Drug Allergy
Latest trends and health care impact
2018 features further evidence that broad spectrum antibiotic use is increasing worldwide, especially in low- and middle-income countries.1 Despite slower growth in high-income countries attributed to implementation of antibiotic stewardship programs and lower infection rates,1 the impact of antibiotic allergy and other drugs remains sizable. Recent prevalence studies, using the Partners Enterprise-wide Allergy Repository within the electronic health record (EHR) in the Greater Boston area, indicate a large health care burden.2,3 The prevalence of hypersensitivity reactions (HSRs) was 14%−20%, with penicillin accounting for the largest proportion of immediate (33%) and delayed (39%) HSRs.2 In both studies, cutaneous reactions such as urticaria and rash (22–48%) and skin pruritus (3–15%) were the most commonly reported, while anaphylaxis constituted 2.5%−6% of reported HSRs.2,3 Within the same patient population, the overall prevalence of patients with non-immunologic reactions to three or more drugs (multiple drug intolerance syndrome, MDIS), and patients with immunologic reactions to two or more drug classes (multiple drug allergy syndrome, MDAS) was reported to be 6.4% and 1.2%, respectively.4
Multiple studies in 2018 quantified the negative health care impact of a β-lactam allergy label, including its cost and effect on quality of life (QOL). Children and adults with a β-lactam allergy label received alternative broad-spectrum antibiotics more frequently and experienced a longer hospital length of stay (LOS), resulting in higher hospital costs.5–7 A retrospective single-center cross-sectional analysis of all pediatric inpatients in a teaching hospital in Australia admitted during 2014 and 2015 showed that children with an antibiotic allergy label, of which β-lactams were the most implicated, were more likely to receive alternative antibiotics such as macrolides, quinolones, lincosamides, nitroimidazoles and cephalosporins than children without any antibiotic allergy label. They also experienced prolonged hospital LOS by 2 days.5 A similar study performed in children with a penicillin allergy label showed prolonged hospital LOS in the allergic group (5 vs. 4 days), a higher comorbidity index, and a tendency towards higher hospitalization costs.6
A retrospective cohort study of adult inpatients with hematologic malignancies at 2 tertiary care hospitals showed worse clinical outcomes among patients with a β-lactam allergy label.7 These included prolonged LOS (11 days vs. 7.6 days), higher mortality rate at 30 and 180 days, higher 30-day readmission rate, increased rate of Clostridioides difficile (C. difficile) infection, and increased hospital costs ($223,000 vs. $173,000).7 A systematic analysis of the cost of a self-reported penicillin allergy suggested an additional inpatient drug cost of $609 per patient, and additional outpatient prescription drug costs ranging $14-$193 for penicillin allergic patients.8
Two retrospective cohort studies from 2018 addressed the higher risk of infection in patients labeled penicillin allergic.9,10 Using data from the EHR of patients registered with the general practices in the United Kingdom, 64,000 patients were identified with a documented penicillin allergy and 237,000 matched comparators. During the mean follow up of 6 years, a penicillin allergy label was associated with a 69% increased risk of methicillin-resistant Staphylococcus aureus (MRSA) and a 26% increased risk of C. difficile infection; this was attributed to administration of alternative, non-β-lactam antibiotics.9 Patients with a penicillin allergy label also possessed a 50% higher odds of a surgical site infection (SSI) regardless of the surgical procedure.10 In the multivariable marginal structural model, SSI development was entirely mediated through the decline of cefazolin use for perioperative prophylaxis and the prescription of β-lactam alternative antibiotics. In addition, patients with MDIS have higher rates of depression and anxiety.4 A diagnosis of MDAS was associated with a 40% increased odds of depression, but not anxiety.4
Advances in disease pathogenesis
In 2018, advances in the pathogenesis of immunologically-mediated drug hypersensitivity focused on the drug specific T cell response and the role of post-transcriptional regulators such as microRNAs (miRNAs). Using piperacillin-specific T cell clones isolated from blood and the skin of hypersensitive patients and healthy controls, factors that induce drug-specific T-cell response were assessed.11 Both CD4+ and CD8+ positive T cell clones derived from the blood and the skin secreted high levels of IL-22 in combination with interferon-γ and IL-13. IL-17 was not detected, pointing to a T helper (TH) 22 phenotype (Figure 1). Furthermore, the differentiation of naïve T cells into drug-specific IL-22 secreting cells depended on aryl hydrocarbon receptor signaling.
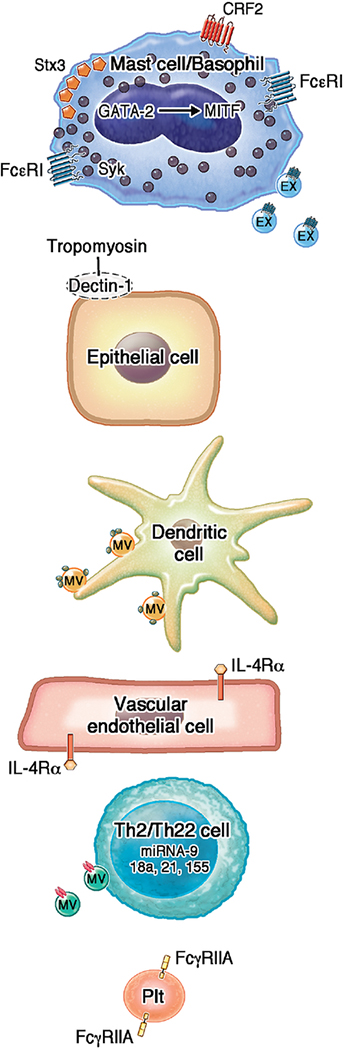
FIGURE I: Key advances in mechanisms of allergy, urticaria and anaphylaxis in 12018.
Within mast cells, GATA 2 induced MITF, CRF2 downregulated IgE-mediated degranulation, and Stx 3 clustering on the plasma membrane induced mast cell exocytosis. Secreted exosomes (EX) also transfered FcεRI and bound to free IgE. Within basophils, SYK expression was modulated by anti-FcεR1. On epithelial cells, anaphylaxis depended on IL-4’s interaction with the VE-specific IL4Rα, and on binding of tropomyosin with dectin-1. Perivascular dendritic cells also captured blood-borne allergens (smallest green circles) and relayed them via MVs to neighboring mast cells and dendritic cells. In primed drug specific T cell clones and naïve T cells, high amounts of IL-22 were secreted, and in peripheral blood mononuclear cells, miRNA-9, miRNA-18a, miRNA21, and/or miRNA-155 were upregulated. MVs derived from activated T cells stimulated mast cell degranulation, in part by carrying miRNA-4443 (red rectangles). FcγRIIA-expressing platelets were activated by aggregated IgG and induced anaphylaxis.
Abbreviations: CRF: corticotropin releasing factor; CSU: Chronic spontaneous urticaria; EX-exosome; FcεRI: high affinity receptor for the Fc region of immunoglobulin E; IL4Rα: Receptor for interleukin 4; MiRNA: microRNA; MITF: microphthalmia-associated transcription factor; PLT: platelet; platelet; STX: syntaxin; SYK: spleen tyrosine kinase Th: T helper; VE: vascular endothelial
Several miRNAs were up-regulated during drug-specific stimulation of CD4+ T-cell clones from patients with different hypersensitivity reactions such as maculopapular exanthema (MPE), toxic epidermal necrolysis (TEN), and Stevens-Johnson syndrome (SJS).12 These included miRNA-18a and miRNA-155 in carbamazepine-specific clones, and miRNA-9 in piperacillin-specific clones. The authors hypothesized that the incidence of carbamazepine-induced SJS/TEN could be related in part to the overexpression of miRNA-18a. Using peripheral blood mononuclear cells (PBMCs) from patients with delayed reactions to sulfamethoxazole, piperacillin, lamotrigine and carbamazepine, miRNA-18a, miRNA-21, miRNA-155 were up-regulated. The effects on miRNA-155 were most sustained, supporting the authors’ conclusion that miRNA-155, a miRNA previously shown to be important Th1 and TH17 immunity, could serve as a potential early biomarker of delayed HSRs.12,13
Approach to diagnosis and management
The basophil activation test (BAT) has been studied as a potential in vitro tool to aid in the diagnosis of IgE-mediated drug allergy where there is a high likelihood of anaphylaxis based on clinical history. In 2018, the BAT was evaluated in the diagnosis of type I hypersensitivity to amoxicillin and clavulanic acid.14 For both amoxicillin and clavulanic acid, the BAT showed a high positive predictive value of 92–93%, but clavulanic acid demonstrated higher sensitivity (62%). Forty two percent of subjects with immediate hypersensitivity to amoxicillin demonstrated negative BAT results at 12 months, compared to 60% of patients in the clavulanic acid group. The authors concluded that the BAT could function as a complementary tool in the diagnosis of amoxicillin and clavulanic acid hypersensitivity if performed within 12 months prior to the presumed clearance of serum IgE. This study also indirectly supported using ‘time since index reaction of more than 1 year’ as part of a ‘low risk’ history when determining the need for skin or other testing prior to a confirmatory oral challenge when evaluating penicillin allergies. In a cohort of 42 patients, 29 (69%) with a history of omeprazole-associated anaphylaxis (and the rest urticaria or angioedema) tested a median of 180 days after their index reactions, the BAT was positive in 8 (57%) of those who were skin test negative but oral challenge positive. These authors concluded that the addition of the BAT to the skin test can correctly diagnose 85% of patients with an omeprazole allergy.15
Management strategies continue to center on the development and implementation of pathways for penicillin allergy de-labeling (Table I). Many institutions have been successful in implementing penicillin allergy de-labeling programs by using a multidisciplinary approach, risk stratification, and EHR that promote a timely penicillin allergy evaluation. One such approach was the use of a clinical decision support (CDS) tool incorporated into the EHR that linked all aztreonam orders for penicillin allergic patients to a pharmacist penicillin skin test consultation.16 This application of the CDS tool was associated with an increase in the utilization of penicillin, a decrease in the use of aztreonam and cephalosporin, and a decrease in antibiotic costs from $1265 to $592 USD per patient.16 Another study demonstrated cost effectiveness among 30 patients undergoing a penicillin allergy evaluation using an independent nurse practitioner and performing a 2-step graded challenge without penicillin skin testing.17
TABLE 1.
