Abstract
Most clinically approved drugs (primarily small molecules or antibodies) are rapidly cleared from circulation and distribute throughout the body. As a consequence, only a small portion of the dose accumulates at the target site, leading to low efficacy and adverse side effects. Therefore, new delivery strategies are necessary to increase organ and tissue-specific delivery of therapeutic agents. Nanoparticles provide a promising approach for prolonging the circulation time and improving the biodistribution of drugs. However, nanoparticles display several limitations, such as clearance by the immune systems and impaired diffusion in the tissue microenvironment. To overcome common nanoparticle limitations various functionalization and targeting strategies have been proposed. This review will discuss synthetic nanoparticle and extracellular vesicle delivery strategies that exploit organ-specific features to enhance drug accumulation at the target site.
Keywords: extracellular vesicles, exosomes, nanoparticles, nanomedicine, organotropic
1. Introduction
A major goal of medicine is to develop treatment strategies that target specific organs, tissues, and cells (Kwon, Lee, Han, & Park, 2012; Tiwari, et al., 2012). Therapeutic agents that are effective in vitro often fail in vivo due to biological barriers that prevent site-specific accumulation (Barua & Mitragotri, 2014; Blanco, Shen, & Ferrari, 2015). In fact, conventional medications are usually subject to short circulation half-lives and rapid renal clearance (Podust, et al., 2016). As a consequence, only around 0.001–0.01% of the administered therapeutic dose accumulates at the target site (K. C. P. Li, Pandit, Guccione, & Bednarski, 2004; Wolfram, Shen, & Ferrari, 2015). Dose limitations due to side effects further restrict drug accumulation in diseased tissues (Tamargo, Le Heuzey, & Mabo, 2015). Thus, new strategies are essential to increase site-specific delivery of therapeutic agents. Nanomedicine, which consists on the application of synthetic or biological nanoparticles (NPs) for medical purposes, is a promising approach for overcoming barriers in the body (Salata, 2004; Wagner, Dullaart, Bock, & Zweck, 2006; Wolfram & Ferrari, 2019; Wolfram, Zhu, et al., 2015). In particular, nanoparticles can act as carriers for the delivery of a wide variety of drugs to diseased tissues.
Nanosized particles can be synthesized from a plethora of materials, such as lipids (Shen, Kim, et al., 2017; Wolfram, et al., 2016; Wolfram, Suri, Huang, et al., 2014), polymers (Molinaro, et al., 2013; Y. Yang, Wolfram, Fang, Shen, & Ferrari, 2014), metals (Mu, et al., 2018; Shen, Kim, Mu, et al., 2014), silica (Shen, Kim, Su, et al., 2014; Shen, Liu, et al., 2017), and silicon (Shen, et al., 2013), or isolated from biological sources. NPs exhibit unique properties that are lacking on the macro and molecular scale (Baer, 2018; Navya & Daima, 2016). For instance, NPs have high surface area to volume ratios (Baer, 2018) that can be exploited for e.g. protein (Saha, Evers, & Prins, 2014) and polymer (Makadia & Siegel, 2011; Thamake, Raut, Gryczynski, Ranjan, & Vishwanatha, 2012) functionalization to prolong circulation and bestow specific targeting abilities (Dhar, Gu, Langer, Farokhzad, & Lippard, 2008; Jokerst, Lobovkina, Zare, & Gambhir, 2011; Paolino, et al., 2014; Scavo, et al., 2015). In addition to the material properties, the shape (Caldorera-Moore, Guimard, Shi, & Roy, 2010; Kinnear, Moore, Rodriguez-Lorenzo, Rothen-Rutishauser, & Petri-Fink, 2017; Longmire, Ogawa, Choyke, & Kobayashi, 2011) and size (Betzer, et al., 2017; Jiang, Kim, Rutka, & Chan, 2008; Prisner, Bohn, Hahn, & Mews, 2017) of nanoparticles can confer specific transport abilities in the body. In particular, NP properties can be tailored to enhance interactions with tissue microenvironments that display distinct characteristics, including structural components, interstitial pressure (Torosean, et al., 2013), pH (Du, Lane, & Nie, 2015), and biomolecules (Friedman, Claypool, & Liu, 2013). Consequently, site-specific delivery of NPs is usually superior to that of small molecules (100 to 1000-fold improvement) (Wolfram, et al., 2017).
Traditionally, NP drug delivery approaches have been divided into active and passive targeting strategies (Bertrand, Wu, Xu, Kamaly, & Farokhzad, 2014; R. Li, Zheng, Yuan, Chen, & Huang, 2017). Active targeting involves utilization of NP surface molecules that preferentially bind to the tissue of interest (Bazak, Houri, El Achy, Kamel, & Refaat, 2015), while passive targeting involves exploitation of physical properties, such as NP shape and size (Bertrand, et al., 2014; E. Gentile, et al., 2013). An example of active targeting is the coupling of transferrin to NPs to target pathological cells that express high levels of transferrin receptors (Gan & Feng, 2010). The most common example of passive targeting is exploitation of immature leaky vasculature and impaired lymphatic drainage for intratumoral accumulation of NPs, known as the enhanced permeability and retention (EPR) effect (Greish, 2010). The EPR effect can be enhanced through NP pegylation, which attracts a protective water shell that hides NPs from macrophages and enhances circulation half-life (Jokerst, et al., 2011; Pasut, et al., 2015; Wolfram, Suri, Yang, et al., 2014). NP shapes can also be optimized for improved biodistribution and site-specific delivery (Kinnear, et al., 2017). For example, discoidal particles pioneered by Dr. Mauro Ferrari mimic the dimensions of platelets to exploit fluid dynamics for adherence to inflamed vasculature (Decuzzi, et al., 2010; Mi, Mu, et al., 2016; Mi, Wolfram, et al., 2016; Shen, et al., 2015; Venuta, Wolfram, Shen, & Ferrari, 2017).
Despite major nanoparticle-mediated improvements in the biodistribution of drugs, analysis of more than 100 nanodelivery studies demonstrated that changing the material, shape, size, and charge of NPs results in minimal improvements in intratumoral accumulation (Wilhelm, 2016). Notably, tumor type, stage, and model had a greater impact on site-specific delivery compared to nanoparticle properties, indicating that the macro and microenvironment play a crucial role in promoting NP adhesion, extravasation, and tissue penetration (Wilhelm, 2016). Additionally, the success of nanomedicine is mainly hampered by the immune system (Boraschi, Castellano, & Italiani, 2017) and anatomical obstacles, such as the endothelium (Blanco, et al., 2015). One of the first barriers encountered upon intravenous injection is the mononuclear phagocyte system (MPS), which is composed of specialized phagocytic cells in the lymph nodes, spleen, and liver. The phagocytes are responsible for rapid clearance of nanosized endogenous and exogenous circulating material (Samuelsson, Shen, Blanco, Ferrari, & Wolfram, 2017). Additionally, the adaptive immune system produces antibodies against immunogenic molecules, leading to a potent immune response against subsequent exposure, which can also compromise NP therapy. For example, the formation of antibodies against molecules used for NP functionalization, such as polyethylene glycol (PEG), has been reported in animal models (Kierstead, et al., 2015). Therefore, nanoparticle design strategies can be combined with other approaches that aid in overcoming biological barriers. For instance, optimization of the administration route can substantially improve biodistribution, as exemplary demonstrated by intranasal administration of nanoparticles to overcome the blood brain barrier (BBB) (Patel, Patel, & Patel, 2018). Additionally, temporary inactivation of resident macrophages in the liver through pretreatment with pharmacological agents was shown to reduce immunological clearance of NPs, leading to improved site-specific delivery (Khalid, et al., 2016; Pelt, et al., 2018; Wolfram, et al., 2017).
Another approach to overcome barriers in the body is exploitation of biological components that have been optimized through evolution for effective navigation within and between compartments in the body. One example is Abraxane, a United States Food and Drug Administration (FDA) approved NP, that contains albumin and paclitaxel (Peer, et al., 2007). Albumin is the most abundant protein in the plasma and a carrier for endogenous hydrophobic molecules (Fanali, et al., 2012). carriers for short and long-distance transportation of biomolecules (Busatto, et al., 2018; Théry, et al., 2018; Vader, Mol, Pasterkamp, & Schiffelers, 2016). It has been shown that EVs exhibit organ-specific targeting abilities that are at least partially due to integrins, but most likely attributed to the interplay of several EV components (Hoshino, et al., 2015). In particular, Hoshino, each EV population mirrored the organ tropism of the secreting cell line promoting the formation of the pre-metastatic niche (Hoshino, et al., 2015). EVs were also able to educate specific tumor cells to colonize to organs that were not preferred sites of metastasis (Hoshino, et al., 2015; Nogues, Benito-Martin, Hergueta-Redondo, & Peinado, 2018). Consequently, exploitation of EVs as drug carriers presents a promising opportunity for obtaining site-specific delivery. The vast majority of synthetic and EV-based nanodelivery strategies have focused on exploiting common characteristics of the tumor microenvironment, and several reviews have discussed this topic in detail (S. Chen, et al., 2016; Chulpanova, Kitaeva, James, Rizvanov, & Solovyeva, 2018; Wilhelm, 2016; S. Yang & Gao, 2017).
