Abstract
Objective:
To define a distinct, dominantly inherited, mild skeletal myopathy associated with prominent and consistent tremor in two unrelated, three-generation families.
Methods:
Clinical evaluations as well as exome and panel sequencing analyses were performed in affected and non-affected members of two families to identify genetic variants segregating with the phenotype. Histological assessment of a muscle biopsy specimen was performed in 1 patient, and quantitative tremor analysis was carried out in 2 patients. Molecular modeling studies and biochemical assays were performed for both mutations.
Results:
Two novel missense mutations in MYBPC1 (p.E248K in family 1 and p.Y247H in family 2) were identified and shown to segregate perfectly with the myopathy/tremor phenotype in the respective families. MYBPC1 encodes slow myosin binding protein-C (sMyBP-C), a modular sarcomeric protein playing structural and regulatory roles through its dynamic interaction with actin and myosin filaments. The Y247H and E248K mutations are located in the NH2-terminal M-motif of sMyBP-C. Both mutations result in markedly increased binding of the NH2 terminus to myosin, possibly interfering with normal cross-bridge cycling as the first muscle-based step in tremor genesis. The clinical tremor features observed in all mutation carriers, together with the tremor physiology studies performed in family 2, suggest amplification by an additional central loop modulating the clinical tremor phenomenology.
Interpretation:
Here, we link two novel missense mutations in MYBPC1 with a dominant, mild skeletal myopathy invariably associated with a distinctive tremor. The molecular, genetic, and clinical studies are consistent with a unique sarcomeric origin of the tremor, which we classify as “myogenic tremor.”
Mutations in genes that encode sarcomere-associated proteins underlie an increasingly diverse and growing number of skeletal and cardiac myopathies. Skeletal sarcomeric myopathies encompass histological manifestations ranging from rods and hyaline bodies to various fiber-type disproportion patterns.1 Remarkably, different mutations in the same sarcomeric protein can lead to strikingly distinct clinical manifestations, ranging from congenital to late onset, and resulting in either muscle weakness or stiffness. These physical symptoms are derived from the molecular repercussions of specific mutations, such as changes in calcium sensitivity or target binding. In addition to mutations causing weakness in conjunction with congenital myopathy, stiffness-inducing mutations have also been described in TPM2, TPM3, and ACTA1.2 In some cases, patients may present with multiple congenital joint contractures (arthrogryposis multiplex) as a result of prenatal restriction of joint movements.3 This diversity in disease manifestation underscores the central role of the sarcomere in muscle function and disease. It also highlights that different mutations in sarcomeric proteins may have highly variable pathophysiological consequences, indicating the importance of elucidating their individual molecular pathogenesis.
Myosin binding protein-C (MyBP-C) comprises a family of sarcomeric accessory proteins and includes three isoforms: cardiac (c), fast skeletal (f), and slow skeletal (s).4 The major roles attributed to the MyBP-C family are stabilization of thick filaments and regulation of cross-bridge cycling.5–7 Slow myosin binding protein-C (sMyBP-C) is encoded by MYBPC1, located on chromosome 12 in humans.8 Alternatively spliced transcripts of the gene give rise to a subfamily of at least 14 variants regulated by phosphorylation, which are co-expressed in different combinations and amounts in both slow- and fast-twitch muscles.6,9–12
sMyBP-C is a modular protein composed of immunoglobulin (Ig) and fibronectin-III (Fn-III) repeats, numbered C1 to C10. The NH2-terminal Ig, C1, is flanked by unique sequences, the Pro/Ala–rich motif and a MyBP-C specific motif, termed M-motif (Fig 1A).6,13 The NH2 terminus of sMyBP-C, including the Pro/Ala–rich motif, C1, and the M-motif, supports binding to actin and myosin subfragment-2 (S2) in a variant-specific manner,14 whereas the COOH-terminal Ig, C10, binds directly to light meromyosin (LMM), and this interaction is further enhanced by the presence of Ig C8 and Fn-III C9.15,16 In addition to binding LMM, the COOH terminus of sMyBP-C supports binding to titin, obscurin, four and a half LIM domains 1 (FHL1) protein, and creatine kinase.16–19
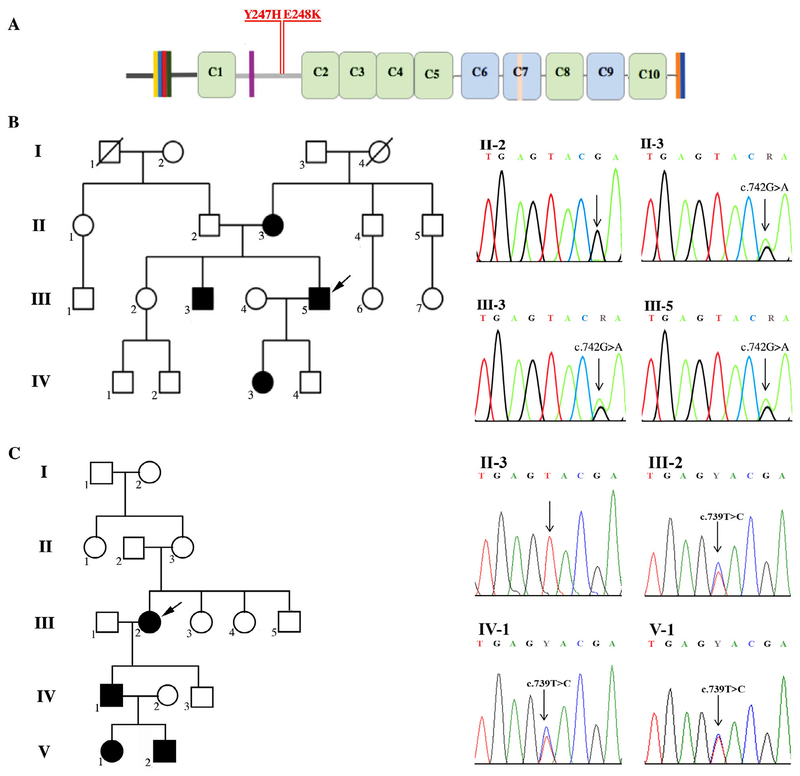
FIGURE 1:
Identification of two novel missense mutations in MYBPC1 underlying the development of skeletal myopathy with tremor. (A) Schematic representation of the structure of sMyBP-C. Dark and light gray horizontal lines correspond to the Pro/Ala–rich region and the M-motif, respectively, whereas vertical colored lines in the NH2 terminus, domain C7, and the extreme COOH terminus represent alternatively spliced segments. Green and blue rectangles denote immunoglobulin (Ig) and fibronectin-III (Fn-III) domains, respectively. The two novel MYBPC1 human mutations, Y247H and E248K, and their location in the sMyBP-C M-motif are indicated. (B) Pedigree of family 1 and sequence chromatograms showing the c.742G>A, p.(E248K) mutation in affected individuals II-3, III-3, and III-5 after exome sequencing and the wild-type sequence in the II-2 unaffected individual. (C) Pedigree of family 2 and sequencing chromatograms showing the c.739T>C, p.(Y247H) mutation in affected individuals III-2, IV-1, and V-1 and wild-type sequence at this position in an unaffected individual (II-3). For both pedigrees, black and white symbols indicate affected and unaffected individuals, respectively, whereas index patients are denoted with arrows.
