Abstract
Intervertebral disc degeneration describes the vicious cycle of the deterioration of intervertebral discs and can eventually result in degenerative disc disease (DDD), which is accompanied by low‐back pain, the musculoskeletal disorder with the largest socioeconomic impact world‐wide. In more severe stages, intervertebral disc degeneration is accompanied by loss of joint space, subchondral sclerosis, and osteophytes, similar to osteoarthritis (OA) in the articular joint. Inspired by this resemblance, we investigated the analogy between human intervertebral discs and articular joints. Although embryonic origin and anatomy suggest substantial differences between the two types of joint, some features of cell physiology and extracellular matrix in the nucleus pulposus and articular cartilage share numerous parallels. Moreover, there are great similarities in the response to mechanical loading and the matrix‐degrading factors involved in the cascade of degeneration in both tissues. This suggests that the local environment of the cell is more important to its behavior than embryonic origin. Nevertheless, OA is widely regarded as a true disease, while intervertebral disc degeneration is often regarded as a radiological finding and DDD is undervalued as a cause of chronic low‐back pain by clinicians, patients and society. Emphasizing the similarities rather than the differences between the two diseases may create more awareness in the clinic, improve diagnostics in DDD, and provide cross‐fertilization of clinicians and scientists involved in both intervertebral disc degeneration and OA.
Keywords: articular joint, degeneration, inflammation, intervertebral disc, mechanical loading, osteoarthritis
1. INTRODUCTION
Low back pain and osteoarthritis (OA) are the two major musculoskeletal causes for disability worldwide.1, 2, 3 Low back pain has several causes and degenerative disc disease (DDD) is one of them.4, 5, 6, 7 The pain, dysfunction, and stiffness characterizing patients with DDD and OA can cause significant loss in work participation and lead to high socio‐economic costs.8, 9 The diseases are caused by degeneration of the intervertebral disc and articular joint, following a vicious circle towards joint deterioration.10, 11 While this process of degeneration in the articular joint carries the same name as its disease (ie, OA), the degenerative process in intervertebral discs is commonly known as intervertebral disc degeneration.12, 13, 14 On plain X‐rays both intervertebral disc degeneration and OA are accompanied by a loss of joint space, formation of osteophytes, subchondral cysts and sclerosis.15, 16 No definitive treatment options are available to halt or reverse intervertebral disc degeneration and OA, but early symptoms of DDD and OA can be relieved with physiotherapy and pain medication, and with spinal fusion and joint arthroplasty in end‐stage DDD and OA, respectively. Inspired by these similarities, we investigated the analogy between both conditions.
In the 19th century, Luschka was the first to suggest that the intervertebral disc is similar to an articular joint, comparing the cartilaginous endplates of the intervertebral disc to articular cartilage (AC).17, 18, 19 Since then, several studies have focused on clinical aspects in DDD and OA, and pathomechanisms and regenerative strategies in intervertebral disc degeneration and OA, but this was often in parallel and not in a comparative way.13, 20, 21 Most research on intervertebral disc degeneration has concentrated on only one aspect of degeneration (in particular the nucleus pulposus [NP]),22, 23, 24 while OA is generally regarded as a whole organ disease,25 rather than just degeneration of the AC. Still, most studies consider the differences between intervertebral disc degeneration and OA rather than the similarities between the two conditions.
In this review, we provide a broad comparison on the human intervertebral disc and articular joint. We investigate similarities and differences in anatomy, embryonic development, cellular behavior and tissue composition. Consecutively, we compare the degeneration of intervertebral discs and articular joints in humans, followed by a clinical perspective on both diseases. Since the NP and AC are believed to be the most affected part of the degenerated intervertebral disc and articular joint, respectively, we focus on the NP and AC.26, 27
2. THE HEALTHY INTERVERTEBRAL DISC AND ARTICULAR JOINT
2.1. Anatomy and embryonic development
The intervertebral disc is a unique structure in the human body. The intervertebral discs are amphiarthrosic joints and consist of: (a) the NP, (b) the cartilaginous endplates that cover the subchondral bone of the adjacent vertebrae, and (c) the annulus fibrosus (AF),28 but the functional spinal unit also involves the facet joints and vertebrae (Figure 1A). The articular joints are diarthrosic joints and consist of: (a) the synovial fluid, (b) hyaline cartilage covering the subchondral bone of the adjacent bones, and (c) the capsule (Figure 1B), and its functional unit also involves tendons, ligaments and the bone of the adjacent bones. While the structural elements of all mammalian intervertebral discs are essentially the same, articular joints may contain additional structures specific to the concerning joint (eg, bursae, tendons, menisci).31
Figure 1.
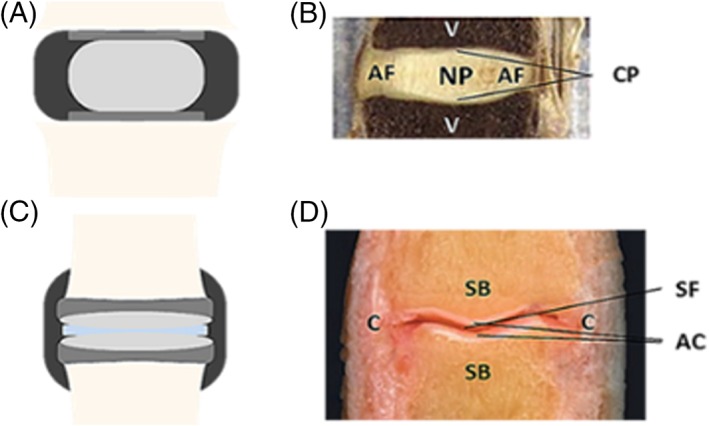
A and B, Anatomy of the human intervertebral disc and articular joint.29, 30 A, The intervertebral disc consists of a nucleus pulposus (light gray), surrounded by an annulus fibrosus (black), and is between two cartilaginous endplates (dark gray) that adhere to the adjacent vertebrae (beige). B, An image of a sagittal section of an intervertebral disc. NP: nucleus pulposus; AF: annulus fibrosus; CP: cartilaginous endplates; V: vertebrae. C, The articular joint consists of articular cartilage (light gray), that lies over the subchondral bone (dark gray) of the adjacent joints, and is divided by synovial fluid (light blue). The capsule (black) surrounds the articular joint. D, An image of a sagittal section of an articular joint. AC: articular cartilage; C: capsule; SB: subchondral bone; SF: synovial fluid
During early human embryonic development, starting in week 5, the intervertebral discs are formed by two structures: the notochord and sclerotome (Figure 2A). The sclerotome, forming the vertebrae and the outer AF, surrounds the expanding notochord, which will partition into the nuclei pulposi. A transition zone of notochord and sclerotome characterizes the inner AF. The NP and AF are unique structures in humans and other mammals,32, 33, 34 but as other vertebrates like birds and reptiles develop articular joints between vertebrae instead of intervertebral discs, more similarity is suggested than one may expect from looking at anatomy and embryology alone.28, 34 For a more extensive description of the anatomy and embryonic development of human intervertebral discs we refer to.32, 33, 35, 36, 37
Figure 2.
