Abstract Abstract
Pristimantis is the most diverse genus of tetrapods comprising 532 described species. It contains a large number of morphologically cryptic species that are being discovered with the assistance of genetic evidence. We use molecular, morphological, bioacoustic, and environmental data to assess the phylogenetic relationships and determine the species within an Andean clade of Pristimantis, which is distributed from central Ecuador to northern Peru. We assign to this clade the name Huicundomantis and propose it as a subgenus. Our results show that Huicundomantis is composed of two large clades which we name as the P. phoxocephalus species group and the P. cryptomelas species group. Huicundomantis is composed of 28 species of which 12 have been described and 16 are new. We describe 11 of these undescribed species. The most effective characters to discriminate among species are DNA sequences, qualitative morphology, and advertisement calls. Morphometric and environmental characters are not very useful to define species limits. We clarify the identity of P. riveti and show that populations from southern Ecuador traditionally ascribed to P. riveti are a new species, P. lutzaesp. nov. We also show that P. prometeii is a junior synonym of P. hampatusami. The current diversity and geographic distribution of Huicundomantis are consistent with a model of allopatric speciation. All species have a restricted distribution range (less than 4330 km2) and are assigned to the Red List categories Data Deficient or threatened with extinction. We provide new reasons to increase conservation efforts for these species and their habitat. Taking our results into account, Pristimantis species richness in Ecuador increases from 211 to 221 species, and the number of species endemic to Ecuador from 119 to 129.
Keywords: Andes, cryptic diversity, integrative taxonomy, Neotropics, Pristimantis phoxocephalus species group, P. cryptomelas species group
Resumen Abstract
Pristimantis es el género más diverso de tetrápodos, contando con 532 especies descritas. Contiene un gran número de especies morfológicamente crípticas que están siendo descubiertas con el uso de evidencia genética. En el presente estudio usamos análisis integrativos, incluyendo información molecular, morfológica, bioacústica y ambiental para determinar el contenido de especies de un clado andino de Pristimantis que se distribuye desde el centro del Ecuador hasta el norte de Perú. Asignamos a este clado el nombre de Huicundomantis y lo proponemos con el rango de subgénero. Nuestros resultados indican que Huicundomantis está compuesto por dos grandes clados que nombramos como los grupos de especies P. phoxocephalus y P. cryptomelas. Huicundomantis contiene 28 especies de las cuales 12 están descritas y 16 son nuevas. En este estudio describimos 11 de las especies nuevas. Los caracteres más efectivos para discriminar entre especies de Huicundomantis son secuencias de ADN, morfología cualitativa y cantos de anuncio. Diferencias morfométricas y ambientales entre especies son de poca utilidad para delimitar especies. En este estudio también clarificamos la identidad de P. riveti y determinamos que poblaciones del sur del Ecuador, tradicionalmente consideradas P. riveti, corresponden a la nueva especie P. lutzaesp. nov. Además, reportamos a P. prometeii como sinónimo junior de P. hampatusami. La diversidad y distribución geográfica de Huicundomantis son consistentes con un modelo de especiación alopatrica. Todas las especies tienen un rango de distribución restringido (menos de 4330 km2) y son asignadas a las categorías de Lista Roja de Datos Insuficientes o amenazadas de extinción. Nuestros resultados son un nuevo argumento para aumentar los esfuerzos de conservación de estas especies y su hábitat. Tomando en cuenta nuestros resultados, la riqueza de especies de Pristimantis en el Ecuador aumenta de 211 a 221 especies y su número de especies endémicas, de 119 a 129.
Introduction
Pristimantis Jiménez de la Espada, 1870 is the most speciose genus of tetrapods with 532 described species (Frost 2019). It is distributed from Honduras in Central America to northern Argentina and southern Brazil, from the sea level to elevations above 4000 m a.s.l. (Frost 2019). In Ecuador, it comprises 34.3% of the anuran diversity with 204 species, of which 114 are endemic to the country (Ron et al. 2019).
Pristimantis taxonomy has been complex and labile because early studies were based almost exclusively on external morphology (e.g., Lynch and Duellman 1997; Lynch and Duellman 1980; Duellman and Lehr 2009), which was frequently insufficient to infer species limits and phylogenetic relationships. Recent studies have shown that species richness of Pristimantis is underestimated due to the existence of morphologically cryptic species (e.g., Elmer and Cannatella 2009; Padial and de la Riva 2009; Hutter and Guayasamin 2015; Ortega et al. 2015). The use of DNA sequences in combination with morphological and behavioral data has allowed objective species delimitation in a framework known as ‘integrative taxonomy’ (Will et al. 2005; Bickford et al. 2007; Padial et al. 2010). This approach has been increasingly applied to Neotropical amphibians resulting in the discovery of large numbers of cryptic species (e.g., Ron et al. 2006; Fouquet et al. 2007; Elmer and Cannatella 2009; Vieites et al. 2009; Funk et al. 2012; Caminer and Ron 2014; Ortega et al. 2015).
Unfortunately, relatively few groups of Pristimantis have been subject of systematic reviews based on genetic characters. Among the species that await such review are Pristimantis phoxocephalus (Lynch, 1979) and its close relatives Pristimantis riveti (Despax, 1911), P. spinosus (Lynch, 1979), and Pristimantis versicolor (Lynch, 1979). Pristimantis phoxocephalus was described from the Pacific versant of the Andes of central and southern Ecuador; its type locality is Pilaló in Cotopaxi Province (central Ecuador) at an elevation of 2340 m (Lynch 1979). Duellman and Wild (1993) provided two records from the Huancabamba Cordillera in northern Peru. Lynch and Duellman (1997) published a species account and hypothesized a close relationship with P. eugeniae, P. nyctophylax, and P. subsigillatus. They also suggested the inclusion of these species in the P. lacrimosus species group. Duellman and Pramuk (1999) commented on the existence of morphological differences between Ecuadorian and Peruvian populations. Duellman and Lehr (2009) provided a species account, noted additional morphological differences between Ecuadorian and Peruvian populations, and recommended the use of molecular characters to test the conspecificity of Ecuadorian and Peruvian populations. As currently defined, P. phoxocephalus has a wide distribution range, occurring in the Andean slopes from central Ecuador to northern Peru between 1800 and 3400 m a.s.l. (Duellman and Lehr 2009; Ron et al. 2019).
Pristimantis riveti was described from a single specimen collected in Ecuador, El Mirador (Despax 1911). This species name was not associated with any population until Lynch (1969) incorrectly identified populations of P. curtipes as “P. riveti” (Lynch 1979). Subsequently, Lynch (1979) provided a species account including a detailed description of morphological variation, comparisons with similar species, and natural history. The species account was based on specimens collected near Cuenca, Azuay Province, in southern Ecuador. Remarkably, Lynch characterized the distribution range of P. riveti as “both Andean Cordilleras and the connecting nudos at elevations of 2620–3420 m surrounding the Cuenca hoya in southern Ecuador”, a range that excludes the type locality. Subsequent references to the species (e.g., Almendáriz and Orcés 2004; Székely et al. 2016) relied on the morphological characterization provided by Lynch (1979) and ascribed the binomen to populations from Azuay Province and the adjacent Cañar and Loja Provinces. Heinicke et al. (2007) included a sample of “P. riveti” in their phylogeny. The sample was also from Azuay Province. Lynch and Duellman (1980) assigned P. riveti to the P. unistrigatus assemblage, and Hedges et al. (2008) later assigned it to the P. unistrigatus species group. Padial et al. (2014) left it unassigned.
Pristimantis spinosus was described by Lynch (1979) with the type locality “Sapote, Provincia Morona-Santiago, Ecuador, 2470 m”. Lynch (1979) hypothesized a close relationship with P. nigrogriseus and P. cryptomelas. Heinicke et al. (2007) included in their phylogeny a sample from Ecuador, Morona-Santiago, 10.6 km west of Plan de Milagro. Hedges et al. (2008) assigned it to the Pristimantis unistrigatus species group, and Padial et al. (2014) left it unassigned.
Pristimantis versicolor was described by Lynch (1979) from specimens collected at “15 km E Loja, 2800 m” in Loja Province, southern Ecuador. Duellman and Pramuk (1999) provided a species account and reported populations from Cordillera del Cóndor in northern Peru. Heinicke et al. (2007) included a sample of P. versicolor in their phylogeny which showed a close relationship with P. riveti and P. phoxocephalus. Lynch (1979) hypothesized a close relationship with P. cajamarcensis and P. unistrigatus. Lynch and Duellman (1980) assigned it to the P. unistrigatus assemblage, and Hedges et al. (2008) later assigned it to the P. unistrigatus species group. As with P. riveti, Padial et al. (2014) left it unassigned.
Our study aims to determine the content, identity, and phylogenetic relationships of species within P. phoxocephalus and closely related species. Our systematic review includes the description of 11 new species of which 10 belong to the P. phoxocephalus species group and one to the P. cryptomelas species group. Our taxonomic review is based on genetic, morphologic, bioacoustic, and environmental information.
Materials and methods
Species sampling
We focused our analysis in P. phoxocephalus and closely related species (Pyron and Wiens 2011; Padial et al. 2014). We expanded our study group to include morphologically similar species and species shown to be closely related to P. phoxocephalus based on an unpublished phylogeny of Pristimantis obtained by SRR as part of a large-scale review of Ecuadorian Pristimantis.
Phylogenetic analyses
For the molecular phylogenetic analyses, we used sequences of 85 individuals ascribed to the species above and added sequences of 48 individuals as the outgroup (46 Pristimantis spp., Craugastor talamancae, Epipedobates boulengeri). Our final dataset consists of sequences of 133 individuals from 68 localities. Sequences of 117 of these individuals were newly generated and obtained from tissues deposited at the genome bank of the Zoology Museum, Pontificia Universidad Católica del Ecuador (QCAZ), representing 25 described and at least 22 undescribed species of Pristimantis. The remaining sequences, representing 16 species, were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/genbank). Vouchers and GenBank accession numbers are shown in Table 1.
Table 1.
Genbank accession numbers for DNA sequences used in the phylogenetic analyses.
| Species | Voucher | 16S | ND1 | RAG1 | GenSeq Nomenclature |
|---|---|---|---|---|---|
| Craugastor talamancae | QCAZ 30675 | MK881414 | MK881414 | MK881322 | genseq-3 |
| Epipedobates boulengeri | QCAZ 42251 | MK881437 | NA | MK881337 | genseq-4 |
| P. appendiculatus | QCAZ 16365 | MK881401 | NA | MK881315 | genseq-4 |
| P. atillo sp. nov. | QCAZ 31946 | MK881419 | NA | MK881324 | genseq-2 |
| QCAZ 40584 | MK881435 | NA | MK881335 | genseq-2 | |
| QCAZ 42485 | MK881438 | NA | MK881338 | genseq-2 | |
| QCAZ 42486 | MK881439 | NA | MK881339 | genseq-2 | |
| QCAZ 42488 | MK881440 | NA | MK881340 | genseq-2 | |
| QCAZ 42489 | MK881441 | NA | MK881341 | genseq-2 | |
| QCAZ 42492 | MK881442 | NA | MK881342 | genseq-2 | |
| QCAZ 42496 | MK881443 | NA | MK881343 | genseq-2 | |
| QCAZ 42498 | MK881444 | NA | MK881344 | genseq-2 | |
| QCAZ 42499 | MK881445 | NA | MK881345 | genseq-2 | |
| QCAZ 42500 | MK881446 | NA | MK881346 | genseq-1 | |
| QCAZ 42501 | MK881447 | NA | MK881347 | genseq-2 | |
| QCAZ 42503 | MK881448 | NA | MK881348 | genseq-2 | |
| QCAZ 42505 | MK881449 | NA | MK881349 | genseq-2 | |
| QCAZ 42506 | MK881450 | NA | MK881350 | genseq-2 | |
| QCAZ 42512 | MK881451 | NA | MK881351 | genseq-2 | |
| QCAZ 42543 | MK881452 | NA | MK881352 | genseq-2 | |
| QCAZ 42548 | MK881453 | NA | MK881353 | genseq-2 | |
| P. atratus | QCAZ 45580 | MK881471 | MK881471 | MK881364 | genseq-4 |
| QCAZ 45645 | MK881473 | MK881473 | MK881366 | genseq-4 | |
| P. bicantus | QCAZ 31860 | MK881417 | NA | NA | genseq-4 |
| QCAZ 31986 | MK881420 | NA | MK881325 | genseq-4 | |
| P. cajamarcensis | KU 217845 | EF493663 | NA | NA | genseq-4 |
| QCAZ 17142 | MK881403 | MK881403 | MK881317 | genseq-4 | |
| QCAZ 24648 | MK881405 | NA | NA | genseq-4 | |
| QCAZ 31474 | MK881416 | NA | NA | genseq-4 | |
| P. ceuthospilus | CORBIDI 4306 | MK881397 | MK881397 | MK881311 | genseq-4 |
| P. chomskyi sp. nov. | QCAZ 45666 | MK881476 | MK881476 | MK881369 | genseq-2 |
| QCAZ 45669 | MK881477 | MK881477 | MK881370 | genseq-1 | |
| P. cryptomelas | QCAZ 45612 | MK881472 | MK881472 | MK881365 | genseq-4 |
| QCAZ 45660 | MK881475 | MK881475 | MK881368 | genseq-4 | |
| P. curtipes | QCAZ 17505 | MK881404 | MK881404 | MK881318 | genseq-4 |
| QCAZ 39568 | MK881429 | MK881429 | NA | genseq-4 | |
| QCAZ 39569 | MK881430 | MK881430 | NA | genseq-4 | |
| P. eremitus | QCAZ 43392 | MK881460 | NA | NA | genseq-4 |
| P. gagliardoi | QCAZ 42575 | MK881456 | NA | MK881355 | genseq-4 |
| QCAZ 46738 | MK881480 | NA | MK881372 | genseq-3 | |
| P. glandulosus | QCAZ 40153 | MK881431 | MK881431 | MK881333 | genseq-4 |
| P. gloria sp. nov. | KU 218035 | EF493348 | NA | NA | genseq-4 |
| QCAZ 16448 | MK881402 | MK881402 | MK881316 | genseq-2 | |
| QCAZ 31455 | MK881415 | NA | NA | genseq-2 | |
| QCAZ 56463 | MK881502 | NA | NA | genseq-2 | |
| QCAZ 57201 | MK881503 | NA | NA | genseq-1 | |
| P. hampatusami | QCAZ 58042 | MK881504 | MK881504 | MK881387 | genseq-3 |
| P. jimenezi sp. nov. | QCAZ 45170 | MK881466 | MK881466 | MK881360 | genseq-1 |
| QCAZ 45178 | MK881468 | MK881468 | MK881362 | genseq-2 | |
| QCAZ 46977 | MK881481 | MK881481 | MK881373 | genseq-2 | |
| QCAZ 46978 | MK881482 | MK881482 | MK881374 | genseq-2 | |
| P. kichwarum | QCAZ 25766 | JN991458 | NA | JQ025196 | genseq-4 |
| P. latidiscus | QCAZ 17101 | EF493354 | NA | EF493440 | genseq-4 |
| QCAZ 25852 | JN991451 | NA | JQ025188 | genseq-4 | |
| P. lividus | QCAZ 54322 | MK881497 | MK881497 | MK881382 | genseq-4 |
| P. lutzae sp. nov. | QCAZ 27471 | MK881410 | NA | NA | genseq-2 |
| QCAZ 27534 | MK881411 | NA | NA | genseq-2 | |
| QCAZ 27597 | MK881412 | NA | NA | genseq-2 | |
| QCAZ 32785 | MK881421 | MK881421 | MK881326 | genseq-2 | |
| QCAZ 32786 | MK881422 | MK881422 | MK881327 | genseq-2 | |
| QCAZ 32791 | MK881424 | MK881424 | MK881329 | genseq-2 | |
| QCAZ 37546 | MK881428 | NA | NA | genseq-2 | |
| QCAZ 47211 | MK881487 | NA | NA | genseq-2 | |
| QCAZ 53728 | MK881495 | NA | NA | genseq-1 | |
| P. multicolor sp. nov. | QCAZ 47213 | MK881488 | NA | NA | genseq-1 |
| QCAZ 47214 | MK881489 | NA | NA | genseq-2 | |
| P. muscosus | QCAZ 54857 | MK881501 | MK881501 | MK881386 | genseq-4 |
| P. nangaritza sp. nov. | QCAZ 41710 | MK881436 | MK881436 | MK881336 | genseq-1 |
| P. omeviridis | QCAZ 10564 | MK881398 | NA | MK881312 | genseq-4 |
| P. petrobardus | KU 212293 | EF493367 | NA | NA | genseq-2 |
| P. philipi | KU 217863 | EF493672 | NA | NA | genseq-3 |
| QCAZ 37528 | MK881425 | MK881425 | MK881330 | genseq-3 | |
| QCAZ 37537 | MK881426 | MK881426 | MK881331 | genseq-3 | |
| QCAZ 37542 | MK881427 | MK881427 | MK881332 | genseq-3 | |
| P. phoxocephalus | QCAZ 58463 | MK881507 | MK881507 | MK881390 | genseq-3 |
| P. pichincha | QCAZ 11673 | MK881399 | MK881399 | MK881313 | genseq-4 |
| P. pycnodermis | KU 218028 | EF493680 | NA | NA | genseq-4 |
| KU 218030 | EF493683 | NA | NA | genseq-4 | |
| P. quaquaversus | QCAZ 25676 | JN991463 | NA | JQ025201 | genseq-4 |
| P. rubicundus | QCAZ 26551 | MK881407 | MK881407 | MK881320 | genseq-4 |
| P. sobetes | QCAZ 11737 | MK881400 | NA | MK881314 | genseq-4 |
| P. spinosus | KU 218052 | EF493673 | NA | NA | genseq-4 |
| P. sternothylax | CORBIDI 13316 | MK881393 | MK881393 | MK881308 | genseq-4 |
| P. teslai sp. nov. | QCAZ 46213 | MK881478 | NA | NA | genseq-1 |
| P. thymelensis | QCAZ 16428 | EF493516 | NA | EF493442 | genseq-4 |
| QCAZ 62801 | MK881508 | MK881508 | MK881391 | genseq-4 | |
| QCAZ 62803 | MK881509 | MK881509 | MK881392 | genseq-4 | |
| P. tinguichaca | QCAZ 31945 | MK881418 | MK881418 | MK881323 | genseq-3 |
| QCAZ 40581 | MK881432 | NA | NA | genseq-3 | |
| QCAZ 40582 | MK881433 | NA | MK881334 | genseq-3 | |
| QCAZ 42570 | MK881454 | NA | NA | genseq-3 | |
| QCAZ 42574 | MK881455 | NA | MK881354 | genseq-3 | |
| QCAZ 42576 | MK881457 | NA | MK881356 | genseq-3 | |
| QCAZ 42578 | MK881458 | NA | MK881357 | genseq-3 | |
| QCAZ 54601 | MK881498 | NA | MK881383 | genseq-3 | |
| QCAZ 54602 | MK881499 | NA | MK881384 | genseq-3 | |
| QCAZ 54603 | MK881500 | NA | MK881385 | genseq-3 | |
| P. torresi sp. nov. | QCAZ 47342 | MK881490 | NA | NA | genseq-1 |
| QCAZ 47397 | MK881492 | MK881492 | MK881380 | genseq-2 | |
| P. totoroi sp. nov. | KU 218025 | EF493349 | NA | NA | genseq-4 |
| QCAZ 25105 | MK881406 | MK881406 | MK881319 | genseq-1 | |
| QCAZ 58425 | MK881505 | MK881505 | MK881388 | genseq-2 | |
| P. unistrigatus | KU 218057 | EF493387 | NA | EF493444 | genseq-4 |
| P. verrucolatus sp. nov. | QCAZ 45174 | MK881467 | MK881467 | MK881361 | genseq-2 |
| QCAZ 45195 | MK881469 | MK881469 | MK881363 | genseq-2 | |
| QCAZ 46982 | MK881483 | MK881483 | MK881375 | genseq-1 | |
| QCAZ 46992 | MK881484 | MK881484 | MK881376 | genseq-2 | |
| QCAZ 46993 | MK881485 | MK881485 | MK881377 | genseq-2 | |
| P. versicolor | KU 218096 | EF493389 | NA | EF493431 | genseq-4 |
| QCAZ 45650 | MK881474 | MK881474 | MK881367 | genseq-4 | |
| QCAZ 46310 | MK881479 | MK881479 | MK881371 | genseq-4 | |
| P. wiensi | KU 219796 | EF493668 | NA | NA | genseq-2 |
| P. yumbo | QCAZ 52241 | MK881494 | NA | MK881381 | genseq-3 |
| QCAZ 58450 | MK881506 | MK881506 | MK881389 | genseq-4 | |
| Pristimantis sp. (CCS1) | QCAZ 32790 | MK881423 | MK881423 | MK881328 | genseq-4 |
| Pristimantis sp. (CCS2) | QCAZ 45129 | MK881462 | MK881462 | MK881358 | genseq-4 |
| QCAZ 45155 | MK881465 | MK881465 | MK881359 | genseq-4 | |
| Pristimantis sp. (UCS1) | QCAZ 53999 | MK881496 | NA | NA | genseq-4 |
| Pristimantis sp. (UCS2) | QCAZ 45029 | MK881461 | NA | NA | genseq-4 |
| Pristimantis sp. (UCS3) | QCAZ 26642 | MK881409 | NA | NA | genseq-4 |
| Pristimantis sp. | CORBIDI 14804 | MK881394 | MK881394 | MK881309 | genseq-4 |
| CORBIDI 14805 | MK881395 | NA | NA | genseq-4 | |
| CORBIDI 2848 | MK881396 | MK881396 | MK881310 | genseq-4 | |
| QCAZ 26586 | MK881408 | NA | MK881321 | genseq-4 | |
| QCAZ 29206 | MK881413 | NA | NA | genseq-4 | |
| QCAZ 40583 | MK881434 | NA | NA | genseq-4 | |
| QCAZ 43024 | MK881459 | NA | NA | genseq-4 | |
| QCAZ 45131 | MK881463 | NA | NA | genseq-4 | |
| QCAZ 45142 | MK881464 | NA | NA | genseq-4 | |
| QCAZ 45210 | MK881470 | NA | NA | genseq-4 | |
| QCAZ 47002 | MK881486 | MK881486 | MK881378 | genseq-4 | |
| QCAZ 47353 | MK881491 | MK881491 | MK881379 | genseq-4 | |
| QCAZ 50732 | MK881493 | NA | NA | genseq-4 |
DNA was extracted from liver or muscle tissue preserved in 95% ethanol or tissue storage buffer using a guanidine thiocyanate protocol (M. Fujita, unpublished) with some modifications. Primers used for the PCR amplification of the below-mentioned genes are listed in Suppl. material 1. Amplicons were sequenced by the Macrogen Sequencing Team (Macrogen Inc., Seoul, Korea).
The phylogenetic analyses were based on a 3199 bp dataset containing DNA sequences of the mitochondrial genes 16S (1318 bp, partial sequence), tRNALeu (90 bp), NADH dehydrogenase subunit 1 ND1 (961 bp), tRNAIle (73 bp), tRNAGln (71 bp), and tRNAMet (31 bp), and the nuclear gene RAG1 (655 bp). The alignment of the sequences was performed in GeneiousPro v. 5.4.6 (GeneMatters Corp.) with the plug-in MAFFT (Katoh et al. 2002) and a posterior manual alignment with Mesquite v. 3.02 (Maddison and Maddison 2014). The aligned matrix is available at http://zenodo.org under https://doi.org/10.5281/zenodo.2555411. The best partitioning scheme and the best-fit model of molecular evolution for each partition were estimated with the software PartitionFinder v. 1.1.1 (Lanfear et al. 2012) under the greedy algorithm and the Bayesian information criterion (BIC).
Phylogenetic analyses were performed under two approaches, Bayesian Inference (BI) and Maximum-likelihood (ML). The Bayesian analysis was carried out with MrBayes v. 3.2.1 (Ronquist et al. 2012) in two independent searches for 3×107 generations; each search had four Monte Carlo Markov Chains. The temperature parameter was set at 0.5. Trees were sampled every 1000 generations. We evaluated the convergence of the chains with software Tracer v. 1.6. (Rambaut et al. 2014) by inspecting plots of parameter values vs generation and confirming stationarity in their values. We also evaluated the Effective Sample Size and considered the search finished when all values were greater than 200. We discarded 25% of the 30000 trees as burn-in. The remaining trees were combined to obtain a 50% majority-rule consensus tree as well as the branch posterior probabilities (BPP).
For the ML analysis, we used Garli v. 2.0 (Zwickl 2006). We run ten replicate searches starting from stepwise trees and 10 from random trees. We finished the search after 100000 generations without topology improvements (genthreshfortopoterm = 100000); we used default values for the remaining parameters. Non-parametric bootstrap values (NPB) were obtained with 500 pseudoreplicates with the same settings of the random search, each with two independent searches. The 50% majority rule consensus for the bootstrap trees was obtained with Mesquite v. 2.75. We used the same settings to run two additional ML analyses, one exclusively for the nuclear gene and one for the mitochondrial genes. Throughout the text, we considered that a node has “strong support” when its bootstrap value is >70 and its Bayesian posterior probability is 0.95 or higher.
In order to identify candidate species, we calculated uncorrected p genetic distances for gene 16S (~1300 bp), within and between clades, with software MEGA v.7.0 (Kumar et al. 2016). See Integrative Analyses section for details.
Morphological analyses
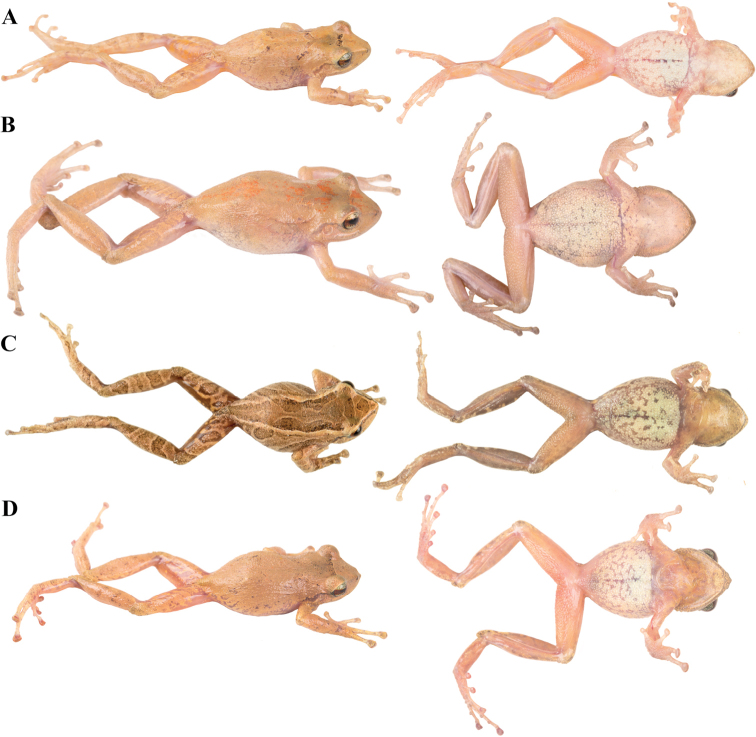
Specimens deposited at the QCAZ collection were preserved in 10% formalin and stored in 70% ethanol. Photographs of preserved specimens were taken while these were submersed in ethanol to avoid reflections. We compared qualitative and quantitative morphological characters between clades. We used only adult specimens for these analyses but, when available, juveniles were considered to describe morphological variation. We examined 750 individuals from central and southern Ecuador ranging from 230 to 4200 m a.s.l. (Figs 1, 2), from collections of the Zoology Museum, Pontificia Universidad Católica del Ecuador (QCAZ), Ecuadorian Museum of Natural Sciences (MECN), Museum of Natural History, University of Kansas, USA (KU), Gustavo Orcés Museum of Natural History, Escuela Politécnica Nacional, Ecuador (MEPN), National Museum of Natural History, Smithsonian Institution, USA (USNM), and French Museum of Natural History of Paris (MNHNP); information for each individual is detailed in Suppl. material 2.
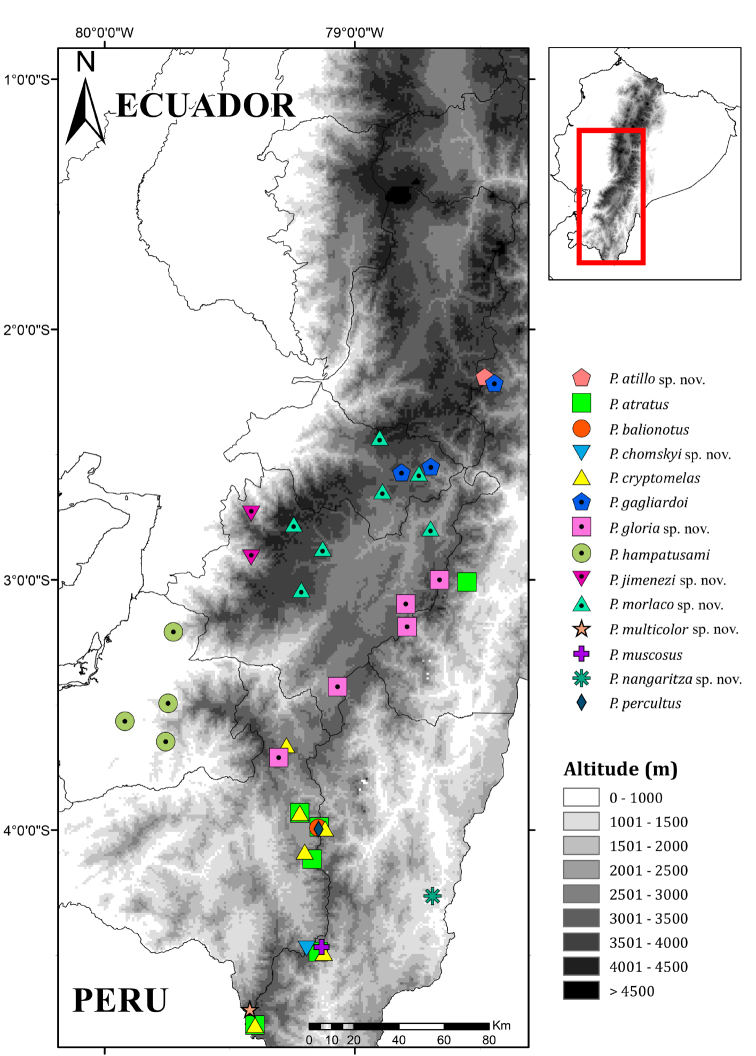
Figure 1.
Distribution of Pristimantis, subgenus Huicundomantis. Records are based on specimens deposited at the Museum of Zoology, Pontificia Universidad Católica del Ecuador (QCAZ). Locality information is shown in Suppl. material 2.
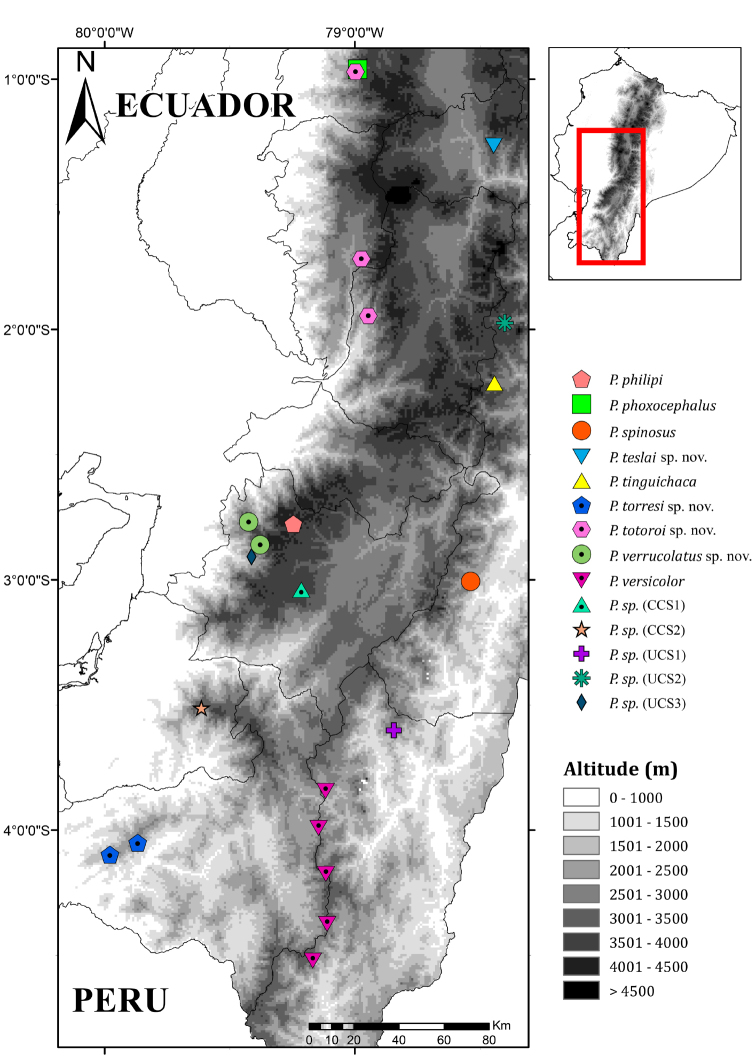
Figure 2.
Distribution of Pristimantis, subgenus Huicundomantis. Records are based on specimens deposited at the Museum of Zoology, Pontificia Universidad Católica del Ecuador (QCAZ). Candidate species numbers correspond to those of the text and Figure 3. Locality information is shown in Suppl. material 2. Abbreviations: CCS = confirmed candidate species, UCS = unconfirmed candidate species.
We determined sex and reproductive condition by looking for vocal sacs, vocal slits, and nuptial pads, and by gonadal inspection. For males, the presence of vocal sac, vocal slits, or nuptial pads was used as indicator of adulthood; when these characters were absent, we examined the gonads and categorized an individual as adult if the testes were swollen and enlarged. For females, we considered the presence of convoluted oviducts and large ovarian eggs as features of adulthood (Duellman and Lehr 2009).
Diagnosis and description of species follow Duellman and Lehr (2009) terminology. We use the term “distinct” for a character that is clearly visible, and “indistinct” for a character that is barely visible. When referring to the tympanum state, we use “prominent” to indicate that it protrudes from the skin surface; when referring to tubercles, we use “prominent” to imply they are conspicuous and elevated. Following Lynch and Duellman (1997), we qualify Toe V as slightly longer than Toe III when the distal end of Toe V does not reach the proximal edge of the distal subarticular tubercle on Toe IV when they are adpressed. Toe V is called longer than Toe III when its distal end reaches the proximal edge of the distal subarticular tubercle in Toe IV and does not extend to its distal edge. Toe V is much longer than Toe III if the distal end of Toe V reaches, or extends beyond, the distal edge of the distal subarticular tubercle on Toe IV. Abbreviations used for quantitative descriptive characters are: snout-vent length (SVL, distance from the tip snout to posterior margin of vent); head width (HW, greatest width of head); head length (HL, distance from the tip of snout to posterior angle of jaw articulation); eye diameter (ED, distance between anterior and posterior borders of eye); interorbital distance (IOD); upper eyelid width (EW); eye-nostril distance (EN); internarial distance (IND); tympanic diameter (TD, distance between external anterior and posterior margins of tympanic annulus); tympanum-eye distance (TED, distance between anterior margin of the tympanum to the closest point of the posterior margin of the eye); tibia length (TL, length of flexed leg from knee to heel); foot length (FL, distance from heel to tip of toe IV). All measurements were taken with a digital caliper with a precision of 0.01 mm.
For morphometric analyses, we measured 232 individuals of the candidate species and P. phoxocephalus and P. versicolor. We performed separate analyses for males and females. As they have been reported to be variable among Pristimantis species (Arroyo et al. 2005), we used SVL, HL, HW, TD, and ED measurements for morphometrics. To assess the degree of morphometric differentiation between candidate species, we performed a Discriminant Function Analysis (DFA) including the variables mentioned above without size correction. For pairwise comparisons, we used the Wilcoxon signed-rank test with corrected measurements. To remove the effect of co-varying body size, we made linear regressions between each variable and SVL. Statistical analyses were performed using software JMP v. 9.0.1 (SAS Institute Inc. 2010).
Bioacoustic analyses
We analyzed recordings of eight individuals belonging to four candidate species of the clade under study, namely Pristimantis jimenezi sp. nov., Pristimantis phoxocephalus, Pristimantis totoroi sp. nov, and Pristimantis verrucolatus sp. nov. (see Results).
Recordings were obtained from the QCAZ audio library and are available in BIOWEB (http://bioweb.bio/faunaweb/amphibiaweb/). Recording temperatures ranged between 8.8 and 12 °C. Recordings of P. phoxocephalus were obtained at its type locality, Pilaló, Cotopaxi Province. We recorded one collected and two non-collected individuals from the same chorus. Their calls had similar properties; however, we did not include the advertisement call of the collected individual in the analysis to avoid accounting for effects of stress on the characteristics of the call given that it was recorded from a plastic bag after the individual’s capture. Advertisement calls for P. jimenezi sp. nov. were recorded from Azuay Province at two localities: at the border of Cajas National Park (QCAZ 45178) and Zadracay River (QCAZ 46977) (see Suppl. material 2 for details on localities); both individuals were sequenced. For P. verrucolatus sp. nov., we had recordings available for two individuals (QCAZ 46984, QCAZ 46981) from Shoupshe, Azuay Province. Finally, recordings for P. totoroi sp. nov. were obtained from Bosque Protector Cashca Totoras, Bolívar Province. We recorded a non-collected and a collected individual (QCAZ 25105), which was subsequently sequenced.
We analyzed calls with the software Raven Pro v. 1.3 (Cornell Lab of Ornithology 2003–2008) using a sampling rate of 44.1 kHz and a frequency resolution of 10.8–11.2 Hz. Temporal data was measured on oscillograms and frequency data on power spectrums (e.g., Caminer and Ron 2014). We randomly chose five advertisement calls from each recording and measured: (1) number of notes per call; (2) number of harmonics; (3) note duration; (4) inter-note interval; (5) peak time of the note (time from beginning of the note to peak energy); (6) dominant frequency; (7) frequency of the second harmonic; (8) initial and (9) final frequency of the note; and (10) frequency change in each note, estimated from the difference in frequency between the beginning and end of a note. Because of the low number of individuals recorded per species, we could not apply statistical comparisons. However, each species had a highly stereotyped call, qualitatively distinct from the others.
Environmental analyses
We considered environmental differentiation as additional evidence to define species boundaries (e.g., Rissler and Apodaca 2007; Ortega et al. 2015). Accordingly, we analyzed differences in bioclimatic and vegetation-related variables between candidate species.
Values at each presence locality were extracted from raster maps using software ArcGIS 10.0 (ESRI). Following Menéndez-Guerrero and Graham (2013), we used eight bioclimatic variables with 30" resolution, downloaded from WorldClim (http://www.worldclim.org). The variables are: (1) annual mean temperature; (2) mean diurnal range; (3) temperature seasonality; (4) maximum temperature of warmest month; (5) minimum temperature of coldest month; (6) annual precipitation; and precipitation of (7) warmest and (8) coldest quarter. We also included three 30 m resolution layers of vegetation characteristics downloaded from Reverb (http://reverb.earthdata.nasa.gov): (1) leaf area index (LAI); (2) normalized difference vegetation index (NDVI); and (3) gross primary production (GPP). We used Principal Component Analysis (PCA) to examine environmental differentiation between candidate species using software JMP v. 9.0.1.
To avoid spatial autocorrelation, we reduced the number of localities using a buffer with a 5 km radius around each point; if two or more localities of the same species were at a distance <10 km from each other, we randomly chose one of them. We obtained a total of 62 localities for the analysis. As species limits in this clade are incorrectly defined, we did not include geographic records from the literature. Locality data are available in Suppl. material 2. A distribution map is presented in Figures 1, 2.
For the description of the habitat and distribution of the species, we assigned natural regions according to Ron et al. (2019); we estimated the Extent of Occurrence and Area of Occupancy of each species with the R package red (Cardoso 2017).
Integrative analyses
To determine species limits, we examined the covariation of independent sets of characters under the framework of integrative taxonomy (Dayrat 2005; Vieites et al. 2009). Our datasets consisted of genetic, morphological, bioacoustic, and environmental characters. We assumed that covariation between independent sets of characters is an indication of evolutionary independence between lineages (Vieites et al. 2009).
