Abstract
Background
Proton pump inhibitors (PPIs) and histamine type 2 receptor blockers (H2Bs) are used for stress ulcer prophylaxis. Although the PPIs have greater potency for acid suppression, their relative effectiveness for preventing clinically important GI bleeding (CIGIB) has not been established. The goal of this study was to determine whether prophylactic PPIs were associated with lower risk of CIGIB than H2Bs among critically ill adults.
Methods
This retrospective cohort study included adults with critical illness from January 1, 2008, to June 30, 2012, who had at least one stress ulcer risk factor and received a PPI or H2B for ≥ 3 days. Cox proportional hazards regression propensity score matching and instrumental variable analyses were used to control for selection bias and confounding by unmeasured factors. The Acute Physiology and Chronic Health Evaluation Score version IV score was used to adjust for differences of acuity. The main outcome and exposure was CIGIB.
Results
Among 70,093 patients at risk, 49,576 (70.7%) received prophylaxis for at least 3 days, and 424 patients (0.6%) met the definition for experiencing CIGIB. The hazard for CIGIB was two times greater for PPI users compared with H2B users (adjusted hazard ratio, 1.82 [95% CI, 1.19-2.78]; hazard ratio, 2.37 [95% CI, 1.61-3.5]). Sensitivity analyses failed to detect any plausible scenario in which PPIs were superior to H2Bs for the prevention of CIGIB.
Conclusions
H2Bs were robustly and consistently associated with significantly lower CIGIB risk compared with PPIs in this population.
Key Words: bleeding, critical care, ICU
Abbreviations: APACHE-IV, Acute Physiology and Chronic Health Evaluation version IV; CIGIB, clinically important GI bleeding; H2B, histamine type 2 receptor blocker; HR, hazard ratio; ICD-9, International Classification of Diseases, Ninth Revision; LOS, length of stay; PPI, proton pump inhibitor; PSM, propensity score matching
Proton pump inhibitors (PPIs) and histamine type 2 receptor blockers (H2Bs) are the main stress ulcer prophylactic agents prescribed by critical care providers. The 1999 American Society of Health-System Pharmacists’ guidelines that recommended H2Bs1 are discordant with subsequent meta-analyses that favored PPIs.2, 3, 4, 5, 6 Methodologic flaws of the studies that were included in these meta-analyses3 raised important concerns regarding which of these alternative therapies are superior in terms of preventing bleeding in critical care practice. Serious questions regarding recommendations that favor PPIs were raised by a 2014 observational study of 35,312 patients.7 This study reported that PPIs were associated with a higher risk of GI bleeding (OR, 2.24 [95% CI, 1.81-2.76]) than H2Bs. However, this study also had some concerning limitations; it included GI bleeding episodes that did not meet accepted definitions for being clinically important.8, 9 In addition, its estimates of the protective effects of H2Bs could have been inflated due to higher acuity of the group that received PPIs and the fact that the type of prophylactic agent clustered according to ICU. In addition to concerns that PPIs may not be superior to H2B for preventing bleeding events, recent cost-effectiveness analyses that assume that PPIs have a better ability to prevent bleeding have found prophylaxis with H2Bs to be more cost-effective than prophylaxis with PPIs.10
We performed a multicenter study in a geographically dispersed population of adults cared for in US nonfederal ICUs that had near-universal adherence to guidelines for stress ulcer prophylaxis (98%).11 Although stress ulcer prophylaxis was nearly always prescribed for high-risk patients, some at-risk patients did not receive 3 days of prophylaxis due to extubation, resolution of sepsis, or for other reasons. The choice of a PPI or an H2B was sufficiently heterogeneous to allow comparative effectiveness analyses of their association with clinically important GI bleeding (CIGIB). The study design included adjustment for acuity that is specific for critically ill adults, analytical methods that balance measured factors which differed among the groups, and techniques which account for unknown factors that cluster with the prophylaxis-prescribing habits of individual ICUs.
Patients and Methods
Data
The data security, structure, sources, and characteristics of the Philips eICU Research Institute data repository have been previously described.12, 13 Briefly, this repository is a de-identified electronic clinical database that contains physical examination, laboratory result, clinical diagnosis, treatment, and vital signs variables from adult patients of geographically dispersed US nonfederal hospitals. Selection of disease diagnoses was performed through a menu of discrete diagnosis strings that are linked to individual International Classification of Diseases, Ninth Revision (ICD-9), codes. Health severity was measured according to the Acute Physiology and Chronic Health Evaluation version IV (APACHE-IV) score.14 Data security was certified by Privacert, Inc, as meeting safe harbor standards. Institutional review board evaluation (Human Subjects Review #12513) resulted in a waiver of the requirement for informed consent in accordance with the 45th Code of Federal Regulations 164.514 (b) (1) (i).
Inclusion and Exclusion Criteria
Between January 1, 2008, and June 30, 2012, patients were included who received a PPI or H2B with at least one of the following stress ulcer risk factors: mechanical ventilation > 24 h, coagulopathy, head injuries, major burns, sepsis, corticosteroid therapy > 250 mg of hydrocortisone or equivalent daily, acute renal failure, hepatic failure, transplantation, neurological injuries, hypotension, surgery, trauma, or ICU length of stay (LOS) > 1 week.
Exclusion criteria included ICU LOS < 72 h, GI bleeding within the first 72 h of admission, receipt of a PPI or H2B for < 3 days prior to an episode of CIGIB, concomitant or consecutive use of PPIs and H2Bs, or patients with missing platelet counts, admission source, or teaching hospital status.