Key advances in drug allergy, urticaria, hereditary angioedema and anaphylaxis in 2018
| Advances | References | ||
|---|---|---|---|
| Treatment recommendations | Drug allergy | Drug allergy pathways should be implemented. | 16, 18 |
| Vigorous penicillin de-labeling is cost effective. | 17 | ||
| Urticaria | Omalizumab is safe and effective in extended dosing. | 33 | |
| Omalizumab is efficacious for several types of inducible urticaria. | 29, 37 | ||
| Guideline based algorithm for treatment is effective in the realworld. | 40 | ||
| Hereditary angioedema | All attacks should be considered for on demand treatment and treated as soon as possible. | 49 | |
| All patients should have on demand therapy available (two doses). | 49 | ||
| Long-term prophylactic treatment is appropriate for patients with HAE who do not achieve adequate benefit from on-demand therapy. | 49 | ||
| Anaphylaxis | Provider education on anaphylaxis diagnostic criteria should be delivered. | 98 | |
| Emergency department patients should be discharged with an EIA device. | 98 | ||
| Emerging therapies | Drug allergy | Telemedicine for penicillin delabeling (observational study). | 19 |
| Penicillin challenge without prior skin testing (observational study + clinical trial). | 20, 21 | ||
| Urticaria | IL-1 inhibitors (case reports). | 41 | |
| Anti-IgE (Ligelizumab) (clinical trials). | 41 | ||
| Syk inhibitors (clinical trials). | 41 | ||
| BTK inhibitors (clinical trials). | 41 | ||
| Hereditary angioedema | Oral kallikrein inhibitors for acute treatment and long-term prophylaxis (clinical trials). | 42, 70, 75–76 | |
| Recombinant C1INH for long-term prophylaxis and in pediatric patients (clinical trials). | 69 | ||
| Human monoclonal antibody against factor XII for long-term prophylaxis (clinical trials). | 72 | ||
| Anti-sense oligonucleotide to reduce the production of prekallikrein (clinical trials). | 73 | ||
| Gene therapy to correct C1-inhibitor deficiency (early development). | 74 | ||
| Oral bradykinin receptor antagonist (early development). | 76 | ||
| Anaphylaxis | Imatinib (mouse studies). | 88 | |
| Immune checkpoint modifiers (proposed). | 105 |
Successful de-labeling strategies also have been investigated in non-immediate β-lactam hypersensitivity. Eighteen children with a history of a MPE to a β-lactam confirmed with an initial drug provocation test (DPT) were rechallenged 3.5 years later (range, 1.5–7.5 years) with a 2-day protocol, with 16 out of the 18 patients (89%) being tolerant at follow up. A larger group of 122 children with a MPE after β-lactam use, but with a negative initial DPT, were followed with a survey instead of a diagnostic rechallenge. Only 3.3% of the 122 children who were reexposed to the incriminated β-lactam reported recurrence of a benign MPE. Based on these results, the authors calculated the negative predictive value of a 2-day DPT protocol to be 96.7%.18
Emerging Therapies
New approaches to drug allergy management in 2018 include the use of telemedicine as part of a penicillin de-labeling strategy (Table 1). Telemedicine resulted in high patient satisfaction marks and a savings of approximately $360 per patient.19 Another emerging theme is the use of an oral penicillin/amoxicillin challenge without preceding penicillin skin testing. This has been demonstrated to be safe and effective in low risk patients.20,21
Urticaria
Latest trends and health care impact
Research published in 2018 focused on chronic urticaria (CU, extending past 6 weeks in duration), and its subtypes - chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CindU), with less focus on acute urticaria. Using the National Health Insurance Service – National Sample Cohort data the estimated cumulative-incidence rate for any episode of urticaria in the general population was 4.9% over 10 years. In 10 years of follow up, 7.8% of those with urticaria developed CU. Of those that developed CU, 52.6% achieved remission at 1 year and 88.9% at 5 years.22 In patients attending an allergy clinic at an academic center, 13% of CU patients developed recurrent CU (return of CU ≥6 months after cessation of controller therapy). Patients who developed recurrent CU were more likely to require treatment other than anti-histamines.23 The overall large impact of CSU included high direct and indirect economic costs, decreased quality of life, greater absenteeism, and an effect on presenteesim.24
Advances in disease pathogenesis
In 2018, the importance of spleen tyrosine kinase (SYK) in the activation of human basophils and CSU were evaluated. Autoantibodies to the high affinity IgE receptor (anti-FᴄεRI) were shown to downregulate SYK expression in basophils (Figure I).25 As only 7% of CSU patients appear to possess anti-FᴄεRI antibodies, this latest research suggested that more mechanistic work needs to be conducted to explain the pathogenesis underlying CSU.25
Several studies examined new biomarkers and assessed the clinical utility of established biomarkers in CSU. These included IgE autoantibodies such as IgE-anti-IL-24.26 In a cross-sectional study, serum from patients with CSU and healthy controls was screened for > 9,000 candidate proteins on an array. Screened proteins were then further selected based on detection of IgE to autoantigen (31 proteins), presence in >70% CSU patients (200 proteins), and expression in the skin (8 proteins), leading to the determination that only IgE autoantibodies against IL-24 were expressed in the skin and found in serum from all CSU patients. The average serum level of IgE-anti-IL-24 was higher in CSU patients and more frequently elevated (>0.33 IU/mL, with cutoff calculated by receiver operating characteristic analyses with specificity and sensitivity 80% each) at 80% compared to 20% of control patients. In vitro studies demonstrated that IL-24 triggered histamine release from mast cells only in CSU patients. Subsequent analyses also showed that higher levels of IgE-anti-IL-24 antibody predicted the presence of CSU with a likelihood ratio of 3.9 and highly correlated with disease activity (measured by the Urticaria Activity Score (UAS) and UAS7). The authors therefore proposed that these properties make IgE-anti-IL-24 a candidate biomarker for both CSU diagnosis and disease activity.26 C-reactive protein (CRP) elevation, a well-established biomarker for systemic inflammation, has been evaluated retrospectively in patients with CSU. CRP was elevated in 31% of 1253 patients with CSU, and levels correlated with urticarial disease activity and impaired QOL. CRP levels were higher in those with CU refractory to antihistamines, suggesting that this may be a potentially useful biomarker to identify patients who will require therapy beyond first-line treatment.27 When initiating omalizumab, some patients achieve rapid clinical response (within 4 weeks) while others need more time to achieve clinical response.28 Data from a small study of 44 patients suggest patients who responded quickly may have higher baseline level of basophil FᴄεRI expression.28
Approach to diagnosis and management
Several tools have been utilized to assess clinical disease activity. International guidelines recommended assessing health-related quality of life using the validated chronic urticaria quality of life questionnaire (CU-Q2oL).29 In 2018, the 10-question Chronic Urticaria Patient Perspective (CUPP) became a newly validated tool to assess health-related QOL in CU patients. Changes in CUPP scores correlated with disease activity changes in (UAS)-Hive count, UAS-Itch severity, and UAS-Total score.30 Two versions of the often utilized UAS– the UAS7 (once daily) and UAS7TD (twice daily), were found to be comparable and highly correlated in 130 patients with CSU; although, the UAS7 questionnaire appeared to have greater ease of its use.31
Omalizumab was the most studied pharmacological treatment for CU in 2018, with publications focusing on applications to the real-word setting, extended dosing, application of personalized algorithms, predictions of disease recurrence, and efficacy in specific phenotypes of CU. A meta-analysis of 67 studies and over 1000 patients treated with omalizumab reported a complete response rate of 72.2% and a partial response rate of 17.8%, confirming that outside of clinical trials omalizumab appears highly effective in treating CSU.32 Due to continued use of omalizumab for refractory CSU, the XTEND-CIU study, a phase IV, multi-center, randomized, double-blind, placebo-controlled trial, evaluated the efficacy of omalizumab after 24 weeks of therapy, 48 weeks of therapy, and the clinical course after discontinuation. Patients tolerated extended omalizumab therapy well; those treated with omalizumab experienced better control of CSU and were less likely to relapse during treatment as compared to those treated with placebo. Relapse rates after discontinuation of omalizumab were the same for those treated for 24 weeks vs. 48 weeks (Table I).33
New research assessed more personalized therapy including alternative dosing regimens of omalizumab (e.g. up-dosing, tapering and spacing of frequency). An algorithm was evaluated in 63 CSU patients that allowed for dose increase (from 400 mg to 450 mg) followed by an increased in frequency of omalizumab for patients who had not achieved complete response. With these adjustments, the overall response rate was 77.8%. After successful therapy, 38% of the patients tolerated extended intervals of 8 weeks between doses followed by eventual discontinuation of the drug.34 This study provided evidence that flexibility in dosing and frequency may be beneficial in achieving disease control as well as in discontinuing therapy.
