This randomized clinical trial compares the effectiveness of telephone-delivered collaborative goal setting and behavioral activation vs enhanced usual care for depression symptoms among US veterans with uncontrolled diabetes.
Key Points
Question
Can a telephone-delivered, collaborative goal-setting intervention improve depression symptoms and glycemic control among high-risk, patients with comorbid diabetes identified using a population screening approach?
Findings
In this randomized clinical trial of 225 US veterans with uncontrolled diabetes and significant depression symptoms, a telephone-delivered intervention using collaborative goal setting and behavioral activation had mixed results compared with enhanced usual care. Secondary analyses found that a significantly higher proportion of intervention participants achieved and maintained clinically significant responses of depression symptoms at 12 months but did not find such improvements for glycemic levels at 12 months.
Meaning
A telephone-delivered, collaborative goal-setting approach can improve depression symptoms among high-risk patients with uncontrolled diabetes who maintain engagement for 12 months.
Abstract
Importance
Depression symptoms are present in one-third of patients with diabetes, contributing to significant adverse consequences. Population screening of high-risk patients coupled with telephone delivery of evidence-based therapies for comorbid diabetes may address barriers to care.
Objective
To evaluate the effectiveness of proactive population screening plus telephone delivery of a collaborative goal-setting intervention among high-risk patients with uncontrolled diabetes and depression.
Design, Setting, and Participants
In this randomized clinical trial, 225 participants (intervention [n = 136] and control [n = 89]) were enrolled from a regional Veterans Healthcare System serving Southeast Texas from November 1, 2012, through June 24, 2016. Data were gathered at baseline and 6 and 12 months after intervention. Patients selected had uncontrolled diabetes (hemoglobin A1c [HbA1c] >7.5%]) and clinically significant depression (Patient Health Questionnaire–9 scores [PHQ-9] ≥10) and were living more than 20 miles from the Veterans Affairs medical center. Data collection was completed on December 6, 2016, and final analyses were completed by January 25, 2018. All analyses were intent to treat.
Interventions
Healthy Outcomes Through Patient Empowerment (HOPE) included 9 telephone sessions with 24 trained health care professionals using collaborative goal-setting and behavioral activation methods. The control group received enhanced usual care (EUC) and notification of high-risk status.
Main Outcomes and Measures
Change in depression symptoms using PHQ-9 and glycemic control using HbA1c from baseline to 6 months and to 12 months. Secondary analyses evaluated clinically significant responses for these measures.
Results
Among 225 participants, 202 (89.8%) were men, the mean (SD) age was 61.9 (8.3) years, 145 (64.4%) were married, and 156 (69.3%) had some education beyond high school. For the overall study, 38 participants (16.9%) were lost to follow-up or withdrew at 6 months and another 21 (9.3%) were lost to follow-up or withdrew at 12 months. Repeated-measures analysis with multiple imputation for missing data assessing the interaction of treatment group (HOPE vs EUC) and time (baseline, 6 months, and 12 months) found no significant improvement in PHQ-9 (β, 1.56; 95% CI, –0.68 to 3.81; P = .17) or HbA1c (β, –0.005; 95% CI, –0.73 to 0.72; P = .82). Analyses using t test for change from baseline to 12 months showed a HOPE vs EUC between-group mean difference for PHQ-9 of 2.14 (95% CI, 0.18 to 4.10; P = .03) and for HbA1c of –0.06% (95% CI, –0.61% to 0.50%; P = .83). A secondary analysis of patients experiencing a clinical response found that 52.1% of HOPE participants had clinically significant responses in PHQ-9 at 12 months vs 32.9% in EUC (difference, 0.19; 95% CI, 0.04-0.33; P = .01).
Conclusions and Relevance
Telephone-delivered, collaborative goal setting produced clinically significant reductions in depression symptoms but not glycemic control among patients who remained engaged at 12 months compared with EUC among a population screened sample of high-risk patients with diabetes and depression. Although the intervention created some lasting effect for depression, additional strategies are needed to maintain engagement of this high-risk population within an interprofessional team approach to primary care.
