Abstract
Background
The aim of this study was to compare completion of the Surviving Sepsis Campaign 3-hour treatment recommendations and patient-centered outcomes between patients with severe sepsis who received a sepsis-specific diagnosis code with those who did not.
Methods
This was a retrospective cohort analysis of adult patients admitted through an academic medical center ED who received an antibiotic and met criteria for severe sepsis. We measured and compared the Surviving Sepsis Campaign 3-hour treatment recommendations along with patient-centered outcomes in patients who were diagnosed with severe sepsis and those who were not.
Results
A total of 5,631 patients were identified (60.6 ± 17.2 years of age; 48.9% women). Less than half (32.8%) received an International Classification of Diseases, ninth revision, diagnosis code of 995.92. Completion of all four bundle components in < 3 hours was low for all patients (8.72%). Therapeutic components (a broad-spectrum antibiotic and IV fluids) were completed more often (31.3%). Those with a diagnosis code received all four bundle components (10.2% vs 7.9%; P < .005), as well as therapeutic components at a higher frequency (36.0% vs 29.0%; P < .001). Patients with a diagnosis code had higher mortality (6.3% vs 2.3%), more frequent ICU admissions (44.7% vs 22.5%), and longer hospitalizations (9.2 ± 6.9 days vs 6.9 ± 6.7 days) than did patients with severe sepsis with no diagnosis code (all P < .001).
Conclusions
Severe sepsis continues to be an underdiagnosed and undertreated condition. Patients who were diagnosed had higher treatment rates yet experienced worse outcomes. Continued investigation is needed to identify factors contributing to diagnosis, treatment, and outcomes in patients with severe sepsis.
Key Words: clinical coding, ICD-9, outcomes, severe sepsis, treatment bundles
Abbreviations: HERON, Healthcare Enterprise Repository for Ontological Narration; ICD-9, International Classification of Diseases, ninth revision; SOFA, Sequential Organ Failure Assessment
Severe sepsis is a syndrome of life-threatening organ dysfunction due to a dysregulated host response to infection. It is the leading cause of death among hospitalized patients with infection.1, 2 Despite evidence that early recognition and interventions decrease severe sepsis mortality and morbidity, patients meeting severe sepsis criteria are underdiagnosed. It is estimated that only one in five patients with severe sepsis received an International Classification of Diseases, ninth revision (ICD-9) code of 995.92.3, 4, 5 We hypothesized that uncoded cases represent an underdiagnosed and undertreated group of patients with severe sepsis.
There are limited data regarding treatment rates and outcomes of patients with severe sepsis who are not specifically diagnosed as having sepsis. Only one study to date has compared outcomes between patients with severe sepsis who received codes for sepsis and those who did not. Whittaker et al5 found that patients with a code of 995.92 had higher mortality rates, longer hospitalizations, and higher measures of organ dysfunction when compared with patients with severe sepsis who received a code. To our knowledge, these findings have not been replicated, and treatment differences between patients who were or were not diagnosed and given a code to indicate they had the condition have not been compared. The primary objective of this study was to compare both treatments and patient-centered outcomes among patients who were diagnosed with severe sepsis and patients who met clinical criteria for severe sepsis but who were not specifically diagnosed with severe sepsis.
Methods
All patients admitted through the University of Kansas Hospital ED from November 1, 2007 (inception of the institution’s electronic medical record) through September 30, 2015 (last date before conversion to the ICD-10 diagnosis codes) were eligible for study inclusion. Study inclusion required that patients were ≥ 18 years of age, received an ICD-9 diagnosis code for acute infection, were given an antibiotic within 8 hours of ED triage defined as an initial nursing evaluation, and had discharge disposition codes available for the encounter. We required an antibiotic to be given within 8 hours of triage to better identify those presenting with the condition rather than acquiring sepsis during the hospitalization. After meeting these inclusion criteria, patients were retained for analysis if they met case definitions for severe sepsis: (1) a 995.92 ICD-9 diagnosis code for severe sepsis or (2) documented presence of an infection plus two or more sites of organ dysfunction. Acute organ dysfunction was defined either by using specific ICD-9 diagnosis codes or by the presence of an abnormal first laboratory or physiological marker of acute organ dysfunction (e-Tables 1, 2). Laboratory threshold values followed those proposed by the American College of Chest Physicians/Society of Critical Care Medicine definitions.6, 7 Any patient who had criteria for shock who did not meet criteria for severe sepsis or who was not given an antibiotic within 8 hours of triage was excluded from subsequent analysis. We required that patients have organ dysfunction at two or more sites to increase the likelihood that patients had severe sepsis and not isolated organ dysfunction associated with the same site of infection.