After successful treatment and then discontinuation of omalizumab urticaria often recurs, either rapidly or slowly. Factors that predict recurrences were not well-understood. Using data from prior Phase III studies of omalizumab, investigators associated high baseline UAS7 and low early area above the curve with a higher probability of rapid recurrence.35
Until 2018, less was known about the efficacy of omalizumab in other phenotypes of CU including CindU. In a retrospective review of 16 patients with cholinergic urticaria treated with omalizumab, 37% had complete response and 31% had a major response. In 4 of the 6 patients who initially had no response or partial response, improved control was achieved with increasing the dose of omalizumab. This small study suggested that omalizumab may have similar efficacy in cholinergic urticaria.36 Similarly, two systematic reviews focusing on CindU found that while there is little evidence for most types of CindU, the evidence for using omalizumab is strongest for symptomatic dermographism, cold urticaria and solar urticaria.29,37
A meta-analysis of 18 studies investigated the efficacy and safety of cyclosporine in treatment of CSU refractory to anti-histamines. Though limited by quality and quantity of prior studies, the authors reported response rates for cyclosporine of up to 73% with moderate dose therapy (4–5 mg/kg/day) for 12 weeks. Adverse event rates occurred in 23% in the low dose group (2 to <4 mg/kg/day) and 57% in the moderate dose group. The number of adverse events increased with increasing dosage; however, rates of major adverse events (hypertension and elevated creatinine) did not significantly differ between the very low-dose group (6%) and the moderate dose group (10%).38
The step-wise treatment of CSU has been described by multiple evidence-based guidelines globally.39 In 2018, a multi-center, triple-blind, prospective randomized clinical trial of 150 patients with CSU compared standard dose daily anti-histamine or guideline-based step-up therapy as needed (e.g. 4x anti-histamine dose, omalizumab or cyclosporine). Clinical response was defined by Dermatology Quality of Life Index (DQLI) ≤5. With first-line therapy of daily oral anti-histamines, 59% achieved control. With second-line therapy of high dose oral anti-histamines (up to 4 times the daily dose), an additional 78% of those without initial response achieved control. The addition of third-line therapy (omalizumab or cyclosporine) achieved control in an additional 15% of those who did not respond to previous therapies.40
Emerging therapies
Potential therapies currently under investigation or on the clinical pathway for investigation include multiple biologics. Though no clinical trials were published in 2018, the current pipeline was reviewed in depth.41 Currently available biologics such as TNF-α antagonists and anti-CD20 therapies have been described in case reports to treat CU with some success, but more research is needed.41 Potential therapeutics currently under investigation include IL-1 inhibitors (anakinra and canakinumab), SYK inhibitors, prostaglandin D2 receptor antagonist and Bruton tyrosine kinase inhibitor (Table I).41
Angioedema
Urticaria is well-understood to be mast cell mediated while angioedema can be mast cell or bradykinin-mediated. In this section, we focus specifically on a bradykinin-mediated form of angioedema, hereditary angioedema (HAE). New research published in 2018 featured a better understanding of the disease pathogenesis and burden of disease, a potential new QOL tool, and a new FDA approved therapy for long-term prophylaxis, lanadelumab.
Latest trends and health care impact
HAE is a very rare autosomal dominant genetic disease with an estimated prevalence of 1:50,000 to 1:100,000 globally,42,43 although this may underrepresent the actual prevalence. Publications from 2018 showed that HAE remains underdiagnosed, with use of inappropriate treatments, multiple physician visits, and even unnecessary surgery between initial onset of symptoms and diagnosis.44,45 Delays in diagnosis have improved, but the lag for some patients is still measured in years from initial onset of symptoms to diagnosis.46
Socioeconomic cost at the level of an individual patient with HAE is high. A French study showed an annual cost of $11,371 ± $19,275, mostly driven by drug costs.47 A US study estimated total annual costs per patient accounting for inflation, in 2017, would be more than $65,000.48 The cost of care for an HAE patient in the US study was driven more by emergency department visits and hospitalization rather than cost of drugs alone. Differences between these two studies highlight the importance of having each patient with HAE followed by an expert that understands evidence-based published guidelines, all available treatment options, and can optimize management and minimize the need for emergency care.49 In addition to the direct cost of medical care, indirect costs such as time missed from school, decreased productivity at work and loss of opportunities must be considered. Patients with HAE face an average of 20 missed days from school or work per year and have poorer health QOL compared to the normal population.42 However, availability of and access to novel therapies is providing clinically meaningful improvements in QOL including decreased anxiety and increased work productivity.50–52
Understanding QOL with HAE specific tools would assist providers immensely in assessing the burden of disease in patients with HAE. An HAEA-QoL tool has been developed to measure objectively the impact of HAE on health-related QoL.53 Additional validation studies and assessment of reliability are ongoing to refine the instrument, but this tool should offer an innovative way to improve patient care.
Advances in disease pathogenesis
The defect in HAE is a deficiency or dysfunction of C1 inhibitor protein (C1-INH). C1-INH inhibits the action of kallikrein, which catalyzes the conversion of high molecular weight kininogen (HMWK) to bradykinin. Without C1-INH, the formation of bradykinin occurs freely in the presence of uninhibited kallikrein activity. Bradykinin then binds to its B2 receptors on endothelial cells, which leads to vasodilation and increased vascular permeability (Figure II).54
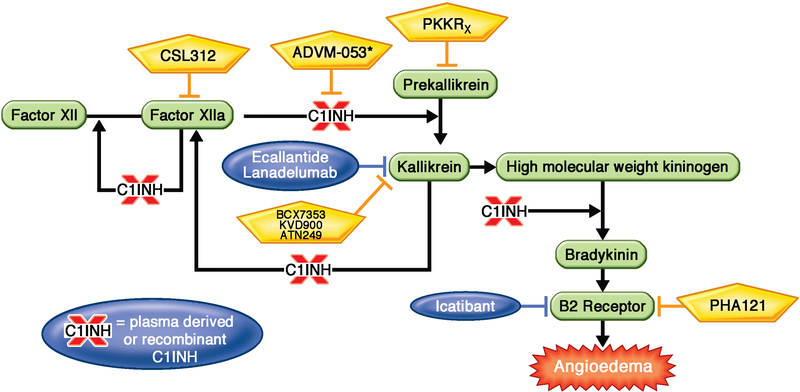
FIGURE II. Key advances in treatment options for patients with HAE.
C21 inhibitor protein inhibits the kallikrein-kininogen pathway in multiple places as shown leading to decreased generation of bradykinin. However, patients with HAE are missing or have dysfunctional C1 inhibitor protein. FDA approved therapies for patients with HAE are shown in blue. Investigational therapies for patients with HAE are shown in yellow. Each of these therapies leads to decreased bradykinin generation by impacting the kallikrein-kininogen pathway as shown. Investigational therapies include CSL312, which is a recombinant fully human monoclonal antibody that blocks Factor XIIa, ADVM-53, which is a gene therapy aimed at correcting the C1-inhibitor deficiency, BCX7353, KVD900 and ATN249 which are oral kallikrein inhibitors, PKKRx which is an anti-sense oligonucleotide to reduce the production of prekallikrein, and PHA121 which is an oral bradykinin receptor antagonist.
Abbreviations: HAE: hereditary angioedema; FDA: food and drug administration; PKK: prekallikrein
Two distinctive types of the disease have been identified: type I HAE, that affects approximately 85% of patients and is characterized by subnormal plasma levels of C1-INH protein (5%–30% of normal), and type II HAE, that affects 15% of patients and is characterized by normal or elevated levels, but functionally inactive, C1-INH. New data in 2018 showed that HAE with normal C1-INH can be categorized into at least four distinct types: HAE with known factor XII mutation (HAE-FXII), HAE with a mutation in the angiopoietin 1 gene (HAE-ANGPT), HAE with a mutation in the plasminogen gene (HAE-PLG) and HAE with unknown mutation (HAE-unknown) that likely includes mutations that have yet to be identified.54–58 Targeted next generation sequencing is looking promising as a novel platform for confirmatory genotyping within HAE with C1-INH deficiency.59
Biomarkers are still needed in HAE. Proteins under evaluation in patients with HAE include factor XII, plasma prekallikrein/kallikrein, HMWK and bradykinin, but each of these has advantages and limitations so further study is needed.60 Early promising data demonstrated that the stimulated plasma kallikrein activity assay could be a valuable tool,61 as activity levels were increased in both HAE with normal labs and idiopathic non-histaminergic angioedema subjects compared to non-swelling controls and histaminergic angioedema subjects.61
Approach to diagnosis and management
Laboratory tests necessary for the diagnosis of HAE are a serum C4 level, quantitative and functional C-INH. These are simple and inexpensive lab tests that can diagnose patients with HAE type 1 and 2. However, even in 2018, diagnostics for the evaluation of patients with recurrent angioedema and normal standard laboratory test results are still lacking.
In the last decade, the management of HAE has improved substantially as evidenced by the distribution of approximately 30 consensus/guidelines worldwide.62 Updated recommendations (Table I) include:
All patients should have at least two doses of on demand medication.
All HAE patients should have an HAE action plan.
All HAE attacks should be considered for on demand treatment.
HAE attacks should be treated as soon as possible.