Earlier reports have linked mutations in MYBPC1 to development of severe and lethal forms of distal arthrogryposis. (DA).20–22 Here, we report on a distinct clinical phenotype in two independent, three-generation families, manifesting as mild myopathy invariably associated with a persistent, posturally pronounced, high-frequency tremor in all affected individuals that segregates with novel, dominant, missense mutations in MYBPC1 in each family. We provide pathophysiological insights into this tremor by showing that altered myo-filament binding of mutant sMyBP-C appears to be the basis of what we refer to as myogenic tremor. A comprehensive literature search revealed that unexplained tremor associated with myopathy is an overlooked feature of diseases involving mutations in genes encoding sarcomeric proteins.
Materials and Methods
Patients and Clinical Evaluation
Examination of family 1 from Latvia was performed under a protocol approved by the Central Medical Ethics Committee of Latvia, and all subjects and/or their parents have given informed consent. The variant, NM_002465.3:c.742G>A p.(E248K), has been submitted to the ClinVar database. Clinical evaluation of patients was performed by a clinical geneticist and a neurologist. Genetic studies in individuals from family 2 from Germany were performed with informed consent, in accord with the Human Genetic Examination Act (Genetic Diagnosis Act-GenDG).
Quantitative Tremor Analysis
Quantitative tremor analysis was performed in subjects III-2 and IV-1 from family 2 using a Dantec Keypoint Workstation (Natus Medical Inc., Pleasanton, CA). Briefly, accelerometers were attached to the dorsum of the hands, and electromyography (EMG) of forearm flexor and extensor muscles was recorded by surface electrodes fixed to the extensor carpi ulnaris and flexor carpi ulnaris muscles, respectively. Power spectra of the accelerometry and EMG data were calculated by fast Fourier transformation of tremor amplitude and frequency time-series recordings.23 Tremor analysis was performed at rest and during posture with outstretched hands, both unloaded and with a weight of 500g.
Sequencing
Family 1.
Exome sequencing of 4 members of family 1 from Latvia (Fig 1B) was performed using the Illumina HiSeq 2500 platform (Illumina, San Diego, CA) and the Nextera Rapid Capture Expanded Exome kit (Illumina) for best variant segregation. Raw sequencing reads were aligned and subsequently processed according to Genome Analysis Toolkit’s (GATK) best practice guidelines using BWA-MEM, Picard-tools, and GATK. Variants were called by GATK Haplotype Caller and then annotated using Annovar. A step-wise approach of filtering was used on the obtained data. Only rare (below 1% in all reference databases, including ExAC, 1000 Genomes, ESP6500, and an in-house next-generation sequencing database from Tartu University Hospital) non-silent variations in autosomal coding regions and splice sites were considered for further analysis. Pathogenicity of variants was evaluated using several in-silico prognostic tools, including PolyPhen2, Mutation Taster, SIFT, and Combined Annotation Dependent Depletion (CADD). Notably, during our analysis, we also evaluated close to 100 previously identified myopathic genes as potential candidates, but they were found to be negative.
Variation validation as well as segregation analysis were performed with nested polymerase chain reaction (PCR; initial synthesis primer set: 5’TCTGGGCTTCAAGTACTCAGG3’ and 5’CTCACCTGCGCTCTTCTTCT3’, and Sanger sequencing primer set: 5’TACCTCGACCTTCGTGGTCT3’ and 5’GCTTGAGTCGCTTGAGCAT3’) using HOT FIREPol polymerase (Solis Biodyne, Tartu, Estonia). In addition to the previously mentioned 4 members, samples from 6 additional family members were used for mutation testing and extended segregation analysis. Amplicons were sequenced with an ABI Prism 3130XL (Applied Biosystems, Waltham, MA) using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems). Sequencing data were analyzed using CLC MainWorkbench (CLCbio, Aarhus, Denmark). Ninety DNA samples from control subjects taken from the Latvian National Genome database were also directly sequenced for presence of the MYBPC1 E248K mutation.
Family 2.
Panel sequencing, using a panel specifically for distal and congenital myopathies, was performed in DNA obtained from the proband III-2 of family 2 from Germany. Confirmation of the observed sequence change in MYBPC1 was performed by PCR amplification of the affected exon followed by Sanger sequencing in the forward and reverse direction using the BigDye terminator (v1.1) cycle sequencing kit from Applied Biosystems. DNA isolated from clinically affected and unaffected family members was also screened for the sequence change identified in subject III-2 using the same method.
Immunohistochemistry
A biopsy was obtained from the deltoid muscle of the index patient (III-2) from family 2, and histochemical staining (hematoxylin and eosin, cytochrome C oxidase, Gomori, van Gieson, NADH dehydrogenase, ATPase 9.4, PAS, and oil-red O) was performed using standard procedures.
Generation and Purification of Recombinant Wild-Type and Mutant Proteins
For overlay assays, human skeletal muscle complementary DNA was used with sense (5’ATGCCAGAACCCACT3’) and antisense (5’TCAATCAAGAATTTTTGC3’) oligonucleotides to amplify the NH2-terminal region of sMyBP-C that contained the Pro/Ala–rich motif, Ig domain C1, and the M-motif (amino acids 1–284, XP_006719468.1). The PCR fragment was subcloned into the pGex4T-1 (Amersham Pharmacia, Piscataway, NJ) vector at EcoRI/XhoI sites to generate a GST-fusion protein. The Y247H mutation was introduced into the resulting plasmid with the following set of primers: sense (5’GCCAAGCCCAGCGAGCACGAGAAGATCG CCTTC) and antisense (5’GAAGGCGATCTTCTCGTGCTCG CTGGGCTTGGC3’), and the E248K mutation was similarly introduced using the primer set: sense (5’AAGCCCAGCGAGTACAAGAAGATCGCCTTCCAG3’) and antisense (5’CTGGAAGGCGATCTTCTTGTACTCGCTGGGCTT3’). Authenticity of the wild-type and mutant constructs was verified by sequence analysis. Recombinant polypeptides were expressed by induction with 1mM isopropyl-β-thioglycopyranoside (IPTG) overnight at 22°C and purified by affinity chromatography on glutathione-agarose columns, according to the manufacturer’s instructions (MilliporeSigma, Bedford, MA).
For circular dichroism experiments, the following set of sense (5’ACTGGAATTCATGCAGGAGGAGGAGCCC) and antisense (5’ACTGCTCGAGTCACTTCTTCTCCTCCCT’) primers was used to amplify the M-motif region of wild-type sMyBP-C. The PCR fragment was subcloned into the pET30a+ vector (MilliporeSigma) at EcoRI/XhoI sites to generate a histidine-tagged fusion protein. The Y247H and E248K mutations were introduced in the wild-type construct using the same primers listed above. Authenticity of the wild-type and mutant constructs was verified by sequence analysis. Recombinant polypeptides were expressed in BL21 (DE3) cells (MilliporeSigma) by induction with 1mM IPTG overnight at 22°C and purified by affinity chromatography on Ni-NTA-agarose columns (MilliporeSigma), according to the manufacturer’s instructions.