Measures
The dependent variable was CIGIB. Episodes of GI bleeding were defined through the ICD-9 code 578 that encompassed hematemesis, blood in stool, and unspecified bleeding. Only one entry with the aforementioned code was required to define a bleeding episode. Diagnosis strings were used to exclude bleeding due to other causes such as “postpartum hemorrhage” within the aforementioned ICD-9 code. CIGIB episodes were defined in accordance with the definition of Cook et al,8, 9 after slight modification, as the occurrence of any of the following: (1) an absolute reduction in systolic blood pressure by at least 20 mm Hg; (2) reduction in diastolic blood pressure by at least 10 mm Hg; (3) heart rate increase by at least 20 beats/min; or (4) administration of a blood transfusion. The main independent variable was receipt of a PPI vs an H2B for at least 3 days before an episode of CIGIB.
The following covariates were included in the multivariable model: demographic characteristics (age, sex, and race); clinical variables (stress ulcer risk factor(s) as defined earlier, cancer, HIV, cirrhosis, enteral nutrition receipt, and intubation in the first day); medications that affect bleeding risk, including antiplatelet agents, anticoagulants, thrombolytics, nonsteroidal antiinflammatory drugs, sucralfate, and antacids; admission source; physician specialty; teaching hospital status; and APACHE-IV score.
Statistical Analyses
Univariable and bivariable analyses were used to describe the variables and their distributions and to compare the two treatment groups by using χ2 tests for categorical variables and t tests for continuous variables, respectively. A Cox proportional hazards model was fit to estimate the relative hazard of CIGB among patients exposed to at least 3 days of a PPI compared with patients exposed to at least 3 days of an H2B using patient-day observations. Patients were censored when they were discharged from the ICU.
Because treatment selection was nonrandom, propensity score matching (PSM) and instrumental analysis were used to make comparisons among groups with similar distributions of measured factors and to account for unmeasured covariates that track with stress ulcer prophylaxis-prescribing habits of their ICU, respectively.
Propensity Score Matching
In a multivariable logistic regression model, the propensity scores for those receiving 3 days of a PPI or 3 days of an H2B were determined by using the demographic characteristics, ICU type, enteral nutrition, cancer, HIV, cirrhosis, neutropenia, platelet count, immunosuppression, stress ulcer risk factors, sucralfate, antacids, anticoagulants, antiplatelets, thrombolytics, nonsteroidal antiinflammatory drugs, admission source, physician specialty, and APACHE-IV score. One-to-one matching with no replacement and a caliper of 0.00001 were then used to create matched groups. Covariate balance prior to and following matching was assessed by using t tests, accounting for matching design, and the standardized mean difference approach.15 If the P value of the t test was < .05 and the standardized mean difference was > 10%, the covariate was then considered imbalanced between the two groups and was therefore included in the final model. Lastly, we estimated exposure effect on the hazard of CIGIB by using Cox modeling.
Instrumental Variable Analyses
An instrumental variable approach that used the two-stage residual inclusion method16 was used to account for some unmeasured variables. The instrumental variable approach facilitates comparisons between intervention and control groups that are otherwise comparable by using an instrument to determine group assignment within a nonrandomized design. A valid instrumental variable has two characteristics: first, it must be strongly correlated with exposure; second, it should not be correlated with the unobserved variables that influence the outcome in the error term.17 Observing that an extremely high proportion of patients from an ICU are exposed to a PPI rather than to an H2B suggests that prescribing decisions may be dependent on ICU prescribing practices rather than individual patient characteristics. Consequently, the preferred therapeutic class for the patient’s ICU can be used as an instrumental variable. We classified ICUs that prescribed PPIs to at least 90% of their patients as PPI preference units. The first stage of the model was validated by demonstrating that the PPI preference variable was strongly correlated with the exposure (ie, the receipt of PPIs for 3 days [adjusted OR, 13.4 (95% CI, 10.9-16.5)]).
Sensitivity Analyses
Multiple case reports have suggested that PPIs may be associated with thrombocytopenia.18, 19, 20, 21, 22 Because coagulopathy from post-PPI thrombocytopenia was identified as a plausible explanation for the higher risk of bleeding associated with PPIs,7 we included testing post-PPI thrombocytopenia as a possible explanatory factor in our prespecified analyses.
Lastly, history of gastric ulcer or bleeding has been identified as a stress ulcer risk factor1 that was not available in our dataset. This unmeasured risk factor could act as a confounder and could also have been a factor influencing treatment selection because patients with these two conditions are more likely to be treated with PPIs than with H2Bs. We used the approach of Lin et al23 for assessing the impact of this unmeasured potential confounder in sensitivity analyses. This analysis was accomplished by adjusting the observed estimate by the prevalence of the unmeasured confounder in the PPI group and the H2B group and the effect of the unmeasured confounder on CIGIB using a simple formula. A series of other sensitivity analyses and their rationale are presented in Table 1.
Table 1.