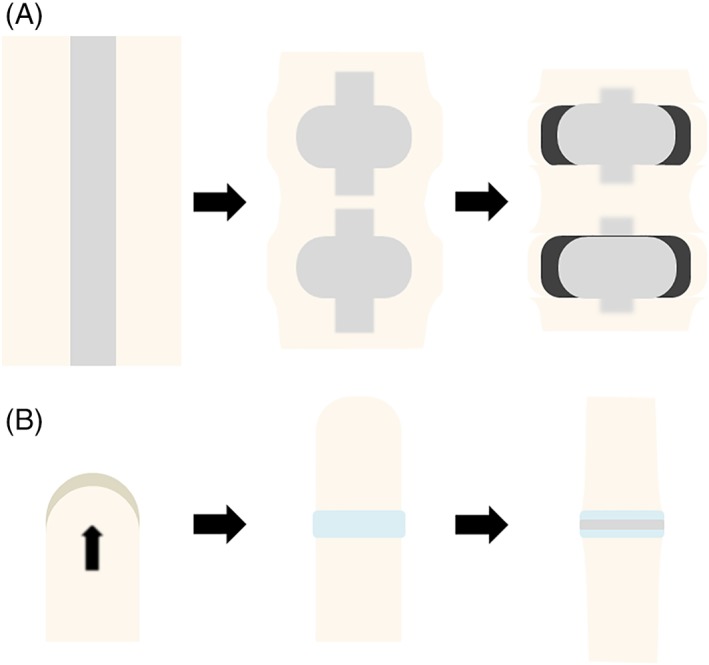
A and B, Embryonic development of the human intervertebral disc and articular joint. A, Starting in week 5, the intervertebral discs are formed by the notochord and sclerotome. The sclerotome (on the outside) forms the outer annulus and surrounds the notochord (inside), which will eventually partition into the nuclei pulposi. A transition zone between notochord and sclerotome characterizes the inner annulus fibrosus. B, In week 6, the limb bud is formed by mesoderm covered by ectoderm and starts to grow outwards in order to form arms and legs. The formation of articular joints occurs later in embryonic development (ie, in week 8), and is characterized by the interzone (light blue), which is a group of mesenchymal cells that form an interspace
The formation of arms and legs occurs slightly later in human embryonic development, in week 6 (Figure 2B). Limbs derive from the limb bud, a structure of mesenchyme covered with ectoderm. The limb bud starts to grow gradually outwards forming limbs over time.38, 39 The formation of articular joints occurs later in embryonic development (ie, in week 8) and becomes morphologically visible with the presence of the interzone, which is a region at the location of the future joint where the mesenchymal cells transform into an interspace in between two outer layers adjacent to the epiphyseal end of the future bones.40 By contrast, intervertebral discs are formed in a process of sequential segmentation. For a more extensive description of the anatomy and embryonic development of articular joints we refer to.38, 39, 41, 42, 43
2.2. Extracellular matrix and biomechanical properties
The intervertebral disc and articular joint absorb and distribute the loads that are imposed by muscle force and gravity on the vertebrae and adjacent bones, respectively. To counterbalance the loads, an intradiscal pressure is found in the NP and an intra‐articular pressure in the AC.44, 45 These pressures are attributed to the high concentration of negatively charged proteoglycans—predominantly aggrecan—in the extracellular matrix (ECM) of the NP and AC.44, 45, 46, 47, 48 Proteoglycans attract water which generates a hydrostatic pressure within the tissue. This hydrostatic pressure is essential for the NP and AC to provide a healthy mechanobiological stimulus to the cells, and dynamic loading is necessary for transport of nutrients and waste products.49, 50
Besides proteoglycans, the ECM of the NP and AC contains collagen, mainly collagen type II.51 The ratio of aggrecan to collagen is more than five times higher in the NP than in AC,35, 52, 53 and it is this high ratio of aggrecan that creates a high intradiscal pressure in the intervertebral disc (ie, 0.43‐0.50 MPa in relaxed standing54), whereas the pressure inside AC is much lower (ie, ± 300 kPa in 30% compression45, 55). Toward the outer regions of the intervertebral disc, the AF becomes a more fibrous structure of mainly collagen type I,51, 56 which is able to resist tensile stresses caused by the intradiscal pressure. The articular joint capsule is also a dense fibrous connective tissue and surrounds the articular joint with a variety in thickness, depending on the applied loads.56
The collagen type I fibers in the AF are organized in oblique lamellae which limits movement of the spinal segment.57 This way, the intervertebral disc provides stability and essentially forms an elastic hinge providing flexibility over a small range of motion by deformation of the tissue, but is not able to articulate. In contrast, articular joints provide low‐friction articulation, only limited by ligaments that stabilize the articular joints.58 Thus, articular joints have a much larger range of motion.
2.3. Cells
At birth, the human NP has a high density of notochordal cells,59 which are large in diameter, contain intracellular vacuoles and appear in clusters.60 However, they are slowly replaced by chondrocyte‐like cells after birth.61, 62 There is some evidence that the cells maintain some of their notochordal characteristics, as human degenerated intervertebral disc cells express brachyury, which is a well‐established notochordal marker.63 Other cell‐specific markers found in NP cells are cytokeratin‐19, FOX‐F1, CA12 and PAX‐1. The chondrocyte‐like cells are much smaller in diameter and lack intracellular vacuoles.60 A lack of innervation and vascularization makes that these cells live in a harsh environment, with a relatively low pH (ie, 7.1).64, 65 Only the outer annulus and endplates have minimal blood supply, which culminates in a suboptimal repair mechanism of the intervertebral disc itself.
The articular joints are surrounded by the joint capsule. The capsule has two parts, an inner synovial membrane, consisting of the intima, which has only one cell layer, and the well‐vascularized subintima surrounding the intima.66, 67 The outer, more fibrous membrane of the joint capsule and subchondral bone in the articular joint has minimal blood supply,68 which is similar to the intervertebral disc. Yet there is an exchange of solutes between the synovial fluid and joint capsule which is incomparable to the intervertebral disc, as there is no distinct exchange between the NP and AF. However, the environment of the chondrocytes, the only cell type present in AC, is also hostile with minimal repair capacity, as blood vessels are absent in the AC.69 The chondrocytes are differentiated from the embryonal mesenchyme, which is derived from the mesoderm, and their gene expression profile contains SOX9, COMP, FGFB, MAPK, WNT, and JNK.70 They are round cells, like NP cells, but their cell density is much higher compared to the NP and their distribution differs over the several distinct layers in AC.60 In adult AC, the chondrocytes are surrounded by the pericellular matrix and they lack cell‐cell interactions. Just like the chondrocyte‐like cells in the NP, their proliferative activity is very low, but they are still important in maintaining the homeostasis and production of ECM.71
For a more detailed description of the cell‐types in the intervertebral disc and articular joint, we refer to more extensive reviews on this subject.60, 69, 70, 72, 73 However, considering the resemblance in some features of cell behavior after birth (eg, produce the same type of ECM proteins), the local environment of the cell (ie, ECM and mechanobiological cues) may have more influence on its behavior than its embryonic origin.35
In summary, there are differences in anatomy and embryology between the healthy intervertebral disc and articular joint in humans, but there are also marked similarities. The NP and AC correspond especially in the production of predominantly proteoglycans and collagen type II, resulting in an ECM with a high hydrostatic pressure, which is able to endure compressive loads. Furthermore, both structures are mostly avascular, limiting their own regenerative capacities (see Table 1). These similarities between the two healthy tissues provide a good base from which the process of degeneration in the human intervertebral disc and articular joint can be further understood.10, 11
Table 1.