Overlay Assay and Immunoblotting
Heavy meromyosin (HMM) and actin purified from skeletal muscle were purchased from Cytoskeleton (Heavy meromyosin Cat. #MH01-A, Actin Cat. #AKL99-A; Cytoskeleton, Inc., Denver, CO). Equivalent amounts (3μg) of purified actin and HMM were separated by 4% to 12% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to nitrocellulose. Equivalent loading and transfer were confirmed by staining the nitrocellulose membrane with Ponceau red. Blots were incubated in buffer A (50mM KCl, 20mM MOPS, 4mM MgCl2, 0.1mM EGTA, 2mM DTT, 3% BSA, 10mM NaN3, 0.5% Tween-20, and 0.5% Nonidet P-40) for 8 hours at room temperature, followed by incubation with buffer A containing 0.5μg/ml of the indicated glutathione S-transferase (GST)-fusion proteins overnight at 4°C. Blots were washed extensively with buffer A, followed by PBS, blocked in 5% milk, and subsequently probed with antibodies to GST (1:10,000; Novagen, Billerica, MA), as described in a previous work.24 Immunoreactive bands were detected with the Tropix Chemiluminescence kit (Applied Biosystems). These experiments were repeated at least three independent times, and quantification of immunoreactive bands was performed using densitometric analysis and ImageJ software (NIH, Bethesda, MD). Statistical significance was evaluated with a t test, p < 0.05.
Modeling of sMyBP-C/Myosin Interaction
Models of the M-motif of sMyB-C were generated using Phyre2, RaptorX, and iTASSER, based on the human cardiac version of this domain (PBD: 2LH4; 80% identical to sMyBP-C).25–27 The model was then allowed to equilibrate using the computer program YASARA for ~90ns, as previously described.28,29 Individual mutations were introduced to the model by the “swap” command in YASARA, and the resulting models were allowed to equilibrate for ~100ns.
Docking of wild-type and mutant sMyBP-C to myosin was performed using equilibrated sMyBP-C models and the S2 fragment of myosin (PDB: 2FXO) with the HADDOCK program.30 The best models, as judged by low z-scores and low root mean square deviation, were then further refined for ~30ns.
Circular Dichroism
Circular dichroism experiments were conducted in a 1-mm pathlength cuvette with wild-type and mutant His-tagged proteins of 15 to 17μM in 20mM phosphate buffer (pH 7.5) and 50mM NaCl. Samples were measured in triplicate at temperatures from 20°C to 90°C in 10°C intervals on a Jasco J-810 spectrophotometer.
Results
Family 1 was ascertained in Latvia by examination of a 30-year-old male (Fig 1B; proband III-5) who presented with muscle weakness and skeletal deformities (Figs 1B and 2A; Supplementary Table 1). The patient had hypotonia and a postural hand tremor since infancy, followed by a delay in gross motor milestone acquisition, with independent walking achieved at 2 years of age. His cognitive functions were normal, and his intellectual development was age appropriate. In early adolescence, symptoms became more noticeable, and the patient developed scoliosis and thoracic asymmetry. Since then, the disease has reached a phase of stability without further deterioration in strength, although the patient reported frequent respiratory infections. Current clinical findings include a high-frequency irregular tremor that is accentuated by posture, most noticeable in the hands (Supplementary Video 1) and, to a lesser extent, in the legs. In addition, the patient presents mild axial muscle weakness, predominantly proximal appendicular weakness, scoliosis with thoracic/sternal deformity, and rigidity of the spine (Fig 2A). Examination of additional family members identified 3 individuals with similar clinical manifestations from three generations, including the index patient’s mother, brother, and daughter (Figs 1B and 2A). All 4 affected family members display a similar, predominantly postural, irregular, high-frequency, low-amplitude tremor (Supplementary Video 2).
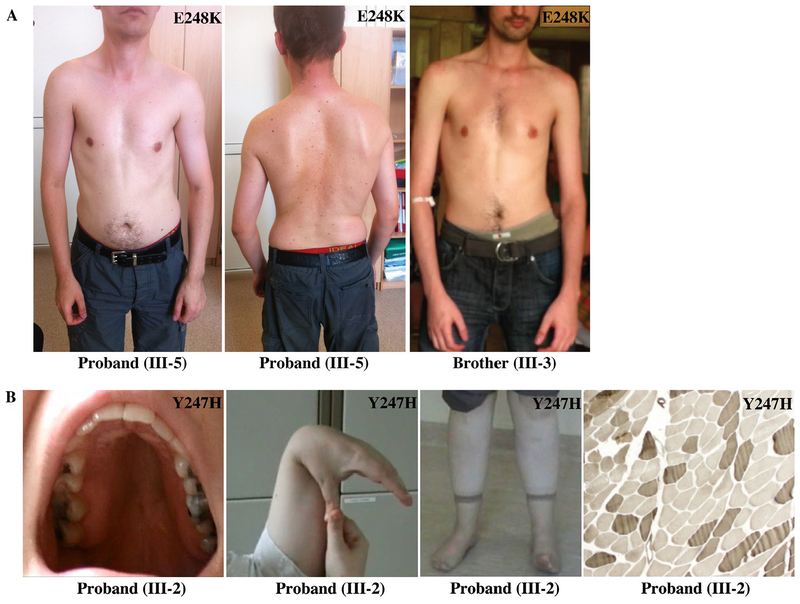
FIGURE 2:
Phenotypic traits of individuals carrying the Y247H and E248K MYBPC1 mutations. (A) Photographs of proband (III-5) and his brother (III-3) from family 1, who carry the E248K mutation, indicating the presence of thoracic and sternum deformities, scoliosis, and elbow contractures. (B) Photographs of the index patient (III-2) from family 2, who harbors the Y247H mutation, showing high arched palate, joint hypermobility, contracture of the ankle joint, and histochemical staining (ATPase, pH 9.4) of a biopsy from the deltoid muscle from III-2 showing type 1 fiber predominance (light fibers are type 1, dark fibers are type 2).
EMG performed in the proband revealed myopathic changes that were most pronounced in the arms. The family did not consent to a muscle biopsy.
To identify disease-associated genetic variants in this family, whole-exome sequencing analysis was performed on 3 of the affected members and 1 unaffected member, revealing the presence of a single, novel heterozygous variation in the MYBPC1 gene c.742G>A, p.(E248K) segregating in all 3 affected individuals, but not in the unaffected individual. Directed Sanger sequencing of DNA obtained from additional unaffected family members as well as of 90 Latvian control samples confirmed that the variant was confined to the 3 symptomatic family members (Fig 1B).
