Abstract
Catalytic difunctionalization of alkenes has been an ideal strategy to generate structurally complex molecules with diverse substitution patterns. Although both phosphonyl and carboxyl groups are valuable functional groups, the simultaneous incorporation of them via catalytic difunctionalization of alkenes, ideally from abundant, inexpensive and easy-to-handle raw materials, has not been realized. Herein, we report the phosphonocarboxylation of alkenes with CO2 via visible-light photoredox catalysis. This strategy is sustainable, general and practical, providing facile access to important β-phosphono carboxylic acids, including structurally complex unnatural α-amino acids. Diverse alkenes, including enamides, styrenes, enolsilanes and acrylates, undergo such reactions efficiently under mild reaction conditions. Moreover, this method represents a rare example of redox-neutral difunctionalization of alkenes with H-P(O) compounds, including diaryl- and dialkyl- phosphine oxides and phosphites. Importantly, these transition-metal-free reactions also feature low catalyst loading, high regio- and chemo-selectivities, good functional group tolerance, easy scalability and potential for product derivatization.
Subject terms: Homogeneous catalysis, Synthetic chemistry methodology, Photochemistry
Phosphonyl and carboxyl groups are valuable functional groups, however their simultaneous incorporation via catalytic difunctionalization of alkenes has not been realized yet. Here the authors report the phosphonocarboxylation of alkenes with CO2 via visible-light photoredox catalysis.
Introduction
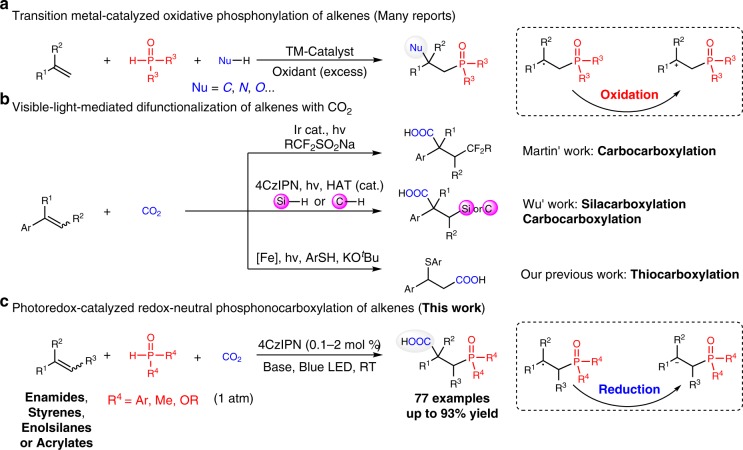
Difunctionalization of alkenes has developed into a powerful tool in organic synthesis for generation of highly functionalized skeletons due to the easy availability of alkenes with different functional groups and diverse substitution modes1–4. Catalytic difunctionalization of alkenes with H-P(O) compounds is an important and ideal method to generate valuable organophosphine derivatives5–11, which are of great importance in agrochemicals12, functional materials13,14, synthetic15, and medicinal16,17 chemistry. Huge progress has been achieved in oxidative transformations (Fig. 1a)18–21, in which the electron-rich alkyl radicals, in situ generated through addition of phosphonyl radicals to alkenes, are oxidized to alkyl cations and then trapped by the nucleophilic components. However, the redox-neutral difunctionalization of alkenes with H-P(O) compounds remains very rare22.
Fig. 1.
Catalytic difunctionalization of alkenes. a Oxidative difunctionalization of alkenes with H-P(O) compounds. b Limited examples for visible-light-mediated difunctionalization of alkenes with CO2 reported by Martin, Wu and our group. c Redox-neutral difunctionalization of alkenes with H-P(O) to generate important β-phosphono carboxylic acids, including structurally complex unnatural α-amino acids
Phosphorus-containing carboxylic acids are highly valuable compounds and widely exist in natural products23, materials24, and pharmaceuticals, which exhibit a diverse range of biological activities, acting as inhibitors of urease, glutamate carboxypeptidase II, neuropeptidase N-acetylated α-linked acidic dipeptidase (NAALADase), and so on25–28. Notably, the β-phosphono α-amino acids are important motifs in peptidic drugs, supramolecular catalysis (artificial metalloenzymes), and organic synthesis29–31. However, synthetic methods for such important compounds are extremely limited to de novo synthesis, which suffers from poor diversity, multiple steps, limited substrate scope, and/or harsh reaction conditions. We envisioned that the simultaneous incorporation of both phosphonyl and carboxyl groups via selective difunctionalization of enamides and other alkenes would serve as an ideal route to deliver important β-phosphono carboxylic acids, including β-phosphono α-amino acids. Different from the above mentioned oxidative functionalization of the generated alkyl radicals18–21, we hypothesized that reduction of such key alkyl radicals to anions, which might undergo nucleophilic attack to CO2, could realize redox-neutral difunctionalization of alkenes with H-P(O) compounds. To the best of our knowledge, this strategy has never been realized to generate such valuable targets.
Carbon dioxide (CO2) has been regarded as a ubiquitous, green and recyclable one carbon (C1) building block in organic synthesis32–38. Although the thermodynamic stability and kinetic inertness of CO2 introduces daunting challenges, a wide range of transformation using this gaseous reagent have been developed to construct important carboxylic acids, which are found in myriad natural products, agrochemicals, and pharmaceuticals39. Notably, catalytic carboxylation of unsaturated compounds with CO2 has attracted much attention of chemists38,40–49. Compared with widely investigated hydrocarboxylation of alkenes, however, catalytic difunctionalization of alkenes with CO2, which is obviously more attractive and cost-effective to obtain structurally complex molecules with diverse substitution patterns, is more challenging and much less investigated38. Although chemists have been mimicking Nature’s ability for long time to harness light in organic transformations50–54 and transform CO2 to value-added products55,56, the visible-light-mediated difunctionalization of alkenes with CO2 is still scarce and yet underdeveloped with limited examples reported by Martin, Wu and our group, independently (Fig. 1b)57–59. Moreover, photocatalytic difucnctionlization of the electron-rich alkenes, such as enamine and enol derivatives, with CO2 has not been reported yet, thus calling for a strategy to generate structurally more diverse α-amino acids60–62 and α-hydroxy acids63.
