Abstract
Objective
Human T cell lymphotropic virus 1 (HTLV‐1)‐associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a chronic, progressive, neurological disease. Chronic activation of CD8+ T cells, as evidenced by increased spontaneous lymphoproliferation and HTLV‐1‐specific cytotoxic T cells, has been demonstrated in HAM/TSP patients. Since IL‐2 and IL‐15 stimulate memory CD8+ T cell activity, these cytokines have been implicated in the immunopathogenesis of HAM/TSP. In this phase I trial, we evaluated the safety, pharmacokinetics, and ability of Hu‐Mikβ1, a humanized monoclonal antibody directed toward the IL‐2/IL‐15 receptor β‐chain (IL‐2/IL‐15Rβ: CD122), to saturate CD122 and regulate abnormal immune responses in patients with HAM/TSP by inhibition of IL‐15 action.
Methods
Hu‐Mikβ1 was administered intravenously at doses of 0.5 mg/kg, 1.0 mg/kg, or 1.5 mg/kg in a total of nine HAM/TSP patients. Five doses of Hu‐Mikβ1 were administered at 3‐week intervals. The clinical response was evaluated using standardized scales. Viral and immunologic outcome measures were examined including HTLV‐1 proviral load, T cell phenotypic analysis and spontaneous lymphoproliferation in HAM/TSP patients.
Results
There was no significant toxicity associated with Hu‐Mikβ1 administration in HAM/TSP patients. Saturation of CD122 by Hu‐Mikβ1 was achieved in five out of nine HAM/TSP patients. Administration of Hu‐Mikβ1 was associated with inhibition of aberrant CD8+ T cell function including spontaneous lymphoproliferation and degranulation and IFN‐γ expression, especially in HAM/TSP patients that achieved CD122 saturation.
Interpretation
The treatment with Hu‐Mikβ1 had a number of immunological effects on HAM/TSP patients although no clinical efficacy was observed. We also did not see any dose‐related toxicity.
Introduction
Human T cell lymphotropic virus 1 (HTLV‐1)‐associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a chronic, progressive, neurological disease.1, 2 Currently, no therapy has been shown to significantly modify the long‐term disability associated with HAM/TSP.3 HTLV‐1 proviral DNA load (PVL) is significantly elevated in the peripheral blood and cerebrospinal fluid (CSF) of HAM/TSP patients and is strongly correlated with disease pathogenesis.4, 5 HAM/TSP is characterized by perivascular inflammatory infiltrates, predominantly CD8+ T cells, in chronic inflammatory lesions of the central nervous system, affecting the spinal cord in particular patients.6, 7 Notably, increased numbers of memory and/or effector CD8+ T cells and HTLV‐1 Tax‐specific cytotoxic CD8+ T cells were found in the peripheral blood, CSF and spinal cord of HAM/TSP patients.8, 9, 10 Chronic immune activation associated with HTLV‐1 infection has been suggested to underlie the pathogenesis of this disorder.
HTLV‐1 expresses a transcriptional trans‐activator protein, Tax, which induces the expression of a number of the common γ chain family of cytokines and their receptors, such as IL‐2/IL‐2R and IL‐15/IL‐15R.11, 12, 13 Both cytokines induce the proliferation and increase the cytolytic activity of NK and CD8+ T cells, and the receptors for IL‐2 and IL‐15 share the IL‐2Rβ and γ chains.13 IL‐2 and IL‐15 could also regulate the expression of inhibitory receptors, such as CD244 and Tim‐3, leading to regulation of CD8+ T cell differentiation and exhaustion.14 In particular, IL‐15 is critical for the development of NK cells and antigen‐specific memory CD8+ T cells and is well characterized for its role in maintaining memory pools of CD8+ T cells.13 HAM/TSP patients showed high frequency of CD4+CD25(IL‐2Rα)+ T cells which contain high HTLV‐1 PVL, express HTLV‐1 tax mRNA and induce various cytokines.15, 16 The treatment of HAM/TSP patients with anti‐Tac, a humanized monoclonal antibody to the IL‐2Rα, demonstrated several inhibitory effects on spontaneous lymphoproliferation and HTLV‐1 PVL in PBMCs.17 In addition to IL‐2, IL‐15 has been suggested to be involved in pathogenesis of HAM/TSP, potentially through upregulation of IL‐15.18, 19, 20 Since increased expressions of these critical immune mediators may directly contribute to cell activation and proliferation observed in HAM/TSP patients, IL‐15/IL‐15R autocrine loop might be a critical therapeutic target for HAM/TSP.
Hu‐Mikβ1 is a humanized monoclonal antibody to the β chain shared by the IL‐2 and IL‐15 receptors (IL‐2/IL‐15Rβ; CD122). Hu‐Mikβ1 was shown to block IL‐15 transpresentation and IL‐15‐induced cell proliferation and was evaluated in a phase I clinical trial in patients with T cell large granular lymphocyte leukemia.21 Previous ex vivo studies demonstrated that Hu‐Mikβ1 inhibited abnormal T cell proliferation and HTLV‐1‐specific cellular immune responses of HAM/TSP patients,19, 20, 22 suggesting that Hu‐Mikβ1 might contribute to blocking IL‐15 action in HAM/TSP patients. Based on its effects, we hypothesized that the administration of Hu‐Mikβ1 in HAM/TSP patients would lead to inhibition of abnormal T cell functions. HAM/TSP patients were intravenously treated with Hu‐Mikβ1 at doses of 0.5 mg/kg (group 1), 1.0 mg/kg (group 2), or 1.5 mg/kg (group 3) based on the previous clinical trials.21, 23 The primary goals of this phase I trial were to evaluate the safety, pharmacokinetics, and ability to saturate the IL‐2/IL‐15Rβ and regulate immune responses in HAM/TSP patients following the administration of Hu‐Mikβ1.
Methods
Patients and treatment plan
Nine patients with clinically defined HAM/TSP by the WHO criteria 24 were enrolled into the clinical trial of Hu‐Mikβ1 in HAM/TSP (NCT00076843). Hu‐Mikβ1 was administered intravenously at doses of 0.5 mg/kg (group 1), 1.0 mg/kg (group 2), or 1.5 mg/kg (group 3). Each patient received five doses of Hu‐Mikβ1, were administered at 3‐week intervals. PBMCs were isolated and cryopreserved in liquid nitrogen until use. CSF was obtained by lumbar puncture and the cells were collected by centrifugation of CSF samples. The study protocol (04‐N‐0071) was reviewed and approved by the National Institute of Neurological Disorders and the Stroke Institutional Review Board. Prior to study inclusion, written informed consent was obtained from all the participants in accordance with the Declaration of Helsinki. Details on this clinical trial are recorded at clinicaltrials.gov (NCT00076843).