The primary outcome measure was completion of the Surviving Sepsis Campaign 3-hour treatment bundle.8 Each component of the protocol (blood culture ordered, serum lactate levels ordered, broad spectrum-antibiotics administered, and appropriate IV fluids administered) was analyzed independently for frequency of completion within 3 hours. If all four components were completed within 3 hours, the patient was considered to have had successful bundle completion. Appropriate fluid was defined as either IV fluids given at 30 mL/kg if the patient was hypotensive or had a lactate level > 4 mmol/L or no IV fluids if the patient was normotensive and lactate levels were < 4 mmol/L.8 Additionally, completion of the therapeutic components (defined as both a broad-spectrum antibiotic and appropriate fluids given within 3 hours of triage) was analyzed separately, as these two components have been shown to individually improve mortality. Fluid times were limited to those initiated within 2½ hours of triage time to prevent overestimating the actual fluid received by a patient, as fluids could have been initiated, but not completed, by the 3-hour mark.
The second study goal was to compare patient-centered outcomes, including hospital mortality, 30-day readmission, and length of stay, between patients with and those without an ICD-9 code specific to severe sepsis (code 995.92). To evaluate for differences in illness at presentation and baseline medical status, Sequential Organ Failure Assessment (SOFA) scores and Charlson Comorbidity Index scores were calculated and compared. SOFA scores were calculated using the first laboratory and physiological values recorded to capture illness status at ED triage.
Data cleaning and sensitivity analysis were performed using SAS software, version 9.4 (SAS Institute Inc.). All binary outcomes were analyzed using the χ2 test, and continuous data were evaluated using the Student t test. Multivariable logistic regression analysis was performed to identify independent predictors of patients receiving complete treatment within the first 3 hours of admission and factors associated with receiving an ICD-9 diagnosis code of 995.92. Overall model fit was assessed using the Hosmer and Lemeshow goodness of fit test, in which a higher P value signifies a better overall fit.
The university’s institutional review board approved this study with a waiver of informed consent (study No. 00001753). Data were collected from the electronic medical record using the i2b2-based interface query tool HERON.9 Flowsheet data not captured by the HERON interface were electronically obtained from the hospital’s SQL database by matching medical record numbers and triage dates with the query software Crystal Reports (SAP Software Solutions).
Results
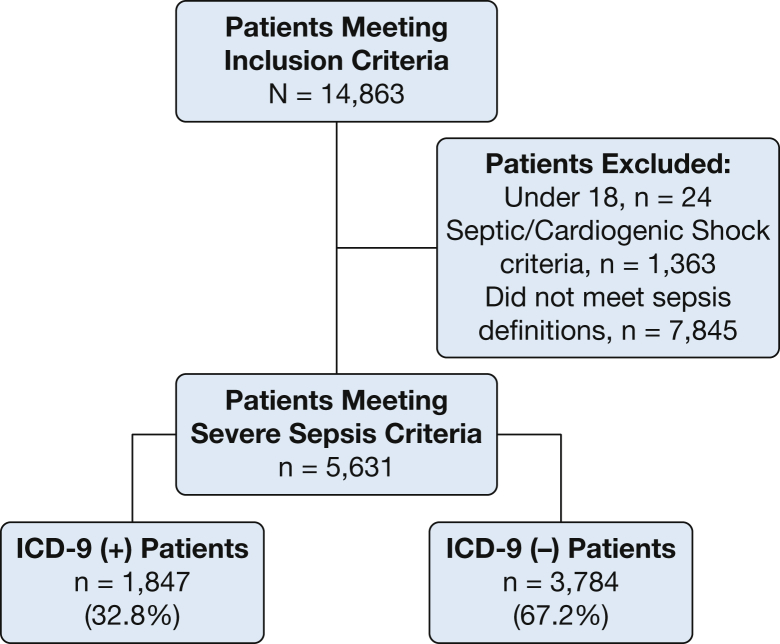
A total of 14,863 patients were identified, 9,232 of whom were excluded for not meeting severe sepsis criteria or for having septic shock. A total of 5,631 patients with severe sepsis were included in the final analysis (Fig 1). The mean age was 60.6 ± 17.2 years, and 48.9% (n = 2,759) were women. The presence of an appropriate 995.92 diagnosis code was found for 32.8% of patients (n = 1,847).
