Key Points
Question
What are the risk factors for physical disability in patients with leprosy?
Findings
This systematic review and meta-analysis of 32 studies found a strong association between the presence of physical disabilities and male sex, multibacillary leprosy, leprosy reactions, and lepromatous presentation.
Meaning
These findings can guide the early identification of individuals at higher risk of developing physical disabilities and the development of targeted preventive interventions.
This systematic review and meta-analysis of 32 studies assesses the strength of evidence linking clinical features and sex of patients with leprosy to disability.
Abstract
Importance
The World Health Organization (WHO) 2016-2020 Global Leprosy Strategy aims to reinvigorate efforts to control leprosy and avert leprosy disability to less than 1 per million population.
Objective
To systematically identify clinical factors associated with physical disability in patients with leprosy.
Data Source
Searches were conducted in Scopus, PubMed, and Web of Science databases to identify studies published from January 23, 1988, to May 23, 2018, using the keywords leprosy and physical disability and related terms.
Study Selection
Studies that evaluated patients using the WHO leprosy disability grading system and reported the number of patients with and without disability by clinical characteristics were included.
Data Extraction and Synthesis
The odds ratio (OR) was used as a measure of association between the clinical features and physical disability. Summary estimates were calculated using random-effects models.
Main Outcomes and Measures
The primary outcome was physical disability according to the WHO disability classification. The association between clinical features and physical disability was evaluated.
Results
The search identified 2447 reports. After screening titles and abstracts, 177 full-text articles were assessed for eligibility, and 32 studies were included in the systematic review; 24 of the 32 studies included sex information (39 571 patients), of whom 24 218 (61.2%) were male. Male patients with leprosy were more likely to have physical disability than female patients with leprosy (pooled OR, 1.66; 95% CI, 1.43-1.93; I2, 81.3%; P < .001). Persons with multibacillary leprosy were 4-fold more likely to have physical disability than those with paucibacillary leprosy (pooled OR, 4.32; 95% CI, 3.37-5.53; I2, 88.9%, P < .001). Patients having leprosy reactions were more likely to have disability (pooled OR, 2.43; 95% CI, 1.35-4.36; I2, 92.1%; P < .001). Patients with lepromatous leprosy experienced 5- to 12-fold higher odds of disability.
Conclusions and Relevance
This systematic review and meta-analysis confirms the association between the presence of physical disabilities and male sex, multibacillary leprosy, leprosy reactions, and lepromatous presentation. These findings can guide the development of targeted interventions for early identification of individuals at greater risk of developing physical disabilities and education campaigns to promote early consultation to institute treatment for leprosy reactions and prevent physical disability.
Introduction
Leprosy is a chronic infectious disease caused by Mycobacterium leprae that affects the skin and peripheral nerves, leading to progressive physical disability and deformities if not diagnosed and treated early.1,2,3 Despite a significant reduction in its global prevalence since the World Health Organization (WHO) implemented the free multidrug therapy program in 1995, leprosy remains a major cause of morbidity owing to its associated long-term disabilities and sequelae4 in an estimated 2 million people worldwide.5,6
The WHO goal is to reduce leprosy disabilities to a target of less than 1 per million population through the strengthening of strategies for the prevention and reduction of deformities.7 These strategies include the early recognition and prioritization of individuals with leprosy with characteristics associated with physical disability and the main focus of control programs and rehabilitation centers is to prevent and manage physical impairment to improve quality of life.8,9 Although clinical features such as multibacillary (MB) leprosy and leprosy reactions are considered to predispose patients to physical disability and deformity,2,5,10,11,12,13 there are no systematic analyses assessing the strength of this evidence. We report here a systematic review and meta-analysis to assess the clinical factors associated with physical disability in leprosy.
Methods
This study followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guideline.14 Institutional review board approval and informed consent were not required because all data were obtained from secondary data sources and data were deidentified.
Search Strategy and Selection Criteria
From April 4, 2018, to May 23, 2018, we systematically searched the PubMed, Scopus, and Web of Science databases to identify studies published from January 23, 1988, to May 23, 2018, using the keywords leprosy and physical disability and related terms, as described in eTable 1 in the Supplement. Two independent reviewers (H.L.de P. and C.D.F.de S.) screened the search results and identified potentially relevant studies based on their title and abstract. The studies were then read in full for consideration for inclusion in the analysis. Disagreements between the 2 reviewers were resolved by discussion. Studies were included if (1) patients had been assessed for physical disability using WHO leprosy disability grading1; (2) the study evaluated the association between the clinical presentation and physical disability; and (3) the clinical factors (exposure) were described according to the presence or absence of physical disability. We excluded publications without original data, such as reviews and opinions, those with overlapping data, and those from which data extraction was not possible. The authors of the latter studies were asked to provide access to the original databases, but none of them responded.