Flow cytometry
For analysis of peripheral blood lymphocyte populations, EDTA‐treated whole blood of HAM/TSP patients and healthy volunteers (HVs) were stained with CD3, CD4, CD8, CD14, CD19, CD25, CD27, CD45, CD45RA, CD45RO, CD56, CD95, CD122 (clone Mikβ2 and Mikβ3), CD197 (CCR7), CD244 (all from BD Biosciences), CD279 (PD‐1; BioLegend), and Tim‐3 (R&D Biosystems). Mikβ1 and Mikβ3 identified noncompeting epitopes on the IL‐2/IL‐15Rβ, but Mikβ1 and Mikβ2 appeared to recognize the same epitope or very closely related epitopes on the β chain.21, 25 Tax 11‐19/HLA‐A201 tetramer was provided by National Institute of Allergy and Infectious Diseases Major Histocompatibility Complex Tetramer Core Facility. CMV pp65/HLA‐A201 tetramer (Beckman Coulter) was used as control. For detection of phosphorylated STAT5 (pSTAT5), EDTA‐treated whole blood were incubated for 15 min at 37°C and lysed with BD PhosflowTM Lyse/Fix buffer (BD Biosciences). After washing and permeabilization with cold 90% methanol on ice for 30 min, the cells were stained with antibodies for CD3, CD4, CD8, and pSTAT5 (all from BD Biosciences). CD107a mobilization assay was performed as previously described.20 All flow cytometric analyses were performed using a FACSCalibur or LSR II (both from BD Biosciences). The data were analyzed using FlowJo 10.2 software (FlowJo LLC).
Lymphoproliferation assay
Lymphoproliferation assay was performed as previously described.26 PBMCs were cultured in triplicate and pulsed after 3 to 5 days of culture with 1 μCi [3H] thymidine. The average cpm from each of the wells was plotted.
HTLV‐1 PVL
HTLV‐1 PVL was measured using ddPCR (Bio‐Rad) as previously described.27 Primers and probe specific to HTLV‐1 tax and human ribonuclease P protein subunit 30 were used.
Statistics
The Mann–Whitney test was used to compare: pSTAT5, PD‐1, CD244 and Tscm cells between HVs and HAM/TSP patients. Paired t test was used to compare: pSTAT5, PD‐1, CD244, and Tscm cells at pretreatment and at post‐Hu‐Mikβ1 treatment of HAM/TSP patients. All statistical analyses were performed using Prism (GraphPad software).
Results
Patients characteristics
Nine HAM/TSP patients were enrolled into this phase I clinical trial of Hu‐Mikβ1. All had a slowly progressive course of neurologic disease. The demographic characteristics of the study population are summarized in Table 1. Mean age of the study population was 52.8 years. The majority of patients were female and were predominantly African American. Mean disease duration of the study population was 11.6 years.
Table 1.
Demographics of HAM/TSP patients for HuMikβ1 trial.
| Group | Patient No. | Age (year) | Gender | Race/Ethnicity | Disease duration (year) |
|---|---|---|---|---|---|
| 1 | HAM #1 | 39 | F | Hispanic | 2 |
| (0.5 mg/kg) | HAM #2 | 60 | F | Hispanic | 23 |
| HAM #3 | 48 | F | Black/African American | 2 | |
| HAM #4 | 53 | F | Black/African American | 11 | |
| 2 | HAM #5 | 47 | F | Black/African American | 18 |
| (1.0 mg/kg) | HAM #6 | 62 | M | Black/African American | 17 |
| HAM #7 | 56 | M | Black/African American | 1 | |
| 3 | HAM #8 | 70 | M | White | 27 |
| (1.5 mg/kg) | HAM #9 | 40 | F | Black/African American | 3 |
Safety and clinical response of Hu‐Mikβ1
Adverse events in HAM/TSP patients during the trial are summarized in Table 2. Two subjects (dosed at 0.5 mg/kg) developed deep vein thrombosis (DVT) of the lower extremities after the protocol‐specified observation period (at three and three and one‐half months following the final administration of Hu‐Mikβ1). One subject (dosed at 1.0mg/kg) developed DVT around 2 months following the last administration of Hu‐Mikβ1. This was asymptomatic and was diagnosed by Doppler of the lower extremities as required per protocol. While this was reported as possibly related to the research, prolonged immobilization secondary to the underlying myelopathy was identified as a major risk factor for DVT in both subjects and was likely contributory. An independent data safety monitoring board concurred with these observations and recommended prophylactic anticoagulation therapy as well as excluding patients that were nonambulatory.
Table 2.
Adverse events occurring in HAM/TSP patients during HuMikβ1 trial.
| Group | Adverse event | Grade | Number of events |
|---|---|---|---|
| 0.5mg | pruritus | 1 | 1 |
| flu‐like syndrome | 1 | 1 | |
| hypotension | 1 | 1 | |
| hypokalemia | 1 | 1 | |
| tachycardia | 1 | 1 | |
| fever | 1 | 1 | |
| palpitation | 1 | 1 | |
| diarrhea | 1 | 1 | |
| infection | 2 | 1 | |
| decubitus ulcer | 2 | 1 | |
| limb edema | 2 | 1 | |
| substernal pain | 2 | 1 | |
| rash/desquamation 1 | 2 | 1 | |
| thrombosis 1 | 3 | 2 | |
| 1.0mg | headache, chills, fatigue | 1 | 1 |
| flu‐like symptoms | 1 | 2 | |
| paresthesias | 1 | 1 | |
| herpes labialis | 1 | 1 | |
| prolonged QTCB on EKG | 1 | 1 | |
| rash | 1 | 1 | |
| tooth abscess | 2 | 1 | |
| infection/UTI | 2 | 1 | |
| elevated ALT | 2 | 1 | |
| shoulder pain | 3 | 1 | |
| thrombosis | 3 | 1 | |
| 1.5 mg | bradycardia | 1 | 1 |
| low b12 | 1 | 1 | |
| elevated RSVP on echo | 1 | 1 | |
| contact dermatitis | 1 | 1 | |
| anemia | 1 | 1 | |
| vaginal spotting | 1 | 1 | |
| lymphopenia | 1 | 1 | |
| lymphopenia | 2 | 1 | |
| elevated troponin | 2 | 1 | |
| elevated troponin | 2 | 1 | |
| post dural puncture headache | 4 | 1 |
Post study
The clinical responses were evaluated using standardized scales including expanded disability status scale (EDSS), Scripps neurologic rating scale (SNRS), Timed 25‐Foot Walk (T25‐FW), ambulation index (AI), and Insituto de Pesquisa Clinica Evandro Chagas (IPEC). Clinical effects of Hu‐Mikβ1 in the study population are summarized in Table 3. For the all doses, there were no changes in the rate of clinical progression during the study period and no serious infusion toxicities. One patient (HAM#5, dosed at 1.0mg/kg) had a subjective improvement in bladder function (nocturia) at week 3 that was sustained throughout the study.
Table 3.
