Abstract
Background
Repeated measurements of spirometry and fractional exhaled nitric oxide (Feno) are recommended as part of the management of childhood asthma, but the evidence base for such recommendations is small. We tested the hypothesis that reducing spirometric indices or increasing Feno will predict poor future asthma outcomes.
Methods
A one-stage individual patient data meta-analysis used data from seven randomized controlled trials in which Feno was used to guide asthma treatment; spirometric indices were also measured. Change in %FEV1 and % change in Feno between baseline and 3 months were related to having poor asthma control and to having an asthma exacerbation between 3 and 6 months after baseline.
Results
Data were available from 1,112 children (mean age, 12.6 years; mean %FEV1, 94%). A 10% reduction in %FEV1 between baseline and 3 months was associated with 28% increased odds for asthma exacerbation (95% CI, 3-58) and with 21% increased odds for having poor asthma control (95% CI, 0-45) 6 months after baseline. A 50% increase in Feno between baseline and 3 months was associated with 11% increase in odds for poor asthma control 6 months after baseline (95% CI, 0-16). Baseline Feno and %FEV1 were not related to asthma outcomes at 3 months.
Conclusions
Repeated measurements of %FEV1 that are typically within the “normal” range add to clinical risk assessment of future asthma outcomes in children. The role of repeated Feno measurements is less certain because large changes were associated with small changes in outcome risk.
Key Words: asthma, child, monitoring, nitric oxide, spirometry
Abbreviations: ERS, European Respiratory Society; FEF25-75, forced expiratory flow at 25-75% of FVC; Feno, fractional exhaled nitric oxide; GLI, Global Lung Initiative; ICS, inhaled corticosteroid; IPD, individual patient data; IQR, interquartile range; LTRA, leukotriene receptor antagonist; NHANES, National Health and Nutrition Examination Survey; RCT, randomized controlled trial
Asthma is a common condition affecting 1 million children in the United Kingdom1 and 6 million in the United States.2 Guidelines recommend that objective markers of respiratory function (eg, FEV1) and airway inflammation (eg, fractional exhaled nitric oxide [Feno]) may be used in conjunction with symptoms to guide asthma preventive treatment in children. These recommendations differ between guidelines, however, and none gives clinicians advice how to interpret changes in spirometry when values fall within the normal range, yet FEV1 (the most commonly used spirometric index) is usually within normal limits.3
One guideline recommends that lung function should be “monitored and recorded” only in children >12 years of age.4 Two guidelines recommend that FEV1 may be useful for monitoring of asthma for children aged between ages 5 and 7 years.5, 6 A fourth guideline7 recommends that lung function should be measured 3 to 6 months after treatment is started and “periodically” thereafter, and that %FEV1 <60% identifies a patient at risk for future asthma exacerbations. A fifth guideline8 recommends that lung function should be measured every 1 to 2 years (more frequently when symptoms are poorly controlled) and suggests that treatment might be stepped up if %FEV1 is <80% or <60%. Some guidelines suggest that a 20% drop in FEV1 relative to personal best identifies an individual at risk for future asthma exacerbations.5, 7
Although Feno is recommended by the US Food and Drug Administration for monitoring asthma,9 there is no consensus on how results should be interpreted; one international guideline states that a change in Feno of 10 ppb or 20% may be clinically relevant.10
To understand the relationship between change in spirometry and Feno and asthma outcomes, we obtained individual patient data (IPD) from seven Feno randomized controlled trials (RCTs) in which details of spirometry, Feno, asthma control, and the occurrence of asthma exacerbations were collected longitudinally.11, 12, 13, 14, 15, 16, 17 Our primary hypothesis was that falling spirometric indices (with %FEV1 as the primary index) and/or rising Feno between randomization and 3-month follow-up will be associated with increased risk for asthma being uncontrolled and for an asthma exacerbation between 3- and 6-month follow-up. The secondary hypothesis was that, at baseline, low spirometric indices and high Feno will be associated with increased risk for asthma being uncontrolled and for an asthma exacerbation between baseline and 3-month follow-up.
