Abstract
Objective
Pharmacological treatment of non-alcoholic fatty liver disease (NAFLD) is still evolving. Probiotics could be a promising treatment option, but their effectiveness needs to be established. The present study aimed to evaluate the efficacy of a high potency multistrain probiotic in adult patients with NAFLD.
Methods
Thirty-nine liver biopsy-proven patients with NAFLD were randomised in a double-blind fashion to either lifestyle modifications plus an oral multistrain probiotic (675 billion bacteria daily, n=19) or identical placebo (n=20) for 1 year. Lifestyle modifications included regular exercise for all and control of overweight/obesity (with additional dietary restrictions), hypertension and hyperlipidaemia in those with these risk factors. Primary objective of the study was the histological improvement in NAFLD activity score (NAS) and its components and secondary objectives were improvement in alanine transaminase (ALT) and cytokine profile.
Results
Thirty (76.9%) out of 39 patients with NAFLD completed the study with 1 year of follow-up. A repeat liver biopsy at 1 year could be done in 10 patients (52.6%) in probiotic group and five patients (25%) in placebo group. In comparison to baseline, hepatocyte ballooning (p=0.036), lobular inflammation (p=0.003) and NAS score (p=0.007) improved significantly at 1 year in the probiotic group. When compared with placebo, the NAS score improved significantly in the probiotic group (p=0.004), along with improvements in hepatocyte ballooning (p=0.05) and hepatic fibrosis (p=0.018). A significant improvement in levels of ALT (p=0.046), leptin (p=0.006), tumour necrosis factor-α (p=0.016) and endotoxins (p=0.017) was observed in probiotic group in comparison to placebo at 1 year. No significant adverse events were reported in the study.
Conclusion
Patients with NAFLD managed with lifestyle modifications and multistrain probiotic showed significant improvement in liver histology, ALT and cytokines.
Trial registration number
The clinical trial is registered with CLINICAL TRIAL REGISTRYINDIA (CTRI); http://ctri.nic.in, No. CTRI/2008/091/000074
Keywords: non-alcoholic steatohepatitis, NASH, hepatic steatosis, fatty liver, metabolic syndrome, bacterial overgrowth, microbiota
Summary Box.
What is already known about this subject?
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease and is becoming a serious public health concern.
The pathogenesis of NAFLD is unclear, but gut microbiota is known to be involved in its pathogenesis.
Probiotics are safe and have been found beneficial in various gastrointestinal conditions like inflammatory bowel disease, irritable bowel syndrome, diarrhoea, hepatic encephalopathy/minimal hepatic encephalopathy, etc but need further exploration in NAFLD.
What are the new findings?
The first human study to show the histological efficacy of a multistrain probiotic in biopsy-proven patients with NAFLD.
The study also demonstrates the beneficial effect of multistrain probiotic on levels of alanine transaminase, cytokines and endotoxins in patients with NAFLD.
How might it impact on clinical practice in the foreseeable future?
The use of probiotics in general clinical practice is not far away. The study provides data suggesting an important role of a multistrain probiotic in decreasing inflammation in patients with NAFLD which could help prevent future complications. Modulation of gut microbiota by probiotics represents a new treatment option in NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is currently the most common cause of chronic liver disease, becoming a serious public health concern as a result of the obesity epidemic, unhealthy dietary patterns and sedentary lifestyles.1 NAFLD represents a spectrum of conditions ranging from fat accumulation alone (steatosis without inflammation) to non-alcoholic steatohepatitis (NASH), with macrovesicular steatosis in hepatocytes, associated with inflammation, fibrosis and scarring, which can lead to cirrhosis and hepatocellular carcinoma.2 The prevalence of NAFLD is rapidly rising and is becoming a worldwide public health problem. NAFLD occurs among all ages, both the genders and various ethnic groups, and its global prevalence among the general population is ~25% with the highest prevalence seen in the Middle East and South America and the lowest in Africa.3–7
The pathogenesis of NAFLD is unclear; however, complex interactions between genetic, epigenetic, inflammatory status, environmental factors and lifestyle all play key roles in its development. The initial two-hit hypothesis has been replaced by the multi-hit hypothesis.8 Visceral adipose tissue accumulation contributing to inflammatory pathways and the development of peripheral insulin resistance are the main mechanisms for the development of NAFLD.6 Inflammatory cells, such as macrophages infiltrate visceral adipose tissue, thus increasing inflammatory adipokine secretion and reducing adiponectin production.9 A growing body of evidence has surfaced in recent past regarding small intestinal bacterial overgrowth (SIBO) or intestinal dysbiosis, which induces liver injury by causing increased intestinal permeability favouring the absorption of gut-derived lipopolysaccharides and endotoxins as a pivotal factor in the development and progression of NAFLD.10–13
The main treatment for NAFLD is lifestyle modification, including weight loss through a combination of decreased energy intake and increased energy expenditure.1 3 14 Pharmacological treatment of NAFLD/NASH is still evolving. Probiotics are a collection of bacteria with a wide range of beneficial effects on the host.15 Although the exact mechanism of probiotics in the treatment of NAFLD is not completely understood, it is thought that probiotics interfere with NAFLD progression through its effect in eradicating pathogenic bacteria in the intestine. Probiotics also reduce ethanol production and reduce inflammation by altering cytokine signalling.16 17
No human study has evaluated the efficacy of this probiotic preparation in a randomised manner for the treatment of NAFLD with liver histology as the endpoints. Hence, we conducted a proof of concept study in a randomised, double-blind, placebo-controlled manner to assess the role of the probiotic on liver histology, liver enzymes and adipocytokines in adult patients with biopsy-proven NAFLD.