Long-term prophylactic treatment is appropriate for patients with HAE who do not achieve adequate benefit from on-demand therapy.
Guidelines published in 2018 continue to focus on individualized care.49 This individualized care was recommended to be based on patient preference, burden of treatment including the need for intravenous access, distance to local emergency department and side effects.49 HAE management remained focused on treating HAE attacks as quickly as possible after onset of swelling (on demand treatment), long-term prophylaxis to decrease the overall number and severity of angioedema attacks, and short-term prophylaxis to decrease the likelihood of an attack secondary to a known trigger (e.g., medical or dental procedures).
In the past decade, a plasma-derived C1-INH concentrate (Berinert), a kallikrein inhibitor (Kalbitor), a bradykinin B2 receptor antagonist (Icatibant), and a recombinant C1-INH (Ruconest) were FDA approved and shown to be highly effective for on-demand treatment of HAE attacks (Figure II).63–66 Prior to 2018, options for long-term prophylaxis included an intravenous formulation of plasma derived C1-INH concentrate (Cinryze) and a subcutaneous formulation (HAEGARDA) that is safe and well-tolerated without dose-dependent safety concerns.67 Anti-fibrinolytics and attenuated androgens are orally available agents for long-term prophylaxis in HAE but are associated with lower efficacy or adverse effects, respectively.49
The newest therapy approved in 2018 for long-term prophylaxis for patients with HAE was lanadelumab, a monoclonal antibody targeting active plasma kallikrein.68 In the pivotal phase III trial, 125 patients were randomized and treated with subcutaneous lanadelumab for 26 weeks and showed a reduction in the mean attack rate (0.26–0.53 attacks/month) compared with placebo (1.97 attacks/month). Patients treatment with lanadelumab also demonstrated a clinically meaningful and statistically significant improvement in QOL compared to placebo. Lanadelumab is approved in patients 12 years and older at a dose of 300 mg subcutaneously once every two weeks that can be extended to once every four weeks.68
Emerging therapies
There are several novel therapies under investigation for the management of patients with HAE, although few clinical trials were published in 2018. Ongoing studies include recombinant C1-INH being evaluated both for long-term prophylaxis in HAE and in children with HAE.69 BCX7353 is a second-generation oral kallikrein inhibitor that is administered once-daily showing promise for acute treatment70 and long-term prophylaxis (Table I).71 Additional medicines in earlier phase trials are a human monoclonal antibody against factor XII (CSL312),72 anti-sense oligonucleotide to reduce the production of prekallikrein (IONIS-PKKRX),73 and gene therapy to correct C1-INH deficiency.74 In very early development are additional oral kallikrein inhibitors (KVD900 and ATN249) and an oral bradykinin receptor antagonist (PHA121).75,76 These investigational therapies have the potential to make a significant impact on the management of patients with HAE.
Anaphylaxis
Latest trends and health care impact
In 2018, retrospective chart reviews, cross-sectional, case-control and population studies, and meta-analyses that published informative data on the prevalence of anaphylaxis were reviewed.77 The latest population studies were published from national health insurance databases in Taiwan and Korea, respectively. These demonstrated that the overall incidence of anaphylaxis increased from 4.79 per 100,000 person-years in 2001 to 8.20 in 2013 in Taiwan,78 and the overall prevalence of anaphylaxis increased 1.7-fold from 2010 to 2014 in Korea.79 The severity of anaphylaxis also may be worsening as observed in a U.S. database of anaphylaxis-related emergency department visits from 2005–2014. This study documented that the proportion of patients admitted to the hospital increased by 37.6% (from 13.2% in 2005 to 18.2% in 2014) and to the intensive care unit increased by 27.4% (from 4.5% in 2005 to 5.8% in 2014). The proportion of endotracheal intubations (from 0.8% in 2005 to 1.9% in 2014) also doubled.80
The continued substantial health care impacts of anaphylaxis became very apparent in recent publications that focused on specific triggers. Trends published after the end of 2018 from EHRs covering two large U.S. tertiary care hospitals from 1995–2013 reported a 1.1% population prevalence of drug-induced anaphylaxis.81 Although, it should be noted that 90% of the EHR anaphylaxis reports were unverified without tryptase and allergy encounters available in the health care system, suggesting that many cases were misreported. U.S. administrative claims from 2005 through 2014 measured an increase in emergency department visits for food-induced anaphylaxis by 214%, from 6.40 per 100 000 enrolled children in 2005 to 20.05 in 2014. Peanuts accounted for the highest rates, followed by tree nuts and seeds.80 The SchoolNuts study in Melbourne, Australia conducted among food allergic adolescents, reported frequent reactions (44.4%) and anaphylaxis (9.7%) to foods, with peanut and tree nuts being the most common food triggers. Adolescents with asthma and those with more than two food allergies were at the highest risk.82
A prediction model for mast cell clonality, modified from the Spanish Network on Mastocytosis score by lowering the serum tryptase limits and adding allele-specific quantitative PCR, yielded a sensitivity and specificity of 75% and 100%, respectively.83 Bovine and pork gelatin were implicated in anaphylaxis to zoster vaccine following tick bite instead of alpha-gal in one 2018 letter to the editor.84
Advances in disease pathogenesis
Mechanisms reported in 2018 featured new regulators and recognition of the importance of extracellular vesicles (Figure I). Within mast cells, the transcription factor GATA 2 induced the expression of microphthalmia-associated transcription factor (MITF) that bound to the enhancer of the histidine decarboxylase gene to restore its expression and ability to catalyze the production of histamine. Mice deficient in connective tissue mast cell specific GATA 2 were protected against anaphylaxis unless MITF was overexpressed.85 Mice deficient in corticotropin releasing factor (CRF) 2 produced over 4-fold higher histamine levels, and exhibited signs of anaphylaxis including greater colonic permeability following both passive systemic anaphylaxis and sensitization and challenge with IgE monoclonal anti-dinitrophenyl (DNP) compared with wildtype mice. Additional experiments showed that CRF2 suppressed store-operated Ca2+ entry (SOCE) signaling and thereby mast cell degranulation.86 A defect in syntaxin 3 (a soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) protein) on the mast cell plasma membrane markedly impaired exocytosis and therefore mast cell degranulation in a mouse model of passive systemic anaphylaxis.87
In studies of the mechanisms that underlie the pulmonary venous vasodilatation and fluid extravasation of anaphylactic shock, mouse models of passive and active oral antigen and IgE-induced anaphylaxis were dependent on IL-4’s interaction with the vascular endothelial (VE) specific IL-4 receptor and abelson murine leukemia viral oncogene homology (ABL1) kinase activity.88 Mice deficient in the Clec7a gene that encodes dectin-1, the pattern-recognition receptor that is expressed on epithelial and other cells and believed to be important in mounting innate and TH2/Th17-mediated immune responses, demonstrated the importance of ligand binding of invertebrate tropomyosin to dectin-1 to protect against the release of IL-33 and the recruitment of IL-13 producing innate lymphoid cells. In a model of shrimp-induced anaphylaxis, mice deficient in the Clec7a gene displayed signs of anaphylactic shock when compared to littermate controls.89
Mast cells also were found to secrete exosomes (EXs) that transfer FcεRI and bind to free IgE, thereby decreasing IgE levels and degranulation through the phospholipase Cγ1-protein kinase C pathway.90 Microvesicles (MVs) derived from activated T cells carried miRNA-4443 and thereby regulated extracellular signal-regulated kinase phosphorylation, IL-8 release, and mast cell degranulation.91 Dendritic cells were shown to capture and relay blood-borne allergens via surfaces of budding MVs from the plasma membrane to neighboring mast cells and dendritic cells in the perivascular space, leading to rapid mast cell degranulation. This was dependent on the adenosine triphosphatase activity of vacuolar protein sorting-associated protein 4.92
Advances also were reported in our understanding of alternative pathways of anaphylaxis that depend on IgG-mediated activation through Fcγ receptors. This included further determination of the importance of the inhibitory receptor FcγRIIB on modulating the suppression of anaphylaxis in mice. Cross-linking of FcγRIIB to stimulatory Fcγ receptors through the Fc domain of an anti-FcγRIIB antibody mildly induced and then inhibited active and passive IgG (but not IgE)-mediated anaphylaxis.93 In addition, platelets were implicated as critical players in IgG-dependent anaphylaxis. In mouse models that utilized platelets that expressed human FcγRIIA, and no other Fcγ receptors, the human FcγRIIA-expressing platelets were directly activated by aggregated human IgG in vitro and in vivo and were sufficient to induce anaphylaxis. The serotonin they released drove the severity of the reaction (i.e. hypothermia). Platelet activation and reductions in circulating platelets in a cohort of 67 (vs case controls) humans during neuromuscular blocking agent (NMBA)-induced active anaphylaxis, but not its recovery, corroborated the critical role of platelets in IgG dependent anaphylaxis.94
New approaches to diagnosis and management
A new mast cell activation test demonstrated utility in experiments involving primary human blood-derived mast cells generated from CD117+ peripheral blood precursors. These were passively sensitized with patients’ sera and then incubated in vitro with allergen (i.e. peanut, grass, or wasp). Dose-dependent increases in the expression of surface activation markers of CD63 and CD107a and release of β-hexosaminidase and prostaglandin D2 (PGD2) among patients with anaphylaxis (vs controls) were observed. Diagnostic cutoffs with high levels of discrimination were validated and found to be more reproducible compared to the BATs and component resolved diagnostics.95