Methods
Study Design
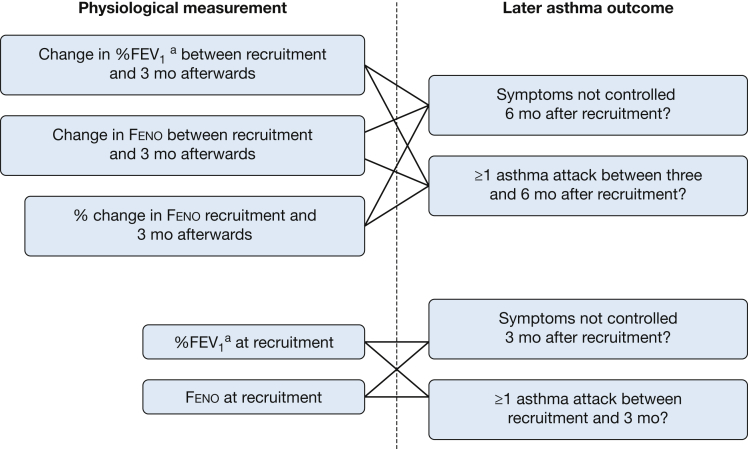
Authors of published RCTs in which measurements of Feno were used to guide asthma treatment in children18 were invited to provide anonymized data for IPD.19 The outcomes were asthma exacerbation (defined as a prescription of prednisolone during the follow-up period and derived using data provided by study authors) and poor asthma control (defined by per trial protocol by symptom score, and including FEV1 cutoff values in some trials11, 12, 16 but not including an asthma exacerbation). e-Appendix 1 provides definitions of being uncontrolled. For all RCTs, prescribing oral corticosteroids for asthma exacerbations was at the discretion of the attending doctor. The explanatory variables between baseline and 3-month follow-up were absolute change and %change in Feno and absolute change in percentage of predicted (%) spirometry; the analysis of change in % spirometry and % change in Feno included the corresponding baseline measurement. The relationship between outcomes and the following explanatory variables at baseline were sought: Feno and %FEV1, %FEV1/FVC, forced expiratory flow at 25% to 75% of FVC (%FEF25-75), and %FVC. Figure 1 shows which physiological measurements (and changes) were linked to later asthma outcomes in this study. Additional covariates collected at baseline and included in the models were: age, sex, height, weight, ethnicity, trial arm, dose of inhaled corticosteroid (ICS, as daily budesonide equivalent dose), prescribed long-acting beta agonist (LABA) or not, prescribed leukotriene receptor agonist (LTRA) or not, asthma control, and treatment compliance. For each follow up visit, the following variables were collected: Feno, FEV1, height, dose of ICS, asthma control, and asthma exacerbation since the previous visit. The focus of this study was follow-up at 3 and 6 months because these are typically used in asthma clinics; for trials in which there was no 3- or 6-month assessment, the assessment closest to these time points was used. In all but two studies,14, 15 absolute spirometry data were available and expressed as percentage of predicted to the Global Lung Initiative (GLI) standard20; where absolute data were not available, the % predicted value provided by the local team was used for analysis. Although %FEV1 was the primary spirometric index of interest, %FEV1/FVC, %FEF25-75, and %FVC were also considered to determine which index had the greatest precision for future outcomes. For completeness, FEV1 was also expressed as z score and centile (standardized to the GLI standard20). Additionally, as a sensitivity analysis, %FEV1 was derived using National Asthma Education and Prevention Program (NHANES) III standard21 to determine whether any relationship between %FEV1 and later outcome was dependent on the standard used. BMI was derived and International Obesity Task Force weight categories created.22 In each trial, Feno was measured in accordance with the 2005 guideline.23 Ethical approval was obtained for each individual study but was not required for the IPD.
Figure 1.
A diagram showing how different physiological measurements were linked to later asthma outcomes in the study’s analyses. The analyses used data collected at recruitment to seven clinical trials and at follow-up assessments 3 and 6 months after recruitment. aAlthough %FEV1 was the primary spirometric index, the following were also considered: %FEV1/FVC, %FEF25-75, and %FVC. Feno = fractional exhaled nitric oxide.
Individual Patient Data Analysis
Demographic and baseline characteristics were obtained for each study. A one-stage IPD meta-analysis was undertaken using the melogit command in STATA with study included as a random effect. All models were adjusted for the baseline variables of age, sex, LABA, LTRA, asthma control, ICS dose, arm of trial and, where relevant, baseline Feno or baseline FEV1. A one-stage approach was used rather than a two-stage approach become some of the studies had low event counts (few asthma exacerbations), and adoption of one-stage in this instance is recommended by Burke et al.24 Sensitivity analyses considered separately outcomes for individuals in Feno intervention and standard care arms of the trials and also excluded data from trials in which %FEV1 was used to guide treatment decisions.11, 12, 16 STATA, version 14, was used for analysis.