Methods
Study design
The study was an investigator-initiated, randomised, double-blind placebo-controlled clinical trial conducted at two major tertiary care hospitals in Northern India. Signed informed consent was obtained from all patients before inclusion in the study. CD Pharma India Private Limited (New Delhi, India) funded the study and supplied the investigational drugs.
Patients
Eligible patients were aged above 18 years with raised liver enzymes (aspartate transaminase (AST) and alanine transaminase (ALT)) of at least 1.5 times the normal for more than 3 months, with no history of alcohol intake or intake <20 g/d (confirmed from at least two family members), negative viral markers (hepatitis B virus surface antigen and anti-hepatitis C virus), negative autoimmune markers (anti-nuclear antibodies (ANA), anti-smooth muscle antibody (ASMA), anti-liver kidney microsomal antibody, anti-mitochondrial antibody), negative Kayser-Fleischer ring with normal ceruloplasmin and normal iron studies and a liver biopsy consistent with features of NAFLD. Exclusion criteria included pregnant or lactating women, subjects with diabetes mellitus (DM) or with cirrhosis on imaging or liver biopsy, or patients with a history of drug intake for chronic conditions likely to cause NAFLD (eg, corticosteroids, methotrexate, tamoxifen, etc). The reason for excluding patients with DM was the possible effect of DM on gut motility leading onto the risk of SIBO.
Clinical evaluation, laboratory assessment and imaging
Clinical evaluation included anthropometric and a thorough general physical examination. Laboratory investigations included a complete haemogram and serum biochemistry including lipid profile, renal and liver function tests (LFTs), fasting and postprandial glucose levels. All patients were then subjected to an ultrasound examination of the abdomen; hepatic steatosis was noted and graded.18
Insulin resistance
Insulin resistance was determined by the homeostasis model assessment for insulin resistance (HOMA-IR). HOMA-IR was calculated as the product of fasting insulin (µU/L) and fasting plasma glucose (in mmol/L) divided by 22.5. An absolute value of HOMA-IR >1.64 was taken as abnormal.19
Metabolic syndrome
Metabolic syndrome was defined as the presence of at least ≥3 out of five modified Adult Treatment Panel III criteria including modified abnormal waist as per the Asia Pacific criteria.20
Adipokine and cytokine measurement
Adipokines (leptin and adiponectin) and cytokines (tumour necrosis factor (TNF)-α, interleukin (IL)−1β, IL-6 and IL-8) levels were measured in plasma using specific ELISA kits (Ray Biotech Life, Norcross, GA), according to the manufacturer’s protocol.
Small intestine bacterial overgrowth
Recruited patients were assessed for the presence of SIBO using non-invasive glucose hydrogen breath test (GHBT) and duodenal fluid culture as per the standard procedure.21 22
Histopathology
Liver biopsy was done under local anaesthesia as per standard protocol and the tissue was subjected to histological examination. Patients were graded as per the NASH-Clinical Research Network NAFLD activity score (NAS).23 Interpretation of liver biopsy was done by the single pathologist at respective centres; however, the same pathologist interpreted the histopathology at baseline and post-intervention and was blinded to the clinical details and the intervention group.
Randomisation and intervention
Randomisation, allocation and blinding
Computer-generated randomisation using permuted blocks of 4 and numbered packing of the intervention were prepared by a person not involved in the study. Individual randomisation codes for each recruited subject were concealed in separate opaque envelopes and marked with the patient number on the outer envelope. The individual sealed opaque envelope method was used to maintain blinding of the investigators and study participants. All the envelopes were passed on to the investigators (at the site) before the study initiation with an instruction that the envelope could be opened only in case of an emergency. Patients were randomised into either group immediately after the availability of the liver biopsy report.
Intervention
All patients were advised lifestyle modifications in the form of control of various risk factors like hypertension, hyperlipidaemia (with statins or fibrates) and overweight/obesity. All patients were advised regular exercise like brisk walking, jogging, running, swimming, cycling, etc for at least 30–45 min/day, for at least 5 days in a week. Patients with overweight/obesity, in addition, were advised 5%–10% of weight reduction from baseline (not more than 1.6 kg/week) with the help of hypocaloric diet (30% reduction in calorie intake) by reducing the intake of both carbohydrates and fats.