Comparison of the healthy intervertebral disc and articular joint
| Intervertebral disc | Articular joint (eg, the knee) | |||
|---|---|---|---|---|
| Anatomy28, 32, 33, 35, 36, 37, 41, 42, 43 | Nucleus pulposus | Synovial fluid | ||
| Cartilaginous endplates covering subchondral bone endplates | Hyaline cartilage covering subchondral bone adjacent bones | |||
| Annulus fibrosus | Capsule | |||
| Amphiarthrosic joint | Diarthrosic joint | |||
| Embryonic development32, 33, 35, 36, 37, 38, 39 | Starts in week 5 in humans | Starts in week 6 in humans | ||
| Notochord | Forms the nuclei pulposi | Mesenchyme and ectoderm | Limb bud | |
| Disappears in vertebrae | ||||
| Sclerotome | Consists of mesenchymal cells | |||
| Forms the vertebrae and outer annulus fibrosus | ||||
| Transition zone of notochord and sclerotome in the inner annulus fibrosus | ||||
| Secondary to somites that form through sequential segmentation | Limb bud forms by appositional growth | |||
| Extracellular matrix 35, 44, 46, 47, 48, 51, 55, 56, 57, 58 | Proteoglycans (mainly aggrecan) in the nucleus pulposus (15%) | Proteoglycans (mainly aggrecan) in the cartilage (10‐15%) | ||
| Elastine in the annulus fibrosus | Elastine in the capsule | |||
| Collagen (20%) | Mainly type II in the nucleus pulposus | Collagen (10‐20%) | Mainly type II in the cartilage | |
| Mainly type I in the annulus fibrosus | Mainly type I in the capsule | |||
| Biomechanics 35, 44, 46, 55, 58 | Hydrostatic pressure in the nucleus pulposus; strain and shear in the annulus fibrosus | Hydrostatic pressure in the synovial fluid and cartilage; longitudinal strain and shear in the capsule | ||
| Normal joint loads transferred to endplates and vertebrae | Normal joint loads transferred to underlying subchondral bone | |||
| Elastic deformation of disc | Articulating surfaces | |||
| Stability by annulus fibrosus, posterior elements, and spinal ligaments | Stability by ligaments and tendons | |||
| Stability by muscular force | Stability by muscular forces | |||
| Cells59, 61, 62, 64, 69 | Notochordal cells | Chondrocytes | ||
| Chondrocyte‐like cells | ||||
3. DEGENERATION IN THE HUMAN INTERVERTEBRAL DISC AND ARTICULAR JOINT
The balance between anabolic and catabolic processes is tenuous in the intervertebral disc and articular joint. When the balance is tipped, there is an inequality between the synthesis and degradation of the ECM due to catabolic cell behavior (58, 74, 75, 76, 77). Within the intervertebral disc and articular joint, this mainly affects the NP and AC. Several systemic inflammatory factors have been described to tip this balance toward degeneration in both diseases, such as diabetes, obesity, smoking, and low‐grade systemic infection.78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 Mechanical overloading is another established factor that induces local inflammation in NP and AC,90, 91 especially in the AC of knee and ankle,86, 92 since the lesions are often localized to weight‐bearing cartilage or to sites of trauma.93, 94
Catabolic cell behavior is characterized by an increase in the expression of cytokines and matrix‐degrading enzymes, and a downregulation of their inhibitors.95, 96, 97, 98, 99, 100 The cytokines that have been well‐documented to have a detrimental effect in intervertebral disc degeneration and OA are tumor necrosis factor alpha (TNFα) and interleukin‐1β (IL‐1β).101, 102, 103, 104, 105 These cytokines generate local inflammation in the NP and AC by an upregulation of enzymes that degrade the ECM: matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS).106, 107, 108, 109, 110, 111, 112 Simultaneously, a downregulation of their inhibitors (eg, tissue inhibitor of metalloproteinase 1‐3, TIMP 1‐3) occurs,100, 113, 114 all together pushing the ECM in a vicious cycle of degradation.10 Several MMPs have been related to degeneration in the NP, including MMP 1‐3, MMP 7‐10, and MMP 12‐14.108, 113, 115, 116 These factors show remarkable similarity to those found in AC, including MMP 1‐3 and 7‐14,93, 117, 118, 119 although their expression levels may differ from those in the NP (see Table 2). Additionally, the ECM in the NP and AC is also cleaved by ADAMTSs 4 and 5.125, 126, 127
Table 2.
Comparison of degeneration in the intervertebral disc and articular joint
| Intervertebral disc | Articular joint (eg, the knee) | |||||
|---|---|---|---|---|---|---|
| Extracellular matrix26, 76, 77, 120, 121, 122, 123 | Degradation of the proteoglycans: Less fluid attracted to nucleus pulposus | Degradation of the proteoglycans: Less fluid attracted to the articular cartilage | ||||
| Decrease in intradiscal pressure | Decrease in intra‐articular pressure | |||||
| Shift to collagen type I: Nucleus pulposus becomes more fibrous | Shift to collagen type I: Articular cartilage becomes more fibrous | |||||
| Biomechanics48, 121, 124 | Reduced disc height | Reduced joint space | ||||
| Increase in shear stresses | Increase in shear stresses | |||||
| Less resistive to compressive loads | Less resistive to compressive loads | |||||
| Cells95, 96, 97, 98, 99, 100 | Inflammatory mediators: Production of catabolic factors | Inflammatory mediators: Production of catabolic factors | ||||
| Catabolism by the chondrocyte‐like cells: Degradation of ECM | Catabolism by the chondrocytes: Degradation of ECM | |||||
| Caused by 58, 74, 75, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94 | Local inflammation | Systemic inflammation by | Diabetes | Local inflammation | Systemic inflammation by | Diabetes |
| Obesity | Obesity | |||||
| Smoking | Smoking | |||||
| Mechanical overloading | Mechanical overloading | |||||
| Inflammatory factors93, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 125, 126, 127 | TNF‐α and IL‐1β | TNF‐α and IL‐1β | ||||
| MMP 1–3, MMP 7–10, and MMP 12–14 | MMP 1‐3 and 7–14 | |||||
| ADAMTSs 4 and 5 | ADAMTSs 4 and 5 | |||||
| TIMP 1–3 | TIMP 1–3 | |||||
| Clinical symptoms12, 13, 14, 128 | Pain | Pain | ||||
| Dysfunction | Dysfunction | |||||
| Morning stiffness | Morning stiffness | |||||
| Radiological findings4, 15, 129, 130 | Formation of cysts and osteophytes | Formation of cysts and osteophytes | ||||
| Loss of joint space | Loss of joint space | |||||
| Subchondral sclerosis | Subchondral sclerosis | |||||
| Adjacent structures involved25, 74, 81, 131 | Vertebrae | Bone of tibia and femur | ||||
| Facet joints | Meniscal tears | |||||
| Modic changes | Synovitis | |||||
| Nerve roots, ligaments and muscles | Nerve roots, ligaments and muscles | |||||
3.1. Effects of ECM degradation
The degradation of the ECM results in a decrease in the production of proteoglycans in the cartilaginous matrices.120 Consequently, less fluid is attracted, leading to a decrease in hydrostatic pressure.121 This causes reduced joint space and an increase in tissue deformation (ie, shear stresses). Shear stress is a mechanical cue for the cells to shift from collagen type II to collagen type I production, resulting in a more fibrous tissue.26, 122, 123 This fibrous tissue has an inferior capacity to resist compressive loads, as there is a loss of the typical poro‐ and viscoelastic biomechanical properties.48, 121, 124 The disruption of ECM not only results in loss of joint space, but also causes subchondral sclerosis and the formation of osteophytes, which is a reaction of the bone to the changing mechanical environment.4, 16, 129
3.2. Genetic aspects
Besides shared environmental factors, intervertebral disc degeneration and OA also share genetic aspects. For example, Bijkerk et al found a strong genetic effect for intervertebral disc degeneration and hand OA,132 and Loughlin describes several genetic polymorphisms that are attributed to both intervertebral disc degeneration and OA.133 They demonstrate that GDF5 polymorphism rs143383 is a risk factor for both intervertebral disc degeneration and knee OA, just as the repeat polymorphism of the asporin gene (ASPN).134 Moreover, polymorphisms of vitamin D receptor (ie, FokI and TaqI) are not only attributed to OA, but also to reduced signal intensity on MRI in lumbar intervertebral discs, suggesting an association with intervertebral disc degeneration.135, 136 Based on these studies, it is likely that intervertebral disc degeneration is more prevalent in patients with OA and vice versa, which further supports the resemblance between both conditions.