We considered age, sex, clinical presentation categories, the presence of leprosy reactions, and the WHO leprosy classification stage as exposure factors. The WHO classification includes paucibacillary leprosy (≤5 skin lesions, only 1 affected nerve trunk, or both; or negative findings on microscopy), MB leprosy (>5 skin lesions, more than 1 affected nerve trunk, or both; or positive findings on microscopy).15 Clinical forms include tuberculoid, borderline, lepromatous, and indeterminate presentations.16 Leprosy reactions include episodes characterized by the acute inflammation of skin lesions or nerves (type 1) and the appearance of inflamed cutaneous nodules with or without neuritis (type 2).17
Our primary outcome was physical disability according to the WHO disability classification.1 In this classification, grade 0 indicates no sensory impairment or disability or damage to the eyes, hands, or feet; grade 1 indicates the presence of eye (visual acuity >6/60 in either eye) or sensory impairment in the hands or feet, without visible deformities or damages; and grade 2 indicates severe visual impairment (visual acuity <6/60 or inability to count fingers at 6 m) or the presence of visible deformity in the eyes (lagophthalmos, iridocyclitis, or corneal opacities) or visible deformity or damage on hands or feet (ie, ulcerations, traumatic injuries, resorption, claw, fallen hand, foot drop, or ankle contracture). We combined physical disability grades 1 and 2 and considered them jointly for statistical purposes.
Data Extraction and Bias Assessment
Data were extracted using standardized tables, including author, country, study design, participant characteristics, clinical setting (specialized health center [specializing in the care of patients with leprosy], general hospital [not specializing in the care of patients with leprosy], primary health care, or data obtained from a health information system) and physical disability (presence or absence). We extracted the number of patients with and without physical disability at the time of diagnosis and stratified for each exposure variable. Not all studies reported all variables and we used percentages to obtain the absolute number of patients by stratum.
The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies of the National Institutes of Health (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) was used to grade the quality of each study. This tool is composed of 14 items that evaluate the representativeness and selection of the sample, description and measurement of exposure, follow-up of participants, and treatment of confounding. After critical appraisal of each item, the studies were rated as good, fair, or poor and the findings were discussed qualitatively. Disagreements were resolved by discussion.
Statistical Analysis
We calculated the pooled odds ratio (OR) for the primary outcome and forest plots to present results with 95% CIs. Not all studies reported data on all exposure variables, and the pooled OR was estimated from the data available for each variable. Pooled estimates were calculated using a random-effects model (DerSimonian and Laird method). Two-sided P < .05 was used to determine statistical significance. Statistical heterogeneity was assessed using the Cochran Q test18 and quantified by the I2 index.19
Subgroup analyses were performed according to the study design, population characteristics (adults, adults and children together, and children) and study setting. Publication bias was assessed by visually inspecting whether larger and smaller studies were asymmetrically distributed in the funnel plot.20 Leave-1-out sensitivity analysis was conducted to examine the influence of each study on the pooled effect size.21 Analyses were performed using Stata version 14.0 (StataCorp LP) and Review Manager, version 5.3 (Cochrane IMS) statistical software.
Results
The search strategy identified 2447 reports. After screening titles and abstracts, 177 full-text articles were assessed for eligibility and 32 were included in the analysis (eFigure 1 in the Supplement). The Table describes the characteristics of the studies included. Twenty-seven of the 32 studies (84%) were cross-sectional, 4 (13%) were from surveillance systems (continuous and routine reporting of cases for monitoring purposes) and 1 (3%) was a cohort study. Nine (28%) studies included adults, 3 (9%) included children, and 20 (63%) enrolled both adults and children and reported combined findings. Eleven (34%) studies were based in general hospitals, 9 (28%) in primary health care settings and 8 (25%) in specialized health care centers, and 4 (13%) were data extracted from health information systems of leprosy control programs, the last of which came from the systematic collection of surveillance services. The racial/ethnic origin of the patients was not reported.
Table. Characteristics of the Included Studies.
| Source | Country | Study Design | Population | Settings | Risk Factors Analyzed | Outcome | Sample Size | Total Disability |
|---|---|---|---|---|---|---|---|---|
| Zhanget et al,22 1993 | China | Cross-sectional | Adults/children | Tertiary health center | Sex, WHO leprosy classification, clinical forms | Combined grades 1 and 2 | 14 257 | 8122 |
| Tiendrebeogo et al,23 1996 | Burkina Faso | Cross-sectional | Adults | Primary care | Sex, WHO leprosy classification | Combined grades 1 and 2 | 554 | 165 |
| Çakiner et al,24 1997 | Turkey | Cross-sectional | Adults | Hospital | Sex | Combined grades 1 and 2 | 711 | 546 |