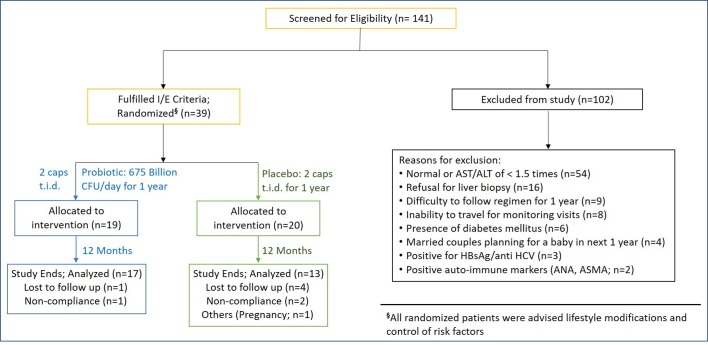
In addition to the lifestyle modifications, patients were randomised in a double-blind fashion to receive the high potency multistrain probiotic preparation (675 billion bacteria daily), or a placebo (identical in appearance and colour) for a period of 12 months (figure 1).
Figure 1.
Consort diagram. ANA, Anti-nuclear antibody; ALT, alanine transaminase; ASMA, Anti smooth muscle antibody; AST, aspartate transaminase; HCV, hepatitis C virus.
Probiotic Group: 2 capsules t.i.d. (Each capsule containing 112.5 billion live, lyophilised, lactic acid bacteria and bifidobacteria, namely Lactobacillus paracasei DSM 24733, Lactobacillus plantarum DSM 24730, Lactobacillus acidophilus DSM 24735 and Lactobacillus delbrueckii subsp. bulgaricus DSM 24734, Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, Bifidobacterium breve DSM 24732, and Streptococcus thermophilus DSM 24731, produced at Danisco-Dupont, WI, USA, currently sold in Europe, Singapore, USA and Korea under the brand VIVOMIXX, VISBIOME and DESIMONE FORMULATION, respectively).
Placebo Group: 2 placebo capsules t.i.d. (capsules containing microcrystalline cellulose).
Outcome and follow-up
Primary endpoint
Histological improvement in NAS and its components or hepatic fibrosis at the completion of the intervention (12 months).
Secondary endpoint
Improvement in ALT and cytokine profile at the completion of the intervention (12 months).
Physical examination, vital signs, haematology and biochemistry including LFTs were done monthly; anthropometry, measurement of adipocytokines, HOMA-IR and components of metabolic syndrome and ultrasound (abdomen) were done at baseline, at 3 months and at the end of study. Repeat assessment of SIBO and histopathology was planned at the end of the study.
Statistical analysis
Since the study was planned by investigators and was designed as a proof of concept study, sample size calculation was not done during initiation; however, it was planned to enrol at least 20 patients in each group. The descriptive statistics (mean±SD; median/range) was presented for continuous variables under each group. The distribution of categorical variables was presented in terms of frequency (percentage), under each group separately. If data did not follow a normal distribution, the non-parametric Kruskal-Wallis test was applied. Statistical significance of categorical variables was carried out by χ2 test/Fisher Exact test between the two groups. For comparison between the two groups, unpaired t-tests for normally distributed variables and non-parametric Mann-Whitney Test were applied for data that did not follow a normal distribution. Within-group comparison of quantitative variables was carried out by paired t-tests for normally distributed variables, and non-parametric Wilcoxon Signed Rank test for data that did not follow a normal distribution. Change in histology and other parameters was correlated with change in body weight and other components of metabolic syndrome using Spearman’s correlation coefficient. The level of statistical significance was taken as p<0.05. The data were analysed using SPSS Statistical Software V.18.0 (IBM, NY, USA) and is shown for those who adhered to the protocol and completed the follow-up (per-protocol analysis).
Results
Study patients
A total of 141 subjects were screened for enrolment, of which 102 patients were excluded for various reasons (figure 1). A total of 39 patients were randomised to receive either probiotic (n=19) or placebo (n=20), of which 30 (76.9%) patients (probiotic=17, placebo=13) completed the study with 1 year of follow-up. Study discontinuations were distributed evenly across both groups and are explained in later section. The patients in both the groups were comparable at baseline with respect to demographic, anthropometric and components of metabolic syndrome (table 1).
Table 1.
Baseline demographic, anthropometric and metabolic syndrome characteristics in the two groups
| Parameters | Probiotic | Placebo | P value |
| Patients, n (%) | 19 (49) | 20 (51) | 0.671 |
| Sex: male, n (%) | 13 (68) | 15 (75) | 0.424 |
| Age in year, mean (SD) | 38 (10) | 33 (6) | 0.228 |
| Weight in kg, mean (SD) | 70 (11) | 74 (14) | 0.263 |
| Height in cm, mean (SD) | 163 (9) | 167 (10) | 0.125 |
| BMI in kg/m2, mean (SD) | 26 (3) | 27 (4) | 0.894 |
| Waist circumference in cm, mean (SD) | 93 (10) | 93 (8) | 0.964 |
| Hip circumference in cm, mean (SD) | 92 (7) | 96 (7) | 0.087 |
| W/H ratio, mean (SD) | 1 (0.1) | 1 (0.1) | 0.121 |
| Hypertension (≥130/≥85 mm Hg); n (%) | 5 (26) | 4 (20) | 0.728 |
| Fasting blood glucose in mg/dL, mean (SD) | 98 (15) | 96 (16) | 0.695 |
| Cholesterol in mg/dL, mean (SD) | 180 (49) | 172 (43) | 0.647 |
| HDL in mg/dL, mean (SD) | 40 (9) | 40 (7) | 0.987 |
| LDL in mg/dL, mean (SD) | 102 (38) | 107 (37) | 0.660 |
| Triglycerides in mg/dL, mean (SD) | 205 (137) | 146 (58) | 0.270 |
| Metabolic syndrome, n (%) | 9 (47.4%) | 8 (40%) | 0.670 |
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; W/H ratio, waist/hip ratio.
