Abstract
Prior studies suggest an association between Vitamin K antagonists (VKA) and cerebral microbleeds (CMBs); less is known about nonvitamin K oral anticoagulants (NOACs). In this observational study we describe CMB profiles in a multicenter cohort of 89 anticoagulation‐related intracerebral hemorrhage (ICH) patients. CMB prevalence was 51% (52% in VKA‐ICH, 48% in NOAC‐ICH). NOAC‐ICH patients had lower median CMB count [2(IQR:1–3) vs. 7(4–11); P < 0.001]; ≥5 CMBs were less prevalent in NOAC‐ICH (4% vs. 31%, P = 0.006). This inverse association between NOAC exposure and high CMB count persisted in multivariable logistic regression models adjusting for potential confounders (OR 0.10, 95%CI: 0.01–0.83; P = 0.034).
Introduction
Cerebral microbleeds (CMBs) are cerebral small vessel disease markers identified in blood‐sensitive brain MRI sequences (gradient‐recalled echo [GRE] or susceptibility‐weighted imaging (SWI).1 They correspond to diverse underlying pathological processes. While hypertensive2 or cerebral amyloid angiopathy‐related microhemorrhages are the most commonly associated conditions, ischemic processes such hemorrhagic transformation of ischemic microinfarcts and extravasation of red thrombi might also have similar imaging appearance CMBs have been linked to increased incident risk of both ischemic and hemorrhagic stroke.2 Although ischemic strokes are more frequent in terms of absolute event rates, the association of CMB presence with incident intracerebral hemorrhage (ICH) for the individual patient is more potent, especially in those with higher CMB burden.3 Thus, CMBs are recognized as a potentially useful prognostic of ICH especially in high‐risk patients exposed to therapeutic oral anticoagulation.3, 4
Less is known regarding the relationship between oral anticoagulation and the pathogenesis of CMBs. Data regarding Vitamin K antagonists (VKAs) and CMBs generally suggest higher risk, especially among those with highly variable international normalized ratio (INR).5, 6 Data regarding nonvitamin K oral antiocoagulants (NOACs) are scarce and suggest no overall association between NOAC exposure and CMB incidence or burden.7 Notably, a recent multicenter study reported higher CMB prevalence in patients experiencing ICH than ischemic stroke while taking NOACs.8 We undertook this observational, cross‐sectional study to characterize the prevalence and burden of CMBs in patients with symptomatic anticoagulation‐associated ICH and examine potential differences in VKA versus NOAC‐exposed patients.
Methods
Patient population
This is a retrospective analysis of two prospectively enrolled consecutive patient cohorts of nontraumatic ICH and positive history of oral anticoagulant intake in 15 participating tertiary‐care stroke centers during a 2‐year period (August 2015–July 2016 and August 2016–July 2017).9, 10 Patient characteristics and outcome have been described in detail previously9, 10 and are available in the online supplement (Table S1). Briefly, the definition of VKA‐related ICH required effective use of a VKA with an international normalized value of >1.5 on hospital admission, while the definition of NOAC‐related ICH required confirmed ingestion of the relevant NOAC during the last 24 h before the index event. Patients with major head trauma or known underlying structural or vascular cause of ICH were excluded from further evaluation. We also excluded patients with hemorrhagic transformation of ischemic infarcts and patients with pure intraventricular hemorrhage. Lastly, we excluded patients without baseline MRI with available blood‐sensitive sequences allowing CMB detection.
Imaging characteristics and criteria
We defined CMBs according to the Standards for Reporting Vascular changes on neuroimaging (STRIVE) consensus criteria.1 We recorded magnet strength (1.5 vs. 3T) and type of sequence used. Further details on CMB reading are available in the online supplement. MRI was performed in the acute phase (during the admission for the index ICH). Images were reviewed by experienced vascular neurologists at each center, blinded to anticoagulation exposure and official Neuroradiologist read for both CMB presence and ICH location. Formal Neuroradiological interpretation was used to corroborate ICH location.