The index patient from family 2, a 50-year-old female (Fig 1C; proband III-2), was first diagnosed at age 46 with a history of slowly progressive, proximal, and axial weakness and myalgia since childhood (Fig 1C; Supplementary Table 1). Moreover, the patient reported myalgia and cramping in the calves at night or following strenuous exercise as well as immediate postural and tongue tremor following muscle exertion and prolonged tongue protrusion. Her symptoms have remained stable since her teenage years until the present, and activities of daily living are only mildly affected with minimal impairment (Supplementary Video 3). On examination, there was mild facial dysmorphia with micrognathia, prominent nasolabial folds, microstomia with pouting lips, and a high-arched palate (Fig 2B; Supplementary Table 1; Supplementary Video 3), in addition to mild facial myopathy with bilateral ptosis (left > right; Supplementary Table 1). Mild, symmetrical weakness (proximal > distal) of the arms and legs, marked axial muscle weakness and atrophy, abdominal muscle weakness, calf pseudohypertrophy, and mild bilateral scapular winging were also noted. The patient had mild axial and appendicular weakness and atrophy (proximal > distal), as well as mild weakness of foot dorsiflexion resulting in stepping gait (Supplementary Table 1). There were no cardiac or ocular symptoms, and hearing was intact.
EMG showed a myopathic pattern without spontaneous activity and a high-frequency postural tremor (10Hz) in the arms and hands. Nerve conduction studies excluded a neuropathy. Histopathological examination of a muscle biopsy obtained from the deltoid muscle revealed mild myopathic changes with NADH and ATPase stains revealing a marked preponderance of type 1 fibers, which, in general, appeared smaller than the few type 2 fibers (Fig 2B).
Examination of additional family members revealed the son (IV-1) and the granddaughter (V-1) of the index patient to be similarly affected, with mild axial and proximal weakness, high- arched palate, and a similar high-frequency, postural hand tremor (Supplementary Table 1; Supplementary Video 3). In all affected adult members, the clinical symptoms progressed slowly until the teen years and then reached a plateau. Patient V-2, a 6-month-old boy, has proximal muscle weakness with poor head control, and the family reported witnessing a hand tremor in this child as well. Power spectral tremor analysis performed in individuals III-2 and IV-1 from family 2 showed no resting tremor (Fig 3A,D). However, with outstretched hands, a narrow frequency peak in the 10 to 11 Hz range was noted by accelerometry in the left hand of both patients with a corresponding EMG peak of flexor and extensor forearm muscles (Fig 3B,E). Recordings with loaded hands showed identical accelerometric peak frequencies compared with the unloaded recording, again with synchronous EMG spectral peaks at 10 Hz (Fig 3C,F). In the right hand of patient IV-1, only a low-amplitude peak (10Hz) was noted with outstretched hands (Fig 3E), but no discernable peak, neither by accelerometry nor in the EMG, could be identified with loaded hands (Fig 3F). This corresponds with the clinical findings (Supplementary Video 3), where the right-sided tremor appeared less prominent and more irregular, thus preventing adequate Fourier analysis. In the right hand of patient III-2, a low-amplitude peak of 13Hz with corresponding EMG peaks of the same frequency was identified, both with unloaded and loaded hands (Fig 3B,C). This constellation of findings essentially excludes the mechanical reflex components of both physiological and enhanced physiological tremor, which are characterized by peripheral limb oscillations with reduction of accelerometric and EMG tremor frequencies after weight loading.31 Instead, the stability of frequency during loading as observed in our patients would typically be compatible with a centrally mediated component to this tremor. The existence of different tremor frequencies between both arms in patient III-2 (Fig 3B,C) implies multiple tremor pacemakers; this is corroborated by dual-channel EMG recordings in patient IV-1, which showed lack of intermuscular tremor synchronicity between both legs (Fig 3G), but retained synchronicity within the same leg (Fig 3H).
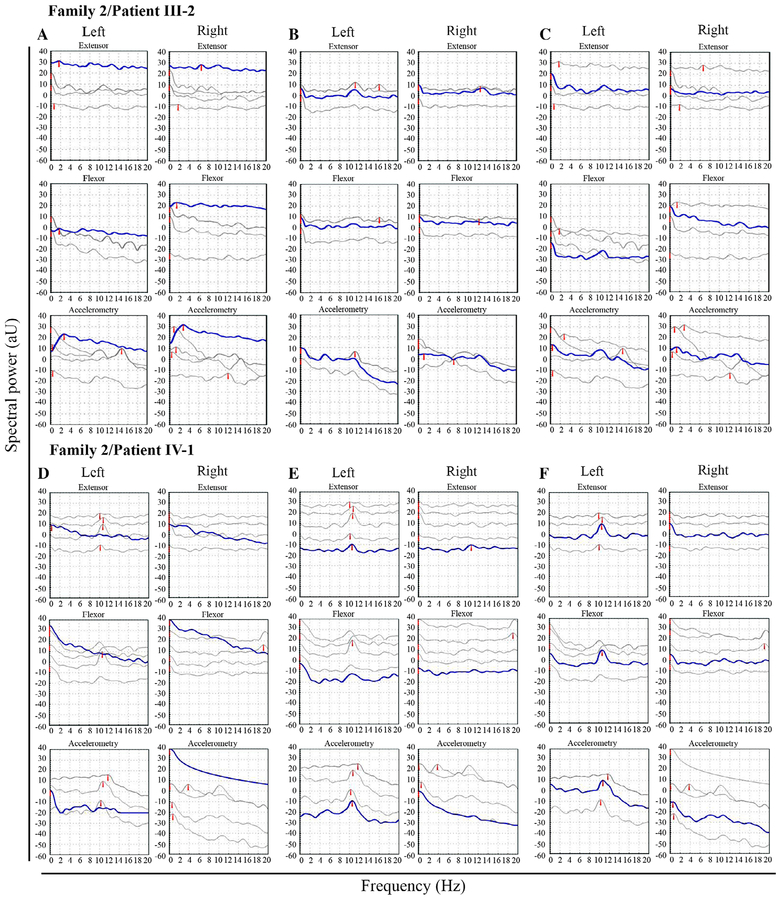
FIGURE 3:
Power spectral tremor analysis of individuals III-2 (A–C) and IV-1 (D–F) from family 2 at rest (A,D), with outstretched hands (B,E), and outstretched and loaded hands (C,F). Top panels in (A) to (F) show the electromyography (EMG) in the extensor muscles, the middle panels show the EMG in the flexor muscles, and the lower panels show the accelerometry recordings. At rest, there is no tremor (A,D); with outstretched hands (B,E), and with loaded hands (C,F), there is a tremor peak at 10 to 11Hz in the left hand of both patients, synchronous in the EMG and accelerometry. In the right hand of patient III-2, there is a low-amplitude peak of 13Hz with corresponding EMG peaks, both with unloaded (B) and loaded (C) hands, whereas no clear peak could be identified with loading in the right hand of patient IV-1 (F). Dual-channel EMG shows lack of intermuscular tremor synchronicity in patient IV-1 between the left and right leg (G), but retained synchronicity within the same leg (H). In panels(A) to (F), the multiple thin individual traces correspond to single measurements, whereas the thick line represents the best fit; please note that the red arrowheads were randomly added by the software. In panels (G) and (H), the subdivisions correspond to 100ms.
Next-generation sequencing was performed on a multi-gene panel, revealing a single, novel, heterozygous c.739T>Cp.(Tyr247His) sequence change in the MYBPC1 gene. This sequence change was identified in all affected individuals (III-2, IV-1, V-1, and V-2), but was not found in any of the unaffected individuals tested for the mutation (Fig 1C).
