Abstract
Behavioural differences among social groups can arise from differing ecological conditions, genetic predispositions and/or social learning. In the past, social learning has typically been inferred as responsible for the spread of behaviour by the exclusion of ecological and genetic factors. This ‘method of exclusion’ was used to infer that ‘sponging’, a foraging behaviour involving tool use in the bottlenose dolphin (Tursiops aduncus) population in Shark Bay, Western Australia, was socially transmitted. However, previous studies were limited in that they never fully accounted for alternative factors, and that social learning, ecology and genetics are not mutually exclusive in causing behavioural variation. Here, we quantified the importance of social learning on the diffusion of sponging, for the first time explicitly accounting for ecological and genetic factors, using a multi-network version of ‘network-based diffusion analysis'. Our results provide compelling support for previous findings that sponging is vertically socially transmitted from mother to (primarily female) offspring. This research illustrates the utility of social network analysis in elucidating the explanatory mechanisms behind the transmission of behaviour in wild animal populations.
Keywords: dolphins, sponging, social learning, network-based diffusion analysis, culture, tool use
1. Introduction
Various mechanisms can be responsible for causing behavioural differences among social groups or populations [1]. The cultural hypothesis states that behavioural variation is the result of social transmission of different behavioural innovations. The ecological hypothesis, on the other hand, proposes that behavioural differences among groups can be attributed to differing ecological conditions. Finally, the genetic hypothesis assumes that different groups are genetically predisposed to behave in different ways [1].
The last few decades have seen increasing interest in animal cultural phenomena, i.e. behaviours that are socially transmitted among conspecifics [1]. Various methods have been used to identify social learning in animal populations. For example, the method of exclusion (also termed group contrast method, or ethnographic method)—commonly used among primatologists in the past, e.g. [2,3]—identifies patterns of variation in the behavioural repertoire of the population in question and infers social transmission as at least partly responsible for differing behaviours by excluding genetic and ecological differences as sufficient explanations [4, p. 132].
The method of exclusion has also been used to assess patterns of transmission of ‘sponging’, a foraging behaviour involving tool use in a population of Indo-Pacific bottlenose dolphins (Tursiops aduncus) in Shark Bay, Western Australia [5]. This behaviour involves dolphins carrying conical sponges as protective ‘gloves’ on their rostra when foraging for buried prey [6]. Sponging is female-biased, and almost all sponging dolphins possess the same mitochondrial haplotype, i.e. belong to the same matriline [5,7]. As the deep-water channels where sponging occurs were used by both ‘spongers’ and ‘non-spongers’, a purely ecological explanation seemed unlikely [5]. By considering 10 different pathways of potential genetic inheritance (x-linked and autosomal), Krützen et al. [5] inferred that sponging was vertically socially transmitted from mother to female offspring.
The method of exclusion has been criticized, however, with considerable debate over its utility [8–10]. Laland & Janik [9] argued that it is impossible to take all plausible explanations for the spread of behaviour into account, and therefore, that social learning can never be inferred with absolute certainty, leading to increased rates of false claims of culture [4]. Furthermore, they argued that social learning, ecology and genetics are not necessarily mutually exclusive [9,10]. Instead, they can simultaneously shape behaviour in a population, warranting a more nuanced approach to disentangle the relative contributions of the three drivers of behavioural variation.
In an attempt to resolve the animal cultures debate, more quantitative methods to infer social learning have been developed. For example, using repertoire-based methods on long-term behavioural data from 11 orangutan (Pongo spp.) populations, Krützen et al. [11] showed that neither uniquely genetic nor ecological components explained the total observed variance with regard to putative cultural elements, corroborating a cultural explanation. Further, ‘network-based diffusion analysis' (NBDA) [12,13], a network-based approach allowing the quantification of the importance of social learning on the spread of behaviour, has been used increasingly in recent years to detect and quantify social learning in animal populations, e.g. [14,15]. NBDA infers social transmission if the spread of a behaviour follows the social network, assuming that more closely associated individuals have more opportunities to learn from each other [13,16]. Multi-network NBDA allows the inclusion of several different networks to quantify the relative importance of transmission along different pathways [17].