Statistical analyses
Continuous variables are presented as median with interquartile range, whereas categorical variables are presented as percentages. Statistical comparisons between different subgroups were performed using the Pearson's χ 2 test and Mann–Whitney U test, where appropriate.
Multivariable logistic regression analyses were performed on the association of baseline characteristics with the presence of five or more cerebral microbleeds in baseline neuroimaging.11 In univariable models of all baseline characteristics a threshold of P < 0.1 was used to identify candidate variables for inclusion in the multivariate regression models that tested statistical significance hypothesis using the likelihood ratio test with an alpha value of 0.05. We also performed sensitivity univariable/multivariable logistic regression analyses after excluding patients with no presence of cerebral microbleeds on baseline neuroimaging. Finally, we also performed adjusted for baseline ICH volume analyses on the probabilities of hematoma expansion and ICH volume more than 30 cm3 at the follow‐up neuroimaging in 24 h according to baseline CMB burden (<5 CMBs or ≥5 CMBs).
We used Stata Statistical Software Release 13 for Windows (College Station, TX, StataCorp LP) for all analyses.
Results
Our two previous cohorts comprised a total of 109 NOAC‐related and 248 VKA‐related ICHs. Of these, a total of 89 patients (25 NOAC‐, 64 VKA‐associated) received brain MRI with GRE or SWI sequences allowing CMB detection. Compared to those who did not receive MRI (Table S1), included patients had higher median BMI (30 vs. 27 kg/m2, P = 0.002), were less likely to have hypertension (89% vs. 97%; P = 0.002), hyperlipidemia (52% vs. 65%; P = 0.03), and coronary artery disease (28% vs. 40%; P = 0.04); otherwise there were no significant differences. Patient characteristics according to anticoagulant type are summarized in Table 1. NOAC‐ICH patients were older (median age 78 [70–81] years vs. 70 [60–77] years; P = 0.005), had higher CHA2DS2‐VASc score (4[4–6] vs. 4[3–5]); P = 0.017, and were less likely to have lobar ICH location (28% vs. 58%; P = 0.001).
Table 1.
Baseline characteristics and outcomes according to the type of oral anticoagulant treatment
| Variable | VKA (n = 64) | NOAC (n = 25) | P‐value |
|---|---|---|---|
| Baseline clinical characteristics | |||
| Age (years, median, IQR) | 70 (60–77) | 78 (70–81) | 0.005 |
| Males (%) | 65.6% | 52.0% | 0.234 |
| BMI (median, IQR) | 30 (25–33) | 27 (17–34) | 0.133 |
| CHA2DS2‐VASc score (median, IQR) | 4 (3–5) | 4 (4–6) | 0.017 |
| HAS‐BLED score (median, IQR) | 3 (2–3) | 2 (2–4) | 0.917 |
| Hypertension (%) | 92.1% | 96.0% | 0.519 |
| Diabetes (%) | 42.2% | 32.0% | 0.376 |
| Hyperlipidemia (%) | 50.0% | 48.0% | 0.865 |
| Heart failure (%) | 21.9% | 12.0% | 0.287 |
| Current smoking (%) | 10.9% | 4.0% | 0.304 |
| Coronary artery disease (%) | 31.2% | 32.0% | 0.945 |
| Chronic kidney disease (%) | 17.2% | 16.0% | 0.893 |