Herein, we report the catalytic phosphonocarboxylation of diverse alkenes, including enamides, styrenes, enolsilanes, and acrylates, with CO2 (Fig. 1c). This strategy is sustainable, general, and practical, representing a rare example of redox-neutral difunctionalization of alkenes with H-P(O) compounds to generate important β-phosphono carboxylic acids with high efficiency and selectivity under mild reaction conditions.
Results
Reaction design
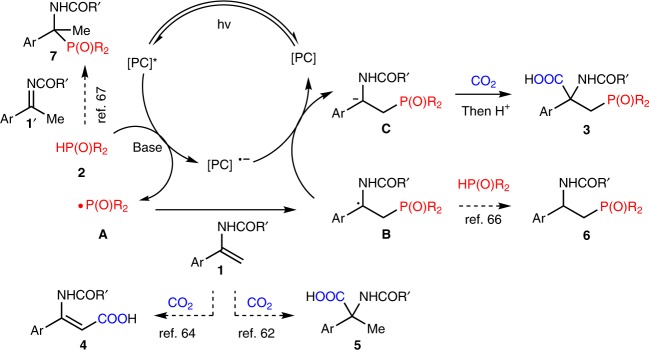
At the beginning of this project, we challenged ourselves with realization of selective phosphonocarboxylation of enamides with CO2 via visible-light photoredox catalysis to generate valuable β-phosphono α-amino acids. As proposed in Fig. 2, the phosphonyl radicals A, generated via single-electron transfer (SET) between H-P(O) compounds 2 and a photo-excited photocatalyst in the presence of a base, might undergo facile addition to the C = C double bonds of enamides 1 to selectively generate the α-amido radicals B, which was stabilized by phenyl and amide groups. A subsequent SET between B and the reduced photocatalyst might give rise to the α-amido carbanions C, which then could react with CO2 to deliver the desired β-phosphono α-amino acids 3. However, the possible hydrocarboxylation62, C-H bond carboxylation64 of enamides as well as hydrophosphinylation (Pudovik reaction)65 via hydrogen atom transfer (HAT)66 between B and 2 could be competitive side reactions and generate 4, 5, and 6, respectively. The tautomerization between enamides 1 with imines 1′ also should be considered, given that the latter species could be attacked by 2 to give α-amidophosphonate 767.
Fig. 2.
Proposed mechanism for selective phosphonocarboxylation of enamides 1 with 2 to give 3. Compounds 4, 5, 6, and 7 are possible byproducts. [PC] = photocatalyst
Investigations of reaction conditions
We began our investigations using N-(1-phenylvinyl)benzamide 1a and diphenylphosphine oxide 2a as model substrates with atmospheric CO2 under visible light irradiation at room temperature (Table 1). To our delight, we detected the formation of β-phosphono α-amino acid 3aa using Ru(bpy)3Cl2 as the catalyst and Cs2CO3 as the base, albeit in trace amounts (Table 1, entry 1). When we tested other photocatalysts, we found that an Ir-based photocatalyst significantly improved the efficiency for generation of 3aa (70%, Table 1, entry 2) and the organic photocatalyst 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) provided the best result (75%, Table 1, entry 3). Importantly, in the absence of such photocatalysts, 4a (19%)64 and 7a (41%)67 were generated instead of 3aa (Table 1, entry 4). Further screening of various bases (Table 1, entries 5–7) showed that K2CO3 was the best choice (Table 1, entry 6), while the use of triethylamine as the base (Table 1, entry 7) would generate 5a (29%)62 and 6a (24%)65,66 along with 3aa (32%). Intriguingly, the amount of photocatalyst could be even reduced to 0.1% without interfering the reaction (89%, Table 1, entry 8), illustrating the high efficiency of the reaction. Control experiments revealed that CO2, light, photocatalyst, and base were all crucial for this transformation (Table 1, entries 9–12).
Table 1.