Different machine learning (ML) techniques for processing data from EHRs in predicting anaphylaxis were explored. These included classical ML algorithms (i.e. efficient classifiers in text classifications) and a Convolutional Neural Network (CNN), which is characterized by deep learning and nonreliance on hand-engineered features. Analyses showed that most classifiers and representations were predictive, although CNN performed slightly better.96 Natural language processing (NLP) algorithms that classify anaphylaxis cases from the free text embedded within EHRs were tested as well. Although, this approach did not demonstrate superiority over human review, it does suggest that applying NLP to unstructured narrative information from medical charts for smarter clinical decision-making warrants more research in the future.97
Advances in the management of anaphylaxis in 2018 focused on the application of clinical pathways and describing the associated barriers with using an epinephrine autoinjector (EAI) (Table 1). For example, application of a clinical pathway in the pediatric emergency department featuring provider education on anaphylaxis diagnostic criteria and discharging patients with the EAI device achieved its goal of reducing length of observation and admission rate without adverse outcomes.98 One survey of barriers for not filling EAI prescriptions cited cost, absence of previous reactions or documented allergy, and for not using the EAI if it was owned, cited unavailability of EAI.99 A population-based survey of food allergic adolescents demonstrated high levels of EAI carriage at school, but not at other domains of their lives.100 Poor adherence to possessing more than one EAI was documented among adult patients and caregivers from a U.S. survey conducted by a third party.101 Additional barriers associated with following guidelines included the absence of full-time nurses on staff at school,102 insufficient rates of EAI prescriptions,103 and nonadherence with visits to an allergists upon hospital discharge.103,104
Emerging therapies
2018 introduced potentially new therapies for the treatment of anaphylaxis. Administration of the ABL kinase inhibitor imatinib prior to the final oral antigen challenge protected mice from further progression of anaphylactic symptoms including shock (Table I).88 Moreover, recent experiments on human basophils derived from different types of allergy patients demonstrated that FCεRI and FCγRIIB expression levels and responses to regulatory signals did not vary by disease, suggestive of fully functional regulatory mechanisms without intrinsic defects. These findings open up possibilities for clinical trials of new immune modulators or immune checkpoint modifiers against allergic reactions.105
Research gaps and future directions for all four disorders
Despite the many advances in 2018, our understanding of these four disorders remains insufficient. The knowledge gaps in health care impacts still appear wide. These challenges particularly pertain to our understanding of the contribution of non-β-lactam antibiotics to the burden of drug allergy, the impact of acute urticaria, the delays in diagnosis for patients with HAE, and the importance of all these diseases on QOL for patients. We are slowly advancing in the understanding of mechanisms that underlie T cell mediated hypersensitivity, as well as those responsible for severe drug reactions (SJS, TEN, DRESS), non-IgE-mediated anaphylaxis, and HAE with normal labs and their underlying genetic predispositions. However, much more research is needed. A theme that appears through 2018 is that the characterization of risk factors and the implementation of molecular diagnostics for better assessment of disease activity for all four disorders, particularly across diverse patient profiles and phenotypes, remains inadequately developed.
Our diagnostic tools remain limited with minimal data validating skin testing to drugs beyond penicillin. The lack of specific biomarkers makes it difficult to distinguish mast cell-mediated urticaria from other forms of urticaria, especially when there is no response to the typical treatment options (i.e., anti-histamines) or a need to differentiate bradykinin-mediated swelling from histamine-mediated swelling in a patient with normal labs but recurrent swelling without prominent symptoms of urticaria or pruritus.
Future directions hopefully will focus on more efficient and accessible clinical pathways towards de-labeling drug allergy, expansion of research into the application of antibiotic stewardship programs and developing new approaches to identifying those at high risk for anaphylaxis and intervening against this risk. For urticaria, an increased focus on underlying disease mechanisms, biomarkers and novel therapies for chronic urticaria and a renewed interest in acute urticaria is needed. For patients with HAE, a final version of the HAEA-QoL will be valuable for patient management as well understanding individual non-response to specific therapies.
Conclusions
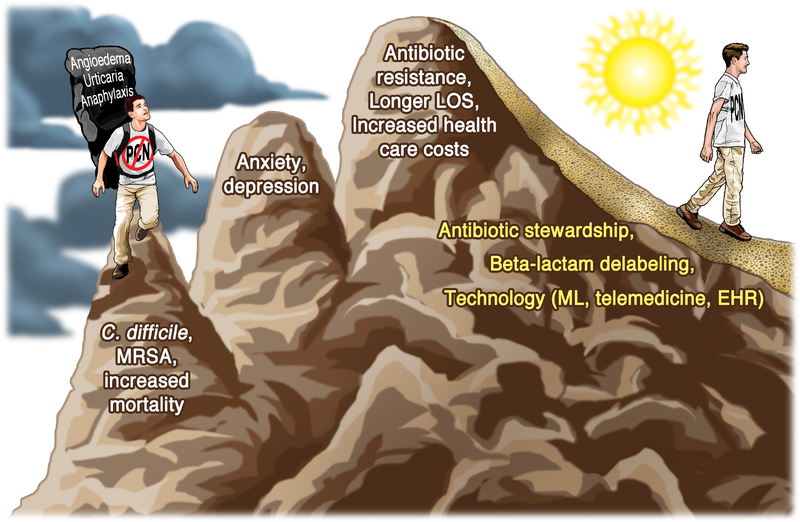
Overall, great progress has been made in the understanding of the health care impacts, diagnostic and therapeutic algorithms, and development of novel therapies for drug allergy, urticaria, angioedema and anaphylaxis. Not only have we identified the burden of these diseases, and of incorrect labeling of these diseases, for both the individual patient ( i.e. C. difficile and MRSA infections, higher mortality) and society (i.e. longer LOS, increased health care costs), but advances in 2018 have pointed to several new paths for better health (Figure III). These feature the application of antibiotic stewardship programs, more accurate risk stratification, and implementation of new technologies and therapies, all leading to better patient care.
FIGURE III. The journey towards overcoming current research gaps to smoother paths and better patient care.
The patient hiking up the mountain on the left is labeled as penicillin-allergic, carrying the heavy burden of real or perceived increased risk for urticaria, mast cell-mediated angioedema, or anaphylaxis. He (and society, represented in the third peak) encounters multiple tough challenges and medical complications ahead as a result of this label. As research advances and our understanding of these disorders deepens, patients (depicted as hiker on the right) should find that their descent follows smoother paths and offers better patient care.
Abbreviations: EHR: electronic health record; LOS: length of stay; ML: machine learning; MRSA: methicillin resistant Staphylococcal aureus; PCN: penicillin
Synopsis.
Many notable advances in drug allergy, urticaria, angioedema and anaphylaxis were reported in 2018. Broad spectrum antibiotic use and consequently antibiotic resistance is widespread, and algorithms to clarify β-lactam allergy and optimize antibiotic use were described. Meaningful data emerged on the pathogenesis of delayed drug hypersensitivity reactions. Progress not only in defining biomarkers, but also in understanding the impact on quality of life and developing better treatments have been made for individuals with chronic idiopathic urticaria. Patients with hereditary angioedema have gained additional access to highly efficacious therapies with associated improvements in quality of life, and some progress was made in our understanding of recurrent angioedema in patients with normal labs. Guidelines have defined clear goals to help providers optimize therapies in patients with hereditary angioedema. The epidemiology and triggers of anaphylaxis, and the mechanisms underlying anaphylaxis, were elucidated further. In summary, these disorders (and labels) cause substantial burdens for individuals and even society. Fortunately, publications in 2018 have informed on advancements in diagnosis and management and have provided better understanding of mechanisms that potentially could yield new therapies. This progress should lead to better health outcomes and paths forward in drug allergy, urticaria, hereditary angioedema and anaphylaxis.
This article will highlight many notable research advances in drug allergy, urticaria, angioedema and anaphylaxis that were reported in the Journal of Allergy and Clinical Immunology and elsewhere in 2018. Studies cited demonstrate that these disorders (and labels) cause substantial burdens for individuals and even society. Fortunately, recent developments have updated our understanding of the diagnosis and management of these disorders, and have provided more insights on mechanisms that potentially could yield novel treatments.
Abbreviations
- BAT
basophil activation test
- C1INH
C1 inhibitor protein
- CDS
clinical decision support
- C. difficile
Clostridioides difficile
- CRF
corticotropin releasing factor
- CRP
C-reactive protein
- CU
chronic urticaria
- CUPP
chronic urticaria patient perspective
- CU-Q2oL
chronic urticaria quality of life questionnaire
- CSU
chronic spontaneous urticaria
- CindU
chronic inducible urticaria
- DNP
dinitrophenyl
- DPT
drug provocation test
- DQLI
dermatology quality of life index
- EAI
epinephrine autoinjector
- EHR
electronic health record
- EX
exosome
- FcγR
Fc gamma receptor
- FcεR1
high affinity receptor for the Fc region of immunoglobulin E
- HAE
hereditary angioedema
- HAE-FXII
HAE with known F12 mutation
- HAE-ANGPT
HAE with a mutation in the angiopoietin 1 gene
- HAE-PLG
HAE with a mutation in the plasminogen gene
- HAE-unknown
HAE with an unknown mutation
- Hdc
histidine decarboxylase
- HMWK
high-molecular-weight kininogen
- HSRs
hypersensitivity reactions
- IL4Rα
receptor for interleukin 4
- LKB1/AMPK
liver kinase B1/adenosine monophosphate-activated protein kinase
- LOS
length of stay
- MDAS
multiple drug allergy syndrome
- MDIS
multiple drug intolerance syndrome
- MiRNA
microRNA
- MITF
microphthalmia-associated transcription factor
- ML
machine learning
- MPE
maculopapular exanthem
- MPV
mean platelet volume
- MRSA
methicillin-resistant Staphylococcus aureus
- MV
microvesicle
- NMBA
neuromuscular blocking agent
- PBMC
peripheral blood mononuclear cells
- PGD2
prostaglandin D2
- PLT
platelets
- QOL
quality of life
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment receptor
- SOCE
store-operated Ca2+ entry
- SJS
Stevens-Johnson syndrome
- SSI
surgical site infection
- SYK
spleen tyrosine kinase
- STX
syntaxin
- TEN
toxic epidermal necrolysis
- Th
T helper
- UAS
urticaria activity score
- VE
vascular endothelial
Footnotes
Conflict of interest statements:
Rachel L. Miller: Consultant: UpTo Date; AstraZeneca; Food Allergy Research Education (FARE): Advisory Board: Food Allergy Fund
Maria Shtessel: No disclosures.