Neither sequence change (p.E248K or p.Y247H) is listed in the NHLBI ESP Exome Variant Server (~13,005 alleles; http://evs.gs.washington.edu/EVS/), the 1000 Genomes server (http://browser.1000genomes.org), or the Exome Aggregation Consortium (ExAC) and Genome Aggregation Database (gnomAD) reference databases, nor were they listed in the ClinVar or HGMD databases of known pathogenic variations preceding our submission of the variants, or in our in-house database of 90 Latvian samples.32–34 Multiple computational tools, including SIFT (http://sift.jcvi.org), PolyPhen2 (http://genetics.bwh.harvard.edu/pph2), Panther-PSEP (http://pantherdb.org/tools/csnpScoreForm.jsp), PaPI (http://papi.unipv.it/help.xhtml), PhDSNP (http://snps.biofold.org/phd-snp/phdsnp.html), SNP&GO (http://snps-and-go.biocomp.unibo.it/snps-and-go), and MutationTaster, supported the deleterious effects of both mutations on the protein, in addition to high phred-scaled CADD scores of 36 for the E248K mutation and 25 for the Y247H mutation.35–38
The Y247H and E248K mutations are located next to each other in the unique M-motif of sMyBP-C, which is constitutively expressed in all splice variants. Notably, the M-motif, along with the Pro/Ala–rich motif and Ig C1, support the direct interaction of sMyBP-C with actin and myosin S2 and modulate the formation of actomyosin cross-bridges in the force-generating cycle.9 To assess the effects of the individual mutations on the ability of the NH2 terminus of sMyBP-C to directly bind actin or myosin, we performed blot overlay assays. To this end, we generated GST-tagged recombinant proteins containing the NH2-terminal Pro/Ala–rich motif, Ig C1 and the M-motif (Pro/Ala-C1-M) in the absence and presence of the Y247H or the E248K mutation (Fig 4A), which we tested for their ability to bind actin or HMM immobilized on nitrocellulose membrane. We observed that both mutant proteins exhibited significantly increased binding to HMM (~2-fold for Y247H and ~3.5-fold for E248K) compared to wild-type protein (Fig 4B,C). Binding to actin appeared slightly increased for both mutations, too; however, this increase in binding did not reach statistical significance (Fig 4B,C).
FIGURE 4:
Examination of the effects of the Y247H and E248K mutations on the ability of the NH2 terminus of sMyBP-C to bind actin and myosin in vitro. (A) Coomassie blue–stained gel showing equivalent amounts (1.5μg) of control glutathione S-transferase (GST), wild-type GST-P/A-C1-M, or mutant GST-P/A-C1-M proteins used in the overlay assays. Full-length GST-P/A-C1-M recombinant proteins (~58kDa) are denoted with an arrowhead; a degradation product at ~50kDa is present in all three protein preparations. (B) Equivalent amounts (3μg) of purified heavy meromyosin (HMM) or actin were separated by SDS-PAGE, transferred to nitrocellulose membrane, and overlaid with 0.5μg/ml of control GST protein, wild-type GST-P/A-C1-M, or mutant GST-P/A-C1-M carrying the Y247H or E248K mutation. Nitrocellulose membranes (Ponceau stain) and films (western blots probed with GST-ab) were cropped to only include the area of expected signal. (C) Quantification of immunoblots following the overlay assays indicated that both mutant proteins exhibit significantly increased binding to HMM (~2-fold for Y247H and ~3.5-fold for E248K) compared to wild-type protein (n = at least three independent repeats; t test; *p < 0.04 and #p < 0.015, respectively); control GST protein did not bind to either myosin or actin. GST-ab = glutathione S-transferase antibody; SDS-PAGE = sodium dodecyl sulfate/polyacrylamide gel electrophoresis.
We next asked what molecular changes underlie the increased binding of the mutant sMyBP-C proteins to HMM, as compared to wild type. Whereas there is no high-resolution structure of the sMyBP-C M-motif available, the cardiac iso-form (80% identical) has been studied extensively.39 Much of the cardiac M-motif is highly dynamic, but a ~35-residue region (amino acids 233–268) folds into a stable three-helix bundle (Fig 5A). Using this structure as a template, we modeled the wild-type and mutant sMyBP-C M-motif and found that neither mutation significantly changed the overall domain fold (Fig 5B). Further analysis of these models showed that one face of the three-helix bundle is enriched in positive charges and both mutation sites expand this basic patch (Fig 5C). Circular dichroism spectra of sMyBP-C confirm that this section of the M-domain is mainly helical, and that the mutations show a similar, predominantly helical structure in agreement with our molecular dynamics (MD) simulations (Fig 5D).
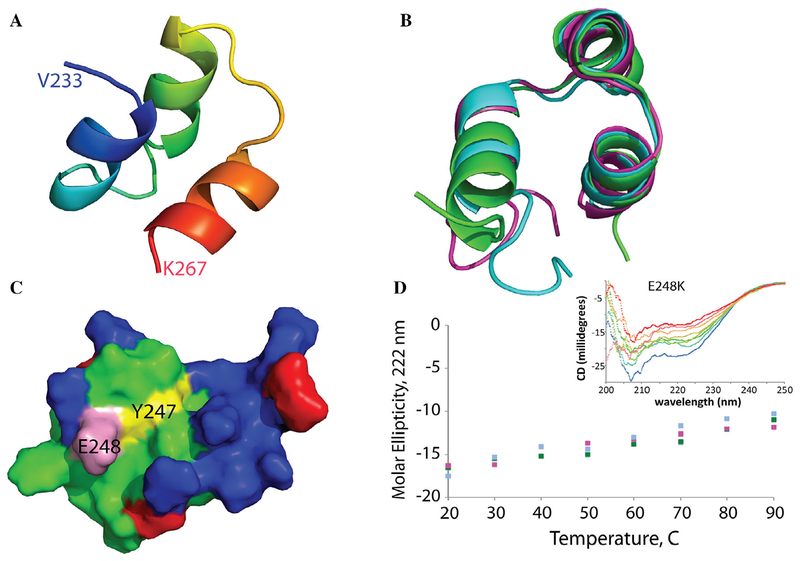
FIGURE 5:
Modeling of the sMyBP-C M-motif and effects of the Y247H and E248K mutations. (A) The NH2-terminal half of the M-motif of sMyBP-C (residues 186–233) is predicted to be partially disordered, whereas the COOH terminus (residues 234–268) folds into a well-ordered three-helix bundle, based on cardiac MyBP-C (80% identical; 92% similar for the M-motif; PDB: 2LHU).59 (B) Structure prediction programs and molecular dynamics (MD) simulations show this three-helix bundle to be stable, with similar structures for the mutants (wild type in green; Y247H in magenta; E248K in cyan). (C) The M-motif is highly charged, with extensive positive charges (colored in blue) on one side of the structure. Mutations are expected to further increase this concentration of positive charges by replacing a negative charge for a positive charge at the E248 position (pink) or adding a protonatable imidazole ring at the Y247 position (yellow). (D) These MD simulations agree with experimental circular dichroism data, which show the wild-type and mutant domains to be predominantly helical over a wide temperature range (wild type in green, Y247H in magenta, E248K in cyan; inset shows representative CD spectra over a 20°C–90°C temperature range).