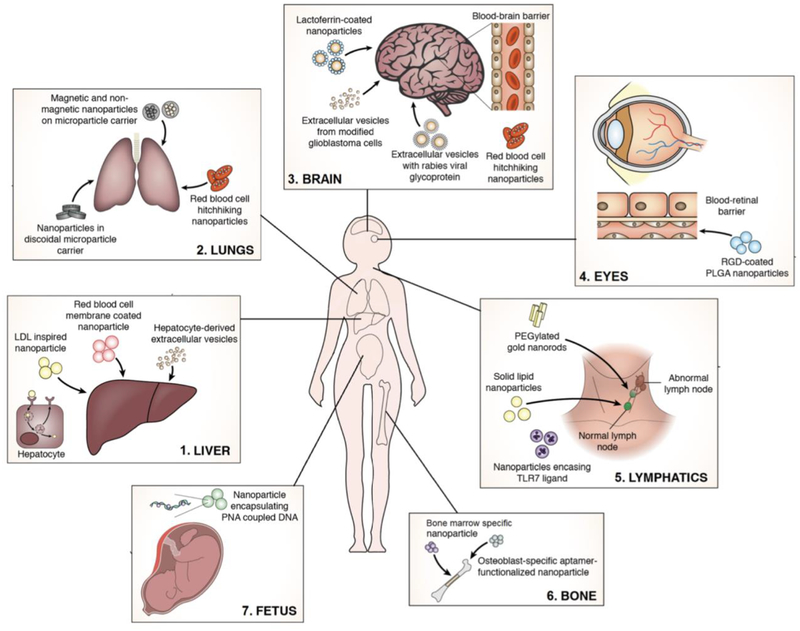
Furthermore, innovative approaches for synthetic NP targeting and novel advances to deliver therapy in utero are emerging. Even though NP modifications augmenting site specific drug delivery has advanced, differences in macro environments make some organs (liver and lungs) more suitable for NP uptake than others (brain, eyes, lymphatics, and bone). For example, resident macrophages in the liver promote organotropic homing of NPs making hepatic tissue an ideal target for NP-based therapeutics (Borrelli, et al., 2018), while the brain and eyes are limited by specialized endothelial barriers (Del Amo & Urtti, 2008; Hersh, et al., 2016). The purpose of this review is to provide an overall perspective of NP-based drug delivery strategies designed to exploit organ-specific properties (Fig. 1).
Figure 1.
Schematic of site-specific delivery strategies for nanotherapeutics to the brain, liver, bone, eye, lymphatic system, and fetus.
2. Liver delivery
A large portion of systemically injected nanoparticles accumulate in the liver due to resident macrophages (Gustafson, Holt-Casper, Grainger, & Ghandehari, 2015). Despite this hepatic tropism, the intra-organ biodistribution is often unfavorable for the treatment of liver diseases. Studies have focused on specifically delivering NPs to hepatocytes for improved therapeutic effects. For example, cationic solid lipid nanoparticles (CSLN) were designed to have a similar lipid composition as natural low-density lipoprotein (LDL), enabling hepatocyte recognition and intake of nanoparticles via lipoprotein receptors (Kim, et al., 2008; Kong, et al., 2013). The CSLN can form a complex with negatively charged siRNA that can silence profibrogenic growth factors genes. This complex protects siRNA from enzymatic degradation during circulation, while increasing specificity for hepatocytes. In vitro, the CSLN/siRNA complex displayed higher uptake and improved gene silencing in HepG.2.2.15 cells compared to transfection with the commercially available agent, Lipofectamine. CSLN/siRNA treatment of rats with liver fibrosis reduced connective tissue growth factor (CTGF) expression and reversed the hepatic cell structure and lobular architecture to resemble that of non-fibrotic rats (Kong, et al., 2013).
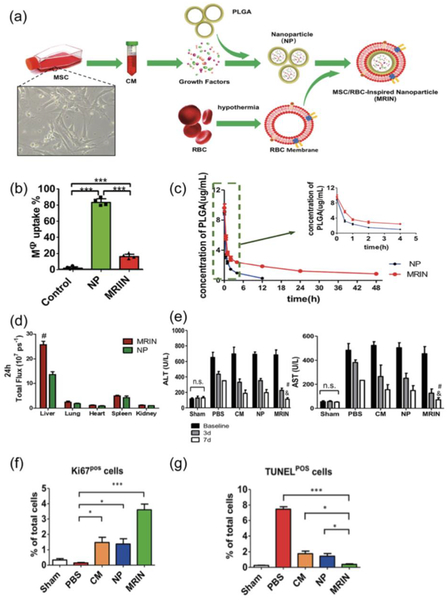
A study by Liang et al. explored the use of lyophilized conditioned media from mesenchymal stem cells (MSCs) in PLGA NPs encased by a red blood cell membrane for treatment of liver failure (Fig. 2a) (H. Liang, et al., 2018). MSCs and the MSC-derived secretome have previously shown to be effective in the treatment of acute liver failure in animal models and in clinical trials (Lin, et al., 2017; Shi, et al., 2017). Previous studies using RBC-coated NPs have demonstrated an increased circulation half-life due to the preservation of membrane proteins essential for long-term RBC blood circulation (Mohandas & Gallagher, 2008; Piao, et al., 2014). The RBC membrane prevents detection and uptake of NPs by macrophages, while the nanoscale size (200 nm) enables the NPs to bypass filtration in the lungs (H. Liang, et al., 2018). In cell culture, it was shown that 20% of the red blood cell membrane-coated NPs were endocytosed by macrophages, while the corresponding value was 80% for noncoated ones (Fig. 2b) (H. Liang, et al., 2018). In animal models, the coated NPs had a prolonged circulation time (Fig. 2c) and increased accumulation in the liver (Fig. 2d) compared to uncoated NPs (H. Liang, et al., 2018). Treatment with the coated NPs also lead to a greater improvement in survival of mice with liver failure induced by carbon tetrachloride (CCU) (H. Liang, et al., 2018). The coated NPs were also superior at improving the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), indicators of liver functionality (Fig. 2e), as well as promoting a normal liver architecture (Fig. 2f, g) (H. Liang, et al., 2018).
Figure 2.
Liver delivery of intravenously injected mesenchymal stem cell/red blood cell (RBC)-inspired nanoparticle (MRIN) in a mouse model of acute liver failure. a Schematic of MRIN fabrication: growth factors are isolated from mesenchymal stem cell-conditioned media (CM) and encapsulated in poly(lacticco-glycolic acid) (PLGA) nanoparticles (NPs). NPs are then coated with cell membranes obtained from RBCs resulting in the final MRIN product. b Macrophage uptake of fluorescently labeled particles (n = 4). c Concentration of uncoated NPs and MRINs in blood (n = 5). d Analysis of particle accumulation in organs 24 hours post treatment (n = 3). Data expressed as mean values ± s.d. #, P < 0.01 compared to uncoated NPs. e Serum concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) following carbon tetrachloride (CCl4)-induced liver injury (n = 3). #, P < 0.05 compared to uncoated NP group; &, P < 0.05 compared to CM group. f Quantitation of proliferative cells via Ki67 staining (n = 3). *. P < 0.05; ***, P < 0.001. g Quantitation of apoptotic cells via terminal deoxynucleotidyl transferase-mediated 2′ -deoxyuridine, 5′-triphosphate (dUTP) nick end labeling (TUNEL) (n = 3). *, P < 0.05; ***; P < 0.001. Reproduced from Liang, et al. 2018 with permission.
EVs from hepatocytes have also been used to promote post-injury liver regeneration by delivery of endogenous biomolecules, such as sphingosine kinase 2 (SK2), which stimulates cell proliferation (Nojima, et al., 2016). Treatment with these self-tropic EVs led to an increase in hepatocyte proliferation in mouse models of ischemia/reperfusion (I/R) injury and partial hepatectomy. In addition to utilizing targeting properties and endogenous therapeutic biomolecules in EVs, exogenous drugs have been loaded into EVs to further improve therapeutic efficacy (Borrelli, et al., 2018). Taken together, various biomimicry approaches have shown promise for liver and hepatocyte-specific targeting.