Summary of Analyses for Studying the Risk of CIGIB Between Patients Who Received PPIs Compared With Patients Who Received H2Bs
| Analysis | Rationale | Results |
|---|---|---|
| Two-day use of PPIs compared with 2-day use of H2Bs n = 477,350 patient-days | Determine if shorter duration has the same effect on the risk of CIGIB Provide comparison to study by MacLaren et al7 |
(HR, 2.10 [95% CI, 1.65-2.67]) |
| Limiting cohort to patients who did not discontinue treatment or discontinued treatment no more than 2 days before discharge (84% of the original sample) n = 298,308 patient-days | The main model considers any patient who received the medications of interest for 3 days as exposed regardless of whether the medications were discounted later. This approach may lead to estimate overestimation | (HR, 1.81 [95% CI, 1.35-2.43]) |
| Removal of patients above the 90th percentile for ICU LOS n = 287,269 patient-days | Observations with extreme LOS may have skewed the results | (HR, 1.90 [95% CI, 1.4-2.6]) |
| Analysis confined to patients who stayed < 6 days in the ICU n = 114,274 patient-days | To examine the effect of occult bleeding during the first 6 days analyses were performed that excluded these patients | (HR, 1.5 [95% CI, 0.94-2.52]) |
| Testing the hypothesis of PPI-induced thrombocytopenia n = 356,147 patient-days | PPIs-induced thrombocytopenia has been reported in few case reports. If true, then posttreatment thrombocytopenia should be a mediator that, if adjusted for, will significantly reduce the observed HR | Model 1: Adjusted for baseline thrombocytopenia, baseline coagulopathy, and other covariates (HR, 1.97 [95% CI, 1.48-2.63]) Model 2: Adjusted for baseline thrombocytopenia, baseline coagulopathy, posttreatment thrombocytopenia, and other covariates (HR, 1.95 [95% CI, 1.44-2.65]) |
CIGIB = clinically important GI bleeding; H2Bs = histamine type 2 receptor blockers; HR = hazard ratio; LOS = length of stay; NSAIDs = nonsteroidal antiinflammatory drugs; PPIs = proton pump inhibitors.
All analyses accounted for ICU clustering effect by using a robust variance estimator. Data preparation was performed by using SAS version 9.3 (SAS Institute, Inc), and Stata version 11 was used for data analyses (StataCorp LP).
Results
In the present study, 70,093 patients contributed a total of 356,147 patient-days of observation. Exposure to 3 days of a PPI (70.7%) was more common than exposure to a H2B (29.3%). Almost 76% of the sample was white, and 54% was male. The most common stress ulcer risk factor was mechanical ventilation (60%). There were 424 cases of new CIGIB (0.6%) among critically ill adults exposed to at least 3 days of a PPI or H2B. The incidence rate of CIGIB in this cohort was 1.2 cases per 1,000 patient-days (95% CI, 1.08-1.31). More than 50% of patients received anticoagulants, antiplatelets, or nonsteroidal antiinflammatory drugs during their ICU stay (Table 2). After exclusion of patients with LOS < 72 h, the average LOS was 9.9 days while the median was 7 days.
Table 2.
Characteristics of Patients Who Received Either PPIs or H2Bs for at Least 3 Days During Their ICU Stay
| Characteristic | Bivariable Analyses |
||||
|---|---|---|---|---|---|
| H2Bs (n = 20,517) |
PPIs (n = 49,576) |
P Value | |||
| Frequency or Mean | Column % or SD | Frequency or Mean | Column % or SD | ||
| Outcome | |||||
| CIGIB | 63 | 0.3 | 361 | 0.7 | < .001 |
| Sex | |||||
| Male | 11,127 | 54.2 | 26,391 | 53.2 | .007 |
| Age, y | |||||
| 18-60 | 8,505 | 41.5 | 18,648 | 37.6 | < .001 |
| 61-70 | 4,552 | 22.2 | 11,380 | 23 | |
| 71-80 | 4,316 | 21 | 11,130 | 22.5 | |
| ≥ 81 | 3,144 | 15.3 | 8,418 | 17 | |
| Race | |||||
| White | 15,271 | 74.4 | 37,952 | 76.6 | < .001 |
| African American | 1,997 | 9.7 | 5,985 | 12.1 | |
| Hispanic | 586 | 2.9 | 1,461 | 2.9 | |
| Native American | 112 | 0.5 | 405 | 0.8 | |
| Asian | 273 | 1.3 | 591 | 1.2 | |
| Others | 2,278 | 11.1 | 3,182 | 6.4 | |
| ICU type | |||||
| Mixed | 12,165 | 59.3 | 20,933 | 42.2 | < .001 |
| Cardiovascular-surgical | 2,020 | 9.8 | 4,004 | 8.1 | |