Here, we used multi-network NBDA to quantify the relative importance of social learning, ecological factors and genetic relatedness on the spread of sponge tool use in the dolphin population of Shark Bay, Western Australia. Furthermore, we distinguished between different pathways of social learning, namely vertical (between mother and offspring) and horizontal/oblique learning (among peers/between older and younger generations, respectively).
2. Material and methods
(a). Field methods
We collected association and behavioural data during boat-based surveys using standardized sampling methods for cetaceans between 2007 and 2018 in the western gulf of Shark Bay, Western Australia. On approach to each dolphin group, we recorded GPS location, determined group composition during the first 5 min of each encounter using long-established photo-identification techniques [18] and recorded predominant group behaviour. All occurrences of sponging were recorded and an individual was deemed a ‘sponger’ once it had been seen carrying a sponge on at least two independent occasions. Biopsy samples were taken on an opportunistic basis using a system designed specifically for sampling cetaceans [19].
(b). Genetic methods
To test for a genetic predisposition for developing sponging behaviour, we obtained a measure of genetic biparental relatedness for each dyad. Individuals for which biopsies were available (N = 295) were genetically sexed [20] and genotypes determined based on 27 microsatellite markers (electronic supplementary material, table S1). Using COANCESTRY 1.0.1.7 [21], we calculated dyadic biparental relatedness based on genotypes for individuals with no more than three microsatellite loci missing (N = 293), using the estimator TrioML [22] (electronic supplementary material). With a cut-off point of seven sightings (see below), genetic data were available on 226 out of 415 individuals, resulting in 25 425 unique dyads. For the remaining 189 individuals where no genetic information was available (60 480 dyads), we used the population average relatedness of 0.043.
We also statistically controlled for a correlation between matriline membership and sponging behaviour by sequencing a 468 bp-long fragment of the mitochondrial DNA (mtDNA) control region (d-loop) to assign dolphins to mtDNA haplotypes [23].
(c). Network constructions and network-based diffusion analysis
To assess the relative importance of social learning, ecological factors and genetics in promoting the spread of sponging, we ran multi-network NBDA [17] using four different networks (NBDA package v. 0.6.1 [24] in R 3.5.1 [25]). The first social network assessed vertical learning between mother and offspring, with entries of 1 between mother and known offspring and all other connections set to 0. We created the network based on behaviourally and genetically identified mother–offspring pairs (N = 294; electronic supplementary material). The second social network allowed for horizontal/oblique (henceforth ‘horizontal’) learning using dyadic association strengths (simple ratio index [26]) among all individuals but excluding mother–offspring associations, which were set to 0. Sightings of the same or a subset of the original group within 2 h were excluded. Association matrices were created using R package ‘asnipe’ [27]. The third, ecological network contained dyadic home range overlap as a proxy of the environmental similarity experienced by individuals. We created home ranges using individual GPS locations based on 95% Epanechnikov kernel density estimates (‘adehabitatHR’ [28]) with a customized smoothing factor (electronic supplementary material). Dyadic home range overlap (95%) was calculated using the ‘utilization distribution overlap index’ (adehabitatHR) [28,29]. Finally, the fourth network contained measures of dyadic biparental genetic relatedness among individuals. Since NBDA infers social learning if a behaviour follows the social network, there is a trade-off between sample size and data quality. Dropping individuals with few sightings can increase certainty about the strengths of connections but, at the same time, decrease the power of NBDA to reliably detect social learning if linking individuals are removed [30]. We ran a simulation to select a threshold that maximizes the power of NBDA to detect social learning, revealing maximum power at seven sightings (electronic supplementary material) [30]. In all networks, we therefore only considered individuals with a minimum of seven observations.
We then applied the ‘order of acquisition diffusion analysis' (OADA) variant of NBDA [13] (electronic supplementary material). For several individuals, the order of acquisition of sponging was unknown, as they were likely already spongers when first encountered. In NBDA models, such individuals can be taken to be ‘informed’ at the start of the diffusion (termed ‘demonstrators’) [13]. We considered all individuals as demonstrators who had been seen carrying a sponge within the first two encounters where predominant group behaviour was foraging. We argue that an individual's information state can be determined with reasonable certainty after two sightings, given spongers carry sponges 96% of the time when foraging [31]. Maternity data were unavailable for nine individuals who acquired sponging after 2007. These nine individuals were excluded as learners, but we allowed for other individuals having learned from these spongers (electronic supplementary material).