Clinical parameters of HAM/TSP patients at baseline and posttreatment of HuMikβ1.
| Group | Patient No. | Time course | EDSS | SNRS | T25‐FW | AI | IPEC |
|---|---|---|---|---|---|---|---|
| 1 | HAM #1 | Baseline | 6.5 | 75 | 11.2 | 3 | 16 |
| Week 12 | 6.5 | 55 | 27.5 | 6 | 18 | ||
| Week 18 | 6 | 64 | 16.8 | 4 | 18 | ||
| HAM #2 | Baseline | 6 | 72 | 10.2 | 4 | 13 | |
| Week 12 | 6 | 72 | 8.1 | 4 | 13 | ||
| Week 18 | 6 | 66 | 8.6 | 4 | 13 | ||
| HAM #3 | Baseline | 6.5 | 68 | 41.2 | 6 | 20 | |
| Week 12 | 6.5 | 64 | na | 6 | 21 | ||
| Week 18 | 7 | 65 | na | 7 | 22 | ||
| HAM #4 | Baseline | 7 | 73 | na | 7 | 24 | |
| Week 12 | 7.5 | 67 | na | 7 | 24 | ||
| Week 18 | 8 | 61 | na | 8 | 26 | ||
| 2 | HAM #5 | Baseline | 6.5 | 47 | 23.9 | 6 | 17 |
| Week 12 | 6.5 | 50 | 20.6 | 6 | 13 | ||
| Week 18 | 6.5 | 54 | 18.8 | 5 | 13 | ||
| HAM #6 | Baseline | 7 | 53 | na | 8 | 21 | |
| Week 12 | 7.5 | 51 | na | 8 | 20 | ||
| Week 18 | 7.5 | 51 | na | 8 | 19 | ||
| HAM #7 | Baseline | 8 | 55 | na | 8 | 24 | |
| Week 12 | 8 | 51 | na | 8 | 23 | ||
| Week 18 | 8 | 56 | na | 8 | 25 | ||
| 3 | HAM #8 | Baseline | 6.5 | 67 | 10.4 | 5 | 19 |
| Week 12 | 6.5 | 67 | 9.1 1 | 5 | 19 | ||
| Week 18 | 6.5 | 67 | 10.4 | 5 | 19 | ||
| HAM #9 | Baseline | 6 | 69 | 33.8 | 5 | 14 | |
| Week 12 | 6 | 69 | 39.7 | 5 | 14 | ||
| Week 18 | 6 | 69 | 33.8 | 5 | 14 |
EDSS, Expanded Disability Status Scale; SNRS, Scripps Neurologic Rating Scale; T25‐FW, Timed 25‐Foot Walk; AI, Ambulation Index; IPEC, Insituto de Pesquisa Clinica Evandro Chagas; na, not applicable.
At 9 weeks
Saturation of CD122 on NK cells of HAM/TSP patients
NK cells express high frequency of CD122. In the nine HAM/TSP patients, CD122 was highly expressed on NK cells (Mikβ2: 81.4–99.1%, Mikβ3: 84.07–99.2%). Mikβ2, shared epitopes with Hu‐Mikβ1, showed saturation of IL‐2/IL‐15Rβ on CD56+ NK cells of a HAM/TSP patient, but Mikβ3 which had noncompeting epitopes with Hu‐Mikβ1 did not (Fig. 1A). This result suggested that Hu‐Mikβ1 could block IL‐2/IL‐15Rβ on CD56+ NK cells but did not deplete CD56+ NK cells of a HAM/TSP patient. Group analysis of frequencies of Mikβ2+ CD56+ NK cells in HAM/TSP patients demonstrated that 25% of group 1 (one of four patients), 66.7% of group 2 (two of three patients), and 100% of group 3 patients (two of two patients) showed saturation of CD122 on CD56+ NK cells at week 12 of Hu‐Mikβ1 treatment (Fig. 1B). The five patients also showed more than 90% saturation of CD122 on CD56+ NK cells (Fig. 1C). These results demonstrated that Hu‐Mikβ1 could saturate IL‐2/IL‐15Rβ in HAM/TSP patients, which might be in a dose‐dependent manner. Consistent saturation of CD122 on CD56+ NK cells were achieved in HAM#5, #6, and #8 during the Hu‐Mikβ1 treatment phase of the trial and were maintained up to 3 weeks after the final dose but lost by 6 weeks after the final dose (Fig. 1D, left graph). HAM#1, #4, and #9 demonstrated delayed or partial saturation of CD122 on CD56+ NK cells during the Hu‐Mikβ1 treatment period (Fig. 1D, center graph). In the other patients (HAM#2, #3, and #7), any consistent saturation was not observed during the Hu‐Mikβ1 treatment phase of the trial (Fig. 1D, right graph). Since the three patients (HAM#2, #3, and #7) demonstrated a transient saturation of CD122 on CD56+ NK cells at week 1 of Hu‐Mikβ1 treatment, it was supposed that saturation of CD122 was not maintained up to 3 weeks after Hu‐Mikβ1 treatment (data not shown). These results demonstrated that consistent or partial saturation of IL‐2/IL‐15Rβ on CD56+ NK cells was achieved in most patients during the Hu‐Mikβ1 treatment phase of the trial.
Figure 1.
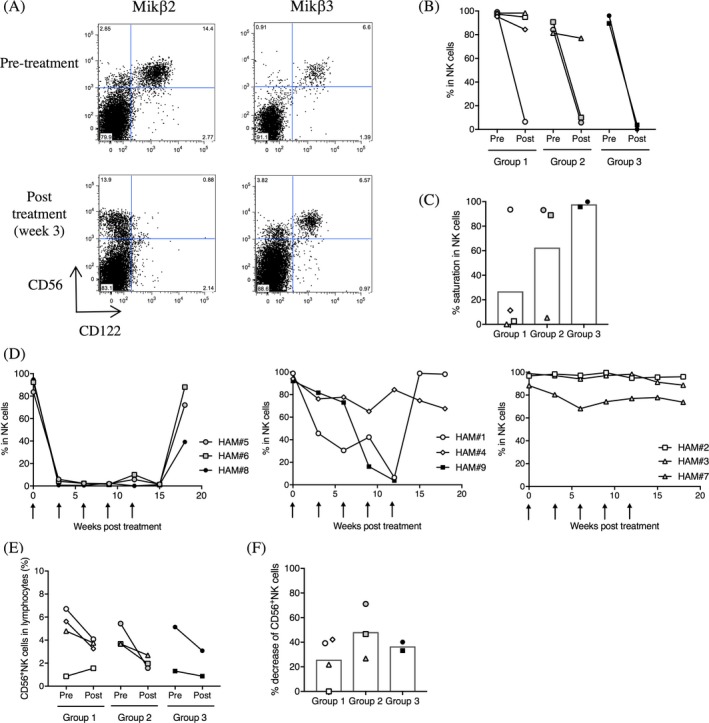
Saturation of CD122 on NK cells. (A) Representative dot plots of CD122 (Mikβ2 and Mikβ3) and CD56 expressions in CD3‐ lymphocytes of a HAM/TSP patient. (B) Frequencies of Mikβ2+ CD56+ NK cells in HAM/TSP patients at pretreatment (pre) and at week 12 of Hu‐Mikβ1 treatment (post). (C) Saturation of Mikβ2+ CD56+ NK cells in HAM/TSP patients (group 1, 2, and 3) at week 12 of Hu‐Mikβ1 treatment. Each bar graph represents the mean. (D) Change of frequencies of Mikβ2+ CD56+ NK cells in HAM/TSP patients (HAM#5, #6 and #8; left graph, HAM#1, #4, and #9; center graph, HAM#2, #3, and #7; right graph) during Hu‐MiKβ1 trial. Arrows indicate Hu‐Mikβ1 dosing. (E) Frequencies of CD56+ NK cells in HAM/TSP patients at pretreatment (pre) and at week 12 of Hu‐Mikβ1 treatment (post). (F) Decrease of CD56+ NK cells in HAM/TSP patients (group 1, 2, and 3) at week 12 of Hu‐Mikβ1 treatment. Each bar graph represents the mean
Since Hu‐Mikβ1 has been shown to block IL‐15‐induced cell proliferation 21, we asked whether administration of Hu‐Mikβ1 might affect frequency of NK cells in HAM/TSP patients. Group analysis showed that the frequencies of CD56+ NK cells were reduced in most HAM/TSP patients after Hu‐Mikβ1 administration (Fig. 1E and F). These results indicated that Hu‐Mikβ1 administration led to decreased frequency of circulating NK cells.