Figure 1.
Cohort organization.
Patients with severe sepsis with a diagnosis code of 995.92 had a higher number of total documented infections (2.3 vs 1.8) and were more likely to receive a diagnosis of septicemia (97.8% vs 64.0%) or a diagnosis of a respiratory infection (51.8% vs 41.2%) when compared with patients with severe sepsis without a diagnosis code (all P < .01) (Table 1). When comparing baseline acute organ dysfunction, no clinically apparent differences were seen based on the first SOFA scores, first lactate measurements, or total number of involved organ dysfunction sites. Patients with a diagnosis code were more likely to have respiratory organ dysfunction (48.3% vs 34.9%; P < .001). No significant differences were found when comparing Charlson Comorbidity Index scores.
Table 1.
Cohort Characteristics for Patients With Severe Sepsis
| Variable | All Patients With Severe Sepsis |
Patients With 995.92 Code |
Patients Without 995.92 Code |
P Valuea |
|---|---|---|---|---|
| N = 5,631 | n = 1,847 (32.8%) | n = 3,784 (67.2%) | ||
| Age, mean ± SD, y | 60.6 ± 17.2 | 61.2 ± 16.7 | 59.5 ± 18.0 | < .001 |
| Sex, female, No. (%) | 2,759 (48.9%) | 914 (49.4%) | 1,845 (48.7%) | .61 |
| Race, No. (%) | ||||
| White | 3,710 (65.9) | 1,198 (64.9) | 2,512 (66.4) | |
| Black | 1,339 (23.8) | 436 (23.6) | 903 (23.9) | |
| Other | 576 (10.2) | 210 (11.4) | 366 (9.67) | |
| Charlson Comorbidity Index score (of 21 ± SD) | 6.03 ± 3.59 | 5.64 ± 5.48 | 6.22 ± 6.11 | .32 |
| Measures of infection | ||||
| Sites of infection, mean ± SD | 1.95 ± 0.88 | 2.31 ± 0.82 | 1.78 ± 0.84 | < .001 |
| Bacteremia/septicemia, No. (%) | 4,229 (75.1) | 1,806 (97.8) | 2,423 (64.0) | .001 |
| Respiratory | 2,516 (44.7) | 957 (51.8) | 1,559 (41.2) | .001 |
| Urinary | 2,176 (38.6) | 741 (40.1) | 1,435 (37.9) | .11 |
| Soft tissue | 986 (17.5) | 336 (18.2) | 650 (17.2) | .35 |
| Abdomen | 523 (9.29) | 194 (10.5) | 329 (8.69) | .03 |
| Measures of acute organ dysfunctionb | ||||
| First serum lactate level, mean ± SD | 2.21 ± 1.61 | 2.33 ± 1.73 | 2.13 ± 1.53 | < .001 |
| Average SOFA score, mean of 24 ± SD | 3.02 ± 2.08 | 2.89 ± 2.17 | 3.07 ± 2.03 | < .001 |
| First MAP, mean ± SD | 92.3 ± 20.3 | 91.8 ± 20.6 | 92.5 ± 20.2 | .23 |
| Organ dysfunction sites, mean of 7 ± SD, No. (%) | 2.58 ± 0.99 | 2.62 ± 1.29 | 2.56 ± 0.82 | .06 |
| Renal | 3,145 (56.8) | 1,024 (58.3) | 2,121 (56.1) | .12 |
| Hematologic | 2,922 (52.7) | 821 (46.7) | 2,101 (55.5) | < .001 |
| Respiratory | 2,170 (39.2) | 849 (48.3) | 1,321 (34.9) | < .001 |
| Cardiovascular | 1,444 (26.1) | 437 (24.9) | 1,007 (26.6) | .17 |
| CNS | 1,665 (30.0) | 529 (30.0) | 1,136 (30.0) | .97 |
MAP = mean arterial pressure; SOFA = Sequential Organ Failure Assessment.