| Wittenhorst et al,25 1998 | Zimbabwe | Surveillance | Adults/children | Information system | Sex, WHO leprosy classification | Grade 2 | 746 | 247 |
| Croft et al,26 1999 | Bangladesh | Cross-sectional | Adults/children | Tertiary health center | Sex, WHO leprosy classification | Combined grades 1 and 2 | 2664 | 415 |
| Ahmad et al,27 2004 | Pakistan | Cross-sectional | Adults | Hospital | Sex, WHO leprosy classification, clinical forms | Combined grades 1 and 2 | 100 | 41 |
| Kar and Job,11 2005 | India | Cross-sectional | Children | Tertiary health center | Sex, WHO leprosy classification, leprosy reaction | Grade 2 | 275 | 29 |
| Rad et al,28 2007 | Iran | Cross-sectional | Adults/children | Hospital | Sex, WHO leprosy classification | Combined grades 1 and 2 | 180 | 79 |
| Silva-Sobrinho et al,29 2007 | Brazil | Cross-sectional | Adults/children | Primary care | Sex | Combined grades 1 and 2 | 99 | 79 |
| Lana et al,30 2008 | Brazil | Surveillance | Adults/children | Information system | Sex, WHO leprosy classification | Combined grades 1 and 2 | 1461 | 672 |
| Soomro et al,31 2008 | Pakistan | Cross-sectional | Adults | Hospital | WHO leprosy classification | Separately grades 1 and 2 | 100 | 55 |
| Ramos and Souto,32 2010 | Brazil | Cross-sectional | Adults | Tertiary health center | Sex, WHO leprosy classification | Separately grades 1 and 2 | 193 | 51 |
| El-Dawela et al,33 2012 | Egypt | Cross-sectional | Adults/children | Hospital | WHO leprosy classification | Grade 2 | 587 | 204 |
| Sarkar et al,34 2012 | India | Cross-sectional | Adults | Hospital | WHO leprosy classification | Separately grades 1 and 2 | 244 | 244 |
| Kumar et al,10 2012 | India | Cohort | Adults/children | Tertiary health center | Sex, WHO leprosy classification, clinical forms | Grade 2 | 293 | 27 |
| Nardi et al,35 2012 | Brazil | Cross-sectional | Adults/children | Primary care | Sex, WHO leprosy classification, clinical forms | Separately grades 1 and 2 | 335 | 71 |
| van Brakel et al,5 2012 | Indonesia | Cross-sectional | Adults | Primary care | Sex, WHO leprosy classification | Separately grades 1 and 2 | 1308 | 1003 |
| Monteiro et al,36 2013 | Brazil | Cross-sectional | Adults/children | Primary care | WHO leprosy classification, leprosy reaction | Separately grades 1 and 2 | 282 | 44 |
| Oliveira et al,37 2013 | Brazil | Cross-sectional | Adults/children | Tertiary health center | Sex | Separately grades 1 and 2 | 494 | 142 |
| Guerrero et al,38 2013 | Colombia | Cross-sectional | Adults/children | Primary care | Sex, WHO leprosy classification | Combined grades 1 and 2 | 333 | 117 |
| de Castro et al,39 2014 | Brazil | Cross-sectional | Adults | Primary care | Sex, WHO leprosy classification | Combined grades 1 and 2 | 225 | 137 |
| Silva et al,40 2015 | Brazil | Cross-sectional | Adults/children | Primary care | Sex, WHO leprosy classification | Grade 2 | 1916 | 366 |
| Monteiro et al,41 2015 | Brazil | Surveillance | Adults/children | Information system | Sex, WHO leprosy classification, leprosy reaction, clinical forms | Grade 2 | 12 328 | 664 |
| Santos et al,2 2015 | Brazil | Surveillance | Adults/children | Information system | Sex, WHO leprosy classification, leprosy reaction, clinical forms | Combined grades 1 and 2 | 2358 | 656 |
| Sethi and Rao,42 2015 | India | Cross-sectional | Children | Hospital | WHO leprosy classification, clinical forms | Separately grades 1 and 2 | 94 | 32 |
| Patel and Modi,43 2016 | India | Cross-sectional | Adults | Tertiary health center | Sex, WHO leprosy classification, leprosy reaction | Separately grades 1 and 2 | 239 | 127 |
| Onyeonoro et al,44 2016 | India | Cross-sectional | Adults/children | Hospital | Sex, WHO leprosy classification | Separately grades 1 and 2 | 287 | 168 |
| Queirós et al,45 2016 | Brazil | Cross-sectional | Adults/children | Hospital | WHO leprosy classification | Separately grades 1 and 2 | 458 | 63 |
| Anjum et al,46 2017 | India | Cross-sectional | Adults/children | Tertiary health center | WHO leprosy classification | Combined grades 1 and 2 | 54 | 48 |
| Rodrigues et al,13 2017 | Brazil | Cross-sectional | Adults/children | Hospital | Sex, WHO leprosy classification | Combined grades 1 and 2 | 182 | 124 |
| Darlong et al,47 2017 | India | Cross-sectional | Children | Hospital | WHO leprosy classification | Grade 2 | 319 | 21 |
| Haefner et al,12 2017 | Brazil | Cross-sectional | Adults/children | Primary care | Sex | Separately grades 1 and 2 | 910 | 262 |
Abbreviation: WHO, World Health Organization.
The risk of bias of the studies is showed in eTable 2 in the Supplement. All studies had clear objectives and eligibility criteria, recruited subjects from the same population, and described the definitions of exposure factors and outcomes. However, 28 of the 32 studies did not report the number of eligible participants recruited into the study. Because 27 of the 32 studies were cross-sectional, the exposure and outcome status (physical disability) of the participants were collected at the same time, introducing potential sources of bias.
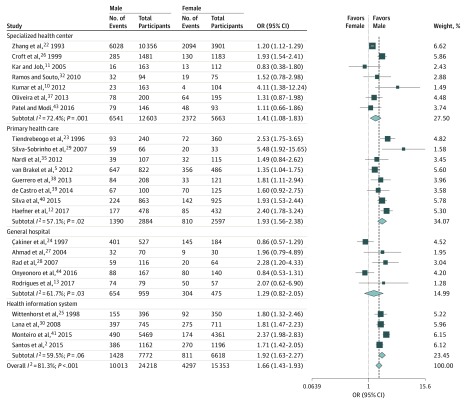
Twenty-four of the 32 studies had sex information (39 571 patients), of whom 24 218 (61.2%) were male.2,5,10,11,12,13,22,23,24,25,26,27,28,29,30,32,35,37,38,39,40,41,43 Male patients were more likely to have physical disability than female patients (pooled OR, 1.66; 95% CI, 1.43-1.93; I2, 81.3%; P < .001) and the odds of physical disability did not depend on the study location (Figure 1 and eFigure 2 in the Supplement).2,5,10,11,12,13,22,23,24,25,26,27,28,29,30,32,35,37,38,39,40,41,43,44
Figure 1. Subgroup Analysis for Sex by Location of Enrollment.