Results
Study Subjects
Data from seven pediatric RCTs were analyzed (Table 1)11, 12, 13, 14, 15, 16, 17; data from an eighth RCT could not be obtained.25 Details of population inclusion and exclusion criteria are presented in e-Appendix 1. The IPD included data on 1,112 participants. In two studies,14, 17 spirometry was measured at baseline and at 12 months only, and change in %FEV1 between baseline and 3 months could not be calculated. There was a predominance of male participants (58%) and mean (SD) age was 12.6 (3.1) years (Table 2). For individual RCTs, median values of Feno varied between 18 and 34 ppb with an overall median (interquartile range [IQR]) of 22 ppb (12, 43). Mean %FEV1 values at baseline varied between 89% and 98% predicted with an overall mean (SD) of 94% (18). Details of mean FEV1 z scores and centile are presented in e-Table 1. The Pearson correlation coefficient between Feno and %FEV1 at baseline was –0.184 (n = 1,025, P < .001) and between % change in Feno (baseline to 3 months) and change in %FEV1 (baseline to 3 months) was –0.127 (n = 759, P < 001). Overall, 7% of participants had an asthma exacerbation during the first 3 months and 12% in the second 3 months, whereas 27% were uncontrolled at baseline, 25% at 3 months, and 23% at 6 months, (Table 3). An asthma exacerbation occurred between baseline and 3 months in 47 (7%) of the 718 participants with controlled symptoms at baseline and in 27 (12%) of 230 with uncontrolled symptoms at baseline.
Table 1.
Details of the Randomized Controlled Trials Included in This Individual Patient Data Analysis
| Study | Mean Age, y |
Intervals at Follow-up After Baseline When Feno Was Measured (mo)a | Intervals at Follow-up After Baseline When Spirometry Was Measured (mo)a | Were Absolute Spirometry Data Available? | Which Spirometric Indices Were Available? | Was There a Run-in Period? | Was Atopy an Inclusion Criteria? | What Measure of Asthma Control Was Used? | |
|---|---|---|---|---|---|---|---|---|---|
| Feno Arm | Control Arm | ||||||||
| Fritsch et al11 | 11.3 | 12.1 | 0, 1.5, 3, 4.5, 6 | 0, 1.5, 3, 4.5, 6 | Yes | FEV1, FVC | Yes | Yes | Unvalidated symptom diary |
| Peirsman et al12 | 10.6 | 10.7 | 0, 3, 6, 9, 12 | 0, 3, 6, 9, 12 | Yes | FEV1 | No | Yes | First 4 (of 7) questions on C-ACTb |
| Petsky et al13 | 9.9 | 10.1 | 0, 1, 2, 3, 4, 6, 8, 10, 12 | 0, 1, 2, 3, 4, 6, 8, 10, 12 | Yes | FEV1, FEF25-75, FVC | Yes | No | Validated symptom diary |
| Pijnenburg et al14 | 11.9 | 12.6 | 0, 3, 6, 9, 12 | 0, 12 | No | FEV1, FEF50, FVC | Yes | Yes | Validated symptom diary |
| Pike et al15 | 10.5 | 11.4 | 0, 2, 4, 6, 7, 10, 12 | 0, 2, 4, 6, 7, 10, 12 | No | FEV1, FVC | No | No | Modified validated symptom diaryb |
| Szefler et al16 | 14.4 | 14.4 | 0, 1.5, 3.2, 5, 7, 8.5, 10.5 | 0, 1.5, 3.2, 5, 7, 8.5, 10.5 | Yes | FEV1, FEF25-75, FVC | Yes | No | ACTc plus FEV1 |
| Voorend-van Bergen et al17 | 10.3 | 10.2 | 0, 4, 8, 12 | 0, 12 | Yes | FEV1, FEF75, FVC | Yes | Yes | ACT and C-ACT |
ACT = asthma control test, C-ACT = Childhood Asthma Control Test; FEF25-75, forced expiratory flow at 25% to 75% of FVC; Feno = fractional exhaled nitric oxide.
Zero corresponds to baseline.
Reliever medication use and FEV1.
FEV1 alone were used in the treatment algorithm for both arms of the RCT but were not used to define being uncontrolled in this study.
Table 2.
