Abstract
Background
MgrA is an important global virulence gene regulator in Staphylococcus aureus. In the present study, the role of mgrA in host-pathogen interactions related to virulence was explored in both methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) strains.
Methods
In vitro susceptibilities to human defense peptides (HDPs), adherence to fibronectin (Fn) and endothelial cells (ECs), EC damage, α-toxin production, expression of global regulator (eg, agr RNAIII) and its downstream effectors (eg, α-toxin [hla] and Fn binding protein A [fnbA]), MgrA binding to fnbA promoter, and the effect on HDP-induced mprF and dltA expression were analyzed. The impact of mgrA on virulence was evaluated using a mouse bacteremia model.
Results
mgrA mutants displayed significantly higher susceptibility to HDPs, which might be related to the decreased HDP-induced mprF and dltA expression but decreased Fn and EC adherence, EC damage, α-toxin production, agr RNAIII, hla and fnbA expression, and attenuated virulence in the bacteremia model as compared to their respective parental and mgrA-complemented strains. Importantly, direct binding of MgrA to the fnbA promoter was observed.
Conclusions
These results suggest that mgrA mediates host-pathogen interactions and virulence and may provide a novel therapeutic target for invasive S. aureus infections.
Keywords: Staphylococcus aureus, mgrA, host factors, virulence and bacteremia
We demonstrate the role of mgrA in host-pathogen interactions and virulence in an experimental bacteremia model. Our data suggest that MgrA might be a potential target to optimize anti–Staphylococcus aureus therapeutic strategies.
Staphylococcus aureus is an important human pathogen that causes a wide range of serious infections, including life-threatening bacteremia [1]. The emergence of methicillin-resistant S. aureus (MRSA) further emphasized this public health issue and has become a global problem [1]. Of concern, up to 30% of patients with S. aureus bacteremia do not respond to antibiotic treatment even when a gold-standard anti-MRSA antibiotic (eg, vancomycin or daptomycin) is used [2, 3]. With the critical shortage of new anti-MRSA antibiotics, there is an urgent need to understand the intersection of host and pathogen factors that contribute to the pathogenesis and to develop novel strategies to prevent and/or treat these severe infections.
The ability of S. aureus to cause infections is attributed to its complex expression of multiple gene products, including surface adhesins and exoproteins [4]. The adhesins allow bacteria to attach to host cells and extracellular matrix during the early colonization phase [5]. Of these adhesins, fibronectin (Fn) binding protein A (FnBPA; encoded by fnbA) has been demonstrated to be involved in binding and internalization into host cells (eg, endothelial cells [ECs]), which is important in S. aureus endovascular infections [6]. Exoproteins may facilitate tissue damage and spread into the bloodstream during later infection [7]. α-toxin (encoded by hla) is a major pore-forming exoprotein produced by the majority of S. aureus strains, and it targets a broad range of host cells (eg, ECs) that ultimately leads to tissue damage and dissemination [8, 9]. MgrA is one of the major master regulators and regulates the expression of many virulence factors in S. aureus, such as α-toxin, capsule, and protease, by binding to their respective promoters [10, 11]. In addition, mgrA is ubiquitous among clinical S. aureus isolates, including MRSA and methicillin-susceptible S. aureus (MSSA) [4]. However, the role of MgrA in host-pathogen interactions has not been well studied. In the current study, we showed that mgrA mediates host-pathogen interactions that might contribute to virulence in a mouse model of bacteremia due to MSSA and MRSA.
METHODS
Bacterial Strain and Plasmids
One representative MSSA strain (RN6390, a laboratory strain related to 8325-4 [11]) and 1 MRSA strain (MW2, a USA400 strain [12]) were used as parental strains. A mgrA deletion in strain RN6390 (ALC2530; a mgrA deletion mutant of RN6390 [∆mgrA::ermC]) was achieved by using a temperature-sensitive erythromycinr shuttle plasmid, pCL52.2 [13]. mgrA was inactivated in MRSA strain MW2 by transducing the ∆mgrA::ermC mutation from ALC2530, using phage ϕ11 [13]. The mgrA mutant strains were complemented with mgrA by transforming it with plasmid pEPSA5::mgrA as described previously [14]. Unless otherwise stated, all S. aureus strains were grown at 37°C in tryptic soy broth (TSB; Difco) or TSB agar plates.
Susceptibility to Human Defense Peptides (HDPs)
Human neutrophil peptide 1 (hNP-1) was purchased from Peptides International (Louisville, KY). Thrombin-induced platelet microbicidal proteins (tPMPs) were prepared from thrombin-stimulated platelets isolated from fresh rabbit blood [15]. The bioactivity of the tPMPs was assessed by a Bacillus subtilis ATCC 6633 susceptibility assay [16, 17]. The HDP susceptibilities of our study strains were tested by exposing 105 colony-forming units (CFU)/mL or 103 CFU/mL of S. aureus cells from the exponential growth phase (incubation time, approximately 3 hours) to hNP-1 at 1.25 μg/mL or tPMPs at 6.25 μg/mL, respectively for 2 hours at 37°C [16–18]. These HDP concentrations were selected on the basis of extensive pilot studies, in which we identified peptide levels that did not rapidly result in S. aureus killing over a 2-hour exposure period (data not shown). The results are expressed as the percentage (±standard deviation [SD]) of the initial inoculum that survived exposure to HDPs.
