Abstract
An impressive body of research over the past 30 years has implicated the human cerebellum in a broad range of functions, including motor control, perception, language, working memory, cognitive control, and social cognition. The relatively uniform anatomy and physiology of the cerebellar cortex has given rise to the idea that this structure performs the same computational function across diverse domains. Here, we highlight evidence from the human neuroimaging literature that documents the striking functional heterogeneity of the cerebellum, both in terms of task-evoked activity patterns and, as measured under task-free conditions, functional connectivity with the neocortex. Building on these observations, we discuss the theoretical challenges that these results present to the idea of a universal cerebellar computation, and consider the alternative concept of multiple functionality, the idea that the same underlying circuit implements functionally distinct computations.
In Brief
Diedrichsen et al. (2019) review recent functional neuroimaging work documenting the engagement of the human cerebellum in a broad range of cognitive tasks, and discuss the implications of these results for theories of cerebellar function.
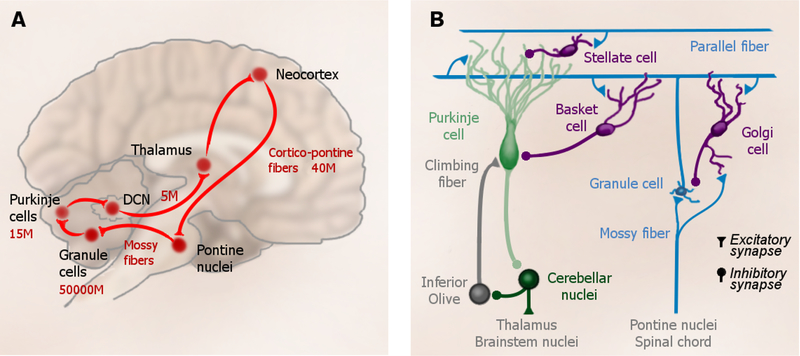
The cortico-cerebellar system is one of the most prominent networks in the human brain (Fig. 1a). Of the 40 million axons that exit the neocortex and traverse the cerebral peduncles, the vast majority send collaterals to the pontine nuclei (Tomasch, 1969), and these, in turn, project to the granule cells of the cerebellar cortex. The 50 billion granule cells constitute more than half of the neurons in the human brain (Azevedo et al., 2009). Thus, this transmission point corresponds to an information expansion of at least 1:1000, creating the system with the largest information bandwidth in the human brain. Each granule cell gives rise to a single axon, the parallel fibers that innervate the Purkinje cells, the main computational elements of the cerebellar cortex. The Purkinje cells deliver their output, via the deep cerebellar nuclei (DCN) and thalamus, back to the neocortex. Unfolded, the cerebellar cortex has approximately the same surface area as one cerebral hemisphere (Sereno et al., 2014) and it accounts for approximate 1/5 of the entire energy budget of the human brain (Howarth et al., 2010). Based on these numbers alone, the ‘Kleinhirn’ (i.e., little brain) is simply too large to ignore.
Figure 1.
Cerebellar circuitry. (a) The main connections of the cortico-cerebellar loop. Numbers indicate rough estimates of the number of projections, or cells in the human brain in millions (M). (b) Local circuit in the cerebellum. Every granule cell receives 4–5 mossy fibers and gives rise to a single parallel fiber. Each Purkinje cell receives input from ~175,000 parallel fibers, as well as from a single climbing fiber that originates in the inferior olive. Purkinje cells send inhibitory projection to cells in the deep cerebellar nuclei (DCN). The inhibitory interneurons (mainly Golgi, Stellate, and Basket cells) complete the circuit.
What is the function of this remarkable system? Based on the clinical symptoms observed in people with cerebellar damage, the historical focus has always been on the fine control of movement (Holmes, 1939). However, a paradigm shift can be associated with the publication of a paper by Leiner, Leiner and Dow (1986) who, based on evolutionary considerations, suggested that the cerebellum may have an important role in human cognition. Leiner et al. were impressed by the expansion of the cerebellar hemispheres in anthropoid apes and humans that paralleled the increase in size of the prefrontal cortex. Subsequent cross-species comparisons suggested that the expansion of the cerebellum disproportionally outstripped that of prefrontal areas (Barton and Venditti, 2013, 2014). More recently, the analysis of intra-cranial volumes indicates that cerebellar size is the most prominent neuroanatomical difference between Homo neanderthalensis and early Homo sapiens (Kochiyama et al., 2018). Noteworthy, these changes are specific to regions of the cerebellum that have been linked to non-motor functions (Balsters et al., 2010), supporting the argument that the cerebellum may have played a key role in the evolution of human cognition.
Anatomical tracing studies have revealed extensive communication between almost the entire neocortex and the cerebellum (Strick et al., 2009). Pontine projections arise not only from motor areas, but also from prefrontal (Schmahmann & Pandya, 1997), parietal, superior temporal (Schmahmann and Pandya, 1991) and parahippocampal areas (Schmahmann and Pandya, 1993). Moreover, projections from the dentate nucleus return to a similar set of cortical association areas (Dum and Strick, 2003; Kelly and Strick, 2003; Middleton and Strick, 1997). The presence of these loops strongly suggests a role of the cerebellum that extends well beyond motor control.
Looking beyond anatomy, two other lines of research have provided a compelling case for the involvement of the cerebellum in cognition. First, the clinical picture of patients with cerebellar disorders, as well as their performance on neuropsychological test, has revealed a broad range of impairments, including deficits in executive function, language, and affect (Kansal et al., 2017; Schmahmann and Sherman, 1998; Tedesco et al., 2011; but see Alexander et al., 2012). Second, functional neuroimaging studies involving healthy individuals have revealed the engagement of the cerebellum in a surprisingly diverse set of tasks (Strick et al., 2009). What remains unclear, however, is how to best characterize the functional role of the cerebellum across all of these domains.
A Single Cerebellar Computation or Multiple Functionality?
The cerebellar circuitry is highly uniform across the entire cerebellar cortex, inspiring the belief that cerebellar function might be conserved when generalized to task domains beyond motor control. In the words of Leiner et al. (1986), “The hypothesis states that in the human brain the newest cerebrocerebellar loops could contribute to skilled mental performance in much the same way that the older loops contribute to skilled motor performance”. Building on this notion, Schmahmann and colleagues (1996) coined the term “universal cerebellar transform” to capture the idea of a single cerebellar computation.
This idea contrasts dramatically with the common conception of the neocortex. Here, different regions can be distinguished based on their unique cytoarchitecture, myelination patterns, and gene expression (Toga et al., 2006). These features suggest a highly specialized organization, with processing modules subserving specific functions that are supported by their local circuitry. Only in the most abstract sense would we ever consider the question “What is the function of the neocortex?”. In contrast, given the uniform circuitry, the question “What is the function of the cerebellum?” seems much more sensible. But does the question have an answer?