Given the charge distribution and location of the mutations, we reasoned that the M-motif likely binds myosin using this basic side of the three-helix domain (Fig 5C). With this information, we docked this face of wild-type and both mutant models to acidic patches on the myosin S2 domain using HADDOCK. Of the four large acidic regions of S2 (Fig 6A, black and red boxes), productive interactions were successfully modeled for the two middle sites (Fig 5A, red boxes). Further MD analysis on these two sites suggested that the more NH2-terminal site, centering around myosin residue 898, moves extensively and is not well defined. In contrast, the more COOH-terminal site, surrounding residue 931, produces a stable complex, driven mostly through electrostatic interactions (Fig 6B). Both the Y247H and E248K mutated sites (Fig 6B, spheres) are proximal to this putative binding interface, and both mutant complexes increase the potential number of favorable electrostatic interactions with myosin. Thus, this model agrees well with our blot overlay data, presents a reasonable binding site on myosin for sMyBP-C, and provides a molecular rationale for why the sMyBP-C Y247H and E248K mutants bind more tightly to myosin. We surmise that this tighter binding to myosin interferes with normal cross-bridge kinetics and serves as the primary generator of the tremor, which, with subsequent central transformation, results in the clinical phenomenology observed in the patients.
FIGURE 6:
Computational modeling supports increased binding to myosin with the Y247H and E248K mutations. (A) Docking and molecular dynamics (MD) simulations predict that the folded M-motif region can bind and form stable interactions with human S2 myosin in two locations, centering around residues 898 and 931 (boxed in red; PDB: 2FXO).60 (B) One of these potential binding sites, around residue 931, forms multiple charge-charge interactions with the M-motif of MyBP-C. Both the Y247H and E248K mutations (shown as red spheres) are within the binding interface and add extra positive charges to stabilize this complex.
Discussion
In this study, we describe the clinical, genetic, and molecular findings in two unrelated, three-generation families, each of which harbors a different novel sequence change in MYBPC1 resulting in a mild skeletal myopathy with marked, unexplained tremor as the most distinguishing feature. Mild dysmorphic features were noted in some affected members of both families. Based on the familial segregation, absence from the general population, in-silico assessment, and increased target binding conferred by the substituted amino acids, the c.739T>C p.(Y247H) and c.742G>A p.(E248K) mutations can be classified as pathogenic according to American College of Medical Genetics and Genomics guidelines.40 Notably, a de-novo mutation in MYBPC1, c.885T>Gp.Leu295Arg, has been identified in a calf with weakness, stiffness, and muscle tremor.41 The c.885T>Gp.Leu295Arg mutation also resides in the M-motif of bovine MYBPC1 and corresponds to amino acid p.Leu263Arg in exon 11 of the human gene, the same exon affected in our families.
All affected individuals from both families presented with early-onset, mild, predominantly axial muscle weakness, contractures, and tremor of high frequency and irregular amplitude, primarily affecting the hands. Tremor was also noted, but to a lesser extent in the legs of some affected members of family 1 and family 2, and in the tongue of all affected members of family 2. Clinically, the tremor was elicited by muscle usage, such as in assuming postures and/or activity. It is of note that presence of the tremor permitted identification of a newborn child in family 2 as clinically affected even before the development of myopathy. Weakness and tremor progressed slowly during childhood until adolescent years, followed by stability at a moderate level of disabilities, preserving ambulation. An increased susceptibility for prolonged respiratory infections was reported by several affected members of family 1.
The joint contractures of the feet and/or elbows in all affected adults were not present at birth, but developed over the course of the disease and remained mild, and thus differ from the congenital distal contractures characteristic of MYBPC1 mutations associated with DA in both time of onset and severity. A muscle biopsy in the index patient from family 2 revealed type 1 fiber predominance with some smallness of type 1 fibers. This is a fairly nonspecific histological finding observed in a number of congenital myopathies, including those resulting from mutations in sarcomeric proteins. It is of interest, though, that muscle biopsies from DA1 patients have also shown type 1 fiber atrophy consistent with fiber-type disproportion.20
The E248K mutation results in substitution of a negatively charged amino acid (glutamic acid, E) by a positively charged one (lysine, K). This change would be predicted to affect the electrostatic interactions mediated by E248K, which, in turn, could alter the binding affinity of mutant sMyBP-C to myosin and actin. Similarly, the Y247H mutation results in substitution of an amino acid with a hydrophobic side chain (tyrosine, Y) by an amino acid with a potentially protonatable imidazole ring (histidine, H), again possibly altering the binding affinity of mutant sMyBP-C to myosin and actin, although likely to a lesser extent. Consistent with this notion, our in-vitro binding assays indicated that both mutations result in markedly increased binding of the NH2 terminus of the corresponding mutant, sMyBP-C, to the HMM portion of myosin, with the E248K mutation eliciting a more-pronounced effect. Molecular simulations of sMyBP-C binding to myosin are consistent with the notion of altered binding activity as a result of the mutations. Given that sMyBP-C functions as a regulator of cross-bridge formation, it will now be crucial to understand what exactly the consequences of the enhanced binding of mutant sMyBP-C to myosin would be on cross-bridge formation and cycling and on myosin ATPase activity. This will require further sophisticated biochemical and biophysical investigation along with the generation of the appropriate animal models.
The most distinctive aspect of this new phenotype associated with specific MYBPC1 mutations is the obligatory tremor in all mutation carriers, which also appeared to be a prominent feature of the spontaneous animal model of the disease.41 Detailed clinical tremor analysis, including physiological studies, in two patients from family 2 argued against both physiological and enhanced physiological tremor generated by mechanical reflex oscillations, although up to 10% of controls and of individuals with enhanced physiological tremor may show a weight-invariant tremor frequency. The lack of tremor frequency change after weight loading would typically be considered to be compatible with a central pacemaker at the origin of the tremor. However, given that the tremor perfectly segregated with the mutation in the two families so that the mutant sMyBP-C could be inferred as the cause of the tremor, and given that sMyBP-C is not expressed in the central nervous system or in peripheral nerves (Fig 7 and The Human Protein Atlas), it is unlikely that the originator or primary pacemaker of the tremor is located centrally.