Screening the reaction conditionsa

| |||
|---|---|---|---|
| Entry | [PC] | Base | Yield (%) |
| 1 | Ru(bpy)3Cl2 | Cs2CO3 | <5 |
| 2 | Ir[(ppy)2(dtbpy)]PF6 | Cs2CO3 | 70 |
| 3 | 4CzIPN | Cs2CO3 | 75 |
| 4 | — | Cs2CO3 | N.D. |
| 5 | 4CzIPN | Na2CO3 | 83 |
| 6 | 4CzIPN | K2CO3 | 89 |
| 7 | 4CzIPN | Et3N | 32 |
| 8b | 4CzIPN | K2CO3 | 89 |
| 9b, c | 4CzIPN | K2CO3 | <5 |
| 10 | — | K2CO3 | N.D. |
| 11b, d | 4CzIPN | K2CO3 | N.D. |
| 12b | 4CzIPN | — | 21 |
LED light-emitting diode, DMF N, N-dimethylformamide, N.D. not detected
aReaction conditions: 1a (0.2 mmol), 2a (1.2 eq.), [PC] (2 mol%), Base (1.5 eq.), DMF (2 mL), RT. The yields are of isolated products
b0.1 mol% of [PC]
cWithout CO2
dWithout light
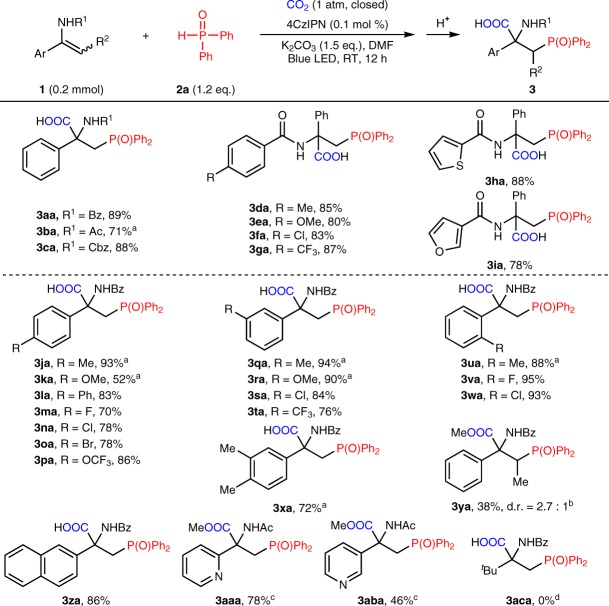
Substrate scope of enamides
With the acceptable reaction conditions in hand, a variety of β-phosphono α-amino acids bearing a quaternary carbon center were obtained in moderate to excellent yields. As illustrated in Fig. 3, a diverse array of protecting groups for the enamines (3aa-3ca), including the readily removed Ac (3ba) and Cbz (3ca) carbamate, proved to be compatible with the light-driven phosphocarboxylation reaction. Notably, the enamides bearing electron-donating (methoxyl, 3ea) or electron-withdrawing groups (trifluoromethyl, 3ga) as well as heteroarenes, such as thiophene (3ha) and furan (3ia), all reacted well. We next turned our attention to the substituents on arenes. As also shown in Fig. 3, a broad range of aryl enamides bearing different functional groups, including methyl (3ja), methoxyl (3ka), phenyl (3la), halogens (3ma-3oa), and trifluoromethoxyl (3pa) at the para-position, afforded the desired products in moderate to excellent yields. The enamides with meta- (3qa-3ta) and ortho-substituted arenes (3ua-3wa) also underwent such a transformation with high efficiency. The current protocol could also be applied to the substrates bearing di-substitution (3xa), internal enamides (3ya) and naphthalene (3za). Enamides containing pyridine (3aaa and 3aba) could also be tolerated in the reaction. Unfortunately, when alkyl enamide (1ac) was used as substrate in the reaction, we did not detected the desired carboxylative product 3aca while the hydrophosphinylation product 6ac was obtained.
Fig. 3.
Substrate scope of enamides. a4CzIPN (0.5 mol%) was used bR2 = Me. cIsolated as methyl ester by treating the reaction mixture with CH3I at 65 °C for 2 h. dThe hydrophosphinylation product 6ac was obtained
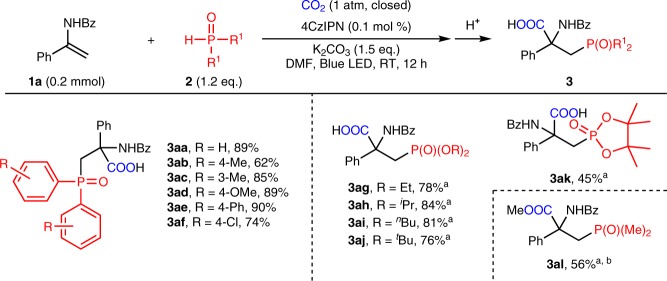
Substrate scope of H-P(O) compounds
Having demonstrated the good functional group compatibility in the enamide substrates, we next investigated the scope of the H-P(O) compounds 2. As illustrated in Fig. 4, a broad range of phosphorus-containing α-amino acids were obtained. Both electron-donating (3ab-3ad) and mildly electron-withdrawing substituents (3ae-3af) on the aryl groups were well tolerated, leading to the desired products in good yields (62–90%). It is important to note that this transformation is not restricted to diarylphosphine oxides, various phosphites (3ag-3ak), which usually did not work well in the photochemical reactions, also could deliver the corresponding products smoothly, illustrating the synthetic utility and flexibility of this approach. Notably, dialkyl phosphine oxide, such as 3al, also took part in the reaction to provide the desired product in moderate yield (56%), which demonstrates the generality of our transformation.
Fig. 4.
Substrate scope of phosphine oxides and phosphites. a4CzIPN (2 mol%) and Cs2CO3 (1.5 eq.) were used. bThe yield for methyl ester of corresponding carboxylic acid is provided
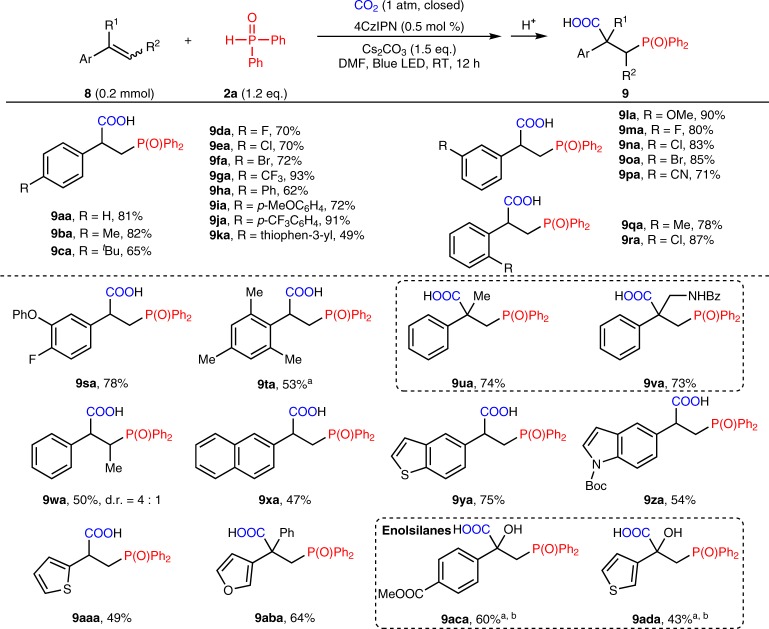
Substrate scope of styrenes
Considering the importance of β-phosphono carboxylic acids23, we wondered whether styrenes 8 could be utilized in this phosphonocarboxylation process. To our delight, this protocol was easily applicable to a range of electronically diverse styrenes (Fig. 5). Diverse functional groups, including halogens (9da-9fa, 9ma-9oa, 9ra, 9ta), trifluoromethyl (9ga), nitrile (9pa), and heteroarenes (9ka, 9va) were tolerated well under the mild reaction conditions. The styrenes bearing disubstituted (9sa) and sterically hindered (2,4,6-trimethyl, 9ta) benzenes also delivered the desired products smoothly. Notably, the reaction also worked well for α-methyl or aminomethyl substituted styrenes (9ua, 9va), generating the products bearing quaternary carbon centers in good yields (74% and 73%, respectively). Other challenging alkenes, such as internal styrene (9wa) and heteroaryl-substituted styrenes (9xa-9aba), proved to be competent substrates in the reaction. Importantly, enolsilanes could also deliver the α-hydroxy acids (9aca-9ada) in moderate yields. In addition, we did not detect the desired product when alkyl olefin, such as dec-1-ene, was subjected to the reaction.