We included several individual-level variables (ILVs) with potential influence on the learning rate: sex; average water depth of each individual's sightings (a proxy for habitat use, since sponging occurs in deep-water channels [32]); average group size (since sponging is a solitary activity [31]) and mitochondrial haplotype as a reduced two-level factor (either haplotype E (=sponging haplotype in the western gulf [7]), or other) to avoid overfitting of models. Sex was determined genetically and/or by the presence of a dependent calf for females. In an NBDA, the strength of transmission through a network (s parameter) is estimated relative to a baseline rate of asocial learning. This baseline was set to the mean of all continuous variables, at the mid-point between males and females, and haplotype E (set as the reference level for this factor).
We fitted OADA with and without transmission through the networks and with all possible combinations of networks and ILVs [13]. Thereby, ILVs were allowed to influence both social and asocial learning rates independently (‘unconstrained’ models [4]; electronic supplementary material). Support for each model was calculated based on the Akaike information criterion corrected for sample size (AICc) [33]. To provide a more robust inference about strength of transmission for the different networks and the influence of ILVs, model averaging methods were employed [33]. We calculated 95% confidence intervals (CI) for model parameters using the profile likelihood method, conditional on the best performing model (electronic supplementary material).
3. Results
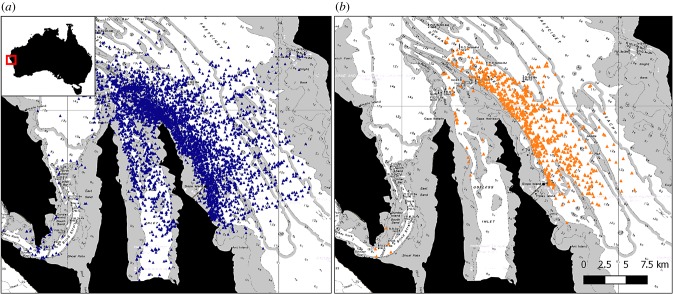
Between 2007 and 2018, 5300 dolphin groups were encountered in the western gulf of Shark Bay and more than 1000 different dolphins identified (figure 1a). Sponging was observed on 825 occasions and restricted to the deep-water channels within the study area (figure 1b). A total of 76 individuals were identified as spongers, of which 49 were confirmed female, 14 male and 13 of unknown sex.
Figure 1.
Locations of (a) all dolphin groups encountered in the western gulf of Shark Bay between 2007 and 2018; and (b) observations of sponging behaviour, which primarily occurred in deep (greater than 10 m) water channels (white areas).
After removal of individuals with fewer than seven sightings, as well as eight offspring that were either dependent calves at the time of analyses or had died before weaning, 415 individuals remained, of which 62 were spongers (18 learners, of which nine were removed due to missing maternity data, and 44 demonstrators). All spongers with maternity data available were born to sponging mothers. All spongers with genetic data available carried haplotype E, with one exception: a male sponger with haplotype H (but see electronic supplementary material).
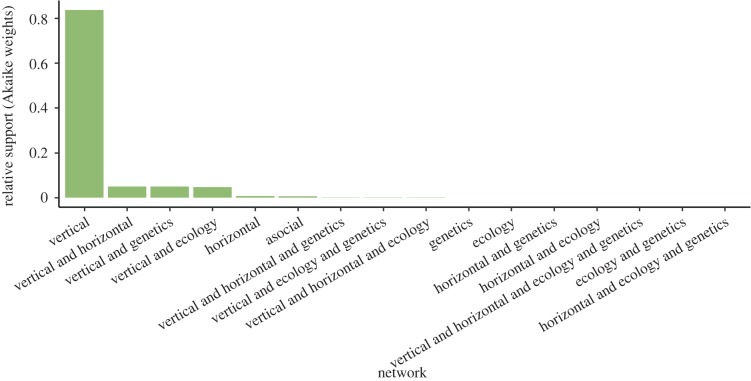
Multi-network NBDA revealed most support for models with transmission through the vertical social network , while asocial learning, and transmission through the horizontal, ecological or genetic network (or any combination of the four networks) received much less support (; figure 2). In the best performing model, which included vertical social transmission and sex influencing social learning, s (the rate of social transmission from mothers to offspring) was estimated to be 1.23 × 1010 times greater than the rate of asocial learning (95% CI [33.1; infinity]; ). The social learning rate was an estimated 126 times higher for females than males (95% CI [9.5; 2897]; ). This corresponds to approximately 100% of spongers learning sponging socially from their mothers (95% CI [98.9; 100]). The average group size, average water depth and haplotype did not influence social or asocial learning rate (all ; electronic supplementary material).