4. CLINICAL PERSPECTIVE ON INTERVERTEBRAL DISC DEGENERATION AND OA
Intervertebral disc degeneration is not just a disease of the cartilaginous structures, but affects the whole intervertebral disc and adjacent structures, like the vertebrae, nerve roots, ligaments, muscles and spinal facets, and there is an association with Modic changes (ie, changes in signal intensity of endplates and subchondral bone on MRI in patients with spinal degenerative diseases).74, 131, 137 The same applies for articular joints: OA not only affects the AC, but involves the whole joint as it is accompanied by bony changes, meniscal tears, synovitis, and muscle weakness.25, 81 Imaging confirms that the whole joint is affected, as both conditions share the same characteristic radiological findings throughout the entire joint: loss of joint space, subchondral sclerosis, and the formation of cysts and osteophytes4, 16, 129 (Figure 3A,B).
Figure 3.
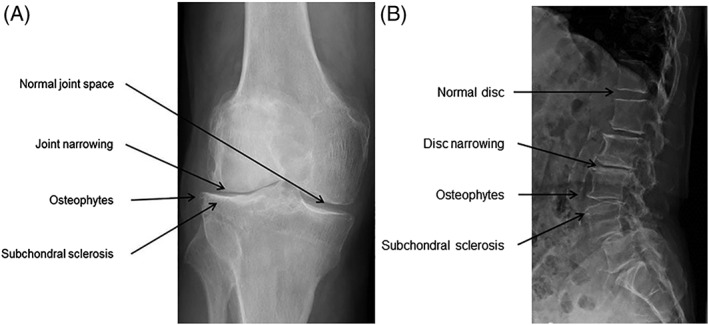
A and B, Radiological examples in the degenerated articular joint and intervertebral disc. Both OA (A) and DD (B) are radiologically characterized by loss of joint space, the formation of osteophytes and subchondral sclerosis
The degree of degeneration on X‐rays, however, in both intervertebral disc degeneration and OA weakly correlates to clinical symptoms.13, 138, 139, 140 Clinical symptoms are similar in both DDD and OA: pain, dysfunction and morning stiffness. For OA, these symptoms are the three most important clinical criteria based on the diagnostic guidelines of the American College of Rheumatology (ACR), the most commonly used classification system for OA.128 These guidelines, combined with patient history, physical examination and X‐ray radiographs, make OA easy to diagnose for clinicians. After diagnosis and treatment following a clinical algorithm based on evidence‐based medicine and expert opinion, patients return to work rather soon.141 OA is even that well‐known, that the same term is used for both the process of degeneration and the accompanying disease by not only clinicians and scientists, but also by patients and in society.
In contrast to OA, DDD is often disregarded as a risk factor for function loss and disability associated with low‐back pain,142 as widely used classification criteria do not exist for DDD. As such, it is difficult for clinicians to find a specific diagnosis when a patient presents itself with low back pain.143 For example, the Dutch society for general practitioners has a guideline for “Non‐traumatic knee problems” (ie, NHG guidelines), which mainly focuses on knee OA as a cause of these problems. The guideline for “Non‐specific low back pain,” however, mentions DDD only briefly as a possible cause, but is overshadowed by other—relatively rare—conditions, like malignancies, fractures, and spondylarthrosis. This lack of wide recognition and consecutive diagnosis means that there is little opportunity to develop effective evidence‐based treatment plans.
4.1. Treatment
In both DDD and OA, no treatment is available that regenerates the original tissue. Current treatments focus on symptomatic relieve, such as the reduction of overloading and inflammation by physiotherapy and anti‐inflammatory drugs in early stage OA.144, 145 Currently, physiotherapy and anti‐inflammatory drugs are only a postponement of surgical treatment in OA and most patients eventually undergo arthroplasty.146, 147 Most patients regain their mobility soon after surgery and remain free of pain and disability for some 10 years.148 Arthroplasty in DD is less typical, but artificial intervertebral discs are starting to show promising results, although spinal fusion is still more common.149, 150, 151
Several regenerative therapies have been proposed for both diseases, such as hydrogels, stem cell therapy or injectable medications,20, 125, 152 but so far none has gained general acceptance. Finding a cure to restore the cartilaginous structures is quite a challenge, but in knee OA there are some advancements in regenerative therapies (eg, by joint unloading), which demonstrate that cartilage does have some intrinsic healing capacity.153, 154, 155, 156, 157 In DDD, however, no regenerative therapies are present, neither clinically nor preclinically.
5. SUMMARY
In this review, we showed that the cartilaginous structures of both the human intervertebral disc and articular joint contain predominantly proteoglycans and collagen type II, resulting in a tissue that is able to create hydrostatic pressure, endure compressive loads and provide flexibility. Their roads to degeneration also show striking parallels: local inflammation takes place as a result of mechanical overloading or low grade systemic inflammation, and is characterized by increased levels of inflammatory cytokines, mainly IL‐1β and TNF‐α. These cause an upregulation of equivalent factors that decompose the ECM (eg, MMPs 1‐3, 7‐10, and 12‐14; and ADAMTSs 4 and 5) and a downregulation of their inhibitors (eg, TIMP 1‐3), which are similar in both tissues. These results in a vicious cycle of tissue damage and inflammation, causing cell death of the NP cells and chondrocytes, increased in shear stress and decreased levels of proteoglycans and collagen type II. When the healthy intervertebral disc and articular joint degenerate, this is accompanied by identical radiological findings such as loss of joint space, subchondral sclerosis, and the formation of osteophytes, which cause pain and stiffness.
We also found some differences, mainly in anatomy, embryonic development, cells and partly in their biomechanical properties. Therefore, it would be inappropriate to claim that intervertebral disc and articular joint and their degenerative conditions are identical. Nevertheless, some important parts of cellular behavior and ECM in the human adult NP and AC show striking resemblance despite their completely different origin, which suggests that the local mechanobiological environment of the cell after birth has greater influence on its behavior than its embryonic origin.
The biggest difference between both conditions, however, lies in its awareness among health care professionals, patients and society. OA is a well‐accepted health problem in society.158 The condition is no longer regarded as a “wear‐and‐tear” disease or patho‐anatomical finding,159, 160 but as a common disease that disables the patient. Clinicians are easily accessible and patients follow a clear, clinical algorithm of diagnosis and treatment, despite the fact that the relation between clinical symptoms in knee OA and radiographic images is also doubtful.140 Still, clinicians often find it easier to relate pain to radiographic changes in a single joint than in the lower back. The reason may be that the relation between imaging and pain in articular joints is thought to be much stronger and that the clear clinical algorithms with knee, hip or ankle pain are specifically designed for diagnosing or excluding OA. The clear, clinical algorithm that elderly patients with joint pain follow in OA despite this weak correlation between symptoms and radiographic images, is in contrast to the unawareness, and thus, undervaluation of DDD as a cause of low‐back pain. The lack of a specific diagnosis for low‐back pain patients causes patients to feel stigmatized.161, 162 Most of the times, they end up consulting a paramedic or psychologist, because no diagnosis is found, nor is there a straight‐forward clinical algorithm to follow for medical professionals in primary care.