Trial Registration
ClinicalTrials.gov identifier: NCT01572389
Introduction
Approximately one-third of patients with diabetes have clinically significant depression symptoms, contributing to substantial adverse consequences.1 Patients with diabetes and depression use more health services and have greater functional impairment and morbidity compared with those without depression.2,3,4 Depression increases mortality risks among patients with diabetes, especially older adults and those who have had recent cardiovascular events.5 Furthermore, there are correlations between the number of diabetes-related symptoms and the number of depression symptoms, suggesting a magnification of physical and affective symptoms in patients with comorbidities.6
Evidence-based psychotherapies, pharmacologic therapy, and coordinated or stepped-care approaches have moderate efficacy in reducing depression symptoms and modest success with improving hemoglobin A1c (HbA1c) and diabetes self-care activities.5,7,8 However, few studies7,9,10 have addressed the needs of complex medically ill populations regarding sustained treatment and follow-up periods.7,9,10 Evidence-based treatments for comorbid diabetes and depression are limited because of scarcity of skilled health professionals, need for frequent visits, and difficulty identifying who could benefit from mental health treatment.9,11 A previous study6 reported that individuals living more than 10 miles from their health care facility had significantly fewer follow-up visits for mental health. Furthermore, identification of at-risk patients is often difficult using customary screening methods.11,12 Learning health care systems using electronic health records linked to large data repositories provide digital infrastructure for screening high-risk populations using more efficient and reliable methods.13 Learning health care systems can use structured clinical, billing, and outcomes data to identify clusters of patients with persistently uncontrolled diabetes and depression risk factors before they present with more serious illness.14
Systems approaches15 to identifying high-risk populations with depression and diabetes are essential11,16 but should be coupled with pathways to evidence-based treatments.5 Structured telephone delivery may address some barriers to accessing effective treatments for diabetes self-care and depression symptoms, especially for rural-living and functionally impaired individuals.17 Three meta-analyses reported that telehealth delivery (call or text message) provides small but significant improvements in glycemic control among patients with diabetes.18,19,20 Furthermore, a few studies10,21,22 incorporating telehealth approaches showed improvements in depression and anxiety symptoms among patients with diabetes. However, additional research is needed to evaluate whether structured, telephone-delivered behavioral interventions can enhance treatment for depression symptoms and glycemic control for high-risk patients with comorbidity.10
A previous study23 showed the effectiveness of collaborative goal setting as a behavioral intervention for glycemic control in chronically ill patients. Collaborative goal setting has 4 key components: (1) identifying what matters most to patients (health-related values); (2) using these values to set specific, measurable, and actionable health outcome goals; (3) communicating outcome goals to the patient’s clinicians; and (4) working with clinicians to align reasonable treatment and self-care recommendations to achieve outcome goals. Collaborative goal setting is an effective, guidelines-concordant strategy in diabetes care.24,25,26,27 Goal setting promotes behavioral activation, an evidence-based psychotherapy,28 which acts synergistically to reduce depression symptoms and enhance self-care.29,30,31
To address barriers to screening, access, and treatment of high-risk patients with diabetes and depression, we developed the Healthy Outcomes Through Patient Empowerment (HOPE) intervention.31 This study used a learning health care systems approach14,15 to screen for and improve treatment of a high-risk subpopulation. The HOPE approach provides a structured, telephone-delivered, collaborative goal-setting intervention for 6 months to enhance behavioral activation targeting depression symptoms and diabetes self-care. The objective of this study was to evaluate the effectiveness of HOPE for clinically significant improvements in depression and glycemic control compared with enhanced usual care (EUC)—usual diabetes and depression care enhanced by a systems approach to screening for high-risk status.13,32,33
Methods
Study Design
This randomized clinical trial, conducted from November 1, 2012, through June 24, 2016, examined a telehealth intervention for patients with uncontrolled diabetes and clinically significant depression.32 The trial protocol can be found in Supplement 1. The Baylor College of Medicine institutional review board and the Michael E. DeBakey VA Medical Center (MEDVAMC) research and development committee, both in Houston, Texas, approved the study protocol. All participants provided verbal informed consent conducted by telephone. The study was reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. Data collection was completed on December 6, 2016, and final analyses were completed by January 25, 2018. All analyses were intent to treat.
The trial used an integrated, regional Veterans Affairs (VA) learning health care system.13,14,33 This approach consists of (1) an electronic data warehouse to identify a specific high-risk population (veterans with persistent uncontrolled diabetes and depression risk factors living at least 20 miles from the medical center), followed by (2) telephone screening for depression using the Patient Health Questionnaire–9 (PHQ-9) and (3) training of clinicians to deliver a structured telehealth intervention that can be counted as a routine clinical encounter. Enrolled participants were randomized to a 6-month blended diabetes and depression behavioral health coaching program, followed by a 6-month maintenance period without coaching (intervention), or received usual clinical care plus educational materials (EUC) for 12 months. Primary outcomes were glycemic control, as measured by HbA1c, and depression control, as measured by the PHQ-9.34,35