| Coronary care | 2,907 | 14.2 | 10,139 | 20.5 | |
| Trauma | 166 | 0.8 | 154 | 0.3 | |
| Surgical | 1,065 | 5.2 | 4,809 | 9.7 | |
| Medical | 1,074 | 5.2 | 5,730 | 11.6 | |
| Neuroscience | 1,120 | 5.5 | 3,807 | 7.7 | |
| Nutrition | |||||
| No feeding | 8,388 | 40.9 | 17,925 | 36.2 | < .001 |
| Enteral nutrition | 11,256 | 54.9 | 27,778 | 56 | |
| Parenteral nutrition | 144 | 0.7 | 578 | 1.2 | |
| Both enteral nutrition and parenteral nutrition | 729 | 3.6 | 3,295 | 6.6 | |
| Cancer | 1,465 | 7.1 | 4,037 | 8.1 | < .001 |
| HIV | 41 | 0.2 | 126 | 0.3 | .154 |
| Cirrhosis | 120 | 0.6 | 697 | 1.4 | < .001 |
| Immunosuppression | 507 | 2.5 | 1,882 | 3.8 | < .001 |
| Intubated in the first day | 11,173 | 54.5 | 25,326 | 51.1 | < .001 |
| Risk factors | |||||
| Coagulopathy | 4,724 | 23 | 13,804 | 27.8 | < .001 |
| Mechanical ventilation > 24 h | 12,686 | 61.8 | 29,668 | 59.8 | < .001 |
| Traumatic brain injury | 1,252 | 6.1 | 2,074 | 4.2 | < .001 |
| Hepatic failure | 78 | 0.4 | 512 | 1 | < .001 |
| Hydrocortisone ≥ 250 md/d or equivalent | 758 | 3.7 | 1,912 | 3.9 | .536 |
| Transplantation | 27 | 0.1 | 129 | 0.3 | < .001 |
| Acute myocardial infarction | 835 | 4.1 | 1,409 | 2.8 | < .001 |
| Sepsis | 4,422 | 21.6 | 13,814 | 27.9 | < .001 |
| Neurologic injuries | 3,823 | 18.6 | 6,672 | 13.5 | < .001 |
| Surgical and multiple trauma | 5,549 | 27 | 10,635 | 21.5 | < .001 |
| Hypotension | 5,060 | 24.7 | 13,689 | 27.6 | < .001 |
| Acute renal failure | 5,031 | 24.5 | 15,308 | 30.9 | < .001 |
| Burns ≥ 30% BSA | 12 | 0.1 | 8 | 0 | .002 |
| ICU LOS > 7 d | 7,270 | 35.4 | 19,337 | 39 | < .001 |
| Medication | |||||
| Sucralfate | 302 | 1.5 | 1,402 | 2.8 | < .001 |
| Antacids | 7,237 | 35.3 | 16,747 | 33.8 | .001 |
| Anticoagulants | 11,500 | 56.1 | 28,014 | 56.5 | .725 |
| Antiplatelets | 12,848 | 62.6 | 30,409 | 61.3 | .003 |
| Thrombolytics | 1,877 | 9.1 | 3,755 | 7.6 | < .001 |
| NSAIDs | 10,805 | 52.7 | 26,024 | 52.5 | .034 |
| Admission source | |||||
| Chest pain center | 82 | 0.4 | 188 | 0.4 | < .001 |
| Direct admission | 1,980 | 9.7 | 4,204 | 8.5 | |
| ED | 10,332 | 50.4 | 25,020 | 50.5 | |
| Floor | 2,580 | 12.6 | 8,662 | 17.5 | |
| Operating room | 3,984 | 19.4 | 7,603 | 15.3 | |
| Other (other hospital or ICU, recovery room, step-down unit) | 1,559 | 7.6 | 3,899 | 7.9 | |
| Year of admission | |||||
| 2008 | 3,065 | 14.9 | 8,226 | 16.6 | < .001 |
| 2009 | 4,258 | 20.8 | 11,120 | 22.4 | |
| 2010 | 5,243 | 25.6 | 12,035 | 24.3 | |
| 2011 | 5,111 | 24.9 | 12,534 | 25.3 | |
| 2012 | 2,840 | 13.8 | 5,661 | 11.4 | |
| Physician specialty | |||||
| Internal medicine | 2,307 | 11.2 | 9,708 | 19.6 | < .001 |
| Pulmonary | 4,025 | 19.6 | 8,170 | 16.5 | |
| Hospitalist | 1,378 | 6.7 | 5,169 | 10.4 | |
| Cardiology | 1,710 | 8.3 | 3,362 | 6.8 | |
| Surgery-general | 1,477 | 7.2 | 3,241 | 6.5 | |
| Critical care medicine | 1,827 | 8.9 | 3,357 | 6.8 | |
| Family practice | 959 | 4.7 | 3,138 | 6.3 | |
| Surgery-cardiac | 1,370 | 6.7 | 2,042 | 4.1 | |
| Others | 5,464 | 26.6 | 11,389 | 23 | |
| Teaching hospital | 9,726 | 47.4 | 11,467 | 23.1 | < .001 |
| APACHE-IV score | 66 | 26.82 | 69 | 26.93 | < .001 |
| Platelet counts | 161 | 78.84 | 154 | 85.57 | < .001 |
APACHE-IV = Acute Physiology and Chronic Health Evaluation version IV; BSA = body surface area; NSAIDs = nonsteroidal antiinflammatory drugs. See Table 1 legend for expansion of other abbreviations.
The Cox model (Table 3) revealed that the risk of CIGIB was nearly twofold higher among the PPI group compared with the H2B group after adjusting for potential confounders (hazard ratio [HR], 1.97 [95% CI, 1.48-2.63]). Other factors that were associated with a higher risk of CIGIB included the following: male sex (HR, 1.27 [95% CI, 1.04-1.54]), acute renal failure (HR, 1.59 [95% CI, 1.28-1.97]), the receipt of sucralfate (HR, 3.25 [95% CI, 2.18-4.85]), the receipt of an antiplatelet agent (HR, 1.35 [95% CI, 1.01-1.79]), and admission to an ICU during 2009 or 2010. On the contrary, having a surgery or being a trauma victim was associated with lower risk of CIGIB (HR, 0.46 [95% CI, 0.25-0.84]).
Table 3.