Based on the correspondence between morphological and genetic divergence, we set a 0.02 threshold of uncorrected p distances for the gene 16S to identify candidate species. We chose a lower threshold than that proposed by Fouquet et al. (2007) for amphibians (0.03) because there is evidence indicating that sister species in Pristimantis (e.g., Ortega et al. 2015; Padial and de la Riva 2009) and other Neotropical frogs (e.g., Caminer and Ron 2014; Jungfer et al. 2013; Coloma et al. 2012; Funk et al. 2012) often differ by genetic distances <0.03. Candidate species categories follow Vieites et al. (2009). Each candidate species was categorized as: (1) unconfirmed candidate species (UCS) when genetic distance was >0.02 and there were no additional character sets available; (2) confirmed candidate species (CCS) when genetic distance was >0.02 and there was covariation between genetic and morphological, bioacoustic and/or environmental characters; (3) deep conspecific linage (DCL) when genetic distance was >0.02 and there was not covariation between genetic and morphological, bioacoustics, and environmental characters. We followed the species concept proposed by de Queiroz (2007) that defines a species as a separately evolving metapopulation lineage.
Results
Phylogeny and genetic variation
The best partition scheme for the concatenated matrix consisted of the following five partitions and models: 16S, ND1 1st position (GTR+I+G); ND1 2nd position (HKY+I+G); ND1 3rd position (TrN+I+G); RAG1 3rd position (K80+G); RAG1 1st and 2nd position (K81uf+I+G).
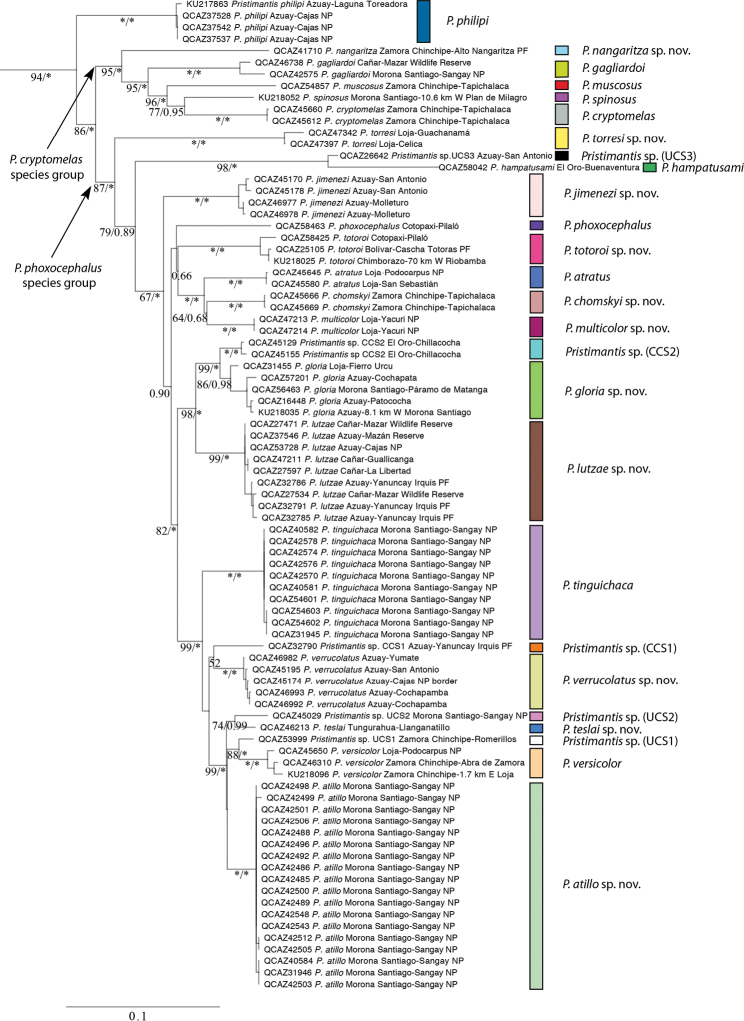
Tree topologies under ML and Bayesian inference were similar except for the order of divergence events of P. tinguichaca, P. sp. CCS1, and P. atillo sp. nov. (see Integrative results). The best ML tree based on nuclear and mitochondrial genes is shown in Figure 3; the consensus tree of the Bayesian analysis is available through zenodo.org (http://doi.org/10.5281/zenodo.2556418). We found a strongly supported clade (94 NPB, 1.0 BBP) that has P. philipi diverging basally and sister to a clade composed of two large subclades with strong support (Fig. 3 and Suppl. material 3). We are referring to these clades as the P. phoxocephalus and P. cryptomelas species groups. Figure 3 shows the species limits identified in the integrative analyses (see below). The topology of the ML tree based on mitochondrial genes was congruent with the one based on the complete dataset for branches with strong support (>75% NBP) (Suppl. material 4). The tree based on the nuclear gene (Suppl. material 5) has low resolution and low branch support.
Figure 3.
Phylogenetic relationships of Pristimantis, sugenus Huicundomantis. Maximum likelihood tree for genes 16S, ND1 and RAG1. Bootstrap values (%) followed by Bayesian posterior probabilities are shown under the corresponding branches. Asterisks indicate support values of 100 (bootstrap) or 1 (posterior probabilities); missing values indicate values below 50 (bootstrap) or 0.5 (posterior probability). The collection number, identification, province and locality of the samples are shown next to each terminal; all samples are from Ecuador. Outgroup is not shown. Abbreviations: CCS = confirmed candidate species, NP = national park, PF = protected forest, UCS = unconfirmed candidate species.
Uncorrected p-genetic distances are summarized in Tables 2, 3. Average interspecific genetic distances (16S) for the ingroup range from 2.0 to 12.9% (1.8 to 5.9% between individuals of sister species). Pristimantis gloria sp. nov. was the species with the highest divergence between two populations; the population from Loja Province presented up to 1.3% of genetic distance with populations from Azuay Province (average intraspecific distance: 0.7%). We found 26 clades (i.e., candidate species) with genetic distances >2.0%. Ten of these clades are formerly described species: P. atratus (Lynch, 1979), P. cryptomelas (Lynch, 1979), P. gagliardoi Bustamante & Mendelson, 2008, P. prometeiiSzékely et al., 2016, P. muscosus (Duellman & Pramuk, 1999), P. philipi, P. phoxocephalus, P. spinosus, P. tinguichacaBrito et al., 2016, and P. versicolor; the remaining 16 clades are candidate species to be confirmed with the following analyses.
Table 2.
Genetic divergence (Gene 16S) between species of the Pristimantis phoxocephalus species group. Mean uncorrected p distances (%) between groups are shown under the diagonal, standard error estimates above the diagonal. Mean uncorrected p distance within each clade and its standard error are shown on the diagonal. Number of samples is given in brackets after the name of each clade. Standard error estimates were obtained by a bootstrap procedure in MEGA 7.0.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P. atillo (18) | 0 | 0.9 | 0.9 | 0.7 | 0.9 | 1.0 | 0.8 | 0.9 | 1.0 | 0.6 | 0.8 | 1.0 | 0.9 | 0.8 | 0.7 | 0.8 | 0.8 | 0.5 | 0.7 | 0.9 |
| 2 | P. atratus (2) | 7.3 | 0 | 0.7 | 0.8 | 1.0 | 0.7 | 0.8 | 0.8 | 0.8 | 1.0 | 1.0 | 0.9 | 0.7 | 0.6 | 0.8 | 0.7 | 0.7 | 0.9 | 1.0 | 1.0 |
| 3 | P. chomskyi (2) | 7.1 | 5.4 | 0.2±0.1 | 0.7 | 1.0 | 0.7 | 0.7 | 0.8 | 0.8 | 1.0 | 1.0 | 0.9 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.9 | 1.0 | 1.0 |
| 4 | P. gloria (5) | 5.1 | 5.8 | 6.3 | 0.7±0.2 | 0.9 | 0.7 | 0.6 | 0.8 | 0.8 | 0.8 | 0.9 | 0.9 | 0.7 | 0.7 | 0.7 | 0.7 | 0.4 | 0.8 | 0.9 | 0.9 |
| 5 | P. hampatusami (1) | 7.0 | 11.1 | 10.9 | 8.7 | NA | 1.0 | 0.9 | 1.0 | 1.0 | 1.0 | 0.9 | 1.0 | 1.0 | 0.9 | 0.9 | 1.0 | 0.9 | 1.0 | 1.1 | 0.7 |
| 6 | P. jimenezi (4) | 7.7 | 6.8 | 7.8 | 5.8 | 10.7 | 0.5±0.2 | 0.7 | 1.0 | 0.9 | 1.0 | 1.0 | 0.9 | 0.8 | 0.6 | 0.7 | 0.7 | 0.6 | 1.0 | 1.1 | 1.0 |
| 7 | P. lutzae (9) | 5.0 | 5.7 | 5.6 | 2.9 | 7.5 | 5.3 | 0.2±0.1 | 0.9 | 0.8 | 0.8 | 0.8 | 0.9 | 0.7 | 0.6 | 0.7 | 0.7 | 0.6 | 0.8 | 0.9 | 0.9 |
| 8 | P. multicolor (2) | 6.7 | 4.4 | 4.9 | 5.5 | 8.9 | 6.7 | 5.5 | 0 | 0.9 | 1.0 | 1.0 | 1.0 | 0.9 | 0.9 | 1.0 | 0.9 | 0.8 | 1.0 | 1.0 | 1.0 |
| 9 | P. phoxocephalus (1) | 7.8 | 5.8 | 6.7 | 5.4 | 11.1 | 6.8 | 5.8 | 6.3 | NA | 1.1 | 1.0 | 1.0 | 0.7 | 0.8 | 0.9 | 0.8 | 0.9 | 1.0 | 1.1 | 1.0 |
| 10 | P. teslai (1) | 2.6 | 7.6 | 7.4 | 5.9 | 7.6 | 7.9 | 5.2 | 7.1 | 8.0 | NA | 0.9 | 1.0 | 1.0 | 0.8 | 0.8 | 0.8 | 0.9 | 0.6 | 0.6 | 1.0 |
| 11 | P. tinguichaca (10) | 4.7 | 7.8 | 7.7 | 6.1 | 7.0 | 7.8 | 5.3 | 7.5 | 7.7 | 5.7 | 0 | 1.0 | 0.9 | 0.8 | 0.8 | 0.8 | 0.9 | 0.8 | 0.9 | 0.9 |
| 12 | P. torresi (2) | 8.7 | 8.7 | 9.5 | 7.3 | 11.1 | 9.1 | 7.5 | 8.3 | 8.6 | 9.1 | 8.6 | 0.9±0.4 | 0.9 | 0.9 | 0.8 | 0.9 | 0.8 | 1.0 | 1.1 | 1.1 |
| 13 | P. totoroi (3) | 7.0 | 6.0 | 6.8 | 6.0 | 11.1 | 6.9 | 5.5 | 6.4 | 5.9 | 7.4 | 7.2 | 8.0 | 1±0.2 | 0.7 | 0.7 | 0.8 | 0.7 | 0.9 | 0.9 | 0.9 |
| 14 | P. verrucolatus (5) | 4.6 | 5.4 | 6.6 | 4.8 | 9.7 | 5.6 | 4.3 | 6.0 | 6.1 | 4.9 | 4.9 | 8.6 | 5.7 | 0.2±0.1 | 0.6 | 0.5 | 0.6 | 0.8 | 0.8 | 0.9 |
| 15 | P. versicolor (3) | 3.9 | 7.2 | 7.4 | 6.0 | 10.2 | 7.5 | 5.6 | 7.9 | 8.1 | 4.4 | 5.8 | 8.7 | 7.1 | 4.7 | 0.38±0.1 | 0.6 | 0.6 | 0.7 | 0.8 | 1.0 |
| 16 | Pristimantis CCS1 (1) | 4.4 | 6.2 | 7.2 | 5.3 | 10.4 | 6.4 | 5.1 | 6.9 | 6.8 | 4.7 | 4.6 | 8.6 | 6.1 | 3.2 | 5.0 | NA | 0.6 | 0.8 | 0.9 | 0.9 |
| 17 | Pristimantis CCS2 (2) | 5.4 | 5.8 | 6.0 | 2.0 | 7.5 | 5.8 | 2.9 | 5.4 | 6.0 | 6.4 | 5.8 | 7.6 | 5.9 | 4.5 | 5.7 | 5.1 | 0.19±0.1 | 0.8 | 0.9 | 0.9 |
| 18 | Pristimantis UCS1 (1) | 2.5 | 7.0 | 7.2 | 5.4 | 7.4 | 7.5 | 5.1 | 6.5 | 7.3 | 3.0 | 5.6 | 8.6 | 7.4 | 4.3 | 2.9 | 4.8 | 5.8 | NA | 0.6 | 0.9 |
| 19 | Pristimantis UCS2 (1) | 3.2 | 7.7 | 7.3 | 6.3 | 8.0 | 8.4 | 5.9 | 6.9 | 8.1 | 2.5 | 5.8 | 9.9 | 7.4 | 4.9 | 4.3 | 4.8 | 6.6 | 2.8 | NA | 1.0 |
| 20 | Pristimantis UCS3 (1) | 6.9 | 8.1 | 7.4 | 6.6 | 3.5 | 7.3 | 6.2 | 8.0 | 7.7 | 7.1 | 6.5 | 8.5 | 7.7 | 6.9 | 7.5 | 6.7 | 6.8 | 7.2 | 7.7 | NA |
Table 3.
Genetic divergence (16S) between species of the Pristimantis cryptomelas species group. Mean uncorrected p distances (%) between groups are shown under the diagonal, standard error estimates above the diagonal. Mean uncorrected p distance within each clade and its standard error are shown on the diagonal. Number of samples is given in brackets after the name of each clade. Standard error estimates were obtained by a bootstrap procedure in MEGA 7.0.
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| 1 | P. cryptomelas (2) | 0 | 1.1 | 0.8 | 0.9 | 0.6 |
| 2 | P. gagliardoi (2) | 8.8 | 0.9±0.4 | 1.1 | 1.1 | 1.0 |
| 3 | P. muscosus (1) | 7.6 | 8.6 | NA | 1.0 | 0.7 |
| 4 | P. nangaritza (1) | 8.1 | 8.3 | 9.8 | NA | 0.9 |
| 5 | P. spinosus (1) | 5.9 | 7.6 | 7.4 | 8.1 | NA |
Morphological analyses
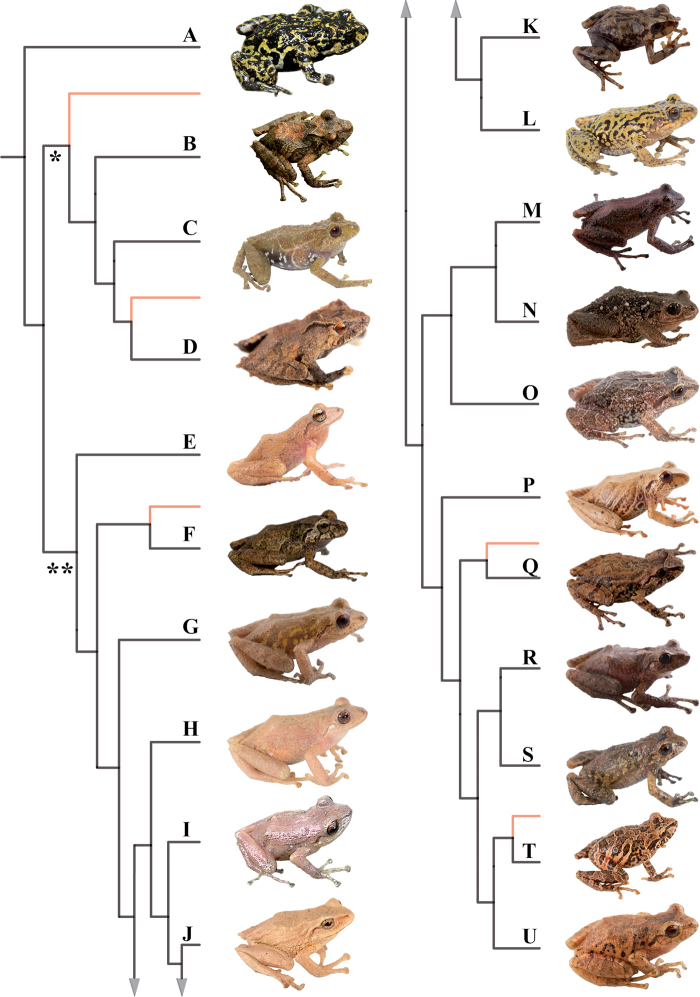
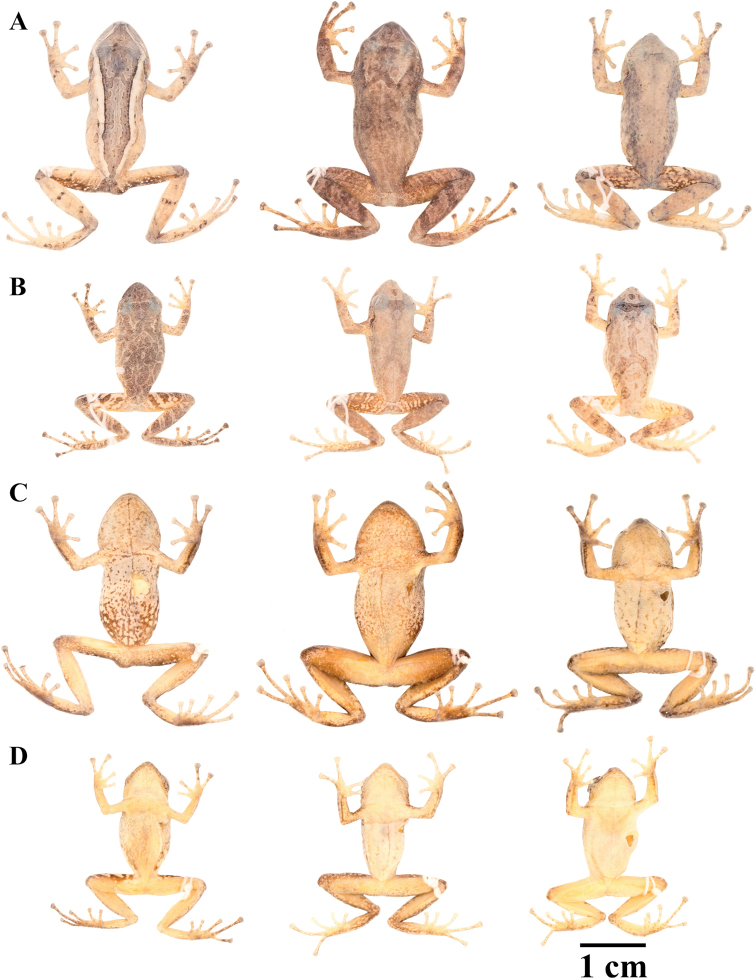
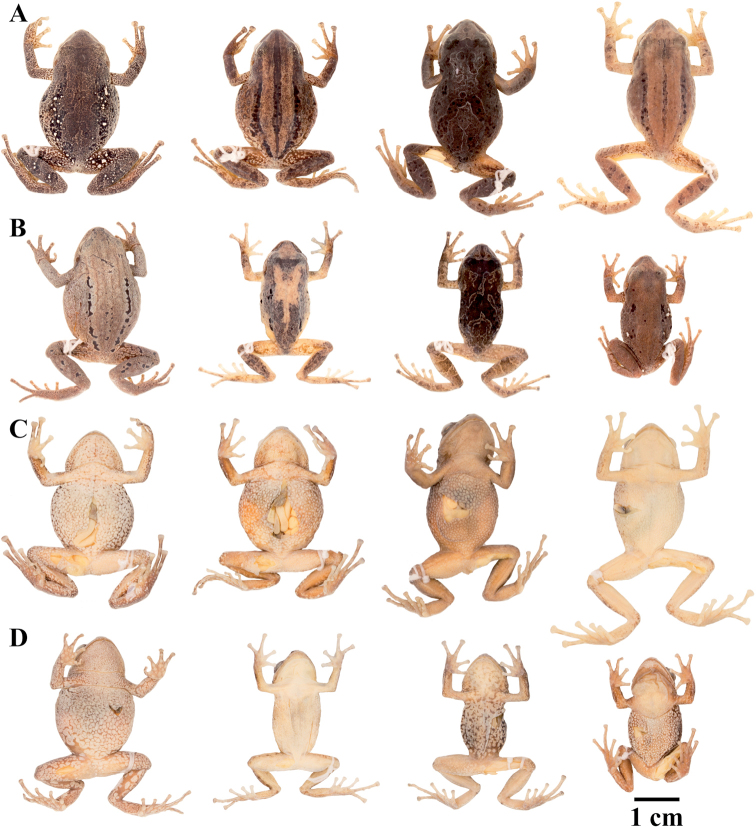
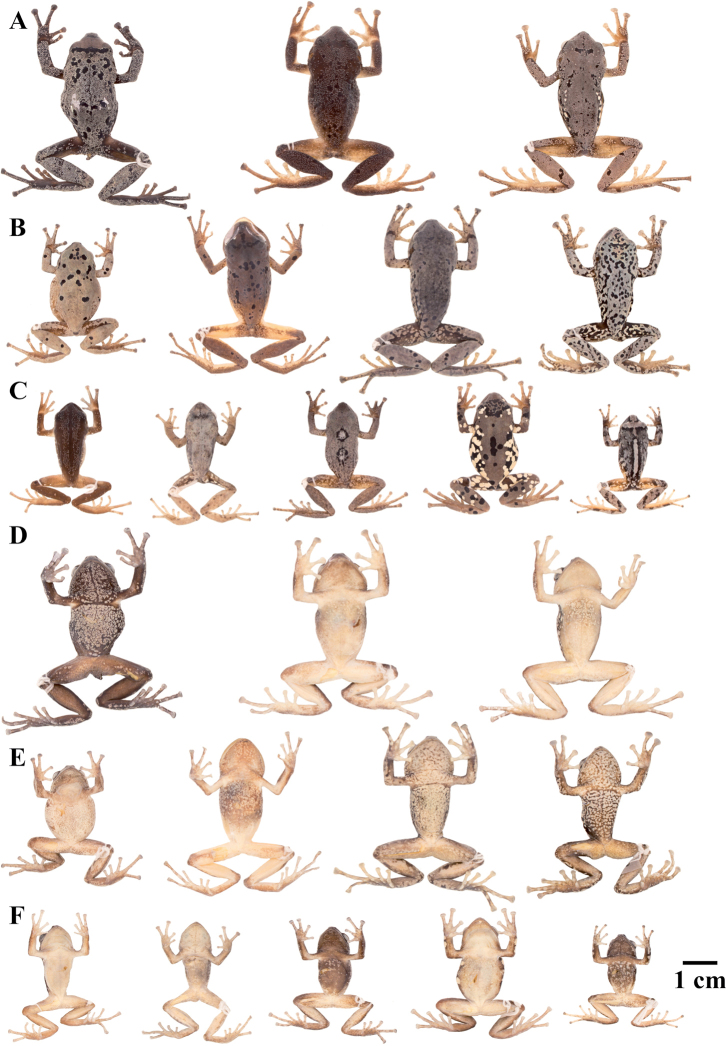
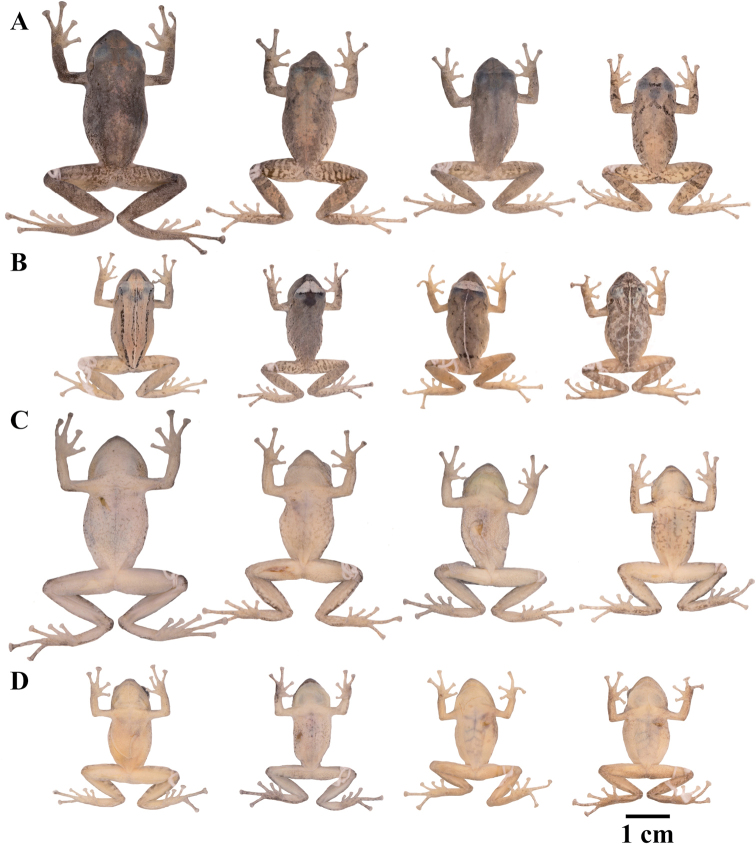
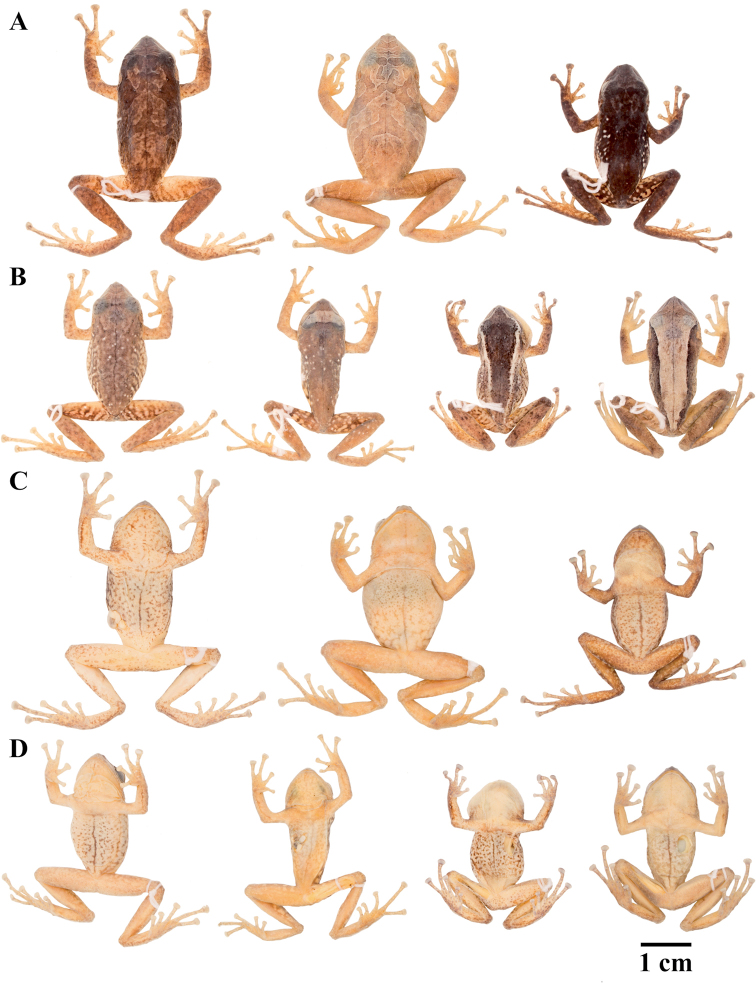
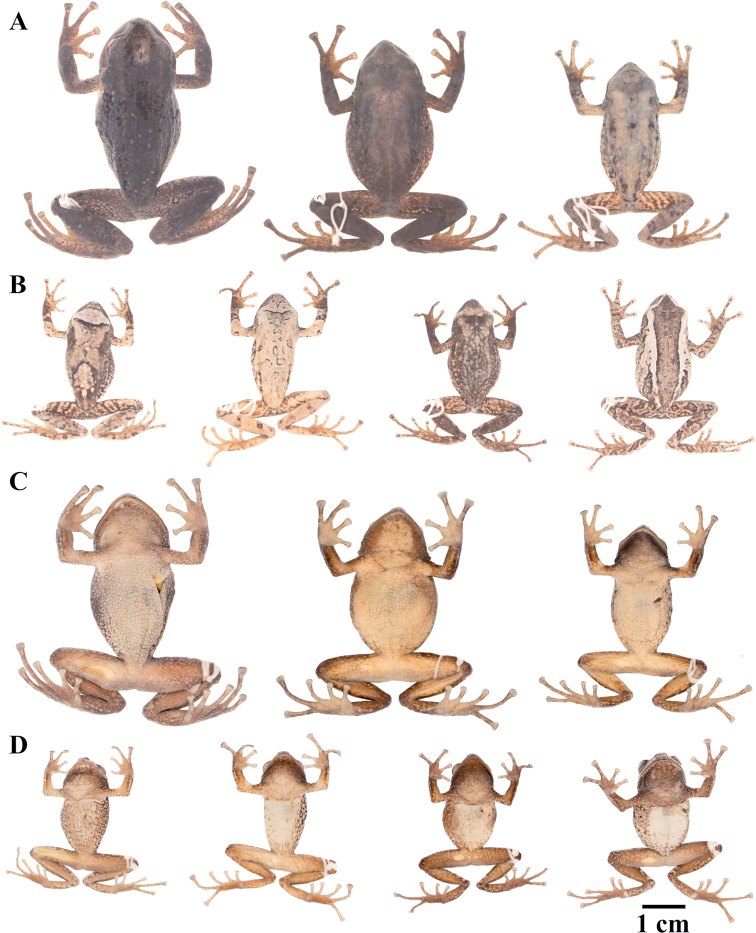
Morphological variation is shown in Figure 4. The ingroup shares the absence of dorsolateral folds (except for P. atratus), presence of a tympanic annulus and membrane (except for P. philipi), dentigerous processes of vomers evident, first finger shorter than the second, presence of lateral fringes on fingers and toes, basal webbing on toes (except for P. philipi and P. prometeii), and small to prominent tubercles on heel. Additionally, all the species of the P. cryptomelas group bear postocular folds and prominent tubercles on eyelids and heels. Because postocular folds and prominent tubercles on eyelids and heels are absent in the P. phoxocephalus group and P. philipi, both characters appear to be synapomorphies for the P. cryptomelas group. Meanwhile, species of the Pristimantis phoxocephalus group have a keel or a papilla at the tip of the snout. The papilla is absent in P. philipi and all species of the P. cryptomelas group, except for P. cryptomelas which has papilla, suggesting that the presence of a papilla is a synapomorphy for the P. phoxocephalus group with an independent origin in P. cryptomelas. Traits that differ the most between species include snout shape, presence or absence of middorsal, lateral and postocular folds, dorsal skin texture, shape and width of discs, and coloration of groins, hidden surfaces of thighs, and iris. Character states for qualitative morphological traits are shown in Table 4.
Figure 4.
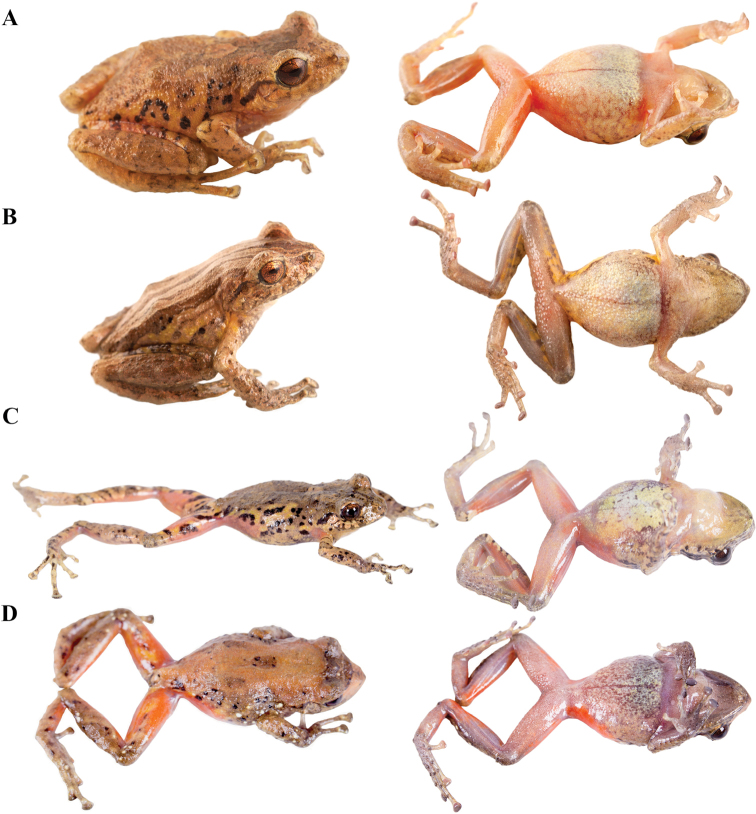
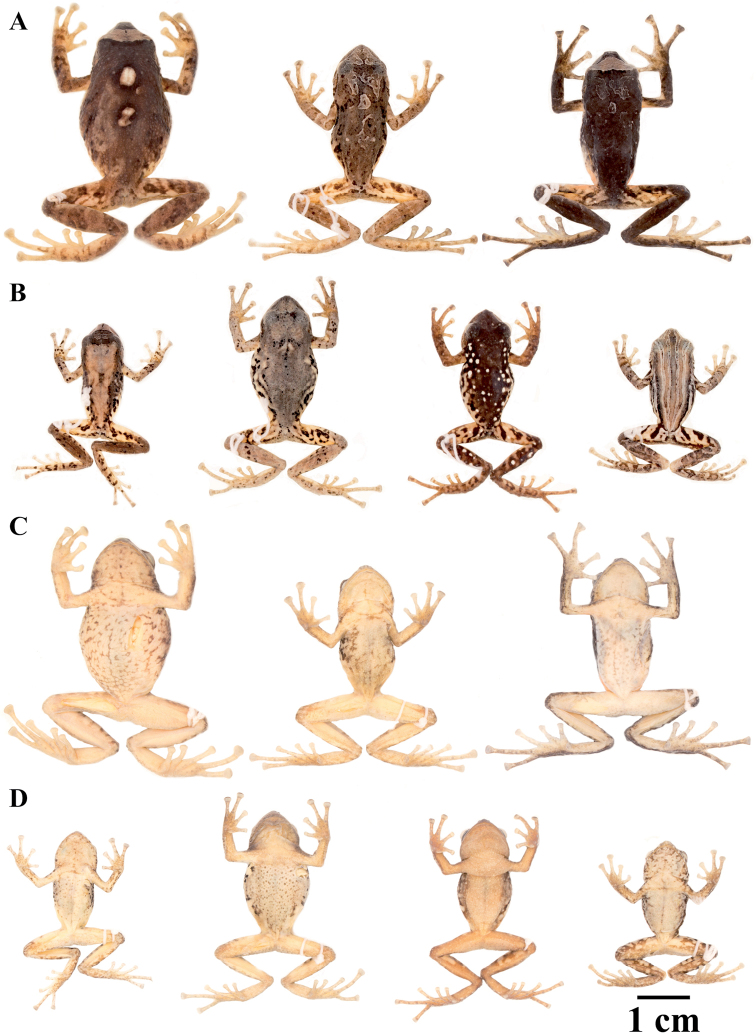
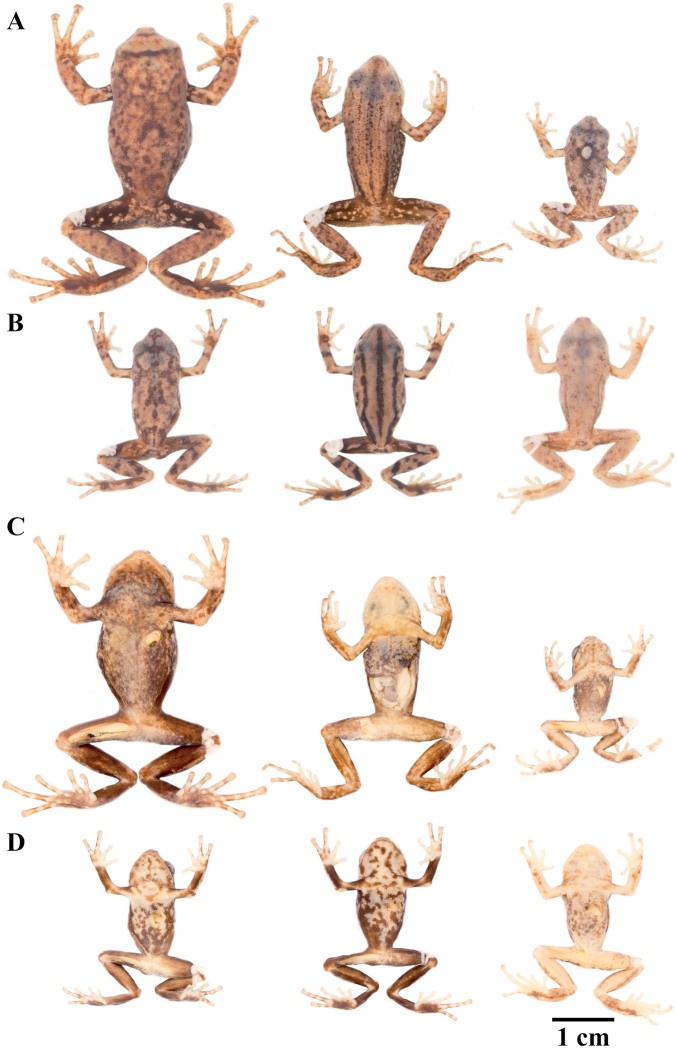
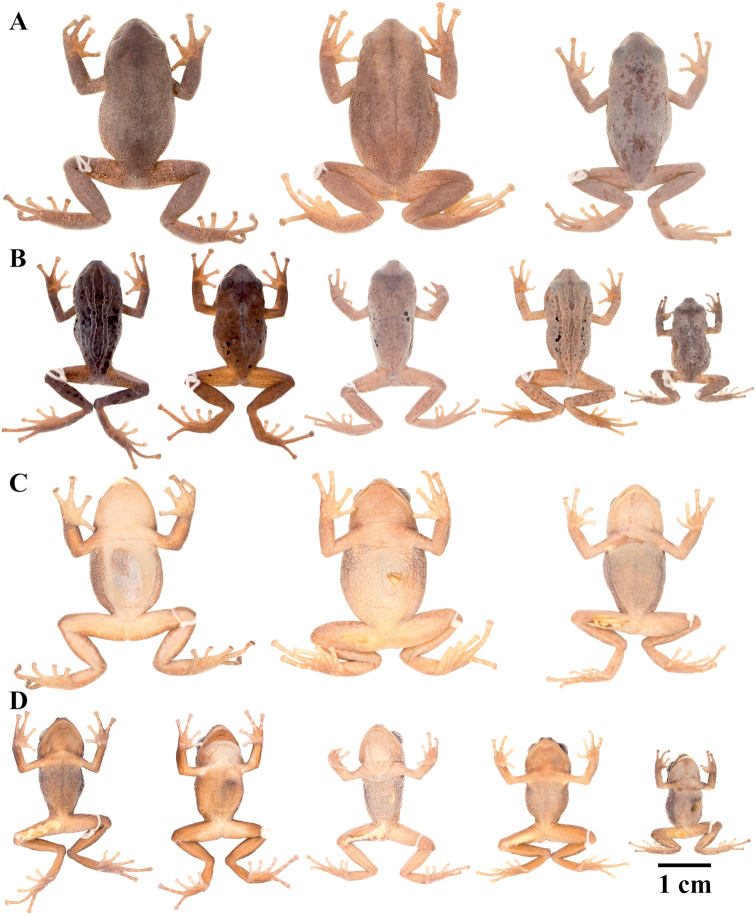
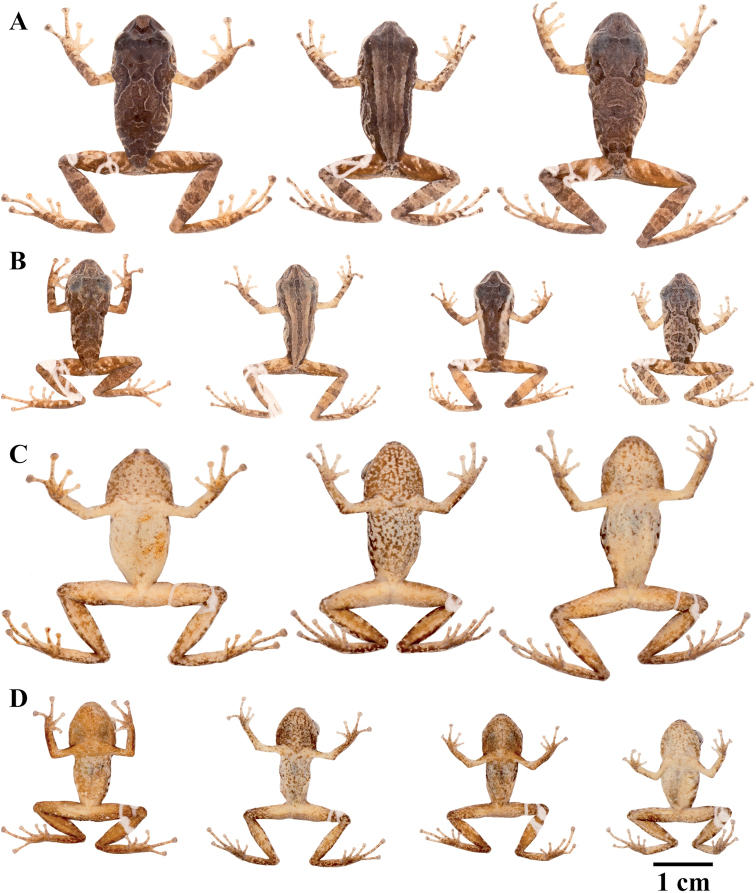
Morphological variation within Pristimantis, subgenus Huicundomantis. APristimantis philipi. Pristimantis cryptomelas group (*): BPristimantis gagliardoiCPristimantis muscosusDPristimantis cryptomelas. Pristimantis phoxocephalus group (**): EPristimantis torresi sp. nov. FPristimantis hampatusamiGPristimantis jimenezi sp. nov. HPristimantis phoxocephalusIPristimantis totoroi sp. nov. JPristimantis atratusKPristimantis chomskyi sp. nov. LPristimantis multicolor sp. nov. MPristimantis sp. (CCS2) NPristimantis gloria sp. nov. OPristimantis lutzae sp. nov. PPristimantis tinguichacaQPristimantis verrucolatus sp. nov. RPristimantis sp. (UCS1) SPristimantis teslai sp. nov. TPristimantis versicolorUPristimantis atillo sp. nov. Red branches are for clades without available photographs of live individuals. Not shown at the same scale.
Table 4.
Qualitative morphological traits of Pristimantis, subgenus Huicundomantis. Coloration corresponds to live individuals unless otherwise noticed. + is for present; – is for absent.
| Dorsal texture | Cranial crests | Postocular/scapular fold/ridge | Middorsal fold | Tubercles row head | Dorsolateral folds | Lateral fold | Snout shape (dorsal; lateral) |
Discs (expansion, shape) |
Basal webbing | Iris coloration | Groin coloration | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. atillo sp. nov. | Shagreen with or without scattered small subconical tubercles | – | – | +/– | + | – | +/– | Acuminate; protruding; with a fleshy keel | Expanded to broadly expanded, rounded to elliptical | + | Copper with a faint medial horizontal darker streak | Bright orange, surrounded or not by yellow spots |
| P. atratus | Shagreen | Low | – | +/– | +/– | + | + | Subacuminate; rounded; with or without a papilla | Broadly expanded, rounded to elliptical | + | Pale yellow to pale bronze, thin brown streak | Black with white or yellow spots |
| P. balionotus | Tuberculate | – | – | – | – | – | +/– | Subacuminate; rounded; with a papilla | Slightly expanded, elliptical | – | Bronze with a red medial streak | In preservative, pale rusty brown with or without faint brown marbling |
| P. chomskyi sp. nov. | Shagreen | – | – | – | – | – | – | Subacuminate; rounded; with or without a papilla | Expanded, rounded to elliptical | + | Orange with a faint reddish brown streak | Dark chocolate brown with or without small cream flecks |
| P. gloria sp. nov. | Tuberculate to warty | – | – | + | – | – | – | Subacuminate; rounded; with or without a papilla | Expanded, truncate to elliptical | + | Light silver to cream with wide black reticulations and a red streak | Pinkish to purplish brown with irregular cream to light brown flecks or spots |
| P. hampatusami | Shagreen with scattered tubercles | – | Low, W-shaped | + | + | – | + | Subacuminate; rounded; with or without a papilla | Broadly expanded, elliptical to truncate | – | Golden to bronze with a red medial horizontal streak | Reddish brown with yellow spots |
| P. jimenezi sp. nov. | Shagreen with or without scattered small subconical tubercles | – | – | + | + | – | + | Acuminate; protruding; with a fleshy keel | Expanded to broadly expanded, elliptical to truncate | + | Copper with red medial streak to completely red | Pinkish, purplish or dark brown with small light brown to yellow spots |
| P. lutzae sp. nov. | Shagreen to tuberculate | – | – | +/– | +/– | – | +/– | Round to subacuminate, with or without a papilla | Expanded, elliptical to truncate | + | Golden to creamy brown with a reddish brown medial streak | Pinkish to reddish brown, suffused or not with orange, with cream or light brown spots |
| P. multicolor sp. nov. | Shagreen to warty | – | – | – | +/– | – | – | Subacuminate; rounded; with or without a papilla | Expanded to broadly expanded, elliptical to truncate | + | Copper to creamy yellow with or without a red to dark brown streak | Cream, orange, brown, or black with or without cream to yellow flecks or spots |
| P. phoxocephalus | Shagreen with scattered small tubercles | – | – | + | + | – | +/– | Acuminate; protruding; with a fleshy keel | Broadly expanded, rounded to elliptical | + | Golden with wide black reticulations | In preservative, brown with cream reticulation or spots |
| P. percultus | Tuberculate | Low | – | – | – | – | – | Subacuminate; acutely rounded; with a keel | Expanded, elliptical | + | Copper with or without a faint red medial streak | Yellow with black reticulations |
| P. teslai sp. nov. | Tuberculate with prominent and rounded tubercles | – | – | – | + | – | – | Acuminate; protruding; with a fleshy keel | Expanded to broadly expanded, elliptical to truncate | + | Copper | Dark brown with yellow irregular blotches |
| P. tinguichaca | Smooth | – | – | + | + | – | + | Subacuminate; rounded; with or without a papilla | Broadly expanded, rounded | + | Red with a dark medial streak | Reddish to dark brown |
| P. torresi sp. nov. | Shagreen | – | – | + | +/– | – | + | Acuminate; protruding; with a fleshy keel | Broadly expanded, elliptical to truncate | + | Golden to beige with red to reddish brown streak | Light purplish brown to brown with or without yellow spots |
| P. totoroi sp. nov. | Shagreen with or without scattered small tubercles | – | – | + | + | – | + | Acuminate; protruding; with a fleshy keel | Broadly expanded, rounded to elliptical | + | Golden with a medial horizontal red streak | In preservative, brown with or without pale spots |
| P. verrucolatus sp. nov. | Shagreen with or without scattered small tubercles | – | – | +/– | +/– | – | + | Acuminate; rounded; with a fleshy keel | Broadly expanded, elliptical to truncate | + | Coppery brown | Reddish brown with small light brown, orangey brown or yellow spots |
| P. versicolor | Shagreen to tuberculate | – | – | +/– | – | – | – | Subacuminate; rounded; with or without a papilla | Expanded, rounded to elliptical | + | Bronze-white with a reddish brown streak | Brown to black with cream to reddish pink flecks or spots |
| P. philipi | Tuberculate with low tubercles and warts | – | W-shaped ridge | +/– | + | – | – | Rounded | Slightly expanded, truncate | – | Grayish bronze | Black with or without white or yellow streaks |
| P. cryptomelas | Shagreen | – | Prominent,) (-shaped | +/– | + | – | + | Subacuminate; rounded; with or without a papilla | Broadly expanded, rounded to elliptical | + | Red, orange or cream with a red or orange streak | Black with or without yellow or white spots |
| P. gagliardoi | Shagreen with scattered tubercles | – | Prominent, W-shaped | +/– | + | – | + | Rounded | Broadly expanded, elliptical to truncate | + | Bronze with or without a brown medial horizontal streak | Pink to orange with or without brown blotches |
| P. muscosus | Smooth to shagreen | – | Low,) (-shaped | – | + | – | + | Rounded | Broadly expanded, elliptical to truncate | + | Reddish brown | Dark brown with orange, yellow or white spots |
| P. nangaritza sp. nov. | Finely tuberculate | – | Low,) (-shaped | +/– | + | – | +/– | Subacuminate; rounded | Broadly expanded, rounded to elliptical | + | Unknown | In preservative, light brown with or without pale flecks |
| P. spinosus | Finely tuberculate | Low | Low,) (-shaped | – | + | – | + | Subacuminate; rounded to truncate | Broadly expanded, rounded | + | Unknown | Black with big white spots |
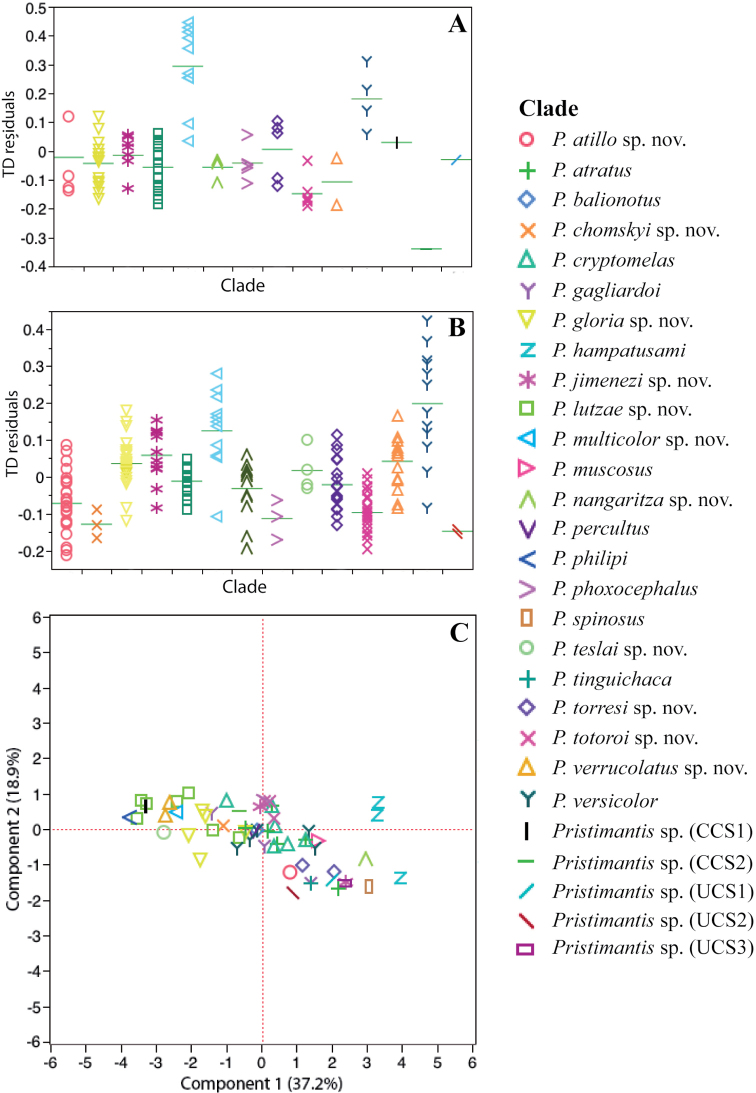
Ranges of quantitative variables widely overlap among species (Fig. 5A, B). However, several species pairs differ significantly from each other according to one or more variables (see Fig. 5A, C for an example); these results will be mentioned when useful to discriminate similar species. The DFA classification assigned the correct species in 77.1% of the specimens in females (64 out of 83), and 68.7% in males (126 out of 182). Although the DFA classification was not perfect, it shows some morphometric differentiation among species. Summarized information of morphometric measurements is shown in Table 5.
Figure 5.
Morphological and environmental comparisons among species of Pristimantis, sugenus Huicundomantis. Intra and interspecific variation in tympanum size, respective to body, in A females and B males. Green horizontal lines represent the mean C Principal components from analysis of eleven environmental variables. See Table 7 for character loadings on each component.
Table 5.
Descriptive statistics for morphometric variables of Pristimantis, subgenus Huicundomantis. Mean ± SD followed by the range of the measurements are given in each cell. ‘n’ is for the number of samples. Numbers in brackets after the species name refer to the bibliographic source of the data: (1) Lynch 1979, (2) Bustamante and Mendelson 2008, (3) Yánez-Muñoz et al. 2016, (4) Duellman and Pramuk 1999, (5) Lynch and Duellman 1995, (6) Brito et al. 2016; otherwise the source is this study. Abbreviations are: SVL = snout-vent length; HL = head length; HW = head width; TD = tympanum diameter; ED = eye diameter. All measurements are in mm.
| Females | |||||
|---|---|---|---|---|---|
| Clade | SVL | HL | HW | TD | ED |
| P. atillo sp. nov. n = 5 | 31.3 ± 2.3; 29.4–35.3 | 11.0 ± 0.5; 10.3–11.7 | 11.5 ± 0.7; 10.9–12.7 | 1.7 ± 0.2; 1.5–1.8 | 3.2 ± 0.1; 3.1–3.3 |
| P. atratus (1) n = 10 | 27.4 ± 2.1; 24.9–29.2 | – | – | – | – |
| P. balionotus (1) n = 7 | 28.1 ± 0.6; 27.1–29.1 | – | – | – | – |
| P. cryptomelas (1) n = 1 | 38.6 | – | – | – | – |
| P. gagliardoi (2) n = 5 | 30.6 ± 3.1; 26.8–33.6 | 10.7 ± 0.6; 9.8–11.4 | 12.2 ± 1.0; 10.7–13.3 | 1.3 ± 0.2; 1.0–1.5 | 3.8 ± 0.3; 3.5–4.1 |
| P. gloria sp. nov. n = 15 | 30.1 ± 3.0; 26.7–35.8 | 11.0 ± 0.7; 10.1–12.4 | 11.3 ± 1.1; 9.8–13.5 | 1.6 ± 0.1; 1.4–1.8 | 3.2 ± 0.2; 2.8–3.6 |
| P. hampatusami (3) n = 11 | 30.6 ± 2.1; 25.9–34.1 | – | – | – | – |
| P. jimenezi sp. nov. n = 9 | 35.0 ± 2.1; 31.1–37.4 | 12.0 ± 0.5; 11.0–12.6 | 13.1 ± 0.6; 13.0–13.9 | 1.9 ± 0.1; 1.7–2.1 | 3.7 ± 0.2; 3.5–4.0 |
| P. lutzae sp. nov. n = 15 | 31.4 ± 1.3; 29.7–33.9 | 11.3 ± 0.4; 10.7–12.1 | 12.2 ± 0.5; 11.4–12.9 | 1.7 ± 0.1; 1.5–1.8 | 3.4 ± 0.2; 3.1–3.8 |
| P. multicolor sp. nov. n = 10 | 35.3 ± 3.5; 29.4–40.5 | 13.4 ± 1.1; 11.6–15.1 | 14.4 ± 1.3; 11.9–16.1 | 2.2 ± 0.2; 1.8–2.5 | 4.0 ± 0.3; 3.6–4.4 |
| P. muscosus (4) n = 4 | 37.8; 29.6–46.1 | – | – | – | – |
| P. nangaritza sp. nov. n = 4 | 29.1 ± 2.7; 25.9–32.4 | 11.5 ± 0.8; 10.6–12.2 | 11.2 ± 1.0; 10.1–12.1 | 1.5 ± 0.1; 1.4–1.7 | 3.7 ± 0.3; 3.4–4.0 |
| P. percultus (1) n = 1 | 38.2 | – | – | – | – |
| P. phillipi (5) n = 6 | 31.2 ± 1.1; 26.5–33.7 | – | – | – | – |
| P. phoxocephalusn = 5 | 36.9 ± 2.2; 34.2–39.9 | 12.9 ± 0.9; 11.5–13.8 | 13.1 ± 1.2; 11.3–14.2 | 1.9 ± 0.1; 1.8–2.1 | 3.8 ± 0.3; 3.5–4.1 |
| Pristimantis CCS1 n = 1 | 37.0 | 12.5 | 13.8 | 2.0 | 3.5 |
| Pristimantis CCS2 n = 1 | 38.3 | 13 | 14.4 | 1.7 | 3.5 |
| Pristimantis UCS1 n = 1 | 31.2 | 12.4 | 12.4 | 1.7 | 3.8 |
| P. spinosus (1) n = 29 | 31.8 ± 1.62; 28.3–34.5 | – | – | – | – |
| P. tinguichaca (6) n = 9 | 29.7 ± 1.5; 28.1–31.7 | 10.6 ± 0.4; 10.1–11.0 | 11.0 ± 0.5; 10.1–11.5 | 1.4 ± 0.1; 1.3–1.7 | 3.3 ± 0.3; 2.8–3.7 |
| P. torresi sp. nov. n = 5 | 34.7 ± 3.7; 30.1–39.5 | 12.2 ± 1.3 10.4–13.8 | 13.1 ± 1.2 11.5–14.6 | 1.9 ± 0.2 1.8–2.2 | 3.6 ± 0.3 3.3–3.9 |
| P. totoroi sp. nov. n = 7 | 33.0 ± 0.9; 31.9–34.3 | 12.1 ± 0.4; 11.5–12.6 | 12.3 ± 0.3; 11.8–12.6 | 1.6 ± 0.1; 1.6–1.8 | 3.3 ± 0.2; 3.1–3.6 |
| P. verrucolatus sp. nov. n = 2 | 43.6 ± 4.5; 40.4–46.8 | 15.0 ± 1.1; 14.2–15.8 | 16.6 ± 1.7; 15.3–17.8 | 2.2 ± 0.1; 2.1–2.3 | 4.2 ± 0.0; 4.2–4.3 |
| P. versicolorn = 4 | 27.8 ± 3.5; 24.9–32.4 | 10.7 ± 1.6; 9.0–12.4 | 10.5 ± 1.0; 9.5–11.4 | 1.7 ± 0.3; 1.4–2.1 | 3.2 ± 0.4; 2.9–3.7 |
| Males | |||||
| P. atillo sp. nov. n = 29 | 24.7 ± 2.5; 17.7–28.1 | 8.7 ± 0.7; 6.4–9.7 | 8.8 ± 0.9; 5.9–10.0 | 1.2 ± 0.1; 0.8–1.4 | 2.7 ± 0.3; 2.0–3.0 |
| P. atratus (1) n = 19 | 21.7 ± 3.71; 17.4–24.0 | – | – | – | – |
| P. balionotus (1) n = 2 | 20.0 ± 0.2; 21.8–22.2 | – | – | – | – |
| P. chomskyi sp. nov. n = 3 | 28.0 ± 4.2; 24.0–32.4 | 9.7 ± 1.2; 8.8–11.1 | 10.6 ± 1.6; 9.4–12.4 | 1.3 ± 0.2; 1.1–1.5 | 3.3 ± 0.4; 2.9–3.6 |
| P. cryptomelas (1) n = 4 | 29.2; 28.2–30.3 | – | – | – | – |
| P. gagliardoi (2) n = 5 | 22.2 ± 2.0; 19.1–24.3 | 7.9 ± 0.9; 6.8–9.1 | 9.0 ± 0.9; 7.7–10.2 | 0.8 ± 0.0; 0.7–0.8 | 2.8 ± 0.2; 2.4–3.0 |
| P. gloria sp. nov. n = 24 | 21.7 ± 2.3; 16.8–24.7 | 8.6 ± 0.7; 6.7–9.4 | 8.4 ± 0.8; 6.5–9.5 | 1.2 ± 0.1; 0.9–1.4 | 2.6 ± 0.2; 2.1–2.9 |
| P. hampatusami (3) n = 28 | 21.0 ± 1.8; 17.0–24.9 | – | – | – | – |
| P. jimenezi sp. nov. n = 12 | 25.5 ± 1.6; 21.8–27.1 | 8.9 ± 0.6; 7.8–9.6 | 9.1 ± 0.6; 7.8–9.7 | 1.4 ± 0.1; 1.3–1.5 | 3.06 ± 0.24; 2.56–3.37 |
| P. lutzae sp. nov. n = 14 | 24.6 ± 1.7; 21.4–27.0 | 8.7 ± 0.5; 7.9–9.4 | 9.3 ± 0.7; 8.2–10.4 | 1.3 ± 0.1; 1.1–1.4 | 2.77 ± 0.19 2.53–3.26 |
| P. multicolor sp. nov. n = 12 | 26.2 ± 3.5; 19.7–29.7 | 9.6 ± 1.0; 8.0–11.0 | 10.1 ± 1.2; 8.0–11.5 | 1.5 ± 1.2; 1.1–1.8 | 3.18 ± 0.49; 2.45–3.82 |
| P. nangaritza sp. nov. n = 13 | 18.8 ± 1.1; 17.4–20.8 | 7.6 ± 0.6; 7.1–8.8 | 7.2 ± 0.5; 6.6–8.3 | 1.0 ± 0.1; 0.8–1.2 | 2.69 ± 0.17 2.49–3.08 |
| P. percultus (1) n = 1 | 29.8 | – | – | – | – |
| P. phillipi (5) n = 10 | 23.0 ± 0.4; 21.1–25.1 | – | – | – | – |
| P. phoxocephalusn = 3 | 24.7 ± 3.2; 20.8–27.9 | 9.0 ± 1.36; 7.4–10.6 | 8.7 ± 1.5; 7.0–10.4 | 1.2 ± 0.2; 1.0–1.3 | 2.66 ± 0.25 2.36–2.90 |
| Pristimantis UCS2 n = 2 | 20.5 ± 1.7; 19.3–21.7 | 7.6 ± 0.7; 7.1–8.1 | 7.3 ± 0.4; 7.0–7.6 | 1.0 ± 0.1; 0.9–1.0 | 2.43 ± 0.23 2.29–2.62 |
| P. spinosus (1) n = 34 | 20.1 ± 1.8; 16.1–25.0 | – | – | – | – |
| P. teslai sp. nov. n = 4 | 25.2 ± 1.8; 23.4–27.3 | 8.9 ± 0.4; 8.4–9.2 | 9.0 ± 0.6; 8.2–9.5 | 1.3 ± 0.1; 1.2–1.5 | 2.89 ± 0.14 2.76–3.07 |
| P. tinguichaca (6) n = 11 | 23.4 ± 1.1; 21.2–24.7 | 8.7 ± 0.3; 8.2–9.2 | 8.6 ± 0.5; 7.9–9.4 | 1.3 ± 0.2; 1.1–1.6 | 2.8 ± 0.4 2.3–3.3 |
| P. torresi sp. nov. n = 18 | 25.9 ± 2.1; 23.3–30.0 | 9.2 ± 0.6; 8.4–10.5 | 9.3 ± 0.7; 8.4–10.5 | 1.3 ± 0.1; 1.2–1.6 | 2.98 ± 0.23 2.64–3.47 |
| P. totoroi sp. nov. n = 21 | 26.8 ± 2.0; 23.2–29.4 | 9.6 ± 0.6; 8.5–10.5 | 9.5 ± 0.6; 8.4–10.3 | 1.3 ± 0.1; 1.1–1.4 | 2.79 ± 0.25 2.27–3.25 |
| P. verrucolatus sp. nov. n = 15 | 29.4 ± 2.7; 25.1–34.5 | 9.7 ± 0.7; 8.5–10.7 | 10.5 ± 1.0; 8.5–12.1 | 1.5 ± 0.1; 1.3–1.8 | 3.21 ± 0.24 2.79–3.64 |
| P. versicolorn = 12 | 20.5 ± 1.6; 18.1–23.3 | 8.2 ± 0.5; 7.3–8.9 | 7.6 ± 0.6; 6.7–8.6 | 1.3 ± 0.2; 1.0–1.6 | 2.84 ± 0.32 2.32–3.36 |
Bioacoustic analyses
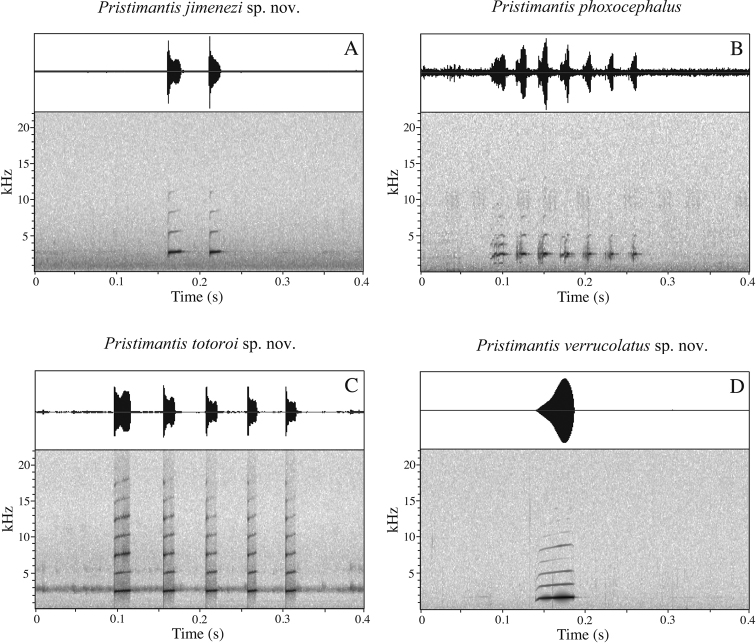
Bioacoustic data are summarized in Table 6. The available advertisement calls of P. phoxocephalus and three candidate species have a similar structure: one to several whistles (Fig. 6). Calls from Cashca Totoras (Bolívar Province, Ecuador) were distinct from those from Pilaló (Cotopaxi Province, Ecuador) even though both populations had historically been ascribed to P. phoxocephalus. The clade from high altitudes of Azuay Province shows, by far, the longest duration of the note, the lowest dominant frequency, and the highest variation between the initial and final frequency of the note. The advertisement call of the clade from lower altitudes of Azuay shows the highest frequency of the first and second harmonic. Pristimantis phoxocephalus shows the shortest interval between notes. All analyzed species are separated by large genetic distances (>5.6%). They also have unequivocally distinct advertisement calls (Fig. 6).
Table 6.
Descriptive statistics for bioacoustic variables of Pristimantis jimenezi sp. nov., P. phoxocephalus, P. totoroi sp. nov. and P. verrucolatus sp. nov. Mean ± SD and range in brackets are given in each cell. The number of samples is given in brackets after species name.
| P. jimenezi (2) | P. phoxocephalus (2) | P. totoroi (2) | P. verrucolatus (2) | |
|---|---|---|---|---|
| Notes per call | 1–2 | 3–9 | 2–6 | 1 |
| No. harmonics | 4 | 4–5 | 3–7 | 4–7 |
| Note duration (s) | 0.182 ± 0.035 | 0.154 ± 0.030 | 0.125 ± 0.038 | 0.433 ± 0.037 |
| (0.157–0.206) | (0.133–0.175) | (0.098–0.152) | (0.407–0.459) | |
| Interval between notes (s) | 0.297 ± 0.088 | 0.135 ± 0.035 | 0.339 ± 0.038 | NA |
| (0.234–0.360) | (0.110–0.159) | (0.312–0.366) | ||
| Peak time (s) | 0.091 ± 0.018 | 0.077 ± 0.014 | 0.063 ± 0.019 | 0.216 ± 0.018 |
| (0.080–0.103) | (0.067–0.087) | (0.049–0.076) | (0.204–0.230) | |
| Dominant frequency (Hz) | 2894.58 ± 167.49 | 2578.12 ± 0 | 2569.65 ± 21.21 | 2114.08 ± 265.17 |
| (2776.15–3013.01) | (2512.22–2627.07) | (1926.58–2301.58) | ||
| Initial frequency (Hz) | 2789.89 ± 232.02 | 2452.15 ± 87.01 | 2550.96 ± 148.20 | 1837.5 ± 212.13 |
| (2625–2953.13) | (2390.63–2513.67) | (2446.16–2655.75) | (1687.5–1987.5) | |
| Final frequency (Hz) | 2976.56 ± 232.02 | 2586.91 ± 12.43 | 2619.88 ± 50.73 | 2231.25 ± 238.65 |
| (2812.5–3140.63) | (2578.13–2595.70) | (2584–2655.75) | (2062.5–2400) | |
| Frequency change (Hz) | 187.5 ± 0 | 134.77 ± 99.44 | 68.92 ± 97.47 | 393.75 ± 26.52 |
| (64.45–205.08) | (0–137.84) | (375–412.5) | ||
| 2nd harmonic frequency (Hz) | 5373.79 ± 412.25 | 4532.23 ± 62.15 | 4714.43 ± 453.59 | 4171.89 ± 596.64 |
| (5446.29–6029.3) | (4488.28–4576.17) | (4393.69–5035.16) | (3750–4593.78) |
Figure 6.
Comparison of advertisement calls of Pristimantis jimenezi sp. nov., P. phoxocephalus, P. totoroi sp. nov. and P. verrucolatus sp. nov. APristimantis jimenezi: QCAZ 46977 BPristimantis phoxocephalus: non-collected individual from Pilaló (type locality). CPristimantis totoroi: non-collected individual from Cashca Totoras (type locality) DPristimantis verrucolatus: QCAZ 46981. Oscillograms are shown above spectrograms. X axis for time (s); Y axis for frequency (kHz) in spectrograms.
Environmental analyses
In the PCA, the eleven environmental variables were reduced to four principal components (PCs) with an eigenvalue >1. Together they accounted for 81.58% of the variation. The highest loadings for each PC were: temperature-associated variables for PC I, vegetation-associated variables and precipitation of the coldest quarter for PC II, precipitation-associated variables for PC III, and NDVI and temperature seasonality for PC IV. Percentage of explained variance and variable loadings for each PC are shown in Table 7.
Table 7.
Character loadings, eigenvalues and percentage of explained variance for Principal Components (PC) I–IV. The analysis was based in eleven environmental variables extracted from presence localities of 26 species of Pristimantis, subgenus Huicundomantis. Bold figures indicate highest loadings.
| Variable | PCI | PCII | PCIII | PCIV |
|---|---|---|---|---|
| Annual mean temperature | 0.956 | 0.014 | -0.139 | 0.153 |
| Mean diurnal temperature range | 0.680 | -0.365 | -0.026 | -0.383 |
| Temperature seasonality | -0.257 | 0.016 | 0.465 | 0.642 |
| Max temperature of warmest month | 0.966 | -0.046 | -0.122 | 0.121 |
| Min temperature of coldest month | 0.930 | 0.050 | -0.153 | 0.201 |
| Annual precipitation | 0.382 | -0.395 | 0.777 | -0.101 |
| Precipitation of warmest quarter | 0.219 | 0.156 | 0.709 | 0.234 |
| Precipitation of coldest quarter | 0.428 | -0.605 | 0.210 | -0.268 |
| Gross primary production | 0.377 | 0.839 | 0.128 | -0.185 |
| Leaf area index | 0.469 | 0.797 | 0.146 | -0.125 |
| Normalized difference vegetation index | 0.339 | -0.229 | -0.378 | 0.611 |
| Eigenvalue | 4.096 | 2.074 | 1.606 | 1.198 |
| Cumulative variance (%) | 37.23 | 56.09 | 70.69 | 81.58 |
Candidate species widely overlap in environmental space (Fig. 5C). However, we found differences between clades occurring in areas with the lowest altitudes and warmest temperatures ((P. hampatusami = P. prometeii), P. nangaritza sp. nov., P. spinosus) relative to those occurring in paramo (P. gloria sp. nov., P. lutzae sp. nov., P. multicolor sp. nov., P. philipi, P. teslai sp. nov., P. verrucolatus sp. nov., Pristimantis sp. CCS1).
Integrative analyses
By combining evidence from the abovementioned character sets, we conclude that our study group is composed of 28 candidate species. Of them, 25 are confirmed candidate species and 3 are unconfirmed. Of the CCS, 12 have available names and the 13 remaining are new species. We describe 11 of them below. We did not describe two CCS because we did not have enough specimens to characterize species variation. Because of their similar morphology to the examined species, and after a thorough bibliographic analysis, we propose P. balionotus (Lynch, 1979) and P. percultus (Lynch, 1979) as part of our clade of study.
In brief, our ingroup is composed of 27 species divided in two species groups and P. philipi. The P. phoxocephalus species group comprises 22 species: P. atillo sp. nov., P. atratus, P. chomskyi sp. nov., P. gloria sp. nov., P. hampatusami, P. jimenezi sp. nov., P. lutzae sp. nov., P. multicolor sp. nov., P. phoxocephalus, P. teslai sp. nov., P. tinguichaca, P. torresi sp. nov., P. totoroi sp. nov., P. versicolor, P. verrucolatus sp. nov., Pristimantis sp. (CCS1), and Pristimantis sp. (CCS2); and three unconfirmed species: Pristimantis sp. (UCS1), Pristimantis sp. (UCS2), and Pristimantis sp. (UCS3). The P. cryptomelas species group comprises P. cryptomelas, P. gagliardoi, P. muscosus, P. nangaritza sp. nov., and P. spinosus. We present the evidence used to define the limits of each species in the following section.
We identified that the recently described P. hampatusamiYánez-Muñoz et al., 2016 and P. prometeiiSzékely et al., 2016 belong to the same species. We compared specimens of both species from the QCAZ and INABIO collections. Our comparisons showed that both species are morphologically indistinguishable. The type locality for both species is the same further suggesting that both represent a single species. With this evidence, we propose P. prometeii as a junior synonym for P. hampatusami given the principle of priority.
We found a misidentification of two Genbank sequences with accession numbers KU217863 and KU218025. GenBank identifications are P. cryophilius and P. phoxocephalus, respectively. They actually are P. philipi (KU217863) and P. totoroi sp. nov. (KU218025). Updated identifications of sequences used in this study are shown in Table 1.
In the following section, we present the descriptions of the new species and new taxa identified in this study. We also include an updated diagnosis of P. phoxocephalus sensu stricto, as diagnoses made in its description and later revisions (e.g., Duellman and Pramuk 1993; Lynch and Duellman 1997) included information of non-conspecific populations.
Systematic accounts
Huicundomantis subgenus nov.
9097e14e-f8a4-53d6-a517-956a8045dae7
http://zoobank.org/A18720A1-FB71-4342-B0B2-8C9C8F06B5F4
Type species.
Pristimantis phoxocephalus (Lynch, 1979).
Definition.
This clade is strongly supported by genetic evidence (Fig. 3). Morphological synapomorphies are unknown. Members of this clade are characterized by: (i) dorsolateral folds absent (except for P. atratus); (ii) cranial crests absent (except for P. atratus, P. percultus, and P. spinosus); (iii) tympanic membrane and tympanic annulus prominent (both absent in P. philipi); (iv) dentigerous processes of vomer present; (v) small to prominent tubercles on heel; (vi) fingers with lateral fringes; (vii) basal webbing between toes (except for P. balionotus, P. hampatusami, and P. philipi which lack webbing); (viii) Toe V longer or much longer than Toe III (Figs 7–9); (ix) in life, groins and concealed surfaces of thighs with distinctive coloration patterns including flash colors and light or bright colored flecks or spots on a darker background; colors, shapes and sizes of these ornaments are variable among species; (x) SVL females 24.9–46.8 mm; SVL males 16.1–34.5 mm. Species of this clade may bear a fleshy keel or a papilla at the tip of the snout, and lateral, middorsal, or postocular folds.
Figure 7.
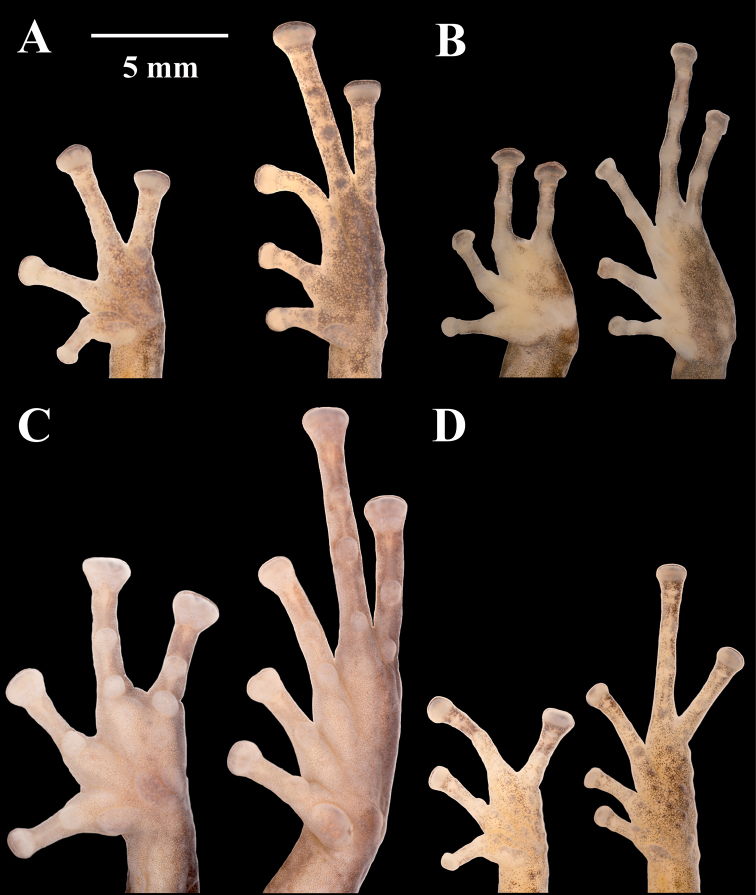
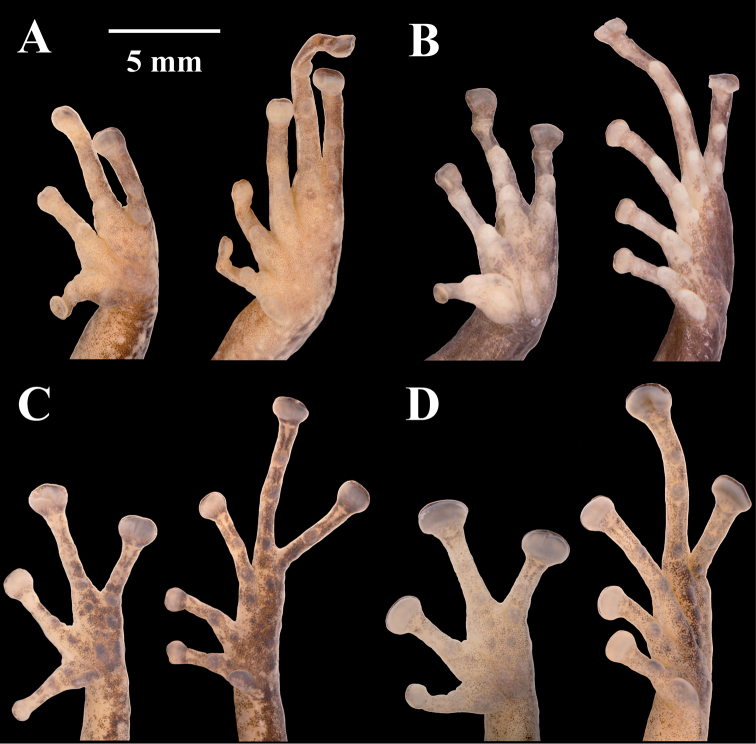
Palmar and plantar surfaces of Pristimantis atillo sp. nov., Pristimantis chomskyi sp. nov., Pristimantis gloria sp. nov., and Pristimantis jimenezi sp. nov. Photographs of left hand and foot of the holotypes APristimantis atillo sp. nov.: QCAZ 42500, male BPristimantis chomskyi sp. nov.: QCAZ 47515, male CPristimantis gloria sp. nov.: QCAZ 57201, female DPristimantis jimenezi sp. nov.: QCAZ 45170, male. All specimens are shown at the same scale.
Figure 9.
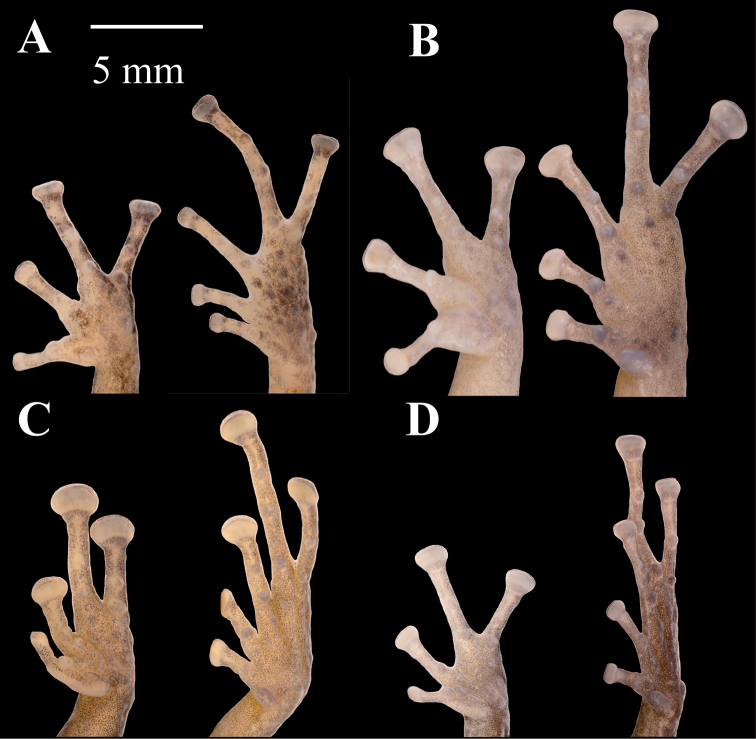
Palmar and plantar surfaces of Pristimantis teslai sp. nov., Pristimantis torresi sp. nov., Pristimantis totoroi sp. nov., and Pristimantis verrucolatus sp. nov. Photographs of hand and foot of the holotypes of the following species: APristimantis teslai sp. nov.: QCAZ 46213, male BPristimantis torresi sp. nov.: QCAZ 47342, female CPristimantis totoroi sp. nov.: QCAZ 25105, male DPristimantis verrucolatus sp. nov.: QCAZ 46982, male. All specimens are shown at the same scale.
Content.
This clade comprises 23 described species (11 of them described below): P. atillo sp. nov., P. atratus, P. balionotus, P. chomskyi sp. nov., P. cryptomelas, P. gagliardoi, P. gloria sp. nov., P. hampatusami, P. jimenezi sp. nov., P. lutzae sp. nov., P. multicolor sp. nov., P. muscosus, P. nangaritza sp. nov., P. percultus, P. philipi, P. phoxocephalus, P. spinosus, P. teslai sp. nov., P. tinguichaca, P. torresi sp. nov., P. totoroi sp. nov., P. versicolor, P. verrucolatus sp. nov. This clade encompasses the P. phoxocephalus and P. cryptomelas species groups.
Distribution.
Huicundomantis occurs in Eastern and Western Andean slopes and Inter-Andean valleys of southern and central Ecuador, and Eastern Andean slopes of northern Peru. They inhabit the following Natural Regions: Deciduous Costa Forest, Western Foothill Forest, Western Montane Forest, Paramo, Inter-Andean Shrub, Eastern Montane Forest, and Eastern Foothill Forest, between elevations of 230 and 4200 m a.s.l.
Etymology.
We name this clade Huicundomantis because these frogs are frequently found inside bromeliad plants. Huicundo is a word in Quechua, an indigenous South American language, locally used to referring to bromeliads.
Pristimantis phoxocephalus species group new taxon
Definition. The P. phoxocephalus species group is strongly supported in our phylogeny. Members of this group share the following morphological traits: (i) dorsolateral folds absent (except for P. atratus); (ii) snout with a fleshy keel or papilla at the tip; (iii) cranial crests absent (except for P. atratus and P. percultus); (iv) tympanic membrane and tympanic annulus prominent; (v) dentigerous processes of vomer present; (vi) males with vocal slits (except for P. versicolor); (vii) fingers and toes with lateral fringes; (viii) basal webbing between toes (except for P. balionotus and P. hampatusami); (ix) Toe V longer or much longer than Toe III (Fig. 7); (x) in life, groins and concealed surfaces of thighs with distinctive coloration, including flash colors and light or bright colored flecks or spots on a darker background; colors, shapes, and sizes of ornaments variable among species; (xi) SVL females 24.9–46.8 mm; SVL males 16.8–34.5 mm.
Content. Currently, the P. phoxocephalus group comprises 17 described species (10 of them are described below): P. atillo sp. nov., P. atratus, P. balionotus, P. chomskyi sp. nov., P. gloria sp. nov., P. hampatusami, P. jimenezi sp. nov., P. lutzae sp. nov., P. multicolor sp. nov., P. percultus, P. phoxocephalus, P. teslai sp. nov., P. tinguichaca, P. torresi sp. nov., P. totoroi sp. nov., P. versicolor, P. verrucolatus sp. nov.
Distribution. Eastern and Western Andean slopes and Inter-Andean valleys of central and southern Ecuador, in ten provinces: Azuay, Bolívar, Cañar, Chimborazo, Cotopaxi, El Oro, Loja, Morona Santiago, Tungurahua, and Zamora Chinchipe. They inhabit Deciduous Costa Forest, Western Foothill Forest, Western Montane Forest, Paramo, Inter-Andean Shrub, and Eastern Montane Forest Natural Regions, between 230 and 4100 m a.s.l.
Remarks. The P. phoxocephalus species group is sister to the P. cryptomelas species group.
Pristimantis atillo sp. nov.
23E6843DA14459C5B4E6D2DAD284E7DA
http://zoobank.org/078D7203-04D4-4509-9769-8DE14FF5B886
Common name.
English: Atillo Rain Frog. Spanish: Cutín de Atillo.
Holotype.
QCAZ 42500, an adult male from Sangay National Park, Ranger Station, Atillo, Morona Santiago Province, Ecuador (2.1867S, 78.4963W, 3400 m), collected by Elicio Tapia on May 19, 2009. Figures 10A, 11A.
Figure 10.
Holotypes of Pristimantis atillo sp. nov. and P. chomskyi sp. nov. Photographs of preserved holotypes of AP. atillo (QCAZ 42500, male) and BP. chompskyi (QCAZ 47515, male). Dorsal view on the left, ventral view on the right. Specimens are shown at the same scale.
Figure 11.
Color variation in live individuals of Pristimantis atillo sp. nov. AQCAZ 42500 (holotype, male, SVL 26.3 mm) BQCAZ 42502 (male, SVL 20.6 mm) CQCAZ 59228 (male, SVL 23.7 mm) DQCAZ 59261 (male, SVL 25.3 mm). Dorsolateral view on the left, ventral view on the right.
Paratypes
(44: 28 males, 5 females, 11 juveniles). All from Sangay National Park, nearby the type locality. Ecuador: Chimborazo Province: QCAZ 2298–302, QCAZ 2304, adult males, QCAZ 2303, QCAZ 2305–306, juveniles (2.1749S, 78.5047W, 3600 m), collected by Giovanni Onore and Luis Coloma in October 1991; QCAZ 3110, adult female, QCAZ 3111–112, adult males (2.1749S, 78.5047W, 3600 m), collected by Luis Coloma in January 1991; QCAZ 40593, QCAZ 40594, adult females (2.2103S, 78.4991W, 3730 m), collected by Diego Almeida, Galo Díaz, Ítalo Tapia, and Martín Bustamante in May 2003. Morona Santiago Province: QCAZ 31946, adult male (2.1989S, 78.4824W, 3185m), collected by Charles M. Kieswetter and Ítalo Tapia in April 2006; QCAZ 40584, adult male (2.2024S, 78.4697W, 3366 m), collected by Diego Almeida, Galo Díaz, Ítalo Tapia and Martín Bustamante in May 2002; QCAZ 42501, adult female, QCAZ 40897, QCAZ 42483–486, QCAZ 42488–492, QCAZ 42499–500, QCAZ 42502–505, adult males, QCAZ 42493, QCAZ 42496, QCAZ 42498, QCAZ 42506, QCAZ 42512, QCAZ 42543, QCAZ 42548, juveniles (2.1867S, 78.4963W, 3400 m), collected by Elicio Tapia on May 18–19, 2009; QCAZ 59274, adult female, QCAZ 59214, QCAZ 59261, adult males (2.1842S, 78.4972W, 3436 m), collected by Francy Mora, David Velalcázar, Javier Pinto, Luis Tipantiza, Keyko Cruz, Daniel Rivadeneira, Freddy Almeida, Pol Pintanel, and Nadia Páez in March 2015. QCAZ 59228, adult male, QCAZ 59276, juvenile (2.1887S, 78.4823W, 3299 m), collected by Francy Mora, David Velalcázar, Javier Pinto, Luis Tipantiza, Keyko Cruz, Daniel Rivadeneira, Freddy Almeida, Pol Pintanel, and Nadia Páez in March 2015.
Diagnosis.
A member of the Pristimantis phoxocephalus group having the following combination of characters: (1) skin on dorsum shagreen with or without scattered small subconical tubercles; middorsal fold ill-defined or absent; head with a middorsal longitudinal row of two or more subconical tubercles; dorsolateral folds absent; skin on venter areolate; discoidal fold present or absent; (2) tympanic membrane and tympanic annulus prominent, its upper and posterior margin concealed by supratympanic fold; (3) snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; (4) upper eyelid with one or more small prominent subconical tubercles surrounded by lower tubercles; cranial crests absent; (5) dentigerous processes of vomers low to prominent, oblique, moderately separated, posteromedial to choanae; (6) males having vocal slits, external vocal sac and white nuptial pads; (7) Finger I shorter than Finger II; discs of digits expanded to broadly expanded, rounded to elliptical; (8) fingers with broad lateral fringes; (9) ulnar tubercles low and rounded, sometimes connected by an ill-defined fold; (10) heel bearing one subconical tubercle surrounded or not by smaller rounded tubercles; outer edge of tarsus bearing low subconical tubercles; inner edge with or without ill-defined tubercles, short inner tarsal fold present; (11) inner metatarsal tubercle ovoid, elevated, five times the size of round outer metatarsal tubercle; supernumerary plantar tubercles numerous; (12) toes with lateral fringes, less conspicuous than those on fingers; basal webbing on feet (Fig. 7A); Toe V longer or much longer than Toe III (disc on Toe III reaches or exceeds distal edge of the penultimate subarticular tubercle on Toe IV, disc on Toe V reaches or exceeds distal edge of the distal subarticular tubercle on Toe IV); toe discs nearly as large as those on fingers; (13) in life, dorsal coloration varies from pale to dark brown; black or brown interorbital stripe, supratympanic stripe, and labial bars present; flanks with black dots and flecks arranged in a diagonal pattern, bordering light reticulations; groins, axils, concealed surfaces of thighs, and shanks orange; venter and throat cream to white; iris copper with a faint medial horizontal darker streak and black reticulations (Fig. 11); (14) average SVL in adult females: 31.3 ± 2.3 mm (29.4–35.3 mm; n = 5); in adult males: 24.7 ± 2.5 mm (17.5–28.1 mm; n = 29).
Comparison with other species.
Pristimantis atillo is similar to other species with acuminate and protruding snouts of this group such as P. jimenezi sp. nov., P. phoxocephalus, P. teslai sp. nov., P. torresi sp. nov., P. totoroi sp. nov., and P. verrucolatus sp. nov. The bright orange coloration of its groins and posterior surfaces of thighs distinguishes it from them (brown with small light brown to yellow spots in P. jimenezi sp. nov.; yellow with black reticulations in P. phoxocephalus; dark brown with yellow irregular blotches in P. teslai sp. nov.; brown with or without yellow spots in P. torresi sp. nov.; reddish brown with small light brown to yellow spots in P. verrucolatus sp. nov.). Pristimantis atillo can be further distinguished from P. jimenezi sp. nov. and P. phoxocephalus by the presence of its characteristic black dots in the flanks (black dots absent in P. jimenezi sp. nov. and P. phoxocephalus). Pristimantis atillo differs from P. teslai sp. nov. by the dorsal skin texture (shagreen in P. atillo; tuberculate in P. teslai sp. nov.). The copper coloration of the iris differentiates P. atillo from P. torresi sp. nov. and P. totoroi sp. nov., whose iris is golden with a red streak. Furthermore, tubercles and folds of P. totoroi sp. nov. are more prominent than those of P. atillo, and its head, relative to the body, is longer (males Wilcoxon’s Z = 4.50124, p < 0.001, HL/SVL = 33.5–38.5% in P. atillo, 34.2–38.7% in P. totoroi sp. nov.; females Z = 2.43599, p = 0.0149, HL/SVL = 33.1–36.6% in P. atillo, 35.2–37.6% in P. totoroi sp. nov.). Pristimantis verrucolatus sp. nov. differs from P. atillo in having large tubercles and warts on its flanks. Pristimantis atillo is similar to P. modipeplus (Lynch 1981) in coloration pattern; P. modipeplus has a subacuminate snout in dorsal view and rounded in profile, lacks basal webbing between toes, and large females bear low cranial crests (Lynch 1981); meanwhile, P. atillo has an acuminate and protruding snout with a keel at the tip, presents basal webbing between toes and has no cranial crests.
Description of the holotype.
Adult male (QCAZ 42500, SC28232). Measurements (in mm): SVL 26.3; TL 12.0; FL 11.9; HL 8.9; HW 9.1; ED 2.6; TD 1.3; IOD 2.8; EW 2.4; IND 2.1; EN 2.7; TED 1.1. Head narrower than body, slightly wider than long; snout moderately long, acuminate with triangular fleshy keel in dorsal view, protruding in profile; nostrils slightly protuberant, directed laterally with slight dorsal inclination; canthus rostralis concave in dorsal view, rounded in cross section; loreal region slightly concave; cranial crests absent; upper eyelid bearing one small but distinct conical tubercle surrounded by few indistinct smaller tubercles; tympanic membrane and annulus prominent, its upper and posterior margins concealed by supratympanic fold; two enlarged conical postrictal tubercle surrounded by indistinct low tubercles. Choanae median, elliptical, non-concealed by palatal shelf of maxilla; dentigerous processes of vomers prominent, oblique, moderately separated, posteromedial to choanae; each vomer bearing several indistinct teeth; tongue slightly longer than wide, posteriorly notched, posterior two-thirds free; vocal slits slightly curved, located at posterior half of mouth floor in between tongue and margin of jaw; vocal sac present.
Skin on dorsum shagreen; head with a row of two middorsal tubercles; dorsolateral folds absent; flanks with slightly larger tubercles than those on dorsum; skin on throat, chest, belly and ventral surfaces of thighs areolate; discoidal fold absent. Two distinct, low and round ulnar tubercles connected by an ill-defined fold; white nuptial pads present; palmar tubercles prominent, outer palmar tubercle bifid, slightly bigger than ovoid thenar tubercle; subarticular tubercles prominent, rounded; distinct, low supernumerary tubercles at base of fingers; fingers with broad lateral fringes; Finger I shorter than Finger II; discs on fingers expanded and elliptical; ventral pads on fingers surrounded by circumferential grooves (Fig. 7A).
Hindlimbs slender; dorsal surfaces of hindlimbs with scattered low round tubercles; posterior surfaces of thighs smooth, ventral surfaces of thighs areolate; heel bearing one low conical tubercle surrounded by some lower rounded tubercles; outer edge of tarsus bearing low subconical tubercles; inner edge of tarsus with ill-defined tubercles; short inner tarsal fold present; inner metatarsal tubercle ovoid, elevated, five times the size of circular, rounded, outer metatarsal tubercle; plantar surface with numerous indistinct supernumerary tubercles; subarticular tubercles prominent, rounded; toes with broad lateral fringes; small basal webbing between toes; discs nearly as large as those on fingers; discs on toes expanded, elliptical; all toes having ventral pads and circumferential grooves; relative lengths of toes: I<II<III<V<IV; Toe V much longer than Toe III (disc on Toe III reach distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches distal edge of distal subarticular tubercle on Toe IV; Fig. 7A). Coloration of the holotype in preservative and life is shown in Figures 10, 11.
Variation.
This section is based on 45 preserved specimens of the type series and photographs available for 11 individuals. Variation in living and preserved individuals is shown in Figures 11, 12. Coloration in life is mentioned in parenthesis. Dorsum varies from pale to dark brown with or without lighter irregular marks or white spots scattered on dorsum, few individuals have several thin parallel longitudinal stripes or a middorsal band (i.e., longitudinal pattern); the interorbital band can be thin to broad, paler or darker than background and is absent in individuals with longitudinal pattern; interscapular blotch is often present. Flanks have pale oblique reticulations usually surrounded by dark stripes formed by rows of black flecks. Groins, anterior and posterior surfaces of thighs cream (orange, in most cases surrounded by yellow blotches; an individual has yellow groins (QCAZ 42502)). Dorsal surfaces of thighs with brown to black oblique stripes. Venter and throat coloration varies from cream to white (cream to white with different levels of transparency, with or without ill-defined yellow blotches) with or without black flecks. Iris is reddish to orangey copper with a faint medial horizontal darker streak and thin black reticulations; sclera varies from white to light blue.
Figure 12.
Color variation in preserved individuals of Pristimantis atillo sp. nov. A Dorsal view of (from left to right): QCAZ 40593 (female), QCAZ 42501 (female), QCAZ 59274 (female) B dorsal view of: QCAZ 42498 (juvenile female), QCAZ 42503 (male), QCAZ 2302 (male), QCAZ 42548 (juvenile female) C ventral view of specimens in (A) D ventral view of specimens in B. See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Coloration of holotype in preservative. Dorsum brown with lighter markings including W-shaped scapular mark, interscapular blotch, and faint irregular reticulations; head with light brown interorbital band; face with faint canthal and labial bars; dark brown supratympanic bar; flanks light brown with pale oblique reticulations surrounded by rows of black flecks; armpits, groins, anterior and posterior surfaces of thighs cream; dorsal surfaces of hindlimbs brown with lighter transversal bands; limbs with scattered black flecks; ventral surfaces cream; plantar and palmar surfaces dirty cream (Fig. 10A).
Coloration of holotype in life. Based on studio photographs (Fig. 11A). Dorsum brown with cream light markings including a W-shaped scapular mark, interscapular blotch, and irregular reticulations; head with light brown interorbital band; face with faint brown canthal and labial bars; dark brown supratympanic bar; flanks light brown with yellow reticulations surrounded by rows of black flecks; armpits, groins, anterior and posterior surfaces of thighs and shanks orange; dorsal surface of hindlimbs with faint yellowish brown transverse bands and scattered black flecks; venter yellowish white; vocal sac, chest, and ventral surfaces of forelimbs surfaces transparent pinkish cream; ventral surface of thighs yellow suffused with orange; plantar and palmar surfaces dirty cream; iris copper with a faint medial horizontal darker streak and thin black reticulations; light blue sclera.
Distribution, natural history, and conservation status.
This species is only known from the type locality, surroundings of Lagunas de Atillo, and nearby locations at Sangay National Park, between 3185 and 3730 m a.s.l (Fig. 1). This corresponds to the Eastern Montane Forest and Paramo biogeographic regions. Most individuals collected at night were found active on low vegetation, from ground level up to 1 m above the ground; an individual was collected from a bromeliad 2.5 m above the ground. During the day, they were found beneath rocks. Most collections were made on roadsides. Calling males have been found in March.
According to available data, this species has a very restricted distribution (Extent of Occurrence 7 km2, Area of Occupancy 16 km2). However, less accessible adjacent areas in Sangay National Park are unexplored and represent potential distribution areas for this species. Therefore, we consider this species to be Data Deficient (IUCN 2017).
Etymology.
The specific epithet refers to the type locality of this species, the surroundings of Lagunas de Atillo, a lake complex in Sangay National Park, a UNESCO World Heritage Site.
Pristimantis chomskyi sp. nov.
E412B6355C165C5F8F76C11949A51BEA
http://zoobank.org/710B5B6E-FECA-46AC-A7F8-A62DF3B7747B
Common name.
English: Chomsky’s Rain Frog. Spanish: Cutín de Chomsky.
Holotype.
QCAZ 47515, an adult male from Tapichalaca Reserve, Zamora Chinchipe Province, Ecuador (4.4730S, 79.1930W, 3366 m), collected by Elicio E. Tapia on September 27, 2009. Figures 10B, 13A.
Figure 13.
Color variation in live individuals of Pristimantis chomskyi sp. nov. AQCAZ 47515 (holotype, male, SVL 24.0 mm) BQCAZ 45669 (juvenile male, SVL 21.4 mm) CQCAZ 45665 (juvenile female, SVL 19.7 mm) DQCAZ 45666 (juvenile female, SVL 21.9 mm). Dorsal view on the left, ventral view on the right.
Paratypes
(11: 2 adult males, 9 juveniles).QCAZ 45670, QCAZ 47733, adult males, QCAZ 45664–669, QCAZ 45677, QCAZ 47730–731, juveniles, collected with the holotype.
Diagnosis.
A species of Pristimantis having the following combination of characters: (1) skin on dorsum shagreen, skin on flanks with large warts, skin on venter areolate to coarsely areolate; discoidal fold absent; dorsolateral folds absent; (2) tympanic membrane and tympanic annulus prominent, its upper and posterolateral margin concealed by thick supratympanic fold; (3) snout short, subacuminate in dorsal view, rounded in lateral view, with or without a small papilla at the tip; (4) upper eyelid lacking tubercles; cranial crests absent; (5) dentigerous processes of vomers low to prominent, oblique, moderately separated, posteromedial to choanae; (6) vocals slits, vocal sac and nuptial pads present in adult males; (7) Finger I shorter than Finger II; discs of digits expanded, rounded to elliptical; (8) fingers with lateral fringes; (9) ulnar tubercles low, diffuse; (10) heel bearing one small low rounded tubercle; inner edge of tarsus with or without a low tubercle or small fold; outer edge of tarsus with or without indistinct tubercles; (11) inner metatarsal tubercle elliptical, elevated, about 4 times the size of round outer metatarsal tubercle; supernumerary tubercles low; (12) toes with lateral fringes; basal webbing on feet; Toe V longer to much longer than Toe III (disc on Toe III reaches or exceeds distal edge of the penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the middle to distal edge of the distal subarticular tubercle on Toe IV); toe discs smaller than those on fingers (Fig. 7B); (13) in life, dorsum dark brown to orange; head bears brown supratympanic and canthal stripes; groins, axils and concealed surfaces of hindlimbs dark chocolate brown suffused or not with orange, with or without small cream flecks; venter cream to dusty brown with or without dark markings; iris orange with a faint reddish brown medial horizontal streak and thin black reticulations (Fig. 13); (14) average SVL in adult males: 28.0 ± 4.2 mm (24.0–32.4 mm; n = 3); females: unknown.
Comparison with other species.
Pristimantis chomskyi is similar to P. balionotus, P. gloria sp. nov., P. lutzae sp. nov., and P. multicolor sp. nov. The most similar is its sister species P. multicolor sp. nov. The region above supratympanic fold is more swollen in P. chomskyi. In addition, P. chomskyi has a smaller tympanum (males Z = 2.38157, p = 0.0172, TD/SVL = 4.5–4.7% in P. chomskyi, 4.9–6% in P. multicolor sp. nov.). Pristimantis balionotus can be distinguished from P. chomskyi because it has a tuberculate dorsal skin, lacks basal webbing between toes, and its iris is bronze with a red medial streak (shagreen dorsal skin, basal webbing between toes, iris orange with a faint reddish-brown streak in P. chomskyi). Pristimantis chomskyi is easy to distinguish from P. gloria sp. nov. by the coloration of the iris (orange with a faint reddish brown streak and thin black reticulations in P. chomskyi; light silver to cream with wide black reticulations and a red streak in P. gloria sp. nov.) and groins (dark chocolate brown with or without small cream flecks in P. chomskyi; pinkish to purplish brown with irregular cream to light brown flecks or spots in P. gloria sp. nov.), and the texture of the dorsal skin (shagreen without a middorsal fold in P. chomskyi; tuberculate to warty with a middorsal fold in P. gloria sp. nov.). Furthermore, P. gloria sp. nov. is smaller (males Z = -2.31490, p = 0.0206, SVL = 24.0–32.4 mm in P. chomskyi, 16.7–24.7 mm in P. gloria sp. nov.), has larger tympanum (males Z = 2.66173, p = 0.0078, TD/SVL = 4.5–4.7% in P. chomskyi, 5.1–6.2% in P. gloria sp. nov.) and smaller eye (males Z = -2.50743, p = 0.0122, ED/SVL = 11.1–12% in P. chomskyi, 11–13.8% in P. gloria sp. nov.), relative to its body length. Pristimantis lutzae sp. nov. differs from P. chomskyi in having a golden to creamy brown iris with a reddish brown streak, a larger tympanum (males Z = 2.45677, p = 0.0140, TD/SVL = 4.5–4.7% in P. chomskyi, 5–5.4% in P. lutzae sp. nov.) and smaller eye (males Z = -2.45677, p = 0.0140, ED/SVL = 11.1–12% in P. chomskyi, 10.3–12.1% in P. lutzae sp. nov.), relative to its body length.
Description of the holotype.
An adult male (QCAZ 47515, SC29227). Measurements (in mm): SVL 24.0; TL 12.0; FL 11.6; HL 8.8; HW 9.4; ED 2.9; TD 1.1; IOD 3.3; EW 2.6; IND 2.4; EN 2.4; TED 1.1. Head wider than long, narrower than body; snout subacuminate in dorsal view, rounded in profile, with a small papilla at the tip; cranial crests absent; nostrils slightly protuberant, directed anterolaterally; canthus rostralis slightly concave in dorsal view, rounded in cross section; loreal region concave; upper eyelid lacking tubercles; tympanic annulus prominent, its upper and posterolateral edge concealed by thick, heavy supratympanic fold; tympanic membrane distinct; two prominent postrictal tubercle not surrounded by smaller tubercles. Choanae median, ovoid, non-concealed by palatal shelf of maxilla; dentigerous processes of vomers prominent, oblique, moderately separated, positioned posteromedial to choanae; each vomer bearing several indistinct teeth; tongue slightly longer than wide, not notched, posterior half free; vocal slits slightly curved, positioned at posterior half of mouth floor in between tongue and margin of jaw; small vocal sac.
Dorsal surfaces of body shagreen; dorsolateral folds absent; skin on flanks bearing low rounded warts; skin on chest and belly areolate, that on throat shagreen, ventral surfaces of limbs smooth, ventral surfaces of thighs coarsely areolate; discoidal fold absent. Diffuse ulnar tubercles; nuptial pads present; outer palmar tubercle bifid, as large as ovoid thenar tubercle; subarticular tubercles prominent, rounded; large supernumerary tubercles at base of fingers, distinct; fingers bearing lateral fringes; Finger I shorter than Finger II; discs on fingers expanded and rounded; pads on fingers surrounded by circumferential grooves on all fingers (Fig. 7B).
Hindlimbs slender; dorsal surfaces of hindlimbs shagreen; posterior surfaces of thighs smooth, ventral surfaces of thighs coarsely areolate; heel bearing one low and rounded tubercle; outer and inner edge of tarsus lacking tubercles; inner metatarsal tubercle elliptical, elevated, 4 times the size of oval outer metatarsal tubercle; plantar surface with small, low and rounded supernumerary tubercles; subarticular tubercles prominent, rounded; toes bearing lateral fringes; basal webbing between toes III and IV, and IV and V; discs on toes smaller than those on fingers, expanded and rounded; all toes having pads surrounded by circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V longer than Toe III (disc on Toe III reaches the distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the distal edge of distal subarticular tubercle on Toe IV; Fig. 7B). Coloration of the holotype in life and preservative is shown in Figures 10A, 13A.
Coloration of holotype in preservative. Dorsal surfaces of body and flanks cream covered by minute brown spots evenly distributed; brown supratympanic and canthal stripes; groins, anterior, and posterior surfaces of thighs, shanks and tarsus brown; venter, belly and throat cream suffused with dusty brown irregular spots; belly with dark brown blotches (this pattern does not correspond with coloration in life, probably effect of preservation); ventral surfaces of limbs dusty cream; ventral surfaces of fingers and toes cream (Fig. 10B).
Coloration of holotype in life. Based on studio photographs. Dorsal surfaces of body and flanks orange-hued brown covered by minute chocolate brown spots evenly distributed; brown supratympanic and canthal stripes; groins, anterior and posterior surfaces of thighs, shanks and tarsus chocolate brown; venter yellowish cream covered by chocolate brown spots coinciding with areolation pattern; throat orange suffused with brown; ventral surfaces of thighs, shanks, and tarsus reddish orange suffused with brown; plantar and palmar surfaces orange; iris orange with a faint reddish brown medial horizontal streak and thin black reticulations (Fig. 13A).
Variation.
Based on the 12 preserved specimens of the type series and photographs from eight individuals. Variation in life and preservative is shown in Figures 13, 14. Coloration in life is given in parenthesis. Dorsum and flank coloration vary from brown to brownish cream (dark brown to orange). Dorsal markings may be absent or present as longitudinal stripes or dark blotches evenly scattered; one individual has a pale blotch on dorsum. All individuals have brown supratympanic and canthal stripes; some also have dark interorbital stripe and labial bars. Color of groins, armpits, dorsal surfaces of thighs, concealed surfaces of thighs, shanks and tarsus is dark brown (dark chocolate brown, suffused or not with orange, with or without small cream flecks), few individuals with small pale flecks; dorsal surfaces of thighs and groins can have irregular pale spots. Limbs bear scattered small dark spots arranged or not as transversal bands. Venter varies from cream to dusty brown with or without dark markings (orange, cream, or dusty brown, with or without darker flecks or mottling). The iris is orange with a faint red medial streak and thin black reticulations. Sclera varies from white to light blue.
Figure 14.
Color variation in preserved individuals of Pristimantis chomskyi sp. nov. A Dorsal view of (from left to right): QCAZ 45670 (male), QCAZ 47731 (juvenile female), QCAZ 45667 (juvenile male), B Dorsal view of: QCAZ 45669 (juvenile male), QCAZ 45670 (male), QCAZ 45666 (juvenile female) C Ventral view of specimens in (A) D Ventral view of specimens in B See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Distribution, natural history, and conservation status.
This species is only known from the type locality, Tapichalaca Reserve, Zamora Chinchipe Province, Ecuador at 3366 m a.s.l (Fig. 1), which corresponds to the Paramo biogeographic region. All individuals were found at night inside bromeliads, mostly of the genus Puya. Tapichalaca Reserve is adjacent to Podocarpus National Park (northward) and Yacuri National Park (southward). Both areas are unexplored and represent potential habitats for this species. Therefore, we assign P. chomskyi to the Data Deficient Red List Category (IUCN 2017).
Etymology.
The specific epithet is a noun in the genitive case and is a patronym for Noam Chomsky, US born theoretical linguist and one of the most cited modern scholars. Chomsky is the founder of modern linguistics. He developed the concept of “universal grammar,” an innate cognitive capacity, shared by all humans, which allows to learn and communicate through complex speech. Chomsky was professor at the Massachusetts Institute of Technology between 1950 and 2017. He is currently professor at Arizona State University.
Pristimantis gloria sp. nov.
0F786388E489523FB2AA9523676E4325
http://zoobank.org/115BC459-FCEA-4950-8B80-7E5B7D6DB8C4
Common name.
English: Gloria’s Rain Frog. Spanish: Cutín de Gloria.
Holotype.
QCAZ 57201, a gravid female from Cochapata, Azuay Province, Ecuador (3.4284S, 79.0699W, 3100 m), collected by Edwin Carrillo on May 1, 2014. Figures 15A, 16A.
Figure 15.
Holotypes of Pristimantis gloria sp. nov. and P. jimenezi sp. nov. Photographs of preserved holotypes of AP. gloria (QCAZ 57201, female) and BP. jimenezi (QCAZ 45170, male). Dorsal view on the left, ventral view on the right. Specimens are shown at the same scale.
Figure 16.
Color variation in live individuals of Pristimantis gloria sp. nov. AQCAZ 57201 (holotype, female, SVL 35.8 mm) BQCAZ 62778 (male, SVL 19.7 mm) CQCAZ 62780 (juvenile female, SVL 23.5 mm) DQCAZ 62789 (juvenile female, SVL 21.8 mm) For A–C dorsolateral view on the left, ventral view on the right. For D dorsolateral view on the left, close up of the eye in the right.
Paratypes
(59: 14 females, 24 males, 21 juveniles). Ecuador: Azuay Province: QCAZ 3051–052, QCAZ 3054, adult males, QCAZ 3053, juvenile, from 7 km E Sigsig (3.0973S, 78.7970W, 2890 m); QCAZ 3055, juvenile, from 8 km E Sigsig (3.1025S, 78.7929W, 2920 m); QCAZ 3056, juvenile, from 3 km Sigsig, Sigsig-Las Minas road (3.0750S, 78.8000W, 2460 m), collected by Luis Coloma, Oscar Delgado and Luis Eduardo López on July 25, 1989; QCAZ 3452, male, QCAZ 3451, juvenile, from 23 km SE Sigsig (3.1794S, 78.7993W, 3318 m), collected by Stella de la Torre, Luis Coloma, and Felipe Campos on November 27, 1992; QCAZ 16449, adult female, QCAZ 16452, adult male, QCAZ 16447–448, juveniles, collected by Luis Eduardo López on September 2001; QCAZ 45289, adult male, collected by Luis Eduardo López on August 28, 2009; QCAZ 56533, QCAZ 56536, adult females, QCAZ 56534, juvenile, collected by Luis Eduardo López on December 6, 2013; QCAZ 62779, QCAZ 62783, QCAZ 62788, QCAZ 62791, adult females, QCAZ 62778, QCAZ 62782, QCAZ 62787, adult male, QCAZ 62780, QCAZ 62789, juveniles, collected by Luis Eduardo López on December 13, 2015, from Patococha (3.0040S, 78.6658W, 3416 m); QCAZ 29133, juvenile, from Matanga (3.1779S, 78.8000W, 3325 m), collected by Luis Coloma on December 1, 1991; QCAZ 30737, female, from 11.1 km W Azuay-Morona Santiago border (2.9480S, 78.7120W, 3110 m), collected by Luis Coloma and John Wiens on April 20, 1990; QCAZ 30738, adult male, QCAZ 30739, juvenile, from 8.1 km W Azuay-Morona Santiago border (2.9640S, 78.7020W, 3140 m), collected by John Wiens on April 20, 1990; QCAZ 30740–745, juveniles from 10 km S Cutchil (3.1340S, 78.8130W, 2900 m), collected by Luis Coloma and John Wiens on April 29, 1990; QCAZ 56463, adult male from Chiguinda, Páramo de Matanga (3.1885S, 78.7922W, 3327 m), collected by Jorge Brito on October, 2013. Loja Province: QCAZ 31455, QCAZ 31457, adult females, from Fierro Urcu (3.7104S, 79.3049W, 3439 m), collected by Ítalo G. Tapia on March 2006. Morona Santiago Province: QCAZ 17143, juvenile female, from Gualaceo-Macas road (3.0097S, 78.6650W, 3462 m), collected by Omar Torres, Jennifer Pramuk and Lena Echelle on September 14, 2001; QCAZ 23933, adult female, QCAZ 23929–932, adult males, from 52 km N El Ideal (3.1831S, 78.7961W, 3525 m), collected by Santiago Ron and Giovanna Romero on April 10, 2003; QCAZ 26367, QCAZ 26370, adult females, QCAZ 26368–369, QCAZ 26372–373, adult males, QCAZ 26371, QCAZ 26375, juveniles, from Gualaceo-Plan de Milagro road (3.0007S, 78.6616W, 3372 m), collected by Andrés Merino, Ítalo Tapia and Erik Wild on August 10, 2003; QCAZ 27280, adult female, QCAZ 27276–279, QCAZ 27281, adult males, from Gualaceo-Plan de Milagro road (3.0029S, 78.6572W, 3096 m), collected by David A. Kizirian and Juan Carlos on November 2, 2000; QCAZ 40784, juvenile, from Sigsig-Gualaquiza road (3.1850S, 78.7962W, 3322 m), collected by Omar Torres, Ernesto Arbeláez, Amaranta Carvajal and David Salazar on June 17, 2008.
Referred specimens.
KU 218035 from 8.1 km W Morona Santiago border, Gualaceo-Limón road (2.9645S, 78.7016W, 3145 m) Azuay Province, Ecuador.
Diagnosis.
A species of Pristimantis having the following combination of characters: (1) skin on dorsum tuberculate to warty; tubercles and warts are more prominent in posterior dorsum; middorsal fold present; dorsolateral folds absent; skin on flanks bears large warts and more prominent tubercles than those on dorsum; skin on venter coarsely areolate; (2) tympanic membrane and tympanic annulus prominent, its upper and posterolateral margin concealed by supratympanic fold; (3) snout moderately long, subacuminate in dorsal view, rounded in profile; with or without a small papilla at the tip; (4) upper eyelid without conspicuous tubercles; cranial crests absent; (5) dentigerous processes of vomers low to prominent, oblique, moderately separated, posteromedial to choanae; (6) vocal slits, vocal sac and nuptial pads present in adult males; (7) Finger I shorter than Finger II; discs of digits expanded, truncate to elliptical; (8) fingers with broad lateral fringes; (9) indistinct ulnar tubercles; (10) heel bearing a low rounded tubercle, surrounded by several smaller; outer edge of tarsus bearing inconspicuous tubercles; inner edge of tarsus bearing a medium to long fold followed by small tubercles; (11) inner metatarsal tubercle ovoid, elevated, six times the size of round outer metatarsal tubercle; supernumerary tubercles low, numerous; (12) toes with broad lateral fringes; basal webbing present; Toe V longer to much longer than Toe III (disc on Toe III reaches or exceeds distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches middle to distal edge of distal subarticular tubercle on Toe IV); discs on toes smaller than those on fingers, truncate to elliptical (Fig. 7C); (13) in life, dorsum light to dark brown with or without shades of cream, yellow or orange; groins and concealed surfaces of thighs brown, creamish brown, pinkish brown, or purplish brown with irregular cream to brown flecks or spots; background color looks like thin reticulations when flecking is dense; ventral surfaces of body cream to brownish cream; iris cream to light silver bearing wide black reticulations and a red medial streak (Fig. 16); (14) average SVL in adult females: 30.1 ± 3.0 mm (26.7–35.8 mm; n = 15); in adult males: 21.7 ± 2.3 mm (16.8–24.7 mm; n = 24).
Comparison with other species.
Similar species are P. balionotus, P. chomskyi, P. lutzae sp. nov., P. multicolor sp. nov., P. percultus, and Pristimantis sp. (CCS2). Pristimantis gloria is readily distinguished from them (except P. percultus) by the presence of wide black reticulations in its iris (thin black reticulations in the other species). Pristimantis gloria can be further distinguished from P. balionotus by having basal webbing between toes (absent in P. balionotus) and warty flanks (tuberculate in P. balionotus). Pristimantis chomskyi is different from P. gloria by having shagreen dorsal skin (tuberculate to warty in P. gloria), lacking a middorsal fold (present in P. gloria), having a larger body (males Z = -2.31490, p = 0.0206, SVL = 23.98–32.38 mm in P. chomskyi, 16.7–24.74 mm in P. gloria), a smaller tympanum (males Z = 2.66173, p = 0.0078, TD/SVL = 4.5–4.7% in P. chomskyi, 5.1–6.2% in P. gloria) and larger eye (males Z = -2.50743, p = 0.0122, ED/SVL = 11.1–12% in P. chomskyi, 11–13.8% in P. gloria sp. nov.), relative to body length. The dorsum and flanks of Pristimantis lutzae sp. nov. are predominantly covered with tubercles, while it is mostly covered with warts in P. gloria; the ratio between the length and width of the head is larger in P. gloria than P. lutzae sp. nov. (males Z = -5.00826, p < 0.0001, HL/HW = 96.8–114.5% in P. gloria, 90–97% in P. lutzae sp. nov.; females Z = -3.77517, p = 0.0002, HL/HW = 92–105% in P. gloria, 90–96% in P. lutzae sp. nov.). Morphometrically, P. multicolor sp. nov. is larger than P. gloria (males Z = 3.07054, p = 0.0021, SVL 16.7–24.7 mm in P. gloria, 19.7–29.7 mm in P. multicolor sp. nov.; females Z = 2.85671, p = 0.0043, SVL 26.7–35.8 mm in P. gloria, 29.3–40.5 mm in P. multicolor sp. nov.), and has a wider head (males Z = 2.23159, p = 0.0256, HW/SVL = 36.1–40.7% in P. gloria, 36.3–40.9% in P. multicolor sp. nov.; females Z = 4.13252, p < 0.0001, HW/SVL = 36.1–39% in P. gloria, 39.6–42.2% in P. multicolor sp. nov.) relative to body length; furthermore, P. multicolor sp. nov. lacks a middorsal fold. Pristimantis percultus, like P. gloria, has wide black reticulations on the iris, and warts and tubercles on flanks. They can be readily recognized because P. percultus has a keel on the snout (absent in P. gloria), cranial crests (absent in P. gloria), and is larger (Table 5). Pristimantis sp. (CCS2) is the closest species to P. gloria (Fig. 3, Table 2). CCS2 has a red dorsum, a golden iris with a red medial streak and thin reticulations, and broadly expanded discs on finger (Fig. 4M); while the dorsum of P. gloria is light to dark brown, its iris is cream to light silver with a red medial streak and wide black reticulations. Though there is only one female available for CCS2, it has a larger body and smaller tympanum than any individual of P. gloria (Table 5). Pristimantis gloria is also similar to the Peruvian species Pristimantis pataikos (Duellman & Pramuk, 1999) from which can be distinguished by the presence of nuptial pads in adult males (absent in P. pataikos), the presence of lateral fringes on fingers and toes (absent in P. pataikos), the presence of vomerine odontophores (absent in P. pataikos), and the presence of pale spots on the groins and concealed surfaces of thighs (uniformly brown in P. pataikos) (Duellman and Pramuk 1999).
Description of the holotype.
An adult female (QCAZ 57201). Measurements (in mm): SVL 35.8; TL 15.6; FL 16.5; HL 12.4; HW 13.5; ED 3.6; TD 1.8; IOD 3.5; EW 3.0; IND 2.5; EN 3.5; TED 1.7. Head wider than long, slightly narrower than body; snout moderately long, subacuminate in dorsal view, rounded in profile; cranial crests absent; nostrils slightly protuberant, narrow, directed anterolaterally; canthus rostralis slightly concave in dorsal view, angular in cross section; loreal region slightly concave; upper eyelid with indistinct tubercles, smaller than those on dorsum; tympanic annulus prominent, upper and posterolateral edge concealed by supratympanic fold; tympanic membrane distinct; two large, low and rounded postrictal tubercles surrounded by smaller tubercles. Choanae median, ovoid, not concealed by palatal shelf of maxillae; dentigerous processes of vomers prominent, large, oblique, moderately separated, positioned posteromedial to choanae; each vomer bearing several teeth; tongue slightly longer than wide, posteriorly notched, posterior half free.
Dorsal surfaces of body tuberculate, dorsum bearing median low rounded tubercles and scattered warts, more prominent posteriorly; skin on head shagreen; middorsal fold present; dorsolateral folds absent; skin on flanks bearing more prominent and larger tubercles and warts than dorsum; skin on chest and belly coarsely areolate, that on throat shagreen; discoidal fold present; ventral surfaces of limbs smooth, ventral surfaces of thighs coarsely areolate. Ulnar tubercles indistinct except for low antebrachial tubercle; outer palmar tubercle bifid, twice the size of ovoid thenar tubercle; subarticular tubercles prominent, rounded; median low supernumerary tubercles at base of fingers and palms; fingers bearing broad lateral fringes; Finger I shorter than Finger II; discs on fingers expanded and truncate; pads on fingers surrounded by circumferential grooves on all fingers (Fig. 7C).
Dorsal surfaces of hindlimbs shagreen with scattered tubercles; posterior surfaces of thighs smooth, ventral surfaces of thighs coarsely areolate; heel bearing a median, low and rounded tubercle surrounded by indistinct ones; outer edge of tarsus bearing indistinct tubercles; inner edge of tarsus bearing a median fold ending in a row of small tubercles; inner metatarsal tubercle elevated, ovoid, six times the size of round outer metatarsal tubercle; plantar surface with several small, indistinct supernumerary tubercles; subarticular tubercles prominent, rounded; toes bearing broad lateral fringes; basal webbing between toes IV and V present; discs on toes smaller than those on fingers, expanded, elliptical; all toes having pads surrounded by circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V longer than Toe III (disc on Toe III reaches distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the middle of distal subarticular tubercle on Toe IV; Fig. 7C). Color of holotype in life and preservative is shown in Figures 15A, 16A, respectively.
Variation.
This section is based in 60 individuals of the type series and photographs available from 15 individuals. Variation in life and preservative is shown in Figures 16, 17. In preservative, dorsal coloration varies from light to dark brown with or without shades of gray. Dorsum uniformly colored or bearing a pattern of parallel longitudinal stripes, irregular reticulations or pale blotches. Most individuals have scattered black spots on dorsum, especially on dorsolateral surfaces; three individuals (QCAZ 57201, QCAZ 26369, QCAZ30741) display scattered white spots. Black or brown canthal bars, supratympanic stripes, labial bars, and interorbital bars or stripes can be present in this species. Flanks have the same or slightly lighter hue than dorsum. Groins and posterior surfaces of thighs bear pale irregularly shaped small spots or flecks on a brown background; when flecking is very dense the background color resembles brown reticulations. One individual lacks these flecks and spots (QCAZ62778). Limbs bear or not transversal bars and black spots. Ventral coloration varies from cream to dirty cream. In life, dorsal coloration varies from light to dark brown with or without shades of cream, yellow or orange. When present, reticulations and longitudinal stripes are brighter colored than the rest of dorsum and bordered by thin black stripes. Dorsum usually bears black spots, rarely, white spots. When present, canthal and supratympanic bars or stripes are brown or black, interorbital bars or stripes are black, brown or yellow. Flanks are the same color as dorsum or lighter. Groins and posterior surfaces of thighs are brown, creamish brown, pinkish brown or purplish brown with irregular flecks or spots; when flecking is dense, the background color resembles thin reticulations (QCAZ 62778 is the only individual that do not present these spots). Venter varies from cream to brownish cream. Iris is cream or light silver with wide black reticulations that can cover almost completely the iris; a faint red to reddish brown medial streak is present (Fig. 16D); sclera varies from cream to light blue.
Figure 17.
Color variation in preserved individuals of Pristimantis gloria sp. nov. A Dorsal view of (from left to right): QCAZ 31457 (female), QCAZ 31455 (female), QCAZ 16449 (female) B Dorsal view of: QCAZ 16448 (juvenile female), QCAZ 56463 (male), QCAZ 16452 (male), QCAZ 16447 (juvenile female), QCAZ 31461 (juvenile female) C Ventral view of specimens in (A) D Ventral view of specimens in (B). See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Coloration of holotype in preservative. Dorsum dark brown with abundant scattered black medium-sized spots, more concentrated on dorsolateral surfaces and a few scattered white spots; flanks slightly lighter than dorsum; black supratympanic stripes; dorsal surface of limbs with same background coloration as dorsum and scattered black spots; groins and concealed surfaces of thighs brown with cream flecks; ventral surface of body pale cream (Fig. 15A).
Coloration of holotype in life. Based on studio photographs (Fig. 16A). Dorsal surfaces of body grayish brown with scattered black medium-sized spots, more abundant on dorsolateral surfaces; black supratympanic stripe; few scattered white spots on dorsum and dorsal surfaces of thighs; flanks lighter than dorsum with scattered black spots; groins and concealed surfaces of thighs reddish brown with cream flecks; venter and ventral surfaces of thighs pinkish cream with cream spots coinciding with areolate pattern; throat cream; ventral surfaces of shanks, tarsus, feet and hands pinkish cream; iris silver with wide black reticulations and a reddish brown medial streak.
Distribution, natural history, and conservation status.
Pristimantis gloria is known from Inter-Andean Shrub and Paramo regions in Azuay, Loja, and Morona Santiago Provinces, between 2460 and 3525 m a.s.l. (Fig. 1). Most individuals were found underneath rocks in paramos or pastures near the roadside, during day or night. Two couples in amplexus were found underneath rocks at night in April. A calling male was found in low vegetation at 19h00 on October 16, 2013.
We consider P. gloria as an Endangered species following B1ab(iii) + 2ab(iii) IUCN criteria because: (i) it is only known from five localities (sensu IUCN 2017) in non-protected areas, partly affected by human settlements, agriculture and cattle raising; (ii) its Extent of Occurrence is estimated to be less than 5000 km2 (617 km2); and (iii) its Area of Occupancy < 500 km2 (44 km2).
Etymology.
The specific epithet is a patronym for Gloria Lorena Rosales Narváez and Gloria María Esmeralda Narváez, mother and grandmother of the leading author. This species is named after them in gratitude for all their love and support.
Remarks.
Because of the previous lack of molecular data, this species has been mistakenly identified as P. riveti (e.g., collections at the QCAZ museum). Here, we recognize it as a different species and assign it to the P. phoxocephalus species group. It has the lowest genetic divergence with its closest species, Pristimantis sp. CCS2; the average uncorrected p distance between them is 2%. The differences in morphology between them are so evident that we consider 2.0% as our reference value to delimit candidate species.
Pristimantis jimenezi sp. nov.
B6C0F4D3E44658619F71535AF5B232CB
http://zoobank.org/7968FB77-1DD1-46DE-956F-3A93E0767F34
Common name.
English: Jiménez de la Espada’s Rain Frog. Spanish: Cutín de Jiménez de la Espada.
Holotype.
QCAZ 45170, an adult male from San Antonio, Cajas National Park border, Azuay Province, Ecuador (2.8835S, 79.4103W, 2099 m), collected by Elicio E. Tapia and Juan Carlos Morocho on August 21, 2009. Figures 15B, 18A.
Figure 18.
Color variation in live individuals of Pristimantis jimenezi sp. nov. AQCAZ 45170 (holotype, male, SVL 24.1 mm) BQCAZ 45179 (female, SVL 32.2 mm) CQCAZ 46977 (male, SVL 26.8 mm) DQCAZ 46978 (female, SVL 34.8 mm). Dorsal view on the left, ventral view on the right.
Paratypes
(24: 11 males, 9 females, 4 juveniles). Ecuador: Azuay Province: QCAZ 45179, QCAZ 45186, adult females, QCAZ 45178, QCAZ 45187–189, adult males, from San Antonio, Cajas National Park border (2.8835S, 79.4103W, 2099 m), collected by Elicio E. Tapia and Juan Carlos Morocho on August 21, 2009; QCAZ 45199, adult female, QCAZ 45196, juvenile, from San Antonio, Cajas National Park border (2.8612S, 79.3787W, 2900 m), collected by Elicio E. Tapia and Juan Carlos Morocho on August 24, 2009; QCAZ 45202–203, QCAZ 45208–209, QCAZ 45212, adult females, QCAZ 45201, QCAZ 45204, QCAZ 45207, QCAZ 45211, adult males, QCAZ 45205–206, juveniles, from San Antonio, Malacatos river (2.9107S, 79.4146W, 1800 m), collected by Elicio E. Tapia and Juan Carlos Morocho on August 24, 2009; QCAZ 46955, adult male, from Chipla river (2.7457S, 79.4089W, 2500 m), collected by Elicio E. Tapia on January 16, 2010; QCAZ 46978, adult female, QCAZ 46977, QCAZ 46980, adult males, QCAZ 46979, juvenile, from Molleturo, Zadracay river (2.7353S, 79.4142W, 2674 m), collected by Elicio E. Tapia on January 20, 2010.
Diagnosis.
A member of the Pristimantis phoxocephalus group characterized by the following combination of characters: (1) skin on dorsum shagreen with or without scattered small subconical tubercles; faint middorsal fold; dorsolateral folds absent; thin lateral folds on anterior half of flanks; skin on venter areolate; discoidal fold absent; (2) tympanic membrane and tympanic annulus prominent, its upper and posterior margin concealed by supratympanic fold; (3) snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; (4) upper eyelid with small, distinct tubercles; cranial crests absent; (5) dentigerous processes of vomers low to prominent, oblique, moderately separated, posteromedial to choanae; (6) males bearing vocal slits and vocal sac, with white nuptial pads; (7) Finger I shorter than Finger II; discs of digits expanded to broadly expanded, elliptical to truncate; (8) fingers with broad lateral fringes; (9) ulnar tubercles low or absent; (10) heel bearing one medium subconical tubercle surrounded or not by lower tubercles; outer edge of tarsus bearing low subconical tubercles; inner edge with or without low tubercles, short inner tarsal fold present; (11) inner metatarsal tubercle ovoid, elevated, three times the size of elliptical, elevated outer metatarsal tubercle; supernumerary tubercles indistinct; (12) toes with broad lateral fringes; basal webbing present; Toe V longer than Toe III (disc on Toe III reaches or exceeds the proximal edge of penultimate subarticular tubercle on Toe IV; disc on Toe V reaches or exceeds the proximal edge of distal subarticular tubercle on Toe IV); toe discs slightly smaller than those on fingers, elliptical to truncate (Fig. 7D); (13) in life, dorsal background light to dark brown with a scapular W-shaped marking and an interorbital stripe; groins and posterior surfaces of thighs salmon, pinkish brown, purplish brown or dark brown with small light brown to yellow spots; venter cream; iris reddish copper with black reticulations with a red medial streak to completely red (Fig. 18); (14) average SVL in adult females: 35.0 ± 2.1 mm (30.9–37.4 mm; n = 9); in adult males: 25.5 ± 1.6 mm (21.9–27.1 mm; n = 12).
Comparison with other species.
Pristimantis jimenezi is similar to P. atillo, P. phoxocephalus, P. teslai sp. nov., P. torresi sp. nov., P. totoroi sp. nov., and P. verrucolatus sp. nov. It differs from P. atillo in the coloration of the groins and posterior surfaces of thighs (orange in P. atillo; pinkish to dark brown with small light brown to yellow spots in P. jimenezi), and flanks (black dots and flecks surrounding light reticulations in P. atillo; lacking black dots in P. jimenezi). Pristimantis jimenezi can be distinguished from P. phoxocephalus by its groin coloration (yellow with black reticulations in P. phoxocephalus) and advertisement call. The call of P. phoxocephalus has shorter inter-note intervals and a lower frequency of the second harmonic and final frequency of the note (Table 6). Pristimantis teslai sp. nov. has tuberculate dorsal skin (shagreen in P. jimenezi) and lacks lateral folds, which are present in P. jimenezi. The most similar species to P. jimenezi is P. torresi sp. nov., which has golden to beige iris with a red medial streak (copper to red in P. jimenezi). P. totoroi sp. nov. has more prominent folds and tubercles than P. jimenezi, and its head length is larger (males Z = 3.05006, p = 0.0023, HL/SVL = 33.4–37.5% in P. jimenezi, 34.2–38.7% in P. totoroi sp. nov.; females Z = 2.85798, p = 0.0043, HL/SVL = 32.8–37.3% in P. jimenezi, 35.2–37.6% in P. totoroi sp. nov.), its tympanum smaller (males Z = -4.24763, p < 0.0001, TD/SVL = 4.8–6% in P. jimenezi, 4.4–5.1% in P. totoroi sp. nov.; females Z = -2,96383, p = 0.003, TD/SVL = 5–5.6% in P. jimenezi, 4.8–5.3% in P. totoroi sp. nov.), and its eyes smaller (males Z = -4.06051, p < 0.001, ED/SVL = 11.1–13.3% in P. jimenezi, 9.2–11.6% in P. totoroi sp. nov.; females Z = -2.43458, p = 0.0149, ED/SVL = 9.5–11.8% in P. jimenezi, 9.5–10.6% in P. totoroi sp. nov.) than those of P. jimenezi. Furthermore, advertisement calls of P. totoroi sp. nov. have shorter notes and lower dominant, second harmonic, and final frequencies of the notes than those of P. jimenezi (Table 6). Pristimantis verrucolatus sp. nov., which has an adjacent distribution to P. jimenezi at high elevations, can be distinguished by the presence of large tubercles and warts on flanks (absent in P. jimenezi) and by its smaller size (males Z = 3.58643, p = 0.0003, SVL = 21.8–27.0 mm in P. jimenezi, 25.1–34.5 mm in P. verrucolatus sp. nov.; females Z = 2.00347, p = 0.0451, SVL = 31.1–37.4 mm in P. jimenezi, 40.4–46.8 mm in P. verrucolatus sp. nov.). Additionally, they can be distinguished by their advertisement calls: the call of P. verrucolatus sp. nov. consists of a single note, longer than the one or two notes of the call of P. jimenezi; the dominant frequency is higher in advertisement calls of P. jimenezi, and the change in frequency along the note is lower in P. jimenezi (Table 6).
Description of the holotype.
An adult male (QCAZ 45170, SC29041). Measurements (in mm): SVL 24.1; TL 12.7; FL 11.0; HL 8.2; HW 8.5; ED 2.8; TD 1.4; IOD 2.7; EW 2.5; IND 2.2; EN 2.6; TED 1.0. Body slender; head as wide as body, slightly wider than long; snout moderately long, acuminate with a vertical fleshy keel in dorsal view, protruding in profile; nostrils slightly protuberant, ovoid, directed laterally with slight dorsal inclination; canthus rostralis concave in dorsal view, angular in cross section; loreal region slightly concave, bearing small tubercles; upper eyelid bearing small distinct tubercles; cranial crests absent; tympanic membrane and annulus prominent with upper and posterior margins concealed by supratympanic fold; two medium-sized, rounded postrictal tubercles surrounded by smaller tubercles outlining supratympanic stripe. Choanae large, circular, not concealed by palatal shelf of maxilla; dentigerous processes of vomers prominent, oblique, moderately separated, posteromedial to choanae; each vomer bearing several indistinct teeth; tongue slightly wider than long, posterior border notched, posterior half free; vocal slits slightly curved, located at posterior half of mouth floor in between tongue and margin of jaw; vocal sac present.
Skin on dorsum and flanks shagreen; faint middorsal fold; row of three small middorsal tubercles on head; dorsolateral folds absent; thin lateral folds on anterior half of the flanks; skin on throat, chest, belly and ventral surfaces of thighs areolate; discoidal fold absent. One low round ulnar tubercle; white nuptial pads present; palmar tubercles prominent, outer palmar tubercle bifid, slightly bigger than ovoid thenar tubercle; subarticular tubercles distinct, rounded; low supernumerary tubercles at base of fingers; fingers with broad lateral fringes extended to outer edge of the palm; Finger I shorter than Finger II; discs on fingers expanded and elliptical; ventral pads on fingers surrounded by circumferential grooves (Fig. 7D).
Hindlimbs slender; dorsal surfaces of hindlimbs with scattered low tubercles; posterior surfaces of thighs smooth, ventral surfaces of thighs areolate; heel bearing one medium prominent subconical tubercle surrounded by few lower rounded tubercles; outer edge of tarsus bearing low subconical tubercles; short inner tarsal fold present; inner metatarsal tubercle ovoid, elevated, three times the size of elliptical, rounded outer metatarsal tubercle; plantar surface with indistinct supernumerary tubercles; subarticular tubercles distinct, rounded; toes with broad lateral fringes; basal webbing present; discs smaller than those on fingers, wider on Toe IV and V; discs on toes expanded, elliptical; all toes having ventral pads and circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V longer than Toe III (disc on Toe III exceeds proximal edge of the distal subarticular tubercle on Toe IV, disc on Toe V exceeds proximal edge of penultimate subarticular tubercle on Toe IV; Fig. 7). Coloration of the holotype in life and preservative is shown in Figures 15B, 18A.
Coloration of holotype in preservative. Dorsum grayish light brown with cream markings including an irregular W-shaped scapular mark and irregular reticulations; middorsal black stripe; head with interorbital stripe; faint canthal and labial bars; dark brown supratympanic stripe; flanks with the same background color as dorsum; flanks, groins, anterior, and posterior surfaces of thighs with cream medium-sized spots and cream oblique reticulations; background color of groins, anterior, and posterior surfaces of thighs lighter than dorsum; venter cream with scattered minute brown flecks on belly and brown midventral stripe (Fig. 15B).
Coloration of holotype in life. Based on studio photographs. Dorsum light brown with cream markings including an irregular W-shaped scapular mark and irregular reticulations; head with interorbital stripe; faint gray canthal stripes and thin labial bars; black supratympanic stripe; flanks, groins, anterior, and posterior surfaces of thighs with yellow medium-sized spots and cream oblique reticulations; background color on groins and posterior surfaces of thighs purplish brown; ventral surfaces of limbs and chest pinkish cream; vocal sac yellowish cream; venter pinkish cream suffused with white; iris copper with thin black reticulations; light-blue sclera (Fig. 18A).
Variation.
Data of preserved individuals is based on 25 specimens of the type series; information of coloration in life is based on photographs from nine individuals. Variation of preserved and live individuals is shown in Figures 18, 19. Coloration in life is provided in parenthesis. Dorsum is creamy to light and dark brown, with or without lighter irregular marks or chevrons; some individuals show a middorsal band; a pale W-shaped mark on scapula is often present; head bears an interorbital stripe or band paler or darker than dorsum; most individuals bear labial bars, and a few, canthal bars (black to brown). Flanks often bear pale (light-brown to yellow) spots or reticulations all over them or confined to the groins and axils. Groins and posterior surfaces of thighs cream with small pale spots (salmon, pinkish brown, purplish, purplish brown or dark brown with small light-brown to yellow spots). Dorsal surfaces of thighs are darker than dorsum bearing pale (yellow or light brown) reticulations. Ventral surfaces of the body are cream (yellowish to pale cream) with or without dark flecks or reticulations on the belly and throat. Iris varies from copper with a red medial streak of variable size to completely red; it bears thin black reticulations; white to light-blue sclera.
Figure 19.
Color variation in preserved individuals of Pristimantis jimenezi sp. nov. A Dorsal view of (from left to right): QCAZ 45204 (male), QCAZ 45178 (male), QCAZ 45189 (male) B Dorsal view of: QCAZ 46978 (female), QCAZ 45203 (female), QCAZ 45199 (female) C Ventral view of specimens in (A) D Ventral view of specimens in B. Ventral view of the same specimens. See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Advertisement call.
Based on recordings of QCAZ 45178 (January 20, 2010; 19h10; 12 °C) and QCAZ 46977 (August 23, 2009; 16h30). The advertisement call of P. jimenezi consists of one or two peep-like notes (Fig. 6A). The average duration of a note is 0.18 s (range 0.016–0.21 s), and the interval between them is 0.30 s (range 0.23–0.36 s). The fundamental and dominant frequencies of the notes are on average 2894 Hz (range 2776–3013 Hz). The peak time of the note occurs exactly in the middle of its duration. There is an increase in frequency along the note (2790–2977 Hz on average). Descriptive statistics for bioacoustic parameters are shown in Table 6.
Distribution, natural history, and conservation status.
This species is known from Western Andean slopes of Azuay Province between elevations of 1800 and 2900 m (Fig. 1); this corresponds to the Western Montane Forest region. Individuals of this species inhabit primary and secondary forest. Specimens were found at afternoon and night on low vegetation up to 1.5 m above the ground and inside bromeliads up to 8 m above the ground. Some individuals were collected from banana plants. Gravid females and calling males were collected in January and August; males were found calling at 16 and 19 h.
Following B1ab(iii) IUCN criteria, we consider P. jimenezi to be Critically Endangered. Available records come from two localities (sensu IUCN 2017) where suitable surrounding habitats are disturbed by human settlements, agriculture, and cattle raising. Its Extent of Occurrence is estimated to be less than 100 km2 (39 km2).
Etymology.
The specific epithet is a noun in the genitive case and is a patronym for Marcos Jiménez de la Espada, a Spanish naturalist who visited South America between 1862 and 1865. During his trip, he made significant collections of amphibians including new species that he described between 1870 and 1875. Among others, he named the genus Pristimantis and 19 species of Ecuadorian amphibians. His species descriptions are remarkably detailed in comparison with those of his contemporaries. He also made accurate and detailed descriptions of the natural history and the external and internal morphology of Neotropical anurans.
Pristimantis lutzae sp. nov.
F72D2FF25DC85EA194427234C92AAE1F
http://zoobank.org/F9014765-D5AC-4B9F-A04B-D1B6303059DE
Common name.
English: Lutz’s Rain Frog. Spanish: Cutín de Lutz.
Holotype.
QCAZ 37546, an adult female from Mazán Reserve, Azuay Province, Ecuador (2.8689S, 79.1148W, 3047 m), collected by Luis A. Coloma, Santiago R. Ron, Ítalo G. Tapia, Ernesto Arbeláez, and Robin Moore on September 2007. Figure 20A.
Figure 20.
Holotypes of Pristimantis lutzae sp. nov. and P multicolor sp. nov. Photographs of preserved holotypes of AP. lutzae (QCAZ 37546, female) and BP. multicolor (QCAZ 47213, male). Dorsal view on the left, ventral view on the right. Specimens are shown at the same scale.
Paratypes
(32: 14 males, 13 females, 5 juveniles). Ecuador: Azuay Province: QCAZ 32785, adult female, QCAZ 32786, QCAZ 32788, QCAZ 32791, adult males, QCAZ 32792, juvenile female, from Yanuncay-Irquis Protected Forest, Páramo de Quimsacocha (3.0398S, 79.2147W, 3758 m), collected by Andrés Merino-Viteri, Lorena E. Falconí, David Salazar-V, Paula Peña, Mónica Páez, Ernesto Arbeláez, and Juan Daniel Jaramillo in December 2006; QCAZ 37509, adult female, from Mazán Reserve (2.8748S, 79.1292W, 3115 m), collected by Luis A. Coloma, Santiago R. Ron, Ítalo G. Tapia, Ernesto Arbeláez and Robin Moore in September 2007; QCAZ 37545, QCAZ 37547, QCAZ 37566, adult females, QCAZ 37561, QCAZ 37564, adult males, QCAZ 37550–551, juvenile females, collected with the holotype; QCAZ 37571, adult female, QCAZ 37570, adult male, from Mazán Reserve (2.8752S, 79.1292W, 3189 m), collected by Luis A. Coloma, Santiago R. Ron, Ítalo G. Tapia, Ernesto Arbeláez and Robin Moore in September 2007; QCAZ 51736, adult female, from San Vicente (2.7953S, 78.6981W, 3044 m), collected by Omar Torres, Vanessa Aguirre, Simón Lobos, Fernando Ayala and Estefanía Boada in March 2011; QCAZ 53728, juvenile, from Cajas National Park, El Capo, Laguna Toreadora (2.7785S, 79.2453W, 4100 m), collected by Santiago R. Ron, Andrés Merino, Fernando Ayala, Teresa Camacho and Martin Cohen on July 19, 2011. Cañar Province: QCAZ 27467, QCAZ 27469, QCAZ 27534, adult females, QCAZ 27470–471, adult males, QCAZ 27472, juvenile female, from Mazar Wildlife Reserve, Rumiloma (2.5746S, 78.7455W, 3400 m), collected by Martín R. Bustamante, Joseph Mendelson and Michelle Cummer in February 2004; QCAZ 27521, adult male, from Mazar Wildlife Reserve, Rumiloma (2,5612S, 78.7336W, 3550 m), collected by Martín R. Bustamante, Joseph Mendelson and Michelle Cummer in February 2004; QCAZ 27596–597, adult females, from La Libertad (2.5466S, 78.6984W, 2895 m), collected by Martín R. Bustamante, Joseph Mendelson and Michelle Cummer in February 2004; QCAZ 47211, adult male, from Guallicanga ravine (2.4321S, 78.9022W, 3960 m), collected by Paola Mafla-Endara, Silvia Aldás-Alarcón and Freddy Velásquez-Alomoto in December 2009; QCAZ 56182, adult female, QCAZ 56183–186, adult males, from Charón Ventanas Community Tourism Center (2.6471S, 78.8905W, 3300 m), collected by Andrea Manzano, Paulina Romero, and Leonardo Negrete in July 2013.
Diagnosis.
A species of Pristimantis having the following combination of characters: (1) skin on dorsum shagreen to tuberculate with scattered low tubercles; thin middorsal fold present or absent; dorsolateral folds absent; flanks tuberculate, tubercles larger than those on dorsum, with or without scattered warts; lateral fold present or absent; skin on venter coarsely areolate; discoidal fold present or absent; (2) tympanic membrane and tympanic annulus prominent, its upper and posterolateral margin covered by supratympanic fold; (3) snout moderately long, round to subacuminate in dorsal, rounded in lateral view, with or without a small papilla at the tip; (4) upper eyelid bearing a small, rounded tubercle, surrounded by several lower tubercles; cranial crests absent; (5) dentigerous processes of vomers prominent, oblique, moderately separated, posteromedial to choanae; (6) vocals slits, vocal sac, and nuptial pads present in adult males; (7) Finger I shorter than Finger II; discs of digits expanded, elliptical to truncate; (8) fingers with broad lateral fringes; (9) ulnar tubercles small, distinct; (10) heel bearing a low rounded tubercle, surrounded by several smaller tubercles; inner and outer edge of tarsus bearing a row of small tubercles; short inner tarsal fold; (11) inner metatarsal tubercle elevated, ovoid, three times the size of round outer metatarsal tubercle; supernumerary tubercles indistinct; (12) toes with broad lateral fringes; basal webbing present; Toe V longer or much longer than Toe III (disc on Toe III reaches the middle of penultimate subarticular tubercle on Toe IV or slightly exceeds its distal edge, disc on Toe V reaches the middle of distal subarticular tubercle on Toe IV or slightly exceeds its distal edge); toe discs smaller than those on fingers, truncate to elliptical (Fig. 8A); (13) in life, dorsum light, orangey or dark brown; head with black or brown supratympanic, canthal, and interorbital stripes; groins, anterior and posterior surfaces of thighs pinkish, purplish or reddish brown, suffused or not with orange, with cream or light brown spots; ventral surfaces of body white to cream with or without brown reticulations; vocal sac in males yellow; iris golden to creamy brown with a reddish brown medial streak (Fig. 21); (14) average SVL in adult females: 35.3 ± 3.5 mm (29.7–33.9 mm; n = 15); in adult males: 24.6 ± 1.7 mm (21.4–27.0 mm; n = 14).
Figure 8.
Palmar and plantar surfaces of Pristimantis lutzae sp. nov., Pristimantis multicolor sp. nov., Pristimantis nangaritza sp. nov., and Pristimantis phoxocephalus. Photographs of hand and foot of the holotypes (except for P. phoxocephalus) of the following species: APristimantis lutzae sp. nov.: QCAZ 37546, female BPristimantis multicolor sp. nov.: QCAZ 47213, male CPristimantis nangaritza sp. nov.: QCAZ 41710, female DPristimantis phoxocephalus: QCAZ 58463, female from the type locality. All specimens are shown at the same scale.
Figure 21.
Color variation in live individuals of Pristimantis lutzae sp. nov. AQCAZ 53728 (juvenile female, SVL 26.5 mm) BQCAZ 56183 (male, SVL 23.3 mm) CQCAZ 56184 (male, SVL 24.8 mm) DQCAZ 56185 (male, SVL 26.1 mm). Dorsolateral view on the left, ventral view on the right.
Comparison with other species.
Pristimantis lutzae is similar to P. balionotus, P. chomskyi, P. gloria, P. multicolor sp. nov., and P. philipi. The most similar is P. balionotus, which occurs at lower elevations and can be recognized by the absence of basal webbing between toes (present in P. lutzae) and having smaller discs on fingers. Pristimantis lutzae can be distinguished from P. chomskyi by the golden to creamy brown iris with a reddish dark brown streak (orange with a faint reddish brown streak in P. chomskyi), and having a bigger tympanum (males Z = 2.45677, p = 0.0140, TD/SVL = 4.5–4.7% in P. chomskyi, 5–5.4% in P. lutzae). Pristimantis gloria differs from P. lutzae in having a wartier skin, wide black reticulations on iris (thin in P. lutzae), and a larger ratio between the length and width of the head (males Z = -5.00826, p < 0.0001, HL/HW = 96.8–114.5% in P. gloria, 90–97% in P. lutzae; females Z = -3.77517, p = 0.0002, HL/HW = 92–105% in P. gloria, 90–96% in P. lutzae). Pristimantis multicolor sp. nov. has a longer head (males Z = 3.67756, p = 0.0002, HL/SVL = 33.4–37% in P. lutzae, 34.1–40.4% in P. multicolor sp. nov.; females Z = 3.9524, p < 0.0001, HL/SVL = 35–37.5% in P. lutzae, 36.5–40.6% in P. multicolor sp. nov.), larger tympanum (males Z = 3.57469, p = 0.0004, TD/SVL = 5–5.4% in P. lutzae, 4.9–6% in P. multicolor sp. nov.; females Z = 3.9524, p < 0.0001, TD/SVL = 4.9–5.6% in P. lutzae, 5.5–6.7% in P. multicolor sp. nov.) and larger eyes (males Z = 2.75174, p = 0.0059, ED/SVL = 10.3–12.1% in P. lutzae, 10.3–13.2% in P. multicolor sp. nov.; females Z = 3.3083, p = 0.0009, ED/SVL = 9.9–12.1% in P. lutzae, 10.7–12.4% in P. multicolor sp. nov.), relative to the body length, than P. lutzae. Pristimantis lutzae is readily distinguished from P. philipi because it has a visible tympanic membrane and annulus, and its males have vocal slits (absent traits in P. philipi).
Description of the holotype.
An adult female (QCAZ 37546, SC 21029). Measurements (in mm): SVL 31.7; TL 13.7; FL 14.7; HL 11.2; HW 12.1; ED 3.7; TD 1.7; IOD 3.8; EW 2.8; IND 2.3; EN 3.5; TED 1.3. Head wider than long, slightly narrower than body; snout moderately long, rounded with a small papilla in dorsal and lateral view; cranial crests absent; nostrils slightly protuberant, narrow, directed laterally with slight dorsal inclination; canthus rostralis slightly concave in dorsal view, rounded in cross section; loreal region slightly concave; upper eyelid bearing a small, low and rounded tubercle surrounded by inconspicuous tubercles; tympanic annulus prominent, its upper and posterior margins concealed by supratympanic fold; tympanic membrane distinct; two low and rounded postrictal tubercles surrounded by lower tubercles. Choanae large, round, not concealed by palatal shelf of maxillae; dentigerous processes of vomers prominent, oblique, moderately separated, positioned posteromedial to choanae; each vomer bearing several teeth; tongue longer than wide, posterior border notched, posterior half free.
Dorsum shagreen with scattered low tubercles, larger posteriorly; dorsolateral folds absent; skin on flanks bearing low rounded tubercles, larger than those on dorsum; skin on chest and belly coarsely areolate, that on throat shagreen, ventral surfaces of limbs smooth, ventral surfaces of thighs coarsely areolate; discoidal fold present. Ulnar tubercles rounded and low; outer palmar tubercle bifid, three times the size of ovoid thenar tubercle; subarticular tubercles prominent, rounded; low supernumerary tubercles; fingers bearing broad lateral fringes; Finger I shorter than Finger II; discs on fingers expanded and truncate; pads on fingers surrounded by circumferential grooves on all fingers (Fig. 8A).
Dorsal surfaces of hindlimbs shagreen with scattered small tubercles; posterior surfaces of thighs smooth, ventral surfaces of thighs coarsely areolate; heel bearing a median, low, rounded tubercle surrounded by smaller ones; outer and inner edge of tarsus bearing a row of small tubercles; small inner tarsal fold present; inner metatarsal tubercle ovoid, elevated, three times the size of round outer metatarsal tubercle; plantar surface with small, indistinct supernumerary tubercles; subarticular tubercles prominent, rounded; toes bearing broad lateral fringes; basal webbing between Toes IV and V present; discs on toes smaller than those on fingers, slightly expanded, truncate; all toes having pads surrounded by circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V much longer than Toe III (disc on Toe III reaches the distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the distal edge of distal subarticular tubercle on Toe IV; Fig. 8A). Coloration of the holotype in preservative is shown in Figure 20A; coloration in life, unknown.
Coloration of holotype in preservative. Dorsum grayish dark brown with lighter irregular reticulations and scattered black spots; dark brown supratympanic stripe, canthal, and interorbital bands; dorsal surfaces of limbs with the same background as dorsum and darker irregular transversal bands and scattered dark brown spots; groins, anterior, and posterior surfaces of thighs reddish brown with cream small spots; ventral surfaces of body cream; soles and palms dusty cream (Fig. 20A).
Coloration of holotype in life. Unknown.
Variation.
Variation in preservative is based on 40 individuals of the type series and photographs from eight individuals. Variation in life and preservative is shown in Figures 21, 22. Coloration in life is provided in parenthesis. Dorsal coloration varies from light to dark gray or brown (light, orangey or dark brown); markings on dorsum (light brown, yellow or orange) are present or absent, most individuals have irregular reticulations, some have a series of parallel longitudinal stripes; dorsum and flanks may bear scattered black or white spots. All individuals bear supratympanic and canthal stripes (black, brown); an interorbital stripe or band is present except for individuals with the longitudinal pattern on dorsum. Flanks have the same background color as dorsum. Groins, posterior and anterior surfaces of thighs are cream to brown with small paler spots (pinkish, purplish or reddish brown, suffused or not with orange, with cream or light brown spots). Limbs bear dark transversal bands or scattered small dark spots. Ventral coloration varies from cream to dusty cream (white to cream); venter with or without brown reticulations. The iris is golden to creamy brown with a reddish dark brown medial horizontal streak. White to light-blue sclera.
Figure 22.
Color variation in preserved individuals of Pristimantis lutzae sp. nov. A Dorsal view of (from left to right): QCAZ 37545 (female), QCAZ 37547 (female), QCAZ 27534 (female), QCAZ 27597 (female) B Dorsal view of: QCAZ 32785 (female), QCAZ 56185 (male), QCAZ 53728 (juvenile female), QCAZ 27471 (male) C Ventral view of specimens in (A) D Ventral view of specimens in B. See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Distribution, natural history, and conservation status.
Pristimantis lutzae is known from Paramo, Inter-Andean Shrub, Western and Eastern Montane Forest in the Andes of Azuay and Cañar Provinces in Ecuador, between 2895–4100 m a.s.l (Fig. 1). Individuals collected at night were found in bunch grasses, pastures, and low vegetation up to 80 cm above the ground. Individuals collected at day were found in pastures or underneath rocks. Calling males have been found on bunch grasses or low vegetation during February, July, and December at night.
Despite the relatively small distribution range of this species (Extent of Occurrence = 2338 km2) we assign it to the Least Concern Red List category because its distribution overlaps with four protected areas, Cajas National Park, Mazán Reserve, Mazar Wildlife Reserve, and Yanuncay Irquis Protected Forest, and it is a common species in these places.
Etymology.
The specific epithet is a noun in the genitive case and is a patronym for Bertha Lutz, who was a Brazilian herpetologist. We name this species after her in recognition of her scientific career and her activism in the fight for gender equality.
Remarks.
Specimens of this species were previously referred as Pristimantis riveti (Despax 2011) based on Lynch (1979) characterization of the species (e.g., Almendáriz and Orcés 2006; Heinicke et al. 2007; Padial et al. 2014). Photographs of the holotype of P. riveti (Fig. 23B) as well as the location of its type locality indicate that P. lutzae is a different species. See Taxonomic status of P. riveti section for details. Through morphological and molecular evidence, we recognize P. lutzae as a different species and assign it to the P. phoxocephalus species group.
Figure 23.
Preserved holotypes of Pristimantis phoxocephalus and P. riveti. Dorsal, ventral and lateral views of: APristimantis phoxocephalus (KU 142075) BPristimantis riveti (MNHNP 1902.357). Shown at the same scale.
Pristimantis multicolor sp. nov.
B59FBA030F035F5B83BC88BD3351F454
http://zoobank.org/8F325BAA-C366-4F73-B767-A19551E5A7F8
Common name.
English: Multicolored Rain Frog. Spanish: Cutín multicolor.
Holotype.
QCAZ 47213, an adult male from Yacuri National Park, Laguna Negra, Loja Province, Ecuador (4.7081S, 79.4322W, 3400 m), collected by Carlos Antonio Rodríguez on December 10, 2009. Figure 20B.
Paratypes
(59: 35 males, 23 females, 1 juvenile). All individuals from Yacuri National Park, nearby the type locality. Ecuador: Loja Province: QCAZ 47214, adult female, collected with the holotype; QCAZ 47215, adult male, from Laguna Negra (4.7095S, 79.4356W, 3348 m), collected by Carlos Antonio Rodríguez on December 10, 2009; QCAZ 61279, QCAZ 61390–391, QCAZ 61404, adult females, QCAZ 61392, QCAZ 61394, QCAZ 61396, adult males, from Jimbura-San Andrés road (4.7325S, 79.4352W, 3300 m); QCAZ 61283, QCAZ 61287, adult females, QCAZ 61284, QCAZ 61286, QCAZ 61297, adult males, from Laguna Negra (4.7121S, 79.4308W, 3186 m); QCAZ 61293, adult male, from Loja-Zamora border (4.7436S, 79.4234W, 3515 m); QCAZ 61299–300, QCAZ 61302–304, adult females, QCAZ 61301, QCAZ 61306, adult males, from entrance to Lagunas Coloradas, Jimbura-San Andrés road (4.7349S, 79.4283W, 3390 m); QCAZ 61310, adult female, QCAZ 61314, QCAZ 61316–317, adult males, from Laguna de los Patos (4.7204S, 79.4286W, 3247 m); QCAZ 61344, QCAZ 61346, QCAZ 61348, adult females, QCAZ 61343, adult male, from Loja-Zamora border (4.7433S, 79.4244W, 3506 m); QCAZ 61349, QCAZ 61352, QCAZ 61361, adult females, QCAZ 61362, QCAZ 61367–368, adult males, QCAZ 61351, juvenile, from Jimbura-San Andrés road (4.7277S, 79.4333W, 3411 m); QCAZ 61358, adult male, from surroundings of refuge (4.7126S, 79.4399W, 3245 m); QCAZ 61359, QCAZ 61363, QCAZ 61369–370, adult males, from Los Picachus (4.7121S, 79.4308W, 3186 m); QCAZ 61373, adult female, from Laguna Negra (4.7154S, 79.4212W, 3242 m); QCAZ 61377, QCAZ 61379–380, adult females, from Jimbura-Zumba road (4.7252S, 79.4353W, 3333 m); QCAZ 61383–384, adult females, QCAZ 61381, adult male, from Jimbura-San Andrés road (4.7309S, 79.4340W, 3332 m), collected by Francy Mora, Keyko Cruz, David Velalcázar, Andrés Calahorrano, Darwin Núñez, Juan Sánchez, Javier Pinto and Daniel Rivadeneira between April 21 and May 2, 2015. Zamora Chinchipe Province: QCAZ 61407, QCAZ 61424–425, adult females, QCAZ 61399, QCAZ 61410, QCAZ 61413, QCAZ 61427, QCAZ 61432, QCAZ 61435–436, adult males, from Laguna Colorada (4.7379S, 79.3958W, 3318 m), collected by Francy Mora, Keyko Cruz, David Velalcázar, Andrés Calahorrano, Darwin Núñez, Juan Sánchez, Javier Pinto and Daniel Rivadeneira between April 21 and May 2, 2015.
Diagnosis.
A species of Pristimantis having the following combination of characters: (1) skin on dorsum shagreen to warty; dorsolateral folds absent; skin on flanks bearing low warts, bigger than those of dorsum; skin on venter coarsely areolate; discoidal fold absent; (2) tympanic membrane and tympanic annulus prominent, its upper and posterior margin concealed by thick supratympanic fold; (3) snout moderately long, subacuminate in dorsal view, rounded in profile, with or without a papilla at the tip; (4) upper eyelid lacking tubercles; cranial crests absent; (5) dentigerous processes of vomers low to prominent, oblique, narrowly to moderately separated, posteromedial to choanae; (6) vocals slits, vocal sac, and nuptial pads present in adult males; (7) Finger I shorter than Finger II; discs of digits expanded to broadly expanded, elliptical to truncate; (8) fingers with lateral fringes; (9) ulnar tubercles present; (10) heel bearing one rounded tubercle, surrounded or not by several smaller tubercles; inner and outer edge of tarsus bearing small and low tubercles; (11) inner metatarsal tubercle ovoid, elevated six times the size of elliptical, elevated outer metatarsal tubercle; supernumerary tubercles numerous; (12) toes with lateral fringes; basal webbing present; Toe V much longer than Toe III (disc on Toe III reaches or exceeds distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches or exceeds distal edge of distal subarticular tubercle on Toe IV); toe discs smaller than those on fingers, elliptical (Fig. 8B); (13) in life, dorsal surfaces vary from yellow to dark brown with or without olive or orange tones; black or brown canthal and supratympanic stripe usually present; groins and posterior surfaces of thighs cream, orange, brown, or black with or without cream to yellow spots; venter varies from cream to dark brown with a few to many small beige to yellow spots; iris copper to creamy yellow with or without a dark brown to red medial horizontal streak, with black reticulations (Fig. 24); (14) average SVL in adult females: 35.3 ± 3.5 mm (29.4–40.5 mm; n = 27); in adult males: 26.2 ± 3.5 mm (19.7–29.7 mm; n = 32).
Figure 24.
Color variation in live individuals of Pristimantis multicolor sp. nov. AQCAZ 61283 (female, SVL 32 mm) BQCAZ 61304 (female, SVL 34.6 mm) CQCAZ 61373 (male, SVL 21.8 mm) DQCAZ 61381 (male, SVL 29.2 mm) EQCAZ 61418 (male, SVL 24.5 mm). Dorsolateral view on the left, ventral view on the right.
Comparison with other species.
It is most similar to P. balionotus, P. chomskyi, P. gloria, P. lutzae, and P. percultus. Pristimantis multicolor is different from P. balionotus by having basal webbing between toes (absent in P. balionotus), larger discs on fingers and toes, and larger tubercles and warts on flanks. Morphometrically, P. chomskyi has a smaller tympanum (males Z = 2.38157, p = 0.0172, TD/SVL = 4.5–4.7% in P. chomskyi, 4.9–6.0% in P. multicolor). Pristimantis multicolor differs from P. gloria by its larger size (males Z = 3.07054, p = 0.0021, SVL 16.7–24.7 mm in P. gloria, 19.7–29.7 mm in P. multicolor; females Z = 2.85671, p = 0.0043, SVL 26.7–35.8 mm in P. gloria, 29.3–40.5 mm in P. multicolor), by lacking a middorsal longitudinal fold (present in P. gloria), and having thinner black reticulations on the iris; additionally, P. multicolor has a proportionally wider head (males Z = 2.23159, p = 0.0256, HW/SVL = 36.1–40.7% in P. gloria, 36.3–40.9% in P. multicolor; females Z = 4.13252, p < 0.0001, HW/SVL = 36.1–39% in P. gloria, 39.6–42.2% in P. multicolor). Compared to P. lutzae, Pristimantis multicolor sp. nov. has a longer head (males Z = 3.67756, p = 0.0002, HL/SVL = 33.4–37% in P. lutzae, 34.1–40.4% in P. multicolor; females Z 3.9524, p < 0.0001, HL/SVL = 35–37.5% in P. lutzae, 36.5–40.6% in P. multicolor), larger tympanum (males Z = 3.57469, p = 0.0004, TD/SVL = 5–5.4% in P. lutzae, 4.9–6% in P. multicolor; females Z = 3.9524, p < 0.0001, TD/SVL = 4.9–5.6% in P. lutzae, 5.5–6.7% in P. multicolor) and larger eyes (males Z = 2.75174, p = 0.0059, ED/SVL = 10.3–12.1% in P. lutzae, 10.3–13.2% in P. multicolor; females Z = 3.3083, p = 0.0009, ED/SVL = 9.9–12.1% in P. lutzae, 10.7–12.4% in P. multicolor), relative to body size. Pristimantis percultus is easily distinguished from P. multicolor by having low cranial crests (absent in P. multicolor) and a red stripe on the upper lip.
Description of the holotype.
An adult male (QCAZ 47213, SC29908). Measurements (in mm): SVL 29.3; TL 14.8; FL 14.5; HL 11.0; HW 11.5; ED 3.5; TD 1.8; IOD 3.7; EW 2.8; IND 2.5; EN 3.1; TED 1.9. Head wider than long, wider than body; snout moderately long, subacuminate in dorsal view, rounded in profile; cranial crests absent; nostrils slightly protuberant, directed anterolaterally; canthus rostralis concave in dorsal view, prominent and rounded in cross section; loreal region concave; upper eyelid with indistinct tubercles; tympanic membrane distinct; tympanic annulus prominent, upper and posterior edge concealed by thick, heavy supratympanic fold; three large rounded postrictal tubercles. Choanae median ovoid, not concealed by palatal shelf of maxillae; dentigerous processes of vomers prominent, oblique, moderately separated, positioned posteromedial to choanae; each vomer bearing several teeth; tongue slightly longer than wide, posterior border notched, posterior three fifths not adherent to floor of mouth; vocal slits slightly curved, located at posterior half of mouth floor in between tongue and margin of jaw; vocal sac present.
Dorsal surfaces of body shagreen with scattered low warts; dorsolateral folds absent; skin on flanks bearing rounded warts, larger than those on dorsum; skin on venter coarsely areolate, ventral surfaces of limbs smooth, ventral surfaces of thighs coarsely areolate; discoidal fold absent. Low, round ulnar tubercles, antebrachial tubercle prominent; white nuptial pads present; outer palmar tubercle bifid, almost twice the size of ovoid thenar tubercle; subarticular tubercles prominent, rounded; ill-defined supernumerary tubercles; fingers bearing lateral fringes; Finger I shorter than Finger II; discs on fingers broadly expanded, truncate; pads on fingers surrounded by circumferential grooves on all fingers (Fig. 8B).
Hindlimbs slender; dorsal surfaces of hindlimbs shagreen; posterior surfaces of thighs smooth, ventral surfaces of thighs coarsely areolate; heel bearing a medium sized, low, rounded tubercle surrounded by several smaller tubercles; outer and inner edge of tarsus bearing low rounded tubercles; inner metatarsal tubercle ovoid, elevated, six times the size of elliptical, elevated outer metatarsal tubercle; plantar surface bearing numerous ill-defined supernumerary tubercles; subarticular tubercles prominent, subacuminate; toes bearing lateral fringes; basal webbing between toes IV and V present; discs on toes smaller than those on fingers, expanded and elliptical; all toes having pads surrounded by circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V much longer than Toe III (disc on Toe III reaches distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the distal edge of distal subarticular tubercle on Toe IV; Fig. 8B). Coloration of the holotype in preservative is shown in Figure 20B; coloration in life, unknown.
Coloration of holotype in preservative. Dorsum dark brown covered with minute light brown irregular spots; dorsal surfaces of forelimbs light brown with scattered minute dark brown flecking; dorsal surfaces of hindlimbs dark brown with light brown spots becoming larger and more abundant posteriorly; groins and concealed surfaces of thighs, shanks and tarsus brown; ventral surfaces of body dusty brown; chest with cream irregular blotches; vocal sac and ventral surfaces of thighs cream; ventral surfaces of fingers and toes pale cream (Fig. 20B).
Coloration of holotype in life. Unknown.
Variation.
Based on 60 preserved specimens and photographs from 57 individuals. Variation of live and preserved individuals is shown in Figures 24, 25. Texture of skin varies among individuals. Dorsum varies from shagreen to warty, with very low warts (not tuberculate); flanks vary from tuberculate to warty, warts on flanks are larger than those of dorsum; tubercles on ulna, shanks, and tarsus can be distinct or inconspicuous. Coloration in life is highly variable (Fig. 24). Dorsal surfaces can be yellow, cream, orange, or pale to dark brown with or without olive or orange tones. They can have few to abundant black spots on dorsum, flanks, and dorsal surfaces of limbs. Dorsum occasionally bears pale scapular or sacral blotches, a middorsal stripe, or white spots. Head usually bears black or brown canthal and supratympanic stripes, sometimes an interorbital bar. Groins and posterior surfaces of thighs are cream, orange, reddish brown, orangey brown, brown, dark brown, or black with or without cream to yellow spots. The background color of the venter varies from cream to dark brown with different levels of transparency; small beige to yellow blotches can be present on the belly, chest, and throat, but in some individuals are restricted to the chest. Iris is copper, golden, creamy yellow or creamy brown with or without a dark brown to red medial horizontal streak; black reticulations are present; sclera can be light brown, cream, white, or light blue.
Figure 25.
Color variation in preserved individuals of Pristimantis multicolor sp. nov. A Dorsal view of (from left to right): QCAZ 61304 (female), QCAZ 61361 (female), QCAZ 61383 (female) B Dorsal view of: QCAZ 61394 (male), QCAZ 47214 (female), QCAZ 61310 (female), QCAZ 61283 (female) C Dorsal view of: QCAZ 61413 (male), QCAZ 61297 (male), QCAZ 61369 (male), QCAZ 61381 (male), QCAZ 61373 (male) D Ventral view of specimens in (A) E Ventral view of specimens in (B) F Ventral view of specimens in (C). See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Distribution, natural history, and conservation status.
Pristimantis multicolor is known from Parque Nacional Yacuri, near the border with Peru, at Loja and Zamora Chinchipe Provinces (Fig. 1). It inhabits Paramo and Eastern Montane Forest between 3186 and 3515 m. During the late afternoon and night, individuals were commonly found beneath or over moss, mat-forming plants, tussock grasses and rocks, perched on branches and leaves of shrubs up to 1.75 m above ground, or inside bromeliads, terrestrial or epiphytes, up to 1 m above the ground. During the day they were found inside bromeliads. Calling males and gravid females were found in December, April, and May; males were calling on low branches of shrubs.
According to available data, P. multicolor has a very restricted distribution (Extent of Occurrence = 10 km2) but is locally abundant. However, we propose assigning it to the Data Deficient Red List Category (IUCN 2017) because extensive areas in Yacuri National Park remain unexplored and represent potential distribution for this species.
Etymology.
The specific epithet comes from the Latin words multus meaning many, and color meaning color. It refers to the wide range of color variation in this species (Fig. 24).
Remarks.
This species was collected for the first time in 2015 by personnel from the QCAZ museum during expeditions to Yacuri National Park. It was identified as Pristimantis aff. riveti. Here, we recognize it as a different species and assign it to the P. phoxocephalus species group.
Pristimantis phoxocephalus
(Lynch, 1979)
B9F89C8F7B745416A627F30BCFF4654B
Eleutherodactylus phoxocephalus Lynch 1979. Holotype KU 142075. Figure 23A.
Pristimantis phoxocephalus Heinicke et al. 2007
Common name.
English: Cotopaxi Rain Frog. Spanish: Cutín silbador.
Diagnosis.
This and the following sections are based on specimens of P. phoxocephalus listed in Suppl. material 2. A species of the Pristimantis phoxocephalus group having the following combination of characters: (1) dorsum shagreen with scattered small tubercles; faint middorsal fold; head with a middorsal row of two or more small tubercles; dorsolateral folds absent; anterior half of flanks with or without inconspicuous longitudinal lateral folds; skin on venter areolate to coarsely areolate; discoidal fold present or absent; (2) tympanic membrane and tympanic annulus prominent, its upper and posterolateral margin covered by supratympanic fold; (3) snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; (4) upper eyelid with small distinct rounded tubercles; cranial crests absent; (5) dentigerous processes of vomers low to prominent, oblique, moderately separated, posteromedial to choanae; (6) vocals slits, vocal sac and large nuptial pads present in adult males; (7) Finger I shorter than Finger II; discs of digits broadly expanded, rounded to elliptical; (8) fingers with lateral fringes; (9) small distinct ulnar tubercles (Fig. 23A); (10) heel bearing a small, subconical tubercle surrounded by smaller tubercles; outer edge of tarsus bearing a row of low subconical tubercles; inner edge of tarsus bearing a small inconspicuous fold followed by small subconical tubercles; (11) inner metatarsal ovoid, elevated, 4–6 times the size of round outer metatarsal tubercle; supernumerary tubercles low, numerous; (12) toes with broad lateral fringes; basal webbing between toes present; Toe V longer or much longer than Toe III (disc on Toe III reaches the middle to distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the middle to distal edge of distal subarticular tubercle on Toe IV); toe discs smaller than those on fingers, rounded to elliptical (Fig. 8D); (13) in life, dorsal coloration is cream, yellow, or brown with or without reddish or olive tones; dorsum with or without a longitudinal middorsal band, scapular W-shaped marking, chevrons, white or black flecking or pale botches; head with or without black or brown interorbital band, canthal stripe, supratympanic stripes, and labial bars; flanks with the same color or lighter than dorsum; groins, anterior and posterior surfaces of thighs, and concealed surfaces of tarsus and shanks yellow with black reticulations; ventral surfaces of thighs yellow; venter white with brown midline and reticulations; iris copper with or without a faint medial horizontal brown to reddish brown streak, with thin black reticulations (Fig. 26); (14) average SVL in adult females: 36.9 ± 2.2 mm (34.2–39.9 mm; n = 5); in adult males: 23.7 ± 2.9 mm (20.9–27.9 mm; n = 4).
Figure 26.
Color variation in live individuals of Pristimantis phoxocephalus. AQCAZ 58461 (male, SVL 29.9 mm) BQCAZ 58463 (female, SVL 36.1 mm) CQCAZ 58470 (male, SVL 26.7 mm) DQCAZ 58472 (female, SVL 34.2 mm). Dorsolateral view on the left, ventral view on the right.
Comparison with other species.
Pristimantis phoxocephalus is most similar to P. atillo, P. jimenezi, P. teslai sp. nov., P. totoroi sp. nov., and P. verrucolatus sp. nov. However, the coloration of the groins and concealed surfaces of thighs (yellow coloration with dark brown to black reticulations) distinguishes it from the species above (orange in P. atillo; different shades of brown with light brown to yellow flecks, spots or blotches in the other species). It can be further distinguished from P. jimenezi by having an advertisement call with shorter inter-note interval and a lower frequency of the second harmonic and final frequency of the note (Table 6). Pristimantis teslai sp. nov. has a tuberculate dorsum while in P. phoxocephalus is shagreen. Pristimantis torresi sp. nov. differs from P. phoxocephalus in having a golden to beige iris with a red to reddish-brown medial streak (copper with or without a faint red streak in P. phoxocephalus), and a wider head relative to its body (males Z = -2.56285, p = 0.0104, HW/SVL = 33.4–34.4% in P. phoxocephalus, 34.2–37.8% in P. torresi sp. nov.; females Z = -2.08893, p = 0.0367, HW/SVL = 33.1–37.3% in P. phoxocephalus, 36.7–38.5% in P. torresi sp. nov.). Pristimantis totoroi sp. nov. is the most similar and geographically closest species; both species differ by the coloration of the groins and iris (copper with or without a brown streak in P. phoxocephalus; golden with a red streak in P. totoroi sp. nov.) and by the presence of more distinctive tubercles and dermal folds in P. totoroi sp. nov. The advertisement call of P. totoroi sp. nov. has shorter inter-note intervals than P. phoxocephalus (Table 6). Pristimantis verrucolatus sp. nov. differs from P. phoxocephalus by having large tubercles and warts on flanks and by its advertisement call. Calls of P. verrucolatus sp. nov. have one note (3–9 in P. phoxocephalus) that lasts more than the notes of the calls of P. phoxocephalus, and the dominant frequency, second harmonic frequency, and final and initial frequency of the notes are lower (Table 6; Fig. 6).
Variation.
Variation in live and preserved individuals is shown in Figures 26, 27. This section is based on 23 individuals of the QCAZ collection collected at the type locality and its surroundings. Photographs are available from 12 individuals. Coloration in life is mentioned in parenthesis. Skin on dorsum is shagreen with scattered prominent tubercles; dorsum bears a faint middorsal fold; a middorsal row of two or more tubercles is present on the head; lateral folds are inconspicuous or absent. Folds and tubercles in juveniles are more prominent and larger than those in adults. In preservative, middorsal fold, lateral folds, and tubercles on dorsum can be lost. Dorsal coloration varies from cream, light gray and light brown to dark brown (cream, pale yellow or light to dark brown with or without reddish or olive tones). Dorsum can have a scapular W-shaped marking, chevrons, longitudinal middorsal band, pale blotches, and black or white flecking. Dorsal markings in juveniles are more evident than those in adults. Groins, anterior and posterior surfaces of thighs, and concealed surfaces of tarsus and shanks are uniformly white (yellow) in juveniles. Adults bear dark brown to black reticulations outlining well-defined pale (yellow) spots. Dorsal surfaces of thighs bear dark oblique bars, well defined on juveniles, ill-defined or absent on adults. Ventral surfaces of thighs are white or cream (yellow). Venter is white with a light brown midline (reddish cream) extending to the throat; throat is white (cream, sometimes yellow in males). Iris is copper with black reticulations in adults; sclera varies from cream to light blue. Juveniles have a faint medial horizontal red streak.
Figure 27.
Color variation in preserved individuals of Pristimantis phoxocephalus. A Dorsal view of (from left to right): QCAZ 58463 (female), QCAZ 58472 (female), QCAZ 58470 (male), QCAZ 58468 (male), QCAZ 58461 (male). B Ventral view of the same specimens. See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Advertisement call.
Based on recordings of two non-collected individuals at the type locality of P. phoxocephalus (November 17, 2014; 19h26; <10 °C). Advertisement calls of P. phoxocephalus consist of a series of 3–9 sharp notes (Fig. 6B). Notes last on average 0.154 s (range 0.133–0.175 s), silences between them, 0.135 s (range 0.110–0.159 s). The exact middle of the note presents the highest energy. The dominant and fundamental frequency of the notes is 2578 Hz. The note increases its frequency from beginning to end (2587–2452 Hz on average). Descriptive statistics for bioacoustic parameters are shown in Table 6.
Distribution, natural history, and conservation status.
Pristimantis phoxocephalus is known from its type locality, Pilaló, Cotopaxi Province, Ecuador, at 2340–2820 m a.s.l. (Fig. 2), which corresponds to Western Montane Forest. Individuals collected for this study were found by day inside bromeliads at 2–4 m above the ground. These bromeliads were on scattered trees within pastures. Lynch (1979) provides more ecological data for specimens collected at the type locality.
Currently, P. phoxocephalus is considered to be a Least Concern species (Rodríguez et al. 2004) under the assumptions: (i) Extent of Occurrence < 20000 km2, (ii) common and adaptable species with a presumed large population, (iii) unlikely to be declining fast enough to qualify for a more threatened category. After assessing the identity of this species, herein we assign it to the Critically Endangered Red List category following the B1ab(iii)+2ab(iii) IUCN criteria because: (i) it is known from one locality whose habitat is constantly degrading due to human settlements and cattle raising, (ii) Extent of Occurrence < 100 km2, (iii) Area of Occupancy < 10 km2.
Remarks.
Until now, P. phoxocephalus has been considered a single highly polymorphic species (e.g., Lynch 1979; Lynch and Duellman 1997; Duellman and Lehr 2009). We show that most populations previously ascribed to P. phoxocephalus represent other species (e.g., Pristimantis atillo, P. jimenezi, P. teslai sp. nov., P. torresi sp. nov., P. totoroi sp. nov., P. verrucolatus sp. nov., Pristimantis sp. (CCS2), Pristimantis sp. (UCS2), Pristimantis sp. (UCS3)). To determine the true identity of P. phoxocephalus, we made collections at the type locality (Pilaló, Cotopaxi Province, see Suppl. material 2 for the list of specimens). Surprisingly, sequences from Pilaló fell in two distinct clades, indicating the presence of two species. One juvenile (QCAZ 58425), collected at 2258 m, is actually P. totoroi sp. nov. All other specimens (e.g., QCAZ 58463) are P. phoxocephalussensu stricto. Examination of photographs of the holotype KU 142075 (Fig. 23A) showed unequivocally that it is conspecific with the specimens from the highland population. We included in our analysis sequences of “P. phoxocephalus” from two localities of Peru (Kañaris in Ferrenafe Province, Warmicocha-Cochapamba road in Chachapoyas Province); they do not belong to Huicundomantis (Suppl. material 3) and likely represent an undescribed species. Hence, P. phoxocephalus is endemic to Ecuador and has a restricted distribution.
Pristimantis teslai sp. nov.
5DB35A0E59D1556DA87A57D10898CF71
http://zoobank.org/1A73A8C1-771E-464A-86DA-F64E7FD30197
Common name.
English: Tesla’s Rain Frog. Spanish: Cutín de Tesla.
Holotype.
QCAZ 46213, an adult male from Llanganatillo, Llanganates National Park border, Tungurahua Province, Ecuador (1.2658S, 78.4459W, 3600 m), collected by Rodrigo Toscano and Silvia Aldás-Alarcón on November 11, 2009. Figure 28A.
Figure 28.
Holotypes of Pristimantis teslai sp. nov. and P. torresi sp. nov. Photographs of preserved holotypes of AP. teslai (QCAZ 46213, male) and BP. torresi (QCAZ 47342, female). Dorsal view on the left, ventral view on the right. Specimens are shown at the same scale.
Paratopotypes
(4: 3 males, 1 juvenile).QCAZ 46108, QCAZ 46208, QCAZ 46211, adult males, QCAZ 46209, juvenile, collected by Elicio E. Tapia, Silvia Aldás-Alarcón, and Rodrigo Toscano in November 2009.
Diagnosis.
A species of the Pristimantis phoxocephalus group having the following combination of characters: (1) dorsal surfaces tuberculate, tubercles on anterior dorsum prominent, small and rounded, those on posterior dorsum larger; middorsal, dorsolateral, and lateral folds absent; head with two small middorsal tubercles; skin on flanks as or more tuberculate than dorsum, bearing scattered warts; skin on venter coarsely areolate; discoidal fold present; (2) tympanic membrane and tympanic annulus prominent, its upper and posterior margin concealed by thick supratympanic fold; (3) snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; (4) upper eyelid with distinct rounded tubercles surrounded by smaller tubercles; cranial crests absent; (5) dentigerous processes of vomers low to prominent, oblique, moderately separated, posteromedial to choanae; (6) vocals slits, vocal sac, and nuptial pads present in adult males; (7) Finger I shorter than Finger II; discs of digits expanded to broadly expanded, elliptical to truncate; (8) fingers with broad lateral fringes; (9) distinct, rounded ulnar tubercles; (10) heel bearing a prominent round tubercle surrounded by smaller tubercles; inner and outer edge of tarsus bearing a row of prominent, rounded tubercles; (11) inner metatarsal tubercle elliptical, elevated four times the size of round, elevated outer metatarsal tubercle; supernumerary tubercles distinct, as large as outer metatarsal tubercle; (12) toes with lateral fringes; basal webbing present; Toe V longer or much longer than Toe III (disc on Toe III reaches distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the proximal to distal edge of distal subarticular tubercle on Toe IV); toe discs smaller than those on fingers, elliptical to truncate (Fig. 9A); (13) in life, dorsum olive to reddish brown with irregular red or cream markings; groins and anterior surfaces of thighs dark brown with yellow blotches irregularly bordered; dorsal surfaces of thighs with irregular oblique yellow stripes, posterior surfaces of thighs brown with yellow flecks; venter dusty white with scattered brown flecks, with or without faint yellow blotches; iris copper with thin black reticulations (Fig. 29); (14) average SVL in adult males: 25.2 ± 1.8 mm (23.4–27.3 mm; n = 4); females: unknown.
Figure 29.
Color variation in live individuals of Pristimantis teslai sp. nov. AQCAZ 46208 (male, SVL 23.4 mm) BQCAZ 46209 (juvenile female, SVL 29.4 mm). Dorsolateral view on the left, ventral view on the right.
Comparison with other species.
Pristimantis teslai is most similar to P. atillo, P. jimenezi, P. percultus, P. phoxocephalus, P. torresi sp. nov., P. totoroi sp. nov., and P. verrucolatus sp. nov. They share an acuminate and protruding snout with a keel at the tip. However, (except for P. percultus) P. teslai is unique among them by having the dorsum covered by prominent rounded tubercles. Additionally, lack of lateral folds distinguishes P. teslai from P. jimenezi, P. totoroi sp. nov., P. torresi sp. nov., and P. verrucolatus sp. nov. Groins of P. teslai are dark brown with yellow blotches irregularly bordered, different from those of P. atillo (groins orange surrounded or not by yellow blotches), P. jimenezi (pinkish, purplish or dark brown with small light brown to yellow spots), P. phoxocephalus (yellow with dark brown to black reticulations), and P. verrucolatus sp. nov. (reddish brown with light brown to yellow spots). In males, the tympanum diameter is significantly larger than that of P. totoroi sp. nov. (males Z = -2.63144, p = 0.0085, TD/SVL = 5.0–5.5% in P. teslai, 4.4–5.1% in P. totoroi sp. nov). Pristimantis teslai is smaller than P. verrucolatus sp. nov. (males Z = 2.35, p = 0.0188, SVL = 23.4–27.3 mm in P. teslai, 25.1–34.5 mm in P. verrucolatus sp. nov). It can be distinguished from P. percultus by the absence of cranial crests (low in P. percultus), the coloration of the iris (copper with thin reticulations in P. teslai; golden with wide black reticulations in P. percultus), and lacking the red labial stripe, characteristic of P. percultus.
Description of the holotype.
Adult male (QCAZ 46213, SC29676). Measurements (in mm): SVL 27.3; TL 12.8; FL 12.9; HL 9.2; HW 9.5; ED 2.9; TD 1.4; IOD 3.1; EW 2.7; IND 2.3; EN 2.7; TED 1.0. Head wider than long, as wide as body; snout moderately long, acuminate in dorsal view, protruding in profile, bearing a fleshy keel; cranial crests absent; nostrils slightly protuberant, ovoid, directed laterally with slight dorsal inclination; canthus rostralis slightly concave in dorsal view, rounded in cross section; loreal region slightly concave; upper eyelid with prominent, rounded, medium sized tubercles surrounded by smaller tubercles; tympanic membrane distinct; tympanic annulus prominent, upper and posterior edge concealed by supratympanic fold; two prominent rounded postrictal tubercles. Choanae large, ovoid, not concealed by palatal shelf of maxillae; dentigerous processes of vomers small, prominent, oblique, narrowly separated, positioned posteromedial to choanae; each vomer bearing several teeth; tongue longer than wide, slightly notched, posterior three fifths not adherent to floor of mouth; vocal slits slightly curved, located at posterior half of mouth floor in between tongue and margin of jaw; vocal sac present.
Dorsal surfaces of body tuberculate, head and anterior dorsum with small prominent rounded tubercles, posterior dorsum with medium sized tubercles; middorsal, dorsolateral and lateral folds absent; head bears two prominent middorsal tubercles; flanks with the same texture as dorsum, bearing scattered warts; skin on venter coarsely areolate, ventral surfaces of limbs smooth, ventral surfaces of thighs coarsely areolate; discoidal fold absent. Low and round ulnar tubercles; white nuptial pads; outer palmar tubercle bifid, almost twice the size of ovoid thenar tubercle; subarticular tubercles prominent, rounded; low supernumerary tubercles at the base of fingers; fingers bearing broad lateral fringes; Finger I shorter than Finger II; discs on fingers expanded, truncate; pads on fingers surrounded by circumferential grooves on all fingers (Fig. 9A).
Hindlimbs slender; dorsal surfaces of hindlimbs tuberculate; posterior surfaces of thighs smooth, ventral surfaces of thighs coarsely areolate; heel bearing a medium sized, prominent and rounded tubercle surrounded by several slightly smaller tubercles; outer and inner edge of tarsus bearing distinct, rounded tubercles; inner metatarsal tubercle elliptical, elevated 4 × the size of round, elevated outer metatarsal tubercle; supernumerary tubercles as large as outer metatarsal tubercle; subarticular tubercles prominent, rounded; toes bearing lateral fringes; basal webbing between toes IV and V present; discs on toes smaller than those on fingers, expanded and elliptical; toes having pads surrounded by circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V much longer than Toe III (disc on Toe III reaches distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches distal edge of distal subarticular tubercle on Toe IV; Fig. 9A). Coloration of the holotype in preservative is shown in Figure 28A.
Coloration of holotype in preservative. Dorsum brown with a light brown W-shaped scapular mark and interscapular blotch; head with dark brown supratympanic stripe, canthal and interorbital bands; dorsal surfaces of forelimbs, shanks and tarsus light brown with dark brown transversal bands; armpits, groins, anterior, and posterior surfaces of thighs cream; posterior surfaces of thighs brown with yellow flecks; venter cream with scattered brown flecks; throat, soles, and palms dusty cream (Fig. 28A).
Coloration of holotype in life. Unknown.
Variation.
Variation in preservative is shown in Figure 30, where all the type series is included. Coloration in life is known for two individuals; their photographs are shown in Figure 29. Coloration in life is in parenthesis. Background coloration of dorsal surfaces vary from gray to brown (olive to reddish brown); dorsum might bear dark brown W-shaped scapular marking, irregular chevrons, or longitudinal stripes (dorsum with red or cream irregular spots or blotches); head bears or not dark brown supratympanic stripe, canthal stripe, interorbital band and labial bars; flanks with or without black or white spots. Groins and anterior surfaces of thighs dark brown with pale (yellow) blotches irregularly bordered; posterior surfaces of thighs brown with pale (yellow) flecks; dorsal surfaces of thighs with irregular oblique pale (yellow) stripes, venter white to dusty white with scattered brown flecks (dusty white with or without faint yellow blotches). Iris is copper with thin black reticulations; light-blue sclera. Tubercles of the individual with the striped color pattern on dorsum are less prominent than those of the other individuals.
Figure 30.
Color variation in preserved individuals of Pristimantis teslai sp. nov. A Dorsal view of (from left to right): QCAZ 46209 (juvenile female), QCAZ 46108 (male), QCAZ 46211 (male), QCAZ 46208 (male) B Ventral view of specimens in A. See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Distribution, natural history, and conservation status.
Pristimantis teslai is known from two Paramo localities in the eastern Andean slopes in Tungurahua Province (Fig. 2). Individuals were found beneath rocks, among moss or bunch grasses at day, or active on low vegetation up to 80 cm above ground at night. Parque Nacional Llanganates is, to a large extent, unexplored. It could have additional populations for this species. Given the scant available information, we assign P. teslai to the Data Deficient Red List Category (IUCN 2017).
Etymology.
The specific epithet is a noun in the genitive case and is a patronym for Nikola Tesla, a revolutionary inventor of the late 19th and early 20th century. It is named after him in recognition of his contributions to physics and his dedication to the ideal of providing free wireless electric power.
Remarks.
Pristimantis teslai has been mistakenly identified as P. phoxocephalus (e.g., collections at the QCAZ museum). Here, we recognize it as a different species and assign it to the P. phoxocephalus species group. Pristimantis teslai is most similar species to UCS1. They are sister species and have a genetic distance of 2.5%. Pristimantis teslai differs from UCS1 by having more prominent tubercles and smaller and fuzzier yellow blotches on the groins and posterior surfaces of thighs. However, these small differences may represent intraspecific variation. Hence, the status of UCS1 will remain tentative until additional specimens and populations of both species are examined.
Pristimantis torresi sp. nov.
4FFD270148D35548960B3C83B72FEC13
http://zoobank.org/1E92D590-2072-4530-88C6-2019A0A6D8F9
Common name.
English: Torres’ Rain Frog. Spanish: Cutín de Torres.
Holotype.
QCAZ 47342, adult female from Guanchanamá, military antennas, Loja Province, Ecuador (4.0511S, 79.8693W, 2729 m), collected by Elicio E. Tapia and Margarita Baquero on February 21, 2010. Figure 28B.
Paratypes
(46: 16 males, 6 females, 24 juveniles). Ecuador: Loja Province: QCAZ 47343, QCAZ 47354, adult females, QCAZ 47344, adult male, QCAZ 47340–341, QCAZ 47345–351, juveniles, collected with the holotype; QCAZ 47397, QCAZ 47405, QCAZ 47409, QCAZ 47414, QCAZ 47416–418, QCAZ 47503, adult males, from Celica-Alamor road (4.0990S, 79.9799W, 2101 m), collected by Elicio E. Tapia and Freddy Velásquez on February 27, 2010; QCAZ 64524, adult male, QCAZ 64525, QCAZ 64527–529, QCAZ 64555, juveniles, from Guanchanamá, military antennas (4.0511S, 79.8693W, 2729 m), collected by Nadia Páez, Fernando Ayala, Pablo Sandoval and Ricardo Gavilanes in August 2016; QCAZ 64533, QCAZ 64538, juveniles, from Celica-Guachanamá road (4.0907S, 79.9509W, 2348 m), collected by Nadia Páez, Fernando Ayala, Pablo Sandoval and Ricardo Gavilanes in August 2016; QCAZ 64544, juvenile from Guachanamá-La Tolera road (4.0321S, 79.8836W, 2736 m), collected by Nadia Páez, Fernando Ayala, Pablo Sandoval and Ricardo Gavilanes in August 2016; QCAZ 64549, adult male, QCAZ 64551, QCAZ 64556, juveniles, from Celica–Alamor road (4.0974S, 79.9796W, 2117 m), collected by Nadia Páez, Fernando Ayala, Pablo Sandoval and Ricardo Gavilanes in August 2016. Nearby Guachanamá, collected by Diego Almeida, Darwin Núñez, Eloy Nusirquia, Santiago Guamán and Guadalupe Calle in November 2016: QCAZ 65762, adult male, (4.0441S, 79.8814W, 2664 m); QCAZ 65766–767, adult females (4.0410S, 79.8827W, 2770); QCAZ 65769, adult male (4.0408S, 79.8829W, 2787 m); QCAZ 65771, adult male (4.0431S, 79.8817W, 2738 m); QCAZ 65774, QCAZ 65776, adult males (4.0441S, 79.8814W, 2646 m). Nearby La Tolera, collected by Diego Almeida, Darwin Núñez, Eloy Nusirquia, Santiago Guamán and Guadalupe Calle in November 2016: QCAZ 65779, adult female (4.0317S, 79.8824W, 2737 m); QCAZ 65782, adult female, QCAZ 65780, juvenile (4.0326S, 79.8836W, 2752 m); QCAZ 65783–786, juveniles (4.0395S, 79.8848W, 2833 m).
Diagnosis.
A member of the Pristimantis phoxocephalus group characterized by the following combination of characters: (1) skin on dorsum shagreen; thin middorsal fold; head with a middorsal small tubercle or row of tubercles; dorsolateral folds absent; flanks with longitudinal lateral folds on anterior half; skin on venter areolate to weakly areolate; discoidal fold present or absent; (2) tympanic membrane and tympanic annulus prominent, its upper and posterior margin concealed by supratympanic fold; (3) snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; (4) upper eyelid with a small and rounded tubercle, surrounded by several lower tubercles; cranial crests absent; (5) dentigerous processes of vomers prominent, oblique, moderately to broadly separated, posteromedial to choanae; (6) vocals slits, vocal sac and nuptial pads present; (7) Finger I shorter than Finger II; discs of digits broadly expanded, elliptical to truncate; (8) fingers with lateral fringes; (9) ulnar tubercles ill-defined; (10) heel bearing a small, rounded tubercle surrounded or not by smaller tubercles; outer and inner tarsal tubercles absent or ill-defined; inner tarsal fold present; (11) inner metatarsal tubercle ovoid, rounded, elevated, six times the size of round outer metatarsal tubercle; supernumerary tubercles numerous; (12) toes with broad lateral fringes; basal webbing present; Toe V longer or much longer than Toe III (disc on Toe III reaches the middle or exceeds distal edge of penultimate subarticular tubercle on Toe IV; disc on Toe V reaches the middle or exceeds distal edge of distal tubercle on Toe IV); toe discs as large or slightly smaller than those on fingers (Fig. 9B); (13) in life, dorsum cream, brown or orangey brown; head bearing a dark supratympanic stripe and interorbital band or stripe; coloration of groins and concealed surfaces of thighs have sexual dimorphism: in females (and juveniles), ground color varies from light purplish brown to medium brown with or without minute cream flecks on; males have the same ground color with yellow, cream or orangey-yellow small to big spots; venter white to dusty cream; iris golden to beige with black reticulations and a red or reddish brown medial streak (Fig. 31); (14) average SVL in adult females: 34.7 ± 3.7 mm (30.1–39.5 mm; n = 5); in adult males: 25.9 ± 2.1 mm (23.3–30.0 mm; n = 18).
Figure 31.
Color variation in live individuals of Pristimantis torresi sp. nov. AQCAZ 64524 (male, SVL 26.7 mm) BQCAZ 65767 (female, SVL 35.8 mm) CQCAZ 65771 (male, SVL 28.7 mm) DQCAZ 65779 (male, SVL 26.4 mm). Dorsolateral view on the left, ventral view on the right.
Comparison with other species.
Pristimantis torresi is similar to P. atillo, P. jimenezi, P. phoxocephalus, P. teslai, P. totoroi sp. nov., and P. verrucolatus sp. nov., which also have an acuminate snout with a fleshy keel. Pristimantis atillo usually has orange groins and black dots on the flanks, instead, P. torresi has brown groins with or without yellow spots, and its flanks lack black dots. The most similar species to P. torresi is P. jimenezi, whose iris varies from copper to red (golden to beige with a red medial streak in P. torresi). Pristimantis torresi differs from P. phoxocephalus in having a golden to beige iris (copper in P. phoxocephalus), brown groins with or without light brown to yellow spots (yellow with black reticulations in P. phoxocephalus), and a wider head relative to its body (males Z = -2.56285, p = 0.0104, HW/SVL = 33.4–34.4% in P. phoxocephalus, 34.2–37.8% in P. torresi; females Z = -2.08893, p = 0.0367, HW/SVL = 33.1–37.3% in P. phoxocephalus, 36.7–38.5% in P. torresi). Pristimantis torresi is easy to distinguish from P. teslai by having shagreen dorsal skin, lateral folds, and golden to beige iris with a red to reddish-brown medial streak (tuberculate dorsal skin, lateral folds absent and copper iris in P. teslai). Pristimantis totoroi sp. nov. has more prominent tubercles and folds than those of P. torresi, and its head is longer (males Z = 3.84623, p = 0.0001, HL/HW = 95.1–102.3% in P. torresi, 99.7–104% in P. totoroi sp. nov; females Z = -2.76079, p = 0.0058, HL/HW = 90.6–94.7% in P. torresi, 95.6–103.2% in P. totoroi sp. nov), relative to head width. Pristimantis torresi differs from Pristimantis verrucolatus sp. nov. in lacking large tubercles and warts on the flanks (present in P. verrucolatus) and having a golden to beige iris (coppery brown in P. verrucolatus).
Description of the holotype.
Adult female (QCAZ 47342, SC32171). Measurements (in mm): SVL 35.8; TL 17.5; FL 16.4; HL 12.4; HW 13.2; ED 3.7; TD 1.8; IOD 3.8; EW 3.1; IND 2.8; EN 3.5; TED 0.8. Head wider than long, narrower than body; snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; nostrils slightly protuberant, directed anterolaterally; canthus rostralis distinct, curved in dorsal view, rounded in cross-section; loreal region concave; upper eyelid bearing a small rounded tubercle surrounded by several lower tubercles; tympanic membrane and tympanic annulus distinct, its upper and posterior margin covered by supratympanic fold; one enlarged subconical postrictal tubercle surrounded by several smaller tubercles. Choanae median, semicircular, not concealed by palatal shelf of maxilla; dentigerous processes of vomers prominent, oblique, moderately separated, posteromedial to choanae; each vomer bearing several indistinct teeth; tongue as wide as long, posteriorly notched, free on posterior half of its length.
Skin on dorsum shagreen; faint middorsal fold; head with a middorsal row of two small tubercles; dorsolateral folds absent; flanks areolate, with thin lateral folds on anterior half; skin on belly and chest areolate, skin on throat, chest and ventral surfaces of limbs smooth; discoidal fold present. Ulnar tubercles present, indistinct; palmar tubercles prominent, outer palmar tubercle bifid, almost twice size of ovoid thenar tubercle; subarticular tubercles prominent, rounded; supernumerary tubercles at base of fingers distinct; fingers with broad lateral fringes; Finger I shorter than Finger II; discs broadly expanded and rounded; pads on fingers surrounded by circumferential grooves on all fingers (Fig. 9B).
Hindlimbs slender; dorsal surfaces of hindlimbs smooth; posterior surfaces of thighs smooth, ventral surfaces of thighs areolate; heel bearing a low rounded tubercle surrounded by some smaller tubercles; outer tarsal tubercles absent; inner tarsal fold extend to half of length of tarsus; inner metatarsal tubercle ovoid, rounded, elevated, six times the size of rounded, ill-defined outer metatarsal tubercle; plantar surface with supernumerary tubercles, those on the base of toes low but distinct; subarticular tubercles prominent, rounded; toes with broad lateral fringes; basal webbing present; discs nearly as large as those on fingers, elliptical; all toes having pads surrounded by circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V much longer than Toe III (disc on Toe III reaches the middle of the penultimate subarticular tubercle on Toe IV, disc on Toe V exceeds distal edge of distal subarticular tubercle on Toe IV; Fig. 9B). Coloration of the holotype in preservative is shown in Figure 28B; coloration in life is unknown.
Coloration of holotype in preservative. Dorsal surface of body light gray; head with cream interorbital band outlined with black, dark brown supratympanic stripes, and faint gray labial bars; limbs with transversal bands slightly darker than background; dorsolateral surfaces with oblique reticulations extending to the flanks, slightly darker than background; groins and concealed surfaces of thighs gray with small cream spots; ventral surfaces of body white, venter with faint gray flecks and midventral longitudinal line; plants and palms dusty cream (Fig. 28B).
Coloration of holotype in life. Unknown.
Variation.
Based on the 47 preserved specimens of the type series and photographs for 24 individuals. Variation in living and preserved individuals is shown in Figures 31, 32. Coloration in life is given in parenthesis. Dorsum coloration varies between cream, light gray and brown (cream, brown or orangey-brown). Some individuals present dark brown reticulations, chevrons, flecks, a middorsal band, an hourglass-shaped band or a thin white middorsal stripe on dorsum. Head always bears brown supratympanic stripes and interorbital stripes or bands; sometimes, it bears brown canthal stripes or labial bars. Limbs present faint or well-defined transversal bands. Flanks may have diagonal bars. Coloration of groins and concealed surfaces of hindlimbs has sexual dimorphism: both sexes have cream, gray or brown backgrounds (light purplish brown to medium brown); females have none or little pale (cream) flecks; males have small or big pale (yellow, cream or orangey yellow) spots, usually more conspicuous in larger males. Venter varies from white to dusty cream, usually with gray or light brown markings and a midline (in males, throat cream, light or bright yellow). Iris is golden to beige with black reticulations and a red or reddish-brown medial streak; sclera varies from cream to light blue. Coloration in life of each individual may change; the ground color of the hidden surfaces of thighs and other markings as the supratympanic stripe can vary from light purplish brown to medium brown in the same individual.
Figure 32.
Color variation in preserved individuals of Pristimantis torresi sp. nov. A Dorsal view of (from left to right): QCAZ 65767 (female), QCAZ 65762 (male), QCAZ 65769 (male), QCAZ 64524 (male) B Dorsal view of: QCAZ 64549 (male), QCAZ 65782 (male), QCAZ 47397 (male), QCAZ 47416 (male) C Ventral view of specimens in (A) D Ventral view of specimens in B. See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Distribution, natural history, and conservation status.
This species is known from the surroundings of Celica, Guachanamá and La Tolera, towns in the western Andean slopes of Loja Province in Ecuador (Fig. 2). It inhabits Inter-Andean Shrub region between 2101 and 2833 m a.s.l. Most individuals were found at night inside terrestrial or arboreal bromeliads up to 3 m above the ground, inside patches of native vegetation, or on low vegetation up to 60 cm above ground. During the day they were only found inside bromeliads. Calling males have been found on low vegetation, 30 cm above the ground in February.
We propose assigning P. torresi to the Critically Endangered Red List category following the B1ab(iii) IUCN criteria. Available records come from two localities (sensu IUCN 2017) whose habitat is being degraded by human settlements, cattle raising and agriculture; the Extent of Occurrence of the species < 100 km2 (21 km2).
Etymology.
The specific epithet is a noun in the genitive case and is a patronym for Omar Torres-Carvajal, curator of reptiles of Museo de Zoología at Pontificia Universidad Católica del Ecuador. The species name is in recognition of his significant contributions to herpetological research in Ecuador and the development of collections at the QCAZ museum.
Pristimantis totoroi sp. nov.
F22567F5929355799C460E404CB4608F
http://zoobank.org/C1E75C61-45D3-41E3-84E8-99721B713E76
Common name.
English: Totoras Rain Frog. Spanish: Cutín de Totoras.
Holotype.
QCAZ 25105, an adult male from Cashca Totoras Protected Forest, Bolívar Province, Ecuador (1.7188S, 78.9749W, 3200 m), collected by Rachel Kosoff and Ítalo G. Tapia on December 14, 2001. Figure 33A.
Figure 33.
Holotypes of Pristimantis totoroi sp. nov. and P. verrucolatus sp. nov. Photographs of preserved holotypes of AP. totoroi (QCAZ 25105, male) and BP. verrucolatus (QCAZ 46982, male). Dorsal view on the left, ventral view on the right. Specimens are shown at the same scale.
Paratypes
(41: 22 males, 5 females, 14 juveniles). Ecuador: Bolívar Province: Cashca Totoras Protected Forest: QCAZ 1021, QCAZ 1024, adult females, QCAZ 1020, QCAZ 1022–1023, juveniles, collected by Giovanni Onore in December 1987; QCAZ 14704–705, adult males, collected by Chris Funk in December 2000; QCAZ 16834–836, adult males, collected by Santiago Ron in January 2001; QCAZ 16916–922, adult males (1.7115S, 78.9781W, 3000 m), collected by Luis Coloma and Martín Bustamante in October 2000; QCAZ 12580, adult female (1.7200S, 78.9590W, 3200 m), collected by Felipe Campos and Stella de la Torre in December 1987; QCAZ 16718, QCAZ 16720–721, QCAZ 16731, adult males, QCAZ 16719, QCAZ 16725, QCAZ 16728, juveniles (1.7235S, 78.9802W, 2923 m), collected by Santiago Ron, Martín Bustamante, Ítalo Tapia and Mónica Guerra in August 2001; QCAZ 25122, QCAZ 25128, QCAZ 25135, adult males, QCAZ 25124, QCAZ 25127, QCAZ 25134, juveniles (1.7167S, 78.9667W, 2960 m), collected by Rachel Kosoff and Ítalo Tapia in December 2001; QCAZ 31505, adult female, QCAZ 31502, adult male, QCAZ 31501, QCAZ 31503–504, juveniles (1.7255S, 78.9806W, 2867 m), collected by Martín Bustamante and Galo Díaz in July 2002; QCAZ 32039, adult female, QCAZ 32040, adult male (1.7255S, 78.9806W, 2847 m), collected by Martín Bustamante in November 2002. Cotopaxi Province: QCAZ 58425, juvenile, from Pilaló surroundings (0.9713S, 78.9987W, 2258 m), collected by Santiago Ron, Pablo Venegas, Nadia Páez and Pamela Baldeón in November 2014. Bolívar Province: QCAZ 49511, adult male, QCAZ 49509, juvenile, from San Vicente (1.7120S, 79.0170W, 2408 m), collected by Luis Coloma and Ernesto Martínez in December 1984.
Referred specimens (1): KU 218025 from 70 km W Riobamba, Riobamba-Pallatanga road (1.9460S, 78.9468W, 2430 m), Chimborazo Province, Ecuador.
Diagnosis.
A species of the Pristimantis phoxocephalus group having the following combination of characters: (1) skin on dorsum shagreen with or without scattered small tubercles; middorsal fold; head with a middorsal row of two or more tubercles; dorsolateral folds absent; lateral folds on anterior half of dorsum; skin on venter coarsely areolate; discoidal fold evident; (2) tympanic membrane and tympanic annulus prominent, its upper and posterior margin covered by supratympanic fold; (3) snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; (4) upper eyelid with small tubercles; cranial crests absent; (5) dentigerous processes of vomers low to prominent, oblique, moderately separated, posteromedial to choanae; (6) vocals slits, vocal sac, and prominent nuptial pads present in adult males; (7) Finger I shorter than Finger II; discs of digits broadly expanded, rounded to elliptical; (8) fingers with broad lateral fringes; (9) small distinct ulnar tubercles; (10) heel bearing a subconical tubercle surrounded by smaller tubercles; outer edge of tarsus bearing subconical tubercles; inner edge of tarsus with a fold, followed or not by a row of small tubercles; (11) inner metatarsal tubercle ovoid, elevated, 5 times the size of round outer metatarsal tubercle; supernumerary tubercles numerous, elevated; (12) toes with broad lateral fringes; basal webbing present; Toe V much longer than Toe III (disc on Toe III reaches the middle of penultimate subarticular tubercle on Toe IV or slightly exceeds its distal edge, disc on Toe V reaches the middle of distal subarticular tubercle on Toe IV or slightly exceeds its distal edge); toe discs smaller than those on fingers, rounded to elliptical (Fig. 9C); (13) in life, dorsal coloration varies from cream to dark brown; flanks with dark diagonal bars; groins and posterior surfaces of thighs cream to brown with or without pale spots; venter white, bearing a midline extended or not to the throat; ventral surfaces of thighs light to dark gray, orange or cream; iris golden with a medial horizontal red streak and fine black reticulations (Fig. 34); (14) average SVL in adult females: 33.0 ± 0.9 mm (31.9–34.3 mm; n = 7); in adult males: 26.8 ± 2.0 mm (23.2–29.4 mm; n = 21).
Figure 34.
Color variation in live individuals of Pristimantis totoroi sp. nov. AQCAZ 16718 (male, SVL 24.7 mm) BQCAZ 16721 (male, SVL 24.1 mm) CQCAZ 16725 (juvenile female, SVL 23.7 mm). Dorsolateral view on the left, ventral view on the right.
Comparison with other species.
Pristimantis totoroi is similar to P. atillo, P. jimenezi, P. phoxocephalus, P. teslai, P. torresi and P. verrucolatus sp. nov., which also have an acuminate snout with a fleshy keel. The texture of its skin is different from the other species. Pristimantis totoroi has a shagreen dorsal skin, which is tuberculate in P. atillo; it lacks the large tubercles and warts on flanks present in P. verrucolatus sp. nov.; its tubercles and lateral folds are more prominent than those of P. atillo, P. jimenezi, P. phoxocephalus, and P. torresi. The coloration of the iris, golden with a red medial streak, helps distinguish P. totoroi from P. atillo (copper), P. jimenezi (copper to red), P. phoxocephalus (copper), P. teslai (copper), and P. verrucolatus sp. nov. (coppery brown). Additionally, the advertisement call of P. totoroi is different from the available calls of other species of the group. Notes of call of P. totoroi are shorter than those of P. jimenezi and P. verrucolatus sp. nov., inter-note intervals are longer than those of P. phoxocephalus, dominant frequency and frequency of the second harmonic are lower than those of P. jimenezi and higher than P. verrucolatus sp. nov. (Table 6).
Description of the holotype.
An adult male (QCAZ 25105, SC7093). Measurements (in mm): SVL 29.4; TL 13.9; FL 13.8; HL 10.2; HW 9.8; ED 3.6; TD 1.3; IOD 3.0; EW 3.0; IND 2.2; EN 2.9; TED 1.3. Head longer than wide, as wide as body; snout moderately long with a fleshy keel at the tip, acuminate in dorsal view, protruding in profile; cranial crests absent; nostrils slightly protuberant, narrow, directed anterolaterally; canthus rostralis concave in dorsal view, rounded in cross section; loreal region slightly concave; upper eyelid with several rounded tubercles, larger posteriorly; tympanic annulus prominent, upper and posterolateral edge concealed by supratympanic fold; tympanic membrane distinct; two prominent, subconical postrictal tubercles surrounded by smaller ones. Choanae median, ovoid, not concealed by palatal shelf of maxillae; dentigerous processes of vomers small, prominent, oblique, moderately separated, positioned posteromedial to choanae; each vomer bearing several indistinct teeth; tongue longer than wide, posteriorly notched, posterior half free; vocal slits slightly curved, located at posterior half of mouth floor in between tongue and margin of jaw; vocal sac present.
Dorsal surfaces of body shagreen with scattered small tubercles; thin middorsal fold; one tubercle between nostrils and eyes, one interorbital, two postocular and two scapular tubercles forming an inverted “V”; skin on head and loreal region bearing small rounded tubercles; dorsolateral folds absent; evident lateral folds on anterior half of flanks; skin on flanks having more prominent and larger tubercles than on dorsum; skin on chest and belly coarsely areolate, that on throat shagreen, ventral surfaces of limbs smooth, ventral surfaces of thighs coarsely areolate; discoidal fold present. Low and rounded ulnar tubercles; outer palmar tubercle bifid, slightly bigger than ovoid thenar tubercle; subarticular and supernumerary tubercles at the base of fingers prominent, rounded; fingers bearing broad lateral fringes; Finger I shorter than Finger II; discs on Fingers broadly expanded, rounded; pads on fingers surrounded by circumferential grooves on all fingers (Fig. 9C).
Hindlimbs slender; dorsal surfaces of hindlimbs shagreen with scattered tubercles; posterior surfaces of thighs smooth, ventral surfaces of thighs coarsely areolate; heel bearing a median, prominent, subconical tubercle surrounded by indistinct tubercles; outer edge of tarsus bearing distinct median subconical tubercles; inner tarsal fold present; inner metatarsal tubercle ovoid, elevated, five times the size of round outer metatarsal tubercle; plantar surface with small but distinct supernumerary tubercles; subarticular tubercles prominent, rounded; toes bearing broad lateral fringes; basal webbing between toes IV and V present; discs on toes smaller than those on fingers, expanded, rounded; all toes having pads surrounded by circumferential grooves; relative lengths of toes: I < II < V < III < IV; Toe V much longer than Toe III (disc on Toe III reaches distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches distal edge of distal subarticular tubercle on Toe IV; Fig. 9C). Coloration of the holotype in preservative is shown in Figure 33A; coloration in life unknown.
Coloration of holotype in preservative. Dorsal surfaces of body dark brown, dorsum with faint middorsal longitudinal line slightly darker than background coloration; brown supratympanic stripe; dorsal surfaces of thighs with cream transversal reticulations; groins, anterior and posterior surfaces of thighs brown with cream spots; ventral surfaces of body dusty cream, venter with a faint brown longitudinal midline and faint brown flecking (Fig. 33A).
Coloration of holotype in life. Unknown.
Variation.
Based on the 43 specimens of the type series and photographs of five individuals. Variation of live and preserved individuals is shown in Figures 34, 35. Coloration in life is given in parenthesis. Dorsal coloration varies from light to dark brown (tan to dark brown); middorsal fold is dark brown to black. Dorsum may bear irregular chevrons, a broad middorsal band or parallel longitudinal stripes; scattered black flecks or white spots may be present on dorsum; head bears a black or brown supratympanic stripe, sometimes an interorbital band or stripe, and labial bars; flanks usually bear dark diagonal bars. Dorsal surfaces of thighs bear pale transversal bands, sometimes formed by rows of pale spots. Groins and hidden surfaces of thighs are cream to brown (reddish cream to brown) with or without small pale flecks or spots. Venter is cream to dusty cream with a brown midline extending from the belly to the throat; brown or gray flecks on belly, chest, and throat may be present (in males, throat varies from white to yellow). Iris is golden with a medial horizontal red streak and fine black reticulations; sclera varies from white to cream. Skin ornamentation, as middorsal fold, dorsolateral folds, and dorsal tubercles, may fade in preservative.
Figure 35.
Color variation in preserved individuals of Pristimantis totoroi sp. nov. A Dorsal view of (from left to right): QCAZ 32039 (female), QCAZ 16918 (female), QCAZ 16834 (male) B Dorsal view of: QCAZ 16920 (male), QCAZ 25124 (juvenile female), QCAZ 25135 (male), QCAZ 31502 (male) C Ventral view of specimens in (A) D Ventral view of specimens in (B) See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Advertisement call.
Based on recordings of QCAZ 25105 (December 14, 2001; 20h00), and a non-collected individual from the type locality (December 14, 2001; 21h40; 10.2 °C). The advertisement call consists of a series of 2–6 sharp peep-like notes (Fig. 6C); each of these notes lasts about 0.13 s (range 0.10–0.15 s) and is separated of the following note by 0.34 s (range 0.31–0.37 s). The peak time occurs exactly in the middle of the note. Fundamental frequency is the same as dominant frequency, on average 2895 Hz (range 2776–3013 Hz). Frequency increases along the note (2551–2620 Hz on average). Descriptive statistics for bioacoustic parameters are shown in Table 6.
Distribution, natural history, and conservation status.
Pristimantis totoroi is known from Western Montane Forest of Bolívar, Chimborazo and Cotopaxi Provinces, between 2258–3200 m (Fig. 2). This species occurs in primary and secondary forests and pastures. Individuals found at night were on low vegetation up to 1.6 m above the ground or inside bromeliads up to 3 m above the ground. During the day they were found only inside bromeliads. Calling males have been found in October, December, and January on low vegetation during the night.
Following the B1ab(iii) + 2ab(iii) IUCN criteria, we consider P. totoroi to be Endangered because: (i) it is only known from three localities (sensu IUCN 2017); (ii) its habitat is gradually degraded by human settlements, cattle raising, and agriculture; (iii) its Extent of Occurrence < 5000 km2 (346 km2); and (iv) its Area of Occupancy <500 km2 (28 km2). Its distribution overlaps with one small protected area, Cashca Totoras Protected Forest. Unfortunately, the protection of the forest is not enforced and, as a result, it is being destroyed by logging.
Etymology.
The specific epithet is a noun in the genitive case that refers to the type locality of this species, the Cashca Totoras Protected Forest. This small reserve contains Western Montane Forest and Paramo natural regions. It is one of few protected areas in the western slopes of the Andes of central Ecuador, which are part of a biodiversity hotspot. Therefore, its effective protection is urgent to preserve unique assemblages of Andean biodiversity.
Remarks.
Pristimantis totoroi has been mistakenly referred to as P. phoxocephalus (e.g., Hedges et al. 2008). Here, we recognize it as a different species and assign it to the P. phoxocephalus species group.
Pristimantis verrucolatus sp. nov.
40B938747BBF51959F1831816CD27B11
http://zoobank.org/AEA32DD5-E2E5-4B83-B0DD-098447EA3FFC
Common name.
English: Warty Flank Rain Frog Spanish: Cutín de flancos verrugosos
Holotype.
QCAZ 46982, an adult male from Yumate, Shoupshe, Azuay Province, Ecuador (2.7696S, 79.4254W, 3504 m), collected by Elicio E. Tapia on January 21, 2010. Figures 33B, 36A.
Figure 36.
Color variation in live individuals of Pristimantis verrucolatus sp. nov. AQCAZ 46982 (holotype, male, SVL 28.4 mm) BQCAZ 45195 (male, SVL 34.5) CQCAZ 46993 (female, SVL 46.8 mm) DQCAZ 46999 (male, SVL 28.4 mm). Dorsal view on the left, ventral view on the right.
Paratypes
(17: 15 males, 1 female, 1 juvenile). Ecuador: Azuay Province: QCAZ 45174, adult female, QCAZ 45175, QCAZ 45195, adult males, from San Antonio, Cajas National Park border (2.8612S, 79.3787W, 2943 m), collected by Elicio E. Tapia and Juan Carlos Morocho in August 2009; QCAZ 46981, QCAZ 46984–989, adult males, collected with the holotype; QCAZ 46993–995, adult males, QCAZ 46992, juvenile, from Cochapamba (2.8020S, 79.4139W, 3662 m), collected by Silvia Aldás and Freddy Velásquez in January 2010; QCAZ 46999–7001, adult males, from Cochapamba (2.7971S, 79.4156W, 3548 m), collected by Elicio E. Tapia in January 2010.
Diagnosis.
A member of the Pristimantis phoxocephalus group characterized by the following combination of characters: (1) skin on dorsum shagreen with or without scattered tubercles, larger on posterior dorsum; with or without a faint middorsal fold; head with or without a middorsal row of two inconspicuous tubercles; flanks with warts and larger tubercles than those on dorsum; dorsolateral folds absent; thick, continuous or fragmented lateral folds on anterior flanks; skin on venter coarsely areolate; discoidal fold present or absent; (2) tympanic membrane and tympanic annulus prominent, its upper and posterior margin concealed by supratympanic fold; (3) snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; (4) upper eyelid with several low rounded tubercles; cranial crests absent; (5) dentigerous processes of vomers prominent, oblique, moderately separated, posteromedial to choanae; (6) males bearing vocal slits, vocal sac, and white nuptial pads; (7) Finger I shorter than Finger II; discs of digits broadly expanded, elliptical to truncate; (8) fingers with lateral fringes; (9) ulnar tubercles low and round sometimes connected by a weak fold; (10) heel bearing one or more small, prominent, subconical tubercles surrounded or not by some lower tubercles; outer and inner edge of tarsus bearing a row of low, rounded tubercles, inner tarsal fold present; (11) inner metatarsal tubercle ovoid, elevated, four times the size of round outer metatarsal tubercle; supernumerary tubercles numerous; (12) toes with broad lateral fringes; basal webbing present; Toe V longer or much longer than Toe III (disc on Toe III reaches the proximal to distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the proximal to distal edge of distal subarticular tubercle on Toe IV); toe discs smaller than those on fingers, elliptical to truncate (Fig. 9D); (13) in life, dorsum light to dark brown; head with an interorbital stripe or band and a dark supratympanic stripe; venter cream to white with or without brown reticulations; groins and posterior surfaces of the thighs reddish brown with small light brown, orangey brown or yellow spots; iris coppery brown (Fig. 36); (14) average SVL in adult females: 44.0 ± 4.5 mm (40.4–47.5 mm; n = 2); in adult males: 29.4 ± 2.7 mm (25.1–34.5 mm; n = 15).
Comparison with other species.
Pristimantis verrucolatus is most similar to P. atillo, P. jimenezi, P. phoxocephalus, P. teslai, P. torresi, and P. totoroi. It differs from all of them by having thick lateral folds. Except for P. teslai, it is the only having large tubercles or warts on flanks; though tubercles on flanks are also present in P. teslai, they are not as large as the ones of P. verrucolatus; furthermore, dorsum of P. teslai is tuberculate, while the dorsum P. verrucolatus is shagreen, with or without scattered tubercles. Pristimantis verrucolatus is further distinguished from P. atillo by the coloration of its groins (orange in P. atillo; reddish brown with light brown to yellow spots in P. verrucolatus), and its larger size (males Z = 2.35, p = 0.0188, SVL = 23.4–27.3 mm in P. teslai, 25.1–34.5 mm in P. verrucolatus). Pristimantis jimenezi, which has an adjacent distribution at lower elevations, is smaller than P. verrucolatus (males Z = 3.58643, p = 0.0003, SVL = 21.8–27.0 mm in P. jimenezi, 25.1–34.5 mm in P. verrucolatus; females Z = 2.00347, p = 0.0451, SVL = 31.1–37.4 mm in P. jimenezi, 40.4–46.8 mm in P. verrucolatus). Additionally, the advertisement call of P. verrucolatus is very distinctive, distinguishing it from all species with available calls: P. jimenezi, P. phoxocephalus, and P. totoroi. The call of P. verrucolatus has a single note, longer than those of the calls of the other species. It also has the lowest dominant frequency and the highest variation in frequency from the beginning to the end of the note (Table 6). Pristimantis sp. (CCS1) is the closest species to P. verrucolatus (average p genetic distance = 3.2%), they are not morphologically alike. CCS1 has a chubbier body, lacks a keel at the tip of the snout (present in P. verrucolatus), has slightly expanded discs on fingers and toes (broadly expanded in P. verrucolatus), and lacks lateral folds (present in P. verrucolatus).
Description of the holotype.
QCAZ 46982, SC29952 adult male. Measurements in mm: SVL 28.4; TL 13.3; FL 12.9; HL 8.8; HW 9.8; ED 3.3; TD 1.4; IOD 2.7; EW 2.5; IND 2.2; EN 2.6; TED 1.0. Head wider than body, wider than long; snout moderately long, acuminate with a fleshy keel in dorsal view, protruding in profile; nostrils slightly protuberant, ovoid, directed dorsolaterally; canthus rostralis weakly concave in dorsal view, angular in cross section; loreal region concave; cranial crests absent; upper eyelid bearing several low rounded tubercles; tympanic membrane and annulus prominent, its upper and posterior margins concealed by supratympanic fold; two enlarged, prominent and rounded postrictal tubercles surrounded by smaller tubercles. Choanae large, rounded, not concealed by palatal shelf of maxillae; dentigerous processes of vomers prominent, oblique, moderately separated, posteromedial to choanae; each vomer bearing several teeth; tongue slightly longer than wide, posterior border notched; posterior three fifths free; vocal slits slightly curved, located at posterior half of mouth floor in between tongue and margin of jaw; vocal sac present.
Skin on dorsum shagreen with scattered low tubercles; thin middorsal fold; thick lateral folds; flanks with large warts; skin on venter coarsely areolate. Chest, throat, and ventral surfaces of thighs areolate; discoidal fold absent. Low and round ulnar tubercles connected by an ill-defined fold; white nuptial pads present; palmar tubercles prominent, outer palmar tubercle bifid, slightly bigger than ovoid thenar tubercle; subarticular tubercles prominent, rounded; supernumerary tubercles at base of fingers prominent, smaller than subarticular tubercles; fingers with lateral fringes; Finger I shorter than Finger II; discs on fingers broadly expanded, elliptical; ventral pads on fingers surrounded by circumferential grooves (Fig. 9D).
Hindlimbs slender; dorsal surfaces of hindlimbs with scattered low tubercles; posterior surfaces of thighs smooth, ventral surfaces of thighs areolate; heel bearing a small subconical tubercle surrounded by some lower rounded tubercles; outer edge of tarsus bearing large low subconical tubercles; inner tarsal fold present followed by small distinct round tubercles on inner edge of tarsus; inner metatarsal tubercle ovoid, elevated, 4 × the size of round outer metatarsal tubercle; distinct, round supernumerary tubercles at the base of toes, indistinct ones on the rest of plantar surface; subarticular tubercles prominent, rounded; toes with broad lateral fringes; basal webbing present; discs on toes expanded, elliptical, smaller than those on fingers; all toes having ventral pads surrounded by circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V much longer than Toe III (disc on Toe III reaches distal edge of the penultimate subarticular tubercle on Toe IV, disc on Toe V reaches distal edge of distal subarticular tubercle on Toe IV; Fig. 9D). Coloration in life and preservative is shown in Figures 33B and 36A.
Coloration of holotype in preservative. Dorsum gray with light gray reticulations bordered by black lines; black interorbital and supratympanic stripes, and black canthal and labial bars; dorsal surfaces of limbs light gray with dark transversal bands bearing scattered black flecks; flanks with oblique light gray reticulations bordered by black spots; groins and posterior surfaces of thighs dark brown with cream spots; venter white; ventral surfaces of limbs dusty cream; throat dusty cream with dark brown mottling near the lips (Fig. 33B).
Coloration of holotype in life. Based on studio photographs. Dorsum brown with light brown reticulations bordered by black lines; black interorbital and supratympanic stripes, and black canthal and labial bars; dorsal surfaces of limbs light brown bearing dark brown transverse bands with scattered black flecks; flanks with oblique light brown reticulations bordered by black spots; groins and posterior surfaces of thighs dark brown with small cream spots; venter white; throat yellowish cream, vocal sac and ventral surfaces of limbs dusty pinkish cream; iris coppery brown with thin black reticulations; white sclera (Fig. 36A).
Variation.
Data are based on 18 preserved specimens and photographs from six living individuals. Variation in life and preservative is shown in Figures 36, 37. Coloration in life is given in parenthesis. Dorsal coloration varies from light gray to dark gray or brown (light to dark brown). Dorsal pattern can be uniformly colored, with irregular reticulations or with a middorsal band. Light colored individuals bear a pale stripe on lips. Head usually bears a dark canthal stripe that continues along the upper eyelid and above the tympanum, and an interorbital stripe. Most specimens have labial bars. Flanks bear or not pale reticulations or black flecks. Groins and posterior surfaces of thighs are brown with cream dots (reddish brown with small light brown or orangey brown spots). Ventral surfaces of limbs are dusty cream to brown; venter is cream or white with or without brown reticulations; throat is cream to dusty brown with or without dark mottling. Iris is coppery brown with thin black reticulations; white sclera. Flanks have medium sized to large tubercles and warts, arranged or not in rows; lateral folds can be thick and continuous or be present as a row of tubercles.
Figure 37.
Color variation in preserved individuals of Pristimantis verrucolatus sp. nov. A Dorsal view of (from left to right): QCAZ 46993 (female), QCAZ 45174 (female), QCAZ 45195 (male) B Dorsal view of: QCAZ 46999 (male), QCAZ 46987 (male), QCAZ 46984 (male), QCAZ 46981 (male) C Ventral view of specimens in (A) D Ventral view of specimens in (B) See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Advertisement call.
Based on recordings of QCAZ 46981 (September 21, 2010; 19h00; 8.8 °C) and QCAZ 46984 (January 20, 2010; 21h10). Advertisement calls consist of a single sharp whistle (Fig. 6D); each lasting on average 0.43 s (range 0.41–0.46 s). The peak time of each note occurs exactly in the middle of its duration. The dominant and fundamental frequencies are the same, on average 2144 Hz (range 1927–2302 Hz). The note increases its frequency from beginning to end (2231–1838 Hz on average). Descriptive statistics for bioacoustic parameters are shown in Table 6.
Distribution, natural history, and conservation status.
This species is known from Western Andean slopes of Azuay Province, between 2943–3662 m a.s.l (Fig. 2). It inhabits Paramo and Western Montane Forest regions. Individuals collected during the day were found beneath rocks or inside arboreal bromeliads up to 2 m above ground; at night they were found active on branches of low shrubs, up to 80 cm above the ground, or inside bromeliads. Gravid females were found in August and January; calling males in January.
Pristimantis verrucolatus is known from only two localities (sensu IUCN) and has a very restricted distribution. These places are adjacent to Parque Nacional Cajas, a protected area with unexplored regions that represent potential distribution for the species. Thus, following the IUCN (2017) guidelines, we assign this species to the Data Deficient Red List category.
Etymology.
The specific epithet is a noun in apposition with masculine gender. It is derived from the Latin words verruca meaning wart, and latus meaning flanks. The name refers to the large and low tubercles or warts on flanks that characterize this species.
Pristimantis cryptomelas species group new taxon
Definition. Monophyly of the P. cryptomelas species group is strongly supported in our phylogeny. Members of this group are characterized by: (i) postocular folds present; (ii) dorsolateral folds absent; (iii) cranial crests absent (except for low crests in P. spinosus); (iv) tympanic membrane and tympanic annulus prominent; (v) eyes bearing prominent tubercles; (vi) dentigerous processes of vomer present; (vii) prominent tubercles on heel and tarsus; (viii) fingers and toes with lateral fringes; (ix) broadly expanded discs on fingers and toes; (x) basal webbing between toes present; (xi) in life, groins and concealed surfaces of thighs have distinctive coloration patterns, including flash colors, and light or bright colored flecks or spots on a darker background; colors, shapes, and sizes of these ornaments are variable among species; (xii) SVL females 25.9–46.1 mm; SVL males 16.1–30.3 mm.
Content. The P. cryptomelas species group comprises four described species, P. cryptomelas, P. gagliardoi, P. muscosus, and P. spinosus, and the newly described P. nangaritza sp. nov. We suspect that the species content of the group will increase with collections and genetic studies of populations from northern Peru.
Distribution. Members of the P. cryptomelas group occur in the eastern Andean slopes of southern Ecuador and northern Peru. In Ecuador, they inhabit the Eastern Foothill and Montane Forest of Cañar, Loja, Morona Santiago, and Zamora Chinchipe Provinces, between elevations of 1800 and 3500 m a.s.l. In Peru, they live between 1770 and 2820 m a.s.l. Distribution based on QCAZ collection specimens, Duellman and Lehr (2009), and Yánez-Muñoz et al. (2012).
Remarks. The P. muscosus specimen included in our phylogeny is from the same and only population of P. muscosus reported for Ecuador (Yánez et al. 2012). The population is 216 km from the type locality in Peru (east slope of Abra Pardo de Miguel). Our results indicate that most species of Huicundomantis have small geographic ranges. The large geographic distance from the type locality of P. muscosus suggests that the Ecuadorian population may represent an undescribed species. The same applies to Peruvian populations of P. cryptomelas, which are widely separated from the type locality in Ecuador.
Pristimantis nangaritza sp. nov.
2E237460391E5375A410AFD27EC11A21
http://zoobank.org/E35A79CF-16A8-4838-90BA-103D0C48A019
Common name.
English: Nangaritza Rain Frog. Spanish: Cutín de Nangaritza.
Holotype.
QCAZ 41710, an adult female from Alto Nangaritza Protected Forest, Las Orquídeas, Tepuy Forest, Zamora Chinchipe Province, Ecuador (4.2620S, 78.6902W, 1809 m), collected by Elicio E. Tapia on April 18, 2009. Figure 38.
Figure 38.
Holotype of Pristimantis nangaritza sp. nov. Photographs of preserved holotype of AP. nangaritza (QCAZ 41710, female). Dorsal view on the left, ventral view on the right.
Paratypes
(17: 13 males, 3 females, 1 juveniles). All from Alto Nangaritza Protected Forest, Las Orquídeas, Tepuy Forest. Ecuador: Zamora Chinchipe Province: QCAZ 41704–708, QCAZ 41725–726, QCAZ 41728–729, QCAZ 41731–733, adult males, QCAZ 41734–736, adult females, QCAZ 41730, juvenile (4.2632S, 78.6911W, 1843 m), collected by Elicio E. Tapia, Jessica Loe Deichmann, Amable Fermín Jiménez and Holger Braun in April 2009; QCAZ 41709, adult male (4.2567S, 78.6780W, 1820 m), collected by Elicio E. Tapia and Jessica Loe Deichmann in April 2009.
Diagnosis.
A species of the Pristimantis cryptomelas group with the following combination of characters: (1) dorsal surfaces finely tuberculate; middorsal fold present or absent; head with a middorsal row of two small tubercles; dorsolateral folds absent; thin lateral folds on anterior half of flanks present or absent; skin on venter coarsely areolate; discoidal fold present or absent;) (-shaped postocular folds; (2) tympanic membrane and tympanic annulus prominent, its upper and posterior margin concealed by thick supratympanic fold; (3) snout moderately long, subacuminate in dorsal view, rounded in profile; (4) upper eyelid with subconical tubercles surrounded by several smaller tubercles; cranial crests absent; (5) dentigerous processes of vomers prominent, oblique, narrowly to broadly separated, posteromedial to choanae; (6) vocals slits and white nuptial pads present in adult males; vocal sac not externally expanded; (7) Finger I shorter than Finger II; discs of digits broadly expanded, rounded to elliptical; (8) fingers with lateral fringes; (9) low ulnar tubercles; (10) heel bearing a subconical tubercle surrounded by smaller tubercles; inner and outer edge of tarsus bearing low tubercles; short inner tarsal fold; (11) inner metatarsal tubercle ovoid, elevated five times the size of round outer metatarsal tubercle; supernumerary tubercles numerous; (12) toes with lateral fringes; basal webbing barely evident; Toe V longer or much longer than Toe III (disc on Toe III reaches the middle or exceeds distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V reaches the middle or exceeds distal edge of distal subarticular tubercle on Toe IV); toe discs smaller than those on fingers, elliptical (Fig. 8C); (13) in preservative, dorsum light to dark brown with darker markings including a scapular W, irregular chevrons and interorbital stripe, or a pattern of longitudinal stripes; head with black or dark brown supratympanic, canthal and labial vertical bars; flanks with oblique pale bars that merge with ventral coloration; posterior surfaces of thighs light brown with or without minute pale flecks; groins with the same coloration as posterior surfaces of thighs or venter; venter and throat cream with different levels of brown mottling (Fig. 39); (14) average SVL in adult females: 29.1 ± 2.7 mm (25.9–32.4 mm; n = 4); in adult males: 18.8 ± 1.1 mm (17.4–20.5 mm; n = 13).
Figure 39.
Color variation in preserved individuals of Pristimantis nangaritza sp. nov. A Dorsal view of (from left to right): QCAZ 41730 (juvenile female), QCAZ 41735 (female), QCAZ 41736 (female) B Dorsal view of: QCAZ 41708 (male), QCAZ 41732 (male), QCAZ 41728 (male), QCAZ 41725 (male) C Ventral view of specimens in (A) D Ventral view of specimens in B. See Suppl. material 2 for locality data. All specimens are shown at the same scale.
Comparison with other species.
It is most similar to P. cryptomelas, P. gagliardoi, P. muscosus, P. spinosus, and P. versicolor. The postocular folds of P. nangaritza are lower than those of P. cryptomelas, the background color of the posterior surfaces of thighs is light brown instead of black, its body is smaller (Table 5), and males have vocal slits (absent in males of P. cryptomelas). Pristimantis gagliardoi can be distinguished from P. nangaritza by having W-shaped postocular folds (“) (”-shaped in P. nangaritza), smaller tympanum (Table 5), and by lacking vocal slits. Pristimantis muscosus has smaller tubercles on the upper eyelid (round in P. muscosus; subconical in P. nangaritza), lacks tubercles on the inner edge of tarsus (present in P. nangaritza), and have dark brown or black groins. Pristimantis spinosus differs from P. nangaritza in having low cranial crests (absent in P. nangaritza) and black posterior surfaces of thighs with white spots. Pristimantis versicolor is readily recognized from P. nangaritza by lacking postocular folds and having a proportionally larger tympanum (females Z = 2.16506, p = 0.0304 TD/SVL = 4.7–5.9% in P. nangaritza, 5.2–7.2% in P. versicolor; males Z = 3.45394, p = 0.0006, TD/SVL = 5.1–5.4% in P. nangaritza, 5.8–6.4% in P. versicolor).
Description of the holotype.
An adult female (QCAZ 41710, SC28142). Measurements (in mm): SVL 32.4; TL 15.5; FL 13.4; HL 12.2; HW 12.1; ED 3.7; TD 1.7; IOD 3.1; EW 3.5; IND 2.5; EN 3.7; TED 1.2. Head longer than wide, wider than body; snout moderately long, subacuminate in dorsal view, rounded in profile; cranial crests absent; nostrils slightly protuberant, narrow, directed laterally with slight dorsal inclination; canthus rostralis concave in dorsal view, sharp in cross section; loreal region slightly concave; upper eyelid with subconical tubercles, those on posterior half larger; tympanic membrane distinct; tympanic annulus prominent, its upper and posterior edge concealed by supratympanic fold; two small prominent postrictal tubercles. Choanae large, circular, not concealed by palatal shelf of maxillae; dentigerous processes of vomers prominent, oblique, narrowly separated, positioned posteromedial to choanae; each vomer bearing several distinct teeth; tongue as long as wide, posteriorly notched; posterior third not adherent to the floor of mouth.
Dorsal surfaces of body finely tuberculate; dorsolateral folds absent; bearing) (-shaped postocular folds; skin on venter coarsely areolate, ventral surfaces of limbs smooth, ventral surfaces of thighs coarsely areolate; weak discoidal fold. Low median ulnar tubercles; outer palmar tubercle bifid, twice the size of ovoid thenar tubercle; subarticular tubercles prominent, rounded; prominent supernumerary tubercles at the base of fingers, slightly smaller than subarticular tubercles; fingers bearing lateral fringes; Finger I shorter than Finger II; discs on fingers broadly expanded, rounded; pads on fingers surrounded by circumferential grooves on all fingers (Fig. 8C).
Hindlimbs slender; dorsal surfaces of hindlimbs finely tuberculate; posterior surfaces of thighs smooth, ventral surfaces of thighs coarsely areolate; heel bearing a medium sized, subconical tubercle surrounded by several smaller tubercles; outer and inner edge of tarsus bearing low tubercles; inner tarsal fold present; inner metatarsal tubercle ovoid, elevated 5 × the size of round outer metatarsal tubercle; supernumerary tubercles prominent at the base of each toe, those on plantar surface distinct, but low; subarticular tubercles prominent, rounded; toes bearing lateral fringes; basal webbing barely evident between toes IV and V; discs on toes smaller than those on fingers, expanded and elliptical; all toes having pads surrounded by circumferential grooves; relative lengths of toes: I < II < III < V < IV; Toe V longer than Toe III (disc on Toe III exceeds distal edge of penultimate subarticular tubercle on Toe IV, disc on Toe V exceeds the distal of distal subarticular tubercle on Toe IV; Fig. 8C). Color of the holotype in preservative is shown in Figure 38; coloration in life is unknown.
Coloration of holotype in preservative. Dorsum light brown with lighter irregular reticulations and a W-shaped scapular mark bordered by dark brown lines; head with dark brown canthal, supratympanic, and labial bars; flanks with cream oblique cream bars bordered by dark brown spots and lines; groins, and concealed surfaces of thighs dark brown with scattered cream flecks; venter cream and ventral surfaces of thighs cream; throat cream with brown mottling; ventral surfaces of limbs dusty cream (Fig. 38).
Coloration of holotype in life. Unknown.
Variation.
This section is based on 18 specimens of the type series. In preservative, dorsum varies from light to dark brown with darker markings including a scapular W, irregular chevrons, and interorbital stripe. Some individuals have a pattern of longitudinal parallel stripes. Posterior surfaces of thighs are brown with or without minute pale flecks. Groins with the same coloration as posterior surfaces of thighs or venter. Venter cream with varying amount of brown mottling. This variation is shown in Figure 39. Coloration in life is unknown.
Distribution, natural history, and conservation status.
Pristimantis nangaritza is only known from its type locality, Alto Nangaritza Protected Forest, Zamora Chinchipe Province, Ecuador, a low vegetation forest with bromeliads, orchids, moss, and Podocarpus trees that belongs to the Eastern Foothill Forest, between 1809 and 1843 m a.s.l. (Fig. 1). Individuals were found active at night on branches, 1–2 m above ground. Calling males were found in April.
We consider it as a Data Deficient species because adjacent areas in Alto Nangaritza Protected Forest are difficult to access and poorly explored.
Etymology.
The specific epithet refers to the type locality of this species, Alto Nangaritza Protected Forest. This protected area preserves native vegetation of the Cordillera del Cóndor and hosts unique geologic formations in Ecuador, called Tepuyes. This reserve is largely unexplored and is currently threatened by the potential opening of roads for mining activities; 80% of its territory is under mining concessions.
On the identity of Pristimantis riveti
Several species described here were previously misidentified as P. riveti, including P. lutzae, P. gloria, P. multicolor, P. chomskyi, and Pristimantis sp. (CCS1) (e.g., Almendáriz and Orcés 2006; Heinicke et al. 2007; Padial et al. 2014). These misidentifications were based on a redescription of “P. riveti” by Lynch (1979). Populations used for Lynch’s redescription (Azuay Province, Cuenca) are geographically distant (400 km to the south) from the type locality of P. riveti, “Mirador”, which, based on the collector itinerary, we infer to be in Carchi Province, Ecuador. Although Despax (1911) did not mention the province for the type locality, the holotype was collected by Paul Rivet during the Second Geodesic Mission which visited “Mirador” in Carchi Province (Perrier 1913). According to Frost (2019), Mirador is in Tungurahua Province, Ecuador, but we could not find a reference to support that statement.
The great distance between the type locality of P. riveti and populations in Azuay Province used in Lynch’s redescription indicate that they are not conspecific. This conclusion is consistent with morphological comparisons of photographs of the holotype of P. riveti, MNHNP 1902.357 (Fig. 23B) with P. lutzae (= “P. riveti” of Lynch 1979) which showed marked differences. The holotype of P. riveti is an adult female whose snout-vent length is 34.2 mm, a size above the range for females of Pristimantis lutzae (29.7–33.9 mm); Pristimantis riveti has distinctive big white irregular blotches dispersed over the dorsal surface, which are absent in P. lutzae; besides, Toe V is slightly longer than toe III in P. riveti while it is longer or much longer than Toe III in P. lutzae. The combined evidence indicates that the populations from Azuay usually ascribed to P. riveti represent a previously undescribed species that we have named here.
Based on information published by Perrier (1913) we were able to infer the position of the type locality of P. riveti. Mirador is a mountain peak at an elevation of 3830 m near the border between Carchi and Sucumbíos Provinces. Recent collections made on that mountain, at an elevation between 3500 and 3792 m, include specimens similar to the holotype of P. riveti (QCAZ 69853, 69855, 69857–859, 69861, 69865–869). We tentatively considered these specimens to be the only documented records of P. riveti sensu stricto besides that of the holotype.
Discussion
Cryptic diversity within Huicundomantis
Herein, we document the existence of 16 new candidate species within a clade of which only 12 species were previously described. These results represent an increase in species richness of 133%. Moreover, several populations not included in our study likely represent additional undescribed species. For example, in the QCAZ collection, specimens from 6 km E Loja, Loja Province (e.g., QCAZ 21708) or Zamorahuaico, Zamora Chinchipe Province (e.g., QCAZ 22529) are morphologically similar to P. phoxocephalus and appear to represent undescribed species. These populations were excluded from our review because they lacked tissues for DNA analyses. Similarly, several populations of “P. phoxocephalus” from southern Ecuador reported by Lynch (1979) (e.g., 10 km SW Victoria del Portete, Azuay Province; 18.4 km NW El Tambo, Cañar Province; Saraguro, Loja Province) and from northern Peru by Duellman and Lehr (2009), Duellman and Pramuk (1999), and Duellman and Wild (1993) are likely undescribed species as indicated by our samples of “P. phoxocephalus” from Peru which fall outside Huicundomantis. Given the branch lengths that separate them, the three Peruvian samples appear two represent two different species (Suppl. material 3). Our results suggest that Andean Pristimantis still hide a large number of cryptic undescribed species. If the content of undescribed species of our group is a representative sample of all Pristimantis, we would expect the discovery of hundreds of new species in the coming decades.
Characters that help diagnose species of Huicundomantis are body tuberculation, the presence of dermal folds (e.g., middorsal, lateral, postocular folds), and the coloration in life of the groins, concealed surfaces of thighs, and iris. These characters are frequently lost in preservative, which could explain why previous taxonomic reviews, mainly based on morphology of preserved specimens, lumped so many species into single binomens. Hence, we highlight the importance of documenting coloration and skin texture in live individuals, prior to preservation. We also found extensive intraspecific and even intrapopulation morphological variation, especially in coloration and skin texture (e.g., P. multicolor; Fig. 24). This variation can only be properly described by sampling a large number of individuals. Future taxonomic reviews of Pristimantis will greatly benefit from documenting both life coloration and genetic variation in a large number of specimens.
Morphometrics were of little help to diagnose species except for several species pairs. For morphometric diagnoses, we compared body size and head proportions, which can be useful taxonomic characters (Arroyo et al. 2005). The addition of other measurements or the use of new approaches such as geometric morphometrics (Kaliontzopoulou 2011; Acevedo et al. 2016) could help to better differentiate cryptic species of Huicundomantis.
Similar to morphometrics, environmental envelopes were of limited help to distinguish closely related species. However, we were able to discriminate among species inhabiting high-altitude Paramo habitats from those inhabiting warmer regions in Eastern Foothill Forest, Deciduous Costa Forest, and Western Foothill Forest. These environmental differences are mirrored by distinct body types that appear to be related to different environments. Species from paramo habitats have a chubby appearance and usually have narrow finger discs while species from lower altitudes have a slenderer body and wider discs.
When available, advertisement calls allowed unequivocally diagnosing of closely related species. This is congruent to previous studies in Pristimantis (Hutter and Guayasamin 2015), and other Neotropical frogs (e.g., Padial et al. 2008; Funk et al. 2012; Caminer et al. 2014,) in which divergence in advertisement calls led to the discovery of cryptic species. Advertisement calls are under sexual selection and were the most variable trait between species. Therefore, it seems likely that sexual selection played an important role in speciation of Huicundomantis.
Huicundomantis, a new subgenus for Pristimantis
We propose Huicundomantis as a subgenus given that it has strong phylogenetic support and morphological distinctiveness. Currently, two subgenera are recognized within the genus Pristimantis: Pristimantis and Hypodictyon. According to recent reviews, Pristimantis is paraphyletic while Hypodictyon (sensu Crawford et al. 2010) is monophyletic (Heinicke et al. 2017). We agree with Heinicke et al. (2017) on the need for the recognition of new subgenera within Pristimantis to convey phylogenetic information among its 532 species. Padial et al. (2014) did not recognize the subgenus Pristimantis given its rampant paraphyly. However, the recognition of Hypodictyon is justified given its well established monophyly. The description of the new monophyletic subgenus Huicundomantis is an additional step to reach the goal of splitting Pristimantis into monophyletic subgenera. Moreover, Huicundomantis does not generate taxonomic instability because the subgenus Pristimantis has been largely ignored as a result of its nearly complete redundancy with its parent genus. Finally, we highlight that, if needed, the species content of Huicundomantis can be redefined to accommodate the requirements of a large-scale taxonomic review of its parent genus.
The name Huicundomantis refers to the association of most of its species to bromeliad plants (huicundos in Quechua). This association has been reported, at least facultatively, in P. atillo, P. atratus, P. chomsky, P. cryptomelas, P. jimenezi, P. multicolor, P. phoxocephalus, P. tinguichaca, P. torresi, P. totoroi, P. verrucolatus, and P. versicolor (Ron et al. 2019 and references therein). Another clade of Pristimantis with a large number of species associated to bromeliads is the P. lacrimosus group (Hedges et al. 2008). Both clades are not closely related (Pyron 2014), which suggests that the bromeliad association evolved independently in both groups. Numerous other species of Pristimantis of uncertain phylogenetic position also occur in bromeliads (e.g., P. huicundo, P. orphnolaimus). Understanding the evolution origin of this ecological character will require a comprenhensive analysis across all species of Pristimantis.
Speciation and genetic divergence
We found seven sister species pairs (including unconfirmed candidate species) within Huicundomantis. In all of them, their distribution ranges do not overlap and do not have significant differences in environmental envelope. Allopatric distribution and lack of differences in environmental envelope suggest that speciation has been mainly driven by incidental divergence in allopatry, rather than ecological divergence along environmental gradients (Peterson et al. 1999; Graham et al. 2004). The fragmented landscape of the Andean slopes, and expansions and contractions of the distribution range due to environmental oscillations should frequently result in geographic isolation, making allopatric speciation the most likely mode of speciation for Huicundomantis. Moreover, Andean slopes allow the existence of high diversity and endemism because they present sharp gradients of elevation, temperature, and precipitation. These heterogeneous conditions should explain the small distribution ranges observed in montane species of frogs (Wollenberg et al. 2008). The pattern of species richness and diversification of Huicundomantis is congruent with other studies from mountainous areas, which report allopatric speciation as the most frequent mode of speciation in the group (e.g., Lynch and Duellman 1997; Coloma et al. 2012; Jungfer et al. 2013).
We found that the 3% genetic distance threshold (gene 16S) proposed to identify candidate species (Fouquet et al. 2007; Vieites et al. 2009) could be too conservative. In Huicundomantis, the minimum genetic distance between confirmed candidate species was 2.0%; applying the 3% threshold would have resulted in 3 confirmed candidate species overlooked. Our results are similar to those of other systematic reviews of Neotropical amphibians (e.g., Padial and de la Riva 2009; Coloma et al. 2012; Funk et al. 2012; Jungfer et al. 2013; Caminer and Ron 2014; Ortega et al. 2015) showing that the 3% genetic distance threshold could result in underestimation of species numbers.
Cryptic diversity and conservation
The discovery of cryptic diversity can impact estimates of the conservation status of species (Bickford et al. 2007). One of the main variables to assess the conservation status of species is the size of its distribution range (IUCN 2017). After dividing what was thought to be a single species into several cryptic species, distribution ranges of the resulting species are smaller than those of the original; therefore, the conservation status should be revaluated after cryptic species are discovered. For example, until now, Pristimantis phoxocephalus was considered to be distributed along the western and eastern Andean slopes of Ecuador and Peru (elevations 1800–3100 m a.s.l; Rodríguez et al. 2004). Given its large distribution range, it was considered Least Concern by the IUCN Red List (Rodríguez et al. 2004). Our review shows that P. phoxocephalus is known from a single locality, Pilaló, in the western Andean slopes of central Ecuador. Therefore, we propose that P. phoxocephalus is Critically Endangered according to the IUCN (2017) guidelines.
Accurate taxonomic assessments contribute to conservation by helping to delimit priority conservation areas. Species of Huicundomantis are distributed along the central and southern Andes of Ecuador between 230 and 4200 m of elevation. Sixteen out of 28 species occur in protected areas: P. atillo (Sangay National Park), P. atratus (Podocarpus National Park, Tapichalaca Reserve, Yacuri National Park), P. chomskyi (Tapichalaca Reserve), P. cryptomelas (Huashapamba Protected Forest, Podocarpus National Park, Tapichalaca Reserve, Yacuri National Park), P. gagliardoi (Sangay National Park, Mazar Wildlife Reserve), P. hampatusami (Buenaventura Reserve), P. lutzae (Cajas National Park, Mazar Wildlife Reserve, Yanuncay-Irquis Protected Forest, Mazán Protected Forest, Sangay National Park), P. multicolor (Yacuri National Park), P. muscosus (Tapichalaca Reserve), P. nangaritza (Alto Nangaritza Protected Forest), P. philipi (Cajas National Park), P. tinguichaca (Sangay National Park), P. totoroi (Cashca Totoras Protected Forest), P. versicolor (Podocarpus National Park, Tapichalaca Reserve), Pristimantis sp. (CCS1) (Yanuncay-Irquis Protected Forest), and Pristimantis sp. (UCS2) (Sangay National Park). Nonetheless, P. balionotus, P. gloria, P. jimenezi, P. percultus, P. phoxocephalus, P. spinosus, P. teslai, P. torresi, P. verrucolatus, Pristimantis sp. (CCS2), Pristimantis sp. (UCS1), and Pristimantis sp. (UCS3) occur in areas currently threatened by agriculture, cattle raising, dam construction, mining, logging and human settlement (Coloma et al. 2004; Rodríguez et al. 2004). All species of Huicundomantis have a restricted distribution range (maximum confirmed Extent of Occurrence is 4330 km2 for P. atratus). As a result, most species are either Data Deficient or threatened with extinction. The recognition of 13–16 new species of Pristimantis for central and southern Andes of Ecuador, all with small distribution ranges (up to 2388 km2 for the new species), provides new reasons for the conservation of this region.
Supplementary Material
Acknowledgements
This research was funded by the Secretaría de Educación Superior, Ciencia, Tecnología e Innovación del Ecuador SENESCYT (Arca de Noé Initiative, Omar Torres and SRR principal investigators), and grants from Pontificia Universidad Católica del Ecuador. We thank Pablo Sandoval Acuña for the edition of most of the photographs and figures and his help with morphometric measurements. We thank Andrés Merino-Viteri for estimating the Area of Occupancy and Extent of Occurrence of the species. Pablo Venegas, through CORBIDI, provided tissues of Peruvian populations previously ascribed to P. phoxocephalus. We thank Mario Yánez-Muñoz (INABIO) and Ana Almendariz (Gustavo Orcés Museum) for granting access to their herpetological collections (MECN, INABIO). William E. Duellman (Natural History Museum, University of Kansas) and Annemarie Ohler (French National Museum of Natural History of Paris) provided photographs of specimens of interest. We thank Pablo Venegas, María José Navarrete, and Marcel Caminer for helpful comments during the research and Gabriela Galarza, Rafael Cárdenas, and Marcel Caminer for comments on the manuscript. We are grateful to the QCAZ Molecular Laboratory, specially to Marcel Caminer, Gabriela Castillo, Sebastián Espinoza, Lien González, Liliana Jaramillo, Andrea Manzano, Gabriella Nichols, Diana Pazmiño, and Sofía Salinas, for providing the sequences used in this study, and with the personal of the QCAZ museum for the preservation and processing of specimens, particularly Diego Paucar, Fernando Ayala, Santiago Guamán, and Yerka Sagredo. We thank Vanessa Aguirre, Silvia Aldás-Alarcón, Diego Almeida, Freddy Almeida, Ernesto Arbeláez, Fernando Ayala, Pamela Baldeón, Margarita Baquero, Carlos Boada, Estefanía Boada, Jorge Brito, Martín Bustamante, Andrés Calahorrano, Guadalupe Calle, Teresa Camacho, Felipe Campos, Edwin Carrillo, Amaranta Carvajal, Martin Cohen, Luis Coloma, Keyko Cruz, Michelle Cummer, Stella de la Torre, Oscar Delgado, Galo Díaz, Lena Echelle, Lorena Falconí, Chris Funk, Ricardo Gavilanes, Santiago Guamán, Mónica Guerra, Juan Daniel Jaramillo, Charles M. Kieswetter, David A. Kizirian, Rachel Kosoff, Simón Lobos, Luis Eduardo López, Paola Mafla-Endara, Andrea Manzano, Ernesto Martínez, Joseph Mendelson, Andrés Merino-Viteri, Robin Moore, Francy Mora, Juan Carlos Morocho, Felipe Moscoso, María José Navarrete, Leonardo Negrete, Darwin Núñez, Eloy Nusirquia, Giovanni Onore, Mónica Páez, Diego Paucar, Paula Peña, Pol Pintanel, Javier Pinto, Jennifer Pramuk, Daniel Rivadeneira, Carlos Antonio Rodríguez, Giovanna Romero, Paulina Romero, David Salazar, Juan Sánchez, Pablo Sandoval, Elicio Tapia, Ítalo Tapia, Luis Tipantiza, Rodrigo Toscano, Omar Torres, David Velalcázar, Freddy Velásquez, Pablo Venegas, John Wiens, and Erik Wild for specimen collections. The Ecuadorian Ministerio de Ambiente provided research and collection permits numbers 001-10 IC-FAU-DNB/MA, 002-16 IC-FAU-DNB/MA, 003-14 IC-FAU-DNB/MA, 003-15 IC-FAU-DNB/MA, 005-12-IC-FAU-DNB/MA, 005-14 IC-FAU-DNB/MA, 008-09 IC-FAU-DNB/MA, MAE-DNB-ARRGG-CM-2014-0002.
Citation
Páez NB, Ron SR (2019) Systematics of Huicundomantis, a new subgenus of Pristimantis (Anura, Strabomantidae) with extraordinary cryptic diversity and eleven new species. ZooKeys 868: 1–112. https://doi.org/10.3897/zookeys.868.26766
Supplementary materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Primers used for DNA PCRs
Data type: molecular data
Explanation note: Primers used for amplification of DNA.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Examined specimens
Data type: occurrence
Explanation note: Museum specimens analyzed in this taxonomic review. *: analyzed only with molecular information. ** = analyzed only with photographic material.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Phylogram of Huicundomantis including outgroup
Data type: phylogram
Explanation note: ML tree for genes 16S, ND1 and RAG1. Bootstrap values (%) are shown under the corresponding branches; missing values indicate values below 50 %. The number of collection, identification, country and locality of the samples are shown next to each terminal. EC is for Ecuador, PE for Peru, NP is for National Park, PF is for Protected Forest. Terminals pointed with an arrow correspond to specimens from Peru previously ascribed to P. phoxocephalus. Figure includes only outgroup within Pristimantis. See vouchers and GenBank accession numbers of all sequences used for molecular analyses in Table 1.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Mitochondrial phylogram depicting relationships within Huicundomantis
Data type: phylogram
Explanation note: ML tree for genes 16S and ND1. Bootstrap values (%) are shown under the corresponding branches; missing values indicate values below 50 %; asterisks indicate support values of 100%. The number of collection, identification, country and locality of the samples are shown next to each terminal. EC is for Ecuador, PE for Peru, PA is for Panama, NP is for National Park, PF is for Protected Forest. Outgroup is shown.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Phylogram depicting relationships within Huicundomantis
Data type: phylogram
Explanation note: ML tree for nuclear gene RAG1. Bootstrap values (%) are shown under the corresponding branches; missing values indicate values below 50 %. The number of collection, identification, country and locality of the samples are shown next to each terminal. EC is for Ecuador, PE for Peru, PA for Panama, NP is for National Park, PF is for Protected Forest. Outgroup is shown.
References
- Acevedo AA, Lampo M, Cipriani R. (2016) The cane or marine toad, Rhinella marina (Anura, Bufonidae): two genetically and morphologically distinct species. Zootaxa 4103: 574–586. 10.11646/zootaxa.4103.6.7 [DOI] [PubMed] [Google Scholar]
- Almendáriz CA, Orcés G. (2004) Distribución de algunas especies de la herpetofauna de los pisos: altoandino, temperado y subtropical. Politécnica 25 Biología 5: 97–150. http://bibdigital.epn.edu.ec/handle/15000/4791
- Arroyo SB, Sánchez PM, Ramírez-Pinilla MP, Suárez HA, Miranda-Esquivel DR. (2005) Morphometric analysis to differentiate taxonomically seven species of Eleutherodactylus (Amphibia: Anura: Leptodactylidae) from an Andean cloud forest of Colombia. Zootaxa 1018: 1–14. 10.11646/zootaxa.1018.1.1 [DOI] [Google Scholar]
- Bickford D, Lohman DJ, Sodhi NS, Ng PK, Meier R, Winker K, Ingram KK, Das I. (2007) Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution 22: 148–155. 10.1016/j.tree.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Brito JM, Ojala-Barbour R, Batallas DR, Almendáriz AC. (2016) A New Species of Pristimantis (Amphibia: Strabomantidae) from the Cloud Forest of Sangay National Park, Ecuador. Journal of Herpetology 50: 337–344. 10.1670/13-103 [DOI] [Google Scholar]
- Bustamante MR, Mendelson JR III. (2008) A new frog species (Strabomantidae: Pristimantis) from the high Andes of southeastern Ecuador. Zootaxa 1820: 49–59. 10.5281/zenodo.182997 [DOI] [Google Scholar]
- Caminer MA, Ron SR. (2014) Systematics of treefrogs of the Hypsiboas calcaratus and Hypsiboas fasciatus species complex (Anura, Hylidae) with the description of four new species. ZooKeys 370: 1–68. 10.3897/zookeys.370.6291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso P. (2017) red-an R package to facilitate species red list assessments according to the IUCN criteria. Biodiversity Data Journal 5: e20530. 10.3897/BDJ.5.e20530 [DOI] [PMC free article] [PubMed]
- Coloma LA, Ron SR, Yánez-Muñoz M, Almeida D. (2004) Pristimantis riveti The IUCN Red List of Threatened Species. Version 2014.2. http://www.iucnredlist.org/species/56919/11553288 [Accessed on: 2017-1-17]
- Coloma LA, Carvajal-Endara S, Dueñas JF, Paredes-Recalde A, Morales-Mite M, Almeida-Reinoso D, Tapia EE, Hutter CR, Toral E, Guayasamín JM. (2012) Molecular phylogenetics of stream frogs of the Hyloscirtus larinopygion group (Anura: Hylidae), and description of two new species from Ecuador. Zootaxa 3364: 1–78. 10.11646/zootaxa.3364.1.1 [DOI] [Google Scholar]
- Cornell Lab of Ornithology (2003–2008) Raven pro. Versión 1.3. Bioacustic Research Program, New York.
- Crawford AJ, Ryan MJ, Jaramillo CA. (2010) A new species of Pristimantis (Anura: Strabomantidae) from the Pacific coast of the Darien Province, Panama, with a molecular analysis of its phylogenetic position. Herpetologica 66: 192–206. 10.1655/09-018R1.1 [DOI] [Google Scholar]
- Dayrat B. (2005) Towards integrative taxonomy. Biological Journal of the Linnean Society 85: 407–415. 10.1111/j.1095-8312.2005.00503.x [DOI] [Google Scholar]
- Despax R. (1911) Mission géodésique de l’Équateur. Collections recueilles par M. le Dr. Rivet. Batraciens anoures. Bulletin du Museum National d’Histoire Naturelle 17: 90–94. [Google Scholar]
- De Queiroz K. (2007) Species concepts and species delimitation. Systematic Biology 56: 879–886. 10.1080/10635150701701083 [DOI] [PubMed] [Google Scholar]
- Duellman WE, Lehr E. (2009) Terrestrial breeding frogs (Strabomantidae) in Peru. Natur- und Tier-Verlag, Naturwissenschaft, Münster, 382 pp. [Google Scholar]
- Duellman WE, Pramuk JB. (1999) Frogs of the genus Eleutherodactylus (Anura: Leptodactylidae) in the Andes of northern Peru. Scientific Papers. Natural History Museum, University of Kansas 13: 1–78. 10.5962/bhl.title.16169 [DOI] [Google Scholar]
- Duellman WE, Wild ER. (1993) Anuran amphibians from the Cordillera de Huancabamba, northern Peru: Systematics, ecology, and biogeography. Occasional Papers of the Museum of Natural History, University of Kansas 157: 1–53. https://biodiversitylibrary.org/page/4469099 [Google Scholar]
- Elmer KR, Cannatella DC. (2009) Three new species of leaflitter frogs from the upper Amazon forests: cryptic diversity within Pristimantis “ockendeni” (Anura: Strabomantidae) in Ecuador. Zootaxa 1784: 11–38. http://nbn-resolving.de/urn:nbn:de:bsz:352-opus-75947 [Google Scholar]
- Fouquet A, Gilles A, Vences M, Marty C, Blanc M, Gemmell NJ. (2007) Underestimation of species richness in neotropical frogs revealed by mtDNA analyses. PLoS ONE 10: e1109. 10.1371/journal.pone.0001109 [DOI] [PMC free article] [PubMed]
- Frost DR. (2019) Amphibian Species of the World: an Online Reference. Version 6.0. American Museum of Natural History, New York, USA. http://research.amnh.org/herpetology/amphibia/index.html. Accessed on: 2019-1-18.
- Funk WC, Caminer M, Ron SR. (2012) High levels of cryptic species diversity uncovered in Amazonian frogs. Proceedings of the Royal Society, Biological Sciences 279: 1806–1814. 10.1098/rspb.2011.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Ron SR, Santos JC, Schneider CJ, Moritz C. (2004) Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution 58: 1781–1793. 10.1111/j.0014-3820.2004.tb00461.x [DOI] [PubMed] [Google Scholar]
- Hedges SB, Duellman WE, Heinicke MP. (2008) New World direct-developing frogs (Anura: Terrarana): molecular phylogeny, classification, biogeography, and conservation. Zootaxa 1737: 1–182. https://www.mapress.com/zootaxa/list/2008/zt01737.html [Google Scholar]
- Heinicke MP, Duellman WE, Hedges SB. (2007) Major Caribbean and Central American frog faunas originated by ancient oceanic dispersal. Proceedings of the National Academy of Sciences 104: 10092–10097. 10.1073/pnas.0611051104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinicke MP, Lemmon AR, Lemmon EM, McGrath K, Hedges SB. (2017) Phylogenomic support for evolutionary relationships of New World direct-developing frogs (Anura: Terraranae). Molecular Phylogenetics and Evolution 118: 145–155. 10.1016/j.ympev.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Hutter CR, Guayasamin JM. (2015) Cryptic diversity concealed in the Andean cloud forests: two new species of rainfrogs (Pristimantis) uncovered by molecular and bioacoustic data. Neotropical Biodiversity 1: 36–59. 10.1080/23766808.2015.1100376 [DOI] [Google Scholar]
- IUCN Standards and Petitions Subcommittee (2017) Guidelines for Using the IUCN Red List Categories and Criteria. Version 13. Prepared by the Standards and Petitions Subcommittee. http://www.iucnredlist.org/documents/RedListGuidelines.pdf. [Accessed on: 2017-12-15]
- Jiménez de la Espada M. (1870) Fauna neotropicalis species quaedam nondum cognitae. Jornal de sciencias mathematicas, physicas e naturaes 3: 57–65. https://www.biodiversitylibrary.org/part/55205 [Google Scholar]
- Jungfer KH, Faivovich JA, Padial JM, Castroviejo-Fisher S, Lyra MM, Berneck BV, Iglesias PP, Kok PJ, Macculloch RD, Rodrigues MT, Verdade VK, Torres CP, Chaparro JC, Valdujo PH, Reichle S, Moravec JR, Ik VA, Gagliardi-Urrutia G, Ernst R, de la Riva I, Means DB, Lima AP, Naris JC, Wheeler WC, Haddad CE. (2013) Systematics of spiny backed-treefrogs (Hylidae: Osteocephalus): an Amazonian puzzle. Zoologica Scripta 42: 351–380. 10.1111/zsc.12015 [DOI] [Google Scholar]
- Katoh K, Misawa K, Kuma KI, Miyata T. (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliontzopoulou A. (2011) Geometric morphometrics in herpetology: modern tools for enhancing the study of morphological variation in amphibians and reptiles. Basic and Applied Herpetology 25: 5–32. 10.11160/bah.11016 [DOI] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Ho SY, Guindon S. (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Lynch JD. (1969) Taxonomic notes on Ecuadorian frogs (Leptodactylidae: Eleutherodactylus). Herpetologica 25: 262–274. https://www.jstor.org/stable/3891217 [Google Scholar]
- Lynch JD. (1979) Leptodactylid frogs of the genus Eleutherodactylus from the Andes of southern Ecuador. Miscellaneous Publication, Museum of Natural History, University of Kansas 66: 1–62. 10.5962/bhl.title.16268 [DOI] [Google Scholar]
- Lynch JD. (1981) Leptodactylid frogs of the genus Eleutherodactylus in the Andes of northern Ecuador and adjacent Colombia. Miscellaneous Publication, Museum of Natural History, University of Kansas 72: 1–46. 10.5962/bhl.title.16289 [DOI] [Google Scholar]
- Lynch JD, Duellman WE. (1980) The Eleutherodactylus of the Amazonian slopes of the Ecuadorian Andes (Anura: Leptodactylidae). Miscelaneous Publication, Museum of Natural History, University of Kansas 69: 1–86. 10.5962/bhl.title.16222 [DOI] [Google Scholar]
- Lynch JD, Duellman WE. (1995) A new fat little frog (Leptodactylidae: Eleutherodactylus) from lofty Andean grasslands of southern Ecuador. Occasional Papers of the Museum of Natural History, University of Kansas 173: 1–7. https://biodiversitylibrary.org/page/4466693 [Google Scholar]
- Lynch JD, Duellman WE. (1997) Frogs of the genus Eleutherodactylus in western Ecuador. Systematics, ecology, and biogeography. Special Publication, Museum of Natural History, University of Kansas, 23: 1–236. 10.5962/bhl.title.7951 [DOI] [Google Scholar]
- Maddison WP, Maddison DR. (2014) Mesquite: a modular system for evolutionary analysis. Version 3.01. http://mesquiteproject.org. Accessed on: 2014-12-01.
- Menéndez‐Guerrero PA, Graham CH. (2013) Evaluating multiple causes of amphibian declines of Ecuador using geographical quantitative analyses. Ecography 36: 756–769. 10.1111/j.1600-0587.2012.07877.x [DOI] [Google Scholar]
- Ortega-Andrade HM, Rojas-Soto OR, Valencia JH, de los Monteros AE, Morrone JJ, Ron SR, Cannatella DC. (2015) Insights from integrative systematics reveal cryptic diversity in Pristimantis frogs (Anura: Craugastoridae) from the Upper Amazon Basin. PloS ONE 10: e0143392. 10.1371/journal.pone.0143392 [DOI] [PMC free article] [PubMed]
- Padial JM, De la Riva I. (2009) Integrative taxonomy reveals cryptic Amazonian species of Pristimantis (Anura: Strabomantidae). Zoological Journal of the Linnean Society 155: 97–122. 10.1111/j.1096-3642.2008.00424.x [DOI] [Google Scholar]
- Padial JM, Grant T, Frost DR. (2014) Molecular systematics of terraranas (Anura: Brachycephaloidea) with an assessment of the effects of alignment and optimality criteria. Zootaxa 3825: 1–132. 10.11646/zootaxa.3825.1.1 [DOI] [PubMed] [Google Scholar]
- Padial JM, Koehler J, Muñoz A, De la Riva I. (2008) Assessing the taxonomic status of tropical frogs through bioacoustics: geographical variation in the advertisement calls in the Eleutherodactylus discoidalis species group (Anura). Zoological Journal of the Linnean Society 152: 353–365. 10.1111/j.1096-3642.2007.00341.x [DOI] [Google Scholar]
- Padial JM, Miralles A, De la Riva I, Vences M. (2010) Review: The integrative future of taxonomy. Frontiers in Zoology 7: 1–14. 10.1186/1742-9994-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AT, Soberón J, Sánchez-Cordero V. (1999) Conservatism of ecological niches in evolutionary time. Science 285: 1265–1267. 10.1126/science.285.5431.1265 [DOI] [PubMed] [Google Scholar]
- Perrier G. (1913) Mission du Service géographique de l’Armée pour la mesure d’un arc de méridien équatorial en Amérique du Sud sous le contrôle scientifique de l’académie des sciences, 1899–1906. Tome II. Fascicle 1. Gauthier-Villars, Imprimeur Libraire du Bureau des Longitudes de l’Ecole Polytechnique, 67 pp 10.5962/bhl.title.980 [DOI] [Google Scholar]
- Pyron RA, Wiens JJ. (2011) A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution 61: 543–583. 10.1016/j.ympev.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Pyron RA. (2014) Biogeographic analysis reveals ancient continental vicariance and recent oceanic dispersal in amphibians. Systematic Biology 63: 779–797. 10.1093/sysbio/syu042 [DOI] [PubMed] [Google Scholar]
- Rambaut A, Suchard MA, Xie D, Drummond AJ. (2014) Tracer v1.6. [Accessed on: 2014-08-01]
- Rissler LJ, Apodaca JJ. (2007) Adding more ecology into species delimitation: ecological niche models and phylogeography help define cryptic species in the Black Salamander (Aneides flavipunctatus). Systematic Biology 56: 924–942. 10.1080/10635150701703063 [DOI] [PubMed] [Google Scholar]
- Rodríguez L, Martinez JL, Ron SR, Coloma LA, Almeida D. (2004) Pristimantis phoxocephalus The IUCN Red List of Threatened Species. Version 2014.2. http://www.iucnredlist.org. [Accessed on: 2014-9-17]
- Ron SR, Santos JC, Cannatella DC. (2006) Phylogeny of the túngara frog genus Engystomops (= Physalaemus pustulosus species group; Anura: Leptodactylidae). Molecular Phylogenetics and Evolution 39: 392–403. 10.1016/j.ympev.2005.11.022 [DOI] [PubMed] [Google Scholar]
- Ron SR, Merino-Viteri A, Ortiz DA. (2019) Anfibios del Ecuador. Version 2019.0. Museo de Zoología, Pontificia Universidad Católica del Ecuador. https://bioweb.bio/faunaweb/amphibiaweb. [Accessed on: 2019-02-03]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. (2010) Using JMP 9. SAS Institute Inc. , Cary, NC, 540 pp. [Google Scholar]
- Székely P, Cogǎlniceanu D, Székely D, Páez-Rosales N, Ron SR. (2016) A new species of Pristimantis from southern Ecuador (Anura, Craugastoridae). ZooKeys 606: 77–97. 10.3897/zookeys.606.9121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieites DR, Wollenberg KC, Andreone F, Köhler J, Glaw F, Vences M. (2009) Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proceedings of the National Academy of Sciences of the United States of America 106: 8267–8272. 10.1073/pnas.0810821106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yánez-Muñoz MH, Toral-Contreras E, Meza-Ramos PA, Reyes-Puig JP, Bejarano-Muñoz EP, Mueses-Cisneros JJ, Paucar D. (2012) New country records for five species of Pristimantis Jiménez de la Espada, 1870 from Ecuador. Check List 8: 286–290. 10.15560/8.2.286 [DOI] [Google Scholar]
- Yánez-Muñoz MH, Sánchez-Nivicela JC, Reyes-Puig C. (2016) Tres nuevas especies de ranas terrestres Pristimantis (Anura: Craugastoridae) de la Provincia de El Oro, Ecuador. ACI Avances en Ciencias e Ingenierías 8: 5–25. 10.18272/aci.v8i1.455 [DOI] [Google Scholar]
- Wollenberg KC, Vieites DR, Van Der Meijden A, Glaw F, Cannatella DC, Vences M. (2008) Patterns of endemism and species richness in Malagasy cophyline frogs support a key role of mountainous areas for speciation. Evolution 62: 1890–1907. 10.1111/j.1558-5646.2008.00420.x [DOI] [PubMed] [Google Scholar]
- Will KW, Mishler BD, Wheeler QD. (2005) The perils of DNA barcoding and the need for integrative taxonomy. Systematic Biology 54: 844–851. 10.1080/10635150500354878 [DOI] [PubMed] [Google Scholar]
- Zwickl D. (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD thesis, University of Texas, Austin, 115 pp http://hdl.handle.net/2152/2666 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Primers used for DNA PCRs
Data type: molecular data
Explanation note: Primers used for amplification of DNA.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Examined specimens
Data type: occurrence
Explanation note: Museum specimens analyzed in this taxonomic review. *: analyzed only with molecular information. ** = analyzed only with photographic material.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Phylogram of Huicundomantis including outgroup
Data type: phylogram
Explanation note: ML tree for genes 16S, ND1 and RAG1. Bootstrap values (%) are shown under the corresponding branches; missing values indicate values below 50 %. The number of collection, identification, country and locality of the samples are shown next to each terminal. EC is for Ecuador, PE for Peru, NP is for National Park, PF is for Protected Forest. Terminals pointed with an arrow correspond to specimens from Peru previously ascribed to P. phoxocephalus. Figure includes only outgroup within Pristimantis. See vouchers and GenBank accession numbers of all sequences used for molecular analyses in Table 1.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Mitochondrial phylogram depicting relationships within Huicundomantis
Data type: phylogram
Explanation note: ML tree for genes 16S and ND1. Bootstrap values (%) are shown under the corresponding branches; missing values indicate values below 50 %; asterisks indicate support values of 100%. The number of collection, identification, country and locality of the samples are shown next to each terminal. EC is for Ecuador, PE for Peru, PA is for Panama, NP is for National Park, PF is for Protected Forest. Outgroup is shown.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Nadia B. Páez, Santiago R. Ron
Phylogram depicting relationships within Huicundomantis
Data type: phylogram
Explanation note: ML tree for nuclear gene RAG1. Bootstrap values (%) are shown under the corresponding branches; missing values indicate values below 50 %. The number of collection, identification, country and locality of the samples are shown next to each terminal. EC is for Ecuador, PE for Peru, PA for Panama, NP is for National Park, PF is for Protected Forest. Outgroup is shown.