Participants and Eligibility Criteria
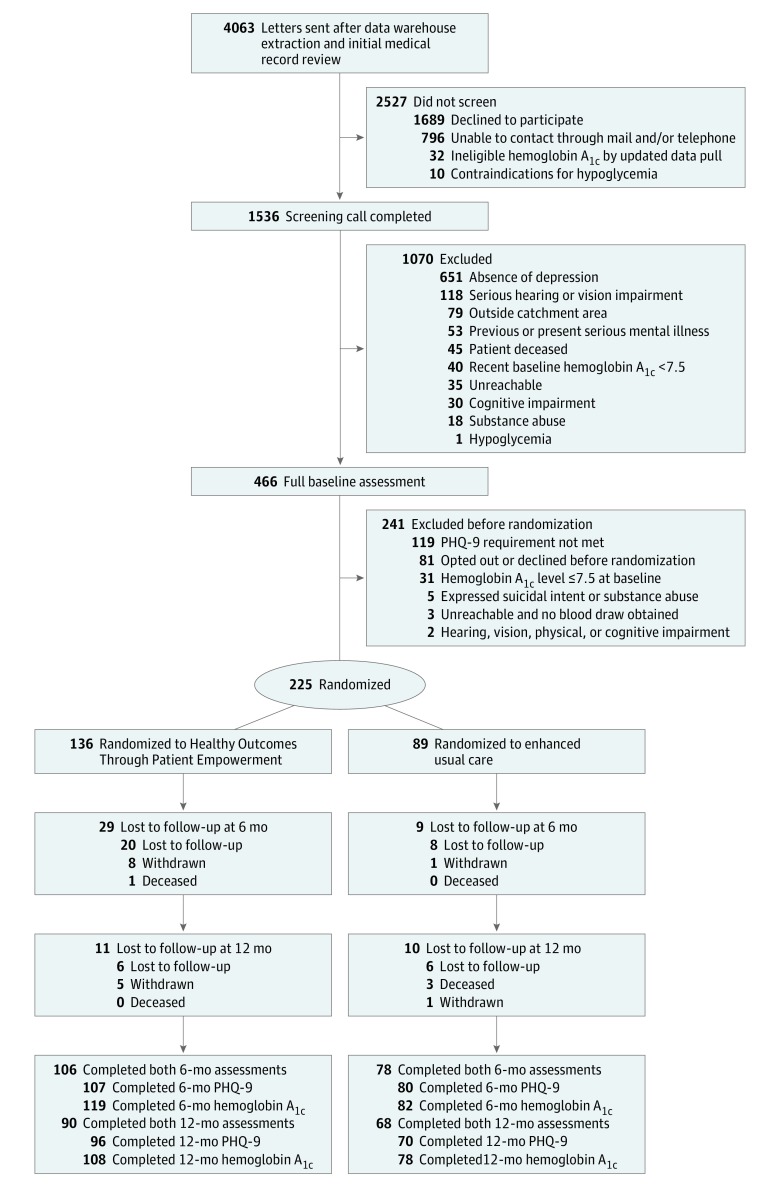
The study was conducted at the MEDVAMC and 6 affiliated community-based outpatient clinics across Southeast Texas. A corporate data warehouse was used to identity 4063 veterans with uncontrolled diabetes (defined by International Classification of Diseases, Ninth Revision diagnosis code 250.XX and HbA1c of ≥7.5% for 1 year before the study) who lived at least 20 miles from the main Veterans Health Administration hospital in Houston, Texas, or who received primary care services within a MEDVAMC satellite community-based clinic across Southeast Texas (Figure 1). Among patients expressing interest, medical record and telephone screening (n = 1536) was conducted. Patients were excluded during this screening if there was an absence of depression symptoms, a telephone-based coaching intervention would be inappropriate (eg, the patient had severe cognitive impairment or mental health condition, hearing or visual impairment, or active suicidal ideation), or presence of significant hypoglycemic events or substance abuse. Among those not initially excluded, 466 completed a full baseline assessment by telephone to confirm inclusion criteria eligibility for clinically significant depression symptoms (score ≥10 on the PHQ-9)4,5 and uncontrolled diabetes at baseline (HbA1c >7.5%).36,37,38 Of these, 225 patients met all eligibility criteria and consented to randomization (Figure 1).
Figure 1. CONSORT Diagram of Patient Flow.
PHQ-9 indicates Patient Health Questionnaire–9.
Randomization and Blinding
To increase statistical power for measuring the intervention and factors related to its telehealth delivery in secondary treatment analyses, the final sample was assigned to treatment groups using unequal block randomization (60% intervention [n = 136] and 40% control [n = 89]), stratified by clinic site. Independent evaluators, blinded to group assignment, gathered baseline, 6-month, and 12-month data by telephone and were not involved in any other study procedures. At each respective time point, evaluators scheduled participants for blood sample collection and HbA1c measurement using standardized methods at a local VA clinical laboratory. If participants could not participate at 1 or more collection point, HbA1c data were extracted from medical records if values were present within 4 weeks of the expected time frame. Research staff enrolled and assigned patients to groups using a blinded randomization procedure.
Interventions
HOPE Participants
During the active intervention, the HOPE group received 9 coaching sessions with a trained health professional: biweekly (for 30-40 minutes) from months 1 to 3 and monthly (for 15 minutes) from months 4 to 6. Twenty-four trained health professionals or coaches (18 female) included psychologists (n = 16), nurses (n = 5), pharmacists (n = 2), and social workers (n = 1). Most (n = 18) were at the MEDVAMC; 6 were at a Veterans Health Administration community-based clinic. Coaches were assigned primarily by similar site of care and availability. Clinician training consisted of facilitated training (two 120-minute teleconference sessions) and subsequent support (mentoring and feedback related to fidelity to the intervention) from a behavioral health expert (N.E.H.), plus monthly peer mentoring with other coaches. Patients and coaches used workbooks that guided telephone conversations and allowed patients to define and track their progress. Primary care physicians received notifications of their patients’ participation, HbA1c results, and PHQ-9 questionnaire outcomes via secure electronic messaging; however, they received no formal training related to the HOPE intervention components.
During the first 2 patient sessions, HOPE coaches focused on building rapport, introducing and clarifying values, collaboratively setting initial goals, identifying potential skill sets to address goals, and empowering patients to advocate for their health through active communication with their clinicians. For sessions 3 through 6, participants focused on discrete skill modules (increasing pleasant activities, using thoughts to improve wellness, diet, physical activity, medication management, and relaxation)3 customized to meet their diabetes and depression goals. Sessions 7 through 9 focused on maintenance skills (reviewing action plans and overcoming barriers).32 Skills emphasized in the modules were designed to improve diabetes- and depression-related outcomes simultaneously. The HOPE modules stressed the importance of the coach-patient relationship as critical to improvement in participant physical and/or emotional self-management. During months 7 to 12, participants received usual primary care without contact from HOPE coaches.
Enhanced Usual Care
In addition to usual care, EUC participants were informed about their high-risk status (uncontrolled diabetes status and clinically significant depression symptoms) and were given related educational materials. Study assessments were conducted for EUC participants via telephone, and educational materials were mailed. Participants were encouraged to address these results with their primary care clinician.
Telehealth
Investigators defined telehealth in concordance with the American Telemedicine Association guideline for delivering clinical services by using remote technology (eg, telephone).39 Because of the difficulty for participants to commute to study sites, all procedures (screening, enrollment, protocol delivery, and follow-up assessments) were conducted by telephone to ease treatment burden. Participants from both study arms visited the study site only to obtain HbA1c measurements and their usual medical care.
Outcomes
Primary outcomes included change in glycemic control (HbA1c) and depression symptoms (PHQ-9) from baseline to 6 months and 12 months. As secondary outcomes, patients with HbA1c response were those with a 0.5% decrease in HbA1c (as the minimal clinically significant improvement) from baseline.40 Those with PHQ-9 response included those with at least 50% decrease from baseline or a PHQ-9 value less than 10.36,37,38 In addition, other variables, including demographics, health care use, self-efficacy, comorbid physical and mental conditions, and other psychological factors, were evaluated.