Adherence to Fibronectin (Fn)
To assay the ability of S. aureus cells to adhere to Fn, 6-well tissue culture plates were coated with purified human Fn (50 μg/mL, Sigma Chemicals) for 18 hours at 4°C and then treated with 3% bovine serum albumin (Sigma Chemicals) for 2 hours to prevent nonspecific adhesion [19]. S. aureus cells grown to exponential phase (incubation time, approximately 3 hours), when MgrA expression is most optimal [20], were added to the plates (5 × 103 CFU/well) and incubated for 1 hour at 37°C [19]. After washing with phosphate-buffered saline, TSB agar was added to each well, and plates were incubated overnight at 37°C. Adherence was expressed as the percentage (±SD) of the initial inoculum bound to Fn [19].
Confocal Microscopy
To confirm adherence to Fn, a fluorescence-labeled Fn (FN-488; Cytoskeleton, Denver, CO) was used according to the manufacturer’s protocol [21, 22]. The maximum excitation and emission spectra of FN-488 are 450 nm and 550 nm, respectively [21]. Briefly, S. aureus exponential-phase cells (108 CFU/mL) were incubated with FN-488 (4 μg/mL) for 30 minutes at 37°C, washed with phosphate-buffered saline, and imaged by confocal microscopy [22].
α-Toxin Production
A semiquantitative assay of α-hemolysin production was performed by dropping 3 μL of 108 CFU/mL of S. aureus exponential-phase cells onto 5% sheep blood agar plates, followed by incubation for 24 hours at 37°C [23]. Clear zones around the bacterial colonies on the blood agar plates indicate α-hemolytic activity.
EC Adherence
Human microvascular ECs (HMEC-1) were obtained from the Centers for Disease Control and Prevention and maintained as previously described [24, 25]. For the EC adherence assay, confluent HMEC-1 in 6-well plates were washed with Hank’s balanced salt solution (HBSS). S. aureus exponential-phase cells were added to the plates at a final inoculum of 5 × 103 CFU/well and incubated for 1 hour at 37oC. Unbound S. aureus cells were removed by washing the wells with HBSS. The number of adherent bacteria was determined by lysing HMEC-1 with cold distilled water, followed by quantitative culture [26]. Adherence was expressed as the percentage (±SD) of the initial inoculum bound to HMEC-1 [26].
EC Damage
A well-established 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to test the EC damage [24, 27]. Briefly, S. aureus exponential-phase cells were added to confluent HMEC-1 in 24-well plates (5 × 105 ECs/well), corresponding to a multiplicity of infection (MOI) of 50 and 500 (as established by pilot studies) for the MW2 and RN6390 strain sets, respectively. After 3 hours of incubation at 37oC, the wells were washed with HBSS, medium containing lysostaphin (10 μg/mL; Sigma Chemicals) was added to lyse extracellular S. aureus cells, and plates were incubated at 37oC [24]. After 18 hours of incubation, 100 μL of MTT (5 mg/mL, Sigma Chemicals) was added, and plates were incubated for 2 hours. The medium was replaced with 150 μL of 0.04 M HCl to stop the reaction. HMEC-1 without S. aureus exposure were considered a 0% damage control. HMEC-1 damage is presented as a percentage (±SD), calculated as 1 − [OD560 of samples/OD560 of control] [24].
RNA Isolation and Real-Time Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from exponential-phase S. aureus cells, as the level of mgrA expression reaches its peak during exponential phase [19], using an RNeasy Kit (Qiagen, Valencia, CA). Then DNase-treated RNA was transcribed into complementary DNA. Real-time qRT-PCR was performed using an ABI Prism 7000 instrument (Applied Biosystems) and the SYBR green PCR master kit (Applied Biosystems). The amplification of agr RNAIII, hla, and fnbA was conducted using primers as described previously [28–30]. gyrB was used to normalize the transcript quantification, and relative expression of the study genes was calculated by the ∆∆CT method [28].
In addition, to assess the impact of mgrA on the expression of genes involved in HDP susceptibility (eg, mprF and dltA), as well as induction of mprF and dltA by HDPs, RNA samples were isolated from exponential-phase S. aureus cells exposed to hNP-1 (5 μg/mL) for 30 minutes as described previously [31]. The concentration of hNP-1 was selected on the basis of our pilot studies to identify the peptide level that did not rapidly kill S. aureus over a 30 minute-exposure period (≥90% survival). Amplification of mprF and dltA was conducted using primers as described previously [32, 33]. Then, the relative levels of mprF and dltA expression, with or without hNP-1 exposure, were calculated as described above.
Gel Shift Analysis
To determine whether MgrA interacts directly with fnbA promoter, purified MgrA and a gel-purified fragment containing the fnbA promoter region were used. Cloning and purification of the His6-tagged fusion MgrA were performed as described elsewhere [13]. A 287-bp fragment containing the fnbA promoter region was amplified by PCR, using previously described primers [34]. A specific competitor DNA representing the promoter fragment of hla [11] and a nonspecific competitor DNA from within the fnbA coding sequence (primers were the same as for the fnbA real-time qRT-PCR) were also used. Briefly, the fnbA promoter DNA (500 ng) with or without the specific or nonspecific competitor DNA (2500 ng) was incubated at room temperature for 20 minutes with various amount of purified MgrA in binding buffer (10 mM Tris-HCl, 150 mM KCl, 0.1 mM DTT, and 0.1 mM ethylenediaminetetraacetic acid) [35]. The reaction mixtures were analyzed in a precast 10% native Tris-glycine gel (Novex, San Diego, CA). The band shifts were stained with a well-studied fluorescence-based SYBR green electrophoretic mobility shift assay (EMSA) kit (Molecular Probes EMSA, Panomics, Fremont, CA) and detected by exposure to UV302 according to the manufacturer’s instructions [36, 37].