Lacey B. Robinson: No disclosures.
Aleena Banerji: Advisory Board: Shire (Takeda), CSL Behring, Biocryst, Pharming, Kalvista; Research: Shire (Takeda), Biocryst
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra SA, Levin H, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A 2018; 115(15): E3463–E70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong A, Seger DL, Lai KH, Goss FR, Blumenthal KG, Zhou L. Drug hypersensitivity reactions documented in electronic health records within a large health system. The Journal of Allergy and Clinical Immunology In Practice 2019; 7(4): 1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss FR, Lai KH, Topaz M, Acker W, Kowalski L, Plasek JM, et al. A value set for documenting adverse reactions in electronic health records. J Am Med Inform Assoc 2018; 25(6): 661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumenthal KG, Li Y, Acker WW, Chang Y, Banerji A, Ghaznavi S, et al. Multiple drug intolerance syndrome and multiple drug allergy syndrome: Epidemiology and associations with anxiety and depression. Allergy 2018; 73(10): 2012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas M, Arnold A, Sommerfield A, Trevenen M, Braconnier L, Schilling A, et al. Antibiotic allergy labels in children are associated with adverse clinical outcomes. The Journal of Allergy and Clinical Immunology In Practice 2018; 7(3): 975–82. [DOI] [PubMed] [Google Scholar]
- 6.Sousa-Pinto B, Araujo L, Freitas A, Delgado L. Hospitalizations in children with a penicillin allergy label: An assessment of healthcare impact. Int Arch Allergy Immunol 2018; 176(3–4): 234–8. [DOI] [PubMed] [Google Scholar]
- 7.Huang KG, Cluzet V, Hamilton K, Fadugba O. The impact of reported beta-lactam allergy in hospitalized patients with hematologic malignancies requiring antibiotics. Clin Infect Dis 2018; 67(1): 27–33. [DOI] [PubMed] [Google Scholar]
- 8.Mattingly TJ 2nd, Fulton A, Lumish RA, Williams MC, Yoon S, Yuen M, et al. The cost of self-reported penicillin allergy: A systematic review. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(5): 1649–54 e4. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK. Risk of methicillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: Population based matched cohort study. BMJ 2018; 361: k2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis 2018; 66(3): 329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan A, Wang E, Farrell J, Whitaker P, Faulkner L, Peckham D, et al. Beta-lactam hypersensitivity involves expansion of circulating and skin-resident TH22 cells. The Journal of Allergy and Clinical Immunology 2018; 141(1): 235–49 e8. [DOI] [PubMed] [Google Scholar]
- 12.Monroy-Arreola A, Duran-Figueroa NV, Mendez-Flores S, Dominguez-Cherit J, Watkinson J, Badillo-Corona JA, et al. Up-regulation of T-cell activation microRNAs in drug-specific CD4(+) T-Cells from hypersensitive patients. Chem Res Toxicol 2018; 31(6): 454–61. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 2010; 33(4): 607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salas M, Fernandez-Santamaria R, Mayorga C, Barrionuevo E, Ariza A, Posadas T, et al. Use of the basophil activation test may reduce the need for drug provocation in amoxicillin-clavulanic allergy. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(3): 1010–8 e2. [DOI] [PubMed] [Google Scholar]
- 15.Laguna JJ, Bogas G, Salas M, Mayorga C, Dionicio J, Gonzalez-Mendiola R, et al. The basophil activation test can be of value for diagnosing immediate allergic reactions to omeprazole. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(5): 1628–36 e2. [DOI] [PubMed] [Google Scholar]
- 16.Chen JR, Tarver SA, Alvarez KS, Wei W, Khan DA. Improving aztreonam stewardship and cost through a penicillin allergy testing clinical guideline. Open Forum Infect Dis 2018; 5(6): ofy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumenthal KG, Li Y, Banerji A, Yun BJ, Long AA, Walensky RP. The cost of penicillin allergy evaluation. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(3): 1019–27 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonson la Tour A, Michelet M, Eigenmann PA, Caubet JC. Natural history of benign nonimmediate allergy to beta-lactams in children: A prospective study in retreated patients after a positive and a negative provocation test. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(4): 1321–6. [DOI] [PubMed] [Google Scholar]
- 19.Staicu ML, Holly AM, Conn KM, Ramsey A. The use of telemedicine for penicillin allergy skin testing. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(6): 2033–40. [DOI] [PubMed] [Google Scholar]
- 20.Trubiano JA, Smibert O, Douglas A, Devchand M, Lambros B, Holmes NE, et al. The safety and efficacy of an oral penicillin challenge program in cancer patients: A multicenter pilot study. Open Forum Infect Dis 2018; 5(12): ofy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iammatteo M, Alvarez Arango S, Ferastraoaru D, Akbar N, Lee AY, Cohen HW, et al. Safety and outcomes of oral graded challenges to amoxicillin without prior skin testing. The Journal of Allergy and Clinical Immunology In Practice 2019; 7(1): 236–43. [DOI] [PubMed] [Google Scholar]
- 22.Eun SJ, Lee JY, Kim DY, Yoon HS. Natural course of new-onset urticaria: results of a 10-year follow-up, nationwide, population-based study. Allergology International 2019; 68(1): 52–8. [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Har D, Brown LS, Khan DA. Recurrence of chronic urticaria: incidence and associated factors. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(2): 582–5. [DOI] [PubMed] [Google Scholar]
- 24.Lacour JP, Khemis A, Giordano-Labadie F, Martin L, Staumont-Salle D, Hacard F et al. The burden of chronic spontaneous urticaria: unsatisfactory treatment and healthcare resource utilization in France (the ASSURE-CSU study). European Journal of Dermatology: 2018; 28(6): 795–802. [DOI] [PubMed] [Google Scholar]
- 25.MacGlashan D Auto-antibodies to IgE and FCeRI and the natural variability of SYK expression in basophils. The Journal of Allergy and Clinical Immunology 2019; 143(3): 1100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK, et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. The Journal of Allergy and Clinical Immunology 2018; 142(3): 876–82. [DOI] [PubMed] [Google Scholar]
- 27.Kolkhir P, Altrichter S, Hawro T, Maurer M. C-reactive protein is linked to disease activity, impact, and response to treatment in patients with chronic spontaneous urticaria. Allergy 2018; 73(4): 940–8. [DOI] [PubMed] [Google Scholar]
- 28.Deza G, Bertolin-Colilla M, Sanchez S, Casale TB. Basophil FCRepsilonRI expression is linked to time to omalizumab response in chronic spontaneous urticaria. J Allergy Clin Immunol 2018; 141(6): 2313–6.e1. [DOI] [PubMed] [Google Scholar]
- 29.Dressler C, Werner RN, Eisert L, Zuberbier T, Nast A, Maurer M. Chronic inducible urticaria: a systematic review of treatment options. The Journal of Allergy and Clinical Immunology 2018; 141(5): 1726–34. [DOI] [PubMed] [Google Scholar]
- 30.Baiardini I, Braido F, Molinengo G, Caminati M, Costantino M, Cristaudo A, et al. Chronic urticaria patient perspective (CUPP): The first validated tool for assessing quality of life in clinical practice. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(1): 208–18. [DOI] [PubMed] [Google Scholar]
- 31.Hawro T, Ohanyan T, Schoepke N, Metz M, Peveling-Oberhag A, Staubach P, et al. Comparison and interpretability of the available urticaria activity scores. Allergy 2018; 73(1): 251–5. [DOI] [PubMed] [Google Scholar]
- 32.Tharp MD, Bernstein JA, Kavati A, Ortiz B, MacDonald K, Denhaerynck K, et al. Benefits and harms of omalizumab treatment in adolescent and adult patients with chronic idiopathic (spontaneous) urticaria: a meta-analysis of “real-world” evidence. JAMA Dermatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurer M, Kaplan A, Rosen K, Holden M, Iqbal A, Trzaskoma BL, et al. The XTEND-CIU study: Long-term use of omalizumab in chronic idiopathic urticaria. The Journal of Allergy and Clinical Immunology 2018; 141(3): 1138–9.e7. [DOI] [PubMed] [Google Scholar]
- 34.Niemeyer-van der Kolk T, van Maaren MS, van Doorn MBA. Personalized omalizumab treatment improves clinical benefit in patients with chronic spontaneous urticaria. The Journal of Allergy and Clinical Immunology 2018; 142(6): 1992–4. [DOI] [PubMed] [Google Scholar]