| Prior history of ischemic stroke (%) | 29.7% | 24.0% | 0.592 |
| Prior history of intracerebral hemorrhage (%) | 6.2% | 0% | 0.201 |
| Statin pretreatment (%) | 67.2% | 48.0% | 0.094 |
| Antiplatelet pretreatment (%) | 43.7% | 36.0% | 0.505 |
| NIHSS admission (median, IQR) | 5 (3–18) | 6 (3–12) | 0.487 |
| GCS admission (median, IQR) | 14 (8–15) | 14 (13–15) | 0.257 |
| SBP admission (mmHg, median, IQR) | 163 (147–190) | 175 (141–200) | 0.435 |
| DBP admission (mmHg, median, IQR) | 94 (80–99) | 91 (74–98) | 0.464 |
| Baseline Laboratory values | |||
| INR admission (median, IQR) | 2.4 (1.8–3.6) | 1.2 (1.1–1.6) | <0.001 |
| aPTT admission (sec, median, IQR) | 39 (33–42) | 30 (28–32) | <0.001 |
| Platelet count ×103/μL (median, IQR) | 192 (159–259) | 218 (184–270) | 0.217 |
| CrCl on admission (ml/min, median, IQR) | 60 (44–70) | 60 (45–75) | 0.291 |
| Baseline neuroimaging findings | |||
| Lobar hemorrhage (%) | 57.8% | 28.0% | 0.001 |
| Intraventricular hemorrhage (%) | 35.9% | 32.0% | 0.726 |
| Baseline ICH volume (cm3, median, IQR) | 11.3 (5.1–26.3) | 4.9 (2.1–22.1) | 0.051 |
| ICH score (median, IQR) | 1 (1–2) | 1 (1–2) | 0.635 |
| Severe ICH (%)a | 14.5% | 16.0% | 0.861 |
| CMB presence (%) | 51.6% | 48.0% | 0.763 |
| CMB number (median, IQR)b | 7 (4–11) | 2 (1–3) | <0.001 |
| CMB ≥5 (%) | 31.2% | 4.0% | 0.006 |
| CMB ≥10 (%) | 17.1% | 4.0% | 0.102 |
| 3T MRI | 39.1% | 36.0% | 0.789 |
| SWI sequence | 6.2% | 0% | 0.201 |
Defined as ICH score ≥2.
After excluding patients without CMB presence.
A total of 45 patients (51%) had ≥ 1 CMB. There was no difference between VKA‐ and NOAC‐ ICH (52% vs. 48%; P = 0.763; Table 1). However, the median CMB number was significantly lower in NOAC‐ICH patients (2 [1–3] vs. 7[4–11]; P < 0.001 (Table 1 and Fig. 1). A total of 21 patients had high CMB burden (≥5 CMB). Their characteristics are summarized in Table S2. High CMB burden (≥ 5 CMBs) was less prevalent in NOAC‐ICH patients (31% vs. 4%; P = 0.006, Table 1). In contrast, high CMB burden was more common in younger patients and in patients who underwent 3 Tesla MRI (Table 2). This inverse association between NOAC (vs. VKA) exposure and high CMB count persisted in multivariable logistic regression models adjusting for potential confounders including demographics, risk factors, laboratory and brain imaging parameters: (OR 0.10, 95%CI:0.01–0.83; P = 0.034; Table 2). In sensitivity analyses, four factors were associated with high CMB burden in this sensitivity analysis: age, NOAC pretreatment, antiplatelet pretreatment and MRI strength (Table S3). Pretreatment with NOACs was independently related to lower odds of high CMB burden (OR 0.02, 95%CI: 0.01–0.25; P = 0.006) in the sensitivity analysis (Table S3).
Figure 1.
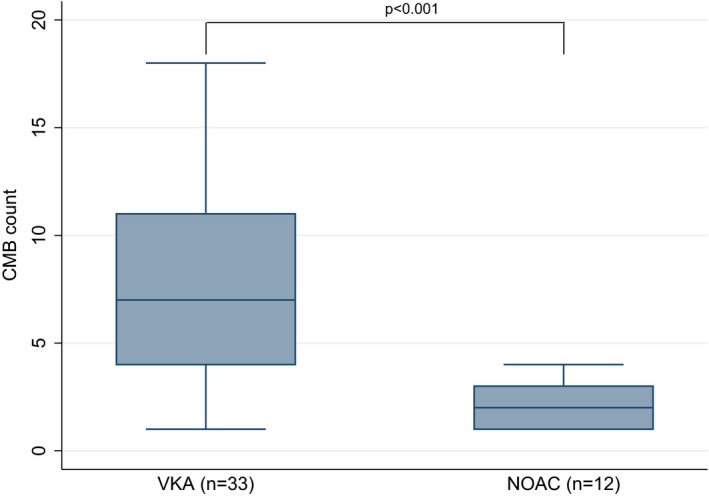
Distribution of cerebral microbleeds in baseline neuroimaging between patients with history of oral anticoagulation pretreatment with either vitamin K antagonists or nonvitamin K antagonist oral anticoagulants.