Fig. 5.
Substrate scope of styrenes. a4CzIPN (2 mol%) was used bEnolsilane was used as substrate, R1 = OTBS, TBS = tert-butyldimethylsilyl. Desilylation occurred upon acidifying the reaction mixture with aq. HCl (2 N), providing α-hydroxycarboxylic acid as the final product
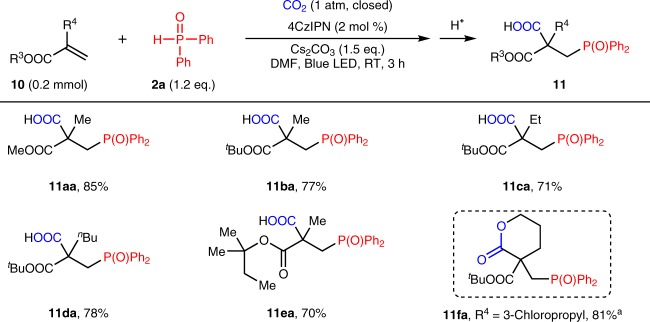
Substrate scope of acrylates
To further demonstrate the generality of the phosphonocarboxylation reaction, we also explored electron-deficient acrylates (Fig. 6). Although the hydrophosphinylation of electron-deficient alkenes with 2 via nucleophilic addition has been well documented68, we were pleased to find that a broad range of acrylates 10 smoothly underwent our photocatalyzed phosphonocarboxylation transformation with diphenylphosphine oxide 2a. It is worth noting that when 3-chloropropylacrylate was used as substrate, a multi-substituted six-membered ring lactone 11fa was obtained in good yield via intramolecular cyclization.
Fig. 6.
Substrate scope of acrylates. aWhen 3-chloropropylacrylate 10f was used as the substrate, a lactone 11fa was obtained via cascade intramolecular cyclization
Synthetic applications
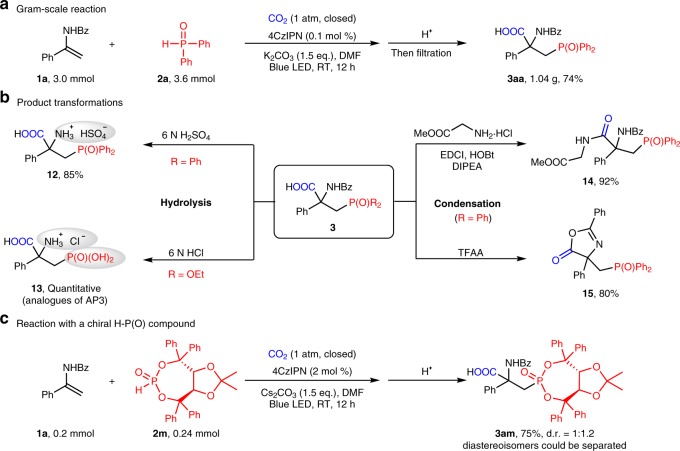
To demonstrate the potential application of the protocol, a gram-scale reaction was carried out to afford 3aa in 74% isolated yield without need of column chromatography (Fig. 7a). Moreover, the utility of the method was validated by facile derivatization of the products (Fig. 7b). For example, the benzoyl group could be removed easily to generate free β-phosphono α-amino acid 12 in good yield (85%). Hydrolysis of the benzamide and phosphite ester moieties in 3ag (R = OEt) quantitatively generated 13, an analogs of bioacitve AP369. Importantly, amino acids condensation between 3aa and methyl glycinate hydrochloride provided the phosphorus-containing dipeptide 14 (92%) and intramolecular condensation of 3aa with the assistance of TFFA easily afforded cyclic oxazolinone 15. Furthermore, since reactions of chiral phosphorus-centered radicals could proceed stereoselectively with retention of configuration70, a chiral H-P(O) compound derived from (4R, 5R)-Taddol derivative was used to achieve an enantioselective photocatalytic method with CO2 (Fig. 7c). Although the current protocol provides poor diasteroselectivity ratio, the diastereoisomers could be completely separated by column chromatography with good yields, which provides an alternative method for obtaining phosphonic acids-containing chiral α-amino acids upon hydrolysis (For more information regarding other types of chiral H-P(O) compounds, see Supplementary Figs. 2 and 3). The success of these experiments indicates the great potential application of the method in designing and synthesis of peptide drugs and ligands.
Fig. 7.