Mouse Bacteremia Model
To better define the role of mgrA in vivo in regard to staphylococcal virulence, a murine bacteremia model was used [38]. The Institutional Animal Care and Use Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center approved all animal study protocols. Female CD1 Swiss mice (weight, 18–23 g) were infected intravenously with 5 × 107 CFU/animal (a 95% effective dose established by pilot studies) of each study S. aureus strain (approximately 8 mice/group). Ten days after infection, mice were euthanized, and their kidneys, spleen, livers, and lungs were removed and quantitatively cultured. S. aureus counts in the target tissues are specified as mean log10 CFU/g of tissue (±SD). In addition, the body weight of animals was monitored during the experimental period.
Statistical Analysis
The 2-tailed Student t test was used to analyze the in vitro data. Differences in S. aureus density in the target tissues were compared by analysis of variance. P values of <.05 were considered statistically significant.
RESULTS
HDPs Susceptibilities
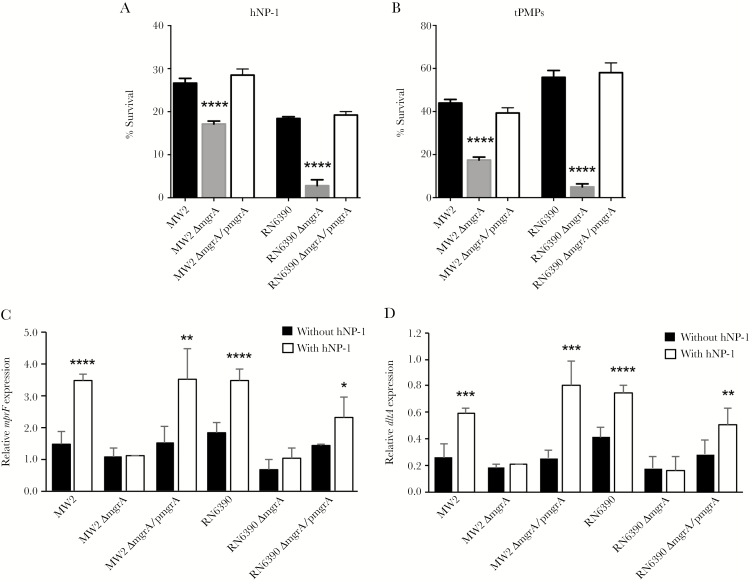
Susceptibility to killing by HDPs from polymorphonuclear leukocytes (eg, hNP-1) and platelets (eg, tPMPs) is a critical factor in the pathogenesis of S. aureus endovascular infection [26]. We demonstrated that both mgrA mutant strains in the MSSA and MRSA backgrounds exhibited significantly reduced survival due to hNP-1 and tPMP killing, compared with their respective parental and mgrA-complemented strains (P < .0001; Figure 1). For instance, the survival percentages among MW2 parental strains in the presence of hNP-1 and tPMPs were 26.6% and 44.0%, respectively, and the percentages among mgrA-complemented strains were 28.5% and 39.3%, respectively; these values were significantly higher than those for mgrA mutant strains (17.1% and 17.4%, respectively; Figure 1).
Figure 1.
In vitro susceptibilities to human neutrophil peptide 1 (hNP-1; A) and thrombin-induced platelet microbicidal proteins (tPMPs; B) and relative expression of mprF (C) and dltA (D) in the absence and presence of sublethal concentration of hNP-1 in MW2 and RN6390 parental Staphylococcus aureus strains and their respective isogenic mgrA mutants and mgrA-complemented strain sets. *P < .05, **P < .01, ***P < .001, and ****P < .0001 vs their respective parental and mgrA-complemented strains or condition without hNP-1 exposure.
Effect of mgrA on Induction of mprF and dltA Expression
It is known that mprF and dltA, which regulate the cell membrane surface charge, are involved in the susceptibility to HDPs [31]. To assess the underlying mechanism of MgrA on susceptibility to HDPs, MRSA and MSSA strain sets were exposed to a sublethal concentration of hNP-1, and transcription levels of mprF and dltA were measured. We demonstrated that mgrA mutants exhibited lower levels of mprF and dltA expression than their isogenic parental and mgrA-complemented strains in the absence of hNP-1, indicating that mgrA positively regulates mprF and dltA expression (Figure 1C and 1D). Importantly, levels of mprF and dltA expression were significantly induced by hNP-1 in the MRSA and MSSA parental and mgrA-complemented strains but not in the mgrA mutant strains (Figure 1C and 1D).