FIGURE 7:
Examination of sMyBP-C expression in brain and spinal cord. Western blot analysis of protein lysates (20μg) prepared from mouse extensor digitorum longus (EDL) muscle, brain, or spinal cord. sMyBP-C is abundantly expressed in EDL muscle lysates, but is absent from all brain parts tested and the spinal cord. GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
Considering the subcellular location of MYBPC1 and based on our molecular and biochemical results, we postulate that the tremor-initiating event is located at the level of the sarcomere, which then, through a central loop, leads to synchronization and oscillation of the effector muscles resulting in the clinically visible and recordable tremor characteristics. This is not unlike the hypothesis put forward for tremor generation in Charcot-Marie-Tooth (CMT) disease, where weight-invariant postural tremors may be present.42 However, even though peripheral, in CMT disease the initial generator does not originate from the muscle itself, but is a result of its denervation, and is hypothesized to lead to an enhancement of the central neurogenic component of physiological tremor. Given that our patients do not have a neuropathy and MYBPC1 is expressed purely in skeletal muscle, we hypothesize instead that the tremor is sarcomeric and hence myogenic in origin, but, similar to the denervation tremor, is then picked up by stretch receptors, where it undergoes centrally looped propagation and enhancement, thereby rendering it clinically visible. This hypothesis is consistent with the tremor recordings in our patient and establishes “myogenic tremor” as a novel tremor class. We propose that the mutant sMyBP-C results in deregulated cross-bridge cycling, which, in addition to causing a mild deficit in force generation, may take on an oscillatory quality upon contraction, resulting in tremor. This concept of a “myogenic tremor” initiated by deregulation of binding cycles of sarcomeric proteins is corroborated by an animal model with a de-novo MYH7 mutation, which also showed a progressive high-frequency postural tremor43 resembling the tremor observed in our patients (Supplementary Table 2). Consistent with this, our group has also observed a similar tremor in a patient with a distinct de-novo MYH7 mutation (C.G.B. and D.H., unpublished observations). A search of the literature for mutations in additional sarcomeric proteins associated with myopathy and tremor revealed further cases of patients with mutations in genes encoding thick (MYH2 and MYL2) and thin (TNNT1, TPM3, and NEB) filament proteins (Supplementary Table 2),44–58 suggesting that cross-bridge dysregulation may emerge as a myogenic tremor generator beyond the MYBPC1 mutations reported here. Additional studies in transgenic animals to further elucidate the physiology of this novel type of tremor and get more insight into tremor-causing mechanisms are in preparation.
In summary, identification of the novel Y247H and E248K mutations in MYBPC1 define a new autosomal-dominant syndrome of mild skeletal myopathy with a distinctive tremor of likely myogenic origin, which we propose to add as a new tremor entity.
Supplementary Material
Acknowledgment
This work was supported by NIH/NIAMS (Training Program in Muscle Biology, T32 AR007592–17 to J.G. and R21AR072981 to A.K.K.), the Muscular Dystrophy Association (Research Grants 313579 and 602614 to A.K.K.), European Regional Development Fund (No. 2010/0223/2DP/2.1.1.1.0/10/APIA/VIAA/025 to B.L.), NSF RUI (MCB-1607024 to N.T.W.), and the Latvian Council of Science State funding (to B.L.). C.G.B. is supported by intramural funds from NIH/NINDS.
We express our deepest gratitude to all the patients and their families.
Footnotes
Potential Conflicts of Interest
Nothing to report.
Additional supporting information can be found in the online version of this article.
References
- 1.North KN, Wang CH, Clarke N, et al. Approach to the diagnosis of congenital myopathies. Neuromuscul Disord 2014;24:97–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravenscroft G, Laing NG, Bönnemann CG. Pathophysiological concepts in the congenital myopathies: blurring the boundaries, sharpening the focus. Brain 2015;138:246–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajsharghi H, Oldfors A. Myosinopathies: pathology and mechanisms. Acta Neuropathol 2013;125:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res 2004;94:1279–1289. [DOI] [PubMed] [Google Scholar]
- 5.Oakley CE, Chamoun J, Brown LJ, Hambly BD. Myosin binding protein-C: enigmatic regulator of cardiac contraction. Int J Biochem Cell Biol 2007;39:2161–2166. [DOI] [PubMed] [Google Scholar]
- 6.Ackermann MA, Kontrogianni-Konstantopoulos A. Myosin binding protein-C slow: an intricate subfamily of proteins. J Biomed Biotechnol 2010;2010:652065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rybakova IN, Greaser ML, Moss RL. Myosin binding protein C interaction with actin. J Biol Chem 2011;286:2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber FE, Vaughan KT, Reinach FC, Fischman DA. Complete sequence of human fast-type and slow-type muscle myosin-binding-protein C (MyBP-C): differential expression, conserved domain structure and chromosome assignment. Eur J Biochem 1993;216:661–669. [DOI] [PubMed] [Google Scholar]
- 9.Ackermann MA, Kontrogianni-Konstantopoulos A. Myosin binding protein-C slow is a novel substrate for protein kinase A (PKA) and C (PKC) in skeletal muscle. J Proteome Res 2011;10:4547–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann MA, Kerr JP, King B, et al. The phosphorylation profile of myosin binding protein-C slow is dynamically regulated in slow-twitch muscles in health and disease. Sci Rep 2015;5:12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann MA, Ward CW, Gurnett C, Kontrogianni-Konstantopoulos A. Myosin binding protein-C slow phosphorylation is altered in Duchenne dystrophy and arthrogryposis myopathy in fast-twitch skeletal muscles. Sci Rep 2015;5:13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geist J, Kontrogianni-Konstantopoulos A. MYBPC1, an emerging myopathic gene: what we know and what we need to learn. Front Physiol 2016;7:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Einheber S, Fischman DA. Isolation and characterization of a cDNA clone encoding avian skeletal muscle C-protein: an intracellular member of the immunoglobulin superfamily. Proc Natl Acad Sci U S A 1990;87:2157–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackermann MA, Kontrogianni-Konstantopoulos A. Myosin binding protein-C slow: a multifaceted family of proteins with a complex expression profile in fast and slow twitch skeletal muscles. Front Physiol 2013;4:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okagaki T, Weber FE, Fischman DA, et al. The major myosin-binding domain of skeletal muscle MyBP-C (C protein) resides in the COOH-terminal, immunoglobulin C2 motif. J Cell Biol 1993;123:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freiburg A, Gautel M. A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur J Biochem 1996;235:317–323. [DOI] [PubMed] [Google Scholar]
- 17.McGrath MJ, Cottle DL, Nguyen MA, et al. Four and a half LIM protein 1 binds myosin-binding protein C and regulates myosin filament formation and sarcomere assembly. J Biol Chem 2006;281: 7666–7683. [DOI] [PubMed] [Google Scholar]