3. Lung delivery
Lung cancer and other pulmonary disorders affect a large portion of the population and have high mortality rates. As is the case for most solid tumors, a small fraction of intravenously injected drugs reach lung cancer cells (Primeau, Rendon, Hedley, Lilge, & Tannock, 2005). The pulmonary administration route is a promising alternative for delivery of NPs to lung tissue, as this method substantially increases site-specific exposure to therapeutic agents while reducing systemic side effects (De Backer, et al., 2015; Rahhal, et al., 2016; Tewes, Gobbo, Ehrhardt, & Healy, 2016). In particular, NPs have shown promise as inhaled drug carriers for chemotherapeutic agents (Y. Chen, et al., 2016; Nagesh, et al., 2016; Norgaard, et al., 2010). A study by Abbas et al. sought to improve inhalation of NPs by incorporating them into larger microparticles (MPs), as previous research has demonstrated the failure of NPs to deposit deep into lung tissue due to size-dependent ease of exhalation. The use of aerodynamically favorable MPs as a carrier can aid in lung deposition (Ali & Lamprecht, 2014; Rytting, Nguyen, Wang, & Kissel, 2008). Aerodynamics can be evaluated with a Next Generation Impactor (NGI), in which particles are dispersed in an air stream traveling through eight stages of NGI based on aerodynamic cutoff diameters. The results of this model suggest that approximately 10% of the MP dose would be retained in the upper respiratory track, while 90% would travel to the lower conducting and respiratory tracts, demonstrating the value of this model in aiding particle design for improved pulmonary delivery (Abbas, et al., 2016). In addition to improving lung delivery, the authors sought to improve drug release through the use of magnetic and non-magnetic NPs, both containing epidermal growth factor receptor (EGFR) antibodies for targeting cancer cells. By administering both nanoparticles via MPs, a sustained basal drug release with intermittent increases in drug release via application of an alternating high frequency magnetic field (HFMF) could be established. This method of combined sustained and intermittent drug release has been shown improve therapeutic results in cancer treatment (Celikoglu, Celikoglu, & Goldberg, 2008; Howell, 2001). Other studies have examined how to optimize NP design for lung targeting trough intravenous administration. A study by Decuzzi et al. examined how the size and shape of NPs and MPs affects their accumulation in specific organs. Mice were injected with particles with varying shapes and sizes. After two to six hours, the mice were euthanized, and organs (liver, spleen, heart, lung, kidneys, and brain) were harvested and examined. The authors noted that discoidal particles were found to be advantageous for lung treatment with the results showing discoidal particle accumulation in the lungs to be four times higher than that of spherical particles (Decuzzi, et al., 2010). Mathematical modeling and experimental studies with discoidal particles have demonstrated improved adherence to the blood vessel wall when compared to other particle shapes (Decuzzi & Ferrari, 2006; F. Gentile, et al., 2008; Lee, Ferrari, & Decuzzi, 2009). Additionally, discoidal particles have been shown to avoid internalization by various cell types, possibly reducing uptake by liver macrophages (Champion & Mitragotri, 2006; Gratton, et al., 2008). A study by Mi et al. examined the use of discoidal MPs as carriers for therapeutic agents encapsulated into NPs for the treatment of melanoma lung metastases (Y. Mi, C. Mu, et al., 2016). The discoidal MPs were selected in order to increase lung accumulation and enable simultaneous delivery of multiple types of NPs for combination therapy. Specifically, liposomes carrying small interfering RNA (siRNA) and docetaxel-encapsulated poly(lactide-coglycolide) (PLGA) PEG NPs were incorporated in the same MP (Y. Mi, C. Mu, et al., 2016). Mice studies revealed that liposome delivery resulted in siRNA retention solely in the kidneys, whereas MP-liposome delivery led to siRNA accumulation predominantly in the lungs after 24 hours (Y. Mi, C. Mu, et al., 2016). Similar results were observed when comparing PLGA-PEG NPs to NPs complexed with MPs; the NP only treatment resulted in significant accumulation in the liver, while the MP delivery system resulted in high lung retention. Analysis of cell-specific accumulation demonstrated that siRNA uptake in lung cancer cells was ten times higher with the MP platform compared to liposome delivery. Treatment with MPs resulted in longer survival times and significant reductions in metastatic nodules (Y. Mi, C. Mu, et al., 2016). MPs were shown to be superior in treating mice when compared to other treatment groups, including combination therapy with siRNA-loaded liposomes and docetaxel-loaded PLGA-PEG NPs.
Another study explored the use of red blood cells (RBCs) as NP carriers for lung delivery. This RBC-hitchhiking (RH) strategy involved intravenous injection of NPs adhered to RBCs through mixing (RH) (Brenner, et al., 2018). As the RBCs pass through the first capillary bed downstream of the injection, i.e. the pulmonary capillary bed, NPs transfer to the blood vessel wall (Brenner, et al., 2018). Mice studies showed a significant improvement in lung accumulation when comparing RH NPs to NP alone. Several NPs were tested using the RH method, all of which showed a substantial increase in lung uptake when compared to free NPs. The fold improvement ranged from 4-fold for albumin-NPs and 40-fold for liposomes (Brenner, et al., 2018). The efficacy of this treatment was demonstrated across multiple species: mice, rats, pigs, and humans. The experiment that determined the performance of RH treatment in humans was carried out ex vivo through injection into the pulmonary artery in human lungs that were unsuitable for transplantation. Analysis of the human lungs revealed that 41% of the injected RH NPs was deposited in the lung compared to 15–20% of the free NPs (Brenner, et al., 2018).
Overall, these studies suggest that injection route and particle dimensions play a major role in lung delivery. In particular, inhaled MPs have been shown to increase drug bioavailability in the lungs, while discoidal MPs and RH improve intravenous treatment of lung pathologies.
4. Brain delivery
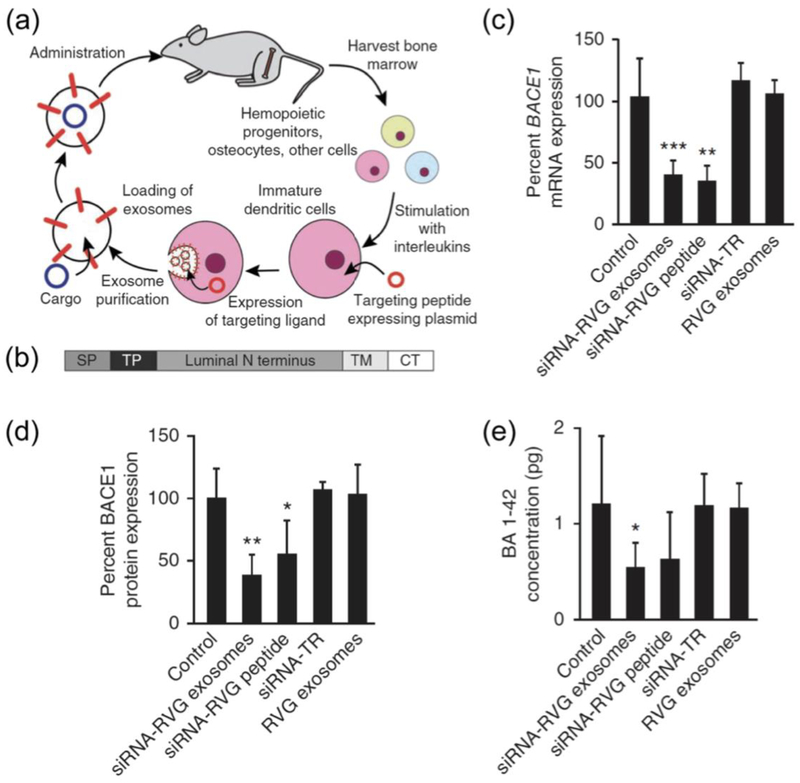
The BBB separates and protects the central nervous system (CNS) from the systemic blood circulation (Daneman & Prat, 2015). This barrier is composed of endothelial cells with tight junctions that line the lumen of cerebral capillaries forming a physical obstacle that permits passage of a select few small molecules (Ballabh, Braun, & Nedergaard, 2004; Pandey, Sharma, & Gupta, 2016). The BBB is an example of a biological barrier that substantially limits organotropic drug delivery (Hersh, et al., 2016). Emphasis has been placed on designing drug delivery vehicles to enable therapeutic agents to reach the CNS. For example, engineered EVs have shown promise in delivering small interfering RNA (siRNA) to the brain following intravenous injection. Dendritic cells were transfected with a plasmid encoding for a fusion protein composed of the EV membrane protein, lysosome-associated membrane protein 2 (Lamp2b), the CNS-specific rabies viral glycoprotein (RVG), and a FLAG epitope (Fig. 3a,b) (Alvarez-Erviti, et al., 2011). EVs with the RVG peptide were purified from cell media and loaded with siRNA through electroporation (Alvarez-Erviti, et al., 2011). The siRNAs were against beta-amyloid cleaving enzyme 1 (BACE1) protease that is involved in the enzymatic cascade that causes B-amyloid peptide aggregation in Alzheimer’s pathogenesis. In vitro results showed that RVG-EVs had a delivery efficiency comparable to cationic liposomes, resulting in an equal and dose-dependent gene knock down without evident cytotoxic effects (Alvarez-Erviti, et al., 2011). Notably, in vivo studies demonstrated that intravenously injected EVs efficiently targeted brain tissue delivering exogenous genetic material without inducing inflammatory responses (Alvarez-Erviti, et al., 2011). Specifically, the EVs caused a substantial decrease in BACE1 mRNA (61%) (Fig. 3c) and protein (62%) (Fig. 3d) levels in cortical tissue (Alvarez-Erviti, et al., 2011). RVG-EV therapy also decreased the concentration of the β-amyloid 1–42 peptide (55%), which is one of the major components of amyloid plaques (Fig. 3e) (Alvarez-Erviti, et al., 2011).
Figure 3.
Brain delivery of intravenously injected extracellular vesicles with small interfering RNA (siRNA) against beta-amyloid cleaving enzyme (BACE1) in a wild type mouse model. a Schematic of engineering, collection and administration of targeted extracellular vesicles for siRNA delivery. b Schematic representation on the fusion protein composed of: Lamp2b protein. SP, signal peptide; TP, targeting peptide; TM, transmembrane domain; CT, C terminus. c BACE1 mRNA levels in the brain cortical region (normalized to 18S RNA levels). d BACE1 protein levels in brain tissue. e Quantification of β-amyloid 1–42 (BA 1–42) in brain tissue samples. Data is presented as mean ± s.d. (n = 5). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to untreated control. Reproduced from Alvarez-Erviti, et al., 2011 with permission. RVG, rabies virus glycoprotein; TR in vivo transfection reagent.
In addition to the use of viral peptides, NPs can be functionalized with endogenous ligands that recognize receptors that are overexpressed in the CNS. For example, neuronal cells of patients affected by age related neurodegenerative pathologies have increased levels of lactoferrin receptors (Qian & Wang, 1998), which can be targeted with NPs. Specifically, lactoferrin-coated pegylated polymeric NPs have been used for delivery of neuroprotective molecules for the treatment of Alzheimer’s disease in animal models (Liu, et al., 2013). The intranasal administration route proved beneficial for bypassing the BBB, and NPs protected cargo against mucus and enzymes that compromise penetration and integrity in the nasal cavity. In mice studies, these intranasally administered lactoferrin-NPs loaded with a neuroprotective agent demonstrated a dose-dependent effect with an amelioration of cognitive defects (Liu, et al., 2013). Notably, the lactoferrin-NPs were superior to the neuroprotective agent alone or untargeted NPs (Liu, et al., 2013).