Adherence to Fn
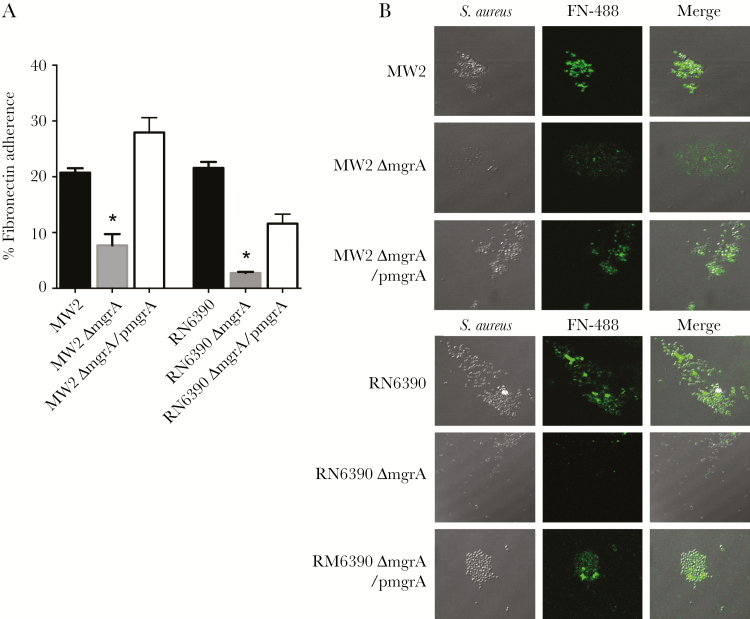
Through bridging the staphylococcal cell wall–anchored FnBPs to the mammalian cell-surface integrin α5β1 (FnBP-Fn-α5β1), Fn-mediated adherence of S. aureus has been implicated in the pathogenesis of S. aureus infections [39]. We found that adhesion to Fn in mgrA mutant strains was significantly reduced (by 2.6–7.8-fold) as compared to their respective parental and mgrA-complemented strains (P < .001; Figure 2A). To confirm the role of mgrA in the ability of S. aureus to adhere to Fn, a well-studied fluorescence-labeled Fn was used [21, 22]. Confocal microscopy of the 2 mgrA strain sets revealed that a substantially lower percentage of MSSA and MRSA mutants had bound to Fn as compared to their respective parental and mgrA-complemented strains (Figure 2B).
Figure 2.
A, In vitro fibronectin (Fn) adherence by the study strain sets (A). B, Confocal microscopy of fluorescence-labeled Fn (FN-488) bound to the Staphylococcus aureus strain sets. *P < .001 vs their respective parental and mgrA-complemented strains.
EC Adherence and Damage
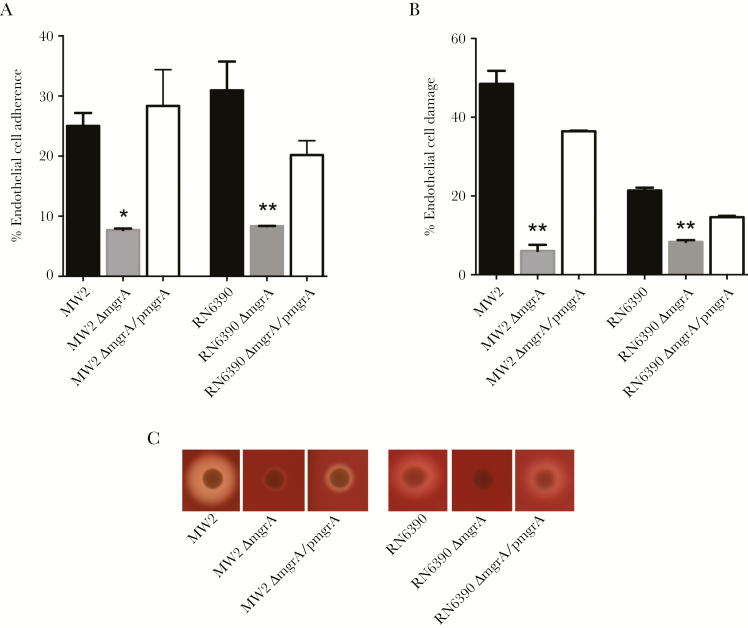
In line with the Fn adherence results described above, we found that the mgrA mutant strains exhibited a significantly reduced capacity for EC adherence, compared with their respective wild-type strains (Figure 3A). For example, mgrA mutants in the MSSA and MRSA backgrounds had an approximately 2.4-fold decrease in EC adherence, compared with their respective parental and mgrA-complemented strains (P < .001; Figure 3A). It has been reported that EC damage is involved in staphylococcal pathogenesis by providing a route for dissemination of S. aureus to distant sites [40]. In the present study, we found that both mgrA mutant strains in the MSSA and MRSA backgrounds caused significantly less EC damage than their respective parental and mgrA-complemented strains (P < .0001; Figure 3B). For instance, the mutant MRSA strain damaged 6% of ECs, which is significantly less damage than that caused by its isogenic parental strain (approximately 48%) and mgrA-complemented strain (approximately 36%; P < .0001; Figure 3B).
Figure 3.
Endothelial cell (EC) adherence (A) and damage (B) and α-toxin activity (zones of clearance on the sheep blood plate; C) among MW2 and RN6390 parental Staphylococcus aureus strains and their respective mgrA mutant and mgrA-complemented strain sets. *P < .001 and **P < .0001 vs their respective parental and mgrA-complemented strains.