Saturation of CD122 in CD8+ T cells of HAM/TSP patients
In the nine HAM/TSP patients, CD122 was also detected on CD8+ T cells (Mikβ2: 16.03–55.1%, Mikβ3: 25.0–60.23%). Similar to NK cells, the loss of Mikβ2 binding, not Mikβ3, indicated saturation of CD122 on CD8+ T cells of a HAM/TSP patient following administration of Hu‐Mikβ1 (Fig. 2A). Group analysis of frequencies of Mikβ2+ CD8+ T cells in HAM/TSP patients demonstrated that 25% of group 1 (one of four patients), 66.7% of group 2 (two of three patients) and 100% of group 3 patients (two of two patients) showed more than 90% saturation of CD122 on CD8+ T cells at week 12 of Hu‐Mikβ1 treatment (Fig. 2B and C). Time course analysis indicated that HAM#1, #5, #6, and #8 sustained the consistent saturation of CD122 on CD8+ T cells during the Hu‐Mikβ1 treatment period followed by gradual loss of CD122 saturation on CD8+ T cells during the posttreatment period (Fig. 2D, left graph). In the other patients (HAM#2, #3, #4, #7, and #9), CD122 was partially or transiently saturated on CD8+ T cells (Fig. 2D, right graph). Notably, HAM/TSP patients without significant saturation of CD122 on NK cells also showed a partial saturation of CD122 on CD8+ T cells after Hu‐Mikβ1 administration (HAM#2 and #3). These results demonstrated that Hu‐Mikβ1 could also saturate IL‐2/IL‐15Rβ on CD8+ T cells of HAM/TSP patients.
Figure 2.
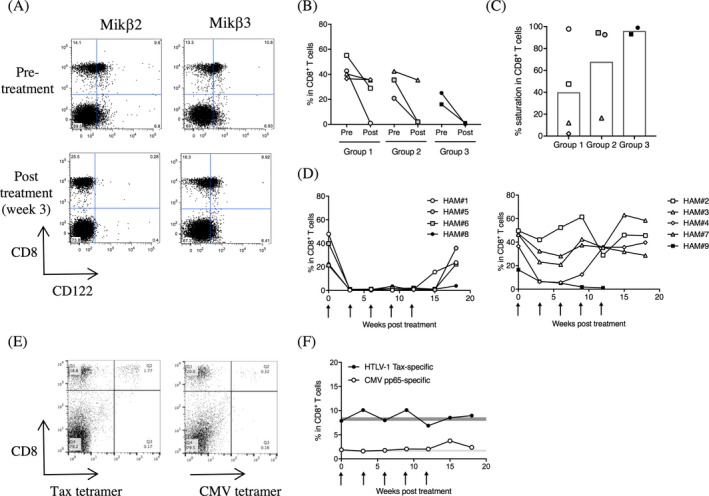
Saturation of CD122 on CD8+ T cells. (A) Representative dot plots of CD122 (Mikβ2 and Mikβ3) and CD8 expressions in CD3+ T lymphocytes of a HAM/TSP patient. (B) Frequencies of Mikβ2+ CD8+ T cells in HAM/TSP patients at pretreatment (pre) and at week 12 of Hu‐Mikβ1 treatment (post). (C) Saturation of Mikβ2+ CD8+ T cells in HAM/TSP patients at week 12 of Hu‐Mikβ1 treatment. Each bar graph represents the mean. (D) Change of frequencies of Mikβ2+ CD8+ T cells in HAM/TSP patients (HAM#1, #5, #6, and #8; left graph, HAM#2, #3, #4, #7, and #9; right graph) during Hu‐Mikβ1 trial. Arrows indicate Hu‐Mikβ1 dosing. (E) Tax11‐19‐ and CMV pp65‐specific CD8+ T cells in a HAM/TSP patient during Hu‐Mikβ1 trial. Representative dot plots of Tax11‐19‐ and CMV pp65‐tetramer staining in CD3+ lymphocytes of a HAM/TSP patient. (F) Frequencies of Tax11‐19‐ and CMV pp65‐specific CD8+ T cells in a HAM/TSP patient during Hu‐Mikβ1 trial. Arrows indicate Hu‐Mikβ1 dosing
Relatively high frequency of HTLV‐1 Tax‐specific CD8+ T cells are detected in the peripheral blood of HAM/TSP patients.10 One HAM/TSP patient (HAM#3, Group 1) expressed the HLA‐A201 allele, allowing for the detection of HTLV‐1‐specific and CMV‐specific CD8+ T cells was able to detect in the peripheral blood using Tax11‐19/HLA‐A201 and CMV pp65/HLA‐A201 tetramers, respectively (Fig. 2E). This allowed for reliable monitoring of changes to the frequencies of HTLV‐1‐ and CMV‐specific CD8+ T cells during Hu‐Mikβ1 treatment. At baseline, 7.88% of CD8+ T cells were HTLV‐1 Tax‐specific and 1.88% of CD8+ T cells were CMV pp65‐specific. The frequencies of both virus‐specific CD8+ T cells did not change significantly over the treatment period (Fig. 2F). Of note, the patient (HAM#3) showed only a partial saturation of CD122 on CD8+ T cells (Fig. 2D, right graph).