All P values < .001 were rounded and reported as P < .001.
Two thousand two hundred thirty-six patients (39.7%) never had a serum lactate measurement, 105 patients (1.9%) had one or more components missing that are necessary to calculate a complete SOFA score, and 51 patients (0.9%) had an unattainable MAP. They were excluded from the respective calculation.
Treatment Differences
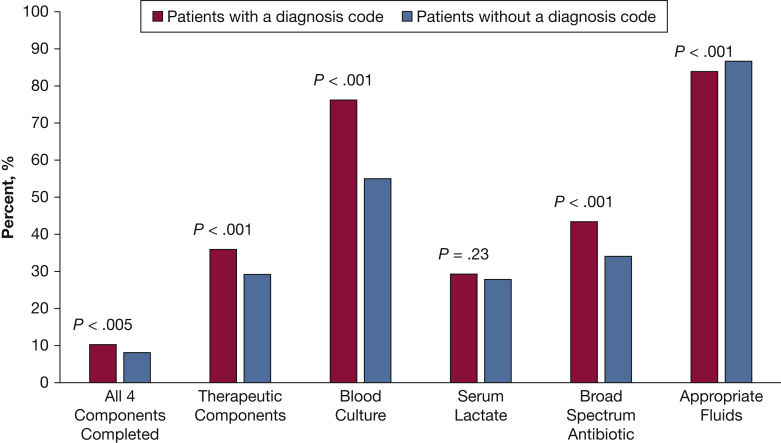
Receipt of all four Surviving Sepsis Campaign components in < 3 hours was low for all patients (8.7%), but those with a diagnosis code of 995.92 had higher completion rates than did patients without a diagnosis code (10.2% vs 7.9%, respectively; P < .005) (Fig 2). Therapeutic component completion, both a broad-spectrum antibiotic and appropriate fluid, was higher than total bundle protocol completion for the whole cohort (31.3% vs 8.7%) and was also higher for those with a diagnosis code compared with those without a diagnosis code (36.0% vs 29.0%; P < .001). The frequency of all individual protocol component completion was higher in those with a diagnosis code. A blood culture was the most common of all four components completed in all patients (n = 3,483 [61.9%]), and serum lactate measurement was completed least often (n = 1,589 [28.2%]). Only 46.1% of patients received an antibiotic within 3 hours of triage, as recommended, but 75.8% of patients appropriately received a broad-spectrum antibiotic as their first dose. The mean time to first antibiotic administration was 3.7 ± 1.9 hours for the whole cohort. Patients with a diagnosis code were given antibiotics sooner than were patients without a diagnosis code (3.2 ± 1.9 hours vs 3.9 ± 2.0 hours; P < .001). Few patients, (16.5% [n = 928]) had a requirement for fluid resuscitation by lactate or BP criteria, as patients with septic shock were excluded from the analysis. Patients with a diagnosis code of 995.92 were more likely to require fluid resuscitation (20.1% vs 14.8%; P < .001) and to receive 30 mL/kg of crystalloid than were patients without a code (19.6% vs 10.6%; P < .001) (e-Table 3).
Figure 2.