Primary endpoint
Overall 30 (76.9%) out of 39 patients included in the study, completed 1 year of follow-up (probiotic=17, placebo=13). Four patients were excluded (reasons detailed in the compliance section) and five patients were lost to follow-up. Ten out of 17 (58.8%) patients in probiotic group and 5 out of 13 (38.5%) patients in placebo group agreed to undergo a repeat liver biopsy. As compared with baseline, even though there was no significant improvement in hepatic steatosis, hepatocyte ballooning (p=0.036), lobular inflammation (p=0.003) and NAS score (4.4±1.6 vs 2.7±1.0; p=0.007) improved significantly at 1 year in the probiotic group (table 2 Online Supplementary figure 1(A-E). When compared with the placebo group after 1 year, NAS score improved significantly in the probiotic group (2.7±1.0 vs 3.2±2.2; p=0.004) with significant improvements in individual components of hepatocyte ballooning (p=0.05) and hepatic fibrosis (p=0.018). Even though the number of patients with NAS score ≥5 were small (40%) in each group, a significant improvement (p=0.026) was also seen in NASH grading in the probiotic group as compared with the placebo group at the end of 1 year (table 2, Online supplementary Figure 1F).
Table 2.
Histological parameters at baseline and after 12 months of intervention in the two groups
| Parameters/groups | 0 Mo | 12 Mo | P value (0 vs 12 mo) |
|||||
| Probiotic (n=19) |
Placebo (n=20) | P value | Probiotic (n=10) |
Placebo (n=5) | P value | Probiotic | Placebo | |
| Steatosis | ||||||||
| ≤5% | 0 | 0 | 0.632* | 0 | 0 | 0.999* | 0.287* | 0.136* |
| 5%–33% | 5 | 5 | 4 | 2 | ||||
| >33%–66% | 9 | 7 | 6 | 3 | ||||
| >66% | 5 | 8 | 0 | 0 | ||||
| Ballooning necrosis | ||||||||
| None | 8 | 11 | 0.526* | 6 | 3 | 0.050* | 0.036* | 0.082* |
| Few balloon cells | 3 | 4 | 4 | 0 | ||||
| Many balloon cells | 8 | 5 | 0 | 2 | ||||
| Lobular inflammation | ||||||||
| 0 or 1 focus | 0 | 4 | 0.104* | 4 | 2 | 0.852* | 0.003* | 0.233* |
| 2–4 foci per 200× field | 13 | 8 | 5 | 2 | ||||
| >4 foci per 200× field | 6 | 8 | 1 | 1 | ||||
| Fibrosis | ||||||||
| None | 9 | 8 | 0.577* | 8 | 1 | 0.018* | 0.732* | 0.544* |
| Perisinusoidal or periportal | 5 | 5 | 2 | 1 | ||||
| Perisinusoidal and portal/periportal | 4 | 3 | 0 | 3 | ||||
| Bridging fibrosis | 1 | 4 | 0 | 0 | ||||
| Cirrhosis | 0 | 0 | 0 | 0 | ||||
| NAS score† | 4.4±1.6 | 4.2±2.0 | 0.227‡ | 2.7±1.0 | 3.2±2.2 | 0.004‡ | 0.007§ | 0.257§ |
| NASH grading | ||||||||
| No NASH (NAS<3) |
2 | 5 | 0.470* | 4 | 3 | 0.026* | 0.197* | 0.233* |
| Borderline NASH (NAS 3–4) |
9 | 7 | 6 | 0 | ||||
| Definite NASH (NAS≥5) | 8 | 8 | 0 | 2 | ||||
*Pearson χ2 test.
†± Values are mean±SD.
‡Kruskal-Wallis test.
§Wilcoxon Signed-Rank test.
NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; mo, months.
bmjgast-2019-000315supp002.pdf (281.1KB, pdf)
Secondary endpoints
Assessment of liver functions
LFT assessment was done in all patients at baseline and at 3 and 12 months in those on follow-up. Even though patients in both placebo and probiotic groups demonstrated a reduction in ALT levels at 12 months, patients in the probiotic group showed a greater improvement in ALT levels (p=0.046) in comparison to the placebo group at the end of 12 months (table 3).
Table 3.