Power Analysis
To achieve 80% power to detect an effect size of 0.45 at α = .05, 242 participants were needed. Unequal randomization (60 intervention to 40 control) was used, and attrition at 12 months was assumed to be 25%.
Statistical Analysis
Analyses were performed based on the randomized treatment assignment and not on the treatment as received. Descriptive statistics were used to characterize differences by study arm at baseline. Means (SDs) were tabulated separately for EUC and HOPE for HbA1c and PHQ-9 scores at baseline, 6 months, and 12 months. Contrasts between baseline, 6 months, and 12 months were tested for differences in outcomes at the follow-up times. A 2-tailed P < .05 was considered to be statistically significant.
To test for differences in the means over time between groups for each outcome, we used a pooled t test for equal variances or Satterthwaite approximation for unequal variances. A significant value indicated that the outcome changed significantly within the group during these periods. We also conducted a repeated measures analysis with participants nested within site, while accounting for all study time points within a single model for HbA1c and a single model for PHQ-9 outcomes. Participants with missing values were included with multiple imputation procedures using Monte Carlo simulations to account for missing values. Separate models for HbA1c and PHQ-9 outcomes account for each of the periods (baseline, 6 months, and 12 months). The independent variables in the model were time, treatment, and the interaction of time and treatment. Significant time-by-treatment interactions indicate a significant difference between the HOPE and EUC groups during the 12 months.
In our secondary analyses, change scores for the outcomes were derived and tested for differences between HOPE and EUC. The 6-month change score for each patient was calculated as the 6-month value minus the baseline value. A negative value indicates that the patient had less depression at 6 months compared with baseline. The success rate difference (SRD) was calculated as p1 – p2, where p1 was the proportion of responders from HOPE and p2 was the proportion of responders in EUC. An SRD more than 0 indicated that HOPE was preferred to EUC.41 Based on previous studies,41,42,43 treatment responses with CIs were categorized as small (SRD, 0.11; number needed to treat [NNT] = 9), medium (SRD, 0.28; NNT = 4), and large (SRD, 0.43; NNT = 2).
Results
Of 225 participants randomized, 136 were assigned to HOPE and 89 were assigned to EUC. For the overall study, 38 (16.9%) participants were lost or withdrew at 6 months and another 21 (9.3%) were lost or withdrew at 12 months. Figure 1 describes differences by study arm. A higher percentage of EUC participants completed assessments at both 6 and 12 months. Participants who completed the study at 12 months reported higher diabetes knowledge, diabetes self-care understanding, and health literacy. There were no differences in sociodemographic or other clinical variables at baseline among participants who completed and did not complete the study at 12 months.
Characteristics of the Sample
Table 1 shows demographic characteristics of participants at baseline. Of 225 participants, most were older (mean age [SD], 61.9 [8.3] years), male (202 [89.8%]), married (145 [64.4%]), educated beyond high school (155 [68.9%]), and retired (74 [32.9%]) or disabled (92 [40.9%]). Most participants rated their health as fair to poor (173 [76.9%]) and had poorly controlled diabetes (mean [SD] HbA1c, 9.3% [1.4%]) and moderately severe depression symptoms (mean [SD] PHQ-9, 15.9 [4.1]). Groups did not significantly differ at baseline in terms of age, race/ethnicity, occupation, HbA1c levels, PHQ-9 severity and categories, Deyo comorbidity scores,44 and insulin use.
Table 1. Demographic Characteristics of Participants 6 Months Before Baseline Measures.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Total (N = 225) | HOPE (n = 136) | EUC (n = 89) | |
| Age ≥65 y | 104 (46.2) | 68 (50.0) | 36 (40.4) |
| Male | 202 (89.8) | 121 (89.0) | 81 (91.0) |
| Marital status | |||
| Single | 17 (7.6) | 10 (7.4) | 7 (7.9) |
| Married | 145 (64.4) | 87 (64.0) | 58 (65.2) |
| Separated or divorced | 63 (28.0) | 39 (28.7) | 24 (27.0) |
| Race/ethnicity | |||
| White | 124 (55.1) | 73 (53.7) | 51 (57.3) |
| Non-Hispanic black | 57 (25.3) | 41 (30.1) | 16 (18.0) |
| Hispanic | 23 (10.2) | 12 (8.8) | 11 (12.4) |
| Other | 21 (9.3) | 10 (7.4) | 11 (12.4) |
| Lives alone at home | 41 (18.2) | 26 (19.1) | 15 (16.9) |
| Education | |||
| High school or less | 69 (30.7) | 38 (27.9) | 31 (34.8) |
| Some college or college graduate | 148 (65.8) | 92 (67.6) | 56 (62.9) |
| Graduate school | 7 (3.1) | 5 (3.7) | 2 (2.2) |
| Household income, $ | |||
| <30 000 | 109 (48.4) | 59 (43.4) | 50 (56.2) |
| 30 000-59 999 | 89 (39.6) | 61 (44.9) | 28 (31.5) |
| ≥60 000 | 24 (10.7) | 15 (11.0) | 9 (10.1) |
| Employment | |||
| Employed full- or part-time | 38 (16.9) | 22 (16.2) | 16 (18.0) |
| Retired | 74 (32.9) | 48 (35.3) | 26 (29.2) |
| Disabled | 92 (40.9) | 56 (41.2) | 36 (40.4) |
| Other | 20 (8.9) | 9 (6.6) | 11 (12.4) |
| Patient-rated health | |||
| Excellent to very good | 5 (2.2) | 3 (2.2) | 2 (2.2) |
| Good | 45 (20.0) | 30 (22.1) | 15 (16.9) |
| Fair to poor | 173 (76.9) | 101 (74.3) | 72 (80.9) |
| Diabetes management | |||
| Insulin only | 60 (26.7) | 36 (26.5) | 24 (27.0) |
| Oral agents | 61 (27.1) | 38 (27.9) | 23 (25.8) |
| Insulin and oral agents | 62 (27.6) | 32 (23.5) | 30 (33.7) |
| Lifestyle only | 42 (18.7) | 30 (22.1) | 12 (13.5) |
| PHQ-9 scores | |||
| 10-14 | 95 (42.2) | 61 (44.9) | 34 (38.2) |
| 15-19 | 80 (35.6) | 42 (30.9) | 38 (42.7) |
| >19 | 50 (22.2) | 33 (24.3) | 17 (19.1) |
| PHQ-9 severity scores, mean (SD) | 15.9 (4.1) | 15.8 (4.2) | 16.2 (4.0) |
| HbA1c level, mean (SD) | 9.3 (1.4) | 9.2 (1.4) | 9.3 (1.5) |
| Deyo comorbidity score, mean (SD) | 2.1 (1.6) | 2.1 (1.5) | 2.1 (1.8) |
Abbreviations: EUC, enhanced usual care; HbA1c, hemoglobin A1c; HOPE, Healthy Outcomes Through Patient Empowerment; PHQ-9, 9-item Patient Heath Questionnaire.