As an approach, it is useful to consider the problem in terms of the three levels of analysis introduced by Marr (1982; see also Dean and Porrill, 2016). We can distinguish between the computational level (What task the brain has to solve), the level of representations and algorithms (How the brain solves the task), and the level of implementation (How the requisite processes are realized in neuronal tissue). Given the heterogeneity of clinical deficits and functional activation patterns observed in the cerebellum, it is clear that commonalities cannot be found at the first level, as each task will demand its own computational description. In contrast, the uniformity of the cerebellar circuitry suggests an invariance at the implementation level in terms of connectivity and plasticity. What is unclear, however, is whether we can identify a theory at the level of algorithms and representation that explains how the uniform cerebellar circuitry supports the diverse set of computations required at the task level. Such a theory would conform to a universal cerebellar transform. Alternatively, it may be that, as the functional domain of the cerebellum diversified over the course of evolution, so too did the computations that are supported by its circuitry (Fig. 2).
Figure 2.
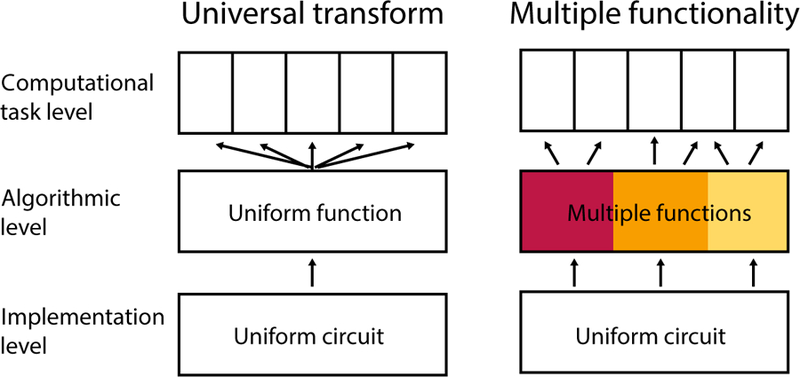
Schematic of the universal transform and multiple functionality hypotheses, considered across Marr’s three levels of analysis. At the computational level, each task demands a different computational description. At the implementation level, the cerebellar circuitry is remarkably uniform. The idea of a universal transform holds that at the algorithmic level we can formulate a general idea of how cerebellar circuits contributes to diverse functions. In contrast, the multiple functionality models posits that different task rely on variable contributions from a number of cerebellar functional modules, each of which requires a distinct algorithmic description.
Candidate hypotheses for a universal transform at the algorithmic level have been motivated by an influential theory of how the cerebellum works at the implementation level. The distinctive anatomical and physiological features of the cerebellar circuitry (Fig. 1b), well- conserved across species, were described in exquisite detail in the middle of the 20th Century (Eccles et al., 1967). This work inspired the development of a circuit-level model of cerebellar function, commonly known as the Marr-Albus-Ito model (Marr, 1969; Albus, 1971; Ito and Kano, 1982). This model builds on two core ideas. First, information coming through the mossy fibers is massively expanded by the projection onto the 50 billion granule cells, providing a detailed representation of the context via its near-infinite set of patterns. For example, the exact duration of a simple tone or light can be encoded in repeatable, time-varying activity pattern that is transmitted to the Purkinje cells via parallel fibers (Medina et al., 2000b, 2000a). Second, the unitary climbing fiber input from the inferior olive to the Purkinje cell serves as a teaching signal for supervised learning. The activation of a climbing fiber causes long-term depression of recently activated parallel fiber-to-Purkinje cell synapses (Ito and Kano, 1982). Thus, when the pattern that previously preceded a climbing fiber input is detected again, the Purkinje cell reduces its firing rate, disinhibiting cells in the DCN cell. The output of the DCN neurons can help to produce or shape the desired behavioral output, as well as to inhibit the corresponding inferior olive neuron, thus reducing climbing fiber input to the cerebellum as learning unfolds. The Marr-Albus-Ito model has proven, with minor modifications, to provide a compelling account of the cerebellar role in simple sensory-motor learning tasks (e.g., Medina and Lisberger, 2008). At a more abstract level, the core tenet of the model is that each Purkinje cell learns to predict its climbing fiber input based on the context signaled by the complex pattern of parallel fiber activity.
The key elements of the model, pattern expansion, supervised learning, and timed prediction, have provided a springboard for theorists considering a general characterization of cerebellar function at the algorithmic level. These ideas include prediction (Miall et al., 1993), internal models (Ito, 2008; Wolpert et al., 1998), timing (Ivry, 1997; Ivry and Keele, 1989), and automatization (Balsters and Ramnani, 2011; Ramnani, 2014). These hypotheses, by their very nature, are very general in order to encompass how a singular computation might apply across motor control, attention, working memory, language, and social cognition. The major challenge in evaluating such domain general hypotheses of cerebellar function has been to translate them into testable experimental predictions.
For example, the timing hypothesis proposes that cerebellar circuits are involved whenever the computations require a representation of the precise temporal relationship between stimuli, events, or motor commands (Ivry, 1997). In some task domains, testing this hypothesis has been relatively straightforward; for example, in motor control, individuals with cerebellar dysfunction would be expected to be impaired when the explicit timing of the movements is required (Spencer et al., 2003). In perception, deficits would be predicted for judgments that are based on temporal properties of the stimuli (Ackermann et al., 1997; Ivry & Keele, 1989). In other domains, especially in those where the cerebellum acts on internal, unobservable representations, it is much more difficult to derive critical tests. For example, while cerebellar activity is consistently observed during semantic retrieval (Petersen et al., 1989), it is not clear why generating a matching verb for the noun “apple” would require the manipulation of information in a precisely-timed manner.
A related conceptualization of cerebellar function is that of a predictive forward model for state estimation (Ito, 2008). This hypothesis has provided an appealing account of a number of phenomena in motor control and learning: Deficits of patients with cerebellar damage in accounting for intersegmental dynamics, or in learning to move in a novel environment, can be understood as a failure of an internal forward model (Diedrichsen and Bastian, 2014; Tseng et al., 2007). Again, there have been some successes in extending this idea to cognitive domains. For example, there is evidence that one way in which the cerebellum supports language is by using forward models to generate semantic expectancies based on the linguistic context (Lesage et al., 2012; Moberget et al., 2014). However, many unresolved questions remain: What is the error signal used to shape a forward model of semantics? How are the predictions of a cerebellar forward model distinct from predictions that can be generated by neocortical circuits alone? Clearly, prediction is not unique to the cerebellum; most neural activity can be understood as some form of prediction (Friston, 2009).
In summary, the uniformity of the cerebellar circuitry has been a powerful argument that, at some level, there exists a common computational principle that applies across various task domains. However, the remarkable heterogeneity of the human cerebellum at the task level poses an important challenge to the universal transform hypothesis, as it requires identifying a common principle that holds across a large number of disparate task domains. Notably, in the 20 years since the formulation of the universal cerebellar transform idea, very little progress has been made towards the systematic evaluation and comparison of domain general hypotheses of cerebellar function.