Inhibitory effects of Hu‐Mikβ1 on T cell function of HAM/TSP patients
Spontaneous lymphoproliferation is a well‐established measure of ex vivo T cell activation for HTLV‐1‐infected subjects.28 Partial inhibition of spontaneous lymphoproliferation was detected in the HAM/TSP patients at week 9 and/or week 15 of Hu‐Mikβ1 treatment (Fig. 3A). In particular, patients that achieved full saturation of CD122 during the Hu‐Mikβ1 treatment period (e.g., HAM#6, #8, and #9) demonstrated greater than 50% inhibition of spontaneous proliferation at week 9 or week 15 of Hu‐Mikβ1 treatment (Fig. 3A).
Figure 3.
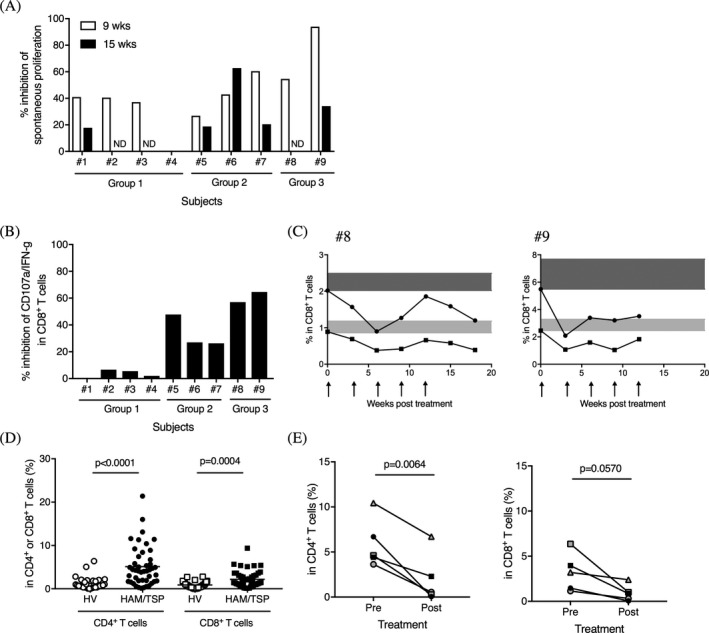
Inhibitory effects of Hu‐Mikβ1 on T cell function in HAM/TSP patients. (A) Inhibition of spontaneous lymphoproliferation in HAM/TSP patients at week 9 and week 15 of Hu‐Mikβ1 treatment. ND; not done. (B) Inhibition of CD107a and IFN‐γ expression in CD8+ T cells of HAM/TSP patients at week 9 of Hu‐Mikβ1 treatment. (C) Inhibitory effects of Hu‐Mikβ1 on CD107a and IFN‐γ expression in CD8+ T cells of HAM/TSP patients (#8 and #9) during Hu‐Mikβ1 trial. Arrows indicate Hu‐Mikβ1 dosing. The baselines of CD107a expression (closed circles) and both CD107a and IFN‐γ expression (closed squires) in PBMC CD8+ T cells of each HAM/TSP patients are highlighted in dark gray and light gray, respectively (D) Comparisons of pSTAT5 in CD4+ and CD8+ T cells of HVs and HAM/TSP patients. The horizontal line represents the mean. (E) Frequencies of pSTAT5 in CD4+ T cells (left) and in CD8+ T cells (right) of HAM/TSP patients (#5‐#9) at pretreatment (pre) and at week 9 of Hu‐Mikβ1 treatment (post)
Spontaneous degranulation and IFN‐γ expression has been reported to be increased in CD8+ T cells of HAM/TSP patients and inhibited by Hu‐Mikβ1 in ex vivo PBMC culture of HAM/TSP patients.20 HAM/TSP patients in group 3 showed 57.0–64.6% inhibition of spontaneous degranulation and IFN‐γ expression in CD8+ T cells at week 9 of Hu‐Mikβ1 treatment (Fig. 3B) and varying degrees of inhibition was sustained over the treatment period (Fig. 3C). HAM/TSP patients in the lower dose groups (group 1 and 2) showed less inhibitory effects of Hu‐Mikβ1 on spontaneous degranulation and IFN‐γ expression in CD8+ T cells (Fig. 3B). These results demonstrated that the administration of Hu‐Mikβ1 led to inhibition of CD8+ T cell function in the HAM/TSP patients in a partly dose dependent manner.
It has been reported that PBMCs from HAM/TSP patients showed increased STAT5 activation, as indicated by STAT5 phosphorylation, in short‐term (20 h) culture, which was partially inhibited by Hu‐Mikβ1.22 In the current study, we examined pSTAT5 in CD4+ and CD8+ T cells of HVs and HAM/TSP patients using fresh whole blood. As shown in Figure 3D, HAM/TSP patients showed significantly increased pSTAT5 in both CD4+ and CD8+ T cells of peripheral blood compared to HV. When we compared the frequencies of pSTAT5 in CD4+ and CD8+ T cells at pretreatment and at week 9 of Hu‐Mikβ1 treatment in the higher dose groups (group 2 and 3), HAM/TSP patients showed significant decreases of pSTAT5 in CD4+ T cells of peripheral blood (Fig. 3E, left) following Hu‐Mikβ1 treatment. In CD8+ T cells, pSTAT5 was decreased after Hu‐Mikβ1 treatment and approached significance (Fig. 3E, right).
Alternative effects of Hu‐Mikβ1 on CD8+ T cell subsets of HAM/TSP patients
Given the inhibitory effects of Hu‐Mikβ1 on T cell function, we asked whether Hu‐Mikβ1 administration also modulated additional aspects of CD8+ T cell function. In HAM/TSP patients, alternative expressions of various inhibitory receptors, such as PD‐1, CD244, and Tim‐3, have been demonstrated on CD8+ T cells.29, 30, 31, 32 To determine whether the cells expressing the inhibitory receptors (PD‐1, CD244, and Tim‐3) were affected by Hu‐Mikβ1 administration, we examined the frequencies of PD‐1+, CD244+, and Tim‐3+ cells in CD8+ T cells of HVs and HAM/TSP patients and compared their frequencies in HAM/TSP patients (group 2 and 3) at pretreatment and at week 9 of Hu‐Mikβ1 treatment. Compared to HVs, HAM/TSP patients showed higher frequencies of PD‐1+ and CD244+ cells, but not Tim‐3+ cells, in CD8+ T cells (Fig 4A–C). Group analysis of HAM/TSP patients demonstrated significant decrease in PD‐1+ cells in CD8+ T cells at week 9 of Hu‐Mikβ1 treatment (Fig 4A). Although there were no significant changes of CD244+ and Tim‐3+ cells in CD8+ T cells of HAM/TSP patients during Hu‐Mikβ1 treatment, some HAM/TSP patients showed decrease in CD244+CD8+ T cells (HAM#5) and Tim‐3+ CD8+ T cells (HAM#6 and 9; Fig 4B and C).
Figure 4.