Square data markers represent odds ratio, with size representing the statistical weight of the study using random-effects analysis. Error bars represent 95% CI. Diamond data marker represents the overall OR and 95% CI for the outcome of interest.
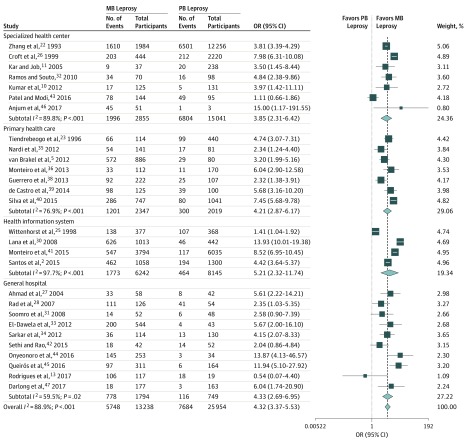
World Health Organization leprosy classification data were obtained from 28 studies including 39 192 patients.2,5,10,11,13,22,23,25,26,27,28,30,31,32,33,34,35,36,37,38,39,41,42,43,44,45,46,47 Paucibacillary leprosy was more frequent than MB leprosy (25 954 [66.2%] vs 13 238 [33.8%], respectively), but patients with MB leprosy were 4-fold more likely to have physical disabilities (pooled OR, 4.32; 95% CI, 3.37-5.53; I2, 88.9%, P < .001) independent of the study location (Figure 2 and eFigure 3 in the Supplement).2,5,10,11,22,23,25,27,28,30,31,32,33,34,35,36,38,39,40,41,42,43,44,45,46,47
Figure 2. Subgroup Analysis for World Health Organization (WHO) Leprosy Classification by Location of Enrollment.
Square data markers represent odds ratio, with size representing the statistical weight of the study using random-effects analysis. Error bars represent 95% CI. Diamond data marker represents the overall OR and 95% CI for the outcome of interest. MB indicates multibacillary; PB, paucibacillary.
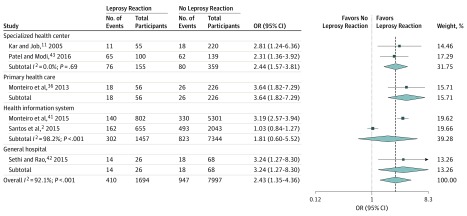
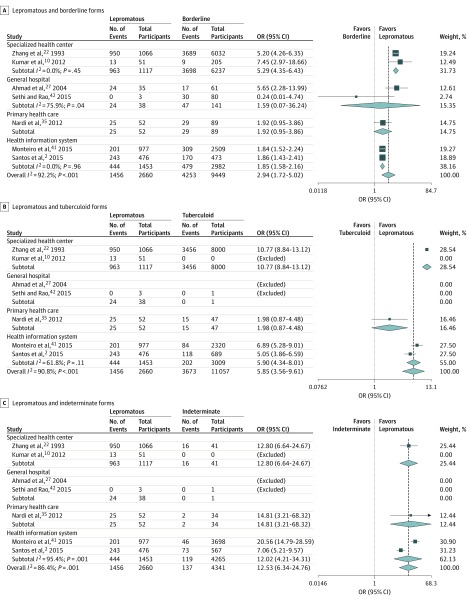
Six studies reported leprosy reactions and disability,2,11,36,41,42,43 including 9691 patients, of whom 1694 (17.5%) had leprosy reactions and 7997 (82.5%) had no reaction, resulting in a pooled OR of 2.43 (95% CI, 1.35-4.36; I2, 92.1%; P < .001) (Figure 3 and eFigure 4 in the Supplement).2,11,36,41,42,43 The clinical presentation was reported in 7 studies. Patients with lepromatous forms were more likely to have disability than patients with borderline forms (pooled OR, 2.94; 95% CI, 1.72-5.02; I2, 92.2%; P < .001), tuberculoid (pooled OR, 5.85; 95% CI, 3.56-9.61; I2, 90.8%; P < .001), or indeterminate leprosy (pooled OR, 12.53; 95% CI, 6.34-24.76; I2, 86.4%; P < .001) and these pooled ORs were not dependent on the study location (Figure 4 and eFigures 5-7 in the Supplement).2,22,27,35,42
Figure 3. Subgroup Analysis for Leprosy Reaction by Location of Enrollment.
Square data markers represent odds ratio (OR), with size representing the statistical weight of the study using random-effects analysis. Error bars represent 95% CI. Diamond data marker represents the overall OR and 95% CI for the outcome of interest.
Figure 4. Forest Plot Showing the Pooled Odds Ratio (OR) for Physical Disability in Patients With Leprosy by Clinical Form.
A, Subgroup analysis for lepromatous and borderline forms. B, Subgroup analysis for lepromatous and tuberculoid forms. C, Subgroup analysis for lepromatous and indeterminate forms. Square data markers represent odds ratio, with size representing the statistical weight of the study using random-effects analysis. Error bars represent 95% CI. Diamond data marker represents the overall OR and 95% CI for the outcome of interest.