Synthetic applications of the method. a Gram-scale synthesis free of isolation with chromatography. b Transformations of the product via hydrolysis and condensation. c Reaction with a chiral H-P(O) compound. The diastereoisomers could be easily separated by column chromatography
Preliminary investigation of reaction mechanism
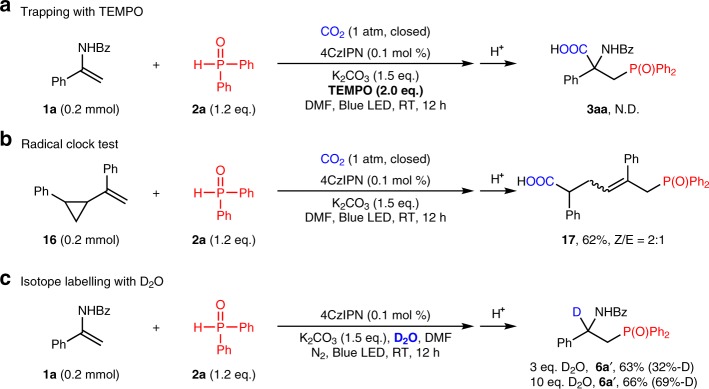
To gain more insight to the reaction mechanism, several control experiments were conducted. As illustrated in Fig. 8a, the reaction was suppressed when the radical scavenger 2,2,6,6-tetramethyl-piperidinyloxyl (TEMPO) was employed. Moreover, the radical clock test (Fig. 8b) also suggested that this transformation might rely on a radical process. Additionally, isotope-labeling studies provided a strong support for α-amino benzylic anionic species acted as key intermediates (Fig. 8c). Besides, Stern-Volmer luminescence studies demonstrated that the excited state of 4CzIPN was quenched by 2a in the presence of base (see Supplementary Page 36) instead of 1a. These results indicate the involvement of a reductive quenching photocatalytic cycle in the reaction.
Fig. 8.
Preliminary mechanistic studies. a The reaction was totally suppressed when the radical scavenger 2,2,6,6-tetramethyl-piperidinyloxyl (TEMPO) was employed. b Ring-opening occurred when cyclopropane substituted styrene was used as the substrate. c When different amounts of deuterated water was added, different degrees of deuterated products were obtained
Discussion
In summary, we have described a general and practical strategy to realize the phosphonocarboxylation of alkenes with CO2 via visible-light photoredox catalysis. This method is suitable for diverse alkenes (enamides, styrenes, enolsilanes, and acrylates) and H-P(O) compounds (diaryl- and dialkyl- phosphine oxides and phosphites), all of which undergo such reactions efficiently to access important and potentially bioactive β-phosphono carboxylic acids, including β-phosphono α-amino acids. Notably, these redox-neutral and transition-metal-free reactions feature low catalyst loading, mild reaction conditions, high regio- and chemo-selectivities, good functional group tolerance, facile scalability, and easy product derivatization. Further application of this strategy is underway in our laboratory.
Methods
General procedure
An oven-dried Schlenk tube (10 mL) containing a stirring bar was charged with the substrates (0.2 mmol). The Schlenk tube was then introduced in a glovebox, where it was charged with H-P(O) compound (49 mg, 0.24 mmol, 1.2 eq.) and K2CO3 (41 mg, 0.3 mmol, 1.5 eq.). The tube was taken out of the glovebox and connected to a vacuum line where it was evacuated and back-filled with CO2 for 3 times. Then DMF (2 mL) and 4CzIPN (32 μL, 0.1 mol%, 5 mg dissolved in 1 mL DMF) were added under CO2 flow. Finally, the reaction mixture in sealed tube was placed at a distance of 2–3 cm from a 30 W blue LED and stirred at room temperature (25 °C) for 12 h. Then, the mixture was quenched with 4.5 mL of H2O and 0.5 mL of 2 N HCl (aq.), extracted with ethyl acetate (EA) for at least 5 times, then concentrated in vacuo. The residue was purified by silica gel flash chromatography (0.2% AcOH in CH2Cl2/MeOH 100/1 ~ 20/1) to give the pure desired product. Note: (1) for styrenes (0.5 mol% 4CzIPN and Cs2CO3 was used), flashed with petroleum ether/AcOEt 1/1 to 0.67% AcOH in petroleum ether/AcOEt 1/1; (2) For acrylates (2 mol% 4CzIPN and Cs2CO3 was used), flashed with petroleum ether/AcOEt 1/1 to 0.67% AcOH in petroleum ether/AcOEt 1/1; (3) for phosphites (2 mol% 4CzIPN and Cs2CO3 was used), before the addition of 0.5 mL 2N HCl (aq.), the quenched reaction mixture was extract three times for removing the inpurity, then 0.5 mL of 2N HCl (aq.) was added, the reaction mixture was extracted for 4 times, then the combined organic phase was concentrated in vacuum to obtain the pure product without chromatography.
Supplementary information
Acknowledgements
We thank Prof. Jason J. Chruma (Sichuan University) for valuable help. Financial support was provided by the National Natural Science Foundation of China (21822108 and 21772129), the Fok Ying Tung Education Foundation (161013), the “973” Project from the MOST of China (2015CB856600), the “1000-Youth Talents Program”, and the Fundamental Research Funds for the Central Universities. We also thank the comprehensive training platform of the Specialized Laboratory in the College of Chemistry at Sichuan University for compound testing.
Author contributions
D.G.Y. and Q.F. conceived and designed the study, and wrote the paper. Q.F., Z.Y.B., J.H.Y., T.J., H.H., and L.L.L. performed the experiments and mechanistic studies. Q.F. performed the crystallographic studies. All authors contributed to the analysis and interpretation of the data.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplemental Information files. Extra data are available from the author upon request. The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1885892 (methyl ester of 3aa) and can be obtained free of charge from www.ccdc.cam.ac.uk/getstructures.
Competing interests
A Chinese Patent on this work has been applied with the number (201910599890.5) on 4 July 2019. The remaining authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11528-8.