Three-hour Surviving Sepsis Campaign treatment rates by presence or absence of International Classification of Diseases, ninth revision diagnosis code specific to severe sepsis. Therapeutic components were defined as both a broad-spectrum antibiotic and appropriate IV fluids administered within 3 hours of triage. Appropriate fluid was defined as crystalloid IV fluids given at a rate of 30 mL/kg within 2.5 hours of triage if the patient was hypotensive or had a lactate level > 4 mmol/L or were appropriately not given IV fluids if the patient had no need for fluids based on mean arterial pressure (MAP) and lactate values. Following the Society of Critical Care Medicine definitions, a need for fluids was met if the first MAP was < 70 mm Hg or the first serum lactate level was ≥ 4 mmol/L.
Multivariate logistic regression did not result in a good overall model fit that could predict receiving all four components of the 3-hour bundle. The most significant predictors of receiving the therapeutic components of the bundle were having a respiratory infection (OR, 1.56; 95% CI, 1.43-1.78), receiving a sepsis-specific ICD-9 diagnosis code (OR, 1.24; 95% CI, 1.10-1.36), presence of respiratory dysfunction (OR, 1.25; 95% CI, 1.12-1.40), and age (OR, 1.006; 95% CI, 1.00-1.01). Sex, race, number of comorbidities, and markers of acute organ dysfunction including SOFA scores, first lactate measurements, and total number of dysfunctional organ sites were not found to be predictive. Components of the final model can be found in e-Table 4.
Due to a higher prevalence of respiratory infection in patients with a diagnosis code (51.8% vs 41.2%) (Table 1) and because respiratory infection was independently predictive of patients receiving treatment in multivariate analysis, a subanalysis of treatment completion for patients with and those without diagnosis codes was performed while controlling for respiratory infections. Differences in overall bundle treatment and therapeutic component treatment completion no longer differed, and completion of individual components continued to be higher in patients with a diagnosis code (e-Fig 1).
Patient-Centered Outcomes
Overall severe sepsis mortality was 3.6% (Table 2). Mortality was significantly higher among those patients with a diagnosis code of 995.92 compared with patients without (6.3% vs 2.3%; P < .001). Patients with a diagnosis code also had more frequent ICU admissions (44.7% vs 22.5%), longer hospital length of stay (9.2 ± 9.4 vs 6.9 ± 6.7 days), and were more frequently discharged with home health-care services (22.4% vs 19.5%) (all P < .01). Patients who were given codes were also less likely to be discharged home (43.6% vs 52.0%; P < .001) and were more likely to be discharged with hospice care (6.1% vs 4.4%; P < .001) or to a long-term care facility (2.5% vs 1.7%; P = .04). Patients without a diagnosis code, however, had higher (30-day) readmission rates (25.2% vs 21.5%; P = .02).
Table 2.
Discharge Outcomes of Patients With Severe Sepsis
| Outcome Measures | All Patients With Severe Sepsis Criteria |
Patients With 995.92 Code |
Patients Without 995.92 Code |
P Valuea |
|---|---|---|---|---|
| N = 5,631 | n = 1,847 (32.8%) | n = 3,784 (67.2%) | ||
| Mortality, % | 204 (3.62) | 117 (6.33) | 87 (2.30) | < .001 |
| Hospital length of stay, mean ± SD, d | 7.63 ± 7.76 | 9.14 ± 9.35 | 6.89 ± 6.73 | < .001 |
| 7.63 ± 7.77b | 9.21 ± 9.42b | 6.89 ± 6.73b | ||
| ICU length of stay, mean ± SD, d | 3.71 ± 4.07 | 3.92 ± 4.32 | 3.50 ± 3.80 | .04 |
| 3.73 ± 4.15b | 3.97 ± 4.45b | 3.50 ± 3.85b | .02 | |
| ICU admission rate, No. (%) | 1,679 (29.8) | 826 (44.7) | 853 (22.5) | < .001 |
| 30-d readmission rate, No. (%) | 1,352 (24.0) | 397 (21.5) | 955 (25.2) | .02 |
| Discharge location, No. (%) | ||||
| Home | 2,771 (49.2) | 805 (43.6) | 1,966 (52.0) | < .001 |
| Home, with home health services | 1,151 (20.4) | 414 (22.4) | 737 (19.5) | .01 |
| Rehabilitation facility | 129 (2.29) | 42 (2.27) | 87 (2.30) | 1.00 |
| Acute nursing care | 855 (15.2) | 269 (14.6) | 586 (15.5) | .38 |
| Long-term care | 109 (1.94) | 46 (2.49) | 63 (1.66) | .04 |
| Hospice | 281 (5.00) | 113 (6.12) | 168 (4.44) | < .001 |
All P values < .001 were rounded and reported as P < .001.