Table 2.
Univariable and multivariable logistic regression analyses on the association of baseline characteristics with the presence of five or more cerebral microbleeds in baseline neuroimaging
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age (years) | 0.94 (0.89, 0.98) | 0.011 | 0.94 (0.89, 0.99) | 0.031 |
| Males (%) | 1.28 (0.48, 3.40) | 0.616 | – | – |
| BMI | 1.03 (0.97, 1.09) | 0.338 | – | – |
| Hypertension | 1.59 (0.17, 14.40) | 0.681 | – | |
| Diabetes | 1.21 (0.45, 3.27) | 0.705 | – | – |
| Hyperlipidemia | 1.50 (0.56, 4.02) | 0.421 | – | – |
| Heart failure | 0.99 (0.29, 3.46) | 0.994 | – | – |
| Current smoking | 2.10 (0.46, 9.64) | 0.340 | – | – |
| Coronary artery disease | 0.61 (0.20, 1.88) | 0.390 | – | – |
| Kidney failure | 1.81 (0.54, 6.06) | 0.335 | – | – |
| Prior history of ischemic stroke | 1.39 (0.48, 3.99) | 0.542 | – | – |
| Prior history of intracerebral hemorrhage | 3.47 (0.46, 26.32) | 0.228 | ||
| Statin pretreatment | 1.32 (0.47, 3.69) | 0.600 | – | – |
| Antiplatelet pretreatment | 0.48 (0.16, 1.38) | 0.172 | – | – |
| NOAC pretreatment | 0.09 (0.01, 0.72) | 0.024 | 0.10 (0.01, 0.83) | 0.034 |
| Admission SBP | 0.99 (0.98, 1.01) | 0.575 | – | – |
| Admission DBP | 1.02 (0.99, 1.05) | 0.191 | – | – |
| Lobar hemorrhage | 1.17 (0.44, 3.11) | 0.758 | – | – |
| 3T MRI | 4.80 (1.68, 13.67) | 0.003 | 6.42 (1.96, 21.03) | 0.002 |
| SWI sequence | 3.47 (0.46, 26.32) | 0.228 | – | – |
Discussion
In this cross‐sectional observational study of anticoagulation‐associated ICH, we documented an overall prevalence of CMBs of 51%. Although CMB prevalence was well‐balanced between VKA and NOAC‐ICH, NOAC‐ICH patients had significantly lower CMB burden, both in median number and when dichotomized as <5 versus ≥5 CMBs. These associations remained significant after adjusting for potential confounders.
The observed CMB prevalence of ~50% is significantly higher than the frequency reported in population‐based studies12, 13, 14 and studies in ischemic stroke patients3 which range from 8.8 to 23%, with tendency to increase with age. However, this prevalence is comparable or lower than CMB frequency reported in ICH cohorts which often exceeds 60%,15 using similar MRI strength and technique. This finding is counterintuitive, as one would expect higher prevalence of cerebral hemorrhagic complications, including CMBs, in those exposed to oral anticoagulation. Moreover, although the absolute CMB count in prior similar studies is not always reported, the proportion of patients with high CMB count (defined as ≥5 CMBs) in a study of pooled hospital‐based ICH cohorts is estimated very similar to our observed frequency of ~24%. Thus, although higher CMB burden is strongly associated with future ICH risk in patients receiving oral anticoagulation, our study does not provide evidence for increased crude CMB prevalence or burden in patients exposed to therapeutic anticoagulation compared to historical controls; this finding should be viewed with caution due to lack of a nonanticoagulated control group in this study.