EV carriers for brain delivery have also been administered through the intranasal route (Sterzenbach, et al., 2017). For example, human glioblastoma cells were transfected with a plasmid encoding Cre recombinase with a WW tag that promotes loading into EVs (Riling, et al., 2015; Sterzenbach, et al., 2017). EVs were then administered to transgenic mice carrying a stop cassette that inhibited expression of a gene encoding for a red fluorescent protein. Analysis of brain tissue demonstrated a high number of red fluorescent neurons (Sterzenbach, et al., 2017), indicating that EV-based intranasal enzyme delivery was successful.
Delivery of NPs to the brain has also been enhanced utilizing RBCs (Brenner, et al., 2018). Brenner et al. achieved improved NP-delivery to the brain after internal carotid artery injection of RH. Specifically, brain-specific uptake was 11.5% of the injected dose and the RH strategy displayed higher brain-to-blood ratios (27 fold) and brain to liver ratios (143 fold) compared to free NPs (Brenner, et al., 2018). Notably, RBCs can efficiently shuttle NPs to several organs through administration of catheters placed into specific blood vessels that supply the target organ (Brenner, et al., 2018).
5. Eye delivery
Macular degeneration is an age-related medical condition that promotes neovascularization of the subretinal space, leading to vision loss (Ambati, Atkinson, & Gelfand, 2013). Presently, the only therapy available is in vitreous administration of anti-vascular endothelial growth factor (VEGF) molecules that block vascularization (Spaide, et al., 2006). In the majority of patients, repeated intraocular injections result in retinal fibrosis or atrophy, consequently promoting eye damage. Intravenous injection of agents is also problematic, as the blood-retina barrier makes it challenging for therapeutic agents to reach the eye. To overcome these challenges, VEGF-targeting NPs have recently been developed (Luo, et al., 2013). Intravenously administered arginylglycylaspartic acid (RGD)-coated PLGA NPs carrying a plasmid encoding for the VEGF intraceptor Flt23K were able to target the fundus of normal and degenerated eyes in three different induced choroid damage models: a gene-silenced and a laser-induced mouse model and a laser-induced monkey model (Luo, et al., 2013). The plasmid encoding for Flt23K intraceptor efficiently targeted and seized VEGF into the endoplasmic reticulum, inhibiting paracrine and autocrine cell-stimulation, leading to decreased angiogenesis and fibrosis (Luo, et al., 2013). Notably, in both mouse models RGD-Flt23K-NPs localized preferentially to the extracellular matrix of lesion-containing eyes and were retained there for longer compared to Flt23K-NPs and Blank-NPs (Fig. 4a). Similarly, in the monkey model, the RGD-Flt23K-NPs were retained for one month after treatment, whereas the Flt23K-NPs and Blank-NPs were not detected (Fig. 4b) (Luo, et al., 2013). Furthermore, RGD-Flt23K-NPs were more effective at reducing the fibrotic lesion volume and choroidal neovascularization volume (CNV) (Fig. 4c,d) compared to Flt23K-NPs, Blank-NPs, and sham groups (Luo, et al., 2013). Both mouse models noted a significant reduction in CNV lesion volume when treated with RGD-Flt23K-NPs compared to the other groups (Fig. 4e, f) (Luo, et al., 2013). Moreover, the RGD-Flt23K-NPs were superior at reducing CNV lesion volume compared to intravitreal administration of a VEGF antibody (Fig. 4g). Furthermore, six weeks after treatment, more than the 85% of the RGD-Flt23K-NPs treated mice showed an improvement in visual acuity greater than 10%, whereas the other groups displayed no significant changes (Fig. 4h) (Luo, et al., 2013).
Figure 4.
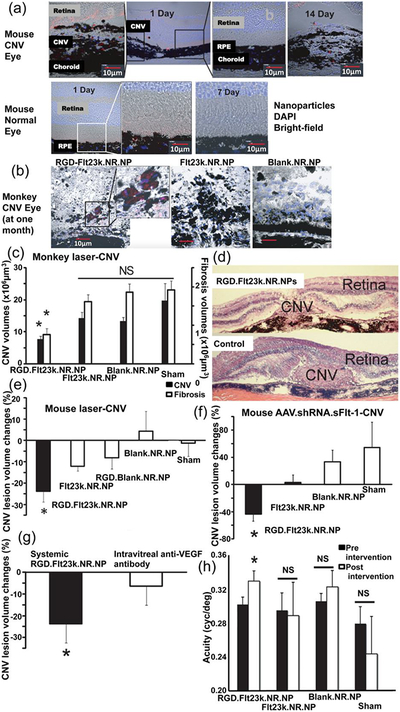
Choroidal neovascularization (CNV) eye lesion delivery of intravenously injected PLGA NPs in mice and monkeys. a Confocal analysis of red-labeled arginylglycylaspartic acid (RGD) functionalized NPs carrying the recombinant vascular endothelial growth factor intraceptor (Flt23k) intraceptor and Nile red (RGD.Flt23k.NR.NPs) in mouse ocular tissue cryosections. b RGD.Flt23k.NR.NPs in CNV eye lesions in a laser-induced monkey model. c Volumes of CNV lesions and fibrosis in a laser-induced CNV monkey model after treatment with RGD.Flt23k.NR.NPs (n = 14), red-labeled bare NPs carrying Flt23k intraceptor (Flt23k.NR.NPs) (n = 16 and n = 8 for CNV volume and fibrosis respectively), red-labeled NPs (Blank.NR.NPs) (n = 8 and n = 19 for CNV volume and fibrosis respectively), and sham control (n = 19 and n = 16 for CNV volume and fibrosis respectively). d Representative images of CNV in a laser-induced CNV monkey model treated with RGD.Flt23k.NR.NP or sham control. e CNV lesion regression in a laser-induced mouse models (n = 11). f CNV lesion volumes in a soluble fms-like tyrosine kinase-1 (sFlt-1) knockdown mouse model treated with RGD.Flt23k.NR.NPs (n = 9), Flt23k.NR.NPs (n = 10), red-labeled blank NPs functionalized with RGD (RGD.Blank.NR.NPs) (n = 10), Blank.NR.NPs (n = 10) and sham group (n = 10). g CNV lesion reduction after RGD.Flt23k.NR.NP treatment and intravitreal injection of anti-VEGF antibody (n = 10, 2 weeks). h Visual acuity in a soluble fms-like tyrosine kinase-1 (sFlt-1) knockdown mouse model treated with RGD.Flt23k.NR.NP, Flt23k.NR.NPs, Blank.NR.NPs, or sham (n = 11, 4 weeks). *, P < 0.05 versus sham control (c, e, f and h) and intravitreal injection (g). Data are presented as mean ± s.e.m. Reproduced from Luo, et al., 2013 with permission. AAV, adeno-associated virus; DAPI, 4’,6-diamidino-2-phenylindole; NS, non-significant; RPE, retinal pigment epithelium; shRNA, short hairpin RNA; VEGF, vascular endothelial growth factor.
6. Lym phatic delivery
The lymphatic system is part of both the circulatory and immune system and is composed by vessels, primary organs (thymus and bone marrow), and secondary organs (lymph nodes and spleen). The function of lymph nodes is to defend against pathogens. Lymph nodes are the location of lymphocytes and antigen-presenting cells that in healthy conditions can start and lead an efficient immune response (Ji, 2016). Lymph nodes are also the primary site of metastasis from adjacent tumors due to their location and structure (Ji, 2016). For these reasons, the lymphatic system is considered a good target for therapy, and several NP-based approaches have recently been proposed. In mice models of lymph node metastasis, the delivery efficiency of direct injection of pegylated gold nanorods (PAuNRs) into the lymph node adjacent to the site of metastasis has been studied (Oladipo, et al., 2017). The NPs drained into the metastasized lymph node and remained there for an extended period of time, making photothermal therapy possible (Oladipo, et al., 2017). Near-infrared irradiation caused a rise in temperature that destroyed surrounding cells and prevented metastatic spread (Oladipo, et al., 2017).
Stimulation of the immune system with ligands that bind to toll-like receptors (TLRs) can drive antitumor responses mediated by a subset of T cells (cytotoxic and natural killer). However, systemic administration of TLR ligands is often inefficient due to dose limitations. To improve lymphatic delivery, polymeric NPs encasing TLR7 ligands have been injected subcutaneously close to the inguinal space (Widmer, et al., 2018). The NPs protected ligands and delivered them to the draining lymph nodes, particularly to monocytes, macrophages and dendritic cells (Widmer, et al., 2018).
In addition to cancer treatment, the lymphatic system is a promising target for NP-based therapies for viral infections. Among other viruses, the human immunodeficiency virus (HIV) mainly proliferates inside macrophages and attacks CD4+ T cells in the lymphatic system (Fevrier, Dorgham, & Rebollo, 2011; Vidya Vijayan, Karthigeyan, Tripathi, & Hanna, 2017). It is challenging for systemically administered anti-retroviral drugs to these reach reservoir sites, causing continuous viral proliferation with poor outcomes for patients (Kumar S., 2018). Therefore, there is a need to develop nanocarriers that are able to deliver antiviral drugs to lymphatic organs. SLNs are stable drug delivery vehicles that can be orally administered, as they resist gastric acidity (Kumar S., 2018) and are taken up by the intestinal lymphatic system that transports lipids and nanosized particle from the gut into systemic blood circulation (Singh, Swami, Khan, & Sistla, 2014). In rats, it has been shown that orally administered poorly water-soluble antiretroviral drugs encapsulated into SLNs accumulate in lymphatic organs (spleen and thymus). In conclusion, there are several promising NP strategies for lymphatic delivery, which will become increasingly important with the rising number of immunotherapies in development. However, transport mechanisms within the lymphatic system are still poorly understood and thought to be characterized by unique and complex processes, hampering the development of NPs that exploit the anatomy and physical parameters of lymph vessels. For this reason, computational models of mass transport in the lymphatic system could be valuable for the design of NPs (Kojic, et al., 2017).