Partly this may be due to the fact that we currently lack a understanding of the computations underlying different mental activities that is detailed enough to generate strong and falsifiable predictions concerning cerebellar function. However, it is also important to consider the more fundamental question of whether we should expect to observe a common theory of cerebellar function at the algorithmic or representational level. The idea of a universal transform is predicated on the assumption that there is a one-to-one relationship between the implementation and algorithmic levels; i.e., that a uniform circuit implies a uniform function. However, this assumption may be incorrect. It is widely recognized that the same algorithmic process can be implemented in many different, but functionally, equivalent ways, a concept referred to as “multiple realizability” (Fodor, 1975; Putnam, 1988). Similarly, we may need to consider the possibility of “multiple functionality”, the idea that the same circuit at the implementation level can be used to realize quite different computations. For example, the predictive forward mode may have great explanatory power to capture the function of the cerebellum in motor control, but an entirely different concept may be needed to describe the role of the cerebellum in language comprehension. Thus, to describe cerebellar involvement in a broad range of tasks, it may require a (finite) set of modules, each of which requires its own functional description (Fig. 2, right).
At this point it is unclear whether a universal transform or set of multiple functions will provide the more useful description of cerebellar processing. We do believe two prerequisites are essential for significant advance to be made on this question. First, we need to obtain a much more detailed picture of the functional heterogeneity within the cerebellum, understanding the importance of different areas for different tasks. Second, we need to understand how activity within each cerebellar sub-regions is coordinated with corresponding neocortical regions. Functional imaging research of the human cerebellum is starting to provide considerable insight into these two important questions. For the remainder of this paper we review these findings in detail, returning in the final section to the question of how this information can help in understanding cerebellar function at a more general level.
Functional Imaging of the Human Cerebellum: Methodological Considerations
An extensive neuroimaging literature has revealed prominent activation of the human cerebellar cortex across diverse task domains. Before reviewing these data, we need to consider two methodological issues. First, it is important to understand which neural processes in the cerebellar cortex lead to changes in the fMRI signal. fMRI measures the blood oxygenation level dependent (BOLD) signal. How this signal relates to neural processing depends, to a large extent, on the processes that govern metabolism and blood flow, and these vary considerably between neocortex and cerebellum (Vaishnavi et al., 2010). The careful work of Martin Lauritzen and his group has revealed important insights concerning the regulation of blood flow in the cerebellum. Mossy fiber input, and the resultant granular cell activity, cause substantial increases in cerebellar blood flow (Caesar et al., 2003; Mathiesen et al., 2000). In contrast, even large increases in the activity of Purkinje cells, either through changes in simple or complex spike firing rates, produce no measureable change in blood flow (Thomsen et al., 2004, 2009), suggesting that Purkinje cells may not be able to trigger a vasodilatory response (Attwell and Iadecola, 2002).
These empirical observations seem reasonable when considering the energy expenditure in the cerebellum. At rest, approximately 80% of the energy use in the cerebellar cortex is related to signal transmission in the granular cell layer (Howarth et al., 2010). Although Purkinje cells contribute another 15% to the composite energy use, the high baseline firing rate of simple spikes (50–80Hz) and low frequency of complex spikes should result in relatively stable energy demands. In contrast, granule cells have a large dynamic range (1 to 600 Hz). Taken together, this body of work suggests that blood flow changes in the cerebellum most directly track activity modulation in the mossy fiber-granule cell system, and likely tells us very little about the activity and computation of the Purkinje cells.
The second important issue is that of spatial resolution. With the advent of high-field imaging and improvements in gradient design, fMRI studies with <2 mm isotropic resolution are now commonplace. In the neocortex, this level of resolution allows for excellent localization of the source of the hemodynamic changes. Based on a standard anatomical image, the surface of the neocortex can be reconstructed, and the activity patterns can be projected onto the surface of the individual brain. Indeed, this approach has become a standard in many laboratories, enabling precise analyses of the functional organization of the cerebral cortex (Dale et al., 1999).
In contrast, a surface-based analysis approach remains elusive for cerebellar fMRI data. Given the intricate folding of the cerebellar folia, a complete unfolding of the human cerebellum is extremely challenging (Fig. 3a), with a complete unfolding of the surface only possible when the scanning resolution is better than 200µ m (Sereno et al., 2014; Fig. 3b). Even if fMRI data are acquired at 1mm resolution, the BOLD signal will mix activation signals across neighboring folia. Thus, a surface-based display of the cerebellar cortex would cause activation of a single folium to appear distributed over various locations on the flattened surface.
Figure 3.
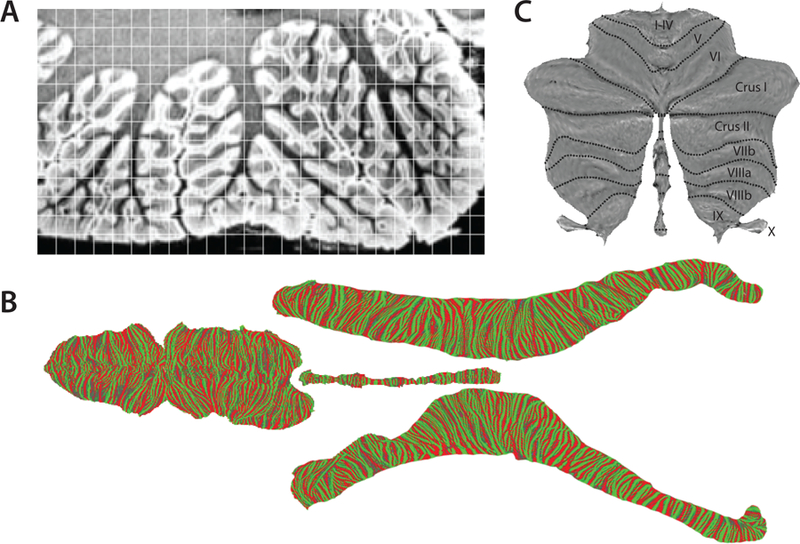
Surface of cerebellar cortex. (a) A section showing inferior lobules VIIIa, VIIIb and IX of the left hemisphere of a human cerebellum scanned at 150μm resolution. The scan clearly visualizes the granular cell layer in medium gray and the molecular layer in white. The superimposed grid indicates a typical sampling of a functional scan at 1.5mm resolution. (b) Unfolded representation of an entire human cerebellum (Sereno et al., 2014). For flattening, the surface is cut into 4 pieces. Color indicates local curvature, with green indicating the crest of a folia. (c) Simplified surface at the level of cerebellar lobules for the display of volume-averaged function data (Diedrichsen and Zotow, 2015).
Given this problem, we developed a hybrid solution to create a surface-based visualization of volume-averaged cerebellar activity data. This flat map averages across neighboring folia that cannot be cleanly resolved (Diedrichsen and Zotow, 2015; Fig. 3c). The display is designed to be proportional, with the surface area of each cerebellar region on the flat map corresponding, approximately, to its gray-matter volume. Note that the actual cerebellar surface is ~15 cm wide and ~1.2 m long (Sereno et al., 2014). This means that, while 1 cm in the horizontal direction on the flat map corresponds to the comparable distance on the surface of the cerebellar cortex, 1 cm distance in the vertical direction on the flat map corresponds to ~10 cm on the cerebellar cortex.