Closer examination stratifying by anticoagulation type reveals significantly lower burden of CMBs in NOAC‐ICH patients, despite older age. This is in line with previous studies in non‐ICH atrial fibrillation Asian patient cohorts that have found no association between NOAC exposure and increased CMB burden, which was similar to nonanticoagulated controls.7, 16 It is also in line with well‐established lower overall hemorrhagic risk of NOACs compared to VKAs.17 Given the significantly higher proportion of lobar ICH in the VKA‐exposed patients, another plausible explanation for this imbalance in CMB burden is that this subgroup had a higher prevalence of cerebral amyloid angiopathy (CAA), resulting in higher CMB burden. On the other hand, it should be noted that patients pretreated with NOACs were older than patients pretreated with VKA and this observation does not support the hypothesis of a higher CAA prevalence in the VKA group.
This finding also suggests that the interaction between CMB and ICH risk might not be clinically consequential in those exposed to NOAC, making NOAC a more suitable choice in patients with higher CMB burden in need for anticoagulation. In the large prospective observational CROMIS‐2 study there was a significantly lower proportion of NOAC‐exposed patients among those who suffered an ICH compared to VKA exposure (14% vs. 86%) over a 2‐year follow‐up period, although this did not reach statistical significance (P = 0.071) due to the low number of events.3 Similarly, in a secondary analysis of the NAVIGATE‐ESUS trial (Shoamanesh et al., oral presentation in International Stroke Conference 2019), the risk of ICH in the rivaroxaban‐allocated group was not affected by CMB presence compared to aspirin.
Our study is not without limitations. It was retrospective, observational, and treatment bias might have affected anticoagulation therapy allocation. The final sample size was small, especially with regards to the NOAC group. External validation in a larger cohort is necessary Given the cross‐sectional nature of this analysis it is not possible to ascertain whether there is a causative link between anticoagulation exposure and CMB formation or whether CMB presence and anticoagulation exposure act synergistically to increase the risk of hemorrhage. Approximately 25% of the entire cohort received MRI and was available for analysis. Despite small imbalances in baseline cardiovascular risk factor prevalence, we found no evidence of a systematic selection bias with regards to parameters of interest, including anticoagulation type, concomitant medications, ICH severity and overall CHA2DS2‐VASc and HAS‐BLED score (Table S1). MRI protocols and strengths which are well‐known to affect the sensitivity of CMB detection were heterogeneous among participating centers. However, both 3T MRI and SWI sequence use were evenly distributed between anticoagulation allocation groups (Table 1) and the association between NOAC‐ICH and lower CMB burden remained significant after adjusting for magnet strength. We had no information regarding duration of exposure to anticoagulation which might be an important factor determining development of CMBs. However, we note that NOAC‐ICH patients were older and with higher CHA2DS2‐VASc score; it is plausible that they might have been exposed to VKA and subsequently changed to NOAC, which would further highlight lower risk of CMB formation associated with NOAC. We do not have information regarding CMB anatomical location, which is a shortcoming subtracting granularity from our findings, given that different CMB location implies differential underlying pathology.13, 14
In conclusion, we documented similar prevalence but significantly higher burden of CMBs in VKA compared to NOAC exposure, in a cohort of 89 patients with anticoagulation‐related ICH. Results of additional ongoing prospective studies (Intracerebral Hemorrhage Due to Oral Anticoagulants: Prediction of the Risk by Magnetic Resonance (HERO) https://clinicaltrials.gov/ct2/show/NCT02238470) and Cerebral Microbleeds During NOACs or Warfarin Therapy in NVAF Patients With Acute Ischemic Stroke (CMB‐NOW) https://clinicaltrials.gov/ct2/show/NCT02356432) are expected to further refine our understanding of the interaction between CMBs and anticoagulant therapy.
Author Contributions
Vasileios Lioutas participated in conception and design of study and first manuscript draft. Aristeidis Katsanos involved in drafting of manuscript and figures/tables. Georgios Tsivgoulis involved in study conception and design, manuscript, and figures drafting. All authors involved in acquisition and analysis of the data.
