Abstract
Animals use cues to find their food, in microhabitats within their physiological tolerances. Termites build and modify their microhabitat, to transform hostile environments into benign ones, which raises questions about the relative importance of cues. Termites are desiccation intolerant and foraging termites are attracted to water, so most research has considered moisture to be a cue. However, termites can also transport water to food, and so moisture may play other roles than previously considered. To examine the role of moisture, we compared Coptotermes acinaciformis termite foraging decisions in laboratory experiments when they were offered dry and moist wood, with and without load. Without load, termites preferred moist wood and ate it without any building, whereas they moistened dry wood after wrapping it in a layer of clay. For the ‘With load’ units, termites substituted some of the wood for load-bearing clay walls, and kept the wood drier than on the unloaded units. As drier wood has higher compressive strength and higher rigidity, it allows more of the wood to be consumed. These results suggest that moisture plays a more important role in termite ecology than previously thought. Termites manipulate the moisture content according to the situational context and use it for multiple purposes: increased moisture levels soften the fibre, which facilitates foraging, yet keeping the wood dry provides higher structural stability against buckling which is especially important when foraging on wood under load.
Keywords: clay building, moisture, static stability, multifunctional material
1. Introduction
Termite nests are designed subterranean, epigeal or arboreal and of complex geometry and material composition [1,2]: chopped-up, pre-digested plant matter mixed with inorganic components into a clay–wood–faeces compound matrix serves as food reserve—within the inner layers, especially the carton material [3–6]. The colony within engineers favourable living conditions by controlling the mound's microclimate, its temperature, relative humidity (RH) and airflow—also a source of inspiration for carbon-neutral house designs [7–10].
Especially water in soils and within wood is attractive to termites [11]. Heavy rainfalls or flooding combined with elevated temperatures promote termite activity [7,12–17]. Yet, the role of moisture in clay and its relation to foraging is poorly understood; it might be interwoven with mechanisms, also responsible to trigger autonomous collective building behaviours including complex depositing, excavation and defence strategies [10,18–20]. To study this potential interrelation, we investigate the role of moist clay on building activities and foraging decisions of C. acinaciformis using bioassays and mechanical experiments. We characterize wood beams with regards to their moisture absorption and study termite foraging activities providing either wet or dry wood or a choice of wood of different tree species. We then run bioassays for wood ‘With load’ and ‘No load’, followed by compression tests, to examine the influence of moisture on foraging and load-bearing capacity.
2. Material and methods
(a). Collection and maintenance of termites
Termites of the mound building, Northern form of C. acinaciformis (Blattodea: Rhinotermitidae) were collected from three mounts near the Darwin Laboratory of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) in Berrimah (Northern Territory, 12°28'52″ S, 131°1'44″ E). Healthy workers and soldiers in their natural ratio (approx. 10 : 1) were isolated from soil and separated from the royal pair, lower instars and brood [21]. We placed 35 g of termites (approx. 8,140 individuals), two 8 × 12 cm slates of Eucalyptus (E.) regnans (119.3 ± 3.8 g) and water-soaked nest carton material into 2 l jars. We gave the termites a 3-day settling-in period to promote acclimatization to minimize transportation damage [21]. The experiments were set up in walk-in environmental chambers (28°C at 80% RH) at CSIRO (Canberra).
(b). Preparation of wood samples
We cut two types of geometries using silver poplar (Populous (Po.) alba), Monterey pine (Pinus (Pi.) radiata) and mountain ash (E. regnans). The wood used consisted only of heartwood sourced from building timber. The block geometry (for pilot tests) was 30 mm long with a 20 × 20 mm2 cross-sectional area (volume V = 12 cm3, n = 15 each wood type). From the timber used for the blocks, we also produced n = 67 beams of each species of length of 20 mm and a 5 × 5 mm2 cross-sectional area (V = 0.5 cm3). We measured the average dry weight (scale: AEA 250 g Adam Equipment Co. Ltd, Milton Keynes, four digits accuracy; oven: XU490 France Etuves, 4 kW, 16 h at 105°C), the humidity absorption from air (environmental chamber: Challenge 600, ACS, Massa Martana; 80 ± 3% RH at 28°C), and the moisture content after soaking (8 h at 21°C). The beams were clustered with respect to their densities and average moisture absorption [22,23].1
(c). Wood reduction under the influence of moisture, the application of clay or load
(i). Pilot
Initially, we studied the clay wrapping behaviour of termites. We used oven-dried and cooled down wood blocks (n = 15), and placed them into the centre of termite jars. We also took soil samples from the jar to measure their humidity absorption through weighing. We checked on the blocks on a daily basis for 60 days and weighed each block after removing any clay attached.