Imaging Studies of Functional Heterogeneity
One of the earliest reports of functional activation in the cerebellum was in the seminal PET study by Petersen and colleagues (1989), who set out to describe the brain’s language network through a series of nested contrasts. One cerebellar region, the superior motor representation, was activated during the overt production of words, relative to passive viewing or listening, consistent with a role in speech production. Surprisingly, a second cerebellar region, localized to crus I/II in the right hemisphere, was more activated when the participants were required to generate a semantic associate to the stimulus word compared to when they simply repeated the stimulus word. Because this contrast controlled for motor demands, this result suggested a role for the human cerebellum in language. Interestingly, this finding was so unexpected, violating preconceptions of cerebellar function, that the authors opted to ignore the cerebellum in their initial report (Petersen et al., 1988). However, subsequent work not only replicated this result, but showed cerebellar involvement is a much broader set of domains, including attentional control (Allen et al., 1997), working memory (Desmond et al., 1997), emotion processing (Baumann and Mattingley, 2012), and social cognition (Svoboda et al., 2006). Indeed, it soon became recognized that it was rare to find task contrasts that did not engage the cerebellum.
In an attempt to make sense of this plethora of results, a number of groups have conducted meta-analyses of the cerebellar activation patterns (E et al., 2014; Van Overwalle et al., 2014; Stoodley and Schmahmann, 2009). One important insight from this work is that there are clearly differentiable loci for motor and non-motor tasks. Motor activity tends to be restricted to two topographic areas, a superior region spanning lobules IV-VI, and an inferior region in lobule VIII. In contrast, lobule VII is activated by more cognitive tasks. While providing a reasonable first-pass overview of the functional organization of the human cerebellum, these meta-analyses have important limitations. First, they are based on the reported foci of highest activity, ignoring information about the extent and shape of the activation. Second, many of the studies included in these analyses provide relatively imprecise information about the coverage of the cerebellum (given that the focus is often on the cerebral cortex), making it hard to interpret the absence of activation. Finally, the fact that each task was studied in different sets of participants with different normalization methods makes direct comparisons across tasks difficult.
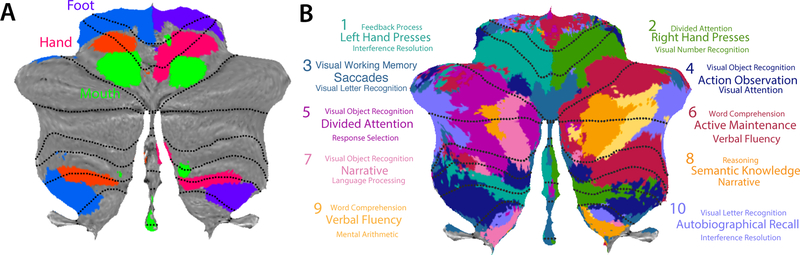
An alternative approach that mitigates these concerns is to study a broad task battery in the same set of participants. Stoodley et al. (2012) conducted the first study of this kind with respect to the cerebellum, testing seven participants on a set of five different tasks. A much larger-scale effort is contained in the Human Connectome Project (HCP), which includes data from 1,000 participants performing seven tasks (Barch et al., 2013). The projection of the activity patterns from these tasks onto the flat map representation of the cerebellar cortex reveals a complex, yet coherent functional organization (Diedrichsen and Zotow, 2015; Guell et al., 2018a). For example, the two motor representations can be shown to have an ordered arrangement for hand, foot, and tongue movement (Fig. 4a). Foot movements occupy lobules I- IV and the lateral aspects of lobules VIIIb. Hand movements elicit activity on the boundary of lobules V/VI and in the medial aspects of lobules VIII, areas that also contain a representation of individual finger movements (Wiestler et al., 2011). Tongue movements produce activity in lobule VI and medial aspects of VIIIa. However, even this extensive data set has important limitations, in that the number of tasks remains relatively small. Furthermore, different tasks are typically performed in different runs, again making direct comparisons across tasks problematic given that a common baseline measurement is lacking.
Figure 4.
Functional organization of the human cerebellum. (a) Somatotopic representation of foot (blue), hand (red) and tongue (green) movement in the cerebellum (Diedrichsen & Zotow, 2015). (b) Functional parcellation of the cerebellum based on multi-domain task battery (King et al., 2018). Parcellation and underlying task contrasts are available at diedrichsenlab.org/imaging/mdtb.htm.
To make a more direct assault on the question of functional heterogeneity in the human cerebellum, we developed a rich, multi-domain battery involving 26 tasks entailing 47 unique task conditions (King et al., 2018). Participants were scanned while performing one of two subsets of 17 tasks during each of the four 80-min sessions, preceded by training to ensure the participants were proficient in performing each task and in flexibly shifting between tasks every 35s.
The diverse task battery resulted in activation patterns that encompassed almost the entire surface of the cerebellar cortex. To provide a concise summary of these data, we subdivided the cerebellum into 10 regions, each with a specific activation profile across all task conditions. This map reveals a picture of both the functional organization of the human cerebellum, as well as its functional heterogeneity (Fig. 4b). Activity in regions 1 and 2 was associated with left and right finger movements, corresponding to the hand areas in lobules IV-VI and VIII. We did not include foot movements in our battery, which likely accounts for the relative absence of activity in lobules I-IV. Region 3 encompasses the human equivalent of the oculomotor vermis observed in the macaque (Nitschke et al., 2005; Ohtsuka and Noda, 1995). Activity in this region strongly correlated with the number of eye movements made in each task (King et al., 2018), and is, as shown recently, retinotopically organised (van Es et al., 2018). Interestingly, the activity level in this region also appeared to be strongly modulated by the demands on visuo-spatial attention, suggesting that the recruitment of this area goes beyond that required by the actual eye movements.
Region 4 was most strongly engaged in tasks involving action observation. Activity here was quite pronounced when participants passively watched videos of knot-tying (Cross et al., 2012; King et al., 2018). Interestingly, this finding seems to contradict a recent meta-analysis (Van Overwalle et al., 2014) that found no evidence of cerebellar activation during “mirroring” tasks. The discrepancy may relate, in part, to incomplete coverage of the cerebellum in some of the studies included in the meta-analysis. Even when studies report “whole brain” imaging, the inferior aspects of lobules VIII and IX are often cut off, and subsequently filled in with smoothing procedures. This can lead to a substantial loss of statistical power in these regions.