Multivariable-adjusted HRs and 95% CIs of Factors for CIGIB Episodes Among ICU Patients
| Factor | HR | 95% CI |
|---|---|---|
| SUP exposure (3 d) | ||
| H2B | Reference | |
| PPI | 1.97 | 1.48-2.63 |
| Sex | ||
| Female | Reference | |
| Male | 1.27a | 1.04-1.54 |
| Age, y | ||
| 18-60 | Reference | |
| 61-70 | 1.12 | 0.87-1.45 |
| 71-80 | 1.10 | 0.84-1.44 |
| ≥ 81 | 1.16 | 0.85-1.58 |
| Race | ||
| White | Reference | |
| African American | 1.04 | 0.77-1.42 |
| Hispanic | 1.58 | 0.80-3.11 |
| Native American | 0.75 | 0.38-1.45 |
| Asian | 1.05 | 0.38-2.93 |
| Others | 1.08 | 0.74-1.58 |
| ICU type | ||
| Medical | Reference | |
| Cardiovascular-surgical | 0.75 | 0.099-5.59 |
| Coronary care | 0.86 | 0.51-1.44 |
| Trauma | 1.30 | 0.91-1.88 |
| Surgical | 0.96 | 0.58-1.61 |
| Mixed | 1.26 | 0.91-1.76 |
| Neuroscience | 0.90 | 0.50-1.63 |
| Nutrition | ||
| No feeding | Reference | |
| Enteral nutrition | 1.17 | 0.93-1.47 |
| Parenteral nutrition | 1.03 | 0.72-1.48 |
| Cancer | 1.29 | 0.93-1.79 |
| HIV | 1.00 | 0.24-4.26 |
| Cirrhosis | 1.38 | 0.77-2.48 |
| Immunosuppression | 0.85 | 0.51-1.42 |
| Intubated in the first day | 0.80 | 0.62-1.05 |
| Risk factors | ||
| Coagulopathy | 1.19 | 0.95-1.49 |
| Mechanical ventilation > 24 h | 0.79 | 0.61-1.02 |
| Traumatic brain injury | 0.64 | 0.28-1.46 |
| Hepatic failure | 1.26 | 0.71-2.23 |
| Hydrocortisone ≥ 250 mg/d or equivalent | 1.10 | 0.71-1.70 |
| Acute myocardial infarction | 1.37 | 0.74-2.53 |
| Sepsis | 1.03 | 0.81-1.31 |
| Neurologic injuries | 0.95 | 0.68-1.33 |
| Surgical and multiple trauma | 0.46a | 0.25-0.84 |
| Hypotension | 1.20 | 0.94-1.53 |
| Acute renal failure | 1.59b | 1.28-1.97 |
| Medication | ||
| Sucralfate | 3.25b | 2.18-4.85 |
| Antacids | 0.93 | 0.76-1.15 |
| Anticoagulants | 0.84 | 0.64-1.10 |
| Antiplatelets | 1.35a | 1.01-1.79 |
| Thrombolytics | 0.86 | 0.60-1.21 |
| NSAIDs | 0.97 | 0.80-1.19 |
| Admission source | ||
| Direct admission | Reference | |
| Chest pain center | 0.86 | 0.12-6.41 |
| ED | 1.31 | 0.90-1.91 |
| Floor | 1.26 | 0.83-1.91 |
| Operating room | 2.01 | 0.96-4.22 |
| Other (other hospital or ICU, recovery room, step-down unit) | 1.07 | 0.65-1.77 |
| Year of admission | ||
| 2008 | Reference | |
| 2009 | 1.42a | 1.02-1.97 |
| 2010 | 1.48a | 1.07-2.06 |
| 2011 | 1.31 | 0.94-1.83 |
| 2012 | 1.20 | 0.80-1.80 |
| Physician specialty | ||
| Internal medicine | Reference | |
| Pulmonary | 1.16 | 0.85-1.57 |
| Hospitalist | 1.06 | 0.72-1.57 |
| Cardiology | 0.77 | 0.46-1.31 |
| Surgery-general | 0.72 | 0.38-1.36 |
| Critical care medicine | 1.40 | 0.95-2.07 |
| Family practice | 1.28 | 0.83-1.96 |
| Surgery-cardiac | 0.82 | 0.43-1.60 |
| Others | 1.05 | 0.76-1.45 |
| Teaching hospital | 1.16 | 0.92-1.46 |
| Continuous variables | ||
| APACHE-IV | 1.00 | 1.00-1.01 |
| Platelet counts (< 1,000/μL) | 1.00b | 1.00-1.00 |
| Observations (patient-days) | 356,147 |
In sensitivity analyses (Table 1), prophylaxis with a PPI for at least 2 days was associated with higher bleeding risk compared with prophylaxis with an H2B after adjusting for potential confounders (HR, 2.10 [95% CI, 1.65-2.67]). Moreover, PPIs were associated with a higher risk of CIGIB compared with H2Bs (HR, 1.81 [95% CI, 1.35-2.43]) when the analysis was confined to patients who did not discontinue treatment or discontinued treatment for no more than 2 days before discharge, which constituted 84% of the original sample. Testing for PPI associated thrombocytopenia as a possible mediator for the increased risk of CIGIB revealed no significant difference in the HR between the model that excluded posttreatment thrombocytopenia (HR, 1.97 [95% CI, 1.48-2.63]) and the model that included it (HR, 1.95 [95% CI, 1.44-2.65]).