References
- 1.Yin G, Mu X, Liu G. Palladium(II)-catalyzed oxidative difunctionalization of alkenes: bond forming at a high-valent palladium center. Acc. Chem. Res. 2016;49:2413–2423. doi: 10.1021/acs.accounts.6b00328. [DOI] [PubMed] [Google Scholar]
- 2.Merino E, Nevado C. Addition of CF3 across unsaturated moieties: a powerful functionalization tool. Chem. Soc. Rev. 2014;43:6598–6608. doi: 10.1039/C4CS00025K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koike T, Akita M. A versatile strategy for difunctionalization of carbon–carbon multiple bonds by photoredox catalysis. Org. Chem. Front. 2016;3:1345–1349. doi: 10.1039/C6QO00139D. [DOI] [Google Scholar]
- 4.Lan X-W, Wang N-X, Xing Y. Recent advances in radical difunctionalization of simple alkenes. Eur. J. Org. Chem. 2017;2017:5821–5851. doi: 10.1002/ejoc.201700678. [DOI] [Google Scholar]
- 5.Leca D, Fensterbank L, Lacôte E, Malacria M. Recent advances in the use of phosphorus-centered radicals in organic chemistry. Chem. Soc. Rev. 2005;34:858–865. doi: 10.1039/b500511f. [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Ji J-X. Catalytic and direct oxyphosphorylation of alkenes with dioxygen and H-phosphonates leading to β-ketophosphonates. Angew. Chem. Int. Ed. 2011;50:9097–9099. doi: 10.1002/anie.201100219. [DOI] [PubMed] [Google Scholar]
- 7.Cai B-G, Xuan J, Xiao W-J. Visible light-mediated C-P bond formation reactions. Sci. Bull. 2019;64:337–350. doi: 10.1016/j.scib.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Li C-X, Tu D-S, Yao R, Yan H, Lu C-S. Visible-light-induced cascade reaction of isocyanides and N-arylacrylamides with diphenylphosphine oxide via radical C-P and C-C bond formation. Org. Lett. 2016;18:4928–4931. doi: 10.1021/acs.orglett.6b02413. [DOI] [PubMed] [Google Scholar]
- 9.Wang N, et al. Catalytic diverse radical-mediated 1,2-cyanofunctionalization of unactivated alkenes via synergistic remote cyano migration and protected strategies. Org. Lett. 2016;18:6026–6029. doi: 10.1021/acs.orglett.6b02960. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Yu X, Song Q. Silver-catalyzed radical-involved cascade cyclization of diphenylphosphine with cinnamamides: access to 2-phosphinoyl-3H-pyrrolo[1,2-a]indoles. Org. Lett. 2017;19:980–983. doi: 10.1021/acs.orglett.6b03713. [DOI] [PubMed] [Google Scholar]
- 11.Buquoi, J. Q., Lear, J. M., Gu, X. & Nagib, D. A. Heteroarene phosphinylalkylation via a catalytic, polarity-reversing radical cascade. ACS Catal. 9, 5330–5335 (2019). [DOI] [PMC free article] [PubMed]
- 12.Murphy, P. Organophosphorus Reagents. (Oxford Univ. Press, Oxford, 2004). .
- 13.Peruzzini, M. & Gonsalvi, L. (ed) Phosphorus Compounds: Advanced Tools In Catalysis And Material Sciences (Springer, Berlin, 2011).
- 14.Duffy MP, Delaunay W, Bouit PA, Hissler M. π-Conjugated phospholes and their incorporation into devices: components with a great deal of potential. Chem. Soc. Rev. 2016;45:5296–5310. doi: 10.1039/C6CS00257A. [DOI] [PubMed] [Google Scholar]
- 15.Börner, A. (ed) Phosphorus Ligands In Asymmetric Catalysis: Synthesis And Applications (Wiley-VCH, Weinheim, 2008).
- 16.Kukhar, V. P. & Hudson, H. R. (eds) Aminophosphonic and Aminophosphinic Acids, Chemistry And Biological Activity (John Wiley & Sons, Ltd., Chichester, U.K., 2000).
- 17.Demmer CS, Krogsgaard-Larsen N, Bunch L. Review on modern advances of chemical methods for the introduction of a phosphonic acid group. Chem. Rev. 2011;111:7981–8006. doi: 10.1021/cr2002646. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Tang G, Zhao Y. Recent progress toward organophosphorus compounds based on phosphorus-centered radical difunctionalizations. Phosphorus Sulfur. Silicon Relat. Elem. 2017;192:589–596. doi: 10.1080/10426507.2017.1295965. [DOI] [Google Scholar]
- 19.Li J-A, et al. Phosphinoyl radical-initiated α, β-aminophosphinoylation of alkenes. Org. Lett. 2017;19:4704–4706. doi: 10.1021/acs.orglett.7b02183. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P-Z, et al. Phosphinoyl radical initiated vicinal cyanophosphinoylation of alkenes. Org. Lett. 2017;19:5537–5540. doi: 10.1021/acs.orglett.7b02621. [DOI] [PubMed] [Google Scholar]
- 21.Yang B, et al. Cerium(IV)-promoted phosphinoylation-nitratation of alkenes. Adv. Synth. Catal. 2018;360:4470–4474. doi: 10.1002/adsc.201800985. [DOI] [Google Scholar]
- 22.Zhang C, et al. Silver-catalyzed radical phosphonofluorination of unactivated alkenes. J. Am. Chem. Soc. 2013;135:14082–14085. doi: 10.1021/ja408031s. [DOI] [PubMed] [Google Scholar]
- 23.Horsman GP, Zechel DL. Phosphonate biochemistry. Chem. Rev. 2017;117:5704–5783. doi: 10.1021/acs.chemrev.6b00536. [DOI] [PubMed] [Google Scholar]
- 24.Lu S-Y, Hamerton I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002;27:1661–1712. doi: 10.1016/S0079-6700(02)00018-7. [DOI] [Google Scholar]