Values account for those who died during hospitalization by removing them from this analysis.
Multivariate logistic regression analysis was performed to identify the role of case documentation (ICD-9 presence or absence), acuteness of cases (organ dysfunction), and treatment intervention on 30-day mortality. We found that the odds of death were independently predicted by the presence of ICD-9 status (OR, 2.53; 95% CI, 1.89-3.39), number of organ dysfunctions on presentation to the ED (OR, 1.85; 95% CI, 1.66-2.08), and receipt of all four components of the bundle protocol (OR, 0.42; 95% CI, 0.22-0.80) (e-Table 5).
Discussion
We have presented a retrospective study of 5,631 patients with severe sepsis admitted through the ED at a single urban medical center. It is the largest study to date investigating treatment and outcome differences between patients with and those without a sepsis-specific ICD-9 diagnosis code. Consistent with previous studies, we found that patients meeting clinical criteria for severe sepsis continue to be underdiagnosed, as evidenced by more than half of the cohort lacking a sepsis-specific ICD-9 diagnosis code (67.2%).1, 4, 10 The ICD-9 code (and now the ICD-10 code) is applied only when providers have recorded the specific diagnosis of severe sepsis in the medical record, being both sensitive when documentation is present and 99.5% specific when validated by chart adjudication.11 These features make the 995.92 code an excellent marker for whether physicians have specifically diagnosed severe sepsis during their treatment.
We demonstrated that all patients, with or without sepsis-specific ICD-9 diagnosis codes, are relatively unlikely to receive treatment according to the 3-hour bundle guidelines. However, those with an ICD-9 diagnosis code received individual bundle components and the complete bundle protocol more often than did those without a code. Having a diagnosis code was also independently associated with receiving treatment after adjusting for other possible factors in multivariate analysis. We believe this signifies that patients without a diagnosis code are not simply patients who have not received a diagnosis code but are a group of patients who go underrecognized and undertreated.
We also hypothesized that patients without an ICD-9 diagnosis would experience worse outcomes. However, similar to Whittaker et al,5 we found that patients with a severe sepsis diagnosis code of 995.92 had worse clinical outcomes compared with patients without a code. All patients given a 995.92 code had a higher number of infections, were more frequently diagnosed with septicemia, and had a higher number of total organ dysfunction sites.4, 5 It is possible that patients with severe sepsis who are not formally diagnosed are a less acutely ill population and, despite being less aggressively treated, experience better outcomes.5 However, unlike previous studies, we demonstrated that patients with and those without ICD-9 diagnosis codes had similar baseline measures of acute organ dysfunction at presentation in the ED. Nationwide surveys of ED providers reported that identification of patients meeting severe sepsis criteria was the greatest barrier to early initiation of treatment bundles.12, 13, 14 This study might suggest that despite similar presentation severity, some patients with severe sepsis will progress more rapidly than others, adding to the challenging aspect of identifying this population.
Our study has several limitations. The data in this study were collected and analyzed before the publication of the Sepsis-3 definitions, which might have increased the specificity of our cohort for mortality or prolonged ICU stay. However, because this study focused on physician identification and treatment of patients with severe sepsis during the time of Sepsis-2 consensus definitions, the selection criteria we chose were appropriate. Our study used patients admitted through a single center's ED, limiting generalizability to other institutions and hospital settings. Our retrospective design also limits our ability to determine when, or by whom, patients were diagnosed. It is possible that not all patients who received codes presented to the ED with criteria for severe sepsis at the initial triage time, and some acquired the condition later during hospitalization. We do believe that requiring patients to have had an antibiotic within 8 hours and two sites or more of organ dysfunction helps increase the likelihood of acute infection and organ dysfunction on presentation. Finally, due to the large size of our cohort, interpretation of statistically significant findings requires clinical judgment when analyzing the effect size of the differences between groups.