α-Toxin Production
Previous studies indicated that α-toxin production is positively associated with EC damage [41]. Consistent with previous findings, we found that mgrA mutants showed substantially weaker α-toxin activity than their respective parental and mgrA-complemented strains (Figure 3C), which is consistent with the EC damage data presented above.
Role of mgrA in agr RNAIII, hla, and fnbA Expression
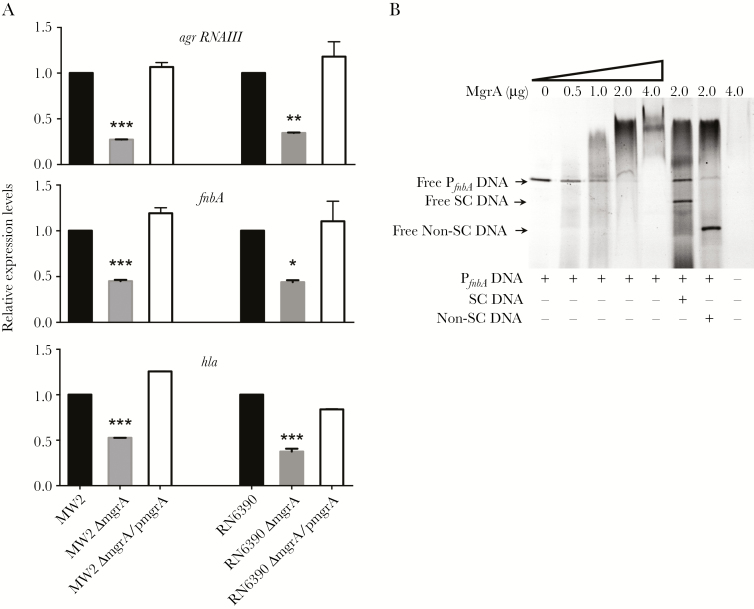
As reported from prior studies, mgrA positively regulates agr RNAIII to promote hla expression and binds to the hla promoter to augment gene expression [11]. Consistently, our study showed that both mgrA mutant strains in the MSSA and MRSA backgrounds had reduced agr RNAIII and hla expression, compared with their respective parental and mgrA-complemented strains (P < .001; Figure 4A). It has been reported that agr downregulates both fnbA expression and Fn binding [19]. However, decreased expression of agr RNAIII and fnbA in the mgrA mutants was observed, compared with findings for their respective wild-type strains in the current study, which is in contrast to findings from previous studies [19]. Gel shift analysis showed that MgrA bound to the fnbA promoter region, which might have contributed to the unexpected enhanced fnbA expression in the mgrA mutants (Figure 4B). For instance, a retarded MgrA-PfnbA complex could be detected with 0.5 μg of MgrA. Notably, as the concentrations of MgrA increased, the retarded MgrA-PfnbA complex showed a more predominant band, with complete conversion at 4.0 μg of MgrA. This binding could be outcompeted by adding the specific competitor DNA (Figure 4B). In addition, adding the nonspecific competitor DNA did not significantly affect MgrA-PfnbA binding (Figure 4B). These results indicate that MgrA-PfnbA binding is specific. Importantly, decreased fnbA expression profiles in the mgrA mutants positively correlated with Fn and EC adherence phenotypes.
Figure 4.
A, Relative expression of agr RNAIII, fnbA, and hla in MW2 and RN6390 parental Staphylococcus aureus strains and their respective mgrA mutant and mgrA-complemented strain sets. Expression levels are relative to that of the parental strain, which is arbitrarily set at 1. *P < .05, **P < .001, and ***P < .0001 vs their respective parental and mgrA-complemented strains. B, Binding of purified MgrA with the 287-bp fnbA promoter DNA (PfnbA), as determined by gel shift assay. The first lane represents the fnbA promoter DNA alone. Increasing amounts of purified MgrA (0.5, 1.0, 2.0, and 4.0 μg) were applied. The last lane represents purified MgrA alone (4.0 μg). In competition assays, MgrA was incubated with a presence of 5-fold excess of specific competitor (SC; 235-bp hla promoter DNA) or nonspecific competitor (non-SC; 162 bp from within the fnbA coding sequence).