Characteristic of Study Participants at the Baseline Visit in Each Study
| Characteristic | Fritsch et al11 | Peirsman et al12 | Petsky et al13 | Pijnenburg et al14 | Pike et al15 | Szefler et al16 | Voorend-van Bergen et al17 | All Populations Combined |
|---|---|---|---|---|---|---|---|---|
| Total No. participants | 47 | 99 | 63 | 86 | 90 | 546 | 181 | 1,112 |
| Men, % (No.) | 60 (28) | 67 (66) | 49 (31) | 65 (56) | 57 (51) | 53 (288) | 68 (123) | 58 (643) |
| Age | ||||||||
| Mean (SD) | 11.5 (3.1) | 10.7 (2.1) | 10.0 (3.2) | 12.3 (2.8) | 10.9 (2.6) | 14.4 (2.1) | 10.2 (3.0) | 12.6 (3.1) |
| Range | 6–17 | 5–14 | 4–16 | 6–18 | 5–16 | 12–19 | 4–18 | 4–19 |
| Trial arm | ||||||||
| Standard | 25 | 50 | 32 | 46 | 46 | 270 | 92 | 561 |
| Feno | 22 | 49 | 31 | 40 | 44 | 276 | 89 | 551 |
| Feno | ||||||||
| No. observations | 46 | 49 | 61 | 86 | 90 | 546 | 179 | 1,057 |
| Median, ppb | 33.9 | 31.3 | 25.6 | 32 | 25.5 | 20.1 | 18.2 | 21.9 |
| IQR, ppb | (18.6, 58.6) | (14, 69) | (12.2, 47.5) | (16.6, 52.5) | (10, 48) | (11.2, 40.6) | (10.2, 30.4) | (11.6, 43.0) |
| % predicted FEV1 | ||||||||
| No. observations | 47 | 98 | 54 | 86 | 90 | 546 | 157 | 1,078 |
| Mean (SD) | 93.5 (15.7) | 91.4 (15.7) | 90.7 (15.6) | 97.5 (17.5) | 89.2 (14.3) | 90.9 (16.6) | 93.8 (13.0) | 93.5 (18.1) |
| % predicted FEV1/FVC | ||||||||
| No. observations | 47 | 0 | 0 | 0 | 0 | 546 | 156 | 749 |
| Mean (SD) | 90.1 (10.6) | - | - | - | - | 91.3 (9.9) | 93.4 (9.4) | 91.7 (9.9) |
| % with positive skin prick test | 100 | 100 | 38 (24/63) | 100 | 76 (68/90) | 88 (467/531) | 100 | 89 (972/1,097) |
| Mean centile BMI (SD) | ||||||||
| No. observations | 47 | 99 | 58 | 86 | 89 | 546 | 181 | 1,106 |
| Mean (SD) | 67.6 (27.0) | 52.1 (30.1) | 48.5 (32.4) | 60.8 (27.3) | 64.2 (32.2) | 83.1 (23.5) | 58.9 (29.9) | 70.7 (29.8) |
| Obese | ||||||||
| No. observations | 47 | 99 | 58 | 85 | 89 | 526 | 181 | 1,085 |
| % (No.) overweight | 28 (13) | 12 (12) | 16 (9) | 14 (12) | 25 (22) | 28 (145) | 20 (36) | 23 (249) |
| % (No.) obese | 8 (4) | 1 (1) | 2 (1) | 4 (4) | 8 (7) | 31 (165) | 3 (5) | 17 (187) |
| LTRA treatment prescribed | ||||||||
| No. observations | 47 | 99 | 58 | 86 | 90 | 546 | 181 | 1,107 |
| % (No.) yes | 28 (13) | 60 (59) | 10 (6) | 0 | 51 (46) | 15 (80) | 13 (23) | 21 (227) |
| LABA treatment prescribed | ||||||||
| No. observations | 47 | 99 | 58 | 86 | 90 | 546 | 181 | 1,107 |
| % (No.) yes | 38 (18) | 32 (32) | 67 (39) | 38 (33) | 76 (68) | 66 (360) | 46 (84) | 57 (634) |
| Median dose of inhaled corticosteroids (IQR) | 400 (0, 800) | 320 (200, 400) | 400 (250, 500) | 800 (400, 1,000) | 800 (400, 1,000) | 1000 (400, 2,000) | 400 (400, 800) | 400 (400, 1,000) |
| Ethnic group, % (No.) | ||||||||
| No. observations | 0 | 84 | 20 | 0 | 90 | 526 | 179 | 889 |
| White | 82 (69) | 92 (83) | 89 (160) | 35 (312) | ||||
| Hispanic | 65 (340) | 38 (340) | ||||||
| Other | 18 (15) | 100 (20) | 8 (7) | 35 (186) | 11 (19) | 28 (247) | ||
| Asthma control status | ||||||||
| No. observations | 47 | 65 | 57 | 77 | 90 | 528 | 181 | 1,045 |
| Asthma controlled, % (No.) | 49 (23) | 75 (49) | 72 (41) | 57 (44) | 68 (62) | 80 (421) | 67 (122) | 73 (762) |
| Asthma not controlled, % (No.) | 51 (24) | 25 (16) | 28 (16) | 43 (33) | 31 (28) | 20 (107) | 33 (59) | 27 (283) |
IQR = interquartile ratio; LABA = long-acting beta agonist; LTRA = leukotriene receptor agonist. See Table 1 legend for expansion of other abbreviations.
Table 3.