Primary Outcomes
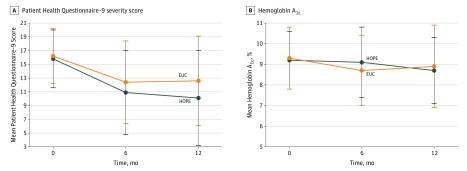
Figure 2 shows the change in primary outcomes for PHQ-9 and HbA1c across 3 time points stratified by HOPE and EUC groups. The HOPE participants experienced a decline in depression symptoms (PHQ-9) from baseline (mean [SD], 15.8 [4.2]) to 6 months (10.9 [6.1]), which persisted at 12 months (10.1 [6.9]). The EUC participants had a similar but more modest change from baseline (16.2 [4.0]) to 6 months (12.4 [6.0]) and 12 months (12.6 [6.5]). The PHQ-9 differences between HOPE and EUC were statistically significant at 6 months (mean difference, 1.74; 95% CI, 0.14-3.33; P = .03) and 12 months (mean difference, 2.14; 95% CI, 0.18-4.10; P = .03). Figure 2B shows that HOPE participants had improved glycemic control (mean [SD] HbA1c) from baseline (9.2% [1.4%]) to 6 months (9.1% [1.7%]) and 12 months (8.7% [1.6%]). The EUC participants experienced a decline in mean (SD) HbA1c from baseline (9.3% [1.5%]) to 6 months (8.7% [1.7%]) and then a gradual regression at 12 months (8.9% [2.0%]). Differences in HbA1c between groups were not significant at 6 months (mean difference, –0.40%; 95% CI, –0.86% to 0.06%; P = .08) or 12 months (mean difference, –0.06%; 95% CI, –0.61% to 0.50%; P = .83). Half (51%) of the HOPE participants attended 6 or more sessions, and 73% attended 3 or more sessions. Session attendance did not have a significant effect on the primary outcomes. Repeated-measures analysis with multiple imputation for missing data assessing the interaction of treatment group and time revealed no significant improvement in PHQ-9 (β, 1.56; 95% CI, –0.68 to 3.81; P = .17) or HbA1c (β, –0.005; 95% CI, –0.73 to 0.72; P = .82).
Figure 2. Mean Quantitative Values for Patient Health Questionnaire–9 and Hemoglobin A1c From Baseline to 12-Month Follow-up.
HOPE indicates Healthy Outcomes Through Patient Empowerment; EUC, enhanced usual care; and error bars, SD.
Secondary Outcomes
Table 2 shows the number of participants experiencing a clinical response in HbA1c and PHQ-9 at 6 months and 12 months and compares success rates between groups. At 6 months, 38% (47.2% in the HOPE group and 35.0% in the EUC group) of all participants had improved PHQ-9 in both groups. Although not statistically significant, this was a small effect size (success rate difference, 0.12; 95% CI, –0.02 to 0.26; P = .09) for HOPE at 6 months, with an NNT of 8. At 12 months, there were significantly more patients with PHQ-9 response in the HOPE group (52.1%) than in the EUC group (32.9%) (success rate difference, 0.19; 95% CI, 0.04 to 0.33; P = .01) with an NNT of 5. At 6 months, there were more patients with an HbA1c response in the EUC group (57.7%) than in the HOPE group (37.7%; success rate difference, –0.20; 95% CI, 0.05 to 0.33; P = .007). By 12 months, there were no significant differences in HbA1c response between the HOPE group (48.9%) and EUC group (51.5%; success rate difference, –0.03; 95% CI, –0.13 to 0.18; P = .75); however, half (50.0%) of all participants, regardless of group, had clinically significant improved glycemic control.
Table 2. Comparison of Participants With HbA1c and PHQ-9 Response at 6 and 12 Months in the HOPE Intervention and EUC Groupsa.
| Measure | No./Total No. (%) | P Value | Success Rate Difference (95% CI)b | No. Needed to Treat | |
|---|---|---|---|---|---|
| HOPE | EUC | ||||
| 6-mo Response | |||||
| HbA1c | 40/106 (37.7) | 45/78 (57.7) | .01 | −0.199 (0.05 to 0.33) | 5 |
| PHQ-9 | 51/108 (47.2) | 28/80 (35.0) | .09 | 0.12 (−0.02 to 0.26) | 8 |
| 12-mo Response | |||||
| HbA1c | 44/90 (48.9) | 35/68 (51.5) | .75 | −0.026 (−0.13 to 0.18) | 38 |
| PHQ-9 | 50/96 (52.1) | 23/70 (32.9) | .01 | 0.19 (0.04 to 0.33) | 5 |
Abbreviations: EUC, enhanced usual care; HbA1c, hemoglobin A1c; HOPE, Healthy Outcomes Through Patient Empowerment; PHQ-9, 9-item Patient Health Questionnaire.