Leave-1-out sensitivity analysis was conducted by means of omitting 1 study at a time and examining the influence of each study on the pooled effect size. Sensitivity analysis showed that the result was robust. No evidence of publication bias was observed (eFigures 8-12 in the Supplement).
Discussion
Factors predisposing to the development of physical disability in leprosy have been reported extensively, providing an excellent opportunity for a comprehensive analysis. This review confirms that male patients, those with MB leprosy, leprosy reactions, and lepromatous presentations are more likely to have physical disabilities.
Male patients were almost 2 times more likely than female patients to have physical disability. This sex difference has been attributed to social behaviors and reluctance and difficulties in accessing health services.48 Men often ignore leprosy symptoms and seek health services at more advanced stages of the disease and with more severe clinical manifestations.49,50,51 Health professionals should be aware of men’s increased risk of physical disability during active case finding activities and contact tracing, to ensure that male contacts and secondary cases are not missed during home visits.
Leprosy disease progression is determined by the cellular immune responses to M leprae, which are expressed through different pathophysiologic mechanisms. The absence of cellular and enhanced humoral immune responses in patients with MB leprosy is associated with high bacilli loads and with development of neuritis and peripheral nerve damage.26,52 Patients with MB leprosy in the present systematic review and meta-analysis were more likely to have physical disabilities, highlighting the importance of good clinical classification and microscopic detection of bacilli.16
Although tuberculoid and indeterminate leprosy are the most frequent clinical presentations, our meta-analysis demonstrates that patients with lepromatous leprosy have 5- to 12-fold greater odds of disability. Lepromatous leprosy is characterized by helper T-cell 2 immune responses with increased production of interleukin 4 and interleukin 10 and activation of regulatory T cells, a robust but ineffective production of antibodies with formation of immune complexes, and a failure to restrict M leprae growth, especially into the Schwann cells.53 The immunologic response to infected Schwann cells is associated with nerve injuries and physical disability.54
Individuals with leprosy reactions are more susceptible to peripheral nerve injuries and sequelae. Type 1 reactions are a reversal or upgrade of the cell-mediated immunity to M leprae antibodies, whereas type 2 reactions are the result of immune complexes attracting granulocytes and activation of complement and cytokine responses.53 Both reactions may damage peripheral nerves with impairment of function and can occur at any time in the clinical course of the disease, independent of treatment. The World Health Organization thus recommends to follow up patients with leprosy for several years after an apparently successful treatment.4,55,56
This systematic review focused on the likelihood of disability among patients with leprosy reactions at the time of diagnosis. However, studies have reported a high risk of leprosy reactions after completion of multidrug therapy, requiring long-term follow-up with neurologic examinations.4,10,57 The early identification of reactions and their prompt management with prednisone (1 to 2 mg/kg/d for ≥90 days) can prevent neuropathies and disability.17
The WHO Global Leprosy Strategy 2010-20207 aims to accelerate action toward a leprosy-free world, with a focus on the early detection of cases, before disabilities occur, and the prevention and early detection of disabilities among higher-risk groups by conducting active case–finding campaigns in highly endemic areas or communities. In this sense, our findings provide information to stakeholders regarding to the characterization of high-risk patients that should be prioritized and targeted to receive preventive interventions for the early detection and reduction of grade 2 disability in endemic areas.
Limitations
Our findings, however, should be interpreted with caution. All studies included were observational, patients were not randomized, and studies were often conducted with other primary objectives; therefore, the studies are susceptible to patient selection bias and the disability information may not have been collected systematically. Moreover, it was not possible to perform meta-analyses to explore whether age, schooling level, and socioeconomic status were associated with physical disability. Most studies, however, indicated that the prevalence of disability increases with age and that disability is inversely proportional to socioeconomic conditions and educational level. Education and income are considered determining factors for disease improvement and protective for the occurrence of disability.2
Conclusions
Despite these limitations, we demonstrate an association between the presence of physical disabilities and sex, MB leprosy, leprosy reactions, and a lepromatous presentation. These findings can guide the development of targeted interventions to identify individuals at early risk of physical disabilities and to inform education campaigns promoting early consultation to institute treatment for leprosy reactions and prevention of further physical disability. Long-term follow-up is necessary to monitor factors associated with development of disabilities, as are the provision of interventions promoting self-care, disability prevention, and the availability of rehabilitation services.
eTable 1. Full Search Strategy
eFigure 1. Flow Diagram of Study Selection
eTable 2. Risk of Bias of the Included Studies Using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies
eFigure 2. Forest Plot Showing the Pooled Odds Ratio for Male and Female
eFigure 3. Forest Plot Showing the Pooled Odds Ratio for Multibacillary and Paucibacillary Leprosy
eFigure 4. Forest Plot Showing the Pooled Odds Ratio for Leprosy Reaction.