Liver function tests at baseline and 12 months of intervention in the two groups
| Parameters/groups | Probiotic | Placebo | P value* | P value† | ||
| 0 mo (n=19) |
12 mo (n=17) |
0 mo (n=20) |
12 mo (n=13) |
|||
| Bilirubin (mg/dL) | 1.1±1.3 | 0.7±0.4 | 1.0±1.1 | 1.1±1.0 | 0.216 | 0.036 |
| P value | 0.025‡ | 0.721‡ | ||||
| AST (U/L) | 68.0±32.3 | 36.0±16.4 | 74.2±30.1 | 44.9±18.5 | 0.305 | 0.119 |
| P value | 0.002‡ | 0.005‡ | ||||
| ALT (U/L) | 101.1±48.0 | 45.1±29.7 | 105.5±51.0 | 68.0±40.7 | 0.613 | 0.046 |
| P value | 0.001‡ | 0.013‡ | ||||
| ALP (IU/L) | 187.1±74.9 | 138.7±55.0 | 239.8±155.0 | 210.3±117.6 | 0.305 | 0.039 |
| P value | 0.011‡ | 0.463‡ |
± Values are mean±SD.
*Between-group comparison at 0 mo by Mann-Whitney test.
†Between-group comparison at 12 mo by Mann-Whitney test.
‡Within-group comparison, 0 mo versus 12 mo by Wilcoxon Signed-Rank test.
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; mo, months.
Adipocytokines and endotoxins
Assessment of adipocytokines and endotoxins was done in all patients at baseline and at 3 and 12 months in those on follow-up. Leptin levels decreased significantly from 5.72±2.11 ng/mL at baseline to 3.95±1.31 ng/mL at 12 mo (p=0.001) in the probiotic group (table 4). Patients in the probiotic group showed a significant reduction in the levels of TNF-α at 12 months (p=0.016) in comparison to the placebo group. The levels of inflammatory cytokines (TNF-α, IL-1β, IL-6) and endotoxins were found to be significantly reduced only in the probiotic group at 12 months (table 4).
Table 4.
Adipocytokines and endotoxins levels at baseline and 12 months of intervention in the two groups
| Parameters/mo | 0 mo (n†=19/20) | 12 mo (n†=17/13) | P value‡ |
| Leptin units (ng/mL) | |||
| Probiotic | 5.7±2.1 | 4.0±1.3 | 0.001 |
| Placebo | 5.1±2.1 | 5.7±2.4 | 0.972 |
| P value§ | 0.384 | 0.006 | |
| Adiponectin units (mcg/mL) | |||
| Probiotic | 5.0±2.2 | 5.5±2.2 | 0.866 |
| Placebo | 4.8±2.5 | 5.3±2.2 | 0.917 |
| P value§ | 0.908 | 0.999 | |
| TNF-α units (pg/mL) | |||
| Probiotic | 207.9±102.2 | 107.8±94.4 | 0.011 |
| Placebo | 190.0±131.1 | 243.15±167.1 | 0.917 |
| P value§ | 0.438 | 0.016 | |
| IL-1β units (pg/mL) | |||
| Probiotic | 99.6±56.4 | 78.8±56.7 | 0.027 |
| Placebo | 90.7±73.8 | 106.2±75.3 | 0.576 |
| P value§ | 0.603 | 0.3 | |
| IL-6 units (pg/mL) | |||
| Probiotic | 125.6±95.3 | 100.6±74.7 | 0.041 |
| Placebo | 112.8±83.7 | 141.4±107.3 | 0.507 |
| P value§ | 0.448 | 0.189 | |
| Endotoxins units (endotoxin units/mL or EU/mL) | |||
| Probiotic | 0.35±0.29 | 0.15±0.16 | 0.021 |
| Placebo | 0.34±0.27 | 0.41±0.27 | 0.866 |
| P value§ | 0.935 | 0.017 | |
± Values are mean±SD.
*Probiotic/placebo.
†Within-group comparison at 0–12 mo by Wilcoxon Signed-rank test.
‡Between-group comparison at 0 mo and at 12 mo by Mann-Whitney Test.
IL, interleukin; TNF, tumour necrosis factor; mo, months.
Other assessments
Metabolic syndrome
Nine (47.4%) and 8 (40.0%) patients in the probiotic and placebo groups, respectively, had metabolic syndrome at baseline (table 1). Even though lifestyle interventions were advised in both the groups, over a period of 1 year, there was no significant change in body weight or components of metabolic syndrome in either group (Online supplementary Table 1a and 1b). Furthermore, the improvement in histology, ALT and cytokine profile did not correlate with change in body weight or other components of metabolic syndrome.
bmjgast-2019-000315supp001.pdf (17.4KB, pdf)
Insulin resistance
In all, 15 (78.9%) and 17 (85%) patients in the probiotic and placebo groups, respectively, had evidence of insulin resistance (HOMA-IR >1.64) at baseline. There was no significant change in the HOMA-IR levels in both groups of patients at 3 and 12 months.