7. Bone delivery
Bone tissue is composed of osteocytes that regulate osteoblast and osteoclast activity; orchestrating a homeostatic process that finely controls bone matrix deposition and adsorption (Raggatt & Partridge, 2010; Schaffler & Kennedy, 2012). Pathological conditions such as fractures or chronic bone damage can impair this homeostasis, resulting in tissue damage. For this reason, anabolic strategies are explored as therapies to improve bone regeneration (Roberts & Ke, 2018). Osteoblast-specific oligonucleotide aptamer-functionalized lipid NPs shielding osteogenic siRNA have been evaluated for bone regeneration (C. Liang, et al., 2015). In a rat model, the aptamer-targeted NPs displayed increased accumulation in the bone (Fig. 5a), specifically in osteoblasts (Fig. 5b), and enhanced gene knockdown (Fig. 5c) compared to NPs with a random sequence aptamer (C. Liang, et al., 2015). Gene knockdown was considerably higher in cells expressing alkaline phosphatase (typically osteoblasts) compared to cells that were positive for an osteoclast-associated receptor (Oscar) (Fig. 5d). After repeated injections, osteopenic rats treated with aptamer-functionalized NPs displayed bone parameters comparable to healthy rats (C. Liang, et al., 2015), indicating successful osteogenic treatment.
Figure 5.
Bone delivery of intravenously injected lipid-based nanoparticles (LNPs) with siRNA against pleckstrin homology domain containing O1 (Plekho1) in rats. a Fluorescent siRNA in organs of rats administered with LNPs-siRNA, random sequence (Rd) functionalized LNPs siRNA, or bone targeting aptamer (CH6) functionalized LNPs-siRNA. b siRNA uptake by primary osteoblasts. c Gene knockdown in primary rat osteoblasts. Data are presented as the mean ± s.d. (n = 3). d Plekho1 knockdown efficiency in alkaline phosphatase (Alp)+, Alp-, and osteoclast-associated receptor (Oscar)+ cells following in vivo administration. Data are presented as means ± s.d. (n = 6). *, P < 0.05 for a comparison of CH6-LNPs siRNA with LNPs-siRNA or Rd-LNPs-siRNA (b,c,d); #, P < 0.05 for a comparison of CH6-LNPs-siRNA with free CH6 + CH6-LNPs-siRNA (b,c). Reproduced from Liang, et al., 2015 with permission.
Bone marrow is a tissue encased by an inner bone structure responsible for the synthesis and secretion of functional circulating blood cells. Bone marrow can be affected by a spectrum of genetic disorders that cause medullary dysplasia or cytopenia, i.e. myelodysplastic syndromes (Wu, et al., 2017). Potential effective therapies consist on chemotherapeutic drugs, which also exhibit systemic toxicity resulting in multiple collateral effects. For this reason, Wu et al. proposed the use of biodegradable NPs loaded with decitabine and arsenic trioxide, chemotherapeutic agents that when combined induce a favorable response in patients affected by myelodysplastic syndromes (Wu, et al., 2017). NPs were functionalized with alendronate-PEG-lipid molecules to enhance targeting abilities. Alendronate is a bisphosphonate that mimics pyrophosphate and is used to treat pathological bone conditions, such as osteoporosis. In a mouse model, the bone-targeted NPs improved tissue-specific accumulation of the chemotherapeutic agents compared to untargeted NPs (up to 8-fold) (Wu, et al., 2017). Four weeks after treatment, the white blood cell (WBC) and platelet counts were normal in 50% of the NP treated animals whereas the corresponding value was 25% in the free drug or untargeted NP groups. More than 200 days post-treatment mice that had received bone-targeted NPs displayed decreased mortality (~40%) compared to those that received the free drug (~70%), untargeted NPs (~75%) or phosphate buffered saline (~90%) (Wu, et al., 2017). Ex vivo analysis of bone marrow cells demonstrated that bone-targeted NPs efficiently delivered chemotherapy to the target site, as evidenced by reduced transcription of target gene DNA methyltransferase (80% reduction) (Wu, et al., 2017). Apoptosis was also increased in the bone marrow of mice injected with bone-targeted NPs (~70%) compared to free drugs (50%), untargeted NPs (~50%), or phosphate buffered saline (~10%). On the contrary, liver cell apoptosis was less prevalent in the bone-targeted NP group compared to the other treatment groups (Wu, et al., 2017). These results indicate that the bone-targeted NPs efficiently deliver chemotherapeutic agents to bone tissue, while reducing chemotherapy-mediated hepatic toxicity.
8. In utero delivery
Pediatric patients affected by hemoglobinopathies, particularly β-thalassemia, are frequently subjected to blood transfusions or bone marrow transplants, which are medical procedures that can lead to life-threatening complications (Modell & Darlison, 2008). Despite increasingly precocious pre-natal diagnoses, there is a lack of efficient in utero gene therapy able to edit the mutated gene before birth (Fan, et al., 2012). Peptide nucleic acids (PNAs) are synthetic polymers that differ from DNA and RNA structures in that the backbone of the molecule is formed by amino acids instead of carbohydrates (Nielsen, Egholm, & Buchardt, 1994). PNAs bind to DNA through a stable bond resulting in an enzyme-resistant carrier for genetic material (McNeer, et al., 2013). PLGA-NPs with PNA coupled with donor DNA were injected via the intravitelline vein or into the amniotic cavity of fetal mice carrying the human β-thalassemia mutation (Ricciardi, et al., 2018). The NPs accumulated preferentially in the fetal liver, where hematopoietic stem cells undergo rapid expansion and transfer to bone marrow (Fig. 6a). The treatment resulted in successful gene editing with consequent improved postnatal outcomes compared to controls (Fig. 6b, c) (Ricciardi, et al., 2018). Treated mice also had an increased hemoglobin concentration (Fig. 6d) along with an improved red blood cell (RBC) phenotype (Fig. 6e) and reduced reticulocyte count (Fig. 6f) (Ricciardi, et al., 2018). After a single NP intravitelline injection, successful gene editing was detected in both fetal hematopoietic stem/progenitor cells and bone marrow cells of pups (Fig. 6g) (Ricciardi, et al., 2018). The overall results confirm that in utero delivered NPs can mediate safe fetal gene editing resulting in normal fetal development and reduced post-natal β-thalassemia morbidity.
Figure 6.
Fetus delivery of intravenously (IV) and intra-amniotic (IA) injected PLGA NPs with γ tail-clamp peptide nucleic acids (γtcPNA) in a mouse model of human β-thalassemia. a Fetus organ biodistribution of NPs 3 h post-injection at indicated embryonic (E) stages (n = 9 for control, n = 10 for E15.5 IV, n = 7 for E16.5 IV and E15.5 IA, n = 8 for E16.5 IA). Scale bars, 2 mm. b Survival to weaning (21 days) (n = 7 for untreated, n = 4 for IV E15.5, n = 7 for IA E15.5). c Survival of fetus at E15.5 (IV) (n = 16). d Blood hemoglobin levels (E15.5 IV) (n = 6), horizontal lines within the boxes indicate the median, the box indicates the first and third quartile, and the whiskers represent the range. e Blood cells (Wright-Giemsa staining) and spleen sections (hematoxylin and eosin staining) (E15.5 IV). Peripheral blood scale bars, 10 μm; spleen scale bars, 150 μm. f Reticulocyte count (E15.5 IV). Scale bar, 10 μm. g Gene edited total bone marrow and isolated hematopoietic progenitor cells (HPCs) (E15.5 IV) (n = 3). Data are presented as mean ± s.d. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Reproduced from Ricciardi, et al., 2018 with permission.
9. Protein Corona
There has been extensive research into the modification of NPs following systemic administration to regulate organotropism, specifically the binding of biomolecules to the surface of NPs resulting in a protein-rich coating known as the protein corona. The protein corona significantly impacts the characteristics of NPs, causing variable changes in NP size, shape, charge, and aggregation capacity (Wolfram, Yang, et al., 2014). Consequently, these changes have implications in NP toxicity, targeting, biodistribution, and drug release, especially when transitioning from in vitro to in vivo environments (Wolfram, Yang, et al., 2014). With respect to biodistribution, the binding of certain plasma proteins to the protein corona can cause NPs to have affinities for specific tissues. A high concentration of opsonins, such as complement factors, fibrinogen, or immunoglobin G, in the protein corona promotes macrophage uptake of NPs (Nguyen & Lee, 2017) and NP accumulation into the liver and spleen (Wolfram, Yang, et al., 2014). Enrichment with dysopsonins, including apolipoproteins (Apo) and albumin, was shown to increase NP circulation life and accumulation of NPs in organs other than the liver and spleen, such as the brain (Nguyen & Lee, 2017; Wolfram, Yang, et al., 2014). Recent studies into the protein corona have explored methods to use this structure to improve therapeutic goals. One method involves the modification of NPs to create specific protein corona profiles from endogenous proteins. For instance, Schöttler et al. demonstrated that the stealth effect of pegylated NPs, referring to the reduction in non-specific cell uptake and increased blood circulation life of these nanoparticles, was due to the formation of protein coronas with high amounts of a clusterin, Apo J, from plasma (Schottler, et al., 2016). Other nanomaterial compositions were shown to favorably bind to serum proteins that improved delivery to specific tissues, such as polysorbate-coated NPs binding to Apo E or Apo AI leading to an increased distribution in brain endothelium (Nguyen & Lee, 2017). In regard to EVs, the characteristic and implications of the protein corona are largely unknown and are likely to be affected by the isolation method. Overall, to improve the efficiency of nanotherapeutics, studies must account for effects of the protein corona on NP functionality.