Frequency of Outcomes Between Baseline and 3 Months and Between 3 and 6 Months post baseline
| Study | Exacerbation Between Baseline and 3 Mo | Exacerbation Between 3 and 6 Mo | Asthma Not Controlled at 3 Mo | Asthma Not Controlled at 6 Mo |
|---|---|---|---|---|
| Fritsch et al11 | 2 (1/47) | 6 (3/47) | 54 (25/46) | 53 (25/47) |
| Peirsman et al12 | 4 (4/99) | 0 | 25 (21/83) | 21 (18/86) |
| Petsky et al13 | 5 (3/63) | 8 (5/63) | NA | NA |
| Pijnenburg et al14 | 9 (8/86) | 8 (7/86) | 40 (32/81) | 40 (31/78) |
| Pike et al15 | 17 (15/90) | 30 (27/90) | 26 (23/90) | 32 (29/90) |
| Szefler et al16 | 7 (35/522) | 15 (78/505) | 21 (111/541) | 17 (86/513) |
| Voorend-van Bergen et al17 | 7 (12/181) | 7 (12/181) | 21 (38/179) | 20 (36/178) |
| Overall | 7 (78/1,088) | 12 (132/1,071) | 25 (250/1,010) | 23 (225/992) |
Data are presented as No. (%). NA = not available.
Relationship Between Change in Spirometry and Percentage Change in Feno Between Baseline and 3 Months and Outcomes Between 3 and 6 Months
Between baseline and 3 months, the mean (SD) change in %FEV1 was –0.17 (10.4), the median absolute change in Feno was 0.6 ppb (IQR, –7.9, 12.2), and the median % change in Feno was 3.7% (IQR, –30.4, 66.7). A fall in % FEV1 was related to increased odds of an asthma exacerbation over the following 3 months (eg, a reduction of 10% FEV1 between baseline and 3 months was associated with increased OR for future exacerbation between 3 and 6 months of 1.3 [1.0, 1.6], P = .027) and loss of future asthma control (eg, a reduction of 10% FEV1 was associated with increased OR for uncontrolled asthma 1.2 [96% CI, 1.0-1.4], P = .046) (Table 4). A reduction of 10% FVC was also associated with increased odds for a future exacerbation (OR, 1.40 [1.04, 1.88], P = .026); e-Table 2. A 50% increase in Feno between baseline and 3 months was associated with 11% increased odds of asthma being uncontrolled at 6 months (95%, 0-16; P = .014) but not odds of an exacerbation between 3 and 6 months (Table 4). When both change in %FEV1 and %change in Feno were considered in the same model, the OR for asthma being uncontrolled remained significant for Feno (P = .036) but not for %FEV1 (P = .061). Neither change in %FEV1/FVC or %FEF25-75 (e-Table 2) nor absolute change in Feno (Table 4) were associated with outcomes. The associations between change in %FEV1 and % change in Feno did not achieve significance when each trial arm was considered separately (e-Table 3). When the RCTs in which %FEV1 was used to guide treatment were excluded, there was an association between rising Feno and future asthma exacerbation (P = .029), but associations between change in FEV1 and outcomes were not significant (e-Table 4). Among the subset of RCTs in which FEV1 z score and centile values could be derived, falling z scores were associated with increased odds for both asthma exacerbations and being uncontrolled and falling centile score with being uncontrolled (e-Table 5). The results seen with change in % FEV1 using the GLI standard were also seen when the NHANES III standard was used (e-Table 6).
Table 4.
Relationship Between Falling %FEV1 or Rising % Change in Feno Over a 3-Mo Period and Odds of Having an Asthma Attack or Uncontrolled Asthma During the Next 3 Mon
| Change in Measurement of Respiratory Function | Asthma Outcome | OR per Unit Change in FEV1 or Fenoa | OR per 5, 10, and 20 Reduction in %FEV1b |
OR per 20% and 50% Increase in Fenob |
OR per 20 and 50 ppb Increase in Fenob |
||||
|---|---|---|---|---|---|---|---|---|---|
| %FEV1 Reduced by 5 | %FEV1 Reduced by 10 | %FEV1 Reduced by 20 | 20% Increase in Feno | 50% Increase in Feno | 20 ppb Increase in Feno | 50 ppb Increase in Feno | |||
| Change (baseline to 3 mo) in% FEV1 | ≥1 asthma attack between 3 and 6 moc | 1.025 (1.003, 1.047), P = .027, n = 716 (5 trials) | 1.131 [1.015, 1.258] | 1.280 [1.030, 1.583] | 1.639 [1.062, 2.506] | ||||
| Asthma uncontrolled at 6 mo | 1.019 (1.000, 1.038), P = .046, n = 693 (4 trials) | 1.099 [1.000, 1.205] | 1.207 [1.000, 1.452] | 1.457 [1.000, 2.108] | |||||
| % change in Feno (baseline to 3 mo) | ≥1 asthma attack between 3 and 6 moc | 1.001 (0.999, 1.003), P = .228, n = 929 (7 trials) | 1.020 [0.980, 1.062] | 1.051 [0.951, 1.162] | |||||
| Asthma uncontrolled at 6 mo | 1.002 (1.000, 1.003), P = .014, n = 897 (6 trials) | 1.041 [1.000, 1.062] | 1.105 [1.00, 1.162] | ||||||
| Absolute change in Feno (baseline to 3 mo), ppb | ≥1 asthma attack between 3 and 6 moc | 1.004 (0.998, 1.010), P = .197, n = 929 (7 trials) | 1.083 [0.961, 1.220] | 1.221 [0.905, 1.645] | |||||
| Asthma uncontrolled at 6 mo | 1.002 (0.997, 1.008), P = .07, n = 897 (6 trials) | 1.041 [0.942, 1.173] | 1.105 [0.861, 1.489] | ||||||
Results are from a 1-stage individual patient data analysis. All models adjusted for sex, age, treatment with long-acting beta agonists at baseline, treatment with leukotriene receptor antagonist at baseline, asthma control at baseline, and change in dose of inhaled corticosteroid between baseline and 3 mo. See Table 1 legend for expansion of abbreviations.