Figure 2.
Multi-network NBDA revealed most support (based on Akaike weights) for transmission of sponging through the vertical social network, while transmission through the horizontal, ecological and relatedness networks (or combinations thereof) received little support.
4. Discussion
We applied multi-network NBDA to sponging behaviour, revealing overwhelming support for social transmission through the vertical mother–offspring network, with little or no support for transmission through the horizontal association, ecological or genetic networks. Moreover, despite the restriction of sponging to channel habitat [32,34], our analysis suggests that ecological factors play only a minor role once vertical social learning has been taken into account.
Low support for transmission through the genetic network confirms previous findings that sponging individuals in the western gulf are not more closely related than expected by chance [7]. This stands in contrast with findings from the eastern gulf of Shark Bay, where spongers show higher relatedness than the population average, suggesting a more recent common ancestry [5].
We further confirm a previously documented female sex-bias [7,31,35], which is presumably due to differing sex-specific reproductive strategies between males and females [31]. After weaning, male dolphins must focus on forming multi-male alliances to coerce and consort oestrous females [36–38]. This requires significant investment in social relationships and is, therefore, largely incompatible with a time-consuming, solitary and difficult-to-master activity like sponging [31,39]. Meanwhile, female offspring are expected to invest more into developing foraging skills to maximize food intake compared to male offspring [40,41]. Alternatively, Zefferman [42] proposed that the female sex-bias could be the result of a maternal teaching strategy, arguing that teaching a daughter would result in higher long-term fitness for a female: a potential advantage of sponging for a son would last only one generation, while a daughter can pass on the behaviour to subsequent generations which all gain potential benefits associated with sponging. Just 22% of spongers with known sex in the western gulf were males, which corresponded to previously suggested proportions of male offspring learning sponging from their mothers in Shark Bay's eastern gulf ([31], but see [43]).
Given haplotype similarity among spongers, some researchers have argued that mitochondrial genes themselves might predispose dolphins to learning the sponging behaviour [9]. However, we find no evidence that being a member of a particular mtDNA matriline has an effect on the rate at which dolphins learn sponging, as per previous research [44]. Our findings instead support the hypothesis that maternal vertical transmission of both the sponging behaviour and mtDNA results in haplotype similarity among spongers, a phenomenon referred to as ‘cultural hitchhiking’—a form of gene–culture coevolution in which a neutral genetic locus is inherited in parallel with a matrilineally transmitted cultural behaviour [45].
McElreath & Strimling's [46] mathematical models predict the conditions for the evolution of purely vertical transmission, concluding that ‘neither [vertical nor oblique] transmission should be expected to dominate the other across all domains'. Sponging is just one foraging strategy exhibited by the dolphins, and other strategies may be transmitted obliquely and horizontally. Following McElreath & Strimling's models [46], we suggest that sponging is transmitted vertically because either (i) the relevant environment (e.g. availability of sponges) may be stable, or (ii) it may only be possible for a dolphin to learn sponging from its mother, if, for example, it requires repeated observations from close quarters.
The application of multi-network NBDA to sponging behaviour in the dolphins of western Shark Bay allowed us to quantify the effects of social learning on behaviour, while explicitly accounting for the influence of ecological and genetic factors for the first time. Documenting a strong effect of vertical social learning from mother to offspring, our findings provide compelling quantitative evidence to support the claim that sponging is a case of vertically transmitted culture in the bottlenose dolphins of Shark Bay [5].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Shark Bay Resources and the Useless Loop community for logistic support, and all researchers and volunteers who have contributed to data collection for the Dolphin Innovation Project.