eFigure 5. Forest Plot Showing the Pooled Odds Ratio for Lepromatous and Borderline Clinical Forms
eFigure 6. Forest Plot Showing the Pooled Odds Ratio for Lepromatous and Tuberculoid Clinical Forms
eFigure 7. Forest Plot Showing the Pooled Odds Ratio for Lepromatous and Indeterminate Clinical Forms
eFigure 8. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Male and Female
eFigure 9. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Multibacillary and Paucibacillary Leprosy
eFigure 10. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Lepromatous and Borderline Clinical Forms
eFigure 11. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Lepromatous and Turberculoid Clinical Forms
eFigure 12. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Lepromatous and Indeterminate Clinical Forms
References
- 1.Brandsma JW, Van Brakel WH. WHO disability grading: operational definitions. Lepr Rev. 2003;74(4):366-373. [PubMed] [Google Scholar]
- 2.Santos VS, de Matos AM, de Oliveira LS, et al. Clinical variables associated with disability in leprosy cases in northeast Brazil. J Infect Dev Ctries. 2015;9(3):232-238. doi: 10.3855/jidc.5341 [DOI] [PubMed] [Google Scholar]
- 3.Santos VS, Santos LC, Lôbo LVR, Lemos LMD, Gurgel RQ, Cuevas LE. Leprosy and disability in children younger than 15 years in an endemic area of northeast Brazil. Pediatr Infect Dis J. 2015;34(3):e44-e47. doi: 10.1097/INF.0000000000000592 [DOI] [PubMed] [Google Scholar]
- 4.Raposo MT, Reis MC, Caminha AVQ, et al. Grade 2 disabilities in leprosy patients from Brazil: Need for follow-up after completion of multidrug therapy. PLoS Negl Trop Dis. 2018;12(7):e0006645. doi: 10.1371/journal.pntd.0006645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Brakel WH, Sihombing B, Djarir H, et al. Disability in people affected by leprosy: the role of impairment, activity, social participation, stigma and discrimination. Glob Health Action. 2012;5:1-11. doi: 10.3402/gha.v5i0.18394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Veen NHJ, Hemo DA, Bowers RL, et al. Evaluation of activity limitation and social participation, and the effects of reconstructive surgery in people with disability due to leprosy: a prospective cohort study. Disabil Rehabil. 2011;33(8):667-674. doi: 10.3109/09638288.2010.506238 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Global Leprosy Strategy 2016-2020: Accelerating towards a Leprosy-Free World. Monitoring and Evaluation Guide. 2016. https://apps.who.int/iris/handle/10665/208824. Accessed February, 10, 2018.
- 8.Santos VS, Oliveira LS, Castro FDN, et al. Functional activity limitation and quality of life of leprosy cases in an endemic area in northeastern Brazil. PLoS Negl Trop Dis. 2015;9(7):e0003900. doi: 10.1371/journal.pntd.0003900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos VS, Santana JCV, Castro FDN, et al. Pain and quality of life in leprosy patients in an endemic area of Northeast Brazil: a cross-sectional study. Infect Dis Poverty. 2016;5(1):18. doi: 10.1186/s40249-016-0113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Girdhar A, Girdhar BK. Risk of developing disability in pre and post-multidrug therapy treatment among multibacillary leprosy: Agra MB Cohort study. BMJ Open. 2012;2(2):e000361. doi: 10.1136/bmjopen-2011-000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kar BR, Job CK. Visible deformity in childhood leprosy—a 10-year study. Int J Lepr Other Mycobact Dis. 2005;73(4):243-248. [PubMed] [Google Scholar]
- 12.Haefner K, Walther F, Ariza L, et al. High occurrence of disabilities caused by leprosy: census from a hyperendemic area in Brazil’s savannah region. Lepr Rev. 2017;88:520-532. [Google Scholar]
- 13.Rodrigues NC, Castro LE, Silva JG, et al. Physical disability and its social and functional repercussions in patients with leprosy after discharge from multidrug therapy. Lepr Rev. 2017;88:85-94. [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. ;Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Chemotherapy of leprosy for control programmes. World Health Organ Tech Rep Ser. 1982;675:1-33. Accessed August 14, 2018. [PubMed] [Google Scholar]
- 16.Santos VS, de Mendonça Neto PT, Falcão Raposo OF, Fakhouri R, Reis FP, Feitosa VLC. Evaluation of agreement between clinical and histopathological data for classifying leprosy. Int J Infect Dis. 2013;17(3):e189-e192. doi: 10.1016/j.ijid.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 17.Jardim MR, Illarramendi X, Nascimento OJM, et al. Pure neural leprosy: steroids prevent neuropathy progression. Arq Neuropsiquiatr. 2007;65(4A):969-973. doi: 10.1590/S0004-282X2007000600009 [DOI] [PubMed] [Google Scholar]
- 18.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101-129. doi: 10.2307/3001666 [DOI] [Google Scholar]
- 19.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 20.Simmonds M. Quantifying the risk of error when interpreting funnel plots. Syst Rev. 2015;4:24. doi: 10.1186/s13643-015-0004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases In: Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care. Meta-Analysis in Context. 2nd ed; Oak Brook, IL: Wiley 2008:189-208. doi: 10.1002/9780470693926.ch11. [DOI] [Google Scholar]