- 25.Ntatsopoulos V, Vassiliou S, Macegoniuk K, Berlicki Ł, Mucha A. Novel organophosphorus scaffolds of urease inhibitors obtained by substitution of Morita-Baylis-Hillman adducts with phosphorus nucleophiles. Eur. J. Med. Chem. 2017;133:107–120. doi: 10.1016/j.ejmech.2017.03.070. [DOI] [PubMed] [Google Scholar]
- 26.Graham K, et al. Radiofluorinated derivatives of 2-(phosphonomethyl)pentanedioic acid as inhibitors of prostate specific membrane antigen (PSMA) for the imaging of prostate cancer. J. Med. Chem. 2012;55:9510–9520. doi: 10.1021/jm300710j. [DOI] [PubMed] [Google Scholar]
- 27.Majer P, et al. Structural optimization of thiol-based inhibitors of glutamate carboxypeptidase II by modification of the p1‘ side chain. J. Med. Chem. 2006;49:2876–2885. doi: 10.1021/jm051019l. [DOI] [PubMed] [Google Scholar]
- 28.Jackson PF, et al. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated α-linked acidic dipeptidase. J. Med. Chem. 1996;39:619–622. doi: 10.1021/jm950801q. [DOI] [PubMed] [Google Scholar]
- 29.Blaskovich MAT. Unusual amino acids in medicinal chemistry. J. Med. Chem. 2016;59:10807–10836. doi: 10.1021/acs.jmedchem.6b00319. [DOI] [PubMed] [Google Scholar]
- 30.Thomas CM, Ward TR. Artificial metalloenzymes: proteins as hosts for enantioselective catalysis. Chem. Soc. Rev. 2005;34:337–346. doi: 10.1039/b314695m. [DOI] [PubMed] [Google Scholar]
- 31.Cowen BJ, Miller SJ. Enantioselective [3 + 2]-cycloadditions catalyzed by a protected, multifunctional phosphine-containing α-amino acid. J. Am. Chem. Soc. 2007;129:10988–10989. doi: 10.1021/ja0734243. [DOI] [PubMed] [Google Scholar]
- 32.Aresta, M. Carbon dioxide as chemical feedstock. (Wiley-VCH, Weinheim, 2010). .
- 33.Tsuji Y, Fujihara T. Carbon dioxide as a carbon source in organic transformation: carbon–carbon bond forming reactions by transition-metal catalysts. Chem. Commun. 2012;48:9956–9964. doi: 10.1039/c2cc33848c. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Wu L, Jackstell R, Beller M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015;6:5933. doi: 10.1038/ncomms6933. [DOI] [PubMed] [Google Scholar]
- 35.Kleij AW, North M, Urakawa A. CO2 catalysis. ChemSusChem. 2017;10:1036–1038. doi: 10.1002/cssc.201700218. [DOI] [PubMed] [Google Scholar]
- 36.Luo J, Larrosa I. C-H carboxylation of aromatic compounds through CO2 fixation. ChemSusChem. 2017;10:3317–3332. doi: 10.1002/cssc.201701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song Q-W, Zhou Z-H, He L-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017;19:3707–3728. doi: 10.1039/C7GC00199A. [DOI] [Google Scholar]
- 38.Tortajada A, Juliá-Hernández F, Börjesson M, Moragas T, Martin R. Transition-metal-catalyzed carboxylation reactions with carbon dioxide. Angew. Chem. Int. Ed. 2018;57:15948–15982. doi: 10.1002/anie.201803186. [DOI] [PubMed] [Google Scholar]
- 39.Maag, H. Prodrugs of carboxylic acids (Springer, New York, 2007).
- 40.Yan S-S, et al. Transition metal-catalyzed carboxylation of unsaturated substrates with CO2. Coord. Chem. Rev. 2018;374:439–463. doi: 10.1016/j.ccr.2018.07.011. [DOI] [Google Scholar]
- 41.Zhang L, Cheng J, Carry B, Hou Z. Catalytic boracarboxylation of alkynes with diborane and carbon dioxide by an N-heterocyclic carbene copper catalyst. J. Am. Chem. Soc. 2012;134:14314–14317. doi: 10.1021/ja3063474. [DOI] [PubMed] [Google Scholar]
- 42.Nogi K, Fujihara T, Terao J, Tsuji Y. Carboxyzincation employing carbon dioxide and zinc powder: cobalt-catalyzed multicomponent coupling reactions with alkynes. J. Am. Chem. Soc. 2016;138:5547–5550. doi: 10.1021/jacs.6b02961. [DOI] [PubMed] [Google Scholar]
- 43.Gaydou M, Moragas T, Juliá-Hernández F, Martin R. Site-selective catalytic carboxylation of unsaturated hydrocarbons with CO2 and water. J. Am. Chem. Soc. 2017;139:12161–12164. doi: 10.1021/jacs.7b07637. [DOI] [PubMed] [Google Scholar]
- 44.Hou J, et al. Visible-light-driven alkyne hydro-/carbocarboxylation using CO2 via iridium/cobalt dual catalysis for divergent heterocycle synthesis. J. Am. Chem. Soc. 2018;140:5257–5263. doi: 10.1021/jacs.8b01561. [DOI] [PubMed] [Google Scholar]
- 45.Butcher TW, et al. Regioselective copper-catalyzed boracarboxylation of vinyl arenes. Org. Lett. 2016;18:6428–6431. doi: 10.1021/acs.orglett.6b03326. [DOI] [PubMed] [Google Scholar]
- 46.Saito N, et al. Enantioselective synthesis of β-amino acid derivatives via nickel-promoted regioselective carboxylation of ynamides and rhodium-catalyzed asymmetric hydrogenation. Org. Biomol. Chem. 2016;14:10080–10089. doi: 10.1039/C6OB01234E. [DOI] [PubMed] [Google Scholar]
- 47.Doi R, Abdullah I, Taniguchi T, Saito N, Sato Y. Nickel-catalyzed hydrocarboxylation of ynamides with CO2 and H2O: observation of unexpected regioselectivity. Chem. Commun. 2017;53:7720–7723. doi: 10.1039/C7CC03127K. [DOI] [PubMed] [Google Scholar]