A participant with HbA1c response was defined as an individual with a decrease of 0.5 in HbA1C from baseline; PHQ-9 response was defined as a change of at least 50% from baseline or a value of PHQ-9 less than 10.
Success rate difference was calculated as p1 – p2, where p1 is the proportion of HOPE participants with response and p2 is the proportion of EUC group participants with response. A success rate difference greater than 0 indicates that, overall, the HOPE treatment was preferred to the EUC condition.
Table 3 shows health care use among participants in the HOPE group compared with the EUC group. At baseline, there was a significant difference between the HOPE and EUC groups for the percentage of patients who were prescribed mental health medications (χ2 = 4.65; P = .03) but no difference for mental health clinic visits, primary care visits, or diabetes medication prescriptions. There was substantially less active medication management (increase, decrease, or stop medications) for mental health compared with diabetes medications for both groups. There were no statistically significant differences in active management between groups. In both, the number of mental health and primary care visits was comparable throughout the study.
Table 3. Health Care Use Among HOPE and EUC Participants at Baseline, 6 Months, and 12 Months.
| Health Care Service | No. (%) | |||||
|---|---|---|---|---|---|---|
| Baseline (n = 225)a | 6 mo (n = 188)b | 12 mo (n = 166)c | ||||
| HOPE (n = 136) | EUC (n = 89) | HOPE (n = 108) | EUC (n = 80) | HOPE (n = 96) | EUC (n = 70) | |
| Mental Health Management | ||||||
| Patients prescribed mental health medicationsd | 49 (36.0) | 20 (22.4) | NA | NA | NA | NA |
| Mental health active managemente | NA | NA | 14 (13.0) | 7 (8.8) | 5 (5.2) | 9 (12.9) |
| Mental health clinic visits | 61 (44.9) | 38 (42.7) | 53 (49.1) | 34 (42.5) | 45 (46.9) | 35 (50.0) |
| Diabetes Management | ||||||
| Patients prescribed diabetes medications | 106 (77.9) | 77 (86.5) | NA | NA | NA | NA |
| Diabetes active managemente | NA | NA | 43 (40.0) | 37 (46.2) | 26 (27.1) | 23 (32.9) |
| Primary care clinic visits | 119 (87.5) | 76 (85.4) | 97 (90.0) | 73 (91.2) | 89 (92.7) | 64 (91.4) |
Abbreviations: EUC, enhanced usual care; HOPE, Healthy Outcomes Through Patient Empowerment; NA, not applicable.
Baseline data represented the period of 181 days before baseline through baseline.
Six-month data represented the period of day 1 through day 181.
Twelve-month data represented the period of day 182 through day 365.
At baseline, there was a significant difference (P = .03) between HOPE and EUC groups.
Active management was defined as any change in medication compared with baseline (ie, switch from 1 medication to another, addition of a new medication, discontinuation of a medication, uptitration, or downtitration).
Discussion
This randomized clinical effectiveness trial showed that a collaborative goal-setting intervention delivered by trained health professionals (HOPE) provided mixed results, with indications of an effect on depression symptoms and little to no effect on glycemic control in a sample of high-risk patients with comorbid uncontrolled diabetes compared with usual care enhanced by a systems approach to screening for high-risk status. Among participants who completed the study at 12 months, we found clinically and statistically significant improvements in depression symptoms compared with EUC. Fifty-two percent of HOPE participants responded to treatment of depression symptoms. Participants in both study arms experienced modest improvements in glycemic control at 12 months, with no difference between groups.
Recent meta-analyses found that psychotherapy was effective (standardized mean differences [SMD], –0.64 to –1.47) at reducing depression symptoms but had mixed efficacy (SMD, 0.4 to –1.40) for glycemic control.5,45,46 A systematic review of collaborative care (ie, case manager and structured treatment plans) for depression found similar results for depression symptoms (SMD, –0.13 to –0.68) and glycemic control (SMD, 0 to –0.54).7 Within this context, the findings of the current study were comparatively less effective but within the range of the existing literature. Although the findings of the multiple imputation analyses were disappointing, the improvements in clinically significant depression response were more positive and consistent with previous literature.5,7,45,46
Among previous studies,10,47 relatively few clinical trials used significant or exclusive telephone delivery of psychotherapy for depression with comorbid diabetes. Piette et al10 found that telephone-delivered psychotherapy for patients with comorbid diabetes was significantly more likely to promote depression remission (58%) at 12 months compared with usual care (39%) but did not improve glycemic control. Furthermore, a meta-analysis of 5 clinical trials found that telephone interventions for glycemic control were not more effective than usual diabetes care (mean HbA1c difference, −0.91% to 0.16%).18 The results of the current study underscore the difficulties of reaching and delivering care to a population by telephone who live at some distance from specialty diabetes and mental health care services. Overall, the telephone-delivered intervention had mixed results, with data showing positive effects on depression with limited effects on glycemic control compared with enhanced usual care. Patients who remained engaged in the intervention at 12 months experienced significantly higher levels of depression response compared with usual care, which is consistent with the aforementioned study.10 Given the consistency in findings across studies, future efforts to address complex, multimorbid patient populations should explore options for more robust intervention efforts and delivery platforms. For example, the HOPE intervention was not closely aligned with ongoing clinical care activities outside the study protocol. Future efforts to systematically align psychosocial and behavioral intervention efforts with ongoing clinical care practices through interprofessional teams may improve immediate and longer-term outcomes for complex patient populations.48,49
As part of these efforts, a collaborative goal-setting approach building on the one used in the present trial may improve alignment between clinicians to address the multifaceted goals and priorities of multimorbid patients with complex conditions.48,50,51,52 In the current trial, EUC participants experienced regression toward the mean for both PHQ-9 scores and HbA1c, HOPE participants experienced sustained or even improved clinical responses at 12 months. These results reproduce findings from other studies23,30 suggesting that collaborative goal setting can produce lasting behavioral change among patients who remain engaged in the intervention.