The remaining regions were associated with higher-level cognitive processes. Regions 5– 9 cover the medial and mid-lateral aspects of Crus I, Crus II and lobule VIIb. Tasks with high demands on attentional processes (e.g., divided attention and active maintenance) contributed substantially to the activation patterns in regions 5 and 6. In contrast, tasks involving language- related processing (e.g., narrative, language processing, semantic knowledge, word comprehension, verbal fluency) figured prominently in the activity profile for regions 7, 8, and 9. Consistent with prior studies, language-related functions were more prominent in Crus I and II in the right cerebellar hemisphere. Regions 7–9 also included the cerebellar component of the default-mode network (Raichle et al., 2001), brain regions that tend to be more active during rest than during task performance. Finally, region 10, located in the most lateral aspects of lobules VII, loaded heavily on tasks that involved autobiographic memory and recall.
We do not intend to suggest that this parcellation provides the “final” functional map of the human cerebellum, nor do we argue that a subdivision into 10 regions has a special status. Rather, the map offers one visualization that captures the functional heterogeneity of the cerebellum. Combined with other meta-analyses and re-analyses of multi-task data sets (Guell et al., 2018b), this functional map raises a number of interesting questions. How do we characterize the apparent asymmetries between the cerebellar hemispheres? Are the lateral aspects of lobules VIII better characterized as a foot or as an action observation region? Do emotional pictures elicit reliable vermal activity (e.g., Region 3), as has been claimed elsewhere (Guell et al., 2018a), or is this activity better explained by the demands on visual attention or eye movements? A combination of condition-rich experimentation and data integration across studies will ultimately provide us with a much more detailed picture of the functional heterogeneity of the cerebellar cortex.
Does the Cerebellum Have Distinct Functional Regions?
Current functional maps of the cerebellum, however, have already revealed a number of important organizational principles. For example, these maps suggest that functional regions within the cerebellum do not respect lobular boundaries. This observation is especially salient in the parcellations derived from our multi-domain task battery (Fig. 4b), where many of the boundaries appear unrelated to the lobular divisions. To quantify this observation, we developed a simple method to evaluate the degree to which a boundary separates functionally distinct regions (King et al., 2018). This method involves comparing the similarity of the functional profiles of two voxels within a region, relative to two voxels across a boundary. Because the functional similarity of two voxels is highly dependent on their spatial distance, with closer voxels being more similar, we matched the distance for within- and between-regions voxel pairs. This novel criterion clearly showed that lobular boundaries only weakly mark functional segregation. Indeed, many lobular boundaries did not demark a functional change that was larger than the corresponding within-lobular change.
Given this finding, it is possible that the cerebellum does not have any distinct functional regions, but rather, is better characterized as continuous gradients of functional differentiation based on slow variation in the inputs (Guell et al., 2018b). To test this idea, we asked if the functional parcellation derived from our multi-domain task battery (Fig. 4b) defined real functional boundaries, or if it simply cut up an underlying continuous map in an arbitrary way. Of course, it is a trivial exercise to show that, given a limited task set, one can always find boundaries across which the activation profiles change more than within a boundary. To be a real, or meaningful, functional boundary, the parcellation needs to be predictive of functional differences when tested with a completely new set of tasks. Using data from independent tasks within the multi-domain task battery, we were able to show this to be the case. This analysis allowed us to conclude that nature of the input to the cerebellum changes rather abruptly across the newly identified functional boundaries. Interestingly, these boundaries neither coincide with lobular boundaries, nor do they resemble Zebrin-zones that have been identified through immune-histological staining in rodents and non-human primates (Sugihara and Shinoda, 2004).
The identification of functional boundaries, and the fact that these do not align with the macro-anatomical landmarks, has important practical implications for studies of the human cerebellum. References to anatomical localization in the cerebellum is, for the lack of other defining criteria, often made by reference to lobules (Schmahmann et al., 2000; Diedrichsen et al., 2009), with within lobular-divisions limited to distinguishing between vermis and hemisphere. Similarly, regions-of-interest for functional and anatomical analyses also tend to be based on the lobules of the cerebellum (e.g., Kansal et al., 2017). Our quantitative analysis of functional variation shows that lobular divisions have minimal predictive utility. Indeed, the functional specialization within the hemispheric aspect of lobule VII changes multiple times between paravermal and the most lateral regions (Fig. 4b). Thus, analyzing data using lobules as the basic modular units, mixes signals from separate functional regions. The use of functionally- defined and carefully evaluated ROIs should therefore lead to clearer insight into functional differences between different cerebellar regions.
Cerebro-Cerebellar Connectivity
Given the cytoarchitectonic homogeneity, the functional diversity of the cerebellum must arise from variation in the inputs to the cerebellar cortex, most prominently, the massive projections from the cerebral cortex via the pontine nuclei (Schmahmann and Pandya, 1997b). For example, Kelly and Strick (2003), using a combination of retrograde and anterograde trans- synaptic tracers, showed that M1 is connected reciprocally to the two motor areas in lobules IV- VI and VIII, while prefrontal area 46 has extensive connections with the lateral aspect of Crus II. While studies such as these provide important insights into cerebro-cerebellar networks, current anatomical methods can only reveal a small piece of the overall connectivity puzzle at a time. Additionally, this work can only be performed in non-human primates; we need to keep in mind that there may be substantial inter-species differences, especially for the cerebellar hemispheres.
An important breakthrough in the study of human cerebro-cerebellar connectivity was provided by “functional connectivity” studies of the human brain. Biswall et al. (1995) made the important observation that fMRI time series of specific pairs of regions correlated with each other, even if participants simply rested in the scanner without performing a task. Common activity fluctuations are interpreted as evidence that these two areas are “functionally connected”, even though they may not share direct anatomical connections. Although this approach was originally developed to explore functional connectivity in the cerebral cortex, a number of studies have employed this method to study the otherwise inaccessible long-range connections between the human neocortex and cerebellum (Habas et al., 2009; Krienen and Buckner, 2009; O’Reilly et al., 2010).
In a now seminal paper, Buckner et al. (2011) used resting-state fMRI data from 1000 participants to produce a detailed map of cerebellar organization based on cortically-defined networks (Fig. 4). The map clearly showed the two motor regions of the cerebellum with body- part dependent connectivity, as well as confirmed the connectivity between lobule VII to prefrontal and parietal association areas. Subsequent studies using slightly different methodologies have shown a similar picture of the organization of the cerebellum (Marek et al., 2018; Spronk et al., 2019).
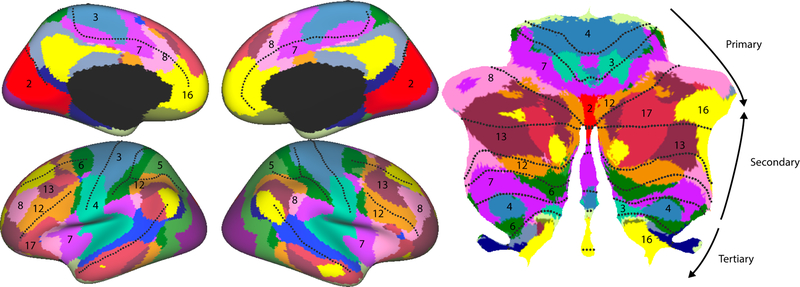
From these resting state studies, a number of novel insights can be deduced that could not be easily anticipated from animal studies. Analyzing the proportion of the cerebellum dedicated to each network, it is remarkable that only 20–30% of the cerebellar territory falls into what might be considered core motor networks. In contrast, fronto-parietal cortical networks (Fig. 5, networks 7, 8, 12, 13) account for a disproportionally (as compared to the neocortex) large part of the cerebellum (Marek et al., 2018), suggesting a prominent role of the cerebellum in higher level aspects of action planning and cognitive control. Furthermore, the hemispheric aspect of lobule IX is consistently correlated with precuneus and anterior cingulate (network 16, Fig. 4), which form part of the default-mode network.