Mouse Model of Bacteremia
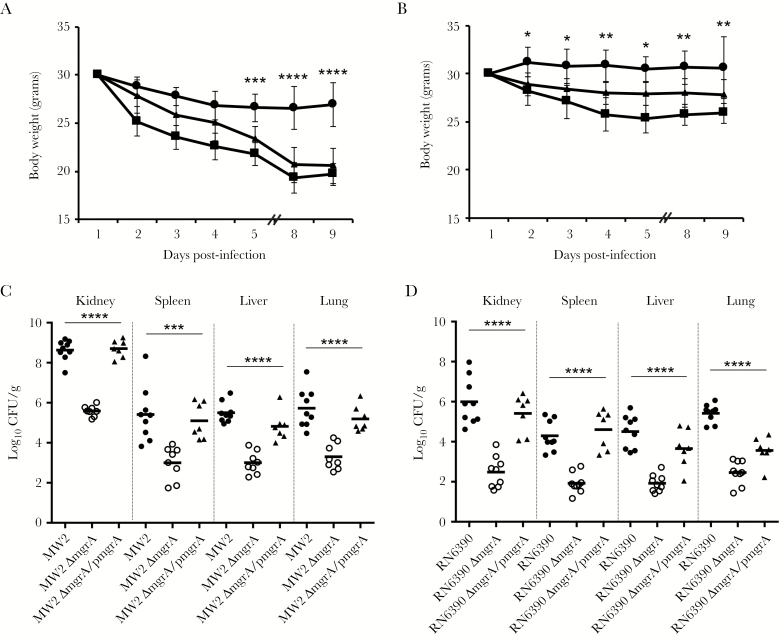
All mice infected with the mgrA mutant strains lost significantly less body weight than mice infected with their respective isogenic parental and mgrA-complemented strains, especially late during the observation period (Figure 5A and 5B). For instance, 5 days after infection, the changes in body weight between mice infected with parental strains and those infected with mgrA mutants became significantly different in the MW2 strain set (P < .05; Figure 5B). Notably, both mgrA mutant strains in the MSSA and MRSA backgrounds exhibited significantly attenuated virulence, with at least 2-log reductions in the S. aureus density in all target tissues (ie, kidney, spleen, liver, and lung), compared with their respective parental strains (P < .05; Figure 5C and 5D). However, for most target tissues, S. aureus densities had partially returned to those of the parental strains when mgrA was complemented (Figure 5C and 5D).
Figure 5.
A and B, Body weight changes among mice after infection of parental (squares), mgrA mutant (solid circles), and mgrA-complemented (triangles) Staphylococcus aureus strains (A, MW2 strain set; B, RN6390 strain set). C and D, S. aureus densities in target tissues in the mouse bacteremia model (C, MW2 strain set; D, RN6390 strain set). Each dot represents 1 animal. Horizontal black bars indicate mean S. aureus densities. *P < .05, **P < .01, ***P < .001, and **** P < .0001 vs their respective parental and mgrA-complemented strains.
DISCUSSION
MgrA, a member of the SarA protein family, is a key global regulator that regulates many virulence genes (eg, hla, spa, and cap5) in S. aureus [10, 11]. It has been reported that MgrA affects biofilm formation, autolysis, and virulence in animal models [4, 10, 11, 42, 43]. The current investigation builds on previous work by exploring the mechanisms of virulence mediated by MgrA through host factors. Several key findings, which shed new light on the role of MgrA in the host-pathogen relationship, emerged from these studies.
The ability of S. aureus to cause disease is at least in part due to evasion of the host’s innate defense response, which includes resistance to HDPs [44]. HDPs, including those of hematogenous origin (eg, neutrophils [for hNP-1] and platelets [for tPMPs]), kill many important blood-borne pathogens, especially S. aureus [45]. Reduced susceptibility to HNP-1 and tPMPs in vitro has been shown to correlate with enhanced in vivo virulence in S. aureus endovascular infections [26]. In the current study, we showed that mgrA mutant strains in both the MSSA and MRSA backgrounds were significantly more susceptible to HDPs than the respective wild-type and mgrA-complemented S. aureus strains. Therefore, MgrA appears to play an important role in HDP susceptibility, which contributes to S. aureus pathogenesis.
Since most HDPs target the bacterial cell membrane to initiate their bactericidal mechanism(s), we hypothesized that MgrA may directly and/or indirectly interact with S. aureus cell membrane factors (eg, mprF and dlt). Peschel et al demonstrated that mprF, a lysyl-phosphatidylglycerol synthase, adds positively charged lysine to the negatively charged PG molecule within the staphylococcal cell membrane. This process results in an increased net positive surface charge, thus reducing the binding of HDPs by S. aureus [46]. In addition, it has been shown that the dltABCD operon also contributes to the net positive surface charge by covalently incorporating d-alanine into cell wall teichoic acids and then promotes resistance to distinct HDPs in S. aureus [47]. In the current study, we tested the effect of mgrA on mprF and dltA expression with or without hNP-1 exposure and demonstrated that mgrA positively regulated mprF and dltA transcription in both the MSSA and MRSA strain backgrounds. Importantly, hNP-1 exposure significantly induced mprF and dltA expression in the parental and mgrA-complemented strains but not in the mgrA mutant strains. These data indicate that mgrA is involved in HDP susceptibility at least in part through its regulation of mprF and dltA expression.
In addition, S. aureus pathogenicity is closely related to its capacity to bind to extracellular matrix (eg, Fn) and host cells (eg, ECs) [6]. In the present study, we observed that significantly fewer mgrA mutant organisms adhered to Fn than their respective isogenic wild-type and mgrA-complemented strains regardless of MRSA or MSSA backgrounds. It is known that host cell adhesion mainly involves Fn forming a bridge between α5β1 integrin on the cellular side and FnBPs on the bacteria [6, 48]. We therefore assume that mgrA may also influence EC adherence. Indeed, significantly decreased EC adherence profiles were found in mgrA mutants, which would theoretically provide a disadvantage for S. aureus pathogenesis. As a component of the FnBP-Fn-α5β1 pathway, FnBPs, especially FnBPA, are essential factors for Fn binding and Fn-mediated EC adherence [6]. Thus, we tested the expression of fnbA (encoding FnBPA). Consistent with the Fn and EC binding results, our results revealed that expression of fnbA in mgrA mutant strains was significantly less than that in parental and mgrA-complemented strains, indicating reduced Fn binding ability, and that subsequently weaker EC adherence might be due to the decreased fnbA expression. However, the observations that mgrA upregulates agr RNAIII [11] and agr RNAIII downregulates fnbA [19] are in contrast to our observations showing that expression of fnbA was decreased in mgrA mutant strains. Thus, we hypothesize that, as a member of the SarA global regulatory protein family, MgrA may bind to target promoter DNA in a similar fashion [49], The regulation of fnbA expression by direct minding of MgrA to the fnbA promoter can thus occur independent of agr [34]. We performed a gel shift assay and found that MgrA bound to the fnbA promoter fragment in a dose-dependent fashion. These data indicate that MgrA could activate fnbA transcription by directly binding to its promoter. To our knowledge, this is the first study to show that mgrA upregulates fnbA expression by directly binding to the fnbA promoter.