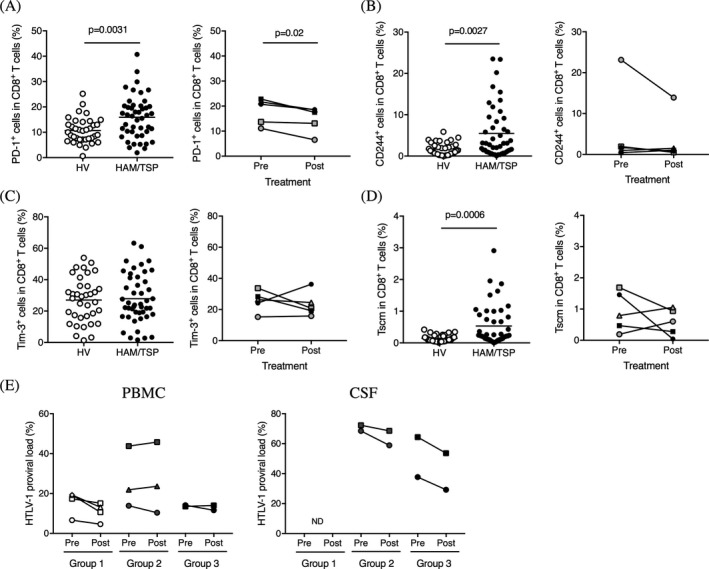
Alternative changes in HAM/TSP patients. (A) Comparison of frequency of PD‐1+ cells in CD8+ T cells of HVs and HAM/TSP patients (left graph). The horizontal line represents the mean. Comparison of frequency of PD‐1+ cells in CD8+ T cells of HAM/TSP patients at pretreatment (pre) and at week 9 of Hu‐Mikβ1 treatment (post) (right graph). (B) Comparison of frequency of CD244+ cells in CD8+ T cells of HVs and HAM/TSP patients (left graph). The horizontal line represents the mean. Comparison of frequency of CD244+ cells in CD8+ T cells of HAM/TSP patients at pretreatment (pre) and at week 9 of Hu‐Mikβ1 treatment (post) (right graph). (C) Comparison of frequency of Tim‐3+ cells in CD8+ T cells of HVs and HAM/TSP patients (left graph). The horizontal line represents the mean. Comparison of frequency of Tim‐3+ cells in CD8+ T cells of HAM/TSP patients at pretreatment (pre) and at week 9 of Hu‐Mikβ1 treatment (post) (right graph). (D) Comparison of frequency of Tscm cells in CD8+ T cells of HVs and HAM/TSP patients (left graph). The horizontal line represents the mean. Comparison of frequency of Tscm cells in CD8+ T cells of HAM/TSP patients at pretreatment (pre) and at week 12 of Hu‐Mikβ1 treatment (post) (right graph). (E) Comparison of HTLV‐1 proviral load in PBMC (left graph) and CSF (right graph) of HAM/TSP patients at pretreatment (pre) and at week 12 of Hu‐Mikβ1 treatment (post)
A new subset of human memory CD8+ T cells, stem cell‐like memory T cells (Tscm) cells, has been reported to be identified based on expression of CD122 and CD95 in naïve phenotypes and constituted a long‐lived, self‐renewing lymphocyte population essential for the maintenance of functional immunity, which might be associated with infectious diseases and autoimmune diseases.33, 34, 35 Compared to HVs, the frequency of Tscm was significantly increased in HAM/TSP patients (Fig. 4D). Although there was no significant difference of Tscm frequency in CD8+ T cells of HAM/TSP patients at pretreatment and at week 12 of Hu‐Mikβ1 treatment by group analysis, two HAM/TSP patients (HAM#6 and #8) showed decrease in Tscm in CD8+ T cells during Hu‐Mikβ1 treatment (Fig. 4D).
HTLV‐1 PVL in HAM/TSP patient during Hu‐Mikβ1 trial
We also analyzed HTLV‐1 PVL in PBMCs of HAM/TSP patients during the trial. HTLV‐1 PVL of the HAM/TSP patients was detected at 6.63–43.77% in PBMC before the treatment and the reduction of HTLV‐1 PVL at week 12 of Hu‐Mikβ1 treatment was 0–44.8% in PBMC of the patients (Fig. 4E, left graph). Of nine HAM/TSP patients, we were able to analyze HTLV‐1 PVL in CSF cells of four HAM/TSP patients (HAM#5, #6, #8, and #9) at pretreatment and at week 12 of Hu‐Mikβ1 treatment (Fig. 4E, right graph). HTLV‐1 PVL was detected at 37.69–72.33% in the CSF cells at pretreatment which was much higher than the PVL in the PBMCs of each patient. At week 12 of Hu‐Mikβ1 treatment, HTLV‐1 PVL in the CSF cells was trending to decrease (5.2–22.4% reduction) but remained higher than that in the PBMCs of the patients (Fig. 4E, right graph). These results suggested that Hu‐Mikβ1 did not directly alter HTLV‐1 PVL in HAM/TSP patients.
Discussion
Activation and dysregulation of CD8+ T cells have been implicated in disease progression and pathogenesis of HAM/TSP. In this study, treatment with a humanized monoclonal antibody to the IL‐2/IL‐15Rβ, Hu‐Mikβ1, had several effects on HAM/TSP patients. First, no patients showed any dose‐related toxicity and manifested progressive disease during the period of therapy. Two subjects (dosed at 0.5 mg/kg) and one subject (dosed at 1.0mg/kg) developed DVT after the protocol‐specified observation period. Although it was not clear whether it was directly related to the treatment, prophylactic anticoagulation therapy was recommended as well as excluding patients that were nonambulatory.
Hu‐Mikβ1 administered at 3‐week intervals achieved greater than 90% saturation of CD122 on NK cells and CD8+ T cells in five of nine HAM/TSP patients. Saturation of CD122 by Hu‐Mikβ1 was more consistently observed among patients receiving the higher doses of Hu‐Mikβ1. The results would be consistent with the first phase I clinical trial of Hu‐Mikβ1 in patients with T cell large granular lymphocyte leukemia.21 Among the five HAM/TSP patients that demonstrated CD122 saturation, three patients demonstrated sustained saturation of CD122 on NK cells and CD8+ T cells during the Hu‐Mikβ1 treatment period and then lost saturation of CD122 by 6 weeks after the final dose. Importantly, the higher doses of Hu‐Mikβ1 administration provided more inhibitory effects on activated T cell functions of HAM/TSP patients, which are the characteristic features of T cell dysregulation in HAM/TSP patients.19, 20, 22, 28 In addition, HAM/TSP patients with longer disease duration (such as HAM#5, #6, and #8) seemed to show more effects of Hu‐Mikβ1 on CD122 saturation and inhibition of activated CD8+ T cells compared to HAM/TSP patients with shorter disease duration (such as HAM#7), which might be related to chronic activation and/or expansion of CD8+ T cells in the patients. It has been previously reported that Hu‐Mikβ1 was able to inhibit the activated T cell functions in ex vivo experiments using PBMCs of HAM/TSP patients.19, 20, 22 In the current study, our results strongly supported the inhibitory effects of Hu‐Mikβ1 on activated T cell functions of HAM/TSP patients in vivo, suggesting that Hu‐Mikβ1 would effectively inhibit IL‐15‐driven T cell dysfunction of HAM/TSP patients when IL‐2/IL‐15Rβ saturation is achieved.