Ethics
Permits for the use of animals for scientific purposes were granted by the Department of Biodiversity, Conservation and Attractions (SF002958; SF010888; SF10388; SF002958; SF010774; 08-000920-1; 08-000706-3) and the Department of Agriculture and Food (U 10/2015-2018). The animal ethics committees of the University of Western Australia, Murdoch University and the University of Zurich provided approvals for the ethical treatment of animals in scientific research (R2649/14; RA/3/100/1449; RA/3/100/1464).
Data accessibility
Code is available in electronic supplementary material, and data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.sc26m6c [47].
Authors' contributions
Conceptualization: S.W., M.K., S.J.A. and W.J.E.H. Funding: M.K. and S.L.K. Data collection: S.W., S.J.A., M.K., L.G. and S.L.K. Formal analysis: S.W. and W.J.E.H. Writing: all. All authors approved of, and agreed to be held accountable for, the final manuscript.
Competing interests
The authors declare no competing interests.
Funding
This research was funded by: Swiss National Science Foundation (31003A_149956), Seaworld Research & Rescue Foundation Inc. (SWRRFI), National Geographic Society, A.H. Schultz Stiftung, Claraz-Schenkung, Julius-Klaus Stiftung and W.V. Scott Foundation, all to M.K. S.L.K. was supported by The Branco Weiss Fellowship—Society in Science.
References
- 1.Boyd R, Richerson PJ. 1995. Why culture is common, but cultural evolution is rare. Proc. Br. Acad. 88, 77–93. (doi:citeulike-article-id:1339814) [Google Scholar]
- 2.van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. 2003. Orangutan cultures and the evolution of material culture. Science 299, 102–105. ( 10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- 3.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CE, Wrangham RW, Boesch C. 1999. Cultures in chimpanzees. Nature 399, 682–685. ( 10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 4.Hoppitt WJE, Laland KN. 2013. Social learning: an introduction to mechanisms, methods, and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Krützen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB. 2005. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl Acad. Sci. USA 102, 8939–8943. ( 10.1073/pnas.0500232102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolker R, Richards A, Connor R, Mann J, Berggren P. 1997. Sponge carrying by dolphins (Delphinidae, Tursiops sp.): a foraging specialization involving tool use? Ethology 103, 454–465. ( 10.1111/j.1439-0310.1997.tb00160.x) [DOI] [Google Scholar]
- 7.Kopps AM, Krützen M, Allen SJ, Bacher K, Sherwin WB. 2014. Characterizing the socially transmitted foraging tactic ‘sponging’ by bottlenose dolphins (Tursiops sp.) in the western gulf of Shark Bay, Western Australia. Mar. Mammal Sci. 30, 847–863. ( 10.1111/mms.12089) [DOI] [Google Scholar]
- 8.Krützen M, van Schaik C, Whiten A. 2007. The animal cultures debate: response to Laland and Janik. Trends Ecol. Evol. 22, 6 ( 10.1016/j.tree.2006.10.011) [DOI] [PubMed] [Google Scholar]
- 9.Laland KN, Janik VM. 2006. The animal cultures debate. Trends Ecol. Evol. 21, 542–547. ( 10.1016/j.tree.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 10.Laland K, Janik VM. 2007. Response to Krützen et al.: Further problems with the ‘method of exclusion’. Trends Ecol. Evol. 22, 7 ( 10.1016/j.tree.2006.10.010) [DOI] [Google Scholar]
- 11.Krützen M, Willems EP, van Schaik CP. 2011. Culture and geographic variation in orangutan behavior. Curr. Biol. 21, 1808–1812. ( 10.1016/j.cub.2011.09.017) [DOI] [PubMed] [Google Scholar]
- 12.Franz M, Nunn CL. 2009. Network-based diffusion analysis: a new method for detecting social learning. Proc. R. Soc. B 276, 1829–1836. ( 10.1098/rspb.2008.1824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoppitt W, Boogert NJ, Laland KN. 2010. Detecting social transmission in networks. J. Theor. Biol. 263, 544–555. ( 10.1016/j.jtbi.2010.01.004) [DOI] [PubMed] [Google Scholar]