If the similarities between DDD and OA would be widely acknowledged, the imbalance between both conditions can be reduced. This may enhance the clinician‐patient communication and reduce the negative stigma on low‐back pain. The knowledge on both conditions could also be enlarged and finding regenerative therapeutics may be accelerated, as it facilitates cross‐fertilization of clinicians and scientists involved in both intervertebral disc degeneration and OA.
In conclusion, the human intervertebral disc and articular joint are not identical, but their composition and process of degeneration are remarkably similar. Intervertebral disc degeneration and OA both follow a vicious cycle of degeneration, eventually resulting in destruction of the intervertebral disc and articular joint, respectively. However, there is a large imbalance between both conditions in knowledge and awareness among patients, clinicians, researchers and society. Acknowledging the similarities between the relatively unknown DDD to its more famous counterpart OA may reduce this imbalance, destigmatize patients by affiliating them with a well‐recognized disease, and lower the threshold for patients to visit a clinician.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Author contributions
All authors have contributed to the manuscript and approved the final version.
ACKNOWLEDGEMENTS
The authors would like to thank Robert Jan Kroeze for his valuable comments to the manuscript. No financial support, grant or other benefits from commercial sources were received for this article.
Rustenburg CME, Emanuel KS, Peeters M, Lems WF, Vergroesen P‐PA, Smit TH. Osteoarthritis and intervertebral disc degeneration: Quite different, quite similar. JOR Spine. 2018;1:e1033. 10.1002/jsp2.1033
REFERENCES
- 1. Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low Back pain. Arthritis Rheum. 2012;64(6):2028‐2037. [DOI] [PubMed] [Google Scholar]
- 2. Buchbinder R, van Tulder M, Öberg B, et al. Low back pain: a call for action. Lancet. 2018;391(10137):2384‐2388. [DOI] [PubMed] [Google Scholar]
- 3. Vos T, Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Schepper EI, Damen J, van Meurs JB, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976). 2010;35(5):531‐536. [DOI] [PubMed] [Google Scholar]
- 5. Pye SR, Reid DM, Smith R, et al. Radiographic features of lumbar disc degeneration and self‐reported back pain. J Rheumatol. 2004;31(4):753‐758. [PubMed] [Google Scholar]
- 6. van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine (Phila Pa 1976). 1997;22(4):427‐434. [DOI] [PubMed] [Google Scholar]
- 7. Raastad J, Reiman M, Coeytaux R, Ledbetter L, Goode AP. The association between lumbar spine radiographic features and low back pain: a systematic review and meta‐analysis. Semin Arthritis Rheum. 2015;44(5):571‐585. [DOI] [PubMed] [Google Scholar]
- 8. Souza NSS, Santana VS, Albuquerque‐Oliveira PR, Barbosa‐Branco A. Work‐related diseases and health‐related compensation claims, northeastern Brazil, 2000. Rev Saude Publica. 2008;42(4):630‐638. [DOI] [PubMed] [Google Scholar]
- 9. Hoy D, March L, Brooks P, et al. Measuring the global burden of low back pain. Best Pract Res Clin Rheumatol. 2010;24(2):155‐165. [DOI] [PubMed] [Google Scholar]
- 10. Vergroesen P‐PA, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057‐1070. [DOI] [PubMed] [Google Scholar]
- 11. Andriacchi TP. Osteoarthritis: probing knee OA as a system responding to a stimulus. Nat Rev Rheumatol. 2012;8(7):371‐372. [DOI] [PubMed] [Google Scholar]
- 12. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976). 1995;20(11):1307‐1314. [DOI] [PubMed] [Google Scholar]
- 13. Scheele J, De Schepper EIT, Van Meurs JBJ, et al. Association between spinal morning stiffness and lumbar disc degeneration: the Rotterdam study. Osteoarthritis Cartilage. 2012;20(9):982‐987. [DOI] [PubMed] [Google Scholar]
- 14. Kean WF, Kean R, Buchanan WW. Osteoarthritis: symptoms, signs and source of pain. Inflammopharmacology. 2004;12(1):3‐31. [DOI] [PubMed] [Google Scholar]
- 15. Gupta KB, Duryea J, Weissman BN. Radiographic evaluation of osteoarthritis. Radiol Clin North Am. 2004;42(1):11‐41. v. [DOI] [PubMed] [Google Scholar]
- 16. Pye SR, Reid DM, Lunt M, Adams JE, Silman AJ, O'Neill TW. Lumbar disc degeneration: association between osteophytes, end‐plate sclerosis and disc space narrowing. Ann Rheum Dis. 2007;66(3):330‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luschka H. Die Halbegelenke des Menschlichen Korpers. Erste Aufl. Berlin: Reimer; 1852. [Google Scholar]
- 18. Luschka H. Die Halbegelenke des Menschlichen Korpers. Zweite Auf. Berlin: Reimer; 1858. [Google Scholar]
- 19. Luschka H. Die altersveranderungen der zwischenwirbelknorpel. Arch Path Anat. 1855;IX:311‐327. [Google Scholar]
- 20. Richardson SM, Kalamegam G, Pushparaj PN, et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods. 2015;99:69‐80. [DOI] [PubMed] [Google Scholar]
- 21. McCann MR, Patel P, Pest MA, et al. Repeated exposure to high‐frequency low‐amplitude vibration induces degeneration of murine intervertebral discs and knee joints. Arthritis Rheumatol. 2015;67(8):2164‐2175. [DOI] [PubMed] [Google Scholar]
- 22. Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol. 2007;53(Ivd):4‐18. [PubMed] [Google Scholar]
- 23. Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(4):652‐655. [DOI] [PubMed] [Google Scholar]
- 24. Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13(3):243‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115‐2126. [DOI] [PubMed] [Google Scholar]
- 26. Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487‐499. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shapiro IM, Vresilovic EJ, Risbud MV. Is the spinal motion segment a diarthrodial polyaxial joint: What is a nice nucleus like you doing in a joint like this? Bone. 2011;50(3):771‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bailey JF, Fields AJ, Liebenberg E, Mattison JA, Lotz JC, Kramer PA. Comparison of vertebral and intervertebral disc lesions in aging humans and rhesus monkeys. Osteoarthritis Cartilage. 2014;22(7):980‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nielsen M, Miller SD. Atlas of Human Anatomy. Hoboken, NJ: Wiley; 2011:352. [Google Scholar]
- 31. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sivakamasundari V, Lufkin T. Bridging the gap: understanding embryonic intervertebral disc development. Cell Dev Biol. 2012;1(2):103. [PMC free article] [PubMed] [Google Scholar]
- 33. Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20(2):107‐121. [DOI] [PubMed] [Google Scholar]
- 34. Bruggeman BJ, Maier JA, Mohiuddin YS, et al. Avian intervertebral disc arises from rostral sclerotome and lacks a nucleus pulposus: implications for evolution of the vertebrate disc. Dev Dyn. 2012;241(4):675‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen S, Fu P, Wu H, Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage‐like tissues in anatomy, development and function. Cell Tissue Res. 2017;370(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raj PP. Intervertebral disc: anatomy‐physiology‐pathophysiology‐treatment. Pain Pract. 2008;8(1):18‐44. [DOI] [PubMed] [Google Scholar]
- 37. Colombier P, Clouet J, Hamel O, Lescaudron L, Guicheux J. The lumbar intervertebral disc: from embryonic development to degeneration. Joint Bone Spine. 2014;81(2):125‐129. [DOI] [PubMed] [Google Scholar]