Chronic viral infection has been reported to induce expression of inhibitory molecules that generate negative signals to downregulate the ensuing T cell responses. Expression of multiple distinct inhibitory receptors is associated with greater T cell exhaustion and rapid disease progression.36 In our cohort, higher frequencies of PD‐1+ cells and CD244+ cells, but not Tim‐3, were detected in CD8+ T cells of HAM/TSP patients compared to HVs. During Hu‐Mikβ1 treatment, PD‐1+ cells were significantly decreased in CD8+ T cells of HAM/TSP patients. We also demonstrated that higher frequency of Tscm cells was detected in CD8+ T cells of HAM/TSP patients compared to HVs. Tscm cells have similar functions to memory T cells including the ability to proliferate rapidly and release inflammatory cytokines in response to antigen reexposure, and a dependence on IL‐15 and IL‐7 for homeostatic turnover.33, 34 To achieve long‐lived protection against chronic HTLV‐1 infection, an adequate number of functionally competent memory CD8+ T cells might be sustained through cytokine‐driven homeostatic proliferation. Recently, it has been reported that frequency of CD8+ Tscm cells was increased in patients with acquired aplastic anemia and uveitis, immune‐mediated diseases associated with autoreactive cytotoxic CD8+ T cells.35, 37 Intriguingly, Tscm cells in CD4+ T cells have been able to sustain themselves through a process of self‐renewal and to reconstitute the identical adult T cell leukemia clones.38 Although there were no significant changes in the frequency of CD244+CD8+ T cells and CD8+ Tscm cells after Hu‐Mikβ1 administration, it is of interest that Hu‐Mikβ1 treatment might be able to modulate CD8+ T cell differentiation and exhaustion in some HAM/TSP patients.
HTLV‐1 PVL in the PBMCs did not change as a result of Hu‐Mikβ1 treatment whereas HTLV‐1 PVL was decreased after treatment with anti‐Tac in a previous clinical trial.17 HTLV‐1 infects mainly CD25+CCR4+CD4+ T cells and induces functional changes in the infected cells.15, 39, 40 A recent report demonstrated that a humanized anti‐CCR4 monoclonal antibody decreased the number of HTLV‐1‐infected cells and the level of inflammatory markers.41 While both anti‐Tac and anti‐CCR4 mainly targeted to CD4+ T cells, Hu‐Mikβ1 might have less efficiency on CD4+ T cells since the frequency of CD122+ cells in CD4+ T cells was much lower than that in CD8+ T cells. Our results suggest that Hu‐Mikβ1 may not directly targeted to HTLV‐1 and HTLV‐1‐infected lymphocytes, but instead modulate T cell dysfunction that characterize HAM/TSP. Combining antiviral therapy with immunotherapies that inhibit T cell dysfunctions might be required to maximize the longevity and effective responses in patients with chronic virus‐associated neuroinflammatory disease.
Author Contribution
YE‐A, UO, JO, TAW, SJ design the study and contributed to discussion and paper writing. JO, BJB, RM, UO, IC, TAW coordinated clinical work and patient care. YE‐A, AV, NN, and BRB performed the experimental works. YE‐A performed statistical analysis. TAW and SJ supervised the project.
Conflict of Interest
The authors have declared that no conflict of interest exists.
Acknowledgments
This research was supported by the Intramural Research Program of the NINDS, National Institutes of Health (NIH). The support of the NIH inpatient and outpatient neurology staff is acknowledged.
Funding Information
This research was supported by the Intramural Research Program of the NINDS, National Institutes of Health (NIH).
Funding Statement
This work was funded by NINDS grant ; National Institutes of Health (NIH) grant .
Contributor Information
Thomas A. Waldmann, Email: tawald@helix.nih.gov
Steven Jacobson, Email: jacobsons@ninds.nih.gov.
References
- 1. Gessain A, Barin F, Vernant JC, et al. Antibodies to human T‐lymphotropic virus type‐I in patients with tropical spastic paraparesis. Lancet 1985;2:407–410. [DOI] [PubMed] [Google Scholar]
- 2. Osame M, Usuku K, Izumo S, et al. HTLV‐I associated myelopathy, a new clinical entity. Lancet 1986;1:1031–1032. [DOI] [PubMed] [Google Scholar]
- 3. Oh U, Jacobson S. Treatment of HTLV‐I‐associated myelopathy/tropical spastic paraparesis: toward rational targeted therapy. Neurol Clin 2008;26(3):781–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV‐I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV‐I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol 1998;4(6):586–593. [DOI] [PubMed] [Google Scholar]
- 5. Nagai M, Yamano Y, Brennan MB, et al. Increased HTLV‐I proviral load and preferential expansion of HTLV‐I Tax‐specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann Neurol 2001;50:807–812. [DOI] [PubMed] [Google Scholar]
- 6. Aye MM, Matsuoka E, Moritoyo T, et al. Histopathological analysis of four autopsy cases of HTLV‐I‐associated myelopathy/tropical spastic paraparesis: inflammatory changes occur simultaneously in the entire central nervous system. Acta Neuropathol. 2000;100:245–252. [DOI] [PubMed] [Google Scholar]
- 7. Umehara F, Izumo S, Nakagawa M, et al. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV‐I‐associated myelopathy. J Neuropathol Exp Neurol 1993;52:424–430. [DOI] [PubMed] [Google Scholar]
- 8. Jacobson S, Shida H, McFarlin DE, et al. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV‐I pX in patients with HTLV‐I associated neurological disease. Nature 1990;348:245–248. [DOI] [PubMed] [Google Scholar]
- 9. Matsuura E, Kubota R, Tanaka Y, et al. Visualization of HTLV‐1‐specific cytotoxic T lymphocytes in the spinal cords of patients with HTLV‐1‐associated myelopathy/tropical spastic paraparesis. J Neuropathol Exp Neurol 2015;74(1):2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nagai M, Kubota R, Greten TF, et al. Increased activated human T cell lymphotropic virus type I (HTLV‐I) Tax11‐19‐specific memory and effector CD8+ cells in patients with HTLV‐I‐associated myelopathy/tropical spastic paraparesis: correlation with HTLV‐I provirus load. J Infect Dis 2001;183(2):197–205. [DOI] [PubMed] [Google Scholar]
- 11. Azimi N, Brown K, Bamford RN, et al. Human T cell lymphotropic virus type I Tax protein trans‐activates interleukin 15 gene transcription through an NF‐kappaB site. Proc Natl Acad Sci USA 1998;95(5):2452–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mariner JM, Lantz V, Waldmann TA, Azimi N. Human T cell lymphotropic virus type I Tax activates IL‐15R alpha gene expression through an NF‐kappa B site. J Immunol 2001;166:2602–2609. [DOI] [PubMed] [Google Scholar]