In support of the role of MgrA in S. aureus virulence that was observed in other studies [10, 42], deletion of mgrA led to decreased virulence in our experimental murine bacteremia model, with significantly less animal weight loss and decreased S. aureus densities in target tissues, compared with respective parental and mgrA-complemented strains, regardless of MSSA or MRSA background. In agreement with our results, other investigations have shown that mutation in mgrA attenuated S. aureus virulence in endocarditis and bacteremia models caused by other S. aureus strain backgrounds [10, 42]. The MgrA-mediated reduced virulence in S. aureus might be related to S. aureus–host interactions, including (1) increased susceptibilities to polymorphonuclear leukocytes and platelet-specific HDPs through regulations of genes (eg, mprF and dltA) involved S. aureus membrane surface charges; (2) lower fnbA expression, which subsequently leads to less adherence to Fn and ECs; and (3) decreased expression of global regulators (eg, agr RNAIII) and virulence factors (eg, hla). These combinatorial pathogen-host interactions could potentially influence virulence in S. aureus.
In conclusion, our studies provide valuable insights into MgrA-mediated pathogen-host interactions, including their effect on susceptibility to HDPs, Fn and EC binding, α-toxin, and EC damage, that are necessarily involved in the virulence in S. aureus. MgrA might be a potential target to optimize anti–S. aureus therapeutic strategies.
Notes
Financial support. This work was supported by the National Institutes of Health (grants AI-136065 and AI-41513 to Y. Q. X. and grant AI-91801 to A. L. C.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Reference
- 1. Klevens RM, Morrison MA, Nadle J, et al. ; Active Bacterial Core surveillance (ABCs) MRSA Investigators Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–71. [DOI] [PubMed] [Google Scholar]
- 2. Fowler VG Jr, Sakoulas G, McIntyre LM, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 2004; 190:1140–9. [DOI] [PubMed] [Google Scholar]
- 3. Xiong YQ, Fowler VG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis 2009; 199:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crosby HA, Schlievert PM, Merriman JA, King JM, Salgado-Pabón W, Horswill AR. The Staphylococcus aureus global regulator mgra modulates clumping and virulence by controlling surface protein expression. PLoS Pathog 2016; 12:e1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hauck CR, Ohlsen K. Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr Opin Microbiol 2006; 9:5–11. [DOI] [PubMed] [Google Scholar]
- 6. Shinji H, Yosizawa Y, Tajima A, et al. Role of fibronectin-binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect Immun 2011; 79:2215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008; 46(Suppl 5):S350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev 1991; 55:733–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwak YK, Vikström E, Magnusson KE, Vécsey-Semjén B, Colque-Navarro P, Möllby R. The Staphylococcus aureus alpha-toxin perturbs the barrier function in Caco-2 epithelial cell monolayers by altering junctional integrity. Infect Immun 2012; 80:1670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta RK, Alba J, Xiong YQ, Bayer AS, Lee CY. MgrA activates expression of capsule genes, but not the α-toxin gene in experimental Staphylococcus aureus endocarditis. J Infect Dis 2013; 208:1841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ingavale S, van Wamel W, Luong TT, Lee CY, Cheung AL. Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus. Infect Immun 2005; 73:1423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota, 1997–1999. JAMA 1999; 282:1123–5. [PubMed] [Google Scholar]
- 13. Ingavale SS, Van Wamel W, Cheung AL. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol Microbiol 2003; 48:1451–66. [DOI] [PubMed] [Google Scholar]
- 14. Forsyth RA, Haselbeck RJ, Ohlsen KL, et al. A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol Microbiol 2002; 43:1387–400. [DOI] [PubMed] [Google Scholar]
- 15. Wu T, Yeaman MR, Bayer AS. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among bacteremic staphylococcal and streptococcal isolates. Antimicrob Agents Chemother 1994; 38:729–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiong YQ, Yeaman MR, Bayer AS. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob Agents Chemother 1999; 43:1111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeaman MR, Sullam PM, Dazin PF, Norman DC, Bayer AS. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J Infect Dis 1992; 166:65–73. [DOI] [PubMed] [Google Scholar]
- 18. Xiong YQ, Mukhopadhyay K, Yeaman MR, Adler-Moore J, Bayer AS. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob Agents Chemother 2005; 49:3114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiong YQ, Bayer AS, Yeaman MR, Van Wamel W, Manna AC, Cheung AL. Impacts of sarA and agr in Staphylococcus aureus strain Newman on fibronectin-binding protein A gene expression and fibronectin adherence capacity in vitro and in experimental infective endocarditis. Infect Immun 2004; 72:1832–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reyes D, Andrey DO, Monod A, Kelley WL, Zhang G, Cheung AL. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J Bacteriol 2011; 193:6020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pankov R, Momchilova A. Fluorescent labeling techniques for investigation of fibronectin fibrillogenesis (labeling fibronectin fibrillogenesis). Methods Mol Biol 2009; 522:261–74. [DOI] [PubMed] [Google Scholar]
- 22. Torr EE, Ngam CR, Bernau K, Tomasini-Johansson B, Acton B, Sandbo N. Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype. J Biol Chem 2015; 290:6951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zell C, Resch M, Rosenstein R, Albrecht T, Hertel C, Götz F. Characterization of toxin production of coagulase-negative staphylococci isolated from food and starter cultures. Int J Food Microbiol 2008; 127:246–51. [DOI] [PubMed] [Google Scholar]
- 24. Seidl K, Zinkernagel AS. The MTT assay is a rapid and reliable quantitative method to assess Staphylococcus aureus induced endothelial cell damage. J Microbiol Methods 2013; 92:307–9. [DOI] [PubMed] [Google Scholar]
- 25. Ades EW, Candal FJ, Swerlick RA, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 1992; 99:683–90. [DOI] [PubMed] [Google Scholar]
- 26. Seidl K, Bayer AS, Fowler VG Jr, et al. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob Agents Chemother 2011; 55:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys 1993; 303:474–82. [DOI] [PubMed] [Google Scholar]
- 28. Seidl K, Chen L, Bayer AS, Hady WA, Kreiswirth BN, Xiong YQ. Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:5631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiong YQ, Willard J, Yeaman MR, Cheung AL, Bayer AS. Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J Infect Dis 2006; 194:1267–75. [DOI] [PubMed] [Google Scholar]
- 30. Beltrame CO, Côrtes MF, Bonelli RR, et al. Inactivation of the autolysis-related genes lrgB and yycI in Staphylococcus aureus increases cell lysis-dependent eDNA release and enhances biofilm development in vitro and in vivo. PLoS One 2015; 10:e0138924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheung AL, Bayer AS, Yeaman MR, et al. Site-specific mutation of the sensor kinase GraS in Staphylococcus aureus alters the adaptive response to distinct cationic antimicrobial peptides. Infect Immun 2014; 82:5336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bertsche U, Weidenmaier C, Kuehner D, et al. Correlation of daptomycin resistance in a clinical Staphylococcus aureus strain with increased cell wall teichoic acid production and D-alanylation. Antimicrob Agents Chemother 2011; 55:3922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang SJ, Bayer AS, Mishra NN, et al. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect Immun 2012; 80:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolz C, Pöhlmann-Dietze P, Steinhuber A, et al. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol Microbiol 2000; 36:230–43. [DOI] [PubMed] [Google Scholar]
- 35. Manna AC, Ingavale SS, Maloney M, van Wamel W, Cheung AL. Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J Bacteriol 2004; 186:5267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jing D, Agnew J, Patton WF, Hendrickson J, Beechem JM. A sensitive two-color electrophoretic mobility shift assay for detecting both nucleic acids and protein in gels. Proteomics 2003; 3:1172–80. [DOI] [PubMed] [Google Scholar]
- 37. Swarup V, Phaneuf D, Dupré N, et al. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor κB-mediated pathogenic pathways. J Exp Med 2011; 208:2429–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Voyich JM, Vuong C, DeWald M, et al. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis 2009; 199:1698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peacock SJ, Foster TJ, Cameron BJ, Berendt AR. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 1999; 145 (Pt 12):3477–86. [DOI] [PubMed] [Google Scholar]
- 40. Seidl K, Bayer AS, McKinnell JA, Ellison S, Filler SG, Xiong YQ. In vitro endothelial cell damage is positively correlated with enhanced virulence and poor vancomycin responsiveness in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Cell Microbiol 2011; 13:1530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Menzies BE, Kourteva I. Staphylococcus aureus alpha-toxin induces apoptosis in endothelial cells. FEMS Immunol Med Microbiol 2000; 29:39–45. [DOI] [PubMed] [Google Scholar]
- 42. Liu CL, Chen ZJ, Wang F, et al. The impact of mgrA on progression of Staphylococcus aureus sepsis. Microb Pathog 2014; 71-72:56–61. [DOI] [PubMed] [Google Scholar]
- 43. Jonsson IM, Lindholm C, Luong TT, Lee CY, Tarkowski A. mgrA regulates staphylococcal virulence important for induction and progression of septic arthritis and sepsis. Microbes Infect 2008; 10:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003; 3:710–20. [DOI] [PubMed] [Google Scholar]
- 45. Yeaman MR, Bayer AS, Koo SP, Foss W, Sullam PM. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Invest 1998; 101:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peschel A, Jack RW, Otto M, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med 2001; 193:1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 1999; 274:8405–10. [DOI] [PubMed] [Google Scholar]
- 48. Herrmann M, Vaudaux PE, Pittet D, et al. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis 1988; 158:693–701. [DOI] [PubMed] [Google Scholar]
- 49. Cheung AL, Nishina KA, Trotonda MP, Tamber S. The SarA protein family of Staphylococcus aureus. Int J Biochem Cell Biol 2008; 40:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]