Small intestine bacterial overgrowth
Two (10.5%) patients and one (5%) patient each in the probiotic and placebo groups as per GHBT and five patients (25%) each as per duodenal fluid culture had evidence of SIBO at baseline, however, none of the patients in both the groups had evidence of SIBO after 12 months.
Ultrasonography
All patients showed evidence of hepatic steatosis at baseline and continued to do so at 3 and 12 months.
Compliance and adverse events
Three subjects, non-compliant to study protocol were excluded from the study (one in the probiotic group and two in the placebo group). A subject in the placebo group got pregnant and had to be excluded from the study for ethical reasons. One patient in the probiotic group and four patients in the placebo group were lost to follow-up. Overall, 30 (76.97%) out of 39 patients included in the study, completed 1 year of follow-up. None of the patients in either group had any serious adverse event that could be corroborated to the usage of study drug or placebo. Mild flatulence was reported by two patients in the probiotic group which got resolved after 1–2 weeks of therapy.
Discussion
In this proof of concept, double-blind, randomised placebo-controlled study, our results have shown for the first time in the world literature the efficacy of a high concentration multistrain probiotic in improving liver histology in adult patients with NAFLD. In addition to improvements in liver histology, patients in the probiotic group showed greater reduction in serum ALT and improvement in cytokine profile in comparison to the patients on placebo. Since there was no significant change in either body weight or components of metabolic syndrome in either group, significant improvement in histological parameters, ALT and cytokine profile in the probiotic group would suggest the beneficial effect of probiotic rather than the effect of the lifestyle interventions.
Pharmacological treatment of patients with NAFLD is still evolving. Most of the guidelines recommend the use of vitamin E or pioglitazone in patients without diabetes and with biopsy-proven NASH.1 3 5 6 However, both these drugs have long-term safety concerns. Lot of new drugs including obeteicholic acid, elafibranor, selonsertib and cenicriviroc have shown promising results in phase II studies but are awaiting results of phase III studies.24–27
Since gut microbiota is known to be involved in the pathogenesis of NAFLD/NASH, there exists a rationale in modulating the gut microbiota in such patients. The gut microbiota can be modulated with antibiotics, faecal microbiota transplantation, etc, however, probiotics are the safest and most studied in patients with NAFLD.28 Probiotics are live microorganisms when administered in adequate amounts confer a health benefit on the host.15 Probiotics have been extensively studied in patients with cirrhosis and its complications like hepatic encephalopathy and use of probiotics have shown improvement in endotoxemia and minimal hepatic encephalopathy in such patients.29 In our own experience, the same multistrain probiotic was shown to reduce liver disease severity and hospitalisations due to hepatic encephalopathy in patients with liver cirrhosis.30 Based on the severity and complexity of the chronic liver disease, earlier studies used a dose of 450 - 900 billion CFU of this probiotic preparation per day.30–32 Taking an average of the available studies, we chose to use 675 billion CFU of the probiotic daily in the present study.
There is sparse data on the use of probiotics in patients with NAFLD. Even though few randomised controlled trials have studied the efficacy of probiotics in both paediatric and adult patients with NAFLD, these studies are limited by the usage of surrogate markers of liver disease like serum ALT, lipids and cytokine profile rather than using the histological endpoints.33–35 Similarly, studies using synbiotics (a combination of prebiotics and probiotics) in biopsy-proven patients with NAFLD assessed its efficacy by surrogate imaging or biochemical markers rather than using the histological endpoints.36 A recent meta-analysis of seven studies suggested probiotics to be a promising option for the treatment of patients with NAFLD.37 Different results with the use of probiotics in NAFLD are related to the usage of varying bacterial strains, doses and treatment duration across studies. However, overall beneficial effects of probiotics in NAFLD are related to improved gut dysbiosis and increased mucin production amounting to a good gut barrier function, increased competitive adhesion and minimising pathogen colonisation, reducing endotoxemia and regulation of gut-associated lymphoid tissue system.16 17
The probiotic used in our study contains one of the highest concentration of bacteria and has been shown to be useful in NAFLD in both animal and human studies in improving biochemical and imaging parameters.38–40 In an earlier study from John Hopkins University, treatment with this probiotic preparation or anti-TNF antibodies in ob/ob mice model of NAFLD, improved liver histology, reduced hepatic total fatty acid content and decreased serum ALT levels. These benefits were associated with decreased hepatic expression of TNF-α mRNA in mice treated with anti-TNF antibodies but not in mice treated with the probiotic. Nevertheless, both treatments reduced the activity of Jun N-terminal kinase, a TNF-regulated kinase that promotes insulin resistance, and decreased the DNA binding activity of nuclear factor kappa B, the target of IκB-kinase, another TNF-regulated enzyme that causes insulin resistance.38 In a methionine-choline deficient diet-induced mice model, the probiotic modulated liver fibrosis but did not protect from inflammation and steatosis in NASH.39 In a recent randomised, placebo-controlled human study in obese children (22 patients each on probiotic and placebo) the probability that children supplemented with probiotic had none, light, moderate or severe fatty liver as assessed on ultrasound at the end of 4 months of study was 21%, 70%, 9% and 0%, respectively, with corresponding values of 0%, 7%,76% and 17% for the placebo group (p<0.001). No between-group differences were detected in triglycerides, HOMA and ALT while BMI decreased and glucagon-like peptide (GLP-1) and activated GLP-1 increased in the probiotic group (p<0.001 for all comparisons).40
In contrast to the available data on the use of this probiotic preparation in NAFLD, the strength of our study includes that it is the first human study to show the histological efficacy in biopsy-proven patients with NAFLD. However, our study is limited by the small number of patients at baseline in both the groups and a further small number on follow-up in who repeat liver biopsies could be performed and interpretation of histopathology by only a single pathologist at respective centres. In addition, most patients had mild liver histology at baseline which may have contributed towards positive results. Even though our numbers were small, our work does provide a proof of concept regarding the efficacy of the high concentration multistrain probiotic preparation in improving the liver histology, ALT and cytokine profile in patients with NAFLD.