In the PSM model, 23,176 patients were matched 1:1, resulting in 11,588 pairs. The groups were matched on all the included covariates in the PSM model. The risk of CIGIB was significantly higher among the PPI group compared with the H2B group (HR, 1.82 [95% CI, 1.19-2.78]).
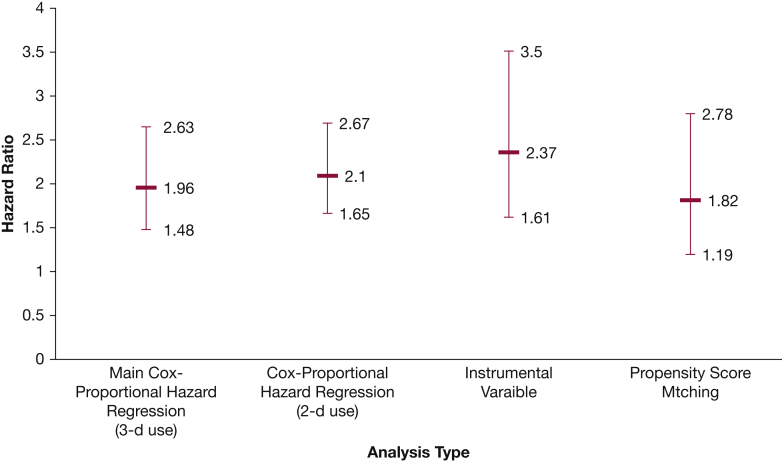
The two-stage instrumental variable analyses also showed that PPIs were associated with higher risk of CIGIB (HR, 2.37 [95% CI, 1.61-3.5]) compared with H2Bs. Table 1 and Figure 1 summarize the association between PPIs, H2Bs, and CIGIB.
Figure 1.
Hazard ratios for stress ulcer prophylaxis (proton pump inhibitors vs histamine type 2 receptor blockers) associated clinically important GI bleeding using different analytical methods.
Lastly, we explored the effect of an unmeasured confounder, such as history of GI ulceration or bleeding, by using the approach of Lin et al.23 A range of possible prevalences is presented in Table 4 along with HRs for an unmeasured confounder. In nearly all scenarios, H2Bs were either superior or equivalent to PPIs in their ability to prevent CIGIB. PPIs were superior to H2Bs only under extreme and clinically implausible scenarios. The HR for an unmeasured confounder (eg, history of GI ulceration or bleeding) must be at least 3.0 and present in at least 90% of patients receiving a PPI while not being present in any patient who received an H2B. In this implausible scenario, the approach of Lin et al23 estimates that PPIs may be associated with lower risk of CIGIB compared with H2Bs (HR, 0.7 [95% CI, 0.52-0.94]), and when the HR of the unmeasured confounder was reduced to 2, PPIs were no longer superior to H2Bs.
Table 4.
HRs and 95% CIs for the Effect of 3-Day Use of PPIs Compared With 3-Day Use of H2Bs Adjusting for an Unmeasured Dichotomous Confounder With an HR of 3
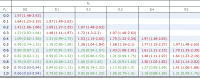 |
Red indicates higher risk of CIGIB with PPIs; green indicates no difference between PPIs and H2Bs; and blue indicates lower risk of CIGIB with PPIs. P0 and P1 are the prevalence of the unmeasured confounder in the H2B group and the PPI group, respectively. See Table 1 legend for expansion of other abbreviations.
Discussion
We found that CIGIB was less common (0.6%) than reported for clinical trial subjects who received stress ulcer prophylaxis.3, 5, 6 The lower event rates may reflect a reduction in hypoperfusion-related factors that lead to the mucosal stress and breakdown that precedes GI bleeding from a stress ulcer. Improvements in critical care practice include more adequate and timely resuscitation, effective and optimally timed enteral nutrition, and more rapid initiation of measures to increase perfusion made possible by newer ICU monitoring systems.1, 24, 25, 26 These improvements may have reduced mucosal damage and contributed to lower rates of CIGIB.
Prophylaxis with a PPI for at least 3 days was associated with higher CIGIB risk (HR, 1.97 [95% CI, 1.48-2.63]) than prophylaxis with an H2B. This study was larger and also supports the veracity of the association of greater effectiveness of H2Bs than PPIs for the prevention of CIGIB observed in a previous epidemiological study.7 Interestingly, outcomes observed in clinical practice seem to be divergent from the findings of five prior reports that compared bleeding prophylaxis efficacy of PPIs and H2Bs.2, 3, 4, 5, 6 Understanding why clinical practice outcomes are different from those expected from methodologically sound and correctly performed analyses of well-done randomized clinical trials requires consideration of several factors. One important difference is that the 0.6% rate of CIGIB noted in our study of clinical practice is substantially lower than the rates of 2.2% to 3.3% reported from clinical trials. It is also lower than the 2.1% bleeding rate reported by a previous epidemiological study.7 This earlier study reported all bleeding rather than selectively reporting CIGIB, as was done in the present article.
Another factor is the relatively small size of the clinical trials compared with the clinical practice outcomes studies. Large clinical outcomes trials include a broader spectrum of patients than randomized trials because they include more sites and use markedly less restrictive exclusion criteria. A third factor relates to advances in resuscitation and organ support that occurred since the completion of the randomized trials that may affect the relative responsiveness of the population to the alternative therapies. This concept is grounded on the hypothesis that better resuscitated patients with less severe mucosal damage may have a greater capacity to respond to H2B prophylaxis than PPI prophylaxis. Because CIGIB events were noted for both groups, future personalized critical care studies will be required to identify individual patients who would achieve better prophylaxis from a PPI than from an H2B. These findings provide the basis for the design of future studies that could allow the selection of a prophylactic agent based on characteristics that are unique to the individual patient.