The results seen in the present study may offer some support for learning health care system approaches.13,14,33 For the present study, this approach used precision algorithms to identify high-risk patients and then link those populations to robust diabetes and depression care within a patient-centered medical home model.5 Within this model, EUC participants achieved similar levels of glycemic control as HOPE participants, and 32.9% had a clinical response for their depression.
Limitations
Participants were largely men and drawn from 1 regional area. This may limit the generalizability of our findings to other patient populations. Similarly, the Veterans Health Administration is a national, integrated health care system with a robust infrastructure for population health and mental health care that may not be available to non–Veterans Health Administration populations. However, further coordination among primary care clinicians and HOPE coaches might have produced better glycemic control. The intervention group had a greater participation burden compared with the EUC group, which may have contributed to higher attrition rates during the follow-up periods in comparison with studies that use attention controls with similar participation requirements across intervention and control groups. The study did not reach its recruitment target at baseline, which may have affected some analyses given dropout and missing data at 12 months.
Conclusions
This study showed that HOPE, a telehealth intervention using collaborative goal setting and behavioral activation, may potentially improve depression symptoms for patients with diabetes and comorbid depression, especially for those who complete the intervention process. Its effects on glycemic control were negative. These results provide some support for population-based screening of high-risk patients with diabetes and comorbid depressive symptoms for telephone delivery of care for chronic conditions. Future studies should build on the approach and findings of HOPE in other low-income or rural settings where access to integrated diabetes and depression care is sparse.
Trial Protocol
Data Sharing Statement
References
- 1.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):-. doi: 10.2337/diacare.24.6.1069 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31(12):2398-2403. doi: 10.2337/dc08-1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30(9):2222-2227. doi: 10.2337/dc07-0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26(10):2822-2828. doi: 10.2337/diacare.26.10.2822 [DOI] [PubMed] [Google Scholar]
- 5.Petrak F, Baumeister H, Skinner TC, Brown A, Holt RIG. Depression and diabetes: treatment and health-care delivery. Lancet Diabetes Endocrinol. 2015;3(6):472-485. doi: 10.1016/S2213-8587(15)00045-5 [DOI] [PubMed] [Google Scholar]
- 6.Cully JA, Tolpin L, Henderson L, Jimenez D, Kunik ME, Petersen LA. Psychotherapy in the Veterans Health Administration: missed opportunities? Psychol Serv. 2008;5(4):320-331. doi: 10.1037/a0013719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atlantis E, Fahey P, Foster J. Collaborative care for comorbid depression and diabetes: a systematic review and meta-analysis. BMJ Open. 2014;4(4):e004706. doi: 10.1136/bmjopen-2013-004706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JA, Al Sayah F, Wozniak L, et al. Controlled trial of a collaborative primary care team model for patients with diabetes and depression: rationale and design for a comprehensive evaluation. BMC Health Serv Res. 2012;12(1):258. doi: 10.1186/1472-6963-12-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebert DD, Nobis S, Lehr D, et al. The 6-month effectiveness of internet-based guided self-help for depression in adults with type 1 and 2 diabetes mellitus. Diabet Med. 2017;34(1):99-107. doi: 10.1111/dme.13173 [DOI] [PubMed] [Google Scholar]
- 10.Piette JD, Richardson C, Himle J, et al. A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Med Care. 2011;49(7):641-648. doi: 10.1097/MLR.0b013e318215d0c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katon WJ, Simon G, Russo J, et al. Quality of depression care in a population-based sample of patients with diabetes and major depression. Med Care. 2004;42(12):1222-1229. doi: 10.1097/00005650-200412000-00009 [DOI] [PubMed] [Google Scholar]
- 12.Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract. 2010;87(3):302-312. doi: 10.1016/j.diabres.2010.01.024 [DOI] [PubMed] [Google Scholar]
- 13.McGinnis JM, Stuckhardt L, Saunders R, Smith M, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 14.Krumholz HM. Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Aff (Millwood). 2014;33(7):1163-1170. doi: 10.1377/hlthaff.2014.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores M, Glusman G, Brogaard K, Price ND, Hood L. P4 medicine: how systems medicine will transform the healthcare sector and society. Per Med. 2013;10(6):565-576. doi: 10.2217/pme.13.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy T, Lloyd CE, Pouwer F, Holt RI, Sartorius N. Screening tools used for measuring depression among people with type 1 and type 2 diabetes: a systematic review. Diabet Med. 2012;29(2):164-175. doi: 10.1111/j.1464-5491.2011.03401.x [DOI] [PubMed] [Google Scholar]
- 17.Sacco WP, Malone JI, Morrison AD, Friedman A, Wells K. Effect of a brief, regular telephone intervention by paraprofessionals for type 2 diabetes. J Behav Med. 2009;32(4):349-359. doi: 10.1007/s10865-009-9209-4 [DOI] [PubMed] [Google Scholar]