Even though the multistrain probiotic used in this study has shown positive results, it should be understood that the actions of individual probiotic strains or multistrain probiotics used in clinical trials are quite specific and therefore should not be generalised to other probiotic formulations differing in the colony forming counts, type of strains, ratio of strains or the manufacturing processes used for formulation of the probiotic product.
In conclusion, results of this proof of concept study suggest that lifestyle modifications along with the multistrain probiotic preparation significantly improve liver histology, serum ALT and cytokine profile in patients with NAFLD. A large randomised controlled trial is warranted to confirm the results of this study.
Acknowledgments
We appreciate the efforts of colleagues, fellows and departmental staff in the conduct of the study and are grateful for their assistance. We acknowledge CD Pharma India Pvt. Ltd. for providing us with the financial and material support towards the conduct of the study.
Footnotes
Presented at: Data presented by Prof. Ajay Duseja, as a poster at the AASLD Liver Meeting on Nov 12, 2016 [Abstract ID 1185, Hepatology 2016; 64(Suppl S1):596A].
Contributors: AD and SKA contributed towards the conception and design of the study, recruited subjects, monitored the conduct and progress of the study, interpreted the results, drafted and reviewed the manuscript. RKD and YC assisted with the study design, subject enrolments, interpretation of results and critical review of the manuscript. MM, SC and S were responsible for management and coordination of the trial, monitoring the study progress, data collection and its entry in database files and drafting of the manuscript. SR, AD and SDG performed laboratory investigations and histopathological assessments, analysed and interpreted the data, and critically reviewed the manuscript. All the authors have read and have approved the final manuscript.
Funding: CD Pharma India Private Limited (New Delhi, India) funded the study and supplied the investigational drugs but did not participate in any part of the study.
Disclaimer: The authors have read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to CONSORT 2010 Statement.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by Institutional Ethics Committee of both hospitals (AIIMS: Ref. No. A-49 and PGIMER: Micro/2007/2582) and was conducted in conformance to ethical guidelines of the 1975 Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on request to researchers who provide a methodically sound proposal and whose proposed use of the data has been approved by an independent review committee identified for this purpose. Such proposals may be directed to the corresponding author (ajayduseja@yahoo.co.in). However, to gain access, data requestors will need to sign and submit a cover letter mentioning the purpose with a list of requested documents along with a statement/undertaking to maintain data confidentiality.
References
- 1. Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, et al. . Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 4. Duseja A, Chalasani N. Epidemiology and risk factors of nonalcoholic fatty liver disease (NAFLD). Hepatol Int 2013;7 Suppl 2:755–64. 10.1007/s12072-013-9480-x [DOI] [PubMed] [Google Scholar]
- 5. Wong VW-S, Chan W-K, Chitturi S, et al. . Asia-Pacific Working Party on non-alcoholic fatty liver disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol 2018;33:70–85. 10.1111/jgh.13857 [DOI] [PubMed] [Google Scholar]
- 6. Duseja A, Singh SP, Saraswat VA, et al. . Non-Alcoholic fatty liver disease and metabolic Syndrome-Position paper of the Indian national association for the study of the liver, endocrine Society of India, Indian College of cardiology and Indian Society of gastroenterology. J Clin Exp Hepatol 2015;5:51–68. 10.1016/j.jceh.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duseja A, Najmy S, Sachdev S, et al. . High prevalence of non-alcoholic fatty liver disease among healthy male blood donors of urban India. JGH Open 2019;3:133–9. 10.1002/jgh3.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010;52:1836–46. 10.1002/hep.24001 [DOI] [PubMed] [Google Scholar]
- 9. Trayhurn P, Bing C. Appetite and energy balance signals from adipocytes. Philos Trans R Soc Lond B Biol Sci 2006;361:1237–49. 10.1098/rstb.2006.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duseja A, Chawla YK.. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis 2014;18:59–71. 10.1016/j.cld.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 11. Leung C, Rivera L, Furness JB, et al. . The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol 2016;13:412–25. 10.1038/nrgastro.2016.85 [DOI] [PubMed] [Google Scholar]
- 12. Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr 2013;56:461–8. 10.1097/MPG.0b013e318284abb5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kapil S, Duseja A, Sharma BK, et al. . Small intestinal bacterial overgrowth and Toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2016;31:213–21. 10.1111/jgh.13058 [DOI] [PubMed] [Google Scholar]