Figure 5.
Functional connectivity between neocortex and cerebellar cortex, using a cortical parcellation into 17 regions (Buckner et al., 2011). It has been proposed that the neocortex is “represented” 3 times in the cerebellum, with each representation running from motor to more cognitive regions.
Based on the pattern of resting-state connectivity, Buckner et al. (2011) suggested a basic organizational principle, by which the neocortex is “represented” three times on the cerebellar cortical sheet. Going from Lobules IV to Crus I, there appears to be a progression from motor (networks 3, 4) to premotor (networks 6, 7, 12) to prefrontal regions (networks 8, 13, 16, 17). This sequence reverses across Crus I to lobule VIII, with a second reversal evident in the representation of more cognitive networks in lobule IX. This triplicate organization has also been observed in task-related data (Guell et al., 2018a).
The idea of a three-fold cortical-to-cerebellar mapping, however, is likely overly simplistic. While the repeated representation of cortical networks looks compelling on a parasagittal slice (see Fig. 6, Buckner, 2013), the projection onto a flattened map of the cerebellum reveals a more complex organization (Fig. 5). For example, two of the areas assigned to “network 12” are actually continuous along the paravermal cerebellum. The tertiary representation in lobules IX is much more limited and dominated by the default-mode network. Furthermore, the neocortex also shows an equally complex repetition of networks, with most networks consisting of a frontal, parietal, and sometimes medial component.
Instead of a three-fold mapping of the cortex onto the cerebellum, it is possible that each cerebellar area receives input from one and only one of the cortical components of each network. To take network 8 as an example, the lateral aspect of Crus I might be dominated by input from dorsolateral prefrontal cortex, whereas VIIb might be dominated by input from the medial or parietal component. Alternatively, each cerebellar region may combine inputs from these anatomically separate regions. Due to the strong correlations of the fMRI data among the cortical components of the same network, this question will not be easily answered using task-free fMRI.
Determining the exact pattern of connectivity has important consequences for understanding cerebellar function. If each cerebellar region is connected, in a reciprocal fashion, with only one cortical region (Kelly and Strick, 2003), the focus of a computational hypothesis would have to be on how the cerebellar circuit modulates the local computations in a single cortical region. In contrast, if it turns out that each cerebellar region integrates information from a combination of cortical regions, theoretical accounts would need to consider how the cerebellum modulates or gates communication between these regions. It is also possible that the basic rules of connectivity vary across cerebellar regions. For example, cerebellar regions that project back to the neocortex through the thalamic nuclei VL and VPL may communicate with a focal cortical area, whereas cerebellar regions that project through the thalamic laminar nuclei may modulate the interactions of many regions (Gornati et al., 2018).
Summary and Outlook
We have reviewed convergent evidence that highlights the functional diversity of the human cerebellum. This diversity makes the formulation of a domain-general theory of cerebellar function, at best, very challenging. It is also important to keep in mind that an algorithmic account of cerebellar function may entail multiple computational concepts, and that these may differ across domains, an idea we termed “multiple functionality”.
The relative merits of the universal transform and multiple functionality hypotheses will, in the end, be an empirical question. For now, we think there is considerable value in carefully developing hypotheses of cerebellar function for specific cognitive domains, without being limited, a priori, by the assumption that the function is somehow analogous to those established for motor control. For example, most of our hypotheses and experiments in the sensorimotor domain focus on the role of the cerebellar circuit in the adult organism. However, in the cognitive domain, the cerebellum may play a more important role in development than in mature function (Badura et al., 2018). Furthermore, whereas damage to the cerebellum in adulthood frequently results in rather subtle symptoms on cognitive and affective measures (Alexander et al., 2012), the same damage in the developing brain may have much more profound consequences. Thus, in cognitive and social domains, the cerebellum may help set up cortical circuitry during certain sensitive phases of development. Once established, the cortical circuits may no longer require substantial cerebellar-based modulation. This hypothesis may be important for understanding why cerebellar dysfunction has been attributed to neuropsychiatric developmental disorders such as autism (Wang et al., 2014) and schizophrenia (Moberget et al., 2018), even though damage to the cerebellum in adulthood will not result in the symptoms associated with these disorders.
When exploring cerebellar function in each task domain, there are two critical issues that must be addressed. First, cerebellar activity should be studied in the context of the activity patterns in the cerebral cortex. In isolation, the study of cerebellar activity may lead to interesting, punctuated insights, such as “the cerebellum represents reward” (Wagner et al., 2017). However, to gain a deeper understanding of cerebellar function, we need to compare cerebellar and cortical representations (Wagner et al., 2019). Do the pontine nuclei simply transmit information from the neocortex to the cerebellum in a non-selective manner? Or, are specific aspects of cortical representations emphasized and other aspects omitted? The circuitry in the pontine nuclei suggests that these subcortical nuclei can perform non-linear integration and gating of cortical input (Schwarz and Thier, 1999). Thus, the information reaching the cerebellum may differ in informative ways from the way it is represented in the neocortex.
Identifying these differences is likely to yield important insight into the role of the cerebellum. For example, if a cerebellar area is especially important in a specific phase of skill activation, we would expect different activity time courses for the relevant cerebellar and cortical regions: Disproportionately higher activity in early phases of learning, if the cerebellum is involved in initial acquisition, and disproportionately higher activity in later phases, if it is important for the performance of automatized behaviors. To perform such experiments and analyses, a full model of cortical-cerebellar connectivity is required, allowing the researcher to identify the relevant pairs of cortical and cerebellar regions.
Second, it will be important to understand what information is carried by the climbing fiber system. According to the Marr-Albus-Ito model, the climbing fiber input specifies the “learning goal” for the cerebellar circuit, and therefore plays a pivotal role in shaping the output of the cerebellum. While the climbing fiber input has traditionally been assumed to represent an error signal, new evidence suggests that it may be better conceptualized as a general teaching signal that may sometimes also relate to reward, rather than error (Heffley et al., 2018). At present, we have virtually no insight concerning the information content of the climbing fiber system in the “cognitive” regions of the human cerebellum. Thus, we do not know what these cerebellar circuits are being instructed to learn. Understanding the learning goal (or cost function) will likely provide an important key to understanding cerebellar function in the domain of cognition.
In summary, the careful investigation of cerebellar function within well-specified task domains will provide a clearer picture of the functional diversity of this major subcortical structure. Looking across domains, we may ultimately discover a universal cerebellar transform. It is likely, however, that this computation will not be easily captured in the functional terms we can intuitively describe, ideas such as timing, automatization, prediction, error correction, or internal models. Rather, a common principle may only emerge in terms of a more abstract language describing the population dynamics of neuronal networks.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (PJT 159520 to J.D.), the Canada First Research Excellence Fund (BrainsCAN to Western University) and the National Institute of Health (NS105839, NS092079 to R.B.I).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Ackermann H, Graeber S, Hentrich I, and Daum I (1997). Categorical speech perception in cerebellar disorder. Brain Lang 60, 323–331. [DOI] [PubMed] [Google Scholar]
- Albus JS (1971). A theory of cerebellar function. Math. Biosci 10, 25–61. [Google Scholar]
- Alexander MP, Gillingham S, Schweizer T, and Stuss DT (2012). Cognitive impairments due to focal cerebellar injuries in adults. Cortex 48, 980–990. [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, and Courchesne E (1997). Attentional activation of the cerebellum independent of motor involvement. Science 275, 1940–1943. [DOI] [PubMed] [Google Scholar]
- Attwell D, and Iadecola C (2002). The neural basis of functional brain imaging signals. Trends Neurosci 25, 621–625. [DOI] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Filho W. Jacob, Lent R, and Herculano-Houzel S (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513, 532–541. [DOI] [PubMed] [Google Scholar]
- Badura A, Verpeut JL, Metzger JW, Pereira TD, Pisano TJ, Deverett B, Bakshinskaya DE, and Wang SS-H (2018). Normal cognitive and social development require posterior cerebellar activity. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, and Ramnani N (2011). Cerebellar plasticity and the automation of first-order rules. J Neurosci 31, 2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Cussans E, Diedrichsen J, Phillips KA, Preuss TM, Rilling JK, and Ramnani N (2010). Evolution of the cerebellar cortex: The selective expansion of prefrontal-projecting cerebellar lobules. Neuroimage 49, 2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C, et al. (2013). Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 80, 169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton RA, and Venditti C (2013). Human frontal lobes are not relatively large. Proc. Natl. Acad. Sci 110, 9001–9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton RA, and Venditti C (2014). Rapid Evolution of the Cerebellum in Humans and Other Great Apes. Curr. Biol 24, 2440–2444. [DOI] [PubMed] [Google Scholar]
- Baumann O, and Mattingley JB (2012). Functional topography of primary emotion processing in the human cerebellum. Neuroimage 61, 805–811. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin F. Zerrin, Haughton VM, and Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2013). The Cerebellum and Cognitive Function: 25 Years of Insight from Anatomy and Neuroimaging. Neuron 80, 807–815. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, and Yeo BT (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106, 2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar K, Gold L, and Lauritzen M (2003). Context sensitivity of activity-dependent increases in cerebral blood flow. Proc Natl Acad Sci U S A 100, 4239–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Cohen NR, Hamilton A.F. de C., Ramsey R, Wolford G, and Grafton ST (2012). Physical experience leads to enhanced object perception in parietal cortex: Insights from knot tying. Neuropsychologia 50, 3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, and Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Dean P, and Porrill J (2016). The importance of Marr’s three levels of analysis for understanding cerebellar function. In Computational Theories and Their Implementation in the Brain, Vaina L, and Passingham RE, eds. (Oxford: Oxford University Press; ), pp. 79–114. [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, and Glover GH (1997). Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J. Neurosci 17, 9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, and Bastian AJ (2014). Cerebellar Function. In The Cognitive Neurosciences, Fifth Edition, Gazzaniga MS, ed. (Cambridge, MA: MIT press; ), pp. 451–460. [Google Scholar]
- Diedrichsen J, and Zotow E (2015). Surface-Based Display of Volume-Averaged Cerebellar Imaging Data. PLoS One 10, e0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, and Ramnani N (2009). A probabilistic MR atlas of the human cerebellum. Neuroimage 46, 39–46. [DOI] [PubMed] [Google Scholar]
- Dum RP, and Strick PL (2003). An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol 89, 634–639. [DOI] [PubMed] [Google Scholar]
- E, K.-H., Chen S-HA, Ho M-HR, and Desmond JE (2014). A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum. Brain Mapp 35, 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Ito M, and Szentagothai J (1967). The cerebellum as a neural machine (Berlin: Springer Verlag; ). [Google Scholar]
- van Es DM, van der Zwaag W, and Knapen T (2018). Retinotopic maps of visual space in the human cerebellum. BioRxiv [DOI] [PubMed]
- Fodor J (1975). The language of thought (New York: Thomas Cromwell; ). [Google Scholar]
- Friston K (2009). The free-energy principle: a rough guide to the brain? Trends Cogn Sci 13, 293–301. [DOI] [PubMed] [Google Scholar]
- Gornati SV, Schäfer CB, Eelkman Rooda OHJ, Nigg AL, De Zeeuw CI, and Hoebeek FE (2018). Differentiating Cerebellar Impact on Thalamic Nuclei. Cell Rep 23, 2690–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X, Gabrieli JDE, and Schmahmann JD (2018a). Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage 172, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X, Schmahmann JD, Gabrieli JDE, and Ghosh SS (2018b). Functional gradients of the cerebellum. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, and Greicius MD (2009). Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29, 8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffley W, Song EY, Xu Z, Taylor BN, Hughes MA, McKinney A, Joshua M, and Hull C (2018). Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat. Neurosci 21, 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G (1939). The cerebellum of man. Brain 62, 1–30. [Google Scholar]
- Howarth C, Peppiatt-Wildman CM, and Attwell D (2010). The Energy Use Associated with Neural Computation in the Cerebellum. J. Cereb. Blood Flow Metab 30, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M (2008). Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci 9, 304–313. [DOI] [PubMed] [Google Scholar]
- Ito M, and Kano M (1982). Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci. Lett 33, 253–258. [DOI] [PubMed] [Google Scholar]
- Ivry R (1997). Cerebellar timing systems. Int. Rev. Neurobiol 41, 555–573. [PubMed] [Google Scholar]
- Ivry RB, and Keele SW (1989). Timing functions of the cerebellum. J. Cogn. Neurosci 1, 136–152. [DOI] [PubMed] [Google Scholar]
- Kansal K, Yang Z, Fishman AM, Sair HI, Ying SH, Jedynak BM, Prince JL, and Onyike CU (2017). Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain 140, 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, and Strick PL (2003). Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23, 8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Hernandez-Castillo CR, Poldrack RR, Ivry R, and Diedrichsen J (2018). A Multi-Domain Task Battery Reveals Functional Boundaries in the Human Cerebellum. BioRxiv [DOI] [PMC free article] [PubMed]
- Kochiyama T, Ogihara N, Tanabe HC, Kondo O, Amano H, Hasegawa K, Suzuki H, Ponce de León MS, Zollikofer CPE, Bastir M, et al. (2018). Reconstructing the Neanderthal brain using computational anatomy. Sci. Rep 8, 6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, and Buckner RL (2009). Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19, 2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage E, Morgan BE, Olson AC, Meyer AS, and Miall RC (2012). Cerebellar rTMS disrupts predictive language processing. Curr. Biol 22, R794–R795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Siegel JS, Gordon EM, Raut RV, Gratton C, Newbold DJ, Ortega M, Laumann TO, Adeyemo B, Miller DB, et al. (2018). Spatial and Temporal Organization of the Individual Human Cerebellum. Neuron 100, 977–993.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1969). A theory of cerebellar cortex. J. Physiol 202, 437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1982). Vision: A computational investigation into the human representation and procesing of visual information (New York: Freeman; ). [Google Scholar]
- Mathiesen C, Caesar K, and Lauritzen M (2000). Temporal coupling between neuronal activity and blood flow in rat cerebellar cortex as indicated by field potential analysis. J Physiol 523, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, and Lisberger SG (2008). Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci 11, 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, and Mauk MD (2000a). Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. J. Neurosci 20, 5516–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Ohyama T, and Mauk MD (2000b). Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol 10, 717–724. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, and Stein JF (1993). Is the Cerebellum a Smith Predictor? J Mot Behav 25, 203–216. [DOI] [PubMed] [Google Scholar]
- Middleton FA, and Strick PL (1997). Cerebellar output channels. In The Cerebellum and Cognition, Schmahmann JD, ed. (San Diego, CA: Academic Press; ), pp. 31–60. [Google Scholar]
- Moberget T, Gullesen EH, Andersson S, Ivry RB, and Endestad T (2014). Generalized Role for the Cerebellum in Encoding Internal Models: Evidence from Semantic Processing. J. Neurosci 34, 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget T, Doan NT, Alnæs D, Kaufmann T, Córdova-Palomera A, Lagerberg TV, Diedrichsen J, Schwarz E, Zink M, Eisenacher S, et al. (2018). Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol. Psychiatry 23, 1512–1520. [DOI] [PubMed] [Google Scholar]
- Nitschke MF, Arp T, Stavrou G, Erdmann C, and Heide W (2005). The cerebellum in the cerebro-cerebellar network for the control of eye and hand movements--an fMRI study. Prog Brain Res 148, 151–164. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, and Johansen-Berg H (2010). Distinct and Overlapping Functional Zones in the Cerebellum Defined by Resting State Functional Connectivity. Cereb. Cortex 20, 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K, and Noda H (1995). Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey. J. Neurophysiol 74, 1828–1840. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K, Mariën P, and Vandekerckhove M (2014). Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. Neuroimage 86, 554–572. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, and et al. (1988). Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 331, 585–589. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, and et al. (1989). Positron emission tomographic studies of the processing of single words. J. Cogn. Neurosci 1, 153–170. [DOI] [PubMed] [Google Scholar]
- Putnam H (1988). Representation and reality (Cambridge, MA: MIT Press; ). [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, and Shulman GL (2001). A default mode of brain function. Proc. Natl. Acad. Sci 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N (2014). Automatic and Controlled Processing in the Corticocerebellar System. In Progress in Brain Research, pp. 255–285. [DOI] [PubMed]
- Schmahmann JD (1996). From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp 4, 174–198. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, and Pandya DN (1991). Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J. Comp. Neurol 308, 224–248. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, and Pandya DN (1993). Prelunate, occipitotemporal, and parahippocampal projections to the basis pontis in rhesus monkey. J. Comp. Neurol 337, 94–112. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, and Pandya DN (1997a). Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J. Neurosci 17, 438–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, and Pandya DN (1997b). The cerebrocerebellar system. In The Cerebellum and Cognition, Schahmann JD, ed. (San Diego, CA: Academic Press; ), pp. 31–55. [Google Scholar]
- Schmahmann JD, and Sherman JC (1998). The cerebellar cognitive affective syndrome. Brain 121, 561–579. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga A, Petrides M, and Evans A (2000). MRI atlas of the human cerebellum (San Diego: Academic Press; ). [DOI] [PubMed] [Google Scholar]
- Schwarz C, and Thier P (1999). Binding of signals relevant for action: towards a hypothesis of the functional role of the pontine nuclei. Trends Neurosci 22, 443–451. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Diedrichsen J, Tachrout M, Silva G, and De Zeeuw CI (2014). Reconstruction and unfolding of the human cerbellar cortex from high-resolution post- mortem MRI. In Annual Meeting of the Society for Neuroscience
- Spencer RM, Zelaznik HN, Diedrichsen J, and Ivry RB (2003). Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science 300, 1437–1439. [DOI] [PubMed] [Google Scholar]
- Spronk M, Ji JL, Kulkarni K, Repovs G, Anticevic A, and Cole MW (2019). Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage 185, 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, and Schmahmann JD (2009). Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44, 489–501. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, and Schmahmann JD (2012). Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59, 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, and Fiez JA (2009). Cerebellum and nonmotor function. Annu Rev Neurosci 32, 413–434. [DOI] [PubMed] [Google Scholar]
- Sugihara I, and Shinoda Y (2004). Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci 24, 8771–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, and Levine B (2006). The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 44, 2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, and Leggio MG (2011). The cerebellar cognitive profile. Brain 134, 3672–3686. [DOI] [PubMed] [Google Scholar]
- Thomsen K, Offenhauser N, and Lauritzen M (2004). Principal neuron spiking: neither necessary nor sufficient for cerebral blood flow in rat cerebellum. J Physiol 560, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen K, Piilgaard H, Gjedde A, Bonvento G, and Lauritzen M (2009). Principal cell spiking, postsynaptic excitation, and oxygen consumption in the rat cerebellar cortex. J Neurophysiol 102, 1503–1512. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Mori S, Amunts K, and Zilles K (2006). Towards multimodal atlases of the human brain. Nat Rev Neurosci 7, 952–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasch J (1969). The numerical capacity of the human cortico-pontocerebellar system. Brain Res 13, 476–484. [DOI] [PubMed] [Google Scholar]
- Tseng Y-W, Diedrichsen J, Krakauer JW, Shadmehr R, and Bastian AJ (2007). Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J. Neurophysiol 98. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, and Raichle ME (2010). Regional aerobic glycolysis in the human brain. Proc. Natl. Acad. Sci 107, 17757–17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, and Luo L (2017). Cerebellar granule cells encode the expectation of reward. Nature 544, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Kadmon J, Nguyen ND, Ganguli S, Schnitzer MJ, and Luo L (2019). Shared Cortex-Cerebellum Dynamics in the Execution and Learning of a Motor Task. Cell [DOI] [PMC free article] [PubMed]
- Wang SS-H, Kloth AD, and Badura A (2014). The Cerebellum, Sensitive Periods, and Autism. Neuron 83, 518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestler T, McGonigle DJ, and Diedrichsen J (2011). Integration of sensory and motor representations of single fingers in the human cerebellum. J Neurophysiol 105, 3042–3053. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, and Kawato M (1998). Internal models in the cerebellum. Trends Cogn. Sci 2, 313–321. [DOI] [PubMed] [Google Scholar]