membrane potentials. These peptides interact with intracellular mitochondria, lead to ATP efflux without cell lysis, block mitochondrial respiration and the oxidation of phospholipids and macro-molecules, eventually lead to the damage of mitochondrial membrane and plasma membrane, and trigger nucleotides efflux and cell death [35,36].
The other mechanisms of action
Among various hypothesized mechanisms of cationic AMPs, membrane damage and permeability is thought to be the most important mechanisms of action of cationic AMPs. However, the same cationic peptides may also play a role under different mechanisms of action. Some cationic AMPs penetrate cells and affect the cellular physiological processes without the permeability of bacterial membranes [4,27]. Thus, besides the action mechanisms mentioned above, scientists also proposed other different mechanisms of AMPs such as the attack of DNA and RNA, the inhibition of the synthesis of protein and cell wall [26]. These peptides penetrate the bacterial membranes, accumulate inside bacteria and then block bacterial functions and induce cell death via interacting with intracellular DNAs and RNAs. The antimicrobial function of these cationic AMPs is mainly to target DNAs and induce DNA damage. Some AMPs such as β-defensin can inhibit the synthesis of protein and cell wall and block the formation of bacterial cell walls, resulting in morphological change of bacteria and further blocking cell growth [4,27]. Cell walls are eventually perforated, leading to bacterial death. This results in outflow of bacterial contents. On the other hand, AMPs can interact with protein macromolecules related to bacterial DNA replication, inhibit DNA replication, and eventually play their bactericidal roles.
The biological functions of antimicrobial peptides
Hundreds of AMPs have been identified to exist in human, animals, plants, bacteria and fungi. These peptides (host defense peptides) act as the first line of defense against microbes, indicating their importance in the innate immune system. Meanwhile, AMPs have a broad spectrum of biological activities including antibacteria, antifungi, antivirus, and anticancer [2,6,14]. These peptides are the important molecules for host cell congenital immunity and are involved in the immune defense systems of human, animals and plants. They play major roles in innate immune defense, chemokine induction, chemotaxis, imflammation and wound healing. They are also capable of enhancing phagocytosis, stimulating prostaglandin release, neutralizing the septic effects of LPS, promoting recruitment and accumulation of various immune cells at inflammatory sites, increasing angiogenesis, and inducing wound repair. Peptides of mammalian origin have also been demonstrated to have an active role in the transition to the adaptive immune response by being chemotactic for human monocytes and T cells, by exhibiting adjuvant and polarizing effects in influencing dendritic cell development. Although such peptides may have a direct effect on the microbes by damaging or destabilizing the bacterial, viral, or fungal membranes, or acting on other targets, they appear to be broadly involved in the orchestration of the innate immune and inflammatory responses [2,4,13]. With their variety of functions, AMPs have displayed broad prospects for clinical applications.
Cytotoxicity
In the cancer treatments, the traditional chemotherapeutic drugs can not tell cancer cells from normal cells and simultaneously kill both of them, resulting in severe side effects. The cationic AMPs can specifically target certain cancer cells and inhibit the growth of these cancer cells while they are not harmful to normal cells [14,21,25]. Most likely, these peptides are potential new anticancer drugs with no or low toxic side effects. These cationic peptides mainly affect the survival of cancer cells via targeting cell membrane, organelle (mainly mitochondria), lysosome, nucleus, chromosomal DNA, and cytoskeleton. In many studies, it has been demonstrated that cancer cells are more sensitive to AMPs than normal cells [13,14,21]. These peptides are associated with the high acidic phospholipids on the outer surfaces of these cancer cells. The high metabolism in cancer cells causes potential changes in cell membrane and the changes in the cytoskeleton or extracellular matrix of cancer cells. The cytoskeleton is not well developed in cancer cells compared to that in normal cells.
Natural immunity
The cationic AMPs play important roles in the natural immunity of the hosts. They not only kill the pathogenic microorganisms that invade the human body, but also show their multiple functions at different stages of the natural immune responses [4,34]. These peptides can stimulate the proliferation of cells including fibroblasts, lymphocytes and vascular endothelial cells. They also promote the growth of wound granulation tissue and enhance wound healing [13,37]. These cationic AMPs are involved in the host defenses associated with acute inflammation. They can induce bacterial lysis, promote phagocytosis of macrophage, prevent infection spreading, stimulate mitosis of fibroblasts and epithelial cells, and promote fibroblast growth to enhance wound healing [4,27,37]. They can activate human lymphocytes to eliminate the cells infected with viruses and bacteria, and the cancer cells. These AMPs also play a role in chronic inflammation. They promote the proliferation of helper T cells and the production of chemokine in these T cells, increase the levels of antibody IgG inside body, promote apoptosis of macrophages, and activate lymphocytes to clear infected cells [34,37,38].
The broad spectrum of antimicrobial activities
AMPs usually form a helix structure, act through the bacterial cell membrane, form ion channels or pores on the microbial membranes, leading to membrane permeability and causing leakage of intracellular substances to result in bacterial death. As well-known, the cationic AMPs display their wider antibacterial spectrum compared to the traditional antibiotics [15]. The traditional antibiotics are usually effective on bacteria. These cationic peptides are against Gram-positive and -negative bacteria, as well as the pathogens fungi and viruses [1,2,13]. Besides these antibacterial activities, different cationic AMPs can be used in combination with traditional antibiotics to improve the therapeutic effects of each, and even broaden the antibacterial spectrum of traditional antibiotics.
Some cationic AMPs displayed their antiviral effects as well. Certain studies identified the obvious inhibitory effects of AMPs on various DNA and RNA viruses including HIV, influenza virus, herpes virus, and hepatitis B virus. AMPs have been found to play their antiviral roles in different manners [4,7].
One is that the peptides are against viruses via directly interacting with virions. The peptides also inhibit the proliferation of viruses, so to mimic the infectious process of the viruses. For example, peptides may result in the viral damage via interfering with the assembly process of the viruses. And also, these cationic peptides can effectively destroy parasites that lead to the parasitic diseases in human and animal, such as malaria, dysentery and other protozoa [7,9]. Many AMPs also displayed their antifungal activities in addition to their antibacterial activity.
For instance, the Cathelicidin family of antimicrobial peptides has a broad spectrum of antimicrobial activity for G+ and G-bacteria, fungi, mold, protozoa and some enveloped viruses [3,7]. Compared with other families such as defensins, the cathelicidins family antibacterial peptides have stronger antibacterial activity at minimum inhibitory concentration. In addition, the cathelicidin family antibacterial peptides have a rapid bactericidal effect. More importantly, some cathelicidins showed their strong effects on a large number of drug-resistant strains clinically isolated, even super-resistant bacteria. The cathelicidin family antibacterial peptides are mainly used for the anti-inflammatory, anti-infective and anti-fungal applications [3,7]. The cathelicidin family antibacterial peptide have a good development prospect in the local treatment of these diseases such as dermatitis, invasive burn sepsis.
The safety of antimicrobial peptides
There are certain problems or concerns of AMPs in clinical applications such as the toxicity, and immunogenicity, drug resistance, hemolytic activity and other side effects [4]. These peptides can exert enormous toxic side effects on mammalian cells in the long-term use [8]. Certain AMPs have also been reported for their hemolytic activity [8,14,19]. For instance, Indolicidin, a 13-residue short cationic peptide rich with tryptophan (Ile-Leu-Pro-Trp-Lys-Trp-Pro-Trp-Trp-Pro-Trp-Arg-Arg-NH), exhibits a broad spectrum of antimicrobial activity (gram-positive and gram-negative bacteria, fungi, viruses), but meanwhile, has hemolytic activity that limits its clinical application [9]. Although these peptides are small molecules and have no immunogenicity or less, the immunogenicity is still concerned and even is the serious problem in peptide drug development. And also, the pathogenic microbes are peptide-resistant after long-term use. Although they showed their strong antibacterial activity, many of these cationic AMPs are more or less toxic to human cells [19,21]. This is one of the reasons why they are rare to be used as drugs. Therefore, how to improve their activity and safety is highly concerned in AMP drug research and development. Scientists are attempting to find new cationic peptides, or modify the natural antimicrobial peptides in order to obtain more effective and safer antibacterial peptides as the potential drug candidates.
The challenge and prospective of antimicrobial peptides in clinical applications
There are more and more accumulated evidences to show that the long-term use of antibiotics result in drug resistance of microbes. Many drug-resistant pathogenic strains have been identified to correspond to each of these traditional antibiotics [15]. Thus, it is becoming much more difficult to find a new antibiotic. AMPs display their broad antimicrobial spectrum and high bactericidal activities [2]. The appearance of these peptides provides us a golden opportunity to develop the potential antimicrobial peptide drug candidates instead of traditional antibiotics [8]. The truth is that, several AMPs have been approved by Food and Drug Administration (FDA) (Table 3). Most of these peptides are usually very limited to be druggable for the clinical applications.
The mechanism of action and the relationship of structure-activity of these antimicrobial peptides are important for scientists to develop new cationic peptide drugs. To design the synthetic cationic AMPs, the unique characteristics of these peptides or cationicity and amphipathicity have to be considered although cationic AMPs exhibit diversity in sequence and structure [4]. The cationicity determines whether AMPs can selectively bind to the outer surface of the negatively charged bacterial cell membranes without interacting with the outer surface of the neutral eukaryotic cell membranes. The amphipathicity determines whether AMP can insert into the bacterial cell membranes to form a hydrophobic channels or pores. Antimicrobial peptides truly play a role by disrupting the integrity of bacterial cell membranes. A difficult is that various microbial membranes have very different susceptibilities to membrane permeability resulting from individual peptides. And antimicrobial peptides may lead to hemolytic activity. It is a tough challenge to design novel and ideal peptides with potent antimicrobial efficacy and without hemolytic activity [4]. And natural AMPs are generally non-stable with a short half-life in circulation. To fix this, we can consider to design a new long-lasting peptide or to modify the natural peptides. The long-term use of AMPs may also lead to inhibition of cell growth, cytotoxicity of host cells and other side effects undisclosed. Finding an ideal peptide is a time-consuming, high expensive and less succeeded task although AMPs provide us a great opportunity and a promising future.
Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province (2007JJ6052), the New Xiangya Talent Projects of the Third Xiangya Hospital of Central South University (20170302), Xiangtan Institute of Industrial Technology Collaborative Innovation and Xiangtan Science and Technology Bureau.
Disclosure of conflict of interest
None.
References
- 1.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals (Basel) 2013;6:1543–75. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosciuczuk EM, Lisowski P, Jarczak J, Strzalkowska N, Jozwik A, Horbanczuk J, Krzyżewski J, Zwierzchowski L, Bagnicka E. Cathelicidins: family of antimicrobial peptides. A review. Mol Biol Rep. 2012;39:10957–70. doi: 10.1007/s11033-012-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moravej H, Moravej Z, Yazdanparast M, Heiat M, Mirhosseini A, Moosazadeh Moghaddam M, Mirnejad R. Antimicrobial peptides: features, action, and their resistance mechanisms in bacteria. Microb Drug Resist. 2018;24:747–67. doi: 10.1089/mdr.2017.0392. [DOI] [PubMed] [Google Scholar]
- 5.Rathinakumar R, Wimley WC. High-throughput discovery of broad-spectrum peptide antibiotics. FASEB J. 2010;24:3232–8. doi: 10.1096/fj.10-157040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau QY, Li J, Sani MA, Sinha S, Li Y, Ng FM, Kang C, Bhattacharjya S, Separovic F, Verma C, Chia CSB. Elucidating the bactericidal mechanism of action of the linear antimicrobial tetrapeptide BRBR-NH2. Biochim Biophys Acta Biomembr. 2018;1860:1517–27. doi: 10.1016/j.bbamem.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 8.Starr CG, Maderdrut JL, He J, Coy DH, Wimley WC. Pituitary adenylate cyclase-activating polypeptide is a potent broad-spectrum antimicrobial peptide: structure-activity relationships. Peptides. 2018;104:35–40. doi: 10.1016/j.peptides.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirski T, Niemcewicz M, Bartoszcze M, Gryko R, Michalski A. Utilisation of peptides against microbial infections - a review. Ann Agric Environ Med. 2017;25:205–10. doi: 10.26444/aaem/74471. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother. 2007;51:1398–406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Som A, Vemparala S, Ivanov I, Tew GN. Synthetic mimics of antimicrobial peptides. Biopolymers. 2008;90:83–93. doi: 10.1002/bip.20970. [DOI] [PubMed] [Google Scholar]
- 12.Jakel CE, Meschenmoser K, Kim Y, Weiher H, Schmidt-Wolf IG. Efficacy of a proapoptotic peptide towards cancer cells. In Vivo. 2012;26:419–26. [PubMed] [Google Scholar]
- 13.Mahlapuu M, Hakansson J, Ringstad L, Bjorn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6:194. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathinakumar R, Walkenhorst WF, Wimley WC. Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J Am Chem Soc. 2009;131:7609–17. doi: 10.1021/ja8093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amso Z, Hayouka Z. Antimicrobial random peptide cocktails: a new approach to fight pathogenic bacteria. Chem Commun (Camb) 2019;55:2007–14. doi: 10.1039/c8cc09961h. [DOI] [PubMed] [Google Scholar]
- 16.Dehsorkhi A, Castelletto V, Hamley IW. Self-assembling amphiphilic peptides. J Pept Sci. 2014;20:453–67. doi: 10.1002/psc.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Park SC, Kim MH, Lim HT, Park Y, Hahm KS. Antimicrobial activity studies on a trypsin-chymotrypsin protease inhibitor obtained from potato. Biochem Biophys Res Commun. 2005;330:921–7. doi: 10.1016/j.bbrc.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 18.Raguse TL, Porter EA, Weisblum B, Gellman SH. Structure-activity studies of 14-helical antimicrobial beta-peptides: probing the relationship between conformational stability and antimicrobial potency. J Am Chem Soc. 2002;124:12774–85. doi: 10.1021/ja0270423. [DOI] [PubMed] [Google Scholar]
- 19.Lee DL, Hodges RS. Structure-activity relationships of de novo designed cyclic antimicrobial peptides based on gramicidin S. Biopolymers. 2003;71:28–48. doi: 10.1002/bip.10374. [DOI] [PubMed] [Google Scholar]
- 20.Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625:190–4. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 21.Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides. 2008;29:963–8. doi: 10.1016/j.peptides.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Huang J, Chen Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell. 2010;1:143–52. doi: 10.1007/s13238-010-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haney EF, Straus SK, Hancock REW. Reassessing the host defense peptide landscape. Front Chem. 2019;7:43. doi: 10.3389/fchem.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Xun M, Han J. A bovine myeloid antimicrobial peptide (BMAP-28) and its analogs kill pan-drug-resistant acinetobacter baumannii by interacting with outer membrane protein A (OmpA) Medicine (Baltimore) 2018;97:e12832. doi: 10.1097/MD.0000000000012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DM, Jiao X, Plotnikoff NP, Griffin N, Qi RQ, Gao XH, Shan FP. Killing effect of methionine enkephalin on melanoma in vivo and in vitro. Oncol Rep. 2017;38:2132–40. doi: 10.3892/or.2017.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graf M, Wilson DN. Intracellular antimicrobial peptides targeting the protein synthesis machinery. Adv Exp Med Biol. 2019;1117:73–89. doi: 10.1007/978-981-13-3588-4_6. [DOI] [PubMed] [Google Scholar]
- 27.Aisenbrey C, Marquette A, Bechinger B. The mechanisms of action of cationic antimicrobial peptides refined by novel concepts from biophysical investigations. Adv Exp Med Biol. 2019;1117:33–64. doi: 10.1007/978-981-13-3588-4_4. [DOI] [PubMed] [Google Scholar]
- 28.Rathinakumar R, Wimley WC. Biomolecular engineering by combinatorial design and high-throughput screening: small, soluble peptides that permeabilize membranes. J Am Chem Soc. 2008;130:9849–58. doi: 10.1021/ja8017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi M, Noda Y, Aida R, Saito K, Ochiai A, Saitoh E, Tanaka T. Cationic peptides from enzymatic hydrolysates of soybean proteins exhibit LPS-neutralizing and angiogenic activities. J Biosci Bioeng. 2019;127:176–82. doi: 10.1016/j.jbiosc.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Koh JJ, Liu S, Lakshminarayanan R, Verma CS, Beuerman RW. Membrane active antimicrobial peptides: translating mechanistic insights to design. Front Neurosci. 2017;11:73. doi: 10.3389/fnins.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Driscoll NH, Cushnie TPT, Matthews KH, Lamb AJ. Colistin causes profound morphological alteration but minimal cytoplasmic membrane perforation in populations of Escherichia coli and Pseudomonas aeruginosa. Arch Microbiol. 2018;200:793–802. doi: 10.1007/s00203-018-1485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Hahm KS. Antimicrobial peptides (AMPs): peptide structure and mode of action. J Biochem Mol Biol. 2005;38:507–16. doi: 10.5483/bmbrep.2005.38.5.507. [DOI] [PubMed] [Google Scholar]
- 34.Zasloff M. Antimicrobial peptides of multicellular organisms: my perspective. Adv Exp Med Biol. 2019;1117:3–6. doi: 10.1007/978-981-13-3588-4_1. [DOI] [PubMed] [Google Scholar]
- 35.Dhir A, Dhir S, Borowski LS, Jimenez L, Teitell M, Rotig A, Crow YJ, Rice GI, Duffy D, Tamby C, Nojima T, Munnich A, Schiff M, de Almeida CR, Rehwinkel J, Dziembowski A, Szczesny RJ, Proudfoot NJ. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature. 2018;560:238–42. doi: 10.1038/s41586-018-0363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Liu H, Yang Y, Xu X, Lv T, Zhang H, Liu K, Zhang S, Chen Y. N-myristoylation of antimicrobial peptide CM4 enhances its anticancer activity by interacting with cell membrane and targeting mitochondria in breast cancer cells. Front Pharmacol. 2018;9:1297. doi: 10.3389/fphar.2018.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taniguchi M, Saito K, Aida R, Ochiai A, Saitoh E, Tanaka T. Wound healing activity and mechanism of action of antimicrobial and lipopolysaccharide-neutralizing peptides from enzymatic hydrolysates of rice bran proteins. J Biosci Bioeng. 2019 doi: 10.1016/j.jbiosc.2019.02.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, Chamilos G, Feldmeyer L, Marinari B, Chon S, Vence L, Riccieri V, Guillaume P, Navarini AA, Romero P, Costanzo A, Piccolella E, Gilliet M, Frasca L. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5:5621. doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]