- 18.Suksomboon N, Poolsup N, Nge YL. Impact of phone call intervention on glycemic control in diabetes patients: a systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2014;9(2):e89207. doi: 10.1371/journal.pone.0089207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai YK, Zhu WJ, Cai YL, Sun DX, Zhao J. Clinical- and cost-effectiveness of telemedicine in type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine (Baltimore). 2014;93(28):e312. doi: 10.1097/MD.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su D, Zhou J, Kelley MS, et al. Does telemedicine improve treatment outcomes for diabetes? a meta-analysis of results from 55 randomized controlled trials. Diabetes Res Clin Pract. 2016;116:136-148. doi: 10.1016/j.diabres.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 21.Hirani SP, Rixon L, Cartwright M, Beynon M, Newman SP; WSD Evaluation Team . The effect of telehealth on quality of life and psychological outcomes over a 12-month period in a diabetes cohort within the Whole Systems Demonstrator Cluster Randomized Trial. JMIR Diabetes. 2017;2(2):e18. doi: 10.2196/diabetes.7128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobis S, Lehr D, Ebert DD, et al. Efficacy of a web-based intervention with mobile phone support in treating depressive symptoms in adults with type 1 and type 2 diabetes: a randomized controlled trial. Diabetes Care. 2015;38(5):776-783. doi: 10.2337/dc14-1728 [DOI] [PubMed] [Google Scholar]
- 23.Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171(5):453-459. doi: 10.1001/archinternmed.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller CK, Bauman J. Goal setting: an integral component of effective diabetes care. Curr Diab Rep. 2014;14(8):509. doi: 10.1007/s11892-014-0509-x [DOI] [PubMed] [Google Scholar]
- 25.Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76(2):174-180. doi: 10.1016/j.pec.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 26.Lafata JE, Morris HL, Dobie E, Heisler M, Werner RM, Dumenci L. Patient-reported use of collaborative goal setting and glycemic control among patients with diabetes. Patient Educ Couns. 2013;92(1):94-99. doi: 10.1016/j.pec.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers MA, Marrero DG. Response to comment on Powers et al. diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care. 2016;39(1):e17. doi: 10.2337/dci15-0009 [DOI] [PubMed] [Google Scholar]
- 28.Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev. 2007;27(3):318-326. doi: 10.1016/j.cpr.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 29.DeWalt DA, Davis TC, Wallace AS, et al. Goal setting in diabetes self-management: taking the baby steps to success. Patient Educ Couns. 2009;77(2):218-223. doi: 10.1016/j.pec.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulter A, Entwistle VA, Eccles A, Ryan S, Shepperd S, Perera R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2015;(3):CD010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naik AD, White CD, Robertson SM, et al. Behavioral health coaching for rural-living older adults with diabetes and depression: an open pilot of the HOPE study. BMC Geriatr. 2012;12(1):37. doi: 10.1186/1471-2318-12-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cully JA, Breland JY, Robertson S, et al. Behavioral health coaching for rural veterans with diabetes and depression: a patient randomized effectiveness implementation trial. BMC Health Serv Res. 2014;14:191. doi: 10.1186/1472-6963-14-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA. 2016;315(18):1941-1942. doi: 10.1001/jama.2016.3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA. 1999;282(18):1737-1744. doi: 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32(9):509-515. doi: 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- 36.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042-1049. doi: 10.1001/archpsyc.61.10.1042 [DOI] [PubMed] [Google Scholar]
- 37.Oxman TE, Dietrich AJ, Schulberg HC. The depression care manager and mental health specialist as collaborators within primary care. Am J Geriatr Psychiatry. 2003;11(5):507-516. doi: 10.1097/00019442-200309000-00005 [DOI] [PubMed] [Google Scholar]
- 38.Williams JW Jr, Gerrity M, Holsinger T, Dobscha S, Gaynes B, Dietrich A. Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry. 2007;29(2):91-116. doi: 10.1016/j.genhosppsych.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 39.American Telemedicine Association About telemedicine. https://www.americantelemed.org. Accessed May 30, 2018.
- 40.Inzucchi SE, Bergenstal RM, Buse JB, et al. ; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-1379. doi: 10.2337/dc12-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraemer HC. Messages for clinicians: moderators and mediators of treatment outcome in randomized clinical trials. Am J Psychiatry. 2016;173(7):672-679. doi: 10.1176/appi.ajp.2016.15101333 [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 43.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873-890. doi: [DOI] [PubMed] [Google Scholar]
- 44.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 45.Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst Rev. 2012;12:CD008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Feltz-Cornelis CM, Nuyen J, Stoop C, et al. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2010;32(4):380-395. doi: 10.1016/j.genhosppsych.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 47.Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75(6):577-587. doi: 10.1007/s40265-015-0347-4 [DOI] [PubMed] [Google Scholar]
- 48.Naik AD, Dindo LN, Van Liew JR, et al. Development of a clinically feasible process for identifying individual health priorities. J Am Geriatr Soc. 2018;66(10):1872-1879. doi: 10.1111/jgs.15437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tinetti M, Dindo L, Smith CD, et al. Challenges and strategies in patients’ health priorities-aligned decision-making for older adults with multiple chronic conditions. PLoS One. 2019;14(6):e0218249. doi: 10.1371/journal.pone.0218249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blaum CS, Rosen J, Naik AD, et al. Feasibility of implementing patient priorities care for older adults with multiple chronic conditions. J Am Geriatr Soc. 2018;66(10):2009-2016. doi: 10.1111/jgs.15465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cully JA, Stanley MA, Petersen NJ, et al. Delivery of brief cognitive behavioral therapy for medically ill patients in primary care: a pragmatic randomized clinical trial. J Gen Intern Med. 2017;32(9):1014-1024. doi: 10.1007/s11606-017-4101-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals–directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1(1):9-10. doi: 10.1001/jamacardio.2015.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement