Abstract
Intervertebral disc (IVD) degeneration is a major contributing factor to chronic low back pain and disability, leading to imbalance between anabolic and catabolic processes, altered extracellular matrix composition, loss of tissue hydration, inflammation, and impaired mechanical functionality. Current treatments aim to manage symptoms rather than treat underlying pathology. Therefore, IVD degeneration is a target for regenerative medicine strategies. Research has focused on understanding the molecular process of degeneration and the identification of various factors that may have the ability to halt and even reverse the degenerative process. One such family of growth factors, the growth differentiation factor (GDF) family, have shown particular promise for disc regeneration in in vitro and in vivo models of IVD degeneration. This review outlines our current understanding of IVD degeneration, and in this context, aims to discuss recent advancements in the use of GDF family members as anabolic factors for disc regeneration. An increasing body of evidence indicates that GDF family members are central to IVD homeostatic processes and are able to upregulate healthy nucleus pulposus cell marker genes in degenerative cells, induce mesenchymal stem cells to differentiate into nucleus pulposus cells and even act as chemotactic signals mobilizing resident cell populations during disc injury repair. The understanding of GDF signaling and its interplay with inflammatory and catabolic processes may be critical for the future development of effective IVD regeneration therapies.
Keywords: annulus fibrosus; bone morphogenetic protein; cartilage derived morphogenetic protein (CDMP); growth differentiation factor (GDF); intervertebral disc degeneration, nucleus pulposus; mesenchymal stem cell
1. INTRODUCTION
Low back pain places a significant socioeconomic burden on society, with ~632 million people affected globally.1 Approximately, 84% of people will experience low back pain during their lifetime, leading to associated annual expenditure of £12 billion in the United Kingdom, with similar costs reported in other developed countries (eg, $85.9 billion in the United States and €16.5‐50 billion in Germany).2, 3 This cost arises from direct medical expenses, work absences and wage compensation1, 4, 5 and surpasses that of many other causes of disability, including arthritis.6, 7 The incidence of low back pain and associated cost are rising dramatically as the current global demographic shifts toward an increasingly aged population.8
Although low back pain is multifactorial and complex in etiology, intervertebral disc (IVD) degeneration has long been identified as a major underlying cause.9, 10, 11 The IVDs are fibrocartilaginous tissues positioned between the vertebrae, contributing to about one‐third of total spinal length.12 Functionally IVDs are crucial structural components responsible for conferring mechanical strength and flexibility to the vertebral column.13, 14 IVD degeneration is thought to arise from cell driven changes to the extracellular matrix (ECM) of the central portion of the disc, the nucleus pulposus (NP), which results in mechanical failure of the NP and annulus fibrosus (AF; a collagenous tissue circumferentially enclosing the NP), progressive AF fissure formation and eventual NP herniation.15 This process is concurrent with an in‐growth of blood vessels and nociceptive nerve fibers into the inflamed disc, facilitating immune cell infiltration and increasing associated pain.16, 17 The progressive obstruction of the IVDs ability to absorb and disperse spinal loads through the motion segment (the structural unit comprising the IVD, facet joints and adjacent vertebral bodies) in degeneration is secondarily linked with facet joint arthritis, spur/osteophyte formation, and vertebral body deformation. These have been associated with degenerative spinal conditions such as spinal cord stenosis, spondylolysthesis, degenerative scoliosis, and other painful pathologies resulting from nerve compression, such as sciatica.9, 18 IVD degeneration can be exacerbated by excessive manual labour, underlying genetic factors, and the aging process.6
As a natural phenomenon of aging, some aspects of IVD degeneration may be difficult to prevent.10, 19 Indeed, the majority of adults over 30 years show some form of structural IVD degeneration without any accompanying symptoms or pain.6 This makes diagnosis and effective early intervention in cases of emerging pathogenic degeneration a priority. Current treatment options are limited and provide predominately symptomatic relief without addressing the underlying pathology. These can be broadly grouped into, first, conservative treatments, ranging from painkillers and anti‐inflammatory medication to physiotherapy, and second, surgical interventional. Surgery is utilized as a last resort, with procedures such as discectomy and spinal fusion costly to perform and resulting frequently in suboptimal healing outcomes and recurrence.
Therefore, there is great demand for a biological treatment aimed at restoring IVD homeostasis and regenerating damaged tissue. Of importance to such strategies is the restoration of both structure and function of the NP and AF tissues. To this end, biological therapies have shown promise in preclinical studies. These could include cellular and acellular therapies delivered with and without instructive biomaterials and in conjunction with bioactive molecules or growth factors (see20 for recent in‐depth review). One such family of factors, growth differentiation factors (GDFs), appear to be an exciting prospect due to their crucial role in chondrogenesis (including differentiation to NP cells, namely, discogenesis) and cartilaginous tissue homeostasis.21, 22, 23, 24 As such, the focus of this review is directed on the continuing development of regenerative strategies for IVD repair employing GDF family members and the potential therapeutic role of GDF6.
2. IVD STRUCTURE, FUNCTION AND DEGENERATION
The IVD can be described as three distinct regions—the NP, AF, and cartilaginous endplates (CEPs)—making up the largest avascular structure in the human body. The properties and functionality of the IVD are dependent on the specific microstructures of its component tissue regions, which in turn are produced by distinct cell populations.
The NP is characterized by an ECM that is rich in anionic proteoglycans (PGs), predominately aggrecan. These are arranged within an irregular type II collagen lattice and are present at a ratio of 27:1 (aggrecan: collagen).18, 25 Additional matrix components consist of small amounts of other collagen types (I, VI, IX, and XI) along with other hydrophilic aggregating PGs, such as versican, and nonaggregating small leucine‐rich PGs such as biglycan, decorin, fibromodulin, keratocan, and lumican.26, 27, 28, 29, 30 The high density of negatively charged PG molecules draws in and retains water allowing the NP to resist compressive loads.
Circumferentially, the NP is enclosed by the AF, a ligamentous structure composed of highly organized collagen fibers arranged in concentric lamellae, with superior and inferior fiber ends rigidly anchored in the CEPs.31 The AF is composed of more than ⅔ collagen and unlike the NP has a small PG component.32, 33 More than 95% of the collagen in the outer AF is type I, decreasing in an almost linear fashion to less than 5% with proximity to the NP. An opposing gradient exists for type II collagen going from the center of the NP to the outer AF.34 In successive lamellae, type I collagen fibers are obliquely oriented at angles of approximately 62 to 47 to the spinal axis, preventing IVD deformation under load.35
The CEPs physically confine the NP and AF to their anatomical boundaries and act as semipermeable barriers, supporting nutrient and fluid exchange. The anchorage of AF fibers to the CEPs superiorly and inferiorly is also critical to the integrity of the motion segment and is strengthened by fiber bundle splitting to increase force distribution.36, 37, 38, 39
During IVD degeneration, type II collagen synthesis by the NP cells is gradually replaced by type I collagen, while PG synthesis is decreased resulting in the boundaries between the NP and AF becoming less distinct. The high PG content is central to healthy NP function, and the ratio of PG‐to‐collagen is one of the defining features sets NP cells apart from chondrocytes. This is important to note for regeneration of the NP, where implantation of chondrocytes rather than NP cells may result in a cartilage‐like matrix with insufficient PG concentrations.40 Deficient water‐retention by the NP results in a decrease in disc height, which in turn leads to a loss of ability to uniformly distribute compressive forces to the AF, creating areas of high pressure.
The cells of the NP are highly specialized, now recognized through detailed transcription profiling and murine cell tracing studies to be developmentally and phenotypically distinct from chondrocytes.41, 42, 43, 44, 45, 46, 47, 48, 49, 50 The Spine Research Interest Group of the Orthopedic Research Society recently defined the NP phenotype to include stabilized expression of hypoxia inducible factor HIF‐1α, the glucose transporter glut‐1, the PG aggrecan (ACAN), type II collagen (COL2A), the signaling factor sonic hedgehog (SHH), the transcription factor Brachyury [T], the keratins KRT18, KRT19, the carbonic anhydrase CA12, and CD24.42
Degenerative NP and AF produce numerous proinflammatory factors including interleukins (IL) −1α, −1β, −2, −4, −6, −8, −10, interferon‐γ, tumor necrosis factor alpha (TNF‐α) and prostaglandin E2. 51, 52, 53, 54, 55, 56, 57, 58 Later, chemokine secretion drives specific recruitment and activation of immune cells to the degenerative IVD (eg, CCL5—macrophage/eosinophil recruitment, CCL2—monocyte recruitment, CCL3 and CCL4—macrophage recruitment.51, 59). Recent work has also implicated mast cell infiltration and proinflammatory action in a similar way as has been observed in other chronic degenerative diseases such as osteoarthritis.60, 61 Wiet et al demonstrated that mast cells were increased in painful discs and induced inflammatory catabolic responses in NP cells and CEP cells but not in AF cells. This may indicate that the degenerative process provides a means for mast cell infiltration to the NP where they actively promote degeneration, making them and this mechanism a potential therapeutic target.62
This environment elicits a range of pathological responses from NP and AF cells including dysregulation of NP‐marker genes such as ACAN and COL2A1 as well as more general processes such as autophagy, senescence, and apoptosis.6, 12, 55, 63, 64, 65 Concurrently, degenerative NP and AF cells increase the expression of matrix degrading enzymes including matrix metalloproteinases (MMPs) −1, −3, −7, −9, −10 −13 and a disintegrin and MMP with thrombospondin motifs (ADAMTS) −1, −4, −5, −9, and −15.52, 55, 65, 66, 67, 68, 69, 70, 71 This further accelerates loss of type II collagen and PG rich ECM and replacement with fibrous, type I collagen scar‐like tissue.
Targeting the cells of the IVD, in particular the NP, to halt the degenerative process and restore healthy ECM production, reverse catabolic processes and reduce inflammatory response is the focus of novel regenerative strategies that have the potential to compliment or replace conventional approaches.
3. CURRENT THERAPIES AND DEVELOPMENT OF REGENERATION STRATEGIES
Conservative treatment of degeneration, such as physiotherapy, may indirectly facilitate self‐repair of mildly degenerated IVDs.72 These therapies can have positive effects on the lives of patients but do not halt the progression of IVD degeneration and may even mask indicators of further damage requiring more rigorous intervention. When conservative treatments have failed, surgery is used as a final option. As all surgical procedures are invasive and irreversible, they are utilized in less than 2% of symptomatic patients59 and are associated with long‐term issues. Where herniation of the IVD has occurred, microdiscectomy remains the gold standard surgical treatment.73 Spinal fusion has been extensively used and shown variable success rates of 32% to 98%.74 Despite good short‐term outcomes, fusion may result in an accelerated degeneration of the IVDs adjacent to the fusion site due to adjusted load bearing in the spine.75, 76, 77 Alternatively, to take load off the AF a nucleoplasty can be performed, allowing the IVD to return to a normal size and decreasing pressure on symptomatic nerve endings by NP tissue removal. However, this technique is frequently linked to subsequent IVD instability.78
In short, the current available surgical treatment options lack the ability to interrupt and correct the degenerative cascade and inflammatory milieu of the degenerative IVD at the necessary cellular and molecular level. Limited use of biologicals in the clinic to enhance surgical procedures such as fusions serve to highlight the potential of these approaches but fall short of providing the regenerative stimulus they may be capable of. The advancement of molecular cell biology and biomaterial science has made the development of effective biologic therapies for IVD regeneration a tangible reality. As our understanding of the biology of the IVD and likewise the pathobiology of degeneration has improved, the number of proposed strategies and molecular targets has grown. These include implantation of cellular and acellular biomaterials, signaling protein‐based strategies such as growth factor delivery and gene therapy. At present, the simplest and most cost‐effective to apply clinically would be local delivery of a bioactive molecule, such as growth factors, morphogens, anticatabolic factors, small molecule inhibitors, cytokines, or chemokines. For example, platelet‐rich plasma, which represents a complex milieu of bioactive factors, has shown promising results both in vitro79 and in an in vivo rabbit model where it was injected into degenerated IVDs in gelatin hydrogel microspheres.80 However, given the potential variability and unpredictability of using complex mixtures of bioactive factors, research has focused on the identification of single biomolecules with therapeutic potential, with several having have been evaluated in animal models or clinical trials (Table 1). The most commonly investigated candidate molecules are anticatabolic/proanabolic proteins, which aim to restore a healthy balance to the IVD and, in particular, the NP. Anticatabolic factors include small molecule inhibitors of inflammatory signaling, including TNF‐α and IL‐1 receptor antagonists, which have been shown to attenuate the phenotypic changes in IVD cells associated with degeneration.93, 94 Numerous growth factors have also been investigated, including transforming growth factor (TGF)β3, insulin growth factor (IGF)‐1, and epidermal growth factor‐1.95, 96, 97, 98 Most notably, however, a range of members of the bone morphogenetic protein (BMP) family have been investigated due to their roles in skeletal tissue development and repair.
Table 1.
Biomolecules evaluated for IVD regeneration in clinical trial or preclinical animal models
| Drug/material | Product name | Development stage | Outcomes/mode of action | Reference | |||
|---|---|---|---|---|---|---|---|
| Preclinical study | Clinical trial | Available on market | |||||
| GDF‐based biological therapies | |||||||
| GDF‐5 | rhGDF5 | ✓ (Phase 1 and 2a completed) n = 40 |
|
NCT011589 Wei et al24 |
|||
| GDF‐6 | ✓ (sheep) |
|
Wei et al81 | ||||
| GDF‐6 | ✓ (rat) (rabbit) |
|
Miyazaki et al82 | ||||
| Other biological therapies | |||||||
| BMP‐7 | Osteogenic protein‐1 | ✓ (rabbit) | ✓ (Phase 1) | ✓ | An et al83 | ||
| HGF | ✓ (rat) |
|
Zou et al84 | ||||
| IL‐6R mAb | Tocilizumab; Actemra; RoActemra | ✓ (Phase 1) n = 31 | ✓ |
|
Sainoh et al85 | ||
| Link‐N | ✓ (rabbit) |
|
Mwale et al86 | ||||
| Hyaluronate hydrogel | ✓ (rabbit) |
|
Nakashima et al87 | ||||
| Chondroitin sulfate hydrogel | ✓ (rabbit) |
|
Nakashima et al87 | ||||
| Simvastatin | Zocor | ✓ (rat) | ✓ |
|
Than et al88 | ||
| Lovastatin | Mevacor | ✓ (rat) | ✓ |
|
Hu et al89 | ||
| Glucocorticoid | Hydrocortancyl (Prednisolone) | ✓ (Phase 4) n = 137 | ✓ |
|
NCT00804531 Nguyen et al90 |
||
| Celecoxib | Celebrex | ✓ (dog) | ✓ |
|
Willems et al91 | ||
| Gefitinib (EGFR inhibitor) | Gefitinib | ✓ (rat) | ✓ (case series) | ✓ |
|
Pan et al92 | |
Abbreviations: DRG, dorsal root ganglion; GDF, growth differentiation factor; IL, interleukins; IVD, intervertebral disc; NGF, Nerve growth factor; NP, nucleus pulposus; sox9, sex determining region Y‐Box9; VEGF, vascular endothelial growth factor.
Despite its role in osteogenesis, BMP2 has been shown to increase ECM production in rat, bovine, and human IVD cells in vitro without increasing osteogenic marker expression.99, 100, 101, 102 Similarly, BMP7 was shown to enhance PG synthesis and cell proliferation in human NP and AF cells,103 with others reporting similar results in rat and rabbit IVD cells.104, 105 However, in human cells, BMP7 was found to be less effective at increasing ECM secretion at similar doses than in rabbit and bovine NP and AF cells.104, 106 In a recent in vitro and organ culture model, BMP2/7 was also shown to increase glycosaminoglycan (GAG) synthesis in bovine NP cells without increasing osteogenic markers.107 However, the majority of these studies failed to investigate NP‐specific or AF‐specific marker genes, often used mixed IVD cell populations and frequently employed only single‐gene analysis to determine osteogenic response. Thus, while they demonstrate the potential of BMP‐based approaches the findings suggest that responses may be cell type specific and be influenced by species, model system, or even local microenvironment, which is further supported by contradictory evidence from in vivo studies. In a rabbit model of degeneration, both BMP2 (adenoviral vector injection) and BMP7 (100‐μg protein injection)‐based therapies demonstrated positive regenerative effects.108, 109, 110 However, a large animal study in goats comparing the efficacy of BMP2, BMP7, and BMP2/7 (1‐5 μg) conjugated to a fibrin/hyaluronic acid carrier showed no evidence of IVD regeneration.111 Similarly, in a canine study of spontaneous IVD degeneration, up to 250‐μg BMP7 was injected per IVD but no regeneration was observed.112 Such data further suggest that species, cell type, and microenvironment may be important factors in growth factor‐based, particular BMP‐based, regenerative approaches. Concerns also exist with the use of extreme supraphysiological doses of BMPs, for example, up to 12 mg BMP2, which has been linked to adverse effects including heterotopic ossification and increased risk of malignancy113, 114 meaning a more sophisticated solution combining biomaterial technology or cell‐mediated secretion may be required to enable lower doses and sustained bioactivity to be achieved.
In addition to BMPs 2 and 7, other BMP family members have been investigated, with recent data suggesting that members of the GDF family (which includes GDF5 [BMP14, cartilage derived morphogenetic protein 1 (CDMP‐1)], GDF6 [BMP‐13, CDMP‐2], and GDF7 [BMP‐12]) may be ideal molecules for IVD regeneration (Figure 1). Not only do GDFs stimulate anabolic ECM gene expression in IVD cells but have specialized and critical roles in IVD development and homeostasis.
Figure 1.
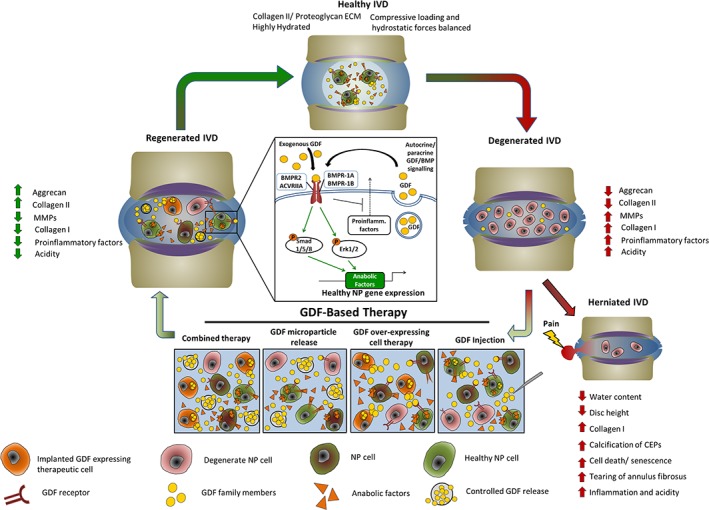
Potential GDF‐based therapeutic cycle for IVD degeneration. A, In the healthy IVD anabolic and catabolic processes are in homeostatic equilibrium. B, In the degenerate IVD cell‐mediated changes, caused by increased inflammatory signaling, shift tissue remodeling processes toward catabolism whilst concomitantly altering ECM production, increasing type I collagen and decreasing proteoglycan production. C, If this process is allowed to proceed, NP herniation can result, causing pain and loss of function. D, Therapeutic intervention involving either injection of GDF, delivery of controlled release GDF microparticles, or combined delivery of GDF with cells has shown promise for readdressing the balance between catabolic and anabolic processes in the degenerate IVD. E, With exogenous GDF delivery the expression of phenotypic and anabolic genes is increased in degenerative NP cells and/or implanted cell populations, thereby restoring disc matrix integrity and function, and reducing pain. F, Summary of GDF signaling pathway through bone morphogenetic protein receptors and smad 1/5/8 which drive anabolic gene expression and inhibit proinflammatory signaling. ECM, extracellular matrix; GDF, growth differentiation factor; IVD, intervertebral disc; MMP, matrix metalloproteinase; NP, nucleus pulposus
4. GDFs IN IVD DEVELOPMENT, HOMEOSTASIS AND POTENTIAL FOR REGENERATION
GDFs were first identified as components of bovine cartilage115 and are members of the BMP family. GDF family members share 80% to 86% amino acid homology with each other and around 50% with the wider BMP family.116, 117, 118 BMP family members have been subdivided, on the basis of similarities in protein structure and amino acid sequence, into the BMP2/4 group, BMP‐5/6/7/8 group (OP‐1 [osteogenic protein‐1] group), BMP‐9/10, and the GDF5/6/7 group. Importantly, while most BMP members such as the BMP‐2/4, BMP‐7 (OP‐1), and BMP‐9/10 show strong bone‐inductive activity, GDF5/6/7 induce the formation of cartilage and tendon‐like but not bone‐like tissue.119
As with other BMP family members, GDF signaling occurs via heteromeric transmembrane serine‐threonine kinase receptor complexes containing both type I and type II receptor molecules.120, 121 GDF5 and GDF6 act through the type I receptors bone morphogenetic protein receptor type 1A (BMPR‐1A) and ‐1B and the type II receptors bone morphogenetic protein receptor 2 (BMPR2), activin A receptor type IIA (ACVRIIA), and activin A receptor type IIB (ACVRIIB)122, 123, 124 On receptor binding, GDFs can recruit and activate SMAD1/5/8. Some BMP molecules are also able to signal through non‐Smad kinase cascades; specifically, p38 mitogen‐activated protein kinase (MAPK), Erk1/2 (extracellular related‐signal kinase), and JNK1/2 (c‐JUN N‐terminal kinase) (Figure 1). Interestingly, these pathways are also linked with inflammatory cytokine signaling and mechanical signaling through integrin activation. Recent evidence has demonstrated that GDFs are also able to activate these non‐Smad pathways, in particular Erk1/2.125 These noncanonical BMP receptor signaling pathways provide mechanisms linking the anabolic action of factors such as GDF6 and the catabolic proinflammatory signaling, which is a feature of IVD degeneration.
This potential interaction of GDFs and proinflammatory molecules hints at the presence of a natural equilibrium that is dysregulated during degeneration, though this relationship appears to be complex. In vitro, GDF6 increases ECM production in degenerate NP cells,126 while in vivo GDF6 treatment prevented ECM degradation in a sheep model.81 GDF5 has been shown to directly inhibit the expression of MMP‐13 and ADAMTS4 in human chondrocytes127 and expression of other proinflammatory markers such as TNF‐α, IL‐1β, and prostaglandinE2 (PGE2) in murine NP cells.128 Meanwhile, IL‐1β and TNF‐α were both found to downregulate GDF5 expression significantly in human AF cells in 3D culture.129 In a mouse model, plasma levels of inflammatory factors were downregulated by overexpression of human GDF6.130 This indicates an indirect role in the decrease of catabolic enzyme production linked to cellular response to proinflammatory cytokine signaling.
GDF5, 6, and 7 play crucial roles in the development of bones, limb joints, the skull, and axial skeleton66 and are expressed in developing cartilage, tendons, and ligaments, as well as in the nervous system.21, 131, 132, 133, 134 In xenopus and zebrafish embryos, GDF6 is expressed in the ectoderm and acts as an epidermal inducer and a neural inhibitor.135, 136 In skeletal tissue, GDF family members have a role independent of alkaline phosphatase expression.122, 137, 138 GDF6‐knockout mice display fusions between specific bones in the wrists and ankles, corresponding with major sites of GDF6 expression. Strikingly, in GDF5/6‐knockouts, the vertebral column shows severe lateral curvature, which develops after birth. Alcian blue staining of the IVDs in these mice shows reduced staining indicating lower PG ECM content, despite chondrocyte‐like cells being present.21, 22 These results suggest that GDF5 and GDF6 are required for normal development and maintenance of the IVD in mice, and correct ECM production by IVD cells. In humans, genomic mutations in the GDF5 gene result in various chondrogenic dysplasias, while polymorphisms in the gene are associated with osteoarthritis.139, 140, 141, 142 Similarly, mutations at the GDF6 gene locus on chromosome 8 are known to cause both familial and sporadic cases of Klippel‐Feil syndrome (KFS) leading to similar carpal, tarsal and vertebral fusions as seen in mice GDF5/6 knockouts.23 The incomplete nature of these carpal and tarsal fusions in GDF6 knockout mice and in some familial cases of KFS in humans indicates a degree of redundancy in GDF signaling. While variability in spinal fusions between regions of the spine, individuals and species may indicate that GDF6 requirements for complete vertebral segmentation may vary, particularly between different regions of the developing spine.23 A recent related report provides evidence that GDF6 is an antiaging factor secreted by young bone marrow‐derived mesenchymal stem cells (MSCs) and its expression can be regulated by miR‐17, a microRNA known to decline with age.130 This could have important implications for the progression of IVD degeneration, which is closely associated with the aging process.
During development, cellular secretion of GDFs forms directional morphogenetic gradients.143, 144, 145, 146, 147 Recently, the expression and localization of GDF6 in developing spinal column of human fetus have been examined.24 The strong expression of GDF6 is especially shown throughout cartilaginous region of vertebrae at early developing period (8‐13 weeks of gestation) and in the developing IVDs (between 8 and 19 weeks of gestation) and is diminished from ossification areas. GDF6 expression is localized to the NP and inner AF, but could not be detected in the outer AF by immunohistochemical staining.24 In adults, GDF5 and GDF6 are detectable in the inner and outer AF129 but are most strongly expressed in the NP.148 The precise role of GDF family members in adult IVD homeostasis is unresolved, though there is strong evidence of their importance for anabolic gene expression.
5. THE EFFECT OF SUPPLEMENTATION WITH GDFs ON DISC CELLS AND CHONDROCYTES IN VITRO
Aside from driving cell differentiation, GDFs have been shown to have an anabolic effect on IVD cells (Table 2), in particular on NP cells, and chondrocytes in vitro. This indicates a homeostatic or protective role in the IVD, which may be dysregulated during degenerative disc disease. In reported in vitro cultures, GDF5 and GDF6 have similar effects on cells. Recombinant GDF5 stimulation of human NP cells in culture increases aggrecan and type II collagen gene expression and PG production148 with similar, dose‐dependent results reported using mouse IVD cells,151 and bovine NP and AF cells in culture.150 In the latter study, while GDF5 upregulated PG and collagen production in both AF and NP cells, NP cells were the most responsive, especially in terms of PG production (+138% in NP, +24% in AF). In pellet culture, GDF5 was also shown to reduce the expression of catabolic enzyme MMP13 in human chondrocytes through dickkopf 1 inhibition of Wnt signaling.127
Table 2.
Effect of GDF family members on intervertebral disc cells in culture
| Cell population | GDF family member/concentration | Culture conditions | Culture duration | Outcomes | Reference |
|---|---|---|---|---|---|
| hNP | GDF5 (10 ng/mL) | 3D alginate bead culture | Up to 14 Days | i) Significantly increased GAG production. ii) Significantly increased collagen II and aggrecan production. |
Le Maitre et al148 |
| Mouse GDF5−/− NP | GDF5 (1‐100 ng/mL) | 3D alginate bead culture | Up to 9 Days | i) Dose‐dependent upregulation of collagen II and aggrecan expression. | Li et al149 |
| Bovine nucleus pulposus | GDF5 (100‐200 ng/mL) | 3D alginate bead culture | Up to 21 days | i) 200 ng/mL increased cell proliferation, collagen synthesis and GAG production. | Chujo et al150 |
| hNP | GDF6 (200‐400 ng/mL) | 3D alginate bead culture | 7 days | i) Significantly increased PG production, collagen synthesis and cell migration. 400 ng/mL most effective concentration. | Gulati et al126 |
| bAF | GDF6 (adenoviral transfection) | 2D | Up to 6 days | i) Significantly increased collagen production, PG secretion and cell proliferation. | Zhang et al74 |
| hAF | GDF6 | 3D alginate bead culture | 7 Days | i) Significantly increased PG production, collagen synthesis and cell migration. 400 ng/mL most effective concentration. | Gulati et al126 |
Abbreviations: bAF, bovine annulus fibrosus; GAG, glycosaminoglycan; hAF, human annulus fibrosus; hNP, human nucleus pulposus; PG, proteoglycan.
Through adenoviral‐mediated overexpression studies, Zhang et al compared the effects overexpressing various BMP family molecules on ECM accumulation in bovine AF cells and demonstrated GDF6 overexpression induced one of the most significant PG and collagen production responses.74 In 3D alginate bead cultures of human NP and AF cells, GDF6 supplementation increased both PG and collagen production.126 Interestingly, at high concentrations (800 ng/mL) GDF6 was also shown to act as a potent chemoattractant for NP cells with similar responses observed to potent chemoattractants, such as fetal calf serum (FCS).126, 152 This result supports evidence that GDF6 can also act as a chemoattractant to chondrocytes.153 In a recent RNA sequencing study investigating regulatory networks controlling ECM synthesis in the human, IVD, GDF6, and GDF5 were identified as important human NP (but not AF) coregulatory gene networks.154 GDF6 supplementation also induces aggrecan expression and PG production in articular chondrocytes155 and human chondrocyte cell line C28/I2.153
Taken together, these results indicate a direct anabolic signaling role for GDF5 and GDF6 in NP and AF cells. Cellular responses and RNAseq data point toward this role being greatest for correct NP cell ECM production. Although AF cells remain responsive to GDF5 and GDF6, their upregulation of PG and collagen production may be a contributory affect, which in vivo supports NP cells to create a functional ECM.
6. THE EFFECT OF GDFs ON THE DIFFERENTIATION OF MSCS TOWARD AN NP‐LIKE PHENOTYPE
Numerous growth factors have been investigated as inducers of NP‐like differentiation in MSCs including TGFβ, IGF‐1, fibroblast growth factor 2 (FGF‐2), and platelet derived growth factor (PDGF).156, 157 However, these studies focus on general chondrogenic markers rather than those markers now associated with specific NP differentiation. Both GDF5 and GDF6 have also been shown to induce the expression of general chondrogenic genes (Sex determining region Y‐Box9 (SOX9); type II collagen; ACAN) and, importantly, to also induce NP‐specific genes (KRT18, KRT19, CA12, Brachyury (T), CD24, HIF1α, Glut‐1 and SHH) in MSCs and adipose‐derived stem cells (ASCs) (Table 3). When GDF5 is added as a media supplement to high‐density cultures of MSCs or ASCs, chondrogenic differentiation is increased; however, several reports also note a significant increase in markers of hypertrophy and ossification including alkaline phosphatase, collagen types I and X, and osteopontin.151, 158, 159, 160, 161, 162, 163 These characteristics indicate a progression in expression reflective of chondrogenic hypertrophy toward endochondral ossification, which is undesirable for NP populations. However, limited evidence of a similar response to GDF5 exists in reports investigating NP cells, meaning at present the implications of this observed hypertrophy in stem cell populations for the use of GDF5 for direct application to degenerative discs are unclear and further in vitro and crucially in vivo investigation is required.
Table 3.
Effect of GDF family members on MSCs in culture
| Cell Population | GDF Family Member/ Concentration | Culture Conditions | Culture Duration | Outcomes | Ref. |
|---|---|---|---|---|---|
| hMSCs | GDF5 | Pellet | i) Significantly increased sGAG and type II collagen production. ii) Increased alkaline phosphatase, types I and X collagen and osteopontin secretion. |
Bai et al151 | |
| hMSCs | GDF5 (50‐500 ng/mL) | Pellet | 21 Days | i) Significantly upregulated collagen II. ii) Potential synergistic relationship with TGFβ1 in driving chondrogenic differentiation. iii) No effect on cell proliferation. iv) Concentration of 300 ng/mL found to be most effective. |
Coleman et al158 |
| Murine Limb bud (E10) + C3H10T1/2 | GDF5 (10‐500 ng/mL) | Pellet | Up to 4 Days | i) Significantly increased collagen II and Sox9 expression ii) Significantly increased cell proliferation (after 24 hours). iii) Increased condensation of limb bud cells at 100‐500 ng/mL, stained positively with alcian blue after 3 days culture. |
Hatakeyama et al159 |
| hMSCs | GDF5 (10‐100 ng/mL) | Woven 3D PLGA scaffolds | 12 Days | i) Significantly increased scaffold cellularity. ii) Did not increased collagen production. |
Jenner et al160 |
| hMSCs and hASCs | GDF5 (10‐1000 ng/mL) | Pellet, 3D collagen I scaffold | 14 Days | i) Significantly upregulated collagen II, sox9 and aggrecan expression. Upregulated NP‐specific marker genes (Keratins 8, 18, 19) though not as dramatically. ii) 100 ng/mL most effective. iii) ASCs more responsive than MSCs, some NP‐specific genes not upregulated (CAXII, Brachyury) |
Clarke et al161 |
| hMSCs | GDF5 (100 ng/mL) | Pellet, followed by agarose mold | 7 Days, followed by up to 28 Days cartilage formation | i) Significantly increased aggrecan expression but not collagen II or Sox9. ii) No evidence of hypertrophic marker expression. iii) Evidence of synergy with TGFβ1 to promote chondrogenic differentiation. |
Murphy et al162 |
| Rabbit ASCs | GDF5 (10‐200 ng/mL) | 2D and 3D collagen I sponge | 21 Days 2D/ 28 Days 3D | i) In 2D, 100 ng/mL induced collagen II, aggrecan and collagen I expression. 100 to 200 ng/mL most effective. ii) Collagen X expression observed after 21 days. iii) In 3D, GDF5 significantly increased collagen II and aggrecan but also collagen I and X. 3D inducti.on was more potent than 2D |
Han et al163 |
| hMSCs | GDF5 (100 ng/mL) | 3D alginate bead | Up to 18 Days | i) Significantly upregulated aggrecan, collagen II. ii) Promoted high expression of aggrecan in relation to collagen. |
Gantenbein‐Ritter et al164 |
| hMSCs and hASCs | GDF6 (10‐1000 ng/mL) | Pellet, 3D collagen I scaffold | 14 Days | i) Significantly upregulated collagen II, sox9 and aggrecan expression. Upregulated NP‐specific marker genes (Keratins 8, 18, 19, CAXII, Brachyury) in MSCs and ASCs and to greater extent than TGFβ1 GDF5. ii) 100 ng/mL most effective. iii) Increased sGAG secretion and NP‐like proteoglycan: collagen ratio. |
Clarke et al161 |
| hMSCs | GDF6 (10 ng/mL) | 3D collagen I gels, PLA constructs | Up to 14 Days | i) No evidence of collagen X or collagen II response to GDF6 stimulus. Ii) No MMP expression response. |
Heckmann et al165 |
| C3H10T1/2 | GDF6 (adenoviral transfection) | 2D | Up to 21 Days | i) Stimulated proliferation. ii) Significantly upregulated collagen II, aggrecan but not collagen X or osteocalcin. iii) 2‐fold increase in GAG secretion vs controls. |
Nochi et al166 |
Abbreviations: GDF, growth differentiation factor; hASCs, human adipose‐derived mesenchymal stem cells; hMSCs, human bone marrow‐derived mesenchymal stem cells; PLGA, poly (lactic‐co‐glycolicacid); sGAG, sulphated glycosaminoglycan; sox9, sex determining region Y‐Box9.
In comparative studies, GDF6 promotes greater expression of NP‐marker genes and stimulates greater PG production than TGFβ3 and GDF5 in both human MSCs and ASCs.161, 164 This increased ratio in PG composition of ECM in comparison to collagenous matrix is a central property of NP tissue and is required for correct functionality. Importantly, reports to date indicate no increase in collagen X production in GDF6 stimulated cultures as seen with other chondrogenic factors.165 This finding is supported further in a study by Nochi et al using adenoviral delivery of a GDF6 construct in mesenchymal progenitors (C3H10T1/2 cells). In this study, GDF6 supported chondrogenic differentiation but not terminal differentiation into hypertrophic chondrocytes.166 This lack of hypertrophy and progression toward endochondral ossification when using GDF6, coupled with the enhanced expression of NP markers, PG production and the higher aggrecan to type II collagen ratio observed in comparison to GDF5 strongly suggests that GDF6 is the most promising candidate to produce implantable NP cell phenotypes from MSCs or particularly ASCs.
7. IN VIVO EFFECTS OF GDFs ON IVD REGENERATION AND DELIVERY METHODS
In preclinical animal models of IVD degeneration treatment with GDF5 and GDF6 have shown promising results. Initial studies investigated delivery of GDF5 and GDF6 through intradiscal injection into models of IVD degeneration. For example, in murine models, IVD recovery after application of static compression was improved with a single GDF5 injection.167 After 4 weeks, a significant increase in disc height and cell number was observed in the NP and inner AF, with cells expressing both aggrecan and type II collagen. Similarly, in stab models of degeneration in mouse168 and rabbit150 delivery of GDF5 improved disc height and histological appearance. Interestingly, GDF5 was found to colocalize with proliferating cells adjacent to the epiphyseal plate in a rabbit model.169 Similarly, in an ovine model of annular injury, GDF6 was found to improve defect healing in the AF and improve the hydration and cellularity of the NP.81 Recently, Miyazaki and coworkers reported the development and use of a novel rat xenograft radiculopathy model, where rabbit NP tissue was transplanted adjacent to the dorsal root ganglia of nude rats.82 The transplanted NP tissue was obtained from annular‐puncture models of degeneration treated either with phosphate buffered saline (PBS) or GDF6 injection. GDF6 injection decreased gene expression, particularly in NP tissue, of proinflammatory factors IL‐6 and TNF‐α (with a trend for decreased IL‐1β) and pain‐associated molecules VEGF, prostaglandin‐endoperoxide synthase 2 (PTGS2), and Nerve growth factor (NGF). More notably, when GDF6‐treated NP was transplanted, transplant‐associated allodynia was significantly reduced. A corresponding decrease in the pain‐related molecules calcium‐binding adaptor molecule‐1 and the nociceptive neuropeptide calcitonin gene‐related peptide in the GDF6‐treated NP associated dorsal root ganglions (DRGs) was also observed.
One potentially interesting role for GDF6 is as a chemotactic factor for stem/progenitor cell populations adjacent or resident in the NP. This could be relevant either as a homeostatic tissue maintenance process or during repair. Other related BMP family members, such as BMP2, have been demonstrated to act as chemotactic signals for mesenchymal progenitor cells170 and as mentioned above GDF6 acts as a chemoattractant to NP cells in vitro.126 Populations of IVD progenitors have been identified in inner AF region of rabbit discs,171, 172, 173 and in both human nondegenerate scoliotic AF174 and degenerate AF tissues.175 This hypothetical mechanism is also supported by the animal studies referred to above, where cells were observed to migrate from the endplates toward the AF and NP injury site. However, the identity and characteristics of these cells, including their secretory profile, remains to be defined.
Injection of supraphysiological doses of growth factors is unlikely to be effective for the long‐term treatment of degenerative IVD disease. This is due to the short half‐life of these growth factors in vivo, while the large doses delivered raise safety concerns due to off‐target effects. Furthermore, repeated injection of the IVD may compromise its mechanical integrity and has been shown to cause an inflammatory reaction at the injection site.167 Thus, the development of alternative GDF delivery methods is desirable. These could include delivery of the GDF gene through adenoviral or plasmid vectors, delivery of siRNAs to block pathways that may downregulate GDF signaling, miRNA delivery/ inhibition or gene editing techniques such as clustered regularly inter spaced short palindromic repeats (CRISPR). Other methods might rely on the controlled delivery of GDF protein, such as microsphere encapsulation and tethering/presentation of GDF coupled with a biomaterial. These options could be used either to manipulate the endogenous cell populations or be coupled with a cellular implant, such as MSCs or ASCs (Figure 1).
In a proof of principle mouse annulus needle puncture model of IVD degeneration, Liang et al injected an adenoviral vector carrying the GDF5 gene into lumbar discs.168 The study demonstrated that the GDF5 gene was successfully expressed and active GDF5 produced, which leads to significant restoration of disc height, histology, and improved disc hydration as assessed through magnetic resonance imaging.168 Adenoviral transduction of murine mesenchymal progenitors was also shown to be feasible for the expression of human GDF6, where GDF6 induced expression of chondrogenic genes and increased PG production without expression of hypertrophic genes.166
Nonviral transfection of GDFs into NP cells or MSCs is perhaps a more attractive option through reduction of safety concerns associated with the use of viral vectors. Nonviral transfection of GDF5 into electroporated MSCs was shown to result in efficient GDF5 expression up to 21 days in culture, upregulate NP‐gene expression and partially recover the GAG/DNA ratio in a bovine IVD degeneration organ culture model.176 In principle, nonviral transfection of primary IVD cells has recently been demonstrated by May et al using the Neon Transfection System to achieve transfections efficiencies ≥47%.177
Alternatively, a controlled GDF delivery system would allow prolonged delivery of GDFs to the degenerate IVD through prevention of degradation, allow the use of lower GDF concentrations, and localize delivery. Encapsulation in polymer microspheres is a commonly used method to control release of growth factors. In a rat needle puncture disc degeneration model, GDF5 encapsulated in poly (lactic‐co‐glycolicacid) (PLGA) microspheres and delivery was sustained over 42 days, which resulted in GAG increases and restoration of disc height.178
These studies indicate an anabolic role for GDF5 and GDF6 in the IVD. Although as mentioned above, GDF5 and GDF6 are present in the degenerate IVD, it is clear that GDF effector signaling is dysregulated, resulting in decreased anabolic gene and protein expression. In these in vivo studies providing an excess of GDF6 can recover this anabolic expression, suggesting a rebalancing of an equilibrium between inflammatory catabolic signaling and GDFs. Indeed, evidence of noncanonical GDF signal transduction supports the direct interaction of these processes. A successful GDF treatment therefore may involve a GDF delivery or overexpression system in concert with an inhibitor of inflammatory response. Delivery period and dosage must be determined through in vivo experiments and perhaps can be tailored with patient specificity through more accurate diagnostic processes. Similarly, whether a cellular or acellular approach is required is dependent on patient‐specific degeneration phase. The stimulation of endogenous cell populations to either increase GDF production or delivery of GDF may only be possible if degeneration remains in the early stages. In this way, IVD regeneration would benefit greatly from a stratified approach to treatment design. Fortunately, hydrogels in combination with microparticle controlled delivery, for example, with or without cells, lend themselves to this kind of modular adaptation for treatment. GDFs, in particular GDF6 with its highly NP‐specific responses and lack of hypertrophic gene expression, are exciting prospects for IVD regeneration.
8. CONCLUSION AND FUTURE DIRECTION
Small molecule therapy, especially in combination with cell implantation, holds promise for the treatment of degenerative disc diseases. The link between ECM composition and tissue functionality in the NP means that it is critical to select the correct bioactive molecule, or combination of molecules. GDF family members have shown great promise both as direct anabolic factors when delivered to NP cells and as NP‐specific differentiation‐stimuli when delivered to MSCs or ASCs. In either case, GDF6 has been shown to increase GAG production relative to type II collagen and upregulate healthy NP‐specific marker gene expression, and critically, to do so to a greater extent than other previously defined chondrogenic factors. Future work will focus on the delivery and controlled release of GDF family members to the disc. The combination of GDF with cell therapy should also be a focus going forward, with work underway to determine optimal cell populations for therapy. In vivo data hinting at a role for GDF6 in modulation of degeneration‐associated pain and the mobilization and localisation of tissue resident stem cells during healing of artificially induced degeneration is exciting. The precise nature of these interactions should be explored in more detail but perhaps they offer a potential avenue for effective cell‐free delivery of GDF family members. Given the proinflammatory environment of the degenerative IVD and the convergence of noncanonical GDF and cytokine signaling on kinase cascades, further research is needed to determine the nature of this interaction, which will likely lead to the identification of novel therapeutic targets that may optimize GDF therapy.
CONFLICTS OF INTEREST
A.D. acts as educational consultant to Nuvasive. Nuvasive provides an unrestricted donation to University of New South Wales for spinal fellow education and research. A.D. is also a patent holder on biologic therapies for spinal disease and may benefit if ever the patents are licensed or commercialized.
ACKNOWLEDGEMENTS
Funding for T.H. is acknowledged from the Biotechnology and Biological Sciences Research Council, the Engineering and Physical Sciences Research Council, and the Medical Research Council via the UK Regenerative Medicine Platform Hubs (grant number MR/K026682/1) “Acellular Approaches for Therapeutic Delivery”, as well as the Medical Research Council via a Confidence‐in‐Concept award to The University of Manchester (MC_PC_14112 v.2). The views expressed in this publication are those of the authors and not necessarily those of the National health service (NHS), the National Institute for Health Research, or the Department of Health.
Author contribution
T.H. and B.S. drafted the manuscript. A.D., J.A.H., and S.M.R. formulated manuscript outline, reviewed, and edited draft versions including final manuscript draft. All authors have read and approved the final submitted article.
Hodgkinson T, Shen B, Diwan A, Hoyland JA, Richardson SM. Therapeutic potential of growth differentiation factors in the treatment of degenerative disc diseases. JOR Spine. 2019;2:e1045. 10.1002/jsp2.1045
Funding information BBSRC/EPSRC/MRC UK Regenerative Medicine Platform, Grant/Award Number: MR/K026682/1; Medical Research Council, Grant/Award Number: MC_PC_14112 v.2; National Institute for Health Research, Grant/Award Number: Manchester Musculoskeletal Biomedical Research Uni; Medical Research Council via a Confidence‐in‐Concept award to The University of Manchester, Grant/Award Number: MC_PC_14112 v.2; Medical Research Council via the UK Regenerative Medicine Platform Hubs, Grant/Award Number: MR/K026682/1; Engineering and Physical Sciences Research Council; Biotechnology and Biological Sciences Research Council
Contributor Information
Judith A. Hoyland, Email: judith.a.hoyland@manchester.ac.uk.
Stephen M. Richardson, Email: s.richardson@manchester.ac.uk.
REFERENCES
- 1. Walker BF. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord Tech. 2000;13:205‐217. [DOI] [PubMed] [Google Scholar]
- 2. Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95‐103. [DOI] [PubMed] [Google Scholar]
- 3. Juniper M, Le TK, Mladsi D. The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature‐based review. Expert Opin Pharmacother. 2009;10:2581‐2592. [DOI] [PubMed] [Google Scholar]
- 4. Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443‐2454. [DOI] [PubMed] [Google Scholar]
- 5. Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain: frequency, clinical evaluation, and treatment patterns from a US national survey. Spine. 1995;20:11‐19. [DOI] [PubMed] [Google Scholar]
- 6. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656‐664. [DOI] [PubMed] [Google Scholar]
- 8. Rizvi M. Novel treatment strategies for intervertebral disc degeneration. Saudi J Health Sci. 2015;4:5. [Google Scholar]
- 9. Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non‐specific low back pain. Lancet. 2012;379:482‐491. [DOI] [PubMed] [Google Scholar]
- 10. Cheung KM, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty‐three individuals. Spine. 2009;34:934‐940. [DOI] [PubMed] [Google Scholar]
- 11. An HS, Thonar EJA, Masuda K. Biological repair of intervertebral disc. Spine. 2003;28:S86‐S92. [DOI] [PubMed] [Google Scholar]
- 12. Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88:10‐14. [DOI] [PubMed] [Google Scholar]
- 13. Takatalo J, Karppinen J, Niinimäki J, et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults? Spine. 2011;36:2180‐2189. [DOI] [PubMed] [Google Scholar]
- 14. Livshits G, Popham M, Malkin I, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UKTwin Spine Study. Ann Rheum Dis. 2011;70:1740‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198‐205. [DOI] [PubMed] [Google Scholar]
- 16. Freemont A, Peacock T, Goupille P, Hoyland JA, O'Brien J, Jayson MIV. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178‐181. [DOI] [PubMed] [Google Scholar]
- 17. Hughes S, Freemont A, Hukins D, et al. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94:1298‐1304. [DOI] [PubMed] [Google Scholar]
- 18. Katz JN. Lumbar disc disorders and low‐back pain: socioeconomic factors and consequences. J Bone Joint Surg. 2006;88:21‐24. [DOI] [PubMed] [Google Scholar]
- 19. Kanayama M, Togawa D, Takahashi C, Terai T, Hashimoto T. Cross‐sectional magnetic resonance imaging study of lumbar disc degeneration in 200 healthy individuals: clinical article. J Neurosurg Spine. 2009;11:501‐507. [DOI] [PubMed] [Google Scholar]
- 20. Moriguchi Y, Alimi M, Khair T, et al. Biological treatment approaches for degenerative disk disease: a literature review of in vivo animal and clinical data. Global Spine J. 2016;6(5):497‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Settle SH, Rountree RB, Sinha A, et al. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003;254:116‐130. [DOI] [PubMed] [Google Scholar]
- 22. Mortlock DP, Guenther C, Kingsley DM. A general approach for identifying distant regulatory elements applied to the Gdf6 gene. Genome Res. 2003;13:2069‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tassabehji M, Fang ZM, Hilton EN, et al. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel‐Feil syndrome. Hum Mutat. 2008;29:1017‐1027. [DOI] [PubMed] [Google Scholar]
- 24. Wei A, Shen B, Williams LA, et al. Expression of growth differentiation factor 6 in the human developing fetal spine retreats from vertebral ossifying regions and is restricted to cartilaginous tissues. J Orthop Res. 2016;34:279‐289. [DOI] [PubMed] [Google Scholar]
- 25. Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:63‐64. [DOI] [PubMed] [Google Scholar]
- 26. Cole T‐C, Burkhardt D, Frost L, et al. The proteoglycans of the canine intervertebral disc. Biochim Biophys Acta Gen Subj. 1985;839:127‐138. [DOI] [PubMed] [Google Scholar]
- 27. McDevitt CA, Billingham ME, Muir H. In‐vivo metabolism of proteoglycans in experimental osteoarthritic and normal canine articular cartilage and the invertebral disc. Semin Arthritis Rheum. 1981;11:17‐18. [Google Scholar]
- 28. DiFabio J, Pearce R, Caterson B, et al. The heterogeneity of the non‐aggregating proteoglycans of the human intervertebral disc. Biochem J. 1987;244:27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams P, Eyre D, Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatology. 1977;16:22‐29. [DOI] [PubMed] [Google Scholar]
- 30. Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159‐171. [DOI] [PubMed] [Google Scholar]
- 31. Ludwinski FE, Gnanalingham K, Richardson SM, Hoyland JA. Understanding the native nucleus pulposus cell phenotype has important implications for intervertebral disc regeneration strategies. Regen Med. 2013;8:75‐87. [DOI] [PubMed] [Google Scholar]
- 32. Buckwalter JA, Smith KC, Kazarien LE, Rosenberg LC, Ungar R. Articular cartilage and intervertebral disc proteoglycans differ in structure: an electron microscopic study. J Orthop Res. 1989;7:146‐151. [DOI] [PubMed] [Google Scholar]
- 33. Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113‐130. [PMC free article] [PubMed] [Google Scholar]
- 34. Berthet‐Colominas C, Miller A, Herbage D, Ronziere MC, Tocchetti D. Structural studies of collagen fibres from intervertebral disc. Biochim Biophys Acta. 1982;706:50‐64. [DOI] [PubMed] [Google Scholar]
- 35. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151‐2161. [DOI] [PubMed] [Google Scholar]
- 36. Tanaka M, Nakahara S, Inoue H. A pathologic study of discs in the elderly. Separation between the cartilaginous endplate and the vertebral body. Spine (Phila Pa 1976). 1993;18:1456‐1462. [PubMed] [Google Scholar]
- 37. Rajasekaran S, Bajaj N, Tubaki V, Kanna RM, Shetty AP. ISSLS Prize winner: The anatomy of failure in lumbar disc herniation: an in vivo, multimodal, prospective study of 181 subjects. Spine (Phila Pa 1976). 2013;38:1491‐1500. [DOI] [PubMed] [Google Scholar]
- 38. Rodrigues SA, Wade KR, Thambyah A, Broom ND. Micromechanics of annulus‐end plate integration in the intervertebral disc. Spine J. 2012;12:143‐150. [DOI] [PubMed] [Google Scholar]
- 39. Rodrigues SA, Thambyah A, Broom ND. A multiscale structural investigation of the annulus‐endplate anchorage system and its mechanisms of failure. Spine J. 2015;15:405‐416. [DOI] [PubMed] [Google Scholar]
- 40. Gorensek M, Joksimovic C, Kregar‐Velikonja N, et al. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett. 2004;9:363‐374. [PubMed] [Google Scholar]
- 41. Minogue BM, Richardson SM, Zeef LA, et al. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Risbud MV, Schoepflin ZR, Mwale F, et al. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res. 2015;33:283‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rutges J, Creemers L, Dhert W, et al. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthr Cartil. 2010;18:416‐423. [DOI] [PubMed] [Google Scholar]
- 44. Gruber HE, Mougeot J‐L, Hoelscher G, Ingram JA, Hanley EN Jr. Microarray analysis of laser capture microdissected‐anulus cells from the human intervertebral disc. Spine. 2007;32:1181‐1187. [DOI] [PubMed] [Google Scholar]
- 45. Power KA, Grad S, Rutges JP, et al. Identification of cell surface–specific markers to target human nucleus pulposus cells: expression of carbonic anhydrase XII varies with age and degeneration. Arthritis Rheumatol. 2011;63:3876‐3886. [DOI] [PubMed] [Google Scholar]
- 46. Choi K‐S, Lee C, Harfe BD. Sonic hedgehog in the notochord is sufficient for patterning of the intervertebral discs. Mech Dev. 2012;129:255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McCann MR, Tamplin OJ, Rossant J, et al. Tracing notochord‐derived cells using a Noto‐cre mouse: implications for intervertebral disc development. Dis Model Mech. 2012;5:73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204:307‐314. [DOI] [PubMed] [Google Scholar]
- 49. Richardson SM, Ludwinski FE, Gnanalingham KK, Atkinson RA, Freemont AJ, Hoyland JA. Notochordal and nucleus pulposus marker expression is maintained by sub‐populations of adult human nucleus pulposus cells through aging and degeneration. Sci Rep. 2017;7:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liebscher T, Haefeli M, Wuertz K, Nerlich AG, Boos N. Age‐related variation in cell density of human lumbar intervertebral disc. Spine. 2011;36:153‐159. [DOI] [PubMed] [Google Scholar]
- 51. Johnson ZI, Schoepflin ZR, Choi H, et al. Disc in flames: roles of TNF‐α and IL‐1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin‐1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732‐R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL‐1β and TNFα expression profile. Arthritis Res Ther. 2007;9:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kepler CK, Markova DZ, Hilibrand AS, et al. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine. 2013;38:E1291‐E1299. [DOI] [PubMed] [Google Scholar]
- 55. Séguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factorα modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30:1940‐1948. [DOI] [PubMed] [Google Scholar]
- 56. Kepler CK, Markova DZ, Dibra F, et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL‐1β in painful human intervertebral discs. Spine. 2013;38:873‐880. [DOI] [PubMed] [Google Scholar]
- 57. Rand N, Reichert F, Floman Y, Rotshenker S. Murine nucleus pulposus‐derived cells secrete interleukins‐1‐β,‐6, and‐10 and granulocyte‐macrophage colony‐stimulating factor in cell culture. Spine. 1997;22:2598‐2601. [DOI] [PubMed] [Google Scholar]
- 58. Studer RK, Vo N, Sowa G, Ondeck C, Kang J. Human nucleus pulposus cells react to IL‐6: independent actions and amplification of response to IL‐1 and TNF‐α. Spine. 2011;36:593‐599. [DOI] [PubMed] [Google Scholar]
- 59. Sharifi S, Bulstra SK, Grijpma DW, Kuijer R. Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. J Tissue Eng Regen Med. 2015;9:1120‐1132. [DOI] [PubMed] [Google Scholar]
- 60. Nigrovic PA, Lee DM. Mast cells in inflammatory arthritis. Arthritis Res Ther. 2004;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakano S, Mishiro T, Takahara S, et al. Distinct expression of mast cell tryptase and protease activated receptor‐2 in synovia of rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 2007;26:1284‐1292. [DOI] [PubMed] [Google Scholar]
- 62. Wiet MG, Piscioneri A, Khan SN, Ballinger MN, Hoyland JA, Purmessur D. Mast cell‐intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in discogenic back pain. Sci Rep. 2017;7:12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shen C, Yan J, Jiang L‐S, Dai LY. Autophagy in rat annulus fibrosus cells: evidence and possible implications. Arthritis Res Ther. 2011;13:R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Le Maitre C, Pockert A, Buttle D, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652‐655. [DOI] [PubMed] [Google Scholar]
- 65. Pockert AJ, Richardson SM, Le Maitre CL, et al. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482‐491. [DOI] [PubMed] [Google Scholar]
- 66. Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47‐54. [DOI] [PubMed] [Google Scholar]
- 67. Richardson SM, Doyle P, Minogue BM, et al. Increased expression of matrix metalloproteinase‐10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gruber HE, Ingram JA, Hoelscher GL, et al. Matrix metalloproteinase 28, a novel matrix metalloproteinase, is constitutively expressed in human intervertebral disc tissue and is present in matrix of more degenerated discs. Arthritis Res Ther. 2009;11(6): R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liang Q‐Q, Cui X‐J, Xi Z‐J, et al. Prolonged upright posture induces degenerative changes in intervertebral discs of rat cervical spine. Spine. 2011;36:E14‐E19. [DOI] [PubMed] [Google Scholar]
- 70. Wang J, Markova D, Anderson DG, et al. TNF‐α and IL‐1β promote a disintegrin‐like and metalloprotease with thrombospondin type I motifs (ADAMTS)‐5 mediated aggrecan degradation through syndecan‐4 in intervertebral disc. J Biol Chem. 2011;286(46):39738‐39749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Patel KP, Sandy JD, Akeda K, et al. Aggrecanases and aggrecanase‐generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine. 2007;32:2596‐2603. [DOI] [PubMed] [Google Scholar]
- 72. Riew KD, Yin Y, Gilula L, et al. The effect of nerve‐root injections on the need for operative treatment of lumbar radicular pain. J Bone Joint Surg Am. 2000;82:1589‐1593. [DOI] [PubMed] [Google Scholar]
- 73. Postacchini F, Postacchini R. Operative Management of Lumbar Disc Herniation. In: Alexandre A., Masini M, Menchetti P, eds. Advancesin Minimally Invasive Surgery and Therapy for Spine and Nerves. Vol 108. Vienna: Springer; 2011. [Google Scholar]
- 74. Zhang Y, Anderson DG, Phillips FM, et al. Comparative effects of bone morphogenetic proteins and Sox9 overexpression on matrix accumulation by bovine anulus fibrosus cells: implications for anular repair. Spine. 2007;32:2515‐2520. [DOI] [PubMed] [Google Scholar]
- 75. Osti O, Vernon‐Roberts B, Fraser R. 1990 Volvo Award in experimental studies: Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762‐767. [DOI] [PubMed] [Google Scholar]
- 76. Lund T, Oxland TR. Adjacent level disk disease—is it really a fusion disease? Orthop Clin North Am. 2011;42:529‐541. [DOI] [PubMed] [Google Scholar]
- 77. Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:S190‐S194. [DOI] [PubMed] [Google Scholar]
- 78. Sharps LS, Isaac Z. Percutaneous disc decompression using Nucleoplasty®. Pain Physician. 2002;5:121‐126. [PubMed] [Google Scholar]
- 79. Pirvu T, Schroeder J, Peroglio M, et al. Platelet‐rich plasma induces annulus fibrosus cell proliferation and matrix production. Eur Spine J. 2014;23:745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nagae M, Ikeda T, Mikami Y, et al. Intervertebral disc regeneration using platelet‐rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13:147‐158. [DOI] [PubMed] [Google Scholar]
- 81. Wei A, Williams LA, Bhargav D, et al. BMP13 prevents the effects of annular injury in an ovine model. Int J Biol Sci. 2009;5:388‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Miyazaki S, Diwan AD, Kato K, et al. ISSLS PRIZE IN BASIC SCIENCE 2018: Growth differentiation factor‐6 attenuated pro‐inflammatory molecular changes in the rabbit anular‐puncture model and degenerated disc‐induced pain generation in the rat xenograft radiculopathy model. Eur Spine J. 2018;27(4):739‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein‐1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25‐31. [DOI] [PubMed] [Google Scholar]
- 84. Zou F, Jiang J, Lu F, et al. Efficacy of intradiscal hepatocyte growth factor injection for the treatment of intervertebral disc degeneration. Mol Med Rep. 2013;8:118‐122. [DOI] [PubMed] [Google Scholar]
- 85. Sainoh T, Orita S, Miyagi M, et al. Interleukin‐6 and interleukin‐6 receptor expression, localization, and involvement in pain‐sensing neuron activation in a mouse intervertebral disc injury model. J Orthop Res. 2015;33:1508‐1514. [DOI] [PubMed] [Google Scholar]
- 86. Mwale F, Masuda K, Pichika R, et al. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nakashima S, Matsuyama Y, Takahashi K, et al. Regeneration of intervertebral disc by the intradiscal application of cross‐linked hyaluronate hydrogel and cross‐linked chondroitin sulfate hydrogel in a rabbit model of intervertebral disc injury. Biomed Mater Eng. 2009;19:421‐429. [DOI] [PubMed] [Google Scholar]
- 88. Than KD, Rahman SU, Wang L, et al. Intradiscal injection of simvastatin results in radiologic, histologic, and genetic evidence of disc regeneration in a rat model of degenerative disc disease. Spine J. 2014;14:1017‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hu M‐H, Yang K‐C, Chen Y‐J, Sun YH, Yang SH. Lovastatin prevents discography‐associated degeneration and maintains the functional morphology of intervertebral discs. Spine J. 2014;14:2459‐2466. [DOI] [PubMed] [Google Scholar]
- 90. Nguyen C, Boutron I, Baron G, et al. Intradiscal glucocorticoid injection for patients with chronic low back pain associated with active discopathy: a randomized trial. Ann Intern Med. 2017;166:547‐556. [DOI] [PubMed] [Google Scholar]
- 91. Willems N, Yang H‐y, Langelaan ML, et al. Biocompatibility and intradiscal application of a thermoreversible celecoxib‐loaded poly‐N‐isopropylacrylamide MgFe‐layered double hydroxide hydrogel in a canine model. Arthritis Res Ther. 2015;17:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pan Z, Sun H, Xie B, et al. Therapeutic effects of gefitinib‐encapsulated thermosensitive injectable hydrogel in intervertebral disc degeneration. Biomaterials. 2018;160:56‐68. [DOI] [PubMed] [Google Scholar]
- 93. Sinclair SM, Shamji MF, Chen J, et al. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine. 2011;36:1190‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Le Maitre CL, Freemont AJ, Hoyland JA. A preliminary in vitro study into the use of IL‐1Ra gene therapy for the inhibition of intervertebral disc degeneration. Int J Exp Pathol. 2006;87:17‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17:441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang S‐z, Chang Q, Lu J, et al. Growth factors and platelet‐rich plasma: promising biological strategies for early intervertebral disc degeneration. Int Orthop. 2015;39:927‐934. [DOI] [PubMed] [Google Scholar]
- 97. Vaudreuil NJ, Vo NV, Sowa GA. Biologic treatments in intervertebral disc degeneration: protein‐based and cell‐based therapies. Oper Tech Orthop. 2016;26:189‐197. [Google Scholar]
- 98. Lv F‐J, Leung VY, Cheung KM. Cell‐based therapies for degenerative disc diseases. Oper Tech Orthop. 2016;26:182‐188. [Google Scholar]
- 99. Kim D‐J, Moon S‐H, Kim H, et al. Bone morphogenetic protein‐2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28:2679‐2684. [DOI] [PubMed] [Google Scholar]
- 100. Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein‐2 (BMP‐2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. Clin Spine Surg. 2004;17:423‐428. [DOI] [PubMed] [Google Scholar]
- 101. Kim H, Lee J‐U, Moon S‐H, et al. Zonal responsiveness of the human intervertebral disc to bone morphogenetic protein‐2. Spine. 2009;34:1834‐1838. [DOI] [PubMed] [Google Scholar]
- 102. Okada M, Kim JH, Yoon ST, et al. Pulsed electromagnetic field (PEMF) plus BMP‐2 upregulates intervertebral disc‐cell matrix synthesis more than either BMP‐2 alone or PEMF alone. Clin Spine Surg. 2013;26:E221‐E226. [DOI] [PubMed] [Google Scholar]
- 103. Imai Y, Miyamoto K, An HS, Thonar EJMA, Andersson GBJ, Masuda K. Recombinant human osteogenic protein‐1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells. Spine. 2007;32:1303‐1309. [DOI] [PubMed] [Google Scholar]
- 104. Masuda K, Takegami K, An H, et al. Recombinant osteogenic protein‐1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922‐930. [DOI] [PubMed] [Google Scholar]
- 105. Takegami K, An HS, Kumano F, et al. Osteogenic protein‐1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC–induced in vitro chemonucleolysis. Spine J. 2005;5:231‐238. [DOI] [PubMed] [Google Scholar]
- 106. Zhang Y, An HS, Song S, et al. Growth factor osteogenic protein‐1: differing effects on cells from three distinct zones in the bovine intervertebral disc. Am J Phys Med Rehabil. 2004;83:515‐521. [DOI] [PubMed] [Google Scholar]
- 107. Li Z, Lang G, Karfeld‐Sulzer LS, et al. Heterodimeric BMP‐2/7 for nucleus pulposus regeneration—In vitro and ex vivo studies. J Orthop Res. 2017;35:51‐60. [DOI] [PubMed] [Google Scholar]
- 108. Masuda K, Imai Y, Okuma M, et al. Osteogenic protein‐1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31:742‐754. [DOI] [PubMed] [Google Scholar]
- 109. Leckie SK, Bechara BP, Hartman RA, et al. Injection of AAV2‐BMP2 and AAV2‐TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12:7‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Imai Y, Okuma M, An HS, et al. Restoration of disc height loss by recombinant human osteogenic protein‐1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine. 2007;32:1197‐1205. [DOI] [PubMed] [Google Scholar]
- 111. Peeters M, Detiger SE, Karfeld‐Sulzer LS, et al. BMP‐2 and BMP‐2/7 heterodimers conjugated to a fibrin/hyaluronic acid hydrogel in a large animal model of mild intervertebral disc degeneration. BioRes Open Access. 2015;4:398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Willems N, Bach FC, Plomp SG, et al. Intradiscal application of rhBMP‐7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res Ther. 2015;17:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off‐label PLIF/TLIF use of bone morphogenetic protein‐2 (BMP‐2). Spine J. 2008;8:1011‐1018. [DOI] [PubMed] [Google Scholar]
- 114. Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP‐2: a cohort controlled study. Spine J. 2011;11:511‐516. [DOI] [PubMed] [Google Scholar]
- 115. Chang SC, Hoang B, Thomas J, et al. Cartilage‐derived morphogenetic proteins. New members of the transforming growth factor‐beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227‐28234. [PubMed] [Google Scholar]
- 116. Reddi AH. Cartilage morphogenesis: role of bone and cartilage morphogenetic proteins, homeobox genes and extracellular matrix. Matrix Biol. 1995;14:599‐606. [DOI] [PubMed] [Google Scholar]
- 117. Wozney JM. The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev. 1992;32:160‐167. [DOI] [PubMed] [Google Scholar]
- 118. Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998;346:26‐37. [PubMed] [Google Scholar]
- 119. Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003;85:1544‐1552. [DOI] [PubMed] [Google Scholar]
- 120. Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231‐250. [PubMed] [Google Scholar]
- 121. Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35‐51. [DOI] [PubMed] [Google Scholar]
- 122. Mazerbourg S, Sangkuhl K, Luo C‐W, Sudo S, Klein C, Hsueh AJW. Identification of receptors and signaling pathways for orphan bone morphogenetic protein/growth differentiation factor ligands based on genomic analyses. J Biol Chem. 2005;280:32122‐32132. [DOI] [PubMed] [Google Scholar]
- 123. Erlacher L, Mccartney J, Piek E, et al. Cartilage‐derived morphogenetic proteins and osteogenic protein‐1 differentially regulate osteogenesis. J Bone Miner Res. 1998;13:383‐392. [DOI] [PubMed] [Google Scholar]
- 124. Kawakami Y, Ishikawa T, Shimabara M, et al. BMP signaling during bone pattern determination in the developing limb. Development. 1996;122:3557‐3566. [DOI] [PubMed] [Google Scholar]
- 125. Daniels J, Binch AA, Le Maitre CL. Inhibiting IL‐1 signaling pathways to inhibit catabolic processes in disc degeneration. J Orthop Res. 2017;35:74‐85. [DOI] [PubMed] [Google Scholar]
- 126. Gulati T, Chung SA, Wei AQ, Diwan AD. Localization of bone morphogenetic protein 13 in human intervertebral disc and its molecular and functional effects in vitro in 3D culture. J Orthop Res. 2015;33:1769‐1775. [DOI] [PubMed] [Google Scholar]
- 127. Enochson L, Stenberg J, Brittberg M, Lindahl A. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthritis Cartilage. 2014;22:566‐577. [DOI] [PubMed] [Google Scholar]
- 128. Shen L, Wu Y, Han L, Zhang H. Overexpression of growth and differentiation factor‐5 inhibits inflammatory factors released by intervertebral disc cells. Exp Ther Med. 2018;15:3603‐3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN Jr. Growth and differentiation factor‐5 (GDF‐5) in the human intervertebral annulus cells and its modulation by IL‐1ß and TNF‐α in vitro. Exp Mol Pathol. 2014;96:225‐229. [DOI] [PubMed] [Google Scholar]
- 130. Hisamatsu D, Ohno‐Oishi M, Nakamura S, Mabuchi Y, Naka‐Kaneda H. Growth differentiation factor 6 derived from mesenchymal stem/stromal cells reduces age‐related functional deterioration in multiple tissues. Aging. 2016;8:1259‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12:3394‐3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lo L, Dormand EL, Anderson DJ. Late‐emigrating neural crest cells in the roof plate are restricted to a sensory fate by GDF7. Proc Natl Acad Sci U S A. 2005;102:7192‐7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF‐beta gene family. J Clin Investig. 1997;100:321‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chuen FS, Chuk CY, Ping WY, Nar WW, Kim HL, Ming CK. Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem. 2004;52:1151‐1157. [DOI] [PubMed] [Google Scholar]
- 135. Rissi M, Wittbrodt J, Délot E, Naegeli M, Rosa FM. Zebrafish Radar: a new member of the TGF‐β superfamily defines dorsal regions of the neural plate and the embryonic retina. Mech Dev. 1995;49:223‐234. [DOI] [PubMed] [Google Scholar]
- 136. Chang C, Hemmati‐Brivanlou A. Xenopus GDF6, a new antagonist of noggin and a partner of BMPs. Development. 1999;126:3347‐3357. [DOI] [PubMed] [Google Scholar]
- 137. Inada M, Katagiri T, Akiyama S, et al. Bone morphogenetic protein‐12 and‐13 inhibit terminal differentiation of myoblasts, but do not induce their differentiation into osteoblasts. Biochem Biophys Res Commun. 1996;222:317‐322. [DOI] [PubMed] [Google Scholar]
- 138. Kang Q, Sun MH, Cheng H, et al. Characterization of the distinct orthotopic bone‐forming activity of 14 BMPs using recombinant adenovirus‐mediated gene delivery. Gene Ther. 2004;11:1312‐1320. [DOI] [PubMed] [Google Scholar]
- 139. Liu J, Cai W, Zhang H, He C, Deng L. Rs143383 in the growth differentiation factor 5 (GDF5) gene significantly associated with osteoarthritis (OA)‐a comprehensive meta‐analysis. Int J Med Sci. 2013;10:312‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Miyamoto Y, Mabuchi A, Shi D, et al. A functional polymorphism in the 5′ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet. 2007;39:529‐533. [DOI] [PubMed] [Google Scholar]
- 141. Syddall CM, Reynard LN, Young DA, Loughlin J. The identification of trans‐acting factors that regulate the expression of GDF5 via the osteoarthritis susceptibility SNP rs143383. PLoS Genet. 2013;9:e1003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Buxton P, Edwards C, Archer CW, et al. Growth/differentiation factor‐5 (GDF‐5) and skeletal development. J Bone Joint Surg Am. 2001;83:S23‐S30. [PubMed] [Google Scholar]
- 143. Cui Y, Hackenmiller R, Berg L, et al. The activity and signalling range of mature BMP‐4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes Dev. 2001;15:2797‐2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Williams LA, Bhargav D, Diwan AD. Unveiling the bmp13 enigma: redundant morphogen or crucial regulator? Int J Biol Sci. 2008;4:318‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sidi S, Goutel C, Peyrieras N, Rosa FM. Maternal induction of ventral fate by zebrafish radar. Proc Natl Acad Sci U S A. 2003;100:3315‐3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Goutel C, Kishimoto Y, Schulte‐Merker S, Rosa F. The ventralizing activity of Radar, a maternally expressed bone morphogenetic protein, reveals complex bone morphogenetic protein interactions controlling dorso‐ventral patterning in zebrafish. Mech Dev. 2000;99:15‐27. [DOI] [PubMed] [Google Scholar]
- 147. Hanel ML, Hensey C. Eye and neural defects associated with loss of GDF6. BMC Dev Biol. 2006;6:43‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Le Maitre CL, Freemont AJ, Hoyland JA. Expression of cartilage‐derived morphogenetic protein in human intervertebral discs and its effect on matrix synthesis in degenerate human nucleus pulposus cells. Arthritis Res Ther. 2009;11:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Li X, Leo BM, Beck G, Balian G, Anderson GD. Collagen and proteoglycan abnormalities in the GDF‐5‐deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine. 2004;29:2229‐2234. [DOI] [PubMed] [Google Scholar]
- 150. Chujo T, An HS, Akeda K, et al. Effects of growth differentiation factor‐5 on the intervertebral disc−in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909‐2917. [DOI] [PubMed] [Google Scholar]
- 151. Bai X, Xiao Z, Pan Y, et al. Cartilage‐derived morphogenetic protein‐1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun. 2004;325:453‐460. [DOI] [PubMed] [Google Scholar]
- 152. Haberstroh K, Enz A, Zenclussen M, et al. Human intervertebral disc‐derived cells are recruited by human serum and form nucleus pulposus‐like tissue upon stimulation with TGF‐β3 or hyaluronan in vitro. Tissue Cell. 2009;41:414‐420. [DOI] [PubMed] [Google Scholar]
- 153. Zhou FY, Wei A‐Q, Shen B, Williams L, Diwan AD. Cartilage derived morphogenetic protein‐2 induces cell migration and its chondrogenic potential in C28/I2 cells. Int J Spine Surg. 2015;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Riester SM, Lin Y, Wang W, et al. RNA sequencing identifies gene regulatory networks controlling extracellular matrix synthesis in intervertebral disk tissues. J Orthop Res. 2018;36:1356‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bobacz K, Gruber R, Soleiman A, Graninger WB, Luyten FP, Erlacher L. Cartilage‐derived morphogenetic protein‐1 and‐2 are endogenously expressed in healthy and osteoarthritic human articular chondrocytes and stimulate matrix synthesis. Osteoarthr Cartil. 2002;10:394‐401. [DOI] [PubMed] [Google Scholar]
- 156. Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus‐like phenotype in vitro: implications for cell‐based transplantation therapy. Spine. 2004;29:2627‐2632. [DOI] [PubMed] [Google Scholar]
- 157. Ehlicke F, Freimark D, Heil B, Dorresteijn A, Czermak P. Intervertebral disc regeneration: influence of growth factors on differentiation of human mesenchymal stem cells (hMSC). Int J Artif Organs. 2010;33:244‐252. [PubMed] [Google Scholar]
- 158. Coleman CM, Vaughan EE, Browe DC, Mooney E, Howard L, Barry F. Growth differentiation factor‐5 enhances in vitro mesenchymal stromal cell chondrogenesis and hypertrophy. Stem Cells Dev. 2013;22:1968‐1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hatakeyama Y, Tuan RS, Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem. 2004;91:1204‐1217. [DOI] [PubMed] [Google Scholar]
- 160. Jenner JT, Van Eijk F, Saris D, et al. Effect of transforming growth factor‐beta and growth differentiation factor‐5 on proliferation and matrix production by human bone marrow stromal cells cultured on braided poly lactic‐co‐glycolic acid scaffolds for ligament tissue engineering. Tissue Eng. 2007;13:1573‐1582. [DOI] [PubMed] [Google Scholar]
- 161. Clarke LE, McConnell JC, Sherratt MJ, et al. Growth differentiation factor 6 and transforming growth factor‐beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Murphy MK, Huey DJ, Hu JC, Athanasiou KA. TGF‐β1, GDF‐5, and BMP‐2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow‐derived stromal cells. Stem Cells. 2015;33:762‐773. [DOI] [PubMed] [Google Scholar]
- 163. Han C, Ren Y, Jia Y, Kong L, Eerdun T, Wu L. The effective mode of growth and differentiation factor‐5 in promoting the chondrogenic differentiation of adipose‐derived stromal cells. Cell Tissue Bank. 2016;17:105‐115. [DOI] [PubMed] [Google Scholar]
- 164. Gantenbein‐Ritter B, Benneker LM, Alini M, Grad S. Differential response of human bone marrow stromal cells to either TGF‐β1 or rhGDF‐5. Eur Spine J. 2011;20:962‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Heckmann L, Fiedler J, Mattes T, Dauner M, Brenner RE. Interactive effects of growth factors and three‐dimensional scaffolds on multipotent mesenchymal stromal cells. Biotechnol Appl Biochem. 2008;49:185‐194. [DOI] [PubMed] [Google Scholar]
- 166. Nochi H, Sung JH, Lou J, Adkisson HD, Maloney WJ, Hruska KA. Adenovirus mediated BMP‐13 gene transfer induces chondrogenic differentiation of murine mesenchymal progenitor cells. J Bone Miner Res. 2004;19:111‐122. [DOI] [PubMed] [Google Scholar]
- 167. Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156‐163. [DOI] [PubMed] [Google Scholar]
- 168. Liang H, Ma S‐Y, Feng G, Shen FH, Joshua Li X. Therapeutic effects of adenovirus‐mediated growth and differentiation factor‐5 in a mice disc degeneration model induced by annulus needle puncture. Spine J. 2010;10:32‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Henriksson HB, Svala E, Skioldebrand E, Lindahl A, Brisby H. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the New Zealand white rabbit. Spine. 2012;37:722‐732. [DOI] [PubMed] [Google Scholar]
- 170. Fiedler J, Röderer G, Günther KP, Brenner RE. BMP‐2, BMP‐4, and PDGF‐bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305‐312. [DOI] [PubMed] [Google Scholar]
- 171. Liu C, Guo Q, Li J, et al. Identification of rabbit annulus fibrosus‐derived stem cells. PloS One. 2014;9:e108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Jin L, Liu Q, Scott P, et al. Annulus fibrosus cell characteristics are a potential source of intervertebral disc pathogenesis. PloS One. 2014;9:e96519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976). 2009;34:2278‐2287. [DOI] [PubMed] [Google Scholar]
- 174. Feng G, Yang X, Shang H, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92:675‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976). 2007;32:2537‐2544. [DOI] [PubMed] [Google Scholar]
- 176. Bucher C, Gazdhar A, Benneker LM, et al. Nonviral gene delivery of growth and differentiation factor 5 to human mesenchymal stem cells injected into a 3D bovine intervertebral disc organ culture system. Stem Cells Int. 2013;2013:326828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. May RD, Tekari A, Frauchiger DA, Krismer A, Benneker LM, Gantenbein B. Efficient nonviral transfection of primary intervertebral disc cells by electroporation for tissue engineering application. Tissue Eng Part C Methods. 2017;23:30‐37. [DOI] [PubMed] [Google Scholar]
- 178. Yan J, Yang S, Sun H, et al. Effects of releasing recombinant human growth and differentiation factor‐5 from poly (lactic‐co‐glycolic acid) microspheres for repair of the rat degenerated intervertebral disc. J Biomater Appl. 2014;29:72‐80. [DOI] [PubMed] [Google Scholar]