The findings of association of greater effectiveness of H2B prophylaxis than PPI prophylaxis are highly internally consistent and robust. We were unable to attribute the difference to variations in acuity by adjustment or PSM analysis. In the present study, 2 days of exposure yielded results similar to 3-day exposure (HR, 2.10 [95% CI, 1.65-2.67]). This result was similar to the study by MacLaren et al,7 which found that PPI use for 2 days was associated with higher odds of GI bleeding compared with H2Bs (OR, 2.24 [95% CI, 1.81-2.76]). Propensity analyses produced similar findings, suggesting that the differences in outcomes were not due to imbalance of the extensive set of measured factors. Additional analyses that examined the role of PPI associated thrombocytopenia, the effects of medications on PPI or H2B pharmacokinetics, and other interactions failed to identify a confounding factor. Furthermore, sensitivity analyses indicated similar results under a wide variety of scenarios for a potent unmeasured confounder such as prior ulceration or bleeding (Table 4).
The present study has strengths and important limitations. First, the use of statistical approaches that assessed for confounding by imbalance of known factors, as well as unknown factors related to the tendency of an ICU to preferentially prescribe a PPI, provided consistent estimates of the comparative effectiveness of the alternative methods of prophylaxis for CIGIB. However, these analyses do not prove that such a factor was not present. The study design does not allow insight into episodes of blood loss that do not meet the criteria of Cook et al8, 9 for being clinically important. However, the availability of physiological measures allowed a more precise definition of clinically significant bleeding events than the ICD-based measures used for previous epidemiological studies. We were unable to perform analyses stratified according to route of administration, and differences in rates of IV administration could have affected effectiveness. IV administration of H2Bs to this group with a nonfeeding rate of 41% may have been modestly higher than the PPIs group, which had a nonfeeding rate of 36%. It does not seem likely that differences attributable to this factor were large because the rapid absorption of enteral H2Bs27 and PPIs28 corresponds to theoretical AUC differences of only 1% of IV PPIs over oral PPIs and a 4% difference for IV H2Bs compared with enteral H2Bs over the 72-h administration period. The key limitation is that the exposure to the prophylactic agent (PPI vs H2B) was not randomly assigned.
Conclusions
CIGIB is an uncommon outcome among critically ill adults who received stress ulcer prophylactic agents. Unlike findings from clinical trials, PPIs were associated with higher risk of CIGIB compared with H2Bs in clinical practice. The robust association of H2Bs with fewer episodes of CIGIB in a second large clinical practice cohort supports the conclusions of cost-effectiveness studies that favor the use of an H2B over a PPI for stress ulcer prophylaxis in at-risk critically ill adults.
Acknowledgments
Author contributions: C. M. L. and O. B. had full access to the data and take responsibility for its integrity and the accuracy of the analyses; M. A., C. M. L., O. B., S. E. T., E. O., and L. S. M. were responsible for study concept and design; M. A., E. O., S. E. T., O. B., and I. H. were responsible for the acquisition and preparation of data; and M. A., C. M. L., O. B., S. E. T., E. O., I. H., and L. S. M. analyzed and interpreted the data. The manuscript was prepared by C. M. L., M. A., O. B., and I. H.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: O. B. is an employee of Philips Healthcare and serves on the Clinical Advisory Board for ICMed. I. H. is an employee of IMPAQ International, Baltimore, MD. None declared (C. M. L., M. A., E. O., S. E. T., L. S. M.).
Other contributions: The authors thank Christine Gill and Jeanne Yang from the Pharmacy Research Computing Center at the School of Pharmacy, University of Maryland, for their efforts in preparing the primary datasets that were used in this study. The manuscript was reviewed and approved by Steven Fuhrman, MD, Louis Gidel, MD, PhD, Corey Scurlock, MD, MBA, Richard Riker, MD, Leo Celi, MD, MPH, Teresa Rincon, RN, BSN, eCCRN, Theresa Davis, PhD, RN, MSN, Michael Waite, MD, and Michael Breslow, MD, of the eICU Research Institute Publications Committee. They were not compensated by the eICU Research Institute for this review.