- 38. Mérida‐Velasco JA, Sanchez‐Montesinos I, Espin‐Ferra J, Rodriguez‐Vazquez JF, Merida‐Velasco JR, Jimenez‐Collado J. Development of the human knee joint. Anat Rec. 1997;248:269‐278. [DOI] [PubMed] [Google Scholar]
- 39. Archer CW, Dowthwaite GP, Francis‐West P. Development of synovial joints. Birth Defects Res Part C—Embryo Today Rev. 2003;69(2):144‐155. [DOI] [PubMed] [Google Scholar]
- 40. Longobardi L, Li T, Tagliafierro L, et al. Synovial joints: from development to homeostasis. Curr Osteoporos Rep. 2015;13(1):41‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. LaPrade RF. The anatomy of the medial part of the knee. J Bone Jt Surg. 2007;89(9):2000‐2010. [DOI] [PubMed] [Google Scholar]
- 42. Blackburn TA, Craig E. Knee anatomy: a brief review. Phys Ther. 1980;60(12):1556‐1560. [DOI] [PubMed] [Google Scholar]
- 43. Flandry F, Hommel G. Normal anatomy and biomechanics of the knee. Sports Med Arthrosc. 2011;19(2):82‐92. [DOI] [PubMed] [Google Scholar]
- 44. Ohshima H, Tsuji HHN. Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine (Phila Pa 1976). 1989;14(11):1234‐1244. [DOI] [PubMed] [Google Scholar]
- 45. Han E, Chen SS, Klisch SM, Sah RL. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys J. 2011;101(4):916‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carter DR, Wong M. Modelling cartilage mechanobiology. Philos Trans R Soc B—Biol Sci. 2003;358(1437):1461‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ishihara H, Warensjo K, Roberts S, Urban JP. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. Am J Physiol. 1997;272:C1499‐C1506. [DOI] [PubMed] [Google Scholar]
- 48. Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30(Pt 6):869‐874. [DOI] [PubMed] [Google Scholar]
- 49. Bleuel J, Zaucke F, Brüggemann GP, Niehoff A. Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review. PLoS One. 2015;10(3):e0119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paul CPL, Zuiderbaan HA, Doulabi BZ, et al. Simulated‐physiological loading conditions preserve biological and mechanical properties of caprine lumbar intervertebral discs in ex vivo culture. PLoS One. 2012;7(3):e33147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roberts S, Menage J, Duance V, Wotton S, Ayad S. Volvo award in basic sciences. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine (Phila Pa 1976). 1991;16(9):1030‐1038. [PubMed] [Google Scholar]
- 52. Yin J, Xia Y, Lu M. Concentration profiles of collagen and proteoglycan in articular cartilage by Fourier transform infrared imaging and principal component regression. Spectrochim Acta—Part A Mol Biomol Spectrosc. 2012;88:90‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iatridis JC, MacLean JJ, O'Brien M, Stokes IAF. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila Pa 1976). 2007;32(14):1493‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilke H‐J, Neef P, Hinz B, Seidel H, Claes L. Intradiscal pressure together with anthropometric data—a data set for the validation of models. Clin Biomech. 2001;16(Suppl 1):S111‐S126. [DOI] [PubMed] [Google Scholar]
- 55. Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6(3):861‐870. [PubMed] [Google Scholar]
- 56. Ralphs JR, Benjamin M. The joint capsule: structure, composition, ageing and disease. J Anat. 1994;184(Pt 3):503‐509. [PMC free article] [PubMed] [Google Scholar]
- 57. Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine (Phila Pa 1976). 1990;15(5):402‐410. [DOI] [PubMed] [Google Scholar]
- 58. Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Phila Pa 1976). 2004;29(23):2724‐2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246(1):129‐137. [DOI] [PubMed] [Google Scholar]
- 60. Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221(6):480‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Trout JJ, Buckwalter J, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204(4):307‐314. [DOI] [PubMed] [Google Scholar]
- 62. Johnson EF, Mitchell R, Berryman H, Cardoso S, Ueal O, Patterson D. Secretory cells in the nucleus pulposus of the adult human intervertebral disc. A preliminary report. Acta Anat. 1986;125(3):161‐164. [DOI] [PubMed] [Google Scholar]
- 63. Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239(8):2141‐2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun‐Shik S et al. Blood supply of the knee joint. Clin Orthop Relat Res. 1986;208:119‐125. [PubMed] [Google Scholar]
- 65. Gilbert HTJ, Hodson N, Baird P, Richardson SM, Hoyland JA. Acidic pH promotes intervertebral disc degeneration: acid‐sensing ion channel‐3 as a potential therapeutic target. Sci Rep. 2016;6:37360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Young B, Lowe JS, Stevens A, Heath JW. Wheater's Functional Histology: A Text and Colour Atlas. London: Churchill Livingstone; 2006. [Google Scholar]
- 67. Firestein GS, Budd RC, Gabriel SE. Kelley & Firestein's textbook of rheumatology Kelley's Textbook of Rheumatology. Amsterdam, The Netherlands: Elsevier; 2016. [Google Scholar]
- 68.RheumatologySmith MD, Wechalekar MD. The synovium. 6th ed. ; 2014.
- 69. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ochi K, Daigo Y, Katagiri T, et al. Expression profiles of two types of human knee‐joint cartilage. J Hum Genet. 2003;48(4):177‐182. [DOI] [PubMed] [Google Scholar]
- 71. Camarero‐Espinosa S, Rothen‐Rutishauser B, Foster EJ, Weder C. Articular cartilage: from formation to tissue engineering. Biomater Sci. 2016;4(5):734‐767. [DOI] [PubMed] [Google Scholar]
- 72. Wuelling M, Vortkamp A. Chondrocyte proliferation and differentiation. Endocr Dev. 2011;21:1‐11. [DOI] [PubMed] [Google Scholar]
- 73. Lv F, Leung VYL, Huang S, Huang Y, Sun Y, Cheung KMC. In search of nucleus pulposus‐specific molecular markers. Rheumatology. 2014;53(4):600‐610. [DOI] [PubMed] [Google Scholar]
- 74. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976). 2006;31(18):2151‐2161. [DOI] [PubMed] [Google Scholar]
- 75. Weber KT, Jacobsen TD, Maidhof R, et al. Developments in intervertebral disc disease research: pathophysiology, mechanobiology, and therapeutics. Curr Rev Musculoskelet Med. 2015;8(1):18‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cs‐Szabo G, Ragasa‐San Juan D, Turumella V, Masuda K, EJ‐MA T, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine (Phila Pa 1976). 2002;27(20):2212‐2219. [DOI] [PubMed] [Google Scholar]
- 77. Gorth DJ, Shapiro IM, Risbud MV. Discovery of the drivers of inflammation induced chronic low back pain: from bacteria to diabetes. Discov Med. 2015;20(110):177‐184. [PMC free article] [PubMed] [Google Scholar]
- 78. Robinson D, Mirovsky Y, Halperin N, Evron Z, Nevo Z. Changes in proteoglycans of intervertebral disc in diabetic patients: a possible cause of increased back pain. Spine (Phila Pa 1976). 1998;23(8):849‐855. [DOI] [PubMed] [Google Scholar]
- 79. Samartzis D, Karppinen J, Chan D, Luk KDK, Cheung KMC. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population‐based study. Arthritis Rheum. 2012;64(5):1488‐1496. [DOI] [PubMed] [Google Scholar]
- 80. Battie MC, Videman T, Gill K, Moneta GB, Nyman R, Kaprio J. 1991 Volvo award in clinical sciences: smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976). 1991;16(9):1015‐1021. [PubMed] [Google Scholar]
- 81. Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham study. Ann Intern Med. 1988;109(1):18‐24. [DOI] [PubMed] [Google Scholar]
- 82. Berenbaum F. Diabetes‐induced osteoarthritis: from a new paradigm to a new phenotype. Postgrad Med J. 2012;88(1038):240‐242. [DOI] [PubMed] [Google Scholar]
- 83. Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta‐analysis. Osteoarthritis Cartilage. 2010;18:24‐33. [DOI] [PubMed] [Google Scholar]
- 84. Urquhart DM, Zheng Y, Cheng AC, et al. Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med. 2015;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rollason J, McDowell A, Albert HB, et al. Genotypic and antimicrobial characterisation of propionibacterium acnes isolates from surgically excised lumbar disc herniations. Biomed Res Int. 2013;2013:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44‐46. [PMC free article] [PubMed] [Google Scholar]
- 87. Goldenberg DL, Cohen AS. Acute infectious arthritis. A review of patients with nongonococcal joint infections (with emphasis on therapy and prognosis). Am J Med. 1976;60(3):369‐377. [DOI] [PubMed] [Google Scholar]
- 88. Zeidler H, Hudson AP. Causality of chlamydiae in arthritis and spondyloarthritis: a plea for increased translational research. Curr Rheumatol Rep. 2016;18(2):9. [DOI] [PubMed] [Google Scholar]
- 89. Zeidler H, Hudson AP. Coinfection of chlamydiae and other bacteria in reactive arthritis and spondyloarthritis: need for future research. Microorganisms. 2016;4(3):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Paul C, Schoorl T, Zuiderbaan H, et al. Dynamic and static overloading induce early degenerative processes in caprine lumbar intervertebral discs. PLoS One. 2013;8(4):e62411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine (Phila Pa 1976). 2000;25(12):1477‐1483. [DOI] [PubMed] [Google Scholar]
- 92. Baliunas AJ, Hurwitz DE, Ryals AB, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(7):573‐579. [DOI] [PubMed] [Google Scholar]
- 93. Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43(9):1916‐1926. [DOI] [PubMed] [Google Scholar]
- 94. Varady NH, Grodzinsky AJ. Osteoarthritis year in review 2015: mechanics. Osteoarthritis Cartilage. 2016;24(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. MacLean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22(6):1193‐1200. [DOI] [PubMed] [Google Scholar]
- 96. Sowa GA, Coelho JP, Vo NV, Pacek C, Westrick E, Kang JD. Cells from degenerative intervertebral discs demonstrate unfavorable responses to mechanical and inflammatory stimuli. Am J Phys Med Rehabil. 2012;91(10):846‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976). 2007;32(25):2812‐2819. [DOI] [PubMed] [Google Scholar]
- 98. Likhitpanichkul M, Torre OM, Gruen J, Walter BA, Hecht AC, Iatridis JC. Do mechanical strain and TNF‐α interact to amplify pro‐inflammatory cytokine production in human annulus fibrosus cells? J Biomech. 2016;49(7):1214‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Carter TE, Taylor KA, Spritzer CE, et al. In vivo cartilage strain increases following medial meniscal tear and correlates with synovial fluid matrix metalloproteinase activity. J Biomech. 2015;48(8):1461‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Naito K, Takahashi M, Kushida K, et al. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases‐1 (TIMP‐1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis. Rheumatology. 1999;38(6):510‐515. [DOI] [PubMed] [Google Scholar]
- 101. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Solovieva S, Kouhia S, Leino‐Arjas P, et al. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology. 2004;15(5):626‐633. [DOI] [PubMed] [Google Scholar]
- 103. Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Spontaneous production of monocyte chemoattractant protein‐1 and interleukin‐8 by the human lumbar intervertebral disc. Spine (Phila Pa 1976). 2002;27(13):1402‐1407. [DOI] [PubMed] [Google Scholar]
- 104. Hamamoto H, Miyamoto H, Doita M, Takada T, Nishida K, Kurosaka M. Capability of nondegenerated and degenerated discs in producing inflammatory agents with or without macrophage interaction. Spine (Phila Pa 1976). 2012;37(3):161‐167. [DOI] [PubMed] [Google Scholar]
- 105. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin‐1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732‐R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kepler CK, Markova DZ, Dibra F, et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL‐1β in painful human intervertebral discs. Spine (Phila Pa 1976). 2013;38(11):873‐880. [DOI] [PubMed] [Google Scholar]
- 107. Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF‐alfa and IL‐1beta promote a disintegrin‐like and metalloprotease with thrombospondin type I motif‐5‐mediated aggrecan degradation through syndecan‐4 in intervertebral disc. J Biol Chem. 2011;286(46):39738‐39749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 109. Poole AR, Alini M, Hollander AP. Cellular biology of cartilage degradation Mechanisms and Models in Rheumatoid Arthritis. Amsterdam, The Netherlands: Elsevier; 1995:163‐204. [Google Scholar]
- 110. Palmer DG, Selvendran Y, Allen C, Revell PA, Hogg N. Features of synovial membrane identified with monoclonal antibodies. Clin Exp Immunol. 1985;59(3):529‐538. [PMC free article] [PubMed] [Google Scholar]
- 111. Mort JS, Dodge GR, Roughley PJ, et al. Direct evidence for active metalloproteinases mediating matrix degradation in interleukin 1‐stimulated human articular cartilage. Matrix. 1993;13(2):95‐102. [DOI] [PubMed] [Google Scholar]
- 112. Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 113. Valdes AM, Hassett G, Hart DJ, Spector TD. Radiographic progression of lumbar spine disc degeneration is influenced by variation at inflammatory genes: a candidate SNP association study in the Chingford cohort. Spine (Phila Pa 1976). 2005;30(21):2445‐2451. [DOI] [PubMed] [Google Scholar]
- 114. Bigg HF, Rowan AD. The inhibition of metalloproteinases as a therapeutic target in rheumatoid arthritis and osteoarthritis. Curr Opin Pharmacol. 2001;1(3):314‐320. [DOI] [PubMed] [Google Scholar]
- 115. Rastogi A, Kim H, Twomey JD, Hsieh AH. MMP‐2 mediates local degradation and remodeling of collagen by annulus fibrosus cells of the intervertebral disc. Arthritis Res Ther. 2013;15(2):R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase. Spine (Phila Pa 1976). 2000;25(23):3005‐3013. [DOI] [PubMed] [Google Scholar]
- 117. Billinghurst RC, Dahlberg L, Ionescu M, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Neuhold LA, Killar L, Zhao W, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase‐3 (MMP‐13) induces osteoarthritis in mice. J Clin Invest. 2001;107(1):35‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Loeser RF. Osteoarthritis year in review 2013: biology. Osteoarthritis Cartilage. 2013;21(10):1436‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004;29(23):2691‐2699. [DOI] [PubMed] [Google Scholar]
- 121. Emanuel KS, Vergroesen P‐PA, Peeters M, Holewijn RM, Kingma I, Smit TH. Poroelastic behaviour of the degenerating human intervertebral disc: a ten‐day study in a loaded disc culture system. Eur Cell Mater. 2015;29:330‐341. [DOI] [PubMed] [Google Scholar]
- 122. Grenier S, Bhargava MM, Torzilli PA. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J Biomech. 2014;47(3):645‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Smith RL, Trindade MCD, Ikenoue T, et al. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37(1–2):95‐107. [PubMed] [Google Scholar]
- 124. Moo EK, Han SK, Federico S, et al. Extracellular matrix integrity affects the mechanical behaviour of in‐situ chondrocytes under compression. J Biomech. 2014;47(5):1004‐1013. [DOI] [PubMed] [Google Scholar]