For change in %FEV1, “per unit” means for each percentage change (eg, from 98% to 97% FEV1); %change in Feno means for each percent change (eg, from 100 to 101 ppb); and absolute change in Feno “per unit” means ppb change (eg, from 35 to 36 ppb).
OR for outcomes were derived from the ORper unit change; for example, OR for asthma attack after a reduction in %FEV1 of 5 is 1.025 to the power of 5.
The model also includes asthma attack between baseline and 3 mo. The change in Feno model included Feno at baseline; the change in FEV1 model included FEV1 at baseline.
Relationship Between Baseline Feno and Spirometric Indices and Outcomes at 3Months
Percentage of predicted FEV1/FVC at baseline (but no other spirometric index) was related to the odds of asthma exacerbation at 3 months during this interval (P = .016; Table 5). No index of spirometry at baseline was related to having uncontrolled asthma at 3 months. Feno at baseline was not related to asthma outcomes at 3 months (Table 5). e-Table 6 demonstrates that when FEV1 was standardized to the NHANES III data, reducing %FEV1 at baseline was associated with increased odds for future asthma exacerbation (P = .033) and a trend for reduced odds for asthma not being controlled in future (P = .055). Baseline FEV1 z score or centile were not related to outcomes (e-Table 7).
Table 5.
Relationship Between Baseline %FEV1 or Baseline Feno and Odds of Asthma Attack or Asthma Being Uncontrolled During the Next 3 Mo
| Measurement of Respiratory Function | Asthma Outcome | OR per Unit Reduction in FEV1 or Rise in Fenoa |
|---|---|---|
| %FEV1 at baseline | ≥1 asthma attack between baseline and 3 mo | 1.011 (0.997, 1.026), P = .118, n = 973 (7 trials) |
| Asthma uncontrolled at 3 mo | 0.993 (0.984, 1.001), P = .098, n = 939 (6 trials) | |
| %FEV1/FVC at baseline | ≥1 asthma attack between baseline and 3 mo | 1.037 (1.007, 1.067), P = .016, n = 706 (3 trials) |
| Asthma uncontrolled at 3 mo | 0.993 (0.973, 1.012), P = .451, n = 715 (3 trials) | |
| Feno (ppb) at baseline | ≥1 asthma attack between baseline and 3 mo | 1.001 (0.995, 1.008), P = .682, n = 966 (7 trials) |
| Asthma uncontrolled at 3 mo | 1.002 (0.997, 1.007), P = .476, n = 929 (6 trials) |
Results are from a 1-stage individual patient data analysis. All models adjusted for sex, age, treatment with long-acting beta agonists at baseline, treatment with leukotriene receptor antagonist at baseline, asthma control at baseline, and change in dose of inhaled corticosteroid between baseline and 3 mo.
For %FEV1, “per unit” means for each percentage reduction (eg, from 98% to 97% FEV1) and for Feno “per unit” means per part per billion change (eg, from 35 to 36 ppb).
Discussion
This study sought to understand the relationship between changes in spirometric measurements and Feno and future asthma outcomes. The first finding was that, independent of all factors that might influence treatment decisions, falling %FEV1 (even within the range of 80% to 120% commonly considered as “normal”) was associated with increased odds for future asthma exacerbation and having uncontrolled asthma. A second finding was that an absolute change in Feno (Table 4) did not predict outcomes, and only a large rise in % change in Feno was related to a small increase in the odds for having uncontrolled asthma in the future. We also observed that at baseline, a “one-off” %FEV1/FVC ratio (but not %FEV1) was associated with future odds for asthma exacerbation. Together, the results suggest that change in %FEV1 can be used as part of risk assessment for asthma outcomes. The role of change in Feno is less clear, and future clinical trials could include % change in Feno as part of a treatment algorithm for children with asthma.