- 13. Waldmann TA. The biology of interleukin‐2 and interleukin‐15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006;6:595–601. [DOI] [PubMed] [Google Scholar]
- 14. Beltra JC, Bourbonnais S, Bedard N, et al. IL2Rbeta‐dependent signals drive terminal exhaustion and suppress memory development during chronic viral infection. Proc Natl Acad Sci USA 2016;113:E5444–E5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamano Y, Araya N, Sato T, et al. Abnormally high levels of virus‐infected IFN‐gamma+ CCR15+ CD4+ CD25+ T cells in a retrovirus‐associated neuroinflammatory disorder. PLoS ONE 2009;4:e6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamano Y, Cohen CJ, Takenouchi N, et al. Increased expression of human T lymphocyte virus type I (HTLV‐I) Tax11‐19 peptide‐human histocompatibility leukocyte antigen A*201 complexes on CD4+ CD25+ T Cells detected by peptide‐specific, major histocompatibility complex‐restricted antibodies in patients with HTLV‐I‐associated neurologic disease. J Exp Med 2004;199:1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehky TJ, Levin MC, Kubota R, et al. Reduction in HTLV‐I proviral load and spontaneous lymphoproliferation in HTLV‐I‐associated myelopathy/tropical spastic paraparesis patients treated with humanized anti‐Tac. Ann Neurol 1998;44:942–947. [DOI] [PubMed] [Google Scholar]
- 18. Ahuja J, Kampani K, Datta S, et al. Use of human antigen presenting cell gene array profiling to examine the effect of human T‐cell leukemia virus type 1 Tax on primary human dendritic cells. J Neurovirol 2006;12:47–59. [DOI] [PubMed] [Google Scholar]
- 19. Azimi N, Jacobson S, Leist T, Waldmann TA. Involvement of IL‐15 in the pathogenesis of human T lymphotropic virus type I‐associated myelopathy/tropical spastic paraparesis: implications for therapy with a monoclonal antibody directed to the IL‐2/15R beta receptor. J Immunol. 1999;163:4064–4072. [PubMed] [Google Scholar]
- 20. Enose‐Akahata Y, Oh U, Grant C, Jacobson S. Retrovirally induced CTL degranulation mediated by IL‐15 expression and infection of mononuclear phagocytes in patients with HTLV‐I‐associated neurologic disease. Blood 2008;112:2400–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waldmann TA, Conlon KC, Stewart DM, et al. Phase 1 trial of IL‐15 trans presentation blockade using humanized Mikbeta1 mAb in patients with T‐cell large granular lymphocytic leukemia. Blood 2013;121:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oh U, McCormick MJ, Datta D, et al. Inhibition of immune activation by a novel nuclear factor‐kappa B inhibitor in HTLV‐I‐associated neurologic disease. Blood 2011;117:3363–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris JC, Janik JE, White JD, et al. Preclinical and phase I clinical trial of blockade of IL‐15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc Natl Acad Sci USA 2006;103:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osame M. Review of Who kagoshima meeting and diagnostic guidelines for Ham/Tsp In:Blattner W, eds. Human Retrovirology: Htlv. 191–197. New York: Raven Press, 1990. [Google Scholar]
- 25. Tsudo M, Kitamura F, Miyasaka M. Characterization of the interleukin 2 receptor beta chain using three distinct monoclonal antibodies. Proc Natl Acad Sci USA 1989;86:1982–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oh U, Yamano Y, Mora CA, et al. Interferon‐beta1a therapy in human T‐lymphotropic virus type I‐associated neurologic disease. Ann Neurol 2005;57:526–534. [DOI] [PubMed] [Google Scholar]
- 27. Brunetto GS, Massoud R, Leibovitch EC, et al. Digital droplet PCR (ddPCR) for the precise quantification of human T‐lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations. J Neurovirol 2014;20:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Itoyama Y, Minato S, Kira J, et al. Spontaneous proliferation of peripheral blood lymphocytes increased in patients with HTLV‐I‐associated myelopathy. Neurology 1988;38:1302–1307. [DOI] [PubMed] [Google Scholar]
- 29. Abdelbary NH, Abdullah HM, Matsuzaki T, et al. Reduced Tim‐3 expression on human T‐lymphotropic virus type I (HTLV‐I) Tax‐specific cytotoxic T lymphocytes in HTLV‐I infection. J Infect Dis 2011;203:948–959. [DOI] [PubMed] [Google Scholar]
- 30. Enose‐Akahata Y, Matsuura E, Oh U, Jacobson S. High expression of CD244 and SAP regulated CD8 T cell responses of patients with HTLV‐I associated neurologic disease. PLoS Pathog 2009;5:e1000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kozako T, Yoshimitsu M, Akimoto M, et al. Programmed death‐1 (PD‐1)/PD‐1 ligand pathway‐mediated immune responses against human T‐lymphotropic virus type 1 (HTLV‐1) in HTLV‐1‐associated myelopathy/tropical spastic paraparesis and carriers with autoimmune disorders. Hum Immunol 2011;72:1001–1006. [DOI] [PubMed] [Google Scholar]
- 32. Manuel SL, Sehgal M, Connolly J, et al. Lack of recall response to Tax in ATL and HAM/TSP patients but not in asymptomatic carriers of human T‐cell leukemia virus type 1. J Clin Immunol 2013;33:1223–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cieri N, Camisa B, Cocchiarella F, et al. IL‐7 and IL‐15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013;121:573–584. [DOI] [PubMed] [Google Scholar]
- 34. Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell‐like properties. Nat Med 2011;17:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med 2017;23:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Attanasio J, Wherry EJ. Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity 2016;44:1052–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hosokawa K, Muranski P, Feng X, et al. Memory stem T cells in autoimmune disease: high frequency of circulating CD8+ memory stem cells in acquired aplastic anemia. J Immunol 2016;196:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagai Y, Kawahara M, Hishizawa M, et al. T memory stem cells are the hierarchical apex of adult T‐cell leukemia. Blood 2015;125:3527–3535. [DOI] [PubMed] [Google Scholar]
- 39. Araya N, Sato T, Ando H, et al. HTLV‐1 induces a Th1‐like state in CD4+CCR39+ T cells. J Clin Invest 2014;124:3431–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamano Y, Takenouchi N, Li HC, et al. Virus‐induced dysfunction of CD4+CD25+ T cells in patients with HTLV‐I‐associated neuroimmunological disease. J Clin Invest 2005;115:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato T, Coler‐Reilly ALG, Yagishita N, et al. Mogamulizumab (Anti‐CCR41) in HTLV‐1‐Associated Myelopathy. N Engl J Med 2018;378:529–538. [DOI] [PubMed] [Google Scholar]