References
- 1.ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis. ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56(4):347–379. doi: 10.1093/ajhp/56.4.347. [DOI] [PubMed] [Google Scholar]
- 2.Pongprasobchai S., Kridkratoke S., Nopmaneejumruslers C. Proton pump inhibitors for the prevention of stress-related mucosal disease in critically-ill patients: a meta-analysis. J Med Assoc Thai. 2009;92(5):632–637. [PubMed] [Google Scholar]
- 3.Barkun A.N., Bardou M., Pham C.Q., Martel M. Proton pump inhibitors vs. histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol. 2012;107(4):507–520. doi: 10.1038/ajg.2011.474. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J.F., Wan X.Y., Huang W., Han L.L. Bleeding and pneumonia in intensive care unit patients given proton pump inhibitor or histamine-2 receptor antagonist for prevention of stress ulcer: a meta analysis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22(4):221–225. [article in Chinese] [PubMed] [Google Scholar]
- 5.Alhazzani W., Alenezi F., Jaeschke R.Z., Moayyedi P., Cook D.J. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41(3):693–705. doi: 10.1097/CCM.0b013e3182758734. [DOI] [PubMed] [Google Scholar]
- 6.Lin P.C., Chang C.H., Hsu P.I., Tseng P.L., Huang Y.B. The efficacy and safety of proton pump inhibitors vs histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta-analysis. Crit Care Med. 2010;38(4):1197–1205. doi: 10.1097/CCM.0b013e3181d69ccf. [DOI] [PubMed] [Google Scholar]
- 7.MacLaren R., Reynolds P.M., Allen R.R. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med. 2014;174(4):564–574. doi: 10.1001/jamainternmed.2013.14673. [DOI] [PubMed] [Google Scholar]
- 8.Cook D.J., Reeve B.K., Scholes L.C. Histamine-2-receptor antagonists and antacids in the critically ill population: stress ulceration versus nosocomial pneumonia. Infect Control Hosp Epidemiol. 1994;15(7):437–442. doi: 10.1086/646948. [DOI] [PubMed] [Google Scholar]
- 9.Cook D.J., Fuller H.D., Guyatt G.H. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994;330(6):377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 10.Hammond D.A., Kathe N., Shah A., Martin B.C. Cost-effectiveness of histamine2 receptor antagonists versus proton pump inhibitors for stress ulcer prophylaxis in critically ill patients. Pharmacotherapy. 2017;37(1):43–53. doi: 10.1002/phar.1859. [DOI] [PubMed] [Google Scholar]
- 11.Lilly C.M., Swami S., Liu X., Riker R.R., Badawi O. Five-year trends of critical care practice and outcomes. Chest. 2017;152(4):723–735. doi: 10.1016/j.chest.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 12.Lilly C.M., Zuckerman I.H., Badawi O., Riker R.R. Benchmark data from more than 240,000 adults that reflect the current practice of critical care in the United States. Chest. 2011;140(5):1232–1242. doi: 10.1378/chest.11-0718. [DOI] [PubMed] [Google Scholar]
- 13.McShea M., Holl R., Badawi O., Riker R.R., Silfen E. The eICU research institute—a collaboration between industry, health-care providers, and academia. IEEE Eng Med Biol Mag. 2010;29(2):18–25. doi: 10.1109/MEMB.2009.935720. [DOI] [PubMed] [Google Scholar]
- 14.Cerner All Together, Welcome to APACHE Foundation. 2015. https://apachefoundations.cernerworks.com/apachefoundations/login/auth. Accessed September 22, 2015.
- 15.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terza J.V., Basu A., Rathouz P.J. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wooldridge J. Introductory Economerics: A Modern Approach. South-Western Cengage Learning; Mason, OH: 2009. Instrumental variables estimation and two stage least squares; p. 506. [Google Scholar]
- 18.Binnetoglu E., Akbal E., Sen H. Pantoprazole-induced thrombocytopenia in patients with upper gastrointestinal bleeding. Platelets. 2015;26(1):10–12. doi: 10.3109/09537104.2014.880108. [DOI] [PubMed] [Google Scholar]
- 19.Korkmaz U., Alcelik A., Eroglu M., Korkmaz A.N., Aktas G. Pantoprazole-induced thrombocytopenia in a patient with upper gastrointestinal bleeding. Blood Coagul Fibrinolysis. 2013;24(3):352–353. doi: 10.1097/MBC.0b013e3283574f2f. [DOI] [PubMed] [Google Scholar]
- 20.Miller J.L., Gormley A.K., Johnson P.N. Pantoprazole-induced thrombocytopenia. Indian J Pediatr. 2009;76(12):1278–1279. doi: 10.1007/s12098-009-0224-9. [DOI] [PubMed] [Google Scholar]
- 21.Watson T.D., Stark J.E., Vesta K.S. Pantoprazole-induced thrombocytopenia. Ann Pharmacother. 2006;40(4):758–761. doi: 10.1345/aph.1G384. [DOI] [PubMed] [Google Scholar]
- 22.Zlabek J.A., Anderson C.G. Lansoprazole-induced thrombocytopenia. Ann Pharmacother. 2002;36(5):809–811. doi: 10.1345/aph.1A303. [DOI] [PubMed] [Google Scholar]
- 23.Lin D.Y., Psaty B.M., Kronmal R.A. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54(3):948–963. [PubMed] [Google Scholar]
- 24.Hurt R.T., Frazier T.H., McClave S.A. Stress prophylaxis in intensive care unit patients and the role of enteral nutrition. JPEN J Parenter Enteral Nutr. 2012;36(6):721–731. doi: 10.1177/0148607112436978. [DOI] [PubMed] [Google Scholar]
- 25.Sesler J.M. Stress-related mucosal disease in the intensive care unit: an update on prophylaxis. AACN Adv Crit Care. 2007;18(2):119–126. doi: 10.1097/01.AACN.0000269254.39967.8e. [DOI] [PubMed] [Google Scholar]
- 26.Ali T., Harty R.F. Stress-induced ulcer bleeding in critically ill patients. Gastroenterol Clin North Am. 2009;38(2):245–265. doi: 10.1016/j.gtc.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Lin J.H. Pharmacokinetic and pharmacodynamic properties of histamine H2-receptor antagonists. Relationship between intrinsic potency and effective plasma concentrations. Clin Pharmacokinet. 1991;20(3):218–236. doi: 10.2165/00003088-199120030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Shin J.M., Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motility. 2013;19(1):25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]