The individuals whose data contributed to this study were participating in RCTs, and this could mean that the results are not necessarily generalizable for at least two reasons. First, participants in RCTs often have to fulfill specific eligibility criteria, receive more clinical contact than standard care, and often have better outcomes such as fewer asthma exacerbations, but these differences are likely to weaken any association between Feno or %FEV1 and asthma outcomes by narrowing the phenotype of participants and improving outcomes. Second, the participants in our study had treatment guided by Feno (and %FEV1 in three studies); thus, the predictive variables in our study may have affected the outcome (eg, rising Feno leading to increased ICS resulting in good asthma control). We justify inclusion of data from these trials because, first, Feno and, in all but one study,14 %FEV1, did not differ between trial arms during the RCT; second, if Feno or %FEV1 did improve asthma outcomes by protocol-driven treatment changes, this would have weakened any association between Feno or %FEV1 and asthma control or asthma exacerbations. Our inclusion of participants in RCTs may therefore have weakened the associations described; in “real life,” change in %FEV1 and % change in Feno may have greater precision for outcomes than indicated by our results.
There are some limitations to our study. First, the methodologies of the RCTs were different; in particular, three of the RCT15, 16, 17 intervals between assessments did not include multiples of 3 months. This heterogeneity may have weakened the associations described, assuming that the relationship between Feno and FEV1 and outcomes changes over time. Different methods were used to assess asthma control; again, this would weaken and not strengthen the associations described between baseline %FEV1 or %change in Feno and being uncontrolled in future. A second limitation is that the range of %FEV1 values was relatively narrow and the incidence of asthma exacerbations was relatively low; this could make the relationship between physiological measurement and clinical outcome difficult to detect. Nonetheless, we were still able to observe an association between %FEV1 and future asthma exacerbations. A third limitation is that we did not have an objective measure of adherence and could not consider how nonadherence may have influenced asthma control and exacerbations; however, this information is not available for most clinicians and thus our study reflects the real world. A fourth limitation is that none of the RCTs included an assessment of short-term variability of pulmonary function, such as PEF variability or bronchodilator response, and we are not able to say how short-term variability in pulmonary function might be related to future asthma outcomes.
The magnitude of the change in OR for an asthma exacerbation or being uncontrolled in future in the context of changing %FEV1 and Feno were relatively small, and this is partly because we included RCT participants as previously discussed and partly because the model considered many other factors that might predict poor asthma outcomes (eg, current symptom control, treatment level, current %FEV1).
In our sensitivity analyses, we excluded the three studies in which %FEV1 was used to guide treatment; the results seen in the whole population were no longer significant, and this is most likely explained by lack of power. When we split results by trial arm, the significant associations seen between change in %FEV1 and %change in Feno for the whole population were also nonsignificant; this is also most likely because of a lack of power in the analysis.
We are not aware of published studies that relate change in spirometry to future asthma outcomes, but there are several studies in which spirometry on a single occasion has been related to subsequent asthma outcomes in children. One study reported an inverse relationship between reduced %FEV1 and increased risk for asthma exacerbation in the next 12 months.26 Two further studies27, 28 (data from one27 contributed to the present IPD) reported that reduced FEV1/FVC ratio was associated with increased risk for future exacerbation.
The 2015 European Respiratory Society Task Force on Monitoring Asthma in Children29 stated that “the meaning of significant changes in Feno in a longitudinal setting is still unclear and needs further attention, and that “the use of ‘personal best’ cut-off points in Feno algorithms requires further investigation.” Our study findings suggest that a relatively large rise (50%) in Feno over 3 months (independent of treatment and initial symptoms) may be a useful predictor of having uncontrolled asthma in future but not for asthma exacerbations. Additionally, our results suggest that a single Feno value and absolute change in Feno over time are unlikely to be clinically useful.
In summary, our results suggest that %FEV1 within the “normal” range over 3-month periods could assist with risk assessment in childhood asthma. These findings now need replicating elsewhere. A fall in %FEV1 and a rise in Feno should prompt an evaluation of medication adherence, inhaler technique, perception of symptoms, and exposure to either allergens or viral infection. The relationship between changes in Feno measurements and asthma outcomes is less clear and requires further evaluation.
Acknowledgments
Author contributions: S. T. conceived the idea for the study, wrote the first draft of the manuscript, and is the guarantor of the study. S. T. and S. F. designed the study. All authors other than S. T. and S. F. contributed data for the analysis. S. F. undertook the analysis. All authors made contributions to the final manuscript.
Financial/nonfinancial disclosure: None declared.
Other contributions: The individual patient data analysis was carried out at the University of Aberdeen. Original data collection took place in the remaining institutions.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Asthma UK. Asthma facts and FAQs. 2017. https://www.asthma.org.uk/about/media/facts-and-statistics/. Accessed August 31, 2017.
- 2.Centers for Disease Control and Prevention. Asthma. Most recent data. 2016. https://www.cdc.gov/asthma/most_recent_data.htm. Accessed August 31, 2017.