This study generates several questions that could amplify its findings. Although educational and quality improvement interventions improve bundle performance and outcomes, it would be of interest to know whether they also alter the assignment of specific ICD diagnoses.15 It would be useful to survey physicians regarding what factors determine whether patients are assigned a diagnosis leading to an ICD code specific to sepsis.
Conclusions
This study highlights a concerning finding that patients with severe sepsis are both underdiagnosed and undertreated. It appears that the diagnosis of severe sepsis was most commonly used to describe patients with more severe illness. Early adverse outcomes, such as mortality, were not affected by a specific diagnosis of severe sepsis, but longer-term outcomes, including hospital readmission, were increased in patients who did not receive the specific diagnosis. Studies of this nature should be repeated as definitions of sepsis evolve.
Acknowledgments
Author contributions: S. Q. S. takes responsibility for the content of the manuscript, including the data and analysis. All authors had full access to all the data in the study and take responsibility for the integrity and accuracy of the analysis. A. D., B. W., and S. S. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. K. S. and A. B. contributed substantially to the data analysis and interpretation.
Financial/nonfinancial disclosures: None declared.
Other contributions: We thank Tamara McMahon, MIS, and Li Huang, MPH, from the Division of Medical Informatics, as well as Eric Howser, BS, and Chris Wittkopp, RHIT, from the Office of Institutional Organization, for their assistance in data capture, making this project possible.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: A. D. received support from a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers [The Heartland Institute for Clinical and Translational Research No. TL1TR000120]. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS. B. B. W. received funding support from the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship scholarship.
Supplementary Data
References
- 1.Angus D.C., Linde-Zwirble W.T., Lidicker J. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality (US); Rockville, MD: 2011. HCUP facts and figures: statistics on hospital-based care in the United States, 2009.https://www.ncbi.nlm.nih.gov/books/NBK91984/ Accessed June 17, 2016. [PubMed] [Google Scholar]
- 3.Iwashyna T.J., Odden A., Rohde J. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52(6):39–43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaieski D.F., Edwards J.M., Kallan M.J. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 5.Whittaker S.A., Mikkelsen M.E., Gaieski D.F. Severe sepsis cohorts derived from claims-based strategies appear to be biased towards a more severely ill patient population. Crit Care Med. 2013;41(4):945–953. doi: 10.1097/CCM.0b013e31827466f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy M.M., Fink M.P., Marshall J.C. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 7.Bone R.C., Balk R.A., Cerra F.B. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger R.P., Levy M.M., Rhodes A. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 9.Waitman L.R., Warren J.J., Manos E.L. Expressing observations from electronic medical record flowsheets in an i2b2 based clinical data repository to support research and quality improvement. American Medical Informatics Association, Annual Symposium Proceedings. 2011:1454–1463. [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar G., Kumar N., Taneja A. Nationwide trends of severe sepsis in the 21st century (2000-2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 11.Brandt B.N., Gartner A.B., Moncure M. Identifying severe sepsis via electronic surveillance. Am J Med Qual. 2015;30(6):559–565. doi: 10.1177/1062860614541291. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez K.L., Burkitt K.H., Sevick M.A. Assessing processes of care to promote timely initiation of antibiotic therapy for emergency department patients hospitalized for pneumonia. Jt Comm J Qual Patient Saf. 2009;35(10):509–518. doi: 10.1016/s1553-7250(09)35070-9. [DOI] [PubMed] [Google Scholar]
- 13.Burney M., Underwood J., McEvoy S. Early detection and treatment of severe sepsis in the emergency department: identifying barriers to implementation of a protocol-based approach. J EmergNurs. 2012;38(6):512–517. doi: 10.1016/j.jen.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Carlbom D.J., Rubenfeld G.D. Barriers to implementing protocol-based sepsis resuscitation in the emergency department–results of a national survey. Crit Care Med. 2007;35(11):2525–2532. doi: 10.1097/01.ccm.0000298122.49245.d7. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer R., Artigas A., Levy M.M. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.