- 14.Allen J, Weinrich M, Hoppitt W, Rendell L. 2013. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485–488. ( 10.1126/science.1231976) [DOI] [PubMed] [Google Scholar]
- 15.Hobaiter C, Poisot T, Zuberbühler K, Hoppitt W, Gruber T. 2014. Social network analysis shows direct evidence for social transmission of tool use in wild chimpanzees. PLoS Biol. 12, e1001960 ( 10.1371/journal.pbio.1001960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coussi-Korbel S, Fragaszy D. 1995. On the relation between social dynamics and social learning. Anim. Behav. 50, 1441–1453. ( 10.1016/0003-3472(95)80001-8) [DOI] [Google Scholar]
- 17.Farine DR, Aplin LM, Sheldon BC, Hoppitt W. 2015. Interspecific social networks promote information transmission in wild songbirds. Proc. R. Soc. B 282, 20142804 ( 10.1098/rspb.2014.2804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Würsig B, Jefferson T. 1990. Methods of photo-identification for small cetaceans. Reports Int. Whal. Comm. 12, 43–52. [Google Scholar]
- 19.Krützen M, Barré L, Möller L, Heithaus M, Simms C, Sherwin W. 2002. A biopsy system for small cetaceans: darting success and wound healing in Tursiops spp. Mar. Mammal Sci. 18, 863–878. ( 10.1111/j.1748-7692.2002.tb01078.x) [DOI] [Google Scholar]
- 20.Gilson A, Syvanen M, Levine K, Banks J. 1998. Deer gender determination by polymerase chain reaction: validation study and application to tissues, bloodstains, and hair forensic samples from California. Calif. Fish Game 84, 159–169. [Google Scholar]
- 21.Wang J. 2011. COANCESTRY: a program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Resour. 11, 141–145. ( 10.1111/j.1755-0998.2010.02885.x) [DOI] [PubMed] [Google Scholar]
- 22.Wang J. 2007. Triadic IBD coefficients and applications to estimating pairwise relatedness. Genet. Res. 89, 135–153. ( 10.1017/S0016672307008798) [DOI] [PubMed] [Google Scholar]
- 23.Krützen M, Sherwin W, Berggren P, Gales N. 2004. Population structure in an inshore cetacean revealed by microsatellite and mtDNA analysis: bottlenose dolphins (Tursiops sp.) in Shark Bay, Western Australia. Mar. Mammal Sci. 20, 28–47. ( 10.1111/j.1748-7692.2004.tb01139.x) [DOI] [Google Scholar]
- 24.Hoppitt WJE. 2018. NBDA: a package for implementing network-based diffusion analysis.
- 25.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/ [Google Scholar]
- 26.Cairns SJ, Schwager SJ. 1987. A comparison of association indices. Anim. Behav. 35, 1454–1469. ( 10.1016/S0003-3472(87)80018-0) [DOI] [Google Scholar]
- 27.Farine DR. 2013. Animal social network inference and permutations for ecologists in R using asnipe. Methods Ecol. Evol. 4, 1187–1194. ( 10.1111/2041-210X.12121) [DOI] [Google Scholar]
- 28.Calenge C.2015. Home range estimation in R: the adehabitatHR Package. 1–60. ( ) [DOI]
- 29.Fieberg J, Kochanny CO. 2005. Quantifying home-range overlap: the importance of the utilization distribution. J. Wildl. Manage. 69, 1346–1359. ( 10.2193/0022-541X(2005)69[1346:QHOTIO]2.0.CO;2) [DOI] [Google Scholar]
- 30.Wild S, Hoppitt W. 2018. Choosing a sensible cut-off point: assessing the impact of uncertainty in a social network on the performance of NBDA. Primates 60, 307–315. ( 10.1007/s10329-018-0693-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann J, Sargeant BL, Watson-Capps JJ, Gibson QA, Heithaus MR, Connor RC, Patterson E. 2008. Why do dolphins carry sponges? PLoS One 3, e3868 ( 10.1371/journal.pone.0003868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sargeant BL, Wirsing AJ, Heithaus MR, Mann J. 2007. Can environmental heterogeneity explain individual foraging variation in wild bottlenose dolphins (Tursiops sp.)? Behav. Ecol. Sociobiol. 61, 679–688. ( 10.1007/s00265-006-0296-8) [DOI] [Google Scholar]
- 33.Burnham K, Anderson D. 2002. Model selection and multi-model inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 34.Tyne J, Loneragan N, Kopps A, Allen S, Krützen M, Bejder L. 2012. Ecological characteristics contribute to sponge distribution and tool use in bottlenose dolphins Tursiops sp. Mar. Ecol. Prog. Ser. 444, 143–153. ( 10.3354/meps09410) [DOI] [Google Scholar]
- 35.Mann J, Sargeant B. 2003. Like mother, like calf: the ontogeny of foraging traditions in wild Indian ocean bottlenose dolphins (Tursiops sp.). In The biology of traditions: models and evidence (eds Fragaszy D, Perry S), pp. 236–266. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 36.Connor RC, Smolker RA, Richards AF. 1992. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl Acad. Sci. USA 89, 987–990. ( 10.1073/pnas.89.3.987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connor RC, Krützen M. 2015. Male dolphin alliances in Shark Bay: changing perspectives in a 30-year study. Anim. Behav. 103, 223–235. ( 10.1016/j.anbehav.2015.02.019) [DOI] [Google Scholar]
- 38.Bizzozzero MR, Allen SJ, Gerber L, Wild S, King SL, Connor RC, Friedman WR, Wittwer S, Krützen M. 2019. Tool use and social homophily among male bottlenose dolphins. Proc. R. Soc. B 286, 20190898 ( 10.1098/rspb.2019.0898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson EM, Krzyszczyk E, Mann J. 2015. Age-specific foraging performance and reproduction in tool-using wild bottlenose dolphins. Behav. Ecol. 27, 401–410. ( 10.1093/beheco/arv164) [DOI] [Google Scholar]
- 40.Gibson QA, Mann J. 2008. Early social development in wild bottlenose dolphins: sex differences, individual variation and maternal influence. Anim. Behav. 76, 375–387. ( 10.1016/j.anbehav.2008.01.021) [DOI] [Google Scholar]
- 41.Krzyszczyk E, Patterson EM, Stanton MA, Mann J. 2017. The transition to independence: sex differences in social and behavioural development of wild bottlenose dolphins. Anim. Behav. 129, 43–59. ( 10.1016/j.anbehav.2017.04.011) [DOI] [Google Scholar]
- 42.Zefferman MR. 2016. Mothers teach daughters because daughters teach granddaughters: the evolution of sex-biased transmission. Behav. Ecol. 27, 1172–1181. ( 10.1093/beheco/arw022) [DOI] [Google Scholar]
- 43.Mann J, Patterson E. 2013. Tool use by aquatic animals. Phil. Trans. R. Soc. B 368, 20120424 ( 10.1098/rstb.2012.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacher K, Allen S, Lindholm AK, Bejder L, Krützen M. 2010. Genes or culture: are mitochondrial genes associated with tool use in bottlenose dolphins (Tursiops sp.)? Behav. Genet. 40, 706–714. ( 10.1007/s10519-010-9375-8) [DOI] [PubMed] [Google Scholar]
- 45.Kopps AM, Ackermann CY, Sherwin WB, Allen SJ, Bejder L, Krützen M. 2014. Cultural transmission of tool use combined with habitat specializations leads to fine-scale genetic structure in bottlenose dolphins. Proc. R. Soc. B 281, 20133245 ( 10.1098/rspb.2013.3245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McElreath R, Strimling P. 2008. When natural selection favors imitation of parents. Curr. Anthropol. 49, 307–316. ( 10.1086/524364) [DOI] [Google Scholar]
- 47.Wild S, Allen SJ, Krützen M, King SL, Gerber L, Hoppitt WJE. 2019. Data from: Multi-network-based diffusion analysis reveals vertical cultural transmission of sponge tool use within dolphin matrilines Dryad Digital Repository. ( 10.5061/dryad.sc26m6c) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wild S, Allen SJ, Krützen M, King SL, Gerber L, Hoppitt WJE. 2019. Data from: Multi-network-based diffusion analysis reveals vertical cultural transmission of sponge tool use within dolphin matrilines Dryad Digital Repository. ( 10.5061/dryad.sc26m6c) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Code is available in electronic supplementary material, and data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.sc26m6c [47].