Conflict of Interest
GT reports advisory board and speaker honoraria from Boehringer Ingelheim, Bayer, Daichii Sankyo, Medtronic, Shire, CSL Behring, Allergan, and Biogen; and an unrestricted research grant from Medtronic.
Supporting information
Table S1. Univariable comparisons of baseline characteristics between patients who received MRI vs those who did not.
Table S2. Baseline characteristics and outcomes according to high cerebral microbleed burden (≥5) on baseline neuroimaging.
Table S3. Univariable and multivariable logistic regression analyses on the association of baseline characteristics with the presence of five or more cerebral microbleeds on baseline neuroimaging, after excluding patients with no cerebral microbleeds on baseline neuroimaging.
Funding Information
None declared.
References
- 1. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsai HH, Pasi M, Tsai LK, et al. Microangiopathy underlying mixed‐location intracerebral hemorrhages/microbleeds: a PiB‐PET study. Neurology 2019;92:e774–e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson D, Ambler G, Shakeshaft C, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS‐2): a multicentre observational cohort study. Lancet Neurol 2018;17:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charidimou A, Shams S, Romero JR, et al. Clinical significance of cerebral microbleeds on MRI: a comprehensive meta‐analysis of risk of intracerebral hemorrhage, ischemic stroke, mortality, and dementia in cohort studies (v1). Int J Stroke 2018;13:454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akoudad S, Darweesh SK, Leening MJ, et al. Use of coumarin anticoagulants and cerebral microbleeds in the general population. Stroke 2014;45:3436–3439. [DOI] [PubMed] [Google Scholar]
- 6. Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke 2010;41:1222–1228. [DOI] [PubMed] [Google Scholar]
- 7. Saito T, Kawamura Y, Sato N, et al. Non‐vitamin k antagonist oral anticoagulants do not increase cerebral microbleeds. J Stroke Cerebrovasc Dis 2015;24:1373–1377. [DOI] [PubMed] [Google Scholar]
- 8. Purrucker JC, Wolf M, Haas K, et al. Microbleeds in ischemic vs hemorrhagic strokes on novel oral anticoagulants. Acta Neurol Scand 2018;138:163–169. [DOI] [PubMed] [Google Scholar]
- 9. Tsivgoulis G, Lioutas VA, Varelas P, et al. Direct oral anticoagulant‐ vs vitamin K antagonist‐related nontraumatic intracerebral hemorrhage. Neurology 2017;89:1142–1151. [DOI] [PubMed] [Google Scholar]
- 10. Lioutas VA, Goyal N, Katsanos AH, et al. Clinical outcomes and neuroimaging profiles in nondisabled patients with anticoagulant‐related intracerebral hemorrhage. Stroke 2018;49:2309–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charidimou A, Karayiannis C, Song TJ, et al. Brain microbleeds, anticoagulation, and hemorrhage risk: meta‐analysis in stroke patients with AF. Neurology 2017;89:2317–2326. [DOI] [PubMed] [Google Scholar]
- 12. Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008;70:1208–1214. [DOI] [PubMed] [Google Scholar]
- 13. Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 2010;41:S103–S106. [DOI] [PubMed] [Google Scholar]
- 14. Poels MM, Ikram MA, van der Lugt A, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke 2011;42:656–661. [DOI] [PubMed] [Google Scholar]
- 15. Charidimou A, Imaizumi T, Moulin S, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: a meta‐analysis. Neurology 2017;89:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soo Y, Abrigo J, Leung KT, et al. Correlation of non‐vitamin K antagonist oral anticoagulant exposure and cerebral microbleeds in Chinese patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2018;89:680–686. [DOI] [PubMed] [Google Scholar]
- 17. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariable comparisons of baseline characteristics between patients who received MRI vs those who did not.
Table S2. Baseline characteristics and outcomes according to high cerebral microbleed burden (≥5) on baseline neuroimaging.
Table S3. Univariable and multivariable logistic regression analyses on the association of baseline characteristics with the presence of five or more cerebral microbleeds on baseline neuroimaging, after excluding patients with no cerebral microbleeds on baseline neuroimaging.