- 22.Zhang G, Li W, Yan L, et al. An epidemiological survey of deformities and disabilities among 14,257 cases of leprosy in 11 counties. Lepr Rev. 1993;64(2):143-149. [PubMed] [Google Scholar]
- 23.Tiendrebeogo A, Toure I, Zerbo PJ. A survey of leprosy impairments and disabilities among patients treated by MDT in Burkina Faso. Int J Lepr Other Mycobact Dis. 1996;64(1):15-25. [PubMed] [Google Scholar]
- 24.Çakiner T, Yüksel A, Eğït AS, Cağri G, Karaçorlu M, Kültür A. The extent of leprosy-related disabilities in Istanbul Leprosy Hospital, Turkey. Lepr Rev. 1997;68(1):43-49. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9121331&dopt=Abstractdoi: 10.5935/0305-7518.19970007 [DOI] [PubMed] [Google Scholar]
- 25.Wittenhorst B, Vree ML, Ten Ham PB, Velema JP. The National Leprosy Control Programme of Zimbabwe a data analysis, 1983-1992. Lepr Rev. 1998;69(1):46-56. doi: 10.5935/0305-7518.19980006 [DOI] [PubMed] [Google Scholar]
- 26.Croft RP, Richardus JH, Nicholls PG, Smith WCS. Nerve function impairment in leprosy: design, methodology, and intake status of a prospective cohort study of 2664 new leprosy cases in Bangladesh (the Bangladesh Acute Nerve Damage Study). Lepr Rev. 1999;70(2):140-159. doi: 10.5935/0305-7518.19990018 [DOI] [PubMed] [Google Scholar]
- 27.Ahmad ML, Khan MS, Hussain I, Kazmi AH. Deformity and disability index in patients with leprosy. J Pak Assoc Dermatol. 2004;14(2):64-69. [Google Scholar]
- 28.Rad F, Ghaderi E, Moradi G, Salimzadeh H. The study of disability status of live leprosy patients in Kurdistan province of Iran. Pak J Med Sci. 2007;23(6):857-861. [Google Scholar]
- 29.Silva Sobrinho RA, Mathias TADF, Gomes EA, Lincoln PB. Evaluation of incapacity level in leprosy: a strategy to sensitize and train the nursing team. Rev Lat Am Enfermagem. 2007;15(6):1125-1130. doi: 10.1590/S0104-11692007000600011 [DOI] [PubMed] [Google Scholar]
- 30.Lana FC, Amaral EP, Lanza FM, Saldanha AN. Physical disabilities resulting from Hansen’s disease in Vale do Jequitinhonha/state of Minas Gerais, Brazil. Rev Lat Am Enfermagem. 2008;16(6):993-997. doi: 10.1590/S0104-11692008000600009 [DOI] [PubMed] [Google Scholar]
- 31.Soomro FR, Pathan GM, Abbasi P et al. Deformity and disability index in patients of leprosy in Larkana region. J Pakistan Assoc Dermatol. 2008;18:18-32. [Google Scholar]
- 32.Ramos JMH, Souto FJD. Disability after treatment among leprosy patients in Várzea Grande, State of Mato Grosso [in Portuguese]. Rev Soc Bras Med Trop. 2010;43(3):293-297. doi: 10.1590/S0037-86822010000300016 [DOI] [PubMed] [Google Scholar]
- 33.El-Dawela RE, Mohamed AS, Yousef F. Analysis of newly detected leprosy in Sohag Governorate, Upper Egypt, 2004-2008. Lepr Rev. 2012;83(1):71-79. [PubMed] [Google Scholar]
- 34.Sarkar J, Dasgupta A, Dutt D. Disability among new leprosy patients, an issue of concern: an institution based study in an endemic district for leprosy in the state of West Bengal, India. Indian J Dermatol Venereol Leprol. 2012;78(3):328-334. doi: 10.4103/0378-6323.95449 [DOI] [PubMed] [Google Scholar]
- 35.Nardi SMT, Paschoal VdelA, Chiaravalloti-Neto F, Zanetta DMT. Leprosy-related disabilities after release from multidrug treatment: prevalence and spatial distribution [in Portuguese]. Rev Saude Publica. 2012;46(6):969-977. doi: 10.1590/S0034-89102013005000002 [DOI] [PubMed] [Google Scholar]
- 36.Monteiro LD, Alencar CHM, Barbosa JC, Braga KP, Castro MD, Heukelbach J. Physical disabilities in leprosy patients after discharge from multidrug therapy in Northern Brazil [in Portuguese]. Cad Saude Publica. 2013;29(5):909-920. doi: 10.1590/S0102-311X2013000500009 [DOI] [PubMed] [Google Scholar]
- 37.Oliveira DT, Sherlock J, Melo EV, et al. Clinical variables associated with leprosy reactions and persistence of physical impairment. Rev Soc Bras Med Trop. 2013;46(5):600-604. doi: 10.1590/0037-8682-0100-2013 [DOI] [PubMed] [Google Scholar]
- 38.Guerrero MI, Muvdi S, León CI. Delay in leprosy diagnosis as a predictor of disability in a cohort of patients in Colombia, 2000-2010 [in Spanish]. Rev Panam Salud Publica. 2013;33(2):137-143. doi: 10.1590/S1020-49892013000200009 [DOI] [PubMed] [Google Scholar]
- 39.de Castro LE, da Cunha AJLA, Fontana AP, de Castro Halfoun VLR, Gomes MK. Physical disability and social participation in patients affected by leprosy after discontinuation of multidrug therapy. Lepr Rev. 2014;85(3):208-217. [PubMed] [Google Scholar]
- 40.Silva MEGC, de Souza CDF, Costa e Silva SP, Costa FM, Carmo RF. Epidemiological aspects of leprosy in Juazeiro-BA, from 2002 to 2012. An Bras Dermatol. 2015;90(6):799-805. doi: 10.1590/abd1806-4841.201533963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteiro LD, Martins-Melo FR, Brito AL, Alencar CH, Heukelbach J. Physical disabilities at diagnosis of leprosy in a hyperendemic area of Brazil: trends and associated factors. Lepr Rev. 2015;86(3):240-250. doi: 10.1590/S0034-8910.2015049005866 [DOI] [PubMed] [Google Scholar]