- 48.Takimoto M, Gholap SS, Hou Z. Cu-catalyzed alkylative carboxylation of ynamides with dialkylzinc reagents and carbon dioxide. Chem. Eur. J. 2015;21:15218–15223. doi: 10.1002/chem.201502774. [DOI] [PubMed] [Google Scholar]
- 49.Saito N, Sun Z, Sato Y. Nickel-promoted highly regioselective carboxylation of aryl ynol ether and its application to the synthesis of chiral β-aryloxypropionic acid derivatives. Chem. Asian J. 2015;10:1170–1176. doi: 10.1002/asia.201403399. [DOI] [PubMed] [Google Scholar]
- 50.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meggers E. Asymmetric catalysis activated by visible light. Chem. Commun. 2015;51:3290–3301. doi: 10.1039/C4CC09268F. [DOI] [PubMed] [Google Scholar]
- 52.Koike T, Akita M. Fine design of photoredox systems for catalytic fluoromethylation of carbon–carbon multiple bonds. Acc. Chem. Res. 2016;49:1937–1945. doi: 10.1021/acs.accounts.6b00268. [DOI] [PubMed] [Google Scholar]
- 53.Xie J, Jin H, Hashmi ASK. The recent achievements of redox-neutral radical C-C cross-coupling enabled by visible-light. Chem. Soc. Rev. 2017;46:5193–5203. doi: 10.1039/C7CS00339K. [DOI] [PubMed] [Google Scholar]
- 54.Marzo L, Pagire SK, Reiser O, König B. Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 2018;57:10034–10072. doi: 10.1002/anie.201709766. [DOI] [PubMed] [Google Scholar]
- 55.Tan F, Yin G. Homogeneous light-driven catalytic direct carboxylation with CO2. Chin. J. Chem. 2018;36:545–554. doi: 10.1002/cjoc.201800011. [DOI] [Google Scholar]
- 56.Yeung C. Photoredox catalysis as a strategy for CO2 incorporation: direct access to carboxylic acids from a renewable feedstock. Angew. Chem. Int. Ed. 2018;58:2–13. doi: 10.1002/anie.201806285. [DOI] [PubMed] [Google Scholar]
- 57.Yatham VR, Shen Y, Martin R. Catalytic intermolecular dicarbofunctionalization of styrenes with CO2 and radical precursors. Angew. Chem. Int. Ed. 2017;56:10915–10919. doi: 10.1002/anie.201706263. [DOI] [PubMed] [Google Scholar]
- 58.Hou J, et al. Visible-light-mediated metal-free difunctionalization of alkenes with CO2 and silanes or C(sp3)−H alkanes. Angew. Chem. Int. Ed. 2018;57:17220–17224. doi: 10.1002/anie.201811266. [DOI] [PubMed] [Google Scholar]
- 59.Ye J-H, et al. Visible-light-driven iron-promoted thiocarboxylation of styrenes and acrylates with CO2. Angew. Chem. Int. Ed. 2017;56:15416–15420. doi: 10.1002/anie.201707862. [DOI] [PubMed] [Google Scholar]
- 60.Seo H, Katcher MH, Jamison TF. Photoredox activation of carbon dioxide for amino acid synthesis in continuous flow. Nat. Chem. 2016;9:453–456. doi: 10.1038/nchem.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan X, Gong X, Ma M, Wang R, Walsh PJ. Visible light-promoted CO2 fixation with imines to synthesize diaryl α-amino acids. Nat. Commun. 2018;9:4936. doi: 10.1038/s41467-018-07351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ju T, et al. Selective and catalytic hydrocarboxylation of enamides and imines with CO2 to generate α,α-disubstituted α-amino acids. Angew. Chem. Int. Ed. 2018;57:13897–13901. doi: 10.1002/anie.201806874. [DOI] [PubMed] [Google Scholar]
- 63.Mita T, Higuchi Y, Sato Y. Carboxylation with CO2 via brook rearrangement: preparation of α-hydroxy acid derivatives. Org. Lett. 2014;16:14–17. doi: 10.1021/ol403099f. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z, et al. Lactonization of C(sp2)-H bonds in enamides with CO2. Chin. J. Chem. 2018;36:430–436. doi: 10.1002/cjoc.201700805. [DOI] [Google Scholar]
- 65.Semenzin D, Etemad-Moghadam G, Albouy D, Diallo O, Koenig M. Dual radical/polar pudovik reaction: application field of new activation methods. J. Org. Chem. 1997;62:2414–2422. doi: 10.1021/jo9622441. [DOI] [PubMed] [Google Scholar]
- 66.Yoo W-J, Kobayashi S. Hydrophosphinylation of unactivated alkenes with secondary phosphine oxides under visible-light photocatalysis. Green Chem. 2013;15:1844–1848. doi: 10.1039/c3gc40482j. [DOI] [Google Scholar]
- 67.Ma J-A. Catalytic asymmetric synthesis of α- and β-amino phosphonic acid derivatives. Chem. Soc. Rev. 2006;35:630–636. doi: 10.1039/B517100H. [DOI] [PubMed] [Google Scholar]
- 68.Enders D, Saint-Dizier A, Lannou M-I, Lenzen A. The phospha-michael addition in organic synthesis. Eur. J. Org. Chem. 2006;2006:29–49. doi: 10.1002/ejoc.200500593. [DOI] [Google Scholar]
- 69.Xu Z-H, et al. Group I mGluR antagonist rescues the deficit of D1-induced LTP in a mouse model of fragile X syndrome. Mol. Neurodegener. 2012;7:24. doi: 10.1186/1750-1326-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jessop CM, Parsons AF, Routledge A, Irvine DJ. Radical addition reactions of chiral phosphorus hydrides. Tetrahedron Asymmetry. 2003;14:2849–2851. doi: 10.1016/j.tetasy.2003.04.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplemental Information files. Extra data are available from the author upon request. The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1885892 (methyl ester of 3aa) and can be obtained free of charge from www.ccdc.cam.ac.uk/getstructures.