10. Conclusion
Many therapeutic agents that show promising results in cell culture fail in vivo due to biological barriers that hinder site-specific accumulation. These barriers consist of the immune system, various anatomical obstacles, and pressure gradients. NPs have been developed as tools to aid in the transport of therapeutic agents within and across compartments in the body. Although some barriers are inherent to all tissues, others are organ-specific, indicating that preconditioning strategies, administration route, and NP properties should be tailored based on the target organ. Despite the development of several targeted nanodelivery strategies, there is still a pressing need to further understand organ-specific macro and microscale obstacles that impede drug delivery. Knowledge of the structure and function of the target organ is necessary to design delivery strategies that lead to favorable drug distributions and therapeutic outcomes.
Besides successfully delivering therapeutic agents to target organs, NPs must also ensure delivery to the appropriate subcellular compartments, as the intracellular environment poses additional obstacles, such as the lysosome that rapidly degrades biomolecules. Multifunctional delivery strategies are necessary to finely orchestrate drug transport across a diverse set of extracellular and subcellular barriers. Both synthetic NPs and extracellular vesicles provide promising multipronged strategies for site-specific delivery.
ACKNOWLEDGEMENTS
This work was mainly supported by Mayo Clinic, the University of Brescia. Among various sources of intramural funding, the authors particularly acknowledge support from the Mayo Clinic in Florida Focused Research Team Program and the Center for Regenerative Medicine. This work was also partially supported by the National Cancer Institute Physical Sciences-Oncology Network of the National Institutes of Health, under award number U54CA210181. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Abbas Y, Azzazy HME, Tammam S, Lamprecht A, Ali ME, Schmidt A, Sollazzo S, & Mathur S (2016). Development of an inhalable, stimuli-responsive particulate system for delivery to deep lung tissue. Colloids and Surfaces B: Biointerfaces, 146, 19–30. [DOI] [PubMed] [Google Scholar]
- Ali ME, & Lamprecht A (2014). Spray freeze drying for dry powder inhalation of nanoparticles. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 87, 510–517. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, & Wood MJ (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol, 29, 341–345. [DOI] [PubMed] [Google Scholar]
- Ambati J, Atkinson JP, & Gelfand BD (2013). Immunology of age-related macular degeneration. Nat Rev Immunol, 13, 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer DR (2018). The Chameleon Effect: Characterization Challenges Due to the Variability of Nanoparticles and Their Surfaces. Front Chem, 6, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, & Nedergaard M (2004). The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis, 16, 1–13. [DOI] [PubMed] [Google Scholar]
- Barua S, & Mitragotri S (2014). Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today, 9, 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazak R, Houri M, El Achy S, Kamel S, & Refaat T (2015). Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol, 141, 769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Wu J, Xu X, Kamaly N, & Farokhzad OC (2014). Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev, 66, 2–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzer O, Shilo M, Opochinsky R, Barnoy E, Motiei M, Okun E, Yadid G, & Popovtzer R (2017). The effect of nanoparticle size on the ability to cross the blood-brain barrier: an in vivo study. Nanomedicine (Lond), 12, 1533–1546. [DOI] [PubMed] [Google Scholar]
- Blanco E, Shen H, & Ferrari M (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnology, 33, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D, Castellano LRC, & Italiani P (2017). Editorial: Interaction of Nanomaterials with the Immune System: Role in Nanosafety and Nanomedicine. Front Immunol, 8,1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli DA, Yankson K, Shukla N, Vilanilam G, Ticer T, & Wolfram J (2018). Extracellular vesicle therapeutics for liver disease. Journal of Controlled Release, 273, 86–98. [DOI] [PubMed] [Google Scholar]
- Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P, Hekierski H, Chatterjee S, Tao JQ, Parhiz H, Bhamidipati K, Uhler TG, Hood ED, Kiseleva RY, Shuvaev VS, Shuvaeva T, Khoshnejad M, Johnston I, Gregory JV, Lahann J, Wang T, Cantu E, Armstead WM, Mitragotri S, & Muzykantov V (2018). Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun, 9, 2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, Bergese P, & Wolfram J (2018). Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldorera-Moore M, Guimard N, Shi L, & Roy K (2010). Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv, 7, 479–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celikoglu F, Celikoglu SI, & Goldberg EP (2008). Bronchoscopic intratumoral chemotherapy of lung cancer. Lung cancer (Amsterdam, Netherlands), 61, 1–12. [DOI] [PubMed] [Google Scholar]
- Champion JA, & Mitragotri S (2006). Role of target geometry in phagocytosis. Proceedings of the National Academy of Sciences of the United States of America, 103, 4930–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yang K, Tuguntaev RG, Mozhi A, Zhang J, Wang PC, & Liang XJ (2016). Targeting tumor microenvironment with PEG-based amphiphilic nanoparticles to overcome chemoresistance. Nanomedicine, 12, 269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Minh LV, Liu J, Angelov B, Drechsler M, Garamus VM, Willumeit-Romer R, & Zou A (2016). Baicalin loaded in folate-PEG modified liposomes for enhanced stability and tumor targeting. In (Vol. 140, pp. 74–82). [DOI] [PubMed] [Google Scholar]
- Chulpanova DS, Kitaeva KV, James V, Rizvanov AA, & Solovyeva VV (2018). Therapeutic Prospects of Extracellular Vesicles in Cancer Treatment. Front Immunol, 9, 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, & Prat A (2015). The blood-brain barrier. Cold Spring Harb Perspect Biol, 7, a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer L, Naessens T, De Koker S, Zagato E, Demeester J, Grooten J, De Smedt SC, & Raemdonck K (2015). Hybrid pulmonary surfactant-coated nanogels mediate efficient in vivo delivery of siRNA to murine alveolar macrophages. In (Vol. 217, pp. 53–63). [DOI] [PubMed] [Google Scholar]
- Decuzzi P, & Ferrari M (2006). The adhesive strength of non-spherical particles mediated by specific interactions. In (Vol. 27, pp. 5307–5314). [DOI] [PubMed] [Google Scholar]
- Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, & Ferrari M (2010). Size and shape effects in the biodistribution of intravascularly injected particles. Journal of Controlled Release, 141, 320–327. [DOI] [PubMed] [Google Scholar]
- Del Amo EM, & Urtti A (2008). Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today, 13, 135–143. [DOI] [PubMed] [Google Scholar]
- Dhar S, Gu FX, Langer R, Farokhzad OC, & Lippard SJ (2008). Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci U S A, 105, 17356–17361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Lane LA, & Nie S (2015). Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J Control Release, 219, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HC, Gu W, Wang J, Blumenfeld YJ, El-Sayed YY, & Quake SR (2012). Non invasive prenatal measurement of the fetal genome. Nature, 487, 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanali G, di Masi A, Trezza V, Marino M, Fasano M, & Ascenzi P (2012). Human serum albumin: from bench to bedside. Molecular Aspects of Medicine, 33, 209–290. [DOI] [PubMed] [Google Scholar]
- Fevrier M, Dorgham K, & Rebollo A (2011). CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses, 3, 586–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AD, Claypool SE, & Liu R (2013). The smart targeting of nanoparticles. Curr Pharm Des, 19, 6315–6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan CW, & Feng SS (2010). Transferrin-conjugated nanoparticles of poly(lactide)-D-alpha tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood-brain barrier. Biomaterials, 31, 7748–7757. [DOI] [PubMed] [Google Scholar]
- Gentile E, Cilurzo F, Di Marzio L, Carafa M, Ventura CA, Wolfram J, Paolino D, & Celia C (2013). Liposomal chemotherapeutics. Future Oncology, 9, 1849–1859. [DOI] [PubMed] [Google Scholar]
- Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM-C, Liu X, Ferrari M, & Decuzzi P (2008). The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. In (Vol. 41, pp. 2312–2318). [DOI] [PubMed] [Google Scholar]
- Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, & DeSimone JM (2008). The effect of particle design on cellular internalization pathways. Proceedings of the National Academy of Sciences, 105, 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greish K (2010). Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol, 624, 25–37. [DOI] [PubMed] [Google Scholar]
- Gustafson HH, Holt-Casper D, Grainger DW, & Ghandehari H (2015). Nanoparticle Uptake: The Phagocyte Problem. Nano Today, 10, 487–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh DS, Wadajkar AS, Roberts N, Perez JG, Connolly NP, Frenkel V, Winkles JA, Woodworth GF, & Kim AJ (2016). Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr Pharm Des, 22, 1177–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, & Lyden D (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SB (2001). Clinical applications of a novel sustained-release injectable drug delivery system: DepoFoam technology. Cancer journal (Sudbury, Mass.), 7, 219–227. [PubMed] [Google Scholar]
- Ji RC (2016). Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. Int J Mol Sci, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Kim BY, Rutka JT, & Chan WC (2008). Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol, 3, 145–150. [DOI] [PubMed] [Google Scholar]