- 3.Bacharier L.B., Strunk R.C., Mauger D., White D., Lemanske R.F.J., Sorkness C.A. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170(4):426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 4.British Thoracic Society and Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. 2016 https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/. Accessed August 31, 2017.
- 5.Papadopoulos N.G., Arakawa H., Carlsen K. International consensus on (ICON) pediatric asthma. Allergy. 2012;67(8):976–997. doi: 10.1111/j.1398-9995.2012.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management. 2017 https://www.nice.org.uk/guidance/ng80. Accessed January 28, 2018. [PubMed] [Google Scholar]
- 7.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2017. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed August 31, 2017.
- 8.National Asthma Education and Prevention Program. Expert Panel Report 3—Guidelines for the Diagnosis and Management of Asthma. 2007. https://www.nhlbi.nih.gov/files/docs/guidelines/asthsumm.pdf. Accessed January 2, 2018.
- 9.Silkoff P.E., Carlson M., Bourke T., Katial R., Ogren E., Szefler S.J. The Aerocrine exhaled nitric oxide monitoring system NIOX is cleared by the US Food and Drug Administration for monitoring therapy in asthma. J Allergy Clin Immunol. 2004;114(5):1241–1256. doi: 10.1016/j.jaci.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Dweik R.A., Boggs P.B., Erzurum S.C. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (Feno) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (Feno) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritsch M., Uxa S., Horak F.J. Exhaled nitric oxide in the management of childhood asthma: a prospective 6-months study. Pediatr Pulmonol. 2006;41(9):855–862. doi: 10.1002/ppul.20455. [DOI] [PubMed] [Google Scholar]
- 12.Peirsman E.J., Carvelli T.J., Hage P.Y. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49(7):624–631. doi: 10.1002/ppul.22873. [DOI] [PubMed] [Google Scholar]
- 13.Petsky H.L., Li A.M., Au C.T., Kynaston J.A., Turner C., Chang A.B. Management based on exhaled nitric oxide levels adjusted for atopy reduces asthma exacerbations in children: a dual centre randomized controlled trial. Pediatr Pulmonol. 2015;50(6):535–543. doi: 10.1002/ppul.23064. [DOI] [PubMed] [Google Scholar]
- 14.Pijnenburg M.W., Bakker E.M., Hop W.C., De Jongste J.C. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2005;172(7):831–836. doi: 10.1164/rccm.200503-458OC. [DOI] [PubMed] [Google Scholar]
- 15.Pike K., Selby A., Price S. Exhaled nitric oxide monitoring does not reduce exacerbation frequency or inhaled corticosteroid dose in paediatric asthma: a randomised controlled trial. Clin Respir J. 2013;7(2):204–213. doi: 10.1111/j.1752-699X.2012.00306.x. [DOI] [PubMed] [Google Scholar]
- 16.Szefler S.J., Mitchell H., Sorkness C.A. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372(9643):1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voorend-van Bergen S., Vaessen-Verberne A.A., Brackel H.J. Monitoring strategies in children with asthma: a randomised controlled trial. Thorax. 2015;70(6):543–550. doi: 10.1136/thoraxjnl-2014-206161. [DOI] [PubMed] [Google Scholar]
- 18.Turner S. Exhaled nitric oxide and the management of childhood asthma – yet another promising biomarker “has been” or a misunderstood gem. Paediatr Respir Rev. 2015;16(2):88–96. doi: 10.1016/j.prrv.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Riley R.D., Lambert P.C., Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. Brit Med J. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 20.Quanjer P.H., Stanojevic S., Cole T.J. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanojevic S., Wade A., Stocks J. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177(3):253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole T.J., Bellizzi M.C., Flegal K.M., Dietz W.H. Establishing a standard definition for child overweight and obesity worldwide: international survey. Brit Med J. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Thoracic Society, European Respiratory Society ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 24.Burke D.L., Ensor J., Riley R.D. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med. 2017;36(5):855–875. doi: 10.1002/sim.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verini M., Consilvio N.P., Di Pillo S. Feno as a marker of airways inflammation: the possible implications in childhood asthma management. J Allergy (Cairo) 2010 doi: 10.1155/2010/691425. pii: 691425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuhlbrigge A.L., Kitch B.T., Paltiel A.D. FEV(1) is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001;107(1):61–67. doi: 10.1067/mai.2001.111590. [DOI] [PubMed] [Google Scholar]
- 27.Teach S.J., Gergen P.J., Szefler S.J. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135(6):1465–1473.e5. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu A.C., Tantisira K., Li L. Predictors of symptoms are different from predictors of severe exacerbations from asthma in children. Chest. 2011;140(1):100–107. doi: 10.1378/chest.10-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pijnenburg M.W., Baraldi E., Brand P.L.P. Monitoring asthma in children. Eur Respir J. 2015;45(4):906–925. doi: 10.1183/09031936.00088814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.