- 18.Ackermann MA, Hu LY, Bowman AL, et al. Obscurin interacts with a novel isoform of MyBP-C slow at the periphery of the sarcomeric M-band and regulates thick filament assembly. Mol Biol Cell 2009; 20:2963–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Zhao TJ, Li J, et al. Slow skeletal muscle myosin-binding protein-C (MYBPC1) mediates recruitment of muscle-type creatine kinase (CK) to myosin. Biochem J 2011;436:437–445. [DOI] [PubMed] [Google Scholar]
- 20.Gurnett CA, Desruisseau DM, McCall K, et al. Myosin binding protein C1: a novel gene for autosomal dominant distal arthrogryposis type 1. Hum Mol Genet 2010;19:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Zhong B, Han W, et al. Two novel mutations in myosin binding protein C slow causing distal arthrogryposis type 2 in two large Han Chinese families may suggest important functional role of immunoglobulin domain C2. PLoS One 2015;10:e0117158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekhilevitch N, Kurolap A, Oz-Levi D, et al. Expanding the MYBPC1 phenotypic spectrum: a novel homozygous mutation causes arthrogryposis multiplex congenita. Clin Genet 2016;90:84–89. [DOI] [PubMed] [Google Scholar]
- 23.Timmer J, Lauk M, Deuschl G. Quantitative analysis of tremor time series. Electroencephalogr Clin Neurophysiol 1996;101:461–468. [PubMed] [Google Scholar]
- 24.Ackermann MA, Patel PD, Valenti J, et al. Loss of actomyosin regulation in distal arthrogryposis myopathy due to mutant myosin binding protein-C slow. FASEB J 2013;27:3217–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Källberg M, Wang H, Wang S, et al. Template-based protein structure modeling using the RaptorX web server. Nat Protoc 2012;7: 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Yan R, Roy A, et al. The I-TASSER suite: protein structure and function prediction. Nat Methods 2014;12:7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 2015;10:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krieger E, Vriend G. YASARA View—molecular graphics for all devices—from smartphones to workstations. Bioinformatics 2014;30: 2981–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu M, He J, Zhao Z, et al. Compressed glassy carbon: an ultrastrong and elastic interpenetrating graphene network. Sci Adv 2017;3:e1603213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Zundert GCP, Rodrigues JPGLM, Trellet M, et al. The HADDOCK2.2 Web Server: user-friendly integrative modeling of biomolecular complexes. J Mol Biol 2016;428:720–725. [DOI] [PubMed] [Google Scholar]
- 31.Raethjen J, Lauk M, Köster B, et al. Tremor analysis in two normal cohorts. Clin Neurophysiol 2004;115:2151–2156. [DOI] [PubMed] [Google Scholar]
- 32.Stenson PD, Ball EV., Mort M, et al. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 2003;21:577–581. [DOI] [PubMed] [Google Scholar]
- 33.Landrum MJ, Lee JM, Benson M, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res 2016; 44:D862–D868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009;4:1073–1081. [DOI] [PubMed] [Google Scholar]
- 36.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kircher M, Witten DM, Jain P, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 2014;46:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz JM, Cooper DN, Schuelke M, Seelow D. Mutationtaster2: mutation prediction for the deep-sequencing age. Nat Methods 2014;11:361–362. [DOI] [PubMed] [Google Scholar]
- 39.Haworth RS, Stathopoulou K, Candasamy AJ, Avkiran M. Neurohormonal regulation of cardiac histone deacetylase 5 nuclear localization by phosphorylation-dependent and phosphorylation-independent mechanisms. Circ Res 2012;110:1585–1595. [DOI] [PubMed] [Google Scholar]
- 40.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiedemar N, Riedi AK, Jagannathan V, et al. Genetic abnormalities in a calf with congenital increased muscular tonus. J Vet Intern Med 2015;29:1418–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saifee TA, Pareés I, Kassavetis P, et al. Tremor in Charcot-Marie-Tooth disease: no evidence of cerebellar dysfunction. Clin Neurophysiol 2015; 126:1817–1824. [DOI] [PubMed] [Google Scholar]
- 43.Murgiano L, Tammen I, Harlizius B, Drögemüller C. A de novo germline mutation in MYH7 causes a progressive dominant myopathy in pigs. BMC Genet 2012;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston JJ, Kelley RI, Crawford TO, et al. A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet 2000;67:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marra JD, Engelstad KE, Ankala A, et al. Identification of a novel nemaline myopathy-causing mutation in the troponin T1 (TNNT1) gene: a case outside of the old order Amish. Muscle Nerve 2015;51: 767–772. [DOI] [PubMed] [Google Scholar]
- 46.Abdulhaq UN, Daana M, Dor T, et al. Nemaline body myopathy caused by a novel mutation in troponin T1 (TNNT1). Muscle Nerve 2016;53:564–569. [DOI] [PubMed] [Google Scholar]
- 47.Donkervoort S, Papadaki M, de Winter JM, et al. TPM3 deletions cause a hypercontractile congenital muscle stiffness phenotype. Ann Neurol 2015;78:982–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans JM, Cox ML, Huska J, et al. Exome sequencing reveals a nebulin nonsense mutation in a dog model of nemaline myopathy. Mamm Genome 2016;27:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darin N, Kyllerman M, Wahlström J, et al. Autosomal dominant myopathy with congenital joint contractures, ophthalmoplegia, and rimmed vacuoles. Ann Neurol 1998;44:242–248. [DOI] [PubMed] [Google Scholar]
- 50.Martinsson T, Oldfors A, Darin N, et al. Autosomal dominant myopathy: missense mutation (Glu-706 → Lys) in the myosin heavy chain IIa gene. Proc Natl Acad Sci U S A 2000;97:14614–14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scoppetta C, Casali C, La Cesa I, et al. Infantile autosomal dominant distal myopathy. Acta Neurol Scand 1995;92:122–126. [DOI] [PubMed] [Google Scholar]
- 52.Voit T, Kutz P, Leube B, et al. Autosomal dominant distal myopathy: further evidence of a chromosome 14 locus. Neuromuscul Disord 2001;11:11–19. [DOI] [PubMed] [Google Scholar]
- 53.Meredith C, Herrmann R, Parry C, et al. Mutations in the slow skeletal muscle fiber myosin heavy chain gene (MYH7) cause laing early-onset distal myopathy (MPD1). Am J Hum Genet 2004;75:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamont PJ, Udd B, Mastaglia FL, et al. Laing early onset distal myopathy: slow myosin defect with variable abnormalities on muscle biopsy. J Neurol Neurosurg Psychiatry 2006;77:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muelas N, Hackman P, Luque H, et al. MYH7 gene tail mutation causing myopathic profiles beyond Laing distal myopathy. Neurology 2010;75:732–741. [DOI] [PubMed] [Google Scholar]
- 56.Lefter S, Hardiman O, McLaughlin RL, et al. A novel MYH7 Leu1453pro mutation resulting in Laing distal myopathy in an Irish family. Neuromuscul Disord 2015;25:155–160. [DOI] [PubMed] [Google Scholar]
- 57.Cullup T, Lamont PJ, Cirak S, et al. Mutations in MYH7 cause Multi-minicore Disease (MmD) with variable cardiac involvement. Neuromuscul Disord 2012;22:1096–1104. [DOI] [PubMed] [Google Scholar]
- 58.Weterman MAJ, Barth PG, van Spaendonck-Zwarts KY, et al. Recessive MYL2 mutations cause infantile type I muscle fibre disease and cardiomyopathy. Brain 2013;136(pt 1):282–293. [DOI] [PubMed] [Google Scholar]
- 59.Howarth JW, Ramisetti S, Nolan K, et al. Structural insight into unique cardiac myosin-binding protein-C motif: a partially folded domain. J Biol Chem 2012;287:8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blankenfeldt W, Thoma NH, Wray JS, et al. Crystal structures of human cardiac beta-myosin II S2-Delta provide insight into the functional role of the S2 subfragment. Proc Natl Acad Sci U S A 2006; 103:17713–17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.