- 35.Ferrer M, Gimenez-Arnau A, Saldana D, Janssens N, Balp MM, Khalil S, et al. Predicting chronic spontaneous urticaria symptom return after omalizumab treatment discontinuation: exploratory analysis. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(4): 1191–7.e5. [DOI] [PubMed] [Google Scholar]
- 36.Altrichter S, Chuamanochan M, Knoth H, Asady A, Ohanyan T, Metz M, et al. Real-life treatment of cholinergic urticaria with omalizumab. The Journal of Allergy and Clinical Immunology 2019; 143(2): 788–91.e8. [DOI] [PubMed] [Google Scholar]
- 37.Maurer M, Metz M, Brehler R, Hillen U, Jakob T, Mahler V, et al. Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. The Journal of Allergy and Clinical Immunology 2018; 141(2): 638–49. [DOI] [PubMed] [Google Scholar]
- 38.Kulthanan K, Chaweekulrat P, Komoltri C, Hunnangkul S, Tuchinda P, Chularojanamontri L, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(2): 586–99. [DOI] [PubMed] [Google Scholar]
- 39.Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018; 73(7): 1393–414. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez J, Zakzuk J, Cardona R. Evaluation of a guidelines-based approach to the treatment of chronic spontaneous urticaria. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(1): 177–82.e1. [DOI] [PubMed] [Google Scholar]
- 41.Deza G, Ricketti PA, Gimenez-Arnau AM, Casale TB. Emerging biomarkers and therapeutic pipelines for chronic spontaneous urticaria. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(4): 1108–17. [DOI] [PubMed] [Google Scholar]
- 42.Aygoren-Pursun E, Magerl M, Maetzel A, Maurer M. Epidemiology of bradykinin-mediated angioedema: a systematic investigation of epidemiological studies. Orphanet Journal of Rare Diseases 2018; 13(1): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanichelli A, Arcoleo F, Barca MP, Borrelli P, Bova M, Cancian M, et al. A nationwide survey of hereditary angioedema due to C1 inhibitor deficiency in Italy. Orphanet Journal of Rare Diseases 2015; 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerji A, Li Y, Busse P, Riedl MA, Holtzman NS, Li HH, et al. Hereditary angioedema from the patient’s perspective: A follow-up patient survey. Allergy and Asthma Proceedings 2018; 39(3): 212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moldovan D, Bara N, Nadasan V, Gabos G, Mihaly E. Consequences of misdiagnosed and mismanaged hereditary angioedema laryngeal attacks: An overview of cases from the Romanian registry. Case Reports in Emergency Medicine 2018; 2018: 6363787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanichelli A, Magerl M, Longhurst HJ, Aberer W, Caballero T, Bouillet L, et al. Improvement in diagnostic delays over time in patients with hereditary angioedema: findings from the Icatibant Outcome Survey. Clin Transl Allergy 2018; 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Javaud N, Bouillet L, Rabetrano H, Bitoun A, Launay D, Lapostolle F, et al. Hereditary angioedema: Clinical presentation and socioeconomic cost of 200 French patients. The Journal of Allergy and Clinical Immunology In Practice 2019; 7(1): 328–30. [DOI] [PubMed] [Google Scholar]
- 48.Lumry W Hereditary angioedema: The economics of treatment of an orphan disease. Front Med (Lausanne) 2018; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maurer M, Magerl M, Ansotegui I, Aygoren-Pursun E, Betschel S, Bork K, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2017 revision and update. Allergy 2018; 73(8): 1575–96. [DOI] [PubMed] [Google Scholar]
- 50.Lumry WR, Weller K, Magerl M, Schranz J, Jain G, Doll H, et al. Lanadelumab markedly improves health-related quality of life in hereditary angioedema patients in the HELP study. Journal of Allergy and Clinical Immunology 2018; 141(2): AB47. [Google Scholar]
- 51.Lumry WR, Craig T, Zuraw B, Longhurst H, Baker J, Li HH, et al. Health-related quality of life with subcutaneous C1-Inhibitor for prevention of attacks of hereditary angioedema. J Allergy Clin Immunol Pract 2018; 6(5): 1733–41.e3. [DOI] [PubMed] [Google Scholar]
- 52.Riedl MA, Aygoren-Pursun E, Baker J, Reshef A, Lumry W, Moldovan D, et al. Evaluation of avoralstat, an oral kallikrein inhibitor, in a Phase 3 hereditary angioedema prophylaxis trial: The OPuS-2 study. Allergy 2018; 73(9): 1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Busse PJ, Christiansen SC, Birmingham JM, Overbey JR, Banerji A, Otani IM, et al. Development of a health-related quality of life instrument for patients with hereditary angioedema living in the United States. The Journal of Allergy and Clinical Immunology In Practice 2018 [DOI] [PubMed] [Google Scholar]
- 54.Cicardi M, Zuraw BL. Angioedema due to bradykinin dysregulation. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(4): 1132–41. [DOI] [PubMed] [Google Scholar]
- 55.Zuraw BL. Hereditary angioedema with normal C1 inhibitor: Four types and counting. The Journal of Allergy and Clinical Immunology 2018; 141(3): 884–5. [DOI] [PubMed] [Google Scholar]
- 56.Dewald G A missense mutation in the plasminogen gene, within the plasminogen kringle 3 domain, in hereditary angioedema with normal C1 inhibitor. Biochemical and Biophysical Research Communications 2018; 498(1): 193–8. [DOI] [PubMed] [Google Scholar]
- 57.Bork K, Wulff K, Steinmuller-Magin L, Braenne I, Staubach-Renz P, Witzke G, et al. Hereditary angioedema with a mutation in the plasminogen gene. Allergy 2018; 73(2): 442–50. [DOI] [PubMed] [Google Scholar]
- 58.Bafunno V, Firinu D, D’Apolito M, Cordisco G, Loffredo S, Leccese A, et al. Mutation of the angiopoietin-1 gene (ANGPT1) associates with a new type of hereditary angioedema. The Journal of Allergy and Clinical Immunology 2018; 141(3): 1009–17. [DOI] [PubMed] [Google Scholar]
- 59.Loules G, Zamanakou M, Parsopoulou F, Vatsiou S, Psarros F, Csuka D, et al. Targeted next-generation sequencing for the molecular diagnosis of hereditary angioedema due to C1inhibitor deficiency. Gene 2018; 667: 76–82. [DOI] [PubMed] [Google Scholar]
- 60.Kaplan AP, Maas C. The search for biomarkers in hereditary angioedema. Frontiers in Medicine 2017; 4: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lara-Marquez ML, Christiansen SC, Riedl MA, Herschbach J, Zuraw BL. Threshold-stimulated kallikrein activity distinguishes bradykinin- from histamine-mediated angioedema. Clinical and Experimental Allergy 2018; 48(11): 1429–38. [DOI] [PubMed] [Google Scholar]
- 62.Giavina-Bianchi P, Kalil J. Improving the management of hereditary angioedema. Clinics (Sao Paulo, Brazil) 2018; 73: e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Craig TJ, Levy RJ, Wasserman RL, Bewtra AK, Hurewitz D, Obtulowicz K, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. The Journal of Allergy and Clinical Immunology 2009; 124(4): 801–8. [DOI] [PubMed] [Google Scholar]
- 64.Levy RJ, Lumry WR, McNeil DL, Li HH, Campion M, Horn PT, et al. EDEMA4: A phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Annals of Allergy, Asthma & Immunology 2010; 104(6): 523–9. [DOI] [PubMed] [Google Scholar]
- 65.Lumry WR, Li HH, Levy RJ, Potter PC, Farkas H, Moldovan D, et al. Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Annals of Allergy, Asthma & Immunology 2011; 107(6): 529–37. [DOI] [PubMed] [Google Scholar]
- 66.Riedl MA, Bernstein JA, Li H, Reshef A, Lumry W, Moldovan D, et al. Recombinant human C1-esterase inhibitor relieves symptoms of hereditary angioedema attacks: Phase 3, randomized, placebo-controlled trial. Annals of Allergy, Asthma & Immunology 2014; 112(2): 163–9.e1. [DOI] [PubMed] [Google Scholar]
- 67.Li HH, Mycroft S, Christiansen S, Wood DN, Feuersenger H, Pawaska D, et al. Subcutaneous C1-esterase inhibitor to prevent hereditary angioedema attacks: Safety findings from the COMPACT trial. Allergy and Asthma Proceedings 2018; 39(5): 365–70. [DOI] [PubMed] [Google Scholar]
- 68.Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, et al. Effect of Lanadelumab compared with placebo on prevention of hereditary angioedema attacks: A randomized clinical trial. JAMA 2018; 320(20): 2108–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pharming Group NV. Pharming announces positive data from paediatric clinical trial with RUCONEST® https://www.pharming.com/news/pharming-announces-positive-data-paediatricclinical-trial-ruconestr (accessed 3/28/19 2018).
- 70.BioCryst presents data highlighting the rapid and sustained plasma concentrations of BCX7353 in HAE patients at the annual scientific meeting of the American College of Allergy, Asthma & Immunology http://ir.biocryst.com/news-releases/news-release-details/biocrystpresents-data-highlighting-rapid-and-sustained-plasma (accessed April 7 2019).