- 14. Cicero A, Colletti A, Bellentani S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): the available clinical evidence. Nutrients 2018;10:pii: E1153 10.3390/nu10091153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Food and Agriculture Organization of the United Nations, World Health Organization . Guidelines for the evaluation of probiotics in food: report of a joint FAO/WHO Working group on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada; 2002. http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf [Accessed 07 May 2019] [Google Scholar]
- 16. Caballero-Franco C, Keller K, De Simone C, et al. . The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 2007;292:G315–G322. 10.1152/ajpgi.00265.2006 [DOI] [PubMed] [Google Scholar]
- 17. Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, et al. . Probiotic mechanisms of action. Ann Nutr Metab 2012;61:160–74. 10.1159/000342079 [DOI] [PubMed] [Google Scholar]
- 18. Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J 1986;292:13–15. 10.1136/bmj.292.6512.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chitturi S, Abeygunasekera S, Farrell GC, et al. . NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002;35:373–9. 10.1053/jhep.2002.30692 [DOI] [PubMed] [Google Scholar]
- 20. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421. 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 21. Rana SV, Sharma S, Kaur J, et al. . Relationship of cytokines, oxidative stress and Gi motility with bacterial overgrowth in ulcerative colitis patients. J Crohns Colitis 2014;8:859–65. 10.1016/j.crohns.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 22. Khoshini R, Dai S-C, Lezcano S, et al. . A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci 2008;53:1443–54. 10.1007/s10620-007-0065-1 [DOI] [PubMed] [Google Scholar]
- 23. Brunt EM, Kleiner DE, Wilson LA, et al. . Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53:810–20. 10.1002/hep.24127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. . Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (Flint): a multicentre, randomised, placebo-controlled trial. The Lancet 2015;385:956–65. 10.1016/S0140-6736(14)61933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ratziu V, Harrison SA, Francque S, et al. . Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016;150:1147–59. 10.1053/j.gastro.2016.01.038 [DOI] [PubMed] [Google Scholar]
- 26. Loomba R, Lawitz E, Mantry PS, et al. . The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 2018;67:549–59. 10.1002/hep.29514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedman SL, Ratziu V, Harrison SA, et al. . A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754–67. 10.1002/hep.29477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu J, Luo H, Jiang Y, et al. . Dietary capsaicin and antibiotics act synergistically to reduce non-alcoholic fatty liver disease induced by high fat diet in mice. Oncotarget 2017;8:38161–75. 10.18632/oncotarget.16975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Q, Duan ZP, Ha DK, et al. . Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004;39:1441–9. 10.1002/hep.20194 [DOI] [PubMed] [Google Scholar]
- 30. Dhiman RK, Rana B, Agrawal S, et al. . Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 2014;147:1327–37. 10.1053/j.gastro.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 31. Pratap Mouli V, Benjamin J, Bhushan Singh M, et al. . Effect of probiotic VSL#3 in the treatment of minimal hepatic encephalopathy: A non-inferiority randomized controlled trial. Hepatol Res 2015;45:880–9. 10.1111/hepr.12429 [DOI] [PubMed] [Google Scholar]
- 32. Gupta N, Kumar A, Sharma P, et al. . Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int 2013;33:1148–57. 10.1111/liv.12172 [DOI] [PubMed] [Google Scholar]
- 33. Vajro P, Mandato C, Licenziati MR, et al. . Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr 2011;52:740–3. 10.1097/MPG.0b013e31821f9b85 [DOI] [PubMed] [Google Scholar]
- 34. Nabavi S, Rafraf M, Somi MH, et al. . Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci 2014;97:7386–93. 10.3168/jds.2014-8500 [DOI] [PubMed] [Google Scholar]
- 35. Famouri F, Shariat Z, Hashemipour M, et al. . Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr 2017;64:413–7. 10.1097/MPG.0000000000001422 [DOI] [PubMed] [Google Scholar]
- 36. Ferolla S, Couto C, Costa-Silva L, et al. . Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients 2016;8:397 10.3390/nu8070397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. S Lavekar A, V Raje D, Manohar T, et al. . Role of probiotics in the treatment of nonalcoholic fatty liver disease: a meta-analysis. Euroasian J Hepatogastroenterol 2017;7:130–7. 10.5005/jp-journals-10018-1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Z, Yang S, Lin H, et al. . Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003;37:343–50. 10.1053/jhep.2003.50048 [DOI] [PubMed] [Google Scholar]
- 39. Velayudham A, Dolganiuc A, Ellis M, et al. . VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology 2009;49:989–97. 10.1002/hep.22711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alisi A, Bedogni G, Baviera G, et al. . Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2014;39:1276–85. 10.1111/apt.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2019-000315supp002.pdf (281.1KB, pdf)
bmjgast-2019-000315supp001.pdf (17.4KB, pdf)