- 42.Sethi M, Rao PSS. Challenges in preventing disabilities among children affected by leprosy: findings from a referral hospital in north India. Lepr Rev. 2015;86(3):296-297. [PubMed] [Google Scholar]
- 43.Patel N, Modi K. A cross sectional study of deformities in patients of leprosy at a tertiary care center of western India. Indian J Lepr. 2016;88:209-215. [Google Scholar]
- 44.Onyeonoro UU, Aguocha GU, Madukwe SO, Nwokeukwu HI, Nwamoh UN, Aguocha BU. Pattern of disabilities among leprosy patients in Abia State, Nigeria—a retrospective review. Indian J Lepr. 2016;88(1):21-28. [PubMed] [Google Scholar]
- 45.Queirós MI, Ramos AN, Alencar CHM, Monteiro LD, Sena AL, Barbosa JC. Clinical and epidemiological profile of leprosy patients attended at Ceará, 2007-2011. An Bras Dermatol. 2016;91(3):311-317. doi: 10.1590/abd1806-4841.20164102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anjum V, Swarupa MSK, Neeluri R. Disability status of the leprosy patients enrolled in a tertiary health centre in a metropolitan city. Indian J Lepr. 2017;89(1):15-22. [Google Scholar]
- 47.Darlong J, Govindharaj P, Darlong F, Mahato N. A study of untreated leprosy affected children reporting with grade 2 disability at a referral centre in West Bengal, India. Lepr Rev. 2017;88:298-305. [Google Scholar]
- 48.Cabral-Miranda W, Chiaravalloti Neto F, Barrozo LV. Socio-economic and environmental effects influencing the development of leprosy in Bahia, north-eastern Brazil. Trop Med Int Health. 2014;19(12):1504-1514. doi: 10.1111/tmi.12389 [DOI] [PubMed] [Google Scholar]
- 49.Henry M, GalAn N, Teasdale K, et al. Factors contributing to the delay in diagnosis and continued transmission of leprosy in Brazil—an explorative, quantitative, questionnaire based study. PLoS Negl Trop Dis. 2016;10(3):e0004542. doi: 10.1371/journal.pntd.0004542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholls PG, Wiens C, Smith WCS. Delay in presentation in the context of local knowledge and attitude towards leprosy—the results of qualitative fieldwork in Paraguay. Int J Lepr Other Mycobact Dis. 2003;71(3):198-209. [DOI] [PubMed] [Google Scholar]
- 51.Zhang F, Chen S, Sun Y, Chu T. Healthcare seeking behaviour and delay in diagnosis of leprosy in a low endemic area of China. Lepr Rev. 2009;80(4):416-423. doi: 10.1016/j.braindev.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 52.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19(2):338-381. doi: 10.1128/CMR.19.2.338-381.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fonseca AB de L, Simon MD, Cazzaniga RA, et al. The influence of innate and adaptative immune responses on the differential clinical outcomes of leprosy. Infect Dis Poverty. 2017;6(1):5. doi: 10.1186/s40249-016-0229-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serrano-Coll H, Salazar-Peláez L, Acevedo-Saenz L, Cardona-Castro N. Mycobacterium leprae-induced nerve damage: direct and indirect mechanisms. Pathog Dis. 2018;76(6). doi: 10.1093/femspd/fty062 [DOI] [PubMed] [Google Scholar]
- 55.Croft RP, Nicholls PG, Steyerberg EW, Richardus JH, Withington SG, Smith WC. A clinical prediction rule for nerve function impairment in leprosy patients—revisited after 5 years of follow-up. Lepr Rev. 2003;74(1):35-41. [PubMed] [Google Scholar]
- 56.Wilder-Smith EP, Van Brakel WH. Nerve damage in leprosy and its management. Nat Clin Pract Neurol. 2008;4(12):656-663. doi: 10.1038/ncpneuro0941 [DOI] [PubMed] [Google Scholar]
- 57.Richardus JH, Nicholls PG, Croft RP, Withington SG, Smith WCS. Incidence of acute nerve function impairment and reactions in leprosy: a prospective cohort analysis after 5 years of follow-up. Int J Epidemiol. 2004;33(2):337-343. doi: 10.1093/ije/dyg225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Full Search Strategy
eFigure 1. Flow Diagram of Study Selection
eTable 2. Risk of Bias of the Included Studies Using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies
eFigure 2. Forest Plot Showing the Pooled Odds Ratio for Male and Female
eFigure 3. Forest Plot Showing the Pooled Odds Ratio for Multibacillary and Paucibacillary Leprosy
eFigure 4. Forest Plot Showing the Pooled Odds Ratio for Leprosy Reaction.
eFigure 5. Forest Plot Showing the Pooled Odds Ratio for Lepromatous and Borderline Clinical Forms
eFigure 6. Forest Plot Showing the Pooled Odds Ratio for Lepromatous and Tuberculoid Clinical Forms
eFigure 7. Forest Plot Showing the Pooled Odds Ratio for Lepromatous and Indeterminate Clinical Forms
eFigure 8. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Male and Female
eFigure 9. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Multibacillary and Paucibacillary Leprosy
eFigure 10. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Lepromatous and Borderline Clinical Forms
eFigure 11. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Lepromatous and Turberculoid Clinical Forms
eFigure 12. Funnel Plot for Evaluation of Publication Bias Among Studies Reporting Lepromatous and Indeterminate Clinical Forms