- Jokerst JV, Lobovkina T, Zare RN, & Gambhir SS (2011). Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond), 6, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid A, Persano S, Shen H, Zhao Y, Blanco E, Ferrari M, & Wolfram J (2016).Strategies for improving drug delivery: nanocarriers and microenvironmental priming. Expert Opin Drug Deliv, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierstead PH, Okochi H, Venditto VJ, Chuong TC, Kivimae S, Frechet JMJ, & Szoka FC (2015). The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J Control Release, 213, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Kim IK, Bae KH, Lee SH, Lee Y, & Park TG (2008). Cationic solid lipid nanoparticles reconstituted from low density lipoprotein components for delivery of siRNA. Mol Pharm, 5, 622–631. [DOI] [PubMed] [Google Scholar]
- Kinnear C, Moore TL, Rodriguez-Lorenzo L, Rothen-Rutishauser B, & Petri-Fink A (2017). Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem Rev, 117, 11476–11521. [DOI] [PubMed] [Google Scholar]
- Kojic M, Milosevic M, Simic V, Koay EJ, Kojic N, Ziemys A, & Ferrari M (2017). Extension of the composite smeared finite element (CSFE) to include lymphatic system in modeling mass transport in capillary systems and biological tissue. J Serbian Soc Comput Mech, 11, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong WH, Park K, Lee MY, Lee H, Sung DK, & Hahn SK (2013). Cationic solid lipid nanoparticles derived from apolipoprotein-free LDLs for target specific systemic treatment of liver fibrosis. Biomaterials, 34, 542–551. [DOI] [PubMed] [Google Scholar]
- Kumar S, N. R, Ahammed V, Nayak Y, Naha A, Nayak UY (2018). Development of ritonavir solid lipid nanoparticles by Box Behnken design for intestinal lymphatic targeting. Journal of Drug Delivery Science and Technology, 44, 181–189. [Google Scholar]
- Kwon IK, Lee SC, Han B, & Park K (2012). Analysis on the current status of targeted drug delivery to tumors. J Control Release, 164, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-Y, Ferrari M, & Decuzzi P (2009). Design of bio-mimetic particles with enhanced vascular interaction. In (Vol. 42, pp. 1885–1890). [DOI] [PubMed] [Google Scholar]
- Li KCP, Pandit SD, Guccione S, & Bednarski MDJBM (2004). Molecular Imaging Applications in Nanomedicine. 6, 113–116. [DOI] [PubMed] [Google Scholar]
- Li R, Zheng K, Yuan C, Chen Z, & Huang M (2017). Be Active or Not: the Relative Contribution of Active and Passive Tumor Targeting of Nanomaterials. Nanotheranostics, 1, 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Guo B, Wu H, Shao N, Li D, Liu J, Dang L, Wang C, Li H, Li S, Lau WK, Cao Y, Yang Z, Lu C, He X, Au DW, Pan X, Zhang BT, Lu C, Zhang H, Yue K, Qian A, Shang P, Xu J, Xiao L, Bian Z, Tan W, Liang Z, He F, Zhang L, Lu A, & Zhang G (2015). Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat Med, 21, 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Huang K, Su T, Li Z, Hu S, Dinh PU, Wrona EA, Shao C, Qiao L, Vandergriff AC, Hensley MT, Cores J, Allen T, Zhang H, Zeng Q, Xing J, Freytes DO, Shen D, Yu Z, & Cheng K (2018). Mesenchymal Stem Cell/Red Blood Cell-Inspired Nanoparticle Therapy in Mice with Carbon Tetrachloride-Induced Acute Liver Failure. ACS Nano, 12, 6536–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, Weng WZ, Peng YW, Cao HJ, Xie JQ, Xie SB, Xiang AP, & Gao ZL (2017). Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology, 66, 209–219. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jiang M, Kang T, Miao D, Gu G, Song Q, Yao L, Hu Q, Tu Y, Pang Z, Chen H, Jiang X, Gao X, & Chen J (2013). Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials, 34, 3870–3881. [DOI] [PubMed] [Google Scholar]
- Longmire MR, Ogawa M, Choyke PL, & Kobayashi H (2011). Biologically optimized nanosized molecules and particles: more than just size. Bioconjug Chem, 22, 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Zhang X, Hirano Y, Tyagi P, Barabas P, Uehara H, Miya TR, Singh N, Archer B, Qazi Y, Jackman K, Das SK, Olsen T, Chennamaneni SR, Stagg BC, Ahmed F, Emerson L, Zygmunt K, Whitaker R, Mamalis C, Huang W, Gao G, Srinivas SP, Krizaj D, Baffi J, Ambati J, Kompella UB, & Ambati BK (2013). Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano, 7, 3264–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia HK, & Siegel SJ (2011). Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel), 3, 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeer NA, Schleifman EB, Cuthbert A, Brehm M, Jackson A, Cheng C, Anandalingam K, Kumar P, Shultz LD, Greiner DL, Mark Saltzman W, & Glazer PM (2013). Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo. Gene Ther, 20, 658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y, Mu C, Wolfram J, Deng Z, Hu TY, Liu X, Blanco E, Shen H, & Ferrari M (2016). A Micro/Nano Composite for Combination Treatment of Melanoma Lung Metastasis. Adv Healthc Mater. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y, Wolfram J, Mu C, Liu X, Blanco E, Shen H, & Ferrari M (2016). Enzyme-responsive multistage vector for drug delivery to tumor tissue. . Pharmacological Research, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell B, & Darlison M (2008). Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ, 86, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N, & Gallagher PG (2008). Red cell membrane: past, present, and future. Blood, 112, 3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro R, Wolfram J, Federico C, Cilurzo F, Di Marzio L, Ventura CA, Carafa M, Celia C, & Fresta M (2013). Polyethylenimine and chitosan carriers for the delivery of RNA interference effectors. Expert Opin Drug Deliv, 10, 1653–1668. [DOI] [PubMed] [Google Scholar]
- Mu C, Wu X, Zhou X, Wolfram J, Shen J, Zhang D, Mai J, Xia X, Holder AM, Ferrari M, Liu X, & Shen H (2018). Chemotherapy Sensitizes Therapy-Resistant Cells to Mild Hyperthermia by Suppressing Heat Shock Protein 27 Expression in Triple-Negative Breast Cancer. Clinical Cancer Research, 24, 4900–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesh PKB, Johnson NR, Boya VKN, Chowdhury P, Othman SF, Khalilzad-Sharghi V, Hafeez BB, Ganju A, Khan S, Behrman SW, Zafar N, Chauhan SC, Jaggi M, & Yallapu MM (2016). PSMA targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer. In (Vol. 144, pp. 8–20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navya PN, & Daima HK (2016). Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, & Lee BJ (2017). Protein corona: a new approach for nanomedicine design. Int J Nanomedicine, 12, 3137–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PE, Egholm M, & Buchardt O (1994). Peptide nucleic acid (PNA). A DNA mimic with a peptide backbone. Bioconjug Chem, 5, 3–7. [DOI] [PubMed] [Google Scholar]
- Nogues L, Benito-Martin A, Hergueta-Redondo M, & Peinado H (2018). The influence of tumour-derived extracellular vesicles on local and distal metastatic dissemination. Mol Aspects Med, 60, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, & Lentsch AB (2016). Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol, 64, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørgaard AW, Larsen S, Hammer M, Poulsen SS, Jensen KA, Nielsen GD, & Wolkoff P (2010). Lung Damage in Mice after Inhalation of Nanofilm Spray Products: The Role of Perfluorination and Free Hydroxyl Groups. Toxicological Sciences, 116, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladipo AO, Oluwafemi OS, Songca SP, Sukhbaatar A, Mori S, Okajima J, Komiya A, Maruyama S, & Kodama T (2017). A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci Rep, 7, 45459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey PK, Sharma AK, & Gupta U (2016). Blood brain barrier: An overview on strategies in drug delivery, realistic in vitro modeling and in vivo live tracking. Tissue Barriers, 4, e1129476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino D, Cosco D, Gaspari M, Celano M, Wolfram J, Voce P, Puxeddu E, Filetti S, Celia C, Ferrari M, Russo D, & Fresta M (2014). Targeting the thyroid gland with thyroid stimulating hormone (TSH)-nanoliposomes Biomaterials, 35, 7101–7109. [DOI] [PubMed] [Google Scholar]
- Pasut G, Paolino D, Celia C, Mero A, Joseph AS, Wolfram J, Cosco D, Schiavon O, Shen H, & Fresta M (2015). Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. Journal of Controlled Release, 199, 106–113. [DOI] [PubMed] [Google Scholar]
- Patel AA, Patel RJ, & Patel SR (2018). Nanomedicine for Intranasal Delivery to Improve Brain Uptake. Curr Drug Deliv, 15, 461–469. [DOI] [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, & Langer R (2007). Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol, 2, 751–760. [DOI] [PubMed] [Google Scholar]
- Pelt J, Busatto S, Ferrari M, Thompson EA, Mody K, & Wolfram J (2018). Chloroquine and nanoparticle drug delivery: A promising combination. Pharmacology & Therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao JG, Wang L, Gao F, You YZ, Xiong Y, & Yang L (2014). Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano, 8, 10414–10425. [DOI] [PubMed] [Google Scholar]
- Podust VN, Balan S, Sim BC, Coyle MP, Ernst U, Peters RT, & Schellenberger V(2016). Extension of in vivo half-life of biologically active molecules by XTEN protein polymers. J Control Release, 240, 52–66. [DOI] [PubMed] [Google Scholar]
- Primeau AJ, Rendon A, Hedley D, Lilge L, & Tannock IF (2005). The Distribution of the Anticancer Drug Doxorubicin in Relation to Blood Vessels in Solid Tumors. Clinical Cancer Research, 11, 8782–8788. [DOI] [PubMed] [Google Scholar]
- Prisner L, Bohn N, Hahn U, & Mews A (2017). Size dependent targeted delivery of gold nanoparticles modified with the IL-6R-specific aptamer AIR-3A to IL-6R-carrying cells. Nanoscale, 9, 14486–14498. [DOI] [PubMed] [Google Scholar]
- Qian ZM, & Wang Q (1998). Expression of iron transport proteins and excessive iron accumulation in the brain in neurodegenerative disorders. Brain Res Brain Res Rev, 27, 257–267. [DOI] [PubMed] [Google Scholar]
- Raggatt LJ, & Partridge NC (2010). Cellular and molecular mechanisms of bone remodeling. J Biol Chem, 285, 25103–25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahhal TB, Fromen CA, Wilson EM, Kai MP, Shen TW, Luft JC, & DeSimone JM (2016). Pulmonary Delivery of Butyrylcholinesterase as a Model Protein to the Lung. Molecular Pharmaceutics, 13, 1626–1635. [DOI] [PubMed] [Google Scholar]
- Ricciardi AS, Bahal R, Farrelly JS, Quijano E, Bianchi AH, Luks VL, Putman R, Lopez-Giraldez F, Coskun S, Song E, Liu Y, Hsieh WC, Ly DH, Stitelman DH, Glazer PM, & Saltzman WM (2018). In utero nanoparticle delivery for site-specific genome editing. Nat Commun, 9, 2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riling C, Kamadurai H, Kumar S, O’Leary CE, Wu KP, Manion EE, Ying M, Schulman BA, & Oliver PM (2015). Itch WW Domains Inhibit Its E3 Ubiquitin Ligase Activity by Blocking E2-E3 Ligase Trans-thiolation. J Biol Chem, 290, 23875–23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SJ, & Ke HZ (2018). Anabolic Strategies to Augment Bone Fracture Healing. Curr Osteoporos Rep, 16, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytting E, Nguyen J, Wang X, & Kissel T (2008). Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert opinion on drug delivery, 5, 629–639. [DOI] [PubMed] [Google Scholar]
- Saha B, Evers TH, & Prins MW (2014). How antibody surface coverage on nanoparticles determines the activity and kinetics of antigen capturing for biosensing. Anal Chem, 86, 8158–8166. [DOI] [PubMed] [Google Scholar]
- Salata O (2004). Applications of nanoparticles in biology and medicine. J Nanobiotechnology, 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson E, Shen H, Blanco E, Ferrari M, & Wolfram J (2017). Contribution of Kupffer cells to liposome accumulation in the liver. Colloids and Surfaces B: Biointerfaces, 158, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavo MP, Gentile E, Wolfram J, Gu J, Barone M, Evangelopoulos M, Martinez JO, Liu X, Celia C, Tasciotti E, Vilar E, & Shen H (2015). Multistage vector delivery of sulindac and silymarin for prevention of colon cancer. Colloids and Surfaces B: Biointerfaces, 136, 694–703. [DOI] [PubMed] [Google Scholar]
- Schaffler MB, & Kennedy OD (2012). Osteocyte signaling in bone. Curr Osteoporos Rep, 10, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottler S, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, Mailander V, & Wurm FR (2016). Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol, 11, 372–377. [DOI] [PubMed] [Google Scholar]
- Shen J, Kim HC, Mu C, Gentile E, Mai J, Wolfram J, Ji L, Ferrari M, Mao Z, & Shen H (2014). Multifunctional gold nanorods for siRNA gene silencing and photothermal therapy. Adv Healthc Mater, 3, 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Kim HC, Su H, Wang F, Wolfram J, Kirui D, Mai J, Mu C, Mao Z, & Shen H (2014). Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics, 4, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Kim HC, Wolfram J, Mu C, Zhang W, Liu H, Xie Y, Mai J, Zhang H, Li Z, Guevara M, Mao ZW, & Shen H (2017). A Liposome Encapsulated Ruthenium Polypyridine Complex as a Theranostic Platform for Triple-Negative Breast Cancer. Nano Letters, 17, 2913–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Liu H, Mu C, Wolfram J, Zhang W, Kim HC, Zhu G, Hu Z, Ji LN, Liu X, Ferrari M, Mao ZW, & Shen H (2017). Multi-step encapsulation of chemotherapy and gene silencing agents in functionalized mesoporous silica nanoparticles. Nanoscale, 9, 5329–5341. [DOI] [PubMed] [Google Scholar]
- Shen J, Wu X, Lee Y, Wolfram J, Mao Z, Ferrari M, & Shen H (2015). Porous silicon microparticles for delivery of siRNA therapeutics. J Vis Exp, 15, 52075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Xu R, Mai J, Kim HC, Guo X, Qin G, Yang Y, Wolfram J, Mu C, Xia X, Gu J, Liu X, Mao ZW, Ferrari M, & Shen H (2013). High capacity nanoporous silicon carrier for systemic delivery of gene silencing therapeutics. ACS Nano, 7, 9867–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Zhang J, Zhou Q, Xin J, Jiang J, Jiang L, Wu T, Li J, Ding W, Li J, Sun S, Li J, Zhou N, Zhang L, Jin L, Hao S, Chen P, Cao H, Li M, Li L, Chen X, & Li J (2017). Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut, 66, 955–964. [DOI] [PubMed] [Google Scholar]
- Singh I, Swami R, Khan W, & Sistla R (2014). Lymphatic system: a prospective area for advanced targeting of particulate drug carriers. Expert Opin Drug Deliv, 11, 211–229. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Laud K, Fine HF, Klancnik JM Jr., Meyerle CB, Yannuzzi LA, Sorenson J, Slakter J, Fisher YL, & Cooney MJ (2006). Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina, 26, 383–390. [DOI] [PubMed] [Google Scholar]
- Sterzenbach U, Putz U, Low LH, Silke J, Tan SS, & Howitt J (2017). Engineered Exosomes as Vehicles for Biologically Active Proteins. Mol Ther, 25, 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamargo J, Le Heuzey JY, & Mabo P (2015). Narrow therapeutic index drugs: a clinical pharmacological consideration to flecainide. Eur J Clin Pharmacol, 71, 549–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewes F, Gobbo OL, Ehrhardt C, & Healy AM (2016). Amorphous Calcium Carbonate Based-Microparticles for Peptide Pulmonary Delivery. ACS Applied Materials & Interfaces, 8, 1164–1175. [DOI] [PubMed] [Google Scholar]
- Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, & Vishwanatha JK (2012). Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials, 33, 7164–7173. [DOI] [PubMed] [Google Scholar]
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach J-M, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman MLD, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DRF, Caruso S, Chamley LW, Chang Y-T, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FAW, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TAP, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DCI, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG-E, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano S. i., Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ, Kornek M, Kosanovic MM, Kovács ÁF, Kramer-Albers E-M, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lazáro-Ibáñez E, Le Lay S, Lee M-S, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li ITS, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lörenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SLN, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen ENM, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostegaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BCH, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IKH, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KMA, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PRM, Silva AM, Skowronek A, Snyder OL, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BWM, van der Grein SG, Van Deun J, van Herwijnen MJC, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ, Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MHM, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yâñez-MÓ M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang J. y., Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, & Zuba-Surma EK (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, & Bannerjee SK (2012). Drug delivery systems: An updated review. Int J Pharm Investig, 2, 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosean S, Flynn B, Axelsson J, Gunn J, Samkoe KS, Hasan T, Doyley MM, & Pogue BW (2013). Nanoparticle uptake in tumors is mediated by the interplay of vascular and collagen density with interstitial pressure. Nanomedicine, 9, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader P, Mol EA, Pasterkamp G, & Schiffelers RM (2016). Extracellular vesicles for drug delivery. Adv Drug Deliv Rev, 106, 148–156. [DOI] [PubMed] [Google Scholar]
- Venuta A, Wolfram J, Shen H, & Ferrari M (2017). Post-nano strategies for drug delivery: multistage porous silicon microvectors. Journal of Materials Chemistry B, 5, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidya Vijayan KK, Karthigeyan KP, Tripathi SP, & Hanna LE (2017). Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front Immunol, 8, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner V, Dullaart A, Bock AK, & Zweck A (2006). The emerging nanomedicine landscape. Nature Biotechnology, 24, 1211–1217. [DOI] [PubMed] [Google Scholar]
- Widmer J, Thauvin C, Mottas I, Nguyen VN, Delie F, Allemann E, & Bourquin C (2018). Polymer-based nanoparticles loaded with a TLR7 ligand to target the lymph node for immunostimulation. Int J Pharm, 535, 444–451. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, & Chan WCW. (2016). Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. [Google Scholar]
- Wolfram J, & Ferrari M (2019). Clinical cancer nanomedicine. Nano Today, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Nizzero S, Liu H, Li F, Zhang G, Li Z, Shen H, Blanco E, & Ferrari M (2017). A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci Rep, 7, 13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Scott B, Boom K, Shen J, Borsoi C, Suri K, Grande R, Fresta M, Celia C, Zhao Y, Shen H, & Ferrari M (2016). Hesperetin Liposomes for Cancer Therapy. Curr Drug Deliv, 13, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Shen H, & Ferrari M (2015). Multistage vector (MSV) therapeutics. Journal of Controlled Release, 219, 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Suri K, Huang Y, Molinaro R, Borsoi C, Scott B, Boom K, Paolino D, Fresta M, Wang J, Ferrari M, Celia C, & Shen H (2014). Evaluation of anticancer activity of celastrol liposomes in prostate cancer cells. Journal of Microencapsulation, 31, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Suri K, Yang Y, Shen J, Celia C, Fresta M, Zhao Y, Shen H, & Ferrari M (2014). Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids and Surfaces B: Biointerfaces, 114C, 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Yang Y, Shen J, Moten A, Chen C, Shen H, Ferrari M, & Zhao Y (2014). The nano-plasma interface: Implications of the protein corona. Colloids and Surfaces B: Biointerfaces, 124, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, Fresta M, Nie G, Chen C, Shen H, Ferrari M, & Zhao Y (2015). Safety of Nanoparticles in Medicine. Current Drug Targets, 16, 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hu Z, Nizzero S, Zhang G, Ramirez MR, Shi C, Zhou J, Ferrari M, & Shen H (2017). Bone-targeting nanoparticle to co-deliver decitabine and arsenic trioxide for effective therapy of myelodysplastic syndrome with low systemic toxicity. J Control Release, 268, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, & Gao H (2017). Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res, 126, 97–108. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wolfram J, Fang X, Shen H, & Ferrari M (2014). Polyarginine Induces an Antitumor Immune Response through Binding to Toll-Like Receptor 4. Small, 10, 1250–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]