- 71.Aygoren-Pursun E, Bygum A, Grivcheva-Panovska V, Magerl M, Graff J, Steiner UC, et al. Oral plasma kallikrein inhibitor for prophylaxis in hereditary angioedema. The New England Journal of Medicine 2018; 379(4): 352–62. [DOI] [PubMed] [Google Scholar]
- 72.A phase 1, safety and pharmacokinetic study of CSL312 in healthy subjects. ANZCTR. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371625 (accessed 06/27/2018 2018).
- 73.ISIS Pharmaceuticals reports positive phase 1 data demonstrating ISIS-PKK Rx produces significant reductions in prekallikrein levels. http://ir.ionispharma.com/newsreleases/news-release-details/isis-pharmaceuticals-reports-positive-phase-1-data-0 (accessed 06/27/2018 2018).
- 74.Qiu T, Chiuchiolo MJ, Whaley AS, Russo AR, Sondhi D, Kaminsky SM, et al. Gene therapy for C1 esterase inhibitor deficiency in a murine model of hereditary angioedema. Allergy 2019, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Attune Pharmaceuticals announces positive phase 1 results for ATN-249, an oral plasma kallikrein inhibitor for the treatment of HAE http://attunepharma.com/assets/AttunePhase-1_2019_01_22.pdf (accessed April 7 2019).
- 76.Oral route for HAE. https://pharvaris.com/wp-content/uploads/2018/06/Pharvaris-fromBioCenturyInnovations053118.pdf (accessed April 7 2019).
- 77.Yu JE, Lin RY. The epidemiology of anaphylaxis. Clinical Reviews in Allergy & Immunology 2018; 54(3): 366–74. [DOI] [PubMed] [Google Scholar]
- 78.Yao TC, Wu AC, Huang YW, Wang JY, Tsai HJ. Increasing trends of anaphylaxis-related events: an analysis of anaphylaxis using nationwide data in Taiwan, 2001–2013. World Allergy Organ J 2018; 11(1): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeong K, Lee JD, Kang DR, Lee S. A population-based epidemiological study of anaphylaxis using national big data in Korea: trends in age-specific prevalence and epinephrine use in 2010–2014. Allergy Asthma Clin Immunol 2018; 14: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. National trends in emergency department visits and hospitalizations for food-induced anaphylaxis in US children. Pediatr Allergy Immunol 2018; 29(5): 538–44. [DOI] [PubMed] [Google Scholar]
- 81.Dhopeshwarkar N, Sheikh A, Doan R, Topaz M, Bates DW, Blumenthal KG, et al. Drug-induced anaphylaxis documented in electronic health records. The Journal of Allergy and Clinical Immunology In Practice 2019; 7(1): 103–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McWilliam VL, Koplin JJ, Field MJ, Sasaki M, Dharmage SC, Tang MLK, et al. Self-reported adverse food reactions and anaphylaxis in the SchoolNuts study: A population-based study of adolescents. The Journal of Allergy and Clinical Immunology 2018; 141(3): 982–90. [DOI] [PubMed] [Google Scholar]
- 83.Carter MC, Desai A, Komarow HD, Bai Y, Clayton ST, Clark AS, et al. A distinct biomolecular profile identifies monoclonal mast cell disorders in patients with idiopathic anaphylaxis. The Journal of Allergy and Clinical Immunology 2018; 141(1): 180–8 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Retterer MKC, Workman LJ, Bacon JR, Platts-Mills TAE. Specific IgE to gelatin as a cause of anaphylaxis to zoster vaccine. The Journal of Allergy and Clinical Immunology 2018; 141(5): 1956–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y, Liu B, Harmacek L, Long Z, Liang J, Lukin K, et al. The transcription factors GATA2 and microphthalmia-associated transcription factor regulate Hdc gene expression in mast cells and are required for IgE/mast cell-mediated anaphylaxis. The Journal of Allergy and Clinical Immunology 2018; 142(4): 1173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Costa S, Ayyadurai S, Gibson AJ, Mackey E, Rajput M, Sommerville LJ, et al. Mast cell corticotropin-releasing factor subtype 2 suppresses mast cell degranulation and limits the severity of anaphylaxis and stress-induced intestinal permeability. The Journal of Allergy and Clinical Immunology 2019; 143(5): 1865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanchez E, Gonzalez EA, Moreno DS, Cardenas RA, Ramos MA, Davalos AJ, et al. Syntaxin 3, but not syntaxin 4, is required for mast cell regulated exocytosis, where it plays a primary role mediating compound exocytosis. J Biol Chem 2018; 294(9): 3012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamani A, Wu D, Waggoner L, Noah T, Koleske AJ, Finkelman F, et al. The vascular endothelial specific IL-4 receptor alpha-ABL1 kinase signaling axis regulates the severity of IgEmediated anaphylactic reactions. The Journal of Allergy and Clinical Immunology 2018; 142(4): 1159–72 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gour N, Lajoie S, Smole U, White M, Hu D, Goddard P, et al. Dysregulated invertebrate tropomyosin-dectin-1 interaction confers susceptibility to allergic diseases. Sci Immunol 2018; 3(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie G, Yang H, Peng X, Lin L, Wang J, Lin K, Cui Z, et al. Mast cell exosomes can suppress allergic reactions by binding to IgE. The Journal of Allergy and Clinical Immunology 2018; 141(2): 788–91. [DOI] [PubMed] [Google Scholar]
- 91.Shefler I, Salamon P, Levi-Schaffer F, Mor A, Hershko AY, Mekori YA. MicroRNA-4443 regulates mast cell activation by T cell-derived microvesicles. The Journal of Allergy and Clinical Immunology 2018; 141(6): 2132–41 e4. [DOI] [PubMed] [Google Scholar]
- 92.Choi HW, Suwanpradid J, Kim IH, Staats HF, Haniffa M, MacLeod AS, et al. Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science 2018; 362(6415). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clay CD, Strait RT, Mahler A, Khodoun MV, Finkelman FD. Anti-FcgammaRIIB mAb suppresses murine IgG-dependent anaphylaxis by Fc domain targeting of FcgammaRIII. The Journal of Allergy and Clinical Immunology 2018; 141(4): 1373–81 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beutier H, Hechler B, Godon O, Wang Y, Gillis CM, de Chaisemartin L, et al. Platelets expressing IgG receptor FcgammaRIIA/CD32A determine the severity of experimental anaphylaxis. Sci Immunol 2018; 3(22). [DOI] [PubMed] [Google Scholar]
- 95.Bahri R, Custovic A, Korosec P, Tsoumani M, Barron M, Wu J, et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. The Journal of Allergy and Clinical Immunology 2018; 142(2): 485–96 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segura-Bedmar I, Colon-Ruiz C, Tejedor-Alonso MA, Moro-Moro M. Predicting of anaphylaxis in big data EMR by exploring machine learning approaches. J Biomed Inform 2018; 87: 50–9. [DOI] [PubMed] [Google Scholar]
- 97.Ball R, Toh S, Nolan J, Haynes K, Forshee R, Botsis T. Evaluating automated approaches to anaphylaxis case classification using unstructured data from the FDA Sentinel System. Pharmacoepidemiol Drug Saf 2018; 27(10): 1077–84. [DOI] [PubMed] [Google Scholar]
- 98.Lee J, Rodio B, Lavelle J, Lewis MO, English R, Hadley S, et al. Improving anaphylaxis care: The impact of a clinical pathway. Pediatrics 2018; 141(5). [DOI] [PubMed] [Google Scholar]
- 99.Warren CM, Zaslavsky JM, Kan K, Spergel JM, Gupta RS. Epinephrine auto-injector carriage and use practices among US children, adolescents, and adults. Annals of Allergy, Asthma & Immunology 2018; 121(4): 479–91. [DOI] [PubMed] [Google Scholar]
- 100.Robinson M, Koplin JJ, Field MJ, Sasaki M, Peters RL, McWilliam V, et al. Patterns of carriage of prescribed adrenaline autoinjectors in 10- to 14-year-old food-allergic students: A population-based study. The Journal of Allergy and Clinical Immunology In Practice 2019; 7(2): 437–43. [DOI] [PubMed] [Google Scholar]
- 101.Song TT, Brown D, Karjalainen M, Lehnigk U, Lieberman P. Value of a second dose of epinephrine during anaphylaxis: A patient/caregiver survey. The Journal of Allergy and Clinical Immunology In Practice 2018; 6(5): 1559–67. [DOI] [PubMed] [Google Scholar]
- 102.Hogue SL, Muniz R, Herrem C, Silvia S, White MV. Barriers to the administration of epinephrine in schools. J Sch Health 2018; 88(5): 396–404. [DOI] [PubMed] [Google Scholar]
- 103.Dubus JC, Le MS, Vitte J, Minodier P, Boutin A, Carsin A, et al. Use of epinephrine in emergency department depends on anaphylaxis severity in children. Eur J Pediatr 2019; 178(1): 69–75. [DOI] [PubMed] [Google Scholar]
- 104.Song TT, Brown D, Lehnigk U, Corvino FA, Lieberman P. Anaphylaxis care guidelines and the effect on health care resource use in the United States. Annals of Allergy, Asthma & Immunology 2018; 121(1): 132–4 e3. [DOI] [PubMed] [Google Scholar]
- 105.Cassard L, Sperber K, Buivan TP, Cotillard A, Bourdet-Sicard R, Alber ML, et al. Basophils from allergic patients are neither hyperresponsive to activation signals nor hyporesponsive to inhibition signals. The Journal of Allergy and Clinical Immunology 2018; 142(5): 1548–57. [DOI] [PubMed] [Google Scholar]