(ii). Main experiment
Then, the pressure trial experiment described in [10] with ‘No load’ (control) or ‘With load’ (treatment) units, was extended in its complexity by using beams of different wood species in a tripod configuration: Po. alba, Pi. radiata and E. regnans which had different densities and moisture absorption coefficients and compressive strengths [23]. Only using three ‘legs’ made of different wood species enabled accurate determination of the load and the foraging preferences. We finished the experiment in two weeks, so that some beams would be still intact, cf. [10]; the small size beams were quickly saturated with water (electronic supplementary material, figure S1).
All beams were sandwiched between concrete pavers, which were separated by water-filled trays and connected via tubing to termite jars (electronic supplementary material, figure S2) [10]. Termites had access to ground carton material and riverbank sand in PVC tubing. Only the beams on the ‘With load’ units carried deadweight of approximately 6.85 kN (60 l water, 10 kg pavers), with gravity acting collinearly to the grain; 21.5 mm long metal spacers carried all load on the ‘No load’ units [10].
Termites substitute loaded wood with clay [10]. To test whether termites not only build more clay on the ‘With load’ units for static stability but also moisten the beams less to increase its structural stability [10,24], we recorded the beams' moisture levels, their mass reduction and the amount of built up clay [10]. Only upright-standing and contacted beams (traces of clay) with no visible fungus were counted as valid samples.
We determined the compressive strengths of 30 random, oven-dried and moistened beams of each wood type using quasi-static compression tests by recording the change in displacement against the force applied [24,25]. Finally, we tested the partially damaged beams taken from the bioassays for their compressive strength. We fitted all beams with the grain collinearly to the force into an adaptor mounted to a compression-testing machine (120 K, JJ Lloyd Instruments, Ametek; 5 kN load cell: Instron IN2519-5 kN, Norwood; laser extensometer, EIR, Model LE-05; 100 Hz, 0.083 mm s−1). In all compression tests, a clear slip plane due to shear mode failure, determined the validity of a test, electronic supplementary material, figure S3. Shear mode failure is the prevalent failure mode in compression tests in grain direction; in our experiments, this was also associated with initial crushing followed by end rolling. If the sample slipped or split during compression, it was assumed that either the end planes were not perfectly parallel or that the sample had a prior weakness at the splitting point caused by an imperfect manufacturing process or grown-in weakness.
3. Results
(a). Pilot
We observed that termites always applied a thick layer of wet clay to all free surfaces of the blocks during an exploration stage (cf. [10]) only displaying nibbling or superficial ‘grazing’ (electronic supplementary material, figure S4). Within these first 18 ± 4 days, we measured increasing wood weights of up to 46.1 ± 18.2%, dominated by moisture absorption (electronic supplementary material, figures S5 and S6).
(b). Main experiment
A significantly larger average amount of solidly built clay per unit volume (Fisher's t-test, n = 13, d.f. = 25, F = 22.84, p < 0.001; electronic supplementary material, figure S7) was found in the ‘With load’ units (57.98 ± 69.35 g) compared to the ‘No load’ units (22.00 ± 15.70 g) which had at most brittle clay sheeting applied.
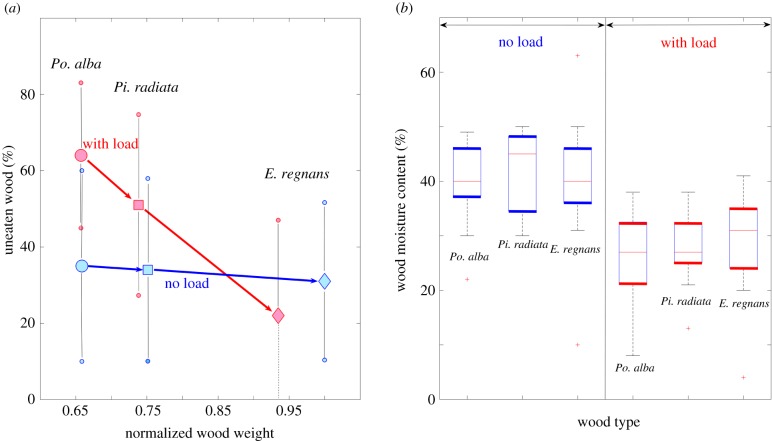
The average relative amount of uneaten wood on the ‘No load’ units was 35 ± 25% (Po. alba), 34 ± 24% (Pi. radiata) and 31 ± 21% (E. regnans); the ‘With load’ units had on average 64 ± 19%, 51 ± 33% and 22 ± 25% left-over (figure 1a). These percentages of uneaten wood are inversely proportional to the average mass reduction, which was found to be 0.12 ± 0.05 g, 0.15 ± 0.06 g and 0.2 ± 0.06 g for the ‘No load’ units and 0.07 ± 0.04 g, 0.12 ± 0.08 g and 0.23 ± 0.07 g for the ‘With load’ units. We applied Fisher's t-test to separate groups of wood and showed that only the wood reduction of the ‘With load’ and ‘No load’ units of Po. alba was significantly different (α = 0.05, Po. alba: d.f. = 25, F = 10.62, p = 0.0033; Pi. radiata: d.f. = 25, F = 1.43, p = 0.2442; E. regnans: d.f. = 25, F = 1.07, p = 0.3116).
Figure 1.
Termites eat on both sides but loaded wood is drier. (a) Mean uneaten wood for the ‘No load’ and the ‘With load’ units against normalized initial beam weight. Large markers indicate the mean value, small markers the standard deviation; arrows show the difference between wood species. (b) Box plots of moisture content for ‘No load’ and ‘With load’ units (n = 13). (Online version in colour.)
The moisture content of the wood species was significantly different between the ‘No Load’ units (Po. alba: 42.15 ± 12.57%, Pi. radiata: 42.00 ± 7.48%, E. regnans: 40.00 ± 12.07%) and the ‘With load’ units (Po. alba: 25.92 ± 8.41%, Pi. radiata: 27.38 ± 6.21%, E. regnans: 31.61 ± 16.51%) as confirmed by Fisher's t-test (α = 0.05, d.f. = 179, F = 62.52, p = 2.67 × 10−13). The moisture differences between the different wood species were also significant (α = 0.05, Po. alba: d.f. = 25, F = 14.97, p = 0.007; Pi. radiata: d.f. = 25, F = 29.36, p = 1.45 × 10−5; E. regnans: d.f. = 25, F = 6.91, p = 0.0147).
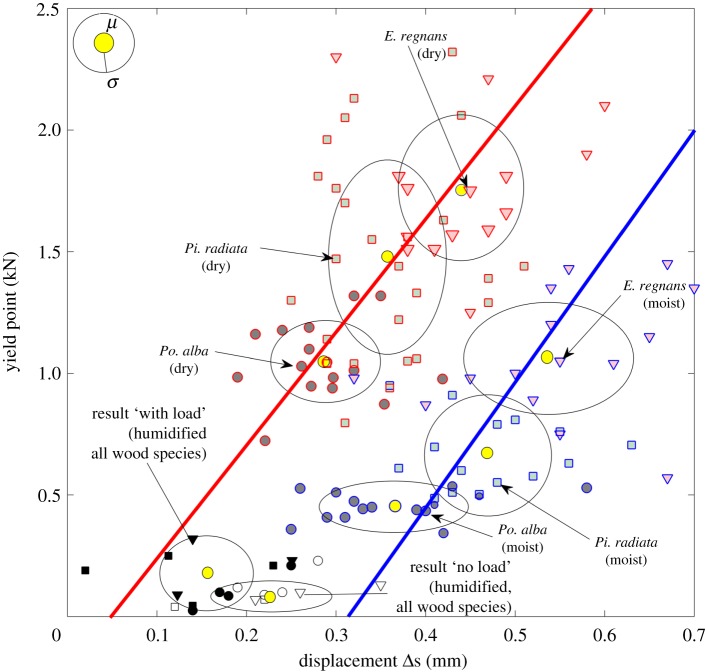
We then studied the beams for their compressive strength (figure 2). Across all wood species, the moistened beams had about half the compressive strength of the dry samples. The yield point of E. regnans was the highest, whereas Po. alba took the least load [26]. Applying linear regression provided a corridor of near parallel lines. The compression tests for beams of the pressure trial bioassay used median moisture levels (figure 1b). We had in total (nWith load, nNo load) = (4,4), (4,2) and (3,3) samples for Po. alba, Pi. radiata and E. regnans, respectively (figure 2). Their average yield points and distances for the ‘No load’ and the ‘With load’ units lie between the undamaged dry (red line) and moist (blue line) beams in figure 2. The average maximum load was about 0.08 ± 0.06 kN (approx. 13.3 ± 10 MPa, approx. 76% cross-section) for the ‘No load’ and 0.21 ± 0.10 kN (approx. 16.8 ± 8.0 MPa, approx. 51% cross-section) for the ‘With load’ units.
Figure 2.
Stability of beams. Results of yield point compression tests on Po. alba, Pi. radiata and E. regnans beams for dry (red) and moist (blue) conditions. The ellipses' minor and major radii indicate the standard deviations (σ) of the displacement and the yielding point relative to their mean values (μ). Results of compression tests of moistened beams of ‘With load’ units (markers, filled black) and ‘No load’ units (blank markers) are both found within the corridor. (Online version in colour.)
4. Discussion
We studied how the application of moisture correlates with feeding and building. Termites are endangered by threats, e.g. by swift spreading of diseases or sporulating fungal pathogens [27,28]. Termite defences make use of antibacterial and spore germination inhibiting faecal pellets [29,30]. Their composition consists of moist clay sands, faeces and saliva; pellets of that kind are also used to cement-in and seal off dead nest-mates or as building material thereby impeding the spread of epizootics [31,32]. The application of a clay–faeces composite to a foreign body triggers building and—similar to fighting pathogens—also acts as a passive defence mechanism against intruders and provides shelter against desiccation.
Once the moist clay is applied its role becomes ambivalent. Cellulose is difficult to digest [3,33] yet termites use their mouthparts and a symbiotic relationship with their gut microorganisms to break up the wood fibres and to extract the energy contained which requires moisture [3,34–37]. Moisture levels are likely to be altered to increase the ease of feeding. While subterranean termites require moisture from the soil with some species being able to transport water in their labial glands to the foraging site, drywood termites take the moisture for the digestive processes directly from the wood and the air [11,16,17,38].
The application of a clay–faeces compound layer acts like a softener and facilitates foraging using the mandibles; it may be used to control humidity to lower required cutting forces and to reduce the wear-rates of the mandibles. We observed that once the moisture levels of around 50% are reached, termites start foraging more extensively (electronic supplementary material, figures S3 and S5). Similarly, the highest foraging activity in C. formosanus has been found for moisture contents of 25–50% [16,17] and 79–103% (as compared to 6–12%) [38]. If the wood is too moist, termites start eating less as observed for 50% in Reticulitermes flavipes [39] or for C. formosanus for vacuum impregnated wood with moisture contents of 140–182% [38]. Termites consumed less of Po. alba compared to Pi. radiata, and even less compared with E. regnans, which was clearly the preferred wood type. While being denser, hence more energetic and also tested to be more compression-stable, E. regnans is known to be less prone to fungal attack than Po. alba, all of which contributing to the foraging preference of termites.
Comparing the wood reduction of the ‘No load’ units with the ‘With load’ units indicates that termites manipulated the wood with moisture levels up to 50% during an initial exploration stage; and that the ‘With load’ units are less moist, therefore able to carry more load (figure 1; electronic supplementary material, figure S5). While the paver underneath the water drum bottom covers 5.5% of the absorbing surface area of the ‘With load’ units, water saturation of these units due to humidity in the air was achieved in 24 h. We, therefore, consider it unlikely that this limitation of absorbing surface area for the ‘With load’ units plays a significant role.
We found on average 41% and 29% moisture levels for the ‘No load’ and ‘With load’ units respectively. The ‘With load’ units took on average per beam about 0.21 ± 0.10 kN compressive strength; hence, in terms of load, for the whole set-up this adds up to about 0.63 kN which is only slightly below the 0.713 kN static load of the ‘With load’ units. The exact point of time when the wood carried most of the load compared with the clay walls is difficult to determine due to natural variation of the dynamic process [10]. From the data available, however, we estimate the clay walls must have already carried on average at least 0.08 kN or about 11% of the static load.
The application of the clay–faeces compound within a dynamic foraging process [10] acts as pathogen control (clay wrapping) and termites moistened all three wood types similarly. Moisture levels, apart from being essential against dehydration of the termite colony, are likely to be altered to increase the ease of feeding. The moisture content was also consistently lower in the ‘With load’ units, which showed a moisture-dependent higher static stability (dry–buckling resistance) of the beams. Whether termites are able to sense stress in timber needs to be tested in future experiments.
In addition, termites employ vibration signals as their dominant means of communication [40]. Having control over the wood's moisture content especially using higher moisture levels may be preferred as they might dampen out chewing and walking noises thus increasing the chances of staying hidden from predators [41]; drier conditions might be advantageous for long-range communication [42] by controlling simultaneously shape (porosity, tunnels) and composition (clays, wood), which needs to be explored in future research.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Endnote
Average moisture and density: 11.86 ± 0.26%; 390 ± 26.95 kg m−3 (Po. alba), 12.89 ± 0.11%; 485.29 ± 67.43 kg m−3 (Pi. radiata) and 12.43 ± 0.07%; 585.70 ± 89.32 kg m−3 (E. regnans).
Data accessibility
The dataset is available from electronic supplementary material, S8.
Authors' contributions
S.O. conceived the idea, designed and performed the experiments, acquired, analysed and interpreted the data, wrote and revised the manuscript; M.L., contributed in discussions with regards to the feeding preferences of termites and the different wood types used; J.C.S.L. and T.A.E. contributed with ideas especially with regards to the initial experimental set-up, provided funding, materials and laboratory space. All authors revised the manuscript and agreed to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was supported under Australian Research Councils Discovery Projects funding scheme (project no. DP110102564).
References
- 1.Grassé PP. 1984. Termitologica tome 2: fondation des sociétiés, construction. Paris, France: Masson. [Google Scholar]
- 2.Lee TRC, Cameron SL, Evans TA, Ho SYW, Lo N. 2015. The origins and radiation of Australian Coptotermes termites: from rainforest to desert dwellers. Mol. Phylogenet. Evol. 82, 234–244. ( 10.1016/j.ympev.2014.09.026) [DOI] [PubMed] [Google Scholar]
- 3.Butler JHA, Buckerfield JC. 1979. Digestion of lignin by termites. Soil Biol. Biochem. 11, 507–513. ( 10.1016/0038-0717(79)90010-5) [DOI] [Google Scholar]
- 4.Jouquet P, Lepage M, Velde B. 2002. Termite soil preference and particle selections: strategies related to ecological requirements. Insect. Soc. 49, 1–7. ( 10.1007/s00040-002-8269-z) [DOI] [Google Scholar]
- 5.Lee KE, Wood TG. 1971. Termites and soils, pp. 251 London, UK: Academic Press. [Google Scholar]
- 6.Lenz M, Amburgey TL, Zi-rong D, Kühne H, Mauldin JK, Preston AF, Westcott M. 1987. Interlaboratory studies on termite-wood decay fungi associations: 1. Determination of maintenance conditions for several species of termites (Isoptera: Mastotermitidae, Termopsidae, Rhinotermitidae, Termitidae). Sociobiology 13, 1–56. [Google Scholar]
- 7.Wood TG. 1988. Termites and the soil environment. Biol. Fert. Soils 6, 228–236. [Google Scholar]
- 8.Korb J. 2003. Thermoregulation and ventilation of termite mounds. Naturwissenschaften 90, 212–219. ( 10.1007/s00114-002-0401-4) [DOI] [PubMed] [Google Scholar]
- 9.French JR, Ahmed BM. 2010. The challenge of biomimetic design for carbon-neutral buildings using termite engineering. Insect Sci. 17, 154–162. ( 10.1111/j.1744-7917.2009.01306.x) [DOI] [Google Scholar]
- 10.Oberst S, Lai JCS, Evans TA. 2016. Termites utilise clay to build structural supports and so increase foraging resources. Sci. Rep. 6, 20990 ( 10.1038/srep20990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadlington S, Staunton I. 2008. Australian termites. Sydney, Australia: University of New South Wales Press Ltd. [Google Scholar]
- 12.Ueckert DN, Bodine MC, Spears BM. 1976. Population density and biomass of the desert termite Gnathamitermes Tubiformans (Isoptera: Termitidae) in a shortgrass prairie: relationship to temperature and moisture. Ecology 57, 1273–1280. ( 10.2307/1935051) [DOI] [Google Scholar]
- 13.Su NY, Puche H. 2003. Tunneling activity of subterranean termites (Isoptera: Rhinotermitidae) in sand with moisture gradients. J. Econ. Entomol. 96, 88–93. ( 10.1093/jee/96.1.88) [DOI] [PubMed] [Google Scholar]
- 14.Houseman RM, Gold RE. 2004. Factors that influence tunnelling in the eastern subterranean termite, Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae). J. Agricult. Urb. Entomol. 20, 69–81. ( 10.1093/jee/97.1.95) [DOI] [Google Scholar]
- 15.Green JM, Scharf ME, Bennett GW. 2005. Impacts of soil moisture level on consumption and movement of three sympatric subterranean termites (Isoptera: Rhinotermitidae) in a laboratory assay. J. Econ. Entomol. 98, 933–937. ( 10.1603/0022-0493-98.3.933) [DOI] [PubMed] [Google Scholar]
- 16.Gautam BK, Henderson G. 2011. Wood consumption by Formosan subterranean termites (Isoptera: Rhinotermitidae) as affected by wood moisture content and temperature. Ann. Entomol. Soc. Am. 104, 459–464. ( 10.1603/AN10190) [DOI] [Google Scholar]
- 17.Gautam BK, Henderson G. 2011. Relative humidity, preference and survival of starved Formosan subterranean termites (Isoptera: Rhinotermitidae) at various temperature and relative humidity conditions. Env. Entomol. 40, 1232–1238. ( 10.1603/EN11062) [DOI] [PubMed] [Google Scholar]
- 18.Bardunias P, Su N-Y. 2009. Opposing headings of excavating and depositing termites facilitate branch formation in the Formosan subterranean termite. Anim. Behav. 78, 755–759. ( 10.1016/j.anbehav.2009.06.024) [DOI] [Google Scholar]
- 19.Werfel J, Peterson K, Nagpal R. 2014. Designing collective behavior in a termite-inspired robot construction team. Science 343, 754–758. ( 10.1126/science.1245842) [DOI] [PubMed] [Google Scholar]
- 20.Gromysz-Kalkowska K, Unkiewicz-Winiarczyk A. 2010. Ethological defence mechanisms in insects. I. Passive defence. Ann. UMCS Biol. 65, 15–27. ( 10.2478/v10067-011-0002-8) [DOI] [Google Scholar]
- 21.Gay FJ, Greaves T, Holdaway FG, Wetherly AH. 1955. Standard laboratory colonies of termites for evaluating the resistance of timber, timber preservatives, and other materials to termite attack. Commonwealth Scientific and Industrial research Organization, Australia, Division of Entomology, Bulletin No. 277, Melbourne, Australia, pp. 60.
- 22.Oberst S, Evans TA, Lai JCS. 2014. Novel method for pairing wood samples for choice tests. PLoS ONE 9, e88835 ( 10.1371/journal.pone.0088835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberst S, Lai JCS, Evans TA. 2018. Key material properties in termite foraging. J. R. Soc. Interface 15, 20180505 ( 10.1098/rsif.2018.0505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csanady E, Magoss E. 2013. Mechanics of wood machining. Berlin, Germany: Springer. [Google Scholar]
- 25.Landau LD, Lifshitz EM. 1970. Theory of elasticity. Institute of physical problems, USSR academy of sciences. Oxford, UK: Pergamon Press. [Google Scholar]
- 26.Thibaut B, Gril J, Fournier M. 2001. Mechanics of wood and trees: some new highlights for an old story. C. R. l'Académie Sci. Series IIB Mech. 329, 701–716. ( 10.1016/S1620-7742(01)01380-0) [DOI] [Google Scholar]
- 27.Delate KM, Grace JK, Tome CHM. 1995. Potential use of pathogenic fungi in baits to control the Formosan subterranean termite (Isopt., Rhinotermitidae). J. Appl. Entom. 119, 429–433. ( 10.1111/j.1439-0418.1995.tb01313.x) [DOI] [Google Scholar]
- 28.Yanagawa A, Fujiwara-Tsujii N, Akino T, Yoshimura T, Yanagawa T, Susumu S. 2012. Odor aversion and pathogen-removal efficiency in grooming behavior of the termite Coptotermes formosanus. PLoS ONE 7, e47412 ( 10.1371/journal.pone.0047412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosengaus RB, Maxmen AB, Coates LE, Traniello JFA. 1998. Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Beh. Ecol. Sociobiol. 44, 125–134. ( 10.1007/s002650050523) [DOI] [Google Scholar]
- 30.Chouvenc T, Efstathion CA, Elliott ML, Su N-Y. 2013. Extended disease resistance emerging from the faecal nest of a subterranean termite. Proc. R. Soc. B 280, 20131885 ( 10.1098/rspb.2013.1885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neoh K-B, Yeap B-K, Tsunoda K, Yoshimura T, Lee C-Y. 2012. Do termites avoid carcasses? Behavioral responses depend on the nature of the carcasses. PLoS ONE 7, e36375 ( 10.1371/journal.pone.0036375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamashiro M, Fujii JK, Lai PY. 1973. A simple method to observe, trap, and prepare large numbers of subterranean termites for laboratory and field experiments. Environ. Entomol. 2, 721–722. ( 10.1093/ee/2.4.721) [DOI] [Google Scholar]
- 33.Norkrans B. 1963. Degradation of cellulose. Annu. Rev. Phytopathol. 1, 325–350. ( 10.1146/annurev.py.01.090163.001545) [DOI] [Google Scholar]
- 34.Tarmadi D, Tobimatsu Y, Yamamura M, Miyamoto T, Miyagawa Y, Umezawa T, Yoshimura T. 2018. NMR studies on lignocellulose deconstructions in the digestive system of the lower termite Coptotermes formosanus Shiraki. Sci. Rep. 8, 1290 ( 10.1038/s41598-018-19562-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Brian RW, Slaytor M. 1982. Role of microorganisms in the metabolism of termites. Austr. J. Biol. Sci. 35, 239–262. ( 10.1071/BI9820239) [DOI] [Google Scholar]
- 36.Eggleton P. 2006. The termite gut habitat: its evolution and co-evolution. In Intestinal microorganisms of termites and other invertebrates, vol. 6 of soil biology (eds König H, Varma A). Berlin, Germany: Springer. [Google Scholar]
- 37.Scharf ME, Karl ZJ, Sethi A, Boucias DG. 2011. Multiple levels of synergistic collaboration in termite lignocellulose digestion. PLoS ONE 6, e21709 ( 10.1371/journal.pone.0021709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama T, Yoshimura T, Imamura Y. 2005. Feeding activities of Coptotermes formosanus Shiraki and Reticulitermes speratus (Kolbe) as affected by moisture content of wood. J. Wood Sci. 51, 60–65. ( 10.1007/s10086-003-0612-0) [DOI] [Google Scholar]
- 39.Esenther GR, Kirk TK. 1974. Catabolism of aspen sapwood in Reticulitermes flavipes (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 67, 989–991. ( 10.1093/aesa/67.6.989) [DOI] [Google Scholar]
- 40.Evans TA, Lai JCS, Toledano E, McDowall L, Rakotonarivo S, Lenz M. 2005. Termites assess wood size by using vibration signals. Proc. Natl Acad. Sci. USA 102, 3732–3737. ( 10.1073/pnas.0408649102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberst S, Bann G, Lai JCS, Evans TA. 2017. Cryptic termites avoid predatory ants by listening to vibrational cues from their footsteps. Ecol. Lett. 20, 212–221. ( 10.1111/ele.12727) [DOI] [PubMed] [Google Scholar]
- 42.Hager F, Kirchner WH. 2013. Vibrational long-distance communication in the termite Macrotermes natalensis and Odontotermes sp. J. Exp. Biol. 216, 3249–3256. ( 10.1242/jeb.086991) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset is available from electronic supplementary material, S8.