- 125. Rodrigues‐Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell‐based tissue regeneration therapies for disc degeneration. Eur Spine J. 2014;23(9):1803‐1814. [DOI] [PubMed] [Google Scholar]
- 126. Wang W‐J, Yu X‐H, Wang C, et al. MMPs and ADAMTSs in intervertebral disc degeneration. Clin Chim Acta. 2015;448:238‐246. [DOI] [PubMed] [Google Scholar]
- 127. Mobasheri A, Bay‐Jensen A‐C, van Spil WE, Larkin J, Levesque MC. Osteoarthritis year in review 2016: biomarkers (biochemical markers). Osteoarthritis Cartilage. 2017;25(2):199‐208. [DOI] [PubMed] [Google Scholar]
- 128. Wu CW, Morrell MR, Heinze E, et al. Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores. Semin Arthritis Rheum. 2005;35(3):197‐201. [DOI] [PubMed] [Google Scholar]
- 129. Kellgren J, Lawrence J. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16(4):494‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pye SR, Reid DM, Lunt M, Adams JE, Silman AJ, O'Neill TW. Lumbar disc degeneration: association between osteophytes, end‐plate sclerosis and disc space narrowing. Ann Rheum Dis [Internet]. 2007;66(3):330‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Määttä JH, MacGregor A, Karppinen J, Williams FMK. The relationship between Modic changes and intervertebral disc degeneration. BMC Musculoskelet Disord. 2016;17(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bijkerk C, Houwing‐Duistermaat JJ, Valkenburg HA, et al. Heritabilities of radiologic osteoarthritis in peripheral joints and of disc degeneration of the spine. Arthritis Rheum. 1999;42(8):1729‐1735. [DOI] [PubMed] [Google Scholar]
- 133. Loughlin J. Knee osteoarthritis, lumbar‐disc degeneration and developmental dysplasia of the hip—an emerging genetic overlap. Arthritis Res Ther. 2011;13(2):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ikegawa S. The genetics of common degenerative skeletal disorders: osteoarthritis and degenerative disc disease. Annu Rev Genomics Hum Genet. 2013;14(1):245‐256. [DOI] [PubMed] [Google Scholar]
- 135. Chan D, Song Y, Sham P, Cheung KMC. Genetics of disc degeneration. Eur Spine J. 2006;15(Suppl 3):S317‐S325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Videman T, Leppävuori J, Kaprio J, et al. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine (Phila Pa 1976). 1998;23(23):2477‐2485. [DOI] [PubMed] [Google Scholar]
- 137. Zhang YH, Zhao CQ, Jiang LS, Chen XD, Dai LY. Modic changes: a systematic review of the literature. Eur Spine J. 2008;17(10):1289‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. van Leeuwen DM, van de Bunt F, de Ruiter CJ, van Schoor NM, Deeg DJH, Emanuel KS. Functioning without cartilage: older people with radiographic knee osteoarthritis who self‐report no functional limitations do score lower on a performance battery. J Aging Phys Act. 2017;25(4):1‐19. [DOI] [PubMed] [Google Scholar]
- 139. Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta‐analysis. AJNR Am J Neuroradiol. 2015;36(12):2394‐2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kuijer PPFM, De Beer MJPM, Houdijk JHP, Frings‐Dresen MHW. Beneficial and limiting factors affecting return to work after total knee and hip arthroplasty: a systematic review. J Occup Rehabil. 2009;19(4):375‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kennedy DJ, Fredericson M. Introduction. PM R. 2012;4(5 Suppl):S1‐S2. [DOI] [PubMed] [Google Scholar]
- 143. Hartvigsen J, Hancock MJ, Kongsted A, et al. Series low back pain 1 what low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356‐2367. [DOI] [PubMed] [Google Scholar]
- 144. Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, SC A. Annals of internal medicine effectiveness of manual physical therapy and exercise in osteoarthritis of the knee a randomized, controlled trial. Ann Intern Med. 2000;132(3):173‐181. [DOI] [PubMed] [Google Scholar]
- 145. Da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non‐steroidal anti‐inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta‐analysis. Lancet. 2016;387(10033):2093‐2105. [DOI] [PubMed] [Google Scholar]
- 146. Michael JW‐P, Schlüter‐Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Meneses SRF, Goode AP, Nelson AE, et al. Clinical algorithms to aid osteoarthritis guideline dissemination. Osteoarthritis Cartilage. 2016;24(9):1487‐1499. [DOI] [PubMed] [Google Scholar]
- 148. Wright R, Sledge C, Poss R. Patient‐reported outcome and survivorship after Kinemax total knee arthroplasty. J Bone Joint Surg Am. 2004;86‐A(11):2464‐2470. [DOI] [PubMed] [Google Scholar]
- 149. Shein D, Shue J, Girardi F. Evaluation of aesculap implant systems activl artificial disc for the treatment of degenerative disc disease. Expert Rev Med Devices. 2016;13(12):1069‐1072. [DOI] [PubMed] [Google Scholar]
- 150. Lackey A, Phan K, Mobbs R. A systematic review and meta‐analysis of outcomes in hybrid constructs for multi‐level lumbar degenerative disc disease. J Clin Neurosci. 2016;34:23‐29. [DOI] [PubMed] [Google Scholar]
- 151. Yajun W, Yue Z, Xiuxin H, Cui C. A meta‐analysis of artificial total disc replacement versus fusion for lumbar degenerative disc disease. Eur Spine J. 2010;19(8):1250‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chan SCW, Gantenbein‐Ritter B. Intervertebral disc regeneration or repair with biomaterials and stem cell therapy—feasible or fiction. Swiss Med Wkly. 2012;142:w13598. [DOI] [PubMed] [Google Scholar]
- 153. Benazzo F, Perticarini L, Padolino A, et al. A multi‐centre, open label, long‐term follow‐up study to evaluate the benefits of a new viscoelastic hydrogel (Hymovis® ) in the treatment of knee osteoarthritis. Eur Rev Med Pharmal Sci. 2016;20:959‐968. [PubMed] [Google Scholar]
- 154. Freitag J, Bates D, Boyd R, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy—a review. BMC Musculoskelet Disord. 2016;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wiegant K, Van Roermund PM, Intema F, et al. Sustained clinical and structural benefit after joint distraction in the treatment of severe knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(11):1660‐1667. [DOI] [PubMed] [Google Scholar]
- 156. Van Valburg AA, Van Roermund PM, Marijnissen ACA, et al. Joint distraction in treatment of osteoarthritis (II): effects on cartilage in a canine model. Osteoarthritis Cartilage. 2000;8(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 157. Blaney Davidson EN, van Caam APM, van der Kraan PM. Osteoarthritis year in review 2016: biology. Osteoarthritis Cartilage. 2017;25(2):175‐180. [DOI] [PubMed] [Google Scholar]
- 158. Wallace IJ, Worthington S, Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid‐20th century. Proc Natl Acad Sci U S A. 2017;114(35):201703856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Thijssen E, Van Caam A, Van Der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity‐induced osteoarthritis. Rheumatology (Oxford). 2015;54(4):588‐600. [DOI] [PubMed] [Google Scholar]
- 160. Koonce RC, Bravman JT. Obesity and osteoarthritis: more than just wear and tear. J Am Acad Orthop Surg. 2013;21(3):161‐169. [DOI] [PubMed] [Google Scholar]
- 161. Jackson JE. Stigma, liminality, and chronic pain: mind‐body borderlands. Am Ethnol. 2005;32(3):332‐353. [Google Scholar]
- 162. Slade SC, Molloy E, Keating JL. Stigma experienced by people with nonspecific chronic low back pain: a qualitative study. Pain Med. 2009;10(1):143‐154. [DOI] [PubMed] [Google Scholar]


